Abstract
Leishmaniasis is a parasitic disease infecting animals and humans. Two clinical forms (Visceral and cutaneous leishmaniasis) and four species are reported to be present in Turkey. Several studies have investigated canine and human leishmaniasis in Turkey but no study was performed to screen the infection among wild rodents, so far. The present study aims to investigate the role of small wild rodents as reservoir animals for Leishmania spp. in different regions of Turkey. Formalin-preserved tissue samples (spleen, liver, lung) of 712 rodents from 30 provinces were screened for the presence of Leishmania spp. DNA. Before DNA extraction, tissues were dried, rehydrated, and homogenated. Leishmania screening in rodent tissues and species determination was performed with a combination of real-time kDNA and ITS1 polymerase chain reaction protocols. Eight (1.12%) out of 712 animals were found to be positive for Leishmania spp. DNA and species typing revealed five L. infantum, two L. tropica and one L. major among positives. Leishmania major and L. infantum DNA were detected in Apodemus spp. from Zonguldak province located in the Western Black Sea Region, while L. tropica DNA was found in Meriones sp. and Gerbillus dasyurus from Adana and Hatay provinces located in Eastern Mediterranean Region of Turkey. The present study is first to report natural infection of L. infantum, L. major and L. tropica in small wild rodents in Turkey, suggesting their possible roles as reservoirs. Further studies are needed for planning epidemiological studies and also for developing rodent control measures in risky endemic areas to break the transmission cycle.
Key words: Leishmaniasis, L. infantum, L. major, L. tropica, reservoir animal, wild rodents
Introduction
Both visceral (VL) and cutaneous leishmaniasis (CL) are endemic diseases in several countries located in Mediterranean Basin as well as in Turkey (Dujardin et al., 2008). Each year around 2000 CL cases are officially reported in Turkey and more than 95% of them were from nine endemic provinces mainly located in Mediterranean and Southeastern Regions (Gürel et al., 2012). Leishmania infantum MON-1 is the main causative agent for zoonotic human and canine visceral leishmaniasis while anthroponotic L. tropica is the main species causing CL in all regions of Turkey. Besides these agents, L. tropica and L. donovani in VL patients while L. infantum, L. major and L. donovani in CL patients were also reported (Koltas et al., 2014; Özbilgin et al., 2019).
Dogs are considered as main reservoirs of L. infantum in Europe as well as in Turkey. So far, canine leishmaniasis (CanL) and reservoir role of the dogs have been studied comprehensively in different VL endemic areas of Turkey. The prevalence of CanL was detected as between 1.45% and 27.5% according to the endemic area (Toz et al., 2009). The possible role of the cats in the transmission of leishmaniasis is another debating topic in endemic countries. The presence of Leishmania DNA is not the conclusive finding to incriminate cats as a reservoir even though their infectiousness to sand fly was shown (Maroli et al., 2007). Up to day, several studies performed to screen Leishmania spp. among cats in Turkey and the positivity were ranging from 7% to 33.3% (Paşa et al., 2015; Can et al., 2016; Karakuş et al., 2019). The possible role of the rodents was also investigated in Italy by sampling the spleen and various tissues and of the sampled rodents, 45% (n = 20) were found to be infected with L. infantum (Di Bella et al., 2003). The presence of L. infantum in natural rodent populations was also investigated in Portugal and the infection rate was found to be 33.3% (Helhazar et al., 2013).
There are several studies performed in the Old-World countries to investigate the role of rodents in the Leishmania transmission cycle especially for zoonotic cutaneous leishmaniasis (ZCL) caused by L. major. Due to the high endemicity of ZCL in Iran, numerous studies related to rodents were performed. The first study was done in the 1950s by Ansari and Mofidi in Iran and followed by several reports up to today stating their importance in the transmission of ZCL caused by L. major (Ansari and Mofidi, 1950; Akhoundi et al., 2013). Leishmania tropica was also demonstrated to infect the Ethiopian wild rodent populations (Kassahun et al., 2015).
According to those findings, four different Leishmania species present in Turkey was reported to be found in sampled tissues of rodents in different countries. The prevalence rate of Leishmania in various populations of humans, dogs and even cats was investigated in Turkey. However, yet no study was performed to screen leishmaniasis infection in small wild rodents even though at least 65 species are present belonging to seven families in Turkey (Kryštufek and Vohralík, 2005, 2009; Yiğit et al., 2006, 2016; Wilson et al., 2016, 2017). Therefore, the potential role of rodents in the Leishmania infection cycle was investigated in the present study.
