Abstract
Factors such as the particular combination of parasite–mosquito species, their co-evolutionary history and the host's parasite load greatly affect parasite transmission. However, the importance of these factors in the epidemiology of mosquito-borne parasites, such as avian malaria parasites, is largely unknown. Here, we assessed the competence of two mosquito species [Culex pipiens and Aedes (Ochlerotatus) caspius], for the transmission of four avian Plasmodium lineages (Plasmodium relictum SGS1 and GRW11 and Plasmodium cathemerium-related lineages COLL1 and PADOM01) naturally infecting wild house sparrows. We assessed the effects of parasite identity and parasite load on Plasmodium transmission risk through its effects on the transmission rate and mosquito survival. We found that Cx. pipiens was able to transmit the four Plasmodium lineages, while Ae. caspius was unable to transmit any of them. However, Cx. pipiens mosquitoes fed on birds infected by P. relictum showed a lower survival and transmission rate than those fed on birds infected by parasites related to P. cathemerium. Non-significant associations were found with the host–parasite load. Our results confirm the existence of inter- and intra-specific differences in the ability of Plasmodium lineages to develop in mosquito species and their effects on the survival of mosquitoes that result in important differences in the transmission risk of the different avian malaria parasite lineages studied.
Key words: Avian malaria parasites, host–parasite interactions, mosquitoes, vector-borne pathogens, vector-competence
Introduction
Parasites of the genus Plasmodium, the causative agent of malaria, are vector-borne Haemosporidians that greatly affect humans and wildlife (Sachs and Malaney, 2002; Valkiūnas, 2005). The avian malaria parasite Plasmodium shows a wide range of competent hosts belonging to different bird orders and families (Fallon et al., 2005; Pérez-Tris et al., 2007; Hellgren et al., 2009). These parasites are transmitted by some competent mosquito species, in which they undergo a sexual reproduction phase (Valkiūnas, 2005). Infective forms of the parasite migrate to the mosquito salivary glands and may be then transmitted to a new avian host. Consequently, mosquito–Plasmodium interactions may play an important role in the dynamics of parasite transmission (Kimura et al., 2010). As stated above, not all mosquito species are competent vectors of avian malaria parasites. The main vectors are mosquitoes of the genus Culex, although other genera such as Aedes or Anopheles could also be involved in their transmission (Santiago-Alarcon et al., 2012). The finding of genetically related parasite lineages/species in different mosquito genera leads to the assumption of a generalist relationship between Plasmodium and mosquitoes (Kimura et al., 2010; Ferraguti et al., 2013; Schoener et al., 2017). However, most information regarding the mosquito species involved in avian Plasmodium transmission is based on the molecular identification of parasite DNA on mosquito pools, without a quantitative evaluation of the vector competence for different mosquito species (Kimura et al., 2010; Ferraguti et al., 2013; Schoener et al., 2017). Indeed, molecular detection of parasite DNA in insects' bodies does not imply these are competent vectors (Valkiūnas, 2011) and interspecific differences in competence for the transmission of avian Plasmodium could be overlooked, as parasite DNA may also be isolated from the body of non-competent mosquito species (Beerntsen et al., 2000; Ishtiaq et al., 2008). Thus, it becomes crucial to evaluate the competence of different mosquito species for the transmission of different Plasmodium lineages to better understand the transmission of avian malaria parasites in the wild.
For the successful transmission of avian Plasmodium, vectors must survive long enough to allow parasites to complete their life cycle (between 8 and 13 days) (Valkiūnas, 2005; LaPointe et al., 2010). The development of Plasmodium in the mosquito may be affected by several environmental factors, such as temperature and humidity (Paaijmans et al., 2010; Lefévre et al., 2013). In addition, the particular species involved in the parasite–vector assemblage and the vertebrate host parasite load may further determine the success of development of parasites in mosquitoes (Cornet et al., 2014). For example, mosquitoes feeding on birds with high parasite loads develop a high density of ookinetes (an initial non-infective phase of Plasmodium) in their abdomen, likely increasing parasite transmission success (Pigeault et al., 2015). However, Plasmodium development in the mosquito produces tissue damage, with potential negative consequences for mosquito survival. Previous studies on the impact of avian malaria parasites on vector survival have reported positive, negative or non-significant effects of parasite infection on mosquito longevity (Vézilier et al., 2012; Lalubin et al., 2014; Delhaye et al., 2016; Pigeault and Villa, 2018; Gutiérrez-López et al., 2019a). However, most of these studies focus on the interaction between Culex pipiens mosquitoes and Plasmodium relictum (lineage SGS1) (Cornet et al., 2013; Pigeault et al., 2015; Martínez-de la Puente et al., 2018). Therefore, studies considering potential differences in virulence (i.e. the cost of the pathogen infections on their host) between parasite species/lineages on different mosquito species are necessary (Lachish et al., 2011).
Here, we experimentally assessed the competence of two mosquito species, Cx. pipiens and Aedes (Ochlerotatus) caspius, for the transmission of four avian Plasmodium lineages. Both mosquito species are common in southern Spain, where they show different feeding patterns. While Cx. pipiens feed mainly on birds (Martínez-de la Puente et al., 2016), Ae. caspius prefers to bite mammals, although birds may represent up to 19% of their diet (Balenghien et al., 2006; Muñoz et al., 2012; Gutiérrez-López et al., 2019b). Avian Plasmodium DNA has been isolated from both mosquito species (Ferraguti et al., 2013; Schoener et al., 2017), and the capacity of Cx. pipiens for the transmission of avian Plasmodium parasites has been previously demonstrated (Kazlauskiené et al., 2013; Gutiérrez-López et al., 2016; Palinauskas et al., 2016). In this study, mosquitoes were allowed to feed on birds naturally infected by Plasmodium to assess the effects of bird parasite load and parasite identity (i.e. different Plasmodium lineages grouped into main clades, see below) on the probability of mosquito infection and parasite transmission. We also analysed the impact of parasite development on mosquito survival. Finally, we estimated the impact of mosquito survival and parasite development on the risk of parasite transmission, based on the quantification of the relative basic reproductive number (R0), modified from Ross (1911) and Macdonald (1955).
