Abstract
The present paper summarizes prevalence, epidemiology and clinical disease of natural Toxoplasma gondii infections in humans and animals from Egypt. The current situation of toxoplasmosis in Egypt is confusing. There is no central laboratory or group of researchers actively investigating toxoplasmosis in humans or animals, and no reports on the national level are available. Based on various serological tests and convenience samples, T. gondii infections appear highly prevalent in humans and animals from Egypt. Living circumstances in Egypt favour the transmission of T. gondii. Up to 95% of domestic cats, the key host of T. gondii, are infected with T. gondii; they are abundant in rural and suburban areas, spreading T. gondii oocysts. Many women have been tested in maternity clinics, most with no definitive diagnosis. Toxoplasma gondii DNA and IgM antibodies have been found in blood samples of blood donors. Clinical toxoplasmosis in humans from Egypt needs further investigations using definitive procedures. Reports on congenital toxoplasmosis are conflicting and some reports are alarming. Although there are many serological surveys for T. gondii in animals, data on clinical infections are lacking. Here, we critically review the status of toxoplasmosis in Egypt, which should be useful to biologist, public health workers, veterinarians and physicians.
Key words: Animals, Egypt, epidemiology, humans, prevalence, Toxoplasma gondii, toxoplasmosis
Introduction
Toxoplasmosis is a worldwide zoonosis caused by the protozoan Toxoplasma gondii, which was first discovered in 1908 in the rodent Ctenodactylus gundi at the Pasteur Institute in Tunisia (Nicolle and Manceaux, 1908). At the same time, the parasite was noted in the domestic rabbit (Oryctolagus cuniculus) from Brazil (Splendore, 1908). Cats (domestic and wild) are the only definitive hosts of T. gondii and are essential in its epidemiology because they are the only hosts that can shed environmentally resistant oocysts (Dubey, 2010).
Approximately one-third of humanity is infected with T. gondii worldwide although this varies markedly between populations (Dubey, 2010; Robert-Gangneux and Dardè, 2012). Most infections appear to be asymptomatic in immunocompetent persons; however, the parasite can cause serious disease in unborn fetus and immunocompromised individuals (Peyron et al., 2016). In many animal host species, the infection is also typically subclinical; however, toxoplasmosis can be fatal in many hosts (Dubey, 2010).
Here, we review the detailed prevalence, epidemiological aspects and clinical disease of natural T. gondii infection in humans and animals, with focus on domestic animals, from Egypt.
Methods for present review
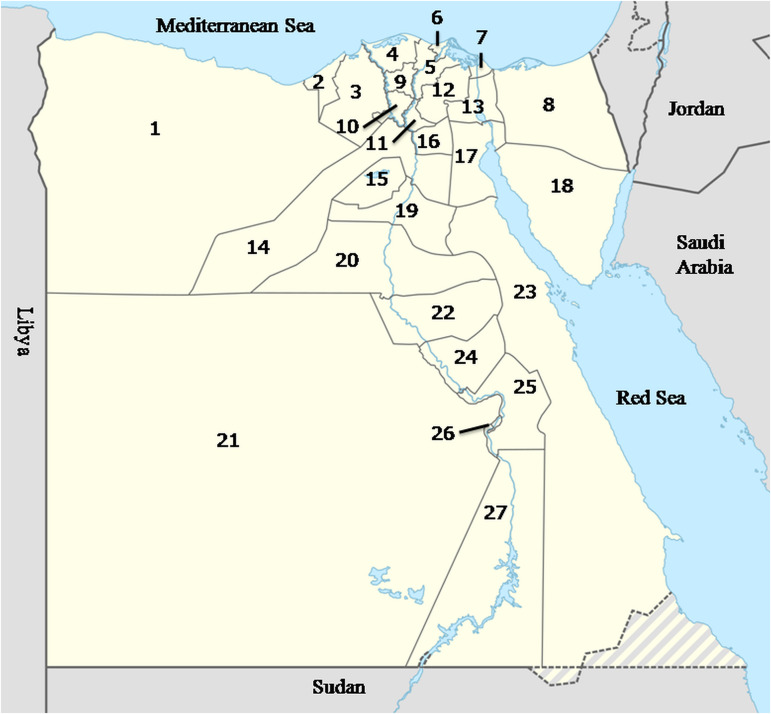
Egypt is a large African country and has a human population >100 million. It is divided into 27 governorates (Fig. 1). The largest city in Egypt is Cairo, the capital, with a population of >8 million people. Nearly 57% of people live in rural areas, whereas 43% live in urbanized cities (World Population Review, 2019). The Egyptian economy is variable and depends largely on agriculture.
Fig. 1.
Map of Egypt including 27 governorates. (1) Matrouh; (2) Alexandria; (3) Beheira; (4) Kafr ElSheikh; (5) Dakahlia; (6) Damietta; (7) Port Said; (8) North Sinai; (9) Gharbia; (10) Menoufiya; (11) Kalubiya; (12) Sharkia; (13) Ismailia; (14) Giza; (15) Fayoum; (16) Cairo; (17) Suez; (18) South Sinai; (19) Beni Suef; (20) Minia; (21) El Wady El Gadeed; (22) Assiut; (23) Red Sea; (24) Sohag; (25) Qena; (26) Luxor; (27) Aswan.
A systematic electronic search of published data was conducted from November 2018 to May 2019. Different databases were consulted including PubMed, Science Direct and Google Scholar using the following keywords: Toxoplasma gondii, toxoplasmosis, Egypt, human and animals. Websites of the local Egyptian journals were also incorporated in our search. Libraries of different Egyptian medical and veterinary faculties and institutes were consulted for the old published papers, which are not available as electronic files. Full texts of some earlier published papers were available in the collection of one of us (JPD).
We found numerous reports (>250) on toxoplasmosis in humans and animals from Egypt. Criteria for inclusion were the full text of papers, abstracts only were excluded. After filtering the collected studies, 170 articles met the criteria to be selected for this review. No statistical methods were employed in this study. In the present review, we attempted to incorporate all published reports available to us on natural T. gondii infections in Egypt. Some reports of toxoplasmosis in Egypt were included in two reviews on T. gondii infections in Africa (Tonouhewa et al., 2017; Rouatbi et al., 2019). The present review is limited to Egypt.
In the present review, detailed serological, parasitological and clinical information on T. gondii infections in humans and animals is summarized in the tables and throughout the text. Different serological techniques used in the Egyptian studies are listed in Table 1. Cut-off values for serological tests are listed wherever the authors provided the information. Superscripts in the tables refer to the details of the serological tests provided in Table 1.
Table 1.
Details of serological tests used for the detection of T. gondii antibodies in animals and humans in Egypt
| Test abbreviation | Antigen | Cut-off | Manufacturer | Citation in the present review |
|---|---|---|---|---|
| Skin test | Soluble | – | In house | Tb2,15,16 |
| Sabin–Feldman dye test DT | Live tachyzoites | Differs | In house | Tb2,3,6,8,11,13,14,15,16,18,19,20,21 |
| Complement fixation test CFT | Soluble | – | NS | Tb 11,19 |
| OnSite Toxo IgG rapid test OTRT | Recombinant | NS | CTK Biotech, CA, USA www.ctkbiotech.com | Tb2,11,13 |
| Slide agglutination test SAT | NS | 1:16 | In house | Tb11 |
| Enzyme linked fluorescence assay ELFA VIDAS Toxo IgG II kit | Membrane and cytoplasmic Toxoplasma antigen (RH strain) | ⩾8 IU ml−1 | Biomērieux, Craponne, France www.biomerieux.com | Tb3 |
| Modified agglutination test | ||||
| MAT | Formalin-treated whole tachyzoites | Differs | In house | Tb3,11,12,13,14,16,17,18,19 |
| Toxoscreen Direct agglutination DAT | Formalin-treated whole tachyzoites | 1:40 | Biomērieux, Craponne, France www.biomerieux.com | Tb11,12 |
| Latex agglutination test LAT | ||||
| 1. Toxocheck-MT | Soluble | 1:64 | Eiken Chemical, Tokyo, Japan www.eiken.co.jp | Tb8,11,13,15,17,20,21 |
| 2. Toxo Latex kit | Soluble | 1:2 | CamTech medical, UK | Tb11,13,14,15,16 |
| 3. LAT | Soluble local antigen | NS | In house | Tb11,13,17 |
| 4. LAT | Soluble | 1:64 | Sigma Scientific Service Co., Cairo, Egypt www.sigmaeg-co.com | Tb3,12 |
| 5. Toxo-LAT fumouze kits | Soluble | NS | Fumouze Diagnostics, France www.fumouze.com | Tb3 |
| Indirect haemagglutination test IHA | ||||
| 1. Toxo-HAI Fumouze kits | Soluble | 1:80 | Fumouze Diagnostics, France www.fumouze.com | Tb2,3,4,7,11,12,13,15,17,18,19 |
| 2. Toxo-HA | Soluble | 1:64 | Biomērieux, Craponne, France www.biomerieux.com | Tb2,8 |
| 3. Toxo-IHA-Fast Kit | Soluble | 1:80 | ABC Diagnostics, New Damietta, Egypt | Tb13,14 |
| 4. IHA | Soluble | 1:16 | In house | Tb11,18 |
| 5. IHA | Soluble | 1:64 | Behringwerke AG, Marburg, Germany (merged into CSL Behring) www.cslbehring.com | Tb2,3,6,11,13,14,15,16,19,20 |
| Indirect fluorescent antibody test IFA | ||||
| 1. IFA | Lyophilized tachyzoites (Biomērieux) | 1:16 | In house | Tb3,4,6,8 |
| 2. Toxo-spot IF slides | Formalin-treated whole tachyzoites | 1:50 | Biomērieux, Craponne, France www.biomerieux.com | Tb2,11,13 |
| 3. IFA | Formalin-treated whole tachyzoites | 1:16 | In house | Tb2,18 |
| 4. IFA | Whole tachyzoites | 1:64 | In house | Tb11,17 |
| 5. IFA | NS | 1:16 | In house | Tb2,3,6,11,13,14,15,16,18 |
| Enzyme linked immunosorbant assay ELISA | ||||
| 1. bioelisaToxo IgG kits | Inactivated | >10 IU ml−1 | Biokit, Barcelona, Spain www.biokit.com | Tb3,4 |
| 2. Toxoplasma IgG ELISA | Whole tachyzoites | ⩾1.2 | Calbiotek, CA, USA www.calbiotech.com | Tb3,4,11,13,14,15,16 |
| 3. ClinotechToxo ELISA IgG Kits | NS | NS | Clinotech Diagnostics and Pharmaceuticals, Richmond, Canada | Tb2,3,4 |
| 4. SeraQuest Toxoplasma IgG | NS | NS | Quest International, Inc., Florida, USA | Tb4 |
| 5. ELISA IgG Kits | NS | ⩾1 | Pre Check, Inc., Housten, USA www.precheck.com | Tb3,4 |
| 6. Toxoplasma IgG ELISA Kits | NS | ⩾1.5 | MyBioSource, CA, USA www.mybiosource.com | Tb3 |
| 7. Toxo IgG ELISA Test Kit | Inactivated | 8 IU ml−1 | Diagnostic Automation/Cortez Diagnostics, Inc., CA, USA www.rapidtest.com | Tb3 |
| 8. Toxoplasma IgG ELISA kit | NS | >1 | BioCheck, Inc., CA, USA www.biocheckinc.com | Tb4,6,7,14 |
| 9. Toxoplasma IgG ELISA Kit | NS | 0.185 | MP Biomedicals Diagnostics Division, Orangeberg, NY, USA | Tb4 |
| 10. Toxo IgG ELISA kit | Sonicated antigen | >0.343 | Human Gesellschaft für Biochemica und Diagnostica mbH, Wiesbaden, Germany | Tb2 |
| 11. DRG® Toxoplasma gondii IgG Kit | Inactivated | NS | DRG internationals, Inc., USA www.drg-international.com | Tb2,7 |
| 12. ID screen toxoplasmosis multispecies indirect ELISA | P30 antigen | NS | ID.Vet, Grabels, France www.id-vet.com | Tb11,13 |
| 13. ELISA | Toxoplasma total lysate antigen | 0.395 | In house | Tb11,13 |
| 14. ELISA | Recombinant GST-TgSAG2t antigen | Differs | In house | Tb3,11,15,19 |
| 15. Indirect ELISA | Recombinant TgGRA7 antigen | NS | In house | Tb3,11,15,17 |
| 16. ELISA | Soluble whole tachyzoites | NS | In house | Tb3,11,13,15,16,17,18,19 |
| 17. ELISA | Soluble crude antigen | NS | In house | Tb11,13,14,15,16,17,19,21 |
| 18. Indirect IgM ELISA | NS | NS | Serion, Würzburg, Germany www.serion-diagnostics.de | Tb3 |
| 19. ELISA-IgG | NS | ⩾1 | Randox, London, UK www.randox.com | Tb3,4 |
| 20. ELISA | NS | NS | Pishtaz Teb Diagnostics, Tehran, Iran. www.old.pishtazteb.com | Tb4 |
| 21. ELISA | NS | NS | Behringwerke AG, Marburg, Germany (merged into CSL Behring) www.cslbehring.com | Tb4 |
| 22. ELISA | NS | ⩾1 | Chemux Bioscience, Inc., CA, USA www.chemux.com | Tb4,7 |
| 23. ETI-TOXO PLUS | NS | NS | DiaSorin, Salugga, Italy www.diasorin.com | Tb2 |
| 24. Novalisa ELISA Kits | NS | NS | Nove Tec immunodiagnostica GmbH, Dietzenbach, Germany www.novatec-id.com | Tb3 |
| 25. ELISA | NS | 10 IU ml−1 | IMMUNOSPEC, California, USA www.immunospec.com | Tb5 |
Tb, table.
History of toxoplasmosis in Egypt
Rifaat and Nagaty (1959) first reported dermal hypersensitivity to T. gondii in 15.6% of 334 hospital patients and technical personnel from Cairo using the T. gondii skin test. The skin test was one of the first tests developed by Frenkel (1948) for a population survey for T. gondii in California, USA; it is a very insensitive test and does not detect acute infection. Subsequently, a highly sensitive and specific test, the dye test (DT), was invented by Sabin and Feldman for the detection of antibodies to T. gondii. Beginning in 1962, Rifaat et al. used the DT to conduct serological surveys for antibodies to T. gondii in humans and other hosts in Egypt. The DT requires the use of live T. gondii and is used now only in few laboratories in the world. Rifaat et al. (1973e) also reported the first case of congenital toxoplasmosis, and they were the first to isolate viable T. gondii in Egypt (Rifaat et al., 1971, 1973a, 1973c, 1976b, 1976c). Currently, there is no central laboratory or group of researchers actively investigating toxoplasmosis in humans or animals, and unfortunately, no studies are available on the awareness of physicians in Egypt about toxoplasmosis.
Toxoplasmosis in humans
Serological prevalence in general population
In Egypt, there are no centralized data on the national prevalence of T. gondii. Most serological reports are based on convenience samples, including in pregnant women, and patients with disorders (Tables 2–5). Generally, little is known of T. gondii infection from Sinai and Red Sea governorates, although habits of people living there promote T. gondii transmission. Most people living there are Bedouins working mainly in livestock rearing. They usually eat undercooked mutton and drink raw goat and camel milk, in addition to the critical deficiency in hygienic measures and health services. Isolated serological reports in the general population and occupational groups are summarized in Table 2.
Table 2.
