Abstract
Costa Rica is near malaria elimination. This achievement has followed shifts in malaria health policy. Here, we evaluate the impacts that different health policies have had on malaria transmission in Costa Rica from 1913 to 2018. We identified regime shifts and used regression models to measure the impact of different health policies on malaria transmission in Costa Rica using annual case records. We found that vector control and prophylactic treatments were associated with a 50% malaria case reduction in 1929–1931 compared with 1913–1928. DDT introduction in 1946 was associated with an increase in annual malaria case reduction from 7.6% (1942–1946) to 26.4% (1947–1952). The 2006 introduction of 7-day supervised chloroquine and primaquine treatments was the most effective health policy between 1957 and 2018, reducing annual malaria cases by 98% (2009–2018) when compared with 1957–1968. We also found that effective malaria reduction policies have been sensitive to natural catastrophes and extreme climatic events, both of which have increased malaria transmission in Costa Rica. Currently, outbreaks follow malaria importation into vulnerable areas of Costa Rica. This highlights the need to timely diagnose and treat malaria, while improving living standards, in the affected areas.
Key words: Breakpoints, El Niño Southern Oscillation, mass drug administration, malaria elimination, regime shifts
Introduction
Costa Rica, together with El Salvador (Bennett and Smith, 2018; Burton et al., 2018) and Belize (WHO, 2018), are very likely to soon achieve malaria elimination in Mesoamerica, a highly vulnerable region to vector-borne tropical diseases, including malaria (Hotez et al., 2014). Plasmodium vivax (Grassi & Feletti) is the most prevalent species in Costa Rica, accounting for more than 95% of the cases since reliable records exist (Vargas, 2001; Marín Rodríguez and Chaves, 2019). Malaria elimination in Costa Rica follows a national (Grupo Técnico Nacional de Enfermedades Vectoriales, 2015), and regional (Herrera et al., 2015), commitment to reduce malaria transmission. Malaria elimination also occurs against a background where only half of Costa Rica area is assumed to have optimal conditions for malaria transmission, but where around 70% of the population lives in areas that are assumed to be protected from malaria by environmental conditions that limit malaria transmission, mainly elevation associated low temperatures (Grupo Técnico Nacional de Enfermedades Vectoriales, 2015; Grupo Técnico Nacional de Enfermedades Vectoriales, 2016). Low temperatures mainly limit malaria parasite development in vectors (Patz and Olson, 2006; Chaves, 2017). Low temperatures might also limit the distribution and abundance of Anopheles albimanus Wiedemann, the main malaria vector in Costa Rica (Vargas, 2001) and Mesoamerica (Rigg et al., 2019).
Costa Rica is a middle-income country (The Economist Intelligence Unit, 2018), where malaria elimination is happening under the context universal health coverage administered through decades-old, and robust, social security trust (Caja Costarricense de Seguro Social, CCSS). This universal coverage health system has achieved exceptional successes in health indicators, for example, life expectancy at birth (Rosero-Bixby and Dow, 2016) and mortality at birth (Kruk et al., 2018), and has health indicators at the level of wealthier nations in Europe and East Asia (Knaul et al., 2015). Along these lines understanding the conditions that have led Costa Rica to the current malaria pre-elimination stage are of great importance, as malaria health policies implemented in Costa Rica could serve to improve regional malaria elimination plans in Mesoamerica. Moreover, the analysis of malaria health policies in Costa Rica could also provide insights for malaria elimination at a global scale, by highlighting health policy shifts associated with malaria transmission reduction and elimination (Greenwood, 2009).
Here, we analyze three historical annual malaria time series that cover most of the historical period from 1913 to 2018. For the analysis, we compiled a list of malaria health policy shifts and natural events that are assumed to have affected malaria transmission during the study period. With these data we test the hypothesis that malaria health policy shifts, and large scale natural events, have impacted malaria transmission in Costa Rica, eventually leading to its near elimination stage in Costa Rica. We then use time series tools to identify breakpoints, i.e. the time when temporal dynamics of a natural system undergo structural and functional changes (Scheffer, 2009). The regime shift analysis allows the identification of different malaria transmission regimes, i.e. time periods when malaria transmission had persistent dynamical behaviour through time (Chaves et al., 2012). Then, we employ regression methods to quantitatively assess the impacts of different health policies on reducing malaria transmission. In other words, an impactful malaria health policy is expected to temporally coincide with, or to be followed by, a regime shift. In contrast, an ineffective malaria health policy is not expected to lead to a regime shift. A successful malaria health policy is expected to be followed by a regime with a reduced number of malaria cases when compared with the regime prior to the policy shift. By contrast, a detrimental health policy shift, or a natural environmental catastrophe, is expected to be followed by a regime with an increased number of malaria cases when compared with the regime prior to the policy shift.
Materials and methods
Large-scale natural events and malaria health policy shifts in Costa Rica
Malaria likely arrived in Costa Rica when its territory was part of the Spanish Empire (Frantzius, 1868; Adams, 1996). However, interest on malaria transmission reduction only started in the 1910s when malaria was a major barrier for the successful commodification of bananas by the United Fruit Company (UFCo) (Lynn, 1913, 1914, 1915; Aliano, 2006). The first malaria survey, which estimated malaria prevalence at 19.0% among UFCo banana plantation workers, was done in 1927 (Clarck, 1928). In the 1930s the Rockefeller Foundation funded preliminary research, evaluating the feasibility of malaria eradication, that allowed the mapping of malaria endemicity (Kumm and Ruiz, 1939) in Costa Rica. The Rockefeller Foundation also promoted joint ventures that led to water drainage and ditching in settlements of Guanacaste province in Northwest Costa Rica (Guzman, 1940), the area with the largest malaria burden in the 1930s (Kumm and Ruiz, 1939). In 1941, the CCSS was created by the government led by Dr Rafael Angel Calderón Guardia (Palmer, 2003), positively impacting health policies in Costa Rica, mainly through the creation of a universal coverage health system (Knaul et al., 2015), but also including the development of a rudimentary epidemiologic surveillance system which recorded malaria cases at the time (Peña Chavarría and Guerrero Arguedas, 1953a,b). In 1954 Costa Rica joined the Pan-American Health Organization (PAHO) Continental Malaria Eradication Program and in 1955 the World Health Organization (WHO) sponsored Global Malaria Eradication Campaign (GMEC) (International Cooperation Administration Expert Panel on Malaria, 1961; Carter et al., 2015), creating in 1957 the ‘Servicio Nacional de Erradicación de la Malaria’ (SNEM), i.e. the national malaria eradication program. Since 1957 all malaria cases have been recorded by ‘Vigilancia de la Salud’ at ‘Ministerio de Salud’, i.e. the health surveillance division at Costa Rica´s Ministry of Health (Vargas, 2001). Table 1 shows a detailed list of the major malaria health policy shifts and large scale natural phenomena associated with malaria changes in Costa Rica between 1913 and 2018. Briefly, we compiled information from the published literature where we employed the terms ‘malaria’ and ‘Costa Rica’ in PubMed, Science Citation Index, Scientific Electronic Library Online and Google Scholar. We also reviewed policy documents available at Costa Rica`s Ministry of Health. In addition to events listed in Table 1, we also considered the impacts of different El Niño Southern Oscillation (ENSO) phases on malaria transmission, provided this large scale climatic phenomenon has been identified as a major driver of malaria epidemics in the region (Bouma et al., 1997; Bouma and Dye, 1997; Hurtado et al., 2014, 2018). We classified every year as belonging to a given ENSO phase (hot, cold, normal) if such phase was present in two or more quarters of the year according to the classification by Wolter and Timlin (2011) for the period between 1913 and 1952. From 1957 to 2018 we classified years by ENSO phase based on more than 4 continuous months of reported cold or hot or normal ENSO activity according to the US National Oceanic and Atmospheric Administration classification (NOAA, 2019)
Table 1.
