Abstract
Definitive diagnosis of hookworm infection is usually based on the microscopic detection of eggs in a stool sample; however, several cases display a low or irregular egg output. Serodiagnosis can be a useful tool to identify these cases, but conventional tests do not differentiate past from active infections. The aim of this study was to obtain and apply egg yolk polyclonal immunoglobulin (IgY) antibodies to detect immune complexes (ICs) in serum samples from patients infected with hookworm. Hens were immunized with Ancylostoma ceylanicum saline extract, their eggs were collected and then IgY antibodies were extracted and purified. Antibody purity was tested by 12% sodium dodecyl sulphate polyacrylamide gel electrophoresis and specificity was assessed by immunoblotting and immunofluorescence. IgY production was evaluated by kinetics enzyme-linked immunosorbent assay (ELISA). Sandwich ELISA tested the ability of IgY to detect ICs in serum samples, from which diagnostic parameters were calculated. Antibody responses increased steadily from day 7 to 42. In the immunoblotting assay, IgY recognized two protein complexes. The immunofluorescence assay showed no staining in control samples. The sandwich ELISA presented a very high diagnostic value, with a sensitivity of 90% and a specificity of 86.7%. Our pioneer strategy highlights the potential use of egg yolk IgY as a diagnostic test to detect active hookworm infection.
Key words: Active infection, hookworm, immune complexes, IgY
Introduction
Soil-transmitted helminthiasis (STH) is the most prevalent neglected tropical disease caused by several species of hookworm (Necator americanus, Ancylostoma duodenale and Ancylostoma ceylanicum), roundworm (Ascaris lumbricoides) and whipworm (Trichuris trichiura). Approximately 1.4 billion people worldwide are estimated to be infected with at least one of the main STHs, and more than 740 million people are infected with hookworm (Pullan et al., 2014; Prieto-Pérez et al., 2016). Infections with N. americanus and A. duodenale remain a major public health problem in several low- and middle-income countries (George et al., 2016).
Diagnosis is mainly based on microscopic examination of faeces, but this method presents sensitivity problems in cases with low parasitic burden, intermittent shedding of eggs, single-sex infections or sexually immature forms (Elsemore et al., 2017). In these cases, other diagnostic tests such as enzyme-linked immunosorbent assay (ELISA) must be applied.
The IgG assay can present false-positive reactions with other parasitic helminths and may not distinguish between past and active infections. To overcome this problem, diagnostic tests based on chicken egg yolk immunoglobulin (IgY) are being developed (Nie et al., 2014). The use of egg yolk IgY was first proposed by Leslie and Clem (1969). IgY reacts with more epitopes on a mammalian antigen, amplifying the signal and improving the sensitivity of ELISA by 10–100 times (Ohnishi et al., 2000). IgY antibodies have many advantages over IgG antibodies, including cost-effectiveness, strong avidity, scalable productivity, low assay background and high yield (Li et al., 2015).
Due to the advantages mentioned above, polyclonal IgY antibodies have been widely used in applications related to parasitic diseases such as toxoplasmosis (Ferreira Júnior et al., 2012), schistosomiasis (Lei et al., 2012), trichinellosis (Liu et al., 2013), strongyloidiasis (Faria et al., 2019) and ascariasis (Lopes et al., 2019). In view of this, we developed the first sensitive, specific and noninvasive IgY sandwich-based ELISA to detect immune complexes (ICs) in serum, indicating active hookworm infection.
Material and methods
Serum samples
Serum samples from 90 individuals were tested and divided into three groups: group 1 consisted of 30 patients infected with hookworm; group 2 included 30 patients who had other parasitic diseases, including infections with Giardia duodenalis (n = 3), Entamoeba histolytica/díspar (n = 3), Ascaris sp. (n = 7), Enterobius vermicularis (n = 4), Hymenolepis nana (n = 3), Schistosoma mansoni (n = 4) and Taenia sp. (n = 6); and group 3 consisted of 30 apparently healthy volunteers. Individuals from all groups provided three faecal samples that were analysed according to Baermann (1917), Lutz (1919) and Moraes (1948).
Preparation of Ancylostoma ceylanicum saline extract
Total saline extract (SE) from A. ceylanicum was produced using approximately 100 000 infective larvae (L3), as previously described by Gonzaga et al. (2013). The protein concentration of SE was determined according to Lowry et al. (1951), then proteins were subjected to 12% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) (Laemmli, 1970) for the characterization of the antigenic profiles after silver staining (Friedman, 1982).
Production of polyclonal IgY antibodies
Four 25-week-old laying hens (Gallus gallus domesticus, Hisex lineage) were immunized in the pectoral muscle with SE using Freund's adjuvant, as described previously by Schwarzkopf et al. (2001). Each hen was firstly immunized with 100 μg s.e. diluted in 250 μL of phosphate-buffered saline (PBS; 0.01 M, pH 7.2) and 250 μL of complete Freund's adjuvant (Sigma Aldrich, USA). Hens then received three booster immunizations at 14-day intervals. These were performed with SE, as in the first immunization, but used incomplete Freund's adjuvant (Sigma Aldrich, USA). The control group was immunized with PBS. All testing groups contained two hens each.