Seroprevalence of T. gondii antibodies in general human population from Egypt
| Population | Governorate | No. tested | No. positive (%) | Test | Important findings | Reference |
|---|---|---|---|---|---|---|
| Hospital patients and technical personnel | Cairo | 334 | 54 (15.6) | Skin test | High prevalence (21%) in older age >20 yrs than in younger 10–19 yrs (6.6%). No positives in children <9 yrs. 15/60 (25%) schizophrenic patients were positive | Rifaat and Nagaty (1959) |
| Students 10–14 yrs | Tahrir provincea | 87 | 12 (13.8) | Skin test | – | Rifaat et al. (1962) |
| Students >12 yrs | El Wady El Gadeed | 356 | 9 (2.5) | Skin test | – | Rifaat et al. (1963) |
| Different sources | Cairo | 505 | 156 (30.9) | IFA5 | High prevalence in 80 emigrants (37.5%) than 110 abattoir workers (30.9%) and 315 hospital attendants (24.4%). 19 had high Ab titres (1:256–1:1024), 147 had low titres (⩽1:64) | Maronpot and Botros (1972) |
| Healthy and hospital attendants of different ages (2–75 yrs) and sexes | Different governorates | 823 | 115 (14.0) | Skin test | Compatibility of the examined population using both tests was not given | Rifaat et al. (1975) |
| 1750 | 293 (16.8) | DT (1:16) | ||||
| Different sources | Dakahlia | 86 | 24 (27.9) | IHA2 | High prevalence in 21 butchers (38%), 29 poultry breeders (24%), 21 nurses (28.6%), 15 laboratory workers (20%) | Aboul-Enein et al. (1983) |
| Occupational workers | Sharkia | 130 | 15 (19.2) | IHA5 | 9 had high Ab titres (1:256–1:512), high rates in abattoir workers and butchers | El-Ridi et al. (1990) |
| Lactating women | Kalubiya | 70 | 22(31.4) | IFA2 | Antibodies in milk of 12 (17.1%) | Azab et al. (1992) |
| Abattoir workers | Gharbia | 21 | 11 (52.3) | IHA5 | 7 had 1:64 Ab titre, 3 had 1:256 and 1 had 1:512 | Ibrahim et al. (1997) |
| Hospital patients | Benha | 500 | 56 (11.2) | IFA | Random samples | Hamadto et al. (1997) |
| NS | Kalubiya | 152 | 88 (57.9) | ELISA | 16 (10.5%) had IgM | Hussein et al. (2001) |
| Blood donors | Dakahlia | 260 | 155 (59.6) | ELISA10 | Risk assessment | Elsheikha et al. (2009) |
| Housewives | Middle Delta | 70 | 13 (43.3) | ELISA-IgG23 | 8 (26.6%) had IgM. Of them, 1/23 in housewives wearing gloves during meat handling and 7/47 in non-glove-users | El-Tras and Tayel (2009) |
| Healthy people | Sharkia | 50 | 12 (24.0) | IHA1 | 2 (4%) had IgM | Awadallah (2010) |
| Blood donors | Dakahlia | 230 | 155 (67.4) | ELISA | 24 (10.4%) positive for IgG avidity | Azab et al. (2012) |
| Blood donors | Kalubiya | 300 | 101 (33.5) | ELISA11 | 93 had IgG, 10 had IgM (2 IgM, 8 IgG and IgM), 18 (6%) positive by PCR | El-Sayed et al. (2016a) |
| Blood donors | Alexandria | 150 | 98 (65.3) | ELISA8 | 15 (10%) positive by PCR (9 ELISA positive and 6 ELISA negative) | El-Geddawi et al. (2018) |
| Occupational workers | Cairo | 127 | 48 (37.8) | IFA3 | Workers from pig farms. 15 had high Ab titres (1:512–1:1024) | Barakat et al. (2011) |
| Humans in contact with chickens | Beni-Suef | 250 | 88 (35.2) | IHA1 | – | Aboelhadid et al. (2013) |
| Occupational workers | NS | 127 | 48 (37.8) | ELISA3 | 17/48 (35.4%) were PCR positive | Hassanain et al. (2013) |
| School children 6–16 yr | El Wady El Gadeed | 1615 | 13 (2.9) | OTRT | – | Bayoumy et al. (2016) |
yrs, year; Ab, antibody.
Now known as Beheira governorate.
Table 5.
Seroprevalence of T. gondii antibodies in patients with several disorders
| Population | Age range | Governorate | No. tested | No. positive (%) | Test | IgM | Reference |
|---|---|---|---|---|---|---|---|
| Meningoencephalitis | NS | Cairo | 42a | 10 (26.0) | IFA | ND | Mabrouk and Dahawi (1991) |
| Cryptogenic epilepsy | 2–46 yr | Zagazig | 72 | 25 (34.7) | ELISA-IgG11 | ND | Abd El-Aal et al. (2016) |
| Non-cryptogenic epilepsy | 40 | 1 (2.5) | |||||
| Depression | 118 | 24 (20.3) | |||||
| Cryptogenic epilepsy | 9 mo-18 yr | Kalubiya | 40 | 8 (20.0) | ELISA-IgG11 | ND | Eraky et al. (2016) |
| Non-cryptogenic epilepsy | 30 | None | |||||
| Schizophrenia | 20–60 yr | Damietta | 100 | 47 (47.0) | ELISA-IgG22 | ND | Saad et al. (2016) |
| Non-schizophrenic neurodevelopmental disorders | < or >20 yr | Alexandria | 188 | 94 (50.0) | ELISA-IgG8 | 31 (16.5) | Shehata et al. (2016) |
| Neurological disorders without chromosomal anomalies | ⩽5 yr | Dakahlia | 30 | 19 (63.3) | ELISA-IgG8 | 11 (36.7) | El-Beshbishi et al. (2018) |
| Down syndrome | 30 | 4 (13.3) | 1 (3.3) | ||||
| Mental retardation | 2 mo-12 yr | Cairo | 200 | 84 (42.0) | IHA1 | ND | Hamed et al. (2018) |
| Chronic liver disease | 50–60 yr | Dakahlia | 120 | 105 (87.5) | ELISA-IgG25 | 16 (13.3) | El-Nahas et al. (2014) |
| Controls | 40 | 6 (15.0) | 3 (7.5) | ||||
| Chronic liver disease | 19–66 yr | Cairo | 70 | 21 (30.0) | PCR-blood | ND | El-Sayed et al. (2016b) |
| Controls | 50 | 3 (6.0) | |||||
| Tonsilitis | 4–20 yr | Zagazig | 100 | 55 (55.0) | IFAT | ND | El-Ridi et al. (1989) |
| Controls | 50 | 12 (24.0) |
ND, not done; NS, not stated; mo, month; yr, year.
Pathogenic bacteria were excluded.
Notable among these early surveys is the 16.3% prevalence determined by the DT (Rifaat et al., 1975). Higher seroprevalence is reported in emigrants (37.5%) and abattoir workers (30.9%) compared with 24.4% in hospital attendants (Maronpot and Botros, 1972). More recently, a very high (33–67%) seroprevalence was reported among blood donors (Table 2). Additionally, T. gondii DNA was found in 10% (15/150) of blood donors from Alexandria (El-Geddawi et al., 2018); of them, nine were IgG seropositive and six were seronegative (no IgM testing was done). Toxoplasma gondii DNA was also noted in 6% (18/300) of blood donors from Kalubiya governorate. Of them, eight were IgM seropositive (El-Sayed et al., 2016a). Authors proposed the acute infection and probability of T. gondii transmission during blood transfusion. This is a very high rate of T. gondii DNA in the blood of asymptomatic individuals. Caution is needed that the accuracy of PCR assay could affect the results. In addition, no further testing was conducted for the positive cases.
Little is known of T. gondii prevalence in children in Egypt. Rifaat et al. (1963) tested 356 school children from El Wady El Gadeed governorate using the skin test. Samples were collected from children ⩾12 years. Nine (2.5%) children were positive. In a recent report, T. gondii antibodies were found in 13 (2.9%) of 6–16 years old 1615 school children (Bayoumy et al., 2016). In these two studies, there is no distinction between acquired infection in childhood and congenital infection.
Data on convenience samples in pregnant women from Egypt attending private health clinics are shown in Table 3. Screening sera for toxoplasmosis is routinely done for pregnant women in Egypt. Unfortunately, this screening is conducted mostly in private diagnostic laboratories, which have no systems for archiving the results. In addition, results of this screening are not conclusive because it is based upon the commercially available tests without efficiency verification. Many published reports on toxoplasmosis in pregnant women from Egypt are of limited sample size and have insufficient information on the studied populations. Results are not comparable among different reports because of sample size, diagnostic test used and living conditions of the women tested. There are few data on seroconversion during pregnancy and before pregnancy.
Table 3.
Seroprevalence of T. gondii antibodies in pregnant women tested in hospitals or private clinics in Egypt
| Governorate | No. tested | No. positive (%) | Test | Additional tests | Remarks | Reference |
|---|---|---|---|---|---|---|
| Assiut | 97 | 26 (26.8) | DT (1:4) | None | OGHA. High Ab titres in 1 (4.8%) of 21 young women (15–20 yrs old) | Rifaat et al. (1972) |
| Cairo | 200 | 32 (16.0) | IFA5 | ELISA | OGHA. 22 of 23 IFA positives were ELISA positive | Azab et al. (1983) |
| Sharkia | 34 | 4 (11.8) | IFA1 | None | – | El-Ridi et al. (1991b) |
| Cairo | 600 | 164 (27.3) | IHA | IFA | Out of IHA positives, 58.5% were IFA positive | Azab et al. (1993) |
| Kalubiya | 150 | 64 (43.0) | IHA5 | IgM | 3 (2%) had IgM. 64 (43%) of their neonates had IgG, 1 (0.6%) had IgM. MFTR 33.3% | El-Nawawy et al. (1996) |
| Dakahlia | 20 | 2 (10.0) | ELISA-IgG19 | IgM | No IgM positives | Soliman et al. (2001) |
| Suez | 358a | 24 (6.7%) | ELISA-IgG | IgM, Mice bioassay, PCR | 46 (12.9%) had IgM. 39 were serconverted. Viable T. gondii was isolated from AF 0f 14 out 85 (46+39) positive women by mice bioassay. 17/85 had T. gondii DNA in AF samples. | Eida et al. (2009) |
| Kalubiya | 181a | 85 (47.0) | LAT5 | IgM | Of positives, 63 (34.8%) had IgM | El-Gozamy et al. (2009)b |
| Dakahlia | 101 | 51 (51.4) | ELISA14 (0.039) | None | – | Ibrahim et al. (2009) |
| Sharkia | 25 | 4 (16.0) | IHA1 | IgM | 2 (8%) had IgM | Awadallah (2010) |
| Fayoum | 59 | 27 (45.8) | ELISA-IgG3 | IgM, PCR | Normal pregnant with bad obstetric history. 18 (30.5%) had IgM, 32.2% were PCR positive | Ghoneim et al. (2010) |
| Sharkia | 100 | 30 (30.0) | IHA-IgG1 | IgM | 10 (10%) had IgM | Abd El-Ghany and Amin (2012)b |
| Menoufiya | 323 | 218 (67.5) | ELFA-IgG | IgM, IgG-avidity, PCR | No seroconversion during pregnancy had occurred. 9 (2.8%) had IgM, of them 1 had low IgG avidity. Viable T. gondii was isolated from this case by mouse bioassay. | El Deeb et al. (2012)b |
| Kalubiya | 60 | 29 (48.3) | LAT4 | PCR | 12 (40%) of seropositives were PCR positive. | Khater et al. (2013) |
| Sharkia | 100 | 71 (71.0) | IHA-IgG1 | IgM | 19 (19%) had IgM | Ahmed et al. (2014)b |
| Dakahlia | 103 | 44 (42.7) | ELISA-IgG2 | IgM | 3 (2.9%) had IgM | El-Tantawy et al. (2014) |
| Minia | 120 | 8 (6.6) | ELISA-IgG2 | IgM | 2 (1.6%) had IgM | Kamal et al. (2015) |
| Alexandria | 382 | 221 (57.9) | ELISA-IgG5 | None | – | Bassiony et al. (2016)b |
| Beni Suef | 300 | 46 (15.3) | ELISA-IgG24 | IgM | Multiparous pregnant women with a history of complication. 26 (8.6%) had IgM | Abdel Gawad et al. (2017)b |
| Cairo | 30 | 5 (16.6) | ELISA-IgM1 | Western-blot-IgM, PCR | History of abnormal pregnancy. 9 (30%) were immunoblot positive, 6 (20%) were PCR positive | Abo Hashim and Attya (2017) |
| Cairo, Kalubiya, Sharkia | 57 | 22 (38.6) | ELISA-IgG6 | IgM | 4 (7%) had IgM | Abou Elez et al. (2017)b |
| Alexandria | 101 | 13 (12.8) | ELFA-IgG | None | – | El-Shqanqery et al. (2017)b |
| Beheira | 34 | 10 (29.4) | ||||
| Gharbiya | 78 | 21 (26.9) | ||||
| Menoufiya | 376 | 124 (32.9) | ||||
| Kalubiya | 78 | 21 (26.9) | ||||
| Fayoum | 26 | 20 (76.9) | ||||
| Total | 693 | 209 (30.1) | ||||
| Kafr ElSheikh | 113 | 5 (4.4) | ELISA-IgM18 | None | – | Elmonir et al. (2017)b |
| Menoufiya, Gharbiya | 364 | 123 (33.7) | ELISA14 (0.039) | RT-PCR | 11.8% were PCR positive | Ibrahim et al. (2017)b |
| Sohag | 350 | 167 (47.7) | ELISA-IgG7 | IgM | 25 (7.1%) had IgM. 138 (39.4%) of their neonates had IgG, while 5 (1.4%) had IgM. MFTR 25% | Hussein et al. (2017) |
| Giza | 388 | 79 (20.4) | ELISA-IgG16 | IgM, IgG avidity | 43 (11.8%) had IgM, of them 28 (7.2%) had low avidity | Hassanain et al. (2018b)b |
MFTR, maternal fetal transmission rate; OGHA, obstetrics and gynaecology hospitals attendants; Ab, antibody; yrs, years.
Including some lymphadenopathy, fever and malaise cases, but the authors did not specify numbers of different cases.
Risk assessment, see Table 6.
Risk factors associated with T. gondii infection
Generally, risk factors of T. gondii infection in humans were discussed by many authors (Table 6). Infections were associated with factors such as contact with cats, contact with soil, residence (rural or urban), socioeconomic standards, educational level, ingestion of ready to eat meat products, consumption of undercooked mutton, consumption of raw vegetables, drinking raw milk and consumption of locally prepared Kareish cheese (Elsheikha et al., 2009; El Deeb et al., 2012; Nassef et al., 2015; Hussein et al., 2017). Reports from occupational workers (Table 2) particularly butchers illustrated high T. gondii seroprevalence (Abou-Elenin et al., 1983; El-Ridi et al., 1990; Ibrahim et al., 1997). In addition, T. gondii antibodies were found in the sera of 48 (37.7%) of 127 workers in pig farms from Cairo and Kalubiya where pigs were raised completely on garbage feeding. Of them, 15 had high (1:512–1:1024) antibody titres (Barakat et al., 2011). Toxoplasma gondii DNA was found in 17 (35.4%) out of 48 seropositive occupational workers; however, the authors did not specify their professions (Hassanain et al., 2013). It seems that they tested the same sera used in Barakat et al. (2011).
Table 6.
Risk factors of T. gondii seroprevalence in human population in Egypt
| Population | No. tested | No. positive (%) | Risk factors | Reference |
|---|---|---|---|---|
| Complication | 100 | 17 (17.0) | Rural areas and previous abortion | Shatat et al. (2006) |
| Complication | 75 | 58 (77.3) | 20–25 years old, urban areas, previous abortion and contact with soil | Abo El Naga et al. (2008) |
| Normal pregnant | 181 | 85 (47.0) | 36–40 years old, rural areas and various disorders | El-Gozamy et al. (2009) |
| Blood donors | 260 | 155 (59.6) | 30 years old or more, rural areas, bad hand hygiene, consumption of meat byproducts and unwashed vegetables, drinking municipal water, no education, and contact with cats, different animals and soil | Elsheikha et al. (2009) |
| Abortion | 100 | 30 (30.0) | Contact with soil and consumption of meat byproducts | Abd El-Ghany and Amin (2012) |
| Normal pregnant | 323 | 218 (67.5) | 30–39 years old, urban areas, low economic status, no knowledge about transmission modes, drinking raw milk, consumption of undercooked meat and unwashed vegetables, and contact with cats, farm animals and soil | El Deeb et al. (2012) |
| Abortion | 76 | 35 (46.1) | <25 years old, rural areas and multigravida | Tammam et al. (2013) |
| Normal pregnant | 100 | 71 (71.0) | 31–35 years old, previous abortion, contact with cats and soil, and consumption of raw milk and homemade cheese | Ahmed et al. (2014) |
| Complication | 120 | 46 (38.3) | 26–30 years old, rural area, low socioeconomic level, housewives, contact with soil, and consumption of undercooked meat and raw vegetables | Kamal et al. (2015) |
| Abortion | 92 | 48 (52.2) | 31–40 years old, rural areas, contact with cats and soil, and consumption of undercooked meat | Nassef et al. (2015) |
| Normal pregnant | 382 | 221 (57.9) | 35–44 years old, contact with cats and multigravida | Bassiony et al. (2016) |
| Normal pregnant | 300 | 46 (15.3) | 30–40 years old, rural areas, 3rd pregnancy trimester and workers | Abdel Gawad et al. (2017) |
| Normal pregnant | 57 | 22 (38.6) | >30 years old and no knowledge about transmission modes | Abou Elez et al. (2017) |
| Normal pregnant | 113 | 5 (4.4) | 17–25 years old, contact with soil and drinking unhygienic water | Elmonir et al. (2017) |
| Normal pregnant | 693 | 209 (30.1) | Previous abortion, contact with cats and soil, and consumption of undercooked meat | El-Shqanqery et al. (2017) |
| Normal pregnant | 350 | 165 (47.1) | 20–30 years old, living in rural areas, unhealthy houses, low socioeconomic level, contact with cats, handling raw meat and consumption of raw milk | Hussein et al. (2017) |
| Normal pregnant | 364 | 123 (33.7) | >25 years old, contact with cats, farm animals and soil, and consumption of undercooked mutton | Ibrahim et al. (2017) |
| Complication | 182 | 97 (53.2) | >30 years old, rural areas, contact with soil, consumption of undercooked meat or viscera and raw milk, and bad hand hygiene | Mandour et al. (2017) |
| Normal pregnant | 388 | 79 (20.4) | 35–39 years old, rural areas, contact with cats and farm animals, previous abortion, taking immunosuppressive drugs and consumption of raw vegetables | Hassanain et al. (2018a) |
Data linking association between T. gondii infection and several disorders such as chronic liver disease and other conditions were too few for a cause–effect relationship (Table 5).