Materials and methods
Sample collection and DNA preparation
The samples collected during epidemiological Hantavirus studies in various provinces of Turkey were used in the present study. For these studies, the wild rodents were collected using Sherman live traps placed with consecutively 10–15 m intervals, and placement points were recorded by GPS. Species identification of rodents was performed according to the phenotypic characteristics of the animals. After taking body measurement of animals, they were sacrificed by cervical luxation method, and tissue samples were preserved in formalin. A total of 906 different tissue samples (spleen, liver, and lung) obtained from 712 rodents caught from 29 provinces were screened. The samples were collected from the individuals belonging to 23 rodent genera and were transferred to Leishmania Laboratory in the Department of Parasitology, Faculty of Medicine of Ege University for molecular screening studies to detect the Leishmania DNA. Samples obtained from Apodemus 431 (47.5%), 68 (7.5%) Mus sp., 68 (7.5%) Myodes sp., 55 (6.0%) Spalax sp., 51 (5.6%) Nannospalax sp., 45 (4.9%) Microtus sp., 42 (4.6%) Meriones sp. and 32 (3.5%) Rattus sp. samples were enrolled in the study. These eight genera made up most of the total samples (87.1%).
Before the DNA extractions, all tissue samples were first cut in small pieces to speed up the evaporation step and dried using Eppendorf Concentrator™ (Thermo Scientific, UK) to remove the formalin. Dried tissue samples were rehydrated using 200-μL of Qiagen® tissue lysis buffer (Qiagen GmbH, Hilden, Germany) and Proteinase K (20-μL) and incubated at 56°C overnight. Tissue samples were later transferred to tubes containing ZR Bashing Bead™ tubes (Zymo Research Corp., USA) and homogenated using Magna Lyser (Roche Molecular Diagnostics, Germany) at 7000 g for 90 s. Homogenates were used in DNA extractions following the manufacturer's instructions of DNeasy Blood & Tissue Kit (Qiagen GmbH).
Realtime kDNA PCR and realtime ITS1 PCR
The detection of Leishmania in rodent tissues and species determination was performed with a combination of Realtime kDNA and ITS1 PCR protocols. The first step was to screen the presence of Leishmania in tissue samples and due to its high copy number, the minicircle region of the kDNA was targeted using genus-specific primers (JW11/JW12) as reported in recent studies (Kassahun et al., 2015). A Realtime PCR was performed using SYBR Green I Master Kit (Roche Diagnostic, France) and positivity determined according to genus-specific melt peaks.
Leishmania spp. positive samples were used in a second step PCR, which was performed to determine the species of the causative agent. Primers targeting the ITS1 region rRNA gene (LITSR/ITS1R-TR1) was used and species determination were made in Leishmania positive samples using melt analysis. A Real-time PCR protocol was applied by the previously published paper (Toz et al., 2013).
Results
During the years of 2005–2017, 906 tissue samples (spleen, liver, and lung) were obtained from 712 rodent species belonging to 23 genera and 41 species. Sampling was done in 73 different locations and samples were divided according to their geographical locations. The majority of animals (56%) were obtained from urban residential locations in CL endemic areas. Based on the sampled rodents, the majority of them (n = 432; 47.6%) were belonging to Apodemus genera. The mean of whole-body length was 188 mm with a range of 171–220 mm.
Of the analysed animal samples, eight (8/712; 1.12%) of them were found to be positive for Leishmania spp. DNA by realtime kDNA-PCR (Suppl Fig. 1). The species typing by real-time ITS1 PCR revealed five L. infantum, two L. tropica and one L. major among the positive samples (Suppl Fig. 2).
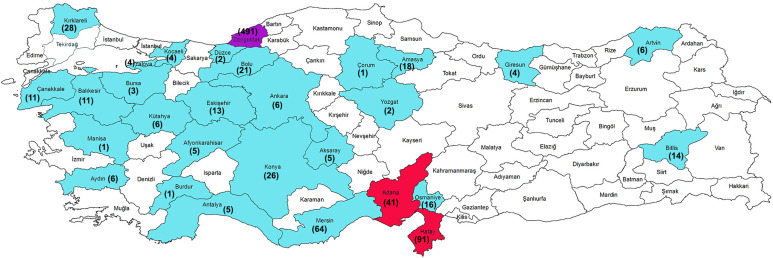
Leishmania major DNA was detected in the specimen belonging to Apodemus genus from Zonguldak province located in the Western Black Sea Region of Turkey. Leishmania infantum DNA was detected in three different rodent species, Apodemus filavicollis, Apodemus mystacinus and Apodemus sp. (four positive rodent specimens could not be detected in species level) from the same province, Zonguldak. Leishmania tropica DNA was detected in Gerbillus dasyurus (the mean of whole-body length was 84 mm with a range of 73–94 mm) from Hatay province and Meriones tristrami (the mean of whole-body length was 120 mm with a range of 100–155 mm) from Adana province. Both provinces are located in the Eastern Mediterranean Region (Table 1; Figure 1). Leishmania infantum positive rodents were all male while L. major and L. tropica positives were female.