Policy shifts and natural events affecting malaria transmission in Costa Rica (1913–2018)
| Number | Year | Policy shift (PS) or Natural event (NE) | Reference |
|---|---|---|---|
| 1 | 1914 | NE: Deforestation in Valle de la Estrella for Banana plantation development by the United Fruit Company | (Lynn, 1915) |
| 2 | 1928 | PS: Vector control through larval source reduction, Paris green treatment of undrainable water bodies, pyrethroid use against adult mosquitoes and unsupervised prophylactic mass treatment, with chloroquine and pamaquine, to banana plantation workers by the United Fruit Company | (Salisbury, 1929; Salisbury, 1930) |
| 3 | 1939 | PS: Water drainage and ditch building, in large endemic settlements of Gunacaste province, implemented as a joint venture between the Rockefeller Foundation and the Costa Rican Government | (Guzman, 1940) |
| 4 | 1941 | PS: Creation of ‘Caja Costarricense de Seguro Social’, i.e. the Costa Rican Social Security Trust (CCSS), universalization of health care and transference of health services to the CCSS | (Palmer, 2003) |
| 5 | 1946 | PS: DDT use in banana plantation associated human settlements by the United Fruit Company | (Lieske, 1950; Peña Chavarría and Guerrero Arguedas, 1953a; ,b) |
| 6 | 1957 | PS: Creation of ‘Servicio nacional de erradicación de la malaria’, i.e. national eradication malaria program (SNEM), and beginning of the nationwide insecticide residual spraying (IRS) with DDT use in endemic areas | (Vargas, 2001; Carter et al., 2015) |
| 7 | 1963 | PS: Operational defunding of the SNEM | (Vargas, 2001) |
| 8 | 1967 | PS: End of the SNEM operational defunding | (Vargas, 2001) |
| 9 | 1985 | PS: End of DDT use for IRS | (Vargas, 2001) |
| 10 | 1990 | PS: IRS and vector control constrained to high risk and malaria transmission areas | (Vargas, 2001) |
| 11 | 1991 | NE: Limón Earthquake | (Sáenz et al., 2012) |
| 12 | 1997 | PS: Change from the 14-day chloroquine (25 mg kg−1 divided in 3 days) plus primaquine (0.25 mg kg−1 day−1) treatment to the 5-day (25 mg chloroquine kg−1 divided in 3 days and 0.25 mg primaquine kg−1 day−1) radical cure | (Castro-Sancho et al., 2002; Ávila-Agüero, 2008) |
| 13 | 2006 | PS: Gradual change to the 7-day supervised chloroquine (25 mg kg-1 divided in 3 days) plus primaquine treatment (0.5 mg kg-1 day-1) and Mass Drug Administrations in Talamanca County | (Ávila-Agüero, 2008; Marín Rodríguez and Chaves, 2019) |
| 14 | 2008 | PS: The 7-day supervised chloroquine plus primaquine treatment becomes established in the Región Huétar Caribe and Región Huétar Norte. | (Marín Rodríguez and Chaves, 2019) |
| 15 | 2009 | PS: IRS and vector control became reactive to malaria outbreaks | (Grupo Técnico Nacional de Enfermedades Vectoriales, 2015, 2016) |
All health policy shifts were implemented by the Costa Rican Government unless otherwise noted. In the table, number indicates the event order, year the time when the event occurred and reference the source describing the event.
Malaria, funding and vector control data
The first dataset spans 1913–1931 (Fig. 1A) and it is based on data published in the Annual Reports of the United Fruit Company Medical Department, which recorded the total number of malaria cases diagnosed at hospitals and dispensaries run by UFCo in areas where the company had banana plantations, which were mainly located on the Caribbean coast of Costa Rica (Aliano, 2006). These data had separated records for ambulatory patients (mild malaria cases) and inpatients (severe malaria cases needing attention in hospitals). The second dataset spans 1942–1952 and is the annual time series of malaria inpatients attended at Hospital San Juan de Dios (HSJD), the main CCSS hospital in Costa Rica (Fig. 1B). Raw data for this period were published in the scientific literature (Peña Chavarría and Guerrero Arguedas, 1953a,b). The third dataset spans 1957–2018 (Fig. 1C) and records are kept by the health surveillance division at Costa Rica`s Ministry of Health. All three datasets are based on malaria cases confirmed by blood slide examination (Salisbury, 1930; Peña Chavarría and Guerrero Arguedas, 1953a,b; Vargas, 2001).
Fig. 1.
Annual malaria cases, funding and people protected against vectors (A) Total (1913–1932) and inpatient (1912–1932) malaria cases reported by the Costa Rica Division in the Annual Reports of the United Fruit Company Medical Department. (B) Inpatient malaria cases (1942–1952) at the Hospital San Juan de Dios, San José, Costa Rica. (C) Total malaria cases in Costa Rica (1957–2019) recorded by the Ministerio de Salud. (D) Budget for malaria-related activities (1959–2017) in Costa Rica. (E) Estimated number of people protected by insecticide residual spraying between 1959 and 2000. (F) Estimated number of people protected by insecticide residual spraying between 2001 and 2018. In panels A, B and C line type indicates the type of malaria cases, for details, see the inset legend of panel A, while dot colour indicates the El Niño Southern Oscillation phase, for details, see the inset legend of panel B. Numbers in some dots refer to the policy shifts and natural events presented in Table 1. In panels D and F, the inset legends indicate, respectively, the funding source and the source of anti-malaria protection.
We were also able to collect more detailed data about the UFCo malaria control program that started in 1928 (Salisbury, 1929, 1930), event No. 2 in Table 1, the Guanacaste water drainage and ditching of 1939 (Guzman, 1940), event No. 3 in Table 1, where the number of cases (or a malaria parasite exposure measurement) prior and after the interventions was published, and the start of DDT fogging in 1946 (event No. 5 in Table 1) in the city of Limón by the UFCo (Lieske, 1950).