Clinical toxoplasmosis
Congenital
The first report of a congenital toxoplasmosis-like illness in Egypt was in a 1.5-year-old child from Giza (Rifaat et al., 1973e). He was admitted to the hospital presenting with marasmus and a mass in the upper part of the abdomen of 1 year's duration. The abdominal examination revealed enlarged liver without ascites or lymph node enlargement. Skull radiography showed microcephaly and bilateral 1–3 mm wide calcification. An extensive central chorio-retinal lesion was also found. The child had a DT T. gondii antibody titre of 1:512. His parents were also seropositive (1:128 and 1:64). Despite anti-Toxoplasma treatment (not specified), the child died 3 weeks later; post-mortem examination was not performed. In another report, Rifaat et al. (1973a) first isolated viable T. gondii from human placenta. A 30-year-old woman aborted an edematous macerated 22 weeks gestational age fetus. The fetus also had hydrocephaly. Viable T. gondii was isolated from the placenta (by mouse inoculation) but not from the fetal brain. Thus, there is no definitive evidence of congenital toxoplasmosis in either of these reports.
Other reports on congenital toxoplasmosis in Egypt are very conflicting and mainly published in local journals which are not widely accessible (Table 4). Most of these reports are based on serological results on single samples from pregnant women. The serologic diagnosis of acute maternal infection based on single serum sample is difficult because IgM antibodies can persist for months and the avidity index might remain low for several months (Peyron et al., 2016), thus definitive diagnosis requires the sequential appearance of specific IgM and IgG antibodies in the same sample. Detection of T. gondii in amniotic fluid can confirm the diagnosis of congenital toxoplasmosis and has been reported by Eida et al. (2009) and El Deeb et al. (2012). However, no clinical follow-up was reported.
Table 4.
Diagnosis of T. gondii associated abortion, complicated pregnancy and congenital infection in women from Egypt
| Population | Pregnancy stage | Governorate | No. tested | No. positive (%) | Test | Additional tests | Remarks | Reference | |
|---|---|---|---|---|---|---|---|---|---|
| IgM | PCR/blood | ||||||||
| Abortion | NS | Sharkia | 62 | 17 (27.4) | IFA1 | ND | ND | 3 had high Ab titre (1:1024), tachyzoites in histological section. No photographs were given | El-Ridi et al. (1991b) |
| Complication | 10 | 3 (30.0) | |||||||
| Complication | NS | Alexandria | 100 | 65 (65.0) | ELISA-IgG21 | ND | ND | – | Hammouda et al. (1993) |
| Repeated abortion | NS | Alexandria | 100 | 37 (37.0) | IHA | 19 (19.0) | ND | – | Sahwi et al. (1995) |
| Complication | NS | Kalubiya | 38 | 17 (44.7) | ELISA | 9 (23.7) | ND | – | Hussein et al. (2001) |
| Complication | NS | Dakahlia | 70 | 57 (81.4) | ELISA-IgG19 | 42 (60.0) | ND | Other complications causes were excluded | Soliman et al. (2001) |
| Abortion | NS | Kalubiya | 40 | 14 (35.0) | ELISA-IgG1 | 12 (30.0) | 8 (20.0) | IgG in 6 (40%) and 2 (13%) of neonates from abortion and early labour groups. | El Fakahany et al. (2002) |
| Early labour | 10 | 5 (50.0) | 3 (33.0) | 5 (50.0) | |||||
| CMF | 5 | 1 (20.0) | None | 3 (60.0) | |||||
| Abortion | 1st | Cairo | 40 | 16 (40.0) | ELISA-IgG | 10 (25.0) | 20 (50.0) | – | Abdel-Hameed and Hassanein (2004) |
| Abortion | 2nd | 33 | 10 (30.3) | 9 (27.2) | 16 (48.0) | ||||
| IUFD | 1st | 27 | 4 (14.8) | 3 (11.1) | 2 (7.4) | ||||
| Abortion | NS | Sharkia | 25 | 10 (40.0) | IHA1 | 3 (12.0) | ND | – | Awadallah (2010) |
| Complication | 1st, 2nd | Assiut | 100 | 17 (17.0) | ELISA-IgG2 | 22 (22.0) | ND | 5 had IgM only; they were primigravida with early abortions | Shatat et al. (2006)a |
| Abortion | NS | Dakahlia | 75 | 75 (100) | NS | 75 (100) | 58 (77.3) | Authors selected positive IgG and IgM cases only | Abo El Naga et al. (2008)a |
| Complication | NS | Zagazig | 100 | 62 (62.0) | ELISA-IgG20 | 47 (47.0) | 73 (73.0) | – | El Gamal et al. (2013) |
| Abortion | NS | NS | 56 | 34 (60.7) | ELISA-IgG3 | ND | 9/34 (26.5) | Seropositive cases only were PCR tested | Hassanain et al. (2013) |
| Abortion | 1st | Qena | 76 | 35 (46.1) | ELISA-IgG4 | 14 (18.4) | ND | A case had tachyzoites in placental sections. Illustrations are not clear. | Tammam et al. (2013)a |
| Abortion | 1st | Beni Suef, Cairo | 56 | 17 (30.4) | ELISA-IgG | 12 (21.4) | 18 (32.1)b | – | Hassanain et al. (2015) |
| 2nd | 30 | 8 (26.7) | 5 (16.7) | 10 (33.3)b | |||||
| 3rd | 15 | 7 (46.7) | 2 (13.3) | 6 (40.0)b | |||||
| CMF | – | 5 | 2 (40.0) | 1 (20.0) | 1 (20.0)b | ||||
| Complication | NS | Minia | 120 | 53 (44.1) | ELISA-IgG2 | 29 (24.1) | ND | Other abortifacient causes were excluded | Kamal et al. (2015)a |
| Complication | Different | Menoufiya | 92 | 48 (52.2) | ELISA-IgG5 | 9 (9.7) | 26 (28.6)b | IgG in aborted women (n = 73) was 63.9% IgG vs 42.9% in those who did not abort (n = 19) | Nassef et al. (2015)a |
| Abortion | NS | Kalubiya | 37 | 22 (59.5) | ELISA-IgG22 | 7 (18.9) | ND | Other abortifacient causes were excluded. The parasite was not detected in placental sections | Hussein et al. (2016) |
| Complication | Different | Zagazig | 100 | ND | NS | 51 (51.0) | 38 (38.0) | 35 had low IgG avidity | El-Settawy et al. (2016) |
| Complication | Different | Assiut | 182 | 97 (53.3) | ELISA-IgG8 | 52 (28.6) | ND | Mandour et al. (2017)a | |
| Abortion | 1st | Cairo | 139 | 62 (44.6) | ELISA-IgG2 | 4/77 (5.1) | 8/77 (10.2) | IgM and PCR on 77 IgG negatives | Abd El Aal et al. (2018) |
| Abortion | 1st | Cairo, Giza | 32 | 12 (37.5) | ELISA-IgG9 | 11 (34.3) | 11 (34.4)b | – | Barakat et al. (2018) |
| 2nd | 21 | 8 (38.1) | 8 (38.1) | 8 (38.1)b | |||||
| 3rd | 16 | 8 (50.0) | 6 (37.5) | 5 (31.1)b | |||||
| CMF | – | 4 | 2 (50.0) | 1 (25.0) | 3 (75.0)b | ||||
| Abortion | Different | Beni-Suef | 35 | 25 (71.4) | ELISA-IgG3 | Done | ND | Authors did not give a separate IgG and IgM prevalence | Hassanain et al. (2018a) |
Ab, antibody; ND, not done; NS, not stated; IUFD, intrauterine fetal death; CMF, congenital malformation.
Numbers in parenthesis are percentages.
Risk assessment, see Table 6.
DNA in the placenta.
Although T. gondii-infected women can abort, toxoplasmosis is not a common cause of habitual abortion in women (reviewed in Dubey and Beattie, 1988). Numerous women in Egypt who aborted fetuses have been tested for toxoplasmosis (Table 4). In some of the reports, T. gondii DNA was detected in placentas or unspecified products of conception. Once again, the accuracy of PCR requires stringent controls to minimize contamination. Caution is needed that the presence of T. gondii DNA in placenta does not equate with congenital infection.
An estimate of the rate of congenital toxoplasmosis can be obtained by data on seroconversion of mothers during pregnancy, serological testing of fetus during pregnancy and after parturition, and clinical follow-up of newborn children. There are no concrete data concerning prevalence of congenital toxoplasmosis in Egypt. To confirm congenital infection, sera would be tested at 12 months showing IgG presence or evidence of neo-synthetized antibodies by Western-blot in children blood from birthday or 3 months of age (Robert-Gangneux and Dardé, 2012).
In summary, there is no definitive evidence of toxoplasmosis abortion or definitive diagnosis of congenital toxoplasmosis in any of these cases.
Post-natal clinical toxoplasmosis
Lymphadenopathy, fever and ocular involvement are some of the common symptoms of acquired toxoplasmosis (Peyron et al., 2016). In addition to the report of these symptoms in pregnant women in Egypt discussed by Eida et al. (2009), there are few other reports of toxoplasmosis-associated lymphadenopathy from Egypt (Azab et al., 1983; Tolba et al., 2014) based on mainly serologic examination. There are also a few reports of ocular toxoplasmosis in Egypt (Table 7). Rifaat et al. (1973b) studied the case of an 18-year-old female student who complained of headache and impaired vision in the right eye. Based on the revealed lesions of uveitis altogether with the positive DT titre (1:128), authors diagnosed the case as toxoplasmic uveitis. This case was treated with pyrimethamine and sulfadiazine for 2 weeks. A month after treatment, lesions regressed, the vision acuity was enhanced. Based on positive serology and the lesion, ocular toxoplasmosis has been reported by others (Azab et al., 1983; El-Ridi et al., 1991a; Safar et al., 1995). Recently, Tolba et al. (2014) reported three chorioretinitis cases from Alexandria; the three cases were IgG-positive, while a single case had IgM antibodies. No test was performed in aqueous or vitrous humour.
Table 7.
Seroprevalence of T. gondii antibodies in suspected ocular patients from Egypt
| Governorate | No. tested | No. positive (%) | Test | Titres | Lesion | Mice inoculation | PCR | Reference |
|---|---|---|---|---|---|---|---|---|
| Cairo | 1 | 1 (100) | DT | 1:128 | Uveitis | ND | ND | Rifaat et al. (1973b) |
| Cairo | 30 | 18 (40.0) | IFA5 | 1:16–1:64 | NS | ND | ND | Azab et al. (1983) |
| Sharkia | 34 | 9 (26.5) | IFA1
IHA5 |
281.6–576.7a | Anterior and posterior uveitis | ND | ND | El-Ridi et al. (1991a) |
| Giza | 70 | 15 (21.1) 36 (51.4) |
IFA IHA |
NS | Retinochoroiditis | ND | ND | Safar et al. (1995) |
| Alexandria | 3 | 3 (100) 2 (66.6) |
ELISA8-IgG ELISA8-IgM |
– | Chorioretinitis | ND | +ve 3/3 | Tolba et al. (2014) |
NS, not stated; ND, not done; +ve, positive.
Antibody titres are given in means.
Toxoplasmosis in animals
Toxoplasmosis can cause severe illness in many domestic and wild animal species. It is a common cause of abortion in sheep and goats worldwide (Dubey, 2010). Many species of animals, such as New World primates, Australasian marsupials, Pallas and Sand cats, are highly susceptible to acute toxoplasmosis, whereas cattle, buffaloes and horses are resistant to toxoplasmosis (Dubey, 2010). Additionally, animals appear reservoirs of T. gondii infection. Humans become infected postnatally by ingesting food and water contaminated with oocysts shed by felids and by eating undercooked meat. Available information on T. gondii infection in domestic animals from Egypt is summarized here.
Cats
The published seroprevalence estimates in cats are highly variable (12.5–97.4%) (Table 8), depending on the life style and age of cats and the serological test. It is noteworthy that five of the six surveys are from Cairo and Giza governorates.
Table 8.
Seroprevalence of T. gondii antibodies in cats from Egypt
| Source of sera | Governorate | No. tested | No. positive (%) | Test (Cut-off) | Reference |
|---|---|---|---|---|---|
| Stray | Cairo and Giza | 318 | 126 (39.6) | DT (1:4) | Rifaat et al. (1976c) |
| Stray | Cairo | 177 | 105 (58.8) | IFA1 | Aboul-Magd et al. (1988) |
| Stray | Gharbiya | 92 | 17 (18.5) 19 (20.7) |
IHA2
IFA1 |
Abu-Zakham et al. (1989) |
| House-hold | 32 | 4 (12.5) 5 (15.6) |
|||
| Stray kittens | Cairo, Giza and Kalubiya | 34 | 24 (70.6) | LAT1 | Hassanain et al. (2008) |
| House-hold Kittens | 63 | 32 (50.8) | |||
| Stray | Giza | 158 | 154 (97.4) | MAT (1:5) | Al-Kappany et al. (2010) |
| Stray | Cairo | 180 | 172 (95.5) | MAT (1:5) | Al-Kappany et al. (2011) |
A very high seroprevalence (>95%) of T. gondii was reported in stray cats. The specificity of the MAT for cats was confirmed by isolation of viable T. gondii (Al-Kappany et al., 2010). Brains, hearts and tongues from 112 seropositive cats were bioassayed individually in mice. Toxoplasma gondii was isolated from 83 hearts, 53 tongues and 36 brains. We are not aware of any report of clinical toxoplasmosis in cats from Egypt.
Cats are the key hosts in the epidemiology of T. gondii because they are the only hosts that can excrete environmentally resistant oocysts in feces. There is limited information on T. gondii oocyst excretion by cats in Egypt (Table 9). Of these, two reports by Rifaat et al. (1976c) and Al-Kappany et al. (2010) need comment. Rifaat et al. (1976c) found T. gondii-like oocysts in feces of 88 (41.3%) of 213 stray cats trapped from Cairo and Giza. A total of 318 cats were trapped, euthanized and blood and feces were collected for T. gondii testing. Antibodies to T. gondii were found in 126 (39.6%) by the DT. Nearly half of the cats were considered adults based on weights of cats. Out of these 318 cats, feces of 213 cats were tested for coccidian oocysts. Feces with T. gondii-like oocysts were bioassayed in mice, and the identity of Toxoplasma oocysts was proven by sub-inoculation of infected mouse tissues to clean mice. Toxoplasma gondii-like oocysts were found in 88 cats (20 in 6–8 weeks old, six in 9–12 weeks old, seven in 4–5 months old and 55 in cats older than 6 months). Serological results and oocyst excretion were compared in 33 cats; 14 (35.7%) of 33 cats excreting oocysts were seropositive, and 19 (15.8%) were seronegative. Thus, both seropositive and seronegative cats were excreting oocysts. From the results presented, it is uncertain whether the results were based solely on the presence of antibodies in mice fed oocysts or demonstration of T. gondii in mouse tissues. If the results were based on serology alone, then data will not exclude the related parasite, Hammondia hammondi infection (Dubey, 2010). There are no archived data or specimens for validation. At any rate, this report from Egypt is the highest prevalence of excretion of T. gondii-like oocysts compared with reports from other countries (Dubey, 2010).
Table 9.
Prevalence of T. gondii-like oocysts in fecal samples from cats in Egypt
| Governorate | No. tested | No. positive (%) | Reference |
|---|---|---|---|
| Cairo and Giza | 213 | 88 (41.3)a | Rifaat et al. (1976c) |
| Cairo, Giza and Kalubiya | 97 | 12 (12.3) | Hassanain et al. (2008) |
| Giza | 158 | Nonea | Al-Kappany et al. (2010) |
| Sharkia | 50 | 25 (50.0)a | Awadallah (2010) |
| Kafr El Sheikh | 113 | 10 (9.0) | Khalafalla (2011) |
| Sharkia | 100 | 2 (2.0)b | Abd El-Ghany and Amin (2012) |
| Kafr El Sheikh | 100 | 2 (2.0) | Elmonir et al. (2017) |
See comments in the text.
T. gondii DNA was isolated from both cases.
Al-Kappany et al. (2010) did not find T. gondii oocysts in feces of 158 stray cats from Giza, probably because most (97.4%) were seropositive to T. gondii and had already excreted oocysts. Awadallah (2010) found T. gondii-like oocysts in 25 (50%) of 50 cat feces from Sharkia; however, oocysts identity was not confirmed by bioassay or PCR.
Toxoplasma gondii oocysts are excreted only for a short period (<2 weeks) in the life of the cat and by the time cats become seropositive, oocysts have already been excreted. However, cats can re-excrete oocysts more than once in life (Dubey, 2010).
Isolation of T. gondii oocysts from the environment
It is technically difficult to isolate T. gondii oocysts from running water (Dubey, 2010). However, Elfadaly et al. (2018) observed T. gondii-like oocysts in seven (2.9%) of 245 water samples collected from ground pumps (water supplies) in rural areas of Giza governorate. The identity of the recovered oocysts was not confirmed. El-Tras and Tayel (2009) tested 30 water samples from irrigation canals by bioassay in mice. It is not clear whether all samples were infected, and if samples were inoculated separately or in pools. After 6 weeks, sera of inoculated mice were tested using direct agglutination test for T. gondii; five were reported to be positives; however, the antibody titres were not stated and mice were not tested for viable T. gondii. They also bioassayed in kittens’ 30 vegetable samples irrigated by the sampled water. Four cats excreted T. gondii-like oocysts; however, oocysts infectivity was not reported, and it is not clear if the kittens were tested for T. gondii antibodies before use in the experiment. Recently, methods for detection and viability measure of T. gondii oocysts were described and they could be employed in Egypt in order to determine the contamination of the environment (Rousseau et al., 2019).
