Abstract
Purpose:
High-risk oropharyngeal squamous cell carcinoma (OPSCC) associated with tobacco exposure remains difficult to treat due to high rates of locoregional recurrence similar to oral cavity squamous cell carcinoma (OCSCC). Current NCCN guidelines allow for surgical management of this disease, but oncologic and functional data in the modern era remain scarce. We sought to compare and contrast oncologic and functional considerations for surgical management of OPSCC and OCSCC in a cohort of Veterans.
Materials and methods:
We conducted a retrospective review of patients treated at the Michael E. DeBakey Veterans Affairs Medical Center between 2017 and 2020, treated using a homogenous, multi-modality algorithm.
Results:
OPSCC tumors presented with a higher rate of perineural invasion (p < 0.05) and extranodal extension (p = 0.02) compared to OCSCC tumors. Compliance with NCCN guidelines for adjuvant treatment were lower for OPSCC patients primarily due to a higher rate of previous irradiation; re-irradiation could be delivered in 75% of patients when recommended by NCCN guidelines. Total glossectomy was accompanied by concomitant total laryngectomy in 100% of OPSCC patients and 0% of OCSCC.
Conclusion:
Surgical resection and free flap reconstruction of high-risk OPSCC generates oncologic outcomes comparable to OCSCC with comparable complication rates but a lower overall functional status. Reconstruction focused on rapid healing allows for high-rates of re-irradiation and minimal treatment delays.
Level of evidence: level 4.
Keywords: Veteran, Oropharynx, Total glossectomy, Re-irradiation, Oral cavity, Free flap reconstruction
1. Introduction
Four decades ago, oral cavity squamous cell carcinoma (OCSCC) and oropharynx squamous cell carcinoma (OPSCC) were generally considered similar diseases, in large part due to an aggressive biological behavior and limited response to radiation-based treatment [1]. Treatment patterns for the two diseases slowly diverged over time, with increased utilization of radiation-based treatment for OPSCC, mainly due to significant post-operative morbidity during an era that preceded the now established utility of microvascular free tissue transfer (MVFTT) [1]. The increased incidence of low-risk, human papillomavirus (HPV) mediated OPSCC accelerated the trend toward non-surgical treatment for this site while OCSCC remained primarily a surgically managed disease in the United States [2–7].
Despite the rapid migration of OPSCC toward a low-risk, HPV-mediated disease, there remains a significant fraction of OPSCC cancers which are associated with extensive tobacco exposure, most often (but not exclusively) in the absence of HPV [8–11]. Management of these cancers remains problematic, since response to conventional chemo-radiation regimens remains suboptimal and minimally invasive surgical approaches (i.e. transoral robotic surgery) are not compatible with the high T-classification that accompanies presentation of most of these cancers [8–10].
Veterans in the United States maintain a high risk of both OCSCC and OPSCC development due to persistently high rates of tobacco exposure which have remained unchanged in recent years [8,9]. As shown by us and others, Veterans often present with HPV negative, advanced T- and N-classification disease, in the presence of tobacco exposure and extensive comorbidities [8,9]. This generates a significant clinical challenge. The advanced stage of the disease and the lack of HPV impact dictates the need for multi-modality treatment delivery with minimal interruptions
Over the last 3 years, our multi-disciplinary treatment team has adopted a homogeneous approach to management of advanced stage OCSCC and high-risk OPSCC. This approach consists of up-front surgical resection followed by adjuvant (chemo)radiation in a manner consistent with NCCN guidelines. Critical to this approach has been the incorporation of MVFTT techniques tailored to achieve maximal wound healing and functional gains, while facilitating the delivery of adjuvant treatment in a timely fashion. We hypothesized that a homogeneous approach to these two similar disease sites would generate similar survival characteristics and functional deficits. Development of a surgical approach for advanced stage disease was also hypothesized to facilitate multi-modality treatment delivery for recurrent disease which has historically proven difficult, particularly in the context of prior irradiation.
2. Materials and methods
Subsequent to receiving approval from Baylor College of Medicine and the Michael E. Debakey Veteran’s Administration (MEDVAMC) Institutional Review Boards, we reviewed the records of Veterans with oral cavity and high-risk oropharyngeal squamous cell carcinoma undergoing surgical resection between January 1, 2017 and January 1, 2020. High-risk oropharyngeal squamous cell carcinoma was defined as p16-negative tumors or p16-positive tumors in patients with a history of prior radiation to the oropharynx. All collection and analysis of the current data was performed in a manner consistent with existing standards for clinical research (Declaration of Helsinki, US Federal Policy for the Protection of Human Subjects). All patients were diagnosed and completed treatment at our institution. Demographic information was recorded including age, gender, race (self-identified), smoking history and alcohol consumption. Clinical-pathologic features were collected including clinical stage according to the American Joint Commission on Cancer (Staging Manual 7th and 8th Editions) staging system.
Functional data (tracheostomy status, diet) were gathered as well as modified barium swallow (MBS) study reports including DIGEST scores, when available. Maximal functional status was defined as the patient’s best functional status at any point post-operatively in terms of tracheostomy status, gastrostomy status and oral intake. Maximal functional status is more informative of the reconstructive outcome since function typically declines if recurrent disease develops. Recurrence and survival were measured from the time of diagnosis. Chi squared analysis was used for categorical variables and t-test or ANOVA analysis was performed for continuous variables. Kaplan-Meier curves were generated for comparison of oncologic outcomes. A p-value of <0.05 was considered significant and all statistical analyses were performed using IBM SPSS Statistics Version 25 (IBM, Armonk, NY).
