Abstract
Apicomplexans, including species of Eimeria, pose a real threat to the health and wellbeing of animals and humans. Eimeria parasites do not infect humans but cause an important economic impact on livestock, in particular on the poultry industry. Despite its high prevalence and financial costs, little is known about the cell biology of these ‘cosmopolitan’ parasites found all over the world. In this review, we discuss different aspects of the life cycle and stages of Eimeria species, focusing on cellular structures and organelles typical of the coccidian family as well as genus-specific features, complementing some ‘unknowns’ with what is described in the closely related coccidian Toxoplasma gondii.
Key words: Cell biology, coccidia, Eimeria, invasion, life cycle stages, secretory organelles
Introduction
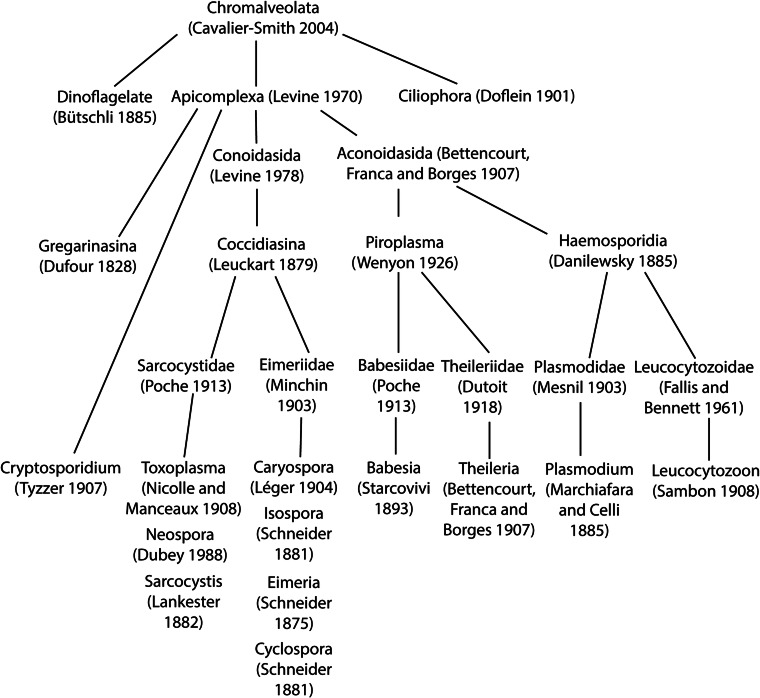
Eimeria species are protozoan parasites belonging to Coccidiasina (Coccidia) (Fig. 1), a group of obligate intracellular parasites of great human and veterinary importance (Shirley et al., 2005). All members of the Coccidia replicate within the intestines of a definitive host progressing through sequential rounds of asexual (schizogony) and sexual (gametogony) reproduction, culminating in the production of oocysts that are shed into the environment with the feces (Kemp et al., 2013). Coccidians of the family Eimeriidae, such as species of Eimeria and Cystoisospora, are monoxenes meaning that their development is restricted to a single host where they replicate rapidly to reach high numbers in the intestine causing acute enteritis of varying severity. This is in contrast to coccidians of the family Sarcocystidae, such as Toxoplasma gondii and Neospora caninum, which are heteroxenes that complete their whole cycle within the intestines of a definitive host and can also undergo asexual replication within a variety of intermediate host species. Here, infection progresses through an acute, rapid-replication phase into a chronic phase where parasites persist as cysts, most commonly in brain and muscle tissues (Wohlfert et al., 2017). Heteroxene Coccidia may also form tissue cysts in the definitive host where they remain dormant throughout the life of the host, but can reactivate into the acute phase and can cause a range of diseases including encephalitis and abortion.
Fig. 1.
Phylogenetic tree showing the relationships between relevant apicomplexan species. The tree was generated based on data from the following sources: Levine, 1984; Tenter and Johnson, 1997; Carreno et al., 1999; Jirku et al., 2002; Tenter et al., 2002; Adl et al., 2007; Lane and Archibald, 2008; Golemansky, 2015; Megia-Palma et al., 2015.
In contrast to the very broad host range of T. gondii (Cowper et al., 2012) species of Eimeria are highly host adapted and are generally capable of parasitizing a specific intestinal location in a single host (Yun et al., 2000a; Augustine, 2001; Cowper et al., 2012). In Dobell's paper titled ‘The Discovery of the Coccidia’ (Dobell, 1922) he concludes that it was Leeuwenhoek who first described an Eimeria specie some 200 years before the conception of the genus by Schneider in 1875 (Schneider, 1875): in one of his letters in 1674, Leeuwenhoek describes numerous microscopic globules in the bile of rabbits which Dobell believes must have been oocysts of Eimeria stiedae. In 1879, Leuckart founded the class Sporozoa, grouping together the Coccidia and similar organisms which are encased within a protective ‘spore’ covering (Leuckart, 1879). In the early 20th century, light microscopists were able to describe in detail the life cycles of Eimeria spp. and reveal their intimate connection with the tissues of the host (Fantham, 1910). By the late 90s many electron microscopy studies had been performed, characterizing organelle and cytoskeletal make-up of Eimeria spp. (Ryley, 1969). Electron microscopy was also instrumental to understanding how apicomplexans enter and reside within host cells (Scholtyseck, 1965; Sheffield and Hammond, 1966; Aikawa et al., 1978).
Eimeria is a large genus, with over 1800 species identified to date (Duszynski, 2001). Despite exquisite host specificity of individual species, the genus as a whole has a highly diverse host range and affects members of all vertebrate classes (Duszynski, 2001). Interestingly, one host not affected by Eimeria is Homo sapiens (Relman et al., 1996). Humans are however specifically infected by Cyclospora cayetanensis, a parasite which is highly similar to Eimeria species in terms of both genetics and pathogenesis (Liu et al., 2016) and is currently considered as the ‘human Eimeria’. As the replication stages of schizogony and gametogony occur within host cells, infection with Eimeria species results in cellular destruction and pathology to the susceptible host. The usual site of this replication is within epithelial cells lining the intestinal tract. This can lead to clinical symptoms of gastrointestinal dysfunction such as diarrhoea, dehydration and failure to gain weight (Yun et al., 2000b). The diseases caused by Eimeria spp. are commonly known as ‘coccidiosis’ or in some cases ‘eimeriosis’.
The following sections predominantly refer to research involving species of Eimeria; where data involving T. gondii is used, for example where equivalent studies in Eimeria species are missing, this is stated within the text.
Chicken coccidiosis
The most economically important disease caused by Eimeria species is coccidiosis in chickens. The estimated global cost of this disease, as stated in a review by Peek and Landman, is more than 2 billion US dollars per year through production losses and costs associated with treatment and prevention measures (Peek and Landman, 2011). There are seven recognized species of Eimeria affecting chickens: E. acervulina, E. brunetti, E. maxima, E. mitis, E. necatrix, E. praecox and E. tenella (Shirley et al., 2005, 2007). Previously described species E. mivati and E. hagani (Edgar and Seibold, 1964) (Levine, 1938) are regarded as nomina dubia by the vast majority of coccidiologists and to our knowledge there are no isolates of these available upon which definitive molecular tests could be applied. There are however a number of hitherto unclassified isolates, referred to as operational taxonomic units X, Y and Z that are widely distributed throughout the world and may represent novel cryptic species (Clark et al., 2016; Jatau et al., 2016). Advances in this area for a final classification would be necessary for accurate control strategies for these isolates.
Each of the seven recognized species has a set of distinct characteristics in terms of prevalence, pathogenicity, site of infection in the intestine and oocyst morphology (Table 1). Eimeria tenella specifically targets the paired caeca, often resulting in fairly extensive haemorrhage; the lesions are primarily caused by second generation schizonts that develop deep to the intestinal epithelium within the lamina propria (Fernando et al., 1983). Infection with E. maxima is likely to cause a thickening of the intestinal lining, with mucoid to bloody exudate, and E. acervulina is described as causing ‘ladder-like’ white bands across the mucosa. Eimeria brunetti and E. necatrix are also capable of causing severe pathology, however they are less commonly encountered compared to E. acervulina, E. maxima and E. tenella (Trees, 2001).
Table 1.
Comparison of the seven known Eimeria species affecting chickens in terms of site of infection, level of pathology and oocyst morphology
| Species | Site of pathology | Lesion score | Oocyst morphology (size range = 15–30 µm) |
|---|---|---|---|
| E. tenella | Caeca 
|
High | Medium round |
| E. maxima | Mid small intestine 
|
Medium | Large oval |
| E. acervulina | Upper small intestine 
|
Medium | Small oval |
| E. necatrix | Mid small intestine 
|
High | Small-medium round |
| E. brunetti | Distal small intestine + colon 
|
High | Medium oval |
| E. mitis | Upper small intestine 
|
Low | Small round |
| E. praecox | Upper small intestine 
|
Low | Medium round |
The life cycle of Eimeria species
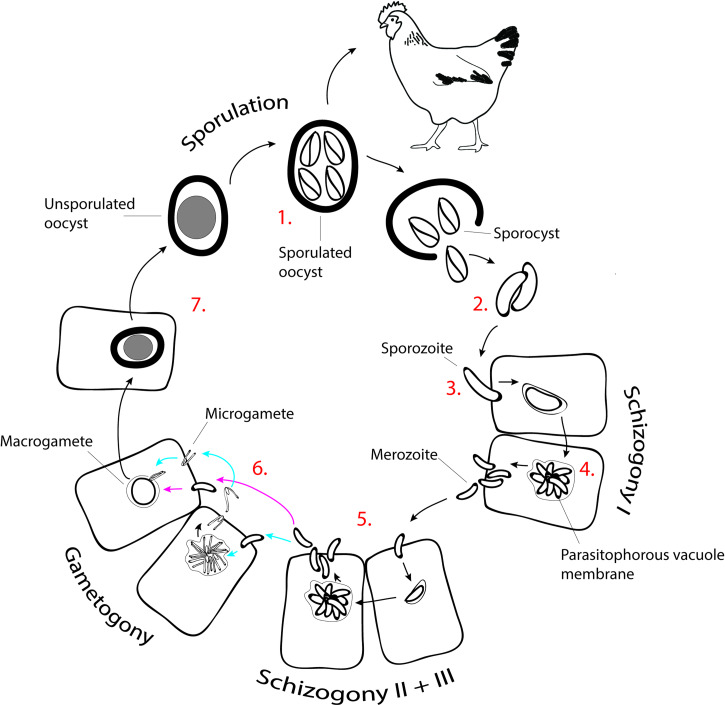
All Eimeria species infecting chickens exhibit a similar life cycle (Lillehoj and Trout, 1993) (Fig. 2). Unsporulated oocysts are released in the feces of an infected animal and persist in the environment for long periods. When exposed to air, moisture and warmth, oocysts go through a developmental process called sporulation (Shirley et al., 2005) [Fig. 2(1)]. If ingested by a chicken, the sporulated oocyst will release sporocysts [Fig. 2(2)]. As these pass into the small intestine, enzymatic digestion releases the sporozoites, which migrate to their preferred site of development to initiate cellular invasion (Jeurissen et al., 1996) [Fig. 2(3)]. The developmental cycle in the host cell begins with two to three rounds of asexual replication known as schizogony. This process involves multiple nuclear divisions to produce a large multinuclear cell called schizont, from which merozoites are formed (Fig. 2.4 and 2.5). After several generations of merozoite production, parasite development proceeds with a single round of sexual replication known as gametogony (Fig. 2.6), forming the dimorphic stages of macrogamete and microgamete. Finally, macrogamete/microgamete fertilization occurs to form a zygote (Ferguson et al., 2003). The zygote will then develop into an oocyst, which, after release in the feces, matures into an infective sporulated oocyst (Jeurissen et al., 1996; Shirley et al., 2005) [Fig. 2(7)].
Fig. 2.
Life cycle of Eimeria tenella. Numbers correlate with subsequent stages of the development. (1) Oocyst sporulation in the environment and oral ingestion by the chicken. (2) Release of sporocysts and sporozoites along the transit in the chicken digestive system. (3) Active invasion of sporozoites in the ceaeca epithelium and formation of the intracellular trophozoite within the parasitophorous vacuole. (4) First round of shyzogony and release of first generation merozoites. (5) Second and third rounds of shyzogony and release of second and third generation merozoites, respectively. (6) Development of microgametes and macrogametes (gametogony) and fecundation. (7) Zygote, development of the oocyst and release to the environment as unsporulated oocyst.
