Abstract
Angiostrongylus vasorum, Crenosoma vulpis and Capillaria aerophila are the most common lungworms of domestic and wild canids. We investigated the short- and long-term lungworm prevalence changes in the Swiss fox population with a focus on A. vasorum. Between 2012 and 2017, lungs and hearts of 533 foxes from north-eastern Switzerland were necropsied and blood samples tested for circulating A. vasorum antigen. Angiostrongylus vasorum prevalence increased steadily from 21.5% in 2012 to 81.8% in 2017. In contrast, C. aerophila and C. vulpis prevalences fluctuated between 41.8 and 74.7%, and 3.6 and 14.9%, respectively. Based on 3955 blood samples collected between 1986 and 2017 from three geographic areas and during four time periods, antigen seropositivity increased from 2.4 to 62.0%. In north-eastern Switzerland, seropositivity was initially low (1.9 and 1.7% in the first two time periods) but increased in the following two decades to 22.2 and 62.0%, respectively. Our findings depict the spectacular expansion of A. vasorum in the past three decades. Regionally, the prevalence in foxes increased 4-fold within 6 years in some regions. This underpins the important role of foxes as reservoir hosts, likely explaining the increasing number of cases of canine angiostrongylosis in Switzerland. Our findings are representative of central Europe and may help anticipating future developments in areas where A. vasorum is present but (still) infrequent.
Key words: Angiostrongylus vasorum, Capillaria aerophila, Crenosoma vulpis, fox, necropsy, nematode, prevalence, serology, Switzerland, worm burden
Introduction
Angiostrongylus vasorum, Crenosoma vulpis and Capillaria aerophila (syn. Eucoleus aerophilus) are the most common lungworms of domestic and wild canids in Europe (Saeed et al., 2006; Taylor et al., 2015; Tolnai et al., 2015; Hermosilla et al., 2017; Maksimov et al., 2017; Martinez-Rondan et al., 2019; Traversa et al., 2019). The red fox (Vulpes vulpes) represents the best known wildlife reservoir host for transmission of parasitic diseases to domestic dogs (Otranto et al., 2015). The cardiopulmonary nematode A. vasorum resides in the right heart and pulmonary arteries of infected definitive hosts; slugs and snails act as intermediate hosts (Guilhon and Cens, 1973). The parasite is endemic in Europe, South America and Newfoundland, Canada (Jefferies et al., 2010) and has also been reported from Uganda (Bwangamoi, 1972). Importantly, canine angiostrongylosis can manifest with respiratory signs, bleeding disorders and other clinical signs; they can be severe, and fatal in 10–15% of cases (Chapman et al., 2004; Koch and Willesen, 2009). Especially in Europe, where the parasite was first discovered (Serres, 1854; Cuillé and Darraspen, 1930), the occurrence of A. vasorum has been increasingly reported in the past decades, in known and in newly recognised endemic areas (Helm et al., 2015; Jolly et al., 2015; Lurati et al., 2015; Maksimov et al., 2017). For instance, in British and Danish fox populations, increasing A. vasorum prevalences have been observed between 2005–2006 and 2013–2014 (Morgan et al., 2008; Taylor et al., 2015) and between 1997–2002 and 2006–2008 (Saeed et al., 2006; Al-Sabi et al., 2014), respectively. However, infected foxes were also reported from countries without previous wildlife host reports (Demiaszkiewicz et al., 2014; Kistler et al., 2014).
Adult C. vulpis are found in the bronchi of canids. Like A. vasorum, this parasite requires gastropods as intermediate hosts (Wetzel, 1940). Capillaria aerophila infects canids, felids, mustelids and hedgehogs; adults reside in the trachea and bronchi of their definitive hosts and can be transmitted directly or via earthworms (Chandler, 1922; Christenson, 1938; Holmes and Kelly, 1973; Deplazes et al., 2016). Capillaria aerophila may cause zoonotic infections on rare occasions (Otranto and Deplazes, 2019). Crenosoma vulpis is endemic in Europe and North America and C. aerophila occurs worldwide (Conboy, 2009). These two parasites have been increasingly reported, but evidence for an actual spread to new areas is lacking, despite the partial overlapping spectrum of definitive and intermediate hosts with A. vasorum (Sreter et al., 2003; Saeed et al., 2006; Morgan et al., 2008; Al-Sabi et al., 2014; Taylor et al., 2015; Tolnai et al., 2015; Maksimov et al., 2017).
In foxes, infections with lungworms are mostly detected by necropsy and, less frequently, by examination of fecal samples processed by the Baermann technique or fecal flotation, or by DNA detection in bronchoalveolar fluid (Willingham et al., 1996; Houpin et al., 2016; Koller et al., 2019). ELISAs for detection of circulating A. vasorum antigen and specific antibodies, developed for dog samples (Schnyder et al., 2011; Schucan et al., 2012), were evaluated for their use in foxes (Gillis-Germitsch et al., 2017). The antibody response in foxes was highly variable, hence, the assay showed low sensitivity. Antigen detection, instead, revealed high sensitivity and specificity, and a positive correlation between worm burdens and optical density values. Since fox necropsies are time-consuming, while blood collection from dead animals is easy and fast, antigen detection was proposed as an efficient method for mass-screening, also in fox populations (Gillis-Germitsch et al., 2017).
The aim of this study was to investigate the short- and long-term lungworm prevalence changes in the Swiss fox population with a focus on the emergence of A. vasorum. To do so, lungs and hearts of foxes from north-eastern Switzerland were necropsied over 6 years and blood samples analysed for the presence of circulating A. vasorum antigen. Additionally, countrywide A. vasorum prevalence changes over a 30-year period of time were investigated in a retrospective analysis of Swiss fox blood samples collected between 1986 and 2017.
Material and methods
Fox lung and heart necropsy
Between 2012 and 2017, 533 red foxes (V. vulpes) were killed during hunting seasons in the frame of control and management measures of fox populations by game wardens and local hunting associations and brought to the Institute of Parasitology in Zurich, Switzerland. All animals originated from the north-east of Switzerland, which, based on defined biogeographic characteristics, is part of the Swiss Plateau (Gonseth et al., 2001). As of 2013, foxes were shot exclusively in the canton of Zurich. Five hundred foxes were killed in rural areas and 33 in the city of Zurich. The animals were necropsied in the first 2 months of the year: 79 in 2012 (of these, 39 foxes were necropsied in October), 87 in 2013, 42 in 2014, 88 in 2015, 83 in 2016 and 154 in 2017. Sex and age were determined [either ‘young’ (<12 months of age) or ‘adult’ (>20 months of age), according to tooth wear]. Lungs and hearts were removed in one piece and frozen at −20°C until further examination. Blood samples, collected directly during necropsy and/or recovered from hearts and lungs before dissection, were stored at −20°C for later mass-screening. As of 2014, the lungs were photographed and scored macroscopically from 0 to 3 according to macroscopic pathological changes. The classification key, adapted from Poli et al. (1991), was as follows: 0 = healthy lung without pathological changes (no discolouration, regular tissue consistency, no adhesions); 1 = mild changes (discolouration, regular tissue consistency, no adhesions); 2 = moderate pathologies (discolouration, nodules and/or increased tissue consistency in lung lobes, no adhesions); and 3 = severe pathologies (discolouration, nodules and/or increased tissue consistency in all lung lobes, adhesions among lung lobes, with pericardium and/or mediastinum). After thawing at 4°C, lungs and hearts were transferred to a conical glass, which was filled with tap water. Ventricles, atria and pulmonary arteries were opened with surgical scissors. Then, bronchi, bronchioles and all visible blood vessels and airways were cut open, until the lung only consisted of a thin layer of tissue. During the dissection process, the organs were repeatedly washed in the water recipient. Lungs and hearts were then removed and parasites were allowed to settle down in the glass for at least 30 min. The supernatant was discarded, the sediment was transferred to a large petri dish and examined under a stereomicroscope. Parasites, if present, were counted; species and sex were determined based on Christenson (1935), Wetzel (1940) and Guilhon and Cens (1973).