The hens were maintained under standard specific pathogen-free conditions and were monitored daily for adverse effects and inflammatory processes. Chicken eggs were collected daily from before the first immunization until after the last boost.
Characterization of polyclonal IgY antibodies
Polyclonal IgY antibodies were extracted from egg yolk using the water-soluble method described by Akita and Nakai (1993). IgY was purified by affinity chromatography (HiTrapIgY Purification HP 5 mL; GE Healthcare, USA) at a flow rate of 3 mL min−1. IgY antibodies were eluted using a linear gradient of 0.02 M sodium phosphate buffer (pH 7.2) in an ÄKTA prime liquid chromatography system (GE Healthcare, USA), then the eluted fraction was dialysed with ultrapure water (Amicon YM 30; Sigma-Aldrich Co., USA) using a 30 kDa cut-off membrane, and the protein concentration was determined at 280 nm (BioDrop, UK).
IgY was analysed by 12% SDS-PAGE under reducing conditions (5% 2-mercaptoethanol; Sigma-Aldrich Co., USA) and the gel was stained with Coomassie Blue (Vetec, Brazil). Purified IgY from eggs produced on day 42 was tested for target specificity by immunoblotting, where 250 μg of SE diluted in PBS (final volume of 200 μL) was transferred to a nitrocellulose membrane after 12% SDS-PAGE according to Towbin et al. (1979). Strips were cut, and non-specific binding sites were blocked with PBS containing 0.05% Tween 20 and 5% skimmed milk (PBS-T-M) for 2 h at room temperature. Membranes were individually incubated with anti-hookworm IgY (15 μg) diluted in 1% PBS-T-M (final volume of 500 μL) overnight at 4°C. Then, 500 μL of HRP-conjugated anti-chicken IgY (whole molecule; Sigma-Aldrich Co., USA) diluted (1: 15 000) in 1% PBS-T-M was used as the second antibody. Specific IgY binding was visualized by staining the membranes with 3,3-diaminobenzidine tetrahydrochloride (DAB SigmaFast tablets; Sigma-Aldrich Co., USA) and 30% hydrogen peroxide (Merck, Brazil) diluted in tris-buffered saline (0.02 M, pH 7.4). Between each step, the strips were washed six times with PBS-T. The reaction was stopped by adding distilled water, and positive reactions were identified by the appearance of defined bands. The relative molecular weights of the recognized protein fractions were determined by comparison with molecular markers (REC001; RBC, Taiwan).
Kinetic evaluation of IgY production
The reactivity and kinetics of anti-hookworm IgY production were monitored by indirect ELISA. In this procedure, samples were used at a final volume of 50 μL per well. Briefly, 96-well low-affinity polystyrene microplates (Greiner Bio-one, Germany) were coated with SE (10 μg mL−1) diluted in carbonate bicarbonate buffer (0.06 M, pH 9.6) and incubated overnight at 4°C. After incubation, microplates were washed three times for 5 min each with PBS-T then blocked with 1% PBS-T-M for 1 h at 37°C. IgY (2 μg mL−1 in PBS-T-M) was added in duplicate and incubated for 1 h at 37°C. Washing was followed by incubation with anti-IgY antibodies conjugated to horseradish peroxidase (Sigma-Aldrich Co., USA) diluted 1:10 000 in 1% PBS-T-M for 45 min at 37°C. After washing, the reaction was revealed by o-phenylenediamine (Sigma-Aldrich Co., USA) with 0.03% hydrogen peroxide (Merck, Brazil) diluted in 0.1 M citrate phosphate buffer (pH 5.5) for 15 min. The reaction was stopped by adding 25 μL of 2N H2SO4 (Vetec, Brazil). The optical density (OD) was determined at 492 nm in an ELISA reader (Thermo plate, China). Data were expressed as the ELISA index (EI) as follows: EI = OD/cut-off of the negative controls (control group − group immunized with PBS) + three standard deviations. To establish the cut-off values for the control group, the OD values from the first 6 weeks were used (Faria et al., 2019; Lopes et al., 2019).
Immunofluorescence assay
IgY was tested for target specificity by immunofluorescence. Prior to staining, parasites were added to tissue freezing medium (Sakura, Netherlands) and frozen at −20°C. Ancylostoma ceylanicum larvae were sectioned at 2 μm thickness with a cryomicrotome (CM 1850 UV; Leica, Germany), placed on glass slides and air dried for 30 min. Sections were incubated with anti-hookworm IgY (35 μg) diluted in PBS (final volume of 50 μL) for 30 min at 37°C. After six washes of 5 min each with PBS, sections were incubated at 37°C for 30 min with 50 μL of the secondary antibody, rabbit anti-chicken IgY conjugated with fluorescein isothiocyanate (FITC; Sigma-Aldrich Co., USA) diluted 1:300 in 3% Evans blue (Vetec, Rio de Janeiro, Brazil) for counterstaining. Possible cross-reactivity was tested using anti-hookworm IgY antibodies on sections of Strongyloides venezuelensis filariform larvae under the same conditions. For the negative control, sections were incubated with IgY from control hens. After washing, the slides were mounted with glycerol/PBS (pH 9.0) and coverslips. FITC fluorescence emission was detected using an LSM 510 confocal microscope (Meta; Carl Zeiss, Germany).
