Abstract
Environmental toxicants are pervasive in nature, but sub-lethal effects on non-target organisms and their parasites are often overlooked. Particularly, studies on terrestrial hosts and their parasites exposed to agricultural toxicants are lacking. Here, we studied the effect of sequence and timing of sub-lethal exposures of the pyrethroid insecticide alpha-cypermethrin on parasite establishment using the tapeworm Hymenolepis diminuta and its intermediate insect host Tenebrio molitor as a model system. We exposed T. molitor to alpha-cypermethrin (LD20) before and after experimental H. diminuta infection and measured the establishment success of larval tapeworms. Also, we conducted in vitro studies quantifying the direct effect of the insecticide on parasite viability. Our results showed that there was no direct lethal effect of alpha-cypermethrin on H. diminuta cysticercoids at relevant concentrations (LD10 to LD90 of the intermediate host). However, we observed a significantly increased establishment of H. diminuta in beetles exposed to alpha-cypermethrin (LD20) after parasite infection. In contrast, parasite establishment was significantly lower in beetles exposed to the insecticide before parasite infection. Thus, our results indicate that environmental toxicants potentially impact host-parasite interactions in terrestrial systems, but that the outcome is context-dependent by enhancing or reducing parasite establishment depending on timing and sequence of exposure.
Key words: Exposure timing, insecticide, multiple stressors, parasite, sub-lethal effects
Introduction
Environmental toxicants, particularly chemical pesticides are prevalent in nature where they may affect a wide range of organisms and ecosystems (Pimentel, 2009; Goulson, 2014). Among these, insecticides are routinely applied to cultivated ecosystems to reduce insect pest populations in agriculture and to control vectors of important diseases of humans and domesticated animals. Continual environmental contamination through water, air and soil increases the risk that non-target organisms are chronically exposed to sub-lethal concentrations of insecticides (Goulson, 2013). The consequences of sublethal insecticide exposure on non-target terrestrial species and their interactions with symbionts or parasites are poorly described, but such exposures have been suggested to impair population dynamics (Guedes et al., 2016), affect insect immunity (James and Xu, 2012), change behaviour or habitat selection (Lalouette et al., 2016; Jiang et al., 2018) and reduce viability and/or reproduction (Müller et al., 2017). A prominent example would be the decline of beneficial insects such as honey bees and other pollinators which may be related to sub-lethal and chronic exposure to insecticides in combination with invading diseases and parasites such as Nosema ceranae (Ratnieks and Carreck, 2010; Doublet et al., 2015; Goulson et al., 2015).
Insecticide-induced stress in insects might affect host-parasite interactions and disease outcome as insects naturally serve as hosts to a broad range of parasites, parasitoids and pathogens (Goulson et al., 2015). For example, in honey bees, chronic exposure to the neonicotinoid imidacloprid increased the abundance of the gut parasite Nosema spp. (Pettis et al., 2012). Similarly, the pyrethroid insecticide alpha-cypermethrin interacted with the entomopathogenic fungus Beauveria bassiana resulting in synergistic mortality in the insect host when B. bassiana exposure was followed by alpha-cypermethrin (Meyling et al., 2018). This latter interaction showed that the order and timing of insecticide and pathogen exposure significantly influence either disease manifestation or insecticide toxicity. Similarly, insecticide exposure prior to ranavirus infection of an amphibian host (wood frog: Lithobates sylvaticus) increased disease-induced mortality, whereas the toxicity of insecticide increased with reversed order of exposure, i.e. prior ranavirus infection (Pochini and Hoverman, 2017). Thus, the context-dependent interaction of several factors highlights the importance of timing and order of exposure when evaluating disease dynamics and insecticide toxicity.
Parasite transmission from one host to the next depends on the survival of parasites and their host until transmission. Therefore, the effect of insecticides is not only expected to affect host population dynamics, but also the long-term survival of host-specific parasite populations in the environment. Studies have mainly focused on the toxic effect of insecticides on host populations while insecticide effects on parasites and resulting disease dynamics have received much less attention and have mainly focused on aquatic ecosystems (Rohr et al., 2008; Hua et al., 2016; Pochini and Hoverman, 2017), with a fewer studies of terrestrial host-parasite interactions such as those involving insects as hosts. Of these, most studies have focused on the potential effects of neonicotinoids particularly in social insect such as honey bees (Goulson et al., 2015; O'Neal et al., 2018; Raymann et al., 2018). Other studies in insect hosts examined interactions between helminths and diatomaceous earth (DE), which induces physical stress via dessication (Shostak, 2012; Shostak et al., 2015; Chin et al., 2017). Here, we investigated the effect of chemical stress from the pyrethroid insecticide alpha-cypermethrin on interactions between an insect and its tapeworm parasite. Pyrethroids represent a relatively old but still very used group of insecticides, which are often substituting the use of neonicotinoids because of their short longevity in the terrestrial environment. Thus, under field conditions, half-life of alpha-cypermethrin in soil is 42.6 days, compared to 174 days for imidacloprid (IUPAC, 2019). Our study is, therefore, one of the first to investigate if and how pyrethroids affect terrestrial host-parasite interactions including parasite establishment.
