Abstract
Tick-borne diseases caused by Theileria are of economic importance in domestic and wildlife ruminants. The majority of Theileria infects a limited number of host species, supporting the concept of host specificity. However, some Theileria seem to be generalists challenging the host specificity paradigm, such as Theileria sp. (sable) reported from various vertebrate hosts, including African buffalo, cattle, dogs and different antelope species. We tested the hypothesis that T. sp. (sable) uses Bovidae as hosts in general using a real-time polymerase chain reaction assay specific for T. sp. (sable) and a closely related genotype: T. sp. (sable-like). Various antelope species from the Tragelaphini (black wildebeest, blesbuck, blue wildebeest, gemsbuck, sable and waterbuck) tested positive for either T. sp. (sable) or T. sp. (sable-like). However, no African buffalo (n = 238) or cattle (n = 428) sampled in the current study tested positive, suggesting that these latter species are not carrier hosts. The results were confirmed using next-generation sequencing which also indicated at least 13 new genotypes or species found in various antelope and giraffes. Genotypes were found in single host species or in evolutionarily related hosts, suggesting that host specificity in Theileria may be a lineage specific phenomenon likely associated with tick-host-parasite co-evolution.
Key words: Bovidae, next-generation sequencing, real-time PCR, Theileria sp. (sable), Theileria sp. (sable-like)
Introduction
Theileria is tick-transmitted apicomplexan parasites of vertebrates that also serves as carrier hosts (Bishop et al., 2004). Tick vector competence and geographic distribution, persistence in the host, as well as host specificity and host geographic distribution determine parasite prevalence (Mans et al., 2015). The question of how host specific Theileria is, given incidental records for Theileria species in atypical hosts, remain (Mans et al., 2015). Host specificity may impact on hypotheses for speciation, epidemiology, geographic distribution and clinical disease etiology.
Theileria sp. (sable) infection causes a high-mortality rate in antelope calves. The infections in these rare and endangered species constrain their translocation and animal breeding. The introduction of naive animals into endemic areas can result in high fatalities (Wilson et al., 1974; Nijhof et al., 2005; Steyl et al., 2012). Theileria sp. (sable) and its disease have mostly been identified in roan and sable antelope.
Using reverse line blot (RLB) analysis, T. sp. (sable) has been detected in African buffalo (Syncerus caffer), cattle, blesbuck (Damaliscus pygargus), blue wildebeest (Connochaetes taurinus), klipspringer (Oreotragus oreotragus), reedbuck (Redunca arundinum), nyala (Tragelaphus angasii), sable antelope (Hippotragus niger), roan antelope (Hippotragus equinus) and dogs (Nijhof et al., 2005; Matjila et al., 2008; Muhanguzi et al., 2010; Yusufmia et al., 2010; Chaisi et al., 2011; Pfitzer et al., 2011; Adamu et al., 2014; Eygelaar et al., 2015; Njiiri et al., 2015; Tembo et al., 2018). It was also detected using sequencing in red hartebeest (Alcelaphus buselaphus caama) (Spitalska et al., 2005). It, therefore, seems to be ubiquitous in a variety of host species and may be described as a host generalist parasite. However, cross reactivity on RLB has been noted between T. sp. (sable) and T. velifera (Brothers et al., 2011; Mans et al., 2011), while direct next-generation sequencing (NGS) did not detect T. sp. (sable) in cattle or buffalo (Mans et al., 2016). Therefore, host specificity and correlations on co-infection with other genotypes remain uncertain (Njiiri et al., 2015).
A closely related genotype, T. sp. (sable-like), was previously detected in a cattle individual with extreme signs of theileriosis, using conventional cloning and sequencing of the 18S rRNA gene (Mans et al., 2011), but was not detected using NGS of larger buffalo and cattle populations (Mans et al., 2016). The host of this genotype and its relationship to T. sp. (sable) remains obscure. In the current study, we investigated the host specificity of two Theileria genotypes with controversial host associations: T. sp. (sable) and T. sp. (sable-like). The prevalence of these two genotypes in various bovids was studied by using a novel real-time hybridization assay capable of distinguishing these two genotypes. The study was conducted to specifically test the hypothesis that African buffalo and cattle are hosts for these genotypes, as reported previously using RLB analysis, where high prevalence ranging from 18–47% was observed (Muhanguzi et al., 2010; Yusufmia et al., 2010; Eygelaar et al., 2015; Njiiri et al., 2015; Tembo et al., 2018). To validate the novel assay, the Theileria diversity in various antelopes was also investigated using a NGS approach previously used to study diversity in cattle and African buffalo (Mans et al., 2016). The study also describes several novel genotypes from antelope and giraffe.
Materials and methods
Samples and DNA extraction
Blood samples (n = 979) submitted to the Agricultural Research Council – Epidemiology, Parasites and Vectors (ARC-EPV) diagnostic laboratory for routine T. parva testing were selected for analysis in this study. These included African buffalo (n = 238), black wildebeest (Connochaetes gnou) (n = 16), blesbuck (n = 22), blue wildebeest (n = 20), bushbuck (Tragelaphus scriptus) (n = 4), cattle (mixed breeds) (n = 428), eland (Taurotragus oryx) (n = 10), gemsbuck (Oryx gazella) (n = 18), giraffe (Giraffa camelopardalis) (n = 31), impala (Aepyceros melampus) (n = 17), kudus of the genus Tragelaphus (n = 10), nyala (n = 14), red hartebeest (n = 8), sable antelope (n = 73), sheep (Ovis aries) (n = 29) from Free State Province, springbok (Antidorcas marsupialis) (n = 35) and waterbuck (Kobus ellipsiprymnus) (n = 6). Cattle and antelope samples originated from the Corridor-disease endemic region in KwaZulu-Natal (Pienaar et al., 2018). The sable antelope originated from Zambia. Buffalo were sampled from the Kruger National Park (KNP) or Hluhluwe-Imfolozi National Park (HIN) and cattle (mixed Nguni breeds) from diptanks adjacent to the parks. DNA was extracted from 200 µL blood and eluted in 100 µL elution buffer using automated MagNa Pure technology (Roche Diagnostics, Mannheim, Germany). Each polymerase chain reaction (PCR) reaction included 2.5 µL of DNA (~15–50 ng µL−1).
Theileria sp. (sable) and T. sp. (sable-like) real-time PCR assay conditions
Hybridization assays for T. sp. (sable) and T. sp. (sable-like) were designed for use on the LightCycler® 480 (Roche Diagnostics, Mannheim, Germany). Each reaction consisted of 0.5 pmol of the T. sp. (sable) forward and reverse primers (TspSF: TGCATTGCCTTTTCTCCT TG, TspSR: CCTACTTTATTATTCCATGCTAA), 0.1 pmol of the T. sp. (sable) anchor (5′-GAGTTGATGCATTGCGGCTTAT-FL) and probe (LC640-TCGGTCATGGTTTTCCTTG-PH) (Fig. 1), 1U uracil deoxy-glycosylase (UDG) (Roche Diagnostics, Mannheim, Germany) and 4 µL of the Hybrid PCR mix in a final volume of 20 µL. The Hybrid PCR mix consists of 2 µL each of the LightCycler® Fast Start DNA Master Plus and LightCycler® Genotyping Master mix (Roche Diagnostics, Mannheim, Germany) (Pienaar et al., 2011b). Cycle conditions were started with 10 min UDG activation at 40 °C, followed by pre-incubation at 95 °C (10 min). An initial 10 cycles of denaturation at 95 °C (10 s), annealing at 57 °C (10 s) and extension at 72 °C (15 s) were followed by a 2-cycle touch-down of 5 °C in annealing temperature to 47 °C. This was followed by 34 cycles of denaturation at 95 °C (10 s), annealing at 47 °C (10 s) and extension at 72 °C (15 s). A positive and negative control was included in each run. Positive controls used was T. sp. (sable) originally isolated and propagated in culture from an infected roan antelope (Hippotragus equinus) (Zweygarth et al., 2009), while for T. sp. (sable-like), the bovine field isolate (28 914) previously confirmed through sequencing and RLB analysis was used (Mans et al., 2011). The negative control was from a bovine born and raised under quarantined tick-free conditions. Samples were also tested using the real-time PCR assays for T. parva, T. sp. (buffalo) and T. sp. (bougasvlei) as previously described (Pienaar et al., 2011a, 2014).