We obtained data about funding used for malaria-related activities, and its sources (Fig. 1D), through the PAHO malaria online database for the Americas (PAHO, 2019), a database where we also collected data on the number of people protected by Insecticide Residual Spraying (IRS) and insecticide-treated bednets (Fig. 1E and F).
To assess the importance of imported malaria cases on the total number of local malaria cases (Ruktanonchai et al., 2016a,b) we estimated their percentage from 1975, when imported malaria cases started to be recorded by Costa Rica´s Ministry of Health, until 2018.
Statistical analysis
To ensure that inpatient malaria cases were a good proxy measurement of all malaria cases we started by estimating the Pearson`s correlation coefficient (Sokal and Rohlf, 1994) between the number of inpatients and total cases in the UFCo time series (Fig. 1A). Since that correlation was high (r̂= 0.50), we will assume parameter estimates for the HSJD time series (Fig. 1B) can be extrapolated to the total number of cases between 1942 and 1952, and we will simply refer to malaria cases through the rest of the manuscript, irrespectively of whether data were from inpatients, like in the HSJD dataset, or the total number of cases, like in the UFCo and Ministerio de Salud datasets.
To identify potential breakpoints for the regime shifts in annual malaria cases, i.e. time points when the average number of malaria cases changed (Chaves et al., 2012, 2008; Hurtado et al., 2014), we plotted the annual difference in the number of cases and estimated the 2.5 and 97.5 percentiles of these annual malaria case change distribution (Sokal and Rohlf, 1994). Values outside these extreme percentiles were identified as potential regime shifts and assumed to be the start and the end of the breakpoints unless the difference time series took longer to reach values near 0, a time-point which was then assumed to be the breakpoint end, the time between start and end of a breakpoint representing transient dynamics between two continuous regimes (Scheffer, 2009; Hastings et al., 2018). We then split the time series into regimes that ended in the identified breakpoints, and for the breakpoints that did not end in the same year, we tested regimes ending at the start and the end of the breakpoints. The significance of the identified regime shifts was then tested by fitting negative binomial models, provided malaria case counts were over-dispersed (Faraway, 2006), with different means for the segments defined by the breakpoints and a covariate for the ENSO phase (Bouma and Van Der Kaay, 1996; Bouma et al., 1997; Bouma and Dye, 1997). Models were then selected by computing the Akaike information criterion (AIC), a metric that trades-off model fit and parameter number and selects the best models based on its minimization(Faraway, 2004). For comparison, we also estimated the AIC of models without regime shifts, with and without considering ENSO phases. For the UFCo and HSJD, we also tested models that considered changes in transmission followed policy changes in those time series. More specifically, we assumed there was a change in transmission in 1928 (event no. 2 in Table 1) in the UFCo time series and in 1946 (event no. 5 in Table 1) in HSJD time series. For the best models for each dataset, parameter estimates different from intercepts were exponentiated in order to be interpreted as proportional changes (Faraway, 2006) in the number of malaria cases.
For the Vigilancia de la Salud dataset we assessed the correlation, using the Spearman coefficient (Sokal and Rohlf, 1994; Venables and Ripley, 2002), between the whole time series and the different significant regimes in malaria cases with funding, and also with the number of people protected by IRS and bednets.
Results
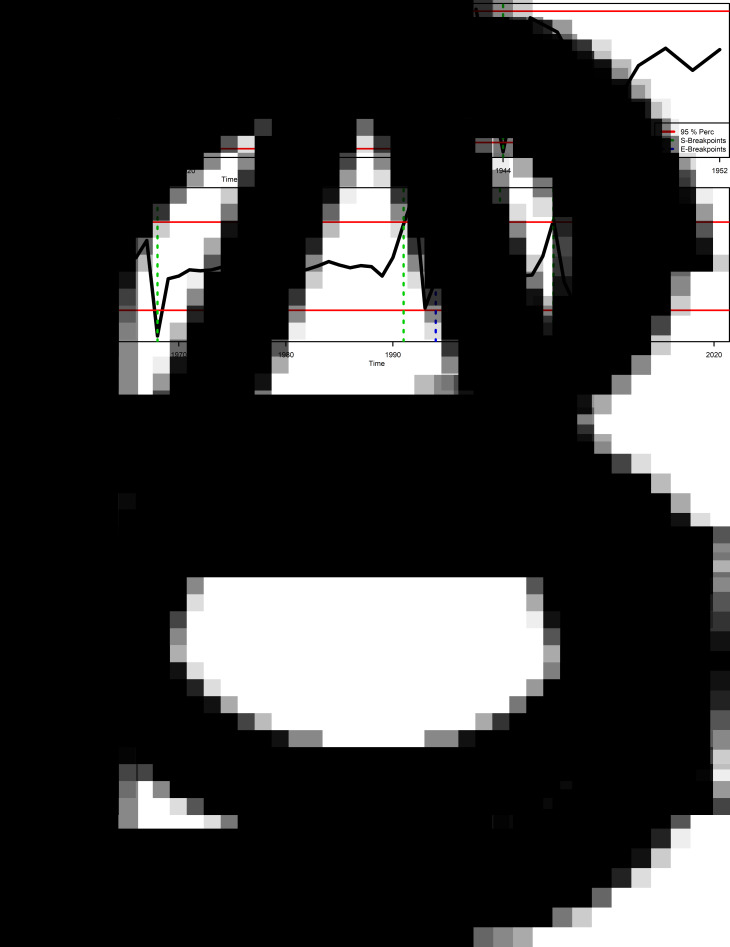
Figure 2 shows the regime shift identification results. For the UFCo dataset (Fig. 2A) 1915 and 1919 were identified as potential regime shift breakpoints, but testing showed them as not significant when compared with models without regime shifts (Table 2). Similarly, ENSO phases did not significantly change the risk of infection in the UFCo dataset period. In contrast, the observed change of 1929, following the 1928 control campaign (event no. 2 in Table 1), had a significant impact on malaria transmission. For the HSJD dataset (Fig. 2B) 1944 was suggested as a breakpoint, which was significant (Table 2), but a model considering a change in 1946, when DDT fogging started to be implemented in settlements associated with UFCo banana plantations (event no. 3 in Table 1), was selected as a better fit (Table 2). For the HSJD dataset, the impacts of ENSO were not tested provided the period was dominated by normal years (Fig. 1B). For the Ministerio de Salud dataset we found 4 potential breakpoints: 1968, 1991–1993, 2000, 2005–2008 (Fig. 2C), where model selection showed the best breakpoints for regime shifts, with transients that lasted more than a year, were 1991 and 2008 (Table 2).
Fig. 2.