We used the Hymenolepis diminuta–Tenebrio molitor model system, which is well known and widely used for ecological studies of host-parasite interactions (Shostak, 2014; Dhakal et al., 2015, 2018). The adult tapeworm lives in the intestine of the rat definitive host (Rattus norvegicus), and infective eggs pass with faeces to the environment. A broad range of insects including T. molitor act as intermediate hosts and acquire the infection through ingestion of infective eggs. The egg hatches in the intestine of the insect, and the emerging oncosphere larva penetrates the gut wall and develops to a cysticercoid in the body cavity of the beetle (Burt, 1980).
Based on previous studies of neonicotenoids in bees, we hypothesized that chemical stressors, such as sublethal doses of a pyrethroid insecticide, will enhance the establishment success of the parasites through chemical-related physiological stress in the host. On the other hand, if the insecticide affects the establishing parasite, sublethal pyrethroid doses could also hamper parasite development to cysticercoids in the exposed host and thereby decrease parasite establishment. The hypotheses were investigated through two experiments: first the indirect effect of exposure to LD20 of alpha-cypermethrin on cysticercoid survival in vivo was measured by quantifying the establishment success of H. diminuta in beetles exposed one or two times to alpha-cypermethrin prior to or following experimental infection with ecologically relevant doses of H. diminuta. Secondly, the direct effect of alpha-cypermethrin at different concentrations (LD10 to LD90 of the intermediate host) on cysticercoid survival was investigated in vitro.
Materials and methods
Management of T. molitor
Tenebrio molitor cultures originally obtained from Avifauna ApS, Denmark were maintained at the laboratory facilities at University of Copenhagen, Department of Plant and Environmental Sciences for at least five generations before experimental use. Cultures were raised in a dark climate cabinet at 26 °C with 50 adult beetles per ventilated plastic box (18 × 12 × 7 cm) provided with 100 g of organic oatmeal, a 10 g fresh organic potato slice and 1 g of chicken-egg white powder. After a week, the adult beetles were transferred to a new plastic box under similar conditions for three consecutive weeks. Larvae produced during the three weeks were provided with a fresh slice of organic potato (10 g) every week and maintained under standard condition until pupation. Pupae were collected and kept in a separate plastic box until eclosion. Adult beetles of age 12–14 days post eclosion were used in the experiment.
Experimental infection of T. molitor with H. diminuta eggs
Hymenolepis diminuta eggs were collected from faeces of experimentally infected rats (R. norvegicus – Wistar strain) maintained at the University of Copenhagen, Denmark (Animal permission No. 2014-15-0201-00387, Section C1). Ten grams of infective faeces were soaked in 25 mL of tap water for an hour, stirred with a wooden spatula to make a uniform paste and wet sieved through a double layer of cotton gauge, followed by stacked 100 and 62 µm metal sieves. Eggs collected on the 62 µm sieve were transferred to a 50 mL falcon tube and centrifuged (Universal 16R, GeminiBV, Apeldoorn, The Netherlands) for 3 min at 300 g. The supernatant was discarded and a mean ± s.d. egg concentration of 433 ± 8 per 10 µL suspension was prepared by diluting the stock solution with tap water (Dhakal et al., 2018).
Beetles were starved for 72 h prior to infection and were exposed individually to a 10 µL droplet of egg suspension for an hour in a dark room (Dhakal et al., 2018). Only beetles that consumed the entire egg suspension were used for the experiment.
Preparation of alpha-cypermethrin
Alpha-cypermethrin (99.8% purity) and the solvent acetone (HPLC grade) were obtained from Sigma Aldrich, Darmstadt, Germany and Merck KGaA respectively. Stock solution of 300 mg L−1 in acetone was prepared and stored at −20 °C. Four different concentrations of alpha-cypermethrin 1.8, 6.2, 52 and 1515 mg L−1 were used in the experiment, which was equivalent to LD10, LD20, LD50 and LD90 in adult T. molitor when exposed to 1 µL volume between the pronotum and elytra individually (Meyling et al., 2018). All the working concentrations were prepared at the time of exposure.
