Abstract
Introduction Wide variations exist in the management of craniopharyngiomas, including pituitary stalk preservation/sacrifice. This study examines the practice patterns over 16 years using the endoscopic endonasal approach for the resection of craniopharyngiomas and it examines the effects of stalk preservation.
Methods Retrospective analysis was conducted for 66 patients who underwent endoscopic transsphenoidal surgery for resection of craniopharyngiomas. Patients were stratified into three epochs: 2005 to 2009 ( N = 20), 2010 to 2015 ( N = 23), and 2016 to 2020 ( N = 20), to examine the evolution of surgical outcomes. Subgroup analysis between stalk preservation/stalk sacrifice was conducted for rate of gross total resection, anterior pituitary function preservation, and development of new permanent diabetes insipidus.
Results Gross total resection rates across the first, second, and third epochs were 20, 65, and 52%, respectively ( p = 0.042). Stalk preservation across epochs were 100, 5.9, and 52.6% ( p = 0.0001). New permanent diabetes insipidus did not significantly change across epochs (37.5, 68.4, 71.4%; p = 0.078). Preservation of normal endocrine function across epochs was 25, 0, and 23.8%; ( p = 0.001). Postoperative cerebrospinal fluid (CSF) leaks significantly decreased over time (40, 4.5, and 0%; [ p = 0.0001]). Stalk preservation group retained higher normal endocrine function (40.9 vs. 0%; p = 0.001) and less normal-preoperative to postoperative panhypopituitarism (18.4 vs. 56%; p = 0.001). Stalk sacrifice group achieved higher GTR (70.8 vs. 28%, p = 0.005). At last follow-up, there was no difference in recurrence/progression rates between the two groups.
Conclusion There is a continuous evolution in the management of craniopharyngiomas. Gross total resection, higher rates of pituitary stalk and hormonal preservation, and low rates of postoperative CSF leak can be achieved with increased surgical experience.
Keywords: craniopharyngioma, endoscopic, pituitary stalk, stalk sacrifice, learning curve
Introduction
Craniopharyngioma is a rare WHO grade I tumor arising from Rathke's pouch epithelium or odontogenic tissue with an age adjusted annual incidence of approximately1.6 per 1,000,000 persons per year. 1 Morbidity from craniopharyngioma resection can be quite high due to the close proximity of tumor to vital structures in the parasellar region. 2 3 4 5 Surgical resection is the primary modality for the management of this tumor. 3 4 However, wide variations exist in the management of craniopharyngiomas, including the choice of surgical approach, extent of resection, use of adjuvant therapy, and strategy regarding pituitary stalk preservation.
Several recent studies have supported the safety and efficacy of endoscopic approaches for craniopharyngioma resection when compared with a traditional transcranial open approach. 6 7 8 9 10 The treatment strategy of gross total resection (GTR) versus subtotal resection with adjuvant therapy remains a point of debate. 9 11 12 13 Additionally, the relative benefits of pituitary stalk preservation and sacrifice have not been fully delineated. 5 11 These practice patterns differ among surgeons and likely evolve with experience during an individual surgeon's career.
Few studies have evaluated the learning curve of endoscopic craniopharyngioma resection. 14 15 We previously published a series evaluating the early learning curve on the endoscopic treatment of craniopharyngioma, demonstrating a threshold of approximately 20 cases to improve rates of GTR. 2 We also noticed marked variation in stalk preservation and subtotal resection (STR) or near total resection (NTR) depending on the time period of patient treatment. The goal of the current study is to examine our institution's practice pattern across 16 years using the endoscopic endonasal approach for the resection of craniopharyngiomas and to examine the effects of stalk preservation or sacrifice.
Materials and Methods
Study Population and Baseline Variables
After Institutional Board Review approval, all patients from November 2005 to December 2020 with a histological diagnosis of craniopharyngioma treated with an endoscopic endonasal approach for primary or recurrent tumors were included in this study. A retrospective chart review was done collecting patients' baseline demographics, presenting symptoms, history of prior treatments, tumor characteristics, surgical outcomes, postoperative complications, length of stay (LOS), discharge status, anterior and posterior hormonal dysfunction, infundibulum status post-surgery, adjuvant therapy, status of recurrence/progression of disease, and overall length of follow-up. Hypothalamic involvement was defined as craniopharyngioma displacing or invading into the hypothalamus. Hypothalamic involvement was determined based upon the preoperative MRI findings.
Surgical Outcome
Preoperative hormonal dysfunction was counted if laboratory value was below reference range or if the patient was already on hormonal replacement. Patients were considered to have panhypopituitarism if patient required three anterior pituitary hormone replacement drugs (thyroid, testosterone/estrogen, and corticosteroids) and partial hypopituitarism if one or two anterior hormone replacement drugs were used at the last follow-up. Patients were considered to have preoperative diabetes insipidus (DI) if desmopressin therapy was initiated prior to surgery. Postoperatively, patients were categorized as new permanent DI if they did not require DI treatment preoperatively but did so at the last follow-up.
Patients were assessed preoperatively and postoperatively for visual acuity and Humphrey visual field testing. Visual grades were classified as improved, stable, or worse. All testing were conducted by independent ophthalmologists.
Extent of resection was assessed on intraoperative documentation and absence of residual on postoperative MRI 3 months after surgery and defined as either GTR, NTR, or STR. In eight patients without 3 months MRI, intraoperative note was used. NTR was defined as 95% or greater resection of the tumor by intraoperative note.
Complications analyzed postoperatively included postoperative cerebrospinal fluid (CSF) leak requiring intervention (surgery or lumbar drain), development of new hydrocephalus requiring additional intervention, symptomatic hyponatremia (defined as postoperative sodium less than 135 mEq/dL) with concurrent signs and symptoms (nausea, vomiting, and seizure), rhinological complications, seizures, meningitis, carotid injury, and stroke.