3. Results
3.1. Patient characteristics
A total of 39 patients were included in the analysis (22 with OCSCC and 17 with OPSCC, (Table 1). All but one reconstruction consisted of MVFTT (submental island flap). The median age of patients was 67.5 (50–75) and 63 (54–79) years for OCSCC and OPSCC, respectively (p = 0.51). All patients were male except for 1 OCSCC (5%) and 1 OPSCC (6%) patient (p = 0.85). The racial demographics were similar for both groups (82% white and 18% black for OCSCC patients; 71% white and 29% black for OPSCC patients; p = 0.41). Both groups had a high rate of tobacco use, 96% and 94%, for OCSCC and OPSCC, respectively (p = 0.85). Median pack-years reported for OCSCC and OPSCC patients were 55 and 40, respectively (p = 0.83). Alcohol use for the two groups was also similar (73% for OCSCC and 65% for OPSCC; p = 0.59). OPSCC patients presented with a higher rate of prior XRT (53% versus 5%, p < 0.05) and recurrent disease (53% versus 5%, p < 0.05) prior to surgical management (Table 1). T and N classifications were similar for the two groups. Only 4 OPSCC tumors were p16 positive. Of these, 3 had a history of prior radiation and one had multifocal disease including a p16+ tonsil SCC and a concurrent and immediately adjacent p16- negative SCC in the tongue base. Charlson comorbidity index (CCI) was similar between OCSCC (2.86) and OPSCC (2.59) patients.
Table 1.
Patient and treatment characteristics.
| Oral cavity (n = 22) | Oropharynx (n = 17) | p-Value | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Number | % | Number | % | |||
| Age (median, years) | 67.5 | 63 | 0.51 | |||
| Sex | Male | 21 | 95 | 16 | 94 | 0.85 |
| Female | 1 | 5 | 1 | 6 | ||
| Race | White | 18 | 82 | 12 | 71 | 0.41 |
| Black | 4 | 18 | 5 | 29 | ||
| Other | 0 | 0 | 0 | 0 | ||
| Presentation | Primary | 21 | 95 | 8 | 47 | <0.05 |
| Recurrent | 1 | 5 | 9 | 53 | ||
| Previous radiation | Yes | 1 | 5 | 9 | 53 | <0.05 |
| No | 21 | 95 | 8 | 47 | ||
| T-stage (7th edition) | 1 | 4 | 18 | 3 | 18 | 0.73 |
| 2 | 7 | 32 | 3 | 18 | ||
| 3 | 4 | 18 | 5 | 29 | ||
| 4 | 7 | 32 | 6 | 35 | ||
| T-stage (8th edition) | 1 | 1 | 5 | 1 | 6 | 0.92 |
| 2 | 6 | 27 | 3 | 18 | ||
| 3 | 8 | 36 | 7 | 41 | ||
| 4 | 7 | 32 | 6 | 35 | ||
| N-stage (7th edition) | 0 | 10 | 45 | 5 | 29 | 0.1 |
| 1 | 5 | 23 | 1 | 6 | ||
| 2 | 7 | 32 | 11 | 65 | ||
| 3 | 0 | 0 | 0 | 0 | ||
| N-stage (8th edition) | 0 | 10 | 45 | 5 | 29 | 0.27 |
| 1 | 5 | 23 | 2 | 12 | ||
| 2 | 3 | 14 | 2 | 12 | ||
| 3 | 4 | 18 | 8 | 47 | ||
| Adjuvant treatment | None | 3 | 14 | 4 | 24 | 0.11 |
| Radiation | 11 | 50 | 3 | 18 | ||
| Chemo-radiation | 8 | 36 | 10 | 59 | ||
| Tobacco use (ever) | Yes | 21 | 95 | 16 | 94 | 0.85 |
| No | 1 | 5 | 1 | 6 | ||
| Tobacco use (pack-years) - median | 55 | 40 | 0.83 | |||
| EtOH use (ever) | Yes | 16 | 73 | 11 | 65 | 0.59 |
| No | 6 | 27 | 6 | 35 | ||
| EtOH (drinks per day) -median | 1 | 1 | 0.89 | |||
3.2. Treatment characteristics (surgical considerations- oncologic)
Following multi-disciplinary evaluation and discussion by the entire treatment team, all patients were dispositioned to definitive, curative intent surgical resection. In order to standardize the description of extent of surgery, we have summarized the oncologic defect for individual patients in Table 2, as a function of distinct functional subunits. The most common functional subsites across both disease sites were the oral tongue and floor of mouth. Five OCSCC patient and 6 OPSCC patients required total glossectomy (p = 0.39). However, all the OPSCC patients that required total glossectomy also required total laryngectomy, while none of the OCSCC patients underwent laryngectomy (p < 0.05) (Table 2). Segmental mandibulectomy was performed in 9 OCSCC patients and 6 OPSCC patients. Positive margins on final pathology were identified for 2 OCSCC patients and 4 OSPCC patients (p = 0.21). Lymphovascular invasion was noted in 10 OCSCC tumors and 12 OPSCC tumors (p = 0.12). There was significantly more perineural invasion (PNI) identified in OPSCC tumor specimens (15/17 versus 9/22, p < 0.05). The rate of extranodal extension (ENE) was also significantly higher for the OPSCC tumors (9/17 versus 4/22, p = 0.02).
Table 2.