In-vivo study
Beetles were exposed to alpha-cypermethrin either before or after parasite infection. Two groups received insecticide either once or twice before parasite infection. Similarly, two other groups received insecticide either once or twice after parasite infection. The control group received only acetone (Table 1) as acetone was used as the solvent of alpha-cypermethrin and did not show a detrimental effect on beetle mortality in a previous study (Meyling et al., 2018). For that reason beetles exposed to acetone served as the control group rather than a completely untreated group of beetles. The resulting five T. molitor groups were referred to as: Single insecticide exposure before parasite infection, double insecticide exposure before parasite infection, single insecticide exposure after parasite infection, double insecticide exposure after parasite infection and control group. Each group consisted of 45, 55, 45, 65 and 40 beetles, respectively and each group were maintained in ventilated plastic box (18 × 12 × 7 cm) as described above. More beetles were included in the groups where higher mortality was expected based on pilot studies. The five groups of beetles each represented a combination of exposure to low dose of alpha-cypermethrin (1 µL per beetle of 6.2 mg L−1 in acetone) applied in different sequences and times in relation to the exposure to H. diminuta eggs. All beetles, including controls, were treated similarly exposing them to acetone if not receiving insecticides at a specific time point, to be sure to expose them to similar levels of handling stress. Times and exposures and number of surviving beetles at each experimental time point of exposure are given in Table 1. The insecticide exposure was done by placing a 1 µL droplet of alpha-cypermethrin solution between the pronotum and elytra (Meyling et al., 2018). All non-exposed beetles received 1 µL droplet of acetone. Insecticide exposures were conducted on day 1, 4, 7 and 10, while exposure to H. diminuta eggs occurred on day 7 for all groups (Table 1). Only beetles that consumed the entire 10 µL of faecal suspension containing H. diminuta eggs were included in the experiment. Also, the remaining uneaten eggs on each glass slide were subsequently counted to estimate the number of eggs consumed by the beetles. Mortality of beetles was recorded on day 1, 4, 7, 10, 14 and 20. On day 20, all remaining beetles were dissected under a dissection microscope (40×) and the numbers of cysticercoids in the haemocoel were counted.
Table 1.
Experimental design for different treatment groups (in-vivo study)
| Treatment group | |||||
|---|---|---|---|---|---|
| Single insecticide exposure before parasite infection | Double insecticide exposure before parasite infection | Single insecticide exposure after parasite infection | Double insecticide exposure after parasite infection | Control | |
| Day 1 | Alpha-cypermethrin 45 |
Alpha-cypermethrin 55 |
Acetone 45 |
Acetone 65 |
Acetone 40 |
| Day 4 | Acetone 43 |
Alpha-cypermethrin 52 |
Acetone 45 |
Acetone 65 |
Acetone 40 |
| Day 7 | 39 | 31 | 45 | 65 | 40 |
| Experimental infection with H. diminuta eggs | |||||
| Refusal to consume droplet | |||||
| 9 (23.1%) | 8 (25.8%) | 4 (8.9%) | 6 (9.2%) | 5 (12.5%) | |
| Acetone 30 |
Acetone 23 |
Alpha-cypermethrin 41 |
Alpha-cypermethrin 59 |
Acetone 35 |
|
| Day 10 | Acetone 29 |
Acetone 18 |
Acetone 36 |
Alpha-cypermethrin 55 |
Acetone 35 |
| Day 14 | 28 | 16 | 24 | 28 | 35 |
| Day 20 | 27 | 16 | 24 | 20 | 33 |
| Counting of H. diminuta cysticercoids | |||||
The number of surviving beetles for each treatment and day combination are indicated. Exposure to alpha-cypermethrin was done after 1 h exposure to H. diminuta eggs.
Calculation of expected cysticercoids
Due to the effect of alpha-cypermethrin exposure, beetles from different treatment groups ingested significantly different numbers of parasite eggs during infection. To calculate the expected number of cysticercoids we used the power function (y = 0.0007 × 1.8548, where y = number of established cysticercoids, and x = consumed egg dose) derived by Dhakal et al. (2018) in the same model system.
In-vitro study
The purpose of this study was to quantify the dose-dependent effect of alpha-cypermethrin on the survival of cysticercoids of H. diminuta. Survival was measured as cysticercoid activation subsequent to enzymatic stimulation mimicking the environment of the definitive host gut. In this in-vitro experiment, our aim was to mimic the conditions experienced by cysticercoids of H. diminuta in-vivo (i.e. in the haemocoel of beetles). Based on a previous study we expected an infection level of 50 cysticercoids to occur in each of the beetles from the egg dose used (Dhakal et al., 2018) and 50 cysticercoids were therefore used per treatment. For in-vitro exposure of cysticercoids, experimental volumes of 1 µL of insecticide, which are used in the in-vivo study, are insufficient to entirely cover the 50 cysticercoids. Based on a pilot study we found that a volume of 100 µL was appropriate to cover the 50 cysticercoids, so the 1 µL of insecticide from each concentration (1.8, 6.2, 52 and 1515 mg L−1) was mixed to 99 µL of 5% acetone (prepared in distilled water) separately as higher concentration of acetone is toxic to cysticercoids. Thus, the active compound of alpha-cypermethrin and the number of cysticercoids per treatment in the in-vitro experiment were expected to be similar to the in-vivo experiment.