Surgical Epoch and Stalk Preservation/Sacrifice
Patients were first divided into three epochs based on the timing of surgery from 2005 to 2020 to assess the learning curve at our institution. The groups consisted of an early epoch (2005–2009), middle epoch (2010–2015), and late epoch (2016–2020).
To further assess the optimal strategy for surgical intervention of craniopharyngiomas, a subgroup analysis of patients with stalk preservation and stalk sacrifice was compared with assessed surgical outcomes, specifically extent of resection, development of new permanent DI, and preservation of anterior pituitary function.
Stalk preservation and sacrifice were identified by the intraoperative surgical findings. In rare cases when surgical notes were not definitive, patients were eliminated from the subgroup analysis. Patients treated for recurrent craniopharyngioma were excluded from the subgroup analysis due to the possible confounding effect of stalk status and alterations in pituitary function. Operative techniques for craniopharyngiomas have been previously described. 2
Statistical Analysis
Chi-square analysis was used to compare categorical values for early, middle, and late epochs. To compare outcomes with respect to pituitary stalk preservation and sacrifice, a student t -test was used to assess continuous variables and Fisher's exact test was used for categorical values. Kaplan-Meier curve was generated to assess recurrence/tumor progression across epochs and log rank test was used to assess significance. p <0.05 was considered significant and two-sided t -tests were conducted for all analysis. SPSS (V26.0) was used for statistical analysis.
Results
Baseline Characteristics and Perioperative Outcomes
A total of 66 patients underwent endoscopic endonasal approach for craniopharyngioma from February 2005 to December 2020. The median age of the study population was 39 (IQR 30.5–56.3) years of age at the time of surgery and 25 (37.9%) patients were female. Vision loss was the most common presenting symptom in 60 (91%) patients, followed by headache in 31 (47%) and endocrinopathy in 18 (27%) patients. Prior surgical intervention with craniotomy and/or transsphenoidal surgery for resection of craniopharyngioma occurred in nine (14%) patients and five patients (7.6%), respectively. The median tumor size was 2.91 cm (IQR 2.1–3.5) with 47 (71%) tumors having both solid and cystic components on preoperative MRI and 55 (83.3%) tumors involving hypothalamus. Majority of the tumors 47 (71.2%) were characterized histologically as adamantinomatous craniopharyngiomas ( Table 1 ).
Table 1. Patient history, demographics, and imaging characteristics in early, middle, and late cohorts.
| Epoch | Total N = 66 |
2005–2010 ( N = 20) | 2011–2015 ( N = 23) |
2016–2020 ( N = 23) | Significance | |
|---|---|---|---|---|---|---|
| Age (median, interquartile range) | 39 (IQR 30.5–56.3) | 46.5 (30–56.8) | 39 (28–64.2) | 39 (32–67) | 0.435 | |
| Sex | Female | 25 (37.9%) | 8 (40%) | 10 (43.5%) | 7 (30.4%) | 0.642 |
| Presenting symptom | Vision loss | 60 (91%) | 20 (100%) | 23 (100%) | 17 (73.9%) | 0.002 |
| Headache | 31 (47%) | 8 (40%) | 13 (56.5%) | 10 (43.5%) | 0.510 | |
| Endocrinopathy | 18 (27%) | 4 (20%) | 9 (39.1%) | 5 (21.7%) | 0.284 | |
| Weight gain | 7 (11%) | 0 (0%) | 3 (13%) | 4 (17.4%) | 0.163 | |
| Fatigue | 10 (15%) | 1 (5%) | 5 (21.7%) | 4 (17.4%) | 0.291 | |
| Memory/cognitive | 6 (9%) | 2 (10%) | 0 (0) | 4 (17.4%) | 0.120 | |
| Prior surgery | Transcranial | 9 (14%) | 5 (25%) | 3 (13%) | 1 (4.3%) | 0.243 |
| Transsphenoidal | 5 (7.6%) | 2 (10%) | 2 (8.7%) | 1 (4.3%) | 0.345 | |
| Prior radiotherapy | 4 (6.0%) | 3 (15%) | 1 (4.3%) | 0 (0) | 0.139 | |
| Prior intracystic therapy | Ommaya reservoir | 1 (1.5%) | 0 (0) | 1 (1.5%) | 0 (0) | |
| Intracystic therapy | 0 (0) | 0 (0) | 0 (0) | |||
| Max tumor diameter, cm (median, interquartile range) | 2.91 (2.1–3.5) | 2.9 (2.3–4.2) | 3.0 (2.1–3.3) | 2.7 (2.1–3.2) | 0.415 | |
| Tumor consistency a | Solid | 6 (9.0%) | 3 (15%) | 2 (8.7%) | 1 (4.3%) | 0.785 |
| Cystic | 13 (20%) | 4 (20%) | 5 (21.7%) | 4 (17.4%) | ||
| Solid and cystic | 47 (71%) | 13 (65%) | 16 (69.6%) | 18 (82.6%) | ||
| Hypothalamic involvement b | 55 (83.3%) | 19 (95%) | 19 (82.6%) | 17 (73.9%) | 0.179 | |
| Pathology | Adamantinomatous | 47 (71.2%) | 14 (70%) | 15 (65.2%) | 18 (78.3%) | 0.501 |
| Papillary | 17 (25.8%) | 5 (25%) | 8 (34.8%) | 5 (21.7%) |
Tumor was graded as primarily cystic or solid if >90% of the tumor was cystic or solid, respectively.
