Abstract
Objective:
To describe cases and estimate subacute sclerosing panencephalitis (SSPE) risk following large-sale measles outbreaks in Georgia. SSPE, a rare, fatal late complication of measles, is often overlooked in assessments focused on the acute illness. Georgia had 8,377 and 11,495 reported measles cases during the 2004–2005 and 2013–2015 outbreaks, respectively, but SSPE burden has not been assessed.
Methods:
SSPE cases diagnosed during 2008–2017 were identified from hospitalization registries in major neurologic departments likely to admit SSPE patients. Information on reported measles cases and deaths was obtained from the national measles surveillance system and published reports. The risk of SSPE (number of measles cases per one SSPE case) was calculated for cases associated with the 2004–2005 outbreak. Crude estimates were adjusted to account for potential underreporting of measles, using 50%, 25% and 10% estimates of completeness of reporting.
Results:
Sixteen SSPE cases diagnosed during 2008–2017 were identified. Eleven (92%) of 12 SSPE cases with known history of measles had infection at ≤2 years and one (8%) at 3 years of age. Crude estimate of SSPE risk for the 2004–2005 outbreak was 1:1,396. Adjusted estimates were 1:2,792, 1:1:5,584, and 1:13,960, assuming 50%, 25% and 10% completeness of reporting measles cases, respectively.
Conclusions:
The review demonstrated substantial risk of SSPE in Georgia, supporting recent data suggesting that risk of SSPE following measles infection is higher than previously thought. To prevent SSPE in Georgia, very high timely immunization coverage for measles should be achieved among children and immunity gap among adults should be closed.
Introduction
Subacute sclerosing panencephalitis (SSPE) is a rare, but very serious late complication of measles resulting from persistent infection of central nervous system with defective forms of measles virus. The disease begins, on average, 6–10 years after acute infection with measles (range, 1–24 years) and is characterized with progressive cognitive and motor deterioration leading to severe disability and eventual fatal outcome within months or years of onset. Most cases of SSPE occur in children infected with measles virus before 2 years of age [1]. SSPE diagnosis can be challenging because often the disease is not suspected due to non-specific nature of presentations, particularly at early stages, and long interval between the onset of symptoms and the original measles infection.
SSPE was considered a very rare complication of measles with 1 case per 1,000,000 measles cases, but recent reports suggest that the risk of SSPE could be much higher than previously estimated [1–7]. Incidence of SSPE varies across countries, with most cases coming from areas where measles is endemic [7]. No estimates of SSPE occurrence were previously available for Georgia, which recently had two major outbreaks of measles, during 2004–2005 (8,377 reported cases) and 2013–2015 (11,495 reported cases) [8–12]. A number of SSPE cases occurred during the last few years in Georgia. To gain better understanding of the full burden of measles and to estimate the risk of SSPE in Georgia, we reviewed SSPE cases diagnosed during 2008–2017.
Methods
SSPE cases diagnosed during 2008–2017 were identified from hospitalization registries in major neurologic departments likely to admit SSPE patients and cross-checked with two laboratories which facilitate laboratory diagnosis of SSPE - testing for the evidence of intrathecal synthesis of anti-measles antibodies (increased level of anti-measles IgG and/or oligoclonal bands in CSF). Information on reported measles cases and deaths was obtained from the national measles surveillance system and published reports [7–12].
For this review, SSPE case definition and criteria for diagnosis, classification and staging of SSPE cases, were adapted from the criteria developed by The International Consortium on Subacute Sclerosing Panencephalitis [13, 14] (Table 1). The information on demographics, dates of onset of SSPE symptoms and diagnosis, history of measles disease and vaccination, SSPE stage at the time of diagnosis, outcome (if deceased, date of death), and clinical and laboratory work-up was abstracted from medical records. Information on history of measles and year of onset was based on parental recall at the time of SSPE diagnosis.
Table 1.