Materials and methods
Mosquito collection and rearing
Larvae of Cx. pipiens and Ae. caspius were collected from April to September in 2014 and 2016 in the natural reserve ‘La Cañada de los Pájaros’ (6°14′W, 36°57′N, Seville Province, Spain) and in marshlands of the Huelva Province (6°53′W, 37°17′N). Larvae were grown in plastic trays with fresh or brackish water, respectively, maintained following Gutiérrez-López et al. (2019a) with food ad libitum. Adult female mosquitoes were identified to the species level following Schaffner et al. (2001), placed in insect cages (BugDorm-43030F, 32.5 × 32.5 × 32.5 cm) and fed ad libitum with 1% sugar solution. One day prior to each experiment, 2–3-week-old female mosquitoes were deprived from sugar solution. Only F0 generation mosquitoes collected in the field were used in the experiment. Laboratory maintained colonies of mosquitoes were not used to minimize the potential effects of artificial selection on vector–host interactions (Franks et al., 2011; Lagisz et al., 2011).
Bird sampling and experimental procedure
A total of 60 wild house sparrows (Passer domesticus) were caught from May to September 2014 in ‘La Cañada de los Pájaros’ and from June to September 2016 in different localities from Huelva Province using mist nets. Birds were individually ringed and weighed. Blood samples (0.2 mL) were obtained by jugular venepuncture using sterile syringes. A drop of blood was smeared, air-dried, fixed with absolute methanol and stained with Giemsa for 45 min (Gering and Atkinson, 2004). The rest of the blood was transferred to non-heparinized Eppendorf tubes to perform molecular detection of parasites (see below). A total of 4000–10 000 erythrocytes from each smear were scanned at high magnification (×1000). Plasmodium parasite load was estimated as the percentage of infected erythrocytes. Although the gametocytaemia (proportion of red blood cells infected by gametocytes, i.e. the sexual stage of the parasite that is transmitted to mosquitoes) may provide a more reliable quantitative measure of parasite infection than parasitaemia, both variables are strongly correlated (Pigeault et al., 2015).
Forty-five individual birds were enclosed for 30 min in an insect cage (BugDorm-43030F, 32.5 × 32.5 × 32.5 cm) containing either Cx. pipiens (mean ± s.d.: 68.48 ± 34.97, range 4–111) or Ae. caspius (57.29 ± 34.04, range 4–126) females. Although we tried to use a similar number of mosquitoes per box, this task was difficult to achieve due to the fact that mosquitoes were obtained from larvae in the field, which limited the number of mosquitoes of similar age/species available for each day/trial. Birds were immobilized to prevent defensive behaviours following Gutierrez-López et al. (2019b). The feeding trials were undertaken from 7:30 to 12:00 h (GMT + 1 h). Because Gutiérrez-López et al. (2019b) did not find any effect of house sparrow sex on Cx. pipiens and Ae. caspius biting rates, we grouped birds from both sexes in this study, including 18 females and 42 males. At the end of each trial, all birds were immediately released at the place of capture, with no apparent sign of damage. In addition, 15 of these 60 birds corresponded to control birds from the study of Yan et al. (2018). These birds were injected with saline solution, maintained in captivity for 24 days before being exposed to mosquitoes in the context of Yan et al. (2018) study. Twenty-four hours after this exposure, we re-exposed these 15 birds to mosquitoes in the context of this study following the same procedure described above.
After trials, engorged mosquitoes (see Table 1) were placed in insect cages, one for all mosquitoes fed on the same individual bird, and maintained under the same conditions detailed above. Mosquito survival was monitored every 12 h until 13 days post-exposure (dpe). At the end of this period, the saliva of surviving mosquitoes was obtained following the protocol detailed by Gutiérrez-López et al. (2016). We chose the isolation of saliva over other conventional methods such as the analysis of mosquito salivary glands because the former allows the use of molecular methods for parasite detection and it has been widely used in studies on the competence of mosquitoes to transmit other pathogens such as viruses (Goddard et al., 2002; Dubrulle et al., 2009; Gutiérrez-López et al., 2019c) and malarial parasites (Golenda et al., 1992; Gutiérrez-López et al., 2019a). Subsequently, the head-thorax of each mosquito, which contains the salivary glands, was separated from the abdomen in a sterile Petri dish. Samples were kept at −80°C until further molecular analyses.
Table 1.
Cx. pipiens and Ae. caspius engorged and analysed for the different Plasmodium lineages found in house sparrows
| Mosquito species | Plasmodium lineages | Clade | N house sparrows | Engorged mosquitoes | Alive until 13 dpe | Head-thorax positive/analysed | Saliva positive/analysed |
|---|---|---|---|---|---|---|---|
| Cx. pipiens | P. relictum SGS1 | A | 15 | 112 | 63a | 21/60 | 2/21 |
| P. relictum GRW11 | A | 4 | 35 | 33 | 14/33 | 2/14 | |
| Plasmodium COLL1 | B | 6 | 23 | 20 | 8/20 | 2/8 | |
| Plasmodium PADOM01 | B | 2 | 13 | 13 | 8/13 | 5/8 | |
| Ae. caspius | P. relictum SGS1 | A | 11 | 58 | 22 | 0/22 | – |
| P. relictum GRW11 | A | 2 | 30 | 14 | 0/14 | – | |
| Plasmodium COLL1 | B | 2 | 17 | 8 | 0/8 | – | |
| Plasmodium PADOM01 | B | 2 | 7 | 1 | 0/1 | – |
The number of individual house sparrows infected with each Plasmodium lineage is shown.
Three mosquitoes fed on birds infected with P. relictum escaped.
Molecular analyses
Genomic DNA was extracted from bird blood samples and the head-thorax of mosquitoes using the MAXWELL® 16 LEV Blood DNA Kit while the Qiagen DNeasy® Kit Tissue and Blood (Qiagen, Hilden, Germany) was used to extract the DNA from mosquito saliva (Gutiérrez-López et al., 2015). The presence of Plasmodium/Haemoproteus parasites was determined following Hellgren et al. (2004). Positive amplifications were sequenced in both directions using the BigDye technology (Applied Biosystems) or with the Macrogen Inc. (Amsterdam, The Netherlands) sequencing service. Sequences were edited using software Sequencher™ v4.9 (Gene Codes Corp., © 1991–2009, Ann Arbor, MI 48108, USA) and assigned to parasite lineages/morphospecies after comparison with public databases [GenBank and MalAvi (Bensch et al., 2009; Kumar et al., 2016)]. The four Plasmodium lineages found in this study were grouped into two main clades (clade A and clade B, see ‘Results’ section) and uncorrected p-distances between lineages/clades were compared using MEGA7 software (Kumar et al., 2016). Five birds showed evidence of coinfection, as revealed by the existence of double peaks in the sequencing chromatogram, and were not included in this study to avoid potential confounding effects of multiple infections on parasite development and mosquito survival (Lover and Coker, 2015). Ten house sparrows uninfected by avian Plasmodium (see ‘Results’ section) were used as control to study the survival of 80 mosquitoes that fed on them.
