Abstract
Swimmer's itch (SI) is a painful rash caused by skin penetration by free-swimming infectious cercariae of avian schistosomes, snail-borne helminth parasites related to the causative agents of human schistosomiasis. The goal of this study was to determine if commonly collected environmental data could be used to predict daily fluctuations in SI incidence at an inland beach in northwestern Michigan. Lifeguards collected daily data over four summers, including the number of self-reported SI cases, total swimmers, water temperature, wind speed and wind direction. Mixed-effects binomial regression revealed that wind direction, wind speed and time of day were the best predictors of daily SI risk. Swimmers entering the water in the morning or on days with direct onshore wind perpendicular to the shoreline had the greatest SI risk. However, there was a negative effect of wind speed after accounting for direction, where SI risk was greatest on days with a gentle breeze originating directly offshore. These results suggest that at this beach, direct onshore winds generate a surface-water current that causes SI cercariae to aggregate in the shallow waters used by swimmers. Data are needed from additional sites to confirm whether the onshore wind is a generally important driver of SI incidence.
Key words: Lymnaea catescopium [ = Stagnicola emarginata], Trematode parasites, Trichobilharzia spp
Introduction
Swimmer's itch (SI), or cercarial dermatitis, is a painful itchy rash caused by avian schistosomes, a group of snail-borne helminth parasites that are closely related to the causative agents of human schistosomiasis (Brant and Loker, 2009). Avian schistosomes (mainly Trichobilharzia spp.) normally have waterfowl definitive hosts and snail intermediate hosts (mostly lymnaeid snails in Northern Michigan; Muzzall et al., 2003). However, the infective cercaria stage (produced by the intermediate host snail) sometimes mistakes humans for birds and attempts to penetrate our skin, resulting in the rash (Talbot, 1936; Verbrugge et al., 2004a). SI is an important problem for communities near inland lakes in northern Michigan, where it has an economic impact on recreational water use (Muzzall et al., 2003; Verbrugge et al., 2004b). Reducing the impact of SI in these communities will require improving our ability to predict when and where SI cercariae will be most abundant.
Factors influencing the distributions of schistosome parasites have been studied for a variety of species at various spatial and temporal scales. Studies investigating spatial patterns have focused on variation at a local scale (Raso et al., 2005; Zhou et al., 2011), at a regional scale (Coady et al., 2006; Rudko et al., 2018) and at a landscape scale (Clements et al., 2006; Brooker, 2007). Studies investigating temporal patterns in schistosomes have largely focused on among-year and among-season variation (Spear et al., 2004; Yang et al., 2006; Ciddio et al., 2015). Most of these studies focused on factors driving either abundance of the parasites' intermediate or definitive hosts at larger spatial and temporal scales or infection prevalence within hosts. For example, increasing temperature in spring is thought to trigger emergence and increased reproduction of overwintering host snails for SI-causing parasites (Clampitt, 1970; Hodasi, 1976), increased light intensity and temperature lead to increased growth of the periphyton eaten by snails (Halstead et al., 2018), and insecticide application can reduce the abundance of arthropod predators that eat snails (Halstead et al., 2014; Sokolow et al., 2015).
In addition to variation on larger temporal scales (e.g. seasonal or annual), SI incidence at Crystal Lake, Michigan is commonly observed to vary widely from one day to the next (Al Flory, pers. comm.). Day-to-day risk assessment is an important endpoint for local lake management (see Bush et al., 2014 for an analysis of daily Escherichia coli risk), and extending that risk assessment to SI could be essential in mitigating the impact of SI cases. However, factors driving variation in SI incidence at daily time scales are less understood than, and are likely different from, factors driving variation at longer time scales. For example, daily fluctuations in SI incidence are probably driven by factors other than local infected snail abundance, which should not fluctuate on such short time scales because lymnaeid snails have multi-year generation times (Sunada et al., 2017), and trematode-infected snails continue producing cercariae for multiple weeks after developing a patent infection (Da Costa et al., 1994; McCreesh and Booth, 2014; Byers et al., 2016). It is possible that short-term fluctuations could be driven by synchronized cercaria emergence from multiple snails due to some random event (e.g. a group of local snails that were simultaneously infected during a single bird visitation). Such random events might result in unpredictable temporal clustering of SI cases, though the underlying cause might be difficult to discern.
Short-term fluctuations in SI incidence might also be driven by environmental factors that vary on short time scales. One factor known to be capable of driving short-term fluctuations in cercaria abundance is temperature variability. Cercaria emergence from schistosome-infected snails is well known to increase in response to a temperature rise, and as a result, controlled temperature shifts are commonly used in laboratory settings to induce or inhibit cercaria production (Kuntz, 1947; McClelland, 1965; Frank, 1966). However, the thermal history of a trematode-infected snail can also influence cercaria production. For example, Paull et al. (2015) found that snails infected with the trematode Ribeiroia ondatrae produced fewer cercariae following past exposure to warmer temperatures, compared to snails that had experienced cooler temperatures. These results suggest that extended exposure to warmer temperatures might be stressful to snails and their trematode parasites, resulting in subsequently decreased cercaria production at any given new temperature. Based on this ‘thermal stress’ hypothesis, we predicted a negative effect of past temperatures on SI incidence (i.e. average water temperature over the past 3–7 days), after controlling for potential positive effects of current-day water temperature on cercaria production rates within infected snails. A second factor that might drive short-term fluctuations in SI incidence is changes in light intensity, which is known to help induce cercaria emergence from snails. For example, SI-causing cercaria emergence from host snails is triggered by the gradual increase in sunlight and temperature starting at dawn, with peak emergence occurring a few hours later (Kuntz, 1947; Anderson et al., 1976; Soldánová et al., 2016; Rudko et al., 2018). We therefore predicted higher SI incidence in the morning than the afternoon, and lower SI incidence on days with more cloud cover (i.e. overcast). A third factor that might influence SI incidence on short time scales is the local density of swimmers, though the directionality of this effect is uncertain. For example, a greater density of swimmers might attract more cercariae to the local swim area (Haas and van der Roemer, 1998; Haeberlein and Haas, 2008), or conversely a larger number of swimmers might mean fewer cercariae are available to attack each individual swimmer (i.e. ‘encounter dilution’ effect; Buck and Lutterschmidt, 2017).
Another set of factors that might drive short-term fluctuations in SI incidence is shifts in wind and water currents, which might influence the movement of cercariae towards swim areas following their emergence from infected snails. Wave action and water currents, which can be affected by changes in wind speed or direction (Weber, 1983), have been shown to carry schistosome cercariae downstream from off-site locations (Upatham, 1974). Furthermore, lymnaeid snails often live in deep-water off-shore habitats not usually associated with recreational swimming (Laman et al., 1984), and avian schistosome cercariae are known to migrate toward the water surface in search of duck definitive hosts (Feiler and Haas, 1988; Rudko et al., 2018), making surface water currents from off-shore a potentially important SI risk factor in deep-water inland lakes. As a result, SI cases might sometimes occur at sites that lack local populations of infected host snails, due to an influx of cercariae from off site (Leighton et al., 2000; Rudko et al., 2018). Anecdotally, beachgoers and lifeguards in Northern Michigan have reported seeing higher SI incidence when winds originate from offshore (Al Flory, pers. comm.). Recent results by Rudko et al. (2018) showed significant wind direction effects at multiple sites on northern Michigan lakes, though their analysis focused on weekly rather than daily variation and on cercaria abundance rather than the resulting SI incidence. Based on these observations and results, we hypothesized that a recreational beach would have a higher SI risk on days with stronger winds originating from offshore, leading to onshore surface currents that could transport cercariae from deep-water snail beds to the shallow-water swimming areas.