Analysis of fox blood samples
Since 1986 foxes had been investigated in the frame of different projects and studies performed at the Institute of Parasitology in Zurich (Ewald, 1993; Hofer et al., 2000; Fischer et al., 2005; Tanner et al., 2006; Hegglin et al., 2007; Guerra et al., 2014). A total of 3955 blood samples were available from Swiss foxes, collected between 1986 and 2017, including samples from the above mentioned 533 foxes necropsied between 2012 and 2017. The samples were kept frozen at −20°C for several years until examination for this study. All samples were tested by ELISA for detection of circulating A. vasorum antigen as described in Schnyder et al. (2011) and cut-off values from fox studies performed by Gillis-Germitsch et al. (2017) were applied. To confirm that antigen can still be detected in old samples, we retested overall 63 8–13 years old stored blood samples from A. vasorum experimentally infected dogs and compared the resulting OD values.
In order to investigate prevalence changes over time, samples were assigned to four different periods of time: 1986–1992 (period 1, n = 1729), 1993–2002 (period 2, n = 1524), 2003–2012 (period 3, n = 249) and 2013–2017 (period 4, n = 453). The coordinates of hunting locations from 3704 foxes were used to create maps with the program Quantum GIS Version 3.6 Noosa (shown in Fig. 1); coordinates were missing for 251 samples. In addition, Fig. 1 displays the three regions from which samples were collected: the north-eastern part of Switzerland (encompassing the canton of Zurich) consisting of the Swiss Plateau and Prealps, the south-eastern part that comprises alpine areas and the southern part of the Alps, and the third area in the western part of Switzerland including different biogeographic regions (Swiss Plateau, Alps and Jura) (Gonseth et al., 2001).
Fig. 1.
Map of Switzerland showing three different areas and origin of foxes hunted between 1986 and 2017. Blood samples were tested for circulating Angiostrongylus vasorum antigen. The coordinates of 3704 out of 3955 fox blood samples were available: red dots (n = 446) indicate seropositive samples, white dots (n = 3258) negative samples.
Statistical analysis
Statistical analysis was performed in R (v. 3.5.1). For prevalence calculation, an animal was considered infected with A. vasorum based on antigen detection above cut-off (Gillis-Germitsch et al., 2017) and/or presence of worms at necropsy. Prevalences for C. vulpis and C. aerophila were calculated based on the necropsy results only. The exact binominal 95% confidence intervals (CI) were calculated according to Clopper and Pearson (1934). Relationships between A. vasorum infection status (‘infected’ or ‘uninfected’) and age (‘young’ or ‘adult’), sex, as well as the hunting years were analysed using a generalised linear (multivariate) model. The data were further stratified to produce odds ratios on significant model predictors. Foxes for which data on sex or age were missing (n = 30/533) were excluded from the analysis.
The relationships between lung scores reflecting pathological changes and A. vasorum, C. aerophila and C. vulpis worm burdens as well as hunting years, age and sex were analysed by ordinal logistic regression. Lung scoring data were missing for foxes hunted in 2012 and 2013. Foxes for which data on sex or age were missing (n = 11/367) were excluded from the analysis. Average worm burden and lung score differences between years were analysed using one-way ANOVA. Mean worm burdens stratified by age groups were tested in a non-parametric Wilcoxon rank test.
Results
Necropsy results
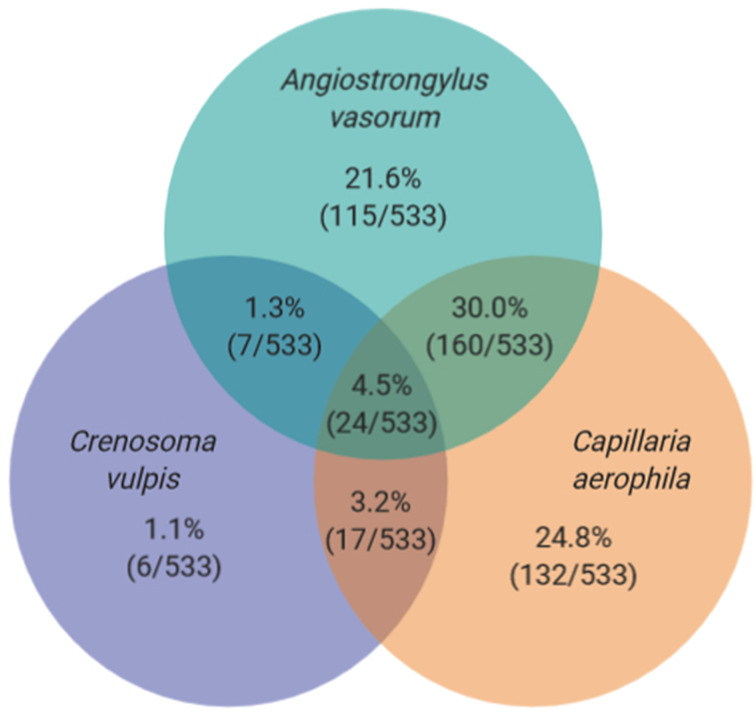
In total, 244 females, 275 males and 14 foxes for which the sex was not recorded were necropsied. Out of the hunted 533 foxes, 312 were adults and 191 were juveniles; the age of 30 foxes was not determined. Yearly lungworm prevalences for A. vasorum, C. vulpis and C. aerophila are displayed in Table 1. Of the 533 foxes, 72 did not have any lungworms, 253 were infected with one lungworm species, 184 with two species and 24 with all three (see Fig. 2). Overall, 3453 intact adult lungworms were recovered: 1572 A. vasorum (1002 females, 570 males), 270 C. vulpis (157 females, 113 males) and 1611 C. aerophila (854 females, 757 males). Exact hunting locations of foxes with the three lungworm species are displayed in Fig. 3a–c.