Statistical analyses
Statistical analyses were performed in R software 3.2.5 (R Core Development Team, 2016). We fitted two similar generalized linear mixed models with binomial error and logit link function using the package lme4 (Bates et al., 2015), to assess the effects of Plasmodium clade identity (fixed factor) and the bird parasite load (covariate) on the status of infection by Plasmodium (infected/uninfected) in the head-thorax or saliva, respectively. The variable bird parasite load was log-transformed to attain normality. In both models, bird identity was included as a random term. We fitted, by maximum likelihood using the package survival (Therneau and Lumley, 2014), a mixed-effects Cox model to test the effects of bird parasite infection status (fixed factor, infected/uninfected birds) and parasite load (covariate) on mosquito survival (measured as the number of mosquitoes alive at each 12-h-period), and bird identity as a random factor, while controlling for the potential effect of mosquito age (2 or 3 weeks old). We also fitted a similar mixed-effects Cox model with parasite identity (clade A, clade B and uninfected) instead of infection status as explanatory variable. We restricted the analyses of survival to Cx. pipiens mosquitoes, as Plasmodium only developed successfully in this species (see ‘Results’ section).
Plasmodium transmission risk
We used a simplified equation of the R0 model proposed by Macdonald (1955) to calculate relative R0 values:
where c represents the probability of a mosquito becoming infected after biting an infected host, P is the daily survival rate of mosquitoes measured as the probability that a mosquito survives for 1 day and v is the pathogen incubation period in the mosquito. In our study, c (hereafter, transmission rate) was considered as the probability of a mosquito carrying Plasmodium DNA in its saliva after feeding on an infected bird. In addition, we considered v as 13 days (Valkiūnas, 2005; LaPointe et al., 2010). The relative R0 value was calculated considering the survival rate until 13 dpe and the proportion of mosquitoes with positive saliva samples infected with lineages of each Plasmodium clade. The relative R0 provides an approach to quantify the impact of differences in the survival rate of mosquitoes or Plasmodium transmission to mosquito saliva on Plasmodium transmission.
Results
Avian malaria parasites in birds
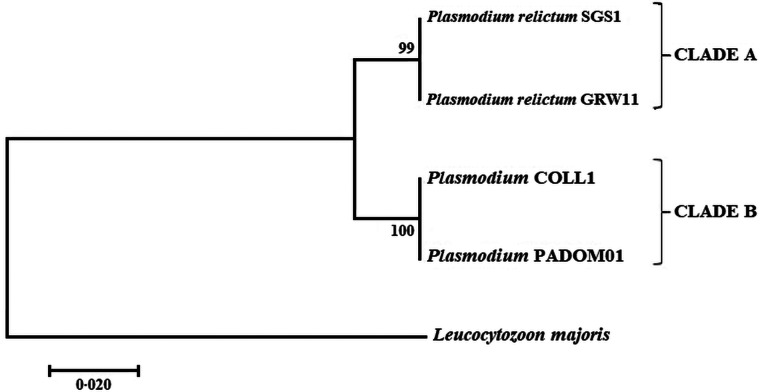
From the 60 birds captured, 10 were uninfected (and the mosquitoes that fed on them were used as controls) and 50 were infected by at least one avian malaria lineage. Five of them showed coinfection by several lineages of haemosporidians and were removed from further analyses. Overall, we exposed 45 birds infected with four parasite lineages, including the P. relictum lineages SGS1 (N = 26) and GRW11 (N = 6), and the lineages COLL1 (N = 8) and PADOM01 (N = 5) (Table 1), to mosquitoes. The morphospecies for COLL1 and PADOM01 are unknown, but these lineages clustered with the lineage SEIAUR01, corresponding to Plasmodium cathemerium, which was already found in house sparrows in the same area (Ferraguti et al., 2018) (Fig. 1). The uncorrected p-distance between lineages SGS1 and GRW1 and between COLL1 and PADOM01 was 0.002 (corresponding to a difference of a single base pair). By contrast, the uncorrected p-distance between lineages SGS1–GRW11 and COLL1–PADOM01 was 0.035. Thus, in further analyses these four lineages were grouped into two different clades: clade A corresponding to the P. relictum lineages SGS1 and GRW11, and clade B corresponding to the Plasmodium spp. lineages COLL1 and PADOM01, which are closely related to P. cathemerium. The parasite load in birds did not differ significantly between clades (mean ± s.d.: clade A: 1.39 ± 0.21, clade B: 1.15 ± 0.35, ANOVA; F1,37 = 0.34, P = 0.56). In addition, the parasite load was similar between parasite lineages within clade A (ANOVA; F1,27 = 0.03, P = 0.87) and clade B (ANOVA; F1,8 = 0.06, P = 0.81).
Fig. 1.
Bootstrap consensus tree inferred from 10 000 replications for the Plasmodium lineages found in house sparrows.
Parasite development in mosquitoes
Overall, 28 and 17 Plasmodium-infected house sparrows were exposed to 1713 Cx. pipiens and 974 Ae. caspius, respectively. Of these, 183 (10.7%) Cx. pipiens and 112 (11.5%) Ae. caspius fed on bird blood (Table 1). One house sparrow infected by the Plasmodium lineage PADOM01 was not bitten by any Cx. pipiens. Plasmodium infection status in the head-thorax was analysed for 126 Cx. pipiens fed on 27 infected birds (19 infected by parasites of clade A and 8 infected by parasites of clade B). Fifty-one (40.5%; N = 126) Cx. pipiens were positive for Plasmodium in the head-thorax. Eleven out of these 51 mosquitoes (21.6%) had Plasmodium DNA in their saliva. For Ae. caspius, Plasmodium infection status was analysed in the head-thorax of 45 mosquitoes fed on 12 infected birds (10 infected by parasites of clade A and 2 infected by parasites of clade B). None of the Ae. caspius that fed on three birds infected by Plasmodium SGS1, one bird infected by Plasmodium COLL1 and one bird infected by Plasmodium PADOM01 survived until 13 dpe. None of the 45 head-thoraxes of Ae. caspius analysed showed evidence of Plasmodium infection (Table 1).