Cysticercoids of H. diminuta were recovered from infected T. molitor by dissecting beetles 15 days after infection under a dissection microscope (40×) (Dhakal et al., 2015). A maximum of 10 cysticercoids from each beetle was collected and a total of 250 cysticercoids were kept in a watch glass (33 mm diameter, 7 mm deep) containing phosphate buffered saline. From these, five watch glasses (15 mm diameter, 1 mm deep) with 50 cysticercoids randomly distributed in each were prepared and 100 µL of the four different concentrations of alpha-cypermethrin or 100 µL of 5% acetone prepared in distilled water were added. Each watch glass was individually covered with a glass slide and incubated for 1 h at 37 °C.
After 1 h of incubation, among the 50 cysticercoids from each group, only 10 cysticercoids were randomly chosen and used for excystation assays to evaluate viability as described by Dhakal et al. (2015) modified from Goodchild and Davis (1972). The experiment was repeated twice.
Statistical analysis
All statistical analyses were performed using SAS® version 9.4 (SAS Institute Inc, Cary, North Carolina). The level of significance was set at α = 0.05. The data from in-vitro excystation of cysticercoids were either alive or dead. So a logistic regression model (PROC GLIMMIX) was fitted using binomial distribution and experimental trial was used as a random factor. The number of beetles survived was analysed using a log-rank test (PROC LIFETEST procedure) with Sidak adjustment (Seid et al., 2019). The number of cysticercoids established in the beetle and the number of uneaten eggs in the treatments were analysed using a generalized linear model (PROC GENMOD procedure). Poisson distribution was assumed in the generalized liner models, but overdispersion was detected, therefore, negative binomial distribution was used. When an overall significant effect was observed, pair-wise comparisons were done using least squares means (PDIFF option).
Results
In-vivo study
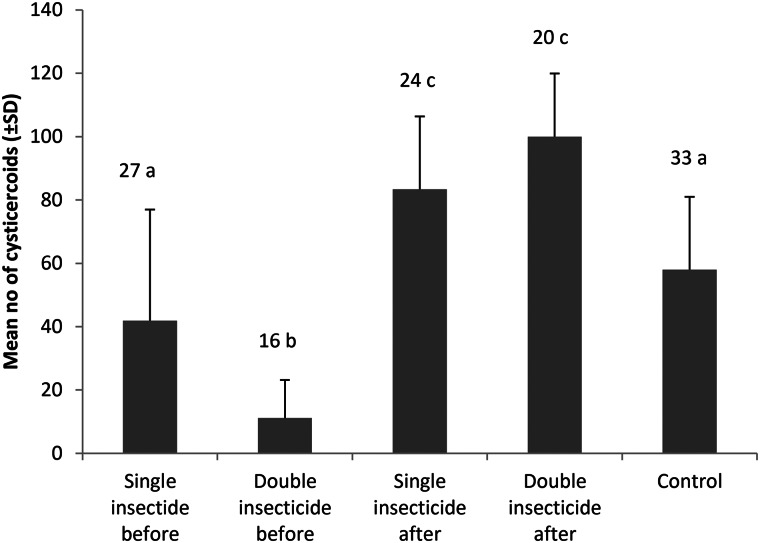
There was a significant effect of treatment on cysticercoid establishment within the different groups of beetles (generalized linear model, χ2 = 62.23, df = 4, P < 0.0001). Establishment of cysticercoids was significantly higher in beetles exposed to alpha-cypermethrin following parasite infection (lsmeans, P < 0.0001), while double exposures of alpha-cypermethrin, before infection, reduced cysticercoid establishment significantly compared to the control group (lsmeans, P < 0.0001) (Fig. 1).
Fig. 1.
Mean number of H. diminuta cysticercoids established (±s.d.) in T. molitor after exposure to infective eggs (433 ± 4) with different exposure combinations of alpha-cypermethrin [0.0062 µg µL−1 per beetle, (LD20)]. Single insecticide before received insecticide at day 1, double insecticide before received insecticide on day 1 and 4, single insecticide after received insecticide on day 7, double insecticide after received insecticide on day 7 and 10, and the control group was exposed to the insecticide solvent only (acetone). Experimental infection with H. diminuta was conducted on day 7 for all treatment groups. Different letters represent statistically different establishment of cysticercoids between groups (α = 0.05), and number above each bar represent sample size.
Beetle survival was significantly different between treatment groups (Proc lifetest, χ2 = 50.47, df = 4, P < 0.0001). Regardless of timing, double insecticide exposure groups displayed a significantly lower survival than the control group within the 20 day experiment (P < 0.0001). Numbers of beetles that survived the different experimental procedures during the 20 day period within different treatment groups are presented in Table 1.
