Abstract
Background
The accurate and rapid identification of mosquito blood meals is critical to study the interactions between vectors and vertebrate hosts and, subsequently, to develop vector control strategies. Recently, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) profiling has been shown to be a reliable and effective tool for identifying single blood meals from mosquitoes.
Methods
In this study, we developed MALDI-TOF MS profiling protocols to identify Anopheles gambiae Giles, Anopheles coluzzii and Aedes albopictus mosquitoes’ mixed blood meals and the last of successive blood meals. The mosquitoes were either successively artificially fed with distinct host bloods or engorged with mixed bloods from distinct vertebrate hosts, such as humans, sheep and dogs.
Results
Blind test analyses revealed a correct identification of mixed blood meals from mosquitoes using MALDI-TOF MS profiling. The 353 MS spectra from mixed blood meals were identified using log score values >1.8. All MS spectra (n = 244) obtained from mosquitoes' successive blood meals were reproducible and specific to the last blood meal, suggesting that the previous blood meals do not have an impact on the identification of the last one.
Conclusion
MALDI-TOF MS profiling approach appears to be an effective and robust technique to identify the last and mixed blood meals during medical entomological surveys.
Key words: Aedes, Anopheles, blood meals, entomological survey, MALDI-TOF MS profiling
Introduction
Mosquitoes are the major arthropod vectors of human infectious diseases across the world (Becker et al., 2010). Aedes mosquitoes are able to transmit arboviral diseases including Yellow Fever, Dengue, Chikungunya and Zika viruses (Gardner and Ryman, 2010; Vasilakis et al., 2011; Caglioti et al., 2013; Gould et al., 2017). As for Anopheles mosquitoes, they include the main vectors of malaria (Carnevale et al., 2009). Malaria is the primary cause of morbidity and mortality in Africa, mainly amongst the children under the age of 5 and pregnant women (WHO, 2016). There is still no effective vaccine for several mosquito-borne diseases; entomological surveys and vector control measures are hence essential to combat them (Deilgat et al., 2014).
Mosquito vectors may feed on multiple hosts, including humans but also various domesticated or wild animals. This may result in successive or interrupted blood meals. A better understanding of the trophic preferences of mosquito vectors is an important factor to evaluate the risk of transmission of zoonotic diseases (Githeko et al., 1994; Shililu et al., 1998; Coulibaly et al., 2016; Ndenga et al., 2016). Studies on the interaction between Anopheles malaria vectors and their trophic preferences are critical to assess the risk of human exposure to Plasmodium parasites (Fyodorova et al., 2006). In Senegal, when the blood meals of 1886 freshly fed Anopheles gambiae Giles were studied, it was shown that most blood meals had been taken on a single host (40.1% on humans and 37.1% on animals). Mixed blood meals were mostly human–animal mix than non-human strictly (Ngom et al., 2013). In Kenya, mixed blood meals were shown to be predominant in culicine mosquitoes such as Culex quinquefasciatus. Up to 94% of the mosquitoes, according to the species, had fed on different hosts, including humans and Rift Valley Fever animal hosts (Muturi et al., 2008). Other reports highlighted that Anopheles mosquitoes fed both on humans and their domestic animals such as cattle and goats (Muriu et al., 2008). The identification of vertebrate hosts in mixed blood meals of mosquitoes may also be critical to adapt control strategies. For example, in 2016, an unusually high frequency of animal and mixed human–animal blood meals in the major malaria vector An. gambiae s.s. was revealed in the western Kenya Highlands. The authors suggested that this was linked with the vectors' response to increased bed net coverage in the area and the close location of livestock as people at night, opening the door for livestock-targeted interventions for malaria control (Ndenga et al., 2016).
Several methods are used to determine the origin of mosquito blood meals, including precipitin tests, counter-current immunoelectrophoresis and enzyme-linked immunosorbent assays (ELISA) (Fyodorova et al., 2006; Thomas et al., 2017). Serological tests are affordable and widely used but limited by the availability of antibodies for uncommon species (Beier et al., 1988) and samples that remain non-reactive (Mucci et al., 2015), for example.
Molecular approaches based on host DNA amplification can be highly specific (Prior and Torr, 2002; Kent and Norris, 2005; Kent, 2009; Munnoz et al., 2011; Logue et al., 2016; Ndenga et al., 2016), although they are curbed by their running costs, turnaround time and the comprehensiveness of online sequence databases such as NCBI GenBank (Oshaghi et al., 2006; Egizi et al., 2013).
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) is a protein profiling-based technique that uses a laser energy absorbing matrix to generate a protein spectrum from an organism of interest (Seng et al., 2010). It has revolutionized clinical microbiology by making it possible to rapidly identify bacteria at a low cost (Seng et al., 2009, 2013; Schubert and Kostrzewa, 2017). In recent years, the effectiveness of MALDI-TOF MS for identifying arthropods (Tandina et al., 2016, 2018a; Yssouf et al., 2016; Diarra et al., 2017; Laroche et al., 2017b; Halada et al., 2018; Mewara et al., 2018) and mosquito blood meals identification (Niare et al. 2016, 2017b; Tandina et al., 2018a,b) has been reported. Indeed, in preliminary studies, MALDI-TOF MS was able to identify the host blood source from An. gambiae Giles and Aedes albopictus mosquitoes artificially fed on human, horse, sheep, rabbit, mouse, rat and dog blood up to 24 h post-feeding (Niare et al., 2016, 2017b; Tandina et al., 2018b).
The goal of the present study was to determine whether MALDI-TOF MS could be extended to the identification of mixed and successive blood meals.
Anopheles gambiae Giles, Anopheles coluzzii and Ae. albopictus mosquitoes were artificially engorged on human blood and subsequently fed on the blood of other vertebrates after complete digestion of the first meal. For the identification of mixed blood meals by MALDI-TOF MS, the mosquitoes were engorged with blood mixed at different concentrations from different vertebrate hosts to mimic interrupted blood meals.
Materials and methods
Ethical statement
Animal studies were conducted in line with Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010, and in compliance with French Government Decree No. 2013-118 of 1 February 2013. The study was approved by the local Animal Ethics Committee (Comité d'Ethique en Expérimentation Animale, Marseille) and the Institutional Animal Care Committee in Marseille, France. Human blood was obtained from the national French blood bank, the ‘Etablissement Français du Sang’ (EFS) accredited by the Institutional Animal Care Committee of IHU Méditerranée Infection.
Mosquito rearing and maintenance
Anopheles gambiae Giles, An. coluzzii and Ae. albopictus mosquitoes were reared in our laboratory (Marseille, France) using standard methods at a temperature of 26 ± 1°C, a relative humidity of 80 ± 10% and a 12 h photoperiod in incubators (Panasonic cooled incubator) (Awono-Ambene et al., 2001). Anopheles gambiae Giles and An. coluzzii colonies were established from laboratory colonies from the ‘Institut de Recherche pour le Développement’ (IRD, Montpellier, France) and IRD Dakar, Senegal, respectively. Aedes albopictus mosquitoes were originally sampled at the larval stage in a pond in Marseille, France in 2011 and reared in our laboratory.
Larvae were reared until the pupal stage in trays containing 1 L of distilled water supplemented with fish food (TetraMinBaby, Tetra Gmbh, Herrenteich, Germany). Pupae were collected daily and transferred into a mosquito cage (Bug Dorm 1 insect rearing cage, BioQuip products, Taiwan). Adults were fed with a 10% glucose solution until the day of the experiment. Three days after emergence, female adult mosquitoes were artificially fed through a Parafilm-membrane (Hemotek membrane feeding systems, Discovery Workshops, UK) using fresh heparinized human blood for 2 h. Engorged female mosquitoes were transferred to another cage and were fed with a 10% glucose solution on cotton.