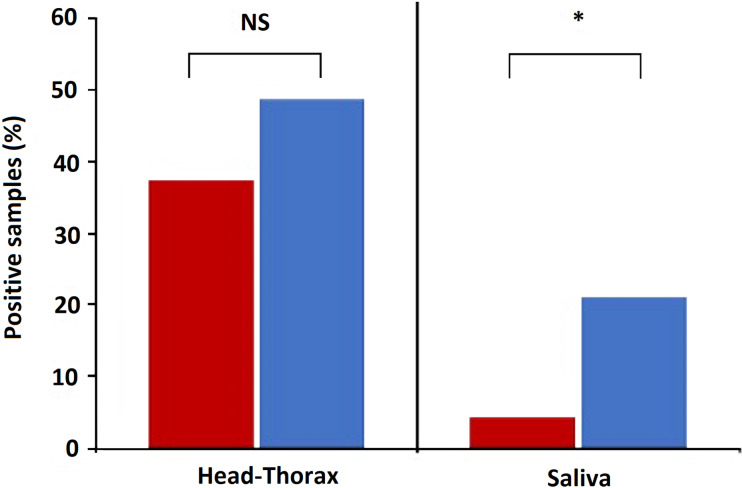
Parasites were detected in the head-thorax of 37.6% (N = 93) and 48.8% (N = 33) Cx. pipiens fed on birds infected by Plasmodium lineages of clades A and B, respectively. Bird parasite load positively affected the prevalence of Plasmodium in the head-thorax of Cx. pipiens [estimate (est) = 0.86, Z = 2.99, P = 0.003], while parasite prevalence did not differ between clades (est = 0.42, Z = 0.98, P = 0.33; Fig. 2). By contrast, a higher prevalence of Plasmodium clade B (21.2%) than clade A (4.3%) was found in Cx. pipiens saliva (est = 1.81, Z = 2.68, P = 0.007; Fig. 2), while non-significant associations were found with parasite load in birds' blood (est = 0.52, Z = 1.40, P = 0.16). All Plasmodium lineages infecting house sparrows were detected in mosquito saliva and the same Plasmodium lineages were found in the head-thorax and saliva of each mosquito.
Fig. 2.
Percentage of Cx. pipiens head-thoraxes and saliva with presence of Plasmodium DNA from parasites of clades A (red) and B (blue). Statistically significant differences are indicated with an asterisk (*). NS means non-significant differences.
Mosquito survival
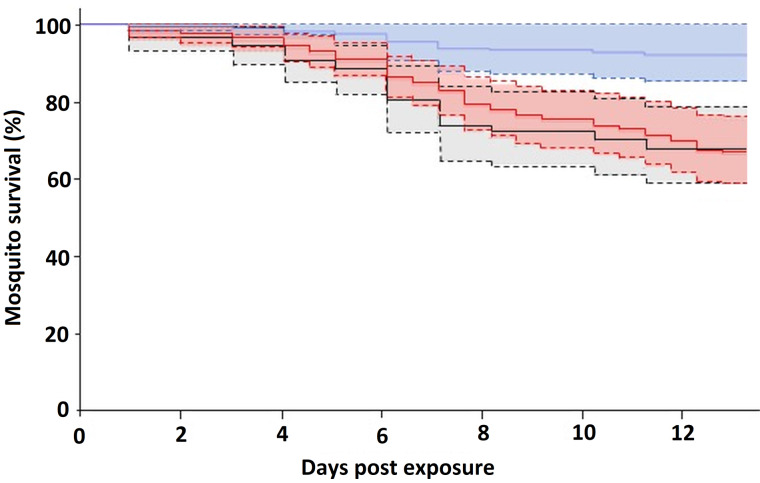
We monitored the survival up to 13 dpe of 180 Cx. pipiens fed on infected birds and 80 Cx. pipiens fed on 10 uninfected control birds. Fifty-one mosquitoes died before 13 dpe. Of them, 33.3% fed on birds infected with Plasmodium parasites of clade A, while only 8.3% fed on birds infected with parasite lineages of clade B. From those mosquitoes fed on uninfected control birds, 32.5% died before 13 dpe. The survival of mosquitoes did not depend on the bird infection status (Z = −1.42, P = 0.16), the host infection intensity (Z = 1.31, P = 0.19) or the mosquito age (Z = 0.9; P = 0.4). However, when considering the identity of Plasmodium parasites instead of the bird infection status, Cx. pipiens fed on birds infected by parasites of clade B survived longer than those fed on birds infected by clade A (Z = 2.23; P = 0.03; Fig. 3), and on un-infected control birds (Z = 2.83; P = 0.005), while parasite load in the bird host (Z = 1.39, P = 0.16) and mosquito age (Z = 0.69; P = 0.49) did not affect mosquito survival.
Fig. 3.
Proportion of Cx. pipiens that survived until 13 dpe to Plasmodium parasites of clade A (red line), clade B (blue line) or control (black line). The shaded areas comprise the standard errors.
Plasmodium transmission risk
Both parameters, i.e. transmission rate and vector survival, were affected by the parasite clade and consequently both clades differed in their risk of transmission. Mosquitoes fed on birds infected by Plasmodium lineages of clade B had a higher daily survival probability (P) than those fed on birds infected by Plasmodium lineages of clade A (daily survival probability = 0.99 and 0.97, respectively). Moreover, the transmission rate (c) of Plasmodium lineages of clade B by Cx. pipiens was higher than the transmission rate of Plasmodium lineages of clade A (transmission rate = 0.21 and 0.04, respectively). Consequently, Plasmodium parasites clade A showed a 20.3 times lower relative transmission risk number than those of clade B (R0,rel = 18.48 and 0.9 for clades B and A, respectively).