Table 1.
The locations where eight positive small wild rodents were caught and PCR results
| No | Sample Code | Province | Town / Village | Long/Lat | Altitude | Total Samples (Province/Town-Village) | Rodent species | Tissue | Real time kDNA PCR | Real time ITS1 PCR |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8604 | Adana | Yumurtalık | 36.772877; 35.789730 | 18 | 41 / 8 | Meriones tristrami | Spleen | POS | L. tropica |
| 2 | 7780 | Hatay | Kırıkhan/KaletepeKöyü | 36.651196; 36.554549 | 226 | 90 / 3 | Gerbillus dasyurus | Spleen | POS | L. tropica |
| 3 | 689 | Zonguldak | Kozlu/OlukyanıKöyü | 41.389786; 31.778748 | 365 | 491 / 88 | Apodemus sp. | Spleen | POS | L. major |
| 4 | 640 | Zonguldak | Kozlu/OlukyanıKöyü | 41.389786; 31.778748 | 365 | 491 / 88 | Apodemus sp. | Spleen | POS | L. İnfantum |
| 5 | 311 | Zonguldak | Kozlu/Değirmenağzı | 41.418801; 31.722165 | 43 | 491 / 15 | Apodemus mystacinus | Spleen | POS | L. infantum |
| 6 | 399 | Zonguldak | Merkez/Kurtköy | 41.493872; 31.985017 | 185 | 491 / 70 | Apodemus flavicollis | Spleen | POS | L. infantum |
| 7 | 359 | Zonguldak | Çaycuma/TemenlerKöyü | 41.518497; 32.065252 | 170 | 491 / 18 | Apodemus sp. | Spleen | POS | L. infantum |
| 8 | 328 | Zonguldak | Çaycuma/SarmaşıkKöyü | 41.547210; 32.122129 | 80 | 491 / 73 | Apodemus sp. | Spleen | POS | L. infantum |
Fig. 1.
The map showing sampling provinces with negative (Blue) and positive results (Red: L. tropica; Purple: L. infantum and L. major DNA positive rodents). Numbers in the bracelets are showing the samples number for each province.
Discussion
Small rodent species in The New World and The Old World are suspicious reservoir hosts for different Leishmania species. Despite the important roles of small rodents for spreading of leishmaniasis, the studies are relatively in low number and mainly carried out in the Middle East and North Africa countries such as Iran, Tunisia, Morocco and Algeria where L. major is the dominant causative agent for CL (Ghawar et al., 2011; Echchakery et al., 2017; Foroutan et al., 2017; Pourmohammadi et al., 2017; Othman et al., 2018). Also, some reports have indicated the infection to L. donovani, L. infantum and L. tropica in some rodents such as Gerbillus spp., Rattus norvegicus and Mus musculus (Helhazar et al., 2013; Kassahun et al., 2015; Navea-Perez et al., 2015) suggesting their potential role to act as a reservoir for another Leishmania species.
The comprehensive studies on canine leishmaniasis were done for many years in Turkey, and results are always showing higher prevalence than human infection in VL endemic areas. Leishmania infantum was found as the main causative agent of CanL as well as human VL in these areas (Toz et al., 2013; Sarı et al., 2015). Because of Leishmania species having zoonotic life cycle have been found in Turkey and no study was carried out on rodents so far (Koltas et al., 2014; Özbilgin et al., 2016), it was necessary to conduct studies for revealing the reservoir potential of the small wild rodents. In the present study, we have investigated the presence of Leishmania DNA in the tissue samples collected from different rodent species in different provinces of Turkey using molecular techniques. Since we had rodent tissue samples that were previously collected and kept in formalin, the microscopical examination of their smears could not be performed for these samples. Our results revealed the presence of two Leishmania species (L. infantum, L. major) in the collected Apodemus spp. Specimens from one province, Zonguldak located in the Western Black Sea Region of Turkey. DNA of L. tropica was also found in two specimens (Meriones tristrami and Gerbillus dasyurus) from two different provinces, Adana and Hatay, located in the East Mediterranean Region of Turkey.