Malaria regime shift analysis (A) Annual difference in total cases (1913–1932) from the Costa Rica division of the United Fruit Company. (B) Annual difference in inpatient malaria cases (1942–1952) at the Hospital San Juan de Dios, San José, Costa Rica. (C) Annual difference in total malaria cases in Costa Rica (1957–2018) recorded by the Ministerio de Salud. In the panels dotted vertical lines indicate significant breakpoints potentially associated with regime shifts, indicating the start (S-breakpoints) and end of the breakpoints (E-breakpoints), while horizontal solid lines indicate the 95% percentiles of the data.
Table 2.
Akaike information criterion (AIC)-based model selection for negative binomial models explaining the number of malaria cases in datasets from Costa Rica as function of different regime segments, El Niño Southern Oscillation (ENSO)
| Dataset | No. Segments | Segments | Covariates | AIC |
|---|---|---|---|---|
| United | 2 | 1913–1918, 1919–1931 | ENSO | 339.63 |
| Fruit | 2 | 1913–1928, 1929–1931 | ENSO | 330.87 |
| Company | 2 | 1913–1918, 1919–1931 | - | 336.01 |
| (UFCo) | 2 | 1913–1928, 1929–1931 | - | 328.72 |
| 1 | 1913–1932 | ENSO | 337.70 | |
| 1 | 1913–1932 | - | 334.31 | |
| Hospital | 2 | 1942–1944, 1945–1952 | Time | 160.15 |
| San Juan | 2 | 1942–1946, 1947–1952 | Time | 155.84 |
| de Dios | 1 | 1942–1952 | Time | 167.62 |
| (HSJD) | 1 | 1942–1952 | - | 188.51 |
| Ministerio de | 5 | 1957–1968, 1969–1991, 1992–2000, 2001–2005, 2006–2018 | ENSO | 987.81 |
| Salud | 5 | 1957–1968, 1969–1994, 1995–2000, 2001–2008, 2009–2018 | ENSO | 969.96 |
| 5 | 1957–1968, 1969–1994, 1995–2000, 2001–2005, 2006–2018 | ENSO | 1001.68 | |
| 5 | 1957–1968, 1969–1991, 1992–2000, 2001–2008, 2009–2018 | ENSO | 952.28 | |
| 4 | 1957–1991, 1992–2000, 2001–2008, 2009–2018 | ENSO | 963.31 | |
| 4 | 1957–1968, 1969–2000, 2001–2008, 2009–2018 | ENSO | 983.81 | |
| 4 | 1957–1968, 1969–1991, 1992–2008, 2009–2018 | ENSO | 954.77 | |
| 4 | 1957–1968, 1969–1991, 1992–2000, 2001–2018 | ENSO | 990.04 | |
| 1 | 1957–2018 | ENSO | 1008.59 | |
| 1 | 1957–2018 | - | 1004.67 |
The best model for each dataset is shown in bold. In the table, dataset indicates the specific dataset studied, no. of segments the number of segments considered in the regression, segments the years spanning each segment, while covariates indicate whether models considered ENSO as covariate or a temporal (Time) trend.
Table 3 shows parameter estimates for each dataset best model. The control campaigns started by UFCo (event 2 in Table 1), decreased by at least 50% malaria transmission during 1929–1931. This figure is in accordance with prevalence changes reported for adults working for UFCo, where malaria prevalence decreased from 25% to 12% (Salisbury, 1929). In the original analysis, however, Salisbury (1929), reported that malaria prevalence in the whole population was reduced from 35% of the population to 14%, and also reported statistics for children where malaria was reduced from 47% to 18%. DDT introduction in 1946 (event 5 in Table 1) was associated with an increase in annual malaria case reduction from 7.6% (1942–1946) to 26.4% (1947–1952). Here, it is worth highlighting that malaria transmission was declining at the national level likely as a product of water drainage and ditching in Guanacaste province (event 3 in Table 1), which according to Guzman (1940) was associated with a 75% decline in the splenic index of children in Liberia and Cañas, the two main human settlements in Guanacaste province. A similar case reduction was observed in the Limón hospital, where raw data reported by Lieske (1950) showed that average number (±s.d.) of malaria cases decreased from 177 ± 20, in 1941–1945, to 58 ± 29, in 1950–1952, a statistically significant difference (Welch`s t = 6.13, df = 3.36, P value <0.006). The end of the operational SNEM defunding, a period when the SNEM was not allocated enough operative funds, in 1967 (event 8 in Table 1), was followed by a 72% decrease in malaria cases that lasted until 1991 when the Limón Earthquake hit the Caribbean coast of Costa Rica (event 11 in Table 1) with malaria cases increasing by 254% between 1992 and 2000 when compared with the baseline period of 1957–1968. Malaria cases decreased again between 2001 and 2008, a time when treatments changed from the 5-day radical cure to the 7-day supervised treatment (events 12 and 13 in Table 1) reducing malaria cases by 8% when compared with the baseline period of 1957–1968. Malaria cases decreased by 98% during the 2009–2018 period when compared with the 1957–1968 baseline period. In the 2009–2018 period Costa Rica reached the pre-elimination period, following the implementation of the 7-day supervised treatment in all areas with the transmission in 2008 (event 14 in Table 1) and was unaffected by a change in policy to a reactive use of IRS and vector control in 2009 (event 15 in Table 1). For the Ministerio de Salud dataset, an interesting phenomenon was that malaria cases doubled during years belonging to the hot ENSO phase (Table 3) when compared with cold ENSO phase years.
Table 3.
Parameter estimates for the best negative binomial models explaining the number of malaria cases in Costa Rica (1913–2018)
| Dataset | Parameter | Proportional Change | Estimate | Std. Error | z value | Pr(>|z|) |
|---|---|---|---|---|---|---|
| United Fruit Company | Intercept (1913–1928) | --- | 8.33 | 0.08 | 101.272 | <2e-16* |
| (1913–1932) | R-1929–1932 | 0.506 | −0.68 | 0.21 | −3.288 | 0.00101* |
| Overdispersion | 9.26 | 2.96 | ||||
| Hospital San Juan de Dios | Intercept (post- sanitation) | --- | 8.10 | 0.10 | 79.073 | <2e-16* |
| (1942–1952) | Time | 0.924 | −0.078 | 0.042 | −1.871 | 0.0613 |
| Intercept Change (post-DDT) | --- | 1.40 | 0.27 | 5.184 | 2.17e-07* | |
| Time-post-DDT | 0.736 | −0.306 | 0.053 | −5.743 | 9.28e-09* | |
| Overdispersion | 58.2 | 25.9 | ||||
| Ministerio de Salud | Intercept (1957–1968, ENSO-Cold) | --- | 7.189 | 0.370 | 19.415 | <2e-16* |
| (1957–2018) | R-1969–1991 | 0.273 | −1.300 | 0.339 | −3.839 | 0.0001* |
| R-1992–2000 | 2.540 | 0.932 | 0.419 | 2.224 | 0.0261* | |
| R-2001–2008 | 0.920 | −0.083 | 0.420 | −0.198 | 0.8429 | |
| R-2009–2018 | 0.020 | −3.916 | 0.395 | −9.905 | <2e-16* | |
| ENSO-Hot | 2.000 | 0.692 | 0.308 | 2.244 | 0.0248* | |
| ENSO-Normal | 1.332 | 0.286 | 0.316 | 0.908 | 0.3641 | |
| Overdispersion | 1.20 | 0.21 |
Parameters starting with “R-” indicate the estimate for a given regime and parameters starting with “ENSO-” indicate the estimate for a given the El Niño Southern Oscillation phase.