Discussion
The successful transmission of mosquito-borne parasites largely depends on the availability of competent vector species in the area (Beerntsen et al., 2000). Although mosquitoes of the genus Culex are considered the main vectors of avian Plasmodium, molecular detection of different Plasmodium lineages in other mosquito genera, such as Anopheles, Aedes and Lutzia (Kimura et al., 2010; Santiago-Alarcón et al., 2012; Ferraguti et al., 2013; Schoener et al., 2017), suggests that avian Plasmodium spp. are not tightly coevolved with mosquito species. However, our results indicate that while Cx. pipiens being capable of transmitting the four Plasmodium lineages we isolated from birds, Ae. caspius does not, which suggests the existence of parasite/insect related mechanisms that prevents the successful development and subsequent transmission of the parasite by some mosquito species (Hardy et al., 1983; Black and Moore, 1996). The insect midgut represents a strong barrier, being able to dramatically reduce the number of viable parasites from those initially ingested (Abraham and Jacobs-Lorena, 2004; Siden-Kiamos et al., 2006). In addition, Plasmodium penetrates the midgut intracellular epithelium by a complex mechanism involving numerous proteins of the membrane (Povelones et al., 2009). Thus, potential differences in the presence of these proteins between mosquito species could explain the inability of avian Plasmodium to develop in Ae. caspius mosquitoes. Furthermore, differences in the immune response against parasites or midgut microbiota between mosquito species may affect parasite development (Azambuja et al., 2005; Weiss and Aksoy, 2011). Our results strongly support that detection of Plasmodium DNA in a particular mosquito species body is not a good indicator of the species' vector competence, since Plasmodium DNA was previously detected, even at high prevalence, in Ae. caspius (Ferraguti et al., 2013; Schoener et al., 2017). Although mosquito head-thorax and saliva were sampled at 13 dpe, a period that exceeds the time needed for different Plasmodium spp. to develop in the mosquito salivary glands (Valkiūnas, 2005; LaPointe et al., 2010), there may exist mosquito–parasite combinations that require different pre-patent periods for parasite development. This could also explain the absence of Plasmodium DNA in Ae. caspius.
Despite Cx. pipiens is a competent vector of avian Plasmodium, we found that its competence for the parasite transmission differed between the two clades. We found a reduced impact on mosquito survival and a higher parasite prevalence in mosquito saliva of clade B lineages (COLL1 and PADOM01), while for clade A (SGS1 and GRW11), we found a higher impact on mosquito survival and lower prevalence in mosquito saliva. As a result, the vector competence of Cx. pipiens for clade B parasites was much higher than for those of clade A. However, these differences could be the result of unequal parasite development in the mosquitoes. It is possible that both clades differ in the time required to develop and reach the salivary glands, as has been reported between Plasmodium parasite species (LaPointe et al., 2010; Palinauskas et al., 2016), with parasites of clade B producing sporozoites faster than those of clade A. This could be due to different pre-patent periods of parasite development in the mosquito. In fact, Maier (1973) found sporozoites of P. cathemerium in the salivary glands of Cx. pipiens mosquitoes at 7 dpe, and P. relictum sporozoites in the salivary glands of mosquitoes have been recorded as early as 4 and 5 dpe (Rosen and Reeves, 1954; Work et al., 1990). However, Kazlauskienė et al. (2013) did not find sporozoites of the P. relictum lineages SGS1 and GRW11 (clade A in this study) until 14 dpe in the salivary glands of Cx. pipiens mosquitoes (presence of sporozoites was analysed at 12 dpe, but not at 13 dpe). In addition to parasite identity, the parasite load of the vertebrate host may largely determine the success of parasite development in the insect vector and, potentially, its ability for being transmitted. In humans, Plasmodium gametocytaemia was positively associated with the mosquito infection rates (Bousema and Drakeley et al., 2011). In avian Plasmodium, however, non-significant associations between host parasitaemia and oocyst prevalence have been found (Pigeault and Villa, 2018). However, the interaction of bird hosts and mosquitoes could potentially affect the within-host dynamics of Plasmodium and therefore, its transmission to mosquitoes. For example, it has been shown that daily variations in mosquito activity are correlated with bird parasitaemia and that mosquito bites increase P. relictum replication in the birds both in the acute and in the chronic phases of the infection (Pigeault et al., 2018). Our results suggest that although Plasmodium load in the avian host facilitates the detection of parasite DNA in the mosquito head-thorax, the successful infection and transmission of Plasmodium into mosquito saliva was not directly related to infection load in the bird host. Consequently, the final development of parasites in mosquito saliva may be modulated by other factors, including specific mosquito–parasite assemblages.
The costs of Plasmodium infection for mosquito survival remain a subject of intense debate (Ferguson and Read, 2002; Martínez-de la Puente et al., 2018). Vézilier et al. (2012) reported a decreased fecundity and an increased longevity of mosquitoes fed on infected birds, while Pigeault and Villa (2018) did not find any association between bird parasite load and mosquito survival. However, these studies focused on the interaction between Cx. pipiens and P. relictum. In our study, we found that mosquitoes fed on birds infected by Plasmodium clade B had higher survival than those fed on birds infected by clade A (P. relictum) or un-infected birds. Differences in the level of virulence between avian Plasmodium lineages on mosquitoes are currently unknown. Nonetheless, a differential impact on bird hosts of parasite lineages/morphospecies has been reported. For instance, Lachish et al. (2011) found that P. relictum had a lower virulence on birds than P. circumflexum. Our results suggest that this may also occur in mosquitoes, with differential cost [i.e. energetic cost (Hurd et al., 2005); survival, this study] imposed by different species/lineages of Plasmodium. Although birds infected by both clades (clades A and B) showed similar Plasmodium intensities of infection, mosquitos feeding on birds with high intensities of infection die sooner (Gutiérrez-López et al., 2019a), probably due to the cost of Plasmodium infection on mosquito survival.
We found that Plasmodium transmission risk differed between parasite clades mainly due to their differential impact on mosquito survival and transmission rate (i.e. presence in saliva). Lineages of P. relictum (clade A) had a higher virulence on mosquitoes and also showed a lower transmission rate than parasites of clade B. Consequently, transmission of Plasmodium was less effective when mosquitoes fed on birds infected by lineages of clade A than when infected by clade B. In addition to the variables measured here, the epidemiology of vector-borne parasites depends on a number of factors, such as host density (Gubbins et al., 2008), host recovery rate (Macdonald, 1955) and vector density (Hartemink et al., 2011). In addition, our estimation of the parasite transmission risk should be considered with caution as we monitored the survival rate of mosquitoes until 13 dpe, while epidemiological models should consider the whole lifespan of individuals. In spite of these limitations, our results provide a first step towards the identification of the consequences of parasite identity for avian malaria epidemiology, a topic that has been traditionally neglected. Interestingly, P. relictum (clade A) is considered a generalist parasite infecting more than 300 species of birds belonging to 11 different orders worldwide, being transmitted by 20 different species of mosquitoes (Valkiūnas et al., 2018). However, recent studies have found that generalist parasites may also show a specialized behaviour in certain, phylogenetically related host species within their host range (Svensson-Coelho et al., 2016; Huang et al., 2018). These findings suggest that the generalist/specialist character of a parasite should not be solely based on its host range, which may in turn vary among different host and parasite communities (Svensson-Coelho et al., 2016), and highlights that phylogenetic relationships among hosts must be considered when determining the specificity of a particular parasite (Huang et al., 2018). However, all such studies refer to the generalist/specialist continuum from a vertebrate host perspective, while little information is available on virulence and specialization on the vectors. Our results showed a higher efficacy of transmission by Cx. pipiens of parasites of clade B as compared with those of clade A. This could be due to the generalist character of P. relictum, which may decrease its fitness when transmitted to Cx. pipiens, but not when infecting birds (Hellgren et al., 2009). Further studies are necessary to understand the range of vectors successfully transmitting the different species of avian Plasmodium.