Criteria for diagnosis, classification and staging of subacute sclerosing panencephalitis (SSPE) cases used for the analysis
| Criteria for SSPE diagnosis* [13, 14] |
|---|
|
| SSPE case classification categories |
| Confirmed case: A person who meets at least three of the above criteria |
| Possible case: A person who meets criteria 2 and 3 but laboratory studies to detect intrathecal synthesis of anti-measles antibodies have not been performed. |
| Criteria for SSPE stages [13, 14] |
| Stage IA Behavioral, cognitive and personality change (decreased school performance, attention deficit/hyperactivity, inappropriate socially, sleep disturbance). Walking |
| Stage IB Myoclonic spasms – aperiodic, focal. Walking. Same mental/behavioral symptoms as IA |
| Stage IIA Further mental-behavioral deterioration. Myoclonic spasms – periodic, generalized and synchronous, frequent. Able to walk independently but does not because of drop spells |
| Stage IIB Apraxias, agnosias, language difficulties. Motor signs – spasticity, ataxia. Ambulatory with assistance |
| Stage IIIA Speaking less, visual diffuculties, no ADLs (activities of daily living). Sits up independently, may stand, no independent ambulation. Myoclonic spasms frequent, multifical, short inter-spasm intervals (3–5 seconds), long duration (3–4 seconds). May have seizures |
| Stage IIIB No spontaneous speech, poor verbal comprehension, may be blind. Myoclonic spasms same as IIIA. Bedridden. Dysphagia, may have to be fed by nasogastric tube. EEG delta background activity, periodic slow wave complexes (PSWC) often obscured in background. Movement disorder may appear (chorea-ballismus-athetosis) |
| Stage IV No myoclonic spasms. EEG very low voltage background activity. No PSWC. Neurovegetative state |
The two supportive criteria (brain biopsy and detection of mutated measles virus) from the original set by the International Consortium are not included because they were not available in Georgia.
The risk of SSPE (number of measles cases per one SSPE case) was calculated for cases associated with the 2004–2005 measles outbreak, since sufficient time for clinical manifestations of SSPE to develop had elapsed since this outbreak. The number of reported measles cases was adjusted to account for underreporting, because patients do not always seek medical care for measles and providers do not always report suspected cases to public health authorities. To assess the degree of underreporting, we reviewed available assessments and published literature from Georgia and elsewhere [15–21]. No measles-specific data on completeness of reporting were available for Georgia. In 2003, completeness of reporting notifiable communicable diseases in Georgia was estimated as 50% [15]. The assessment of Georgia’s Electronic Integrated Disease Surveillance System revealed that healthcare providers reported 60% of rubella cases diagnosed in 2014, low activity year for rubella (NCDC, unpublished data). Because of the integrated nature of measles and rubella surveillance, we considered rubella estimate applicable to measles, but used 50% to account for potentially lower reporting rate during the large-scale outbreak. In summary, we assumed, that 50% of measles patients during 2004–2005 did not seek medical care because of traditional perception of measles as a mild illness and unfavorable conditions at healthcare facilities during that period likely to discourage patients from seeking care, unless seriously ill. We also assumed that providers reported to public health authorities 50% of measles cases who sought medical care. This resulted in 25% estimated overall completeness of measles reporting – the proportion of actual measles cases reported to public health authorities, derived by multiplying the proportion of cases seeking medical care (50%) by the proportion of cases reported by providers to NCDC (50%). Because of the lack of measles-specific data on surveillance completeness for Georgia and great variations in completeness of measles reporting in various settings [16–21], we also obtained estimates for lower (10%) and higher (50%) levels of completeness.
The present review was determined by United States Centers for Disease Control and Prevention and the National Center for Disease Control and Public Health (NCDC) of Georgia to represent non-research public health activity and not human subject research.
Results
Sixteen SSPE cases with onset during 2008–2016 and diagnosis during 2008–2017 were identified via record search at the Iashvili Children’s Hospital (Figure 1). No additional cases were found by cross-checking with other major hospitals or laboratories facilitating specialized diagnostic testing for SSPE. All cases met the criteria for confirmed SSPE (Table 1).
Figure 1.