IgY-based sandwich ELISA to detect immune complexes in serum samples
Detection of circulating ICs by sandwich ELISA using anti-hookworm IgY was performed as previously described (Gonçalves et al., 2012) with some modifications. Preliminary tests with all the reagents were carried out to determine the high signal/noise ratio conditions and samples were diluted to a final volume of 50 μL. Briefly, high-binding 96-well microplates (Corning-Costar, USA) were coated with purified IgY (10 μg mL−1; collected during the sixth week) in 0.06 M carbonate bicarbonate buffer (pH 9.6) and incubated overnight at 4°C. Serum samples were diluted 1:80 in PBS-T and incubated for 45 min at 37°C. Subsequently, goat anti-human IgG (Fc specific) HRP-conjugated antibody (Sigma-Aldrich Co., USA) diluted 1:2000 in PBS-T containing 1% bovine serum albumin was added and incubated for 45 min at 37°C. Between each incubation step, plates were washed three times for 5 min each with PBS-T. The following steps of the reaction were as previously described. Serum samples were tested in duplicate. The cut-off point was established from the receiver operating characteristic (ROC) curve using OD values from the negative and other parasite groups as negative controls.
Statistical analysis
Analyses were performed using GraphPad software package 6.0 (GraphPad Software Inc., San Diego, USA). Statistical analysis was performed by calculating the lower limits of positivity (cut-off value) established for optimal sensitivity (Se) and specificity (Sp) using the ROC curve. The diagnostic parameters calculated were area under the curve (AUC), Se, Sp and positive likelihood ratio (LR+) according Ribeiro et al. (2013). Confidence intervals (CI) of 95% were provided for Se, Sp and AUC statistical calculations.
Results
Antigen preparation and IgY collection
The IgY yield at day 42 was 4750 μg mL−1 before purification. Samples were purified by affinity chromatography and pooled to give an adequate volume and concentration for the following experiments. The pooled samples reached a protein concentration of 700 μg mL−1. In experiments with immunized hens, the final IgY concentration was found to vary. To overcome this variation, a standard concentration was established for each test.
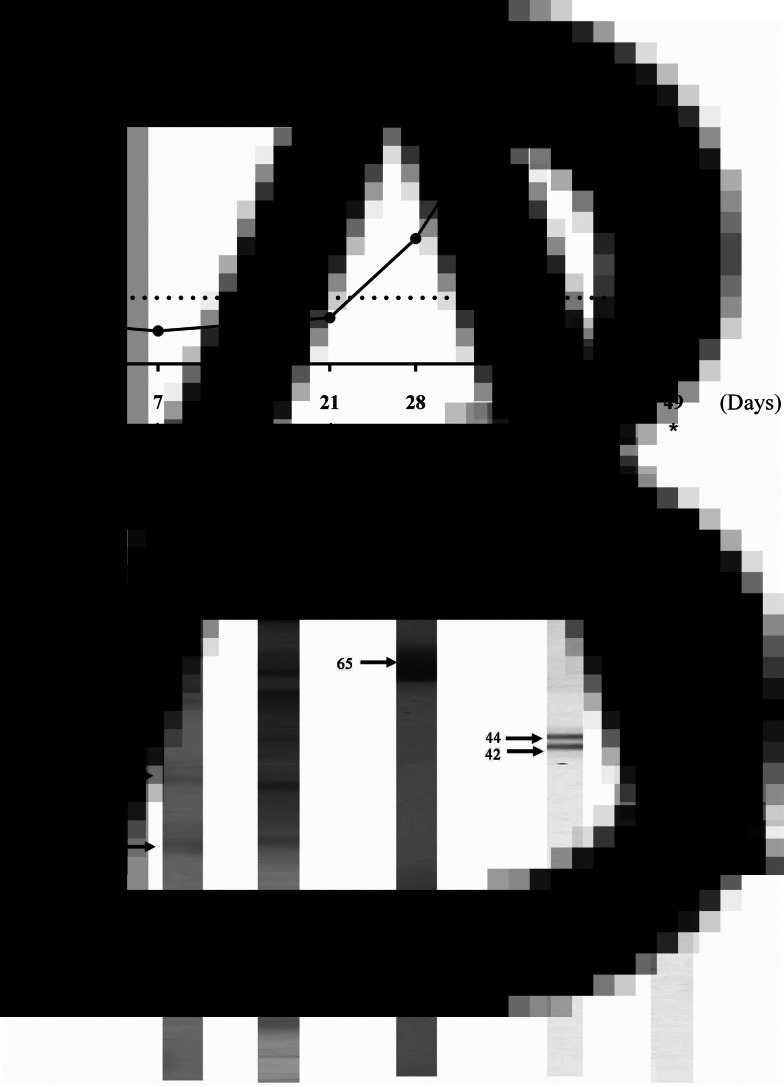
After the second booster immunization, the levels of anti-hookworm IgY reached a peak (Fig. 1A). The antibody response increased steadily from day 7 to 42. The electrophoretic profiles of the s.e. showed bands with apparent molecular weights varying from 7 to 240 kDa (Fig 1B-2).