The infective oocyst
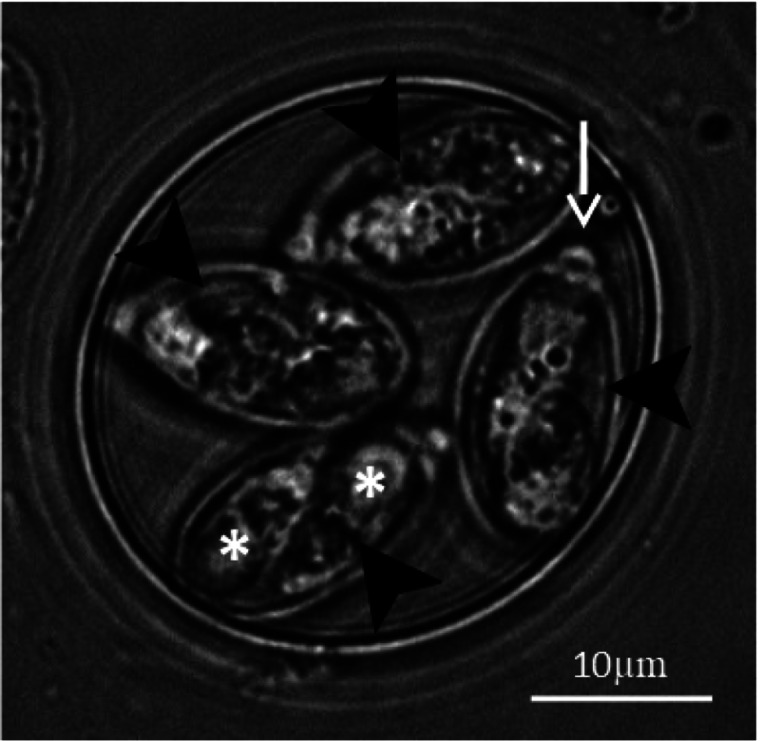
The oocyst, which has important roles in both parasite development and disease propagation (Lillehoj and Trout, 1993), can persist in the environment for long periods, even in the presence of disinfectants (Peek and Landman, 2011). This is largely due to protection afforded by the oocyst wall, measuring around 100 nm in thickness and formed from two opposed but easily separated layers and composed of a mixture of lipids and glycoproteins (Stotish et al., 1978; Belli et al., 2006; Mai et al., 2009). The final stage of oocyst development (sporulation) occurs outside of the host and involves a single meiotic division followed by a round of mitosis, resulting in eight infective parasite stages (sporozoites) arranged as pairs inside four individual casings (sporocysts) within each oocyst (Canning and Anwar, 1968) [Figs 2(1) and 3]. As well as containing two sporozoites, each sporocyst contains a micropyle at the apex and a lipid-rich residual body which remains within the sporocyst following sporozoite excystation. The stieda and sub-stieda bodies are found at the anterior pole of the sporocyst, acting as a barrier to sporozoite release until their degradation in the presence of trypsin (Roberts et al., 1970b).
Fig. 3.
Sporulated oocysts of E. tenella. The oocysts contain four sporocysts (arrowheads), each containing two sporozoites (asterisks) and a micropyle at each sporocysts apex (arrow).
The invasive sporozoite
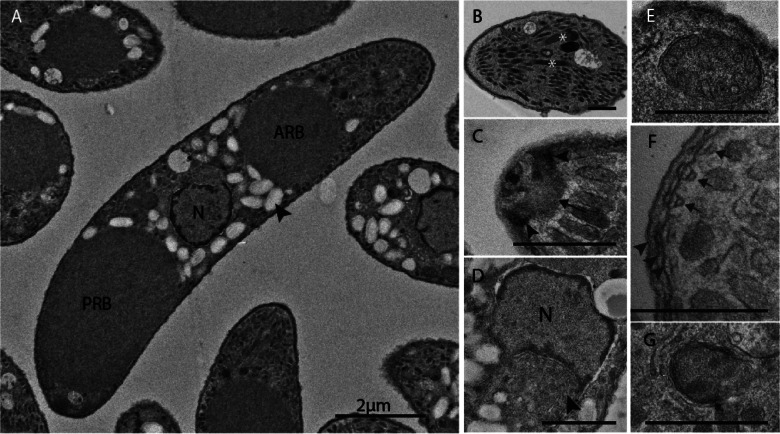
Most knowledge on the sporozoite comes from E. tenella, the most extensively studied of the avian Eimeria species due to its amenability for sporozoite invasion and schizont development in vitro, as well as its potential for genetic manipulation. The E. tenella sporozoite is a distinctly polarized cell, of sickle shape and around 10 µm in length (Figs 4A and 5B). Sporozoites contain many classical features and organelles of typical eukaryotic cells such as the nucleus, mitochondrion, endoplasmic reticulum and Golgi apparatus (Fig. 4D and E). They also contain several features specific to the phylum (such as the conoid, apicoplast, micronemes and rhoptries; Figs 4B, C, G and 5A), and even structures which appear to be unique to this genus [refractile bodies (RBs); Figs 4A and 5B]. The E. tenella sporozoite nucleus is situated in roughly the centre of the parasite (Fig. 4A), contains a nucleoplasm of fine granular consistency and is surrounded by a pore-containing double membrane (Strout and Scholtyseck, 1970; Pacheco et al., 1975). Adjacent to the nucleus is the Golgi apparatus (Fig. 4D), which contains material described as ‘small vesicles in a finely granular matrix’ (Vetterling et al., 1973). The mitochondrion of a T. gondii tachyzoite, reconstructed from serial electron microscopy data, was found to be a single elongated structure distributed throughout the cell (Melo et al., 2000) (Fig. 5A).
Fig. 4.
TEMs of E. tenella sporozoite ultrastructures (A. Burrell, unpublished). (A) Sporozoites with two large non-membrane bound organelles known as anterior and posterior refractile bodies (ARB and PRB) situated at either side of the nucleus (N) as well as numerous amylopectin granules (arrowhead). (B) Micronemes and rhoptries (asterisks) occupying most of the cytoplasm in the anterior quarter of the cell. (C) Apex of the cell with a cone-shaped structures composed of helical fibres known as the conoid (arrow) sitting within the APRs (arrowheads). (D) Centrally located nucleus (N) next to which the Golgi apparatus can be observed (arrowhead). (E) Mitochondrion cross-section showing plump cristae. (F) Triple-layered pellicle (arrowheads) consisting of plasma membrane and IMC beneath which sits an array of sub-pellicular microtubules (arrows). (G) Apicoplast with four membrane layers. Scale bars: (A) ~2 µm; (B–G) ~500 nm.
Fig. 5.
Cell structure and organelle content of different coccidian cells. (A) Tachyzoite of Toxoplasma gondii. (B) Sporozoite of Eimeria species. (C). Merozoite of Eimeria species.
Several early investigations into the structure and composition of the Eimeria cell surface revealed the presence of an inner membrane complex (IMC) composed of a meshwork of flattened sacs found directly beneath the outer plasma membrane (Fig. 4F) (Dubremetz, 1975; Dubremetz and Torpier, 1978). Building on the work by Dubremetz (1975) and Dubremetz and Torpier, (1978), studies of T. gondii tachyzoites have shown that the IMC is connected to the subpellicular microtubules via a network of intermediate filaments and alveolin (Morrissette and Sibley, 2002). They have an important role in maintaining cell rigidity, critical for assembly and stabilization of the actin-myosin motor that powers parasite gliding motility (Sibley, 2010), a point of attachment for organelles (Kudryashev et al., 2010) and apparently originates from the Golgi apparatus (Francia and Striepen, 2014).
Beneath the pellicle there is a set of longitudinally running microtubules. One of the first depictions of this cytoskeletal organization was achieved in E. acervulina through ‘critical point drying’ (a method of dehydrating a biological sample without distorting morphology), allowing the isolation and electron microscopy examination of the outer cytoskeletal microtubules (Russell and Sinden, 1982). More recently, several reviews have focused on the architecture and role in invasion of the cytoskeleton in coccidian parasites (Morrissette and Sibley, 2002; Frenal and Soldati-Favre, 2009; Sibley, 2010). The 24 subpellicular microtubules of Eimeria species are arranged as an evenly spaced spiral and extend to roughly half of the parasite length, acting as a support scaffold to maintain cell shape and rigidity (Vetterling et al., 1973; Russell and Sinden, 1982; Morrissette and Sibley, 2002). The apical ends of these microtubules are fixed in a ring around the conoid, forming the apical polar ring (APR), however it is not clear whether their posterior ends are fixed or free (Vetterling et al., 1973; Russell and Sinden, 1982).
Refractile bodies, amylopectin granules and acidocalcisomes
In light and electron micrographs of E. tenella sporozoites, the most striking structures are the RBs (Figs 4A and 5B). In transmission electron micrographs (TEMs) these appear as spherical or ovoid structures with homogeneous electron dense content and no obvious limiting membrane. Usually a spherical RB is found anterior to the nucleus (around 1–2 µm in diameter) and an ovoid RB is found posterior to the nucleus (around 5 µm in length). Despite their striking appearance, the functions of RBs remain unknown; the most commonly hypothesized roles are protein storage and metabolism (de Venevelles et al., 2006). Work by Fayer described an interesting dynamic, whereby after host cell invasion the anterior and posterior RB merge into a single RB that localizes centrally in the schizont (Fayer, 1969). Twenty years later a monoclonal antibody (1209-C2) specific for RBs (and able to bind related E. tenella proteins) was used to show that the merged RB separates into globules that diffuse through the schizont and re-concentrate as a refractile ‘dot’ within each first-generation merozoite (Danforth and Augustine, 1989). The protein target of the 1209-C2 was identified independently by two research groups seeking to validate vaccine antigens for control of coccidiosis (Miller et al., 1989; Crane et al., 1991). This RB protein, termed variously GX3262, ‘B’ antigen and SO7, contain large numbers of repetitive amino acid tracts (Liberator et al., 1989; Reid et al., 2014) and induce partial protection against challenge with up to four different species of Eimeria (Bhogal et al., 1989; Karkhanis et al., 1991). SO7 (most common name) has been used as a candidate antigen in a variety of vaccinology studies (Song et al., 2015; Yang et al., 2017) although the mechanism of immune protection, and the biological function of this family of RB proteins, that are unique to the genus, remain unknown. A few additional proteins have been localized to the RBs including an aspartyl protease (Laurent et al., 1993), a putative nucleotide transhydrogenase (Vermeulen et al., 1993) and some other up-represented in a RB enriched proteome such as a lactate dehydrogenase, a carbonyl reductase, a subtilisin 2 protease and a haloacid dehalogenase-like hydrolase (de Venevelles et al., 2006).
Surrounding the larger (posterior) RB and dispersed throughout the posterior two-thirds of the parasite, are several tens of amylopectin granules (Vetterling et al., 1973; Pacheco et al., 1975) (Fig. 5B). These are smaller than the RBs and easily identified in TEMs, appearing electron dense or lucent dependent on the staining technique (Ryley et al., 1969; Strout and Scholtyseck, 1970; Fernando and Remmler, 1974). Amylopectin is an important carbohydrate source for Eimeria, being present in the oocyst, sporozoite and merozoite stages, as well as in the residual body of the schizont (Ryley et al., 1969; Coombs et al., 1997). In T. gondii, amylopectin granules are much more numerous in bradyzoites (the latent tissue cyst stage) than tachyzoites (the rapid proliferation stage) suggesting they could be used to fuel transition between these two stages (Guerardel et al., 2005). Eimeria species contain another group of organelles that appear by TEM as membrane bound spherical structures roughly half a micron in diameter and filled to varying extents with highly electron dense material. In T. gondii analogous structures are classified as acidocalcisomes: acidic organelles containing high concentrations of various physiologically important ions (Miranda et al., 2008; Soares Medeiros et al., 2011) (Fig. 5A).
Conoid
The conoid is a movable cone-shaped structure which sits within the APR, from which the subpellicular microtubules emanate (Ryley, 1969; Vetterling et al., 1973) (Fig. 5). In T. gondii, the conoid was found to be composed of a novel tubulin polymer, arranged as in a tight spiral of ‘microtubule-like’ fibres (Hu et al., 2002). Studies on the molecular composition of the T. gondii conoid revealed the presence of several additional novel proteins (Hu et al., 2006), including three calmodulin-like proteins involved in motility, invasion and egress, but not required for conoid extrusion or microneme/rhoptry secretion (Long et al., 2017b), as well as an essential protein (CPH1) involved in conoid stability (Long et al., 2017a). In several species of Eimeria, the conoid has been observed in both extended and retracted states (Roberts and Hammond, 1970). However, in electron micrographs depicting extracellular, invading or intracellular Eimeria sporozoites, the conoid is rarely seen in the extended position (Roberts et al., 1970a, 1971; Vetterling et al., 1973; Jensen, 1975). In T. gondii the conoid has been shown to rotate, tilt, extend and retract (Bommer et al., 1969), often seen in the extended state immediately prior to invasion (Chiappino et al., 1984; Carruthers and Boothroyd, 2007). This range of movement has led to the hypothesis that the conoid has a mechanical role in invasion (Morrissette and Sibley, 2002).
Micronemes and rhoptries
Micronemes are small (20 nm by 50 nm) rod-shaped organelles located at the anterior end of the zoite (Fig. 4B). Their protein content is well defined in several coccidians, including E. tenella and T. gondii. There are genus-specific differences in the precise portfolio of MICs but overall these are generally conserved, comprising a mix of soluble and membrane-spanning proteins, many with domains orthologous to known adhesins (reviewed by Tomley and Soldati, 2001; Carruthers and Tomley, 2008; Cowper et al., 2012). Surface-expressed MICs are able to bind host ligands (essential for gliding motility, attachment and invasion of host cells) and also bind parasite-derived RON ligands to form the moving junction (discussed later).
Rhoptries are club-shaped organelles (up to around 2000 nm in length) often seen with the narrow neck portion extending longitudinally into the conoidal channel (Fig. 5A and B). It is not clear precisely how many rhoptries each sporozoite contains, although up to eight have been seen for E. tenella (Pacheco et al., 1975). Rhoptry proteins are housed in two compartments: RONs are stored in the rhoptry necks and ROPs come from the bulbous part of the organelle (Besteiro et al., 2011).
