Abstract
Cyclospora cayetanensis, a coccidian parasite that causes protracted and relapsing gastroenteritis, has a short recorded history. At least 54 countries have documented C. cayetanensis infections and 13 of them have recorded cyclosporiasis outbreaks. Cyclospora cayetanensis infections are commonly reported in developing countries with low-socioeconomic levels or in endemic areas, although large outbreaks have also been documented in developed countries. The overall C. cayetanensis prevalence in humans worldwide is 3.55%. Among susceptible populations, the highest prevalence has been documented in immunocompetent individuals with diarrhea. Infections are markedly seasonal, occurring in the rainy season or summer. Cyclospora cayetanensis or Cyclospora-like organisms have also been detected in food, water, soil and some other animals. Detection methods based on oocyst morphology, staining and molecular testing have been developed. Treatment with trimethoprim–sulfamethoxazole (TMP–SMX) effectively cures C. cayetanensis infection, whereas ciprofloxacin is less effective than TMP–SMX, but is suitable for patients who cannot tolerate co-trimoxazole. Here, we review the biological characteristics, clinical features, epidemiology, detection methods and treatment of C. cayetanensis in humans, and assess some risk factors for infection with this pathogen.
Key words: Biological characteristic, clinical feature, Cyclospora cayetanensis, detection method, epidemiology, treatment
Introduction
Nearly 1.7 billion cases of diarrheal disease are reported globally every year, and its socioeconomic burden on health services has been estimated at 72.8 million disability-adjusted life years annually (Ryan et al., 2017). Enteric protozoan parasites are among the major contributors to this diarrheal disease load (Fletcher et al., 2012; Di Genova and Tonelli, 2016). Cyclospora cayetanensis is an important global pathogen in humans, typically causing prolonged diarrhea accompanied by anorexia, malaise, nausea and cramping, among other symptoms (Shields and Olson, 2003; Giangaspero and Gasser, 2019). Many large cyclosporiasis outbreaks have been documented in industrialized nations (Ortega and Sanchez, 2010). In these, food has been identified as the main vehicle for Cyclospora transmission, according to source-tracing studies (Herwaldt and Ackers, 1997; Ortega and Sanchez, 2010). Cilantro from Mexico was identified as one of the possible sources of a cyclosporiasis outbreak in the United States (USA) in 2013, with more than 600 cases of infection (Abanyie et al., 2015). More recently, prepackaged vegetable trays and vegetable salads sold at a fast food chain have been the suspected sources of cyclosporiasis outbreaks in June and July, 2018, according to trace-back investigations (Casillas et al., 2018).
Up to 31 December 2018, more than one thousand papers have been published on Cyclospora. Numerous studies of Cyclospora infections among travelers, immunodeficient patients, diarrheal and asymptomatic patients and the residents of disease-endemic areas have been reported. In this study, we review the biological characteristics, clinical features, epidemiology, detection methods and treatment of C. cayetanensis, and assess some risk factors for human infection with this foodborne pathogen.
Biological characteristics
History of discovery and research
The genus Cyclospora, created by Schneider in 1881, was first described by Eimer in 1870 (Ortega and Sanchez, 2010). Until the 1990s, the genus only included species that infect animals, such as rodents, insectivores and reptiles (Casemore, 1994). The earliest description of human infection with Cyclospora was from Papua New Guinea in 1979 (Ashford, 1979). Oocysts were subsequently observed in the faeces of patients from Haiti and Peru in 1983–1985, American travelers returning from Haiti and Mexico in 1986, British travelers who became ill in Nepal in 1989 and travelers and foreign residents in Nepal in 1993 (Herwaldt, 2000), although the identity of the pathogen was uncertain at that time. In 1994, Ortega et al. named this human causative organism C. cayetanensis (Ortega and Sanchez, 2010).
Cyclospora cayetanensis has received further attention since the first outbreak of Cyclospora-associated diarrheal illness in the USA in 1990 (Huang et al., 1995). In 1996, more than 1400 cases of cyclosporiasis were reported in the USA and Canada (Herwaldt and Ackers, 1997). Since then, very large studies of Cyclospora infection among travelers, immunodeficient patients, diarrheal patients and asymptomatic individuals have been reported, as have studies of detection methods and treatment measures for Cyclospora.
Morphology and taxonomy
Cyclospora cayetanensis is the only documented Cyclospora species infecting humans, and it is widely accepted that among common mammals, only humans are susceptible to infection by this microbe (Ortega and Sanchez, 2010).
Under light microscopy, C cayetanensis oocysts have a spheroid shape, 8–10 µm in diameter, with indistinguishable protoplasm (Fig. 1). When sporulated, each oocyst contains two ovoid sporocysts that, in turn, contain two sporozoites each (Ortega and Sanchez, 2010). Cyclospora oocysts are modified with Ziehl–Neelsen acid-fast stain in different ways: some stain dark red with a mottled appearance, some stain pink, whereas others do not stain all and appear as non-refractile glassy spheres against the blue-green background (Clarke and McIntyre, 1996; Zhou et al., 2011). Their autofluorescence makes C. cayetanensis oocysts readily visible in clinical samples with epifluorescence microscopy under a 330–380 nm ultraviolet (UV) filter (Zhou et al., 2011).
Fig. 1.
Morphology of C. cayetanensis oocysts under microscopy. Oocysts in stool smears stained with modified acid-fast stain under light microscopy; two oocysts are stained with different intensities (A); differential interference contrast microscopy of wet mounts, a partially sporulated oocyst can be seen (B); epifluorescence microscopy with a 330–380 nm UV excitation filter (C).
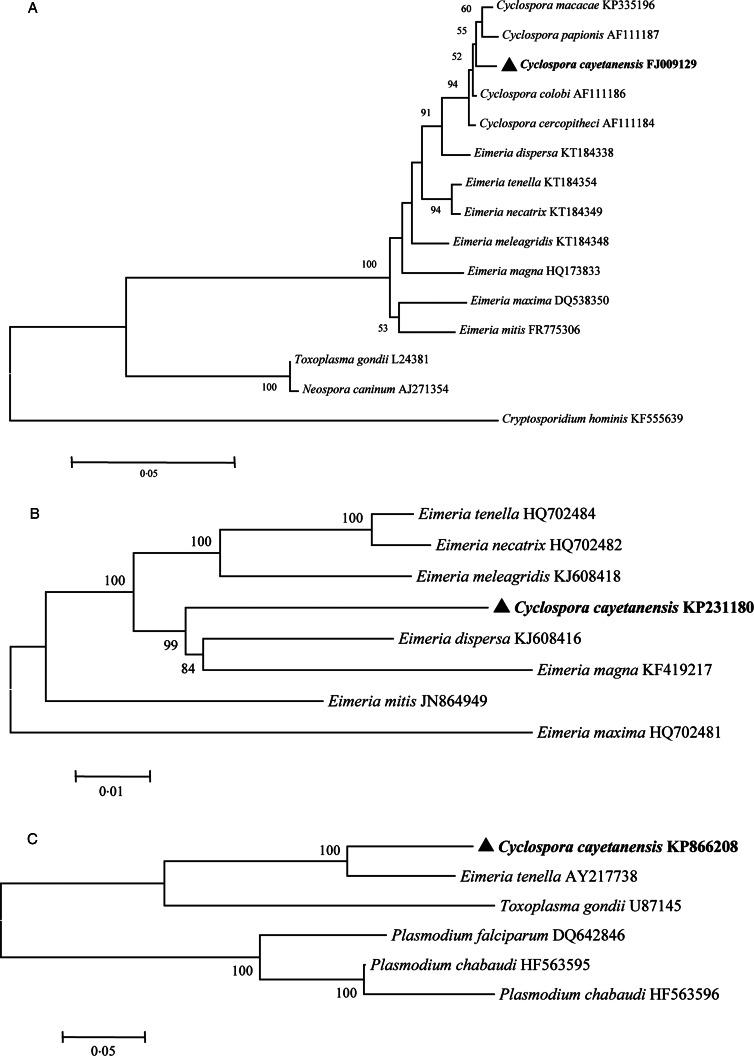
Cyclospora cayetanensis belongs to the subphylum Apicomplexa, subclass Coccidiasina, family Eimeriidae and genus Cyclospora (Ortega and Sanchez, 2010). Phylogenetic analyses have shown that human-associated Cyclospora is closely related to members of the genus Eimeria (Fig. 2) (Relman et al., 1996; Liu et al., 2016). Cyclospora cercopitheci in vervet monkeys (Cercopithecus aethiops), C. colobi in colobus monkeys (Colobus guereza) and C. papionis in olive baboons (Papio anubis) were characterized in 1999 (Eberhard et al., 1999); C. macacae was described in rhesus monkeys (Macaca mulatta) in 2015 (Li et al., 2015); and C. duszynskii and C. yatesi were characterized in moles (Scalopus aquaticus) in 2018 (McAllister et al., 2018). A total of 22 Cyclospora species have so far been described in vipers, moles, myriapodes, rodents, monkeys and humans (Lainson, 2005; Li et al., 2015; McAllister et al., 2018). However, Cyclospora-like organisms have also been described in dogs, cattle, chickens, rats, house mice, birds, monkeys, shellfish, etc., and even in environmental samples (Sherchand and Cross, 2001; Chu et al., 2004; Li et al., 2007; Cordón et al., 2008; Aksoy et al., 2014; Helenbrook et al., 2015; Ghozzi et al., 2017).