Dogs
Dogs are considered a source of infection for humans because they roll over and eat cat feces among other foods ingested (Frenkel et al., 2003). Antibodies to T. gondii have been demonstrated in the sera of dogs and viable T. gondii has been isolated from naturally infected dog tissues (Table 10). Nothing is known of clinical toxoplasmosis in dogs from Egypt.
Table 10.
Seroprevalence of T. gondii antibodies in stray dogs from Egypt
| Governorate | No. tested | No. positive (%) | Test | Cut-off | Mice bioassay | Reference |
|---|---|---|---|---|---|---|
| Cairo | 45 | 11 (24.4) | DT | 1:16 | ND | Rifaat et al. (1970) |
| Cairo | 82 | 40 (46.5) | DT | 1:4 | Yesa | Rifaat et al. (1977a) |
| Cairo | 43 | 12 (27.9) | DT | 1:16 | ND | Khaled et al. (1982) |
| Giza | 51 | 50 (98.0) | MAT | 1:4 | Yesb | El Behairy et al. (2013) |
ND, not done.
Viable T. gondii was isolated from the brains of two dogs.
Viable T. gondii was isolated from 22 out of 43 hearts of seropositive dogs.
Food animals
Sheep
The estimated sheep population in Egypt is 5.5 million (Food and Agriculture Organization, 2015). Sheep meat is widely consumed in Egypt, especially during religious holidays. The consumption of undercooked dish ‘Kabob and kofta’ is popular (Hassan-Wassef, 2004), which favours T. gondii transmission to humans. Most reports used sera from sheep at abattoirs, while few studies were conducted on sheep in farms (Table 11). In a histological study, T. gondii tissue cysts were noted in brain sections of two out of 60 sheep from a herd in Suez governorate (Anwar et al., 2013); we consider the two tissue cysts illustrated in Figure 4 of their paper as Sarcocystis cysts (J.P. Dubey, own opinion).
Table 11.
Seroprevalence of T. gondii antibodies in sheep from Egypt
| Governorate | Source of sera | No. tested | No. positive (%) | Test | Reference |
|---|---|---|---|---|---|
| Different | Abattoir, farms | 398 | 47 (12.1) | IFA5 | Maronpot and Botros (1972) |
| Beheira | Abattoir | 21 | 7 (33.3) | DT (1:8) | Rifaat et al. (1977b) |
| Sharkia | 34 | 11 (32.3) | |||
| Port Said | Abattoir | 7 | 2 (28.5) | DT (1:8) | Rifaat et al. (1977c) |
| Ismailia | 21 | 4 (19.0) | |||
| Suez | 24 | 8 (33.3) | |||
| Cairo | NS | 100 | 37 (37.0), 51 (51.0) | SAT, DT | Michael (1977) |
| 100 | 40 (40.0), 9 (9.0) | SAT, CFT | |||
| 90 | 26 (28.8), 23 (25.5) | SAT, IHA4 | |||
| Menoufiya | Abattoir | 54a | 9 (16.6) | DT (1:8) | Rifaat et al. (1978) |
| Assiut | Abattoir, veterinary hospital | 169 | 115 (67.9) | DT (1:4) | Fahmy et al. (1979b) |
| Alexandria | Abattoir | 40 | 29 (72.5) | DT (1:8) | Rifaat et al. (1979) |
| Sharkia | Abattoir | 17 | 5 (29.4) | IHA5 | El-Ridi et al. (1990) |
| Kafr ElSheikh | Ewes from a farm | 102 | 47 (46.0), 51 (50.0), 50 (49.0) | ELISA17, IFA2, DAT | El-Ghaysh and Mansour (1994) |
| Gharbia | Abattoir | 105 | 52 (49.5) | IHA5 | Ibrahim et al. (1997) |
| Cairo | Abattoir | 300 | 131 (43.7), 125 (41.7), 110 (37.0), 102 (34.0) | MAT (1:25), ELISA17, IFA4, DT | Shaapan et al. (2008) |
| Giza | Farms | 320 | 152 (47.5), 141 (44.0) | IHA, ELISA | Barakat et al. (2009) |
| Sharkia | Abattoir | 50 | 9 (18.0) | IHA1 | Awadallah (2010) |
| Fayoum | NS | 62 | 61 (98.4), 56 (90.3) | ELISA16, DT | Ghoneim et al. (2010) |
| Cairo | Abattoir | 280 | 141 (50.4), 172 (61.4) | LAT1, ELISA17 | Hassanain et al. (2011) |
| Sharkia | Farms | 100 | 85 (85.0) | IHA1 | Abd El-Ghany and Amin (2012) |
| NS | NS | 280 | 172 (61.4) | ELISA16 | Hassanain et al. (2013) |
| Dakahlia | NS | 292 | 122 (41.7), 193 (66.1), 181 (62.0) | LAT3, IHA1, ELISA16 | Younis et al. (2015) |
| Qena | Individual, small farms | 37 | 18 (48.7), 21(56.8) | LAT1, ELISA15 | Fereig et al. (2016) |
| Kafr ElSheikh | 46 | 32 (69.6), 32 (69.6) | |||
| Menoufiya | 28 | 3 (10.7), 4 (14.3) | |||
| Assiut | Rural areas | 50 | 22 (44.0), 43 (86.0) | LAT2, ELISA2 | Kuraa and Malek (2016) |
| Cairo, Giza, Kalubiya | NS | 254 | 163 (64.2) | ELISA17 | El Fadaly et al. (2017) |
| Menoufiya, Gharbia | Public market | 170 | 88 (51.7) | ELISA14 (0.096) | Ibrahim et al. (2017) |
| Cairo | Ewes from small farms | 25 | 10 (40.0), 7 (28.0) | OTRT, ELISA12 | Abd El-Razik et al. (2018) |
| Giza | 33 | 20 (60.6), 17 (51.5) | |||
| Skarkia | 55 | 36 (65.4), 34 (61.8) | |||
| Cairo | Abattoir | 193 | 105 (54.4), 9 (48.7) | ||
| Cairo | Abattoir | 100 | 12 (12.0), 20 (20.0) | ELISA13, IFA2 | Al-Kappany et al. (2018) |
| Dakahlia | 100 | 27 (27.0), 38 (38.0) | |||
| Sharkia | 99 | 17 (17.1), 34 (34.3) | |||
| Giza | 99 | 26 (26.2), 32 (32.3) | |||
| Ismailia | Abattoir | 100 | 34 (34.0), 33 (33.0) | ELISA, MAT | El-Gawady et al. (2018) |
Fifty-four were examined: 27 from Menoufiya governorate and 37 from Tahrir province (currently known as Beheira governorate).
Toxoplasma gondii is an important cause of abortion in sheep worldwide but little is known of its occurrence in sheep from Egypt (Dubey, 2010). Direct evidence of ovine congenital toxoplasmosis was provided by Rifaat et al. (1977a) who isolated viable T. gondii by mouse bioassay from tissues of an aborted lamb. Toxoplasma gondii DNA has been demonstrated in aborted fetal tissues (Table 12). Finding T. gondii parasites or T. gondii DNA only indicates congenital transmission. Histopathological evaluation and exclusion of other causes of abortion are necessary to establish cause–effect relationship. Serological testing of ewes is of little help because high levels of T. gondii IgG can persist for months and IgM antibodies have already peaked in aborted ewes (Dubey, 2010).
Table 12.
Diagnosis of T. gondii in pregnant or aborted sheep and goats from Egypt
| Animal | Governorate | No. tested | Serological test | No. positive (%) | Antibody titres range | Mice bioassay | PCR | Other abortifacient agents | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Pregnant sheep 15 days before parturition with a history of late pregnancy abortions | NS | 10 | IHA | 10 (100) | 1:512–1:2048 | ND | ND | −ve Brucella abortus | Hassanain et al. (1992) |
| Pregnant goats at different stages of pregnancy | Kalubiya | 48 | IHA-IgG MAT-IgM |
17 (35.4) 11 (22.9) |
1:128–1:512 | Donea | ND | ND | Ramadan et al. (2007) |
| Aborted sheep and goats at late stage of pregnancy | Giza | NS | LAT | (100) | NS | ND | +ve 8 lambs and 4 kids | −ve other abortifacient agentsb | Ahmed et al. (2008) |
| Pregnant sheep from 3 flocks with history of previous abortions | Sharkia | 100 | IHA1-IgG IHA1-IgM |
85 (85.0) None |
1:160–1:2560 | ND | ND | ND | Abd El-Ghany and Amin (2012) |
| Pregnant sheep from a flock suffering from abortion | Kalubiya | 30 | LAT4 | 16 (53.3) | ⩾1:64 | Donec | 12 (40.0) | ND | Khater et al. (2013) |
| Pregnant sheep with history of abortion | Nile Delta | 416 | IHA1-IgM | 129 (31.0) | NS | ND | ND | +ve Brucella melitensis in 51 (12.2)d | Mahboub et al. (2013) |
| Pregnant goats with history of abortion | 76 | 13 (17.1) | +ve Brucella melitensis in 28 (36.8)d | ||||||
| Aborted goats | Cairo, Giza and kalubiya | 35 | DAT | 28 (80.0)e | 1:25–1:400 | ND | ND | ND | Attia et al. (2017) |
ND, not done; NS, not stated; +ve, positive; −ve, negative.
Numbers in parenthesis are percentages.
Viable T. gondii was isolated from tissues of two stillborns, see comment in the text.
Brucella, Salmonella, Chlamydia and Neospora caninum
Details were not given.
Data are not separated between T. gondii and Brucella melitensis.
Tachyzoites were found in placental sections, however neither details nor illustrations were given.
Goats
Goat population in Egypt is ~4 million. Goats are usually reared within sheep herds. In a popular system in Egypt, particularly in suburban areas, small numbers of goats are kept in houses, and can roam to feed on the garbage along with cats and dogs. Using different serological tests, high T. gondii seroprevalence was reported from goats in Egypt (Table 13).
Table 13.
Seroprevalence of T. gondii antibodies in goats from Egypt
| Governorate | Source of sera | No. tested | No. positive (%) | Test | Reference |
|---|---|---|---|---|---|
| Different | Abattoir, farms | 234 | 111 (47.4) | IFA5 | Maronpot and Botros (1972) |
| Assiut | Abattoir, veterinary hospital | 98 | 53 (54.1) | DT (1:4) | Fahmy et al. (1979b) |
| Sharkia | Abattoir | 14 | 4 (28.6) | IHA5 | El-Ridi et al. (1990) |
| Gharbia | Abattoir | 78 | 38 (48.7) | IHA5 | Ibrahim et al. (1997) |
| Giza | Small farms | 306 | 182 (59.4), 170 (55.4) | IHA, ELISA | Barakat et al. (2009) |
| Sharkia | Abattoir | 50 | 8 (16.0) | IHA1 | Awadallah (2010) |
| Fayoum | NS | 24 | 10 (41.7), 5 (20.8) | ELISA16, DT | Ghoneim et al. (2010) |
| Giza | Abattoir | 230 | 102 (44.3) | MAT (1:25) | Shaapan et al. (2010) |
| Cairo, Beni-Suef, Sharkia | Herds | 182 | 77 (42.3) | IHA3 | Abdel-Rahman et al. (2012) |
| Minia | Abattoir | 100 | 64 (64.0) | IHA1 | Abdel-Hafeez et al. (2015) |
| Dakahlia | NS | 81 | 40 (49.4), 52 (64.2), 41 (50.6) | LAT3, IHA1, ELISA16 | Younis et al. (2015) |
| Qena | Individual, small farms | 27 | 10 (37.0), 13 (48.2) | LAT1, ELISA15 | Fereig et al. (2016) |
| Kafr ElSheikh | 30 | 30 (66.7), 30 (66.7) | |||
| Menoufiya | 37 | 33 (8.1), 37 (10.8) | |||
| Assiut | Rural areas | 57 | 27 (47.4), 50 (87.7) | LAT2, ELISA2 | Kuraa and Malek (2016) |
| Cairo, Giza, Kalubiya | NS | 293 | 127 (43.3) | ELISA17 | El Fadaly et al. (2017) |
| Cairo | Does from small farms | 32 | 10 (31.2), 9 (28.1) | OTRT, ELISA12 | Abd El-Razik et al. (2018) |
| Giza | 22 | 9 (40.1), 8 (36.3) | |||
| Skarkia | 41 | 24 (56.1), 22 (53.6) | |||
| Cairo | Abattoir | 51 | 28 (53.0), 22 (43.1) | ||
| Dakahlia | Abattoir | 100 | 59 (59.0), 54 (54.0) | ELISA13, IFA2 | Al-Kappany et al. (2018) |
| Ismailia | Abattoir | 100 | 32 (32.0), 31 (31.0) | ELISA, MAT | El-Gawady et al. (2018) |
Like sheep, little is known of toxoplasmal abortion in goats from Egypt; available information is summarized in Table 12. Ramadan et al. (2007) found IgG antibodies in 17 (35.4%) of 48 pregnant Balady goats from Kalubiya governorate; 11 (22.9%) of them had IgM. Three goats in the mid pregnancy stage were sulfadimidine-treated for 5 successive days, while another three kept untreated as controls. No abortions had occurred in the treated group and the delivered kids were seronegative, while one of the untreated goats delivered two seropositive-stillborns (IgG and IgM). Viable T. gondii was isolated from tissues of the stillborns.
Transmission of T. gondii to humans by consumption of raw goat milk is of public health significance (Dubey et al., 2014). Consumption of goat milk is popular in Egyptian rural areas. Abdel-Rahman et al. (2012) fed eight cats raw milk from eight seropositive goats (four IgG and four IgM positive goats); we are not aware of the validity of the used commercial kits. Toxoplasma gondii-like oocysts were found in feces from all cats of the IgM group and one cat from the IgG group; however, oocysts infectivity was not proven. Sadek et al. (2015) found T. gondii tachyzoites, respectively, in five of 58 and six of 47 milk samples from sheep and goats; this is a very high proportion and the illustrations are not clear. In addition, Ahmed et al. (2014) found T. gondii DNA in four (8%) of 50 milk samples from goats. The presence of T. gondii DNA in milk does not mean the viability of the parasite.
Camels
In Egypt, camel meat is inexpensive and consumed mainly in some governorates such as Cairo, Kalubiya, Sharkia and Assiut. It seems that the published reports of toxoplasmosis in camels from Egypt do not reflect the true prevalence in Egyptian camels because most of the sampled camels were imported, particularly those slaughtered at the official abattoir in Cairo (El Basateen). Seroprevalence data are summarized in Table 14. Moreover, T. gondii oocysts were revealed from cats fed pooled meat samples from camels (Abdel-Gawad et al., 1984). Toxoplasma gondii DNA was not found in 50 raw camel milk samples (Saad et al., 2018).
Table 14.
Seroprevalence of T. gondii antibodies in camels from Egypt
| Governorate | Source of sera | No. tested | No. positive (%) | Test | Reference |
|---|---|---|---|---|---|
| Different | Abattoir, farms | 49 | 3 (6.1) | IFA5 | Maronpot and Botros (1972) |
| Ismailia | Abattoir | 43 | 29 (67.4) | DT (1:8) | Rifaat et al. (1977c) |
| Assiut | Individual owners | 80 | 12 (15.0) | DT (1:16) | Michael et al. (1977) |
| Menoufiya | 80 | 15 (18.7) | |||
| Matrouh | 80 | 40 (50.0) | |||
| Menoufiya | Abattoir | 30 | 17 (56.7) | DT (1:8) | Rifaat et al. (1978) |
| Assiut | Abattoir, veterinary hospital | 119 | 30 (24.4) | DT (1:4) | Fahmy et al. (1979a) |
| Sharkia | Abattoir | 19 | 5 (26.3) | IHA5 | El-Ridi et al. (1990) |
| Gharbia | Abattoir | 36 | 6 (16.7) | IHA5 | Ibrahim et al. (1997) |
| Cairo | Abattoir | 166 | 29 (17.4) | MAT (1:25) | Hilali et al. (1998) |
| Cairo | Abattoir | 150 | 127 (18.0), 230 (20.0), 346 (30.7), 441 (27.3) | MATa (1:25) | Shaapan and Khalil (2008) |
| Assiut | Rural areas | 56 | 20 (35.7), 54 (96.4) | LAT2, ELISA2 | Kuraa and Malek (2016) |
| Kalubiya | Abattoir | 120 | 6 (5.0), 63 (52.6) | IHA3, ELISA8 | Ahmed et al. (2017) |
| Cairo, Giza, Kalubiya | NS | 34 | 9 (26.5) | ELISA17 | El Fadaly et al. (2017) |
MAT was conducted using formalin-treated whole tachyzoites from different antigen; 1RH strain, 2local equine strain, 3local camel strain and 4local sheep strain.
Cattle and water buffaloes
Both cattle and buffaloes are considered resistant to T. gondii infection (Dubey, 2010). Apparently, they can clear the infection in their tissues and their role in transmission to humans is uncertain; however, some reports indicated the substantial role of beef in T. gondii transmission (Opsteegh et al., 2011; Belluco et al., 2018). Although antibodies to T. gondii have been reported in both species in Egypt (Tables 15 and 16), viable parasite has not been isolated from beef.