Successive blood meals
To assess the ability of MALDI-TOF MS to identify the last blood meal taken, 60 An. gambiae Giles, 60 An. coluzzii and 124 Ae. albopictus were firstly engorged on human blood. Only one host donor was used per species to limit intraspecific variability. Twelve hours after feeding, 10 Anopheles and 20 Aedes abdomens were submitted to MALDI-TOF MS analysis, while, for each mosquito species, the remaining specimens were dispatched equally in six cages. Three days after the first blood meal (after complete digestion), the mosquitoes from each cage were engorged for a second time on six distinct types of vertebrate blood (goat, cow, chicken, dog, sheep and rabbit). The abdomens of all fully engorged mosquitoes were submitted to MALDI-TOF MS analysis 12 h after feeding.
Mixed blood meals
To determine the ability of MALDI-TOF MS to identify mixed blood meals from mosquito abdomens, the mosquitoes were fed on human blood mixed with dog blood or sheep blood at ratios of 75/25%, 50/50% and 25/75% (Table 1). To prevent the coagulation of blood mixtures, the various mixtures of blood were previously heated at 56°C in a dry bath for 30 min, as previously described (Nossel and Niemetz, 1965). Only one host donor was used per species to limit intraspecific variability.
Table 1.
Number of mosquito samples submitted to MS analysis
| Concentration | Samples | Anopheles gambiae | Anopheles coluzzii | Aedes albopictus | Total | |
|---|---|---|---|---|---|---|
| Mixed blood meals Human–Dog | 75%Human/25%Dog | Number of samples | 14 | 10 | 23 | 47 |
| Number of specimens for DB upgrading | / | 1 | 2 | 3 | ||
| 50%Human/50%Dog | Number of samples | 14 | 10 | 23 | 47 | |
| Number of specimens for DB upgrading | 4 | 1 | 3 | 8 | ||
| 25%Human/75%Dog | Number of samples | 14 | 10 | 23 | 47 | |
| Number of specimens for DB upgrading | / | 1 | 2 | 3 | ||
| Mixed blood meals Human–Sheep | 75%Human/25%Sheep | Number of samples | 4 | 10 | 22 | 36 |
| Number of specimens for DB upgrading | / | 2 | 2 | 4 | ||
| 50%Human/50%Sheep | Number of samples | 5 | 14 | 23 | 42 | |
| Number of specimens for DB upgrading | 5 | 2 | 3 | 10 | ||
| 25%Human/75%Sheep | Number of samples | 4 | 13 | 23 | 40 | |
| Number of specimens for DB upgrading | / | 2 | 2 | 4 | ||
| Mixed blood meals Human–Dog–sheep | Equal concentration | Number of samples | 20 | 10 | 23 | 53 |
| Number of specimens for DB upgrading | 5 | 1 | 3 | 9 | ||
| Total | / | / | 89 | 87 | 177 | 353 |
The mosquitoes' abdomens were collected 12 h after feeding and then submitted to MALDI-TOF-MS analysis. Human, sheep and dog blood was mixed at equal concentrations and given to 25 An. gambiae Giles, 10 An. coluzzii and 23 Ae. albopictus (Table 1).
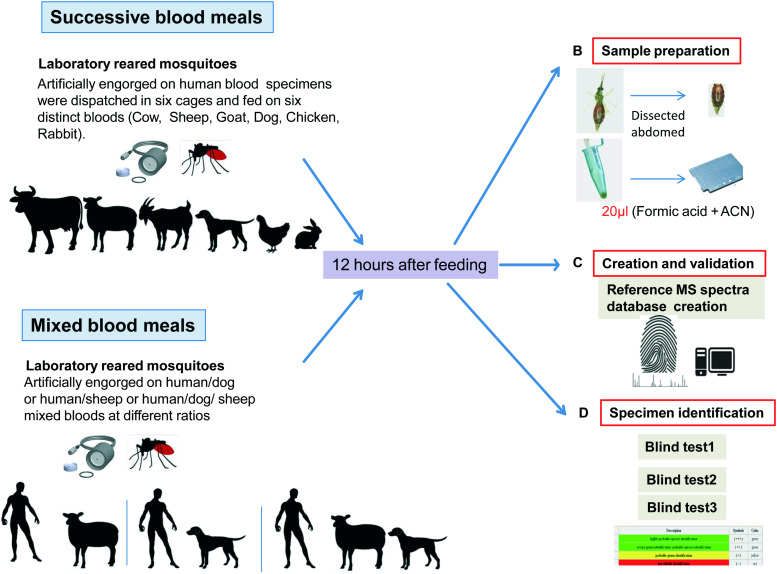
A flowchart illustrating the main steps in the experimental workflow for the arthropod spectra database creation and blood meal identification by MALDI-TOF MS is presented in Fig. 1.
Fig. 1.
Experimental workflow for mixed and successive blood meal identification using MALDI-TOF MS.
Sample preparation for MALDI-TOF MS analysis
Only fully engorged mosquitoes were included in the MS analysis. Engorged females were killed at −20°C prior to dissection. Each mosquito abdomen was separated from the thorax using a sterile scalpel for each sample. The dissected abdomens were manually crushed using sterile pestles (Fischer Scientific, Strasbourg, France) in a 1.5 mL tube containing 50 μL of HPLC-grade water. Ten microlitres of the supernatant of the crushed abdomens were homogenized in 20 μL of 70% formic acid and 20 μL of 50% acetonitrile (Fluka, Buchs, Switzerland) and centrifuged at 10 000 rpm for 20 s. One microlitre of supernatant of each sample was deposited on the MALDI-TOF target plate in quadruplicate (Bruker Daltonics, Wissembourg, France) and covered with 1 μL of CHCA matrix solution composed of saturated α-cyano-4-hydroxycynnamic acid (Sigma, Lyon, France), 50% acetonitrile (v/v), 2.5% trifluoroacetic acid (v/v) (Sigma) and HPLC-grade water. After drying for several minutes at room temperature, the target was introduced into the Microflex LT MALDI-TOF Mass Spectrometer (Bruker Daltonics, Germany) for analysis. To control loading on mass spectra steel, matrix quality and MALDI-TOF MS apparatus performance, the matrix solution was loaded in duplicate onto each MALDI-TOF MS plate with or without a bacterial test standard (Bruker protein Calibration Standard I), as previously described (Niare et al., 2016).
MALDI-TOF MS parameters
Protein mass profiles were acquired using a Microflex LT MALDI-TOF Mass Spectrometer, with detection in the linear positive-ion mode at a laser frequency of 50 Hz within a mass range of 2–20 kDa. The acceleration voltage was 20 kV, and the extraction delay time was 200 ns. Each spectrum corresponded to ions obtained from 240 laser shots performed in six regions of the same spot and automatically acquired using the AutoXecute function of the Flex Control v.2.4 software (Bruker Daltonics, Germany). The spectrum profiles obtained were visualized using Flex analysis v.3.3 software and exported to ClinProTools version v.2.2 (Bruker Daltonics, Germany) and MALDI-Biotyper v.3.0. (Bruker Daltonics, Germany) for data processing (smoothing, baseline subtraction and peak picking) and evaluation with cluster analysis.
MALDI-TOF MS spectra analyses
The reproducibility and specificity of MALDI-TOF MS spectra from the abdomens of mosquitoes which had taken successive or mixed blood meals were evaluated by comparing the four spectra of each sample tested using the Flex Analysis and ClinProTools 2.2 software. The gel view tool of ClinProTools 2.2 software was used to control intraspecific reproducibility. As a control, 1 μL of host blood was mixed with 20 μL of 70% formic acid and 20 μL of 50% acetonitrile and deposited on the MALDI-TOF target plate in quadruplicate and covered with 1 μL of CHCA matrix solution.