Fig. 2.
Phylogenetic relationships of C. cayetanensis and other apicomplexan protozoa. Phylogeny inferred with a neighbour-joining analysis of small-subunit ribosomal RNA gene sequences (A) reported by Li et al. (2017); mitochondrial genomes (B) reported by Cinar et al. (2015) and apicoplast genomes (C) reported by Tang et al. (2015), based on distances calculated with the Kimura 2-parameter model. Bootstrap values >50% from 1000 replicates are shown at the nodes. Scale bars indicate estimated substitutions per site.
Life cycle of C. cayetanensis
Infections of C. cayetanensis mainly occur via the faecal–oral transmission route. Fresh (unsporulated) oocysts are excreted in stools. Oocysts are spheroid, 8–10 µm in diameter, and contain indistinguishable protoplasm (Brown and Rotschafer, 1999). In the environment outside the host, freshly excreted oocysts are not infectious until their sporulation is complete, which occurs within a few days to weeks (at maximum) at temperatures between 22 and 30 °C. Storage at either 4 or 37 °C retards sporulation (Smith et al., 1997). The sporulation of the oocysts occurs irrespective of whether they are stored in deionized water or potassium dichromate solution, and results in the division of the sporont into two sporocysts, each containing two elongated sporozoites (Smith et al., 1997). During this time, food or water can act as the vehicle for Cyclospora transmission. Once the sporulated oocysts in food, water or soil are ingested by a new host, the mature oocysts usually excyst in the small bowel, and sporozoites are released to invade the epithelial cells of the upper small intestine (duodenum or jejunum) (Ortega and Sanchez, 2010).
The presence of asexual and sexual stages in the same host suggests that the life cycle of this microorganism can be completed within one host (Ortega et al., 1997). The intracellular developmental stages begin with the formation of intracytoplasmic parasitophorous vacuoles in the intestinal epithelium cells (Sun et al., 1996; Ortega and Sanchez, 2010), which are sometimes also observed in biliary epithelium cells (Zar et al., 2001). Asexual multiplication results in type I and II meronts (Ortega et al., 1997). Type I meronts give rise to 8–12 merozoites that then infect neighbouring epithelial cells, and this type of asexual reproduction is often quite prolific. Type II meronts form later, releasing four merozoites to invade neighbouring cells. Some of these meronts form macrogametes, whereas others undergo multiple fission events to form microgametocytes containing flagellated microgametes (Ortega et al., 1997). The macrogametocyte is fertilized by the microgametocyte, producing a zygote, in the sexual stages. Once fertilization occurs, an environmentally resistant wall is formed, and the oocyst is excreted from the host into the environment as an unsporulated oocyst in the faeces (Shields and Olson, 2003; Ortega and Sanchez, 2010).
Molecular characteristics
The characteristics of the polymorphic regions of the Cyclospora genome have been studied to better understand the microorganism's mode of infection and epidemiology. Small subunit ribosomal RNA (SSU rRNA) gene sequences show minimal genetic diversity among C. cayetanensis isolates from around the world (Sulaiman et al., 2014), and a phylogenetic analysis showed that C. cayetanensis is genetically related to members of the genus Eimeria (Fig. 2A) (Relman et al., 1996).
However, the internal transcribed spacer (ITS) sequences in C. cayetanensis are highly variable within and between samples, and this variability does not correlate with the geographic origins of the samples (Olivier et al., 2001). It has been demonstrated that this ITS sequence variability occurs at the individual-genome level and approaches or exceeds the variability observed among oocysts (Riner et al., 2010).
No genetic polymorphism has been observed in regions of the 70 kilodalton heat shock protein (HSP70) locus characterized in a previous study (Sulaiman et al., 2013). These results also support the lack of geographic segregation and the existence of genetically homogeneous population of C. cayetanensis parasites at this genetic locus (Sulaiman et al., 2014).
Genome characteristics
Tracing the source of infection is facilitated by the genomic comparison of isolates. Cyclospora can also be clearly identified and differentiated from other protozoan parasites involved in foodborne or waterborne outbreaks by their genomic differences. The mitochondrial genome of C. cayetanensis is ~6200 bp in length, with 33% GC content (Cinar et al., 2015; Ogedengbe et al., 2015; Tang et al., 2015). It contains three protein-coding genes (cytb, cox1 and cox3) and 14 large subunit (LSU) and nine SSU fragmented rRNA genes (Cinar et al., 2015; Ogedengbe et al., 2015). The mitochondrial genome of C. cayetanensis has a linear concatemeric or circular mapping topology (Tang et al., 2015). A comparative genomic analysis showed strong similarity between the C. cayetanensis and E. tenella genomes, with 90.4% nucleotide sequence similarity and complete synteny in gene organization (Tang et al., 2015). Phylogenetic analyses of the mitochondrial genomic sequences have confirmed the genetic similarities between avian Eimeria spp. and C. cayetanensis (Fig. 2B).
The apicoplast genome of C. cayetanensis is ~34 000 bp in size and encodes ~65 genes, with 22% GC content (Tang et al., 2015; Liu et al., 2016). The apicoplast genome is circular, encodes the complete machinery for protein biosynthesis and contains two inverted repeats that differ slightly in the LSU rRNA gene sequences (Tang et al., 2015). A comparative genomic analysis revealed high-nucleotide sequence similarity (85.6%) between C. cayetanensis and E. tenella, and a phylogenetic analysis of apicoplast genomic sequences also confirmed the genetic similarities between avian Eimeria spp. and C. cayetanensis (Fig. 2C).
The whole genome of C. cayetanensis is estimated to have a total length of 44 Mbp, with 52% GC content and ~7500 gene (Liu et al., 2016). A comparative genomic analysis indicated that C. cayetanensis shares a coccidia-like metabolism and invasion components, but has unique surface antigens (Liu et al., 2016). There are also some major differences in the amino acid metabolism and the posttranslational modification of proteins between C. cayetanensis and other apicomplexans (Liu et al., 2016). A multilocus sequence typing tool for C. cayetanensis has been developed based on its whole genome, which involves five microsatellite loci (Guo et al., 2016). Noticeable geographic clustering has been observed in human C. cayetanensis isolates from around the world (Li et al., 2017). Quantitative polymerase chain reaction (PCR) (Guo et al., 2019) and PCR assays (Nascimento et al., 2019), both targeting the polymorphic region in the mitochondrial genome, have been developed to genotype C. cayetanensis isolates. Two novel similarity-based classification algorithms for C. cayetanensis have been developed, including a Bayesian and heuristic component that infer the relatedness of pathogen isolates (Barratt et al., 2019). These useful genotyping tools should be helpful in initial source-tracking studies and in distinguishing different case clusters, especially during cyclosporiasis outbreaks.
Clinical features
The clinical symptoms of cyclosporiasis in humans typically manifest as periodic profuse watery diarrhea, together with malaise, nausea, anorexia, cramping and periods of apparent remission (Shields and Olson, 2003). Mild-to-moderate self-limiting diarrhea is common among healthy individuals who have ingested sporulated oocysts (Mansfield and Gajadhar, 2004). However, patients with immune dysfunction can experience severe intestinal injury and prolonged diarrhea (Shields and Olson, 2003; Mansfield and Gajadhar, 2004). In some cases, low-grade fever and the malabsorption of d-xylose may be present (Shields and Olson, 2003). Asymptomatic infections also occur frequently in disease-endemic areas.
Striking intestinal histological changes are observed during C. cayetanensis infection, including acute or chronic inflammation, disruption of the surface epithelium, villous atrophy, crypt hyperplasia (Connor et al., 1993) and intense lymphocytic infiltration within the lamina propria and epithelial cells (Ortega et al., 1997; Wiwanitkit, 2006). The inflammatory changes associated with C. cayetanensis infection may persist beyond the eradication of the parasite (Connor et al., 1999). Reactive hyperaemia with vascular dilatation and congestion of the villous capillaries has also been observed (Ortega et al., 1997).