Hypothalamic involvement was present if the tumor displaced or invaded the medial or inferior hypothalamus on at least one side. Bold values indicate significant level less than 0.05.
GTR was achieved in 31 (46.9%) patients, NTR in 19 (28.7%) patients, and STR in 16 (24.2%) patients. Common postoperative complications were rhinological in nine (13.6%) patients, including epistaxis ( n = 1), anterior septal perforation ( n = 4), sinusitis ( n = 3), and saddle nose deformity ( n = 1). Postoperative CSF leak required further intervention in eight (12.1%) patients. Preservation of full anterior endocrine function was achieved in nine (15.5%) patients at the last follow-up. In patients without prior DI, new permanent DI developed in 34 (58.6%) patients. The median LOS for all patients was 6 days (range 2–115) and 51 (86%) patients were discharged home after surgery. Recurrence/progression of disease occurred in 13 patients (22.4%). The median follow-up was 41.5 months (range 1–183) for all patients ( Tables 2 3 4 ).
Table 2. Perioperative outcomes.
| Epoch | 2005–2010 ( N = 20) | 2011–2015 ( N = 23) |
2016–2020 ( N = 23) | Significance | ||
|---|---|---|---|---|---|---|
| Extent of resection | STR | 16 (24.2%) | 9 (45.0%) | 3 (13.0%) | 4 (17.4%) | 0.042 |
| NTR | 19 (28.7%) | 7 (35.0%) | 5 (21.7%) | 7 (30.4%) | ||
| GTR | 31 (46.9%) | 4 (20%) | 15 (65.2%) | 12 (52.2%) | ||
| Vision a | Stable | 11 (20%) | 1 (6.3%) | 4 (19%) | 6 (33%) b | 0.264 |
| Improved | 42 (76%) | 14 (87.5%) | 17 (81%) | 11 (61.1%) | ||
| Worsened | 2 (3.6%) | 1 (6.3%) | 0 (0%) | 1 (5.6%) | ||
| Complication | New hydrocephalus | 6 (9.1%) | 4 (20%) | 1 (4.3%) | 1 (4.3%) | 0.127 |
| Meningitis | 2 (3.0%) | 2 (10%) | 0 | 0 | – | |
| Postoperative CSF leak | 9 (13.6%) | 8 (40%) | 1 (4.5%) | 0 (0) | 0.0001 | |
| Hyponatremia | 5 (7.6 %) | 0 | 1 (4.3%) | 4 (17.4%) | 0.076 | |
| Seizure | 1 (1.5%) | 0 | 1 (4.3%) | 0 | – | |
| Rhinological complication c | 9 (13.6%) | 2 (10%) | 4(17.4%) | 3 (13%) | 0.824 | |
| Carotid artery injury | 1 (1.5%) | 1 (5%) | 0 | 0 | – | |
| Stroke | 1 (1.5%) | 1 (5%) | 0 | 0 | – | |
| Median LOS | 6 (2–115) | 6 | 6 | 4 | 0.03 | |
| Discharge status | Home | 51 (86%) | 9 (45%) | 21 (95.7%) | 21 (91.3%) | 0.001 |
| Rehab or NSF | 8 (14%) | 5 (25%) | 1 (4.3%) | 2 (8.7%) |
Abbreviations: CSF, cerebrospinal fluid; GTR, gross total resection; LOS, length of stay; NTR, near total resection; STR, subtotal resection.
Eleven patients without vision assessment.
Six patients in the latest cohort without preoperative vision deficits who remained stable on postoperative assessment.
Epistaxis ( n = 1), anterior septal perforation ( n = 4), sinusitis ( n = 3), and saddle nose deformity ( n = 1). Bold values indicate significant level less than 0.05.
Table 3. Stalk preservation and postoperative endocrine outcomes.
| Epoch | Total N = 58 a | 2005–2010 ( N = 16) | 2011–2015 ( N = 23) |
2016–2020 ( N = 21) | ||
|---|---|---|---|---|---|---|
| Anterior pituitary function | Normal endocrine function | 9 (15.5%) | 4 (25.0%) | 0 (0) | 5 (23.8%) | 0.001 |
| Normal to partial | 15 (25.8%) | 0 (0) | 7 (30.4%) | 8 (38.1%) | ||
| Normal to pan-hypopituitarism | 19 (32.7%) | 3 (18.8%) | 8 (34.8%) | 8 (38.1) | ||
| Partial to pan-hypopituitarism | 2 (3.4%) | 1 (6.3%) | 1 (4.3%) | 0 | ||
| Stable pan-hypopituitarism | 9 (15.5%) | 3 (18.8%) | 6 (26.1%) | 0 | ||
| Stable partial hypopituitarism | 7 (12.1%) | 6 (37.5%) | 1 (4.3%) | 0 | ||
| DI | Preoperative DI | 6 (10.3%) | 2 (12.5%) | 4 (17.3) | 0 (0%) | 0.12 |
| New permanent | 34 (58.6%) | 6 (37.5%) | 13 (68.4%) | 15 (71.4%) | 0.078 | |
| Infundibulum status b | Preserved | 24 (49%) | 13 (100%) | 1 (5.9%) | 10 (52.6%) | 0.0001 |
| Sacrificed | 25 (51%) | 0 (0) | 16 (94.1%) | 9 (47.4%) |
Eight patients without follow-up for endocrine functions.
Seventeen patients were excluded from infundibulum status analysis due to stalk not identified/prior sacrifice/indeterminate. Forty-nine patients were included.
Bold values indicate significant level less than 0.05.