We tested these hypotheses using a dataset of SI reports collected at Congressional Summer Assembly (CSA) Beach on Crystal Lake, Michigan, where the dominant SI parasite is thought to be Trichobilharzia stagnicolae and the dominant host snail is Lymnaea catescopium [ = Stagnicola emarginata; Laman et al., 1984; Correa et al., 2010] (Rudko et al., 2018). While traditional methods for determining SI risk are labour-intensive and require specialized expertise to identify parasites (Kolářová et al., 2010), recent DNA detection and quantification methods are faster and more accurate (Rudko et al., 2018, 2019). However, the distinctive rash caused by SI makes it amenable to data collection by citizen scientists, and public beaches have staff (e.g. lifeguards and park managers) during the summer months with the ability to collect SI incidence data and measurements of possible environmental drivers such as temperature, wind speed and wind direction. This study focused on using these kinds of data collected by lifeguards at CSA beach during each summer season over a 4-year period from 2013 to 2016.
Materials and methods
Data collection
Lifeguards at CSA collected daily data for 7 weeks from late June to early August each year during the 2013–2016 period (Table 1). Lifeguards counted the number of self-reported SI cases (by swimmers) each day. Lifeguard staff also counted the total number of swimmers twice daily, once per session. The morning sessions ran until 1100 h, and counts were performed at ~1000 h. Afternoon sessions ran from 1300 to 1700 h, and counts were performed at ~1600 h. The beach is open at all times, but lifeguards are on duty only during these sessions. Lifeguards also collected surface water temperature using a Pentair R141036-127 floating tube thermometer, wind speed using an Ambient Weather WM-2 handheld anemometer (miles per h) and wind direction to the nearest 8-way direction (i.e. N, E, S, W, NE, SE, SW or NW) using known landmarks oriented from a map of Crystal Lake. However, CSA beach is located in the western part of Crystal Lake and oriented such that northeast winds are directly onshore and perpendicular to the shoreline. We were unable to find a complete dataset for cloud cover at our survey site for the entirety of our sampling period. We therefore used precipitation as a proxy for cloud cover, where days with precipitation are more likely than not to have cloud cover. Precipitation data for all sampling dates was obtained from the NOAA Online Weather Data (NOWData) tool from a National Weather Service station located near CSA beach in Frankfort, MI. Precipitation was quantified using two methods: (1) presence/absence of precipitation as a binary variable (NWSprecip1) and (2) total precipitation per day in inches as a continuous variable (NWSprecip2). Days with trace precipitation (i.e. days with observed precipitation below the minimum reportable interval) were treated as having the presence of precipitation (NWSprecip1) as any day with precipitation might have a greater probability of being partially cloudy or overcast but having 0 inches of precipitation (NWSprecip2). All variables used in statistical analyses were generated based on these data.
Table 1.
Summary statistics for the dataset over the 4-year sampling period at CSA beach
| Year | Days sampled | Total swimmers | Total SI cases |
|---|---|---|---|
| 2013 | 49 | 3003 | 114 |
| 2014 | 53 | 3727 | 244 |
| 2015 | 52 | 6896 | 381 |
| 2016 | 52 | 5112 | 253 |
Analyses based on self-reporting data are potentially unreliable due to the possibility of misidentified or unreported cases. However, CSA beach is a private community that is only open to members and their guests, Crystal Lake has a long history and familiarity with SI and many members have been visiting CSA beach for generations. CSA members were informed about the study via regular meetings and newsletters, and they were encouraged to report cases to the lifeguard on duty. They were provided with informational brochures describing SI and its symptoms, and a large sign was posted at the beach informing people about potential SI risk (Figs S1 and S2). Brochures and a publicly available newsletter were also distributed to members and posted throughout Benzie County, Michigan. Based on the high level of local awareness and education about SI risks and symptoms, we decided it was reasonable to analyse changes in relative risk through time within this dataset, making the implicit assumption that any errors due to misidentification or under-reporting were randomly distributed across time.
To allow analysis of temporal autocorrelations and lagged effects of past temperatures, we calculated the average of previous 1-, 3-, 5- and 7-day SI cases and temperatures (PrevTotal1, 3, 5, 7 and AvgPrevTemp1, 3, 5, 7), and we restricted the analysis to days when these data were available (i.e. removing the first 6 days of each year). The PrevTotal variables also served the purpose of accounting for other sources of temporal autocorrelation, including well-described summer peaks in trematode cercaria abundance for many species (Kube et al., 2002; Valdovinos and Balboa, 2007; Galaktionov et al., 2015) or the potential for a random event to cause synchronized emergence of cercariae from multiple snails (e.g. simultaneous snail infections from a single bird visitation). We excluded a single day that was missing temperature data, as well as subsequent days for which previous average temperatures could not be calculated. We also removed one datapoint from the analysis due to missing wind speed data. There was a single day where the recorded number of SI cases was greater than the recorded total number of swimmers; in this case, we adjusted the total number of swimmers to equal the reported number of SI cases (i.e. proportion of SI that day = 1.0).
Statistical methods – environmental factors and time of day
We used generalized linear mixed-effects models within program R (‘glmer’ function in the lme4 package) to determine the best predictors of the SI cases on a daily time scale (Bates et al., 2015; R Core Team, 2019). We used a binomial distribution in these models to test for effects of environmental factors on the probability of a given swimmer contracting swimmer's itch, based on the count of swimmers positive (i.e. number of reported SI cases) and negative (i.e. the difference between total swimmers and number of reported SI cases) for SI. We included a random effect of ‘Day’ in each model to account for daily variation and to ensure the day was treated as the unit of replication for treatment variables measured on a daily timescale, rather than individual swimmer (which would have resulted in sample size inflation). To test for a time of day (TimeOfDay) effect, we ran a separate set of models in which the morning and afternoon counts were coded separately in the dataset; these models also included a random effect of ‘Day’.
We had trouble achieving convergence for complex models that included large numbers of predictor variables, precluding the use of backward selection procedures to explore parameter space. However, most models converged readily with fewer predictors included. Therefore, we used a forward selection approach to explore contributions of various environmental factors to SI risk. In forward selection, we start with simple models that only include one predictor variable at a time, select the predictor that explains the most variation, and systematically add predictors to the model until no additional variables significantly improve the model. We used a P value threshold of 0.05 from a likelihood-ratio test (‘Anova’ function in the package car) as our criterion for variable inclusion in each final model (Fox and Weisberg, 2011). We encountered convergence warnings in ~15% of all models run during model selection. Warnings (unlike errors) indicate that the model converged successfully but one or more convergence criteria exceeded default tolerances. To confirm whether outcomes from models with convergence warnings were reasonable, we conducted alternative models using a traditional linear regression approach, with the arcsine-square root transformed proportion of swimmers contracting SI (on a given day or session) as the response variable. We chose to use the traditional arcsine-square-root transformation over a logit transformation because our dataset contained several 0's and 1's, which would have required separate transformations to these data points. Additionally, all final models were compared to arcsine-square root linear models with the same predictors.