Table 1:
Prevalence and worm burden of Angiostrongylus vasorum, Crenosoma vulpis and Capillaria aerophila and circulating A. vasorum antigen detection in 533 necropsied foxes from Switzerland over six consecutive years (CI: confidence intervals).
| Year (number of foxes) |
2012 (n=79) |
2013 (n=87) |
2014 (n=42) |
2015 (n=88) |
2016 (n=83) |
2017 (n=154) |
Total (n=533) |
|---|---|---|---|---|---|---|---|
| Angiostrongylus vasorum | |||||||
| Necropsy positive (n) | 8 | 31 | 19 | 52 | 59 | 110 | 279 |
| Necropsy prevalence % (95% CI) | 10.1 (4.5–19.0) |
35.6 (25.6–46.6) |
45.2 (29.8–61.3) |
59.1 (48.1–69.5) |
71.1 (63.6–78.4) |
71.4 (63.6–78.4) |
52.3 (48.0–56.7) |
| Mean worm burden (range) | 7.1 (1–30) |
5.0 (1–18) |
5.0 (1–17) |
5.9 (1–42) |
8.5 (1–44) |
10.3 (1–126) |
8.4 (1–126) |
| Seropositive (n) | 16 | 32 | 18 | 56 | 57 | 118 | 297 |
| Seropositivity % (95% CI) | 20.3 (12.0–30.8) |
36.8 (26.7–47.8) |
42.9 (27.7–59.0) |
64.4* (53.4–74.4) |
68.7 (57.6–78.4) |
76.6 (69.1–83.1) |
55.8 (51.5–60.1) |
| Necropsy and seropositive (n) | 17 | 36 | 21 | 59 | 64 | 126 | 323 |
| Combined prevalence % (95% CI) | 21.5 (13.1–32.2) |
41.4 (30.7–52.9) |
50.0 (34.2–65.8) |
67.0 (56.2–76.7) |
77.1 (66.6–85.6) |
81.8 (74.8–87.6) |
60.6 (56.3–64.8) |
| Crenosoma vulpis | |||||||
| Necropsy positive (n) | 6 | 13 | 5 | 7 | 3 | 20 | 54 |
| Prevalence % (95% CI) | 7.6 (2.8–15.8) |
14.9 (8.2–24.2) |
11.9 (4.0–25.6 |
8.0 (3.3–15.7) |
3.6 (0.8–10.2) |
13.0 (8.1–19.3) |
10.1 (7.7–13.0) |
| Mean worm burden (range) | 2.7 (1–5) |
6.6 (1–33) |
3.3 (1–9) |
10.4 (1–48) |
1.3 (1–2) |
6.7 (1–49) |
6.3 (1–49) |
| Capillaria aerophila | |||||||
| Necropsy positive (n) | 33 | 65 | 24 | 65 | 54 | 94 | 335 |
| Prevalence % (95% CI) | 41.8 (30.8–53.4) |
74.7 (64.3–83.4) |
57.1 (41.0–72.3) |
73.9 (63.4–82.7) |
65.1 (53.8–75.2) |
61.0 (52.9–68.8) |
62.9 (58.6–67.0) |
| Mean worm burden (range) | 2.0 (1–6) |
5.7 (1–51) |
10.7 (1–99) |
9.2 (1–39) |
4.8 (1–30) |
4.5 (1–48) |
6.2 (1–99) |
Of one fox no blood samples were collected (and therefore no ELISA result).
Fig. 2.
Single and multiple infections with Angiostrongylus vasorum, Crenosoma vulpis and Capillaria aerophila of 533 necropsied Swiss foxes examined for lungworms. Seventy-two foxes (13.5%) were negative for lungworms.
Fig. 3.
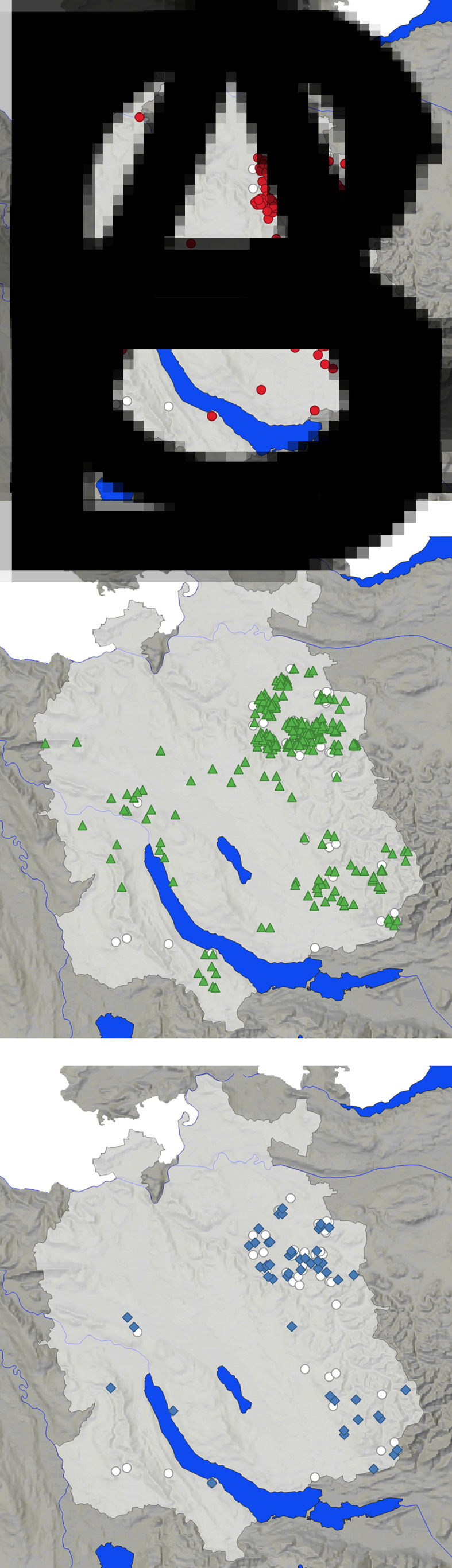
(a–c) Map of the canton of Zurich (light grey) showing the location of 475 foxes hunted and necropsied between 2012 and 2017 and examined for lungworms (of 58 foxes locations were not available). (a) Red dots: Angiostrongylus vasorum-positive foxes (n = 269); (b) green triangles: Capillaria aerophila-positive foxes (n = 313); (c) blue diamonds: Crenosoma vulpis-positive foxes (53). White dots: lungworm-free foxes (n = 53).
Between 2012 and 2017, based on necropsy, the prevalence of A. vasorum infection in the fox population within the study area increased steadily and significantly (P < 0.001) from 10.1% (8 out of 79; 95% CI 4.5–19.0) to 71.4% (110 out of 154; 95% CI 63.6–78.4) (Table 1). Differences in particular were significant between 2012 and 2014 (and the following years), between 2013 and 2015 (and the following years), and between 2014 and 2016 and 2017. The yearly arithmetic mean A. vasorum worm burden per infected animal increased by trend, especially between 2014 and 2017 (Fig. 4): on average, foxes harboured 5.0 adult A. vasorum specimens in 2014 (n = 19), 5.9 in 2015 (n = 52), 8.5 in 2016 (n = 59) and 10.3 in 2017 (n = 110) (P = 0.06). During the period under examination, neither the infection status [odds ratio (OR) = 1.19, P = 0.39] nor the average worm burden (P = 0.77) differed across age groups (young vs adult). Males were more often infected than females, however below the statistical significance level (OR = 1.32, P = 0.14). No worm burden differences were observed between sexes.
Fig. 4.
Angiostrongylus vasorum, Crenosoma vulpis and Capillaria aerophila worm burdens in 533 red foxes dissected between 2012 and 2017. Solid line: median worm burden; dashed line: mean worm burden.