In conclusion, results from this study confirm the existence of inter- and intra-specific differences in the ability of Plasmodium lineages to develop in different mosquito species. While some mosquitoes such as Ae. caspius were refractory to parasite development, Cx. pipiens play a key role in the transmission of avian Plasmodium by regulating, for instance, its temporal (e.g. Lalubin et al., 2014) and spatial dynamics (Martínez-de la Puente et al., 2016) in both natural and anthropized environments. Here, we add valuable information on the competence of Cx. pipiens for the transmission of four Plasmodium lineages with active circulation in Europe. Nevertheless, the identity of each vector–parasite assemblage may modulate the transmission success of Plasmodium lineages through differences in the parasite transmission rate in the mosquito and the costs of infection on mosquito survival. Consequently, Cx. pipiens was better at transmitting lineages related to P. cathemerium than P. relictum due to a lower impact on mosquito survival and higher ability to replicate and reach mosquito saliva of the former. Understanding how Plasmodium virulence in the host and the vector interact may be crucial to understand the maintenance and abundance of the different Plasmodium species and lineages in the wild.
Acknowledgements
Alberto Pastoriza, Alazne Díez, Esmeralda Pérez and Santiago Ruiz helped with bird and mosquito capture and identification. Isabel Martín and Laura Gómez helped in the laboratory. We would also like to thank the two anonymous reviewers for their constructive comments on the manuscript.
Author contributions
All authors designed the study. RGL and JMP conducted the experimental work. RGL and JMP performed the laboratory analyses. RGL, JMP, LG and JF performed the statistical analyses. All authors read and approved the final manuscript.
Financial support
This study was funded by projects CGL2015-65055-P and PGC2018-095704-B-100 from the Spanish Ministry of Science, Innovation and Universities and from the European Regional Development Fund (FEDER). RGL was supported by a FPI grant (BES-2013-065274). JMP was partially supported by a 2017 Leonardo Grant for Researchers and Cultural Creators, BBVA Foundation. The Foundation accepts no responsibility for the opinions, statements and contents included in the project and/or the results thereof, which are entirely the responsibility of the authors. LG was supported by a Marie Sklodowska-Curie Fellowship of the European Commission (grant number 747729 ‘EcoEvoClim’). Fieldwork facilities were provided by Doñana ICTS-RBD.
Conflict of interest
The authors declare that they have no competing interests.
Ethical standards
All experiments involving birds adhered to the requirements of the Spanish Legislative Decree ‘Real Decreto 53/2013 de 1 de Febrero’ on protection of animals used for experimentation and other scientific purposes, the European Community Council Directive no. 2010/63/UE on Laboratory Animal Protection. Regional Authorities and the CSIC Ethics Committee approved this project (ref. CEBA-EBD-12-40). Mosquito sampling was performed with all the necessary permissions from landowners and regional Department of Environment (Consejería de Medio Ambiente, Junta de Andalucía).
References
- Abraham EG and Jacobs-Lorena M (2004) Mosquito midgut barriers to malaria parasite development. Insect Biochemistry and Molecular Biology 34, 667–671. [DOI] [PubMed] [Google Scholar]
- Azambuja P, Garcia ES and Ratcliffe NA (2005) Gut microbiota and parasite transmission by insect vectors. Trends in Parasitology 21, 568–572. [DOI] [PubMed] [Google Scholar]
- Balenghien T, Fouque F, Sabatier P and Bicout DJ (2006) Horse-, bird-, and human-seeking behavior and seasonal abundance of mosquitoes in a West Nile virus focus of southern France. Journal of Medical Entomology 43, 936–946. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B and Walker S (2015) Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1–48. [Google Scholar]
- Beerntsen BT, James AA and Christensen BM (2000) Genetics of mosquito vector competence. Microbiology and Molecular Biology Reviews 64, 115–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensch S, Hellgren O and Pérez-Tris J (2009) Malavi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Molecular Ecology Resources 9, 1353–1358. [DOI] [PubMed] [Google Scholar]
- Black WCIV and Moore CG (1996) Population biology as a tool for studying vector-borne diseases. In Beaty BJ and Marquardt WC (eds), The Biology of Disease Vectors. Colorado, USA: University Press of Colorado, pp. 393–416. [Google Scholar]
- Bousema T and Drakeley C (2011) Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clinical microbiology reviews 24, 377–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornet S, Nicot A, Rivero A and Gandon S (2013) Both infected and uninfected mosquitoes are attracted toward malaria infected birds. Malaria Journal 12, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornet S, Nicot A, Rivero A and Gandon S (2014) Evolution of plastic transmission strategies in avian malaria. PLoS Pathogens 10, e1004308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaye J, Aletti C, Glaizot O and Christe P (2016) Exposure of the mosquito vector Culex pipiens to the malaria parasite Plasmodium relictum: effect of infected blood intake on immune and antioxidant defences, fecundity and survival. Parasites and Vectors 9, 616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrulle M, Mousson L, Moutailler S, Vazeille M and Failloux AB (2009) Chikungunya virus and Aedes mosquitoes: saliva is infectious as soon as two days after oral infection. PLoS ONE 4, e5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon SM, Bermingham E and Ricklefs RE (2005) Host specialization and geographic localization of avian malaria parasites: a regional analysis in the Lesser antilles. American Naturalist 165, 466–480. [DOI] [PubMed] [Google Scholar]
- Ferguson HM and Read AF (2002) Why is the effect of malaria parasites on mosquito survival still unresolved? Trends in Parasitology 18, 256–261. [DOI] [PubMed] [Google Scholar]
