Abstract
Background:
The current drugs for Chagas disease treatment present several limitations
Methods:
The sesquiterpene lactone goyazensolide (GZL) was evaluated regarding to cytotoxicity and trypanocidal activity against amastigotes, selectivity index (SI) in vitro, acute toxicity and anti-Trypanosoma cruzi activity in vivo.
Results:
The in vitro cytotoxicity in H9c2 cells was observed at doses >250 ng mL−1 of GZL and the SI were of 52.82 and 4.85 (24 h) and of 915.00 and 41.00 (48 h) for GZL and BZ, respectively. Nephrotoxicity and hepatotoxicity were not verified. Treatment with GZL of mice infected with CL strain led to a significant decrease of parasitaemia and total survival at doses of 1 and 3 mg kg−1 day−1 by oral and IV, respectively. This last group cured 12.5% of the animals (negativation of HC, PCR, qPCR and ELISA). Animals infected with Y strain showed significant decrease of parasitaemia and higher negativation in all parasitological tests in comparison to BZ and control groups, but were ELISA reactive, as well as the BZ group, but mice treated with 5.0 mg kg−1 day−1 by oral were negative in parasitological tests and survived.
Conclusion:
GZL was more active against T. cruzi than benznidazole in vitro and presented important therapeutic activity in vivo in both T. cruzi strains.
Key words: Goyazensolide, in vitro, in vivo, toxicity, treatment, Trypanosoma cruzi
Introduction
Chagas disease (CD), discovered by Carlos Chagas in 1909 (Chagas, 1909), is a parasitic disease caused by the protozoan haemoflagellate Trypanosoma cruzi. CD is endemic in 21 Latin American countries where the most important epidemiological mechanism of transmission is by the haematophagous insects of the Reduviidae family, Triatominae subfamily. However, today CD is a new global challenge due to its expansion to several non-endemic countries of different continents, as a consequence of the migration of infected individuals (Schmunis and Yadon, 2010; Coura, 2013a, 2013b). In these countries, the parasite is transmitted by insect vector-independent mechanisms, such as blood transfusion, vertical, transplants and shared use of syringes. Unfortunately, and after almost 60 years of the first active drugs discovery, benznidazole (BZ) and nifurtimox (NF), used for human treatment (Coura and Borges-Pereira, 2012), none new compound more advantageous has been discovered by the scientific community. Unfortunately, the expectation regarding the recent clinical essays with the azolic derivatives was not achieved. It was demonstrated that although the posaconazole and E1224 have presented a favourable profile of safety and toxicity, they failed in the induction of parasitological cure in approximately 90% of the treated patients (Pérez-Molina et al., 2015). BZ and NF are highly effective only in the acute phase and recent chronic infections (<14 years), but during the later chronic disease the efficacy of both is considered low (0 to 19%) (Guedes et al., 2008). Moreover, they are associated with important side effects, which lead to limitations of patient's compliance (Bern, 2015; Bermudez et al., 2016). Even with an estimation of 8 to 8.5 million people infected worldwide with T. cruzi and about 10 000 deaths per year (WHO, 2018), CD treatment remains neglected, although a wide variety of compounds have been investigated for the etiological treatment, always seeking for a safe and effective chemotherapeutic agent (Bahia et al., 2014).
Plant-derived products represent a vast source of compounds that may be potentially active against protozoans (Croft et al., 2005; Salem and Werbovetz, 2006). Although they have been studied, there are still many compounds to be discovered and evaluated. Asteraceae family is the largest plant family studied to date, which has sesquiterpene lactones (SL) as chemical markers (Bohlmann et al., 1980; Schmidt et al., 2013). Some of these lactones have been tested in vitro and in vivo and have shown significant anti-T. cruzi activities (Sulsen et al., 2008, 2013). Studies using natural products of Asteraceae family were performed previously in vitro (Chiari et al., 1991, 1996; Oliveira et al., 1996). More recently, our team (Branquinho et al., 2014b) developed a study using sesquiterpene lactone (SL) lycnopholide (LYC) nanoencapsulated for treatment of mice experimentally infected with the CL and Y strains of T. cruzi, sensitive and partially sensitive to BZ and NF treatments, respectively (Filardi and Brener, 1987). Animals infected with both strains and treated with nanoencapsulated LYC in pelleted polymer formulations were able to promote 100% of parasitological cure in mice infected with both T. cruzi strains during the acute phase of the infection. Another study carried out by Mello et al. (2016) also demonstrated the efficacy of LYC nanocapsules (LYC-PLA-PEG-NC) in mice infected with Y strain of T. cruzi treated by oral and intravenous routes during the acute and chronic phases of infection.
In the present study, we invested in the evaluation of another sesquiterpene lactone, the goyazensolide (GZL), with the same alkylating functional groups as LYC. The focus was to evaluate the toxicity and anti-T. cruzi activity of GZL in vitro, followed by in vivo study in mice infected with this parasite.
Material and methods
Compounds
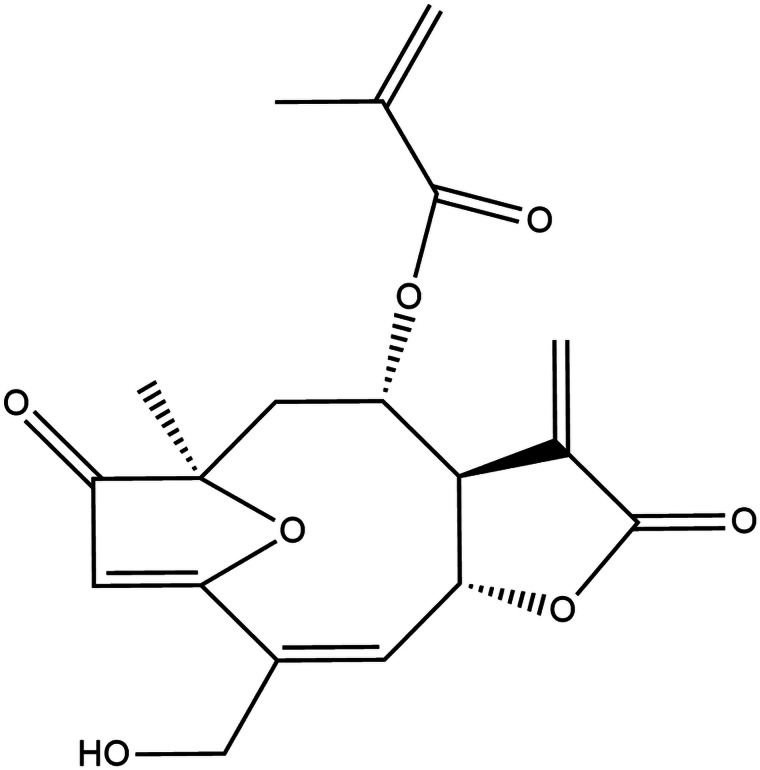
The sesquiterpene lactone Goyazensolide (GZL) (Fig. 1), colourless solid and with melting point 168.7–169.5 °C, the main metabolite present in the chloroformic extract from the aerial parts of the plant Lychnophora passerina was isolated and quantified by the HPLC-DAD method developed and validated by Ugoline et al. (2017) in the Laboratório de Plantas Medicinais (LAPLAMED), Escola de Farmácia (CiPHARMA), Universidade Federal de Ouro Preto (UFOP) and provided for the accomplishment of this work. Benznidazole (2-nitroimidazole-(N-benzyl-2-nitro-1-imidazoleacetamide)), purchased from LAFEPE/PE, was used as a reference drug in comparison to GZL.
Fig. 1.
Chemical structure of the sesquiterpene lactone goyazensolide.
T. cruzi strains
CL strain, classified as TcVI (Zingales et al., 2009), and Y strain classified as TcII (Zingales et al., 2009), both obtained from mice in the acute phase of the infection were used. The strains were previously checked genetically as TcVI and TcII, respectively (de Oliveira et al., 2017).
Animals and ethic aspects
Female Swiss mice, 28–30 days old, were used and obtained from the Centro de Ciência Animal of the UFOP (CCA-UFOP), MG, Brazil. The animals were kept in the CCA-UFOP following the rules established by the Conselho Nacional de Controle de Experimentação Animal (CONCEA) according to the international guidelines. Animals were kept in a conventional room at 20 to 24 °C, 12–12 h light–dark cycle and receiving filtered water and balanced commercial feed ‘ad libitum’. All procedures carried out in mice were approved by the institutional Comitê de Ética em Experimentação Animal (CEUA-UFOP), MG, Brazil, protocol 2016-45.
Preparation of GZL and BZ solutions for in vitro and in vivo studies
For the in vitro experiments, purified GZL was dissolved in DMSO at a concentration of 1 mg mL−1, followed by subsequent dilutions in DMEM high glucose, 10% FBS medium, with the final concentration of DMSO always lower than 1%.
For the in vivo experiments GZL was initially dissolved in DMSO (13%) and further diluted in Cremophor® (25%) and distilled water (62%) according to (Acuna et al., 2013). For oral administrations, doses of 1.0, 5.0 and 25.0 mg kg−1 day−1 of GZL were prepared in an administration volume of 0.2 mL animal−1 day−1. For animals treated by IV route, doses of 1.0 and 3.0 mg kg−1 day−1 were prepared in a volume of 0.06 mL animal−1 day−1.
The isolation and purification of BZ for in vitro tests were prepared as described by (Branquinho et al., 2014a). Briefly, BZ suspension was obtained from Rochagan tablets dissolved in MeOH. The suspension was filtered through Whatman filter paper and concentrated in a rotary evaporator, followed by recrystallization in MeOH–H20. The crystals were filtered and dried under a vacuum in a desiccant containing anhydrous silica. For the in vivo procedures, a suspension of benznidazole (tablet) in gum Arabic and distilled water was used.
In vitro cytotoxicity and anti-T. cruzi assays
To determine the cytotoxicity of GZL and BZ and evaluate their toxic effects on host cells, the cardiac H9c2 (American Type Culture Collection, ATCC: CRL 1446) line cells derived from neonate rats were treated with different concentrations of both compounds.
