Abstract
Human visceral leishmaniasis (HVL) cases are important public health problems due to their zoonotic aspect, with high rates of morbidity and mortality in Brazil. The aim of this this study was to identify spatial patterns in both rates of HVL cases in Brazilian states during the period from 2006 to 2015. This is an ecological study, using geoprocessing tools to create choropleth maps, based on secondary data from open access platforms, to identify priority areas for control actions of the disease. Data were collected in 2017 and analysed according to the global and local Moran's I, using TerraView 4.2.2 software. Similar clusters were observed in neighbouring municipalities in thematic maps of HVL, suggesting spatial similarity in the distribution of the disease in humans mainly in the North and Northeast Regions, which concentrate the states with the highest rates of HVL. Heterogeneous spatial patterns were observed in the distribution of HVL, which show municipalities that need higher priority in the intensification of disease surveillance and control strategies.
Key words: Brazil, human visceral leishmaniasis, leishmaniasis, spatial analysis
Introduction
Leishmaniasis occupies the ninth position in the world ranking among the priority infectious diseases (World Health Organization., 2015; Machado et al., 2016; Carvalho et al., 2018). Visceral leishmaniasis (VL) is present in more than 80 countries (Mehrjou et al., 2016), nevertheless 90% of these cases are concentrated in 10 of them (Brazil, Bangladesh, Ethiopia, China, Kenya, Nepal, India, Sudan, Somalia and South Sudan) (World Health Organisation, 2009; Arruda et al., 2019). In the Americas, it is present in 12 countries, and 95% of the cases are reported in Brazil (Mehrjou et al., 2016).
In the 1990s, the highest index of Brazilian notifications was around 90% in the Northeast Region. The unplanned expansion of the peripheries in small and large cities, associated with the lack ok of adequate infrastructure (Albuquerque et al., 2014; Silva and Abud, 2016), and the presence of dog, the main reservoir of Leishmania infantum (Mehrjou et al., 2016), favoured environments conducive to the proliferation and adaptation of the vector, as well as the consequent expansion of the disease to other regions, as Midwest and Southeast. Thus, the percentage in the Northeast Region decreased to 77% (Brasil, 2014, 2015).
In Brazil the disease is more prevalence in Maranhão, Ceará, Bahia, Piauí, Tocantins, Pará, Minas Gerais, Mato Grosso do Sul and São Paulo State (Brasil, 2015). The cases are often related to poor quality of life and child malnutrition (Duarte-Cunha et al., 2012).
Epidemiological and socioeconomic situations and ecological processes can reduce the impact of control programmes (Otranto and Dantas-Torres, 2013). The Secretariat of Health Surveillance of Ministry of Health coordinates the VL control and surveillance activities in Brazil (Mehrjou et al., 2016). However, the control strategies currently applied were not successful in decreasing the incidence of the disease to acceptable levels (Costa et al., 2013), exposing the vulnerabilities of such measures (Araújo et al., 2013; Arruda et al., 2019).
Using spatial analysis tools and those from Geographic Information System (GIS) allows the creation of thematic maps that assist in the checking and offer a better understanding of the spatial patterns of data distribution, making it possible to detect risk areas and associated factors, as well as indicate the regions with greater need to intensify and/or prioritize control measures, in addition to implementing control strategies, both for the disease and the limited financial resources (Arruda et al., 2019).
A model of geographic distribution has been used in the human visceral leishmaniasis (HVL) (Barbosa and Werneck, 2011; Karagiannis-Voules et al., 2013; Barbosa et al., 2014; Fontoura et al., 2016) and is widely used to analyse the spatial distributions of other studies, as dengue (Rodrigues et al., 2016), Zika virus (de Oliveira et al., 2017), tuberculosis (Santos Neto et al., 2017), diarrhoea (Fontoura et al., 2018a), among others.
Although spatial analyses have already been used in other Brazilian research studies, this study was necessary to identify spatial patterns during the period of last 10-years in order to determine areas that must be prioritized regarding planning disease surveillance and control actions in the country, mainly taking into account the HVL rates in the Brazilian states.
Materials and methods
Study area
Brazil is located in South America, and its area comprises 8.5 million km2. Its population was estimated in 211 million residents in 2020. It is divided into five regions, i.e. Northeast, North, Midwest, Southeast and South, with 27 federated states (1 Federal District and 26 States) and 5570 cities (IBGE, 2020).