Extent of surgical resection.
| Resected surgical subunits | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | Lip | Oral tongue | FOM | Mandible | Hard palate | Soft palate | BOT | Pharynx | Larynx |
| OC1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 |
| OC2 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| OC3 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| OC4 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| OC5 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| OC6 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| OC7 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| OC8 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| OC9 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| OC10 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| OC11 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| OC12 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| OC13 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| OC14 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| OC15 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| OC16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| OC17 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| OC18 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| OC19 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| OC20 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| OC21 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| OC22 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| OP1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 |
| OP2 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 |
| OP3 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| OP4 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 |
| OP5 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| OP6 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 |
| OP7 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| OP8 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 |
| OP9 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| OP10 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| OP11 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 |
| OP12 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| OP13 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| OP14 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| OP15 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 |
| OP16 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| OP17 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 |
Median duration of surgery was 10.8 (range 7.7–17.3) and 11.6 h (8.9–13) for the OCSCC and OPSCC patients (p = 0.59), respectively (Table 3). Median lengths of hospitalization for OCSCC and OPSCC patients were 13.5 (range 7–31) and 10 days (range 8–18; p = 0.06). There was 1 perioperative death in an OCSCC patient. Twelve (55%) OCSCC patients and 7 (41%) OPSCC patients encountered a 30-day complication (p = 0.41), with donor site wound complication (15%) being the most common, followed by pneumonia (10%). There was 1 total flap loss in an OCSCC patient and 1 partial loss in an OPSCC patient. The total loss occurred in a patient with a forearm flap for a floor of mouth defect. He returned to the clinic with a devitalized flap, however, the wound had granulated in well at this point without fistula formation. There were no returns to the operating room for anastomotic complications or acute flap distress and no orocutaneous or pharyngocutaneous fistulas in the entire series.
Table 3.
Peri-operative course characteristics.
| Oral cavity (n = 22) | Oropharynx (n = 17) | p-Value | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Number | % | Number | % | |||
| Length of surgery (hours) - median | 10.8 | 11.6 | 0.59 | |||
| Length of hospitalization (days)- median | 13.5 | 10 | 0.06 | |||
| 30 day mortality | 1 | 5 | 0 | 0 | 0.37 | |
| 30 day readmission or emergency center visit | 0 | 0 | 1 | 6 | 0.25 | |
| 30 day morbidity | 12 | 55 | 7 | 41 | 0.41 | |
| Complication (medical) | Any | 7 | 32 | 3 | 18 | 0.31 |
| Pulmonary embolus | 2 | 9 | 0 | 0 | ||
| Pneumonia | 3 | 14 | 1 | 6 | ||
| Complication (surgical) | Any | 8 | 36 | 4 | 24 | 0.39 |
| Hematoma/seroma | 2 | 9 | 0 | 0 | ||
| Total flap failure | 1 | 5 | 0 | 0 | ||
| Partial flap failure | 0 | 0 | 1 | 6 | ||
| Fistula | 0 | 0 | 0 | 0 | ||
| SSI (head/neck) | 0 | 0 | 0 | 0 | ||
| Flap dehiscence | 2 | 9 | 2 | 12 | ||
| Donor site complication | 4 | 18 | 2 | 12 | ||
| Flap | Soft tissue | 13 | 59 | 17 | 100 | <0.05 |
| Bony | 9 | 41 | 0 | 0 | <0.05 | |
| Alt | 6 | 27 | 14 | 82 | ||
| Forearm (radial or ulnar) | 7 | 32 | 2 | 12 | ||
| Fibula | 7 | 32 | 0 | 0 | ||
| Subscapular system | 2 | 9 | 0 | 0 | ||
| Submental | 0 | 0 | 1 | 6 | ||
3.3. Treatment characteristics (surgical consideration- reconstructive)
The distribution of free flaps used for patients is delineated in Table 3. While the fibula free flap was the bony flap of choice for oral cavity mandibular reconstruction, subscapular system flaps were performed in 2 patients that required mandibulectomy and also required a large volume of soft tissue for reconstruction. One of these patients had an advanced oral cavity SCC that required mandibulectomy, total glossectomy, and resection of a large area of facial/neck skin. A chimeric scapular tip, myocutaneous latissimus and fasciocutaneous transverse scapular free flap was used for reconstruction (Fig. 1).
Fig. 1.