The spectra from An. gambiae Giles mixed blood meals of human/dog blood (n = 4), human/sheep blood (n = 5) and the triple mixture of human/dog/sheep blood (n = 5) were selected to upgrade our arthropod spectra database (Table 1). As for Ae. albopictus, the reference spectra of human/dog blood (n = 7), human/sheep blood (n = 7) and the triple mixture of human/dog/sheep blood (n = 3), and An. coluzzii the reference spectra of human/dog blood (n = 3), human/sheep blood (n = 6) and the triple mixture of human/dog/sheep blood (n = 1) were selected to upgrade the database (Table 1). All these 41 spectra were selected for the database on the basis of their high intensity, reproducibility and global spectrum quality. Details of all spectra selected to upgrade the database are presented in Table 1. This database includes the spectra obtained from various arthropod species and mosquitoes' abdomens fed on 25 distinct host bloods (Homo sapiens, Equus caballus, Ovis aries, rabbit, Balb/C mouse, Rattus norvegicus, Canis familiaris, Bos taurus, Capra hircus, Gallus gallus, Equus asinus, Tapirus indicus, Tapirus terrestris, Carollia perspicillata, Thraupis episcopus, Erythrocebus patas, Callithrix pygmaea, Leopardus pardalis, Arctictis binturong, Antidorcas marsupialus, Panthera onca, Papio hamadryas, Camelus dromedarius, Ammotragus lervia and Sus scrofa) (Additional file: Table S1).
Main Spectrum Profiles (MSPs) were automatically created from the four spectra per sample based on an algorithm using the information on peak position, intensity and frequency from the BioTyper MSP Creation Standard Method. The maximum mass error of each single spectrum was 2000 Da, and the desired mass error for the MSP was 200 Da.
Blind tests
Blind test 1
The abdomen-derived spectra from An. gambiae Giles which had taken successive blood meals were submitted for a blind test analysis. Similarly, the spectra of the abdomens of the mosquitoes fed with mixed blood meals (without those selected to be entered in the MS database) were subjected to a blind test analysis against the database (DB1) (Table 1). The results are presented in the MALDI-Biotyper software v.3.3. as log score values (LSVs) that consists of the degree of signal intensity matching between the queried mass spectra and the reference spectra. LSVs range from 0 to 3. LSVs allow a good assessment of the reproducibility between a queried spectrum and a reference spectrum, as they are the result of the thorough comparison of peak positions and intensity between those two spectra (MALDI BioTyper Help, Bruker). An LSV was obtained for each spectrum of the blind tested samples. The samples were considered to be correctly and significantly identified when the spectrum queried had an LSV ≥1.8 (Laroche et al., 2017a; Niare et al., 2016).
Blind test 2
MS spectra obtained from engorged abdomens of Ae. albopictus and An. coluzzii after successive and mixed blood meals were queried against the arthropod spectra database upgraded with reference spectra of An. gambiae Giles (DB1). Aedes albopictus and An. coluzzii reference spectra (Table 1) for successive and mixed blood meals were then added to DB1 to create DB2 (Additional file: Table S2).
Blind test 3
The spectra of the abdomens of the Ae. albopictus and An. coluzzii fed with mixed blood meals (with the exception of those selected to be entered in the database) were tested against DB2. An LSV was obtained for each of the four spots of each specimen tested. A flowchart of blind test analyses is available as supplementary data (Additional file: S3)
Results
Successive blood meals
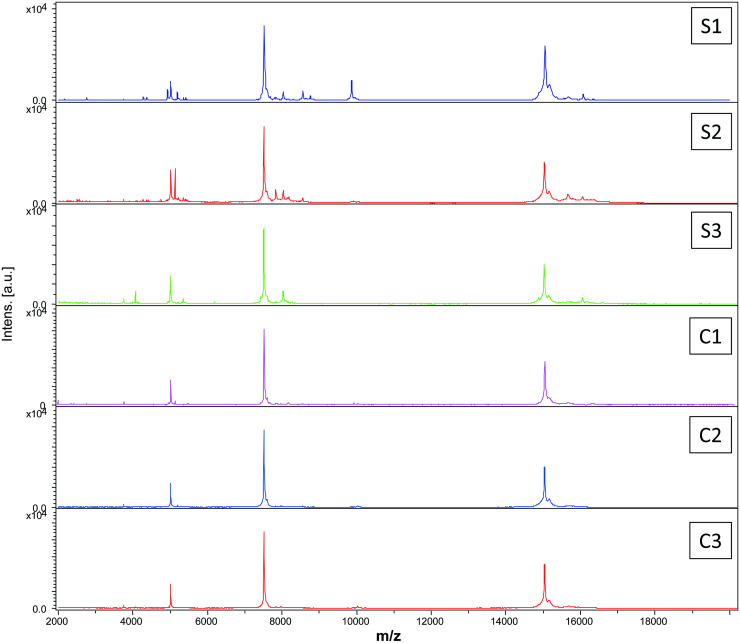
Comparison of the MS spectra from An. gambiae Giles (n = 60), Ae. albopictus (n = 124) and An. coluzzii (n = 60) abdomens after successive blood meals using the Flex Analysis software indicated a high quality (Fig. 2) and reproducibility of the spectra, allowing the inclusion of all spectra in the study. Querying An. gambiae Giles against DB1 (Additional file: Table S1) after successive blood meals, showed reliable and 100% accurate identification of the last blood meal (n = 60) (Table 2). The LSVs for the specimens ranged between 1.826 and 2.764. According to preliminary studies, the samples are considered to be correctly and significantly identified when the spectrum queried had an LSV ≥1.8 (Niare et al., 2016; Laroche et al., 2017a). The spectra from the engorged abdomens of Ae. albopictus and An. coluzzii were then queried against DB1. We observed 80% correct identification with a mean score of 2.242. When tested against DB2, 100% of the blood meals were correctly identified, with a mean score of 2.593 (Table 3). The LSVs for the specimens ranged between 1.855 and 2.873.
Fig. 2.
Representative abdomen-derived MALDI-TOF MS spectra from Anopheles gambiae Giles, Anopheles coluzzii and Aedes albopictus fed on sheep (S1,S2,S3) and cow (C1,C2,C3). All mosquitoes were collected 12 h post-feeding. Au = arbitrary units; m/z = mass-to-charge ratio.
Table 2.
MALDI-TOF MS identification of An. gambiae successive blood meals
| Mosquito species | First blood meal | Second blood meal | Number of specimens used for blind test | Results of MS identification [range LSVs] |
|---|---|---|---|---|
| An. gambiae | Human | / | 10 | Human [1.834–2.320] |
| An. gambiae | Human | Goat | 10 | Goat [2.173–2.670] |
| An. gambiae | Human | Dog | 10 | Dog [2.139–2.764] |
| An. gambiae | Human | Cow | 10 | Cow [1.810–2.396] |
| An. gambiae | Human | Sheep | 10 | Sheep [1.802–2.296] |
| An. gambiae | Human | Rabbit | 10 | Rabbit [1.826–2.101] |
| Total | / | / | 60 |
All mosquitos were firstly engorged on human blood and then fed on goat, dog, cow, sheep and rabbit blood.
Table 3.