Study design and population
We carried out an ecological spatial analysis based on secondary data and time series related to HVL cases in Brazilian cities between 2006 and 2015. We analysed the epidemiological characteristics, spatial patterns with time trends of the HVL distribution, as well as the identification of risk areas.
Data sources
Populational data collected in 2017 originated from the 2010 Demographic Brazilian Census (IBGE, 2017a) carried out by the Brazilian Institute of Geography and Statistics (IBGE, 2017b). HVL data were obtained from Information System of Disease Notification (SINAN, acronym in Portuguese) from 2006 (Brasil, 2017a) and between 2007 and 2015 (Brasil, 2017b), which includes standardized forms that are completed by the physicians in charge of notification. The disease notification form provides demographic data (gender, skin colour/ethnicity, age range, years of study, data regarding region and states), and clinical information (HIV coinfection, evolution, entrance type, diagnosis examinations, confirmatory criteria). These data are available in the website of Computing Department of the Brazilian Unified Health System (DATASUS, acronym in Portuguese) and they are of public domain, therefore they can be accessed for free. We included all data available between 2006 and 2015 regarding HVL in Brazil.
The selection of indicators was based on the distribution of HVL cases reported and their association with risk factors for its occurrence. The analysis was based on indicators that determine the HVL (Araújo et al., 2013; Arruda et al., 2019). The HVL epidemiological characteristics were compared with gender, skin colour/ethnicity, age range, evolution, type of entrance, diagnosis examinations, years of study grouped in a biennial form and data regarding region and states.
Statistical analysis
We described the available variables of the studied population: gender, skin colour/ethnicity, age range, disease evolution, entrance type, HIV coinfection, parasitological diagnosis, immunofluorescent diagnosis, confirmatory criterion, region, and states. The descriptive statistics included absolute number, 95% confidence interval (95% CI) for categorical variables, and average annual rate (AAR), standard deviation (s.d.) and 95% CI for continuous variables.
Gross rate and AAR were calculated by dividing the HVL number in each year through the direct method using the 2010 Brazilian population, multiplied by 100 000 residents.
Prais–Winsten linear regressions were used between 2006 and 2015, a statistical procedure for the analysis of prevalence trend regression and autocorrelation in time series (Falavina et al., 2019). It was used for annual increment rates and respective confidence intervals (95%). Based on these parameters, they were classified as increasing (positive rate), stable (regression coefficient not significant between its value and zero, P > 0.05) or decreasing (negative rate) (Brilhante et al., 2017; Costa et al., 2019).
Finally, we analysed the spatial patterns of HVL distribution in Brazil using the home cities (n = 5570; 2010 territorial division). The geographic units were analysed per tool of the GIS, which are useful in the geographic distribution assessment, as well as in the spatial dependence of the HVL rates.
The development of thematic maps occurred based on gross rates (number of HVL/population living in Brazil in 2010 × 100 000 residents) (Martins-Melo et al., 2014b). The gross rates of HVL were grouped in every 2 years (2006–2007; 2008–2009; 2010–2011; 2012–2013; 2014–2015) and in the total period (2006–2015).
After the descriptive analysis of data, we estimated global and local Moran's I indices (Local Indicators of Spatial Association – LISA), which estimate the spatial correlation and local self-correlation by helping to identify sub-regions with the occurrence of spatial self-correlation. We used a first-order neighbourhood criterion to concretize calculations, in which the cities defined as neighbours were those in the borders (Barbosa and Werneck, 2011; Fontoura et al., 2016). Moran's I global index is defined between −1 and 1, in a way that values close to 0 suggest absence of spatial correlation or randomness and next to 1, positive spatial dependence with more similarity between the adjacent cities (grouping). Negative spatial dependence is pointed as −1, which indicates dissimilarity (dispersion) and negative spatial self-correlation (Martins-Melo et al., 2014a; INPE, 2015).
Data available between 2006 and 2015 were analysed in order to observe a potential overlap between HVL (Fontoura et al., 2016). It was defined in quantiles (form in which the classes are divided, each one receives the same number of occurrences), because this is the best configuration to represent data using the intervals: 0,0 for absence of cases; >0,1 to 5,0, very low; >5,0 to 10,0, low; >10,0 to 20,0, medium; >20,0, high (this format was used to classify the gross rate per 100 000 residents). Choropleth maps were developed to better visualize the attribute variation (Barbosa and Werneck, 2011; INPE, 2015).