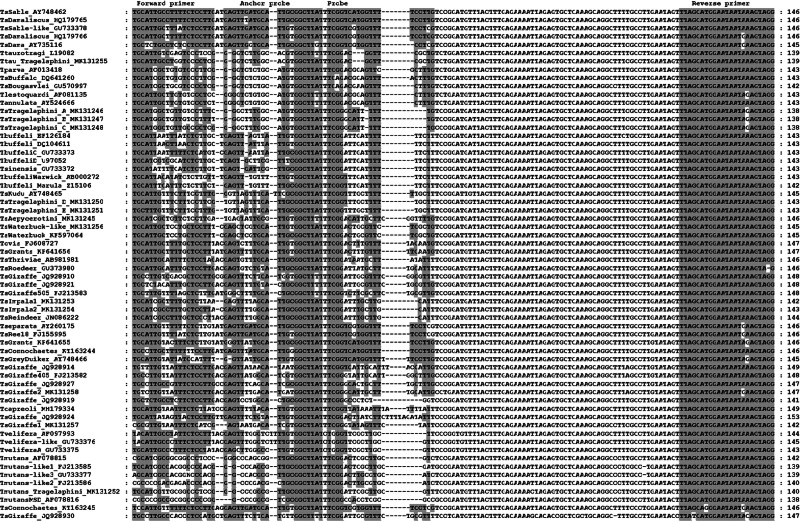
Fig. 1.
Multiple sequence alignment of the 18S hypervariable region for various Theileria species. Conserved regions for the primer, anchor and donor sequences for T. sp. (sable) are shaded in grey.
Analytical sensitivity of the real-time PCR assays
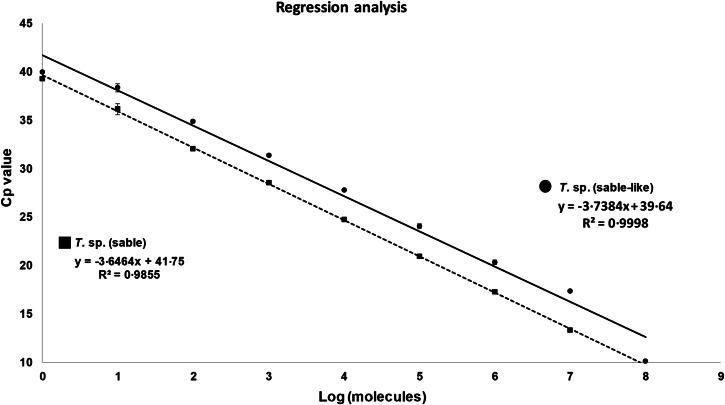
A T. sp. (sable) and T. sp. (sable-like) 18S DNA template was prepared by a conventional PCR from the positive controls using the 18S primers from Allsopp et al. (1993) that yielded a ~1100 bp product. The PCR products were fractionated by agarose electrophoresis and the bands cut out before purification using Wizard® SV Gel and PCR Clean-Up System (Promega). The purified products were quantified spectrophotometrically using an ND-1000 NanoDrop spectrophotometer (NanoDrop Technologies, Inc) and concentrations confirmed by agarose electrophoresis against quantified size standards. A tenfold serial dilution was made from quantified 18S templates and tested in triplicate using the various assays to determine the analytical sensitivity. Crossing point (Cp) values were generated using the software methodology of the LightCycler for qualitative detection. The percentage efficiency of the PCR reactions was determined from the slopes of the regression lines of the Log [C]/Cp value plots using the formula, Efficiency = 100( − 1 + 10(−1/slope)) according to Pfaffl (2004).
Specificity of the real-time PCR assays
Samples identified as positive for various Theileria genotypes using Sanger sequencing was used to confirm the analytical specificity of the T. sp. (sable) and T. sp. (sable-like) real-time PCR assays. The various genotypes tested are indicated in the Results section.
Next-generation sequencing of antelope samples
The procedures to sequence and analyse the 18S V4 hypervariable region using Roche GS-Junior NGS technology was followed as described (Mans et al., 2016). This included amplification of the 18S V4 hypervariable region using universal RLB primers, tagging with sample specific identification tags, quality processing, using Basic Local Alignment Search Tool (BLAST) analysis (Altschul et al., 1990) and pattern searching using electronic signatures to identify and count specific genotypes (Supplementary material). Unique genotypes were also confirmed using conventional ABI sequencing of the 18S gene as previously described, by sequencing 10 random clones from each sample (Mans et al., 2011). Sequences were deposited in Genbank under the accession numbers MK131245–MK131258.
Bioinformatic analysis of the 18S rRNA V4 hypervariable region
Sequences were retrieved from Genbank using BLAST analysis (Altschul et al., 1990), using the novel sequences to retrieve closely related sequences. A curated non-redundant Theileria sequence dataset (Mans et al., 2015) was also included that represent the Theileria sensu strictu clade (Oosthuizen et al., 2009). Theileria equi was used as an appropriate root for the tree since it generally groups outside the Theileria sensu strictu clade (Mans et al., 2015). Sequences were aligned using multiple alignment using fast Fourier transform with parameters Q-INS-I that takes RNA secondary interaction into consideration and a 20PAM/k = 2 nucleotide scoring matrix (Katoh and Standley, 2013). The alignment was trimmed to yield an alignment size of 264 bp that included the V4 hypervariable region. Phylogenetic analysis was performed with Mega 5 (Tamura et al., 2011), using neighbour-joining with 10 000 bootstraps and the Kimura 2-parameter nucleotide substitution model. Uniform rates among sites and homogenous patterns among lineages were used and gaps or missing data were treated as partial deletion at 90% site coverage cutoff resulting in 224 sites used in the final analysis.
Results
Development of the T. sp. (sable) and T. sp. (sable-like) real-time PCR assay
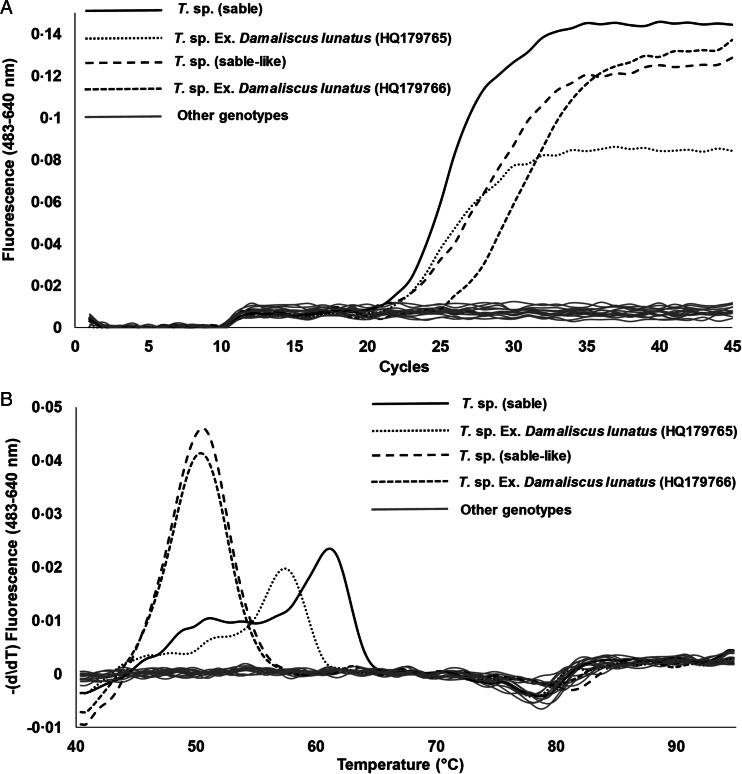
Primers that specifically amplify both T. sp. (sable) and T. sp. (sable-like) were designed with a single set of anchor–donor hybridization probes (Fig. 1). The anchor–donor hybridization probes can differentiate both genotypes, but with different melting profiles and Tm values, for T. sp. (sable-like) at 50 ± 2 °C and T. sp. (sable) at 60 ± 2 °C (Fig. 2). During screening, another genotype was also detected that corresponded with T. sp. Ex. Damaliscus lunatus (HQ179765) (Brothers et al., 2011), which gave a Tm at 58 ± 2 °C (Fig. 2). However, the difference in sequence of the V4 hypervariable region consists of only a single bp with T. sp. (sable) and we consider this a variant of the same genotype (Mans et al., 2011). Conversely, T. sp. (sable) and T. sp. (sable-like) differ by four nucleotides in the V4 hypervariable region, suggesting they are different species (Mans et al., 2011, 2015). Theileria sp. (sable-like) also differs from T. sp. Ex. Damaliscus lunatus (HQ179766) (Brothers et al., 2011), with one nucleotide difference outside the probe area. We consider this to be a variant of the same genotype as well, based on the arguments of Mans et al. (2011). The latter showed that single nucleotide polymorphisms occur in the V4 hypervariable region for T. parva, while established species always differ by greater than three nucleotide differences in the V4 hypervariable region. One to three differences in the V4 hypervariable region may therefore be indicative of variation within a species.