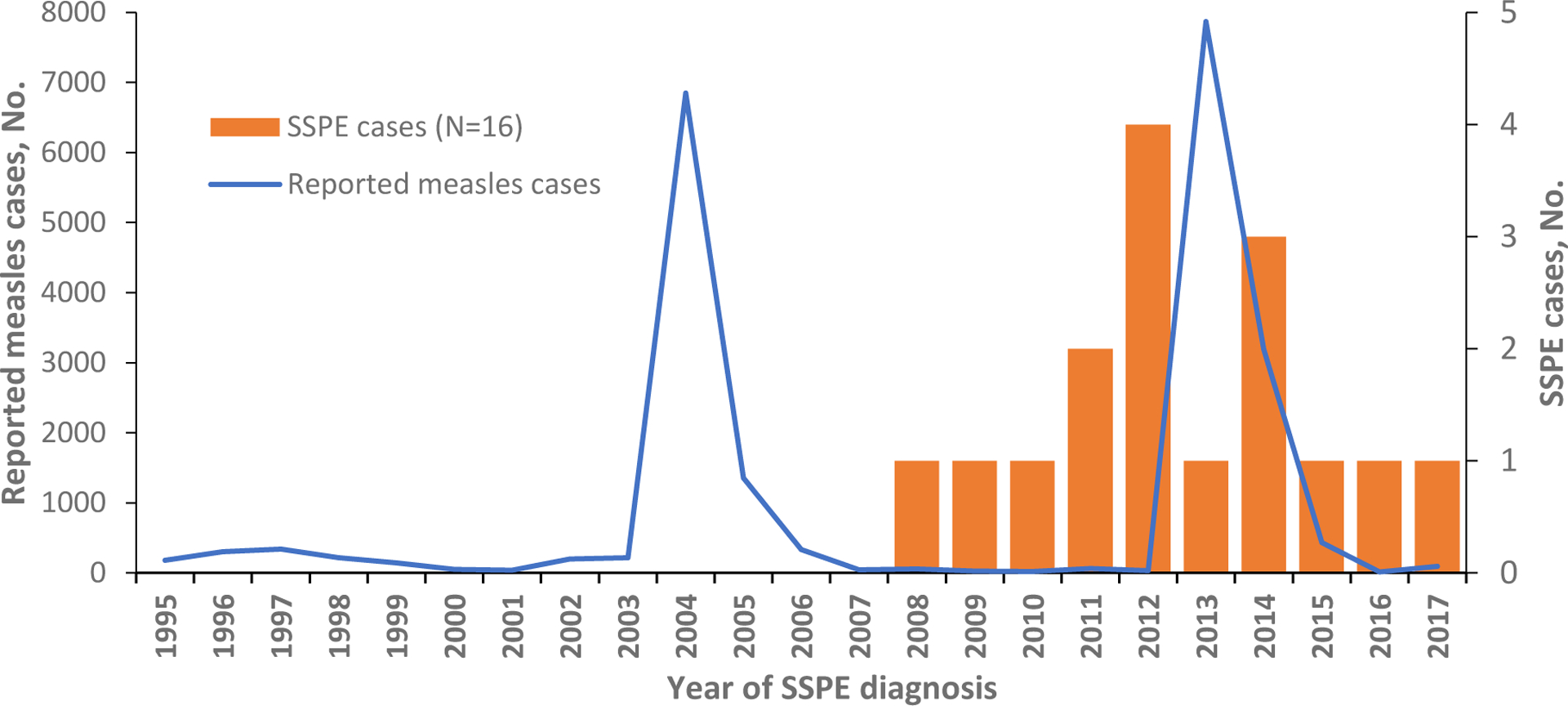
Subacute sclerosing panencephalitis (SSPE) cases identified during 2008–2017 and measles cases reported during 1995–2017, Georgia
Eleven (69%) cases were born during 2002–2005, shortly before or during the 2004–2005 outbreak; three (19%) cases were born before 2002; and two (12%) after 2005. Twelve cases (75%) had known history of measles with onset during 1998–2014: three (25%) cases had measles during 1998–2003, six (50%) during the 2004–2005 outbreak, two (17%) in 2006, and one case (8%) in 2014 (Figure 2). Four cases (25%) had no known history of measles or febrile rash illness, but based on the year of birth, three of them could have been exposed during the 2004–2005 outbreak. In eleven (92%) of 12 SSPE cases with known history of measles, the infection was acquired at age ≤2 years, for one (8%) - at three years. For these cases, median interval between acute measles and SSPE onset was 6 years (Table 2).
Figure 2.