MALDI-TOF MS identification of successive blood meals of Aedes albopictus and Anopheles coluzzii
| Mosquito species | First blood meal | Second blood meal | Identification of blood origin before upgrade (DB 1) | LSVs obtained before upgrade | Number of specimens used for DB upgrade | Identification of blood origin after upgrade (DB2) | LSVs obtained after upgrade |
|---|---|---|---|---|---|---|---|
| Ae. albopictus | Human | / | Ae. albopictus Human (22) | [2.148–2.602] | / | Ae. albopictus-Human(22) | [2.148–2.602] |
| Ae. albopictus | Human | Cow | An. gambiae-Cow (12) | [1.889–2.387] | 3 | Ae. albopictus-Cow(17) | [1.941–2.873] |
| An. coluzzii-Mouflon (8) | [2.225–2.645] | ||||||
| Ae. albopictus | Human | Sheep | An. gambiae-Sheep(2) | [1.714–1.745] | 3 | Ae. albopictus-Sheep(17) | [1.972–2.754] |
| An. coluzzii-Mouflon(18) | [1.749–2.719] | ||||||
| Ae. albopictus | Human | Goat | An. gambiae-Goat(13) | [1.762–1.961] | 3 | Ae. albopictus-Goat(17) | [2.206–2.752] |
| An. coluzzii-Mouflon(5) | [2.085–2.492] | ||||||
| Insufficient(2) | [1.549–1.696] | ||||||
| Ae. albopictus | Human | Chicken | An. gambiae-Chicken (20) | [1.894–2.294] | 2 | Ae. albopictus-Chicken(18) | [2.117–2.636] |
| Ae. albopictus | Human | Rabbit | An. gambiae-Rabbit(21) | [1.429–1.843] | 3 | Ae. albopictus-Rabbit(19) | [2.196–2.535] |
| An. gambiae-Ocelot(1) | [1.767] | ||||||
| An.cColuzzii | Human | / | An. gambiae-Human(10) | [1.964–2.421] | 1 | An. coluzzii-human(9) | [2.217–2.421] |
| An. coluzzii | Human | Cow | An. gambiae-Cow (10) | [1.648–2.543] | 1 | An. coluzzii-Cow(9) | [2.125–2.543] |
| An. coluzzii | Human | Sheep | An. gambiae-Sheep(10) | [1.836–2.584] | 1 | An. coluzzii-Sheep(9) | [1.855–2.584] |
| An. coluzzii | Human | Goat | An. gambiae-Goat (10) | [1.637–1.943] | 1 | An. coluzzii-Goat(9) | [2.014–2.239] |
| An. coluzzii | Human | Chicken | An. gambiae-Chicken(4) | [1.236–1.38] | 1 | An. coluzzii-Chicken(9) | [1.948–2.819] |
| An. gambiae-Ocelot(4) | [1.286–1.373] | ||||||
| An. gambiae-Cow(1) | [1.467] | ||||||
| An. gambiae-Goat(1) | [1.341] | ||||||
| An. coluzzii | Human | Rabbit | An. gambiae-Rabbit(10) | [1.581–1.851] | 1 | An. coluzzii-Rabbit(9) | [2.120–2.643] |
| Total | 184 | 80% | 20 | 164 | 100% |
(x)Number of specimens included in each analysis.
DB, database
Mixed blood meal identification by MALDI-TOF MS
Regarding the human/dog and human/sheep blood mixtures, a correct identification was observed for all 25 An. gambiae Giles mosquitoes that fed on mixed blood meals, with LSVs ranging from 2.055 to 2.771 (Table 4).
Table 4.
MALDI-TOF MS identification of Anopheles gambiae Giles mixed blood meals
| Mosquito species | Concentration of blood meal | Number of Specimens for 1st BT | Results of MS identification before DB upgrade [scores] | Number of specimens used for DB upgrade | Results of MS identification after upgrade (DB 1) [scores] |
|---|---|---|---|---|---|
| Anopheles gambiae | 75%Human/25%Dog | 14 | 14/14 Human [1.819–2.23] | / | 14/14 Human/Dog [2.055–2.529] |
| 50%Human/50%Dog | 14 | 9 Human + 5 Dog [1.828–2.004] | 4 | 10/10 Human/Dog [2.115–2.477] | |
| 25%Human/75%Dog | 14 | 10/10 Dog [1.615–2.017] | / | 14/14 Human/Dog [2.161–2.614] | |
| 75%Human/25%Sheep | 4 | 3 Human + 1 Sheep [1.897–2.047] | / | 4/4 Human/Sheep [2.399–2.493] | |
| 50%Human/50%Sheep | 5 | 5/5 Sheep [1.146–2.216] | 5 | N/A♦ | |
| 25%Human/75%Sheep | 4 | 4/4 Sheep [2.09–2.372] | / | 4/4 Human/Sheep [2.405–2.771] | |
| Human/Dog/Sheep Equal concentration | 20 | 6 Human + 4 Human/Dog + 10 Human Sheep [1.791–2.38] | 5 | 15/15 Human/Dog/Sheep [2.258–2.846] | |
| Total | 75 | 14 | 61/61 correct identifications |
DB, database; BT, blind test.
♦No spectra blind tested because all were used as reference.
The query of spectra from engorged abdomens of Ae. albopictus and An. coluzzii against DB1 containing reference spectra of engorged An. gambiae Giles only revealed 51.51% and 51.42% correct identification of blood mixtures from human/dog and human/sheep blood, respectively. After upgrading the database to DB2, we obtained accurate identification for 100% of the blood meals, with LSVs ranging from 1.762 to 3 (Table 5 for human/dog mixed blood meals, Table 6 for human/sheep mixed blood meals).
Table 5.
Influence of mosquito species on MALDI-TOF MS identification of Aedes albopictus and Anopheles coluzzii fed on human/dog mixed blood meals human/dog mixed blood meals
| Mosquito species | Concentration of blood meal | Results of MS identification before upgrade (DB 1) | LSVs obtained before upgrade | Number of specimens used for DB upgrade | Results of MS identification after upgrade (DB 2) | LSVs obtained after upgrade |
|---|---|---|---|---|---|---|
| Ae. albopictus | 75%Human/25%Dog | An. gambiae-Human–Dog (2) | [1.739–1.763] | 2 | Ae. albopictus-Human–Dog(21) | [1.762–2.665] |
| Ae. albopictus-Human(20) | [1.700–2.140] | |||||
| Ae. albopictus (1) | [1.702] | |||||
| Ae. albopictus | 50%Human/50%Dog | An. gambiae-Human–Dog (21) | [1.686–2.006] | 3 | Ae. albopictus-Human–Dog(20) | [1.945–2.428] |
| An. gambiae-Human–Dog–Sheep (2) | [1.746–1.853] | |||||
| Ae. albopictus | 25%Human/75%Dog | An. gambiae-Human–Dog (13) | [1.736–2.269] | 2 | Ae. albopictus-Human–Dog(21) | [2.067–2.724] |
| An. gambiae-Human–Dog–Sheep (5) | [1.899–2.136] | |||||
| Ae. albopictus-Human(5) | [2.039–2.156] | |||||
| An. coluzzii | 75%Human/25%Dog | Ae .albopictus-Human–Dog(4) | [1.739–1.929] | 1 | An. coluzzii-Human–Dog(9) | [1.948–2.932] |
| Ae. albopictus-Human(5) | [1.927–2.345] | |||||
| An. coluzzii-Human (1) | [2.027] | |||||
| An. coluzzii | 50%Human/50%Dog | Ae. albopictus-Human–Dog(4) | [1.810–1.964] | 1 | An. coluzzii-Human–Dog(9) | [1.928–2.722] |
| An. coluzzii-Human(4) | [1.876–2.264] | |||||
| An. gambiae-Human(2) | [2.036–2.215] | |||||
| An. coluzzii | 25%Human/75%Dog | Ae. albopictus-Human–Dog(8) | [1.722–2.216] | 1 | An. coluzzii-Human–Dog(9) | [2.111–2.835] |
| An. gambiae-Human–Dog–Sheep(2) | [1.860–1.904] | |||||
| Total | 99 | 51.51% | 10 | 89 | 100% |
(x)Number of specimens used for blind test analysis.