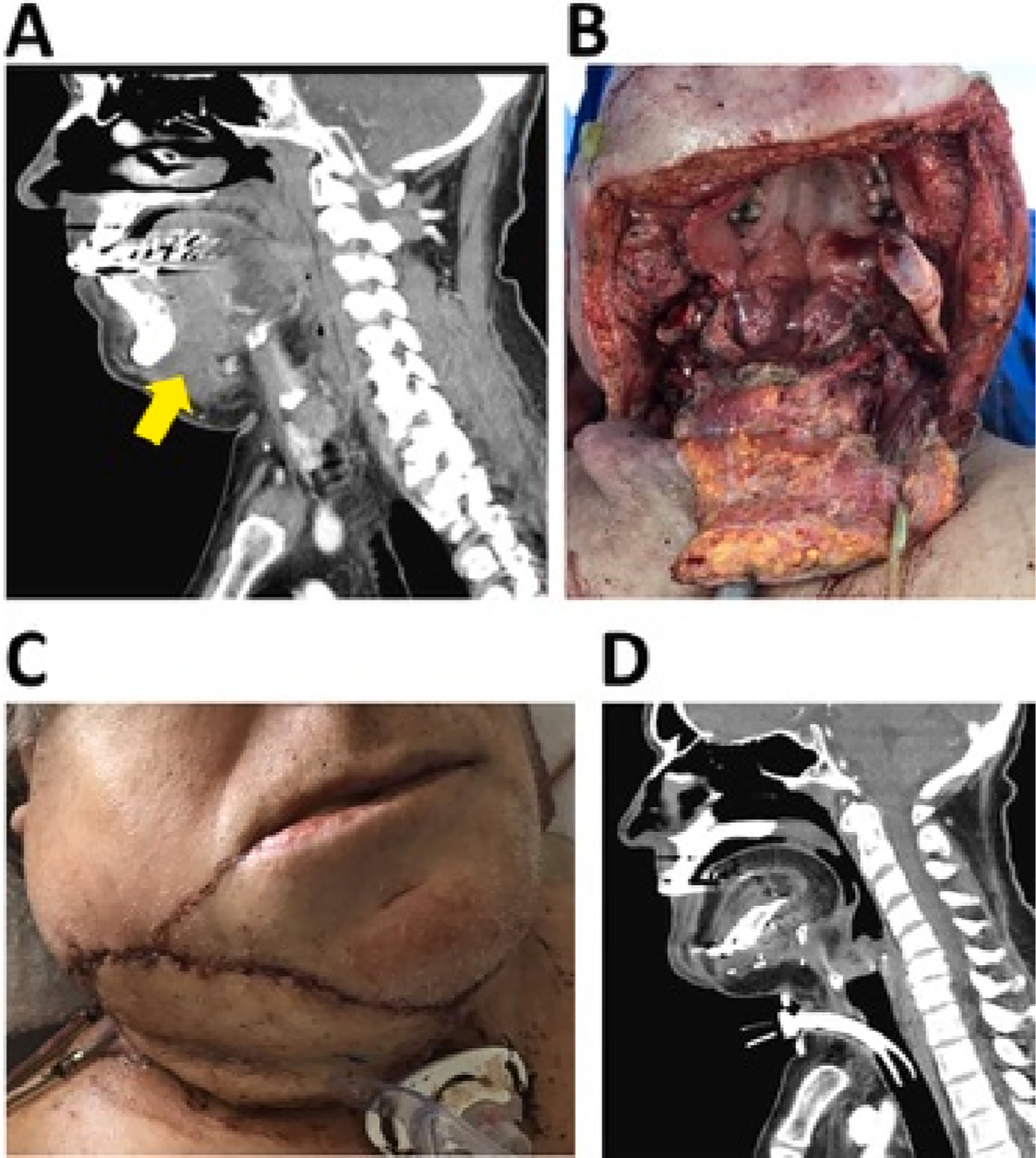
Composite resection and reconstruction (OCSCC). A) CT sagittal image of patient presented with a T4a OCSCC tumor involving the oral tongue, tongue root/hyoid, mandible and soft tissue (arrow demonstrates soft tissue submental extension. B) Surgical defect after mandibulectomy, glossectomy, soft tissue resection. C) Immediate (day 1) post-reconstruction with scapular free flap. D) Follow-up post-operative CT sagittal image.
OPSCC related defects were reconstructed (Table 3) with an ALT free flap in 14 patients (82%), forearm free flap in 2 patients (12%) and submental pedicled flap in 1 patient (6%) (Fig. 2). All 9 mandible defects were reconstructed with a bony free flap for the OCSCC patients (7 fibula and 2 scapular tip), while none of the 6 bone defects were reconstructed with a bone flap for the OPSCC patients (p < 0.05).
Fig. 2.
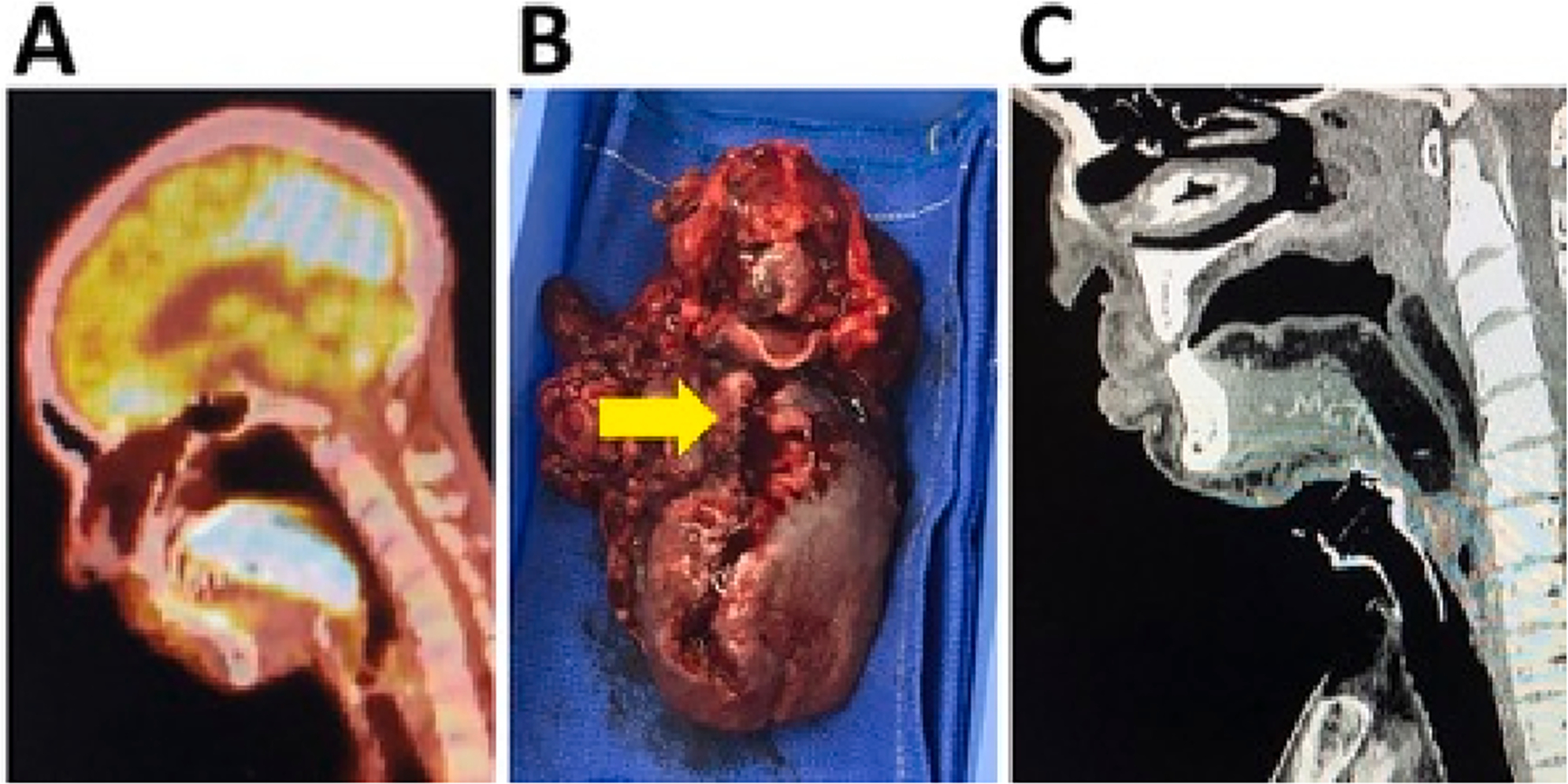
Total glossectomy- total laryngectomy resection and reconstruction (OPSCC). A) Pre-treatment FDG-PET sagittal image demonstrating a T4a OPSCC tumor involving the oral tongue, tongue root/hyoid. B) Surgical specimen. Arrow indicates the ulcerative lesion in the midline of the oral tongue and tongue base with extension into the valeculla. D) Follow-up post-operative CT sagittal image.
3.4. Treatment characteristics (adjuvant treatment)