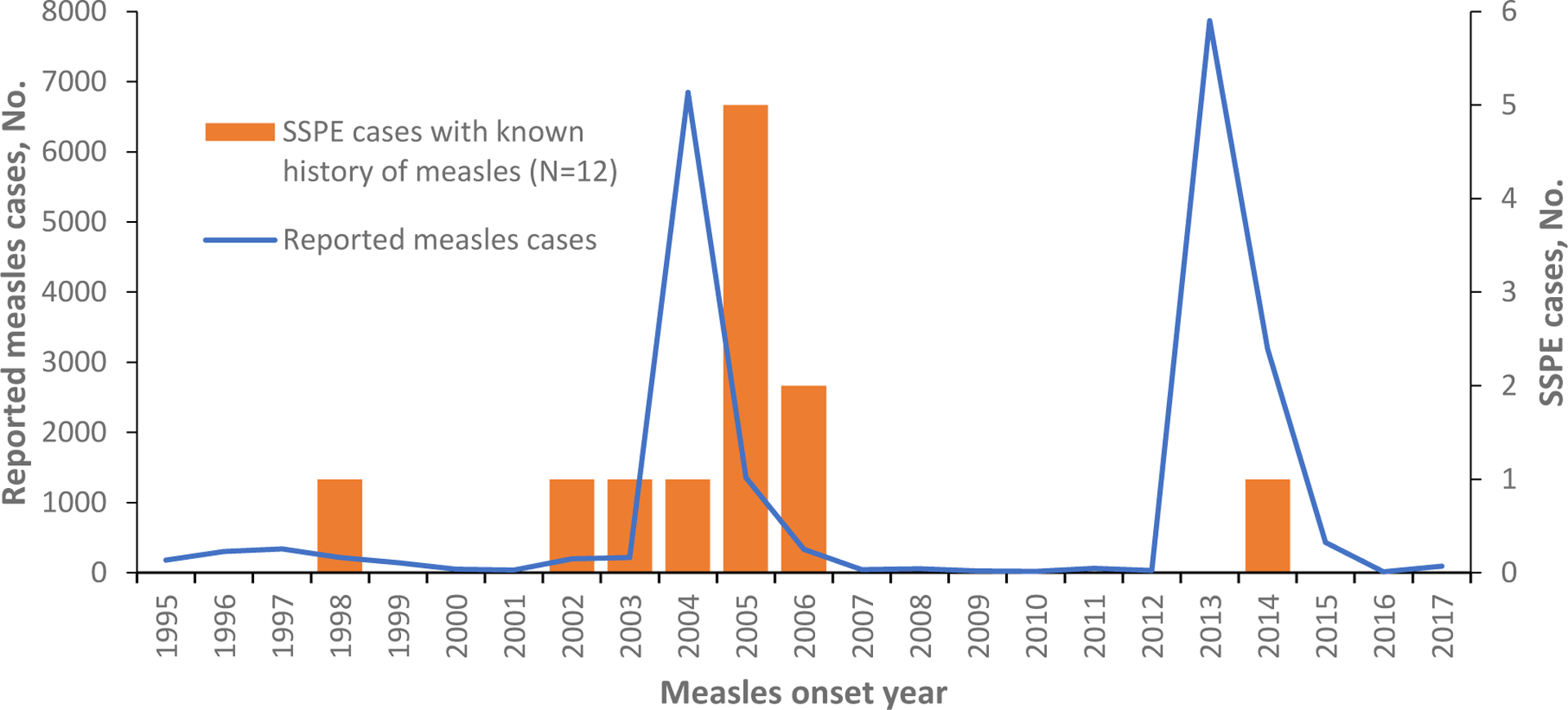
Subacute sclerosing panencephalitis (SSPE) cases with known history of measles by measles onset year, Georgia
Table 2.
Characteristics of subacute sclerosing panencephalitis (SSPE) cases identified in Georgia, 2008–2017
| Variable | All cases | Cases associated with the 2004–2005 outbreak | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| Total cases | 16 | 100 | 6 | 100 |
| Male sex | 12 | 75 | 3 | 50 |
| Age at SSPE onset, median (range) | 9 (3–16) years | 8 (4–10) years | ||
| Age at SSPE diagnosis, median (range) | 9 (4–16) years | 9 (6–11) years | ||
| SSPE stage at the time of diagnosis | ||||
| IA | 0 | 0 | 0 | 0 |
| IB | 4 | 25 | 1 | 17 |
| IIA | 5 | 31 | 1 | 17 |
| IIB | 2 | 13 | 2 | 33 |
| IIIA | 1 | 6 | 1 | 17 |
| IIIB | 4 | 25 | 1 | 17 |
| IV | 0 | 0 | 0 | 0 |
| Known fatal outcome | 5 | 31 | 3 | 50 |
| Reported age of measles onseta | ||||
| <1 year | 5 | 42 | 3 | 50 |
| 1 year | 1 | 8 | 0 | 0 |
| 2 years | 5 | 42 | 3 | 50 |
| 3 years | 1 | 8 | 0 | 0 |
| Unknown | 4 | 25 | 0 | 0 |
| Interval measles to SSPE, median (range)b | 6 (2–14) years | 6 (4–8) years | ||
| Measles immunization status | ||||
| Unvaccinated/likely unvaccinatedc | 5 | 31 | 3 | 50 |
| Vaccinated | 2 | 13 | 1 | 17 |
| Unknown | 9 | 56 | 2 | 33 |
Note. Percentages are rounded to the nearest integer.
Per parental recall at the time of SSPE diagnoses for all cases except the one with measles onset in 2014.
For cases with known year of measles onset;
Likely unvaccinated cases include those with unknown immunization history and those with measles onset before 1 year of age, i.e. too young to be eligible for measles vaccination. In Georgia, the 1st dose is recommended at 12 months of age.