For the cytotoxicity test, uninfected cardiac cells were incubated in the presence or absence of each compound (GZL and BZ), diluted in DMEM, for 24 and 48 h at 37 °C. The viable cell rates were assessed by MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide; Sigma-Aldrich] (Mosmann et al., 1983) colorimetric test. The absorbance was measured at 570 nm in a spectrophotometer (Bio-Rad Model 680 – microplate manager). Considering the values of LC50 feasible doses were calculated to proceed the trypanocidal effect tests in vitro.
For the analysis of the trypanocidal effect, H9c2 cells were infected with Y strain and washed 24 h after the infection to remove free trypomastigotes. The initial concentration of parasites/cell was 10:1. The culture was then incubated for 24 or 48 h in the presence of increasing and non-toxic concentrations of GZL (0.039 to 2.78 µm) and BZ (6 to 192 µm) diluted in DMEM and maintained at 37 °C under a 5% of CO2 atmosphere. After incubation, the cells were fixed and stained with Giemsa's solution and the mean number of infected cells, as well as the mean number of parasites per infected cell was recorded. The activity of the substances was obtained by the infection index (percentage of infected cells × average number of intracellular amastigotes/infected host cell). This percentage of inhibition was then used to determine the dose effect curve and IC50 using the graphic PAD Prism 6.0 and CompuSyn software. The selectivity index (SI) was obtained by calculating the ratio between LC50 value of MTT essay and the EC50 (concentration of the active substance that reduces the number of parasites in 50%) value of the anti-T. cruzi activity test (Romanha et al., 2010). The tests were performed in triplicate. Control groups of infected and not treated cells by both compounds were evaluated in parallel.
Acute toxicity for mice: biochemical and haematological evaluations
The parameters used for the evaluation of acute toxicity included: clinical signs evaluation (NOAEL effect – no observed adverse effect level), mortality, haematological and biochemical analysis.
Therefore, five male and five female Swiss mice, 20–25 g of body weight were used. Haematological and biochemical examinations were performed 14 days after GZL treatment. Haematological parameters evaluated included red blood cell count, leukocytes (total and differential), platelets, dosages of haemoglobin and haematocrit. To determine hepatic function, dosages of the enzymes alanine amino transferase (ALT) and aspartate amino transferase (AST) were performed: Nephrotoxicity was verified by determination of serum concentrations of urea and creatinine. All evaluations for the toxicity experiments followed the OECD guidelines (Organization for Economic Co-operation and Development, 2001; Brasil, 2004). The not treated (NT) animals were evaluated in parallel as the control group.
For the maximum tolerated dose (MTD) test of GZL, the animals were divided into groups containing one male and one female mice, and single doses of GZL (50, 100, 150, 200 and 250 mg kg−1 of body weight) were administered by oral route (Zimmermann et al., 2012). Mice were then evaluated regarding toxicity signals as recommended by OECD. Forty-eight h after administration of GZL, the MTD values were obtained by the observation of survival rates of the animals.
Evaluation of the activity of goyazensolide in the murine model
Mice infection and treatment schemes
These evaluations were performed in groups of eight mice inoculated with CL and Y T. cruzi strains. Animals were inoculated by intraperitoneal route (IP) with an inoculum of 10 000 blood trypomastigotes obtained from Swiss mice in the day of parasitaemia peak counted according to Brener (1962).
Therapeutic activity in vivo
Treatments of the mice were performed by oral route with the GZL solutions at doses of 1.0, 5.0 and 25.0 mg kg−1 day−1 by oral, and by IV route with doses of 1.0 and 3.0 mg kg−1 day−1. Treatment started on the first day of patent parasitaemia, 4th and 9th days after inoculation in animals infected with Y and CL strains, respectively. Volumes of 0.2 mL of the solutions with GZL or BZ were administered for the animals treated by the oral route and a volume of 0.06 mL for those IV treated as already described. Control groups treated with BZ/100 mg mouse−1 day−1 and INT (infected and not treated) were included and evaluated in parallel.
Evaluations of treated animals
Parasitaemia: The evaluation of the parasitaemia curve (PAR) was performed daily by fresh blood examination (FBE) according to the methodology of Brener (1962). The count of the parasites in mice inoculated with the CL and Y strains started on the 9th and 5th day after infection, respectively. FBE was performed throughout the patent period, until no more parasites were observed in the blood for at least five consecutive days or until the occurrence animal's death. The PAR curve was plotted using the daily mean count of the number of parasites/0.1 mL of blood.
Hemoculture (HC): Blood collection for HC evaluation was performed 90, 180 and 240 days post-treatment (d.p.t.) only in animals with negative FBE according to Filardi and Brener (1987). The tubes were homogenized daily and microscopically examined at 30, 60, 90 and 120 days to verify the presence of parasites.
PCR (polymerase chain reaction): For PCR, 0.2 mL of blood were collected by the orbital plexus of each animal at 90, 180 and 240 d.p.t., which were immediately added to 0.4 mL of guanidine solution (Guanidin-HCl) 6 M/ethylenediethylnitrite-acetic acid (EDTA) 0.2 M/pH 8.0 (Avila et al. 1991). Samples were kept at room temperature and treated for DNA cleavage (Britto et al., 1993). An aliquot of 0.2 ml of the lysate was subjected to DNA extraction using the WizardTM Genomic DNA Purification kit (Promega) following all manufacturer's recommendations. PCR was performed using the primers 5 = −AAATAATGTACGGG[T/G]GAGATGCATGA-3 = and 5 = −GGTTCGATTGGGGTTGGTGTAATATA-3 = (Invitrogen, São Paulo, Brazil), which amplify a 330-bp sequence from kDNA as previously described (Avila et al., 1991). Amplification of the DNA was processed in a PTC-150 thermocycler (MJ Research, Ramsey, MA, USA) in 35 cycles as described by Gomes et al. (1998) modified.
Enzyme-linked immunosorbent assay (ELISA): Serum samples from all surviving animals were collected 90, 180 and 240 (d.p.t.). The ELISA reaction for detection of anti-T. cruzi IgG antibodies was performed and standardized in the Laboratory of Chagas Disease, UFOP (Voller et al., 1976; Santos et al., 2012). Sera samples were diluted 1:80 for analysis and peroxidase-labelled anti-mouse IgG conjugate diluted 1:2000 (SIGMA, St. Louis, USA) in buffered saline with 0.05% Tween 20 (PBS-Tween) were used. The reaction was read in a spectrophotometer (Bio-Rad Model 680 – microplate manager) using the filter at 490 nm. Samples containing absorbance values above the cut-off point (mean absorbance of 10 standard non-reactive serum + 2 times the standard deviation) were considered reactive, and those with absorbance values below the cut-off point were considered non-reactive. All samples were tested in duplicate and the final absorbance value was the average of the duplicates reading.
qPCR in heart tissue
The molecular technique of real time PCR was performed in cardiac tissue at 240 d.p.t. after necropsy of the animals according to the protocol of Caldas et al. (2012). The heart tissue previously stored at −80 °C was cut in fragments of 15–30 mg. DNA extraction was performed using the WizardTM Genomic DNA Purification kit (Promega) following the manufacturer's recommendations. For enzymatic digestion of the heart fragments, 30.0 µL of Proteinase K (Sigma-Aldrich®, USA) at the concentration of 20.0 mg mL−1 were added. To standardize the measure of parasite DNA detection in mouse heart fragments by qPCR, a standard curve was constructed in order to determine the number of T. cruzi DNA copies, which was used as reference. DNA dilutions were submitted to the same protocol of DNA extraction already described and were amplified as described: Samples were analysed in duplicate for amplification of T. cruzi DNA. For each qPCR reaction 3.0 µL of the diluted extracted sample containing 30.0 ng of genomic DNA, 5.0 µL of GoTaq® qPCR Master Mix (Promega®) and 10 µm of each primer were used: TCZ-F 5′-GCTCTTGCCCACAMGGGTGC-3′, wherein M = A or C, and TCZ-R 5′-CCAAGCAGCGGATAGTTCAGG-3′. In the same plaque, the reaction was also performed in unicate to assess the murine-specific tumour necrosis factor alpha (TNF-α), used as endogenous control, using the primers: TNF-5241 5′-TCCCTCTCATCAGTTCTATGGCCCA-3′, and TNF-5411 5′-CAGCAAGCATCATAGCACTTAGACCCC-3′ – R 5′ – CCAAGCAGCGGATAGTTCAGG-3′ according to Cummings and Tarleton (2003). All plates contained the test samples, negative and positive controls of the reaction. Each DNA sample was analysed in triplicate (duplicate for T. cruzi and unicate for TNF-α). PCR reactions were performed on 96 well plates – MicroAmp®Optical 96 – Well Reaction Plate (Applied Biosystems by Life Technologies, USA), coated with Optical Adhesive Covers (Applied Biosystems by Life Technologies, USA) and processed in ABI Prism 7500 Sequence Detection System Thermal Cycler (Applied Biosystems, USA).
Survival rates: The survival rates of the animals were evaluated daily and expressed in cumulative percentage.
Cure criteria. The classical cure criterion according to II Brazilian Consensus on Chagas Disease, 2015 (Dias, 2016) was used. The susceptibility or resistance to treatments was defined according to the percentage of negative results obtained by the interpretation of the set of parasitological (FBE, HC and PCR) and serological (ELISA) methods used in the evaluation. Animals that were negative in all parasitological and serological tests were considered cured.
Additionally, the heart qPCR test of high sensibility and specificity (Caldas et al., 2012) was performed for better evaluation of the therapeutic activity.