For the entire cohort, compliance with NCCN guidelines with respect to number of treatment modalities was 90% vs 65%, OC vs OP (p < 0.05). Among patients which previously received head and neck radiation, re-irradiation was performed in 75% (6/8) of those patients where NCCN guidelines recommended post-operative radiation. Adjuvant treatment was administered to 19/22 OCSCC patients (radiation n = 11; chemo-radiation n = 8) and 13/17 OPSCC patients (radiation n = 3; chemo-radiation n = 10). Six OPSCC patients underwent re-irradiation. Mean and median days from surgery to radiation start were 41 and 40.5, respectively. Excluding re-irradiation patients, mean and median days from surgery to radiation start were 38 and 36.5, respectively. Four OCSCC patients and 5 OPSCC patients had a delayed (>42 days) start to XRT. Eighteen OCSCC and 12 OPSCC patients completed adjuvant post-operative XRT. Of these patients that completed their adjuvant XRT, none of the OCSCC and only 1 OPSCC patient had a total treatment package time (date of surgery to date of XRT completion) greater than 100 days. Five of the 9 OCSCC patients and 8 of the 10 OPSCC patients were able to tolerate their planned chemotherapy regimens without de-escalation or dose modification.
3.5. Clinical outcomes
Mean and median follow-up was 18.6 and 17 months, respectively. Five (23%) OCSCC and 7 (41%) OPSCC patients developed recurrent disease (p = 0.21). There were 3 (14%) local recurrences in the OCSCC cohort and 3 (18%) in the OPSCC cohort (p = 0.34). Two (9%) OCSCC and 1 (6%) OPSCC patients developed regional recurrence (p = 0.71). Two (9%) OCSCC and 3 (18%) OPSCC patients developed distant metastases (p = 0.43). Disease free (DFS) and overall survival (OS) did not differ between the two patient cohorts (Fig. 3).
Fig. 3.