In the analysis of wind direction effects in the full binomial model, we initially treated wind direction as a single categorical predictor with eight categories (resulting in seven dummy variables added to each model). However, this model converged with warnings, apparently due to high autocorrelation among dummy variables. We therefore generated new wind-direction variables in which adjacent wind directions were paired (e.g. N + NE, E + SE, etc.). Pairing directions generated two possible wind direction variables, which we considered as possible predictors in model selection (N + NE for WindDirGroup1 vs NW + N for WindDirGroup2). This reduced the number of categories to four per predictor (i.e. three dummy variables per predictor), greatly reducing model complexity and allowing model convergence without warnings. We found that WindDirGroup1 was the better predictor of the two wind-direction variables during model selection, and the two variables explained much of the same variation leading to exclusion of WindDirGroup2, likely due to directly perpendicular northeast winds paired with north or east winds hitting the site at similar angles. Other predictors considered in model selection included: current daily water temperature (WaterTempC), wind speed (WindVel), a wind speed and direction interaction (WindDirGroup1 × WindVel), and the average of previous 1-, 3-, 5- and 7-day temperatures and number of SI cases (AvgPrevTemp1, 3, 5, 7 and PrevSi1, 3, 5, 7), the number of swimmers per day (SwimTotal) and two metrics of precipitation (NWSprecip1 and NWSprecip2). To investigate the time of day effect, we used an alternative version of the dataset separated into two rows per day (i.e. AM and PM counts for total swimmers and SI cases) and conducted forward selection considering the same predictors as above plus TimeOfDay and number of swimmers per session.
No temporal predictors (i.e. previous temperature or SI cases) were significant throughout model selection in either the daily or the time-of-day analysis, so we re-ran our final models using a full version of each dataset (i.e. adding back days for which previous-day temperature or SI data were unavailable). To explore the proportion of variation ‘explained’ by each of the final binomial models, we calculated the generalized coefficient of determination (R2 for likelihood statistics) as detailed by Nagelkerke (1991).
Post-hoc comparisons – wind direction
To further investigate the wind direction effect, we conducted post-hoc comparisons of each combination of adjacent wind directions, by running binomial mixed-effects models on subsets of the data (e.g. N vs NE, NE vs E, etc.). P values from this analysis were then corrected to control for the false discovery rate of multiple comparisons (FDR = 0.05). We also used binomial mixed models on data subsets to investigate the effect of wind speed (WindVel) on the number of SI cases for each individual wind direction (N, NW, W, etc.).
Data availability
Data are available on GitHub: https://github.com/jasonsckrabulis/sckrabulis_etal_wind_predicts_si.
Results
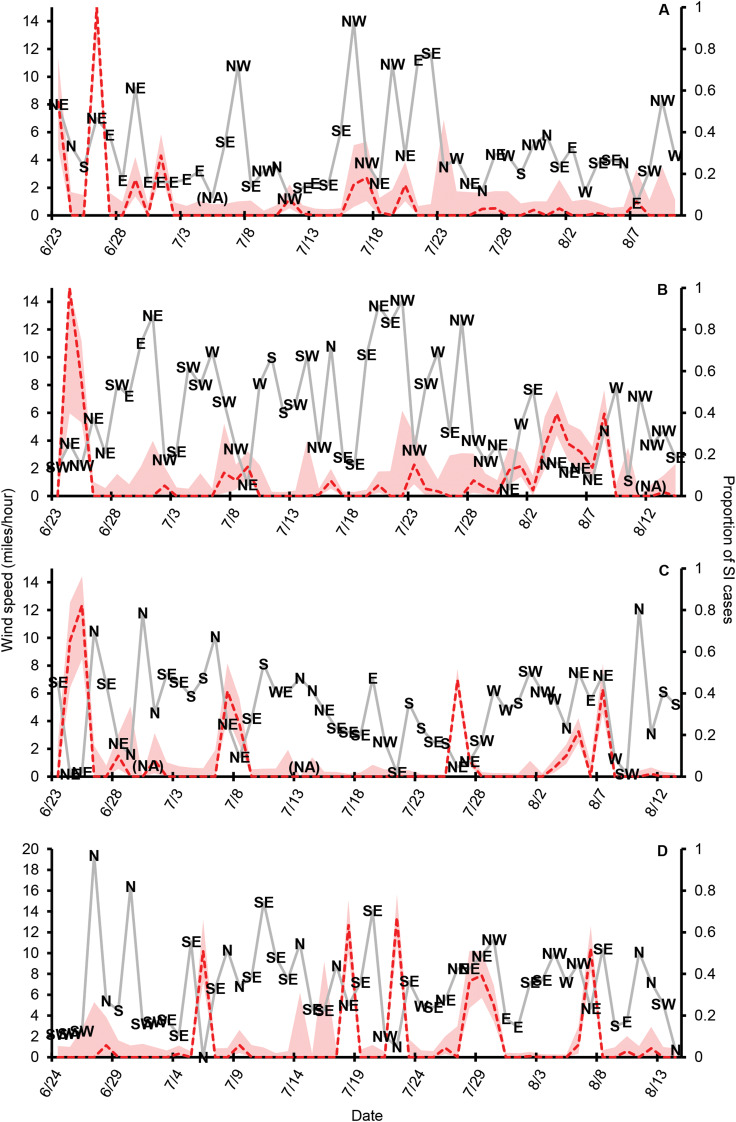
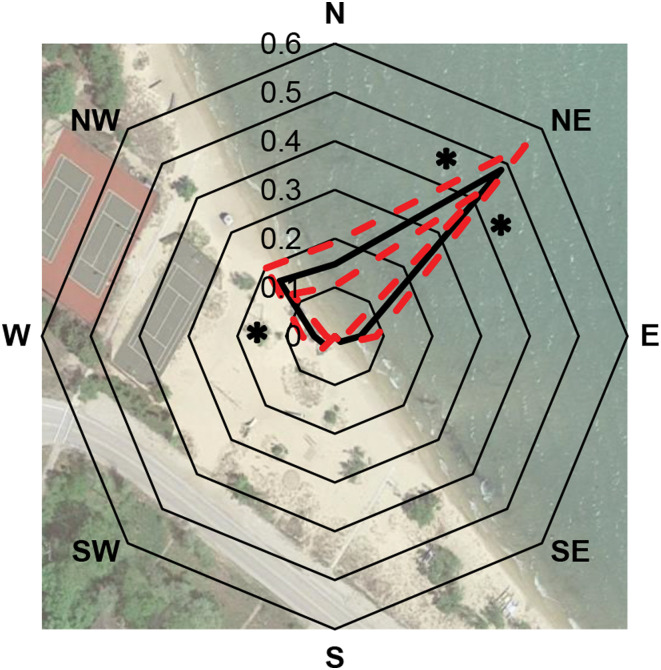
Consistent with anecdotal reports, the CSA data documents large day-to-day variation in the proportion of swimmers reporting SI (Fig. 1). The best binomial mixed-effects model for predicting daily SI risk included wind direction, wind speed and total number of swimmers, all of which were significant in the full model (P < 0.05; R2 = 0.11; Table 2). The wind direction explained more variation as a single predictor (R2 = 0.09) than either wind speed (R2 = 0.005) or number of swimmers (R2 = 0.009). Multiple comparisons analysis of adjacent wind directions revealed a significant increase in the probability of a swimmer contracting SI on days when winds originated from the northwest, north or northeast, with the highest risk of SI on days with northeasterly winds (Padj < 0.05; Fig. 2). No other wind directions were significantly different from adjacent directions (Padj > 0.05; Table 3). We found no significant effects of precipitation or current-day temperature in any statistical model, and none of the temporal variables (past temperatures or SI cases) was significant predictors in any model at any of the temporal scales we examined (P > 0.05). Temperature was not autocorrelated with the wind direction, wind speed or either precipitation variable (P > 0.05).
Fig. 1.