Prevalences of C. vulpis and C. aerophila fluctuated each year. Capillaria aerophila prevalence ranged between 41.8 and 74.7% but the year-to-year variation did not show any trend (Table 1). Similarly, the mean worm burden oscillated between a minimum of 2.0 (2012) and a maximum of 10.7 (2014), and ranged from 1 to 99 specimens (Fig. 4). Male foxes tended to be more often infected with C. aerophila than females (OR = 2.5, P < 0.001) and with a higher worm burden (P < 0.01); there was no correlation between the infection status and age. Among C. aerophila-infected animals, the mean worm burden was not significantly different between both age groups (P = 0.27). Crenosoma vulpis was less commonly found, infecting between 3.6% (2016) and 14.9% (2013) of the fox population. Mean worm burdens fluctuated between 1.3 (2016) and 10.4 (2015), and ranged from 1 to 49 adult specimens (Fig. 4). Male foxes tended to be more often infected than females (OR = 4.4, P < 0.001), as did young animals (OR = 1.8, P = 0.06). Worm burdens were not different between sexes or age groups.
Macroscopic lung scores
A total of 366 lungs were scored according to their macroscopic appearance and were distributed as follows: score 0, n = 7; score 1, n = 39; score 2, n = 153; score 3, n = 167, indicating different degrees of pathological alterations. For illustrative images of lungs scoring 0–3, see Supplementary Fig. S1a–d. Among A. vasorum-infected foxes (n = 269), three scored 0 (1.1%), 17 scored 1 (6.3%), 85 lunges scored 2 (31.6%) and 164 lungs scored 3 (61.0%). Mean lung scores increased from 2.21 (±0.7) to 2.77 (±0.46) between 2014 and 2017 (P = 0.06). Angiostrongylus vasorum infection was significantly associated with high macroscopic lung scoring (P < 0.001). For every increase in A. vasorum worm burden, the odds of being more likely to show a higher lung score is multiplied by 1.21 (95% CI 1.15–1.28). For the other lungworms, there was no significant association.
Serological results
In line with necropsy data, prevalence rates determined by the antigen ELISA for foxes shot between 2012 and 2017 in the north-east of Switzerland increased year by year, from overall 20.3 to 76.6%. Differences were significant between 2012 and 2015 (and following years), between 2013 and 2015 (and following years) and between 2014 and 2017. The comparison between necropsy and serological results showed that both procedures were in agreement (Table 1). Combined necropsy and serology results lead to an increasing prevalence from 21.5% (17 out of 79; 95% CI 13.1–32.2) in 2012 to 81.8% (126 out of 154; 95% CI 74.8–87.6) in 2017.
Out of overall 3955 fox blood samples, originating from across the whole country, 463 (11.7%) were positive for circulating A. vasorum antigen (Fig. 1). Antigen seropositivity increased over time periods 1–4 from 2.4 to 3.9, 32.9 and 62.0%, respectively (Fig. 5a–d). North-eastern Switzerland is the only area represented in all four time intervals: a drastic increase was observed when comparing time periods 1–4, from 1.9% (25/1343), to 1.7% (14/833), to 22.2% (22/99) and to 62.0% (281/453). Samples from south-eastern Switzerland were available from three time periods (1, 2 and 3), where seroprevalences were 7.9% (13/164), 6.5% (45/691) and 38.5% (52/135), respectively, while those from western Switzerland were collected during periods 1 and 3 only, with prevalences of 1.4% (3/222) and 53.3% (8/15), respectively.
Fig. 5.
(a–d) Maps of Switzerland indicating the time period of collection, the location of the examined fox blood samples of which coordinates were available, the number of positive vs the number of totally analysed samples, seropositivity and 95% confidence intervals. Samples were collected (a) between 1986 and 1992 (of 103 foxes no coordinates were available); (b) between 1993 and 2002 (of 120 foxes no coordinates were available); (c) between 2003 and 2012 (of 15 foxes no coordinates were available); (d) between 2013 and 2017 (of 13 foxes no coordinates were available). Red dots: samples tested seropositive, white dots: tested negative for Angiostrongylus vasorum antigen.
The retested 8–13 years old blood samples from A. vasorum experimentally infected dogs showed slight to moderate deviations in their OD values in both directions (decrease or increase) when compared to initial values. The greatest variations were observed in samples with high initial OD values. A decrease in highly positive samples did not lead to values below the cut-off (Supplementary Results S2).
Discussion
Our necropsy data show that A. vasorum prevalence increased 4-fold in the fox population in the canton of Zurich in only 5 years. The parasite burdens increased as well, with higher mean worm loads per infected fox each year. This suggests that infected foxes may get continuously reinfected, accumulating worms in their cardiopulmonary tissues. In line with this, experimental trials showed that foxes do not develop protective immunity and that A. vasorum worm burdens increase in animals upon reinfection (Woolsey et al., 2017), which also resulted in continuous and increasing numbers of excreted first-stage larvae (L1). Our results support the hypothesis that infected foxes are a continuous source for environmental contamination with larval stages that allow the infection of gastropod intermediate hosts (Woolsey et al., 2017). This, on the one hand, explains the successful spread and establishment of A. vasorum in novel areas, and on the other hand, confirms the important role of foxes as wildlife reservoir hosts, likely contributing to dog infection dynamics in the same areas.
In contrast, the prevalences of other common lungworms of foxes, i.e. C. vulpis and C. aerophila, were not increasing, but rather fluctuated from year to year. Also, contrasting with A. vasorum, the C. vulpis and C. aerophila worm burdens did not increase over time, despite the fact that A. vasorum and C. vulpis share the same intermediate hosts (Lange et al., 2018). Hence, one may have expected parallel increasing prevalences for both parasites. In agreement with our results, in Hungary, A. vasorum prevalence in foxes increased from 5 to 18% between 2002 and 2013/2014, while prevalences of C. vulpis (24% in both studies) and C. aerophila (66 vs 62%) were constant (Sreter et al., 2003; Tolnai et al., 2015). Similarly, in Zealand, Denmark, a long known endemic region for A. vasorum, the parasite's prevalence was of 49% in 1997–2002 and of 80% in 2006–2008, while the prevalences of other lungworms remained approximately constant (C. vulpis: 17 vs 23%; C. aerophila: 74.5 vs 87%) (Saeed et al., 2006; Al-Sabi et al., 2014). In Great Britain, A. vasorum prevalence increased from 7 to 18% in 8 years, while C. vulpis increased from 2 to 11% and C. aerophila remained at high levels of 39 and 32%, respectively (Morgan et al., 2008; Taylor et al., 2015). Overall, C. aerophila was the most abundant lungworm in these three countries and prevalences were comparable with the present Swiss prevalence. In Switzerland, C. vulpis was the least common lungworm recovered (10.1%), closely paralleling the prevalence in Great Britain (11%), but below the observed proportions in Danish and Hungarian fox populations (17–23 and 24%, respectively).