Fig. 1.
Anti-hookworm IgY characterization. A: Kinetics of IgY antibodies production. * indicate immunizations. Electrophoretical profiles in SDS-PAGE 12%. B: 1 – molecular weight, 2 – A. ceylanicum saline extract, 3 – IgY antibodies after HPLC fractionation. Immunoblotting to evaluate antigenic recognition of IgY antibodies from 4 – hens immunized with A. ceylanicum s.e. and 5 – hens immunized with PBS.
SDS-PAGE analysis of the recovered polyclonal IgY after HPLC fractionation confirmed the integrity of the antibodies, meaning they were not degraded (Fig 1B-3). This finding indicates that hens, as a host for the production of anti-hookworm IgY, show a good ability to rapidly and efficiently generate specific polyclonal IgY in a non-invasive way.
Immunoblot analysis indicated that IgY recognized two protein complexes of high (200 kDa) and medium (40–50 kDa) molecular weights (Fig. 1B-4). These bands were not recognized by control group IgY (Fig. 1B-5).
Immunofluorescence
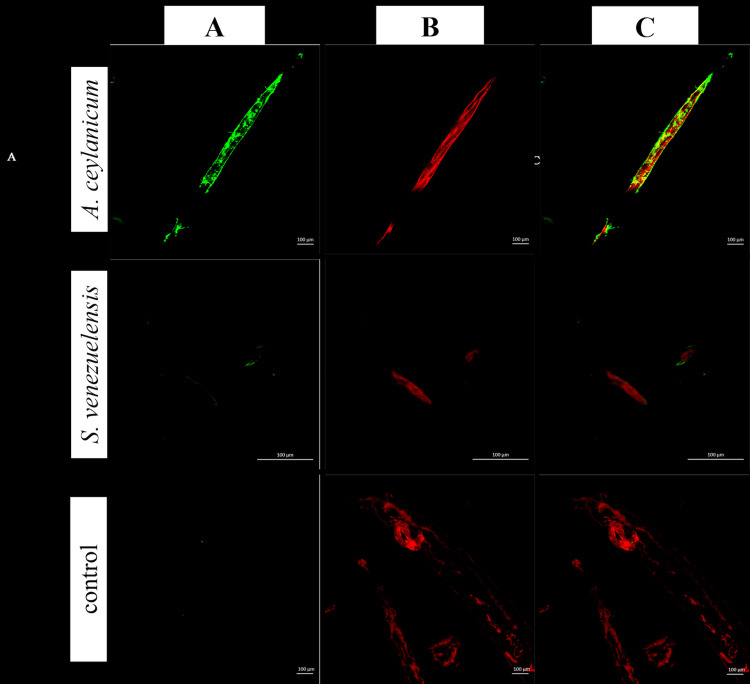
To confirm reactivity and determine recognition patterns, IgY antibodies were separately incubated with A. ceylanicum and S. venezuelensis, and the bound antibodies were visualized by indirect fluorescence. The staining pattern observed in A. ceylanicum sections was not uniform, but rather distributed in small, interspersed textural patches with no apparent regularity. No fluorescence was detected for S. venezuelensis sections or the negative control (Fig. 2).
Fig. 2.
Immunofluorescence antibody test using fractionated polyclonal IgY antibodies in A. ceylanicum sections and other parasitic sample (S. venezuelensis section) to evaluate the possibility of cross reaction. Ancylostoma ceylanicum and S. venezuelensis sections were incubated with anti-A. ceylanicum IgY or with IgY from the control group (immunized with PBS). Column A (green): anti-chicken IgY FITC conjugated; B (red): Evans blue counterstained; C (merged): from column 1 to 2.