Table 15.
Seroprevalence of T. gondii antibodies in cattle from Egypt
| Governorate | Source of sera | No. tested | No. positive (%) | Test | Reference |
|---|---|---|---|---|---|
| Port Said | NS | 35 | None | Skin test | Rifaat et al. (1968) |
| Different | Abattoir, farms | 207 | 52 (25.1) | IFA5 | Maronpot and Botros (1972) |
| Beheira | Abattoir | 15 | 7 (46.6) | DT (1:8) | Rifaat et al. (1977b) |
| Dakahlia | 60 | 44 (30.1) | |||
| Sharkia | 8 | None | |||
| Fayoum | 132 | 28 (21.2) | |||
| Port Said | Abattoir | 16 | 5 (31.2) | DT (1:8) | Rifaat et al. (1977c) |
| Ismailia | 16 | 4 (25.0) | |||
| Suez | 34 | 11 (32.2) | |||
| Kalubiya | Abattoir | 84 | 16 (19.0) | DT (1:8) | Rifaat et al. (1978) |
| Gharbia | 171 | 37 (21.6) | |||
| Menoufiya | 68 | 24 (35.2) | |||
| Kafr ElSheikh | 50 | 22 (44.0) | |||
| Dameitta | Abattoir | 40 | 22 (55.0) | DT (1:8) | Rifaat et al. (1979) |
| Alexandria | 65 | 14 (21.5) | |||
| Assiut | Abattoir, veterinary hospital | 106 | 50 (47.0) | DT (1:4) | Fahmy et al. (1979b) |
| Sharkia | Abattoir | 19 | 4 (21.4) | IHA5 | El-Ridi et al. (1990) |
| Gharbia | Abattoir | 39 | 18 (46.2) | IHA5 | Ibrahim et al. (1997) |
| Sharkia | Veterinary station | 93 | 10 (10.7) | ELISA14 | Ibrahim et al. (2009) |
| Sharkia | Abattoir | 50 | 6 (12.0) | IHA1 | Awadallah (2010) |
| NS | NS | 88 | 17 (19.3) | ELISA16 | Hassanain et al. (2013) |
| Minia | Abattoir | 100 | None | IHA1 | Abdel-Hafeez et al. (2015) |
| Qena | Individual, small farms | 225 | 66 (29.3), 55 (24.4) | LAT1, ELISA15 | Fereig et al. (2016) |
| Sohag | 76 | 22 (29.0), 16 (21.1) | |||
| Assiut | Rural areas | 56 | 18 (32.1), 41 (73.2) | LAT2, ELISA2 | Kuraa and Malek (2016) |
| Cairo, Giza, Kalubiya | NS | 45 | 16 (35.5) | ELISA17 | El Fadaly et al. (2017) |
Table 16.
Seroprevalence of T. gondii antibodies in water buffaloes from Egypt
| Governorate | Source of sera | No. tested | No. positive (%) | Test | Reference |
|---|---|---|---|---|---|
| Port Said | NS | 51 | 5 (9.8) | Skin test | Rifaat et al. (1968) |
| Gharbia | 60 | 3 (5.0) | |||
| Different | Abattoir, farms | 211 | 59 (28.0) | IFA5 | Maronpot and Botros (1972) |
| Beheira | Abattoir | 14 | 4 (28.5) | DT (1:8) | Rifaat et al. (1977b) |
| Dakahlia | 60 | 18 (30.0) | |||
| Sharkia | 24 | 4 (9.5) | |||
| Fayoum | 280 | 83 (29.6) | |||
| Port Said | Abattoir | 48 | 16 (33.3) | DT (1:8) | Rifaat et al. (1977c) |
| Ismailia | 109 | 13 (11.9) | |||
| Suez | 85 | 16 (18.8) | |||
| Kalubiya | Abattoir | 92 | 39 (42.4) | DT (1:8) | Rifaat et al. (1978) |
| Menoufiya | 98 | 22 (24.4) | |||
| Assiut | Abattoir, veterinary hospital | 212 | 93 (43.9) | DT (1:8) | Fahmy et al. (1979b) |
| Dameitta | Abattoir | 193 | 76 (34.2) | DT (1:4) | Rifaat et al. (1979) |
| Alexandria | 80 | 9 (11.2) | |||
| Sharkia | Abattoir | 15 | 3 (20.0) | IHA5 | El-Ridi et al. (1990) |
| Cairo | Abattoir | 75 | 12 (16.0) | MAT (1:25) | Dubey et al. (1998) |
| Giza | Abattoir | 160 | 36 (22.5) | MAT (1:25) | Shaapan et al. (2010) |
| NS | NS | 32 | 11 (34.4) | ELISA16 | Hassanain et al. (2013) |
| Assiut | Rural areas | 55 | 11 (20.0), 41 (74.5) | LAT2, ELISA2 | Kuraa and Malek (2016) |
| Cairo, Giza, Kalubiya | NS | 41 | 7 (17.1) | ELISA17 | El Fadaly et al. (2017) |
El-Tras and Tayel (2009) isolated viable T. gondii from tissues of two out of 30 buffaloes; however, it needs confirmation because there are no valid reports on the isolation of T. gondii from buffalo meat (Dubey, 2010), and the parasite was not found in tissues of three calves experimentally infected with 200 000 T. gondii oocysts (de Oliviera et al., 2001). Moreover, T. gondii DNA was not found in 50 milk samples from cows (Ahmed et al., 2014). The report of the presence of T. gondii DNA in 6% (3/50) of buffalo bull semen samples from Egypt needs confirmation (Abd El-Razik et al., 2017).
Pigs
Due to religious concerns, pork is not popular in Egypt. Pigs are reared in small holdings mainly in Cairo and Kalubiya within a complete garbage feeding system including food remnants, rodents, and dead animals and birds. Thus, they are excellent indicators for the spread of T. gondii infection. Reports on the seroprevalence of T. gondii in pigs from Egypt are given in Table 17.
Table 17.
Seroprevalence of T. gondii antibodies in pigs from Egypt
| Governorate | Source of sera | No. tested | No. positive (%) | Test | Reference |
|---|---|---|---|---|---|
| Cairo | Abattoir | 142 | 31 (21.8) | IFA5 | Maronpot and Botros (1972) |
| Alexandria | Abattoir | 50 | 25 (50.0) | DT (1:8) | Rifaat et al. (1979) |
| Cairo | Abattoir | 100 | 14 (14.0) | IHA | Ibrahim (1990) |
| Cairo | Abattoir | 150 | 74 (49.3) | MAT | Ghattas (1999)a |
| Cairo | Farms | 230 | 172 (74.7) | IFA3 | Barakat et al. (2011) |
| NS | NS | 230 | 185 (80.4) | ELISA16 | Hassanian et al. (2013) |
| Cairo | Farms | 180 | 102 (56.6) | MAT | El Moghazy et al. (2011) |
| 94 (52.2) | ELISA | ||||
| 77 (42.7) | IHA4 | ||||
| 64 (35.5) | DT | ||||
| El Minia | Abattoir | 100 | None | IHA1 | Abdel-Hafeez et al. (2015)b |
Viable T. gondii was isolated by both mice and cat bioassay.
Forty (40.0%) had IgM antibodies.
Viable T. gondii was isolated from two seropositive pigs (Botros et al., 1973) and seven (23.3%) of 30 pigs by mouse bioassay (Ghattas, 1999). Ghattas (1999) fed cats (T. gondii-seronegative) meats from seropositive pigs. Cats excreted T. gondii-like oocysts. The identity of the recovered oocysts was confirmed by oral inoculation in mice.
Equines
Generally, high T. gondii seroprevalences were reported in horses and donkeys from Egypt (Table 18). However, equine meat is not consumed by humans in Egypt. Anti-T. gondii antibodies were noted in seven out of 15 donkey milk samples (Haridy et al., 2010).
Table 18.
Seroprevalence of T. gondii antibodies in equines from Egypt
| Species | Governorate | Source of sera | No. tested | No. positive (%) | Test | Reference |
|---|---|---|---|---|---|---|
| Donkeys | Menoufiya | Rural areas | 121 | 79 (65.6) | ELISA17 | El-Ghaysh (1998) |
| Horses | NS | Farms | 420 | 1160 (38.1), 2133 (31.7), 3217 (51.7), 170 (40.5), 202 (48.1) | ELISA-LA17, ELISA-LAunb17, ELISA-Lab17, IFAT4, MAT (1:25) | Ghazy et al. (2007)a |
| Donkeys | Giza | Zoo abattoir | 200 | 189 (44.5), 2104 (52.0), 372 (36.0), 478 (39.0) | MAT (1:25) | Shaapan and Khalil (2008) b |
| Draught horses | Cairo | Individual owners | 100 | 25 (25.0) | ELISA17 | Haridy et al. (2009) |
| Working donkeys | Cairo | Individual owners | 100 | 45 (45.0) | ELISA17 | Haridy et al. (2010) |
| Sport horses | Cairo | Main farm | 240 | 125 (52.1), 122 (50.8), 94 (39.2) | LAT3, MAT (1:25), ELISA17 | Shaapan et al. (2012) |
| Donkeys | Dakahlia | NS | 79 | 35 (44.3), 53 (67.1), 54 (68.4) | LAT3, IHA1, ELISA16 | Younis et al. (2015) |
| Horses | 54 | 27 (50.0), 39 (72.2), 39 (72.2) | ||||
| Donkeys | Giza | Individual owners | 58 | 16 (27.6), 22 (37.9) | LAT1, ELISA15 | Fereig et al. (2016) |
| Menoufiya | 43 | 13 (30.2), 11 (25.6) | ||||
| Matrouh | 45 | 10 (22.2), 9 (20.0) |
ELISA were carried out using 1crude antigen (LA) prepared from local horse strain, and its purified immunogenetic fractions; 2bound (LAb) and 3unbound (LAunb) fractions.
MAT was carried out using formalin-treated whole tachyzoites from different antigen; 1RH strain, 2local equine strain, 3local camel strain and 4local sheep strain.
Viable T. gondii has been isolated from tissues of 25 slaughtered donkeys at Giza zoo abattoir. Toxoplasma gondii-like oocysts were found in nine of 25 cats fed donkey tissues (Younis et al., 2015); however, the identity of these oocysts was not confirmed. Moreover, viable T. gondii were isolated from horses slaughtered at the same zoo (Shaapan and Ghazy, 2007). Donkeys and horses are slaughtered in the zoo for feeding of wild felids which can excrete T. gondii oocysts.
Chickens and other avian species
Chickens and ducks are widely consumed in Egypt due to their relatively cheap prices in comparison to red meats. Free range (FR) system of rearing birds is common in rural areas particularly in villages of Upper Egypt. FR birds are considered as a common source of human infection (Dubey, 2010). High seroprevalence was reported from FR chicken in Egypt, indicating high oocyst-environmental contamination (Table 19). Hassanain et al. (1997) stated a direct correlation between T. gondii seroprevalence and the decrease in egg production, although the seropositives were at low titres (⩽1:64) and the parasite isolation was not done. Viable T. gondii was isolated from both FR and commercially farmed chicken in Egypt (Table 21).
Table 19.
Seroprevalence of T. gondii antibodies in chickens from Egypt
| Governorate | Source of sera | No. tested | No. positive (%) | Test | Reference |
|---|---|---|---|---|---|
| NS | NS | 30 | 15 (50.0) | DT (1:8) | Rifaat et al. (1969) |
| Kalubiya | C Laying hensa | 600 | 320 (53.3), 200 (33.3) | CFT (1:8), IHA5 | Hassanain et al. (1997) |
| Giza | M | 108 | 51 (47.4) | MAT (1:25) | El-Massry et al. (2000) |
| Menoufiya, Beheira | FR | 121 | 49 (40.4) | MAT (1:5) | Dubey et al. (2003) |
| Assiut | C | 90 | 10 (11.1) | MAT (1:50) | Deyab and Hassanein (2005) |
| H | 60 | 18 (30.0) | |||
| Kafr ElSheikh | FR | 84 | 32 (38.1) | IHA1 | Harfoush and Tahoon (2010) |
| Different | FR | 108 | 75 (69.5) | ELISA16 | Barakat et al. (2012) |
| C | 331 | 227 (68.5) | |||
| Beni Suef | FR | 90 | 18 (20.0) | IHAb | Aboelhadid et al. (2013) |
| SH | 125 | 12 (9.6) | |||
| Delta region | FR | 97 | 16 (16.4) | ELISA14 | Ibrahim et al. (2016) |
| SH | 207 | 18 (8.6) | |||
| Cairo, Giza, kalubiya | FR | 88 | 33 (37.5) | ELISA17 | El Fadaly et al. (2017) |
FR, free range chickens; H, house-bred chickens; M, market chickens; C, commercially farmed chickens; SH, slaughterhouse.
Six hundred laying hens from three flocks (each of 12000 birds) suffered from drop in eggs production and high percent of embryonic mortalities.
This test is wrongly identified in the report as MAT.
Table 21.
Trials to isolate viable T. gondii from tissues of food animals and birds in Egypt by mice bioassay
| Host | Governorate | Serological test | Samples | No. tested | No. positive (%) | References |
|---|---|---|---|---|---|---|
| Pig | Cairo | IFA | Heart, liver, kidney, brain | 1 | 1 (100) | Botros et al. (1973)a |
| Pig | Cairo | MAT | Diaphragm | 30 | 7 (23.3) | Ghattas (1999)a |
| Chicken | Beheira | MAT | Heart, brain | 49 | 19 (38.7) | Dubey et al. (2003)a |
| Duck | 3 | 1 (33.3) | ||||
| Buffalo | Middle Delta | ND | NS | 30 fresh | 2 (6.6) | El-Tras and Tayel (2009) |
| 30 frozen | None | |||||
| Sheep | Cairo | LAT1 | Diaphragm | 28 | 28 (100) | Hassanain et al. (2011)a |
| FR chicken | Different | ND | Hear, brain, breast | 60 | NC | El-Newishy et al. (2012) |
| C chicken | 170 | |||||
| Sheep | Cairo, Giza, Kalubiya | ELISA17 | Diaphragm, thigh muscles | 75 | 8 (10.7) | El Fadaly et al. (2017)a |
| Goat | 49 | 4 (8.2) | ||||
| Cattle | 16 | None | ||||
| Buffalo | 7 | None | ||||
| Camel | 4 | 2 (50.0) | ||||
| FR chicken | 9 | 2 (22.2) | ||||
| Sheep | Cairo | OTRT | Diaphragm | 34 | 15 (44.1) | Abd El-Razik et al. (2018)a |
| Goat | 3 | 3 (100) |
ND, not done; NC, not clear; FR, free range; C, commercially farmed.
The studied samples were from seropositive animals.
Little is known of toxoplasmosis in ducks. In Egypt, T. gondii seroprevalence ranges from 10.5 to 55% using different serological tests in different duck breeds (El-Massry et al., 2000; Dubey et al., 2003; Harfoush and Tahoon, 2010; AbouLaila et al., 2011; Ibrahim et al., 2018). Viable T. gondii was isolated from one of three seropositive FR ducks from Beheira governorate (Dubey et al., 2003).
In other avian species, T. gondii seroprevalence was reported from 29.8% of 188 quails (Shaapan et al., 2011), and 59.5% of 173 turkeys (El-Massry et al., 2000) and 12.5% of 120 Ostriches (El-Madawy and Metawea, 2013). The latter found T. gondii DNA in the blood of nine ostriches. Additionally, T. gondii antibodies were reported from pigeons (Rifaat et al., 1969; Ibrahim et al., 2018).
Rabbits
Prevalence of T. gondii in rabbits from different Egyptian governorates is variable and ranges from 0 to 37.5% (Hilali et al., 1991; Ibrahim et al., 2009; Harfoush and Tahoon, 2010; Ashmawy et al., 2011; Abou Elez et al., 2017). Despite some reports placing the rabbit as a major source for human infection (Almeria et al., 2004), we think that the role of rabbits is not of such importance because 90% of rabbits in Egypt are fed commercial pellets in small farms and kept in hutches or cages, which limit the chances of oocyst ingestion.
Rodents
Rodents are important for T. gondii epidemiology because they serve as a source of infection for cats (Dubey, 2010). Reports on the seroprevalence of T. gondii in different species of rodents from Egypt are given in Table 20. Viable T. gondii was isolated by mouse bioassay (Rifaat et al., 1971, 1973d, 1976a) and/or cat bioassay (El Fadaly et al., 2016).
Table 20.
Seroprevalence of T. gondii antibodies in rodents from Egypt
| Species | Governorate | No. tested | No. positive (%) | Test | Reference |
|---|---|---|---|---|---|
| Rattus norvegicus | Cairo | 100 | 34 (34.0) | DT | Rifaat et al. (1971)a |
| Rattus alexandrinus | Cairo | 110 | 47 (42.7) | DT | Rifaat et al. (1973d)a |
| Acomys cahirinus | Different | 101 | 36 (36.3) | DT (1:8) | Rifaat et al. (1976a)a |
| Rattus norvegicus | Port Said | 104 | 32 (30.8) | IHA | Morsy et al. (1981) |
| Rattus norvegicus | Ismailia | 150 | 21 (12.6) | IHA5 | Morsy et al. (1982) |
| Rattus rattus | 150 | 15 (10.0) | |||
| Rattus norvegicus | Dakahlia | 200 | 26 (13.0) | IHA5 | El-Shazly et al. (1991) |
| Rattus rattus | 228 | 20 (8.8) | |||
| Mus musculus | 87 | None | |||
| Acomys cahirinus | 69 | 4 (5.8) | |||
| Rattus norvegicus | Cairo, Giza | 74 | 34 (45.9) | LAT1 | El Fadaly et al. (2016)b |
| Rattus rattus | 108 | 21 (19.4) | |||
| Rattus frugivorus | 96 | 13 (13.5) | |||
| Rattus norvegicus | Giza | 79 | 3 (3.8) | ELISA | Mikhail et al. (2017) |
| Rattus rattus | 46 | 2 (4.3) |
Viable T. gondii was isolated from brain pools by mice bioassay.