Prior data on A. vasorum and other lungworms in Swiss foxes are scant. In a single Swiss-wide investigation from 2010 to 2012 performed on fox feces, an overall A. vasorum prevalence of 8.8% was established (Koller et al., 2019). This low prevalence obtained before 2012 is in line with the hypothesis of increasing prevalences over a relatively short time but also supports the superiority of necropsy data over fecal examinations performed on frozen samples. Surprisingly, Capillaria spp. were less prevalent (8.3%) and C. vulpis more prevalent (21.4%) (Koller et al., 2019), compared to the present observations (62.9 and 10.1%, respectively). This is likely due to the ability of C. vulpis L1 to resist deep freeze, up to −80°C (Saeed et al., 2006), unlike A. vasorum L1 and Capillaria eggs. Both A. vasorum and C. vulpis were detected frequently in the Swiss Plateau, while both were less commonly detected in the southern (warmer) part of Switzerland, also suggesting that these parasites may be more adapted to survive in colder climates (Koller et al., 2019).
Clustered distributions of A. vasorum and C. vulpis were observed in several countries, with either overlapping or diverging occurrences. This is likely due to occurrence of A. vasorum and C. vulpis in local endemic spots, which may also be defined by local gastropod populations (Aziz et al., 2016; Lange et al., 2018). Findings on the influence of climate on A. vasorum and C. vulpis occurrence in fox populations are conflicting: this was attributed to distinct temperature and precipitation optima favouring e.g. intermediate hosts and therefore the life cycles (Jeffery et al., 2004; Taylor et al., 2015; Tolnai et al., 2015; Cabanova et al., 2018).
In 2009, Morgan et al. used a model that relied on long-standing known endemic foci of A. vasorum and on climatic data to predict potential areas in which A. vasorum could establish in the future, even without climate change. Switzerland was entirely included, with differing levels of predicted suitability (Morgan et al., 2009). The spread of A. vasorum, as predicted by the model, has also been recently described from previously parasite-free areas (or areas with unknown presence) such as Romania (Deak et al., 2017), Belgium (Jolly et al., 2015), the Czech Republic (Hajnalová et al., 2017), Slovakia (Hurnikova et al., 2013) and even mainland North America (Priest et al., 2018). Here we show that A. vasorum has successfully established in the Swiss fox population, reaching regional prevalences of more than 80%. Particularly interesting is the marked emergence of A. vasorum in the Swiss fox population at around the start of the new millennium (from study time span 2–3). The timing of this marked increase correlates with first accumulations of cases of canine angiostrongylosis occurring between 1999 and 2004 in southern and northern Switzerland (Staebler et al., 2005). The very first cases of A. vasorum in the country were nonetheless already reported in the 1960s from a dog breeding facility in the canton of Zurich (Wolff et al., 1969). Although after these first cases there were no further reports for approximately 40 years, we demonstrate here that since 2013 the whole canton of Zurich represents a hot spot. In addition, evidence in support of a spread in the dog population has been accumulating (Glaus et al., 2010; Lurati et al., 2015; Sigrist et al., 2017).
In Switzerland, a drastic increase in the number of foxes in rural and urban areas has been observed in the 1980s and 1990s (Breitenmoser et al., 2000). In the city of Zurich, fox density is estimated at more than 10 adult foxes per square kilometre (Gloor, 2002). This increased number of foxes could result in more contact between foxes and humans and pets (Gloor, 2002; Deplazes et al., 2004), increasing the risk of parasite transmission (Saeed et al., 2006; Otranto et al., 2015). It can be hypothesised that transmission of A. vasorum among foxes started to increase at the end of the 20th century due to higher density of foxes and increasing environmental contamination, leading to higher infection rates of intermediate and definitive hosts, including dogs. Fox populations were suggested to reliably reflect the parasite occurrence because unbiased by factors such as increased disease awareness or anthelmintic treatments (Taylor et al., 2015). The same authors advanced that fox data on parasite distribution and infection intensity over time will promote our understanding of the epidemiology and anticipate future trends (Taylor et al., 2015).
In general, the lung necropsy findings observed in foxes are similar to the ones observed in experimentally (Schnyder et al., 2010) and naturally (Bourque et al., 2008) infected dogs. Angiostrongylus vasorum-infected foxes necropsied in our study displayed severe lung pathology, such as partial lung fibrosis, lung lobe consolidation and adhesions. Despite the fact that we necropsied hunted animals, and that post-mortem changes such as haemorrhages, discolouration and trauma may macroscopically affect some lungs, a more severe degree of lung pathology was nevertheless associated with higher worm burdens. In Italy, Poli et al. (1991) described similar findings in necropsied foxes and confirmed slight changes in 6.5% of A. vasorum-positive foxes, mild alterations in 92.5% of infected foxes and severe pathology in 1% of the animals. Eleni et al. (2014) recently scored 27 lungs of A. vasorum-infected Italian foxes and identified no lesions in 18.5%, light changes in 33.3%, mild changes in 22.2% and severe pathology in 25.9% of the lungs, respectively. Unlike in dogs (Stockdale and Hulland, 1970), foxes infected with C. vulpis showed little pathological lesion in the lungs (Jeffery et al., 2004), explaining the lack of correlation between C. vulpis infection and higher lung scoring. Generally, reports from naturally infected foxes with apparent clinical illness are rare (Simpson, 1996; Philbey and Delgado, 2013), and experimentally infected foxes did not show any clinical signs (Webster et al., 2017). However, affected fitness in foxes with high worm burdens and severe lung pathology cannot be excluded.
There was no significant correlation between age and any lungworm infection, indicating that foxes get infected at a young age and remain infected. Male foxes were significantly more frequently infected with C. vulpis and C. aerophila and were by trend more frequently infected with A. vasorum than females. Males of several mammalian species were described to be more often infected with nematodes than females (Poulin, 1996). This is, among others, attributed to testosterone-induced immunosuppression in male individuals (Folstad and Karter, 1992) and/or different behaviour between the sexes (Klein, 2004): male foxes tend to have larger home ranges and therefore are more likely to forage in endemic areas, explaining more frequent infections. Worm burdens, however, were comparable in male or female hosts (Poulin, 1996), while we observed a sex bias with C. aerophila worm burdens (males harboured more worms).
In the present study, calculated prevalences determined by necropsy and antigen detection are similar. The sensitivity for necropsy stated by Houpin et al. (2016) was slightly lower (84.1%) than what we achieved by detecting circulating A. vasorum antigen by ELISA in fox blood (91.2%) (Gillis-Germitsch et al., 2017). In foxes with low worm burden, A. vasorum specimens may be missed upon necropsy, whilst these animals may be antigen-positive. On the other hand, if foxes are necropsied during the prepatent period (5–10 weeks post inoculation), one may already find subadult specimens in the heart and pulmonary arteries whilst the animal will still show seronegative. This may explain the slightly diverging prevalences by necropsy and serology. Therefore, without necropsy expertise, A. vasorum serology has higher sensitivity and can fully substitute fox necropsy. Although for both procedures foxes are usually killed, serology is more efficient, as it is less time-consuming; it is compatible with whole blood samples and even bloody fluids of hunted foxes (Houpin et al., 2016; Gillis-Germitsch et al., 2017). The age of the stored samples could have represented a limitation. However, we showed that antigen can still be reliably detected even after several years of storage. Given that a clear decrease in OD values of highly positive samples did not lead to values below the cut-off, we assume that the age of samples should not influence the overall findings of this study.