Patient age at the time of onset of SSPE symptoms was between 4 and 16 years (median, 9 years). Median interval between onset of SSPE symptoms and diagnosis was 4 months (range: 1–68 months). In 11 (69%) cases, SSPE diagnosis was made within six months after onset of symptoms; in four (25%) cases, 6–18 months after onset; and in one (6%) case, 68 months after onset. In most instances, (11 cases, 69%) SSPE was diagnosed at stage I or II of the disease (Table 2). Five (31%) cases died before January 1, 2019, three (19%) were alive, and the status of eight (50%) cases was unknown.
Three patients were unvaccinated. Immunization history was unknown for 11 cases, but two of them, who had measles at <1 year of age, would have been ineligible for measles vaccine before disease onset since the first dose of measles-containing vaccine in Georgia is recommended at 12 months. Two cases had received two doses of measles-mumps-rubella vaccine (MMR). For both, the 2nd dose was given years after they had measles.
The risk of SSPE in Georgia was estimated based on six cases associated with the 2004–2005 outbreak (Table 2). Overall, the crude estimate of SSPE risk based on 8,377 reported cases of measles of all ages was 1 per 1,396 cases of measles. Depending on the assumed level of completeness of reporting, adjusted estimates ranged between 1 per 2,792 and 1 per 13,960 (Table 3). SSPE risk among persons with measles onset at <1 year of age was extremely high, with crude estimate of risk 1 per 158, and adjusted estimates between 1 per 316 and 1 per 1,580. SSPE risk for persons with measles onset at ≥1 years of age was 16.7-fold lower than the risk for those who acquired measles before their 1st birthday, with crude estimate of risk 1 per 2,634 and adjusted estimates between 1 per 5,268 and 1 per 26,340 cases of measles (Table 3).
Table 3.
Estimated risk of subacute sclerosing panencephalitis during the 2004–2005 measles outbreak in Georgia
| Measles onset at any age | |
|---|---|
| Measles cases of all ages, reported number | 8,377 |
| SSPE cases with measles onset at any age, number | 6 |
| SSPE risk (SSPE cases : measles cases) | |
| Unadjusted | 1:1,396 |
| Adjusted for incomplete reporting of measles cases | |
| Assumed completeness of reporting - 10% | 1:13,960 |
| Assumed completeness of reporting - 25% | 1:5,584 |
| Assumed completeness of reporting - 50% | 1:2,792 |
| Measles onset at age <1 year | |
| Measles cases aged <1 year, reported number | 474 |
| SSPE cases with measles onset age <1 year, number | 3 |
| SSPE risk | |
| Unadjusted (SSPE cases : measles cases) | 1:158 |
| Adjusted for incomplete reporting of measles cases | |
| Assumed completeness of reporting - 10% | 1:1,580 |
| Assumed completeness of reporting - 25% | 1:632 |
| Assumed completeness of reporting - 50% | 1:316 |
| Measles onset at age >1 years | |
| Measles cases aged ≥1 year, reported number | 7,903 |
| SSPE cases with measles onset age ≥1 year, number | 3 |
| SSPE risk | |
| Unadjusted (SSPE cases : measles cases) | 1:2,634 |
| Adjusted for incomplete reporting of measles cases | |
| Assumed completeness of reporting - 10% | 1:26,340 |
| Assumed completeness of reporting - 25% | 1:10,536 |
| Assumed completeness of reporting - 50% | 1:5,268 |
Discussion
The present review draws attention to substantial hidden burden of SSPE from measles infection in Georgia that is usually overlooked in assessments of measles-related morbidity and deaths focused on the acute illness. We found 16 cases of this invariably fatal condition diagnosed over the past decade, a high number for a country with 3.7 million population. During 2004–2017, 13 measles-related deaths were reported in Georgia - nine during the 2004–2005 outbreak and four during 2013–2015 outbreak [8–12]. The number of SSPE cases identified in this review already exceeds the reported number of deaths caused by acute measles in Georgia during this period and indicates that overall mortality from measles is at least twice as high as previously recognized.
The overall risk of SSPE in Georgia appears high. Even when adjusted for underreporting of measles cases, the risk estimates from this review were closer to the range of recent high estimates from other settings (1 per 5,000–10,000 cases of measles of any age, and 1 per 1,700–3,300 cases when measles was contracted before 5 years of age) [2–5], than to older estimates in the range of 1 per 100,000 measles cases [1, 3, 4, 7].
The present study, the first to attempt to assess the SSPE risk in Georgia, had certain limitations, such as potential recall bias for past history of measles obtained from parents/guardians at the time of SSPE diagnosis, incomplete availability of measles immunization history, and the absence of measles-specific data on surveillance completeness in Georgia. To address the latter limitation, we calculated risks for various levels of potential underreporting, which demonstrated the robustly high risk of SSPE even when the worst-case scenario (10% completeness) was assumed.