Biogenesis of the microneme and rhoptry
The apical organelles are part of the parasite endomembrane system and in E. tenella are generated de novo late in the formation of sporozoites (Ryan et al., 2000) and merozoites (Brown et al., 2000; Tomley and Soldati, 2001). From studies in T. gondii and Plasmodium species it is well established that apicomplexans have re-purposed the exocytic and endocytic pathways of higher eukaryotes into a partially ‘merged’ vesicle and protein trafficking system in order to generate their specialized regulatory secretory organelles (reviewed by Tomavo et al., 2013; McGovern and Carruthers, 2016). Microneme (MIC) and rhoptry (ROP/RON) proteins enter the early exocytic pathway at the endoplasmic reticulum and traffic to the Golgi and trans-Golgi network (TGN), from where they are sorted to novel endosome-like compartments (ELC) bearing early (Rab5) or late (Rab7) endosome markers. In T. gondii, over-expression of Rab5A/5C causes defective trafficking of some (but not all) MIC and all ROP/RON proteins presumably by saturating receptors and dysregulating vesicle transport (Kremer et al., 2013).
Several additional molecules are essential for TGN to ELC trafficking of MIC and ROP/RON proteins in T. gondii and as gene orthologues are present in Eimeria species (www.toxodb.org) it is likely that this trafficking pathway is conserved across the Coccidia. These molecules include vacuolar sorting protein 9, an activator of Rab5 (Sakura et al., 2016); syntaxin 6 (Stx6), a SNARE found mainly in the TGN (Jackson et al., 2013); dynamin-related protein B (DrpB) and clathrin, both found in the TGN and ELC (Breinich et al., 2009; Pieperhoff et al., 2013); the clathrin adaptor complex AP1 (Venugopal et al., 2017); the sortilin-like receptor TgSORTLR (Sloves et al., 2012) and several components of CORVET (class C core vacuole/endosome tethering) and HOPS (homotypic fusion and vacuolar protein sorting) complexes associated with early and late endosomes respectively (Morlon-Guyot et al., 2015, 2018). TgSORTLR loads MIC and ROP/RON proteins at the TGN and escorts them to the ELC (Sloves et al., 2012; Sangare et al., 2016). In higher eukaryotes, sortilin is a transmembrane endosomal receptor with a major role in anterograde transport of lysosomal enzymes from TGN to endosomes (Bonifacino and Rojas, 2006), and in retrograde trafficking where it binds retromer, an evolutionarily conserved protein complex that selects and recycles proteins from endosomes to the TGN or the plasma membrane (Pan et al., 2017). Recent characterization of retromer interactomes in T. gondii confirms that retromer-dependent retrograde transport is essential for apical organelle biogenesis, probably because recycling of TgSORTLR to the TGN is needed to maintain anterograde trafficking of MIC and ROP/RON proteins to the ELC (Sangare et al., 2016).
Because not all MIC proteins depend on Rab5A/5C trafficking, it is suggested there is more than one trafficking pathway leading to either distinct populations of micronemes containing different MICs, or a single population having different sub-compartments (Kremer et al., 2013). More evidence for dual trafficking emerged recently with the observation that vacuolar protein sorting 8 (TgVps8, a CORVET component), whilst essential for organelle biogenesis, completely blocks trafficking of most MICs but only partially affects that of TgAMA1, TgMIC2 and TgMIC6 (Morlon-Guyot et al., 2018). No work on MIC trafficking has been carried out in Eimeria species, but lack of co-localization of MIC proteins has been observed, for example between EtMIC3/EtAMA1, and between EtMIC3/EtMIC5 in sporozoites (Lai et al., 2011), and between EtMIC2/EtAMA2 in second generation merozoites (McGovern et al., 2018; Pastor-Fernandez et al., 2018). These studies used non-quantitative imaging and whilst strongly suggestive that zoites contain distinct populations of micronemes, it cannot be ruled out that they reflect differences in timing and levels of individual MIC protein expression.
By merging and adapting exocytosis and endocytosis, apicomplexans have conserved an endomembrane system that is much reduced compared to higher eukaryotes, but efficient to perform the protein trafficking, targeting, processing and re-cycling needed for their obligate intracellular lifestyles. How MIC and ROP/RON proteins are differentially sorted and trafficked beyond the ELC to their target organelles, and how the endomembrane system efficiently segregates exocytosis from endocytosis are major questions. In T. gondii divergence between MIC and RON/ROP proteins at the ELC has been noted, with immature MICs (TgM2AP and TgMIC5) located in ELC that also contain endocytosed host protein whilst an immature RON (TgRON4) is in ELC lacking ingested host protein (McGovern et al., 2018). Thus endocytosis appears to intersect directly with MIC exocytosis and there must be a mechanism for directing MICs from ELC to micronemes and away from ingested host proteins that are en route to the parasite lysosome-like vacuole (VAC, Dou et al., 2014). In contrast, trafficking of RON/ROP proteins appears to avoid contact with endocytosed host protein, proceeding via an immature pro-rhoptry compartment (McGovern et al., 2018). Nevertheless, successful rhoptry biogenesis requires the endosomal CORVET protein TgVps9, and a novel parasite BEACH domain-containing protein that is also essential for VAC formation (Morlon-Guyot et al., 2018).
An essential step in late exocytosis of MIC and RON/ROP proteins is proteolytic processing (Nishi et al., 2008) that most likely occurs within, or during exit from the ELC (McGovern et al., 2018) mediated by proteinases that include aspartyl protease 3 (Dogga et al., 2017). Failure to undergo proteolytic maturation results in impaired organelle formation. Interestingly, apicomplexans possess a family of four phylogenetically related transporters belonging to the major facilitator superfamily and termed transporter family protein 1–4 (TFP1–4) (Besteiro et al., 2011; Hammoudi et al., 2018). In T. gondii TFP1 localizes to micronemes and ELC and is critical for condensation of microneme content, presumably by allowing the transport of molecules that are essential for this process such as maturases, or chaperones. Knock down of TFP1 impairs microneme formation and completely blocks MIC exocytosis; TFP2 and 3 localize to the rhoptries and knock down of TFP2 results in elongated rhoptries, again suggesting that defects in condensation/compaction have an impact on the late stages of organelle biogenesis (Hammoudi et al., 2018).
Zoite invasion of host cells
A widely researched aspect of coccidian biology is the mechanism by which zoites (sporozoites, merozoites, tachyzoites and bradyzoites) invade the host cell to occupy a unique intracellular niche, the PV. Much of the molecular detail of this process has been described in the T. gondii tachyzoite, a model cell for coccidian parasites. Considering the biological differences between tachyzoites and sporozoites (tachyzoites are formed when a sporozoite or bradyzoite stage converts within the cells of a host, rapidly replicates and disseminates throughout the host, then converts back into bradyzoites) (Dubey et al., 1998), we must be careful when inferring knowledge from T. gondii tachyzoites to Eimeria where there is no such stage-conversion (to tachyzoite or bradyzoite) or persistent infection. However, at the genomic and cellular levels much of the complex invasion machinery used by the coccidia is conserved so it is useful to supplement rather sparse data on Eimeria species with knowledge from T. gondii.
The process of invasion
Following initial contact with a host cell in vitro, the E. tenella sporozoite glides across the cell surface in a helical motion, possibly in a search of an appropriate location to invade (Russell and Sinden, 1981; Entzeroth et al., 1989). Before invasion, the sporozoite re-orientates itself so that the apical tip, at which the conoid is located, makes contact with the host cell plasma membrane forming a ‘moving junction’ (MJ) between parasite and host cell membranes through which the parasite propels itself to enter the newly forming intracellular vacuole. As the parasite pushes itself into the cell, it causes an invagination of the membrane. The MJ remains fixed at the point of attachment to the host cell but is translocated backwards over the surface of the invading parasite from apex to posterior. This invagination continues until the whole parasite length has passed through the MJ, at which point the host membrane pinches together behind the parasite posterior enclosing the parasite within a PV. This process has been well documented in several species of Eimeria as well as T. gondii and other apicomplexans (Suss-Toby et al., 1996; Entzeroth et al., 1998; Beyer et al., 2002). According to this model, the parasite does not enter the host cell cytoplasm, although there is evidence that some of the rhoptry content enters in the form of e-vacuoles (Hakansson et al., 2001), a process that may be critical for the early release of rhoptry neck proteins (RONs) and formation of the MJ (Besteiro et al., 2011). In addition to stabilizing the site of invasion, the MJ has a role as a molecular sieve, removing non-Glycosylphosphatidylinositol (GPI)-anchored host membrane proteins from the newly formed PV membrane, including the key immune signalling/effector molecules MHC class I, MHC class II and FcR (Mordue and Sibley, 1997). The PV of T. gondii differs from phagosomes in that it does not acquire the host-derived proteins involved in endosome fusion thereby protecting the parasite from lysosomal destruction (Mordue and Sibley, 1997; Mordue et al., 1999; Beyer et al., 2002). Although assumed to be similar, the fusion capacities of the PV harbouring Eimeria species has not yet been demonstrated (Entzeroth et al., 1998).
Signalling pathways involved in invasion
Waves of regulated protein secretion from the microneme (MIC) and rhoptry (ROP/RON) apical organelles are essential for parasite movement, invasion, formation of the intracellular parasitophorous vacuole, control of host gene expression and egress of daughter zoites from infected cells. These processes are key virulence determinants for most species of the Apicomplexa (Dubremetz et al., 1998; Keeley and Soldati, 2004; Besteiro et al., 2011).
Rapid secretion and surface capping of microneme proteins (MICs) from the apical tip of E. tenella sporozoites is induced when sporozoites are allowed to glide over a substrate and during invasion of host cells in cell culture (Bumstead and Tomley, 2000). In the absence of host cells, secretion and capping can be induced by exposure of freshly purified sporozoites to serum, or purified albumin at temperatures of 37 or 41 °C (Brown et al., 2000; Bumstead and Tomley, 2000). At lower temperatures or in the absence of serum or albumin, no MIC secretion or capping is detected. Both parasite invasion and albumin-induced MIC secretion is blocked in E. tenella by treatment with a compound that directly inhibits protein kinase G (Wiersma et al., 2004) indicating the likely importance of cyclic GMP (cGMP) signalling in coccidian secretion. PKG-dependent microneme secretion has also been shown in T. gondii (Brown et al., 2000) and using a novel auxin-inducible degron tagging system for conditional protein depletion in T. gondii alongside CRISPR-Cas9 genome editing, signalling was shown to go through PKGI, a myristoylated isoform of PKG localized at the parasite plasma membrane (Brown et al., 2017). In addition to cGMP signalling, it is known that calcium (Ca2+) fluxes provide crucial signals for gliding motility, microneme secretion, conoid extrusion, invasion and egress (Lourido et al., 2010; Pu and Zhang, 2012). These pathways operate through specific members of a calcium-dependent protein kinase (CDPK) family that is conserved in Eimeria (Dunn et al., 1996). A detailed chemical genetics (mutation) approach shows that the pathways linked to parasite invasion and egress, and the secretion of specific MIC proteins are differentially controlled by different CDPKs, and intersect cGMP signalling (Besteiro et al., 2011; Lourido et al., 2012). A variety of treatments that cause transient fluxes of cytosolic Ca2+ induce MIC secretion in T. gondii tachyzoites including calcium ionophores (Carruthers and Sibley, 1999), ethanol and acetaldehyde, but this is dependent upon the presence of albumin and cGMP signalling (Brown et al., 2016). Similarly in E. tenella, acetaldehyde and ethanol stimulate Ca2+-dependent MIC secretion and premature egress of sporozoites from cultured cells in the presence of serum (Yan et al., 2015, 2016), however in the absence of serum or albumin neither ionophores nor ethanol/acetaldehyde are effective (F. M. Tomley and J. M. Bumstead, unpublished). Thus it appears that cGMP and Ca2+-signalling pathways work co-operatively in MIC signalling, with PKGI at the plasma membrane being the essential ‘master’ regulator (Brown et al., 2017) whilst members of the CDPK family provide the selectivity and specificity needed to carry out specific biological functions such as invasion or egress.
Signalling pathways leading to the exocytosis of rhoptry contents are not yet defined however these must allow the selective secretion of RON proteins early in readiness for their role in formation of the MJ (Besteiro et al., 2011). It has also been reported that T. gondii is able to inject ROP proteins into host cells that it does not invade, allowing the parasite to manipulate uninfected cells (Koshy et al., 2012) suggesting that e-vacuole (Hakansson et al., 2001) deployment is an important virulence factor.
The role of micronemes and the glideosome
Microneme proteins (MICs) are secreted from the parasite apex either singly or as protein complexes onto the parasite surface (Tomley et al., 1996; Brown et al., 2000; Bumstead and Tomley, 2000; Lai et al., 2009) a process mediated in T. gondii by DOC2 proteins that recruit the necessary membrane-fusion machinery (Farrell et al., 2012). ‘Capping’ models of motility, whereby parasite molecules are rapidly translocated backwards over the surface to promote forward motion, were proposed over 40 years ago for Plasmodium (circumsporozoite precipitation reaction, Vanderberg, 1974), Eimeria nieschulzi (capping of ferritin, Dubremetz and Torpier, 1978) and gregarines (capping of conA-coated latex beads, King, 1981). The importance of the actin in motility was recognized in both Eimeria (Jensen and Edgar, 1976; Russell and Sinden, 1982) and Plasmodium (Miller et al., 1979) and a later study in E. tenella showed that material secreted when sporozoites were allowed to glide on a substrate emanated from the apical tip (Entzeroth et al., 1989). Subsequently, a large number of studies, mainly in T. gondii, has led to definition of ‘glideosomes’ (Opitz and Soldati, 2002), protein complexes that lie between the parasite plasma membrane and the IMC and power substrate-dependent gliding motility (reviewed in detail by Frenal and Soldati-Favre, 2009).