Calculation of expected cysticercoids
Overall, there was a significant difference in egg consumption between five T. molitor groups (generalized linear model, χ2 = 52.83, df = 4, P = <0.0001) (Table 2). Adults of T. molitor exposed to alpha-cypermethrin twice before being presented with H. diminuta eggs consumed a significantly smaller proportion of eggs compared to the other four groups of beetles (lsmeans, P < 0.0001). Due to a significant difference in the egg consumption, we calculated the expected parasite establishment to range between 46 to 49 cysticercoids. The observed number of cysticercoids (11 cysticercoids) was therefore 76 to 78% lower than expected. The observed number of cysticercoids in beetles receiving single and double exposure to alpha-cypermethrin after parasite infection was 48–58%, and 78–90% higher than predicted, respectively (Table 2).
Table 2.
Mean number (±s.d.) of uneaten H. diminuta eggs by Tenebrio molitor after experimental exposure to 433 ± 8 eggs for 1 h
| Treatment group | Uneaten eggs | Expected number of cysticercoid | Observed number of cysticercoids | Difference between expected and observed cysticercoid |
|---|---|---|---|---|
| Single insecticide exposure before parasite infection | 28 ± 10* | 53–56 | 42 ± 35a | ↓20 – 25% |
| Double insecticide exposure before parasite infection | 58 ± 19** | 46–49 | 11 ± 12b | ↓76 – 78% |
| Single insecticide exposure after parasite infection | 27 ± 13* | 53–56 | 83 ± 23c | ↑58 – 48% |
| Double insecticide exposure after parasite infection | 27 ± 10* | 53–56 | 100 ± 20c | ↑90 – 78% |
| Only parasite infection (control group) | 28 ± 14* | 53– 56 | 58 ± 23a | ↑10 – 3% |
The total number of beetles used for counting uneaten eggs in each treatment group were 30, 23, 20, 20 and 20 from top to bottom row, respectively. Predicted number vs observed mean number (±s.d.) of H. diminuta cysticercoids in T. molitor in the different treatment groups by using formula (y = 0.0007x1.8548), where y = number of established cysticercoids, and x = exposed egg number (Dhakal et al., 2018). Different numbers of asterisks, and different letters represent statistically different ingestion of eggs and establishment between groups, respectively (α = 0.05).
In-vitro study
The average excystation percentage (±s.d.) of cysticercoids from two experimental trials was 95 ± 7, 95 ± 7, 90 ± 0, 90 ± 0 and 90 ± 14 after exposure to alpha-cypermethrin with 1 µL volume of 0.0, 1.8, 6.2, 52 and 1515 mg L−1, respectively. Each experimental trial consisted of 10 cysticercoids. There was no significant difference in the cysticercoid excystation between groups (logistic regression, F4,1 = 0.17, P = 0.95).
Discussion
Our study showed that the timing and sequence of host exposure to alpha-cypermethrin played an important role in parasite establishment. As hypothesized, exposure of sub-lethal concentration (LD20) of alpha-cypermethrin immediately after parasite infection significantly increased the parasite establishment to almost twice the level observed in the unexposed control group. Mode of action of pyrethroids in insects is neurotoxicity, preventing closure of voltage-sensitive sodium channels in the axonal membrane (Soderlund, 2010). The direct effect of pyrethroids in exposed insects varies from impaired locomotory function to complete paralysis and indirectly the chemical affects insects by causing starvation and increased risk of predation. The reductions in mobility and feeding also play a role in disease outcome (Bradbury and Coats, 1989; James and Xu, 2012). Our findings showed that exposure to alpha-cypermethrin after H. diminuta egg infection caused increased parasite burden. Beetles became immobilized for approximately four to eight hours following exposure due to the knock-out effect of alpha-cypermethrin (S. Dhakal, personal observation). Evidently no feeding was possible during that time, and a starvation response reducing the investment of energy into mounting the initial immune response may explain the increase in parasite establishment. This phenomenon has also been observed in another study where larvae of Colorado potato beetles that were starved for 24 h immediately after Beauveria bassiana application showed higher mortality than larvae with access to food following B. bassiana application (Furlong and Groden, 2001). We did not examine the effect of alpha-cypermethrin applied to T. molitor at a later stage of infection than three days after parasite exposure, but Shostak (2012) reported that DE exposure one day after parasite infection and continued throughout the H. diminuta infection did not affect cysticercoid abundance in adults of Tribolium confusum. Although alpha-cypermethrin and DE have different modes of action (DE desiccates the exposed insects and does not affect the nervous system), our results indicated that early exposure to insecticide is important for parasite establishment, while later exposure has limited effects. Development of H. diminuta cysticercoids requires hatching of ingested eggs in the gastro-intestinal tract of the host, penetration of the gut wall by the emerging oncosphere and establishment in the body cavity of the host (Burt, 1980; Lethbridge, 1971). All these processes occur within 45–75 min after egg ingestion (Lethbridge, 1971), and stressed conditions that arise from insecticide exposure immediately after parasite exposure may create favourable conditions for the establishment process, resulting in an increased number of cysticercoids. Also, this timeframe may be the key period in determining the establishment success of cysticercoids, as there is no evidence that established cysticercoids are recognized, melanized and killed by the host immune system (Lackie, 1976), which is the most common defence mechanism of insects towards invading parasites (Schmid-Hempel, 2005).