- Ferraguti M, Martínez-de la Puente J, Muñoz J, Roiz D, Ruiz S, Soriguer RC and Figuerola J (2013) Avian Plasmodium in Culex and Ochlerotatus mosquitoes from southern Spain: effects of season and host-feeding source on parasite dynamics. PLoS ONE 8, e66237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraguti M, Martínez-de la Puente J, Bensch S, Roiz D, Ruiz S, Viana DS, Soriguer RC and Figuerola J (2018) Ecological determinants of avian malaria infections: an integrative analysis at landscape, mosquito and vertebrate community levels. Journal of Animal Ecology 87, 727–740. [DOI] [PubMed] [Google Scholar]
- Franks SJ, Pratt PD and Tsutsui ND (2011) The genetic consequences of a demographic bottleneck in an introduced biological control insect. Conservation Genetics 12, 201–211. [Google Scholar]
- Gering E and Atkinson CT (2004) A rapid method for counting nucleated erythrocytes on stained blood smears by digital image analysis. Journal of Parasitology 90, 879–881. [DOI] [PubMed] [Google Scholar]
- Goddard LB, Roth AE, Reisen WK and Scott TW (2002) Vector competence of California mosquitoes for West Nile virus. Emerging Infectious Diseases 8, 1385–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golenda CF, Burge R and Schneider I (1992) Plasmodium falciparum and P. berghei: detection of sporozoites and the circumsporozoite proteins in the saliva of Anopheles stephensi mosquitoes. Parasitology Research 78, 563–569. [DOI] [PubMed] [Google Scholar]
- Gubbins S, Carpenter S, Baylis M, Wood JL and Mellor PS (2008) Assessing the risk of bluetongue to UK livestock: uncertainty and sensitivity analyses of a temperature-dependent model for the basic reproduction number. Journal of the Royal Society Interface 5, 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-López R, Martínez-de la Puente J, Gangoso L, Soriguer RC and Figuerola J (2015) Comparison of manual and semi-automatic DNA extraction protocols for the barcoding characterization of hematophagous louse flies (Diptera: Hippoboscidae). Journal of Vector Ecology 40, 11–15. [DOI] [PubMed] [Google Scholar]
- Gutiérrez-López R, Martínez-de la Puente J, Gangoso L, Yan J, Soriguer RC and Figuerola J (2016) Do mosquitoes transmit the avian malaria-like parasite Haemoproteus? An experimental test of vector competence using mosquito saliva. Parasites and Vectors 9, 609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-López R, Martínez-de la Puente J, Gangoso L, Yan J, Soriguer RC and Figuerola J (2019a) Infection load influences Plasmodium transmission risk due to their effects on mosquito survival. Scientific Reports 9, 8782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-López R, Martínez-de la Puente J, Gangoso L, Soriguer RC and Figuerola J (2019b) Effects of host sex, body mass and infection by avian Plasmodium on the biting rate of two mosquito species with different feeding preferences. Parasites and Vectors 12, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-López R, Bialosuknia SM, Ciota AT, Montalvo T, Martínez-de la Puente J, Gangoso L, Figuerola J and Kramer LD (2019c) Vector competence of Aedes caspius and Ae. albopictus mosquitoes for Zika virus, Spain. Emerging Infectious Diseases 25, 346–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy JL, Houk EJ, Kramer LD and Reeves WC (1983) Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annual Review of Entomology 28, 229–262. [DOI] [PubMed] [Google Scholar]
- Hartemink N, Vanwambeke SO, Heesterbeek H, Rogers D, Morley D, Pesson B, Davies C, Mahamdallie S and Ready P (2011) Integrated mapping of establishment risk for emerging vector-borne infections: a case study of canine leishmaniasis in southwest France. PLoS ONE 6, e20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellgren O, Waldenström J and Bensch S (2004) A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. Journal of Parasitology 90, 797–802. [DOI] [PubMed] [Google Scholar]
- Hellgren O, Pérez-Tris J and Bensch S (2009) A jack-of-all-trades and still a master of some: prevalence and host range in avian malaria and related blood parasites. Ecology 90, 2840–2849. [DOI] [PubMed] [Google Scholar]
- Huang X, Ellis VA, Jönsson J and Bensch S (2018) Generalist haemosporidian parasites are better adapted to a subset of host species in a multiple host community. Molecular Ecology 27, 4336–4346. [DOI] [PubMed] [Google Scholar]
- Hurd H, Taylor PJ, Adams D, Underhill A and Eggleston P (2005) Evaluating the costs of mosquito resistance to malaria parasites. Evolution 59, 2560–2572. [PMC free article] [PubMed] [Google Scholar]
- Ishtiaq F, Guillaumot L, Clegg SM, Phillimore AB, Black RA, Owens IPF, Mundy NI and Sheldon BC (2008) Avian haematozoan parasites and their associations with mosquitoes across Southwest Pacific Islands. Molecular Ecology 17, 4545–4555. [DOI] [PubMed] [Google Scholar]
- Kazlauskienė R, Bernotienė R, Palinauskas V, Iezhova TA and Valkiūnas G (2013) Plasmodium relictum (lineages pSGS1 and pGRW11): complete synchronous sporogony in mosquitoes Culex pipiens pipiens. Experimental Parasitology 133, 454–461. [DOI] [PubMed] [Google Scholar]
- Kimura M, Darbro JM and Harrington LC (2010) Avian malaria parasites share congeneric mosquito vectors. Journal of Parasitology 96, 144–151. [DOI] [PubMed] [Google Scholar]
- Kumar S, Stecher G and Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33, 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachish S, Knowles SC, Alves R, Wood MJ and Sheldon BC (2011) Infection dynamics of endemic malaria in a wild bird population: parasite species-dependent drivers of spatial and temporal variation in transmission rates. Journal of Animal Ecology 80, 1207–1216. [DOI] [PubMed] [Google Scholar]
- Lagisz M, Port G and Wolff K (2011) Living in a jar: genetic variation and differentiation among laboratory strains of the red flour beetle. Journal of Applied Entomology 135, 682–692. [Google Scholar]
- Lalubin F, Delédevant A, Glaizot O and Christe P (2013) Temporal changes in mosquito abundance (Culex pipiens), avian malaria prevalence and lineage composition. Parasites & vectors 6, 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalubin F, Deledevant A, Glaizot O and Christe P (2014) Natural malaria infection reduces starvation resistance of nutritionally stressed mosquitoes. Journal of Animal Ecology 83, 850–857. [DOI] [PubMed] [Google Scholar]
- LaPointe DA, Goff ML and Atkinson CT (2010) Thermal constraints to the sporogonic development and altitudinal distribution of avian malaria Plasmodium relictum in Hawai'i. Journal of Parasitology 96, 318–324. [DOI] [PubMed] [Google Scholar]