Daily variation in the wind direction, wind speed (solid grey line; primary axis) and proportion of swimmers reporting SI (dashed red line; secondary axis) at CSA beach in (A) 2013, (B) 2014, (C) 2015 and (D) 2016. The wind direction is indicated by text labels (N, NE, E, SE, S, SW, W, NW). The shaded area represents 95% Clopper–Pearson exact confidence intervals for binomial proportion data (‘exactci’ function in the package PropCIs; Scherer, 2018). In Panel A, the ‘(NA)’ label indicates missing a wind direction datapoint. In panels B and C, the ‘(NA)’ labels indicate no confidence interval calculation due to zero swimmers those days.
Table 2.
Likelihood ratio statistics indicating the significant contribution of environmental predictors in the final best fit models for environmental effects and time of day on SI cases
| Predictors | Coef ± s.e. | χ2 | P value (χ2) | F | df | P value (F) |
|---|---|---|---|---|---|---|
| Environmental predictors model (among-day variation) | ||||||
| WindDirGroup1 | NAa | 67.62 | <0.0001* | 22.92 | 3, 196 | <0.0001* |
| WindVel | −0.24 ± 0.08 | 9.48 | 0.002* | 11.17 | 1, 196 | 0.001* |
| SwimTotal | −0.01 ± 0.004 | 6.39 | 0.011* | 2 | 1,196 | 0.15 |
| Time of day model (within-day variation)b | ||||||
| TimeOfDay | −1.73 ± 0.10 | 301.73 | <0.0001* | 14.70 | 1, 384 | 0.0001* |
| WindDirGroup1 | NAa | 75.34 | <0.0001* | 41.40 | 3, 384 | <0.0001* |
| WindVel | −0.22 ± 0.08 | 7.26 | 0.007* | 12.56 | 1, 384 | 0.0004* |
All statistics were based on both binomial mixed-effects models (χ2 test) that incorporated a random effect of ‘Day’, which was the replication unit in the environmental predictors model, and arcsine-square root transformed linear models (F test). All other analysed predictors failed to significantly improve the model (P > 0.05) and were therefore excluded from the final model. χ2 degrees of freedom in the binomial models were equal to the numerator degrees of freedom in the linear models. Coefficients were derived from the binomial models.
Categorical predictors comprised multiple ‘“dummy variables’” and therefore do not have a single meaningful coefficient.
Model ran but with convergence warnings.
* Significant effect (P < 0.05).
Fig. 2.
Proportion of swimmers reporting SI on days with winds originating from each direction at CSA beach. The solid black line represents the average proportion of SI cases per day (arcsine-square root transformed to improve normality). Dashed red lines indicate ± 1 s.e.. Significant differences between adjacent directions are indicated by * (Padj < 0.05 after adjusting to control the false discovery rate; paired comparisons using binomial mixed-effects models). CSA beach image© 2017 Google map data, altered by J.P. Sckrabulis.
Table 3.
Multiple comparison statistics indicating the significant contribution of wind speed to SI cases in models by individual direction
| Directions | χ2 | P value (χ2) | Adj. P value (χ2) | F | df | P value (F) | Adj. P value (F) |
|---|---|---|---|---|---|---|---|
| N + NE | 14.96 | 0.0001 | 0.0004* | 14.04 | 1, 62 | 0.0004 | 0.0016* |
| NE + E | 27.31 | <0.0001 | <0.0001* | 20.81 | 1, 52 | <0.0001 | 0.0003* |
| E + SE (as a direction, not S.E.) | 0.34 | 0.5596 | 0.9756 | 1.72 | 1, 32 | 0.1992 | 0.2655 |
| SE (as a direction, not S.E.) + Sa | <0.01 | 0.9756 | 0.9756 | 2.17 | 1, 58 | 0.1459 | 0.2655 |
| S + SWa | 0.01 | 0.9057 | 0.9756 | 1.72 | 1, 32 | 0.1992 | 0.2655 |
| SW + W | 0.02 | 0.8783 | 0.9756 | 0.27 | 1, 32 | 0.6095 | 0.6966 |
| W + NW | 6.24 | 0.0125 | 0.0332* | 5.13 | 1, 39 | 0.2091 | 0.0777 |
| NW + N | 0.16 | 0.6936 | 0.9756 | 0.04 | 1, 52 | 0.8360 | 0.8360 |
All statistics were based on both binomial mixed-effects models (χ2 test) that incorporated a random effect of ‘Day’, which was the replication unit in the environmental predictors model, and arcsine-square root transformed linear models (F test). χ2 degrees of freedom in the binomial models were equal to the numerator degrees of freedom in the linear models. All predictors are categorical; therefore, all dummy variables do not have meaningful coefficients. Original and adjusted for false discovery rate (0.05) P values are reported.
Model ran but with convergence warnings.
* Significant effect P < 0.05.
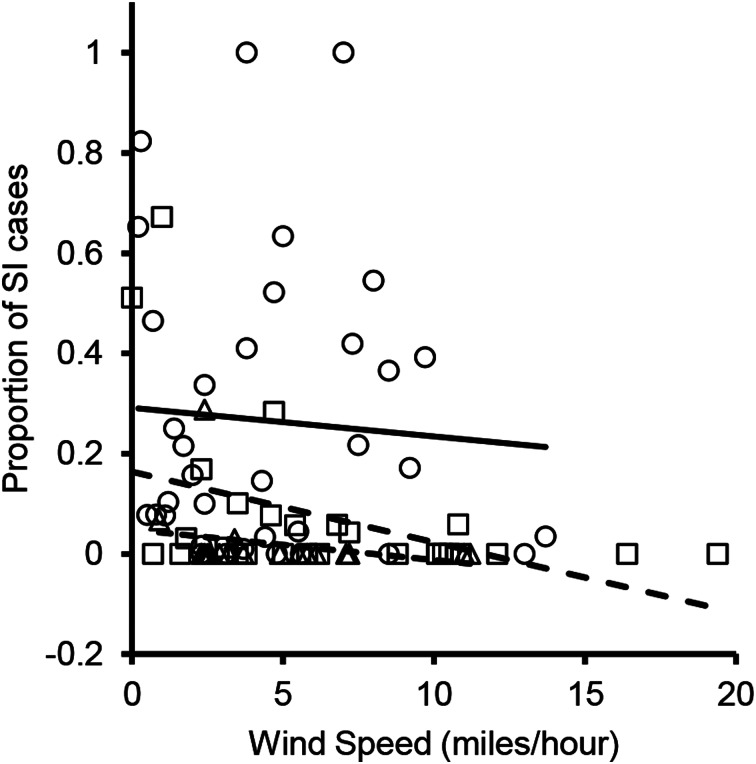
The overall effect of the wind speed on SI risk had a negative coefficient, with higher wind speeds resulting in a lower probability that an individual swimmer would report SI on a given day (P < 0.001; Table 2). A follow-up analysis of wind-speed effects for individual wind directions revealed that this effect was largely driven by winds coming from the north (Fig. 3). The wind speed had a significant negative effect on the proportion of SI cases when the wind came from the north (P = 0.0142; Table 4) but not from other directions (P > 0.05; Table 4).
Fig. 3.
Proportion of swimmers reporting SI as a function of wind speed, for days with wind originating from one of three directions: North (open squares; dashed line), Northeast (open circles; solid line) or East (open triangles; dotted line).
Table 4.