Statistical analyses: Statistical analyses of data were carried out using Prism software v5.02 (GraphPad Software, San Diego, CA). Data were initially assessed by one-way analysis of variance (ANOVA). When interactions were significant, a Tukey test was used to determine specific differences between mean values. The Kolmogorov–Smirnov test was used to compare parasitaemia between infected groups that were either treated or not treated. One-way ANOVA or Mann–Whitney U tests were used to compare maximum peak values of parasitaemia among the different groups. The log-rank (Mantel–Cox) method was used to estimate the average survival for the different experimental groups. Values were expressed as means of the standard deviations. Differences in mean values were considered significant at P < 0.05.
Results
Results in vitro
Cytotoxicity of goyazensolide
The activities of GZL and BZ were evaluated in vitro against intracellular amastigotes of Y T. cruzi strain. GZL showed a high trypanocidal activity, exhibiting an EC50 at 24 h (EC50/24 h) of 0.181 µm and EC50/48 h of 0.020 µm, when compared to BZ, which showed EC50/24 h of 0.268 µm and EC50/48 h of 0.200 µm ~1.1 and 13.4 times higher than GZL, respectively (Table 1). Cytotoxicity analysis of not infected cardiac cell showed that GZL presented lower toxicity than BZ showing LC50/24 h of 9.56 µm, compared to 1.3 µm for BZ (7.5 times less toxic than BZ). After 48 h of treatment, the cytotoxicity was also lower for GZL than BZ (LC50 of 18.3 and 8.2 µm, respectively), 2.2 times less toxic than BZ (Table 1). When the SI/24 h for amastigotes in mammalian cells was analysed, GZL showed a higher value of SI when compared to BZ (52.82 and 4.85, respectively), and this also occurred with the SI/48 h (915.00 and 41.00, respectively) (Table 1).
Table 1.
Trypanocidal effects and selectivity index of the sesquiterpene lactone goyazensolide and benznidazole against T. cruzi Y strain
| Substance | Time | LC50
(MTT) |
EC50
(Amastigotes) |
SI (Amastigotes) |
|---|---|---|---|---|
| GZL | 24 h | 9.56 µm | 0.181 µm | 52.82 |
| BZ | 1.30 µm | 0.268 µm | 4.85 | |
| GZL | 48 h | 18.30 µm | 0.020 µm | 915.00 |
| BZ | 8.20 µm | 0.200 µm | 41.00 |
EC50, compound concentration that reduces the number of parasites in 50%; SI, selectivity index (LC50/EC50 ratio for intracellular parasites) calculated on LC50 after 24 and 48 h of incubation at 37 °C.
Results in vivo
Acute toxicity
According to OECD, no clinical signs of acute toxicity were observed in mice after 48 h of GZL administration when compared to control groups. No behavioural differences were observed in the animals treated with doses of 50 to 100.0 mg kg−1 after 48 h of GZL administration. On the other hand, no survival was observed in male mice treated with a single dose higher than 100.0 mg kg−1. By the way, female mice treated with all doses had no apparent adverse effects up to 48 h after treatment, and mortality was not observed (Table 2).
Table 2.
Acute toxicity analysis of male and female Swiss mice treated with different doses of goyazensolide by the oral route.
| Compound | Route of administration | Sex | GZL doses (mg kg−1) | MTD (mg kg−1) | ||||
|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 150 | 200 | 250 | ||||
| Goyazensolide | Oral | M | NDE | END | Death | Death | Death | 100 |
| F | NDE | NDE | NDE | NDE | NDE | NDE | ||
NDE, no detectable effect; MTD, maximum tolerated dose; GZL, goyazensolide; M, male; F, female.
After the knowledge of the safe doses for mice treatment, the evaluation of acute toxicity with biochemical and haematological parameters after 14 days of GZL administration were performed, according to ANVISA. Results of haematological and biochemical parameters after oral and intravenous administration of GZL at different doses showed that, in general, no signs of toxicity were observed after administration of GZL formulations and during the 14 days of evaluation when compared to control not treated groups (Table 3).
Table 3.
Plasma biochemical and blood count analysis of mice after 14 days after goyazensolide administration
| Parameter | Values for GZL doses (mg kg−1) | ||||||
|---|---|---|---|---|---|---|---|
| Control | GZL 5 Oral |
GZL 25 Oral |
GZL 50 Oral |
GZL 5 IV |
GZL 25 IV |
GZL 50 IV |
|
| Leuc (x 103 mL−1) | 6.9 ± 2.5 | 6.5 ± 1.8 | 6.1 ± 1.9 | 5.1 ± 1.9 | 6.8 ± 2.8 | 4.9 ± 1.9 | 4.8 ± 1.6 |
| RBC (x 106 mL−1) | 7.1 ± 1.4 | 5.5 ± 2.9 | 7.3 ± 5.8 | 6.6 ± 1.2 | 6.9 ± 7.3 | 5.5 ± 2.3 | 6.1 ± 1.8 |
| Hb (g dL−1) | 13.7 ± 1.4 | 13.2 ± 0.9 | 14.1 ± 2.0 | 12.6 ± 1.8 | 13.1 ± 1.4 | 11.4 ± 3.7 | 12.0 ± 2.4 |
| Ht (%) | 40.0 ± 8.5 | 40.1 ± 4.8 | 40.1 ± 2.8 | 36.3 ± 6.4 | 37.6 ± 4.3 | 30.2 ± 12.1 | 30.4 ± 11.3 |
| Plaq (x 105 L−1) | 6.6 ± 2.2 | 8.6 ± 1.2 | 8.2 ± 2.5 | 6.8 ± 2.6 | 7.2 ± 2.2 | 6.5 ± 4.9 | 8.1 ± 1.0 |
| Creatinine (mg dL−1) | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.2 | 0.3 ± 0.2 | 0.8 ± 0.5 |
| Urea (mg dL−1) | 53.3 ± 15.9 | 58.8 ± 21.9 | 100.9 ± 118.7 | 59.4 ± 11.1 | 61.6 ± 17.6 | 66.6 ± 24.9 | 53.1 ± 21.7 |
| ALT (U L−1) | 168.1 ± 70.5 | 126.6 ± 52.4 | 139.3 ± 33.1 | 152.6 ± 54.0 | 130.3 ± 31.3 | 187.4 ± 88.6 | 154.4 ± 94.5 |
| AST (U L−1) | 80.7 ± 44.1 | 53.9 ± 30.5 | 106.8 ± 104.0 | 102.4 ± 72.5 | 74.8 ± 41.4 | 115.2 ± 45.0 | 87.5 ± 60.4 |
GZL, goyazensolide; Leuc, leucocytes; RBC, red blood cells; Hb, haemoglobin; Ht, haematocrit; Plat, platelets; Alt, alanine aminotransferase; AST, aspartate aminotransferase; IV, intravenous route.
Evaluation of goyazensolide activity in T. cruzi
Susceptibility to treatment of mice infected with CL and Y T. cruzi strains
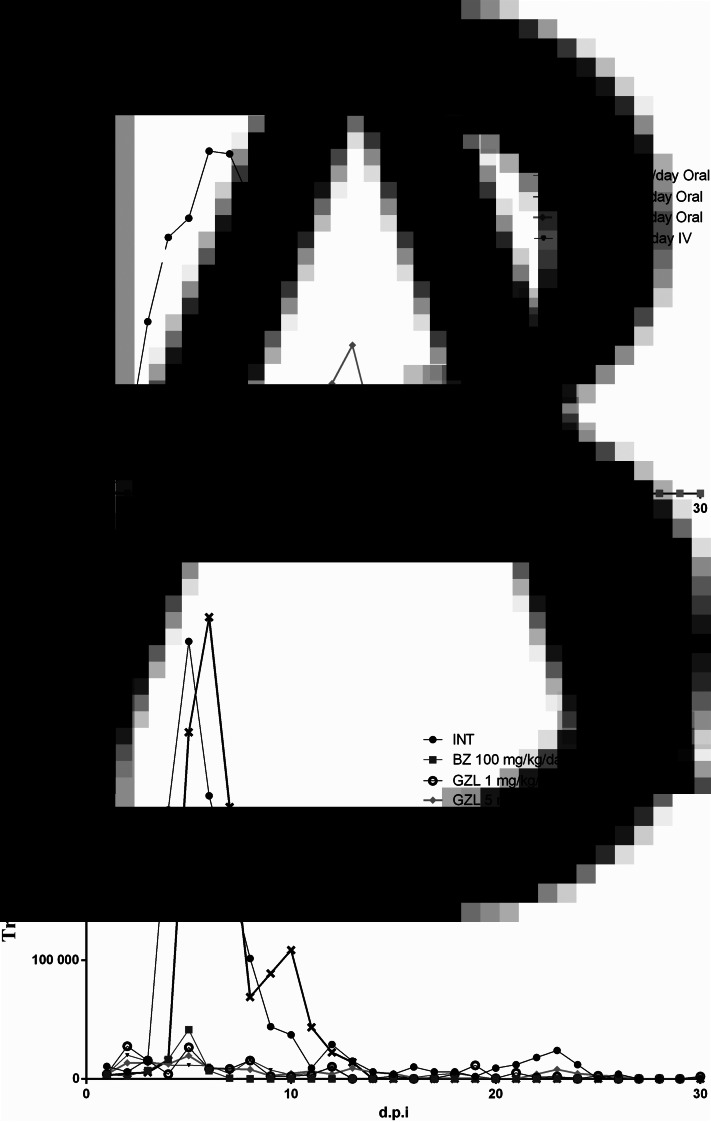
Parasitaemia curve: Parasitaemia curves of animals infected with CL strain treated with GZL by oral and IV routes are shown in Fig. 2. Animals of the groups treated with GZL 1.0 and 3.0 mg kg−1 day−1 by IV, and BZ showed a similar area under the parasitaemia curve (AUC) (18.9 ± 19.5; 84.2 ± 1.9; 0.2 ± 0, respectively). Groups treated with BZ and GZL 1 mg kg−1 day−1 by oral showed parasitaemia values significantly lower than the control group INT and BZ group showed the lowest AUC than all treated and INT animals (0.2 ± 0; 18.9 ± 1.5; 615.7 ± 462.7, respectively). No differences in AUC were observed between the GZL treated groups.