Fig. 2.
The hybridization assay for T. sp. (sable) and T. sp. (sable-like). (A) Amplification profiles for the positive controls and other genotypes tested in the study (section ‘Specificity of the assay’). (B) Melting curves for the positive controls and other genotypes tested in the study (section ‘Specificity of the assay’).
Analytical sensitivity of the real-time PCR assays
Analytical sensitivity was determined using ten-fold serial dilutions of a quantified 18S rRNA template. Both assays had similar sensitivity capable of detecting up to ~10 molecules per reaction with an efficiency of 85–88% (Fig. 3). For each assay a cut-off was instated at 37 cycles.
Fig. 3.
Sensitivity of the T. sp. (sable) and T. sp. (sable-like) assays. A ten-fold dilution series were performed in triplicate using a quantified 18S DNA template for each genotype. Indicated regression analysis of Cp values for triplicate samples and the equation for the regression analysis, its R2 value and the efficiency calculated from the slope. s.d. values are indicated by error bars.
Specificity of the assay
The assay only detected T. sp. (sable) and T. sp. (sable-like) genotypes and did not detect any genotypes found in cattle and buffalo including T. annulata (AY524666), T. buffeli (Warwick) (AB000272), T. cf. buffeli C (GU733373), T. lestoquardi (AF081135), T. mutans (AF078815), T. mutans-like 1 (FJ213585), T. mutans-like 2 (FJ213586), T. mutans-like 3 (GU733377), T. mutans MSD (AF078816), T. parva (AF013418), T. taurotragi (L19082), T. sp. (buffalo) (DQ641260), T. sp. (bougasvlei) (GU570997), T. velifera (AF097993), T. velifera A (GU733375) and T. velifera B (GU733376) (Fig. 2). In addition, 22 genotypes unique to antelope identified in samples from the current study using NGS were also not detected (Fig. 2). This included T. mutans-like (Tragelaphini) (MK131252), T. ovis (FJ608727), T. separata (AY260175), T. sp. (Aepycerotini) (MK131245), T. sp. (giraffe) 1 (MK131257), T. sp. (giraffe) 2 (MK131258), T. sp. (giraffe) 405 (FJ213582), T. sp. (giraffe) 505 (FJ213583), T. sp. (giraffe) (JQ928914), T. sp. (giraffe) (JQ928927), T. sp. (impala) Cervidae-like 1 (MK131253), T. sp. (impala) Cervidae-like 2 (MK131254), T. sp. (kudu) (AY748465), T. sp. Ree (FJ155995), T. sp. (Tragelaphini) A (MK131246), T. sp. (Tragelaphini) B (MK131247), T. sp. (Tragelaphini) C (MK131248), T. sp. (Tragelaphini) D (MK131250), T. sp. (Tragelaphini) E (MK131251), T. sp. (waterbuck) (KF597064), T. sp. (waterbuck-like) (MK131256) and T. taurotragi-like (Tragelaphini) (MK131255).
Real-time PCR assay results for antelope, buffalo, cattle and sheep
A variety of animals that included antelope, buffalo, cattle and sheep were screened using the T. sp. (sable) and T. sp. (sable-like) assays (Table 1). Animals positive for T. sp. (sable) included black wildebeest, blue wildebeest, gemsbuck, sable and sheep, while animals positive for T. sp. (sable-like) included black wildebeest, blesbuck, blue wildebeest, sable and waterbuck. Neither buffalo nor cattle were positive for T. sp. (sable) or T. sp. (sable-like). Conversely, none of the antelope tested were positive for T. parva, T. sp. (buffalo) or T. sp. (bougasvlei) (results not shown).
Table 1.
Summary of results for T. sp. (sable) and T. sp. (sable-like) obtained from the real-time hybridization assay
| T. sp. (sable) | T. sp. (sable-like) | |||||||
|---|---|---|---|---|---|---|---|---|
| Animal | POS | NEG | Total | Percentage | POS | NEG | Total | Percentage |
| Buffalo | 0 | 238 | 238 | 0 | 0 | 238 | 238 | 0 |
| Cattle | 0 | 428 | 428 | 0 | 0 | 428 | 428 | 0 |
| Black wildebeest | 12 | 4 | 16 | 75 | 10 | 6 | 16 | 63 |
| Blesbuck | 7 | 15 | 22 | 32 | 13 | 9 | 22 | 59 |
| Blue wildebeest | 18 | 2 | 20 | 90 | 12 | 8 | 20 | 60 |
| Bushbuck | 0 | 4 | 4 | 0 | 0 | 4 | 4 | 0 |
| Eland | 0 | 10 | 10 | 0 | 0 | 10 | 10 | 0 |
| Gemsbuck | 14 | 4 | 18 | 78 | 0 | 18 | 18 | 0 |
| Giraffe | 0 | 31 | 31 | 0 | 0 | 31 | 31 | 0 |
| Impala | 0 | 17 | 17 | 0 | 0 | 17 | 17 | 0 |
| Kudu | 0 | 10 | 10 | 0 | 0 | 10 | 10 | 0 |
| Nyala | 0 | 14 | 14 | 0 | 0 | 14 | 14 | 0 |
| Redhartbeest | 0 | 8 | 8 | 0 | 0 | 8 | 8 | 0 |
| Sable | 9 | 64 | 73 | 12 | 35 | 38 | 73 | 48 |
| Sheep | 16 | 13 | 29 | 55 | 0 | 29 | 29 | 0 |
| Springbuck | 0 | 35 | 35 | 0 | 0 | 35 | 35 | 0 |
| Waterbuck | 0 | 6 | 6 | 0 | 6 | 0 | 6 | 100 |
Relative parasitaemia of T. sp. (sable) and T. sp. (sable-like)
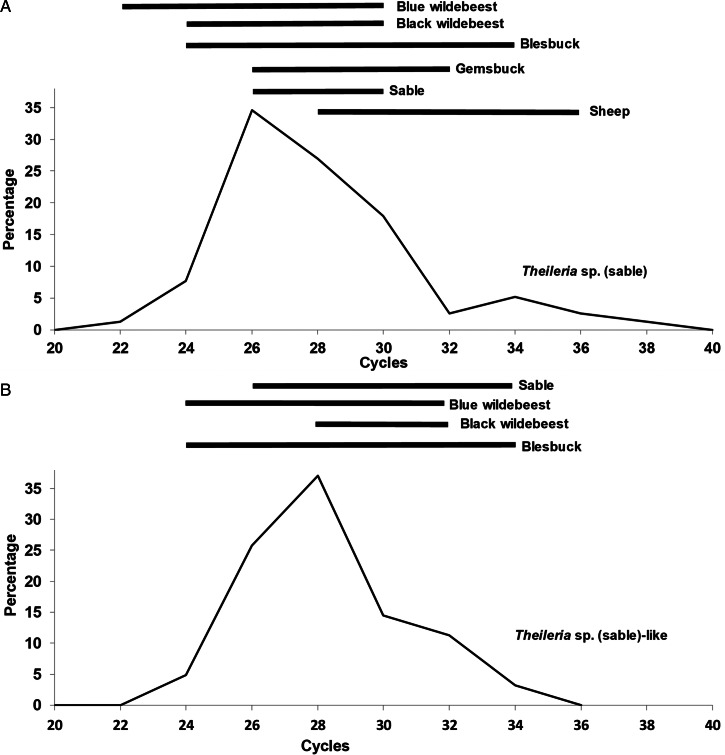
Frequency distribution curves of the crossing-point values indicate that for both T. sp. (sable) and T. sp. (sable-like), a normal distribution is observed that range from 22–34 cycles (Fig. 4). Within this range, the Cp value distribution is similar for different antelope species, suggesting that the parasitaemia range for these species lies well within the detection range of the real-time PCR assays. As such, the majority of field carrier animals should be detectable.