Likelihood ratio statistics indicating the significant contribution of wind speed to SI cases in models by individual direction
| Direction | Coef ± s.e. | χ2 | P value (χ2) | F | df | P value (F) |
|---|---|---|---|---|---|---|
| E | −2.12 ± 2.34 | 0.82 | 0.3649 | 2.02 | 1, 17 | 0.1732 |
| N | −0.44 ± 0.003 | 23 721a | <0.0001 | 6.85 | 1, 27 | 0.0142* |
| NE | −0.07 ± 0.003 | 685.56a | <0.0001 | 0.39 | 1, 33 | 0.5383 |
| NW | −0.09 ± 0.14 | 0.44 | 0.5071 | 0.85 | 1, 23 | 0.3654 |
| S | NAb | NAb | NAb | NAb | 1, 14 | NAb |
| SE (as a direction, not S.E.) | −0.16 ± 2.34 | 0.55 | 0.4594 | 1.25 | 1, 42 | 0.2702 |
| SW | 0.50 ± 0.73 | 0.47 | 0.4932 | 1.11 | 1, 16 | 0.3085 |
| W | 0.26 ± 0.66 | 0.16 | 0.6937 | 0.15 | 1, 14 | 0.7059 |
All statistics were based on both binomial mixed-effects models (χ2 test) that incorporated a random effect of ‘Day’, which was the replication unit in the environmental predictors model, and arcsine-square root transformed linear models (F test). χ2 degrees of freedom in the binomial models were equal to the numerator degrees of freedom in the linear models.
Model ran but with convergence warnings.
Model failed to run due to an absence of swimmer's itch cases on days with southerly winds.
* Significant effect (P < 0.05)
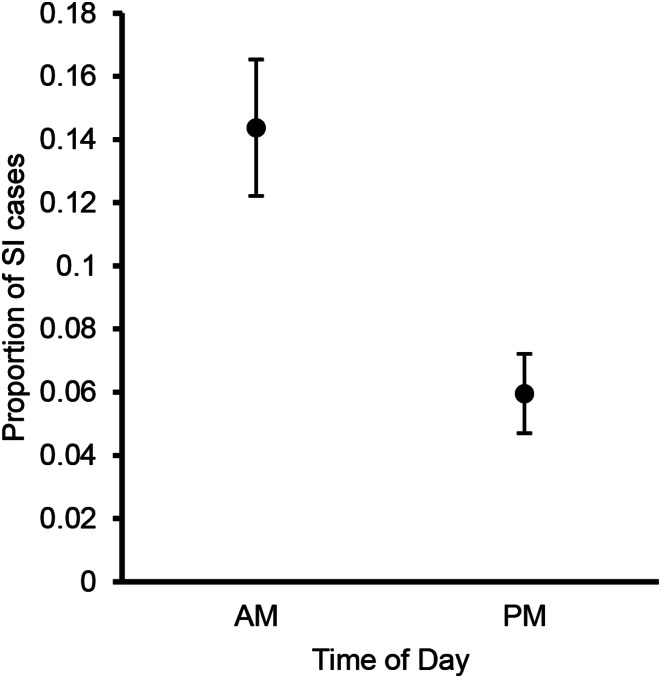
In the model describing within-day variation, time of day had a significant negative effect on the number of SI cases reported, with afternoon swimmers being less likely to report SI (Fig. 4; Table 2). The best binomial mixed-effects model for predicting the risk of SI in the time-of-day model also contained significant wind direction and wind speed effects, consistent with the final model from the analysis of daily variation (all P < 0.01; R2 = 0.24; Table 2). The final time-of-day model converged with warnings; however, the time of day effect remained significant when the other daily predictors were removed from the model (Table 2). Time of day explained more variation as a single predictor (R2 = 0.19) than either wind direction (R2 = 0.04) or wind speed (R2 = 0.002). Unlike in the among-day variation model (Table 2), the number of swimmers per day did not emerge as a significant predictor. This result implied that the SwimTotal effect in the among-day analysis was entirely accounted for by incorporating TimeOfDay into the statistical model, potentially indicating an autocorrelation between these two predictor variables. Upon investigation, we confirmed a relationship between these predictors, where days with more total swimmers also had a significantly smaller proportion of swimmers in the morning session (Fig. S3).
Fig. 4.
Average proportion of swimmers reporting SI in the morning and afternoon sessions at CSA beach. Error bars indicate ± 1 s.e..
The results from linear models (with arc-sine-transformed SI prevalence as the response variable) were qualitatively consistent with binomial model outputs (Tables 2–4), with one exception. There was no significant interaction between the wind direction and wind speed interaction in the binomial mixed-effects models, but linear regression indicated a significant interaction between these predictors in both of among day and within day analyses (both P < 0.05). An interaction between these two variables would indicate that the wind speed effect depended on the wind direction. However, wind speed effects for individual wind directions were consistently negative or non-significant (Table 4), suggesting that any interaction between these variables was driven by quantitative rather than qualitative differences (i.e. changes in the strength of the wind-speed effect).
Discussion
We found that the wind direction had highly significant effects on the probability of a swimmer reporting SI at the CSA beach, with the greatest SI risk occurring on days with direct onshore winds from the northeast (i.e. perpendicular to the shoreline; Fig. 2). The wind direction was also highly variable at CSA beach from day to day, helping to account for the large fluctuations in SI incidence from one day to the next (Fig. 1). Our findings corroborate anecdotal evidence from the lifeguards at CSA beach, where onshore wind is a commonly cited SI risk factor, as well as field monitoring conducted by Rudko et al. (2018), where wind direction was a significant predictor of cercaria abundance. This might be because most cercariae that attack swimmers at CSA beach are produced by infected snails living in offshore beds and are transported to shore via wind-driven surface currents (Upatham, 1974). Lymnaeid snails are known for preferring deep-water habitat (Laman et al., 1984), and SI-causing cercariae are known to move towards the water surface to seek out definitive hosts (Price, 1931; Talbot, 1936; Najim, 1956; Rudko et al., 2018). Direct onshore winds can cause surface currents perpendicular to the shoreline (Weber, 1983), and a combination of wind-driven onshore surface currents and surface-seeking cercaria behaviour likely results in cercariae aggregating in the shallow water where most recreational swimming occurs (Leighton et al., 2000; Verburgge et al., 2004a). While there was a significant negative effect of swimmer numbers in the among-day variation model, this effect disappeared when we accounted for the time of day in the within-day analysis (Table 2). This was likely caused by a confoundment between the number of swimmers and time of day, where days with more total swimmers had a smaller proportion of swimmers in the morning session (Fig. S3).
Northeasterly winds led to dramatically increased SI risk relative to northerly or easterly winds, despite each of these wind directions being arguably ‘on-shore’. At CSA beach, northerly or easterly winds hit the shore at an angle (Fig. 2), which might generate surface currents parallel to the beach rather than directly toward the beach, carrying cercariae away from the site and preventing an aggregation by the shoreline. This might account for northerly and easterly winds resulting in so many fewer SI cases per swimmer than direct northeasterly winds (Fig. 2). Wind from the north or northwest also generated significantly more SI cases than wind from the west or south (Fig. 2), which hints at the possible presence of an important source of cercariae located to the north of the CSA beach.