Fig. 2.
Mean parasitaemia curves of Swiss mice infected with T. cruzi CL (A) and Y (B) strains, treated by oral and intravenous routes for 20 consecutive days during the acute phase of infection. INT, group infected and not treated; BZ, group infected and treated with benznidazol 1000 mg kg−1 day−1; GZL oral, groups treated with goyazensolide 1.0, 5.0 or 25.0 mg kg−1 day−1 by oral route; GZL IV, group treated with goyazensolide with doses 1.0, 3.0 or 5.0 mg kg−1 day−1 by intravenous route; d.p.i., days post infection.
Groups of mice infected with Y strain and treated with GZL 1.0 mg kg−1 day−1 by oral, 5.0 mg kg−1 day−1 by oral, 1.0 mg kg−1 day−1 by IV and BZ presented similar AUC (area under the curve of parasitaemia) (15.0 ± 10.3; 14.7 ± 18.9; 13.2 ± 6.4 and 7.6 ± 5.1, respectively) (Fig. 2) with significant reduction (P < 0.05) of parasitaemia when compared to INT and GZL 25.0 mg kg−1 day−1 by oral group which had also similar parasitaemia (123.8 ± 61.6 and 122.4 ± 39.5, respectively). No statistical differences were observed between the GZL or BZ treated groups, except for the group of animals treated with GZL 25 mg kg−1 day−1 by oral, which showed the highest AUC compared to other GZL treated groups.
Hemoculture: In animals infected with CL strain HC was negative in 85.7% of the animals treated with BZ and in 87.5%, of mice treated with GZL 3 mg kg−1 day−1 by IV, followed by 83.3% of negative animals in the groups treated with GZL 5 mg kg−1 day−1 by oral and 75% in the GZL 1 mg kg−1 day−1 by oral group (Table 4). All animals of the INT group died before the first evaluation by HC as a consequence of the acute infection.
Table 4.
Global results of the post-treatment evaluation according to the classic and alternative cure criteria of mice infected with CL and Y T. cruzi strains treated with goyazensolide by oral and IV routes for 20 consecutive days during the acute phase.
|
T. cruzi
Strain |
Route | Experimental groups (mg kg−1 day−1) | Survival rate (%) | Negativation of laboratorial tests (%) | Cure (%) classic criterion | Negative qPCR (%) | Agreement PCR and qPCR (%) | Parasitological negativation (%) Negative HC, PCR and qPCR | ||
|---|---|---|---|---|---|---|---|---|---|---|
| HC | PCR | ELISA | ||||||||
| CL | Oral | BZ 100 | 87.5 (7/8) | 85.7 (6/7) | 85.7 (6/7) | 85.7 (6/7) | 85.7% | 85.7 (6/7) | 100 (7/7) | 86 (6/7) |
| GZL 1 | 100 (8/8) | 75.0 (6/8) | 87.5 (7/8) | 0 (0/8) | 0% | 75 (6/8) | 87.5 (7/8) | 75 (6/8) | ||
| GZL 5 | 75.0 (6/8) | 83.3 (5/6) | 66.7 (4/6) | 0 (0/6) | 0% | 66.7 (4/6) | 100 (6/6) | 67 (4/6) | ||
| IV | GZL 3 | 100 (8/8) | 87.5 (7/8) | 87.5 (7/8) | 12.5 (1/8) | 12.5 | 62.5 (5/8) | 75 (6/8) | 62.5 (5/8) | |
| – | INT | 0 (0/8) | 0 (0/0) | 0 (0/0) | 0 (0/0) | 0 | – | – | – | |
| Y | Oral | BZ 100 | 87.5 (7/8) | 42.9 (3/7) | 85.7 (6/7) | 0.0 (0/7) | 0 | 71.4 (5/7) | 86 (6/7) | 43 (3/7) |
| GZL 1 | 75 (6/8) | 66.6 (4/6) | 66.6 (4/6) | 0.0 (0/6) | 0 | 66.6 (4/6) | 100 (6/6) | 67 (4/6) | ||
| GZL 5 | 100 (8/8) | 100 (8/8) | 100 (8/8) | 0.0 (0/8) | 0 | 62.5 (5/8) | 62.5 (5/8) | 62.5 (5/8) | ||
| GZL 25 | 50 (4/8) | 50.0 (2/4) | 100 (4/4) | 0.0 (0/4) | 0 | 50 (2/4) | 50 (2/4) | 50 (2/4) | ||
| IV | GZL 1 | 87.5 (7/8) | 85.7 (6/7) | 100 (7/7) | 0.0 (0/7) | 0 | 85.7 (6/7) | 86 (6/7) | 86 (6/7) | |
| – | INT | 12.5 (1/8) | 0.0 (0/1) | 0.0 (0/1) | 0.0 (0/1) | 0 | 0.0 (0/1) | 100 (1/1) | 0 (0/1) | |
HC, hemoculture; PCR, polymerase chain reaction; ELISA, enzyme-linked immunosorbent assay; qPCR, quantitative polymerase chain reaction; BZ, group of animals infected and treated with benznidazole 100.0 mg kg−1 day−1; GZL mg kg−1 day−1, groups of animals infected and treated with goyazensolide at doses of 1.0, 3.0, 5.0 or 25.0 mg kg−1 day−1 by oral or intravenous route; INT, control group of infected not treated animals.
Regarding the animals infected with Y T. cruzi strain and treated with GZL, the HC was 100% negative only in the groups of mice treated with GZL 5.0 mg kg−1 day−1 by oral. The animals treated with GZL 1.0 mg kg−1 day−1 by IV presented 85.7% of negativation in HC, followed by the groups GZL 1.0 and 25.0 mg kg−1 day−1 by oral, which presented HC negativation in 66.6 and 50% of the animals, respectively. The group of animals treated with BZ 100.0 mg kg−1 day−1 by oral showed 42.9% of negative HC, while 100% of the INT group were positive in this test (Table 4).
PCR in blood eluate: PCR was 87.5% negative in animals infected with CL T. cruzi strain treated with GZL 1 mg kg−1 day−1 by oral and GZL 3 mg kg−1 day−1 by IV (Table 4), followed by the group treated with GZL 5 mg kg−1 day−1 by oral, that presented 66.7% of PCR negative. BZ group was 85.7% PCR negative. All INT control group died before all the analysis of PCR due to infection.
Animals infected with Y strain and treated with GZL 5.0 mg kg−1 day−1 by oral and 1.0 mg kg−1 day−1 by IV were 100% PCR negative. Mice treated with 1 mg kg−1 day−1 by oral showed the lowest rate of PCR negative (66.6%), while the group treated with GZL 25 mg kg−1 day−1 by oral was also 100% negative in PCR. The majority (7/8) of the mice of the INT group died during the acute phase and the only surviving animal was PCR positive. BZ treated group was 85.7% PCR negative (Table 4).
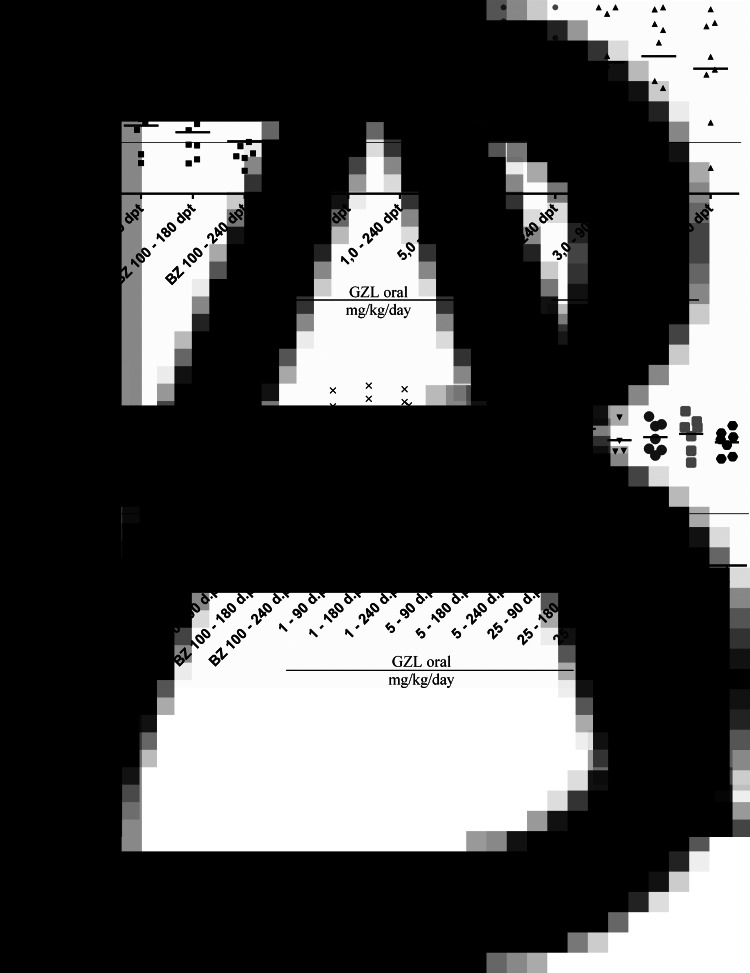
ELISA: Fig. 3 shows the optical density related to the concentration of IgG anti-T. cruzi in sera collected 90, 180 and 240 days post treatment (d.p.t.) of the animals infected with the CL strain of T. cruzi. In the group of animals treated with GZL 3.0 mg kg−1 day−1 by IV, 1/8 (12.5%) was negative in ELISA 90, 180 and 240 d.p.t. All animals of the other groups treated with GZL were ELISA positive. In the control group treated with BZ it was observed that 2/7 animals at 90 d.p.t., 4/7 at 180 d.p.t. and 6/7 (85.7%) at 240 d.p.t. were negative.
Fig. 3.