Table 4. Tumor control.
| Epoch | Total N = 66 | 1 ( N = 20) | 2 ( N = 23) | 3 ( N = 23) | ||
|---|---|---|---|---|---|---|
| Adjuvant therapy for STR/NTR | 18 (27.3%) | 7 (35.0%) | 5 (21.7%) | 6 (26.1%) | 0.62 | |
| Recurrence/Progression a | 13 (22.4%) | 6 (33.3%) | 5 (21.7%) | 2 (9.5%) | 0.189 | |
| Median time to recurrence (months, range) | 11 (3–172) | 10 (4–172) | 10 (3–52) | 18 (12–24) | 0.776 | |
| Treatment of recurrence/progression | Ommaya reservoir | 4 | 1 | 0 | ||
| Intracystic P32 | 3 | 0 | 0 | |||
| Reoperation | 2 | 3 | 1 | |||
| Radiotherapy (FSRT) | 3 | 3 | 1 | |||
| Observed | 0 | 0 | 0 |
Eight patients without follow-up data.
Baseline Characteristics by Epoch
The earliest epoch included 20 patients ranging from 2005 to 2009, the middle epoch included 23 patients from 2010 to 2015, and the latest epoch consisted of 23 patients from 2016 to 2020. Across epochs, there were no significant differences in age, sex, prior surgical status, prior radiotherapy, or prior intracystic therapy for the treatment of craniopharyngiomas. There was a significantly higher number of patients presenting with vision loss as the initial symptom in the 2005 to 2009 and 2010 to 2015 epochs compared with 2016 to 2020 (100 vs. 100 vs. 73.9%, p = 0.002). No other significant differences were observed across epochs in terms of presenting symptoms of headache, endocrinopathy, weight gain, fatigue, and memory or cognitive complaints. Additionally, across epochs, no statistical differences were seen in max tumor diameter, tumor consistency, hypothalamic involvement, or histologic subtype of craniopharyngioma ( Table 1 ).
Surgical Outcomes by Epoch
GTR was significantly higher in the second and third epoch compared with the first (20 vs. 65.2 vs. 52.2%; p = 0.042). No significant difference in complications related to vision was seen across the epochs ( Table 2 ). Postoperative complications across different epochs showed no significant differences in the development of new hydrocephalus after surgery, meningitis, hyponatremia, seizures, rhinological complications, carotid injury, or stroke. Eight patients in the first epoch had postoperative CSF leak and one patient in the middle epoch had a postoperative CSF leak, while no patients had CSF leak in the latest epoch (40 vs. 4.5 vs. 0%, p = 0.0001). There was a significant reduction in LOS for the latest epoch (6 vs. 6 vs. 4, p = 0.03) and discharge to rehab across epochs (25 vs. 4.3 vs. 8.7%, p = 0.001) ( Table 2 ).
Stalk Status and Postoperative Endocrine Outcomes
Preservation of pituitary stalk was highest in the first cohort, followed by the third cohort. (100 vs. 5.9 vs. 52.6%; p = 0.0001) ( Table 3 ). New development of permanent DI did not significantly change across epochs (37.5 vs. 68.4 vs. 71.4%; p = 0.078). Preservation of normal endocrine function was significantly higher in the first and third epochs compared with the second epoch (25 vs. 0 vs. 23.8%; p = 0.001) ( Table 3 ).
Tumor Control
Across epochs, there was no significant difference in adjuvant therapy for STR/NTR after surgical intervention (35.0 vs. 21.7 vs. 26.1%; p = 0.62). Additionally, no differences were seen in tumor recurrence/progression or median time to recurrence (33.3 vs. 21.7 vs. 9.5%; p = 0.189) ( Table 4 ). No differences were seen with Kaplan-Meier progression-free survival across epochs with log rank test ( p = 0.368) ( Fig. 1 ).
Fig. 1.
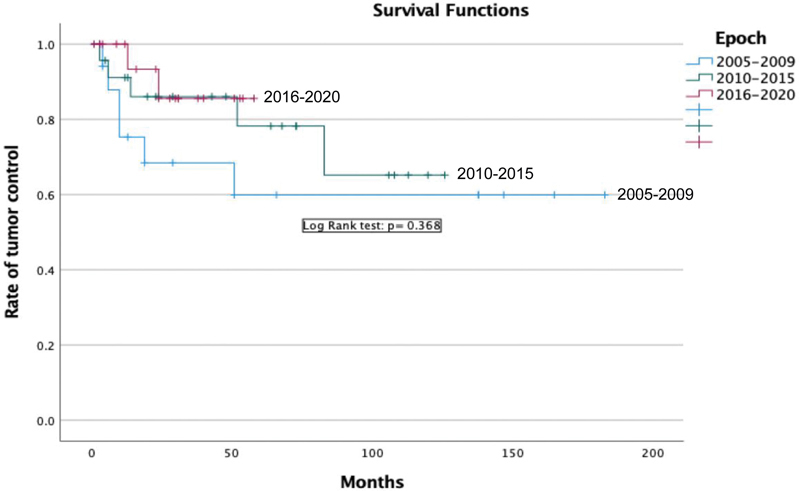
Progression-free survival across three epochs after endoscopic endonasal resection.
Effects of Stalk Preservation/Sacrifice on Clinical Outcomes
A total of 49 patients were included in the subgroup analysis after exclusion of patients with prior surgery and unclear documentation regarding stalk sacrifice or preservation. Patients were divided based on stalk sacrifice ( N = 25) and stalk preservation ( N = 24). In patients with stalk preservation, there was expectedly a significantly higher number retaining normal endocrine function (40.9 vs. 0%; p = 0.001) and less patients with normal preoperative hormonal functions becoming panhypopituitarism (18.2 vs. 56.0% p = 0.001). New permanent postoperative DI developed in 75% of the patients with stalk sacrifice versus 41.7% in the stalk preservation group ( p = 0.039).