The wind speed had a significant negative effect on SI risk in our statistical models, contrary to our a priori prediction. There are a few possible explanations for this result. Relatively slow surface currents have been shown to bring cercariae many times further than they could swim on their own (Upatham, 1974), so perhaps even slow onshore winds are sufficient to induce cercaria aggregation in the shallows. Higher wind speeds likely increase wave action and turbulence, which might decrease the ability of trematode cercariae to locate and find a suitable host by continuously changing their orientations to various stimuli (Jousson and Bartoli, 2000; Fingerut et al., 2003). We also noticed that high-speed winds (greater than 10 mph) nearly always came from the north (Figs 1 and 3), and it is possible that this correlation between wind speed and wind direction influenced the overall negative effect of the wind speed in the primary statistical model (Table 2). As discussed above, northerly winds likely drive currents parallel to the shore that might prevent cercaria aggregation in the shallows, and these currents might be stronger on days with higher wind speeds. Further research is thus needed to determine whether the wind speed has a generally negative effect on SI incidence, rather than an effect specific to the orientation or hydrology of the CSA beach.
The absence of any hypothesized current or past temperature effects on SI risk was initially surprising to us, given the strong temperature effects reported in past laboratory studies of snail-borne parasites. However, relatively low local snail abundance on the CSA beach (Al Flory, pers. comm.), combined with the highly significant effect of wind direction on SI risk, suggests that a large proportion of the cercariae causing SI at this site are drifting into shore from deeper water. If this is true, then local temperatures at the CSA beach might have little effect on the deep-water infected snails that actually produced most of the cercariae. Lymnaeid snails are known for preferring deeper, colder waters far from shore and for migrating in and out of the shallows (Clarke, 1981; Correa et al., 2010). Some species of snails have also been shown to exhibit a sort of reverse behavioural fever, seeking out cool microhabitats in response to trematode infection or injury, which might result in avian schistosome-infected snails moving into even deeper water than uninfected snails (Hodasi, 1976; Żbikowska and Cichy, 2012; Żbikowska and Marszewska, 2018). Most hypotheses regarding temperature effects (i.e. the thermal stress hypothesis; Paull et al., 2015) focus on cercaria production within snails, where direct temperature effects are well documented for schistosomes and non-schistosome trematodes (Ataev, 1991; Paull et al., 2012; Studer and Poulin, 2013). Since the current dataset is limited to environmental data collected in the nearshore environment, it is poorly suited to test for hypothesized temperature effects on deep-water snail hosts. Therefore, these temperature hypotheses remain to be rigorously tested as potential drivers of day-to-day variation in trematode infection risk.
As predicted, SI risk was elevated in the morning swimming session. This was likely due to light- or temperature-induced diurnal patterns of schistosome cercaria emergence from infected host snails at sunrise (Kuntz, 1947; Horák et al., 2015) or increased recreational swimming activity in the morning (Verbrugge et al., 2004a; 2004b). Exposure to light after prolonged darkness (i.e. night) has been shown to induce high rates of cercaria emergence in the morning (Soldánová et al., 2016). Some SI-causing cercariae also have a strong attraction to light (Talbot, 1936; Wright, 1974). Avian schistosome cercariae have short lifespans due to a limited energy reserve (Islam, 1986; Żbikowska, 2005), so any cercariae still present later in the day probably have depleted energy reserves and limited capacities to seek or infect hosts (Lawson and Wilson, 1980; Whitfield et al., 2003). These results are consistent with the findings of Rudko et al. (2018), who found higher abundance of avian schistosome cercariae in water samples collected in the morning, compared to afternoon samples collected on the same day from the same sites.
As with any correlational study, the day-to-day patterns of SI risk we detected in this study would need to be further studied to determine if predictor variables are truly causal. This is particularly true for conclusions drawn from the current dataset, which was based on self-reporting data on SI incidence and collected by lay scientists. Furthermore, SI symptoms can sometimes take more than one day to appear (Augustine and Weller, 1949); thus, it is possible for SI reports to come from people who contracted SI the previous day, or after swimming at another beach. It is also important to note that the scope of this study was restricted to CSA beach, which may have unique biological or hydrological characteristics that increase the importance of wind direction compared to other locations in northern Michigan. However, the wind direction effects observed in this study were strong and consistent across multiple years, suggesting that there is a real biological connection between wind direction and SI incidence at the CSA site. Furthermore, our results support the more recent findings from Rudko et al. (2018) across multiple Michigan lakes, including CSA beach. However, further study of daily SI incidence at additional sites is needed to determine if our results are generalizable to other beaches on Crystal Lake or other lakes.
Conclusions/future directions
Overall, the wind direction was a key predictor of SI risk at CSA beach. On days when winds originated from the northeast, the risk of swimmers contracting SI was significantly increased, likely due to transport of cercariae from offshore lymnaeid snail beds via wind-induced surface currents. We also found that wind speed was a significant negative predictor in the overall model, possibly because high-speed winds create more turbulent water that decrease the ability of cercariae to find and penetrate hosts. However, this pattern was largely driven by a negative effect of high-speed winds from the north, which was less associated with high SI risk than northeasterly winds and more likely to generate water currents parallel to the shoreline. Time of day was also a key predictor of SI risk at CSA beach, with morning swimmers being more at risk for SI than afternoon swimmers, likely due to light-induced emergence of infectious cercaria at sunrise (Soldánová et al., 2016).
The results of this study highlight the importance of understanding factors driving day-to-day variation in parasite exposure, and the potential utility of this information for developing local control strategies. Indeed, CSA staff already use this information to generate recommendations for ways swimmers can mitigate their exposure to SI, by warning swimmers of potentially elevated SI risk on days with direct onshore wind. Few studies have examined drivers of day-to-day variation in exposure to trematode parasites, including the causative agents of human schistosomiasis, in part because the scientific tools needed to measure cercaria abundance in the water have only been developed in the past decade (Jothikumar et al., 2015; Rudko et al., 2018, 2019). Future studies aimed at reducing incidence of these parasitic diseases should consider the potential importance of factors driving daily or diurnal fluctuations in exposure risk.
Acknowledgements
We would like to thank Ted Fisher, Joel Buzzell, Jana Way, Ellen Herscher, the Congregational Summer Assembly, Crystal Lake Watershed Association and especially Leslie Buntin Ritter and the CSA Waterfront Staff for their efforts in collecting data for this study.
Financial support
This work was partially supported by a personal financial contribution by A.R.F. and his wife Monica Schultz. Partial support was also provided by an NSF-CAREER grant to T.R.R. (IOS-1651888) and a Teaching Assistantship from Oakland University to J.P.S.
Ethical standards
The study protocol was reviewed by the Oakland University Institutional Review Board for the Protection of Human Subjects and it was determined that the project does not meet the definition of human subjects research under the purview of the IRB according to federal regulations. More specifically, the study used a deidentified data set that cannot be linked back to individuals. It complies with the Helsinki Declaration of 1975, as revised in 2008.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182020000074.
click here to view supplementary material
Conflict of interest
None.