Optical density dispersion plots for enzyme-linked immunosorbent assay (ELISA) in serum samples from mice infected with CL (A) and Y (B) strains of T. cruzi and treated with goyazensolide and benznidazole orally and intravenously for 20 consecutive days during the acute phase of infection. O.D., optic density; NC, negative control; PC, positive control; INT, infected and not treated control group; BZ, control group of animals treated with benznidazol; GZL, groups of animals treated with goyazensolide.
Figure 3 also shows that all samples from animals infected with Y strain treated with GZL and BZ, as well as all animals of the INT group were reactive in ELISA. No significant differences of T. cruzi anti-IgG reactivity were observed at 90, 180 and 240 d.p.t. among the treated and not treated animals.
Heart qPCR: The evaluation of parasitism in cardiac tissue (Table 4) by the qPCR method revealed absence of T. cruzi kDNA genetic material in 75% of animals infected with CL strain and treated with GZL 1.0 mg kg−1 day−1 by oral, followed by GZL 5.0 mg kg−1 day−1 by oral and GZL 3.0 mg kg−1 day−1 by IV that showed 66.7 and 62.5% of negative results, respectively, while the control group BZ was 85.7% negative. In mice infected with Y strain, 85.7% of the animals treated with GZL 1.0 mg kg−1 day−1 by IV had negative qPCR, followed by the control group BZ, 71.4% negative. Animals treated with GZL 1.0, 5.0 and 25.0 mg kg−1 day−1 by oral were 66, 62.5 and 50% negative, respectively. The only surviving animal of the INT control group infected with Y strain was positive in heart qPCR.
The agreement between the results of blood PCR and heart qPCR of animals of all groups infected with CL and Y strains and treated with GZL was of 86.4 and 76%, respectively.
In animals infected with CL strain treated with BZ 100.0 mg kg−1 day−1 the agreement between PCR and qPCR was 100%. Only one discrepancy occurred in the experimental group with Y strain treated with BZ 100 mg kg−1 day−1 that was negative in 85.7 × 71.4% of the animals when evaluated by PCR and qPCR, respectively (Table 5).
Table 5.
Parasitological evaluations of each experimental group of mice infected with CL and Y T. cruzi strains treated with goyasenzolide and benznidazole
| Strain | Animal/treatment mg kg−1 day−1 | HC | PCR | qPCR | Concordance PCR and qPCR (%) |
Negativation (HC, PCR, qPCR) |
Strain | Animal/treatment mg kg−1 day−1 | HC | PCR | qPCR | Concordance PCR and qPCR (%) |
Negativation (HC, PCR, qPCR) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CL | BZ 100–1 | − | − | − | 100% (7/7) |
86% (6/7) |
Y | BZ 100–1 | + | − | + | 86% (6/7) |
43% (3/7) |
| BZ 100–2 | − | − | − | BZ 100–2 | − | − | − | ||||||
| BZ 100–3 | − | − | − | BZ 100–3 | − | − | − | ||||||
| BZ 100–4 | + | + | + | BZ 100–4 | − | − | − | ||||||
| BZ 100–5 | − | − | − | BZ 100–5 | + | + | + | ||||||
| BZ 100–6 | − | − | − | BZ 100–6 | + | − | − | ||||||
| BZ 100–7 | − | − | − | BZ 100–7 | + | − | − | ||||||
| CL | GZL 1 OR – 1 | − | − | − | 87.5% (7/8) |
75% (6/8) |
Y | GZL 1 OR – 1 | − | − | − | 100% (6/6) |
67% (4/6) |
| GZL 1 OR – 2 | + | + | + | GZL 1 OR – 2 | − | − | − | ||||||
| GZL 1 OR – 3 | + | − | + | GZL 1 OR – 3 | − | − | − | ||||||
| GZL 1 OR – 4 | − | − | − | GZL 1 OR – 4 | + | + | + | ||||||
| GZL 1 OR – 5 | − | − | − | GZL 1 OR – 5 | + | + | + | ||||||
| GZL 1 OR – 6 | − | − | − | GZL 1 OR – 6 | − | − | − | ||||||
| GZL 1 OR – 7 | − | − | − | Y | GZL 5 OR – 1 | − | − | − | 62.5% (5/8) |
62.5% (5/8) |
|||
| GZL 1 OR – 8 | − | − | − | GZL 5 OR – 2 | − | − | + | ||||||
| CL | GZL 5 OR – 1 | + | + | + | 100% (6/6) |
67% (4/6) |
GZL 5 OR – 3 | − | − | − | |||
| GZL 5 OR – 2 | − | − | − | GZL 5 OR – 4 | − | − | − | ||||||
| GZL 5 OR – 3 | − | − | − | GZL 5 OR – 5 | − | − | − | ||||||
| GZL 5 OR – 4 | − | − | − | GZL 5 OR – 6 | − | − | + | ||||||
| GZL 5 OR – 5 | − | + | + | GZL 5 OR – 7 | − | − | − | ||||||
| GZL 5 OR – 6 | − | − | − | GZL 5 OR – 8 | − | − | + | ||||||
| CL | GZL 3 IV – 1 | − | − | + | 75% (6/8) |
62.5% (5/8) |
Y | GZL 25 OR – 1 | + | − | + | 50% (2/4) |
50% (2/4) |
| GZL 3 IV – 2 | − | − | − | GZL 25 OR – 2 | − | − | − | ||||||
| GZL 3 IV – 3 | − | − | − | GZL 25 OR – 3 | + | − | + | ||||||
| GZL 3 IV – 4 | − | − | + | GZL 25 OR – 4 | − | − | − | ||||||
| GZL 3 IV – 5 | − | − | − | Y | GZL 1 IV – 1 | − | − | − | 86% (6/7) |
86% (6/7) |
|||
| GZL 3 IV – 6 | + | + | + | GZL 1 IV – 2 | − | − | − | ||||||
| GZL 3 IV – 7 | − | − | − | GZL 1 IV – 3 | − | − | − | ||||||
| GZL 3 IV – 8 | − | − | − | GZL 1 IV – 4 | + | − | + | ||||||
| CL | INT | All mice died before the post-treatment evaluations | GZL 1 IV – 5 | − | − | − | |||||||
| GZL 1 IV – 6 | − | − | − | ||||||||||
| GZL 1 IV – 7 | − | − | − | ||||||||||
| Y | INT | + | + | + | 100% (1/1) | 0% | |||||||
HC, hemoculture; PCR, polymerase chain reaction; qPCR, quantitative polymerase chain reaction; BZ, group of animals infected and treated with benznidazole 100.0 mg kg−1 day−1; GZL, groups of animals infected and treated with goyazensolide at doses of 1.0, 3.0, 5.0 or 25.0 mg kg−1 day−1 by oral (OR) or intravenous (IV) route; INT, control group of infected not treated animals.
For animals infected with CL strain, treated with GZL, the negativation of all parasitological tests (HC, PCR and qPCR) occurred in a range of 62.5 to 75% in the different experimental groups, whereas the animals infected with the Y strain were parasitologically negative in a range of 50 to 86% in the distinct experimental groups (Table 5).
Survival of the animals: The survival rates of the animals infected with the CL strain and treated with GZL 1.0 mg kg−1 day−1 by oral and GZL 3.0 mg kg−1 day−1 by IV were 100%, followed by the BZ group that presented survival of 87.5% (Table 4). Animals treated with GZL 5.0 mg kg−1 day−1 by oral presented 75% of survival. All animals of the INT group died until the end of the evaluation (240 d.p.t.).
The survival of the animals experimentally infected with Y strain (Table 4) and treated with GZL 5.0 mg kg−1 day−1 by oral was 100%, followed by GZL 1.0 mg kg−1 day−1 by IV and BZ group, which presented survival rate of 87.5%. Animals treated with GZL 1.0 mg kg−1 day−1 by oral presented 75% of survival. Only 12.5% (1/8) of the animals of the INT group survived until 240 d.p.t.
Efficacy of the treatment of animals infected with CL and Y strains of T. cruzi
The evaluation of the results obtained in the ESF, HC, PCR and ELISA (Table 4) in animals infected with the CL strain, both GZL and BZ compounds were able to achieve cure in 12.5% (1/8) of the mice treated with GZL 3 mg kg−1 day−1 by IV, and in 57 and 85.7% in the group treated with BZ at 180 and 240 d.p.t, respectively.
On the other hand, in animals infected with Y strain the negativation of the parasitological tests were higher in animals treated with GZL than the verified in mice treated with BZ. However, considering the results of ELISA, which was always reactive, no cure was observed in any group treated with both compounds, following the classic cure criterion.
On the other hand, considering the negativation of only the results of HC, blood PCR throughout the infection and the heart qPCR (240 d.p.t.), the parasitological negativation assessed was of 68.2% and of 68% in the animals infected with CL and Y strain treated with GZL, respectively, while the negativation of HC, PCR and qPCR were of 86% and 43%, respectively, when the animals were treated with BZ (Table 5).
Discussion
Several advances were achieved in the control of CD. However, it is still necessary to develop a new, more efficient, safe and accessible etiological treatment for this infection (Urbina, 2009), still present in about eight million of patients in the Latin American countries and even in non-endemic continents (Schmunis and Yadon, 2010; WHO, 2018). The need for identifying new compounds and/or therapeutic strategies more effective and accessible for the treatment of T. cruzi infection, particularly in the chronic phase of the disease (Urbina, 2009) when the parasite reproduces slowly within the host cell and in general unreachable by the available compounds. For these reasons, more rational screening methodology for new chemotherapeutic compounds was purposed in order to standardize the procedures (Romanha et al., 2010).
In the initial in vitro studies of several SL, it was verified that GZL presented as the most active lactone on epimastigote and trypomastigote forms (Chiari et al., 1991, 1996; Oliveira et al., 1996). During the last decade, some research groups have evaluated the anti-T. cruzi activities of sesquiterpene lactones (Sulsen et al., 2008; Da Silva et al., 2013), and our group studied the lycnopholide (LYC) isolated from L. trichocarpha in nanocapsule formulations showing total cure of the infection in mice infected with sensitive and partially resistant T. cruzi strains to BZ and NF and dramatic reduction of parasitaemia and parasitological tests in animals infected with resistant strain treated with low doses of LYC (Branquinho et al., 2014b; Mello et al., 2016).