Viable T. gondii was isolated by both mice and cat bioassay.
Isolation of viable T. gondii from food animals
Viable T. gondii was isolated from tissues of different food animals and birds in Egypt by mouse bioassay (Table 21). Cat bioassay was also used in some studies, and T. gondii-like oocysts were excreted from cats fed pooled meat samples. However, no further definitive procedures for theses oocysts were done in many studies (Abdel-Gawad et al., 1984; El-Massry et al., 1990; Hassanain et al., 2011).
Perspective
There are many reports on toxoplasmosis in animals and humans from Egypt, but there is no statistically-valid prevalence study on the national level. Little is known concerning clinical toxoplasmosis in humans or livestock in Egypt. Toxoplasmosis is usually considered by the physicians in Egypt as a cause of abortions and complications in pregnant women; however, the published studies are not well-structured and lack definitive diagnosis. There is a great need to establish a well-planned study concerning congenital toxoplasmosis in Egypt. Reports on toxoplasmosis in animals were based on commercial kits with unconfirmed validity. A large-scale study is needed employing validated serological methods and includes procedures for isolation of the parasite to critically evaluate the role of different food animals from Egypt in the transmission of T. gondii to humans.
Acknowledgements
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the U.S. Department of Agriculture. This work is dedicated to late Professor Mosaad Hilali, Parasitology Department, Faculty of Veterinary Medicine, Cairo University, Egypt.
Financial support
Ibrahim Abbas is the recipient of a junior visit grant (USC17: 141) supported by the US-Egypt Science and Technology (STDF) Joint Fund.
Conflict of interest
None.
Ethical standards
Not applicable.
References
- Abd El-Aal NF, Saber M, Fawzy N and Ashour WR (2016) Sero-prevalence of anti-Toxoplasma gondii antibodies among patients with neuropsychiatric disorders: epilespy and depression. Journal of the Egyptian Society of Parasitology 46, 729–736. [PubMed] [Google Scholar]
- Abd El-Ghany AM and Amin MAM (2012) Epidemiology and molecular detection of zoonotic Toxoplasma gondii in cat feces and seroprevalence of anti-Toxoplasma gondii antibodies in pregnant women and sheep. Life Science Journal 9, 133–146. [Google Scholar]
- Abd El-Razik KA, Mahmoud KM, Sakr AM, Sosa ASA, Hasanain MH, Ahmed YF and Nawito MF (2017) Evaluation of buffalo bull semen for some venereal diseases using PCR. Egyptian Journal of Veterinary Science 48, 73–79. [Google Scholar]
- Abd El-Razik KA, Barakat AMA, Hussein HA, Younes AM, Elfadaly HA, Eldebaky HA and Soliman YA (2018) Seroprevalence, isolation, molecular detection and genetic diversity of Toxoplasma gondii from small ruminants in Egypt. Journal of Parasitic Diseases 42, 527–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El Aal AA, Nahnoush RK, Elmallawany MA, El-Sherbiny WS, Badr MS and Nasr GM (2018) Isothermal PCR for feasible molecular diagnosis of primary toxoplasmosis in women recently experienced spontaneous abortion. Open Access Macedonian Journal of Medical Sciences 6, 982–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Gawad AM, Nassar AM and Hilali M (1984) Isolation of Toxoplasma gondii, Isospora felis and Isospora revolta from the meat of some farm animals. Journal of the Egyptian Veterinary Medical Association 49, 405–414. [Google Scholar]
- Abdel-Hafeez EH, Kamal AM, Abdelgelil NH and Abdel-Fatah M (2015) Parasites transmitted to human by ingestion of different types of meat, El-Minia city, El-Minia governorate, Egypt. Journal of the Egyptian Society of Parasitology 45, 671–680. [DOI] [PubMed] [Google Scholar]
- Abdel-Hameed DM and Hassanein O (2004) Evaluation of semi-quantitative PCR and IgG & IgM ELISA in diagnosis of toxoplasmosis in females with miscarriage. Journal of the Egyptian Society of Parasitology 34, 559–570. [PubMed] [Google Scholar]
- Abdel-Rahman MAM, El-Manyawe SM, Khateib AM and Saba S (2012) Occurrence of Toxoplasma antibodies in caprine milk and serum in Egypt. Assiut Veterinary Medical Journal 58, 145–152. [Google Scholar]
- Abdel Gawad SS, Gheith MA, Kamel NM and Shawky SM (2017) Sero-prevalence of toxoplasmosis among multiparous pregnant women attending antenatal care at Beni-Suef university's hospital, Egypt. Journal of the Egyptian Society of Parasitology 47, 689–694. [Google Scholar]
- Abo El Naga AM, Shaltot AA, El-Baz R and Fayad M (2008) PCR detection of Toxoplasma gondii DNA versus serological diagnosis in women suffering from repeated abortion. Egyptian Journal of Hospital Medicine 33, 577–586. [Google Scholar]
- Abo Hashim AH and Attya AA (2017) Screening test to detect recent Toxoplasma gondii infections in pregnant women. Journal of the Egyptian Society of Parasitology 47, 131–136. [PubMed] [Google Scholar]
- Aboelhadid SM, Abdel-Ghany AE, Ibrahim MA and Mahran HA (2013) Seroprevalence of Toxoplasma gondii infection in chickens and humans in Beni Suef, Egypt. Global Veterinaria 11, 139–144. [Google Scholar]
- Abou-Elenin E, Abdel-Wahab F, El-Bestar SF and Abdel-Aal AM (1983) Toxoplasmosis among people at risk of exposure to infection using indirect haemagglutination test. Journal of the Egyptian Society of Parasitology 13, 435–440. [PubMed] [Google Scholar]
- Abou Elez RMM, Hssanen EAA, Tolba HMN and Elsohaby I (2017) Seroprevalence and risk factors associated with Toxoplasma gondii infection in domestic rabbits and humans. Veterinary Parasitology: Regional Studies and Reports 8, 133–137. [DOI] [PubMed] [Google Scholar]
- AbouLaila M, El-Bahy N, Hilali M, Yokoyama N and Igarashi I (2011) Serodiagnosis of Toxoplasma gondii in ducks from Behere Governorate, Egypt. Journal of Protozoology Research 21, 45–49. [Google Scholar]
- Aboul-Magd LA, Tawfik MS, Arafa MS and El-Ridi AMS (1988) Toxoplasma infection of cats in Cairo area as revealed by IFAT. Journal of the Egyptian Society of Parasitology 18, 403–409. [PubMed] [Google Scholar]
- Abu-Zakham AA, El-Shazly AM, Yossef ME, Romeia SA and Handoussa AE (1989) The prevalence of Toxoplasma gondii antibodies among cats from Mahalla El-Kobra, Gharbia Governorate. Journal of the Egyptian Society of Parasitology 19, 225–229. [PubMed] [Google Scholar]
- Ahmed YF, Sokkar SM, Desouky HM and Soror AH (2008) Abortion due to toxoplasmosis in small ruminants. Global Veterinaria 2, 337–342. [Google Scholar]
- Ahmed HA, Shafik SM, Ali MEM, Elghamry ST and Ahmed AA (2014) Molecular detection of Toxoplasma gondii DNA in milk and risk factor analysis of seroprevalence in pregnant women at Sharkia, Egypt. Veterinary World 7, 594–600. [Google Scholar]
- Ahmed NE, Al-Akabway LM, Ramadan MY, Abd El-Gawad SM and Moustafa MMA (2017) Serological and PCR-sequencing assays for diagnosis of Toxoplasma gondii and Neospora caninum infecting camels in Egypt. Benha Veterinary Medical Journal 33, 200–210. [Google Scholar]
- Al-Kappany YM, Rajendran C, Ferreira LR, Kwok OCH, Abu-Elwafa SA, Hilali M and Dubey JP (2010) High prevalence of toxoplasmosis in cats from Egypt: isolation of viable Toxoplasma gondii, tissue distribution, and isolate designation. Journal of Parasitology 96, 1115–1118. [DOI] [PubMed] [Google Scholar]
- Al-Kappany YM, Lappin MR, Kwok OCH, Abu-Elwafa SA, Hilali M and Dubey JP (2011) Seroprevalence of Toxoplasma gondii and concurrent Bartonella spp., feline immunodeficiency virus, feline leukemia virus, and Dirofilaria immitis infections in Egyptian cats. Journal of Parasitology 97, 256–258. [DOI] [PubMed] [Google Scholar]
- Al-Kappany YM, Abbas IE, Devleesschauwer B, Dorny P, Jennes M and Cox E (2018) Seroprevalence of anti-Toxoplasma gondii antibodies in Egyptian sheep and goats. BMC Veterinary Research 14, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almería S, Calvete C, Pagés A, Gauss C and Dubey JP (2004) Factors affecting the seroprevalence of Toxoplasma gondii infection in wild rabbits (Oryctolagus cuniculus) from Spain. Veterinary Parasitology 123, 265–270. [DOI] [PubMed] [Google Scholar]
- Anwar S, Mahdy E, El-Nesr KA, El-Dakhly KM, Shalaby A and Yanai T (2013) Monitoring of parasitic cysts in the brains of a flock of sheep in Egypt. Revista Brasileira de Parasitologia Veterinária 22, 323–330. [DOI] [PubMed] [Google Scholar]
- Ashmawy KI, Abuakkada SS and Awad AM (2011) Seroprevalence of antibodies to Encephalitozoon cuniculi and Toxoplasma gondii in farmed domestic rabbits in Egypt. Zoonoses and Public Health 58, 357–364. [DOI] [PubMed] [Google Scholar]
- Attia MM, Saad MF and Abdel-Salam AB (2017) Milk as a substitute for serum in diagnosis of toxoplasmosis in goats. Journal of the Egyptian Society of Parasitology 47, 227–234. [PubMed] [Google Scholar]
- Awadallah MAI (2010) Endoparasites of zoonotic importance. Global Veterinaria 5, 348–355. [Google Scholar]
- Azab ME, Rifaat MA, Khalil HM, Safer EH and Nabaweya MK (1983) Evaluations of antibody levels of Toxoplasma infection by the immunofluorescent antibody test and ELISA test. Folia Parasitologica 30, 303–307. [PubMed] [Google Scholar]
- Azab ME, Kamel AM, Makled KM, Khattab H, El-Zayyat EA, Abo-Amer EA and Samy G (1992) Naturally occurring Toxoplasma antibodies in serum and milk of lactating women. Journal of the Egyptian Society of Parasitology 22, 561–568. [PubMed] [Google Scholar]
- Azab ME, El-Shenawy SF, El-Hady HM and Ahmad MM (1993) Comparative study of three tests (indirect haemagglutination, direct agglutination, and indirect immunofluorescence) for detection of antibodies to Toxoplasma gondii in pregnant women. Journal of the Egyptian Society of Parasitology 23, 471–476. [PubMed] [Google Scholar]
- Azab MS, Abousamra MK, Rahbar MH, Elghannam D and Raafat D (2012) Prevalence of, risk factors for, and oxidative stress associated with Toxoplasma gondii antibodies among asymptomatic blood donors in Egypt. Retrovirology 9, P27. [DOI] [PubMed] [Google Scholar]
- Barakat AMA, Abd Elaziz MM and Fadaly HA (2009) Comparative diagnosis of toxoplasmosis in Egyptian small ruminants by indirect hemagglutination assay and ELISA. Global Veterinaria 3, 9–14. [Google Scholar]
- Barakat AM, El Fadaly HA, Shaapan RM and Khalil FAM (2011) Occupational health hazard of Egyptian employees in contact with wastage nourished swine. Journal of American Science 7, 808–903. [Google Scholar]
- Barakat AM, Salem LM, El-Newishy AM, Shaapan RM and El-Mahllawy EK (2012) Zoonotic chicken toxoplasmosis in some Egyptian governorates. Pakistan Journal of Biological Sciences 15, 821–826. [DOI] [PubMed] [Google Scholar]
- Barakat AMA, Ahmed SO, Zaki MS, El Fadaly HA, Abd El-Razik KA, El-Hariri HM and Johar D (2018) New approach to differentiate primary from latent Toxoplasma gondii abortion through immunoglobulin and DNA interpretation. Microbial Pathogenesis 125, 66–71. [DOI] [PubMed] [Google Scholar]
- Bassiony H, Soliman NK, El Tawab S, Eissa S and Eossa A (2016) Sero-prevalence and risk factors associated with Toxoplasma gondii infection among pregnant women in Alexandria, Egypt. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 5, 4220–4227. [Google Scholar]
- Bayoumy AMS, Ibrahim WLF, Abou El Nour BM and Said AAA (2016) The parasitic profile among school children in El-wadi El-gadded governorate, Egypt. Journal of the Egyptian Society of Parasitology 46, 605–612. [PubMed] [Google Scholar]
- Belluco S, Patuzzi I and Ricci A (2018) Bovine meat versus pork in Toxoplasma gondii transmission in Italy: a quantitative risk assessment model. International Journal of Food Microbiology 269, 1–11. [DOI] [PubMed] [Google Scholar]
- Botros BAM, Moch RW and Barsoum IS (1973) Toxoplasmosis in Egypt. Isolation of Toxoplasma gondii from a pig. Journal of Tropical Medicine and Hygiene 76, 259–261. [PubMed] [Google Scholar]
- Deyab AK and Hassanein R (2005) Zoonotic toxoplasmosis in chicken. Journal of the Egyptian Society of Parasitology 35, 341–350. [PubMed] [Google Scholar]
- de Oliviera FCR, da Costa AJ, Bechara GH and Sabatini GA (2001) Distribuição e viabilidade de cistos de Toxoplasma gondii (Apicomplexa: Toxoplasmatinae) em tecidos de bos indicus, Bos taurus e Bubalus bubalis infectados com oocistos. Revista Brasileira de Medicina Veterinária 23, 28–34. [Google Scholar]
- Dubey JP (2010) Toxoplasmosis of Animals and Humans, 2nd Edn. Boca Raton, Florida, USA: CRC Press. [Google Scholar]
- Dubey JP and Beattie CP (1988) Toxoplasmosis of Animals and Man. Boca Raton, Florida, USA: CRC Press. [Google Scholar]
- Dubey JP, Romand S, Hilali M, Kwok OCH and Thulliez P (1998) Seroprevalence of antibodies to Neospora caninum and Toxoplasma gondii in water buffaloes (Bubalus bubalis) from Egypt. International Journal for Parasitology 28, 527–529. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Graham DH, Dahl E, Hilali M, El-Ghaysh A, Sreekumar C, Kwok OCH, Shen SK and Lehmann T (2003) Isolation and molecular characterization of Toxoplasma gondii from chickens and ducks from Egypt. Veterinary Parasitology 114, 89–95. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Verma SK, Ferreira LR, Oliveira S, Cassinelli AB, Ying Y, Kwok OCH, Tuo W, Chiesa OA and Jones JL (2014) Detection and survival of Toxoplasma gondii in milk and cheese from experimentally infected goats. Journal of Food Protection 77, 1747–1753. [DOI] [PubMed] [Google Scholar]
- Eida OM, Eida MM and Ahmed AB (2009) Evaluation of polymerase chain reaction on amniotic fluid for diagnosis of congenital toxoplasmosis. Journal of the Egyptian Society of Parasitology 39, 541–550. [PubMed] [Google Scholar]
- El-Beshbishi SN, El-Tantawy NL, Elzeky SM, Abdalaziz KF and Atia RA (2018) Seroprevalence of Toxoplasma gondii infection in children with central nervous system disorders in Mansoura, Egypt: a case-control study. Transactions of the Royal Society of Tropical Medicine and Hygiene 112, 555–560. [DOI] [PubMed] [Google Scholar]
- El-Gawady HM, Abdel-Aal AA, Sallam NH and Youissif EM (2018) Serological and molecular studies on Toxoplasma gondii infection in sheep and goats in Ismailia Province. Archives of Infectious Diseases and Therapy 2, 1–5. [Google Scholar]
- El-Geddawi OA, El-Sayad MH, Sadek NA, Hussien NA and Ahmed MA (2018) Detection of T. gondii infection in blood donors in Alexandria, Egypt, using serological and molecular strategies. Parasitologists United Journal 9, 24–30. [Google Scholar]
- El-Ghaysh A (1998) Seroprevalence of Toxoplasma gondii in Egyptian donkeys using ELISA. Veterinary Parasitology 80, 71–73. [DOI] [PubMed] [Google Scholar]
- El-Ghaysh AA and Mansour MM (1994) Detection of antibodies to Toxoplasma gondii in an Egyptian sheep-herd using modern serological techniques. Journal of the Egyptian Association of Immunologists 1, 117–121. [Google Scholar]
- El-Gozamy BR, Mohamed SA and Mansour HA (2009) Toxoplasmosis among pregnant women in Qualyobia Governorate, Egypt. Journal of the Egyptian Society of Parasitology 39, 389–401. [PubMed] [Google Scholar]
- El-Madawy SR and Metawea FY (2013) Serological assay and PCR for detection of Toxoplasma gondii infection in an ostrich farm at Ismailia Province, Egypt. IOSR Journal of Agriculture and Veterinary Science 2, 56–60. [Google Scholar]
- El-Massry AA, Abdel-Gawad AM and Nassar AM (1990) Isolation of Toxoplasma gondii, Isospora felis and Isospora revolta from sheep, goats and chicken in Egypt. Journal of the Egyptian Veterinary Medical Association 2, 275–284. [Google Scholar]
- El-Massry A, Mahdy OA, El-Ghaysh A and Dubey JP (2000) Prevalence of Toxoplasma gondii antibodies in sera of turkeys, chickens, and ducks from Egypt. Journal of Parasitology 86, 627–628. [DOI] [PubMed] [Google Scholar]
- El-Nahas HA, El-Tantawy NL, Farag RE and Alsalem AM (2014) Toxoplasma gondii infection among chronic hepatitis C patients: a case-control study. Asian Pacific Journal of Tropical Medicine 7, 589–593. [DOI] [PubMed] [Google Scholar]
- El-Nawawy A, Soliman AT, El Azzouni O, Amer E, Karim MA, Demian S and El Sayed M (1996) Maternal and neonatal prevalence of Toxoplasma and cytomegalovirus (CMV) antibodies and hepatitis-B antigens in an Egyptian rural area. Journal of Tropical Pediatrics 42, 154–157. [DOI] [PubMed] [Google Scholar]
- El-Newishy AMA, Salem LMA, Barakat AM and El Mahallawy EKA (2012) Zoonotic importance of Toxoplasma gondii tissue cysts in chickens. Benha Veterinary Medical Journal 23, 53–60. [Google Scholar]
- El-Ridi AMS, El-Gamal RLR, Farghaly AM, Ramadan ME, Hassan AA and Ramadan AS (1989) Toxoplasmosis among cases of chronic tonsillitis. Journal of the Egyptian Society of Parasitology 19, 85–91. [PubMed] [Google Scholar]
- El-Ridi AM, Nada SM, Aly AS, Habeeb SM and Aboul-Fattah MM (1990) Serological studies on toxoplasmosis in Zagazig slaughterhouse. Journal of the Egyptian Society of Parasitology 20, 677–681. [PubMed] [Google Scholar]
- El-Ridi AM, Nada SM, Abdul-Fattah MM, Habeeb YS and Awad MB (1991a) Prevalence of congenital and acquired toxoplasmic uveitis as evidenced by two serologic methods. Journal of the Egyptian Society of Parasitology 21, 357–361. [PubMed] [Google Scholar]
- El-Ridi AM, Nada SM, Aly AS, Ramadan ME, Hagar EG and Taha TA (1991b) Toxoplasmosis and pregnancy: an analytical study in Zagazig, Egypt. Journal of the Egyptian Society of Parasitology 21, 81–85. [PubMed] [Google Scholar]
- El-Sayed NM, Abdel-Wahabl MM, Kishik SM and Alhusseini NF (2016a) Do we need to screen Egyptian voluntary blood donors for toxoplasmosis? Asian Pacific Journal of Tropical Disease 6, 260–264. [Google Scholar]
- El-Sayed NM, Ramadan ME and Ramadan ME (2016b) Toxoplasma gondii infection and chronic liver diseases: evidence of an association. Tropical Medicine and Infectious Disease 1, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Settawy MA, Fathy GM and Dahrog ME (2016) Comparison between avidity test and real-time PCR in diagnosis of recent toxoplasmosis in pregnancy. Life Science Journal 13, 65–71. [Google Scholar]
- El-Shazly AM, Romia SA, El Ganayni GA, Abou-Zakham AA, Sabry AH and Morsy TA (1991) Antibodies against some zoonotic parasites in commensal rodents trapped from Dakahila Governorate, Egypt. Journal of the Egyptian Society of Parasitology 21, 169–177. [PubMed] [Google Scholar]
- El-Shqanqery HE, Ibrahim HM, Mohamed AH and El-Sharaawy AA (2017) Seroprevalence of Toxoplasma gondii infection and associated risk factors among asymptomatic pregnant females in Egypt. Journal of the Egyptian Society of Parasitology 47, 93–100. [PubMed] [Google Scholar]
- El-Tantawy N, Taman A and Shalaby H (2014) Toxoplasmosis and female infertility: is there a co-relation? American Journal of Epidemiology and Infectious Disease 2, 29–32. [Google Scholar]
- El-Tras WF and Tayel AA (2009) Housewives exposure to Toxoplasma gondii in kitchens. 6th International Science Conference of Veterinary Medicine. Egypt: Mansoura University, pp.643–655.