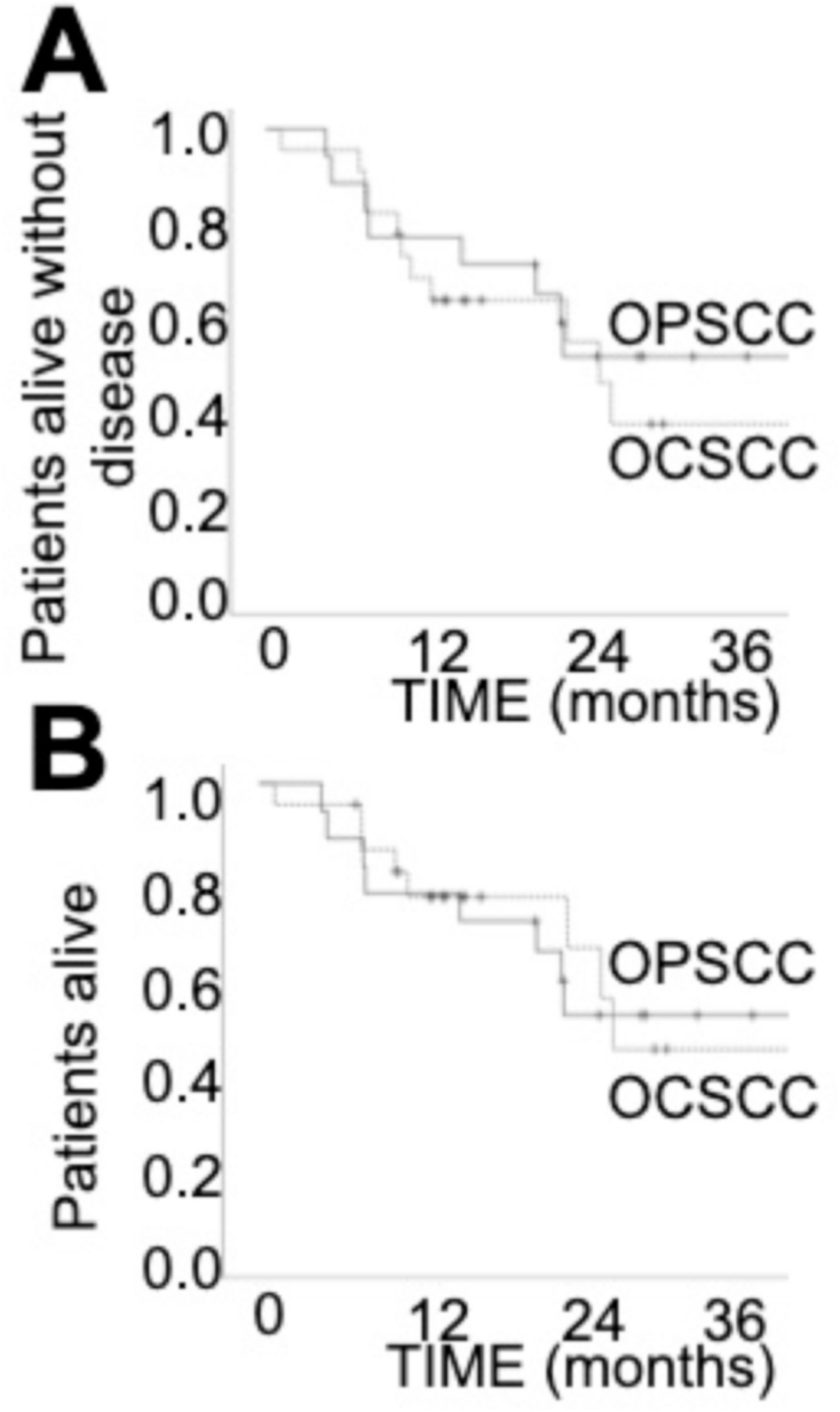
Clinical outcomes. A) Disease free survival. B) Overall survival.
Twenty (91%) OCSCC patients were managed with a tracheostomy. Six (35%) OPSCC patients underwent laryngectomy (Table 4) and the other OPSCC patients required a tracheostomy. Only four (18%) OCSCC patients and 1 (6%) OPSCC patient did not undergo gastrostomy placement. At the point of maximal function, 5 (24%) OCSCC and 10 (59%) OPSCC patients had a tracheostomy or laryngectomy (p < 0.05). At the point of maximal function, 11 (52%) OCSCC and 14 (82%) OPSCC patients had a gastrostomy tube (p > 0.05). Available DIGEST scores showed significantly better safety scores for OCSCC patients but no significant difference in efficiency scores (Table 4). There was also a similar rate of 100% gastrostomy dependence at the point of maximal function (14% and 17%, OC and OP). Similar times to achieve maximal swallowing function were measured for patients able to return to some oral diet (171.3 vs 163.1 days, OC and OP, p = 0.85). Excluding patients with a history of radiation, maximal function gastrostomy utilization rate is non-significant for OC vs OP (50% vs 62.5%, p = 0.55) and the tracheostomy/laryngectomy rate is non-significant (20% vs 37.5%, p = 0.33). The rate of 100% gastrostomy dependence is 10% and 0% for the non-radiated OC and OP patients (p = 0.35). The rate of 100% oral intake (or gastrostomy-independence) for these patients is 60% for the OC and 37.5% for the OP patients (p = 0.28).
Table 4.
Functional outcomes.
| OC |
|
OP |
|
p-Value | ||
|---|---|---|---|---|---|---|
| Number | % | Number | % | |||
| Pre-treatment | Tracheostomy | 0 | 0 | 1 | 6 | 0.25 |
| Gastrostomy | 6 | 27 | 10 | 59 | <0.05 | |
| During treatment | Tracheostomy | 20 | 91 | 17 | 100 | 0.20 |
| Gastrostomy | 17 | 81 | 16 | 94 | 0.23 | |
| Post-treatment (30 day) | Tracheostomy | 10 | 48 | 13 | 76 | 0.07 |
| Gastrostomy | 17 | 81 | 16 | 94 | 0.23 | |
| Post-treatment | DIGEST (safety) | 0.31 | 1.67 | <0.05 | ||
| MBS | DIGEST (efficiency) | 0.62 | 1.44 | 0.11 | ||
| Maximal function | Tracheostomy | 5 | 24 | 10 | 59 | <0.05 |
| Gastrostomy | 11 | 52 | 14 | 82 | >0.05 | |
| 100% gastrostomy | 3 | 14 | 3 | 18 | ||
| Pleasure PO | 1 | 5 | 6 | 35 | ||
| <50% PO | 2 | 10 | 1 | 6 | ||
| >50% PO | 3 | 14 | 2 | 12 | ||
| 100% PO | 12 | 57 | 4 | 24 | ||
| Mean timeto maximal diet (days) | 171 | 163 | 0.85 | |||
| Last follow-up | Tracheostomy | 8 | 38 | 9 | 53 | 0.36 |
| Gastrostomy | 11 | 52 | 14 | 82 | >0.05 | |
4. Discussion
Despite the justified enthusiasm for the improving oncologic outcomes associated with HPV-associated OPSCC, high-risk OPSCC remains a significant clinical challenge, not dissimilar to OCSCC. In the current series, consistent with previous reports, locoregional recurrence was the primary driver of disease-free and overall survival [9,10]. This supports the need for enhanced locoregional control, achieved ideally through maximizing the intensity of the treatment delivered. In reality, this remains difficult to achieve. On the one hand, empiric radiation sensitivity for HPV-negative OPSCC remains low, as it does for OCSCC resulting in suboptimal rates of disease control with radiation-based regimens [1,12,13]. This is consistent with data from pre-clinical models which highlight the deleterious impact of common, non-HPV associated oncogenic events such as mutations in TP53 on chemotherapy and radiation sensitivity [7,14–18]. On the other hand, most patients with high-risk OPSCC present with advanced T-classification which makes surgical resection challenging [9]. The current cohort is instructive to the multiple aspects which generate this complex clinical scenario. The aggressive nature of the disease was confirmed at the time of oncologic surgical resection by the presence of increased PNI as well as a higher rate of ENE compared to the OCSCC cohort. Overall, the positive margin fraction was not-insignificant and was driven overwhelmingly by soft tissue primary tumor extension, particularly in OPSCC patients as opposed to mucosal disease. In all 4 cases of OPSCC tumors for which final pathologic margins were positive, all had been previously radiated.