Although L. tropica is known as anthroponotic species, it is found in different mammalian species as dogs, cats and wild mammals, which play a reservoir role in nature (Töz et al., 2013; Paşa et al., 2015; Echchakery et al., 2017; Baneth et al., 2017). However, confirmation of these hosts as reservoirs requires xenodiagnostic studies as performed in Italy and Spain for L. infantum (Maroli et al., 2007; Jimenez et al., 2014). Adana and Hatay provinces are highly endemic areas for CL caused by L. tropica and L. infantum (Serin et al., 2005), and recently L. major and L. donovani were also reported as causative agents in both provinces (Koltas et al., 2014; Özbilgin et al., 2019). According to the Ministry of Health records, a total of 616 and 207 CL cases were reported from Adana and Hatay provinces between 2013 and 2017, respectively (Özbel Y, personal comm). In a recent study, 21 out of 25 strains (84%) from Adana province were detected as L. tropica, and there were only two isolates from Hatay province and one of them is also found as L. tropica by Realtime ITS1 PCR (Özbilgin et al., 2019). Dog studies carried out in these provinces revealed a high prevalence of CanL as 27.18% by IFAT and 41.74% by conjunctival swap nested PCR in rural areas of Adana (Karakuş et al., 2015). We analyzed 41 and 90 rodent tissue samples from Adana and Hatay, respectively. Nineteen out of 41 were from Meriones tristrami, caught in five different towns, and one (5.26%; 1/19) of them was found positive for L. tropica. For Hatay province, 12 out of 90 samples from Gerbillus dasyurus, caught in four different towns, and one (8.33%; 1/12) of them was found positive for L. tropica. Although the number of samples included in the present study is not very high, the detection of L. tropica in small rodents in Adana and Hatay provinces will make the control of CL more complicated in these endemic areas. Applying control measures for small wild mammals are more difficult because of the geographical and climatic conditions in the area. More studies are needed to understand better its epidemiological importance. Hatay and Adana provinces are among the six highly endemic provinces in Turkey. Because of four Old World Leishmania species causing CL and suitable vector sand fly species (Alten et al., 2015) are present in both provinces, small wild rodents need to be included to the epidemiological studies related to reservoirs planned in these areas.
Among 65 rodent species recorded in Turkey (Kryštufek and Vohralík, 2005, 2009; Yiğit et al., 2006, 2016; Wilson et al., 2016, 2017), six of them belonging to genus Meriones (M. persicus, M. tristrami, M. vinogradovi, M. crassus, M. libycus, M. dahli) playing important role as reservoir animal in different countries (Saliba and Oumeish, 1999), are available in most regions of Turkey except Black Sea and Marmara regions (Yiğit et al., 2006). One endemic species classified in Acomys genus (A. cilicicus) lives only around Silifke and Erdemli towns of Mersin province (Çetintaş et al., 2017), where CL caused by L. tropica is endemic. The wide-spreading of these small wild mammals in many areas enhancing the risk for human and canine leishmaniasis, and keeping the circulation of the parasite in nature.
Leishmaniasis tropica was detected in five (0.85%) out of 586 specimens belonging to Gerbillus genus by molecular techniques in Ethiopia (Kassahun et al., 2015). We also found L. tropica DNA in Gerbillus dasyurus collected from Hatay province, where CL caused by L. tropica and L. infantum is highly endemic (Table 1).
In Central Tunisia, it was reported that the prevalence of L. major in Psammomys obesus and Meriones shawi species ranged from 5% to 33% according to the methods used (Ghawar et al., 2011). Three Leishmania species (L. infantum, L. major, L. tropica) coexist in Morocco and the small rodent Meriones shawi is found to be a rodent reservoir for L. major (Kahime et al., 2014). In a recent study carried out in western Morocco, 18 (9.13%) out of 197 animals belonging to ten species were found positive for Leishmania DNA. Sixteen (six Rattus rattus, nine Mus musculus, and one Rattus norvegicus) and two (Mus musculus) positive samples were identified as L. infantum and L. tropica, respectively (Echchakery et al., 2017). In the present study, we used Realtime kDNA PCR for Leishmania DNA detection and a second Realtime PCR targeting ITS1 region was performed only for positive samples. Eight positives (0.88%) were found among 906 samples. Only one Meriones specimen (M. tristami) was found positive while none of the Mus and Rattus specimens was found positive (Table 1).