In addition to gastrointestinal symptoms, C. cayetanensis can infect the biliary tract (Sifuentes-Osornio et al., 1995), resulting in acalculous cholecystitis in people with acquired immunodeficiency syndrome (AIDS), and the presence of oocysts in gallbladder epithelial cells (Zar et al., 2001). Although no C. cayetanensis respiratory infection has yet been identified, C. cayetanensis oocysts were detected in the sputum of two patients with tuberculosis (Di Gliullo et al., 2000; Hussein et al., 2005). Cyclospora cayetanensis infection has been associated with a variety of sequelae, including reactive arthritis syndrome, Reiter syndrome and Guillain-Barre syndrome (Connor et al., 2001; Shields and Olson, 2003; Abanyie et al., 2015).
Epidemiology
Outbreaks of human cyclosporiasis
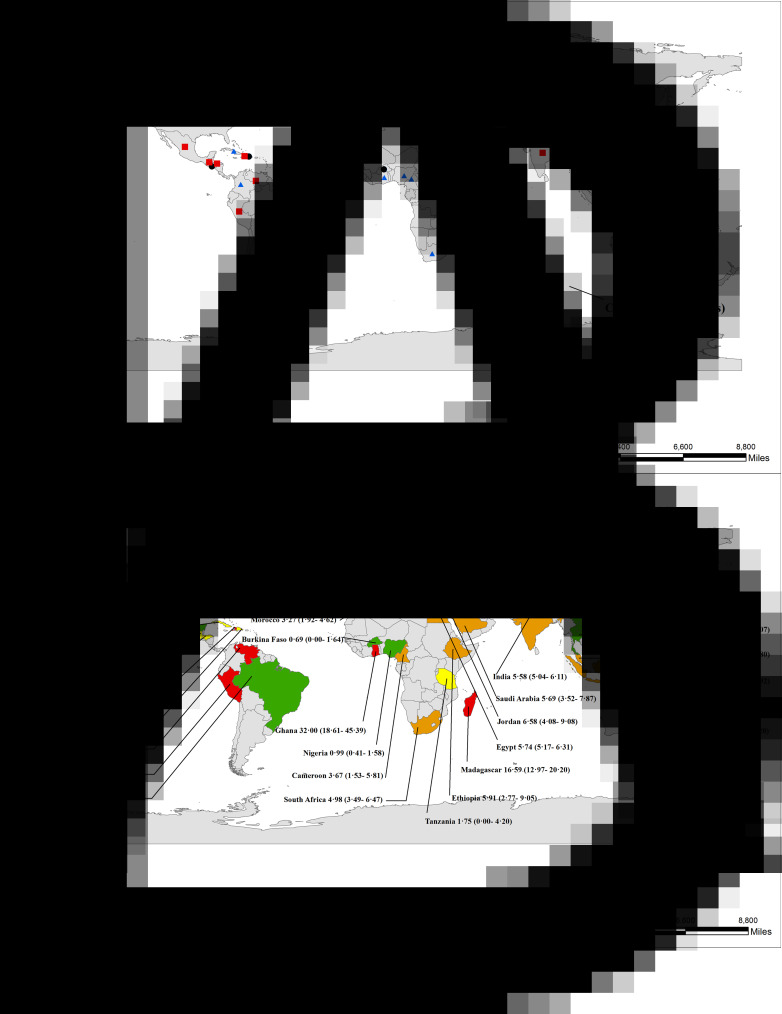
Cyclospora cayetanensis infections in humans have been documented in over 56 countries worldwide, distributed across all five human-inhabited continents (Fig. 3; Table 1S). The first recorded outbreak of C. cayetanensis infection (called an ‘alga-like organism’ at the time) occurred among 55 British expatriates with prolonged diarrhea in Nepal between June and November, 1989 (Shlim et al., 1991). The first reported outbreak of diarrheal illness associated with Cyclospora infection in the USA was in 1990 (Huang et al., 1995).
Fig. 3.
Number of documented human C. cayetanensis infections and prevalence worldwide. Number of documented infection cases (A) and prevalence (B) worldwide (95% confidence intervals are shown in brackets).
Up to 1996, more than 1400 cases of cyclosporiasis were recorded in multistate outbreaks in the USA and Canada (Herwaldt and Ackers, 1997). The most recent large outbreaks were documented in 2013 and 2018 concerning multistate outbreaks in the USA (Abanyie et al., 2015; Casillas et al., 2018). Up to December 2018, at least 13 countries documented cyclosporiasis outbreaks, involving ~6557 cases (Table 2S). Among these countries, cyclosporiasis has mainly been documented in the Americas and Europe, including Peru, Mexico, the USA, Canada and the United Kingdom (Table 2S).
Prevalence and case reports of C. cayetanensis in humans
A total of 13 845 C. cayetanensis cases have been recorded in humans, either during epidemiological studies (5478), during outbreak investigations (6557), or in case reports (1810) (Table 2S; Table 3S; Table 4S). The overall prevalence of C. cayetanensis among humans worldwide is 3.55% (5478/1 54 410). Asia (5.63%, 2771/49 254) and Africa (5.33%, 554/10 401) have shown greater prevalence than the Americas (3.03%, 1625/53 775) and Europe (1.28%, 528/41 186). A high prevalence of C. cayetanensis and large numbers of cases have been recorded in Nepal (13.68%) and India (5.58%) in Asia; Madagascar (16.59%) and Egypt (5.74%) in Africa and Venezuela (9.90%), Peru (6.87%) and Haiti (8.47%) in the Americas (Fig. 3).
Transmission risk factor assessment
A marked seasonality (rainy season or summer) has been observed in human C. cayetanensis infections in the northern hemisphere, including in China (Zhou et al., 2011; Jiang et al., 2018), Nepal (Sherchand and Cross, 2001; Kimura et al., 2005; Bhandari et al., 2015), Turkey (Ozdamar et al., 2010), Honduras (Kaminsky et al., 2016) and Mexico (Orozco-Mosqueda et al., 2014). The consistent pattern of the seasonal distribution of C. cayetanensis infections probably reflects the optimal environmental conditions (temperature and humidity) that are required for oocysts to sporulate. The major risk factors for Cyclospora transmission are probably the consumption of or contact with oocysts in contaminated food, water or soil; contact with animals and poor sanitation. These findings are typically documented in Peru (contaminated water sources) (Burstein Alva, 2005), Nepal (contaminated drinking water) (Bhattachan et al., 2017), Venezuela (contact with soil contaminated with human faeces) (Chacín-Bonilla et al., 2007), Nepal (livestock kept near households and the consumption of raw vegetables and fruits) (Bhandari et al., 2015) and Turkey (consumption of tap water or eating in unsanitary establishments) (Erdogan et al., 2012), among others. In summary, the epidemiological determinants and risk factors for human cyclosporiasis are shown in Table 1.
Table 1.
Epidemiological determinants and risk factors for human cyclosporiasis
| Factors | Main points |
|---|---|
| Sources of transmission: infection oocysts | Suitable environmental temperature and humidity (rainy or summer season) Infectious (sporulated) Cyclospora cayetanensis oocysts |
| Routes of transmission: Biology vectors or mechanical vehicle | Produce (fresh vegetables or fruits) as the vehicle Travel to or residence in endemic areas Water/soil as the vehicle Poor sanitary conditions |
| Susceptible human populations: clinical symptoms and immune status | Residents in low-income communities or endemic areas Patients with diarrhea or gastroenteritis symptoms Immunodeficient patients with diarrhea Immunodeficient patients |
Susceptible populations and risk factors
Cyclospora cayetanensis is recognized as an opportunistic protozoan pathogen of humans (Wiwanitkit, 2006). Immunodeficiency and diarrhea in the host are two major risk factors for C. cayetanensis infection. Notable distributions of infection have been documented in Nigeria (human immunodeficiency virus (HIV) patients with diarrhea) (Alakpa et al., 2002), Mexico (patients with diarrhea) (Jiménez-González et al., 2012), Honduras (patients with diarrhea or liquid stools) (Kaminsky et al., 2016) and Turkey (immunosuppressed patients) (Karaman et al., 2015), among others.
The statistics for C. cayetanensis infection in different human populations demonstrate that diarrhea is a major risk factor for Cyclospora infection: immunocompromised and immunocompetent individuals with diarrhea (7.38 vs 9.14%, respectively) both had a significantly higher prevalence of infection than patients with other symptoms (4.91 vs 2.09%, respectively; P = 0.0001).
Poor sanitation conditions are another risk factor for infection with C. cayetanensis. It should be noted that people from low-income communities living in areas with poor sanitation have the highest prevalence of infection. Remarkably high-prevalence rates have been reported in Peru (54.88 and 41.58%), Venezuela (24.20%) and India (22.27%), together with poor sanitary conditions (Burstein Alva, 2005; Nundy et al., 2011; Cazorla et al., 2012; Jeevitha et al., 2014). In one study in Nepal, the members of a family that kept livestock at home had higher Cyclospora infection rates than families who did not (Bhandari et al., 2015).