In contrast to the increased parasite establishment in beetles exposed to the insecticide after the exposure to the parasite, an almost 10-fold reduction in establishment of cysticercoids was observed when the insecticide was applied twice, three and seven days before parasite infection. This finding contrasts the finding of Shostak (2012), who demonstrated a higher cysticercoid establishment in T. confusum when infected after two weeks of exposure with DE. The ability to consume and process food may be negatively influenced by insecticide exposure by affecting the nervous system, which was also indicated by the larger proportion of beetles that refused to drink the entire droplet in comparison with the untreated group. Even beetles that consumed the droplet left behind more eggs on the glass slide after the droplet was consumed than the controls. We did not observe a direct in-vitro lethal effect of alpha-cypermethrin on fully mature H. diminuta cysticercoids within the spectrum of environmentally relevant concentrations. In addition, our data did not indicate a negative effect of alpha-cypermethrin on the developing stages of cysticercoids. In contrast, in-vivo exposure of beetles to alpha-cypermethrin after egg exposure significantly increased the number of cysticercoids. It is therefore unlikely that the lower parasite establishment success observed when the insecticide was first applied was due to the direct effect of the insecticide on H. diminuta cysticercoids, as initially hypothesized. Our results of the in vitro experiment are in contrast to studies on trematode cercariae, which are directly susceptible to a range of commonly used pesticides including alpha-cypermethrin (Rohr et al., 2008; Hua et al., 2016). Taken together, our results suggest that the decreased number of cysticercoids in beetles exposed to insecticide prior to parasite infection is neither due to direct lethal effects towards cysticercoids nor due to the reduced consumption of H. diminuta eggs as this was taken into account by counting the remaining un-eaten eggs and calculating the expected cysticercoids load. Behaviour changes such as cessation of feeding, hyperactivity and restlessness in the host after sub-lethal insecticide exposure (Bradbury and Coats, 1989) may affect egg hatching and penetration of the beetle gut wall by the oncosphere, which could have a direct negative effect on parasite establishment. Around a quarter of the beetles refused to consume the entire droplet of egg suspension and were removed from the experiment. Considering these beetles, the adverse effect on parasite establishment at the population level would be even larger than what we report in this study.
In addition to the importance of sequence of exposure, the number of exposures to low dose of alpha-cypermethrin seemed to have an impact on parasite establishment. A single exposure of alpha-cypermethrin seven days before the parasite infection did not significantly affect cysticercoid establishment, while a double exposure seven and three days before the parasite infection reduced the cysticercoid establishment by 76–78%. It is uncertain whether the reduced number of cysticercoids was due to the number of pesticide exposures or the length of time since the last exposure. However, the observed effect might be caused by impairment of beetle feeding behaviour by alpha-cypermethrin exposure. Thus, starvation of T. molitor seven days prior to and four days after H. diminuta infection reduce cysticercoid establishment (Dhakal et al., 2018).
Beetle mortality caused by exposure to LD20 of alpha-cypermethrin was expected due to its toxic properties (Meyling et al., 2018). Hence, mortality was higher in the double exposure groups compared to the single exposure groups. In other studies the combinatory exposure to insecticide and parasite increased host mortality (Alaux et al., 2010; Pochini and Hoverman, 2017; Meyling et al., 2018). If that had been the case we would have expected heavily infected hosts to suffer the greatest mortality. In contrast, we observed that the parasite load almost doubled in surviving beetles receiving both stressors (parasite and insecticide) in comparison with one stressor (parasite). Our results therefore indicate that exposure to sub-lethal concentrations of alpha-cypermethrin may enhance parasite establishment success with no evidence that infected hosts are more likely to die from subsequent exposure to the insecticide.
The implications of sub-lethal insecticide exposure to host and parasite populations are not readily predicted from this study. Hosts may experience a greater parasite establishment success when infected hosts are subsequently exposed to insecticide. In this study, the increased parasite load did not incur additional mortality compared to the insecticide only group. However, it is possible that increasingly larger parasite intensity would eventually induce intensity-dependent host mortality as usually observed for helminths (Crofton, 1971). We only exposed beetles to alpha-cypermethrin for a maximum of two times in this study, and it is possible that chronic exposure to sub-lethal concentrations of alpha-cypermethrin may decrease host tolerance to parasite infection and increase host mortality (Dhakal, unpublished data). On the other hand, insecticide exposure increases host mortality, which may reduce the window of opportunity for larval parasite stages relying on trophic transmission to the next host in the life cycle.