IgY-based sandwich ELISA for the detection of immune complexes
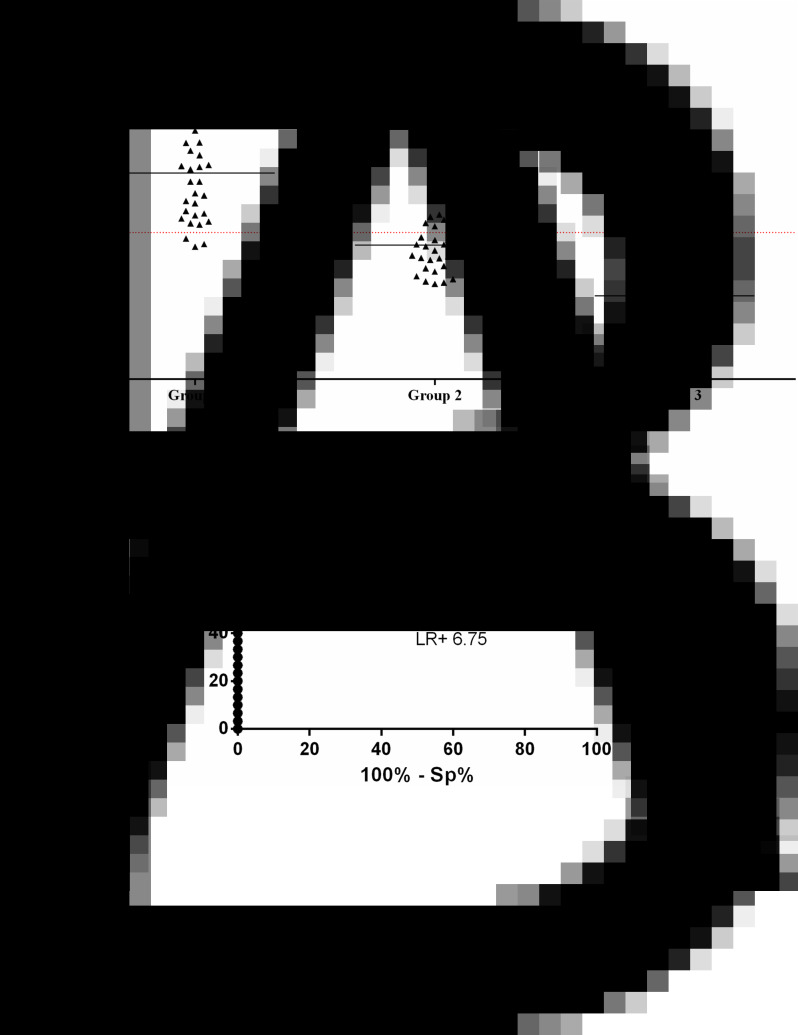
Positivity in group 1 reached 90%. The cross-reactivity observed in group 2 (26.6%, n = 8) was due to samples harbouring other parasites including G. duodenalis (1/8), E. histolytica/díspar (1/8), E. vermicularis (2/8), H. nana (1/8), S. mansoni (2/8) and Taenia sp. (1/8), whereas no reactivity was observed in group 3 (Fig. 3A).
Fig. 3.
ELISA to detect ICs in serum samples from patients with hookworm active infection (group 1, n = 43), other parasitic infections (group 2, n = 30) and apparently healthy individuals (group 3, n = 30) using anti-hookworm IgY. A: scatter dot plots. Dotted line indicates cut off, samples were considered positive when OD >0.4, horizontal bars indicate median. B: ROC curve indicating AUC, sensitivity (Se), specificity (Sp) and positive LR (95% CI indicated).
Test performance, indicated by AUC (0.9539), almost reached the maximum value (1.00) of efficiency. The LR conferred a very high diagnostic value for this test (6.75), representing a high probability of a true positive case of active hookworm infection, and ROC curve analysis (Fig. 3B) confirmed high-efficiency values for the ELISA assays.
Discussion
Diagnostic tests with high sensitivity for detecting neglected helminth tropical diseases are necessary; however, the analysis of faecal specimens by microscopy remains the most commonly used technique (Truscott et al., 2016). This test is limited by egg release and the distribution of eggs in stool samples, which might provide false-negative results, especially in low-intensity infections and following treatment (Nikolay et al., 2014). Therefore, improved diagnostic tools are needed to facilitate the detection of active hookworm infections, considering that a lack of specificity, cross-reactivity and the inability to distinguish between past and current infections are limitations of many immunodiagnostic tests.
Given the feasibility of using congeneric species to diagnose patients with parasites that share epitopes, such as Taenia solium and Taenia saginata (Oliveira et al., 2007) and Strongyloides stercoralis and S. venezuelensis (Feliciano et al., 2010; Faria et al., 2019), we evaluated the use of anti-A. ceylanicum IgY to detect hookworm infections caused by A. duodenale or N. americanus in Brazil, as A. ceylanicum is closely related to these species and therefore shares various antigenic determinants. In addition, unlike the major hookworm species, A. ceylanicum can infect dogs and hamsters. The ability of A. ceylanicum to parasitize laboratory animals has made it a useful model for the study of human hookworm infection (Hu et al., 2013).
Tests to detect antibodies often present cross-reactivity with other parasitic nematodes, especially in tropical countries where infection with STH is common. Furthermore, detection of IgG antibodies may be attributed to current infection, previous infection or exposure to the parasite and not always to an active infection (Werkman et al., 2018). Therefore, the presence of antigen and antibody immunocomplexes may serve as markers of disease activity, while antibody detection may be indicative of a resolved disease with residual antibodies (Gonçalves et al., 2016).