Fig. 4.
Frequency distribution plots of the Cp values for (A) T. sp. (sable) and (B) T. sp. (sable-like). Horizontal bars indicate the distribution range found in different hosts that covers >80% of the distribution values for that host.
Validation of the T. sp. (sable) and T. sp. (sable-like) assays using next-generation sequencing
Previously, the Theileria diversity in cattle and buffalo was validated using conventional and NGS approaches, to allow confidence when assessing specificity of the T. parva, T. sp. (buffalo), T. sp. (bougasvlei) and T. taurotragi real-time PCR assays (Mans et al., 2011, 2016; Pienaar et al., 2011a, 2011b, 2014, 2018). In the case of antelopes, a large-scale diversity assessment has never been performed, making the assessment of potential false positive detection difficult when implementing new assays. To address this and assist in the estimation of specificity of the T. sp. (sable) and T. sp. (sable-like) assays, the V4 hypervariable region of the 18S rRNA gene from various antelopes was sequenced using a NGS approach.
The majority of common genotypes found in cattle or buffalo were not found in any antelope using NGS. This included T. parva, T. sp. (buffalo), T. sp. (bougasvlei), T. mutans 1, T. mutans 2, T. mutans 3, T. mutans, T. mutans MSD, T. buffeli Warwick, T. buffeli C, T. velifera and T. velifera B. Genotypes previously detected in cattle or buffalo were T. taurotragi and T. velifera A. Theileria taurotragi was found in eland and kudu (Fig. 5). Theileria velifera A was found in eland, kudu and nyala (Fig. 5).
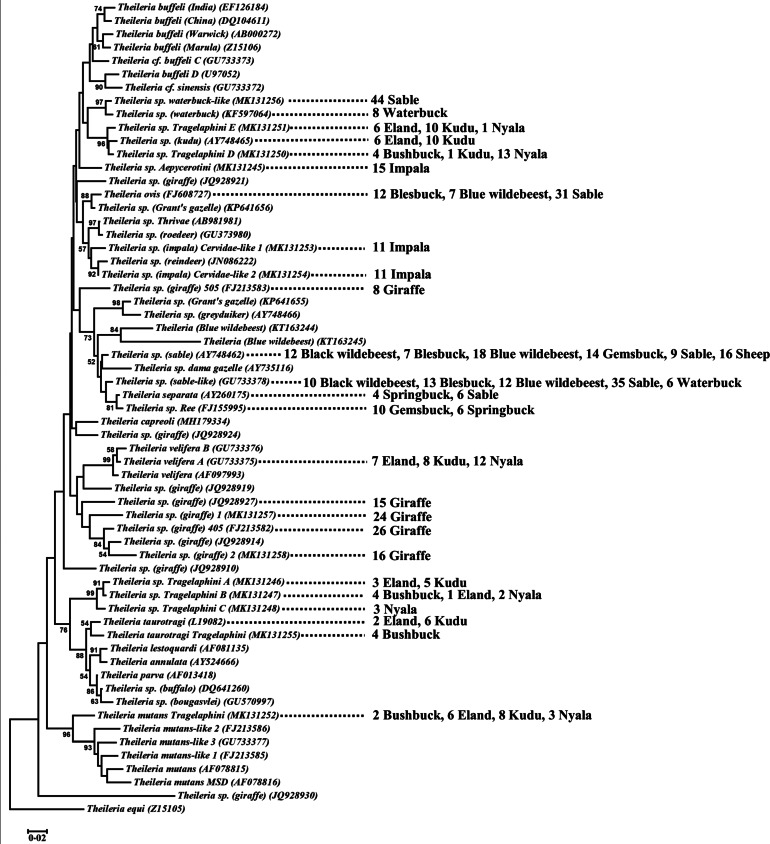
Fig. 5.
Phylogenetic analysis of the Theileria sensu strictu clade. Indicated are various Theileria genotypes or species, their Genbank accession numbers in brackets and the number of animals in which Theileria were detected for various host species using next-generation or Sanger sequencing. The neighbour-joining tree was constructed using Mega 5 (Tamura et al., 2011). Theileria equi was used to root the tree. Nodal support is for 10 000 bootstraps and only support above 50% is shown.
NGS confirmed the positive status of those samples detected with the hybridization assay, while no T. sp. (sable) or T. sp. (sable-like) sequences were detected in samples that tested negative for these genotypes using the real-time PCR assays (Table 2). Conversely, thirteen novel genotypes not previously published were detected in various antelopes and confirmed using conventional sequencing (Fig. 5; Table 3). This included novel genotypes found in bushbuck, eland, giraffe, impala, kudu, nyala and sable, namely T. sp. (Aepycerotini) (MK131245), T. sp. (giraffe) 1A (MK131257), T. sp. (giraffe) 2A (MK131258), T. sp. (impala) Cervidae-like 1 (MK131253), T. sp. (impala) Cervidae-like 2 (MK131254), T. mutans-like (Tragelaphini) (MK131252), T. sp. (Tragelaphini) A (MK131246), T. sp. (Tragelaphini) B (MK131247), T. sp. (Tragelaphini) C (MK131248), T. sp. (Tragelaphini) D (MK131250), T. sp. (Tragelaphini) E (MK131251), T. sp. (waterbuck-like) (MK131256) and T. taurotragi-like (Tragelaphini) (MK131255).
Table 2.
Summary of results for T. sp. (sable) and T. sp. (sable-like) obtained from NGS.
| T. sp. (sable) | T. sp. (sable-like) | |||||||
|---|---|---|---|---|---|---|---|---|
| Animal | POS | NEG | Total | Percentage | POS | NEG | Total | Percentage |
| Buffaloa | 0 | 672 | 672 | 0 | 0 | 672 | 672 | 0 |
| Cattlea | 0 | 478 | 478 | 0 | 0 | 478 | 478 | 0 |
| Black wildebeest | 11 | 5 | 16 | 69 | 12 | 4 | 16 | 75 |
| Blesbuck | 7 | 12 | 19 | 37 | 13 | 6 | 19 | 68 |
| Blue wildebeest | 7 | 0 | 7 | 100 | 6 | 1 | 7 | 86 |
| Bushbuck | 0 | 4 | 4 | 0 | 0 | 4 | 4 | 0 |
| Eland | 0 | 10 | 10 | 0 | 0 | 10 | 10 | 0 |
| Gemsbuck | 10 | 1 | 11 | 91 | 0 | 11 | 11 | 0 |
| Giraffe | 0 | 27 | 27 | 0 | 0 | 27 | 27 | 0 |
| Impala | 0 | 15 | 15 | 0 | 0 | 15 | 15 | 0 |
| Kudu | 0 | 10 | 10 | 0 | 0 | 10 | 10 | 0 |
| Nyala | 0 | 13 | 13 | 0 | 0 | 13 | 13 | 0 |
| Red hartebeest | 0 | 8 | 8 | 0 | 0 | 8 | 8 | 0 |
| Sable | 2 | 46 | 48 | 4 | 24 | 24 | 48 | 50 |
| Springbuck | 0 | 35 | 35 | 0 | 0 | 35 | 35 | 0 |
| Waterbuck | 0 | 6 | 6 | 0 | 6 | 0 | 6 | 100 |
Indicated are the number of positive and negative animals for each animal species tested.
Results collated from Mans et al. (2016).
Table 3.