Besides dogs are the main reservoirs for L. infantum, CanL itself is an important veterinary health problem in southern European countries. The studies on the reservoir potential of rodents for leishmaniasis are very limited in European countries.
In Greece, 97 small rodents in urban and rural areas were collected and liver, spleen, and blood samples were taken for investigation by parasitological, serological, and molecular techniques. Sixteen (16/66; 24%) Mus musculus and 3 (3/12; 25%) Rattus rattus were found positive by molecular and serological tests while they were negative in smears. The researchers pointed out that the parasite load could be very low in the tissues (Tsakmakidis et al., 2017). In Portugal, the presence of L. infantum DNA in M. musculus and R. norvegicus with infection rates of 33.3% in both animals was reported. The authors pointed out that Leishmania DNA was found especially in the skin samples taken from the ear lobe and this was a suitable place for sand flies to get infected (Helhazar et al., 2013). In a similar study in Italy, the positivity rate was reported as 15.5% among 78 captured R. rattus samples in Montecristo island (Zanet et al., 2014). In our study, among 100 specimens belonging to Mus and Rattus genera, all of them were found negative. Because of 56% of these animals were from urban sites of CL endemic areas, the possibility to detect Leishmania DNA was very low. On the other hand, the rodent species of both genera were found positive in different countries, are very wild spread in all regions of Turkey, and this should be considered for the risk estimations and management. In rural areas, considering that the rodent burrows are one of the most suitable living and breeding places for sand flies, it should be known that the parasite is constantly circulated in the environments that can allow limited interventions, and it has a zoonotic cycle among small rodents.
In a study conducted in Granada, Spain, the tissue samples (liver, spleen, blood, skin and bone marrow) from 37 rodent specimens were investigated and ten (27%) animals were found positive by parasitological and/or molecular techniques. Besides three R. rattus and two M. musculus specimens, five Apodemus sylvaticus were also reported as positive (Navea-Perez et al., 2015). In the present study, 431 Apodemus spp. (62 A. mystacinus) were included in the analysis and only 66 (44 A. mystacinus) of them were from CL endemic areas. However, five L. infantum and one L. major positive Apodemus sp. samples were from CL non-endemic but VL sporadic province, Zonguldak. Only eight CL cases were reported from this province in the last 22 years (species and origin of the cases were unclear, Özbel Y, personal comm) while sporadic human VL cases have been reporting for decades. The prevalence of CanL was also reported as 8% from that region previously (Özbel et al., 2002). In Turkey as in other endemic countries, VL circulation among humans continues together with higher percentages of CanL. Therefore, this area is accepted as endemic for CanL. The positivity rate was 1.39% (6/431) in total and 1.70% (6/351) among the samples collected only in Zonguldak province suggesting Apodemus species probably has a role as reservoir animal of Leishmania spp. in the region; even it needs to be supported by further studies such as isolation of the parasites and experimental studies for showing reservoir potentials.
Concluding remarks
This study reports for the first time in Turkey, the natural infection of L. infantum, L. major and L. tropica in small wild rodents, suggesting these animals possibly have a role as reservoirs in the life cycle of all three Leishmania species in Turkey. The molecular detection of three Leishmania species in the present study in highly endemic areas for CL such as Adana and Hatay provinces and also in an endemic area for VL such as Zonguldak shows the possible zoonotic cycle including small wild rodents in addition to the zoonotic cycle of L. infantum in the dogs. Infected rodents with Leishmania may increase the risk not only for humans but also for dogs in rural and urban areas. And this suggests the possible involvement of other wide-spread rodent species in the studies that will be carried out in other endemic areas in Turkey. Further studies are needed not only for planning epidemiological studies but also for developing rodent control measures in potential endemic areas to break the transmission cycle.
Financial support
This study is supported by The Scientific and Technological Research Council of Turkey (TÜBİTAK) Project No: 114S999.
Ethical standards
Ethical approval was obtained from Dokuz Eylül University Animal Ethical Committee under the registration number of 2011-24-28/12 at the date of December 28, 2011.