The generation of LISA map showed clusters of HVL and CVL cases, suggesting places with higher and lower need of interventions, in which 0 indicated non-significant (P > 0.05) that showed inexistence of self-correlation; 1 had low self-correlation, with a 95% confidence level (P = 0.05); 2, medium self-correlation and 99% confidence (P = 0.01); and 3 indicated existence of high self-correlation and 99.9% (P = 0.001) (Barbosa et al., 2014; Carvalho and Nascimento, 2014; Fontoura et al., 2016).
Data for Moran's I Map construction were generated indicating a significance level in the interface (>95% confidence) and suggested places with priority of intervention (INPE, 2015), considering as criteria: zero for non-significant (absence of data); quadrant 1, Q1 – high–high, high priority (positive values, positive means); quadrant 2, Q2 – low–low, low priority (negative values, negative means); quadrant 3, Q3 – high–low (high variable values and low of neighbours) and quadrant 4, Q4 – low–high (low variable values and high of neighbours), which are considered of medium priority (negative spatial association) (Barbosa and Werneck, 2011; Fontoura et al., 2016). Random oscillations were minimized, considering that several consecutive years were analysed according to each variable.
For the spatial analysis, cartographic data presentation, calculation of spatial and local self-correlation indicators and construction of thematic maps, we used the TerraView 4.2.2 software (Instituto Nacional de Pesquisas Espaciais, INPE, São José dos Campos, SP, Brazil – INPE, 2013). The descriptive analysis of the data, as well as the Prais–Winsten regression tests, with 5% significance, was performed using the IBM SPSS 24 programme (IBM SPSS Statistics, 2016).
Results
During the study period, 37 411 cases of VL were reported between 2006 and 2015, representing AAR of 1.95 case/100 000 inhabitants (s.d. ±0.14; 95% CI 2.05–1.88). The Prais–Winsten regression showed that the incidence rate of the total number of cases remained stable −4.9/100 000 inhabitants (P = 0.12).
Male gender (23 510; 63%; AAR of 2.51/100 000 inhabitants) and mixed-race (72%) corresponded to the predominant characteristics of HVL. The highest incidence was found among indigenous people (AAR of 4.28/100 000 inhabitants). The highest proportion was in the age group of children between 1 and 4 years old (27.73%), but with a higher incidence in children under 1 year old (AAR 2.42/100 000 inhabitants). We found 2408 deaths reported (AAR of 0.13/100 000 inhabitants), 26 857 (AAR of 1.39/100 000 inhabitants) were considered cured, 33 916 were new cases (AAR of 1.77/100 000 inhabitants), and 1534 (AAR of 0.08/100 000 inhabitants) presented recurrences.
Among the diagnostic tests performed, 13 260 cases (AAR of 0.68/100 000 inhabitants) were confirmed by parasitological examination, and 15 641 by the indirect immunofluorescence test (AAR of 0.80/100 000 inhabitants). The LVH/HIV co-infection was present in 2229 people (AAR of 0.13/100 000 inhabitants) (Table 1).
Table 1.