In brief, binding of surface-bound MIC adhesins to host ligands provides traction, linkage of these parasite–host surface membrane complexes to the underlying action-myosin motor is needed for their translocation (capping). This is achieved by the glideosome-associated connector (GAC), an armadillo repeat-containing protein that accumulates under the plasma membrane at the apical tip and stabilizes freshly polymerized short F-actin filaments that are nucleated at the tip by parasite formins (Jacot et al., 2016). GAC binds directly to the cytosolic tails of surface-bound transmembrane MICs (Jacot et al., 2016) and in a two-stage process, stabilized actin-GAC-MIC complexes are rapidly shuttled backwards through the interaction of the actin tracks initially with MyoH glideosomes, that are restricted to the conoid region (Graindorge et al., 2016), and thereafter with MyoA glideosomes positioned along the length of the zoite (Herm-Gotz et al., 2002). The complexes are shed from the posterior of zoites by the action of an intramembrane rhomboid-like serine protease, ROM4, which cleaves MIC transmembrane spanning regions (Buguliskis et al., 2010). To generate the force needed for forward motion, glideosomes need to be linked fluidly at the parasite plasma membrane and immobilized onto the cytoskeleton, a feat achieved by glideosome protein GAP45 which has its acylated N-terminus embedded in the plasma membrane and its C-terminus cross-linked to the IMC (Gilk et al., 2009). Additional glideosome proteins GAP40 and GAP50 are further involved in anchoring MyoA firmly the IMC (Harding et al., 2016) and a family of multi-membrane spanning GAPM proteins connect the glideosome right through to the subpellicular microtubules, via interaction with alveolins (Bullen et al., 2009). The regular positioning and complex molecular architecture of the glideosomes suggests that these structures are equivalent to the intramembrane particles visible in scanning electron micrographs of freeze-fractured IMC from sporozoites of Eimeria taken over 40 years ago (Dubremetz and Torpier, 1978).
By virtue of their host-binding activity, MICs are major contributors to parasite host-range and specificity; for example, MAR (microneme adhesive repeat)-domain containing MICs of E. tenella contain a single type (type 1) of MAR (Lai et al., 2011) whereas T. gondii and N. caninum possess MICs with both type 1 and 2 MAR. MARs bind sialyl-terminated oligosaccharides from many types of vertebrate tissue so expressing only a single type effectively narrows the range of sialylated receptors that E. tenella can bind, contributing to the very specific tropism of this parasite (Cowper et al., 2012). In T. gondii secretion of perforin from micronemes is essential for tachyzoite egress from vacuoles (Roiko and Carruthers, 2013). A role for perforin in egress has not been confirmed for Eimeria parasites; a gene encoding a conserved membrane-attack complex/perforin is expressed in E. tenella sporozoites but appears to be down-regulated in the later merozoite and gamete stages (Reid et al., 2014; Walker et al., 2015).
Secretion of rhoptries and dense granules proteins
RONs (rhoptry neck protein) and ROPs (rhoptry bulb proteins) are believed to discharge from the apex of the parasite; rhoptry ducts run through the conoid and terminate at the very apical tip. In T. gondii, RONs act in concert with apical membrane antigens (AMA, secreted from the micronemes) at the early stage of invasion, assembling at the parasite–host interface to form the irreversible MJ (Besteiro et al., 2011; Lamarque et al., 2011; Tyler and Boothroyd, 2011) actively recruiting host proteins to the MJ, subverting their function to enhance invasion efficiency (Guerin et al., 2017). Proteomic and genomic analysis readily identified several families of RONs in E. tenella orthologous to those of T. gondii, along with stage-regulated expression of specific AMA and EtRON2 family members (Oakes et al., 2013), suggesting that the mechanism by which the MJ is built by different coccidians is conserved. In contrast there is only limited conservation of ROPs between T. gondii and E. tenella including significant divergence in the families of ROP kinases that are the major component of the ROP proteome (Oakes et al., 2013; Talevich and Kannan, 2013) and which are known to be key virulence factors in T. gondii. Little is known of the specific function of individual ROP proteins in Eimeria. Among the Eimeria species affecting chickens, rhoptry proteins offer little immunological cross-reactivity between the various species or even between different life cycle stages within the same species (Kawazoe et al., 1992; Tomley, 1994).
Another group of secretory organelles related to host cell interactions in T. gondii, and other heteroxenic, cyst-forming coccidian, are the dense granules. These are roughly spherical structures larger than micronemes but smaller than rhoptries (Paredes-Santos et al., 2012). The contents of dense granules are secreted into the PV during and immediately after invasion, and dense granule proteins (GRA) are targeted to a variety of final locations including the PV cavity, PV membrane, host cell cytoplasm and host cell nucleolus (Mercier and Cesbron-Delauw, 2015). However, the presence of dense granules as an independent organelle in the zoites of Eimeria spp. is uncertain as there is a lack of structural evidence (Vetterling et al., 1973; Entzeroth et al., 1998) and moreover only a very small number of GRA orthologues are found in their genomes (Reid et al., 2014).
Post-invasion events
For Eimeria species, the newly-formed PV is small and closely surrounds the parasite but later enlarges, possibly contain membranous material or projections from the vacuolar membrane (Strout and Scholtyseck, 1970; Lee and Long, 1972; Vetterling et al., 1973; Pacheco et al., 1975). In electron micrographs of recently invaded sporozoites and merozoites, the PV is often not visible. It is unclear whether this is because there is no vacuole present or because the vacuole membrane is so closely opposed to the parasite that it cannot be distinguished (McLaren, 1969; Lee and Long, 1972; Mota and Rodriguez, 2001). Between 24 and 35 h after invasion, the intracellular sporozoite becomes ovoid in shape [Fig. 6(1)]. At this stage, the parasite is known as a trophozoite and loses most of its apical complex and inner membrane (McLaren, 1969; Pacheco et al., 1975).
Fig. 6.
Schizogony (adapted from Francia and Striepen, 2014). (1) Trophozoite development after sporozoite invasion. (2) Immature schizont, nuclei multiply by several rounds of mitosis. (3) Mature schizont, the last round of division coincides with the merozoites budding at the parasite surface. Merozoites release and initiate a new round of schizogony (or gametogony).
Fairly soon following inoculation of in vitro cell cultures, some Eimeria species sporozoites have been seen to leave their invaded host cell without undergoing further development and replication. It has been hypothesized that some of this cell traversal may involve penetration through the host cell plasma membrane rather than formation of a PV (Itagaki et al., 1974; Behrendt et al., 2004). This hypothesis is supported by the observation that Plasmodium yoelii sporozoites will sometimes invade hepatocytes by breaching the host cell membrane (Mota et al., 2001). Breaching of the host cell membrane has been described for E. bovis (Behrendt et al., 2004), however studies using E. magna demonstrated no breach to plasma membrane on parasite invasion (Jensen, 1975; Jensen and Edgar, 1976). This suggests that invasion of cells by Eimeria species does indeed follow the generally accepted model for apicomplexan invasion.
Initial invasion of E. acervulina, E. maxima, E. necatrix and E. tenella in vivo occurs at the villus epithelium (Lillehoj and Trout, 1993; Shirley et al., 2005). Before initiating endogenous development however, these species travel to the intestinal crypts where they invade another cell of the intestinal epithelium (Jeurissen et al., 1996; Shirley et al., 2005). Although the mechanism by which sporozoites travel from the villi to the crypts is not fully understood, it is believed to occur through the interaction with intestinal lymphocytes (Lawn and Rose, 1982). There are some species of Eimeria whose life cycles involve migration out of the gastrointestinal tract. Sporozoites of the rabbit coccidium, E. stiedae, migrate from the duodenum to the liver (Pakandl, 2009). Two species of Eimeria which infect cranes, E. reichenowi and E. gruis, produce a disease known as disseminated visceral coccidiosis, where zoites can be found in diverse organs such as the lungs, liver and heart (Novilla and Carpenter, 2004). The specific mechanisms involved in this type of migration are not fully understood; traffic via portal vein as well as the lymphatic system was an initial hypothesis (Fitzgerald, 1970); alternatively, spread throughout the host organism until settle in the liver has been suggested (Durr, 1972).
Schizogony
The next phase of development in Eimeria species consists of two to five rounds of asexual replication known as schizogony, where nuclear divisions and cellular expansion occurs to produce a multinuclear schizont [Figs 2(4), 2(5) and 6(2)]. The number of rounds of schizogony, the number of nuclear divisions and the specific site of development are specific characteristic to each species of Eimeria parasite. Eimeria tenella has three generations of schizogony, all located in the caecal crypts, whereas E. maxima has four-to-five generations mostly located in the villi of the small intestine (McDonald and Rose, 1987; Dubey and Jenkins, 2018). In the early stages of schizogony (up to 35 h post invasion) proliferation of the parasite endoplasmic reticulum occurs and nuclear divisions result in multiple granular nuclei, each enclosed by a perforated double membrane. During these divisions, intranuclear spindles, centrocones and centrioles can all be seen (Pacheco et al., 1975). Centrioles of Eimeria have a 9 + 1 singlet microtubule pattern, as opposed to the nine triplet symmetry found in mammalian cells (Dubremetz and Elsner, 1979). As the replicating schizont forms, the parasite significantly increases in size and may occupy up to half of the host cell content (McLaren, 1969).
After nuclear division, individual merozoites begin to develop in the form of protrusions of the schizont cytoplasm that develop a conoid at the apex [Fig. 6(3)]. Each merozoite then elongates and receives a single nucleus from the schizont. The mitochondria and apicoplasts (non-photosynthetic plastid organelle) of zoites of Eimeria and other coccidian species contain nucleic acid genomes that must be replicated and segregated into each of the forming merozoites. Apicoplast replication in Eimeria species occurs via a different mechanism to that in T. gondii tachyzoites, which divide by endodyogeny (rather than schizogony) with apicoplasts dividing in close association with centrosomes and in synchrony with nuclear division (Striepen et al., 2000). In E. tenella, over 95% of sporozoites contain a single apicoplast with up to 5% having two or three of them, whereas to 20% of merozoites have multiple apicoplasts (Ferguson et al., 2007). After zoite invasion, E. tenella apicoplasts enlarge to form pleomorphic-shaped structures that divide several times during the proliferative phase of schizogony. This is not associated with centrosomes and occurs independently of nuclear division by an unknown mechanism (Ferguson et al., 2007). Correct segregation of daughter mitochondria during schizogony is also poorly understood. Once schizonts are fully formed, the posterior poles of merozoites undergo constriction by cytoskeletal rings until they separate from what remains of the schizont, known as the residual body (McLaren, 1969; Pacheco et al., 1975). The events that occur during the second round of schizogony in E. tenella and E. necatrix are particularly interesting since parasite infection causes the host cells to detach from adjacent cells and migrate deeper into the underlying tissue (Stockdale and Fernando, 1975; Fernando et al., 1983; del Cacho et al., 2004). In E. tenella, the second generation schizonts are larger than first generation schizonts, and the third generation schizonts are significantly smaller, containing less than 16 merozoites per structure (McLaren and Paget, 1968; Lee and Long, 1972; McDonald and Rose, 1987).
Merozoites share many characteristics and features of the sporozoite (Fig. 5C). They are bound by a triple bilayer pellicle (plasma membrane and double-layered IMC) and contain a posteriorly located nucleus of similar appearance to that of the sporozoite (McLaren and Paget, 1968). Amylopectin granules, endoplasmic reticulum, Golgi and an apical complex are also present; however they do not contain RBs, just a refractile dot. Second generation merozoites have been reported to have more micronemes but fewer rhoptries than their sporozoite counterparts (McLaren and Paget, 1968; Pacheco et al., 1975). A couple of ‘extra structures’ have also been reported in newly formed merozoites, namely rod-shaped mitochondria and a vacuole with an electron dense outer membrane (McLaren, 1969).
Gametogony and fertilization
Upon re-invasion, the final generation of merozoites initiates a single round of sexual replication, however due to limitations of in vitro development little is known about this stage in Eimeria species. Despite some studies reporting a complete reproduction of the life cycle in vitro, this system is still very deficient, meaning that investigation of the sexual stages requires the use of a host animal (Hermosilla et al., 2002). Microscopy of tissue infected in vivo, revealed that Eimeria species develop two sexually dimorphic stages; the macrogamete and the microgamete (Walker et al., 2013). Macrogametes are large cells, measuring over 9 µm by 16 µm, and contain numerous polysaccharide storage granules for providing nutrients to the developing oocyst (McLaren, 1969). They also contain multiple structures known as wall forming bodies and veil forming bodies which are important for production of the oocyst wall (Ferguson et al., 2003). Microgametes are considerably smaller, around 0.5 µm by 5 µm, and possess two flagella which enhance their motility needed for reaching and fertilizing a macrogamete (Madden and Vetterling, 1977). The formation of microgametes occurs in a similar way to the formation of merozoites by schizogony. Multiple nuclear divisions are performed followed by differentiation of mature flagellated microgametes, roughly 100 from each initial cell. This is markedly different from the process of microgamete formation that occurs in the haemosporines (including Plasmodium species) where microgametogenesis occurs as a result of chemical cues from the insect vector and involves extremely rapid exflagellation (the whole process taking around 8–15 min) (Sinden and Croll, 1975; Billker et al., 1998).