Conflicts of interest
The authors declare there are no conflicts of interest.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182020000803.
click here to view supplementary material
References
- Akhoundi M, Mohebali M, Asadi M, Mahmodi MR, Amraei K and Mirzaei A (2013) Molecular characterization of Leishmania spp. in reservoir hosts in endemic foci of zoonotic cutaneous leishmaniasis in Iran. Folia Parasitologia (Praha) 60, 218–224. [DOI] [PubMed] [Google Scholar]
- Alten B, Maia C, Afonso O, Campino L, Jimenez M, Gonzalez E, Molina R, Banuls AL, Prudhomme J, Vergnes B, Toty C, Cassan C, Thierry M, Sereno D, Bongiorno G, Bianchi R, Khouty C, Tsirigotakis N, Dokianakis E, Antoniou M, Christodoulou V, Mazeris A, Karakus M, Ozbel Y, Arserim SK, Erisoz Kasap O, Gunay F, Oguz G, Kaynas S, Tsetsvadze N, Tskhvaradze L, Giorgobiani E, Gramiccia M, Volf P and Gradoni L (2015) Seasonal dynamics of phlebotomine sand fly species proven vectors of Mediterranean leishmaniasis caused by Leishmania infantum. PLOS Neglected Tropical Diseases 10, e0004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari N and Mofidi S (1950) Contribution l’étude des forms humides de leishmaniose cutané. Bulletin de la Societe de PathologieExotique 43, 601–607. [Google Scholar]
- Baneth G, Yasur-Landau D, Gilad M and Nachum-Biala Y (2017) Canine leishmaniosis caused by Leishmania major and Leishmania tropica: comparative findings and serology. Parasites and Vectors 10, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can H, Döşkaya M, Özdemir HG, Şahar EA, Karakavuk M, Pektaş B, Karakuş M, Töz S, Caner A, Döşkaya AD, İz SG, Özbel Y and Gürüz Y (2016) Seroprevalence of Leishmania infection and molecular detection of Leishmania tropica and Leishmania infantum in stray cats of İzmir, Turkey. Experimental Parasitology 167, 109–114. [DOI] [PubMed] [Google Scholar]
- Çetintaş O, Matur F and Sözen M (2017) Distribution and conservation of Acomys cilicicus (mammalia: rodentia) in Turkey. Turkish Journal of Zoology 41, 1059–1068. [Google Scholar]
- Di Bella C, Vitale F, Russo G, Greco A, Milazzo G, Aloise G and Cagnin M (2003) Are rodents a potential reservoir for Leishmania infantum in Italy? Journal of Mountain Ecology 7, 125–129. [Google Scholar]
- Dujardin JC, Campino L, Canavate C, Dedet JP, Gradoni L, Soteriadou K, Mazeris A, Özbel Y and Boelaert M (2008) Spread of vector-borne diseases and neglect of leishmaniasis, Europe. Emerging Infectious Diseases 14, 1013–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echchakery M, Chicharro C, Boussaa S, Nieto J, Carrillo E, Sheila O, Moreno J and Bouzmezzough A (2017) Molecular detection of Leishmania infantum and Leishmania tropica in rodent species from endemic cutaneous leishmaniasis areas in Morocco. Parasites and Vectors 10, 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forotuan M, Khademvatan S, Majidiani H, Khalkhali H, Hedayati-Rad F, Khashaveh S and Mohammadzadeh H (2017) Prevalence of Leishmania species in rodents: a systematic review and meta-analysis in Iran. ActaTropica 172, 164–172. [DOI] [PubMed] [Google Scholar]
- Ghawar W, Toumi A, Snoussi MA, Chlif S, Zaatour A, Boukthir A, Hamida NB, Chemkhi J, Diouani MF and Ben-Salah A (2011) Leishmania major infection among Psammomys obesus and Meriones Shawi: reservoirs of zoonotic cutaneous leishmaniasis in Sidi Bouzid (central Tunisia). Vector Borne and Zoonotic Diseases 11, 1561–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gürel MS, Yeşilova Y, Olgen MK and Ozbel Y (2012) Cutaneous leishmaniasis in Turkey. Türkiye Parazitoloji Dergisi 36, 121–129, Review, in Turkish. [DOI] [PubMed] [Google Scholar]