- El Behairy AM, Choudhary S, Ferreira LR, Kwok OCH, Hilali M, Su C and Dubey JP (2013) Genetic characterization of viable Toxoplasma gondii isolates from stray dogs from Giza, Egypt. Veterinary Parasitology 193, 25–29. [DOI] [PubMed] [Google Scholar]
- El Deeb HK, Salah-Eldin H, Khodeer S and Allah AA (2012) Prevalence of Toxoplasma gondii infection in antenatal population in Menoufia governorate, Egypt. Acta Tropica 124, 185–191. [DOI] [PubMed] [Google Scholar]
- El Fadaly HA, Ahmad SO, Barakat AMA, Soror AH and Zaki MS (2016) Ecological and pathological study of T. gondii Egyptian rat isolates reference to biological & genetic typescripts. International Journal PharmTech, Research 9, 128–140. [Google Scholar]
- El Fadaly HA, Hassanain NA, Shaapan RM, Hassanain MA, Barakat AM and Abdelrahman KA (2017) Molecular detection and genotyping of Toxoplasma gondii from Egyptian isolates. Asian Journal of Epidemiology 10, 37–44. [Google Scholar]
- El Fakahany AF, Abdel-Maboud AI, El-Garhy MF and Eraky MA (2002) Comparative study between ELISA IgG, IgM and PCR in diagnosing and studying toxoplasmosis in Qualyobia Governorate, Egypt. Journal of the Egyptian Society of Parasitology 32, 475–486. [PubMed] [Google Scholar]
- El Gamal RL, Selim MA, Mohamed SMA, Fathy GM and Abdel Rahman SA (2013) Comparison of PCR with ELISA in diagnosis of recent toxoplasmosis in pregnant women. Journal of American Science 9, 824–832. [Google Scholar]
- El Moghazy FM, Kandil OM and Shaapan RM (2011) Toxoplasma gondii: comparison of some serological tests for detection in sera of naturally infected pigs. World Journal of Zoology 6, 204–208. [Google Scholar]
- Elfadaly HA, Hassanain NA, Hassanain MA, Barakat AM and Shaapan RM (2018) Evaluation of primitive ground water supplies as a risk factor for the development of major waterborne zoonosis in Egyptian children living in rural areas. Journal of Infection and Public Health 11, 203–208. [DOI] [PubMed] [Google Scholar]
- Elmonir W, Harfoush M, El-Tras WF and Kotb SA (2017) Toxoplasmosis in stray cats and pregnant women in Egypt: association between socio-demographic variables and high-risk practices by pregnant women. Life Science Journal 14, 1–5. [Google Scholar]
- Elsheikha HM, Azab MS, Abousamra NK, Rahbar MH, Elghannam DM and Raafat D (2009) Seroprevallence of and risk factors for Toxoplasma gondii antibodies among asymptomatic blood donors in Egypt. Parasitology Research 104, 1471–1476. [DOI] [PubMed] [Google Scholar]
- Eraky MA, Abdel-Hady S and Abdallah KF (2016) Seropositivity of Toxoplasma gondii and Toxocara spp. in children with cryptogenic epilepsy, Benha, Egypt. Korean Journal of Parasitology 54, 335–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy MA, Mandour AM, Arafa MS and Abdel Rahman BM (1979a) Toxoplasmosis of camels in Assiu governorate. Journal of the Egyptian Veterinary Medical Association 39, 27–31. [Google Scholar]
- Fahmy MAM, Arafa MS, Mandour AM and Rahman AMA (1979b) Toxoplasmosis in ruminants in Assiut governorate, Upper Egypt. Journal of the Egyptian Veterinary Medical Association 39, 119–126. [Google Scholar]
- Fereig RM, Mahmoud HYAH, Mohamed SGA, Mohamed AEA and Nishikawa Y (2016) Seroprevalence and epidemiology of Toxoplasma gondii in farm animals in different regions of Egypt. Veterinary Parasitology: Regional Studies and Reports 3–4, 1–6. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization (2015) Africa sustainable livestock 2050 report. Country brief Egypt. Available at http://www.fao.org/3/a-i7312e/pdf.
- Frenkel JK (1948) Dermal hypersensitivity to Toxoplasma antigens (Toxoplasmins). Proceedings of the Society for Experimental Biology and Medicine 68, 634–639. [DOI] [PubMed] [Google Scholar]
- Frenkel JK, Lindsay DS, Parker BB and Dobesh M (2003) Dogs as possible mechanical carriers of Toxoplasma, and their fur as a source of infection of young children. International Journal of Infectious Diseases 7, 292–293. [DOI] [PubMed] [Google Scholar]
- Ghattas SS (1999) Studies on Toxoplasma Gondii Infecting Slaughtered Pigs in Egypt (MSc. thesis), Cairo University, Cairo, Egypt. [Google Scholar]
- Ghazy AA, Shaapan RM and Abdel-Rahman EH (2007) Comparative serological diagnosis of toxoplasmosis in horses using locally isolated Toxoplasma gondii. Veterinary Parasitology 145, 31–36. [DOI] [PubMed] [Google Scholar]
- Ghoneim NH, Shalaby SI, Hassanain NA, Zeedan GS, Soliman YA and Abdalhamed AM (2010) Comparative study between serological and molecular methods for diagnosis of toxoplasmosis in women and small ruminants in Egypt. Foodborne Pathogens and Disease 7, 17–22. [DOI] [PubMed] [Google Scholar]
- Hamadto HA, Rashid SM, El-Fakahany AF and Lashin AH (1997) Seroepidemiological studies for toxoplasmosis among out- and inpatients in Benha University Hospitals, Qualyobia Governorate. Journal of the Egyptian Society of Parasitology 27, 223–231. [PubMed] [Google Scholar]
- Hamed AMR, El-Gebaly NSM, Abdelmegeid AK and Elsebaei ES (2018) Seroprevalence of Toxoplasma gondii infection in mentally retarded children in Egypt. Parasitologists United Journal 11, 155–161. [Google Scholar]
- Hammouda NA, El-Gebaly WM and Sadaka SM (1993) Seroprevalence of Toxoplasma and cytomegalovirus in complicated pregnancies. Journal of the Egyptian Society of Parasitology 23, 865–870. [PubMed] [Google Scholar]
- Harfoush M and Tahoon AE (2010) Seroprevalence of Toxoplasma gondii antibodies in domestic ducks, free-range chickens, turkeys and rabbits in Kafr El-Sheikh Governorate Egypt. Journal of the Egyptian Society of Parasitology 40, 295–302. [PubMed] [Google Scholar]
- Haridy FM, Shoukry NM, Hassan AA and Morsy TA (2009) ELISA-seroprevalence of Toxoplasma gondii in draught horses in Greater Cairo, Egypt. Journal of the Egyptian Society of Parasitology 39, 821–826. [PubMed] [Google Scholar]
- Haridy FM, Saleh NMK, Khalil HH and Morsy TA (2010) Anti-Toxoplasma gondii antibodies in working donkeys and donkey's milk in greater Cairo, Egypt. Journal of the Egyptian Society of Parasitology 40, 459–464. [PubMed] [Google Scholar]
- Hassan-Wassef H (2004) Food habits of the Egyptians: newly emerging trends. Eastern Mediterranean Health Journal 10, 898–915. [PubMed] [Google Scholar]
- Hassanain MA, Ezzo OH and Deghidy BS (1992) Some biochemical and hormonal changes in Toxoplasma-infected and aborted ewes. Egyptian Journal of Comparative Pathology and Clinical Pathology 5, 221–227. [Google Scholar]
- Hassanain MA, Zayed AA, Derbala AA and Kutkat MA (1997) Serological diagnosis of Toxoplasma gondii (Apicomplexa:Toxoplasminae) infection in laying hens. Egyptian Journal of Applied Sciences 12, 1–8. [Google Scholar]
- Hassanain MA, Barakat AM, Elfadaly HA, Hassanain NA and Shaapan RM (2008) Zoonotic impact of Toxoplasma gondii sero-prevalence in naturally infected Egyptian kittens. Journal of the Arab Society for Medical Research 3, 243–248. [Google Scholar]
- Hassanain MA, Elfadaly HA, Shaapan RM, Hassanain NA and Barakat AM (2011) Biological assay of Toxoplasma gondii Egyptian mutton isolates. International Journal of Zoological Research 7, 330–337. [Google Scholar]
- Hassanain MA, El-Fadaly HA, Hassanain NA, Shaapan RM, Barakat AM and Abd El Razik KA (2013) Serological and molecular diagnosis of toxoplasmosis in human and animals. World Journal of Medical Sciences 9, 243–247. [Google Scholar]
- Hassanain MA, El Bolaky HAA, Younis AIH, Abd El-Razik KA, El Fadaly HA and Abd El Wahab WM (2015) Serological and molecular diagnosis of T. gondii in complicated pregnant Egyptian women. Basic Research Journal of Medicine and Clinical Sciences 9, 231–236. [Google Scholar]
- Hassanain MA, Elfadaly HA, Abd El Wahab WM and Abo El-Maaty AM (2018a) Comparative hormonal and immunoglobulin profiles of aborted women with or without toxoplasmosis. Journal of Pregnancy and Reproduction 2, 1–4. [Google Scholar]
- Hassanain NA, Shaapan RM and Hassanain MA (2018b) Associated antenatal health risk factors with incidence of toxoplasmosis in Egyptian pregnant women. Pakistan Journal of Biological Sciences 21, 463–468. [DOI] [PubMed] [Google Scholar]
- Hilali M, Nassar AM and Ramadan EI (1991) Detection of encephalitozoonosis and toxoplasmosis among rabbits by carbon immunoassay. Veterinary Medical Journal Giza 39, 129–135. [Google Scholar]
- Hilali M, Romand S, Thulliez P, Kwok OCH and Dubey JP (1998) Prevalence of Neospora caninum and Toxoplasma gondii antibodies in sera from camels from Egypt. Veterinary Parasitology 75, 269–271. [DOI] [PubMed] [Google Scholar]
- Hussein AH, Ali AE, Saleh MH, Nagaty IM and Rezk AY (2001) Prevalence of Toxoplasma infection in Qualyobia governorate, Egypt. Journal of the Egyptian Society of Parasitology 31, 355–563. [PubMed] [Google Scholar]
- Hussein AH, Alawamy W, Abd El-Maboud AI, Elghareeb AS and Hamadto HA (2016) The role of toxoplasmosis and coincidental placental inflammation and Fas ligand expression as a cause of spontaneous abortion in pregnant women from Benha city, Egypt. Egyptian Journal of Medical Sciences 37, 181–197. [Google Scholar]
- Hussein SMM, Elshemy AS, Abd El-Mawgod MM and Mohammed AS (2017) Seroprevalence of Toxoplasma gondii among primigravida women and their neonates in Sohag governorate, Egypt. Journal of the Egyptian Society of Parasitology 47, 381–388. [PubMed] [Google Scholar]
- Ibrahim MHS (1990) Studies on Parasitic Infections Among Egyptian Pigs (MSc. thesis), Benha University, Benha, Egypt. [Google Scholar]
- Ibrahim BB, Salama MM, Gawish NI and Haridy FM (1997) Serological and histopathological studies on Toxoplasma gondii among the workers and the slaughtered animals in Tanta Abattoir, Gharbia Governorate. Journal of the Egyptian Society of Parasitology 27, 273–278. [PubMed] [Google Scholar]
- Ibrahim HM, Huang P, Salem TA, Talaat RM, Nasr MI, Xuan X and Nishikawa Y (2009) Short report: prevalence of Neospora caninum and Toxoplasma gondii antibodies in northern Egypt. American Journal of Tropical Medicine and Hygiene 80, 263–267. [PubMed] [Google Scholar]
- Ibrahim HM, Abdel-Ghaffar F, Osman GY, El-Shourbagy SH, Nishikawa Y and Khattab RA (2016) Prevalence of Toxoplasma gondii in chicken samples from delta of Egypt using ELISA, histopathology and immunohistochemistry. Journal of Parasitic Diseases 40, 485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim HM, Mohamed AH, El-Sharaawy AA and El-Shqanqery HE (2017) Molecular and serological prevalence of Toxoplasma gondii in pregnant women and sheep in Egypt. Asian Pacific Journal of Tropical Medicine 10, 996–1001. [DOI] [PubMed] [Google Scholar]
- Ibrahim HM, Osman GY, Mohamed AH, Al-Selwi AGM, Nishikawa Y and Abdel-Ghaffar F (2018) Toxoplasma gondii: prevalence of natural infection in pigeons and ducks from middle and upper Egypt using serological, histopathological, and immunohistochemical diagnostic methods. Veterinary Parasitology: Regional Studies and Reports 13, 45–49. [DOI] [PubMed] [Google Scholar]
- Kamal AM, Ahmed AK, Abdellatif MZ, Tawfik M and Hassan EE (2015) Seropositivity of toxoplasmosis in pregnant women by ELISA at Minia University Hospital, Egypt. Korean Journal of Parasitology 53, 605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalafalla RE (2011) A survey study on gastrointestinal parasites of stray cats in northern region of Nile Delta, Egypt. PLoS ONE 6, e20283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaled MLM, Morsy TA, Sadek MSM and Salama MMI (1982) The presence of antibodies against toxoplasmosis, leishmaniasis and amoebiasis in stray dogs in Cairo, Egypt. Journal of the Egyptian Society of Parasitology 12, 341–347. [PubMed] [Google Scholar]
- Khater H, Khalifa N and Barakat A (2013) Serological and molecular studies of ovine and human toxoplasmosis with a trial of treatment of infected ewe. Scientific Journal of Veterinary Advances 2, 157–168. [Google Scholar]
- Kuraa HM and Malek SS (2016) Seroprevalence of Toxoplasma gondii in ruminants by using latex agglutination test (LAT) and enzyme-linked immunosorbent assay (ELISA) in Assiut governorate. Tropical Biomedicine 33, 711–725. [PubMed] [Google Scholar]
- Mabrouk MA and Dahawi HS (1991) Toxoplasma antibodies in patients with meningoencephalitis. Journal of the Egyptian Society of Parasitology 21, 547–551. [PubMed] [Google Scholar]
- Mahboub HD, Helal MA, Abd Eldaim MA, Abd El-Razek EM and Elsify AM (2013) Seroprevalence of abortion causing agents in Egyptian sheep and goat breeds and their effects on the animal's performance4. Journal of Agricultural Science 5, 92–101. [Google Scholar]
- Mandour AM, Mounib MEM, Eldeek HEM, Ahmad AAR and Abdel-Kader ARMM (2017) Prevalence of congenital toxoplasmosis in pregnant women with complicated pregnancy outcomes in Assiut governorate, Egypt. Journal of Advances in Parasitology 4, 1–8. [Google Scholar]