Epidemiological characteristics of HVL in Brazil, from 2006 to 2015
| Characteristic | n | % | 95% CI | AAR | 95% CI | ±s.d. |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 23 510 | 62.84 | 62.17–63.83 | 2.57 | 2.42–2.61 | 0.15 |
| Female | 13 901 | 37.16 | 36.32–37.68 | 1.35 | 1.34–1.52 | 0.14 |
| Total | 37 411 | 100.00 | – | 1.95 | 1.88–2.05 | 0.14 |
| Skin colour/ethnicity | ||||||
| White/Caucasian | 6032 | 17.00 | 16.48–17.52 | 0.49 | 0.58–0.74 | 0.13 |
| Black/Afro-descendant | 2991 | 9.00 | 8.69–17.31 | 2.12 | 1.95–2.17 | 0.18 |
| Yellow/Asian descendant | 290 | 0.78 | 0.78–1.12 | 1.20 | 1.15–1.63 | 0.39 |
| Mixed | 24 479 | 72.00 | 71.17–72.83 | 3.26 | 2.82–3.33 | 0.25 |
| Indigenous | 280 | 0.75 | 0.56–1.14 | 4.28 | 2.80–5.05 | 1.01 |
| Age group, years | ||||||
| <1 | 3516 | 9.40 | 8.63–9.47 | 2.42 | 2.05–4.26 | 0.98 |
| 1–4 | 10 373 | 27.73 | 27.37–28.63 | 2.35 | 1.56–3.05 | 0.39 |
| 5–9 | 3861 | 10.32 | 9.63–10.37 | 0.69 | 0.63–0.86 | 0.11 |
| 10–14 | 1875 | 5.01 | 4.77–5.23 | 0.32 | 0.24–0.39 | 0.05 |
| 15–19 | 1874 | 5.01 | 4.75–5.25 | 0.23 | 0.19–0.39 | 0.08 |
| 20–39 | 8012 | 21.42 | 20.52–21.48 | 0.90 | 0.51–0.95 | 0.19 |
| 40–59 | 5500 | 14.70 | 14.58–15.42 | 1.06 | 0.68–1.39 | 0.17 |
| 60–64 | 762 | 2.04 | 1.85–2.15 | 0.21 | 0.13–0.27 | 0.03 |
| 65–69 | 580 | 1.55 | 1.46–2.14 | 0.31 | 0.15–0.34 | 0.08 |
| 70–79 | 739 | 1.98 | 1.84–2.16 | 0.81 | 0.32–0.91 | 0.23 |
| >80 | 300 | 0.80 | 0.78–1.12 | 0.41 | 0.29–0.42 | 0.10 |
| Deaths | 2408 | 8.23 | 6.18–9.82 | 0.14 | 0.12–0.14 | 0.01 |
| Cure | 26 857 | 91.77 | 85.96–98.04 | 1.29 | 1.32–1.50 | 0.15 |
| New case | 33 916 | 95.67 | 89.16–102.84 | 1.77 | 1.70–1.85 | 0.12 |
| Recurrence | 1534 | 4.33 | 2.56–5.44 | 0.10 | 0.07–0.12 | 0.02 |
| Positive parasitological | 13 260 | 78.24 | 73.82–82.18 | 0.58 | 0.53–0.76 | 0.11 |
| Negative parasitological | 3688 | 21.76 | 19.75–24.25 | 0.18 | 0.18–0.21 | 0.02 |
| IF positive | 15 641 | 85.86 | 81.27–90.73 | 0.66 | 0.65–0.89 | 0.12 |
| IF negative | 2575 | 14.14 | 12.08–15.92 | 0.14 | 0.12–0.15 | 0.02 |
| HIV coinfection | 2229 | 5.96 | 4.02–7.98 | 0.17 | 0.08–0.19 | 0.05 |
95% CI, 95% confidence interval; AAR, average annual rate per 100 000 inhabitants; s.d., standard deviation; IF, indirect immunofluorescence.
Ignored or unspecified values were not considered.
Regarding sex over the years, both AAR of total and of male and female sex were similar, with a slight decrease between 2012 and 2013, then increasing again (Table 2). Rates related to colour skin or race and to indigenous race had significant increase between 2010 and 2011, with a slight decrease between 2012 and 2013, then increasing again. Concerning the age, rates continued similarly, with an increase between the years 2008 and 2011, and then they decreased. Mortality and cure rates remained almost the same, with a slight growth in 2015. The rates of new cases increased significantly between 2010 and 2011, with an important decrease in the period between 2012 and 2013, increasing again between 2014 and 2015, unlike recurrences that were remained the same between 2006 and 2011, with an increase between 2012 and 2013, getting higher between 2014 and 2015. The diagnosis rates confirmed by parasitological method presented with significant regression over the years, unlike the rates of positive diagnoses confirmed by the indirect immunofluorescence test that significantly increased over the years until 2010 and 2011, with an important decrease between 2012 and 2013 and an increase between 2014 and 2015. The HVL/HIV coinfection rates gradually increased over the years, especially after 2010.
Table 2.