Sequencing results for NGS and Sanger sequencing
| Tribe | Antelope (NGS/Sanger) | Genotype | NGS | Sanger |
|---|---|---|---|---|
| Alcelaphini | Blesbuck (19/2) | Tovis_FJ608727 | 12 | 0 |
| Tsable_AY748462 | 11 | 1 | ||
| TspSable_like_GU733378 | 11 | 1 | ||
| Alcelaphini | Blue wildebeest (7/4) | Tovis_FJ608727 | 7 | 2 |
| Tsable_AY748462 | 4 | 4 | ||
| TspSable_like_GU733378 | 6 | 3 | ||
| Alcelaphini | Black wildebeest (15/2) | Tsable_AY748462 | 11 | 1 |
| TspSable_like_GU733378 | 12 | 1 | ||
| Hippotragini | Gemsbuck (11/3) | TspRee18_FJ155995 | 10 | 2 |
| Tsable_AY748462 | 10 | 3 | ||
| Hippotragini | Sable (48/5) | Tovis_FJ608727 | 31 | 4 |
| Tseparata_AY260175 | 6 | 2 | ||
| Tsable_AY748462 | 2 | 0 | ||
| TspSable_like_GU733378 | 25 | 2 | ||
| TspWaterbuck-like_MK131256 | 44 | 3 | ||
| Caprini | Sheep (0/2) | Tsable_AY748462 | NA | 2 |
| Reduncini | Waterbuck (6/2) | Tsp_waterbuck_KF597072 | 6 | 1 |
| TspSable_like_GU733378 | 6 | 2 | ||
| Antelopini | Springbuck (8/1) | Tseparata_AY260175 | 4 | 1 |
| TspRee18_FJ155995 | 6 | 1 | ||
| Aepycerotini | Impala (15/2) | TspAepycerotini_MK131245 | 15 | 3 |
| TspImpala_Cervidae-like1_MK131253 | 11 | 2 | ||
| TspImpala_Cervidae-like2_MK131254 | 11 | 2 | ||
| Tragelaphini | Bushbuck (4) | TspTragelaphini_B_MK131247 | 4 | 1 |
| TspTragelaphini_D_MK131250 | 4 | 1 | ||
| Tmut-like_Tragelaphini_MK131252 | 2 | 0 | ||
| Ttau-like_Tragelaphini_MK131255 | 4 | 2 | ||
| Tragelaphini | Eland (7/3) | TspTragelaphini_A_MK131246 | 3 | 1 |
| TspTragelaphini_B_MK131247 | 0 | 1 | ||
| TspTragelaphini_E_MK131251 | 6 | 6 | ||
| Tmut-like_Tragelaphini_MK131252 | 6 | 1 | ||
| TspKudu_AY748465 | 6 | 1 | ||
| TcfveliferaA_GU733375 | 7 | 2 | ||
| Ttau_L19082 | 2 | 2 | ||
| Tragelaphini | Kudu (10/2) | TspTragelaphini_A_MK131246 | 5 | 1 |
| TspTragelaphini_D_MK131250 | 1 | 0 | ||
| TspTragelaphini_E_MK131251 | 10 | 1 | ||
| Tmut-like_Tragelaphini_MK131252 | 8 | 2 | ||
| TspKudu_AY748465 | 10 | 1 | ||
| TcfveliferaA_GU733375 | 8 | 2 | ||
| Ttau_L19082 | 6 | 0 | ||
| Tragelaphini | Nyala (13/2) | TspTragelaphini_B_MK131247 | 2 | 0 |
| TspTragelaphini_C_MK131248 | 3 | 1 | ||
| TspTragelaphini_D_MK131250 | 13 | 2 | ||
| TspTragelaphini_E_MK131251 | 1 | 0 | ||
| Tmut-like_Tragelaphini_MK131252 | 3 | 0 | ||
| TcfveliferaA_GU733375 | 12 | 2 | ||
| Giraffidae | Giraffe (28/3) | TspGiraffe0405_FJ213582 | 26 | 3 |
| TspGiraffe0505_FJ213583 | 8 | 2 | ||
| TspGiraffe1A_MK131257 | 24 | 3 | ||
| TGiraffe2A_MK131258 | 16 | 1 | ||
| TspGiraffe_NG2012b_JQ928927 | 15 | 0 | ||
| TspGiraffe_NG-2012b_JQ928914 | 17 | 2 |
Indicated are the number of animals that was positive using either NGS or Sanger sequencing. Genotypes shows an abbreviated name with its Genbank accession number.
Known genotypes were also found that included T. ovis (FJ608727), T. separata (AY260175), T. sp. (giraffe) 405 (FJ213582), T. sp. (giraffe) 505 (FJ213583), T. sp. (giraffe) (JQ928914), T. sp. (giraffe) (JQ928927), T. sp. (kudu) (AY748465), T. sp. Ree (FJ155995), T. sp. (waterbuck) (KF597064) in blesbuck, black wildebeest, blue wildebeest, eland, gemsbuck, giraffe, kudu, sable, sheep, springbuck and waterbuck. The majority of antelope species were infected with more than one Theileria species or genotype.
Discussion
Accurate diagnostics and epidemiological data depend on sensitive and specific assays to be of any practical or scientific utility (Mans et al., 2015). Accurate quantitative real-time hybridization PCR assays have been developed for T. parva, T. sp. (buffalo) and T. sp. (bougasvlei) (Sibeko et al., 2008; Pienaar et al., 2011b, 2014). Hybridization probe assays can differentiate genotypes based on differences in anchor and probe regions (Mans et al., 2011). The current study describes the development of a sensitive and specific real-time hybridization probe assay capable of differentiating T. sp. (sable) and T. sp. (sable-like).
The analytical sensitivity of the assays is comparable to other Theileria real-time PCR assays (Sibeko et al., 2008; Papli et al., 2011; Pienaar et al., 2011b, 2014, 2018). The frequency distribution of the Cp values follows normal distributions with parasitaemia levels in carrier hosts spanning detectable ranges of the assay. Ranges observed are similar to T. parva, T. sp. (buffalo), T. sp. (bougasvlei) and T. taurotragi (Pienaar et al., 2011b, 2014, 2018). Parasitaemia levels range from 0.001–0.1% and seem to be the norm for the carrier state in Theileria sensu strictu (Pienaar et al., 2011a, 2011b).
The assays for T. sp. (sable) and T. sp. (sable-like) detected no positive cattle (n = 428) or buffalo (n = 238), while antelope showed prevalence's of 12–90% for much smaller sample sizes. Prevalence estimates ranged from 18–47% for buffalo and cattle using RLB analysis (Muhanguzi et al., 2010; Yusufmia et al., 2010; Eygelaar et al., 2015; Njiiri et al., 2015; Tembo et al., 2018). The presence of T. sp. (sable) in cattle and buffalo reported using RLB is therefore probably erroneous (Mans et al., 2011, 2016).
The current study highlights a pitfall of RLB analysis, i.e. cross-hybridization may lead to erroneous detection of species. The probe for T. velifera differs in three nucleotides from T. sp. (sable) suggesting this similarity level may result in cross-hybridization under non-stringent hybridization conditions. Cross-hybridization may not be restricted to T. sp. (sable) and T. velifera, since the probe for T. mutans differs with 2–3 nucleotides for members of the T. mutans clade and cross-reactivity was observed for members (T. mutans-like 1, T. mutans-like 2 and T. mutans-like 3) exclusive to African buffalo (Mans et al., 2011, 2016). Cross-reactivity may also occur with T. mutans-like (Tragelaphini) identified in bushbuck. The probe for T. buffeli is identical for the majority of clade members including T. buffeli C and T. sinensis-like that seem to be specific for African buffalo (Mans et al., 2011, 2016). Since many genotypes show host specificity, RLB analysis could lead to under- or overestimation of prevalence reducing the impact of epidemiological studies (Mans et al., 2016). Other drawbacks of RLB such as PCR competition for the universal primers may lead to suppression of low-abundance templates and underestimation of genotypes since the majority of hosts are infected by multiple species (Mans et al., 2011, 2016), PCR suppression is a major factor limiting the use of RLB for epidemiological studies. The classic example is T. parva in African buffalo, where 27–64% infection was detected using RLB, while real-time PCR assays detected ~70% positive samples (Pienaar et al., 2011a). Alternatives to RLB would be species-specific real-time PCR assays (Mans et al., 2015). Conversely, RLB has been successful in detecting novel genotypes when present as single infections but still needs sequencing for identification and confirmation (Nijhof et al., 2003, 2005; Oosthuizen et al., 2008, 2009; Chaisi et al., 2013, 2014). An alternative to this would be a direct sequencing approach as described in the current and previous studies (Mans et al., 2011, 2016). While NGS may replace real-time PCR applications in the long term (Mans et al., 2016), the latter remains cheaper and faster for routine diagnostics making species-specific PCR assays the current preferred choice within a diagnostic setting.