- Helhazar M, Leitao J, Duarte A, Tavares L and da Fonseca IP (2013) Natural infection of synathropic rodent species Mus musculus and Rattus norvegicus by Leishmania Infantum in Sesimbra and Sintra – Portugal. Parasites and Vectors 6, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez M, Gonzalez E, Martin-Martin I, Hernandez S and Molina R (2014) Could wild rabbits (Oryctolagus cuniculus) be reservoirs for Leishmania Infantum in the focus of Madrid, Spain. Veterinary Parasitology 202, 296–300. [DOI] [PubMed] [Google Scholar]
- Kahime K, Boussaa S, Bounoua L, Ouanaimi F, Messouli M and Boumezzough A (2014) Leishmaniasis in Morocco: diseases and vectors. Asian Pacific Journal of Tropical Diseases 4, S530–S534. [Google Scholar]
- Karakuş M, Töz S, Ertabaklar H, Paşa S, Atasoy A, Arserim SK, Ölgen MK, Alkan MZ, Durant C and Özbel Y (2015) Evaluation of conjunctival swab sampling in the diagnosis of canine leishmaniasis: a two-year follow-up study in Çukurova plain, Turkey. Veterinary Parasitology 214, 295–302. [DOI] [PubMed] [Google Scholar]
- Karakuş M, Arserim SK, Erişöz Kasap Ö, Pekağırbaş M, Aküzüm D, Alten B, Töz S and Özbel Y (2019) Vector and reservoir surveillance study in a canine and human leishmaniasis endemic area in most western part of Turkey, Karaburun. Acta Tropica 190, 177–182. [DOI] [PubMed] [Google Scholar]
- Kassahun A, Sadlova J, Dvorak V, Kostalova T, Rohousova I, Frynta D, Aghova T, Yasur-Landau D, Lemma W, Hailu A, Baneth G, Warburg A, Volf P and Votypka J (2015) Detection of L. donovani And L. tropica In Ethiopian wild rodents. Acta Tropica 145, 39–44. [DOI] [PubMed] [Google Scholar]
- Koltas IS, Eroglu F, Alabaz D and Uzun S (2014) The emergence of Leishmania major and Leishmaniadonovani in southern Turkey. Transactions of the Royal Society of Tropical Medicine and Hygiene 108, 154–158. [DOI] [PubMed] [Google Scholar]
- Kryštufek B and Vohralík V (2005) Mammals of Turkey and Cyprus (Rodentia Volume I: Sciuridae, Dipodidae, Gliridae, Arvicolinae). Koper: Annales Majora, pp. 149–162. [Google Scholar]
- Kryštufek B and Vohralík V (2009) Mammals of Turkey and Cyprus (Rodentia Volume II: Cricetinae, Muridae, Spalacidae, Calomyscidae, Capromyidae, Hystricidae, Castoridae), Koper: Annales Majora, pp. 71–252. [Google Scholar]
- Maroli M, Pennisi MG, Di Muccio T, Khoury C, Gradoni L and Gramiccia M (2007) Infection of sandflies by a cat naturally infected with Leishmania infantum. Veterinary Parasitology 145, 357–360. [DOI] [PubMed] [Google Scholar]
- Navea-Perez HM, Diaz-Saez V, Corpas-Lopez V, Merino-Espinosa G, Morillas-Marquez F and Martin-Sanchez J (2015) Leishmania Infantum in wild rodents: reservoirs or just irrelevant incidental hosts? Parasitology Research 114, 2363–2370. [DOI] [PubMed] [Google Scholar]
- Othman B, Ghawar W, Chaouch M, Ayari C, Chemkhi J, Cancino-Faure B, Tomas-Perez M, Alcover MM, Riera C, Ben-Salah A, Fisa R, Ben-Ismail R and Ben-Abderrezzak S (2018) First detection of leishmania DNA in Psammomys obesus and Psammomys vexillaris: their potential involvement in the epidemiology of leishmaniasis in Tunisia. Infection. Genetics and Evolution 59, 7–15. [DOI] [PubMed] [Google Scholar]
- Özbel Y, Turgay N, Alkan MZ, Babaoğlu A, ÖzensoyTöz S and Babalıoğlu N (2002) A focus of zoonotic visceral leishmaniasis in West Black Sea Region of Turkey: Karabuk. Türkiye Parazitoloji Dergisi 26, 362–366. [Google Scholar]
- Özbilgin A, Çulha G, Uzun S, Harman M, GünaştıTopal S, Okudan F, Zeyrek F, Gündüz C, Östan İ, Karakuş M, Töz S, Kurt Ö, Akyar I, Erat A, Güngör D, Kayabaşı Ç, Çavuş İ, Bastien P, Pratlong F, Kocagöz T and Özbel Y (2016) Leishmaniasis in Turkey: first clinical isolation of Leishmania major from 18 autochthonous cases of Cutaneous Leishmaniasis from four geographical regions of Turkey. Tropical Medicine & International Health 21, 783–791. [DOI] [PubMed] [Google Scholar]