References
- Anderson PA, Nowosielski JW and Croll NA (1976) The emergence of cercariae of Trichobilharzia ocellata and its relationship to the activity of its host snail Lymnaea stagnalis. Canadian Journal of Zoology 54, 1481–1487. [DOI] [PubMed] [Google Scholar]
- Ataev G (1991) Temperature influence on the development and biology of rediae and cercariae of Philophthalamus rhionica (Trematoda). Parazitologija 25, 349–359. [Google Scholar]
- Augustine DL and Weller TH (1949) Experimental studies on the specificity of skin tests for the diagnosis of schistosomiasis. Journal of Parasitology 35, 461–466. [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B and Walker S (2015) Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67, 1–48. [Google Scholar]
- Brant SV and Loker ES (2009) Molecular systematics of the avian schistosome genus Trichobilharzia (Trematoda: Schistosomatidae) in North America. Journal of Parasitology 95, 941–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker S (2007) Spatial epidemiology of human schistosomiasis in Africa: risk models, transmission dynamics and control. Transactions of the Royal Society of Tropical Medicine and Hygiene 101, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck JC and Lutterschmidt WI (2017) Parasite abundance decreases with host density: evidence of the encounter-dilution effect for a parasite with a complex life cycle. Hydrobiologia 784, 201–210. [Google Scholar]
- Bush KF, Fossani CL, Li S, Mukherjee B, Gronlund CJ and O'Neill MS (2014) Extreme precipitation and beach closures in the Great Lakes region: evaluating risk among the elderly. International Journal of Environmental Research and Public Health 11, 2014–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers JE, Holmes ZC and Blakeslee AMH (2016) Consistency of trematode infection prevalence in host populations across large spatial and temporal scales. Ecology 97, 1643–1649. [DOI] [PubMed] [Google Scholar]
- Ciddio M, Mari L, Gatto M, Rinaldo A and Casagrandi R (2015) The temporal patterns of disease severity and prevalence in schistosomiasis. Chaos: An Interdisciplinary Journal of Nonlinear Science 25, 1–10. [DOI] [PubMed] [Google Scholar]
- Clampitt PT (1970) Comparative ecology of the snails Physa gyrina and Physa integra (Basommatophora: Physidae). Malacologia 10, 113–151. [Google Scholar]
- Clarke AH (1981) The Freshwater Molluscs of Canada. Ottawa, Canada: National Museum of Natural Sciences, National Museums of Canada. [Google Scholar]
- Clements ACA, Moyeed R and Brooker S (2006) Bayesian Geostatistical prediction of the intensity of infection with Schistosoma mansoni in east Africa. Parasitology 133, 711–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coady NR, Muzzall PM, Burton TM, Snider RJ, Saxton J, Sergeant M and Sommers A (2006) Ubiquitous variability in the prevalence of Trichobilharzia stagnicolae (Schistosomatidae) infecting Stagnicola emarginata in three northern Michigan lakes. Journal of Parasitology 92, 10–15. [DOI] [PubMed] [Google Scholar]
- Correa AC, Escobar JS, Durand P, Renaud F, David P, Jarne P, Pointeir J and Hurtrez-Bousses S (2010) Bridging gaps in the molecular phylogeny of the Lymnaeidae (Gastropoda: Pulmonata), vectors of fascioliasis. BMC Evolutionary Biology 10, 1–12. doi: 10.1186/1471-2148-10-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Costa C, Dreyfuss G, Rakotondravao and Rondelaud D (1994) Several observations concerning cercarial sheddings of Fasciola gigantica from Lymnaea natalensis. Parasite 1, 44. [DOI] [PubMed] [Google Scholar]
- Feiler W and Haas W (1988) Host-finding in Trichobilharzia ocellata cercariae: swimming and attachment to the host. Parasitology 96, 493–505. [DOI] [PubMed] [Google Scholar]
- Fingerut JT, Zimmer CA and Zimmer RK (2003) Larval swimming overpowers turbulent mixing and facilitates transmission of a marine parasite. Ecology 84, 2502–2515. [Google Scholar]
- Fox J and Weisberg S (2011) An R Companion to Applied Regression, 2nd Edn. Thousand Oaks, California: Sage. http://socserv.socsci.mcmaster.ca/jfox/Books/Companion. [Google Scholar]
- Frank GH (1966) The effect of temperature on the rate of development and emergence of schistosome cercariae. Zoologica Africana 2, 211–221. [Google Scholar]
- Galaktionov KV, Bustnes JO, Bårdsen B, Wilson JG, Nikolaev KE, Sukhotin AA, Skírnisson K, Saville DH, Ivanov MV and Regel KV (2015) Factors influencing the distribution of trematode larvae in blue mussels Mytilus edulis in the North Atlantic and Arctic Oceans. Marine Biology 162, 193–206. [Google Scholar]
- Haas W and van der Roemer A (1998) Invasion of the vertebrate skin by cercariae of Trichobilharzia ocellata: penetration processes and stimulating host signals. Parasitology Research 84, 787–795. [DOI] [PubMed] [Google Scholar]
- Haeberlein S and Haas W (2008) Chemical attractants of human skin for swimming Schistosoma mansoni cercariae. Parasitology Research 102, 657–662. [DOI] [PubMed] [Google Scholar]
- Halstead NT, McMahon TA, Johnson SA, Raffel TR, Romansic JM, Crumrine PW and Rohr JR (2014) Community ecology theory predicts the effect of agrochemical mixtures on aquatic biodiversity and ecosystem properties. Ecology Letters 17, 932–941. [DOI] [PubMed] [Google Scholar]
- Halstead NT, Hoover CM, Arakala A, Civitello DJ, De Leo GA, Gambhir M, Johnson SA, Jouanard N, Loerns KA, McMahon TA, Ndione RA, Nguyen K, Raffel TR, Remais JV, Riveau G, Sokolow SH and Rohr JR (2018) Agrochemicals increase risk of human schistosomiasis by supporting higher densities of intermediate hosts. Nature Communications 9, 1–10. doi: 10.1038/s41467-018-03189-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodasi JKM (1976) The effects of low temperature on Lymnaea truncatula. Zeitschrift Für Parasitenkunde 48, 281–286. [DOI] [PubMed] [Google Scholar]
- Horák P, Mikeš L, Lichtenbergová L, Skála V, Soldánová M and Brant SV (2015) Avian schistosomes and outbreaks of cercarial dermatitis. Clinical Microbiology Reviews 28, 165–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam KS (1986) The morphology and life-cycle of Trichobilharzia arcuata n. sp. (Schistosomatidae: Bilharziellinae) a nasal schistosome of water whistle ducks (Dendrocygna arcuata) in Australia. Systematic Parasitology 8, 117–128. [Google Scholar]
- Jothikumar N, Mull BJ, Brant SV, Loker ES, Collinson J, Secor WE and Hill VR (2015) Real-time PCR and sequencing assays for rapid detection and identification of avian schistosomes in environmental samples. Applied and Environmental Microbiology 81, 4207–4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jousson O and Bartoli P (2000) The life cycle of Opecoeloides columbellae (Pagenstecher, 1863) n. comb. (Digenea, Opecoelidae): evidence from molecules and morphology. International Journal for Parasitology 30, 747–760. [DOI] [PubMed] [Google Scholar]
- Kolářová L, Horák P and Skírnisson K (2010) Methodical approaches in the identification of areas with a potential risk of infection by bird schistosomes causing cercarial dermatitis. Journal of Helminthology 84, 327–335. [DOI] [PubMed] [Google Scholar]
- Kube J, Kube S and Dierschke V (2002) Spatial and temporal variations in the trematode component community of the mudsnail Hydrobia ventrosa in relation to the occurrence of waterfowl as definitive hosts. Journal of Parasitology 88, 1075–1086. [DOI] [PubMed] [Google Scholar]
- Kuntz RE (1947) Effect of light and temperature on emergence of Schistosoma mansoni cercariae. Transactions of the American Microscopical Society 66, 37–49. [PubMed] [Google Scholar]
- Laman TG, Boss NC and Blankespoor HD (1984) Depth distribution of seven species of gastropods in Douglas Lake, Michigan. The Nautilus 98, 20–25. [Google Scholar]
- Lawson JR and Wilson RA (1980) The survival of the cercariae of Schistosoma mansoni in relation to water temperature and glycogen utilization. Parasitology 81, 337–348. [DOI] [PubMed] [Google Scholar]
- Leighton BJ, Zervos S and Webster JM (2000) Ecological factors in schistosome transmission, and an environmentally benign method for controlling snails in a recreational lake with a record of schistosome dermatitis. Parasitology International 49, 9–17. [DOI] [PubMed] [Google Scholar]
- McClelland WJF (1965) The production of cercariae by Schistosoma mansoni and S. haematobium and methods for estimating the numbers of cercariae in suspension. Bulletin World Health Organization 33, 270–276. [PMC free article] [PubMed] [Google Scholar]
- McCreesh N and Booth M (2014) The effect of increasing water temperatures on Schistosoma mansoni transmission and Biomphalaria pfeifferi population dynamics: an agent-based modelling study. PLoS One 9, e101462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzzall PM, Burton TM, Snider RJ and Coady NR (2003) Occurrence, Distribution and Control of the Parasites that Cause Swimmer's Itch in Michigan, Extension Bulletin WQ 58. Michigan State University, Lansing, Michigan: Michigan State University Extension.