*Statistically significant (P<0.05)
For the Ministerio de Salud dataset we found that overall, during the 1959–2018 period (Table 4), funding was negatively associated with malaria cases. In other words, increasing investments in malaria transmission reduction led to a decrease in malaria cases. By contrast, during 1959–2018, the deployment of malaria protection tools had a very low association with the case number. When the associations were analyzed through the different regimes of the 1958–2018 period (Table 4), investment was positively associated with malaria cases until 1991, suggesting that investment increased in response to malaria cases, the relationship became negative in 1992–2018, suggesting an increased investment was associated with a reduction in malaria cases. Regarding the impact of protective anti-malaria measurements (nets and IRS), these were negatively associated with malaria cases only between 1969 and 1991, suggesting that only during that period IRS effectively reduced malaria cases (Table 4).
Table 4.
Spearman correlation between malaria case number and funding for malaria and the number of people protected by insecticide residual spraying (IRS) and bednets (insecticide-treated nets, ITN, and long-lasting insecticide-treated nets, LLITN) between 1959 and 2018
| Period/Regime | Funding | IRS-ITN-LLITN | |
|---|---|---|---|
| 1959–2018 | −0.20 | 0.09 | |
| 1959–1968 | 0.43 | 0.28 | |
| 1969–1991 | 0.44* | −0.37 | |
| 1992–2000 | −0.63 | 0.13 | |
| 2001–2008 | −0.29 | 0.49 | |
| 2009–2018 | −0.33 | 0.40 |
Results are shown for the full period and significant regimes.
*Statistically significant (P < 0.05)
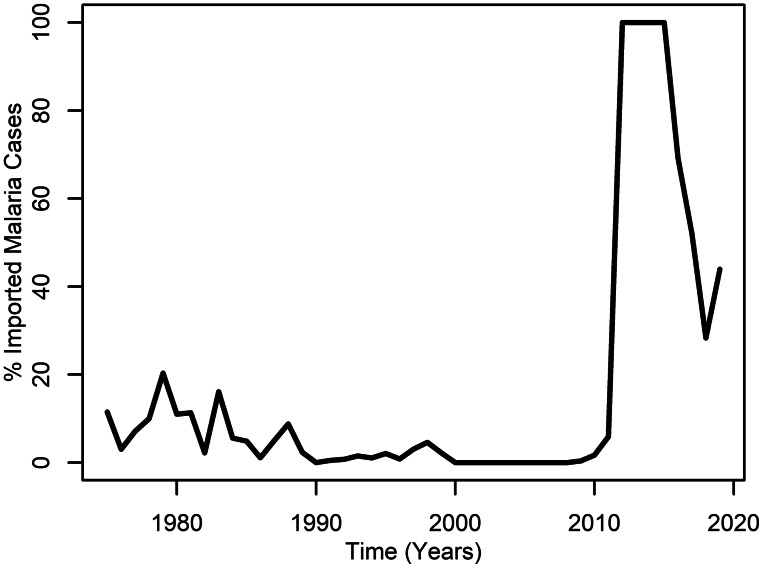
Figure 3 shows the percentage of imported malaria cases between 1975 and 2018. The figure shows that historically these numbers were below 20% of the total cases recorded in the country. There was a peak in the early 1980s when Costa Rica received an important group of refugees from the armed conflict in El Salvador (Rivera Funes, 2005). After that time the per cent of imported cases was very small, until 2012 when the percentage of imported malaria cases started to proportionally rise as Costa Rica reached the pre-elimination stage. Currently, imported cases are being increasingly associated with malaria outbreaks occurring in the country since 2017 (Vigilancia de la Salud, 2019).
Fig. 3.
Percentage of imported malaria cases in Costa Rica (1975–2019) based on data recorded by the Ministerio de Salud.
Discussion
Malaria case data from 1913–2018 in Costa Rica show that most of the studied events presented in Table 1 had an impact on malaria transmission. Our methods were only unable to show a lasting impact for (i) the Valle de la Estrella deforestation where data suggest deforestation impacts on big malaria epidemics were transient (Lynn, 1915). Nevertheless, deforestation had the long-term cost of creating malaria-endemic areas (Kumm and Ruiz, 1939; Vargas, 2001) as observed elsewhere where deforestation has driven malaria transmission emergence (Wallace et al., 2018; Castro et al., 2019). (ii) The creation and operational defunding of the SNEM, which basically reflects the problems faced by the SNEM to become fully operational after its creation (Warren et al., 1975; Weinstock et al., 1979; Vargas, 2001). (iii) The end of DDT use, which was successfully replaced by pyrethroids that more quickly degrade in the natural environment not having the side effects of DDT (Vargas, 2001). (iv) The change from 14-day chloroquine plus primaquine treatment to a 5-day radical cure that delivered a pharmacokinetically insufficient primaquine dose (Marín Rodríguez and Chaves, 2019). This treatment change likely led to an improvement in terms of increasing treatment coverage in remote areas (Bergonzoli and Rivers Cuadra, 2000), but was unable to prevent malaria relapses in endemic villages (Marín Rodríguez and Chaves, 2019). These detrimental effects became evident when other issues exacerbating malaria transmission were controlled since malaria reached zero transmission only once a pharmacokinetically sufficient treatment was used to treat all malaria cases across Costa Rica (Marín Rodríguez and Chaves, 2019).