Epidemiological characteristics over the years of HVL in Brazil, from 2006 to 2015
| 2006–2007 (n) | % | Ratea | 2008–2009 (n) | % | Ratea | 2010–2011 (n) | % | Ratea | 2012–2013 (n) | % | Ratea | 2014–2015 (n) | % | Ratea | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | |||||||||||||||
| Male | 4.568 | 61 | 2.49 | 4.967 | 63 | 2.66 | 4.819 | 62 | 2.56 | 4.306 | 64 | 2.29 | 4.850 | 65 | 2.57 |
| Female | 2.920 | 39 | 1.54 | 2.915 | 37 | 1.51 | 2.985 | 38 | 1.52 | 2.430 | 36 | 1.24 | 2.651 | 35 | 1.35 |
| Totalb | 7.488 | 20 | 2.01 | 7.882 | 21 | 2.07 | 7.804 | 21 | 2.03 | 6.736 | 18 | 1.75 | 7.501 | 20 | 1.95 |
| Skin colour/ethnicity | |||||||||||||||
| White/Caucasian | 1.489 | 22 | 0.82 | 1.394 | 19 | 0.77 | 1.203 | 17 | 0.66 | 1.046 | 17 | 0.57 | 900 | 13 | 0.49 |
| Black/Afro-descendant | 597 | 9 | 2.06 | 600 | 8 | 2.07 | 636 | 9 | 2.19 | 543 | 9 | 1.87 | 615 | 9 | 2.12 |
| Yellow/Asian descendant | 75 | 1 | 1.80 | 53 | 1 | 1.27 | 74 | 1 | 1.78 | 38 | 1 | 0.91 | 50 | 1 | 1.20 |
| Mixed | 4.483 | 67 | 2.72 | 5.034 | 72 | 3.06 | 5.091 | 72 | 3.09 | 4.508 | 73 | 2.74 | 5.363 | 76 | 3.26 |
| White/Caucasian | 37 | 1 | 2.26 | 51 | 1 | 3.12 | 67 | 1 | 4.10 | 55 | 1 | 3.36 | 70 | 1 | 4.28 |
| Indigenous | 37 | 0 | 4.52 | 51 | 1 | 6.24 | 67 | 1 | 8.19 | 55 | 1 | 6.72 | 70 | 1 | 8.56 |
| Age group, years | |||||||||||||||
| <1 | 720 | 10 | 3.63 | 780 | 10 | 4.20 | 684 | 9 | 4.05 | 671 | 10 | 3.97 | 661 | 9 | 3.91 |
| 1–4 | 2.413 | 32 | 2.94 | 2.315 | 28 | 2.97 | 2.158 | 27 | 3.24 | 1.683 | 25 | 2.53 | 1.804 | 24 | 2.71 |
| 5–9 | 922 | 12 | 0.88 | 912 | 12 | 0.90 | 756 | 10 | 0.83 | 610 | 9 | 0.67 | 661 | 9 | 0.73 |
| 10–14 | 403 | 5 | 0.38 | 421 | 5 | 0.42 | 395 | 5 | 0.38 | 322 | 5 | 0.31 | 334 | 4 | 0.32 |
| 15–19 | 404 | 5 | 0.37 | 402 | 5 | 0.39 | 399 | 5 | 0.39 | 313 | 5 | 0.30 | 356 | 5 | 0.35 |
| 20–39 | 1473 | 20 | 0.55 | 1568 | 19 | 0.57 | 1718 | 22 | 0.61 | 1517 | 23 | 0.54 | 1736 | 23 | 0.61 |
| 40–59 | 818 | 11 | 0.61 | 1069 | 14 | 0.74 | 1179 | 15 | 0.78 | 1082 | 16 | 0.72 | 1352 | 18 | 0.90 |
| 60–64 | 111 | 1 | 0.12 | 141 | 2 | 0.14 | 169 | 2 | 0.15 | 162 | 2 | 0.15 | 179 | 2 | 0.16 |
| 65–69 | 92 | 1 | 0.16 | 88 | 1 | 0.14 | 130 | 2 | 0.19 | 121 | 2 | 0.18 | 149 | 2 | 0.22 |
| 70–79 | 93 | 1 | 0.29 | 135 | 2 | 0.39 | 155 | 1 | 0.41 | 161 | 2 | 0.42 | 195 | 3 | 0.51 |
| >80 | 38 | 1 | 0.28 | 44 | 1 | 0.27 | 56 | 1 | 0.32 | 89 | 1 | 0.50 | 73 | 1 | 0.41 |
| Death | 470 | 7 | 0.12 | 455 | 8 | 0.12 | 499 | 8 | 0.13 | 448 | 9 | 0.12 | 536 | 10 | 0.14 |
| Cure | 5825 | 93 | 1.53 | 5517 | 92 | 1.45 | 5841 | 92 | 1.53 | 4743 | 91 | 1.24 | 4931 | 90 | 1.29 |
| New case | 6851 | 96 | 1.80 | 6935 | 96 | 1.82 | 7244 | 96 | 1.90 | 6132 | 95 | 1.61 | 6754 | 95 | 1.77 |