DB, database.
Table 6.
Influence of mosquito species on MALDI-TOF MS identification of Aedes albopictus and Anopheles coluzzii fed on human/sheep mixed blood meals
| Mosquito species | Concentration of blood meal | Results of MS identification before upgrade (DB 1) | LSVs obtained before upgrade | Number of specimens used for DB upgrade | Results of MS identification after upgrade (DB 2) | LSVs obtained after upgrade |
|---|---|---|---|---|---|---|
| Ae. albopictus | 75%Human/25%Sheep | An. gambiae-Human–Sheep (18) | [1.716–1.991] | 2 | Ae. albopictus-Human–Sheep(20) | [2.197–2.482] |
| Ae. albopictus-Human (1) | [1.68] | |||||
| An. gambiae-Human–Dog–Sheep(3) | [1.837–1.864] | |||||
| Ae. albopictus | 50%Human/50%Sheep | An. gambiae-Human–Sheep (22) | [1.96–2.4] | 3 | Ae. albopictus-Human–Sheep(20) | [2.285–2.445] |
| An. gambiae-Sheep (1) | [1.922] | |||||
| Ae. albopictus | 25%Human/75%Sheep | An. gambiae-Human–Sheep (1) | [1.975] | 2 | Ae. albopictus-Human–Sheep(21) | [2.323−2.567] |
| An. coluzzii-Sheep (20) | [1.637–1.958] | |||||
| Ae. albopictus-cow (1) | [2.319] | |||||
| An. coluzzii-mouflon(1) | [1.707] | |||||
| An. coluzzii | 75%Human/25%Sheep | An. coluzzii-Human(1) | [2.096] | 2 | An. coluzzii-Human–Sheep (8) | [2.134–3.000] |
| Aedes albopictus-Human(7) | [2.020–2.272] | |||||
| Aedes albopictus-Cow(2) | [2.168–2.193] | |||||
| An. coluzzii | 50%Human/50%Sheep | An. gambiae-Human–Sheep(3) | [1.855–1.969] | 2 | An. coluzzii-Human–Sheep(12) | [2.312–2.608] |
| Ae. albopictus-Human–Sheep(7) | [1.805–1.993] | |||||
| An. coluzzii-Sheep(1) | [1.826] | |||||
| Ae. albopictus-Sheep(2) | [2.084–2.088] | |||||
| An. gambiae-Human–Dog–Sheep(1) | [2.048] | |||||
| An. coluzzii | 25%Human/75%Sheep | Ae. albopictus-Human–Sheep(3) | [1.736–1.748] | 2 | An. coluzzii-Human–Sheep(11) | [2.222–2.475] |
| An. coluzzii-Sheep(4) | [1.689–1.932] | |||||
| An. coluzzii-Mouflon(2) | [1.714–2.084] | |||||
| Ae. albopictus-Sheep(3) | [1.770–2.215] | |||||
| Ae. albopictus-Cow (1) | [2.146] | |||||
| Total | 105 | 51.42% | 13 | 92 | 100% |
(x)Number of specimens used for blind test analysis.
Finally, the 20 An. gambiae which had been fed with a triple mixture of blood (human/dog/sheep) were subjected to MS analyses. Their abdomens provided good quality spectra and the following blind test led to unambiguous, accurate identification with LSVs ranging from 2.258 to 2.846 (Table 4). Aedes albopictus and An. coluzzii mosquitoes fed with the same triple mixture revealed 36.36% correct identification when blind tested against DB1. After upgrading the database, we observed 100% correct identification of the blood meals with LSVs ranging from 2.070 to 2.725 in the blind test against DB2 (Table 7).
Table 7.
Influence of mosquito species on MALDI-TOF MS identification of Aedes albopictus and Anopheles coluzzii triple blood meals
| Mosquito species | Concentration of blood meal | Results of MS identification before upgrade (DB 1) | LSVs obtained before upgrade | Number of specimens used for DB upgrade | Results of MS identification after upgrade (DB 2) | LSVs obtained after upgrade |
|---|---|---|---|---|---|---|
| Ae. albopictus | Human/Dog/Sheep Equal concentration | An. gambiae-Human–Dog–Sheep (2) | [1.85–2.301] | 3 | Ae. albopictus-Human–Dog–Sheep(20) | [2.070–2.421] |
| An. gambiae-Human–Sheep (21) | [1.875–2.49] | |||||
| An. coluzzii | Human/Dog/Sheep Equal concentration | An. gambiae-Human–Dog–Sheep (10) | [1.743–2.303] | 1 | An. coluzzii-Human–Dog–Sheep (9) | [2.391–2.725] |
| Total | 33 | 36.36% | 4 | 29 | 100% |
(x)Number of specimens used for blind test analysis.
DB, database.
Discussion
MALDI-TOF MS consists of three major steps. Firstly, the organism of interest is mixed with a suitable matrix material and applied to a metal plate. Secondly, the co-crystallized sample is irradiated with laser pulses, effecting desorption and ‘soft’ ionization of the organism. Thirdly, molecules are accelerated in an electric field and separated through a flight tube in linear or reflectron mode, according to their mass-to-charge ratio. The MS protein profiles obtained are used to discriminate between each specimen tested (Yssouf et al., 2016).
MALDI-TOF MS has been shown to be reliable for the identification of single blood meals both in laboratory and field conditions (Tandina et al., 2018a; Tandina et al., 2018b). The purpose of the present study was to artificially mimic what happens in the field when a blood meal is completed on another animal host before (mixed blood meal design) or after (successive blood meals design) digestion of the first meal. Livestock and domestic animals were included in the study as they can be the source of several vector-borne pathogens such as Rift Valley Fever (RVF) (Linthicum et al., 2016), Crimean-Congo haemorrhagic virus (CCHV) (Bente et al., 2013), ehrlichiosis (Unver et al., 2001) and tularaemia for rabbits (Petersen et al., 2009). In this proof-of-concept, only mosquitoes were included but further study is conceivable to assess the ability of MALDI-TOF MS to successfully identify the blood meals of other arthropods of medical and veterinary interest.
MALDI-TOF MS was also able to successfully identify mosquito blood meals up to 24 h post feeding using engorged abdomens (Niare et al., 2016; Tandina et al., 2018b). This time point may limit MS identification but Sella's index allows selection of fully engorged mosquitoes, which can be numerous when caught in houses in the morning or in early-collected traps (Tandina et al., 2018a). The quality of the spectra is stable until blood digestion begins. Spectra from freshly but partially engorged mosquitoes should be identifiable too using MALDI-TOF MS but may not be stable up to 24 h.
Artificial feeding laboratory conditions will probably never mimic the real conditions of mosquitoes feeding in the field or the reality of variable stages of blood digestion at the time of mosquito sampling. However, the MALDI-TOF MS approach was previously validated on field samples collected in Comoros and Mali (Niare et al., 2017a; Tandina et al., 2018a).
The 24 h limit for relevant identification can be circumvented by picking up the mosquito traps early. The blood meals were correctly identified from mosquitoes' abdomens crushed on Whatman filter papers (WFP) by MALDI-TOF MS (Niare et al., 2017a; Tandina et al., 2018a).
However, some studies have shown that mixed or multiple blood meals can amount to up to 10% of the mosquitoes in the field (Logue et al., 2016; Moreno et al., 2017).
This is considered significant in terms of influencing the transmission of pathogens and maintaining residual infectious diseases. For the identification of mixed blood meals using a proteomic approach, Önder et al. developed an experimental design using blacklegged ticks (Ixodes scapularis) (Onder et al., 2013). Their approach successfully identified mixed blood meals from two vertebrates, depending on the concentration ratios. As for the identification of mixed bacterial species, Lin Zhang et al. reported that the major peaks in multi-bacterial samples could be assigned to those found in the respective spectra of each bacterium (Zhang et al., 2015).