In the present study, the antiparasitic activity of the LS goyazensolide was investigated in vitro in H9c2 cells, and in vivo in murine model infected with CL and Y T. cruzi strains.
For initial knowledge of anti-T. cruzi activity of GZL in vitro, experiments were performed to analyse its cytotoxicity in mammalian heart cells (H9c2) and to find safe concentrations for its use in tests in amastigote forms of the parasite. Thus, it was verified that GZL showed low cytotoxicity up to 0.641 µm at 24 and 48 h of treatment, being considered less toxic when compared to the reference drug BZ (LAFEPE/PE Lot: 16070001), which presented cytotoxicity at a concentration of 4.553 µm, 50 times higher than that observed with GZL. When the LC50 of both substances was compared, the values of this variable for GZL and BZ after 24 h of treatment were 7.3 times greater for GZL (9.56 and 1.3 µm, respectively), and 2.2 times greater after 48 h of treatment (18.3 and 8.2 µm, respectively).
From this experiment, the safe concentrations of GZL were identified for use in infected cells with the T. cruzi Y strain. The higher concentrations of GZL (0.641 and 0.769 µm) induced a reduction of about 85% of the EI (% infected cells/number of amastigotes in the infected cells) when the amastigotes of the infected H9c2 cell were counted. This result was similar to that found for the reference drug BZ in its active concentrations in vitro, when a reduction of 93% in its EI was observed (data not shown). The EC50 for amastigotes observed at 24 h of treatment was approximately the same between GZL and BZ (0.181 and 0.268 µm, respectively). However, it was observed that GZL presented EC50 almost 10 times lower to amastigotes at 48 h of treatment when compared to BZ (0.020 and 0.200, respectively). This indicates that GZL was extremely active against T. cruzi in vitro, reaching good levels of parasite elimination when used in lower concentrations than the BZ.
In fact, in this study, the in vitro activity of GZL against T. cruzi was evaluated, with an LC95 of 1.739 µg mL−1 (4.459 µm) in amastigotes, which was several times lower than that found in literature (Oliveira et al., 1996), that showed elimination of 100% of blood trypomastigotes of Y strain, when GZL was used at concentration of 240 µg mL−1 (615 µm). It is important to highlight the difficulty in working with GZL in these models due to its high NF-kB modulation activity, which resulted in a sudden reduction of cell adhesion in vitro (Acuna et al., 2013). Thus, we may suppose that higher concentrations of GZL could be used without changing the cell viability and probably with a better trypanocidal effect, according to the relative dose effect observed at the essayed concentrations, 0.256 to 0.769 µm.
When the SI values for amastigotes in H9c2 cells under the same experimental conditions in both experiments (cytotoxicity and trypanocidal activity tests) were considered, a higher SI for GZL was observed when compared to BZ (52.82 and 4.85, respectively). According to Nwaka and Hudson (2006) and Romanha et al. (2010) a new substance under study needs to show an SI > 50 to advance from in vitro to in vivo experiments. Thus, GZL has shown to be safe enough to advance to animal experiments if the SI for amastigotes is considered.
The values obtained of SI in amastigotes for BZ and two other SL, Cynaropicrin and psilostachyn A, were 274 and 8 for BZ and Cynaropicrin, respectively, and it was not possible to evaluate the SI of psilostachyn A (da Silva et al., 2013). Experiments were performed using primary culture of cardiomyocytes infected or not by the T. cruzi Y strain, which may explain the large difference between the values of SI obtained for BZ when compared to the present work in H9C2 cells. Thus, GZL has shown to be safer when its SI in amastigotes was compared to the obtained for other SL, including in relation to BZ. The work carried out by Guedes-da-Silva et al. (2016) observed SI values in amastigotes for BZ of 51, using primary culture of embryonic cardiac cells. These results, together to those obtained here and by other authors (da Silva et al., 2013), demonstrate the high variation of the BZ effect in different cell lineages and experimental protocols.
As GZL is a new compound that had never been studied in vivo against T. cruzi, no reports of its intravenous (IV) or oral administration, as well as the dose and diluents used in its formulation exist. Considering the parameters of acute toxicity obtained in vivo, greater sensitivity was observed in male mice when compared to females treated with GZL, moreover mortality was observed only in males. No significant differences were observed in the biochemical and haematological parameters between GZL treated mice.
Following this investigation, the post-treatment evaluation of mice infected with the CL strain and treated with GZL at different doses, a significant decrease in parasitaemia levels was observed in relation to the INT group, mainly in those treated with the lowest doses (1.0 mg kg−1 day−1 by oral). This result suggests the strong relationship between the high GZL dosages and its harmful effect on the immune system by the action in NF-kB. This fact may be related to the potent modulatory activity of NF-kB by GZL in higher doses, which may be causing a negative anti-T. cruzi effect of the GZL due to the simultaneous effect on the host immune system, since TNF, IL-1beta, IL-2, IL-6, IL-12 cytokines, adhesion molecules (ICAM), and enzymes (iNOS) were affected by GZL in immunocompetent cells. Activation of this pathway induces the transcription of a range of genes, such as cytokines (COX – cyclooxygenase), all essential for the assembly of an immune response, both innate and adaptive (O'Neill and Kaltschmidt, 1997). It was also observed that two of the seven treated groups (GZL 1.0 mg kg−1 day−1 by oral and GZL 3.0 mg kg−1 day−1 by IV) had survival of 100% of the animals, while in the BZ treated group the survival was of 85.7%. Although some animals had positive parasitological results during the evaluations, in all groups tested, one animal from the group treated with GZL 3.0 mg kg−1 day−1 by IV was considered cured according to the classic cure criterion (Dias, 2016), corresponding to 12.5% of cure in this group. This animal was the only that presented patent parasitaemia, but only in the first two days of treatment. Thereafter the parasitaemia was persistently suppressed in this animal. The treatment with BZ evaluated in parallel, presented cure in 85.7% (6/7) of the animals. It was also observed that two of the seven treated groups (GZL 1.0 mg kg−1 day−1 by oral and GZL 3.0 mg kg−1 day−1 by IV) had survival of 100% of the animals, while 85.7% of the BZ treated group survived. Considering the negativation of HC, blood PCR and heart qPCR in animals infected with CL strain treated with GZL as indication of parasitological cure, we verified that it was achieved in 68.2% of the animals.
In animals infected by the T. cruzi Y strain, the better effect observed on the reduction of parasitaemia was also verified in animals treated with lower doses of GZL 5.0 mg kg−1 day−1 by oral and 1.0 mg kg−1 day−1 by IV. Thus, the lower doses of GZL resulted in the best therapeutic activity against T. cruzi infection, possibly because its trypanocidal effect was higher than the exerted by NF-kB modulatory effect. In the post-therapeutic evaluation of the animals, considering the classic cure criterion (Dias, 2016), linked to the simultaneous negativation of the conventional serological test (ELISA) (Krettli et al., 1982) and parasitological exams, none animal was considered cured. However, it was observed that GZL administered at doses of 5.0 mg kg−1 day−1 by oral and 1.0 mg kg−1 day−1 by IV resulted in higher reduction of the parasitaemia (ASC and PMP), higher negativation of the parasitological tests (HC and PCR) and survival of 100% of the animals, different from the observed in the INT animals with only 12.5% of survival and 100% of HC and PCR positive. Particularly in the group treated with GZL 5.0 mg kg−1 day−1 by oral, 100% of all parasitological parameters (HC and blood PCR) were negative, both usual laboratorial methods to determine the therapeutic failure (Coura and de Castro, 2002), especially in the mouse model, which is the most used in tests of new therapeutic options for Chagas disease (Brener, 1962). Thus, these data demonstrated the ability of GZL for reducing or eliminating the T. cruzi infection in animals infected with T. cruzi Y strain that was resistant to BZ according to the classic cure criterion (0% of cure), but partially resistant to BZ considering the parasitological results (HC, blood PCR and hearth qPCR), negative in 43% of the animals. Considering the parasitological negativation of these same tests in animals infected with Y strain and treated with GZL as indicative of parasitological cure, the global results of all animals treated with GZL, 68% of the mice were parasitologicaly cured.
Results of blood PCR and qPCR agreement here obtained are similar to the observed in a mouse model where the authors verified that qPCR in tissue is an important method to be considered in the post-treatment evaluation (Caldas et al., 2012). What can be considered so far, is that GZL in the absence of more elaborate formulations, presented an effective trypanocidal action in murine model in animals infected with CL and Y strains, which showed evident therapeutic activity against T. cruzi infection, demonstrated by high percentages of the parasitological tests negativation employed in the post-treatment evaluations. Together these results show the potential anti-T. cruzi therapeutic action of GZL that deserves to be better explored.
The survival results of the GZL-treated animals found here are similar to those described by the treatment with LS lychnopholide (free LYC) (Branquinho et al., 2014b), which demonstrated a strong activity of this lactone against T. cruzi using nanostructured formulations (nanocapsules). Together, these results stimulate the continuity and improvement of studies with GZL, using more elaborate formulations. It is important to highlight that the several studies with free SL lychnopholide developed by our group (Branquinho et al., 2014b; Mello et al., 2016), so active against T. cruzi in nanostructured formulations (nanocapsules), never registered parasitological cure in murine model infected with strains of different resistance/susceptibility profiles to treatment with BZ and NF, including in mice infected with CL strain. This observation reveals the potential therapeutic effect of GZL for Chagas disease treatment. Cumanin, substance that has α,β-unsaturated lactone ring, was also evaluated at a dose of 1.0 mg kg−1 day−1 (Sulsen et al., 2013), and was obtained similar results to those found here with the T. cruzi Y strain, but without cure demonstration according to the classic criteria (Dias, 2016). When psilostachyn A and cynaropicrin were evaluated, the activity of these SL, in mice infected with Y and Colombian strains of T. cruzi, were not significant in comparison to the reference drug BZ and did not avoid the mortality of the animals (da Silva et al., 2013).