| Recurrence | 261 | 4 | 0.07 | 252 | 4 | 0.07 | 280 | 4 | 0.07 | 354 | 5 | 0.09 | 387 | 5 | 0.10 |
| Positive parasitologicalb | 3195 | 80 | 0.84 | 2984 | 80 | 0.78 | 2612 | 77 | 0.68 | 2244 | 76 | 0.59 | 2225 | 77 | 0.58 |
| Negative parasitologicalb | 789 | 20 | 0.21 | 752 | 20 | 0.20 | 774 | 23 | 0.20 | 698 | 24 | 0.18 | 675 | 23 | 0.18 |
| IF positiveb | 3122 | 88 | 0.82 | 3511 | 86 | 0.92 | 3676 | 86 | 0.96 | 2807 | 86 | 0.74 | 2525 | 82 | 0.66 |
| IF negativeb | 428 | 12 | 0.11 | 549 | 14 | 0.14 | 597 | 14 | 0.16 | 453 | 14 | 0.12 | 548 | 18 | 0.14 |
| HIV coinfectionab | 112 | 5 | 0.03 | 385 | 17 | 0.10 | 493 | 22 | 0.13 | 609 | 28 | 0.16 | 630 | 28 | 0.14 |
IF, indirect immunofluorescence.
Ignored or unspecified values were not considered.
Average biennial rate per 100 000 inhabitants; †percentage values compared horizontally and total vertically.
Calculated percentage between positive and negative in parasitological examination and positive and negative indirect immunofluorescence test.
The Prais–Winsten regression showed that the incidence rate of the total number of cases remained stable −4.9/100 000 inhabitants (P = 0.12), in the period from 2006 to 2015, without significant changes over the years, conferring a stable trend, without statistical significance (P > 0.05). However, in some variables, the incidence rates presented an increasing trend with positive values among indigenous peoples 61.44/100 000 inhabitants (3.75–151.19; P = 0.04), in the age groups between 40 and 59 years 6.41/100 000 in inhabitants (1.81–11.22; P = 0.01), 60–64 years old 0.93/100 000 inhabitants (0.37–1.48; P 0.00), 65–69 years old 1.86/100 000 inhabitants (0.74–2.99; P = 0.01), 70–79 years old 5.68/100 000 inhabitants (3.37–8.04; P < 0.001), >80 years old 5.44/100 000 inhabitants (−0.23 to 11.43; P 0.05), relapses 1.16/100 000 inhabitants (0.60 to 1.72; P 0.01) and co-infected HVL/HIV patients HIV 3.99/100 000 inhabitants (2.28 to 5.73; P 0.00). In other variables, it was possible to observe a decrease (P < 0.05), with negative values, such as female gender −7.1/100 000 inhabitants (−12.2 to −1.7; P = 0.02), individuals with white colour/ethnicity −9.0/100 000 inhabitants (−10.00 to −7.99; P < 0.001), Asian descendants −17.59/100 000 inhabitants (−32.45 to 0.55; P = 0.05), aged between 5 and 9 years −5.59/100 000 inhabitants (−8.67 to −2.41; P = 0.00), cure −7.10/100 000 inhabitants (−12.58 to −1.28; P = 0.03 inhabitants parasitological tests positive −7.74/100 000 inhabitants (−9.76 to −5.68; P < 0.001) and negative −0.69/100 000 inhabitants (−1.24 to −0.14; P = 0.03). The other variables remained stable (P > 0.05) (Table 3).
Table 3.