NGS of antelope confirmed that the primers and probes for the T. sp. (sable) and T. sp. (sable-like) hybridization assays are specific, while also allowing discovery of a number of unique genotypes specific to antelopes. A number of novel genotypes were exclusive to the Tragelaphini (bushbuck, eland, kudu and nyala) suggesting that these are unique species that may infect the Tragelaphini in general. Theileria sp. (kudu) (AY748465) was found in the greater kudu (Nijhof et al., 2005) and the current study found this genotype in eland, suggesting that Tragelaphini may be general hosts. Theileria cf. velifera A was shown to be prevalent in the Tragelaphini while extensive screening showed its presence in cattle but not in African buffalo (Mans et al., 2011, 2016). Conversely, the related genotypes T. velifera and T. velifera B were extensively detected in cattle and African buffalo (Mans et al., 2016), but not in any wild antelope from the current study.
With regard to host specificity in antelope, it was indicated that T. taurotragi infect the Tragelaphini, possibly due to evolution of this species in the last common ancestor, with only a more recent adaptation to cattle (Pienaar et al., 2018). It was suggested that Tragelaphini in general may be hosts for T. taurotragi (Pienaar et al., 2018) and was confirmed for the mountain bongo (Tragelaphus eurycerus isaaci) (Bishop et al., 2019). NGS of the wildlife samples confirmed this again.
The discovery of the related novel T. sp. taurotragi-like genotype is of interest since it was unique to bushbuck, a member of the Tragelaphini, suggesting speciation due to geographic isolation of bushbuck. It is of interest that bushbuck in the current study was negative for T. taurotragi, but positive for T. sp. taurotragi-like, although bushbuck was found to be carriers of T. taurotragi in Uganda (Oura et al., 2011). Genetic incompatibility has been suggested as a mechanism for speciation in Theileria as observed for T. sp. (buffalo) and T. sp. (bougasvlei) (Pienaar et al., 2014), and may operate here as well, but will need a larger sampling of bushbuck to confirm this. Mitochondrial analysis indicated that bushbuck may consist of different species in Central and South Africa (Hassanin et al., 2012), so that host specificity may also play a role in this instance. Alternatively, the possibility exists that the bushbuck from Uganda was carriers of T. sp. taurotragi-like and not T. taurotragi, since these genotypes differ by one nucleotide in the RLB probe area and would probably show cross-reactivity. In this case, host specificity will exist within the Tragelaphini for T. taurotragi and related genotypes. An important question is whether the hydrolysis probe assay for T. taurotragi (Pienaar et al., 2018), would also detect T. sp. taurotragi-like, since these genotypes differ by one nucleotide in the hydrolysis probe region. However, the T. taurotragi forward primer differs in three positions towards the 3′end of the primer and linked with the touchdown PCR conditions employed ensure specificity, since none of the bushbuck samples tested positive with this assay (Pienaar et al., 2018). Piroplasms previously observed in bushbuck from South Africa were named Theileria tragelaphi (Neitz, 1931; Bigalke et al., 1972). The current study indicated at least four different genotypes associated with bushbuck that would obscure the identity of the original named piroplasm species.
Antelope infected by T. sp. (sable) and T. sp. (sable-like) includes Alcelaphini (blesbuck, blue wildebeest, black wildebeest, red hartebeest and tsessebe) and Hippotragini (gemsbuck and sable antelope) which form a monophyletic clade. They also form a larger monophyletic group with the Caprini (goats and sheep) (Hassanin et al., 2012), suggesting that these species may also be infected by T. sp. (sable) and T. sp. (sable-like). Screening of 29 sheep indicated 55% infected with T. sp. (sable) and was confirmed for two sheep using conventional Sanger sequencing. Theileria sp. (sable) was also reported for sheep in South Africa using RLB (Berggoetz et al., 2014). Extensive screening of small ruminants (chamois, sheep and goats) from the northern hemisphere did not report T. sp. (sable) (Altay et al., 2007; García-Sanmartín et al., 2007; Torina et al., 2007; Iqbal et al., 2013; Aydin et al., 2015; Ozubek and Aktas, 2017; Chaligiannis et al., 2018). This is likely due to Rhipicephalus appendiculatus and Rhipicephalus evertsi evertsi, considered tick vectors for T. sp. (sable) (Steyl et al., 2012), only occurring in sub-Saharan Africa (Walker et al., 2000). The Alcelaphini, Hippotragini and Caprini with the Oreotragini (klipspringer), Cephalotragini (duiker), Reduncini (waterbuck, Leche, Reedbuck), Antelopini (Gazelle and Springbuck), Neotragini (Suni, Royal antelope) and Aepycerotini (Impala) form the Antilopinae (Hassanin et al., 2012). The waterbuck screened in the current study showed the presence of T. sp. (sable-like). Theileria sp. (sable) was also previously detected in klipspringer and reedbuck (Nijhof et al., 2005). All Antilopinae may therefore be potential hosts. The original description of T. sp. (sable-like) in a single cattle sample seemed to have been incidental, since it has not been found in other cattle sampled to date (Mans et al., 2011, 2016), but has now been extensively found in various antelope species.
Additional Theileria genotypes detected in the Antilopinae included T. ovis, T. sp. Ree, T. sp. waterbuck, T. separata and T. sp. waterbuck-like (sable). These genotypes do not seem to be host specific within the Antilopinae (Hassanin et al., 2012).
The only genotypes that fall outside this picture are specific for impala, including T. sp. (Aepycerotini), T. sp. (Impala) Cervidae-like 1 and T. sp. (Impala) Cervidae-like 2. Impala did not present any of the genotypes found in other Antilopinae. The impala (with the Neotragini) group basals to all other Antilopinae (Hassanin et al., 2012). Their relationship to the Antilopinae has been considered uncertain leading to the elevation of its own evolutionary lineage, Aepycerotinae (Ansell, 1971; Vrba, 1979; Gentry, 1992; Matthee and Davis, 2001). Their unique makeup of Theileria genotypes would support this. An unnamed Theileria species serologically distinct from Theileria isolated from Bovidae in East Africa, not infective to cattle but infective to impala by piroplasm inoculation was described (Grootenhuis et al., 1975). Using RLB analysis T. bicornis, T. buffeli and T. sp. (sable) were detected in impala from South Africa (Berggoetz et al., 2014). While these species may also infect impala, the current study suggests that further investigation need to confirm this.
The Theileria sp. (Aepycerotini) group with no defined clade, while T. sp. (Impala) Cervidae-like 1 and T. sp. (Impala) Cervidae-like 2 groups within a well-supported clade with T. sp. (reindeer), a genotype related to Theileria from North Texas white-tail deer (Garner et al., 2012). Reindeer and white-tail deer belong to the Odocoileini (Cervidae) and are genetically distant from Bovidae (Hassanin et al., 2012). The high sequence similarity observed between Theileria from Cervidae and impala is of interest, suggesting that the presence of these parasites (either in impala or reindeer/white-tail deer) was due to a recent introduction into Africa or into North America. It is tempting to speculate that impala is the ancestral host since their origin could be dated to ~16–18 MYA, while the Odocoileini (reindeer/white-tail deer) has originated only ~5.7 MYA (Hassanin et al., 2012). However, the diversity of Theileria genotypes in antelope from various continents and their evolutionary origins still needs elucidation.
The current study identified numerous genotypes described in giraffe from South Africa and Kenya (Oosthuizen et al., 2009; Githaka et al., 2013). In addition, two novel genotypes (T. sp. giraffe 1 and T. sp. giraffe 2) were detected, the former identical to a giraffe genotype sampled from a zoo in China, originating from South Africa (Zhang et al., 2016). As such, Theileria diversity in giraffes from southern Africa is as extensive as in East Africa (Githaka et al., 2013).
Lineage specificity seems to emerge as a theme for Babesia and Theileria species, suggesting co-evolution of parasite and host with origins in the last common host ancestors. This may enable dating of the origin of parasite lineages using fossil and molecular clock records for various antelopes (Hassanin et al., 2012). For T. sp. (sable) and T. sp. (sable-like) this implies that they originated ~16–12 MYA in the ancestral lineage of the Alcelaphini, Caprini, Hippotragini and Reduncini (Hassanin et al., 2012). This is similar to the origin estimated for the T. taurotragi clade that occurred after divergence of the Bovini and Tragelaphini (Pienaar et al., 2018).
In conclusion, the current study indicated host specificity for T. sp. (sable) and T. sp. (sable-like) within the Tragelaphini, with no conclusive evidence of infection of African buffalo or cattle. NGS indicates that Theileria genotypes are specific to either antelope or bovines, even though host specificity may be for lineages and not necessarily individual species. While a variety of novel Theileria genotypes have been detected, their tick vectors remain unknown, impacting on a full understanding of their epidemiology, geographical ranges and veterinary significance.