Fertilization of the macrogamete by a microgamete results in the formation of the zygote, which is encased by the forming oocyst wall prior to excretion with the feces [Fig. 2(6) and 2(7)] (Jeurissen et al., 1996; Shirley et al., 2005). Ferguson et al. examined the ultrastructure of T. gondii microgametes (Ferguson et al., 1974): these contain a dense nucleus, a single mitochondrion, two flagella which arise from basal bodies located within the cytoplasm and an osmophilic plate under the plasma membrane at the anterior of the cell. It was also observed that the number of microgametes produced were much lower than expected, meaning that there is no room for wastage if every macrogamete is to be fertilized (Ferguson, 2002). Two hypotheses have been presented to explain this phenomenon: (1) viable oocysts can be produced in absence of fertilization, and (2) the adaptive sex ratio theory, where, due to the high likelihood of inbreeding, selection pressure leads to production of only the minimal number of microgametes required for fertilization of the macrogametes (Ferguson, 2002; West et al., 2003).
Parasite manipulation of the host cell
With most of their development occurring within a vacuole in the cytoplasm of another cell, coccidian parasites have an intimate relationship with the host (Jeurissen et al., 1996). Eimeria tenella is incapable of de novo synthesis of purines and therefore must salvage these in a pre-formed state, relying on the host metabolism for this compound (LaFon and Nelson, 1985). With E. bovis, infection has been shown to significantly modify the host cell, altering gene expression relating to cell metabolism, cell structure, protein synthesis and gene transcription, suggesting that the parasite is able to manipulate the host cell in multiple ways that are advantageous to its survival (Lutz et al., 2011). In T. gondii there is evidence that the parasite uses multiple mechanisms to intercept the normal apoptotic pathways of the host cell and thereby prevent destruction of its immediate environment. One such mechanism used by T. gondii involves activation of the transcription factor nuclear factor κB (NF-κB). Results of immune-histochemical staining of parasitized chicken tissue suggest that this pathway is also utilized by species of Eimeria in avoiding host cell apoptosis (del Cacho et al., 2004). The intracellular development of E. bovis is particularly slow, compared to other Eimeria species, taking around 2 weeks to complete the first round of replication, forming exceptionally large schizonts (300 µm) known as macromeronts (Lutz et al., 2011). In order to maintain host cell viability for this time, despite the pressures of parasitism, it seems especially likely that E. bovis is able to disrupt the apoptotic pathways of the host; indeed in cultured cells heavily infected by E. bovis, it is ultimately the uninfected cells which are seen to die off, whilst the infected cells survive (Lang et al., 2009). These infected cells were shown to have increased expression of anti-apoptotic factors such as cellular Flice inhibitory protein (c-FLIP) and cellular inhibition of apoptosis protein 1 (c-IAP1).
Discussion
Parasites of the Eimeria genus are highly complex organisms, containing numerous structures and exhibiting complex life cycle and processes, some of which are markedly different from higher eukaryotes. Regardless of their high impact and wide prevalence, there are many mechanisms and morphological features that remain completely uncharacterized in Eimeria spp. Although T. gondii is an invaluable resource for inferring information, in particular regarding early endogenous replication, where proteins from specific secretory organelles (micronemes, rhoptries and dense granules) are essential for attachment, invasion, formation and modification of the intracellular parasitophorous vacuole and modulation of host cell pathways; it is a different organism and the pathogenesis of the two parasites differs slightly. Whereas the mechanisms of invasion are similar, the intracellular development is significantly different. Eimeria has an acute, monoxenous life cycle, with no parasite stages persist within host tissue. In the other hand, T. gondii has a heteroxenous life cycle with acute and chronic phases; when ingested by intermediate hosts, parasites transform into tachyzoites that are found transiently in many tissues before they migrate to neural and muscle tissues, where they convert to the tissue cyst bradyzoites that remain in host tissues for life.
Therefore, potential targets and strategies for the control of toxoplasmosis would differ to those to control coccidiosis. For example, previous work on genomics and proteomics of E. tenella (Oakes et al., 2013; Reid et al., 2014) has shown an excellent conservation between Eimeria and Toxoplasma micronemes and rhoptry neck proteins involved in the first stages of endogenous development. However, there is much more limited conservation of rhoptry bulb proteins. In addition, there is very little conservation of genes encoding dense granules proteins (GRA). The different aim of the parasitophorous vacuole created by sporozoites (residing for short term leading to sexual reproduction) vs tachyzoites (establishing a chronic infection, eventually) could be one of the answers to this variable composition in ROPs and GRAs. This together with the lack of electron microscope evidence raise the significant question of whether Eimeria parasites contain organelles equivalent to dense granules of Toxoplasma and other cyst-forming coccidian. It is also interesting that the largest organelle of the parasite cell (RBs) with potential compounds that could serve as a target for disease control, still have an undetermined function.
Since the boom of molecular biology towards the end of the 20th century, there has been a decrease in microscopy-led biological research. However, both light and electron microscopes are invaluable tools for studying organisms such as Eimeria, which have limited in vitro systems and molecular tools for gene editing. For many biological structures, morphology is closely linked to function. Microscopy-derived data can therefore help answer questions about the function of subcellular structures and even individual proteins. In organisms with extensive genetic toolkits (such as T. gondii. or Trypanosoma spp.) microscopy can still be used to determine protein location and function, following the use of fluorescent tagging and protein synthesis disruption. As development of genetic techniques for species of Eimeria progresses, it is likely that these techniques will also play an important part in unravelling the biology of this species. Additionally, advances in cell culture systems and in genetic modification tools for Eimeria species (e.g. CRISPR/Cas9) could play an important role in answering some of the many questions regarding the functions and properties of eimerian subcellular structures and organelles such as the RBs, secretory organelles, apicoplast and conoid, each of which could potentially contain molecules that for targeting by novel drugs due to their absence in the cells of higher eukaryote host species.
Conclusions/future directions
In this paper, we have reviewed what is known about the life cycle and developmental stages of members of the Eimeria genus. An overarching aim in apicomplexan disease research is the production of affordable and sustainable vaccines and there is therefore a wealth of studies focused on the identification and testing of possible immunoprotective antigens. However, the identification of new candidates will not be possible without a complete understanding of eimerian biology. Investment in in vitro systems to get further in the parasite life cycle and testing alternative compound to control the disease are paramount, together with the development of new molecular tools for gene edition in Eimeria spp.
Financial support
Alana Burrell was funded by the RVC Ph.D. studentship programme. This manuscript has been assigned the reference PPS_02029 by the Royal Veterinary College.
Conflict of interest
None.
Ethical standards
Not applicable.
References
- Adl SM, Leander BS, Simpson AG, Archibald JM, Anderson OR, Bass D, Bowser SS, Brugerolle G, Farmer MA, Karpov S, Kolisko M, Lane CE, Lodge DJ, Mann DG, Meisterfeld R, Mendoza L, Moestrup O, Mozley-Standridge SE, Smirnov AV and Spiegel F (2007) Diversity, nomenclature, and taxonomy of protists. Systematic Biology 56, 684–689. [DOI] [PubMed] [Google Scholar]
- Aikawa M, Miller LH, Johnson J and Rabbege J (1978) Erythrocyte entry by malarial parasites. A moving junction between erythrocyte and parasite. Journal of Cell Biology 77, 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine PC (2001) Cell: sporozoite interactions and invasion by apicomplexan parasites of the genus Eimeria. International Journal for Parasitology 31, 1–8. [DOI] [PubMed] [Google Scholar]
- Behrendt JH, Clauss W, Zahner H and Hermosilla C (2004) Alternative mechanism of Eimeria bovis sporozoites to invade cells in vitro by breaching the plasma membrane. Journal of Parasitology 90, 1163–1165. [DOI] [PubMed] [Google Scholar]
- Belli SI, Smith NC and Ferguson DJ (2006) The coccidian oocyst: a tough nut to crack!. Trends in Parasitology 22, 416–423. [DOI] [PubMed] [Google Scholar]
- Besteiro S, Dubremetz JF and Lebrun M (2011) The moving junction of apicomplexan parasites: a key structure for invasion. Cellular Microbiology 13, 797–805. [DOI] [PubMed] [Google Scholar]
- Beyer TV, Svezhova NV, Radchenko AI and Sidorenko NV (2002) Parasitophorous vacuole: morphofunctional diversity in different coccidian genera (a short insight into the problem). Cell Biology International 26, 861–871. [DOI] [PubMed] [Google Scholar]
- Bhogal BS, Miller GA, Anderson AC, Jessee EJ, Strausberg R, McCandliss R and Strausberg S (1989) Vaccination of chickens with recombinant E. tenella antigen alone or in combination with a subclinical exposure induces cross protective immunity against coccidiosis. Progress in Clinical and Biological Research 307, 131–146. [PubMed] [Google Scholar]
- Billker O, Lindo V, Panico M, Etienne AE, Paxton T, Dell A, Rogers M, Sinden RE and Morris HR (1998) Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature 392, 289–292. [DOI] [PubMed] [Google Scholar]
- Bommer W, Heunert HH and Milthaler B (1969) Cinematographic studies on the movement of Toxoplasma gondii. Zeitschrift fuer Tropenmedizin und Parasitologie 20, 450–458. [PubMed] [Google Scholar]
- Bonifacino JS and Rojas R (2006) Retrograde transport from endosomes to the trans-Golgi network. Nature Reviews Molecular Cell Biology 7, 568–579. [DOI] [PubMed] [Google Scholar]
- Breinich MS, Ferguson DJ, Foth BJ, van Dooren GG, Lebrun M, Quon DV, Striepen B, Bradley PJ, Frischknecht F, Carruthers VB and Meissner M (2009) A dynamin is required for the biogenesis of secretory organelles in Toxoplasma gondii. Current Biology 19, 277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown PJ, Billington KJ, Bumstead JM, Clark JD and Tomley FM (2000) A microneme protein from Eimeria tenella with homology to the Apple domains of coagulation factor XI and plasma pre-kallikrein. Molecular and Biochemical Parasitology 107, 91–102. [DOI] [PubMed] [Google Scholar]
- Brown KM, Lourido S and Sibley LD (2016) Serum albumin stimulates protein kinase G-dependent microneme secretion in Toxoplasma gondii. Journal of Biological Chemistry 291, 9554–9565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KM, Long S and Sibley LD (2017) Plasma membrane association by N-acylation governs PKG function in Toxoplasma gondii. MBio 8(3), e00375-17. doi: 10.1128/mBio.00375-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buguliskis JS, Brossier F, Shuman J and Sibley LD (2010) Rhomboid 4 (ROM4) affects the processing of surface adhesins and facilitates host cell invasion by Toxoplasma gondii. PLoS Pathogens 6, e1000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen HE, Tonkin CJ, O'Donnell RA, Tham WH, Papenfuss AT, Gould S, Cowman AF, Crabb BS and Gilson PR (2009) A novel family of Apicomplexan glideosome-associated proteins with an inner membrane-anchoring role. Journal of Biological Chemistry 284, 25353–25363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumstead J and Tomley F (2000) Induction of secretion and surface capping of microneme proteins in Eimeria tenella. Molecular and Biochemical Parasitology 110, 311–321. [DOI] [PubMed] [Google Scholar]
- Canning EU and Anwar M (1968) Studies on meiotic division in coccidial and malarial parasites. The Journal of Protozoology 15, 290–298. [DOI] [PubMed] [Google Scholar]
- Carreno RA, Martin DS and Barta JR (1999) Cryptosporidium is more closely related to the gregarines than to coccidia as shown by phylogenetic analysis of apicomplexan parasites inferred using small-subunit ribosomal RNA gene sequences. Parasitology Research 85, 899–904. [DOI] [PubMed] [Google Scholar]