Age may be another factor that affects the occurrence of cyclosporiasis in humans. Many studies have reported that children show a higher prevalence of C. cayetanensis infection than the general populations, including in Guatemala, Nepal, Turkey and Honduras, among others (Bern et al., 1999; Kimura et al., 2005; Erdogan et al., 2012; Bhandari et al., 2015; Kaminsky et al., 2016). However, unexpectedly, children had a lower infection rate than the general population (4.90 vs 9.36%, respectively) of immunocompetent individuals with diarrhea, according to epidemiological statistics (P < 0.0001) (Table 2). This may be because the general population has more opportunity to consume raw produce than children.
Table 2.
Cyclospora cayetanensis prevalence in different human population groups
| Population groups | Number of investigation samples | Number of positive | Prevalence (95 CI) |
|---|---|---|---|
| HIV/AIDS or immunodeficient patients with diarrhea | 3863 | 285 | 7.38% (6.55–8.20) |
| Children | 0 | 0 | 0 |
| General | 3863 | 285 | 7.38% (6.55–8.20) |
| HIV/AIDS or immunodeficient patients without diarrhea | 5661 | 278 | 4.91% (4.35–5.47) |
| Children | 364 | 17 | 4.67% (2.49–6.85) |
| General | 5297 | 261 | 4.93% (4.34–5.51) |
| Individuals with diarrhea | 26 852 | 2453 | 9.14% (8.79–9.48) |
| Children | 1347 | 66 | 4.90% (3.75–6.05) |
| General | 25 505 | 2387 | 9.36% (9.00–9.72) |
| Individuals without diarrhea | 118 034 | 2462 | 2.09% (2.00–2.17) |
| Children | 25 077 | 439 | 1.75% (1.59–1.91) |
| General | 92 957 | 2023 | 2.18% (2.08–2.27) |
| Total | 154 410 | 5478 | 3.55% (3.46–3.64) |
Note: Summarized in ‘Table 3S: Epidemiology investigation of Cyclospora cayetanensis prevalence in humans’.
Cyclospora cayetanensis is also an important pathogen causing traveler's diarrhea, especially in industrialized regions (Shields and Olson, 2003; Mansfield and Gajadhar, 2004). International travel or expatriate relocation to developing countries with disease-endemic areas or poor sanitation might be a risk factor for cyclosporiasis in humans (Fryauff et al., 1999; Pandey et al., 2011; Kłudkowska et al., 2017).
Animal reservoirs
Several Cyclospora species or Cyclospora-like organisms have been reported in various animals (Table 5S), including five Cyclospora species identified in primates (Eberhard et al., 1999, 2001; Ortega and Sanchez, 2010; Li et al., 2015). Cyclospora-like organisms have been documented in dogs, cattle, chickens, rats, house mice, birds and even shellfish. The Asian freshwater clam (Corbicula fluminea) can recover the oocysts of C. cayetanensis during artificial contamination, and could therefore be used as a biological indicator of water contaminated with oocysts (Graczyk et al., 1998).
Another study attempted to develop an animal model of C. cayetanensis in which to study human cyclosporiasis. Various types of animals (various strains of mice, rats, sand rats, chickens, ducks, rabbits, birds, hamsters, ferrets, pigs, dogs, owl monkeys, rhesus monkeys and cynomolgus monkeys) were inoculated with human C. cayetanensis oocysts by gavage. None of the animals had developed patent infection or signs of infection 4–6 weeks after inoculation. It was concluded that none of the mammals tested are susceptible to infection by C. cayetanensis (Graczyk et al., 1998). Combined with the unpublished observation and personal communication data, great efforts had been made to attempts to infect various animals, the animal models of C. cayetanensis infections were still unsuccessfully.
A pilot study sought to infect human volunteers with C. cayetanensis, but no oocysts were detected in any stool sample from any of the seven volunteers during the 16-week trial (Alfano-Sobsey et al., 2004). These results suggest that the conditions necessary for Cyclospora to become infectious were not maintained during the preparation or storage of the oocysts. Future studies are required to assess the effects of temperature, humidity, storage conditions and disinfection on the survival, viability and infectivity of stored Cyclospora oocysts.
Food, water and soil sample contamination
In industrialized countries or regions, cyclosporiasis is most often linked to foodborne outbreaks (Rose and Slifko, 1999). In developing countries or disease-endemic areas, recorded C. cayetanensis infections have been associated with contact with contaminated food, water or soil (Burstein Alva, 2005; Chacín-Bonilla, 2008; Bhandari et al., 2015). In a community in Venezuela, a strong association between environmental contact with faecal-contaminated soil and the occurrence of cyclosporiasis was detected, suggesting that contact with soil may be an important mode of transmission (Chacín-Bonilla, 2008).
There are many records of vegetables, fruits, water and soil contaminated with Cyclospora oocysts in countries as diverse as Italy (Giangaspero et al., 2015), Malaysia (Bilung et al., 2017), Peru (Sturbaum et al., 1998), Nepal (Sherchand and Cross, 2001) and Vietnam (Tram et al., 2008), among others (Table 6S). Numerous methods have been developed for the recovery and analysis of Cyclospora oocysts in contaminated food, water and soil samples (Robertson et al., 2000; Shields et al., 2012).
Detection methods
A laboratory diagnosis of C. cayetanensis infection can be made simply by examining wet-mount preparations of faeces under light microscopy or by the autofluorescence of oocysts under UV epifluorescence microscopy. A more-automated flow-cytometric detection assay for C. cayetanensis in human faecal specimens was developed based on the morphology and autofluorescence characteristics of oocysts (Dixon et al., 2005). Modified Ziehl–Neelsen acid-fast staining is recommended for the detection of Cyclospora oocysts (Brennan et al., 1996; Clarke and McIntyre, 1996). Some other staining methods, such as (modified) Kinyoun acid-fast staining (Gonçalves et al., 2005; Hussein, 2007; Behera et al., 2008; Dillingham et al., 2009; Bhandari et al., 2015), trichrome staining (Turgay et al., 2007; Al-Megrin, 2010), carbol fuchsin staining (Alakpa et al., 2002; Chacín-Bonilla et al., 2007), (modified) safranin staining (Visvesvara et al., 1997) and lactophenol cotton blue staining (Parija et al., 2003), have been used in the past to identify Cyclospora oocysts in faecal smears, with variable degree of sensitivity and specificity. However, these morphology-based detection methods need more parasites burden, and may lead to frequent false positive results or false negatives. There are large differences in the performance between the different microscopy techniques. Direct detection using epifluorescence is actually the very best option, followed by the safranin-stain. In practice, two or more techniques could be used together to detect the presence of parasites.
Several PCR-based detection methods that amplify specific genes of C. cayetanensis have been developed. The first PCR method used for the clinical identification of C. cayetanensis, based on SSU rRNA gene sequences, was developed by Relman et al. (1996). Many other different PCR assays have since been developed. The real-time PCR based on the SSU rRNA gene has been optimized to specifically detect DNA from as few as one C. cayetanensis oocyst (Varma et al., 2003; Verweij et al., 2003). Another method uses the real-time quantitative PCR with a melting curve analysis to detect, identify and differentiate C. cayetanensis from other coccidian species of concern in animal health, zoonotic diseases and food safety (Lalonde and Gajadhar, 2011). Several other assays have been developed based on sequences other than the SSU rRNA gene, such as a PCR-based ITS assay, which is highly sensitive in oocyst detection (Olivier et al., 2001; Lalonde and Gajadhar, 2008), and an hsp70-gene-based nested PCR protocol for the detection of C. cayetanensis, which was developed in 2013 (Sulaiman et al., 2013). Many molecular methods have also been used to recover and detect Cyclospora oocysts in environmental water samples and agricultural products (Quintero-Betancourt et al., 2002; Steele et al., 2003; Murphy et al., 2018). Generally speaking, molecular-based detection methods can reliably detect a smaller parasites burden than other methods, even a single oocyst, and they thus overcome many of the limitations of microscopic diagnoses (Lalonde and Gajadhar, 2008).
Serological screening tests for Cyclospora would support epidemiological studies, and would be especially useful in the investigation of outbreaks (Ortega and Sanchez, 2010). However, no serological assays to determine human exposure to Cyclospora are yet available. Specific antibodies for the diagnosis of C. cayetanensis infection are not easily obtained, which greatly restricts immunological testing. Another serious limitation of serological assays is the lack of a laboratory culture method with which Cyclospora can be propagated in vitro (Eberhard et al., 2000; Cinar et al., 2015).