One of the most relevant issues in assessing the therapeutic efficacy of CD is the lack of tools to identify and certify definitive parasitological cure (De Lana and Martins-Filho, 2015). Therefore, new methodologies of high sensibility such as qPCR should be used in those animals with repeated negative parasitological tests and only positive in serological tests in order to verify the presence of T. cruzi kDNA in host tissues. Its known that conventional serology remains positive for a long time after treatment without necessarily indicating the presence of active T. cruzi infection, since this reactivity may be due to several other different reasons (Krettli, 2009), being the most common reason, the memory antibodies and the remaining T. cruzi antigens present in dendritic cells (Andrade et al., 1991). Moreover, alternative serological methods such as research of anti-live trypomastigotes antibodies by flow-cytometry (ALTA-FC) (Andrade et al., 1991; Martins-Filho et al., 1995) presents early negative results than the conventional serology used in parallel in the post-treatment evaluation of mice (Martins et al., 2008; de Oliveira et al., 2017) and humans (Vitelli-Avelar et al., 2007; Wendling et al., 2011).
So, the conclusion is that GZL was strongly active in vitro and showed important therapeutic activity in mice infected with CL and Y T. cruzi strains demonstrated by the high percentage of negative parasitological tests employed in the successive post-treatment evaluations. Together, these results confirm the potential trypanocidal activity of GZL for Chagas disease treatment and stimulate the evolution of studies with this SL in better drug formulations in order to improve the achieved percentage of cure in animal experimentation.
Financial support
We would like to thank the Brazilian agencies CNPq (Process 431413/2016-9) and FAPEMIG (APQ-00766/16 and APQ-01160-15) for the financial support. We thank the UFOP and CAPES undergraduate and postgraduate scholarships. Marta de Lana is a research fellow of CNPq.
Conflict of interest
There are no conflicts of interest between authors.
Ethical standards
Mice were obtained from the Centro de Ciência Animal of the UFOP (CCA-UFOP), MG, Brazil and kept following the rules established by the Conselho Nacional de Controle de Experimentação Animal (CONCEA) according to the international guidelines. All procedures carried out in mice were approved by the institutional Comitê de Ética em Experimentação Animal (CEUA-UFOP), MG, Brazil, protocol 2016-45.
References
- Acuna UM, Shen Q, Ren Y, Lantvit DD, Wittwer JA, Kinghorn A D, Swanson SM and Carcache de Blanco EJ (2013) Goyazensolide induces apoptosis in cancer cells in vitro and in vivo. Section Title: Pharmacology 9, 18, 36–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade SG, Freitas LA, Peyrol S, Pimentel AR and Sadigursky M (1991) Experimental chemotherapy of Trypanosoma cruzi infection: persistence of parasite antigens and positive serology in parasitologically cured mice. Bulletin of the World Health Organization 69, 191–197. [PMC free article] [PubMed] [Google Scholar]
- Avila HA, Sigman DS, Cohen LM, Millikan RC and Simpson L (1991) Polymerase chain reaction amplification of Trypanosoma cruzi kinetoplast minicircle DNA isolated from whole blood lysates: diagnosis of chronic Chagas’ disease. Molecular and Biochemical Parasitology 48, 211–221. [DOI] [PubMed] [Google Scholar]
- Bahia MT, de Diniz LF and Mosqueira VCF (2014) Therapeutical approaches under investigation for treatment of Chagas disease. Expert Opinion on Investigational Drugs 23, 1225–1237. [DOI] [PubMed] [Google Scholar]
- Bermudez J, Davies C, Simonazzi A, Pablo Real J and Palma S (2016) Current drug therapy and pharmaceutical challenges for Chagas disease. Acta Tropica 156, 1–16. [DOI] [PubMed] [Google Scholar]
- Bern C (2015) Chagas’ disease. New England Journal of Medicine 373, 456–466. [DOI] [PubMed] [Google Scholar]
- Bohlmann F, Zdero C, Robinson H and King RM (1980) Caryophyllene derivatives and a heliangolide from Lychnophora species. Phytochemistry 19, 2381–2385. [Google Scholar]
- Branquinho RT, Mosqueira VCF, Kano EK, de Souza J, Dorim DDR, Saude-Guimaraes DA and de Lana M (2014a) HPLC-DAD and UV-spectrophotometry for the determination of lychnopholide in nanocapsule dosage form: validation and application to release kinetic study. Journal of Chromatographic Science 52, 19–26. [DOI] [PubMed] [Google Scholar]
- Branquinho RT, Mosqueira VCF, de Oliveira-Silva JCV, Simões-Silva MR, Saúde-Guimarães DA and de Lana M (2014b) Sesquiterpene lactone in nanostructured parenteral dosage form is efficacious in experimental Chagas disease. Antimicrobial Agents and Chemotherapy 58, 2067–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRASIL (2004) Ministério da Saúde, Agência Nacional de Vigilância Sanitária. Resolução de Diretoria Colegiada (RDC) n° 48 de 16/03/2004. Diário Oficial da União.
- Brener Z (1962) Therapeutic activity and criterion of cure on mice experimentally infected with Trypanosoma cruzi. Revista do Instituto de Medicina Tropical de Sao Paulo 4, 389–396. [PubMed] [Google Scholar]
- Britto C, Cardoso MA, Wincker P and Morel CM (1993) A simple protocol for the physical cleavage of Trypanosoma cruzi kinetoplast DNA present in blood samples and its use in polymerase chain reaction (PCR)-based diagnosis of chronic Chagas disease. Memórias do Instituto Oswaldo Cruz 88, 171–172. [DOI] [PubMed] [Google Scholar]
- Caldas S, Caldas IS, de Diniz LF, de Lima WG, de Oliveira RP, Cecílio AB, Ribeiro I, Talvani A and Bahia MT (2012) Real-time PCR strategy for parasite quantification in blood and tissue samples of experimental Trypanosoma cruzi infection. Acta Tropica 123, 170–177. [DOI] [PubMed] [Google Scholar]
- Chagas C (1909) Nova tripanozomiaze humana: estudos sobre a morfolojia e o ciclo evolutivo do Schizotrypanum cruzi n. gen., n. sp., ajente etiolojico de nova entidade morbida do homem. Memórias do Instituto Oswaldo Cruz 1, 159–218. [Google Scholar]
- Chiari E, de Oliveira AB, Raslan DS, Mesquita AAL and Tavares KG (1991) Screening in vitro of natural products against blood forms of Trypanosoma cruzi. Transactions of the Royal Society of Tropical Medicine and Hygiene 85, 372–374. [DOI] [PubMed] [Google Scholar]
- Chiari E, Duarte DS, Raslan DS, Saúde DA, Perry KSP, Boaventura MAD, Grandi TSM, Stehmann JR, Anjos AMG and De Oliveira AB (1996) In vitro screening of Asteraceae plant species against Trypanosoma cruzi. Phytotherapy Research 10, 636–638. [Google Scholar]
- Coura JR (2013a) Chagas disease: control, elimination and eradication. Is it possible? Memorias do Instituto Oswaldo Cruz 108, 962–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coura JR (2013b) The discovery of Chagas disease (1908-1909): great successes and certain misunderstandings and challenges. Revista da Sociedade Brasileira de Medicina Tropical 46, 389–390. [DOI] [PubMed] [Google Scholar]
- Coura JR and Borges-Pereira J (2012) Doença de Chagas. O que é conhecido e o que deve ser melhorado: Uma visão sistêmica. Revista da Sociedade Brasileira de Medicina Tropical 45, 286–296. [DOI] [PubMed] [Google Scholar]
- Coura JR and de Castro SL (2002) A critical review on Chagas disease chemotherapy. Memórias do Instituto Oswaldo Cruz 97, 3–24. [DOI] [PubMed] [Google Scholar]
- Croft S, Barrett M and Urbina J (2005) Chemotherapy of trypanosomiases and leishmaniasis. Trends in Parasitology 21, 508–512. [DOI] [PubMed] [Google Scholar]
- Cummings KL and Tarleton RL (2003) Rapid quantitation of Trypanosoma cruzi in host tissue by real-time PCR. Molecular and Biochemical Parasitology 129, 53–59. 10.1016/S0166-6851(03)00093-8. [DOI] [PubMed] [Google Scholar]
- da Silva CF, da Batista DGJ, De Araújo JS, Batista MM, Lionel J, de Souza EM, Hammer ER, da Silva PB, De Mieri M, Adams M, Zimmermann S, Hamburger M, Brun R, Schühly W and de Soeiro MNC (2013) Activities of psilostachyn A and cynaropicrin against Trypanosoma cruzi in vitro and in vivo. Antimicrobial Agents and Chemotherapy 57, 5307–5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lana M and Martins-Filho OA (2015) Revisiting the posttherapeutic cure criterion in Chagas disease: time for new methods, more questions, doubts, and polemics or time to change old concepts? BioMed Research International 2015, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira MT, Branquinho RT, Alessio GD, Mello CGC, Nogueira-de-Paiva NC, Carneiro CM, de Toledo MJO, Reis AB, Martins-Filho OAM and de Lana M (2017) Tci, TcII and TcVI Trypanosoma cruzi samples from Chagas disease patients with distinct clinical forms and critical analysis of in vitro and in vivo behavior, response to treatment and infection evolution in murine model. Acta Tropica 167, 108–120. [DOI] [PubMed] [Google Scholar]