- Özbilgin A, Töz S, Harman M, Günaşti Topal S, Uzun S, Okudan F, Güngör D, Erat A, Ertabaklar H, Ertuğ S, Gündüz C, Çavuş İ, Karakuş M, Östan Ural İ, Ölgen MK, Kayabaşi Ç, Kurt Ö and Özbel Y (2019) The current clinical and geographical situation of cutaneous leishmaniasis based on species identification in Turkey. ActaTropica 190, 59–67. [DOI] [PubMed] [Google Scholar]
- Paşa S, Tetik Vardarlı A, Erol N, Karakuş M, Töz S, Atasoy A, Balcıoğlu İC, Emek Tuna G, Ermiş ÖV, Ertabaklar H and Özbel Y (2015) Detection of Leishmania major and Leishmania tropica in domestic cats in the Ege region of Turkey. Veterinary Parasitology 212, 389–395. [DOI] [PubMed] [Google Scholar]
- Pourmohammadi B, Mohammadi-Azni S and Kalantari M (2017) Natural infection of Nesokia indica with leishmania major and Leishmania infantum parasites in Damghan city, Northern Iran. Acta Tropica 170, 134–139. [DOI] [PubMed] [Google Scholar]
- Saliba EK and Oumeish OY (1999) Reservoir hosts of cutaneous leishmaniasis. Clinics in Dermatology 7, 275–277. [DOI] [PubMed] [Google Scholar]
- Sarı B, Limoncu ME, Balcıoğlu İC, Aldemir A, Tasçı GT, Kiliç Y, Toz S, Demirci B, Demir S, Erisoz Kasap O, Olgen MK and Ozbel Y (2015) Seroepidemiological and entomological survey in a new focus of zoonotic visceral leishmaniasis in Kars province, northeastern Turkey. Veterinary Parasitology 209, 179–187. [DOI] [PubMed] [Google Scholar]
- Serin MS, Daglioglu K, Bagirova M, Allahverdiyev A, Uzun S, Vural Z, Kayar B, Tezcan S, Yetkin M, Aslan G, Emekdas G and Koksal F (2005) Rapid diagnosis and genotyping of Leishmania Isolates from cutaneous and visceral leishmaniasis by microcapillary cultivation and polymerase chain reaction-restriction fragment length polymorphism of Miniexon region. Diagnostic Microbiology and Infectious Diseases 53, 209–214. [DOI] [PubMed] [Google Scholar]
- Toz SO, Nasereddin A, Ozbel Y, Ertabaklar H, Culha G, Sevil N, Alkan MZ and Jaffe CL (2009) Leishmaniasis in Turkey: molecular characterization of Leishmania From human and canine clinical samples. Tropical Medicine and International Health 14, 1401–1406. [DOI] [PubMed] [Google Scholar]
- Toz SO, Culha G, Zeyrek FY, Ertabaklar H, Alkan MZ, Vardarlı AT, Gunduz C and Ozbel Y (2013) A real-time ITS1-PCR based method in the diagnosis and species identification of leishmania parasite from human and dog clinical samples in Turkey. PLOS Neglected Tropical Diseases 7, e2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakmakidis Ι, Angelopoulou K, Dovas CI, Dokianakis Ε, Tamvakis Α, Symeonidou I, Antoniou Μ and Diakou Α (2017) Leishmania infection in rodents in Greece. Tropical Medicine and International Health 22, 1523–1532. [DOI] [PubMed] [Google Scholar]
- Wilson DE, Lacher TE, Jr. and Mittermeier RA (2016) Handbook of the Mammals of the World, vol 6. Barcelona: Lagomorphs and Rodents I. Lynx Edicion. [Google Scholar]
- Wilson DE, Lacher TE, Jr. and Mittermeier RA (2017) Handbook of the Mammals of the World, vol 7. Barcelona: Rodents II. Lynx Edicion. [Google Scholar]
- Yiğit N, Çolak E, Sözen M and Karataş A (2006). Rodents of Türkiye: Türkiye Kemiricileri, 1st Edn. Ankara: Meteksan Yayınevi. [In Turkish]. [Google Scholar]
- Yiğit N, Çolak E and Sözen M (2016) A new species of voles, Microtus elbeyli sp. nov., from Turkey with taxonomic overview of social voles distributed in southeastern Anatolia. Turkish Journal of Zoology 40, 73–79. [Google Scholar]
- Zanet S, Sposimo P, Trisciuoglio A, Giannini F, Strumia F and Ferroglio E (2014) Epidemiology of Leishmania Infantum, Toxoplasma Gondii, and Neospora caninum in Rattus Rattus in absence of domestic reservoir and definitive hosts. Veterinary Parasitology 199, 247–249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182020000803.
click here to view supplementary material