Treatment
Treatment with trimethoprim–sulfamethoxazole (TMP–SMX) (160 mg trimethoprim, 800 mg sulfamethoxazole) twice daily for 7–10 days is reported to be effective in curing Cyclospora infection (Hoge et al., 1995; Escobedo et al., 2009). This is also an effective therapy for Cyclospora infections in HIV patients (Pape et al., 1994; Verdier et al., 2000) and AIDS patients with biliary disease (Sifuentes-Osornio et al., 1995). TMP–SMX (also known as co-trimoxazole) is an effective treatment, and a low recurrence rate has been reported in many studies (Hoge et al., 1995; Madico et al., 1997; Goldberg and Bishara, 2012).
Ciprofloxacin is less effective than TMP–SMX, but is suitable for patients who are intolerant of sulfonamide drugs (Verdier et al., 2000). Successful treatment of C. cayetanensis infections with nitazoxanide has only been reported in a small number of patients (Diaz et al., 2003). However, nitazoxanide is an important treatment option for patients with a sulfa allergy or for whom treatment with sulfa or ciprofloxacin has failed (Zimmer et al., 2007). However, norfloxacin, metronidazole, tinidazole and quinacrine have been shown to be ineffective in several studies of human cyclosporiasis (Escobedo et al., 2009).
Conclusions
Since the earliest reported cases of human Cyclospora infection in Papua New Guinea in 1979, at least 54 countries have documented C. cayetanensis infections (involving 13 845 cases) up to December 2018. Of these countries, more than 13 have recorded cyclosporiasis outbreaks (including 6557 cases). The overall C. cayetanensis prevalence in humans worldwide is 3.55% (5478/1 54 410). Cyclospora cayetanensis infections are commonly reported in developing countries with low-socioeconomic levels or disease-endemic areas, such as Madagascar, Nepal, Indonesia, Peru and Haiti, among others. However, large outbreaks have also been documented in developed countries in Europe and the Americas, and among travelers from these countries and those returning from tropical endemic areas. Among susceptible populations, the highest prevalence has been documented in immunocompetent individuals with diarrhea. The marked seasonality of C. cayetanensis infection, which occurs predominantly during the rainy season or summer, is well documented. Infection with C. cayetanensis is mainly transmitted through the ingestion of food contaminated with oocysts. Cyclospora cayetanensis or Cyclospora-like organisms have also been detected in food, water, soil and faecal material from some animals. Detection methods based on oocyst morphology, staining and molecular testing have been developed. Treatment with TMP–SMX effectively cures C. cayetanensis infection. Ciprofloxacin is less effective than TMP–SMX, but is suitable for patients who cannot tolerate co-trimoxazole.
Despite many recent advances in research, our understanding of human cyclosporiasis is hampered by several technical difficulties. It will be necessary to establish an in vitro or animal model of C. cayetanensis in the near future, in which to study human cyclosporiasis. Rapid, convenient, precise and economic detection methods for its diagnosis and genotype in humans, and effective tracing methods, must also be developed to monitor the transmission of C. cayetanensis. More importantly, the proper disposal of faeces to avoid the contamination of soil and food, boiling and filtering drinking water and improved personal hygiene will go a long way toward preventing enteric parasitic infections.
Search strategy and selection criteria
We searched PubMed, Web of Science, ScienceDirect, Wangfang and the China National Knowledge Infrastructure, with no language restriction, using the following search terms to screen for relevant articles: ‘Cyclospora’ or ‘Cyclospora-like organisms’ or ‘cyclosporiasis’ or ‘cyanobacterium-like body’ or ‘alga-like organism’. For articles without the full text or published in other languages, the titles and abstracts in English were screened for mention of Cyclospora infection. We included articles published up to 31 December 2018, when calculating the epidemiology data and summarizing the cases of infection. Articles published in English, Spanish, Portuguese, French, Turkish, Chinese, Czech, Dutch, Japanese, Rumanian and German were included.
Acknowledgements
We thank Janine Miller, PhD, of Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Financial support
This study was partly supported the National Key Research and Development Program of China (2017|YFD0501305, 2017YFD0500405), the National Natural Science Foundation of China (31330079, 30600603, 31672548) and the Natural Science Foundation of Henan Province (162300410129).
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical standards
Not applicable.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182019001471.
click here to view supplementary material
References
- Abanyie F, Harvey RR, Harris JR, Wiegand RE, Gaul L, Desvignes-Kendrick M, Irvin K, Williams I, Hall RL, Herwaldt B, Gray EB, Qvarnstrom Y, Wise ME, Cantu V, Cantey PT, Bosch S, DA Silva AJ, Fields A, Bishop H, Wellman A, Beal J, Wilson N, Fiore AE, Tauxe R, Lance S, Slutsker L, Parise M and Multistate Cyclosporiasis Outbreak Investigation Team (2015) 2013 multistate outbreaks of Cyclospora cayetanensis infections associated with fresh produce: focus on the Texas investigations. Epidemiology & Infection 143, 3451–3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy U, Marangi M, Papini R, Ozkoc S, Bayram Delibas S and Giangaspero A (2014) Detection of Toxoplasma gondii and Cyclospora cayetanensis in Mytilus galloprovincialis from Izmir Province coast (Turkey) by real time PCR/high-resolution melting analysis (HRM). Food Microbiology 44, 128–135. [DOI] [PubMed] [Google Scholar]
- Alakpa G, Fagbenro-Beyioku AF and Clarke SC (2002) Cyclospora cayetanensis in stools submitted to hospitals in Lagos, Nigeria. International Journal of Infectious Diseases 6, 314–318. [DOI] [PubMed] [Google Scholar]
- Alfano-Sobsey EM, Eberhard ML, Seed JR, Weber DJ, Won KY, Nace EK and Moe CL (2004) Human challenge pilot study with Cyclospora cayetanensis. Emerging Infectious Diseases 10, 726–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Megrin WA (2010) Intestinal parasites infection among immunocompromised patients in Riyadh, Saudi Arabia. Pakistan Journal of Biological Sciences 13, 390–394. [DOI] [PubMed] [Google Scholar]
- Ashford RW (1979) Occurrence of an undescribed coccidian in man in Papua New Guinea. Annals of Tropical Medicine and Parasitology 73, 497–500. [DOI] [PubMed] [Google Scholar]
- Barratt JLN, Park S, Nascimento FS, Hofstetter J, Plucinski M, Casillas S, Bradbury RS, Arrowood MJ, Qvarnstrom Y and Talundzic E (2019) Genotyping genetically heterogeneous Cyclospora cayetanensis infections to complement epidemiological case linkage. Parasitology 31, 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera B, Mirdha BR, Makharia GK, Bhatnagar S, Dattagupta S and Samantaray JC (2008) Parasites in patients with malabsorption syndrome: a clinical study in children and adults. Digestive Diseases and Sciences 53, 672–679. [DOI] [PubMed] [Google Scholar]
- Bern C, Hernandez B, Lopez MB, Arrowood MJ, de Mejia MA, de Merida AM, Hightower AW, Venczel L, Herwaldt BL and Klein RE (1999) Epidemiologic studies of Cyclospora cayetanensis in Guatemala. Emerging Infectious Diseases 5, 766–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari D, Tandukar S, Parajuli H, Thapa P, Chaudhary P, Shrestha D, Shah PK, Sherchan JB and Sherchand JB (2015) Cyclospora infection among school children in Kathmandu, Nepal: prevalence and associated risk factors. Tropical Medicine and Health 43, 211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattachan B, Sherchand JB, Tandukar S, Dhoubhadel BG, Gauchan L and Rai G (2017) Detection of Cryptosporidium parvum and Cyclospora cayetanensis infections among people living in a slum area in Kathmandu valley, Nepal. BMC Research Notes 10, 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilung LM, Tahar AS, Yunos NE, Apun K, Lim YA, Nillian E and Hashim HF (2017) Detection of Cryptosporidium and Cyclospora oocysts from environmental water for drinking and recreational activities in Sarawak, Malaysia. BioMed Research International 2017, 4636420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan MK, MacPherson DW, Palmer J and Keystone JS (1996) Cyclosporiasis: a new cause of diarrhea. Canadian Medical Association Journal 155, 1293–1296. [PMC free article] [PubMed] [Google Scholar]