- Nagelkerke NJD (1991) A note on the general definition of the coefficient of determination. Biometrika 78, 691–692. [Google Scholar]
- Najim AT (1956) Life history of Gigantobilharzia huronensis Najim, 1950. A dermatitis-producing bird blood-fluke (Trematoda-Schistosomatidae). Parasitology 46, 443–469. [DOI] [PubMed] [Google Scholar]
- Paull SH, LaFonte BE and Johnson PTJ (2012) Temperature-driven shifts in a host-parasite interaction drive nonlinear changes in disease risk. Global Change Biology 18, 3558–3567. [Google Scholar]
- Paull SH, Raffel TR, LaFonte BE and Johnson PTJ (2015) How temperature shifts affect parasite production: testing the roles of thermal stress and acclimation. Functional Ecology 29, 941–950. [Google Scholar]
- Price HF (1931) Life history of Schistosomatium douthitti (Cort). American Journal of Hygiene 13, 685–727. [Google Scholar]
- R Core Team (2019) R: A Language and Environment for Statistical Computing. [computer software]. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Raso G, Matthys B, N'Goran EK, Tanner M, Vounatsou P and Utzinger J (2005) Spatial risk prediction and mapping of Schistosoma mansoni infections among schoolchildren living in western Côte d'Ivoire. Parasitology 131, 97–108. [DOI] [PubMed] [Google Scholar]
- Rudko SP, Reimink RL, Froelich K, Gordy MA, Blankespoor CL and Hanington PC (2018) Use of qPCR-based cercariometry to assess swimmer's itch in recreational lakes. Ecohealth 15, 827–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudko SP, Turnbull A, Reimink RL, Froelich K and Hanington PC (2019) Species-specific qPCR assays allow for high-resolution population assessment of four species avian schistosome that cause swimmer's itch in recreational lakes. International Journal for Parasitology: Parasites and Wildlife 9, 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer R (2018) Propcis: Various confidence interval methods for proportions. [computer software].
- Sokolow SH, Huttinger E, Jouanard N, Hsieh MH, Lafferty KD, Kuris AM, Riveau G, Senghor S, Thiam C, N'Diaye A, Sarr Faye D and De Leo GA (2015) Reduced transmission of human schistosomiasis after restoration of a native river prawn that preys on the snail intermediate host. Proceedings of the National Academy of Sciences 112, 9650–9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldánová M, Selbach C and Sures B (2016) The early worm catches the bird? Productivity and patterns of Trichobilharzia szidati cercarial emission from Lymnaea stagnalis. PLoS One 11, e0149678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear RC, Zhong B, Mao Y, Hubbard A, Birkner M, Remais J and Qiu D (2004) Spatial and temporal variability in schistosome cercarial density detected by mouse bioassays in village irrigation ditches in Sichuan, China. The American Journal of Tropical Medicine and Hygiene 71, 554–557. [PubMed] [Google Scholar]
- Studer A and Poulin R (2013) Differential effects of temperature variability on the transmission of a marine parasite. Marine Biology 160, 2763–2773. [Google Scholar]
- Sunada H, Totani Y, Nakamura R, Sakakibara M, Lukowaik K and Ito E (2017) Two strains of Lymnaea stagnalis and the progeny from their mating display differential memory-forming ability on associative learning tasks. Frontiers in Behavioral Neuroscience 11, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot SB (1936) Studies on schistosome dermatitis: II. Morphological and life history studies on three dermatitis-producing schistosome cercariae, C. elvae Miller, 1923, C. stagnicolae N. sp., and C. physellae n. sp. American Journal of Epidemiology 23, 372–384. [Google Scholar]
- Upatham ES (1974) Dispersion of St. Lucian Schistosoma mansoni cercariae in natural standing and running waters determined by cercaria counts and mouse exposure. Annals of Tropical Medicine and Parasitology 68, 343–352. [DOI] [PubMed] [Google Scholar]
- Valdovinos C and Balboa C (2007) Cercarial dermatitis and lake eutrophication in south-central Chile. Epidemiology and Infection 136, 391–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbrugge LM, Rainey JJ, Reimink RL and Blankespoor HD (2004a) Prospective study of swimmer's itch incidence and severity. Journal of Parasitology 90, 697–704. [DOI] [PubMed] [Google Scholar]
- Verbrugge LM, Rainey JJ, Reimink RL and Blankespoor HD (2004b) Swimmer's itch: incidence and risk factors. American Journal of Public Health 94, 738–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JE (1983) Steady wind- and wave-induced currents in the open ocean. Journal of Physical Oceanography 13, 524–530. [Google Scholar]
- Whitfield PJ, Bartlett A, Khammo N and Clothier RH (2003) Age-dependent survival and infectivity of Schistosoma mansoni cercariae. Parasitology 127, 29–35. [DOI] [PubMed] [Google Scholar]
- Wright DGS (1974) Responses of cercariae of Trichobilharzia ocellata to white light, monochromatic light, and irradiance reduction. Canadian Journal of Zoology 52, 575–579. [DOI] [PubMed] [Google Scholar]
- Yang GJ, Gemperli A, Vounatsou P, Tanner M, Zhou XN and Utzinger J (2006) A growing degree-days based time-series analysis for prediction of Schistosoma japonicum transmission in Jiangsu province, China. The American Journal of Tropical Medicine and Hygiene 75, 549–555. [PubMed] [Google Scholar]
- Żbikowska E (2005) Do larvae of Trichobilharzia szidati and Echinostoma revolutum generate behavioral fever in Lymnaea stagnalis individuals? Parasitology Research 97, 68–72. [DOI] [PubMed] [Google Scholar]
- Żbikowska E and Cichy A (2012) Symptoms of behavioural anapyrexia – reverse fever as a defence response of snails to fluke invasion. Journal of Invertebrate Pathology 109, 269–273. [DOI] [PubMed] [Google Scholar]
- Żbikowska E and Marszewska A (2018) Thermal preferences of bird schistosome snail hosts increase the risk of swimmer's itch. Journal of Thermal Biology 78, 22–26. [DOI] [PubMed] [Google Scholar]
- Zhou YB, Yang MX, Yihuo WL, Liu GM, Wang HY, Wei JG and Jiang QW (2011) Effect of habitat fragmentation on the schistosome-transmitting snail Oncomelania hupensis in a mountainous area of China. Transactions of the Royal Society of Tropical Medicine and Hygiene 105, 189–196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182020000074.
click here to view supplementary material
Data Availability Statement
Data are available on GitHub: https://github.com/jasonsckrabulis/sckrabulis_etal_wind_predicts_si.