The risk of SSPE will remain high in Georgia until endemic transmission of measles virus is interrupted and measles elimination is achieved [22]. SSPE burden is expected to increase in coming years with the appearance of cases linked to the large-scale measles outbreak during 2013–2015. Despite the relatively short time since this outbreak, one SSPE case with measles onset in 2014 has already been identified. If we apply estimated age-specific risks of SSPE from the 2004–2005 outbreak (Table 3), we can estimate that between 22 (assuming 50% completeness) and 44 (assuming 25% completeness) cases of SSPE could occur following the 2014–2015 outbreak, including 14–28 cases among those who had measles during the 1st year of life and 8–16 cases among those who contracted measles at ≥1 years of age. Because of substantial improvement of surveillance quality since 2004–2005, when case-based surveillance in Georgia was just beginning [8], the 10% level of assumed completeness was not applied to these estimates.
Most SSPE cases occur among children infected before 5 years of age; the risk is highest among persons infected with measles virus at a very early age, particularly during the first year of life [1–6]. The current epidemiology of measles in Georgia is consistent with high potential risk of SSPE [national measles surveillance data for 2013–2017; 8–12]. In both recent outbreaks, infants aged <1 year had much higher incidence of measles than any other age group even though most cases occurred among young adults, followed by children aged 1–4 years. Also, the proportion of infants aged <1 year among reported measles cases increased from 6% during 2004–2005 to 10% during 2013–2014. The proportion of children aged <1 year among cases for non-outbreak years was even higher, between 12% and 23%.
Increasing proportion of infants aged <1 year among measles cases in Georgia reflects persistent immunity gap in cohorts born during mid-1980s to late 1990s [8, 23, 24]. These cohorts, with historically low coverage because of past problems with immunization services in Georgia, include substantial numbers of persons who were not vaccinated in childhood and escaped infection until adulthood, as overall incidence of measles declined with the vaccine introduction. As a result, in Georgia, similar to many other countries [8, 12, 25–28], measles cases are occurring at older ages. Young adults (aged 15–39 years) accounted for 39% and 55% of measles cases in the 2004–2005 and 2013–2015 outbreaks, respectively [8, 12]. The serosurvey among adults conducted after the 2013–2015 outbreak revealed that 10.1% of persons aged 18–24 years were still seronegative for measles [23]. As these inadequately vaccinated cohorts enter reproductive age, higher proportion of babies are born to susceptible mothers and lack protection by maternal antibodies. In outbreak settings, large numbers of very young children unprotected by maternal antibodies are at risk of acquiring measles during the 1st year of life, when the risk of subsequent SSPE is highest.
In Georgia, all identified cases of SSPE had measles at ≤3 years of age, with the vast majority infected at ≤2 years. Therefore, the key to preventing SSPE is preventing measles among infants and young children. According to national immunization schedule of Georgia, the 1st dose of measles-mumps-rubella (MMR) vaccine is given at 12 months and the 2nd dose at 5 years. Of note, there is no evidence of measles vaccine virus causing SSPE, as wild-type measles virus was identified in all instances of molecular characterization of viruses detected from SSPE cases [1, 3].
To protect children too young to be vaccinated, measles immunity gap among young adults in Georgia should be closed. Parents and other adults in contact with young infants should be vaccinated with MMR. To help reduce SSPE risk among young children age-eligible for MMR vaccination, it is crucial to achieve very high coverage and ensure that children receive 1st dose of MMR on time. Very high (≥95%) coverage with both doses of MMR is needed to achieve very high level of population immunity needed for interrupting measles transmission [22]. However, despite recent substantial improvements in immunization coverage in Georgia, the 95% national coverage target for MMR has not been achieved in most years [29]. Recent immunization coverage survey identified widespread vaccination delays as major contributor to suboptimal coverage in Georgia [30]. Unjustified delays in administration of the 1st dose of MMR unnecessarily prolong susceptibility and leave children vulnerable to acquiring measles during the period of the greatest risk for SSPE. Improving timeliness of MMR vaccination in Georgia is needed to ensure that children are protected from measles at earliest possible age, thereby reducing the risk of subsequent SSPE.