Polyclonal antibodies produced in chickens (IgY) offer several important advantages over antibodies produced in mammals. For example, production in chickens avoids interference in immunological assays caused by the complement system (Larsson et al., 1992), chicken antibodies do not react with the rheumatoid factor, they have poor cross-reactivity to mammalian IgGs due to immunological differences (Amro et al., 2018) and they react with more epitopes on a mammalian antigen due to evolutionary differences between mammals and birds, thereby amplifying the signal (Ohnishi et al., 2000).
IgY has been successfully used in different immunoassay formats, mainly ELISA and lateral flow immunoassays, for the detection of veterinary drugs, herbicides, mycotoxins, drugs of abuse, proteins and bacteria (Zhang et al., 2015) for both diagnostic (Ferreira Júnior et al., 2012; Faria et al., 2019) and immunotherapeutic (Thirumalai et al., 2019) purposes.
In the present study, IgY production reached the cut-off point after the first booster immunization (day 21), as chickens transfer maternal antibodies to the egg yolk to protect the developing embryos through passive immunity. Thus, the lag time observed in the first 2 weeks may be attributed to the time taken for specific antibodies to be produced and transferred from the serum to the egg yolk (Esmailnejad et al., 2019).
In this study, the detection of circulating ICs in serum was significant (90%) in group 1. ICs are important because they neutralize disease pathogenesis and are rapidly eliminated from the bloodstream by the innate immune system; therefore, they act as a biomarker of active infection. In addition, ICs are produced at increased frequency in parasitic diseases (Ohyama et al., 2016). The absence of detection in some individuals from group 1 (10%) may be due to the IC size, as smaller ICs generally remain in the circulation or are rapidly cleared or dissociated, intermediate-sized ICs can deposit in tissue while larger ones are generally rapidly cleared by the phagocytic system (Rojko et al., 2014).
The positive detection of ICs in group 2 (26.6%, n = 8) can be explained by the lack of sensitivity of faecal tests, as the tested individuals all live in areas where hookworms are prevalent, so they could possibly be coinfected with this parasite. Parasitological investigation of a single stool specimen detects about 30% of infections, and the sensitivity is increased to 50% if three faecal samples are tested (Sudré et al., 2006).
To our best of our knowledge, this is the first report of an IC detection assay based on polyclonal IgY to detect active hookworm infection in human serum samples. The IgY obtained here was successfully tested and can be used as a direct reagent in serological assays. However, it must be continuously tested to demonstrate its potential use, as novel diagnostic methods are expected to improve epidemiological studies and control efforts for the prevention and treatment of hookworm infection.
Financial support
The study was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES – NO 3200601204P-8), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG – CBB-PPM-00396-1) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq – 303843/2015-2 and CNPq–404816/2016-9).
Conflicts of interest
None.
Ethical standards
The evaluation of the serological diagnosis was performed according to the ethical guidelines of the Brazilian Health Ministry and was approved by the Comitê de Ética em Pesquisas com Seres Humanos (CEP) of the Universidade Federal de Uberlândia (CAAE: 48492315.8.00005152 and 04/2008). All procedures using experimental animals (hens, Gallus gallus domesticus) were conducted after being approved by the Comitê de Ética na Utilização de Animais from the Universidade Federal de Uberlândia, State of Minas Gerais, Brazil (CEUA/UFU No 097/2014).
References
- Akita EM and Nakai S (1993) Comparison of four purification methods for the production of immunoglobulins from eggs laid by hens immunized with an enterotoxigenic E. coli strain. Journal of Immunological Methods 160, 207–214. [DOI] [PubMed] [Google Scholar]
- Amro WA, Al-Qaisi W and Al-Razem F (2018) Production and purification of IgY antibodies from chicken egg yolk. Journal of Genetic Engineering and Biotechnology 16, 99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baermann G (1917) Eine Einfache Methode zur Auffindung von Ankylostomum (Nematoden) Larven in Erdproben. In Mededeel. Mith H. Geneesk. Batavia. Lab Weltevreden Feestbundel 57, 41–47. [Google Scholar]