About the specific impacts of different malaria transmission reduction strategies, it is worth highlighting that (i) a limited scope on target populations curtail potential impacts even on the target populations. For example, UFCo control campaigns, although able to reduce malaria transmission by up to 50%, were focused on keeping a healthy labour workforce, ignoring other populations living around the banana plantations (Salisbury, 1929, 1930; Aliano, 2006). This might explain the limited success of UFCo control campaigns at reducing malaria in children when compared with working adults, who received free treatments. This is very important given that access to treatment was limited by income during the 1920s and quinine shortages were suggested as a cause for the exacerbation of malaria epidemics in Costa Rica (Núñez, 1926). Similarly, (ii) the emphasis on unique tools proves insufficient to reduce malaria transmission. Even though the SNEM focus on IRS had a major impact on reducing malaria from 1969 to 1991, by the mid-1970s it was clear there were issues of parasite persistence in some villages that were unrelated to the impact of vector control and little to no attention was given to improving treatment in those areas (Warren et al., 1975; Weinstock et al., 1979). Equally, (iii) some policy shifts might synergistically act with ongoing policies accelerating malaria transmission reduction. In Costa Rica, although DDT introduction unequivocally helped to reduce malaria transmission (Lieske, 1950; Peña Chavarría and Guerrero Arguedas, 1953a,b; Vargas, 2001), it did so against a background where malaria was already going down as part of the health system strengthening via the CCSS creation (Palmer, 2003) and major infrastructure transformations, some related to water management (Guzman, 1940), that were also reducing malaria transmission. (iv) Some policy shifts also produce impacts over longer time periods than others. While shifts in vector control policy produced quick changes in malaria transmission, as illustrated in this study, policy shifts on drug use take longer to produce an impact. For example, only once the 7-day full dose primaquine and chloroquine treatment was established in areas with transmission in Costa Rica (Ávila-Agüero, 2008; Marín Rodríguez and Chaves, 2019) its impact in reducing malaria transmission became evident. In that sense, it is very important that (v) policy shifts should be grounded on robust scientific evidence. The 5-day radical cure was implemented based on a very limited study (Bergonzoli and Rivers Cuadra, 2000) criticized at the time of its publication (Kroeger, 2001; Ruebush et al., 2001) in light of the scientific evidence available at the time about the problems of pharmacokinetically insufficient primaquine doses to cure P. vivax infections (John et al., 2012). However, it took several years to change that policy despite evidence from the Costa Rican surveillance system about common relapses and repeated transmission in the same households (Ávila-Agüero, 2008; Marín Rodríguez and Chaves, 2019). As shown by our analysis, the change to treatment with a pharmacokinetically sufficient primaquine dose was the most impactful malaria health policy change in Costa Rica, reducing cases by 98% when compared with the baseline.
In that sense, as a country close to elimination, and based on previous successes (Marín Rodríguez and Chaves, 2019), Costa Rica is aiming to systematically use mass drug administration (MDA) to eliminate the last remaining malaria foci in the country. This is done considering encouraging results about MDA community wise protection from South East Asia (Parker et al., 2019), mathematical modelling (Brady et al., 2017) and long-term elimination results from proof of concept trials in islands of Oceania (Kaneko et al., 2000; Kaneko, 2010). This choice also relies on the functional universal health care system administered by the CCSS and the strong epidemic surveillance system of Costa Rica. The biggest challenge for malaria elimination in Costa Rica is the prediction, and eventual prevention, of new outbreaks driven by human movement (Ruktanonchai et al., 2016a). To confront that challenge, mathematical modelling and statistical and computational tools (Ruktanonchai et al., 2016b) could help to establish guidelines for MDA implementation, optimizing MDA deployment (Smith et al., 2014). Modelling could also help to further assess the implications of the vulnerability to extreme climatic events associated with ENSO, which was associated with malaria transmission increases in Costa Rica during the ENSO hot phase. This same phenomenon has been observed in neighbouring nations (Bouma et al., 1997; Bouma and Dye, 1997; Hurtado et al., 2014, 2018). These results highlight the importance of studying the impacts of ENSO, and more generally natural catastrophes (Wallace et al., 2018), especially in the Costa Rica-Nicaragua border, where the largest malaria outbreaks have been concentrated over recent times (Vigilancia de la Salud, 2019). This border concentrates the most socio-economically disadvantaged counties in Costa Rica (Ministerio de Planificación Nacional y Política Económica., 2018).
Conclusion
Despite the implementation of successful policies, that reduced malaria transmission, in Costa Rica outbreaks currently follow malaria importation into vulnerable areas. This epidemiological pattern highlights the need to timely diagnose and treat malaria in both local and migrant populations, aiming to also improve their overall living conditions.
Acknowledgements
The authors thank Ms. Urania Obando whose personal initiative to carefully keep copies of all reports and statistics related to malaria through her career at Vigilancia de la Salud made possible this study. The authors also thank Mr. Scott Martin, Librarian at the University of Michigan, for his help accessing the United Fruit Company Annual Medical Reports kept at the University of Michigan Library.
Financial support
This study was funded by Proyecto B6 601 Vicerrectoría de Investigación, Universidad de Costa Rica, with additional support from Dirección de Vigilancia de la Salud, Ministerio de Salud de Costa Rica.
Ethical standards
This research is based on publicly available non-identifiable data.
Conflict of interest
All authors declare that no conflicts of interest exist.
References
- Adams DP (1996) Malaria, labor, and population distribution in Costa Rica: a biohistorical perspective. The Journal of Interdisciplinary History 27, 75–85. [Google Scholar]
- Aliano D (2006) Curing the ills of Central America: the united fruit company´s medical department and corporate America´s mission to civilize (1900–1940). Estudios Interdisciplinarios de America Latina y el Caribe 17, 35–60. [Google Scholar]
- Ávila-Agüero ML (2008) Epidemiología de la malaria en Costa Rica. Acta Médica Costarricense 50, 72–74. [Google Scholar]
- Bennett A and Smith JL (2018) Malaria elimination: lessons from El Salvador. The American Journal of Tropical Medicine and Hygiene 99, 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergonzoli G and Rivers Cuadra JC (2000) Eficacia terapéutica de diferentes regímenes antimaláricos en la región fronteriza de Costa Rica y Nicaragua. Revista Panamericana de Salud Pública 7, 366–370. [DOI] [PubMed] [Google Scholar]
- Bouma MJ and Dye C (1997) Cycles of malaria associated with El Niño in Venezuela. Journal of the American Medical Association 278, 1772–1774. [PubMed] [Google Scholar]