In conclusion, there is no direct lethal effect of the insecticide towards mature cysticercoids in vitro within the spectrum of environmentally relevant concentrations. We demonstrated that exposure to the pyrethroid insecticide alpha-cypermethrin significantly affected the establishment of H. diminuta cysticercoids in its host T. molitor and that the sequential order of host exposure to insecticide and parasite has a central role in parasite establishment. Exposure to low doses of insecticide prior to parasite infection decreased parasite load whereas infection prior to insecticide increased parasite load. The observed effects of exposure timing and sequence on parasite establishment suggest that modifications in host body condition induced by the insecticide exposure play an important role for parasite development.
Acknowledgements
We thank Elizabeth Jane Cassidy for her help in the laboratory.
Financial support
This study was supported by a research grant (No. 00007457) from the Villum Foundation.
Conflict of interest
None.
Ethical standards
Use of animals adhered to Animal permission No. 2014-15-0201-00387, Section C1.
References
- Alaux C, Brunet JL, Dussaubat C, Mondet F, Tchamitchan S, Cousin M, Brillard J, Baldy A, Belzunces LP and Le Conte Y (2010) Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Environmental Microbiology 12, 774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury SP and Coats JR (1989) Comparative toxicology of the pyrethroid insecticides. In Ware GW (ed.), Reviews of Environmental Contamination and Toxicology. New York, NY: Springer New York, pp. 133–177. [DOI] [PubMed] [Google Scholar]
- Burt MDB (1980) Aspects of the life history and systematics of Hymenolepis diminuta. In Arai HP (ed.), Biology of the Tapeworm Hymenolepis Diminuta. United States: Academic Press, pp. 1–57. [Google Scholar]
- Chin HMH, Luong LT and Shostak AW (2017) Longitudinal study of parasite-induced mortality of a long-lived host: the importance of exposure to non-parasitic stressors. Parasitology 144, 1943–1955. [DOI] [PubMed] [Google Scholar]
- Crofton HD (1971) A quantitative approach to parasitism. Parasitology 62, 179–193. [Google Scholar]
- Dhakal S, Meyling NV, Williams AR, Mueller-Harvey I, Fryganas C, Kapel CMO and Fredensborg BL (2015) Efficacy of condensed tannins against larval Hymenolepis diminuta (Cestoda) in vitro and in the intermediate host Tenebrio molitor (Coleoptera) in vivo. Veterinary Parasitology 207, 49–55. [DOI] [PubMed] [Google Scholar]
- Dhakal S, Micki Buss S, Cassidy EJ, Meyling NV and Fredensborg BL (2018) Establishment success of the beetle tapeworm Hymenolepis diminuta depends on dose and host body condition. Insects 9, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doublet V, Labarussias M, Miranda JR, Moritz RFA and Paxton RJ (2015) Bees under stress: sublethal doses of a neonicotinoid pesticide and pathogens interact to elevate honey bee mortality across the life cycle. Environmental Microbiology 17, 969–983. [DOI] [PubMed] [Google Scholar]
- Furlong MJ and Groden E (2001) Evaluation of synergistic interactions between the Colorado potato beetle (Coleoptera: Chrysomelidae) pathogen Beauveria bassiana and the insecticides, imidacloprid, and cyromazine. Journal of Economic Entomology 94, 344–356. [DOI] [PubMed] [Google Scholar]
- Goodchild CG and Davis BO (1972) Hymenolepis microstoma cysticercoid activation and excystation in vitro (Cestoda). The Journal of Parasitology 58, 735–741. [PubMed] [Google Scholar]
- Goulson D (2013) REVIEW: an overview of the environmental risks posed by neonicotinoid insecticides. Journal of Applied Ecology 50, 977–987. [Google Scholar]
- Goulson D (2014) Pesticides linked to bird declines. Nature 511, 295. [DOI] [PubMed] [Google Scholar]
- Goulson D, Nicholls E, Botías C and Rotheray EL (2015) Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1255957. [DOI] [PubMed] [Google Scholar]
- Guedes RNC, Smagghe G, Stark JD and Desneux N (2016) Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Annual Review of Entomology 61, 43–62. [DOI] [PubMed] [Google Scholar]
- Hua J, Buss N, Kim J, Orlofske SA and Hoverman JT (2016) Population-specific toxicity of six insecticides to the trematode Echinoparyphium sp. Parasitology 143, 542–550. [DOI] [PubMed] [Google Scholar]
- IUPAC (2019) International union of pure and applied chemistry website. Available at https://sitem.herts.ac.uk/aeru/iupac/atoz.htm (Accessed 17 May 2019).