- Elsemore DA, Geng J, Cote J, Hanna R, Lucio-Forster A and Bowman DD (2017) Enzyme-linked immunosorbent assays for coproantigen detection of Ancylostoma caninum and Toxocara canis in dogs and Toxocara cati in cats. Journal of Veterinary Diagnostic Investigation 29, 645–653. [DOI] [PubMed] [Google Scholar]
- Esmailnejad A, Abdi-Hachesoo B, Hosseini Nasab E and Shakoori M (2019) Production, purification, and evaluation of quail immunoglobulin Y against Salmonella typhimurium and Salmonella enteritidis. Molecular Immunology 107, 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria LS, de Souza DLN, Ribeiro RP, de Sousa JEN, Borges IP, Ávila VMR, Ferreira-Júnior Á, Goulart LR and Costa-Cruz JM (2019) Highly specific and sensitive anti-Strongyloides venezuelensis Igy antibodies applied to the human strongyloidiasis immunodiagnosis. Parasitology International 72, 101933. [DOI] [PubMed] [Google Scholar]
- Feliciano ND, Gonzaga HT, Gonçalves-Pires MdR, Gonçalves AL, Rodrigues RM, Ueta MT and Costa-Cruz JM (2010) Hydrophobic fractions from Strongyloides venezuelensis for use in the human immunodiagnosis of strongyloidiasis. Diagnostic Microbiology and Infectious Diseases 67, 153–161. [DOI] [PubMed] [Google Scholar]
- Ferreira Júnior Á, Santiago FM, Silva MV, Ferreira FB, Macêdo Júnior AG, Mota CM, Faria MS, Silva Filho HH, Silva DA, Cunha-Júnior JP, Mineo JR and Mineo TW (2012) Production, characterization and applications for Toxoplasma gondii-specific polyclonal chicken egg yolk immunoglobulins. PLoS ONE 7, e40391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman RD (1982) Comparison of four different silver-staining techniques for salivary protein detection in alkaline polyacrylamide gels. Analytical Biochemistry 126, 346–349. [DOI] [PubMed] [Google Scholar]
- George S, Levecke B, Kattula D, Velusamy V, Roy S, Geldhof P, Sarkar R and Kang G (2016) Molecular identification of hookworm isolates in humans, dogs and soil in a tribal area in Tamil Nadu, India. PLoS Neglected Tropical Diseases 10, e0004891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves AL, Nunes DS, Gonçalves-Pires MR, Ueta MT and Costa-Cruz JM (2012) Use of larval, parasitic female and egg antigens from Strongyloides venezuelensis to detect parasite-specific IgG and immune complexes in immunodiagnosis of human strongyloidiasis. Parasitology 139, 956–961. [DOI] [PubMed] [Google Scholar]
- Gonçalves AL, de Araújo KC, Carvalho EF, Ueta MT and Costa-Cruz JM (2016) Specific IgG and immune complex responses to parthenogenetic females and eggs of nematode Strongyloides venezuelensis for the diagnosis of immunosuppression in infected rats. Journal of Helminthology 90, 342–346. [DOI] [PubMed] [Google Scholar]
- Gonzaga HT, Vila-Verde C, Nunes DS, Ribeiro VS, Cunha-Júnior JP and Costa-Cruz JM (2013) Ion-exchange protocol to obtain antigenic fractions with potential for serodiagnosis of strongyloidiasis. Parasitology 140, 69–75. [DOI] [PubMed] [Google Scholar]
- Hu Y, Ellis BL, Yiu YY, Miller MM, Urban JF, Shi LZ and Aroian RV (2013) An extensive comparison of the effect of anthelmintic classes on diverse nematodes. PLoS ONE 8, e70702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Larsson A, Wejåker PE, Forsberg PO and Lindahl T (1992) Chicken antibodies: a tool to avoid interference by complement activation in ELISA. Journal of Immunology Methods 156, 79–83. [DOI] [PubMed] [Google Scholar]
- Lei JH, Guan F, Xu H, Chen L, Su BT, Zhou Y, Wang T, Li YL and Liu WQ (2012) Application of an immunomagnetic bead ELISA based on IgY for detection of circulating antigen in urine of mice infected with Schistosoma japonicum. Veterinary Parasitology 187, 196–202. [DOI] [PubMed] [Google Scholar]
- Leslie GA and Clem LW (1969) Phylogeny of immunoglobulin structure and function 3. Immunoglobulins of the chicken. Journal of Experimental Medicine 130, 1337–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang L, Zhen Y, Li S and Xu Y (2015) Chicken egg yolk antibodies (IgY) as non-antibiotic production enhancers for use in swine production: a review. Journal of Animal Science and Biotechnology 26, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LN, Jing FJ, Cui J, Fu GY and Wang ZQ (2013) Detection of circulating antigen in serum of mice infected with Trichinella spiralis by an IgY-IgM mAb sandwich ELISA. Experimental Parasitology 133, 150–155. [DOI] [PubMed] [Google Scholar]
- Lopes CA, de Faria LS, de Sousa JEN, Borges IP, Ribeiro RP, Bueno LL, Rodrigues Ávila VM, Ferreira-Júnior Á and Costa-Cruz JM (2019) Anti-Ascaris suum immunoglobulin Y as a novel biotechnological tool for the diagnosis of human ascariasis. Journal of Helminthology 94, e71. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL and Randall RJ (1951) Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry 193, 265–275. [PubMed] [Google Scholar]