- Brown GH and Rotschafer JC (1999) Cyclospora: review of an emerging parasite. Pharmacotherapy 19, 70–75. [DOI] [PubMed] [Google Scholar]
- Burstein Alva S (2005) Cyclosporosis: an emergent parasitosis. (I) Clinical and epidemiological aspects. Revista de Gastroenterologia del Peru 25, 328–335. [PubMed] [Google Scholar]
- Casemore DP (1994) Cyclospora: another ‘new’ pathogen. Journal of Medical Microbiology 41, 217–219. [DOI] [PubMed] [Google Scholar]
- Casillas SM, Bennett C and Straily A (2018) Notes from the field: multiple cyclosporiasis outbreaks – United States, 2018. American Journal of Transplantation 18, 3072–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazorla D, Acosta ME, Acosta ME and Morales P (2012) Clinical and epidemiological study of intestinal coccidioses in a rural population of a semiarid region from Falcon state, Venezuela. Journal of Clinical Investigation 53, 273–288. [PubMed] [Google Scholar]
- Chacín-Bonilla L (2008) Transmission of Cyclospora cayetanensis infection: a review focusing on soil-borne cyclosporiasis. Transactions of the Royal Society of Tropical Medicine and Hygiene 102, 215–216. [DOI] [PubMed] [Google Scholar]
- Chacín-Bonilla L, Barrios F and Sanchez Y (2007) Epidemiology of Cyclospora cayetanensis infection in San Carlos Island, Venezuela: strong association between socio-economic status and infection. Transactions of the Royal Society of Tropical Medicine and Hygiene 101, 1018–1024. [DOI] [PubMed] [Google Scholar]
- Chu DM, Sherchand JB, Cross JH and Orlandi PA (2004) Detection of Cyclospora cayetanensis in animal fecal isolates from Nepal using an FTA filter-base polymerase chain reaction method. American Journal of Tropical Medicine and Hygiene 71, 373–379. [PubMed] [Google Scholar]
- Cinar HN, Gopinath G, Jarvis K and Murphy HR (2015) The complete mitochondrial genome of the foodborne parasitic pathogen Cyclospora cayetanensis. PLoS One 10, e0128645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SC and McIntyre M (1996) Modified detergent Ziehl-Neelsen technique for the staining of Cyclospora cayetanensis. Journal of Clinical Pathology 49, 511–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor BA, Shlim DR, Scholes JV, Rayburn JL, Reidy J and Rajah R (1993) Pathologic changes in the small bowel in 9 patients with diarrhea associated with a coccidia-like body. Annals of Internal Medicine 119, 377–382. [DOI] [PubMed] [Google Scholar]
- Connor BA, Reidy J and Soave R (1999) Cyclosporiasis: clinical and histopathologic correlates. Clinical Infectious Diseases 28, 1216–1222. [DOI] [PubMed] [Google Scholar]
- Connor BA, Johnson EJ and Soave R (2001) Reiter syndrome following protracted symptoms of Cyclospora infection. Emerging Infectious Diseases 7, 453–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordón GP, Prados AH, Romero D, Sánchez Moreno M, Pontes A, Osuna A, Rosales MJ (2008) Intestinal parasitism in the animals of the zoological garden ‘Peña Escrita’ (Almuñecar, Spain). Veterinary Parasitology 156, 302–309. [DOI] [PubMed] [Google Scholar]
- Di Genova BM and Tonelli RR (2016) Infection strategies of intestinal parasite pathogens and host cell responses. Frontiers in Microbiology 7, 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Gliullo AB, Cribari MS, Bava AJ, Cicconetti JS and Collazos R (2000) Cyclospora cayetanensis in sputum and stool samples. Revista do Instituto de Medicina Tropical de Sao Paulo 42, 115–117. [DOI] [PubMed] [Google Scholar]
- Diaz E, Mondragon J, Ramirez E and Bernal R (2003) Epidemiology and control of intestinal parasites with nitazoxanide in children in Mexico. American Journal of Tropical Medicine and Hygiene 68, 384–385. [PubMed] [Google Scholar]
- Dillingham RA, Pinkerton R, Leger P, Severe P, Guerrant RL, Pape JW and Fitzgerald DW (2009) High early mortality in patients with chronic acquired immunodeficiency syndrome diarrhea initiating antiretroviral therapy in Haiti: a case-control study. American Journal of Tropical Medicine and Hygiene 80, 1060–1064. [PMC free article] [PubMed] [Google Scholar]
- Dixon BR, Bussey JM, Parrington LJ and Parenteau M (2005) Detection of Cyclospora cayetanensis oocysts in human fecal specimens by flow cytometry. Journal of Clinical Microbiology 43, 2375–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard ML, da Silva AJ, Lilley BG and Pieniazek NJ (1999) Morphologic and molecular characterization of new Cyclospora species from Ethiopian monkeys: C. cercopitheci sp.n., C. colobi sp.n., and C. papionis sp.n. Emerging Infectious Diseases 5, 651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhard ML, Ortega YR, Hanes DE, Nace EK, Do RQ, Robl MG, Won KY, Gavidia C, Sass NL, Mansfield K, Gozalo A, Griffiths J, Gilman R, Sterling CR and Arrowood MJ (2000) Attempts to establish experimental Cyclospora cayetanensis infection in laboratory animals. Journal of Parasitology 86, 577–582. [DOI] [PubMed] [Google Scholar]
- Eberhard ML, Njenga MN, DaSilva AJ, Owino D, Nace EK, Won KY and Mwenda JM (2001) A survey for Cyclospora spp. in Kenyan primates, with some notes on its biology. Journal of Parasitology 87, 1394–1397. [DOI] [PubMed] [Google Scholar]
- Erdogan DD, Kurt O, Mandiracioglu A, Ahmet U, Mucide A and Hande D (2012) Prevalence and associated factors of Cryptosporidium spp. and Cyclospora cayetanensis in Izmir province, Turkey. Kafkas Üniversitesi Veteriner Fakültesi Dergisi 18, A105–A110. [Google Scholar]
- Escobedo AA, Almirall P, Alfonso M, Cimerman S, Rey S and Terry SL (2009) Treatment of intestinal protozoan infections in children. Archives of Disease in Childhood 94, 478–482. [DOI] [PubMed] [Google Scholar]
- Fletcher SM, Stark D, Harkness J and Ellis J (2012) Enteric protozoa in the developed world: a public health perspective. Clinical Microbiology Reviews 25, 420–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryauff DJ, Krippner R, Prodjodipuro P, Ewald C, Kawengian S, Pegelow K, Yun T, von Heydwolff-Wehnert C, Oyofo B and Gross R (1999) Cyclospora cayetanensis among expatriate and indigenous populations of West Java, Indonesia. Emerging Infectious Diseases 5, 585–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghozzi K, Marangi M, Papini R, Lahmar I, Challouf R, Houas N, Ben Dhiab R, Normanno G, Babba H and Giangaspero A (2017) First report of Tunisian coastal water contamination by protozoan parasites using mollusk bivalves as biological indicators. Marine Pollution Bulletin 117, 197–202. [DOI] [PubMed] [Google Scholar]
- Giangaspero A and Gasser RB (2019) Human cyclosporiasis. The Lancet Infectious Diseases 19, e226–e236. [DOI] [PubMed] [Google Scholar]
- Giangaspero A, Marangi M, Koehler AV, Papini R, Normanno G, Lacasella V, Lonigro A and Gasser RB (2015) Molecular detection of Cyclospora in water, soil, vegetables and humans in southern Italy signals a need for improved monitoring by health authorities. International Journal of Food Microbiology 211, 95–100. [DOI] [PubMed] [Google Scholar]
- Goldberg E and Bishara J (2012) Contemporary unconventional clinical use of co-trimoxazole. Clinical Microbiology and Infection 18, 8–17. [DOI] [PubMed] [Google Scholar]
- Gonçalves EM, Uemura IH, Castilho VL and Corbett CE (2005) Retrospective study of the occurrence of Cyclospora cayetanensis at Clinical Hospital of the University of São Paulo Medical School, SP. Revista da Sociedade Brasileira de Medicina Tropical 38, 326–330. [DOI] [PubMed] [Google Scholar]
- Graczyk TK, Ortega YR and Conn DB (1998) Recovery of waterborne oocysts of Cyclospora cayetanensis by Asian freshwater clams (Corbicula fluminea). American Journal of Tropical Medicine and Hygiene 59, 928–932. [DOI] [PubMed] [Google Scholar]