- Carruthers V and Boothroyd JC (2007) Pulling together: an integrated model of Toxoplasma cell invasion. Current Opinion in Microbiology 10, 83–89. [DOI] [PubMed] [Google Scholar]
- Carruthers VB and Sibley LD (1999) Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii. Molecular Microbiology 31, 421–428. [DOI] [PubMed] [Google Scholar]
- Carruthers VB and Tomley FM (2008) Microneme proteins in apicomplexans. Subcellular Biochemistry 47, 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiappino ML, Nichols BA and O'Connor GR (1984) Scanning electron microscopy of Toxoplasma gondii: parasite torsion and host-cell responses during invasion. The Journal of Protozoology 31, 288–292. [DOI] [PubMed] [Google Scholar]
- Clark EL, Macdonald SE, Thenmozhi V, Kundu K, Garg R, Kumar S, Ayoade S, Fornace KM, Jatau ID, Moftah A, Nolan MJ, Sudhakar NR, Adebambo AO, Lawal IA, Alvarez Zapata R, Awuni JA, Chapman HD, Karimuribo E, Mugasa CM, Namangala B, Rushton J, Suo X, Thangaraj K, Srinivasa Rao AS, Tewari AK, Banerjee PS, Dhinakar Raj G, Raman M, Tomley FM and Blake DP (2016) Cryptic Eimeria genotypes are common across the southern but not northern hemisphere. International Journal for Parasitology 46, 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs GH, Denton H, Brown SM and Thong KW (1997) Biochemistry of the coccidia. Advances in Parasitology 39, 141–226. [DOI] [PubMed] [Google Scholar]
- Cowper B, Matthews S and Tomley F (2012) The molecular basis for the distinct host and tissue tropisms of coccidian parasites. Molecular and Biochemical Parasitology 186, 1–10. [DOI] [PubMed] [Google Scholar]
- Crane MS, Goggin B, Pellegrino RM, Ravino OJ, Lange C, Karkhanis YD, Kirk KE and Chakraborty PR (1991) Cross-protection against four species of chicken coccidia with a single recombinant antigen. Infection and Immunity 59, 1271–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danforth HD and Augustine PC (1989) Eimeria tenella: use of a monoclonal antibody in determining the intracellular fate of the refractile body organelles and the effect on in vitro development. Experimental Parasitology 68, 1–7. [DOI] [PubMed] [Google Scholar]
- de Venevelles P, Francois Chich J, Faigle W, Lombard B, Loew D, Pery P and Labbe M (2006) Study of proteins associated with the Eimeria tenella refractile body by a proteomic approach. International Journal for Parasitology 36, 1399–1407. [DOI] [PubMed] [Google Scholar]
- del Cacho E, Gallego M, Lopez-Bernad F, Quilez J and Sanchez-Acedo C (2004) Expression of anti-apoptotic factors in cells parasitized by second-generation schizonts of Eimeria tenella and Eimeria necatrix. Veterinary Parasitology 125, 287–300. [DOI] [PubMed] [Google Scholar]
- Dobell C (1922) The discovery of the coccidia. Parasitology 14, 342–348. [Google Scholar]
- Dogga SK, Mukherjee B, Jacot D, Kockmann T, Molino L, Hammoudi PM, Hartkoorn RC, Hehl AB and Soldati-Favre D (2017) A druggable secretory protein maturase of Toxoplasma essential for invasion and egress. Elife 6, e27480. doi: 10.7554/eLife.27480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Z, McGovern OL, Di Cristina M and Carruthers VB (2014) Toxoplasma gondii ingests and digests host cytosolic proteins. MBio 5, e01188–e01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP and Jenkins MC (2018) Re-evaluation of the life cycle of Eimeria maxima Tyzzer, 1929 in chickens (Gallus domesticus). Parasitology 145, 1051–1058. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Lindsay DS and Speer CA (1998) Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology and development of tissue cysts. Clinical Microbiology Reviews 11, 267–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubremetz JF (1975) Genesis of merozoites in the coccidia, Eimeria necatrix. Ultrastructural study. The Journal of Protozoology 22, 71–84. [DOI] [PubMed] [Google Scholar]
- Dubremetz JF and Elsner YY (1979) Ultrastructural study of schizogony of Eimeria bovis in cell cultures. The Journal of Protozoology 26, 367–376. [DOI] [PubMed] [Google Scholar]
- Dubremetz JF and Torpier G (1978) Freeze fracture study of the pellicle of an eimerian sporozoite (Protozoa, Coccidia). Journal of Ultrastructure Research 62, 94–109. [DOI] [PubMed] [Google Scholar]
- Dubremetz JF, Garcia-Reguet N, Conseil V and Fourmaux MN (1998) Apical organelles and host-cell invasion by Apicomplexa. International Journal for Parasitology 28, 1007–1013. [DOI] [PubMed] [Google Scholar]
- Dunn PP, Bumstead JM and Tomley FM (1996) Sequence, expression and localization of calmodulin-domain protein kinases in Eimeria tenella and Eimeria maxima. Parasitology 113(Pt 5), 439–448. [DOI] [PubMed] [Google Scholar]
- Durr U (1972) Life cycle of Eimeria stiedai. Acta Veterinaria Academiae Scientiarum Hungaricae 22, 101–103. [PubMed] [Google Scholar]
- Duszynski DW (2001) Eimeria, In: eLS. John Wiley & Sons, Ltd, Chinchester, UK. [Google Scholar]
- Edgar SA and Seibold CT (1964) A new coccidium of chickens, Eimeria mivati Sp. N. (Protozoa: Eimeriidae) with details of its life history. Journal of Parasitology 50, 193–204. [PubMed] [Google Scholar]
- Entzeroth R, Zgrzebski G and Dubremetz JF (1989) Secretion of trials during gliding motility of Eimeria nieschulzi (Apicomplexa, Coccidia) sporozoites visualized by a monoclonal antibody and immuno-gold-silver enhancement. Parasitology Research 76, 174–175. [DOI] [PubMed] [Google Scholar]
- Entzeroth R, Mattig FR and Werner-Meier R (1998) Structure and function of the parasitophorous vacuole in Eimeria species. International Journal for Parasitology 28, 1015–1018. [DOI] [PubMed] [Google Scholar]
- Fantham H (1910). The Morphology and Life-History of Eimeria (Coccidium) avium: a Sporozoö causing a fatal disease among young Grouse. In: Proceedings of the Zoological Society of London, pp. 672–691.
- Farrell A, Thirugnanam S, Lorestani A, Dvorin JD, Eidell KP, Ferguson DJ, Anderson-White BR, Duraisingh MT, Marth GT and Gubbels MJ (2012) A DOC2 protein identified by mutational profiling is essential for apicomplexan parasite exocytosis. Science 335, 218–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayer R (1969) Refractile body changes in sporozoites of poultry coccidia in cell culture. In: Proceedings of the Helminthological Society of Washington, pp. 224–231.
- Ferguson DJ (2002) Toxoplasma gondii and sex: essential or optional extra? Trends in Parasitology 18, 355–359. [PubMed] [Google Scholar]
- Ferguson DJ, Hutchison WM, Dunachie JF and Siim JC (1974) Ultrastructural study of early stages of asexual multiplication and microgametogony of Toxoplasma gondii in the small intestine of the cat. Acta Pathologica Et Microbiologica Scandinavica. Section B: Microbiology and Immunology 82, 167–181. [DOI] [PubMed] [Google Scholar]
- Ferguson DJ, Belli SI, Smith NC and Wallach MG (2003) The development of the macrogamete and oocyst wall in Eimeria maxima: immuno-light and electron microscopy. International Journal for Parasitology 33, 1329–1340. [DOI] [PubMed] [Google Scholar]
- Ferguson DJ, Campbell SA, Henriquez FL, Phan L, Mui E, Richards TA, Muench SP, Allary M, Lu JZ, Prigge ST, Tomley F, Shirley MW, Rice DW, McLeod R and Roberts CW (2007) Enzymes of type II fatty acid synthesis and apicoplast differentiation and division in Eimeria tenella. International Journal for Parasitology 37, 33–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando MA and Remmler O (1974) Fine structure of the first and second generation merozoites of Eimeria necatrix. Zeitschrift Fur Parasitenkunde (Berlin, Germany) 44, 133–137. [DOI] [PubMed] [Google Scholar]
- Fernando MA, Lawn AM, Rose ME and Al-Attar MA (1983) Invasion of chicken caecal and intestinal lamina propria by crypt epithelial cells infected with coccidia. Parasitology 86(Pt 3), 391–398. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PR (1970) New findings on the life cycle of Eimeria stiedae. Journal of Parasitology 56, 100–101. [Google Scholar]
- Francia ME and Striepen B (2014) Cell division in apicomplexan parasites. Nature Reviews Microbiology 12, 125–136. [DOI] [PubMed] [Google Scholar]
- Frenal K and Soldati-Favre D (2009) Role of the parasite and host cytoskeleton in apicomplexa parasitism. Cell Host & Microbe 5, 602–611. [DOI] [PubMed] [Google Scholar]
- Gilk SD, Gaskins E, Ward GE and Beckers CJ (2009) GAP45 phosphorylation controls assembly of the Toxoplasma myosin XIV complex. Eukaryotic Cell 8, 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golemansky V (2015) Checklist of haemosporidian and piroplasmid parasites (Apicomplexa: Haemospororida and Pirolasmorida) of man and animals in Bulgaria. Acta Zoologica Bulgarica 67, 453–460. [Google Scholar]
- Graindorge A, Frenal K, Jacot D, Salamun J, Marq JB and Soldati-Favre D (2016) The conoid associated motor MyoH is indispensable for Toxoplasma gondii entry and exit from host cells. PLoS Pathogens 12, e1005388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerardel Y, Leleu D, Coppin A, Lienard L, Slomianny C, Strecker G, Ball S and Tomavo S (2005) Amylopectin biogenesis and characterization in the protozoan parasite Toxoplasma gondii, the intracellular development of which is restricted in the HepG2 cell line. Microbes and Infection 7, 41–48. [DOI] [PubMed] [Google Scholar]
- Guerin A, Corrales RM, Parker ML, Lamarque MH, Jacot D, El Hajj H, Soldati-Favre D, Boulanger MJ and Lebrun M (2017) Efficient invasion by Toxoplasma depends on the subversion of host protein networks. Nature Microbiology 2, 1358–1366. [DOI] [PubMed] [Google Scholar]
- Hakansson S, Charron AJ and Sibley LD (2001) Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole. EMBO Journal 20, 3132–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammoudi PM, Maco B, Dogga SK, Frenal K and Soldati-Favre D (2018) Toxoplasma gondii TFP1 is an essential transporter family protein critical for microneme maturation and exocytosis. Molecular Microbiology 109, 225–244. [DOI] [PubMed] [Google Scholar]
- Harding CR, Egarter S, Gow M, Jimenez-Ruiz E, Ferguson DJ and Meissner M (2016) Gliding associated proteins play essential roles during the formation of the inner membrane complex of Toxoplasma gondii. PLoS Pathogens 12, e1005403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herm-Gotz A, Weiss S, Stratmann R, Fujita-Becker S, Ruff C, Meyhofer E, Soldati T, Manstein DJ, Geeves MA and Soldati D (2002) Toxoplasma gondii myosin A and its light chain: a fast, single-headed, plus-end-directed motor. EMBO Journal 21, 2149–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermosilla C, Barbisch B, Heise A, Kowalik S and Zahner H (2002) Development of Eimeria bovis in vitro: suitability of several bovine, human and porcine endothelial cell lines, bovine fetal gastrointestinal, Madin-Darby bovine kidney (MDBK) and African green monkey kidney (VERO) cells. Parasitology Research 88, 301–307. [DOI] [PubMed] [Google Scholar]
- Hu K, Roos DS and Murray JM (2002) A novel polymer of tubulin forms the conoid of Toxoplasma gondii. Journal of Cell Biology 156, 1039–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Johnson J, Florens L, Fraunholz M, Suravajjala S, DiLullo C, Yates J, Roos DS and Murray JM (2006) Cytoskeletal components of an invasion machine – the apical complex of Toxoplasma gondii. PLoS Pathogens 2, e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itagaki K, Hirayama N, Tsubokura M, Otsuki K and Taira Y (1974) Development of Eimeria tenella, E. brunetti and E. acervulina in cell cultures. Nihon Juigaku Zasshi 36, 467–482. [DOI] [PubMed] [Google Scholar]
- Jackson AJ, Clucas C, Mamczur NJ, Ferguson DJ and Meissner M (2013) Toxoplasma gondii Syntaxin 6 is required for vesicular transport between endosomal-like compartments and the Golgi complex. Traffic 14, 1166–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacot D, Tosetti N, Pires I, Stock J, Graindorge A, Hung YF, Han H, Tewari R, Kursula I and Soldati-Favre D (2016) An apicomplexan actin-binding protein serves as a connector and lipid sensor to coordinate motility and invasion. Cell Host & Microbe 20, 731–743. [DOI] [PubMed] [Google Scholar]
- Jatau ID, Lawal IA, Kwaga JK, Tomley FM, Blake DP and Nok AJ (2016) Three operational taxonomic units of Eimeria are common in Nigerian chickens and may undermine effective molecular diagnosis of coccidiosis. BMC Veterinary Research 12, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen JB (1975) Ultrastructure of the invasion of Eimeria magna sporozoites into cultured cells. The Journal of Protozoology 22, 411–415. [DOI] [PubMed] [Google Scholar]
- Jensen JB and Edgar SA (1976) Possible secretory function of the rhoptries of Eimeria magna during penetration of cultured cells. Journal of Parasitology 62, 988–992. [PubMed] [Google Scholar]
- Jeurissen SH, Janse EM, Vermeulen AN and Vervelde L (1996) Eimeria tenella infections in chickens: aspects of host-parasite: interaction. Veterinary Immunology and Immunopathology 54, 231–238. [DOI] [PubMed] [Google Scholar]