Because SSPE only becomes clinically apparent several years after acute measles and is not a reportable condition, the disease is rarely viewed in the context of overall burden of measles despite its devastating nature for the patient and the family and substantial direct or indirect costs. In Georgia, where measles-related risks are often underestimated by general population and many health care providers, as demonstrated by the knowledge, attitudes and practices survey to assess health care provider and adult population perceptions towards measles and rubella (NCDC,unpublished data), and awareness of SSPE in population is likely even lower, there has been low uptake of outbreak response immunization offered to adults free of charge by the national public health authorities since 2013. Achieving the goal of measles elimination in Georgia without increasing acceptance and demand for MMR vaccination will be challenging. Efforts to heighten awareness about serious consequences of measles, including SSPE, and the high risk of its development in Georgia, could help with increasing vaccine uptake among population.
Funding:
No external funding was received for conducting this work
Footnotes
Transparency declaration:The present work has not been published previously, is not under consideration for publication elsewhere, its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright-holder. All authors made substantial contributions to the conception and design of the study, acquisition of data, and/or data analysis and interpretation, contributed to writing the manuscript and provided final approval of the version to be submitted. None of the authors have declared any conflict of interest.
Disclaimer:The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry.
References
- 1.Griffin DE. Measles virus and the nervous system. Handb Clin Neurol. 2014;123:577–90. [DOI] [PubMed] [Google Scholar]
- 2.Schönberger K, Ludwig MS, Wildner M, Weissbrich B. Epidemiology of subacute sclerosing panencephalitis (SSPE) in Germany from 2003 to 2009: a risk estimation. PLoS One. 2013;8:e68909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellini WJ, Rota JS, Lowe LE, Katz RS, Dyken PR, Zaki SR, et al. Subacute sclerosing panencephalitis: more cases of this fatal disease are prevented by measles immunization than was previously recognized. J Infect Dis. 2005;192:1686–93. [DOI] [PubMed] [Google Scholar]
- 4.Rota PA, Rota JS, Goodson JL. Subacute Sclerosing Panencephalitis. Clin Infect Dis. 2017;65:233–4. [DOI] [PubMed] [Google Scholar]
- 5.Wendorf KA, Winter K, Zipprich J, Schechter R, Hacker JK, Preas C, et al. Subacute Sclerosing Panencephalitis: The Devastating Measles Complication That Might Be More Common Than Previously Estimated. Clin Infect Dis. 2017;65:226–32. [DOI] [PubMed] [Google Scholar]
- 6.Gutierrez J, Issacson RS, Koppel BS. Subacute sclerosing panencephalitis: an update. Dev Med Child Neurol. 2010;52:901–7 [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Subacute sclerosing panencephalitis surveillance – United States. MMWR Morb Mortal Wkly Rep 1982;31:585–8. [PubMed] [Google Scholar]
- 8.Doshi S, Khetsuriani N, Zakhashvili K, Baidoshvili L, Imnadze P, Uzicanin A. Ongoing measles and rubella transmission in Georgia, 2004–05: implications for the national and regional elimination efforts. Int J Epidemiol. 2009;38:182–91 [DOI] [PubMed] [Google Scholar]
- 9.National Center for Disease Control and Public Health. Annual Report for 2013 (in Georgian: ). Available at: http://ncdc.ge/Handlers/GetFile.ashx?ID=ffc0ae92-5be6-4e8e-a604-f7b61fb279d8. Accessed April 17, 2018 [Google Scholar]
- 10.National Center for Disease Control and Public Health, Ministry of Health, Labour and Social Affairs of Georgia. Healthcare in Georgia, 2014—Highlights (in Georgian: ). Available from URL: http://ncdc.ge/Handlers/GetFile.ashx?ID=d29f8b90-1be5-4e58-9486-a6c2f6ee8f49. Accessed April 4, 2018 [Google Scholar]
- 11.National Center for Disease Control and Public Health, Ministry of Health, Labour and Social Affairs of Georgia. Healthcare in Georgia, 2015—Highlights (in Georgian: ). Available from URL: http://ncdc.ge/Handlers/GetFile.ashx?ID=d29f8b90-1be5-4e58-9486-a6c2f6ee8f49. Accessed April 4, 2018 [Google Scholar]
- 12.Muscat M, Shefer A, Ben Mamou M, Spataru R, Jankovic D, Deshevoy S, et al. The state of measles and rubella in the WHO European Region, 2013. Clin Microbiol Infect 2014;20 (Suppl 5):12–8. [DOI] [PubMed] [Google Scholar]