The fact that a considerable number of samples originated from the city and surroundings of Zurich and that some areas of Switzerland were not sampled may represent a bias of the study. Relying on samples collected previously in the frame of independent projects, the 30-year retrospective analysis was not based on equal numbers of samples for all four time periods and on all areas of Switzerland. However, due to the large number of examined fox blood samples (3955) originating mainly from the Swiss Plateau (which is also the most densely populated area) but also from alpine areas, from the south of the Alps and the Jura, we hypothesise that our findings are representative for the whole country.
In conclusion, serological antigen detection by ELISA applied on fox blood samples from 1986 to 2017 and across the country represented a unique opportunity to analyse the spread of A. vasorum in Switzerland retrospectively. Our findings for the Swiss fox population stand for a prime example of a drastic A. vasorum emergence from 2.4% to 62.0% in the past three decades. Locally, the prevalence increased 4-fold in only 6 years. This underpins the important role of foxes as reservoir hosts and explains the concomitantly increased number of cases of canine angiostrongylosis in Switzerland at the turn of the millennium. Our study may serve as a model for A. vasorum expansion and also helps to understand the increasing number of dog cases along with significant prevalences in the fox populations in other European countries in the last decade. The reasons behind such prevalence developments and why these are not observed for other lungworms of foxes like C. vulpis and C. aerophila are still under debate. Our findings may anticipate future developments and support disease awareness in areas where A. vasorum is indeed present but (still) in low prevalence.
Acknowledgements
The authors would like to thank Maria Teresa Armua, Deborah Joekel, Philipp Kronenberg and Francesca Gori for their participation in fox necropsies.
Financial support
We would like to acknowledge Bayer Vital GmbH, Business Unit Animal Health, Germany, for the financial support of Nina Gillis-Germitsch in the form of a doctoral fellowship.
Ethical standards
Ethical standards were fulfilled.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182020000700.
click here to view supplementary material
Conflict of interest
None.
References
- Al-Sabi MN, Halasa T and Kapel CM (2014) Infections with cardiopulmonary and intestinal helminths and sarcoptic mange in red foxes from two different localities in Denmark. Acta Parasitologica 59, 98–107. [DOI] [PubMed] [Google Scholar]
- Aziz NA, Daly E, Allen S, Rowson B, Greig C, Forman D and Morgan ER (2016) Distribution of Angiostrongylus vasorum and its gastropod intermediate hosts along the rural-urban gradient in two cities in the United Kingdom, using real time PCR. Parasites & Vectors 9, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque AC, Conboy G, Miller LM and Whitney H (2008) Pathological findings in dogs naturally infected with Angiostrongylus vasorum in Newfoundland and Labrador, Canada. The Journal of Veterinary Diagnostic Investigation 20, 11–20. [DOI] [PubMed] [Google Scholar]
- Breitenmoser U, Müller U, Kappeler A and Zanoni R (2000) Die Endphase der Tollwut in der Schweiz. Schweizer Archiv für Tierheilkunde 147, 447–453. [PubMed] [Google Scholar]
- Bwangamoi O (1972) Angiostrongylus vasorum and other worms in dogs in Uganda. Veterinary Record 91, 267. [DOI] [PubMed] [Google Scholar]
- Cabanova V, Miterpakova M, Druga M, Hurnikova Z and Valentova D (2018) GIS-based environmental analysis of fox and canine lungworm distribution: an epidemiological study of Angiostrongylus vasorum and Crenosoma vulpis in red foxes from Slovakia. Parasitology Research 117, 521–530. [DOI] [PubMed] [Google Scholar]
- Chandler WL (1922) Lungworms of foxes. American Fox and Fur Farmer 1, 21. [Google Scholar]
- Chapman PS, Boag AK, Guitian J and Boswood A (2004) Angiostrongylus vasorum infection in 23 dogs (1999–2002). The Journal of Small Animal Practice 45, 435–440. [DOI] [PubMed] [Google Scholar]
- Christenson RO (1935) Studies on the morphology of the common fox lungworm, Capillaria aërophila (Creplin, 1839). Transactions of the American Microscopical Society 54, 145–154. [Google Scholar]
- Christenson RO (1938) Life history and epidemiological studies on the fox lungworm, Capillaria aerophila (Creplin, 1839). Livro Jubilar Prof. L. Travassos. Rio de Janeiro: Instituto Oswaldo Cruz, 119–186. [Google Scholar]
- Clopper CJ and Pearson ES (1934) The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26, 404–413. [Google Scholar]
- Conboy G (2009) Helminth parasites of the canine and feline respiratory tract. The Veterinary Clinics of North America 39, 1109–1126, vii. [DOI] [PubMed] [Google Scholar]
- Cuillé J and Darraspen E (1930) De la Strongylose cardio-pulmonaire du chien. Revue Générale de Médecine Vétérinaire 466, 625–639, 694–710. [Google Scholar]
- Deak G, Gherman CM, Ionica AM, Vezendan AD, D'Amico G, Matei IA, Daskalaki AA, Marian I, Damian A, Cozma V and Mihalca AD (2017) Angiostrongylus vasorum in Romania: an extensive survey in red foxes, Vulpes vulpes. Parasites & Vectors 10, 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demiaszkiewicz AW, Pyziel AM, Kuligowska I and Lachowicz J (2014) The first report of Angiostrongylus vasorum (Nematoda; Metastrongyloidea) in Poland, in red foxes (Vulpes vulpes). Acta Parasitologica 59, 758–762. [DOI] [PubMed] [Google Scholar]
- Deplazes P, Hegglin D, Gloor S and Romig T (2004) Wilderness in the city: the urbanization of Echinococcus multilocularis. Trends in Parasitology 20, 77–84. [DOI] [PubMed] [Google Scholar]
- Deplazes P, Eckert J, Mathis A, von Samson-Himmelstjerna G and Zahner H (2016) Parasitology in Veterinary Medicine. Wageningen, The Netherlands: Wageningen Academic Publishers. [Google Scholar]
- Eleni C, Grifoni G, Di Egidio A, Meoli R and De Liberato C (2014) Pathological findings of Angiostrongylus vasorum infection in red foxes (Vulpes vulpes) from Central Italy, with the first report of a disseminated infection in this host species. Parasitology Research 113, 1247–1250. [DOI] [PubMed] [Google Scholar]
- Ewald D (1993) Prävalenz von Echinococcus multilocularis bei Rotfüchsen (Vulpes vulpes L.) in der Nord-, Ost-und Südschweiz sowie im Fürstentum Liechtenstein (PhD thesis, Phil. II). University of Zurich, Zurich, Switzerland. [Google Scholar]