Overall, our cohorts, although small, demonstrate the feasibility of a homogeneous approach to OCSCC and high-risk OPSCC using an up-front surgical approach by demonstrating equivalent oncologic outcomes and acceptable functional outcomes for the high-risk OPSCC patients. The locoregional recurrence rates for both cohorts were equivalent despite the OPSCC being enriched for recurrent disease/new primaries in the setting of previous radiation. Despite the aggressive nature of the OPSCC tumors outlined here, compliance with NCCN guidelines was high as was the ability to deliver timely adjuvant treatment. Of note, among the patients with a prior radiation history undergoing salvage surgical management, 75% were able to receive adjuvant re-irradiation based on NCCN guidelines, demonstrating the importance of up-front surgery with robust reconstruction. More than half of the OPSCC patients treated here had prior radiation; re-irradiation in the definitive setting for these patients has been associated with a high risk of excessive normal tissue toxicity including life-threatening hemorrhage, osteoradionecrosis and soft tissue necrosis [19,20].
Although a similar disease biology and presumed radiation insensitivity formed the initial impetus for our approach to these two disease sites, the overall therapeutic approach has been facilitated by advances in MVFTT, further tailored to address OPSCC oncologic defects. We have found that reconstructive approaches can be tailored to large OPSCC oncologic defects in a manner which is focused on oncologic principles related to maximal ability to heal in a timely fashion and initiate adjuvant treatment. The reconstructive approach for each site (OC and OP) remained consistent. Bony reconstruction was pursued for all mandibular/bony defects for OCSCC patients, and this often involved reconstruction of the mandibular symphysis. However, bony reconstruction was not performed for any of the 6 patients that underwent mandibulectomy for OPSCC. Mandible defects for OPSCC patients were posterior-lateral, did not involve the symphysis and did not affect occlusion since patients were edentulous. Soft tissue mucosal reconstruction and carotid protection were prioritized for these cases. This approach avoided the need to place hardware and risk a hardware-related complication, potentially allowed for deeper penetration of adjuvant radiation or re-irradiation if pursued, and aided in avoiding osteoradionecrosis (ORN) to a high-risk area. The ALT free flap was a work-horse flap for the OPSCC cohort given the opportunity for multi-layered wound reinforcement with skin, fascia and muscle. Using these principles, we were able to avoid any orocutaneous or pharyngocutaneous fistulas in either cohort of patients, which allowed for timely delivery of curative-intent adjuvant treatment based on NCCN guidelines.
Although we are able to reconstruct defects with a low overall complication rate, functional recovery remains challenging, and demonstrably more so for OPSCC tumors compared to their OCSCC counterparts. In contrast to the OCSCC tumors, OPSCC tumors required resection of more functional subunits, particularly the larynx. The difference between performing a total glossectomy for advanced OCSCC compared to a total glossectomy for advanced OPSCC is clearly demonstrated in this series. None of the 5 OCSCC patients had a laryngectomy in conjunction with their glossectomy but all 6 of the patients undergoing total glossectomy for OPSCC also had a total laryngectomy. While laryngectomy allows for safe swallowing, the glossectomy makes oral preparation of any bolus nearly impossible. Thus, MVFTT is integral in the reconstruction of these patients, as the added bulk may allow for improved oral bolus transit. Tracheoesophageal puncture is not recommended for the total glossectomy-total laryngectomy patients due to the lack of any meaningful articulation. This generates significant post-operative challenges with communication and requires extensive pre-operative patient counseling.
While the tracheostomy status at maximal function was significantly better for the OCSCC patients, this is largely attributable to the need for laryngectomy in 6 OPSCC patients. The differences observed in functional outcomes between the two cohorts of patients are at least partially attributable to the significantly higher rate of prior radiation in the OPSCC cohort given the lack of significant differences when we exclude these patients from this analysis. Swallowing DIGEST safety scores showed worse outcomes for OPSCC patients. However, laryngectomy patients were excluded since their DIGEST safety score is maximal given their inability to aspirate. DIGEST efficiency scores were not significantly different between the two cohorts but trended worse for OPSCC patients. This trend is likely related to multiple factors including the increased incidence of prior radiation for this group.