GTR was achieved at a significantly higher rate in the stalk sacrifice group (28 vs. 70.8%, p = 0.005) whereas NTR was significantly higher in the stalk preservation group (52 vs. 12.5%; p = 0.005). The stalk preservation group underwent adjuvant radiotherapy more often (48 vs. 12.5%; p = 0.012). The median follow-up is significantly longer in the stalk sacrifice group (38 vs. 31.5 months; p = 0.005). At last follow-up, there was no difference in recurrence/progression rate between stalk preservation and sacrifice group ( Table 5 ).
Table 5. Effects of stalk preservation on hormonal outcomes, recurrences, and usage of adjuvant radiotherapy.
| Stalk status N = 49 patients |
Stalk sacrifice ( N = 25) | Stalk preservation ( N = 24) | ||
|---|---|---|---|---|
| Tumor diameter, cm (median, quartile) | 3.0 (2.3–3.3) | 2.8 (2.0–3.0) | 0.415 | |
| Anterior pituitary function a | Normal endocrine function | 0 (0%) | 9 (40.9%) | 0.001 |
| Normal to partial hypopituitarism | 7 (28.0%) | 5 (22.7%) | ||
| Stable hypopituitarism | 3 (12.0%) | 0 (0%) | ||
| Partial to hypopituitarism | 1 (4.0%) | 1 (4.5%) | ||
| Normal to panhypopituitarism | 14 (56.0%) | 4 (18.2%) | ||
| Stable partial hypopituitarism | 0 (0%) | 3 (13.6%) | ||
| DI b | New permanent | 18 (78.3%) | 11 (45.8%) | 0.036 |
| No permanent DI | 5 (21.7%) | 13 (54.2%) | ||
| Resection | STR | 3 (12.0) | 7 (29.2%) | 0.001 |
| NTR | 3 (12.0%) | 12 (50.0%) | ||
| GTR | 19 (76.0%) | 5 (20.8%) | ||
| Recurrence/progression | 4 (16.0%) | 6 (25.0%) | 0.496 | |
| Adjuvant radiotherapy | 2 (8.0%) | 11 (45.8%) | 0.004 | |
| Median follow-up duration, month (range) | 38 (22–75.5) | 31.5 (12.3–96) | 0.005 |
Abbreviations: DI, diabetes insipidus; GTR, gross total resection; NTR, near total resection; STR, subtotal resection.
Three patients in stalk preservation group do not have hormonal outcomes.
Two patients in the stalk sacrifice group do not have follow-up data.
Bold values indicate significant level less than 0.05.
Discussion
In this study, we examined practice patterns across 16 years using the endoscopic endonasal approach for the resection of craniopharyngiomas, divided into three epochs: 2005 to 2009, 2010 to 2015, and 2016 to 2020. Of note, this period also corresponded to numerous innovations and advancements in the field of endoscopic skull base surgery itself, as well as the development of new technologies. The initial epoch consisted of a period of early experience and learning, development of endonasal cranial base repair techniques, and more conservative resection rates with more frequent stalk preservation. This led to reduced rates of postoperative hypopituitarism, but also high rates of subtotal/NTR. The middle epoch, during which increasing surgical experience was gained, involved more aggressive resections, with higher rates of GTR and stalk sacrifice. The more recent epoch likely reflects a stable high-volume practice with incorporation of surgical experience and modern endoscopic techniques, resulting in high rates of GTR with improved stalk preservation. This in turn led to increased rates of preserving normal endocrine function and no worsened recurrence rate when compared with the middle cohort.
Learning Curve of Craniopharyngioma
The learning curve for expertise in the endoscopic management of craniopharyngiomas can be lengthy, with optimal surgical outcomes requiring extensive surgical experience. 2 14 16 17 18 19 20 In an early and late cohort of patients with craniopharyngioma, Ding et al found that neurosurgeons achieved a reduced rate of tumor recurrence and significant reduction in operative time after 17 patients. The cohorts showed no significant differences regarding GTR, panhypopituitarism, and postoperative CSF leak. 14 In a case series of 103 craniopharyngioma patients, Cavallo et al highlighted that careful selection of patients is paramount in the management of this disease. In their early cohort, lack of experience with the endonasal view led to two patients requiring further transcranial resection a week after surgery. 21 Younus et al studied the learning curve of endoscopic approach for skull base tumors in 1,000 cases, of which there were 73 craniopharyngiomas. Patients were split into two cohorts. When the authors examined learning curve in craniopharyngiomas ( N = 32 in first and N = 41 in second cohort), they found that GTR was significantly increased in the second cohort and lumbar drain usage was decreased. 15 Furthermore, our previous study examining the learning curve found that after 20 cases there is a significant reduction in postoperative CSF leak and major neurological complications. 2
In our current study, we report an overall GTR rate of 47%, similar to the average rate of 55% GTR across the literature. 22 The institutional surgical philosophy in 2005 to 2009 was conservative resection to preserve the pituitary stalk . This was reflected in our low rate of GTR (20%) but high rate of stalk preservation (100%). During the middle epochs from 2010 to 2015, the surgical goal transitioned to aim for GTR (65%). The transition led to a lower rate of stalk preservation (5.9%) within our institution. However, our latest epoch shows a significant higher rate of GTR when compared with first epoch (20 vs. 52%; p = 0.04) and higher rate of stalk preservation (5.9 vs. 52%) along with improved preservation of normal pituitary endocrine function (23.8 vs. 0%) when compared with second epoch. The latter finding supports our improvement in the management of craniopharyngiomas with experience, specifically regarding the preservation of the pituitary stalk and endocrine function in GTR. Despite the higher stalk preservation in the latest cohort when compared with the middle cohort (5.9 vs. 52.6%) there was no difference in the rate of permanent DI (68.4 vs. 71.4%). We believe that although the pituitary stalk was structurally preserved, the increased aggressiveness to achieve GTR could have comprised small feeding arteries to the pituitary stalk. In a review by Grewal et al, authors found that higher postoperative DI is consistently associated with GTR when compared with STR in different case series. 9 A recent study published in 2021, Godil et al found that DI developed in 85.3% of patients with GTR and 50% of the patients with STR. 23
We achieved and maintained a low rate of postoperative CSF leak with the refinement of the “button graft” repair technique, innovated at our institution and described in a prior publication. 24 We report an overall postoperative CSF leak of 12%, which is lower than a previously reported rate of 18.4% in a meta-analysis of 149 patients treated with the endoscopic transsphenoidal approach. 10 Our middle cohort showed one patient (4.3%) with postoperative CSF leak and no patients (0/23) with leaks in the latest cohort, again supporting a progressive improvement in technique. In summary, our institution's postoperative CSF leak rate is 1/46 (2.2%) since 2010 and no patients had CSF leaks in the latest epoch (2016–2020). This also likely reflects the increased use of vascularized pedicled nasoseptal flaps in the middle and later epochs.