Financial support
The research project was funded and approved by the Department of Agriculture, Forestry and Fisheries (DAFF) (Project number: P10000062).
Conflict of interest
None.
Ethical standards
Not applicable.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S003118201900132X.
click here to view supplementary material
References
- Adamu M, Troskie M, Oshadu DO, Malatji DP, Penzhorn BL and Matjila PT (2014) Occurrence of tick-transmitted pathogens in dogs in Jos, Plateau State, Nigeria. Parasites & Vectors 7, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsopp BA, Baylis HA, Allsopp MTPE, Cavalier-Smith T, Bishop RP, Carrington DM, Sohanpal B and Spooner P (1993) Discrimination between six species of Theileria using oligonucleotide probes which detect small subunit ribosomal RNA sequences. Parasitology 107, 157–165. [DOI] [PubMed] [Google Scholar]
- Altay K, Dumanli N and Aktas M (2007) Molecular identification, genetic diversity and distribution of Theileria and Babesia species infecting small ruminants. Veterinary Parasitology 147, 161–165. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW and Lipman DJ (1990) Basic local alignment search tool. Journal of Molecular Biology 215, 403–410. [DOI] [PubMed] [Google Scholar]
- Ansell WFH (1971) Order artiodactyla. In Meester J and Setzer HW (eds), The Mammals of Africa: an Identification Manual. Washington, DC: Smithsonian Institution Press, pp. 15–83. [Google Scholar]
- Aydin MF, Aktas M and Dumanli N (2015) Molecular identification of Theileria and Babesia in ticks collected from sheep and goats in the Black Sea region of Turkey. Parasitology Research 114, 65–69. [DOI] [PubMed] [Google Scholar]
- Berggoetz M, Schmid M, Ston D, Wyss V, Chevillon C, Pretorius AM and Gern L (2014) Tick-borne pathogens in the blood of wild and domestic ungulates in South Africa: interplay of game and livestock. Ticks and Tick Borne Diseases 5, 166–175. [DOI] [PubMed] [Google Scholar]
- Bigalke RD, Keep ME and Schoeman JH (1972) Some protozoan parasites of tragelaphine antelopes in South Africa with special reference to a Babesia sp. in a bushbuck and a Trypanosoma theileri-like parasite in a nyala. Onderstepoort Journal of Veterinary Research 39, 225–228. [PubMed] [Google Scholar]
- Bishop R, Musoke A, Morzaria S, Gardner M and Nene V (2004) Theileria: intracellular protozoan parasites of wild and domestic ruminants transmitted by ixodid ticks. Parasitology 129, S271–S283. [DOI] [PubMed] [Google Scholar]
- Bishop RP, Odongo DO, Dolan TT, Dolan RB, Skilton RA and Sayer PD (2019) Theileriosis in mountain bongo repatriated to Kenya: a clinical and molecular investigation. Journal of Zoo and Wildlife Medicine 50, 342–349. [DOI] [PubMed] [Google Scholar]
- Brothers PS, Collins NE, Oosthuizen MC, Bhoora R, Troskie M and Penzhorn BL (2011) Occurrence of blood-borne tick-transmitted parasites in common tsessebe (Damaliscus lunatus) antelope in Northern Cape Province, South Africa. Veterinary Parasitology 183, 160–165. [DOI] [PubMed] [Google Scholar]
- Chaisi ME, Sibeko KP, Collins NE, Potgieter FT and Oosthuizen MC (2011) Identification of Theileria parva and Theileria sp. (buffalo) 18S rRNA gene sequence variants in the African Buffalo (Syncerus caffer) in Southern Africa. Veterinary Parasitology 182, 150–162. [DOI] [PubMed] [Google Scholar]
- Chaisi ME, Janssens ME, Vermeiren L, Oosthuizen MC, Collins NE and Geysen D (2013) Evaluation of a real-time PCR test for the detection and discrimination of Theileria species in the African buffalo (Syncerus caffer). PLoS ONE 8, e75827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaisi ME, Collins NE and Oosthuizen MC (2014) Phylogeny of Theileria buffeli genotypes identified in the South African buffalo (Syncerus caffer) population. Veterinary Parasitology 204, 87–95. [DOI] [PubMed] [Google Scholar]
- Chaligiannis Ι, Fernández de Mera IG, Papa A, Sotiraki S and de la Fuente J (2018) Molecular identification of tick-borne pathogens in ticks collected from dogs and small ruminants from Greece. Experimental and Applied Acarology 74, 443–453. [DOI] [PubMed] [Google Scholar]
- Eygelaar D, Jori F, Mokopasetso M, Sibeko KP, Collins NE, Vorster I, Troskie M and Oosthuizen MC (2015) Tick-borne haemoparasites in African buffalo (Syncerus caffer) from two wildlife areas in Northern Botswana. Parasites & Vectors 8, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Sanmartín J, Aurtenetxe O, Barral M, Marco I, Lavin S, García-Pérez AL and Hurtado A (2007) Molecular detection and characterization of piroplasms infecting cervids and chamois in Northern Spain. Parasitology 134, 391–398. [DOI] [PubMed] [Google Scholar]
- Garner BC, Holman P and Berent LM (2012) Theileriosis in a reindeer (Rangifer tarandus tarandus) associated with a potentially novel Theileria sp. Veterinary Clinical Pathology 41, 497–501. [DOI] [PubMed] [Google Scholar]
- Gentry AW (1992) The subfamilies and tribes of the family Bovidae. Mammal Review 22, 1–32. [Google Scholar]
- Githaka N, Konnai S, Skilton R, Kariuki E, Kanduma E, Murata S and Ohashi K (2013) Genotypic variations in field isolates of Theileria species infecting giraffes (Giraffa camelopardalis tippelskirchi and Giraffa camelopardalis reticulata) in Kenya. Parasitology International 62, 448–453. [DOI] [PubMed] [Google Scholar]
- Grootenhuis JG, Young AS, Kimber CD and Drevemo SA (1975) Investigations on a Theileria species from an impala. Journal of Wildlife Diseases 11, 122–127. [DOI] [PubMed] [Google Scholar]
- Hassanin A, Delsuc F, Ropiquet A, Hammere C, Jansen van Vuuren B, Matthee C, Ruiz-Garcia M, Catzefli F, Areskoug V, Thanh Nguyen T and Couloux A (2012) Pattern and timing of diversification of Cetartiodactyla (Mammalia, Laurasiatheria), as revealed by a comprehensive analysis of mitochondrial genomes. Comptes Rendus Biologies 335, 32–50. [DOI] [PubMed] [Google Scholar]
- Iqbal F, Khattak R, Ozubek S, Khattak M, Rasul A and Aktas M (2013) Application of the reverse line blot assay for the molecular detection of Theileria and Babesia sp. in sheep and goat blood samples from Pakistan. Iran Journal of Parasitology 8, 289–295. [PMC free article] [PubMed] [Google Scholar]
- Katoh K and Standley DM (2013) MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30, 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mans BJ, Pienaar R, Latif AA and Potgieter FT (2011) Diversity in the 18S SSU rRNA V4 hyper-variable region of Theileria in bovines and African buffalo (Syncerus caffer) from Southern Africa. Parasitology 138, 766–779. [DOI] [PubMed] [Google Scholar]
- Mans BJ, Pienaar R and Latif AA (2015) A review of Theileria diagnostics and epidemiology. International Journal of Parasitology: Parasites and Wildlife 4, 104–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mans BJ, Pienaar R, Ratabane J, Pule B and Latif AA (2016) Investigating the diversity of the 18S SSU rRNA hyper-variable region of Theileria in cattle and Cape buffalo (Syncerus caffer) from Southern Africa using a next generation sequencing approach. Ticks and Tick-borne Diseases 7, 869–879. [DOI] [PubMed] [Google Scholar]
- Matjila PT, Leisewitz AL, Oosthuizen MC, Jongejan F and Penzhorn BL (2008) Detection of a Theileria species in dogs in South Africa. Veterinary Parasitology 157, 34–40. [DOI] [PubMed] [Google Scholar]
- Matthee CA and Davis SK (2001) Molecular insights into the evolution of the family Bovidae: a nuclear DNA perspective. Molecular Biology and Evolution 18, 1220–1230. [DOI] [PubMed] [Google Scholar]
- Muhanguzi D, Matovu E and Waiswa C (2010) Prevalence and characterization of Theileria and Babesia species in cattle under different husbandry systems in Western Uganda. International Journal of Animal Veterinary Advances 2, 51–58. [Google Scholar]
- Neitz WO (1931) Blood parasites of game in Zululand. Preliminary report. 17th Report of the Director of Veterinary Service and Animal Industry, Union of South Africa, pp. 45–60.