- Guo Y, Roellig DM, Li N, Tang K, Frace M, Ortega Y, Arrowood MJ, Feng Y, Qvarnstrom Y, Wang L, Moss DM, Zhang L and Xiao L (2016) Multilocus sequence typing tool for Cyclospora cayetanensis. Emerging Infectious Diseases 22, 1464–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Wang Y, Wang X, Zhang L, Ortega Y and Feng Y (2019) Mitochondrial genome sequence variation as a useful marker for assessing genetic heterogeneity among Cyclospora cayetanensis isolates and source-tracking. Parasites & Vectors 12, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenbrook WD, Wade SE, Shields WM, Stehman SV and Whipps CM (2015) Gastrointestinal parasites of Ecuadorian Mantled Howler Monkeys (Alouatta palliata aequatorialis) based on fecal analysis. Journal of Parasitology 101, 341–350. [DOI] [PubMed] [Google Scholar]
- Herwaldt BL (2000) Cyclospora cayetanensis: a review, focusing on the outbreaks of cyclosporiasis in the 1990s. Clinical Infectious Diseases 31, 1040–1057. [DOI] [PubMed] [Google Scholar]
- Herwaldt BL and Ackers ML (1997) An outbreak in 1996 of cyclosporiasis associated with imported raspberries. The Cyclospora Working Group. The New England Journal of Medicine 336, 1548–1556. [DOI] [PubMed] [Google Scholar]
- Hoge CW, Shlim DR, Ghimire M, Rabold JG, Pandey P, Walch A, Rajah R, Gaudio P and Echeverria P (1995) Placebo-controlled trial of co-trimoxazole for Cyclospora infections among travellers and foreign residents in Nepal. The Lancet 345, 691–693. [DOI] [PubMed] [Google Scholar]
- Huang P, Weber JT, Sosin DM, Griffin PM, Long EG, Murphy JJ, Kocka F, Peters C and Kallick C (1995) The first reported outbreak of diarrheal illness associated with Cyclospora in the United States. Annals of Internal Medicine 123, 409–414. [DOI] [PubMed] [Google Scholar]
- Hussein EM (2007) Molecular identification of Cycospora spp. using multiplex PCR from diarrheic children compared to others conventional methods. Journal of the Egyptian Society of Parasitology 37, 585–598. [PubMed] [Google Scholar]
- Hussein EM, Abdul-Manaem AH and El-Attary SL (2005) Cyclospora cayetanensis oocysts in sputum of a patient with active pulmonary tuberculosis, case report in Ismailia, Egypt. Journal of the Egyptian Society of Parasitology 35, 787–793. [PubMed] [Google Scholar]
- Jeevitha D, Pushparaj SP and Kanchana M (2014) Comparative study of the prevalence of intestinal parasites in low socioeconomic areas from South Chennai, India. Journal of Parasitology Research 2014, 630968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Yuan Z, Zang G, Li D, Wang Y, Zhang Y, Liu H, Cao J and Shen Y (2018) Cyclospora cayetanensis infections among diarrheal outpatients in Shanghai: a retrospective case study. Frontiers of Medicine 12, 98–103. [DOI] [PubMed] [Google Scholar]
- Jiménez-González GB, Martínez-Gordillo MN, Caballero-Salazar S, Peralta-Abarca GE, Cárdenas-Cardoz R, Arzate-Barbosa P and Ponce-Macotela M (2012) Microsporidia in pediatric patients with leukemia or lymphoma. Revista de Investigacio Clinica 64, 25–31. [PubMed] [Google Scholar]
- Kaminsky RG, Lagos J, Raudales Santos G and Urrutia S (2016) Marked seasonality of Cyclospora cayetanensis infections: ten-year observation of hospital cases, Honduras. BMC Infectious Diseases 16, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaman U, Daldal N, Ozer A, Enginyurt O and Erturk O (2015) Epidemiology of Cyclospora species in humans in Malatya Province in Turkey. Jundishapur Journal of Microbiology 8, e18661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Rai SK, Rai G, Insisiengmay S, Kawabata M, Karanis P and Uga S (2005) Study on Cyclospora cayetanensis associated with diarrheal disease in Nepal and Loa PDR. Southeast Asian Journal of Tropical Medicine and Public Health 36, 1371–1376. [PubMed] [Google Scholar]
- Kłudkowska M, Pielok Ł, Frąckowiak K and Paul M (2017) Intestinal coccidian parasites as an underestimated cause of travellers' diarrhoea in Polish immunocompetent patients. Acta Parasitologica 62, 630–638. [DOI] [PubMed] [Google Scholar]
- Lainson R (2005) The genus Cyclospora (apicomplexa: Eimeriidae), with a description of Cyclospora schneideri n. sp. in the snake Anilius scytale scytale (Aniliidae) from Amazonian Brazil – a review. Memórias do Instituto Oswaldo Cruz 100, 103–110. [DOI] [PubMed] [Google Scholar]
- Lalonde LF and Gajadhar AA (2008) Highly sensitive and specific PCR assay for reliable detection of Cyclospora cayetanensis oocysts. Applied and Environmental Microbiology 74, 4354–4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde LF and Gajadhar AA (2011) Detection and differentiation of coccidian oocysts by real-time PCR and melting curve analysis. Journal of Parasitology 97, 725–730. [DOI] [PubMed] [Google Scholar]
- Li G, Xiao S, Zhou R, Li W and Wadeh H (2007) Molecular characterization of Cyclospora-like organism from dairy cattle. Parasitology Research 100, 955–961. [DOI] [PubMed] [Google Scholar]
- Li N, Ye J, Arrowood MJ, Ma J, Wang L, Xu H, Feng Y and Xiao L (2015) Identification and morphologic and molecular characterization of Cyclospora macacae n. sp. from rhesus monkeys in China. Parasitology Research 114, 1811–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chang Y, Shi KE, Wang R, Fu K, Li S, Xu J, Jia L, Guo Z and Zhang L (2017) Multilocus sequence typing and clonal population genetic structure of Cyclospora cayetanensis in humans. Parasitology 144, 1890–1897. [DOI] [PubMed] [Google Scholar]
- Liu S, Wang L, Zheng H, Xu Z, Roellig DM, Li N, Frace MA, Tang K, Arrowood MJ, Moss DM, Zhang L, Feng Y and Xiao L (2016) Comparative genomics reveals Cyclospora cayetanensis possesses coccidia-like metabolism and invasion components but unique surface antigens. BMC Genomics 17, 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madico G, McDonald J, Gilman RH, Cabrera L and Sterling CR (1997) Epidemiology and treatment of Cyclospora cayetanensis infection in Peruvian children. Clinical Infectious Diseases 24, 977–981. [DOI] [PubMed] [Google Scholar]
- Mansfield LS and Gajadhar AA (2004) Cyclospora cayetanensis, a food- and waterborne coccidian parasite. Veterinary Parasitology 126, 73–90. [DOI] [PubMed] [Google Scholar]
- McAllister CT, Motriuk-Smith D and Kerr CM (2018) Three new coccidians (Cyclospora, Eimeria) from eastern moles, Scalopus aquaticus (Linnaeus) (Mammalia: Soricomorpha: Talpidae) from Arkansas, USA. Systematic Parasitology 95, 271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy HR, Cinar HN, Gopinath G, Noe KE, Chatman LD, Miranda NE, Wetherington JH, Neal-McKinney J, Pires GS, Sachs E, Stanya KJ, Johnson CL, Nascimento FS, Santin M, Molokin A, Samadpour M, Janagama H, Kahler A, Miller C and da Silva AJ (2018) Interlaboratory validation of an improved method for detection of Cyclospora cayetanensis in produce using a real-time PCR assay. Food Microbiology 69, 170–178. [DOI] [PubMed] [Google Scholar]
- Nascimento FS, Barta JR, Whale J, Hofstetter JN, Casillas S, Barratt J, Talundzic E, Arrowood MJ and Qvarnstrom Y (2019) Mitochondrial junction region as genotyping marker for Cyclospora cayetanensis. Emerging Infectious Diseases 25, 1314–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nundy S, Gilman RH, Xiao L, Cabrera L, Cama R, Ortega YR, Kahn G and Cama VA (2011) Wealth and its associations with enteric parasitic infections in a low-income community in Peru: use of principal component analysis. American Journal of Tropical Medicine and Hygiene 84, 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogedengbe ME, Qvarnstrom Y, da Silva AJ, Arrowood MJ and Barta JR (2015) A linear mitochondrial genome of Cyclospora cayetanensis (Eimeriidae, Eucoccidiorida, Coccidiasina, Apicomplexa) suggests the ancestral start position within mitochondrial genomes of eimeriid coccidia. International Journal for Parasitology 45, 361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivier C, van de Pas S, Lepp PW, Yoder K and Relman DA (2001) Sequence variability in the first internal transcribed spacer region within and among Cyclospora species is consistent with polyparasitism. International Journal for Parasitology 31, 1475–1487. [DOI] [PubMed] [Google Scholar]
- Orozco-Mosqueda GE, Martínez-Loya OA and Ortega YR (2014) Cyclospora cayetanensis in a pediatric hospital in Morelia, México. American Journal of Tropical Medicine and Hygiene 91, 537–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega YR and Sanchez R (2010) Update on Cyclospora cayetanensis, a food-borne and waterborne parasite. Clinical Microbiology Reviews 23, 218–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega YR, Nagle R, Gilman RH, Watanabe J, Miyagui J, Quispe H, Kanagusuku P, Roxas C and Sterling CR (1997) Pathologic and clinical findings in patients with cyclosporiasis and a description of intracellular parasite life-cycle stages. The Journal of Infectious Diseases 176, 1584–1589. [DOI] [PubMed] [Google Scholar]