- Bouma MJ and Van Der Kaay HJ (1996) The El Niño Southern Oscillation and the historic malaria epidemics on the Indian subcontinent and Sri Lanka: an early warning system for future epidemics? Tropical Medicine and International Health 1, 86–96. [DOI] [PubMed] [Google Scholar]
- Bouma MJ, Poveda G, Rojas W, Chavasse D, Quiñones M, Cox J and Patz J (1997) Predicting high-risk years for malaria in Colombia using parameters of El Niño Southern Oscillation. Tropical Medicine and International Health 2, 1122–1127. [DOI] [PubMed] [Google Scholar]
- Brady OJ, Slater HC, Pemberton-Ross P, Wenger E, Maude RJ, Ghani AC, Penny MA, Gerardin J, White LJ, Chitnis N, Aguas R, Hay SI, Smith DL, Stuckey EM, Okiro EA, Smith TA and Okell LC (2017) Role of mass drug administration in elimination of Plasmodium falciparum malaria: a consensus modelling study. The Lancet Global Health 5, e680–e687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton RA, Chévez JER, Sauerbrey M, Guinovart C, Hartley A, Kirkwood G, Boslego M, Gavidia ME, Alemán Escobar JE, Turkel R, Steketee RW, Slutsker L, Schneider K and Campbell CC (2018) Factors associated with the rapid and durable decline in malaria incidence in El Salvador, 1980–2017. The American Journal of Tropical Medicine and Hygiene 99, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter KH, Singh P, Mujica OJ, Escalada RP, Ade MP, Castellanos LG and Espinal MA (2015) Malaria in the Americas: trends from 1959 to 2011. The American Journal of Tropical Medicine and Hygiene 92, 302–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Sancho JI, Munguía-Ramírez MdR and Ávila-Agüero ML (2002) Malaria: una actualización. Acta Médica Costarricense 44, 107–112. [Google Scholar]
- Castro MC, Baeza A, Codeço CT, Cucunubá ZM, Dal'Asta AP, De Leo GA, Dobson AP, Carrasco-Escobar G, Lana RM, Lowe R, Monteiro AMV, Pascual M and Santos-Vega M (2019) Development, environmental degradation, and disease spread in the Brazilian Amazon. PLOS Biology 17, e3000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves, LF (2017) Climate change and the biology of insect vectors of human pathogens. In Johnson S and Jones H (eds), Invertebrates and Global Climate Change, pp. 126–147. Chichester, UK: Wiley. [Google Scholar]
- Chaves LF, Kaneko A, Taleo G, Pascual M and Wilson ML (2008) Malaria transmission pattern resilience to climatic variability is mediated by insecticide-treated nets. Malaria Journal 7, 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves LF, Hashizume M, Satake A and Minakawa N (2012) Regime shifts and heterogeneous trends in malaria time series from Western Kenya highlands. Parasitology 139, 14–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarck HC (1928) The field parasite rate for malaria in the banana divisions. United Fruit Company Medical Department Annual Report 17, 72–76. [Google Scholar]
- Faraway JJ (2004) Linear Models with R. Boca Raton: CRC Press. [Google Scholar]
- Faraway JJ (2006) Extending the Linear Model with R: Generalized Linear, Mixed Effects and Nonparametric Regression Models. Boca Raton: CRC Press. [Google Scholar]
- Frantzius AV (1868) Über die Verbreitung der Malariafieber in Costa Rica. Virchows Archiv 43, 315–329. [Google Scholar]
- Greenwood B (2009) Can malaria be eliminated? Transactions of The Royal Society of Tropical Medicine and Hygiene 103, S2–S5. [DOI] [PubMed] [Google Scholar]
- Grupo Técnico Nacional de Enfermedades Vectoriales (2015). Plan de Eliminación de la Malaria en Costa Rica, 2015–2020. San José, Costa Rica: Ministerio de Salud de Costa Rica. [Google Scholar]
- Grupo Técnico Nacional de Enfermedades Vectoriales (2016) Norma de Malaria. San José, Costa Rica: Ministerio de Salud de Costa Rica. [Google Scholar]
- Guzman AA (1940) Malaria in Costa Rica. Nature 146, 330. [Google Scholar]
- Hastings A, Abbott KC, Cuddington K, Francis T, Gellner G, Lai Y-C, Morozov A, Petrovskii S, Scranton K and Zeeman ML (2018) Transient phenomena in ecology. Science (New York, N.Y.) 361, eaat6412. [DOI] [PubMed] [Google Scholar]
- Herrera S, Ochoa-Orozco SA, González IJ, Peinado L, Quiñones ML and Arévalo-Herrera M (2015) Prospects for malaria elimination in Mesoamerica and Hispaniola. PLoS Neglected Tropical Diseases 9, e0003700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez PJ, Woc-Colburn L and Bottazzi ME (2014) Neglected tropical diseases in Central America and Panama: review of their prevalence, populations at risk and impact on regional development. International Journal for Parasitology 44, 597–603. [DOI] [PubMed] [Google Scholar]
- Hurtado LA, Cáceres L, Chaves LF and Calzada JE (2014) When climate change couples social neglect: malaria dynamics in Panamá. Emerging Microbes & Infections 3, e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado LA, Calzada JE, Rigg CA, Castillo M and Chaves LF (2018) Climatic fluctuations and malaria transmission dynamics, prior to elimination, in Guna Yala, República de Panamá. Malaria Journal 17, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Cooperation Administration Expert Panel on Malaria (1961) Report and recommendations on malaria: a summary. The American Journal of Tropical Medicine and Hygiene 10, 451–502. [Google Scholar]
- John GK, Douglas NM, von Seidlein L, Nosten F, Baird JK, White NJ and Price RN (2012) Primaquine radical cure of Plasmodium vivax: a critical review of the literature. Malaria Journal 11, 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A (2010) A community-directed strategy for sustainable malaria elimination on islands: short-term MDA integrated with ITNs and robust surveillance. Acta Tropica 114, 177–183. [DOI] [PubMed] [Google Scholar]
- Kaneko A, Taleo G, Kalkoa M, Yamar S, Kobayakawa T and Björkman A (2000) Malaria eradication on islands. The Lancet 356, 1560–1564. [DOI] [PubMed] [Google Scholar]
- Knaul FM, Bhadelia A, Ornelas HA, de Lima L and del Rocio Sáenz Madrigal M (2015) Closing the pain divide: the quest for effective universal health coverage. The Lancet Global Health 3, S35. [Google Scholar]
- Kroeger A (2001) ¿Propicia la resistencia medicamentosa y las recaídas el tratamiento breve con antimaláricos? Revista Panamericana de Salud Pública 9, 202–203. [DOI] [PubMed] [Google Scholar]
- Kruk ME, Gage AD, Joseph NT, Danaei G, García-Saisó S and Salomon JA (2018) Mortality due to low-quality health systems in the universal health coverage era: a systematic analysis of amenable deaths in 137 countries. The Lancet 392, 2203–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumm, H. W. and Ruiz, S. H. (1939). A malaria survey of the Republic of Costa Rica, Central America. The American Journal of Tropical Medicine and Hygiene s1–19, 425–445. [Google Scholar]
- Lieske H (1950) Malaria in Costa Rica. Zeitschrift für Tropenmedizin und Parasitologie 5, 270–272. [PubMed] [Google Scholar]
- Lynn WJ (1913) Annual report 1913 Costa Rica division. United Fruit Company Medical Department Annual Report 2, 28–31. [Google Scholar]
- Lynn WJ (1914) Annual report, 1914, Costa Rica division. United Fruit Company Medical Department Annual Report 3, 26. [Google Scholar]
- Lynn WJ (1915) Annual report, 1915, Costa Rica division. United Fruit Company Medical Department Annual Report 4, 26. [Google Scholar]
- Marín Rodríguez R and Chaves LF (2019) Parasite removal for malaria elimination in Costa Rica. Trends in parasitology 35, 585–588. [DOI] [PubMed] [Google Scholar]
- Ministerio de Planificación Nacional y Política Económica (2018) Índice de Desarrollo Social 2017. San José, CR: Mideplan. [Google Scholar]
- NOAA (2019). ENSO Cold & Warm Episodes by Season. Available at www.cpc.ncep.noaa.gov/products/analysis_monitoring/ensostuff/ensoyears.shtml (Accessed 23 December 2019).