A natural question is whether this locoregionally-focused approach is overly simplistic. The introduction of checkpoint inhibitors into management of head and neck cancer calls into question the utility of extensive, often mutilating surgeries for advanced stage disease [21]. Unfortunately, although clearly effective in a subset of patients, the broad applicability of checkpoint inhibitors for high-risk OPSCC and OCSCC remains unclear [21,22]. Conversely, it is clear that for most patients locoregional treatment with surgery and adjuvant radiation will remain mainstays of treatment.
5. Conclusion
Veterans with high risk oropharyngeal squamous cell carcinoma are able to tolerate surgical resection with MVFTT followed by appropriate adjuvant therapy. This approach is well tolerated, leads to acceptable functional outcomes when compared to those of OCSCC patients, and parallels the well-accepted NCCN guideline-based approach to OCSCC.
Funding source
This material is the result of work supported with resources and the use of facilities of the Michael E. DeBakey VA Medical Center. This work is supported in part by a Career Development Award from the Veterans Administration Clinical Science Research and Development division (1IK2CX001953) (VCS).
Footnotes
Declaration of competing interest
The authors have no conflicts of interest to disclose for this manuscript.
References
- [1].Shah JP, Gil Z. Current concepts in management of oral cancer–surgery. Oral Oncol 2009;45:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dahlstrom KR, Calzada G, Hanby JD, et al. An evolution in demographics, treatment, and outcomes of oropharyngeal cancer at a major cancer center: a staging system in need of repair. Cancer 2013;119:81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dahlstrom KR, Garden AS, William WN Jr, Lim MY, Sturgis EM. Proposed staging system for patients with HPV-related oropharyngeal cancer based on nasopharyngeal cancer N categories. J Clin Oncol 2016;34:1848–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol 2015;33:3235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gillison ML, Trotti AM, Harris J, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet 2019;393:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].O’Sullivan B, Huang SH, Su J, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the international collaboration on oropharyngeal cancer network for staging (ICON-S): a multicentre cohort study. Lancet Oncol 2016;17:440–51. [DOI] [PubMed] [Google Scholar]
- [7].Sandulache VC, Michikawa C, Kataria P, et al. High-risk TP53 mutations are associated with extranodal extension in Oral cavity squamous cell carcinoma. Clin Cancer Res 2018;24:1727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sandulache VC, Lei YL, Heasley LE. Innovations in risk-stratification and treatment of Veterans with oropharynx cancer; roadmap of the 2019 field based meeting. Oral Oncol et al. 2019. [DOI] [PMC free article] [PubMed]
- [9].Sandulache VC, Hamblin J, Lai S, et al. Oropharyngeal squamous cell carcinoma in the veteran population: association with traditional carcinogen exposure and poor clinical outcomes. Head Neck 2015;37:1246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fakhry C, Zhang Q, Gillison ML, Nguyen-Tân PF, Rosenthal DI, Weber RS, Lambert L, Trotti 3rd AM, Barrett WL, Thorstad WL, Yom SS, Wong SJ, Ridge JA, Rao SSD, Spencer S, Fortin A, Raben D, Harris J, Le QT. Validation of NRG oncology/RTOG-0129 risk groups for HPV-positive and HPV-negative oropharyngeal squamous cell cancer: Implications for risk-based therapeutic intensity trials. Cancer 2019;125(12):2027–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Elhalawani H, Mohamed ASR, Elgohari B, et al. Tobacco exposure as a major modifier of oncologic outcomes in human papillomavirus (HPV) associated oropharyngeal squamous cell carcinoma. BMC Cancer 2020;20:912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ow TJ, Myers JN. Current management of advanced resectable oral cavity squamous cell carcinoma. Clin Exp Otorhinolaryngol 2011;4:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Liao CT, Wen YW, Lee SR, et al. Clinical outcomes of taiwanese patients with cT4 Oral cavity squamous cell carcinoma: toward the identification of the optimal initial treatment approach for cT4b patients. Ann Surg Oncol 2017;24:785–93. [DOI] [PubMed] [Google Scholar]
- [14].Sano D, Xie TX, Ow TJ, et al. Disruptive TP53 mutation is associated with aggressive disease characteristics in an orthotopic murine model of oral tongue cancer. Clin Cancer Res 2011;17:6658–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Skinner HD, Sandulache VC, Ow TJ, et al. TP53 disruptive mutations lead to head and neck cancer treatment failure through inhibition of radiation-induced senescence. Clin Cancer Res 2012;18:290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhou G, Wang J, Zhao M, et al. Gain-of-function mutant p53 promotes cell growth and cancer cell metabolism via inhibition of AMPK activation. Mol Cell 2014;54: 960–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Neskey DM, Osman AA, Ow TJ, et al. Evolutionary action score of TP53 identifies high-risk mutations associated with decreased survival and increased distant metastases in head and neck cancer. Cancer Res 2015;75:1527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Osman AA, Neskey DM, Katsonis P, et al. Evolutionary action score of TP53 coding variants is predictive of platinum response in head and neck cancer patients. Cancer Res 2015;75:1205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Clancy K, Melki S, Awan M, et al. Outcomes of microvascular free tissue transfer in twice-irradiated patients. Microsurgery 2017;37:574–80. [DOI] [PubMed] [Google Scholar]
- [20].Ruhle A, Sprave T, Kalckreuth T, et al. The value of moderate dose escalation for re-irradiation of recurrent or second primary head-and-neck cancer. Radiat Oncol 2020;15:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ferris RL, Haddad R, Even C, et al. Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol 2020;31:942–50. [DOI] [PubMed] [Google Scholar]