- James RR and Xu J (2012) Mechanisms by which pesticides affect insect immunity. Journal of Invertebrate Pathology 109, 175–182. [DOI] [PubMed] [Google Scholar]
- Jiang X, Wang Z, He Q, Liu Q, Li X, Yu L and Cao H (2018) The effect of neonicotinoid insecticide and fungicide on sugar responsiveness and orientation behavior of honey bee (Apis mellifera) in semi field conditions. Insects 9, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackie AM (1976) Evasion of the haemocytic defence reaction of certain insects by larvae of Hymenolepis diminuta (Cestoda). Parasitology 73, 97–107. [DOI] [PubMed] [Google Scholar]
- Lalouette L, Pottier MA, Wycke MA, Boitard C, Bozzolan F, Maria A, Demondion E, Chertemps T, Lucas P, Renault D, Maibeche M, Siaussat DJES and Research P (2016) Unexpected effects of sublethal doses of insecticide on the peripheral olfactory response and sexual behavior in a pest insect. Environmental Science and Pollution Research 23, 3073–3085. [DOI] [PubMed] [Google Scholar]
- Lethbridge RC (1971) The hatching of Hymenolepis diminuta eggs and penetration of the hexacanths in Tenebrio molitor beetles. Parasitology 62, 445–456. [DOI] [PubMed] [Google Scholar]
- Meyling NV, Arthur S, Pedersen KE, Dhakal S, Cedergreen N and Fredensborg BL (2018) Implications of sequence and timing of exposure for synergy between the pyrethroid insecticide alpha-cypermethrin and the entomopathogenic fungus Beauveria bassiana. Pest Management Science 74, 2488–2495. [DOI] [PubMed] [Google Scholar]
- Müller T, Prosche A and Müller C (2017) Sublethal insecticide exposure affects reproduction, chemical phenotype as well as offspring development and antennae symmetry of a leaf beetle. Environmental Pollution 230, 709–717. [DOI] [PubMed] [Google Scholar]
- O'Neal ST, Anderson TD and Wu-Smart JY (2018) Interactions between pesticides and pathogen susceptibility in honey bees. Current Opinion in Insect Science 26, 57–62. [DOI] [PubMed] [Google Scholar]
- Pettis JS, vanEngelsdorp D, Johnson J and Dively G (2012) Pesticide exposure in honey bees results in increased levels of the gut pathogen Nosema. Naturwissenschaften 99, 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel D (2009) Environmental and economic costs of the application of pesticides primarily in the United States. In Peshin R, Dhawan AK (eds), Integrated Pest Management: Innovation-Development Process: Volume 1. Dordrecht: Springer Netherlands, pp. 89–111. [Google Scholar]
- Pochini KM and Hoverman JT (2017) Reciprocal effects of pesticides and pathogens on amphibian hosts: the importance of exposure order and timing. Environmental Pollution 221, 359–366. [DOI] [PubMed] [Google Scholar]
- Ratnieks FLW and Carreck NL (2010) Clarity on honey bee collapse? Science 327, 152. [DOI] [PubMed] [Google Scholar]
- Raymann K, Motta EVS, Girard C, Riddington IM, Dinser JA and Moran NA (2018) Imidacloprid decreases honey bee survival rates but does not affect the gut microbiome. Applied and Environmental Microbiology 84, e00545–e00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr JR, Raffel TR, Sessions SK and Hudson PJ (2008) Understanding the net effects of pesticides on amphibian trematode infections. Ecological Applications 18, 1743–1753. [DOI] [PubMed] [Google Scholar]
- Schmid-Hempel P (2005) Evolutionary ecology of insect immune defenses. Annual Reiview of Entomology 50, 529–551. [DOI] [PubMed] [Google Scholar]
- Seid AM, Fredensborg BL, Steinwender BM and Meyling NV (2019) Temperature-dependent germination, growth and co-infection of Beauveria spp. isolates from different climatic regions. Biocontrol Science and Technology 29, 411–426. [Google Scholar]
- Shostak AW (2012) Sequential and concurrent exposure of flour beetles (Tribolium confusum) to tapeworms (Hymenolepis diminuta) and pesticide (diatomaceous earth). Journal of Parasitology 98, 453–459. [DOI] [PubMed] [Google Scholar]
- Shostak AW (2014) Hymenolepis diminuta infections in Tenebrionid beetles as a model system for ecological interactions between helminth parasites and terrestrial intermediate hosts: a review and meta-analysis. Journal of Parasitology 100, 46–58. [DOI] [PubMed] [Google Scholar]
- Shostak AW, Van Buuren KG and Cook R (2015) Response of flour beetles to multiple stressors of parasitic (Hymenolepis diminuta), environmental (Diatomaceous Earth), and host (reproduction) origin. The Journal of Parasitology 101, 405–417. [DOI] [PubMed] [Google Scholar]
- Soderlund DM (2010) Toxicology and mode of action of pyrethroid insecticides. In Krieger R (ed.), Hayes' Handbook of Pesticide Toxicology, 3rd Edn. New York: Academic Press, pp. 1665–1686. [Google Scholar]