- Maronpot RR and Botros BAM (1972) Toxoplasma serologic survey in man and domestic animals in Egypt. Journal of the Egyptian Public Health Association 47, 58–67. [PubMed] [Google Scholar]
- Michael SA (1977) Comparative studies on Toxoplasma antibody titers obtained by the new slide agglutination test and other serological test. Journal of the Egyptian Society of Parasitology 7, 73–79. [Google Scholar]
- Michael SA, El Reaii AH and Morsy TA (1977) Incidence of Toxoplasma antibodies among camels in Egypt. Journal of the Egyptian Society of Parasitology 7, 129–132. [Google Scholar]
- Mikhail MW, Hasan AH, Ali Allam K and Mohammed NM (2017) Seroprevalence of Toxoplasma gondii among commensal rodents from Giza governorate, Egypt. Journal of the Egyptian Society of Parasitology 47, 147–156. [PubMed] [Google Scholar]
- Morsy TA, Michael SA and Musallam RA (1981) Antibodies against some parasites of zoonotic importance in rodents caught in Port Said governorate, A.R.E. Journal of the Egyptian Society of Parasitology 11, 147–156. [PubMed] [Google Scholar]
- Morsy TA, Michael SA, Bassili WR and Saleh MS (1982) Studies on rodents and their zoonotic parasites, particularly Leishmania, in Ismailiya Governorate, A. R., Egypt. Journal of the Egyptian Society of Parasitology 12, 565–855. [PubMed] [Google Scholar]
- Nassef NE, Abd El-Ghaffar MM, El-Nahas NS, Hassanain ME, Shams El-Din SA and Ammar AIM (2015) Seroprevalence and genotyping of Toxoplasma gondii in Menoufia governorate. Menoufa Medical Journal 28, 617–626. [Google Scholar]
- Nicolle C and Manceaux L (1908) Sur une infection à corps de Leishman (ou organismes voisins) du gondi. Comptes Rendus des Séances de l'Academie des Sciences 147, 763–766. [Google Scholar]
- Opsteegh M, Prickaerts S, Frankena K and Evers EG (2011) A quantitative microbial risk assessment for meatborne Toxoplasma gondii infection in The Netherlands. International Journal of Food Microbiology 150, 103–114. [DOI] [PubMed] [Google Scholar]
- Peyron F, Wallon M, Kieffer F and Graweg G (2016) Toxoplasmosis. In Remington JS, Klein JO, Wilson CB, Nizet V and Maldonado YA (8th eds), Infectious Diseases of the Fetus and Newborn Infant. Philadelphia, USA: Elsevier Saunders, pp. 949–1042. [Google Scholar]
- Ramadan MY, Abdel-Mageed AD and Khater HF (2007) Seroprevalence and preliminary treatment of toxoplasmosis of pregnant goats in Kalubyia Governorate, Egypt. Acta Scientiae Veterinariae 35, 295–301. [Google Scholar]
- Rifaat MA and Nagaty HF (1959) Toxoplasmosis in Egypt. A toxoplasmin-skin-testing survey among a group of Cairo population. Journal of the Egyptian Public Health Association 34, 121–135. [Google Scholar]
- Rifaat MA, Salem SA and Morsy TA (1962) Toxoplasmosis in Egypt. A toxoplasmin skin testing survey among a group of population in Tahrir Province, UAR. Journal of the Egyptian Public Health Association 37, 163–166. [Google Scholar]
- Rifaat MA, Schafia A, Salem SA, Morsy TA and Khalid MLM (1963) A toxoplasmin skin-test survey in El Waady El Gadeed, United Arab Republic. Transactions of the Royal Society of Tropical Medicine and Hygiene 57, 134–135. [DOI] [PubMed] [Google Scholar]
- Rifaat MA, Michael SA and Morsy TA (1968) Toxoplasmin skin test survey among buffaloes and cattle in U.A.R. (Preliminary Report.). Journal of Tropical Medicine and Hygiene 71, 297–298. [PubMed] [Google Scholar]
- Rifaat MA, Morsy TA and Sadek MSM (1969) Toxoplasmosis in chickens and pigeons in U.A.R. Journal of Tropical Medicine and Hygiene 72, 193–194. [PubMed] [Google Scholar]
- Rifaat MA, Morsy TA, Salem SA and Sadek MSM (1970) Serological pattern of toxoplsmosis in stray dogs and cats collected from Cairo. Pakistan Medical Review, Karachi 5, 11. [Google Scholar]
- Rifaat MA, Mahdi AH, Arafa MS, Nasr NT and Sadek MSM (1971) Isolation of Toxoplasma from Rattus norvegicus in Egypt. Transactions of the Royal Society of Tropical Medicine and Hygiene 65, 788–789. [DOI] [PubMed] [Google Scholar]
- Rifaat MA, Arafa MS, Sadek MS, Sabri A and Mandour AM (1972) A preliminary note on toxoplasmosis among inpatients of the obstetrics department, university hospital at Assiut, Egypt. Journal of the Egyptian Public Health Association 47, 36–42. [PubMed] [Google Scholar]
- Rifaat MA, Sadek MSM and Elazghal HI (1973a) Isolation of Toxoplasma from a human placenta in Egypt. Journal of Tropical Medicine and Hygiene 76, 90. [PubMed] [Google Scholar]
- Rifaat MA, Sadek MSM, Elnaggar BA and Munir AM (1973b) Case of toxoplasmic uveitis treated with pyrimethamine and sulfa drugs in Egypt. Journal of Tropical Medicine and Hygiene 76, 252–253. [PubMed] [Google Scholar]
- Rifaat MA, Hanna SM, Abdallah A, Moch RW and Botros BAM (1973c) Isolation of Toxoplasma gondii from man in Egypt. Journal of the Egyptian Public Health Association 47, 36–44. [PubMed] [Google Scholar]
- Rifaat MA, Nasr NT, Sadek MSM, Arafa MS and Mahdi AH (1973d) The role of domestic rat, Rattus alexandrinus as a reservoir host of Toxoplasma gondii in Egypt. Journal of Tropical Medicine and Hygiene 76, 257–258. [PubMed] [Google Scholar]
- Rifaat MA, Wishahy AG, Sadek MSM, Elkhalek KA and Munir AM (1973e) Case of congenital toxoplasmosis in Egypt. Journal of Tropical Medicine and Hygiene 76, 255–256. [PubMed] [Google Scholar]
- Rifaat MA, Salem SA, Khalil HM, Khaled MLM, Sadek MSM, Azab ME and Hanna SM (1975) Toxoplasmosis serological surveys among inhabitants of some governorates of Egypt. Transactions of the Royal Society of Tropical Medicine and Hygiene 69, 118–120. [DOI] [PubMed] [Google Scholar]
- Rifaat MA, Arafa MS, Sadek MSM and Nasr NT (1976a) Natural infection of the Cairo spiny mouse, Acomys cahirinus with Trypanosoma and Toxoplasma in Egypt. Journal of the Egyptian Society of Parasitology 6, 45–52. [Google Scholar]
- Rifaat MA, Salem SA, Sadek MSM, Azab ME, Abdel-Ghaffar FM and Abdel-Baki MH (1976b) Toxoplasmosis in sheep and dogs in Egypt. I. Isolation of local strains. Journal of the Egyptian Society of Parasitology 6, 135–139. [Google Scholar]
- Rifaat MA, Arafa MS, Sadek MSM, Nasr NT, Azab ME, Mahmoud W and Khalil MS (1976c) Toxoplasma infection of stray cats in Egypt. Journal of Tropical Medicine and Hygiene 79, 67–70. [PubMed] [Google Scholar]
- Rifaat MA, Morsy TA, Sadek MSM, Arafa MS, Azab ME and Abdel Ghaffar FM (1977a) Isolation of Toxoplasma parasite from animals in Egypt (review). Journal of the Egyptian Society of Parasitology 7, 219–223. [Google Scholar]
- Rifaat MA, Morsy TA, Sadek MSM, Azab ME, Safar EH and Nour El-Din OM (1977b) Serological surveys for toxoplasmosis among farm animals in Egypt. Journal of the Egyptian Society of Parasitology 7, 229–233. [Google Scholar]
- Rifaat MA, Morsy TA, Sadek MSM, Khalid MLM, Azab ME, Makled MK, Safar EH and Nour El-Din OM (1977c) Incidence of toxoplasmosis among farm animals in Suez Canal Governorates. Journal of the Egyptian Society of Parasitology 7, 135–140. [Google Scholar]
- Rifaat MA, Morsy TA, Sadek MSM, Khalid MLM, Azab ME and Safar EH (1978) Prevalence of Toxoplasma antibodies among slaughtered animals in lower Egypt. Journal of the Egyptian Society of Parasitology 8, 339–345. [Google Scholar]
- Rifaat MA, Morsy TA, Sadek MSM, Azab ME, Khaled MLM and Safar EH (1979) Incidence of toxoplasmosis among farm animals in north coastal zone of Egypt. Journal of the Egyptian Society of Parasitology 9, 193–197. [Google Scholar]
- Robert-Gangneux F and Dardé ML (2012) Epidemiology of and diagnostic strategies for toxoplasmosis. Clinical Microbiology Reviews 25, 264–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouatbi M, Amairia S, Amdouni Y, Boussaadoun MA, Ayadi O, Al-Hosary AAT, Rekik M, Ben Abdallah R, Aoun K, Darghouth MA, Wieland B and Gharbi M (2019) Toxoplasma gondii infection and toxoplasmosis in North Africa: a review. Parasite 26, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau A, Villena I, Dumètre A, Escotte-Binet S, Favennec L, Dubey JP, Aubert D and La Carbona S (2019) Evaluation of propidium monoazide-based qPCR to detect viable oocysts of Toxoplasma gondii. Parasitology Research 118, 999–1010. [DOI] [PubMed] [Google Scholar]
- Saad MY, Temsah KA, Abdel Daym M, Soliman WA and Abu Albas M (2016) Immunoblot assay for toxoplasmosis in schizophrenic patients. European Journal of Pharmaceutical and Medical Research 3, 685–689. [Google Scholar]
- Saad NM, Hussein AAA and Ewida RM (2018) Occurrence of Toxoplasma gondii in raw goat, sheep, and camel milk in Upper Egypt. Veterinary World 11, 1262–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadek OA, Abdel-Hameed ZM and Kuraa HM (2015) Molecular detection of Toxoplasma gondii DNA in raw goat and sheep milk with discussion of its public health importance in Assiut Governorate. Assiut Veterinary Medical Journal 61, 166–177. [Google Scholar]
- Safar EH, Abd-el Ghaffar FM, Saffar SA, Makled KM, Habib KS, El Abiad R and El Shabrawy E (1995) Incidence of Toxoplasma and Toxocara antibodies among out-patients in the Ophthalmic Research Institute, Egypt. Journal of the Egyptian Society of Parasitology 25, 839–852. [PubMed] [Google Scholar]
- Sahwi SY, Zaki MS, Haiba NY, Elsaid OK, Anwar MY and AbdRabbo SA (1995) Toxoplasmosis as a cause of repeated abortion. Journal of Obstetrics and Gynaecology 21, 145–148. [DOI] [PubMed] [Google Scholar]
- Shaapan RM and Ghazy AA (2007) Isolation of Toxoplasma gondii from horse meat in Egypt. Pakistan Journal of Biological Sciences 10, 174–177. [DOI] [PubMed] [Google Scholar]
- Shaapan RM and Khalil AMF (2008) Evaluation of different Toxoplasma gondii isolates as antigens used in the modified agglutination test for the detection of toxoplasmosis in camels and donkeys. American-Eurasian Journal of Agriculture and Environmental Sciences 3, 837–841. [Google Scholar]
- Shaapan RM, El-Nawawi FA and Tawfik MAA (2008) Sensitivity and specificity of various serological tests for the detection of Toxoplasma gondii infection in naturally infected sheep. Veterinary Parasitology 153, 359–362. [DOI] [PubMed] [Google Scholar]
- Shaapan RM, Hassanain MA and Khalil FAM (2010) Modified agglutination test for serologic survey of Toxoplasma gondii infection in goats and water buffaloes in Egypt. Research Journal of Parasitology 5, 13–17. [Google Scholar]
- Shaapan RM, Khalil FAM and Abu El Ezz NMT (2011) Cryptosporidiosis and toxoplasmosis in native quails of Egypt. Research Journal of Veterinary Sciences 4, 30–36. [Google Scholar]
- Shaapan RM, Abo-ElMaaty AM, Abd El-Razik KA and Abd El-Hafez SM (2012) PCR and serological assays for detection of Toxoplasma gondii infection in sport horses in Cairo, Egypt. Asian Journal of Animal and Veterinary Advances 7, 158–165. [Google Scholar]
- Shatat MA, El-Darwish AG, Samie MA and Hassan MA (2006) Seroprevalence study of anti-Toxoplasma antibodies in complicated pregnancies in Assiut governorate. Al-Azhar Assiut Medical Journal 4, 24–30. [Google Scholar]
- Shehata AI, Hassanein FI and Abdul-Ghani R (2016) Seroprevalence of Toxoplasma gondii infection among patients with non-schizophrenic neurodevelopmental disorders in Alexandria, Egypt. Acta Tropica 154, 155–159. [DOI] [PubMed] [Google Scholar]
- Soliman M, Nour-Eldin MS, Elnaggar HM, El-Ghareb ME and Ramadan NI (2001) Toxoplasma antibodies in normal and complicated pregnancy. Journal of the Egyptian Society of Parasitology 31, 637–646. [PubMed] [Google Scholar]
- Splendore A (1908) Un nuovo protozoo parassita de conigli incontrato nelle lesioni anatomiche d'une malattia che ricorda in molti punti il Kala-azar dell' uomo. Nota preliminare. Revista de Sociedade Scientífica de São Paulo 3, 109–112. [Google Scholar]
- Tammam AE, Haridy MA, Abdellah AH, Ahmed SR, Fayed HM and Alsammani MA (2013) Seroepidemiology of Toxoplasma gondii infection in women with first trimester spontaneous miscarriage in Qena governorate, Egypt. Journal of Clinical and Diagnostic Research 7, 2870–2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolba MM, El-Taweel HA, Khalil SS, Hazzah WA and Heshmat MG (2014) Genotype analysis of T. gondii strains associated with human infection in Egypt. Parasitology Research 113, 1563–1569. [DOI] [PubMed] [Google Scholar]
- Tonouhewa AB, Akpo Y, Sessou P, Adoligbe C, Yessinou E, Hounmanou YG, Assogba MN, Youssao I and Farougou S (2017) Toxoplasma gondii infection in meat animals from Africa: systematic review and meta-analysis of sero-epidemiological studies. Veterinary World 10, 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Population Review (2019) Egypt population. Available at http://www.worldpopulationreview.com/countries/egypt-population.
- Younis EE, Abou-Zeid NZ, Zakaria M and Mahmoud MR (2015) Epidemiological studies on toxoplasmosis in small ruminants and equine in Dakahlia Governorate, Egypt. Assiut Veterinary Medical Journal 61, 22–31. [Google Scholar]