In our study, the last animal blood meal was correctly and unambiguously identified, although mosquitoes had firstly fed on human blood. This may be due to the interval of days (three) between the first and second blood meals of mosquitoes. The first blood meal was indeed digested by the mosquitoes. We obtained accurate identification with high LSVs (100%>1.8). This threshold is congruent with previously published studies on the use of MALDI-TOF MS to identify arthropods and their blood meals (Yssouf et al., 2016). Interestingly, for closely related mosquito species, such as An. gambiae Giles and An. coluzzii, the mosquito species had little influence on blood meal identification, especially for successive blood meals. Indeed, with the exception of chicken blood, all An. coluzzii mosquito blood meals were correctly identified using An. gambiae Giles reference spectra. This was, however, not the case for Ae. albopictus, for which the identification of the last blood meal was more random if the reference spectra of the engorged abdomen of the corresponding species were not present in the database. These data suggest that mosquito proteins can have an impact on the identification of the blood meal, although the impact appears to be low between species belonging to closely related genera.
For mixed blood meals, the spectra from Ae. albopictus and even An. coluzzii fed with human/dog and human/sheep blood mixtures, respectively, revealed 51.51% and 51.42% correct identification if the database only contains the reference spectra of the engorged abdomen of An. gambiae mosquitoes. This could be explained by the diversity of reference spectra needed for accurate identification of mixed blood meal identification. Greater identification was obtained for Aedes and An. coluzzii mosquitoes when reference spectra corresponding to several blood mix ratios were available in the database (data not shown). The only reference spectra available for An. gambiae mixed blood meals were obtained from mosquitoes which were fed 50/50 blood mixtures. This bias could explain the low percentages of identification for mixed blood meals. This probably means that a larger database may be needed to identify blood meals from several mosquito species. However, an increasing number of laboratories (Raharimalala et al., 2017; Halada et al., 2018; Mewara et al., 2018; Arfuso et al., 2019; Chavy et al., 2019; Rothen et al., 2016; Sambou et al., 2015; Yssouf et al., 2016) are applying MALDI-TOF to MS medical entomology and Raharimalala et al. have also highlighted the need for sharing and have expressed interest in an international database (Raharimalala et al., 2017). Many reference spectra are also available on request or directly online (Lawrence et al., 2019), substantially facilitating database creation. The MALDI-TOF mass spectrometry approach may also appear to be limited by the cost of the device but when the MALDI-TOF MS device is bought for clinical microbiology purposes, it can also be used for medical entomology at no additional cost. For example, in Senegal, Masse et al. have successfully identified the Culicoides spp. using protein-based MALDI-TOF MS, which is economically advantageous compared to molecular tools, some sequences of which are not available in Genbank (Sambou et al., 2015).
ELISA is a widely used approach that can be reliable for identifying frequently encountered hosts but much more challenging for wild hosts for which enzyme-labelled antibodies are not yet commercially available (Beier et al., 1988). In previous studies on Culicidae, up to 35% of the blood meals could not be identified using ELISA or were attributed to an unidentified mammal or bird (Apperson et al., 2002). As for molecular approaches, DNA prepared from the engorged abdomen not only can be used for blood meal identification but also can be used for arthropod identification or microorganism detection by PCR (Maleki-Ravasan et al., 2009).
The major advantages of using MALDI-TOF MS are its rapidity, effectiveness and reliability in identifying successive blood meals and mixed blood meals. In this study, only a small quantity of host blood was used for MALDI-TOF MS analysis, and the remaining material can be used for other studies, including the legs to identify mosquitoes at the species level (Yssouf et al., 2013; Nebbak et al., 2016).
In this study, we concluded that the species of mosquito has very little impact on blood meal identification if they are closely related. Anopheles coluzzi and An. gambiae Giles belong to the same complex, and are therefore not representative of the diversity of the Anopheles genus. More species of this genus should be included to draw conclusions regarding the impact of mosquito species on blood meal identification. Furthermore, as illustrated with Ae. albopictus mosquitoes in this study, identification of blood meals using a database created with a very different mosquito species can be unreliable. Nevertheless, the databases can be easily shared in order to circumvent this drawback.
Under an epidemiological perspective for the transmission of human pathogens, mosquito vectors should feed on a person or an animal and subsequently, on other human beings. In this preliminary study, we used a first human blood meal and the second blood meal on different vertebrate species (mostly domestic animals). These hosts were selected as some may act as reservoirs for zoonotic pathogens, and were available in our laboratory. To date, the performance of MALDI-TOF MS has not been determined yet for the distinction of human bloods, challenging the interpretation of MS identification of successive or mixed human blood meals.
Conclusion
Herein this paper, we provide more data to support the fact that MALDI-TOF MS appears as a rapid, reliable and cost-effective method for identifying An. gambiae Giles, An. coluzzii and Ae. albopictus blood meals. Correct identification was observed in all samples tested after the database was upgraded. Although MS profiling has been shown to be effective in this regard, our technique still needs to be tested on specimens collected in the field where feeding parameters are less controlled than in laboratory conditions. All spectra of the arthropod spectra database are available on request for other collaborative research teams.
Conflict of interest
The authors declare that they have no competing interests.
Ethical standards
Human blood was obtained from the national French blood bank, the ‘Etablissement Français du Sang’ (EFS) accredited by the human and animal ethics committees of the Institutional Animal Care Committee of IHU Méditerranée Infection. Consents for blood donation were collected at EFS. Animal studies were conducted in line with Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010, and in compliance with French Government Decree No. 2013-118 of 1 February 2013. The study was approved by the local Ethics Committee (Comité d'Ethique en Expérimentation Animale, Marseille) and the Institutional Animal Care Committee in Marseille, France.
Availability of data and materials
All datasets regarding this study are included in the main paper and the additional supporting files.
Financial support
This work has been carried out with the support of the Investissements d'Avenir programme of the French government, managed by the French National Research Agency, including ANR-11-IDEX-0001-02 (A*MIDEX project) and ANR-10-IAHU-03.
Author contributions
AL, PP and ML designed the experiments. FT and SN performed the experiments. FT, SN, ML and LA analysed the data. PP, OKD, BD and DR provided reagents/materials/analysis tools. FT, ML and NS wrote the paper. ML and PP coordinated the writing of the paper. All authors reviewed the final version.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S003118201900163X.