- Jirku M, Modry D, Slapeta JR, Koudela B and Lukes J (2002) The phylogeny of Goussia and Choleoeimeria (Apicomplexa; Eimeriorina) and the evolution of excystation structures in coccidia. Protist 153, 379–390. [DOI] [PubMed] [Google Scholar]
- Karkhanis YD, Nollstadt KA, Bhogal BS, Ravino O, Pellegrino R, Crane MS, Murray PK and Turner MJ (1991) Purification and characterization of a protective antigen from Eimeria tenella. Infection and Immunity 59, 983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawazoe U, Tomley FM and Frazier JA (1992) Fractionation and antigenic characterization of organelles of Eimeria tenella sporozoites. Parasitology 104(Pt 1), 1–9. [DOI] [PubMed] [Google Scholar]
- Keeley A and Soldati D (2004) The glideosome: a molecular machine powering motility and host-cell invasion by Apicomplexa. Trends in Cell Biology 14, 528–532. [DOI] [PubMed] [Google Scholar]
- Kemp LE, Yamamoto M and Soldati-Favre D (2013) Subversion of host cellular functions by the apicomplexan parasites. FEMS Microbiology Reviews 37, 607–631. [DOI] [PubMed] [Google Scholar]
- King CA (1981) Cell surface interaction of the protozoan Gregarina with concanavalin A beads – implications for models of gregarine gliding. Cell Biology International Reports 5, 297–305. [DOI] [PubMed] [Google Scholar]
- Koshy AA, Dietrich HK, Christian DA, Melehani JH, Shastri AJ, Hunter CA and Boothroyd JC (2012) Toxoplasma co-opts host cells it does not invade. PLoS Pathogens 8, e1002825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer K, Kamin D, Rittweger E, Wilkes J, Flammer H, Mahler S, Heng J, Tonkin CJ, Langsley G, Hell SW, Carruthers VB, Ferguson DJ and Meissner M (2013) An overexpression screen of Toxoplasma gondii Rab-GTPases reveals distinct transport routes to the micronemes. PLoS Pathogens 9, e1003213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudryashev M, Lepper S, Stanway R, Bohn S, Baumeister W, Cyrklaff M and Frischknecht F (2010) Positioning of large organelles by a membrane- associated cytoskeleton in Plasmodium sporozoites. Cellular Microbiology 12, 362–371. [DOI] [PubMed] [Google Scholar]
- LaFon SW and Nelson DJ (1985) Purine metabolism in the intact sporozoites and merozoites of Eimeria tenella. Molecular and Biochemical Parasitology 14, 11–22. [DOI] [PubMed] [Google Scholar]
- Lai L, Simpson P, Bumstead J, Tomley F and Matthews S (2009) Complete NMR assignments for the second microneme adhesive repeat (MAR) domain from Eimeria tenella microneme protein EtMIC3. Biomolecular NMR Assignments 3, 175–177. [DOI] [PubMed] [Google Scholar]
- Lai L, Bumstead J, Liu Y, Garnett J, Campanero-Rhodes MA, Blake DP, Palma AS, Chai W, Ferguson DJ, Simpson P, Feizi T, Tomley FM and Matthews S (2011) The role of sialyl glycan recognition in host tissue tropism of the avian parasite Eimeria tenella. PLoS Pathogens 7, e1002296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarque M, Besteiro S, Papoin J, Roques M, Vulliez-Le Normand B, Morlon-Guyot J, Dubremetz JF, Fauquenoy S, Tomavo S, Faber BW, Kocken CH, Thomas AW, Boulanger MJ, Bentley GA and Lebrun M (2011) The RON2-AMA1 interaction is a critical step in moving junction-dependent invasion by apicomplexan parasites. PLoS Pathogens 7, e1001276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane CE and Archibald JM (2008) The eukaryotic tree of life: endosymbiosis takes its TOL. Trends in Ecology & Evolution 23, 268–275. [DOI] [PubMed] [Google Scholar]
- Lang M, Kann M, Zahner H, Taubert A and Hermosilla C (2009) Inhibition of host cell apoptosis by Eimeria bovis sporozoites. Veterinary Parasitology 160, 25–33. [DOI] [PubMed] [Google Scholar]
- Laurent F, Bourdieu C, Kaga M, Chilmonczyk S, Zgrzebski G, Yvore P and Pery P (1993) Cloning and characterization of an Eimeria acervulina sporozoite gene homologous to aspartyl proteinases. Molecular and Biochemical Parasitology 62, 303–312. [DOI] [PubMed] [Google Scholar]
- Lawn AM and Rose ME (1982) Mucosal transport of Eimeria tenella in the cecum of the chicken. Journal of Parasitology 68, 1117–1123. [PubMed] [Google Scholar]
- Lee DL and Long PL (1972) An electron microscopal study of Eimeria tenella grown in the liver of the chick embryo. International Journal for Parasitology 2, 55–58. [DOI] [PubMed] [Google Scholar]
- Leuckart R (1879) Die Parasiten des Menschen und die von ihnen herführenden Krankheiten, 2nd Edn. Winter, Leipzig und Heidelberg. [Google Scholar]
- Levine P (1938) Eimeria haganin sp. (Protozoa: Eimeriidae) a new coccidium of the chicken. The Cornell Veterinarian 28, 263–266. [Google Scholar]
- Levine ND (1984) Nomenclatural corrections and new taxa in the apicomplexan protozoa. Transactions of the American Microscopical Society 103, 195–204. [Google Scholar]
- Liberator PA, Hsu J and Turner MJ (1989) Tandem trinucleotide repeats throughout the nucleotide sequence of a cDNA encoding an Eimeria tenella sporozoite antigen. Nucleic Acids Research 17, 7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillehoj HS and Trout JM (1993) Coccidia: a review of recent advances on immunity and vaccine development. Avian Pathology 22, 3–31. [DOI] [PubMed] [Google Scholar]
- Liu S, Wang L, Zheng H, Xu Z, Roellig DM, Li N, Frace MA, Tang K, Arrowood MJ, Moss DM, Zhang L, Feng Y and Xiao L (2016) Comparative genomics reveals Cyclospora cayetanensis possesses coccidia-like metabolism and invasion components but unique surface antigens. BMC Genomics 17, 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S, Anthony B, Drewry LL and Sibley LD (2017a) A conserved ankyrin repeat-containing protein regulates conoid stability, motility and cell invasion in Toxoplasma gondii. Nature Communications 8, 2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S, Brown KM, Drewry LL, Anthony B, Phan IQH and Sibley LD (2017b) Calmodulin-like proteins localized to the conoid regulate motility and cell invasion by Toxoplasma gondii. PLoS Pathogens 13, e1006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourido S, Shuman J, Zhang C, Shokat KM, Hui R and Sibley LD (2010) Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature 465, 359–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourido S, Tang K and Sibley LD (2012) Distinct signalling pathways control Toxoplasma egress and host-cell invasion. EMBO Journal 31, 4524–4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz K, Schmitt S, Linder M, Hermosilla C, Zahner H and Taubert A (2011) Eimeria bovis-induced modulation of the host cell proteome at the meront I stage. Molecular and Biochemical Parasitology 175, 1–9. [DOI] [PubMed] [Google Scholar]
- Madden PA and Vetterling JM (1977) Scanning electron microscopy of Eimeria tenella microgametogenesis and fertilization. Journal of Parasitology 63, 607–610. [PubMed] [Google Scholar]
- Mai K, Sharman PA, Walker RA, Katrib M, De Souza D, McConville MJ, Wallach MG, Belli SI, Ferguson DJ and Smith NC (2009) Oocyst wall formation and composition in coccidian parasites. Memorias do Instituto Oswaldo Cruz 104, 281–289. [DOI] [PubMed] [Google Scholar]
- McDonald V and Rose ME (1987) Eimeria tenella and E. necatrix: a third generation of schizogony is an obligatory part of the developmental cycle. Journal of Parasitology 73, 617–622. [PubMed] [Google Scholar]
- McGovern OL and Carruthers VB (2016) Toxoplasma retromer is here to stay. Trends in Parasitology 32, 758–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGovern OL, Rivera-Cuevas Y, Kannan G, Narwold AJ Jr and Carruthers VB (2018) Intersection of endocytic and exocytic systems in Toxoplasma gondii. Traffic 19, 336–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren DJ (1969) Observations on the fine structural changes associated with schizogony and gametogony in Eimeria tenella. Parasitology 59, 563–574. [DOI] [PubMed] [Google Scholar]
- McLaren DJ and Paget GE (1968) A fine structural study on the merozoite of Eimeria tenella with special reference to the conoid apparatus. Parasitology 58, 561–571. [DOI] [PubMed] [Google Scholar]
- Megía-Palma R, Martínez J, Acevedo I, Martín J, García-Roa R, Ortega J, Peso-Fernández M, Albaladejo G, Cooper RD and Paranjpe DA (2015) Phylogeny of the reptilian Eimeria: are Choleoeimeria and Acroeimeria valid generic names? Zoologica Scripta 44, 684–692. [Google Scholar]
- Melo EJ, Attias M and De Souza W (2000) The single mitochondrion of tachyzoites of Toxoplasma gondii. Journal of Structural Biology 130, 27–33. [DOI] [PubMed] [Google Scholar]
- Mercier C and Cesbron-Delauw MF (2015) Toxoplasma secretory granules: one population or more? Trends in Parasitology 31, 60–71. [DOI] [PubMed] [Google Scholar]
- Miller LH, McAuliffe FM and Johnson JG (1979) Invasion of erythrocytes by malaria merozoites. Progress in Clinical and Biological Research 30, 497–502. [PubMed] [Google Scholar]
- Miller GA, Bhogal BS, McCandliss R, Strausberg RL, Jessee EJ, Anderson AC, Fuchs CK, Nagle J, Likel MH, Strasser JM and Strausberg S (1989) Characterization and vaccine potential of a novel recombinant coccidial antigen. Infection and Immunity 57, 2014–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda K, de Souza W, Plattner H, Hentschel J, Kawazoe U, Fang J and Moreno SN (2008) Acidocalcisomes in Apicomplexan parasites. Experimental Parasitology 118, 2–9. [DOI] [PubMed] [Google Scholar]
- Mordue DG and Sibley LD (1997) Intracellular fate of vacuoles containing Toxoplasma gondii is determined at the time of formation and depends on the mechanism of entry. Journal of Immunology 159, 4452–4459. [PubMed] [Google Scholar]
- Mordue DG, Hakansson S, Niesman I and Sibley LD (1999) Toxoplasma gondii resides in a vacuole that avoids fusion with host cell endocytic and exocytic vesicular trafficking pathways. Experimental Parasitology 92, 87–99. [DOI] [PubMed] [Google Scholar]
- Morlon-Guyot J, Pastore S, Berry L, Lebrun M and Daher W (2015) Toxoplasma gondii Vps11, a subunit of HOPS and CORVET tethering complexes, is essential for the biogenesis of secretory organelles. Cellular Microbiology 17, 1157–1178. [DOI] [PubMed] [Google Scholar]
- Morlon-Guyot J, El Hajj H, Martin K, Fois A, Carrillo A, Berry L, Burchmore R, Meissner M, Lebrun M and Daher W (2018) A proteomic analysis unravels novel CORVET and HOPS proteins involved in Toxoplasma gondii secretory organelles biogenesis. Cellular Microbiology 20, e12870. [DOI] [PubMed] [Google Scholar]
- Morrissette NS and Sibley LD (2002) Cytoskeleton of apicomplexan parasites. Microbiology and Molecular Biology Reviews 66, 21–38; table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota MM and Rodriguez A (2001) Migration through host cells by apicomplexan parasites. Microbes and Infection 3, 1123–1128. [DOI] [PubMed] [Google Scholar]
- Mota MM, Pradel G, Vanderberg JP, Hafalla JC, Frevert U, Nussenzweig RS, Nussenzweig V and Rodriguez A (2001) Migration of Plasmodium sporozoites through cells before infection. Science 291, 141–144. [DOI] [PubMed] [Google Scholar]
- Nishi M, Hu K, Murray JM and Roos DS (2008) Organellar dynamics during the cell cycle of Toxoplasma gondii. Journal of Cell Science 121, 1559–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novilla MN and Carpenter JW (2004) Pathology and pathogenesis of disseminated visceral coccidiosis in cranes. Avian Pathology 33, 275–280. [DOI] [PubMed] [Google Scholar]
- Oakes RD, Kurian D, Bromley E, Ward C, Lal K, Blake DP, Reid AJ, Pain A, Sinden RE, Wastling JM and Tomley FM (2013) The rhoptry proteome of Eimeria tenella sporozoites. International Journal for Parasitology 43, 181–188. [DOI] [PubMed] [Google Scholar]
- Opitz C and Soldati D (2002) 'The glideosome': a dynamic complex powering gliding motion and host cell invasion by Toxoplasma gondii. Molecular Microbiology 45, 597–604. [DOI] [PubMed] [Google Scholar]
- Pacheco ND, Vetterling JM and Doran DJ (1975) Ultrastructure of cytoplasmic and nuclear changes in Eimeria tenella during first-generation schizogony in cell culture. Journal of Parasitology 61, 31–42. [PubMed] [Google Scholar]
- Pakandl M (2009) Coccidia of rabbit: a review. Folia Parasitologica (Praha) 56, 153–166. [DOI] [PubMed] [Google Scholar]
- Pan M, Zhou Y, Wang Y, Li L, Song Y, Hou L and Zhao J (2017) Screening and identification of the host proteins interacting with Toxoplasma gondii rhoptry protein ROP16. Frontiers in Microbiology 8, 2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes-Santos TC, de Souza W and Attias M (2012) Dynamics and 3D organization of secretory organelles of Toxoplasma gondii. Journal of Structural Biology 177, 420–430. [DOI] [PubMed] [Google Scholar]
- Pastor-Fernandez I, Kim S, Billington K, Bumstead J, Marugan-Hernandez V, Kuster T, Ferguson DJP, Vervelde L, Blake DP and Tomley FM (2018) Development of cross-protective Eimeria-vectored vaccines based on apical membrane antigens. International Journal for Parasitology 48, 505–518. [DOI] [PubMed] [Google Scholar]