- Lutz A (1919) O Schistosomum mansoni e a schistosomatose segundo observaçoões feitas no Brasil. Memórias do Instituto Oswaldo Cruz 11, 121–125. [Google Scholar]
- Moraes RG (1948) Contribuição para o estudo do Strongyloides Stercoralis e da estrongiloidíase no Brasil. Revista do Serviço Especial de Saúde Pública 1, 507–624. [Google Scholar]
- Nie G, Wang T, Lu S, Liu W, Li Y and Lei J (2014) Detection of Clonorchis sinensis circulating antigen in sera from Chinese patients by immunomagnetic bead ELISA based on IgY. PLoS ONE 9, e113208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolay B, Brooker SJ and Pullan RL (2014) Sensitivity of diagnostic tests for human soil-transmitted helminth infections: a meta-analysis in the absence of a true gold standard. International Journal for Parasitology 44, 765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T, Kakimoto K, Hashida S, Fujii M, Hirono S, Nishiyama K, Amita Y, Ishikawa E, Tsubouchi H and Daikuhara Y (2000) Development of highly sensitive enzyme-linked immunosorbent assays for hepatocyte growth factor/scatter factor (HGF/SF): determination of HGF/SF in serum and urine from normal human subjects. Journal of Immunology Methods 244, 163–173. [DOI] [PubMed] [Google Scholar]
- Ohyama K, Huy NT, Yoshimi H, Kishikawa N, Nishizawa JE, Roca Y, Revolvo Guzmán RJ, Velarde FU, Kuroda N and Hirayama K (2016) Proteomic profile of circulating immune complexes in chronic Chagas disease. Parasite Immunology 38, 609–617. [DOI] [PubMed] [Google Scholar]
- Oliveira HB, Machado GA, Cabral DD and Costa-Cruz JM (2007) Application of Taenia saginata Metacestodes as an alternative antigen for the serological diagnosis of human neurocysticercosis. Parasitology Research 101, 1007–1013. [DOI] [PubMed] [Google Scholar]
- Prieto-Pérez L, Pérez-Tanoira R, Cabello-Úbeda A, Petkova-Saiz E and Górgolas-Hernandéz-Mora M (2016) Geohelminths. Enfermedades Infecciosas y Microbiología Clínica 34, 384–389. [DOI] [PubMed] [Google Scholar]
- Pullan RL, Smith JL, Jasrasaria R and Brooker SJ (2014) Global numbers of infection and disease burden of soil transmitted helminth infections in 2010. Parasites and Vectors 7, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro VdS, Araújo TG, Gonzaga HT, Nascimento R, Goulart LR and Costa-Cruz JM (2013) Development of specific scFv antibodies to detect neurocysticercosis antigens and potential applications in immunodiagnosis. Immunology Letters 156, 59–67. [DOI] [PubMed] [Google Scholar]
- Rojko JL, Evans MG, Price SA, Han B, Waine G, DeWitte M, Haynes J, Freimark B, Martin P, Raymond JT, Evering W, Rebelatto MC, Schenck E and Horvath C (2014) Formation, clearance, deposition, pathogenicity, and identification of biopharmaceutical-related immune complexes: review and case studies. Toxicologic Pathology 42, 725–764. [DOI] [PubMed] [Google Scholar]
- Schwarzkopf, C, Staak, C, Behn, I and Erhard M (2001) Immunisation. In Schade R, Behn I, Erhard M, Hlinak A and Staak C (eds), Chickens Egg Yolk Antibodies, Production and Applications-IgY-Technology. Berlin, Heidelberg: Springer-Verlag, pp. 25–64. [Google Scholar]
- Sudré AP, Macedo HW, Peralta RHS and Peralta JM (2006) Diagnóstico da estrongiloidíase humana: importância e técnicas. Revista de Patologia Tropical 35, 173–184. [Google Scholar]
- Thirumalai D, Visaga Ambi S, Vieira-Pires RS, Xiaoying Z, Sekaran S and Krishnan U (2019) Chicken egg yolk antibody (IgY) as diagnostics and therapeutics in parasitic infections – A review. International Journal of Biological Macromolecules 136, 755–763. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T and Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proceedings of the National Academy of Sciences of the United States of America 76, 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truscott JE, Turner HC, Farrell SH and Anderson RM (2016) Soil-transmitted helminths: mathematical models of transmission, the impact of mass drug administration and transmission elimination criteria. Advances in Parasitology 94, 133–198. [DOI] [PubMed] [Google Scholar]
- Werkman M, Wright JE, Truscott JE, Easton AV, Oliveira RG, Toor J, Ower A, Ásbjörnsdóttir KH, Means AR, Farrell SH, Walson JL and Anderson RM (2018) Testing for soil-transmitted helminth transmission elimination: analysing the impact of the sensitivity of different diagnostic tools. PLoS Neglected Tropical Diseases 12, e0006114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Mi T, Khan OY, Sheng Y, Eremin SA, Beier RC, Zhang S, Shen J and Wang Z (2015) Fluorescence polarization immunoassay using IgY antibodies for detection of valnemulin in swine tissue. Analytical and Bioanalytical Chemistry 407, 7843–7848. [DOI] [PubMed] [Google Scholar]