- Núñez S (1926) The occurrence and Non-occurrence of certain diseases in Costa Rica. The American Journal of Tropical Medicine and Hygiene s1–6, 347–356. [Google Scholar]
- PAHO (2019) Interactive Malaria Statistics. Available at http://www.paho.org/hq/index.php?option=com_content&view=article&id=2632:2010-interactive-malaria-statistics&Itemid=2130&lang=en (Accessed 23 December 2019).
- Palmer, S. (2003) From Popular Medicine to Medical Populism: Doctors, Healers, and Public Power in Costa Rica, 1800–1940. Durham, NC: Duke University Press Books. [Google Scholar]
- Parker DM, Tun STT, White LJ, Kajeechiwa L, Thwin MM, Landier J, Chaumeau V, Corbel V, Dondorp AM, von Seidlein L, White NJ, Maude RJ and Nosten F (2019) Potential herd protection against Plasmodium falciparum infections conferred by mass antimalarial drug administrations. eLife 8, e41023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patz JA and Olson SH (2006) Malaria risk and temperature: influences from global climate change and local land use practices. Proceedings of the National Academy of Sciences of the United States of America 103, 5635–5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña Chavarría A and Guerrero Arguedas J (1953a) Influencia de la lucha antipaludica, especialmente la dedetización eb la Hospitalización por Malaria en el Hopsital San Juan de Dios de San José de Costa Rica, en el periodo 1942–1952. Revista Médica Costarricense 20, 54–60. [PubMed] [Google Scholar]
- Peña Chavarría A and Guerrero Arguedas J (1953b) La influencia del DDT en la incidencia del paludismo en Costa Rica. Boletin de la Oficina Sanitaria Panamericana 35, 487–493. [PubMed] [Google Scholar]
- Rigg CA, Hurtado LA, Calzada JE and Chaves LF (2019) Malaria infection rates in Anopheles albimanus (Diptera: Culicidae) at ipetí-guna, a village within a region targeted for malaria elimination in panamá. Infection, Genetics and Evolution 69, 216–223. [DOI] [PubMed] [Google Scholar]
- Rivera Funes OF (2005) Las migraciones internacionales y sus efectos económicos en El Salvador. Población y Salud en Mesoamérica 2, 5. [Google Scholar]
- Rosero-Bixby L and Dow WH (2016) Exploring why Costa Rica outperforms the United States in life expectancy: a tale of two inequality gradients. Proceedings of the National Academy of Sciences 113, 1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruebush I, Trenton K, Marquiño W, Cabezas C and Neyra D (2001) Régimen antimalárico de un día: riesgo de resistencia frente a utilidad. Revista Panamericana de Salud Pública 10, 217–219. [DOI] [PubMed] [Google Scholar]
- Ruktanonchai NW, Bhavnani D, Sorichetta A, Bengtsson L, Carter KH, Córdoba RC, Le Menach A, Lu X, Wetter E, zu Erbach-Schoenberg E and Tatem AJ (2016a) Census-derived migration data as a tool for informing malaria elimination policy. Malaria Journal 15, 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruktanonchai NW, DeLeenheer P, Tatem AJ, Alegana VA, Caughlin TT, zu Erbach-Schoenberg E, Lourenço C, Ruktanonchai CW and Smith DL (2016b) Identifying malaria transmission foci for elimination using human mobility data. PLOS Computational Biology 12, e1004846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáenz R, Bissell RA and Paniagua F (2012) Post-Disaster malaria in Costa Rica. Prehospital and Disaster Medicine 10, 154–160. [DOI] [PubMed] [Google Scholar]
- Salisbury EI (1929) Control of malaria in Costa Rica. United Fruit Company Medical Department Annual Report 18, 88–97. [Google Scholar]
- Salisbury EI (1930) The malaria index in the Costa Rican division as affected by control measures. United Fruit Company Medical Department Annual Report 19, 33–39. [Google Scholar]
- Scheffer M (2009) Critical Transitions in Nature and Society. Princeton: Princeton University Press. [Google Scholar]
- Smith DL, Perkins TA, Reiner JRC, Barker CM, Niu T, Chaves LF, Ellis AM, George DB, Le Menach A, Pulliam JRC, Bisanzio D, Buckee C, Chiyaka C, Cummings DAT, Garcia AJ, Gatton ML, Gething PW, Hartley DM, Johnston G, Klein EY, Michael E, Lloyd AL, Pigott DM, Reisen WK, Ruktanonchai N, Singh BK, Stoller J, Tatem AJ, Kitron U, Godfray HCJ, Cohen JM, Hay SI and Scott TW (2014) Recasting the theory of mosquito-borne pathogen transmission dynamics and control. Transactions of The Royal Society of Tropical Medicine and Hygiene 108, 185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal RR and Rohlf FJ (1994) Biometry: The Principles and Practices of Statistics in Biological Research, 3rd Edn, New York, NY: W. H. Freeman. [Google Scholar]
- The Economist Intelligence Unit (2018). Costa Rica Country Report. Available at http://country.eiu.com/costa-rica (Accessed 2 January 2019).
- Vargas M (2001) Diagnóstico Situacional de la Malaria Y el uso del DDT en Costa Rica. San José, Costa Rica: Organización Panamericana de la Salud. [Google Scholar]
- Venables WN and Ripley BD (2002) Modern Applied Statistics with S. New York: Springer. [Google Scholar]
- Vigilancia de la Salud (2019) Boletín Epidemiológico No. 20. San José: Ministerio de Salud de Costa Rica. [Google Scholar]
- Wallace R, Chaves LF, Bergmann L, Ayres Lopes CfJ, Hogerwerf L, Kock R and Wallace RG (2018) Clear-Cutting Disease Control: Capital-Led Deforestation, Public Health Austerity, and Vector-Borne Infection. New York: Springer. [Google Scholar]
- Warren M, Collins WE, Jeffery GM and Skinner JC (1975) The seroepidemiology of malaria in Middle America II. Studies on the pacific coast of Costa Rica. The American Journal of Tropical Medicine and Hygiene 24, 749–754. [DOI] [PubMed] [Google Scholar]
- Weinstock H, Garcés JL, Soto JB and Rodríguez J (1979) Immunodiagnosis of malaria infections by the national malaria eradication service (SNEM) of Costa Rica. Bulletin of Pan American Health Organization 13, 257–263. [PubMed] [Google Scholar]
- WHO (2018) Update on the E-2020 Initiative of 21 Malaria-Eliminating Countries. Geneva: World Health Organization. [Google Scholar]
- Wolter K and Timlin MS (2011) El Niño/Southern Oscillation behaviour since 1871 as diagnosed in an extended multivariate ENSO index (MEI.ext). International Journal of Climatology 31, 1074–1087. [Google Scholar]