Regression analysis with annual percentage of incidence rates of characteristics of HVL in Brazil, from 2006 to 2015
| Variables | Annual incidence rates | Annual rate of change % (IC95%) | P* | Situation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | ||||
| Sex | |||||||||||||
| Male | 2.61 | 2.37 | 2.66 | 2.66 | 2.43 | 2.69 | 2.20 | 2.37 | 2.57 | 2.58 | −2.1 (−10.3 to 7.0) | 0.58 | Stable |
| Female | 1.69 | 1.40 | 1.56 | 1.45 | 1.44 | 1.60 | 1.21 | 1.26 | 1.33 | 1.37 | −7.1 (−12.2 to −1.7) | 0.02 | Descending |
| Total | 2.14 | 1.87 | 2.09 | 2.04 | 1.92 | 2.13 | 1.69 | 1.80 | 1.93 | 1.95 | −4.9 (−11.0 to 1.6) | 0.12 | Stable |
| Skin colour/ethnicity | |||||||||||||
| White/Caucasian | 0.87 | 0.76 | 0.82 | 0.71 | 0.63 | 0.69 | 0.58 | 0.57 | 0.52 | 0.47 | −9.0 (−10.00 to −7.99) | <0.001 | Descending |
| Black/Afro-descendant | 2.30 | 1.81 | 1.96 | 2.17 | 2.14 | 2.25 | 1.76 | 1.98 | 2.25 | 1.99 | −0.23 (−10.17 to 10.82) | 0.97 | Stable |
| Yellow/Asian descendant | 1.78 | 1.82 | 1.49 | 1.06 | 1.82 | 1.73 | 1.20 | 0.62 | 1.39 | 1.01 | −17.59 (−32.45 to 0.55) | 0.05 | Descending |
| Mixed | 2.75 | 2.70 | 3.07 | 3.05 | 2.87 | 3.32 | 2.60 | 2.88 | 3.17 | 3.35 | 9.40 (−4.19 to 24.91) | 0.15 | Stable |
| Indigenous | 1.96 | 2.57 | 3.06 | 3.18 | 4.65 | 3.55 | 2.45 | 4.28 | 3.18 | 5.38 | 61.44 (3.75 to 151.19) | 0.04 | Growing |
| Age group, years | |||||||||||||
| <1 | 3.96 | 3.30 | 4.66 | 3.74 | 3.85 | 4.25 | 3.37 | 4.57 | 3.84 | 3.99 | 5.20 (−8.88 to 21.45) | 0.43 | Stable |
| 1–4 | 3.10 | 2.78 | 3.15 | 2.79 | 3.09 | 3.38 | 2.33 | 2.72 | 2.71 | 2.70 | −9.64 (−21.73 to 4.33) | 0.13 | Stable |
| 5–9 | 0.90 | 0.86 | 0.91 | 0.89 | 0.76 | 0.91 | 0.63 | 0.72 | 0.77 | 0.68 | −5.59 (−8.67 to −2.41) | 0.00 | Descending |
| 10–14 | 0.35 | 0.42 | 0.42 | 0.42 | 0.38 | 0.38 | 0.30 | 0.32 | 0.34 | 0.31 | −2.28 (−4.94 to 0.46) | 0.06 | Stable |
| 15–19 | 0.37 | 0.37 | 0.37 | 0.42 | 0.36 | 0.42 | 0.34 | 0.27 | 0.33 | 0.36 | −1.37 (−4.06 to 1.39) | 0.22 | Stable |
| 20–39 | 0.56 | 0.54 | 0.56 | 0.58 | 0.59 | 0.63 | 0.53 | 0.54 | 0.59 | 0.64 | 1.39 (−0.83 to 3.66) | 0.23 | Stable |
| 40–59 | 0.65 | 0.57 | 0.69 | 0.80 | 0.73 | 0.84 | 0.74 | 0.70 | 0.90 | 0.90 | 6.41 (1.81 to 11.22) | 0.01 | Growing |
| 60–64 | 0.13 | 0.12 | 0.14 | 0.14 | 0.16 | 0.14 | 0.16 | 0.13 | 0.18 | 0.15 | 0.93 (0.37 to 1.48) | 0.00 | Growing |
| 65–69 | 0.16 | 0.17 | 0.13 | 0.16 | 0.16 | 0.22 | 0.17 | 0.18 | 0.20 | 0.23 | 1.86 (0.74 to 2.99) | 0.01 | Growing |
| 70–79 | 0.30 | 0.29 | 0.38 | 0.39 | 0.34 | 0.48 | 0.43 | 0.41 | 0.50 | 0.52 | 5.68 (3.37 to 8.04) | <0.001 | Growing |