click here to view supplementary material
References
- Apperson CS, Harrison BA, Unnasch TR, Hassan HK, Irby WS, Savage HM, Aspen SE, Watson DW, Rueda LM, Engber BR and Nasci RS (2002) Host-feeding habits of Culex And other mosquitoes (Diptera: Culicidae) in the Borough of Queens in New York City, with characters and techniques for identification of Culex mosquitoes. Journal of Medical Entomology 39, 777–785. [DOI] [PubMed] [Google Scholar]
- Arfuso F, Gaglio G, Abbate JM, Caracappa G, Lupia A, Napoli E, Giarratana F, Latrofa MS, Giannetto S, Otranto D and Brianti E (2019) Identification of phlebotomine sand flies through MALDI-TOF mass spectrometry and in-house reference database. Acta Tropica 94, 47–52. [DOI] [PubMed] [Google Scholar]
- Awono-Ambene HP, Diawara L and Robert V (2001) Comparison of direct and membrane feeding methods to infect Anopheles arabiensis with Plasmodium falciparum. American Journal of Tropical Medicine and Hygiene 64, 32–34. [DOI] [PubMed] [Google Scholar]
- Becker N, Petriae D, Zgomba M, Baose C, Madon M and Kaiser A (2010) Mosquitoes and Their Control, 2nd edn, Heildelberg, Germany: Springer. [Google Scholar]
- Beier JC, Perkins PV, Wirtz RA, Koros J and Diggs D (1988) Gargan TP 2nd and Koech DK (1988) bloodmeal identification by direct enzyme-linked immunosorbent assay (ELISA), tested on Anopheles (Diptera: Culicidae) in Kenya. Journal of Medical Entomology 25, 9–16. [DOI] [PubMed] [Google Scholar]
- Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA and Bray M (2013) Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antiviral Research 100, 159–189. [DOI] [PubMed] [Google Scholar]
- Caglioti C, Lalle E, Castilletti C, Carletti F, Capobianchi MR and Bordi L (2013) Chikungunya virus infection: an overview. New Microbiology 36, 211–222. [PubMed] [Google Scholar]
- Carnevale P, Rovert V, Manguin S, Corbel V, Fontenille D, Garros C, Rogier C and Roux J (2009) Les Anophèles – biologie, transmission du Plasmodium Et Lutte Antivectorielle. IRD ed. Marseille 2009, 391. [Google Scholar]
- Chavy A, Nabet C, Normand AC, Kocher A, Ginouves M, Prévot G, Vasconcelos Dos Santos T, Demar M, Piarroux R and de Thoisy B (2019) Identification of French Guiana sand flies using MALDI-TOF mass spectrometry with a new massspectra library. PLoS Neglected Tropical Diseases 13, e0007031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulibaly B, Kone R, Barry MS, Emerson B, Coulibaly MB, Niare O, Beavogui AH, Traore SF, Vernick KD and Riehle MM (2016) Malaria vector populations across ecological zones in Guinea Conakry and Mali, West Africa. Malaria Journal 15, 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deilgat M, Geduld J and Drebot M (2014) Chikungunya outbreak in the Caribbean 2013–2014. Canada Communicable Diseases Report 40, 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diarra AZ, Almeras L, Laroche M, Berenger JM, Kone AK, Bocoum Z, Dabo A, Doumbo O, Raoult D and Parola P (2017) Molecular and MALDI-TOF identification of ticks and tick-associated bacteria in Mali. PLoS Neglected Tropical Diseases 11, e0005762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egizi A, Healy SP and Fonseca DM (2013) Rapid blood meal scoring in anthropophilic Aedes Albopictus and application of PCR blocking to avoid pseudogenes. Infection, Genetics and Evolution 16, 122–112. [DOI] [PubMed] [Google Scholar]
- Fyodorova MV, Savage HM, Lopatina JV, Bulgakova TA, Ivanitsky AV, Platonova OV and Platonov AE (2006) Evaluation of potential West Nile Virus vectors in Volgograd region, Russia, 2003 (Diptera: Culicidae): species composition, bloodmeal host utilization, and virus infection rates of mosquitoes. Journal of Medical Entomology 43, 552–556. [DOI] [PubMed] [Google Scholar]
- Gardner CL and Ryman KD (2010) Yellow fever: a reemerging threat. Clinics in Laboratory Medicine 30, 237–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Githeko AK, Service MW, Mbogo CM, Atieli FK and Juma FO (1994) Origin of blood meals in indoor and outdoor resting malaria vectors in Western Kenya. Acta Tropica 58, 307–331. [DOI] [PubMed] [Google Scholar]
- Gould E, Pettersson J, Higgs S, Charrel R and de Lamballerie X (2017) Emerging arboviruses: why today? One Health (Amsterdam, Netherlands) 4, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halada P, Hlavackova K, Risueno J, Berriatua E, Volf P and Dvorak V (2018) Effect of trapping method on species identification of phlebotomine sandflies by MALDI-TOF MS protein profiling. Medical and Veterinary Entomology 32, 388–392. [DOI] [PubMed] [Google Scholar]
- Kent RJ (2009) Molecular methods for arthropod bloodmeal identification and applications to ecological and vector-borne disease studies. Molecular Ecology Ressources 9, 4–18. [DOI] [PubMed] [Google Scholar]
- Kent RJ and Norris DE (2005) Identification of mammalian blood meals in mosquitoes by a multiplexed polymerase chain reaction targeting cytochrome B. American Journal of Tropical Medicine and Hygiene 73, 336–334. [PMC free article] [PubMed] [Google Scholar]
- Laroche M, Almeras L, Pecchi E, Bechah Y, Raoult D, Viola A and Parola P (2017a) MALDI-TOF MS as an innovative tool for detection of Plasmodium parasites in Anopheles mosquitoes. Malaria Journal 16, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche M, Berenger JM, Gazelle G, Blanchet D, Raoult D and Parola P (2017b) MALDI-TOF MS protein profiling for the rapid identification of Chagas disease triatomine vectors and application to the triatomine fauna of French Guiana. Parasitology 145, 1–11. [DOI] [PubMed] [Google Scholar]
- Lawrence AL, Batovska J, Webb CE, Lynch SE, Blacket MJ, Šlapeta J, Parola P and Laroche M (2019) Accurate identification of Australian mosquitoes using protein profiling. Parasitology 146, 462–471. [DOI] [PubMed] [Google Scholar]
- Linthicum KJ, Britch SC and Anyamba A (2016) Rift valley fever: an emerging mosquito-borne disease. Annual Reviews of Entomology 61, 395–415. [DOI] [PubMed] [Google Scholar]
- Logue K, Keven JB, Cannon MV, Reimer L, Siba P, Walker ED, Zimmerman PA and Serre D (2016) Unbiased characterization of Anopheles mosquito blood meals by targeted high-throughput sequencing. PLoS Neglected Tropical Diseases 10, e00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki-Ravasan N, Oshaghi M, Javadian E, Rassi Y, Sadraei J and Mohtarami F (2009) Blood meal identification in field-captured sand flies: comparison of PCR-RFLP and ELISA assays. Iran Journal ofArthropod Borne Diseases 3, 8–18. [PMC free article] [PubMed] [Google Scholar]
- Mewara A, Sharma M, Kaura T, Zaman K, Yadav R and Sehgal R (2018) Rapid identification of medically important mosquitoes by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Parasites & Vectors 11, 281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno M, Saavedra MP, Bickersmith SA, Prussing C, Michalski A, Tong RC, Vinetz JM and Conn JE (2017) Intensive trapping of blood-fed Anopheles Darlingi in Amazonian Peru reveals unexpectedly high proportions of avian blood-meals. PLoS Neglected Tropical Diseases 11, e00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucci LF, Júnior RP, de Paula MB, Scandar SA, Pacchioni ML, Fernandes A and Consales CA (2015) Feeding habits of mosquitoes (Diptera: Culicidae) in an area of sylvatic transmission of yellow fever in the state of São Paulo. Brazil. Journal of Venomous Animals and Toxins including Tropical Diseases 21, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnoz J, Eritja R, Alcaide M, Montalvo T, Soriguer RC and Figuerola J (2011) Host-feeding patterns of native Culex Pipiens and invasive Aedes albopictus mosquitoes (Diptera: Culicidae) in urban zones from Barcelona, Spain. Journal of Medical Entomology 48, 956–996. [DOI] [PubMed] [Google Scholar]