- Nijhof AM, Penzhorn BL, Lynen G, Mollel JO, Morkel P, Bekker CP and Jongejan F (2003) Babesia bicornis sp. nov. and Theileria bicornis sp. nov.: tick-borne parasites associated with mortality in the black rhinoceros (Diceros bicornis). Journal of Clinical Microbiology 41, 2249–2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhof AM, Pillay V, Steyl J, Prozesky L, Stoltsz WH, Lawrence JA, Penzhorn BL and Jongejan F (2005) Molecular characterization of Theileria species associated with mortality in four species of African antelopes. Journal of Clinical Microbiology 43, 5907–5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njiiri NE, Bronsvoort BM, Collins NE, Steyn HC, Troskie M, Vorster I, Thumbi SM, Sibeko KP, Jennings A, van Wyk IC, Mbole-Kariuki M, Kiara H, Poole EJ, Hanotte O, Coetzer K, Oosthuizen MC, Woolhouse M and Toye P (2015) The epidemiology of tick-borne haemoparasites as determined by the reverse line blot hybridization assay in an intensively studied cohort of calves in western Kenya. Veterinary Parasitology 210, 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosthuizen MC, Zweygarth E, Collins NE, Troskie M and Penzhorn BL (2008) Identification of a novel Babesia sp. from a sable antelope (Hippotragus niger Harris, 1838). Journal of Clinical Microbiology 46, 2247–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosthuizen MC, Allsopp BA, Troskie M, Collins NE and Penzhorn BL (2009) Identification of novel Babesia and Theileria species in South African giraffe (Giraffa camelopardalis, Linnaeus, 1758) and roan antelope (Hippotragus equinus, Desmarest 1804). Veterinary Parasitology 163, 39–46. [DOI] [PubMed] [Google Scholar]
- Oura CA, Tait A, Asiimwe B, Lubega GW and Weir W (2011) Theileria parva genetic diversity and haemoparasite prevalence in cattle and wildlife in and around Lake Mburo National Park in Uganda. Parasitology Research 108, 1365–1374. [DOI] [PubMed] [Google Scholar]
- Ozubek S and Aktas M (2017) Molecular and parasitological survey of ovine piroplasmosis, including the first report of Theileria annulata (Apicomplexa: Theileridae) in sheep and goats from Turkey. Journal of Medical Entomology 54, 212–220. [DOI] [PubMed] [Google Scholar]
- Papli N, Landt O, Fleischer C, Koekemoer JO, Mans BJ, Pienaar R, Josemans A, Zweygarth E, Potgieter F and Latif AA (2011) Evaluation of a TaqMan real-time PCR for the detection of Theileria parva in buffalo and cattle. Veterinary Parasitology 175, 356–359. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW (2004) Quantification strategies in real-time PCR. In Bustin SA (ed.), A-Z of Quantitative PCR. La Jolla, CA, USA: International University Line (IUL), pp. 87–112. [Google Scholar]
- Pfitzer S, Oosthuizen MC, Bosman AM, Vorster I and Penzhorn BL (2011) Tick-borne blood parasites in nyala (Tragelaphus angasii Gray 1849) from KwaZulu-Natal, South Africa. Veterinary Parasitology 176, 126–131. [DOI] [PubMed] [Google Scholar]
- Pienaar R, Potgieter FT, Latif AA, Thekisoe OMM and Mans BJ (2011a) Mixed Theileria infections in free-ranging buffalo herds: implications for diagnosing Theileria parva infections in Cape buffalo (Syncerus caffer). Parasitology 138, 884–895. [DOI] [PubMed] [Google Scholar]
- Pienaar R, Potgieter FT, Latif AA, Thekisoe OMM and Mans BJ (2011b) The Hybrid II assay: a sensitive and specific real-time hybridization assay for the diagnosis of Theileria parva infection in Cape buffalo (Syncerus caffer) and cattle. Parasitology 138, 884–895. [DOI] [PubMed] [Google Scholar]
- Pienaar R, Potgieter FT, Latif AA, Thekisoe OMM and Mans BJ (2014) Geographic distribution of Theileria sp. (buffalo) and Theileria sp. (bougasvlei) in Cape buffalo (Syncerus caffer) in Southern Africa: implications for speciation. Parasitology 141, 411–424. [DOI] [PubMed] [Google Scholar]
- Pienaar R, Latif AA and Mans BJ (2018) Investigations into the host specificity of Theileria taurotragi. Veterinary Parasitology 254, 30–35. [DOI] [PubMed] [Google Scholar]
- Sibeko KP, Oosthuizen MC, Collins NE, Geysen D, Rambritch NE, Latif AA, Groeneveld HT, Potgieter FT and Coetzer JA (2008) Development and evaluation of a real-time polymerase chain reaction test for the detection of Theileria parva infections in Cape buffalo (Syncerus caffer) and cattle. Veterinary Parasitology 155, 37–48. [DOI] [PubMed] [Google Scholar]
- Spitalska E, Riddell M, Heyne H and Sparagano OA (2005) Prevalence of theileriosis in Red Hartebeest (Alcelaphus buselaphus caama) in Namibia. Parasitology Research 97, 77–79. [DOI] [PubMed] [Google Scholar]
- Steyl JC, Prozesky L, Stoltsz WH and Lawrence JA (2012) Theileriosis (Cytauxzoonosis) in Roan antelope (Hippotragus equinus): field exposure to infection and identification of potential vectors. Onderstepoort Journal of Veterinary Research 79, E1–E8. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M and Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution 28, 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tembo S, Collins NE, Sibeko-Matjila KP, Troskie M, Vorster I, Byaruhanga C and Oosthuizen MC (2018) Occurrence of tick-borne haemoparasites in cattle in the Mungwi District, Northern Province, Zambia. Ticks and Tick Borne Diseases 9, 707–717. [DOI] [PubMed] [Google Scholar]
- Torina A, Vicente J, Alongi A, Scimeca S, Turlá R, Nicosia S, Di Marco V, Caracappa S and de la Fuente J (2007) Observed prevalence of tick-borne pathogens in domestic animals in Sicily, Italy during 2003–2005. Zoonoses and Public Health 54, 8–15. [DOI] [PubMed] [Google Scholar]
- Vrba ES (1979) Phylogenetic analysis and classification of fossil and recent Alcelaphini Mammalia: Bovidae. Biological Journal of the Linnean Society 11, 207–228. [Google Scholar]
- Walker JB, Keirans JE and Horak IG (2000) The Genus Rhipicephalus (Acari, Ixodidae): a Guide to the Brown Ticks of the World. Cambridge: Cambridge University Press. [Google Scholar]
- Wilson DE, Bartsch RC, Bigalke RD and Thomas SE (1974) Observations on mortality rates and disease in roan and sable antelope on nature reserves in the Transvaal. South African Journal of Wildlife Research 4, 203–206. [Google Scholar]
- Yusufmia SB, Collins NE, Nkuna R, Troskie M, Van den Bossche P and Penzhorn BL (2010) Occurrence of Theileria parva and other haemoprotozoa in cattle at the edge of Hluhluwe-iMfolozi Park KwaZulu-Natal, South Africa. Journal of the South African Veterinary Association 81, 45–49. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li T, Cui Y, Wang J, Lv Y, Wang R, Jian F, Zhang L, Wang J, Yang G and Ning C (2016) The first report of Anaplasma phagocytophilum and a novel Theileria spp. co-infection in a South African giraffe. Parasitology International 65, 347–351. [DOI] [PubMed] [Google Scholar]
- Zweygarth E, Benade J, Steyl J, Prozesky L, Koekemoer O and Josemans AI (2009) In vitro cultivation of a Theileria species from a roan antelope (Hippotragus equinus). Parasitology Research 105, 1755–1757. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S003118201900132X.
click here to view supplementary material