- Peek HW and Landman WJ (2011) Coccidiosis in poultry: anticoccidial products, vaccines and other prevention strategies. Veterinary Quarterly 31, 143–161. [DOI] [PubMed] [Google Scholar]
- Pieperhoff MS, Schmitt M, Ferguson DJ and Meissner M (2013) The role of clathrin in post-Golgi trafficking in Toxoplasma gondii. PLoS One 8, e77620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu YH and Zhang DL (2012) Research progress on invasion mechanism and immunology of Toxoplasma gondii. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 30, 480–485, 490. [PubMed] [Google Scholar]
- Reid AJ, Blake DP, Ansari HR, Billington K, Browne HP, Bryant J, Dunn M, Hung SS, Kawahara F, Miranda-Saavedra D, Malas TB, Mourier T, Naghra H, Nair M, Otto TD, Rawlings ND, Rivailler P, Sanchez-Flores A, Sanders M, Subramaniam C, Tay YL, Woo Y, Wu X, Barrell B, Dear PH, Doerig C, Gruber A, Ivens AC, Parkinson J, Rajandream MA, Shirley MW, Wan KL, Berriman M, Tomley FM and Pain A (2014) Genomic analysis of the causative agents of coccidiosis in domestic chickens. Genome Research 24, 1676–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relman DA, Schmidt TM, Gajadhar A, Sogin M, Cross J, Yoder K, Sethabutr O and Echeverria P (1996) Molecular phylogenetic analysis of Cyclospora, the human intestinal pathogen, suggests that it is closely related to Eimeria species. Journal of Infectious Diseases 173, 440–445. [DOI] [PubMed] [Google Scholar]
- Roberts WL and Hammond DM (1970) Ultrastructural and cytologic studies of the sporozoites of four Eimeria species. The Journal of Protozoology 17, 76–86. [DOI] [PubMed] [Google Scholar]
- Roberts WL, Hammond DM and Speer CA (1970a) Ultrastructural study of the intra- and extracellular sporozoites of Eimeria callospermophili. Journal of Parasitology 56, 907–917. [PubMed] [Google Scholar]
- Roberts WL, Speer CA and Hammond DM (1970b) Electron and light microscope studies of the oocyst walls, sporocysts, and existing sporozoites of Eimeria callospermophili and E. larimerensis. Journal of Parasitology 56, 918–926. [PubMed] [Google Scholar]
- Roberts WL, Speer CA and Hammond DM (1971) Penetration of Eimeria larimerensis sporozoites into cultured cells as observed with the light and electron microscopes. Journal of Parasitology 57, 615–625. [PubMed] [Google Scholar]
- Roiko MS and Carruthers VB (2013) Functional dissection of Toxoplasma gondii perforin-like protein 1 reveals a dual domain mode of membrane binding for cytolysis and parasite egress. Journal of Biological Chemistry 288, 8712–8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DG and Sinden RE (1981) The role of the cytoskeleton in the motility of coccidian sporozoites. Journal of Cell Science 50, 345–359. [DOI] [PubMed] [Google Scholar]
- Russell DG and Sinden RE (1982) Three-dimensional study of the intact cytoskeleton of coccidian sporozoites. International Journal for Parasitology 12, 221–226. [DOI] [PubMed] [Google Scholar]
- Ryan R, Shirley M and Tomley F (2000) Mapping and expression of microneme genes in Eimeria tenella. International Journal for Parasitology 30, 1493–1499. [DOI] [PubMed] [Google Scholar]
- Ryley JF (1969) Ultrastructural studies on the sporozoite of Eimeria tenella. Parasitology 59, 67–72. [DOI] [PubMed] [Google Scholar]
- Ryley JF, Bentley M, Manners DJ and Stark JR (1969) Amylopectin, the storage polysaccharide of the Coccidia Eimeria brunetti and E. tenella. Journal of Parasitology 55, 839–845. [PubMed] [Google Scholar]
- Sakura T, Sindikubwabo F, Oesterlin LK, Bousquet H, Slomianny C, Hakimi MA, Langsley G and Tomavo S (2016) A critical role for Toxoplasma gondii Vacuolar Protein Sorting VPS9 in secretory organelle biogenesis and host infection. Scientific Reports 6, 38842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangare LO, Alayi TD, Westermann B, Hovasse A, Sindikubwabo F, Callebaut I, Werkmeister E, Lafont F, Slomianny C, Hakimi MA, Van Dorsselaer A, Schaeffer-Reiss C and Tomavo S (2016) Unconventional endosome-like compartment and retromer complex in Toxoplasma gondii govern parasite integrity and host infection. Nature Communications 7, 11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A (1875) Note sur la psorospermies oviformes du poulpe. Archives de Zoologie Experimentale et Generale 4, 387–404. [Google Scholar]
- Scholtyseck E (1965) Elektronenmikroskopische Untersuchungen über die Schizogonie bei Coccidien (Eimeria perforans und E. stiedae). Parasitology Research 26, 50–62. [DOI] [PubMed] [Google Scholar]
- Sheffield HG and Hammond DM (1966) Fine structure of first-generation merozoites of Eimeria bovis. Journal of Parasitology 52, 595–606. [PubMed] [Google Scholar]
- Shirley MW, Smith AL and Tomley FM (2005) The biology of avian Eimeria with an emphasis on their control by vaccination. Advances in Parasitology 60, 285–330. [DOI] [PubMed] [Google Scholar]
- Shirley MW, Smith AL and Blake DP (2007) Challenges in the successful control of the avian coccidia. Vaccine 25, 5540–5547. [DOI] [PubMed] [Google Scholar]
- Sibley LD (2010) How apicomplexan parasites move in and out of cells. Current Opinion in Biotechnology 21, 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinden RE and Croll NA (1975) Cytology and kinetics of microgametogenesis and fertilization in Plasmodium yoelii nigeriensis. Parasitology 70, 53–65. [DOI] [PubMed] [Google Scholar]
- Sloves PJ, Delhaye S, Mouveaux T, Werkmeister E, Slomianny C, Hovasse A, Dilezitoko Alayi T, Callebaut I, Gaji RY, Schaeffer-Reiss C, Van Dorsselear A, Carruthers VB and Tomavo S (2012) Toxoplasma sortilin-like receptor regulates protein transport and is essential for apical secretory organelle biogenesis and host infection. Cell Host & Microbe 11, 515–527. [DOI] [PubMed] [Google Scholar]
- Soares Medeiros LC, Gomes F, Maciel LR, Seabra SH, Docampo R, Moreno S, Plattner H, Hentschel J, Kawazoe U, Barrabin H, de Souza W, Damatta RA and Miranda K (2011) Volutin granules of Eimeria parasites are acidic compartments and have physiological and structural characteristics similar to acidocalcisomes. Journal of Eukaryotic Microbiology 58, 416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Xu L, Yan R, Huang X and Li X (2015) Construction of Eimeria tenella multi-epitope DNA vaccines and their protective efficacies against experimental infection. Veterinary Immunology and Immunopathology 166, 79–87. [DOI] [PubMed] [Google Scholar]
- Stockdale PH and Fernando MA (1975) The development of the lesions caused by second generation schizonts of Eimeria necatrix. Research in Veterinary Science 19, 204–208. [PubMed] [Google Scholar]
- Stotish RL, Wang CC and Meyenhofer M (1978) Structure and composition of the oocyst wall of Eimeria tenella. Journal of Parasitology 64, 1074–1081. [PubMed] [Google Scholar]
- Striepen B, Crawford MJ, Shaw MK, Tilney LG, Seeber F and Roos DS (2000) The plastid of Toxoplasma gondii is divided by association with the centrosomes. Journal of Cell Biology 151, 1423–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strout RG and Scholtyseck E (1970) The ultrastructure of first generation development of Eimeria tenella (Railliet and Lucet, 1891) Fantham, 1909 in cell cultures. Zeitschrift Fur Parasitenkunde (berlin, Germany) 35, 87–96. [DOI] [PubMed] [Google Scholar]
- Suss-Toby E, Zimmerberg J and Ward GE (1996) Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fission pore. Proceedings of the National Academy of Sciences of the United States of America 93, 8413–8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talevich E and Kannan N (2013) Structural and evolutionary adaptation of rhoptry kinases and pseudokinases, a family of coccidian virulence factors. BMC Evolutionary Biology 13, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenter AM and Johnson AM (1997) Phylogeny of the tissue cyst-forming coccidia. Advances in Parasitology 39, 69–139. [DOI] [PubMed] [Google Scholar]
- Tenter AM, Barta JR, Beveridge I, Duszynski DW, Mehlhorn H, Morrison DA, Thompson RC and Conrad PA (2002) The conceptual basis for a new classification of the coccidia. International Journal for Parasitology 32, 595–616. [DOI] [PubMed] [Google Scholar]
- Tomavo S, Slomianny C, Meissner M and Carruthers VB (2013) Protein trafficking through the endosomal system prepares intracellular parasites for a home invasion. PLoS Pathogens 9, e1003629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomley FM (1994) Characterization of rhoptry proteins of Eimeria tenella sporozoites: antigenic diversity of rhoptry epitopes within species of the genus Eimeria and among three asexual generations of a single species, E. tenella. Infection and Immunity 62, 4656–4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomley FM and Soldati DS (2001) Mix and match modules: structure and function of microneme proteins in apicomplexan parasites. Trends in Parasitology 17, 81–88. [DOI] [PubMed] [Google Scholar]
- Tomley FM, Bumstead JM, Billington KJ and Dunn PP (1996) Molecular cloning and characterization of a novel acidic microneme protein (Etmic-2) from the apicomplexan protozoan parasite, Eimeria tenella. Molecular and Biochemical Parasitology 79, 195–206. [DOI] [PubMed] [Google Scholar]
- Trees AJ (2001) Parasitic diseases. In Jordan F, Pattison M, Alexander D and Faragher T (eds), Poultry Diseases, 5th Edn. Vol Poultry diseases. W.B. Saunders Ltd., London, UK, 405–433 pp. [Google Scholar]
- Tyler JS and Boothroyd JC (2011) The C-terminus of Toxoplasma RON2 provides the crucial link between AMA1 and the host-associated invasion complex. PLoS Pathogens 7, e1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderberg JP (1974) Studies on the motility of Plasmodium sporozoites. The Journal of Protozoology 21, 527–537. [DOI] [PubMed] [Google Scholar]
- Venugopal K, Werkmeister E, Barois N, Saliou JM, Poncet A, Huot L, Sindikubwabo F, Hakimi MA, Langsley G, Lafont F and Marion S (2017) Dual role of the Toxoplasma gondii clathrin adaptor AP1 in the sorting of rhoptry and microneme proteins and in parasite division. PLoS Pathogens 13, e1006331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen AN, Kok JJ, van den Boogaart P, Dijkema R and Claessens JA (1993) Eimeria refractile body proteins contain two potentially functional characteristics: transhydrogenase and carbohydrate transport. FEMS Microbiology Letters 110, 223–229. [DOI] [PubMed] [Google Scholar]
- Vetterling JM, Pacheco ND and Madden PA (1973) Ultrastructure of dormant, activated, and intracellular sporozoites of Eimeria adenoeides and E. tenella. Journal of Parasitology 59, 15–27. [PubMed] [Google Scholar]
- Walker RA, Ferguson DJ, Miller CM and Smith NC (2013) Sex and Eimeria: a molecular perspective. Parasitology 140, 1701–1717. [DOI] [PubMed] [Google Scholar]
- Walker RA, Sharman PA, Miller CM, Lippuner C, Okoniewski M, Eichenberger RM, Ramakrishnan C, Brossier F, Deplazes P, Hehl AB and Smith NC (2015) RNA seq analysis of the Eimeria tenella gametocyte transcriptome reveals clues about the molecular basis for sexual reproduction and oocyst biogenesis. BMC Genomics 16, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West SA, Reece SE and Read AF (2003) Toxoplasma gondii, sex and premature rejection. Trends in Parasitology 19, 155–157, discussion 157–158. [DOI] [PubMed] [Google Scholar]
- Wiersma HI, Galuska SE, Tomley FM, Sibley LD, Liberator PA and Donald RG (2004) A role for coccidian cGMP-dependent protein kinase in motility and invasion. International Journal for Parasitology 34, 369–380. [DOI] [PubMed] [Google Scholar]
- Wohlfert EA, Blader IJ and Wilson EH (2017) Brains and brawn: toxoplasma infections of the central nervous system and skeletal muscle. Trends in Parasitology 33, 519–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Liu X, Ji Y, Tao G and Suo X (2015) Ethanol and isopropanol trigger rapid egress of intracellular Eimeria tenella sporozoites. Parasitology Research 114, 625–630. [DOI] [PubMed] [Google Scholar]
- Yan X, Tao G, Liu X, Ji Y and Suo X (2016) Calcium-dependent microneme protein discharge and in vitro egress of Eimeria tenella sporozoites. Experimental Parasitology 170, 193–197. [DOI] [PubMed] [Google Scholar]
- Yang G, Yao J, Yang W, Jiang Y, Du J, Huang H, Gu W, Hu J, Ye L, Shi C, Shan B and Wang C (2017) Construction and immunological evaluation of recombinant Lactobacillus plantarum expressing SO7 of Eimeria tenella fusion DC-targeting peptide. Veterinary Parasitology 236, 7–13. [DOI] [PubMed] [Google Scholar]
- Yun CH, Lillehoj HS and Choi KD (2000a) Eimeria tenella infection induces local gamma interferon production and intestinal lymphocyte subpopulation changes. Infection and Immunity 68, 1282–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun CH, Lillehoj HS and Lillehoj EP (2000b) Intestinal immune responses to coccidiosis. Developmental & Comparative Immunology 24, 303–324. [DOI] [PubMed] [Google Scholar]