- Dias JCP, Novaes Ramos A, Dias Gontijo E, Luquetti A, Aparecida Shikanai-Yasuda M, Rodrigues Coura J, Morais Torres R, Renan da Cunha Melo J, Antonio de Almeida E, de Oliveira W Jr, Carlos Silveira A, Marcondes de Rezende J, Scalabrini Pinto F, Walter Ferreira A, Rassi A, Augusto Fragata Filho A, Silvestre de Sousa A, Correia Filho D, Maria Jansen A, Manzan Queiroz Andrade G, Felícia De Paoli de Carvalho Britto C, Yecê das Neves Pinto A, Rassi A Jr, Elisabeth Campos D, Abad-Franch F, Eloi Santos S, Chiari E, Marcel Hasslocher-Moreno A, Furtado Moreira E, Seila de Oliveira Marques D, Seila de Oliveira Marques D, Lages Silva E, Antonio Marin-Neto J, Maria da Cunha Galvão L, Salles Xavier S, Aldo da Silva Valente S, Barbosa Carvalho N, Viana Cardoso A, Albuquerque e Silva R, Maia da Costa V, Monzani Vivaldini S, Mamede Oliveira S, da Costa Valente V, Maia Lima M and Vieira Alves R (2016) II consenso Brasileiro em Doença de Chagas, 2015. Epidemiologia e Serviços de Saúde 25, 1–10. [DOI] [PubMed] [Google Scholar]
- Filardi LS and Brener Z (1987) Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Transactions of the Royal Society of Tropical Medicine and Hygiene 81, 755–759. [DOI] [PubMed] [Google Scholar]
- Gomes ML, Macedo AM, Vago AR, Pena SDJ, Galvão LMC and Chiari E (1998) Trypanosoma cruzi: optimization of polymerase chain reaction for detection in human blood. Experimental Parasitology 88, 28–33. [DOI] [PubMed] [Google Scholar]
- Guedes P, Fietto J, Lana M and Bahia M (2008) Advances in Chagas disease chemotherapy. Anti-Infective Agents in Medicinal Chemistry 5, 175–186. [Google Scholar]
- Guedes-da-Silva FH, Batista DGJ, Meuser MB, Demarque KC, Fulco TO, Araújo JS, Da Silva PB, Da Silva CF, Patrick DA, Bakunova SM, Bakunov SA, Tidwell RR, Oliveira GM, Britto C, Moreira OC and Soeiro MNC (2016) In Vitro and In vivo trypanosomicidal action of novel arylimidamides against Trypanosoma cruzi. Antimicrobial Agents and Chemotherapy 60, 2425–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krettli AU (2009) The utility of anti-trypomastigote lytic antibodies for determining cure of Trypanosoma cruzi infections in treated patients: an overview and perspectives. Memórias do Instituto Oswaldo Cruz 104, 142–151. [DOI] [PubMed] [Google Scholar]
- Krettli AU, Brener Z and Cançado JR (1982) Effect of specific chemotherapy on the levels of lytic antibodies in Chagas's disease. Transactions of the Royal Society of Tropical Medicine and Hygiene 76, 334–340. [DOI] [PubMed] [Google Scholar]
- Martins HR, Figueiredo LM, Valamiel-silva JCO, Carneiro CM, Machado-Coelho GLL, Vitelli-Avelar DM, Bahia MT, Martins-Filho OA, Macedo AM and Lana M (2008) Persistence of PCR-positive tissue in benznidazole-treated mice with negative blood parasitological and serological tests in dual infections with Trypanosoma cruzi stocks from different genotypes. Journal of Antimicrobial Chemotherapy 61, 1319–1327. [DOI] [PubMed] [Google Scholar]
- Martins-Filho OA, Pereira ME, Carvalho JF, Cançado JR and Brener Z (1995) Flow cytometry, a new approach to detect anti-live trypomastigote antibodies and monitor the efficacy of specific treatment in human Chagas’ disease. Clinical and Diagnostic Laboratory Immunology 2, 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CGC, Branquinho RT, Oliveira MT, Milagre MM, Saúde-Guimarães DA, Mosqueira VCF and de Lana M (2016) Efficacy of lychnopholide polymeric nanocapsules after oral and intravenous administration in murine experimental Chagas disease. Antimicrobial Agents and Chemotherapy 60, 5215–5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods 65, 55–63. [DOI] [PubMed] [Google Scholar]
- Nwaka S and Hudson A (2006) Innovative lead discovery strategies for tropical diseases. Nature Reviews Drug Discovery 5, 941–955. [DOI] [PubMed] [Google Scholar]
- O'Neill LA and Kaltschmidt C (1997) NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends in Neurosciences 20, 252–258. [DOI] [PubMed] [Google Scholar]
- OECD/OCDE (2001) Acute oral toxicity – acute toxic class method. Guideline for Testing of Chemicals 423, 1–14. [Google Scholar]
- Oliveira AB, Saúde DA, Perry KSP, Duarte DS, Raslan DS, Boaventura MAD and Chiari E (1996) Trypanocidal sesquiterpenes from Lychnophora species. Phytotherapy Research 10, 292–295. [Google Scholar]
- Pérez-Molina JA, Perez AM, Norman FF, Monge-Maillo B and López-Vélez R (2015) Old and new challenges in Chagas disease. The Lancet Infectious Diseases 15, 1347–1356. [DOI] [PubMed] [Google Scholar]
- Romanha AJ, De Castro SL, Soeiro MDNC, Lannes-Vieira J, Ribeiro I, Talvani A, Bourdin B, Blum B, Olivieri B, Zani C, Spadafora C, Chiari E, Chatelain E, Chaves G, Calzada JE, Bustamante JM, Freitas-Junior LH, Romero LI, Bahia MT, Lotrowska M, Soares M, Andrade SG, Armstrong T, Degrave W and Andrade ZDA (2010) In vitro and in vivo experimental models for drug screening and development for Chagas disease. Memórias do Instituto Oswaldo Cruz 105, 233–238. [DOI] [PubMed] [Google Scholar]
- Salem MM and Werbovetz KA (2006) Natural products from plants as drug candidates and lead compounds against leishmaniasis and trypanosomiasis. Current Medicinal Chemistry 13, 2571–2598. [DOI] [PubMed] [Google Scholar]
- Santos LS, Torres RM, Machado-de-Assis GF, Bahia MT, Martins HR, Teixeira-Carvalho A, Coelho-dos-Reis JGA, Albajar-Viñas P, Martins-Filho OA and de Lana M (2012) In-house ELISA method to analyze anti-Trypanosoma cruzi IgG reactivity for differential diagnosis and evaluation of Chagas disease morbidity. Revista da Sociedade Brasileira de Medicina Tropical 45, 35–44. [DOI] [PubMed] [Google Scholar]
- Schmidt TJ, Da Costa FB, Lopes NP, Kaiser M and Brun R (2013) In silico prediction and experimental evaluation of furanoheliangolide sesquiterpene lactones as potent agents against Trypanosoma brucei rhodesiense. Antimicrobial Agents and Chemotherapy 58, 325-332. https://aac.asm.org/content/58/1/325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmunis GA and Yadon ZE (2010) Chagas disease: a Latin American health problem becoming a world health problem. Acta Tropica 115, 14–21. [DOI] [PubMed] [Google Scholar]
- Sulsen VP, Frank FM, Cazorla SI, Anesini CA, Malchiodi EL, Freixa B, Vila R, Muschietti LV and Martino VS (2008) Trypanocidal and leishmanicidal activities of sesquiterpene lactones from Ambrosia tenuifolia sprengel (Asteraceae). Antimicrobial Agents and Chemotherapy 52, 2415–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulsen VP, Cazorla SI, Frank FM, Laurella LC, Muschietti LV, Catalán CA, Martino VS and Malchiodi EL (2013) Natural terpenoids from ambrosia species are active in vitro and in vivo against human pathogenic trypanosomatids. PLoS Neglected Tropical Diseases 7, e2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugoline BCA, de Souza J, Ferrari FC, Ferraz-Filha ZS, Coelho GB and Saúde-Guimarães DA (2017) The influence of seasonality on the content of goyazensolide and on anti-inflammatory and anti-hyperuricemic effects of the ethanolic extract of Lychnophora passerina (Brazilian arnica). Journal of Ethnopharmacology 198, 444–450. [DOI] [PubMed] [Google Scholar]
- Urbina JA (2009) New advances in the management of a long-neglected disease. Clinical Infectious Diseases 49, 1685–1687. [DOI] [PubMed] [Google Scholar]
- Vitelli-Avelar DM, Sathler-Avelar R, Wendling APB, Rocha RDR, Teixeira-Carvalho A, Martins NÉ, Dias JCP, Rassi A, Luquetti AO, Elói-Santos SM and Martins-Filho OA (2007) Non-conventional flow cytometry approaches to detect anti-Trypanosoma cruzi immunoglobulin G in the clinical laboratory. Journal of Immunological Methods 318, 102–112. [DOI] [PubMed] [Google Scholar]
- Voller A, Bidwell DE and Bartlett A (1976) Enzyme immunoassays in diagnostic medicine. Theory and practice. Bulletin of the World Health Organization 53, 55–65. [PMC free article] [PubMed] [Google Scholar]
- Wendling APB, Vitelli-Avelar DM, Sathler-Avelar R, Geiger SM, Teixeira-Carvalho A, Gontijo ED, Elói-Santos SM and Martins-Filho OA (2011) The use of IgG antibodies in conventional and non-conventional immunodiagnostic tests for early prognosis after treatment of Chagas disease. Journal of Immunological Methods 370, 24–34. [DOI] [PubMed] [Google Scholar]
- WHO (2018) Chagas disease – american trypanosomiasis. Polish Annals of Medicine 18, 156–167. [Google Scholar]
- Zimmermann S, Thomi S, Kaiser M, Hamburger M and Adams M (2012) Screening and HPLC-based activity profiling for new antiprotozoal leads from European plants. Scientia Pharmaceutica 80, 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingales B, Andrade S, Briones M, Campbell D, Chiari E, Fernandes O, Guhl F, Lages-Silva E, Macedo A, Machado C, Miles M, Romanha A, Sturm N, Tibayrenc M and Schijman A (2009) A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Memórias do Instituto Oswaldo Cruz 104, 1051–1054. [DOI] [PubMed] [Google Scholar]