GTR versus STR and Radiotherapy
There is an ongoing debate regarding GTR versus STR plus radiotherapy in the management of craniopharyngiomas. 13 25 26 27 The goal of surgery is to perform maximal safe resection while minimizing postoperative morbidity and maximizing function and quality of life. 21 28 29 30 Hypothalamic invasion and lack of a surgical plane between the tumor and hypothalamus are the main limiting factors in attaining GTR. 31 GTR in craniopharyngiomas is associated with a higher risk of postoperative morbidity compared with patients undergoing STR and radiotherapy. 32 A meta-analysis of 759 cases of adult craniopharyngioma by Dandurand et al demonstrated that the rates of recurrence were significantly higher when GTR was compared to STR, and when STR with radiation was compared with STR alone. However, no difference was found in the recurrence rate between GTR group when compared to STR with radiation (17 vs. 27%). 33
Lack of clear advantages of GTR in terms of recurrence rate and overall survival, higher postoperative morbidity with GTR, and impact on patients' quality of life have led to some surgeons' trend toward performing STR and radiotherapy in recent years. 30 32 34 35 36 A recent systematic review and consensus statement of the European Association of Neurosurgical Societies recommended performing GTR in tumors without hypothalamic infiltration and performing STR in combination with adjuvant radiotherapy in tumors with infiltration of the hypothalamus (Level C of evidence).
In our cohort, GTR was achieved in 31 (47%) patients while 18 (27.3%) patients underwent adjuvant radiation after STR. Recurrence after complete resection and progression after STR/NTR occurred in 13 (22.4%) patients. When compared across cohorts, there was no significant difference in the usage of adjuvant radiation (35 vs. 22 vs. 30%; p = 0.13) or progression-free survival ( p = 0.368) ( Fig. 1 ). The above results indicate that both strategies can be appropriate for the treatment of craniopharyngiomas.
Stalk Preservation versus Stalk Sacrifice
Stalk preservation and sacrifice are two distinct strategies for the management of craniopharyngiomas. 5 11 37 In a modern series of craniopharyngioma patients undergoing the endoscopic approach, Ordóñez-Rubiano et al examined the effects of stalk preservation on the rate of GTR and pituitary function. 5 The authors found that stalk preservation was associated with increased STR, tumor progression, and usage of radiation. In patients with stalk preservation, 33% reported no anterior pituitary dysfunction and 50% were free from DI. Our data reveals similar findings. Specifically, 40% of patients in our stalk preservation cohort maintained normal anterior pituitary function and 54% were free of DI at last follow-up. Furthermore, only four patients (18.2%) in the stalk preservation group with normal preoperative anterior pituitary function developed panhypopituitarism in comparison with 14 patients (56%) with stalk sacrifice ( p = 0.001). As expected, more patients with stalk preservation received adjuvant radiation after surgery (45.8 vs. 8.0%; p = 0.004) and there was no significant difference in tumor recurrence at the last follow-up.
Limitations
The limitations of this study include small patient cohorts when divided across the three epochs, as well as the fact this this was a single-institution retrospective experience which may decrease the generalizability of the findings. The patients included have limited follow-up, especially in the latest cohort, which may affect the true recurrence rate in this later epoch with greater stalk preservation. Due to rapid advancement of endoscopic experience and equipment in treating sellar and parasellar pathology, the surgical outcomes across epochs could also be confounded by the available technology at the time. While we adjusted for confounding effects of repeated surgeries on pituitary stalk function, we used operative findings and notes to designate stalk status, which could be unreliable. We did not use a craniopharyngioma classification system. Additionally, in the subgroup analysis between stalk sacrifice and preservation group, we did not account for the significant rate of adjuvant radiotherapy in the stalk preservation group on long-term DI and anterior pituitary function. However, studies have suggested radiotherapy to be safe with minimal hormonal deterioration. 38 39
Despite these limitations, we believe our results could help guide a surgeon's approach in the treatment of craniopharyngiomas.
Conclusion
Our results indicate that there is a continuous learning curve in the management of craniopharyngioma. Higher GTR with greater pituitary stalk preservation, normal hormonal function, and lower rates of postoperative CSF leak can be achieved with increased surgical experience.
Conflict of Interest J.J.E. receives royalties for Evans Rotatable Instrument set and M.K. receives royalties for his book titled “The Surgical Handbook.” The remaining authors report no conflict of interest.