- Lefévre T, Vantaux A, Dabire KR, Mouline K and Cohuet A (2013) Non-genetic determinants of mosquito competence for malaria parasites. PLoS Pathogens 9, e1003365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lover AA and Coker RJ (2015) Do mixed infections matter? Assessing virulence of mixed-clone infections in experimental human and murine malaria. Infection, Genetics and Evolution 36, 82–91. [DOI] [PubMed] [Google Scholar]
- Macdonald G (1955) The measurement of malaria transmission. Proceedings of the Royal Society of Medicine 48, 295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier WA (1973) Über die mortalität von Culex pipiens Fatigans nach infektion mit Plasmodium cathemerium. Zeitschrift fur Parasitenkunde 41, 11–28. [DOI] [PubMed] [Google Scholar]
- Martínez-de la Puente J, Ferraguti M, Ruiz S, Roiz D, Soriguer RC and Figuerola J (2016) Culex pipiens forms and urbanization: effects on blood feeding sources and transmission of avian Plasmodium. Malaria Journal 15, 589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-de la Puente J, Gutiérrez-López R and Figuerola J (2018) Do avian malaria parasites reduce vector longevity? Current Opinion in Insect Science 28, 113–117. [DOI] [PubMed] [Google Scholar]
- Muñoz J, Ruiz S, Soriguer RC, Alcaide M, Viana DS, Roiz D, Vázquez A and Figuerola J (2012) Feeding patterns of potential West Nile virus vectors in south-west Spain. PLoS ONE 7, e39549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paaijmans KP, Imbahale SS, Thomas MB and Takken W (2010) Relevant microclimate for determining the development rate of malaria mosquitoes and possible implications of climate change. Malaria Journal 9, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinauskas V, Žiegytė R, Iezhova TA, Ilgūnas M, Bernotienė R and Valkiūnas G (2016) Description, molecular characterisation, diagnostics and life cycle of Plasmodium elongatum (lineage pERIRUB01), the virulent avian malaria parasite. International Journal for Parasitology 46, 697–707. [DOI] [PubMed] [Google Scholar]
- Pérez-Tris J, Hellgren O, Križanauskienė A, Waldenström J, Secondi J, Bonneaud C, Fjeldså J, Hasselquist D and Bensch S (2007) Within-host speciation of malaria parasites. PLoS ONE 2, e235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigeault R and Villa M (2018) Long-term pathogenic response to Plasmodium relictum infection in Culex pipiens mosquito. PLoS ONE 13, e0192315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigeault R, Vézilier J, Cornet S, Zélé F, Nicot A, Perret P, Gandon S and Rivero A (2015) Avian malaria: a new lease of life for an old experimental model to study the evolutionary ecology of Plasmodium. Philosophical Transactions of the Royal Society B: Biological Sciences 370, 20140300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigeault R, Caudron Q, Nicot A, Rivero A and Gandon S (2018) Timing malaria transmission with mosquito fluctuations. Evolution Letters 2, 378–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povelones M, Waterhouse RM, Kafatos FC and Christophides GK (2009) Leucine-rich repeat protein complex activates mosquito complement in defense against Plasmodium parasites. Science 324, 258–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2016) R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rosen L and Reeves WC (1954) Studies on avian malaria in vectors and hosts of encephalitis in Kern County, California. American Journal of Tropical Medicine and Hygiene 3, 704–708. [DOI] [PubMed] [Google Scholar]
- Ross R (1911) The Prevention of Malaria. London, UK: John Murray. [Google Scholar]
- Sachs J and Malaney P (2002) The economic and social burden of malaria. Nature 415, 680. [DOI] [PubMed] [Google Scholar]
- Santiago-Alarcon D, Palinauskas V and Schaefer HM (2012) Diptera vectors of avian Haemosporidian parasites: untangling parasite life cycles and their taxonomy. Biological Reviews 87, 928–964. [DOI] [PubMed] [Google Scholar]
- Schaffner E, Angel G, Geoffroy B, Hervy JP, Rhaiem A and Brunhes J (2001) The Mosquitoes of Europe: An Identification and Training Programme. Montpellier, France: IRD Editions. [Google Scholar]
- Schoener E, Uebleis SS, Butter J, Nawratil M, Cuk C, Flechl E, Kothmayer M, Obwaller AG, Zechmeister T, Rubel F, Lebl K, Zittra C and Fuehrer HP (2017) Avian Plasmodium in Eastern Austrian mosquitoes. Malaria Journal 16, 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siden-Kiamos I, Ecker A, Nybäck S, Louis C, Sinden RE and Billker O (2006) Plasmodium berghei calcium-dependent protein kinase 3 is required for ookinete gliding motility and mosquito midgut invasion. Molecular Microbiology 60, 1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson-Coelho M, Loiselle BA, Blake JG and Ricklefs RE (2016) Resource predictability and specialization in avian malaria parasites. Molecular Ecology 25, 4377–4391. [DOI] [PubMed] [Google Scholar]
- Therneau TM and Lumley T (2014) Survival: survival analysis, including penalised likelihood. Available at http://cran.r-project.org/package=survival.
- Valkiūnas G (2005) Avian Malaria Parasites and Other Haemosporidia. Florida, USA: CRC Press. [Google Scholar]
- Valkiūnas G (2011) Haemosporidian vector research: marriage of molecular and microscopical approaches is essential. Molecular Ecology 20, 3084–3086. [DOI] [PubMed] [Google Scholar]
- Valkiūnas G, Ilgūnas M, Bukauskaitė D, Fragner K, Weissenböck H, Atkinson CT and Iezhova TA (2018) Characterization of Plasmodium relictum, a cosmopolitan agent of avian malaria. Malaria Journal 17, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vézilier J, Nicot A, Gandon S and Rivero A (2012) Plasmodium infection decreases fecundity and increases survival of mosquitoes. Proceedings of the Royal Society B: Biological Sciences 279, 4033–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss B and Aksoy S (2011) Microbiome influences on insect host vector competence. Trends in Parasitology 27, 514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Work TM, Washino RK and Van Riper C III (1990) Comparative susceptibility of Culex tarsalis, Anopheles franciscanus and Culiseta inornata (Diptera: Culicidae) to Plasmodium relictum (Haemosporidia: Plasmodiiae). Journal of Medical Entomology 27, 68–71. [DOI] [PubMed] [Google Scholar]
- Yan J, Martínez-de la Puente J, Gangoso L, Gutiérrez-López R, Soriguer R and Figuerola J (2018) Avian malaria infection intensity influences mosquito feeding patterns. International Journal for Parasitology 48, 257–264. [DOI] [PubMed] [Google Scholar]