- 13.Gascon G, Yamani S, Crowell J, Stigsby B, Nester M, Kanaan I, et al. Combined oral isoprinosine-intraventricular alpha-interferon therapy for subacute sclerosing panencephalitis. Brain Dev. 1993;15:346–55. [DOI] [PubMed] [Google Scholar]
- 14.Gascon GG; International Consortium on Subacute Sclerosing Panencephalitis. Randomized treatment study of inosiplex versus combined inosiplex and intraventricular interferon-alpha in subacute sclerosing panencephalitis (SSPE): international multicenter study. J Child Neurol. 2003;18:819–27. [DOI] [PubMed] [Google Scholar]
- 15.Ministry of Labor Health and Social Affairs of Georgia. Surveillance and Control of Communicable Diseases: Guidelines for Public Health Services in Georgia. Third Edition, November 2005. MOHLSA, 2005, p.p. 1–175 [Google Scholar]
- 16.Mette A, Reuss AM, Feig M, Kappelmayer L, Siedler A, Eckmanns T, et al. Under-reporting of measles: an evaluation based on data from north rhine-westphalia. Dtsch Arztebl Int. 2011;108:191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harpaz R Completeness of measles case reporting: review of estimates for the United States. J Infect Dis. 2004. May 1;189 Suppl 1:S185–90. [DOI] [PubMed] [Google Scholar]
- 18.Ewert DP, Westman S, Frederick PD, Waterman SH. Measles reporting completeness during a community-wide epidemic in inner-city Los Angeles. Public Health Rep. 1995. Mar-Apr;110(2):161–5. [PMC free article] [PubMed] [Google Scholar]
- 19.van Isterdael CE, van Essen GA, Kuyvenhoven MM, Hoes AW, Stalman WA, de Wit NJ. Measles incidence estimations based on the notification by general practitioners were suboptimal. J Clin Epidemiol. 2004;57:633–7 [DOI] [PubMed] [Google Scholar]
- 20.Takla A, Wichmann O, Rieck T, Matysiak-Klose D. Measles incidence and reporting trends in Germany, 2007–2011. Bull World Health Organ. 2014. Oct 1;92(10):742–9. doi: 10.2471/BLT.13.135145. Epub 2014 Aug 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trottier H, Carabin H, Philippe P. Measles, pertussis, rubella and mumps completeness of reporting. Literature review of estimates for industrialized countries. Rev Epidemiol Sante Publique. 2006. Feb;54(1):27–39. [DOI] [PubMed] [Google Scholar]
- 22.WHO. Eliminating measles and rubella. Framework for the verification process in the WHO European Region. WHO, 2014. Available at: http://www.euro.who.int/__data/assets/pdf_file/0009/247356/Eliminating-measles-and-rubella-Framework-for-the-verification-process-in-the-WHO-European-Region.pdf?ua=1. Accessed Dec 20, 2017 [Google Scholar]
- 23.Khetsuriani N, Chitadze N, Steven R, Mamou Ben, Measles M. and rubella seroprevalence among adults in Georgia: helping guide the elimination efforts – manuscript submitted to peer-reviewed journal, currently under review, once the decision is made, reference will be updated as appropriate [Google Scholar]
- 24.Khetsuriani N, Imnadze P, Baidoshvili L, Jabidze L, Tatishili N, Kurtsikashvili G, et al. Impact of unfounded vaccine safety concerns on the nationwide measles-rubella immunization campaign, Georgia, 2008. Vaccine. 2010;28:6455–62 [DOI] [PubMed] [Google Scholar]
- 25.O’Connor P, Jankovic D, Muscat M, Ben-Mamou M, Reef S, Papania M,et al. Measles and rubella elimination in the WHO Region for Europe: progress and challenges. Clin Microbiol Infect. 2017;23:504–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.CDC. Increased transmission and outbreaks of measles - European Region, 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1605–10. [PubMed] [Google Scholar]
- 27.Kidd S, Ouedraogo B, Kambire C, Kambou JL, McLean H, Kutty PK, et al. Measles outbreak in Burkina Faso, 2009: a case-control study to determine risk factors and estimate vaccine effectiveness. Vaccine. 2012;30:5000–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogbuanu IU, Muroua C, Allies M, Chitala K, Gerber S, Shilunga P, et al. Measles outbreak reveals measles susceptibility among adults in Namibia, 2009 – 2011. S Afr Med J. 2016;106:715–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. Immunisation, vaccines and biologicals. Data, statistics and graphics. Official country reported coverage estimates time series. Available from URL: http://www.who.int/immunization/monitoring_surveillance/data/en/. [Google Scholar]
- 30.Khetsuriani N, Wannemuehler K, Geleishvili M, and Komakhidze T. Final report - Immunization coverage survey in Georgia, 2015–2016. US Centers for Disease Control and Prevention, Ministry of Labour, Health and Social Affairs of Georgia. Tbilisi, 2017. Available at: http://ncdc.ge/Pages/User/Documents.aspx?ID=1d6e5458-6504-4e5c-88a1-e547b79d5b47 Accessed 30 January 2019. [Google Scholar]