Previous Presentation
This manuscript was presented as a podium presentation at the 31 st annual NASBS meeting on February 19 th , 2022 in Phoenix, Arizona.
References
- 1.Momin A A, Recinos M A, Cioffi G. Descriptive epidemiology of craniopharyngiomas in the United States. Pituitary. 2021;24(04):517–522. doi: 10.1007/s11102-021-01127-6. [DOI] [PubMed] [Google Scholar]
- 2.Kshettry V R, Do H, Elshazly K. The learning curve in endoscopic endonasal resection of craniopharyngiomas. Neurosurg Focus. 2016;41(06):E9. doi: 10.3171/2016.9.FOCUS16292. [DOI] [PubMed] [Google Scholar]
- 3.Karavitaki N, Cudlip S, Adams C B, Wass J A. Craniopharyngiomas. Endocr Rev. 2006;27(04):371–397. doi: 10.1210/er.2006-0002. [DOI] [PubMed] [Google Scholar]
- 4.Varlotto J, DiMaio C, Grassberger C. Multi-modality management of craniopharyngioma: a review of various treatments and their outcomes. Neurooncol Pract. 2016;3(03):173–187. doi: 10.1093/nop/npv029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ordóñez-Rubiano E G, Forbes J A, Morgenstern P F.Preserve or sacrifice the stalk? Endocrinological outcomes, extent of resection, and recurrence rates following endoscopic endonasal resection of craniopharyngiomas J Neurosurg 2018131041–9.(e-pub ahead of print). 10.3171/2018.6.JNS18901 [DOI] [PubMed] [Google Scholar]
- 6.Fong R P, Babu C S, Schwartz T H. Endoscopic endonasal approach for craniopharyngiomas. J Neurosurg Sci. 2021;65(02):133–139. doi: 10.23736/S0390-5616.21.05097-9. [DOI] [PubMed] [Google Scholar]
- 7.Almeida J P, DE Andrade E J, Vescan A. Surgical anatomy and technical nuances of the endoscopic endonasal approach to the anterior cranial fossa. J Neurosurg Sci. 2021;65(02):103–117. doi: 10.23736/S0390-5616.20.05086-9. [DOI] [PubMed] [Google Scholar]
- 8.Fan J, Liu Y, Pan J.Endoscopic endonasal versus transcranial surgery for primary resection of craniopharyngiomas based on a new QST classification system: a comparative series of 315 patients J Neurosurg 2021135051–12.(e-pub ahead of print). 10.3171/2020.7.JNS20257 [DOI] [PubMed] [Google Scholar]
- 9.Grewal M R, Spielman D B, Safi C. Gross total versus subtotal surgical resection in the management of craniopharyngiomas. Allergy Rhinol (Providence) 2020;11:2.152656720964158E15. doi: 10.1177/2152656720964158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Komotar R J, Starke R M, Raper D M, Anand V K, Schwartz T H. Endoscopic endonasal compared with microscopic transsphenoidal and open transcranial resection of craniopharyngiomas. World Neurosurg. 2012;77(02):329–341. doi: 10.1016/j.wneu.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Yaşargil M G, Curcic M, Kis M, Siegenthaler G, Teddy P J, Roth P. Total removal of craniopharyngiomas. Approaches and long-term results in 144 patients. J Neurosurg. 1990;73(01):3–11. doi: 10.3171/jns.1990.73.1.0003. [DOI] [PubMed] [Google Scholar]
- 12.Spoudeas H A, Saran F, Pizer B. A multimodality approach to the treatment of craniopharyngiomas avoiding hypothalamic morbidity: a UK perspective. J Pediatr Endocrinol Metab. 2006;19 01:447–451. doi: 10.1515/jpem.2006.19.4.447. [DOI] [PubMed] [Google Scholar]
- 13.Samii M, Tatagiba M. Surgical management of craniopharyngiomas: a review. Neurol Med Chir (Tokyo) 1997;37(02):141–149. doi: 10.2176/nmc.37.141. [DOI] [PubMed] [Google Scholar]
- 14.Ding H, Gu Y, Zhang X. Learning curve for the endoscopic endonasal approach for suprasellar craniopharyngiomas. J Clin Neurosci. 2017;42:209–216. doi: 10.1016/j.jocn.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 15.Younus I, Gerges M M, Uribe-Cardenas R. How long is the tail end of the learning curve? Results from 1000 consecutive endoscopic endonasal skull base cases following the initial 200 cases. J Neurosurg. 2020;134(03):750–760. doi: 10.3171/2019.12.JNS192600. [DOI] [PubMed] [Google Scholar]
- 16.Barkhoudarian G, Laws E R. Craniopharyngioma: history. Pituitary. 2013;16(01):1–8. doi: 10.1007/s11102-012-0402-z. [DOI] [PubMed] [Google Scholar]
- 17.Koc K, Anik I, Ozdamar D, Cabuk B, Keskin G, Ceylan S.The learning curve in endoscopic pituitary surgery and our experience Neurosurg Rev 20062904298–305., discussion 305 [DOI] [PubMed] [Google Scholar]
- 18.Qureshi T, Chaus F, Fogg L, Dasgupta M, Straus D, Byrne R W. Learning curve for the transsphenoidal endoscopic endonasal approach to pituitary tumors. Br J Neurosurg. 2016;30(06):637–642. doi: 10.1080/02688697.2016.1199786. [DOI] [PubMed] [Google Scholar]
- 19.Robins J MW, Alavi S A, Tyagi A K, Nix P A, Wilson T M, Phillips N I. The learning curve for endoscopic trans-sphenoidal resection of pituitary macroadenomas. A single institution experience, Leeds, UK. Acta Neurochir (Wien) 2018;160(01):39–47. doi: 10.1007/s00701-017-3355-1. [DOI] [PubMed] [Google Scholar]