- Muriu SM, Muturi EJ, Shililu JI, Mbogo CM, Mwangangi JM, Jacob BG, Irungu LW, Mukabana RW, Githure JI and Novak RJ (2008) Host choice and multiple blood feeding behaviour of malaria vectors and other anophelines in Mwea rice scheme, Kenya. Malaria Journal 29, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muturi EJ, Muriu S, Shililu J, Mwangangi JM, Jacob BG, Mbogo C, Githure J and Novak RJ (2008) Blood-feeding patterns of Culex Quinquefasciatus and other culicines and implications for disease transmission in Mwea rice scheme, Kenya. Parasitology Research 102, 1329–1335. [DOI] [PubMed] [Google Scholar]
- Ndenga BA, Mulaya NL, Musaki SK, Shiroko JN, Dongus S and Fillinger U (2016) Malaria vectors and their blood-meal sources in an area of high bed net ownership in the Western Kenya highlands. Malaria Journal 15, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebbak A, Willcox AC, Bitam I, Raoult D, Parola P and Almeras L (2016) Standardization of sample homogenization for mosquito identification using an innovative proteomic tool based. Proteomics 16, 3148–3160. [DOI] [PubMed] [Google Scholar]
- Ngom el HM, Ndione JA, Ba Y, Konaté L, Faye O, Diallo M and Dia I (2013) Spatio-temporal analysis of host preferences and feeding patterns of malaria vectors in the sylvo-pastoral area of Senegal: impact of landscape classes. Parasites & Vectors 6, 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niare S, Berenger JM, Dieme C, Doumbo O, Raoult D, Parola P and Almeras L (2016) Identification of blood meal sources in the main African Malaria mosquito vector by MALDI TOF MS. Malaria Journal 15, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niare S, Almeras L, Tandina F, Yssouf A, Bacar A, Toilibou A, Doumbo O, Raoult D and Parola P (2017a) MALDI-TOF MS identification of Anopheles Gambiae Giles blood meal crushed on Whatman filter papers’. PLoS ONE 12, e0183238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niare S, Tandina F, Davoust B, Doumbo O, Raoult D, Parola P and Almeras L (2017b) Accurate identification of Anopheles Gambiae Giles trophic preferences by MALDI-TOF MS. Infection, Genetics and Evolution 63, 410–419. [DOI] [PubMed] [Google Scholar]
- Nossel HL and Niemetz J (1965) A normal inhibitor of the blood coagulation contact reaction product. Blood 25, 712–772. [PubMed] [Google Scholar]
- Onder O, Shao W, Kemps BD, Lam H and Brisson D (2013) Identifying sources of tick blood meals using unidentified tandem mass spectral libraries. Nature Communication 4, 1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshaghi MA, Chavshin AR, Vatandoost H, Yaaghoobi F, Mohtarami F and Noorjah N (2006) Effects of post-ingestion and physical conditions on PCR amplification of host blood meal DNA in mosquitoes. Experimental Parasitology 112, 232–232. [DOI] [PubMed] [Google Scholar]
- Petersen JM, Mead PS and Schriefer ME (2009) Francisella tularensis: an arthropod-borne pathogen. Veterinary Research 40, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior A and Torr SJ (2002) Host selection by Anopheles arabiensis and Anopheles quadriannulatus feeding on cattle in Zimbabwe. Medical and Veterinary Entomology 16, 207–221. [DOI] [PubMed] [Google Scholar]
- Raharimalala FN, Andrianinarivomanana TM, Rakotondrasoa A, Collard JM and Boyer S (2017) Usefulness and accuracy of MALDI-TOF mass spectrometry as a supplementary tool to identify mosquito vector species and to invest in development of international database. Medical and Veterinary Entomology 31, 289–298. [DOI] [PubMed] [Google Scholar]
- Rothen J, Githaka N, Kanduma EG, Olds C, Pflüger V, Mwaura S, Bishop RP and Daubenberger C (2016) Matrix-assisted laser desorption/ionization time of flight mass spectrometry for comprehensive indexing of East African ixodid tick species. Parasites & Vectors 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambou M, Aubadie-Ladrix M, Fenollar F, Fall B, Bassene H, Almeras L, Sambe-Ba B, Perrot N, Chatellier S, Faye N, Parola P, Wade B, Raoult D and Mediannikov O (2015) Comparison of matrix-assisted laser desorption ionization–time of flight mass spectrometry and molecular biology techniques for identification of culicoides (Diptera: Ceratopogonidae) biting midges in Senegal. Journal of Clinical Microbiology 53, 410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert S and Kostrzewa M (2017) MALDI-TOF MS in the microbiology laboratory: current trends. Current Issues in Molecular Biology 23, 17–20. [DOI] [PubMed] [Google Scholar]
- Seng P, Drancourt M, Gouriet F, La SB, Fournier PE, Rolain JM and Raoult D (2009) Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clinical Infectious Diseases 49, 543–555. [DOI] [PubMed] [Google Scholar]
- Seng P, Rolain JM, Fournier PE, La SB, Drancourt M and Raoult D (2010) MALDI-TOF-mass spectrometry applications in clinical microbiology. Future Microbiology 5, 1733–1717. [DOI] [PubMed] [Google Scholar]
- Seng P, Abat C, Rolain JM, Colson P, Lagier JC, Gouriet F, Fournier PE, Drancourt M, La SB and Raoult D (2013) Identification of rare pathogenic bacteria in a clinical microbiology laboratory: impact of matrix-assisted laser desorption ionization-time of flight mass spectrometry. Journal of Clinical Microbiology 51, 2182–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shililu JI, Maier WA, Seitz HM and Orago AS (1998) Seasonal density, sporozoite rates and entomological inoculation rates of Anopheles Gambiae and Anopheles funestus in a high-altitude sugarcane growing zone in Western Kenya. Tropical Medicine and International Health 3, 706–710. [DOI] [PubMed] [Google Scholar]
- Tandina F, Almeras L, Koné AK, Doumbo OK, Raoult D and Parola P (2016) Use of MALDI-TOF MS and culturomics to identify mosquitoes and their midgut microbiota. Parasites & Vectors 9, 495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandina F, Laroche M, Davoust B, Doumbo O K and Parola P (2018a) Blood meal identification in the cryptic species Anopheles Gambiae and Anopheles coluzzii using MALDI-TOF MS. Parasite 25, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandina F, Niaré S, Laroche M, Koné AK, Diarra AZ, Ongoiba A, Berenger JM, Doumbo OK, Raoult D and Parola P (2018b) Using MALDI-TOF MS to identify mosquitoes collected in Mali and their blood meals. Parasitology 145, 1170–1182. [DOI] [PubMed] [Google Scholar]
- Thomas S, Ravishankaran S, Justin NA, Asokan A, Mathai MT, Valecha N, Montgomery J, Thomas MB and Eapen A (2017) Resting and feeding preferences of Anopheles stephensi in an urban setting, perennial for malaria. Malaria Journal 16, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unver A, Perez M, Orellana N, Huang H and Rikihisa Y (2001) Molecular and antigenic comparison of Ehrlichia canis isolates from dogs, ticks, and a human in Venezuela. Journal of Clinical Microbiology 39, 2788–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilakis N, Cardosa J, Hanley KA, Holmes EC and Weaver SC (2011) Fever from the forest: prospects for the continued emergence of sylvatic dengue virus and its impact on public health. Nature Reviews Microbiology 9, 532–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2016) World Malaria Report 2016. Geneva: World Health Organization. [Google Scholar]
- Yssouf A, Socolovschi C, Flaudrops C, Ndiath MO, Sougoufara S, Dehecq JS, Lacour G, Berenger JM, Sokhna CS, Raoult D and Parola P (2013) Matrix-assisted laser desorption ionization-time of flight mass spectrometry: an emerging tool for the rapid identification of mosquito vectors. PLoS ONE 8, e72380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yssouf A, Almeras L, Raoult D and Parola P (2016) Emerging tools for identification of arthropod vectors. Future Microbiology 11, 549–566. [DOI] [PubMed] [Google Scholar]
- Zhang L, Smart S and Sandrin TR (2015) Biomarker- and similarity coefficient-based approaches to bacterial mixture characterization using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS). Science Reports 5, 15834. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S003118201900163X.
click here to view supplementary material
Data Availability Statement
All datasets regarding this study are included in the main paper and the additional supporting files.