- Fischer C, Reperant LA, Weber JM, Hegglin D and Deplazes P (2005) Echinococcus multlocularis infections of rural, residential and urban foxes (Vulpes vulpes) in the canton of Geneva, Switzerland. Parasite 12, 339–346. [DOI] [PubMed] [Google Scholar]
- Folstad I and Karter AJ (1992) Parasites, bright males, and the immunocompetence handicap. The American Naturalist 139, 603–622. [Google Scholar]
- Gillis-Germitsch N, Kapel CMO, Thamsborg SM, Deplazes P and Schnyder M (2017) Host-specific serological response to Angiostrongylus vasorum infection in red foxes (Vulpes vulpes): implications for parasite epidemiology. Parasitology 144, 1144–1153. [DOI] [PubMed] [Google Scholar]
- Glaus T, Schnyder M, Dennler M, Tschuor F, Wenger M and Sieber-Ruckstuhl N (2010) Natural infection with Angiostrongylus vasorum: characterisation of 3 dogs with pulmonary hypertension. Schweizer Archiv für Tierheilkunde 152, 331–338. [DOI] [PubMed] [Google Scholar]
- Gloor S (2002) The rise of urban foxes (Vulpes vulpes) in Switzerland and ecological and parasitological aspects of a fox population in the recently colonised city of Zurich (PhD thesis). University of Zurich, Zurich, Switzerland. [Google Scholar]
- Gonseth Y, Wohlgemuth T, Sansonnens B and Buttler A (2001) Die Biogeographischen Regionen der Schweiz. Erläuterungen und Einteilungsstandard. Umwelt Materialien. Bern, Switzerland: Bundesamt für Umwelt, Wald und Landschaft, 137. [Google Scholar]
- Guerra D, Hegglin D, Bacciarini L, Schnyder M and Deplazes P (2014) Stability of the southern European border of Echinococcus multilocularis In the Alps: evidence that Microtus arvalis is a limiting factor. Parasitology 141, 1593–1602. [DOI] [PubMed] [Google Scholar]
- Guilhon J and Cens B (1973) Angiostrongylus vasorum (Baillet, 1866): Étude biologique et morphologique. Annales de Parasitologie 48, 567–596. [PubMed] [Google Scholar]
- Hajnalová M, Svobodová V, Schnyder M, Schaper R and Svoboda M (2017) Faecal detection of the lungworms Crenosoma vulpis and Angiostrongylus vasorum and serological detection of A. vasorum in dogs from the Czech Republic. Acta Veterinaria Brno 86, 393–398. [Google Scholar]
- Hegglin D, Bontadina F, Contesse P, Gloor S and Deplazes P (2007) Plasticity of predation behaviour as a putative driving force for parasite life-cycle dynamics: the case of urban foxes and Echinococcus multilocularis tapeworm. Functional Ecology 21, 552–560. [Google Scholar]
- Helm J, Roberts L, Jefferies R, Shaw SE and Morgan ER (2015) Epidemiological survey of Angiostrongylus vasorum in dogs and slugs around a new endemic focus in Scotland. The Veterinary Record 177, 1–6. [DOI] [PubMed] [Google Scholar]
- Hermosilla C, Kleinertz S, Silva LM, Hirzmann J, Huber D, Kusak J and Taubert A (2017) Protozoan and helminth parasite fauna of free-living Croatian wild wolves (Canis lupus) analyzed by scat collection. Veterinary Parasitology 233, 14–19. [DOI] [PubMed] [Google Scholar]
- Hofer S, Gloor S, Muller U, Mathis A, Hegglin D and Deplazes P (2000) High prevalence of Echinococcus multilocularis in urban red foxes (Vulpes vulpes) and voles (Arvicola terrestris) in the city of Zurich, Switzerland. Parasitology 120, 135–142. [DOI] [PubMed] [Google Scholar]
- Holmes P and Kelly J (1973) Capillaria aerophila in the domestic cat in Australia. Australian Veterinary Journal 49, 472–473. [DOI] [PubMed] [Google Scholar]
- Houpin E, McCarthy G, Ferrand M, De Waal T, O'Neill EJ and Zintl A (2016) Comparison of three methods for the detection of Angiostrongylus vasorum in the final host. Veterinary Parasitology 220, 54–58. [DOI] [PubMed] [Google Scholar]
- Hurnikova Z, Miterpakova M and Mandelik R (2013) First autochthonous case of canine Angiostrongylus vasorum in Slovakia. Parasitology Research 112, 3505–3508. [DOI] [PubMed] [Google Scholar]
- Jefferies R, Shaw SE, Willesen J, Viney ME and Morgan ER (2010) Elucidating the spread of the emerging canid nematode Angiostrongylus vasorum between Palaearctic and Nearctic ecozones. Infection, Genetics and Evolution 10, 561–568. [DOI] [PubMed] [Google Scholar]
- Jeffery RA, Lankester MW, McGrath MJ and Whitney HG (2004) Angiostrongylus vasorum and Crenosoma vulpis in red foxes (Vulpes vulpes) in Newfoundland, Canada. Canadian Journal of Zoology 82, 66–74. [Google Scholar]
- Jolly S, Poncelet L, Lempereur L, Caron Y, Bayrou C, Cassart D, Grimm F and Losson B (2015) First report of a fatal autochthonous canine Angiostrongylus vasorum infection in Belgium. Parasitology International 64, 97–99. [DOI] [PubMed] [Google Scholar]
- Kistler WM, Brown JD, Allison AB, Nemeth NM and Yabsley MJ (2014) First report of Angiostrongylus vasorum and Hepatozoon from a red fox (Vulpes vulpes) from West Virginia, USA. Veterinary Parasitology 200, 216–220. [DOI] [PubMed] [Google Scholar]
- Klein SL (2004) Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunology 26, 247–264. [DOI] [PubMed] [Google Scholar]
- Koch J and Willesen JL (2009) Canine pulmonary angiostrongylosis: an update. The Veterinary Journal 179, 348–359. [DOI] [PubMed] [Google Scholar]
- Koller B, Hegglin D and Schnyder M (2019) A grid-cell based fecal sampling scheme reveals: land-use and altitude affect prevalence rates of Angiostrongylus vasorum and other parasites of red foxes (Vulpes vulpes). Parasitology Research 118, 2235–2245. [DOI] [PubMed] [Google Scholar]
- Lange MK, Penagos-Tabares F, Hirzmann J, Failing K, Schaper R, Van Bourgonie YR, Backeljau T, Hermosilla C and Taubert A (2018) Prevalence of Angiostrongylus vasorum, Aelurostrongylus abstrusus and Crenosoma vulpis larvae in native slug populations in Germany. Veterinary Parasitology 254, 120–130. [DOI] [PubMed] [Google Scholar]
- Lurati L, Deplazes P, Hegglin D and Schnyder M (2015) Seroepidemiological survey and spatial analysis of the occurrence of Angiostrongylus vasorum in Swiss dogs in relation to biogeographic aspects. Veterinary Parasitology 212, 219–226. [DOI] [PubMed] [Google Scholar]
- Maksimov P, Hermosilla C, Taubert A, Staubach C, Sauter-Louis C, Conraths FJ, Vrhovec MG and Pantchev N (2017) GIS-supported epidemiological analysis on canine Angiostrongylus vasorum and Crenosoma vulpis infections in Germany. Parasites & Vectors 10, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Rondan FJ, Ruiz de Ybanez MR, Lopez-Beceiro AM, Fidalgo LE, Berriatua E, Lahat L, Sacristan I, Oleaga A and Martinez-Carrasco C (2019) Cardiopulmonary nematode infections in wild canids: Does the key lie on host-prey-parasite evolution? Research in Veterinary Science 126, 51–58. [DOI] [PubMed] [Google Scholar]