- Ozdamar M, Hakko E and Turkoglu S (2010) High occurrence of cyclosporiasis in Istanbul, Turkey, during a dry and warm summer. Parasites & Vectors 3, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey P, Bodhidatta L, Lewis M, Murphy H, Shlim DR, Cave W, Rajah R, Springer M, Batchelor T, Sornsakrin S and Mason CJ (2011) Travelers' diarrhea in Nepal: an update on the pathogens and antibiotic resistance. Journal of Travel Medicine 18, 102–108. [DOI] [PubMed] [Google Scholar]
- Pape JW, Verdier RI, Boncy M, Boncy J and Johnson WD Jr (1994) Cyclospora infection in adults infected with HIV. Clinical manifestations, treatment and prophylaxis. Annals of Internal Medicine 121, 654–657. [DOI] [PubMed] [Google Scholar]
- Parija SC, Shivaprakash MR and Jayakeerthi SR (2003) Evaluation of lacto-phenol cotton blue (LPCB) for detection of Cryptosporidium, Cyclospora and Isospora in the wet mount preparation of stool. Acta Tropica 85, 349–354. [DOI] [PubMed] [Google Scholar]
- Quintero-Betancourt W, Peele ER and Rose JB (2002) Cryptosporidium parvum and Cyclospora cayetanensis: a review of laboratory methods for detection of these waterborne parasites. Journal of Microbiological Methods 49, 209–224. [DOI] [PubMed] [Google Scholar]
- Relman DA, Schmidt TM, Gajadhar A, Sogin M, Cross J, Yoder K, Sethabutr O and Echeverria P (1996) Molecular phylogenetic analysis of Cyclospora, the human intestinal pathogen, suggests that it is closely related to Eimeria species. The Journal of Infectious Diseases 173, 440–445. [DOI] [PubMed] [Google Scholar]
- Riner DK, Nichols T, Lucas SY, Mullin AS, Cross JH and Lindquist HD (2010) Intragenomic sequence variation of the ITS-1 region within a single flow-cytometry-counted Cyclospora cayetanensis oocysts. Journal of Parasitology 96, 914–919. [DOI] [PubMed] [Google Scholar]
- Robertson LJ, Gjerde B and Campbell AT (2000) Isolation of Cyclospora oocysts from fruits and vegetables using lectin-coated paramagnetic beads. Journal of Food Protection 63, 1410–1414. [DOI] [PubMed] [Google Scholar]
- Rose JB and Slifko TR (1999) Giardia, Cryptosporidium, and Cyclospora and their impact on foods: a review. Journal of Food Protection 62, 1059–1070. [DOI] [PubMed] [Google Scholar]
- Ryan U, Paparini A and Oskam C (2017) New technologies for detection of enteric parasites. Trends in Parasitology 33, 532–546. [DOI] [PubMed] [Google Scholar]
- Sherchand JB and Cross JH (2001) Emerging pathogen Cyclospora cayetanensis infection in Nepal. Southeast Asian Journal of Tropical Medicine And Public Health 32(suppl. 2), 143–150. [PubMed] [Google Scholar]
- Shields JM and Olson BH (2003) Cyclospora cayetanensis: a review of an emerging parasitic coccidian. International Journal for Parasitology 33, 371–391. [DOI] [PubMed] [Google Scholar]
- Shields JM, Lee MM and Murphy HR (2012) Use of a common laboratory glassware detergent improves recovery of Cryptosporidium parvum and Cyclospora cayetanensis from lettuce, herbs and raspberries. International Journal of Food Microbiology 153, 123–128. [DOI] [PubMed] [Google Scholar]
- Shlim DR, Cohen MT, Eaton M, Rajah R, Long EG and Ungar BL (1991) An alga-like organism associated with an outbreak of prolonged diarrhea among foreigners in Nepal. American Journal of Tropical Medicine and Hygiene 45, 383–389. [DOI] [PubMed] [Google Scholar]
- Sifuentes-Osornio J, Porras-Cortés G, Bendall RP, Morales-Villarreal F, Reyes-Terán G and Ruiz-Palacios GM (1995) Cyclospora cayetanensis infection in patients with and without AIDS: biliary disease as another clinical manifestation. Clinical Infectious Diseases 21, 1092–1097. [DOI] [PubMed] [Google Scholar]
- Smith HV, Paton CA, Mitambo MM and Girdwood RW (1997) Sporulation of Cyclospora sp. oocysts. Applied and Environmental Microbiology 63, 1631–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele M, Unger S and Odumeru J (2003) Sensitivity of PCR detection of Cyclospora cayetanensis in raspberries, basil, and mesclun lettuce. Journal of Microbiological Methods 54, 277–280. [DOI] [PubMed] [Google Scholar]
- Sturbaum GD, Ortega YR, Gilman RH, Sterling CR, Cabrera L and Klein DA (1998) Detection of Cyclospora cayetanensis in wastewater. Applied and Environmental Microbiology 64, 2284–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulaiman IM, Torres P, Simpson S, Kerdahi K and Ortega Y (2013) Sequence characterization of heat shock protein gene of Cyclospora cayetanensis isolates from Nepal, Mexico, and Peru. Journal of Parasitology 99, 379–382. [DOI] [PubMed] [Google Scholar]
- Sulaiman IM, Ortega Y, Simpson S and Kerdahi K (2014) Genetic characterization of human-pathogenic Cyclospora cayetanensis parasites from three endemic regions at the 18S ribosomal RNA locus. Infection Genetics and Evolution 22, 229–234. [DOI] [PubMed] [Google Scholar]
- Sun T, Ilardi CF, Asnis D, Bresciani AR, Goldenberg S, Roberts B and Teichberg S (1996) Light and electron microscopic identification of Cyclospora species in the small intestine. Evidence of the presence of asexual life cycle in human host. American Journal of Clinical Pathology 105, 216–220. [DOI] [PubMed] [Google Scholar]
- Tang K, Guo Y, Zhang L, Rowe LA, Roellig DM, Frace MA, Li N, Liu S, Feng Y and Xiao L (2015) Genetic similarities between Cyclospora cayetanensis and cecum-infecting avian Eimeria spp. in apicoplast and mitochondrial genomes. Parasites & Vectors 8, 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tram NT, Hoang LM, Cam PD, Chung PT, Fyfe MW, Isaac-Renton JL and Ong CS (2008) Cyclospora spp. in herbs and water samples collected from markets and farms in Hanoi, Vietnam. Tropical Medicine & International Health 13, 1415–1420. [DOI] [PubMed] [Google Scholar]
- Turgay N, Yolasigmaz A, Erdogan DD, Zeyrek FY and Uner A (2007) Incidence of cyclosporiasis in patients with gastrointestinal symptoms in western Turkey. Medical Science Monitor 13, CR34–CR39. [PubMed] [Google Scholar]
- Varma M, Hester JD, Schaefer FW III, Ware MW and Lindquist HD (2003) Detection of Cyclospora cayetanensis using a quantitative real-time PCR assay. Journal of Microbiological Methods 53, 27–36. [DOI] [PubMed] [Google Scholar]
- Verdier RI, Fitzgerald DW, Johnson WD Jr and Pape JW (2000) Trimethoprim-sulfamethoxazole compared with ciprofloxacin for treatment and prophylaxis of Isospora belli and Cyclospora cayetanensis infection in HIV-infected patients. A randomized, controlled trial. Annals of Internal Medicine 132, 885–888. [DOI] [PubMed] [Google Scholar]
- Verweij JJ, Laeijendecker D, Brienen EA, van Lieshout L and Polderman AM (2003) Detection of Cyclospora cayetanensis in travellers returning from the tropics and subtropics using microscopy and real-time PCR. International Journal of Medical Microbiology 293, 199–202. [DOI] [PubMed] [Google Scholar]
- Visvesvara GS, Moura H, Kovacs-Nace E, Wallace S and Eberhard ML (1997) Uniform staining of Cyclospora oocysts in fecal smears by a modified safranin technique with microwave heating. Journal of Clinical Microbiology 35, 730–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiwanitkit V (2006) Intestinal parasite infestation in HIV infected patients. Current HIV Research 4, 87–96. [DOI] [PubMed] [Google Scholar]
- Zar FA, El-Bayoumi E and Yungbluth MM (2001) Histologic proof of acalculous cholecystitis due to Cyclospora cayetanensis. Clinical Infectious Diseases 33, E140–E141. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Lv B, Wang Q, Wang R, Jian F, Zhang L, Ning C, Fu K, Wang Y, Qi M, Yao H, Zhao J, Zhang X, Sun Y, Shi K, Arrowood MJ and Xiao L (2011) Prevalence and molecular characterization of Cyclospora cayetanensis, Henan, China. Emerging Infectious Diseases 17, 1887–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer SM, Schuetz AN and Franco-Paredes C (2007) Efficacy of nitazoxanide for cyclosporiasis in patients with sulfa allergy. Clinical Infectious Diseases 44, 466–467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182019001471.
click here to view supplementary material