- 20.Chi F, Wang Y, Lin Y, Ge J, Qiu Y, Guo L. A learning curve of endoscopic transsphenoidal surgery for pituitary adenoma. J Craniofac Surg. 2013;24(06):2064–2067. doi: 10.1097/SCS.0b013e3182a24328. [DOI] [PubMed] [Google Scholar]
- 21.Cavallo L M, Frank G, Cappabianca P. The endoscopic endonasal approach for the management of craniopharyngiomas: a series of 103 patients. J Neurosurg. 2014;121(01):100–113. doi: 10.3171/2014.3.JNS131521. [DOI] [PubMed] [Google Scholar]
- 22.Schmidk And Sweet Operative Neurosurgical Techniques7th ed. Philadelphia, PA 19103-2899: ELSEVIER, 197–200
- 23.Godil S S, Tosi U, Gerges M.Long-term tumor control after endoscopic endonasal resection of craniopharyngiomas: comparison of gross-total resection versus subtotal resection with radiation therapy J Neurosurg 2021136051–9.(e-pub ahead of print). 10.3171/2021.5.JNS202011 [DOI] [PubMed] [Google Scholar]
- 24.Luginbuhl A J, Campbell P G, Evans J, Rosen M. Endoscopic repair of high-flow cranial base defects using a bilayer button. Laryngoscope. 2010;120(05):876–880. doi: 10.1002/lary.20861. [DOI] [PubMed] [Google Scholar]
- 25.Müller H L.The diagnosis and treatment of craniopharyngioma Neuroendocrinology 2020110(9-10):753–766. [DOI] [PubMed] [Google Scholar]
- 26.Tang B, Xie S H, Xiao L M. A novel endoscopic classification for craniopharyngioma based on its origin. Sci Rep. 2018;8(01):10215. doi: 10.1038/s41598-018-28282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hankinson T C, Palmeri N O, Williams S A. Patterns of care for craniopharyngioma: survey of members of the American Association of Neurological Surgeons. Pediatr Neurosurg. 2013;49(03):131–136. doi: 10.1159/000357783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koutourousiou M, Gardner P A, Fernandez-Miranda J C, Tyler-Kabara E C, Wang E W, Snyderman C H. Endoscopic endonasal surgery for craniopharyngiomas: surgical outcome in 64 patients. J Neurosurg. 2013;119(05):1194–1207. doi: 10.3171/2013.6.JNS122259. [DOI] [PubMed] [Google Scholar]
- 29.Patel K S, Raza S M, McCoul E D. Long-term quality of life after endonasal endoscopic resection of adult craniopharyngiomas. J Neurosurg. 2015;123(03):571–580. doi: 10.3171/2014.12.JNS141591. [DOI] [PubMed] [Google Scholar]
- 30.Puget S, Garnett M, Wray A. Pediatric craniopharyngiomas: classification and treatment according to the degree of hypothalamic involvement. J Neurosurg. 2007;106 01:3–12. doi: 10.3171/ped.2007.106.1.3. [DOI] [PubMed] [Google Scholar]
- 31.Prieto R, Pascual J M, Rosdolsky M. Craniopharyngioma adherence: a comprehensive topographical categorization and outcome-related risk stratification model based on the methodical examination of 500 tumors. Neurosurg Focus. 2016;41(06):E13. doi: 10.3171/2016.9.FOCUS16304. [DOI] [PubMed] [Google Scholar]
- 32.Sughrue M E, Yang I, Kane A J. Endocrinologic, neurologic, and visual morbidity after treatment for craniopharyngioma. J Neurooncol. 2011;101(03):463–476. doi: 10.1007/s11060-010-0265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dandurand C, Sepehry A A, Asadi Lari M H, Akagami R, Gooderham P. Adult craniopharyngioma: case series, systematic review, and meta-analysis. Neurosurgery. 2018;83(04):631–641. doi: 10.1093/neuros/nyx570. [DOI] [PubMed] [Google Scholar]
- 34.Schoenfeld A, Pekmezci M, Barnes M J. The superiority of conservative resection and adjuvant radiation for craniopharyngiomas. J Neurooncol. 2012;108(01):133–139. doi: 10.1007/s11060-012-0806-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang E W, Zanation A M, Gardner P A.ICAR: Endoscopic Skull-Base Surgery Wiley Online Library; 2019:145–365 [Google Scholar]
- 36.Yang I, Sughrue M E, Rutkowski M J. Craniopharyngioma: a comparison of tumor control with various treatment strategies. Neurosurg Focus. 2010;28(04):E5. doi: 10.3171/2010.1.FOCUS09307. [DOI] [PubMed] [Google Scholar]
- 37.Xiao G, Yuan X, Yuan J. Pituitary stalk management during the microsurgery of craniopharyngiomas. Exp Ther Med. 2014;7(05):1055–1064. doi: 10.3892/etm.2014.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harrabi S B, Adeberg S, Welzel T. Long term results after fractionated stereotactic radiotherapy (FSRT) in patients with craniopharyngioma: maximal tumor control with minimal side effects. Radiat Oncol. 2014;9:203. doi: 10.1186/1748-717X-9-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Astradsson A, Munck Af Rosenschöld P, Feldt-Rasmussen U. Visual outcome, endocrine function and tumor control after fractionated stereotactic radiation therapy of craniopharyngiomas in adults: findings in a prospective cohort. Acta Oncol. 2017;56(03):415–421. doi: 10.1080/0284186X.2016.1270466. [DOI] [PubMed] [Google Scholar]