- Morgan ER, Tomlinson A, Hunter S, Nichols T, Roberts E, Fox MT and Taylor MA (2008) Angiostrongylus vasorum and Eucoleus aerophilus in foxes (Vulpes vulpes) in Great Britain. Veterinary Parasitology 154, 48–57. [DOI] [PubMed] [Google Scholar]
- Morgan ER, Jefferies R, Krajewski M, Ward P and Shaw SE (2009) Canine pulmonary angiostrongylosis: the influence of climate on parasite distribution. Parasitology International 58, 406–410. [DOI] [PubMed] [Google Scholar]
- Otranto D and Deplazes P (2019) Zoonotic nematodes of wild carnivores. International Journal of Parasitology: Parasites and Wildlife 9, 370–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otranto D, Cantacessi C, Dantas-Torres F, Brianti E, Pfeffer M, Genchi C, Guberti V, Capelli G and Deplazes P (2015) The role of wild canids and felids in spreading parasites to dogs and cats in Europe. Part II: Helminths and arthropods. Veterinary Parasitology 213, 24–37. [DOI] [PubMed] [Google Scholar]
- Philbey AW and Delgado D (2013) Detection of Angiostrongylus vasorum in red foxes in Scotland. Veterinary Record 173, 148–148. [DOI] [PubMed] [Google Scholar]
- Poli A, Arispici M, Mancianti F and Abramo F (1991) Pathology of naturally acquired Angiostrongylus vasorum infection in the red fox (Vulpes vulpes). Angewandte Parasitologie 32, 121–126. [PubMed] [Google Scholar]
- Poulin R (1996) Sexual inequalities in helminth infections: a cost of being a male? The American Naturalist 147, 287–295. [Google Scholar]
- Priest JM, Stewart DT, Boudreau M, Power J and Shutler D (2018) First report of Angiostrongylus vasorum in coyotes in mainland North America. Veterinary Record 183, 747. [DOI] [PubMed] [Google Scholar]
- Saeed I, Maddox-Hyttel C, Monrad J and Kapel CM (2006) Helminths of red foxes (Vulpes vulpes) in Denmark. Veterinary Parasitology 139, 168–179. [DOI] [PubMed] [Google Scholar]
- Schnyder M, Fahrion A, Riond B, Ossent P, Webster P, Kranjc A, Glaus T and Deplazes P (2010) Clinical, laboratory and pathological findings in dogs experimentally infected with Angiostrongylus vasorum. Parasitology Research 107, 1471–1480. [DOI] [PubMed] [Google Scholar]
- Schnyder M, Tanner I, Webster P, Barutzki D and Deplazes P (2011) An ELISA for sensitive and specific detection of circulating antigen of Angiostrongylus vasorum in serum samples of naturally and experimentally infected dogs. Veterinary Parasitology 179, 152–158. [DOI] [PubMed] [Google Scholar]
- Schucan A, Schnyder M, Tanner I, Barutzki D, Traversa D and Deplazes P (2012) Detection of specific antibodies in dogs infected with Angiostrongylus vasorum. Veterinary Parasitology 185, 216–224. [DOI] [PubMed] [Google Scholar]
- Serres E (1854) Entozoaires trouvés dans l'oreille droite, le ventricule correspondant et l'artère pulmonaire d'un chien. Journal des Vétérinaires du Midi 7, 70. [Google Scholar]
- Sigrist NE, Hofer-Inteeworn N, Jud Schefer R, Kuemmerle-Fraune C, Schnyder M and Kutter APN (2017) Hyperfibrinolysis and hypofibrinogenemia diagnosed with rotational thromboelastometry in dogs naturally infected with Angiostrongylus vasorum. Journal of Veterinary Internal Medicine 31, 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson VR (1996) Angiostrongylus vasorum infection in foxes (Vulpes vulpes) in Cornwall. Veterinary Record 139, 443–445. [DOI] [PubMed] [Google Scholar]
- Sreter T, Szell Z, Marucci G, Pozio E and Varga I (2003) Extraintestinal nematode infections of red foxes (Vulpes vulpes) in Hungary. Veterinary Parasitology 115, 329–334. [DOI] [PubMed] [Google Scholar]
- Staebler S, Ochs H, Steffen F, Naegeli F, Borel N, Sieber-Ruckstuhl N and Deplazes P (2005) Autochthone Infektionen mit Angiostrongylus vasorum bei Hunden in der Schweiz und Deutschland. Schweizer Archiv für Tierheilkunde 147, 121–127. [DOI] [PubMed] [Google Scholar]
- Stockdale PH and Hulland TJ (1970) The pathogenesis, route of migration, and development of Crenosoma vulpis in the dog. Pathologica Veterinaria 7, 28–42. [DOI] [PubMed] [Google Scholar]
- Tanner F, Hegglin D, Thoma R, Brosi G and Deplazes P (2006) Echinococcus multilocularis in Graubünden: Verbreitung bei Füchsen und Vorkommen potentieller Zwischenwirte. Schweizer Archiv für Tierheilkunde 148, 501–510. [DOI] [PubMed] [Google Scholar]
- Taylor CS, Garcia Gato R, Learmount J, Aziz NA, Montgomery C, Rose H, Coulthwaite CL, McGarry JW, Forman DW, Allen S, Wall R and Morgan ER (2015) Increased prevalence and geographic spread of the cardiopulmonary nematode Angiostrongylus vasorum in fox populations in Great Britain. Parasitology 142, 1190–1195. [DOI] [PubMed] [Google Scholar]
- Tolnai Z, Szell Z and Sreter T (2015) Environmental determinants of the spatial distribution of Angiostrongylus vasorum, Crenosoma vulpis and Eucoleus aerophilus in Hungary. Veterinary Parasitology 207, 355–358. [DOI] [PubMed] [Google Scholar]
- Traversa D, Morelli S, Cassini R, Crisi PE, Russi I, Grillotti E, Manzocchi S, Simonato G, Beraldo P, Viglietti A, De Tommaso C, Pezzuto C, Pampurini F, Schaper R and Frangipane di Regalbono A (2019) Occurrence of canine and feline extra-intestinal nematodes in key endemic regions of Italy. Acta Tropica 193, 227–235. [DOI] [PubMed] [Google Scholar]
- Webster P, Monrad J, Kapel CMO, Kristensen AT, Jensen AL and Thamsborg SM (2017) The effect of host age and inoculation dose on infection dynamics of Angiostrongylus vasorum in red foxes (Vulpes vulpes). Parasites & Vectors 10, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel R (1940) Zur Biologie des Fuchslungenwurmes Crenosoma vulpis. Archiv für Wissenschaftliche und Praktische Tierheilkunde 75, 445–450. [Google Scholar]
- Willingham AL, Ockens NW, Kapel CMO and Monrad J (1996) A helminthological survey of wild red foxes (Vulpes vulpes) from the metropolitan area of Copenhagen. Journal of Helminthology 70, 259–263. [DOI] [PubMed] [Google Scholar]
- Wolff K, Eckert J and Leemann W (1969) Beitrag zur Angiostrongylose des Hundes. In: Congress of the Dtsch. Vet-med Ges., Fachgr. ‘Kleintierkrankheiten’, Zürich.
- Woolsey ID, Webster P, Thamsborg S, Schnyder M, Monrad J and Kapel CMO (2017) Repeated inoculations with the lung and heartworm nematode Angiostrongylus vasorum result in increasing larval excretion and worm burden in the red fox (Vulpes vulpes). International Journal of Parasitology: Parasites and Wildlife 6, 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182020000700.
click here to view supplementary material