| >80 | 0.29 | 0.26 | 0.29 | 0.26 | 0.29 | 0.34 | 0.62 | 0.38 | 0.43 | 0.39 | 5.44 (−0.23 to 11.43) | 0.05 | Growing |
| Deaths | 0.15 | 0.10 | 0.12 | 0.12 | 0.12 | 0.14 | 0.11 | 0.12 | 0.13 | 0.15 | 0.46 (−0.09 to 1.02) | 0.26 | Stable |
| Cure | 1.69 | 1.36 | 1.48 | 1.41 | 1.43 | 1.64 | 1.23 | 1.26 | 1.29 | 1.30 | −7.10 (−12.58 to −1.28) | 0.03 | Descending |
| New case | 1.91 | 1.68 | 1.77 | 1.86 | 1.80 | 2.00 | 1.55 | 1.67 | 1.76 | 1.78 | −2.50 (−9.26 to 4.76) | 0.41 | Stable |
| Recurrence | 0.09 | 0.04 | 0.06 | 0.08 | 0.07 | 0.08 | 0.10 | 0.09 | 0.09 | 0.11 | 1.16 (0.60 to 1.72) | 0.01 | Growing |
| Positive parasitological | 0.92 | 0.76 | 0.81 | 0.75 | 0.68 | 0.69 | 0.59 | 0.58 | 0.62 | 0.54 | −7.74 (−9.76 to −5.68) | <0.001 | Descending |
| Negative parasitological | 0.21 | 0.20 | 0.18 | 0.21 | 0.19 | 0.21 | 0.16 | 0.21 | 0.17 | 0.18 | −0.69 (−1.24 to −0.14) | 0.03 | Descending |
| IF positive | 0.78 | 0.86 | 0.94 | 0.90 | 0.89 | 1.04 | 0.77 | 0.70 | 0.65 | 0.68 | −4.50 (−12.58 to 4.33) | 0.25 | Stable |
| IF negative | 0.12 | 0.11 | 0.14 | 0.15 | 0.17 | 0.15 | 0.11 | 0.13 | 0.15 | 0.14 | 0.46 (−1.19 to 2.14) | 0.54 | Stable |
| HIV coinfection | 0.00 | 0.06 | 0.09 | 0.11 | 0.12 | 0.14 | 0.17 | 0.15 | 0.16 | 0.17 | 3.99 (2.28 to 5.73) | 0.00 | Growing |
*Prais–Winsten regression (P < 0.05).
The highest proportion of cases of HVL was in the Northeast (19 908; 53%), but the highest incidence was in the North Region (AAR of 7.35/100 000 inhabitants). Among the states with the highest rates of HVL, Ceará was the prevalent, with 5654 (15%) of the reported cases, but the highest rate was in the state of Tocantins (AAR of 24.18/100 000 inhabitants) (Table 4).
Table 4.
Epidemiological characteristics of HVL in Brazilian regions and states, from 2006 to 2015
| 2006–2007 (n) | % | Ratea | 2008–2009 (n) | % | Ratea | 2010–2011 (n) | % | Ratea | 2012–2013 (n) | % | Ratea | 2014–2015 (n) | % | Ratea | Total | % | AAR | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Region | ||||||||||||||||||
| North | 1563 | 21 | 8.72 | 1642 | 21 | 8.95 | 1605 | 21 | 8.31 | 1209 | 18 | 6.26 | 961 | 13 | 2.81 | 6980 | 19 | 7.35 |
| Northeast | 3828 | 51 | 6.16 | 3854 | 48 | 6.06 | 3913 | 49 | 6.10 | 3549 | 53 | 5.53 | 4764 | 64 | 10.83 | 19 908 | 53 | 6.20 |
| Southeast | 1463 | 20 | 1.54 | 1689 | 21 | 1.75 | 1570 | 19 | 1.62 | 1222 | 17 | 1.26 | 1266 | 17 | 4.60 | 7210 | 19 | 1.50 |
| South | 8 | 0 | 0.02 | 13 | 1 | 0.04 | 13 | 1 | 0.04 | 9 | 1 | 0.03 | 12 | 0 | 0.04 | 55 | 0 | 0.04 |
| Midwest | 625 | 8 | 3.91 | 684 | 9 | 4.16 | 705 | 9 | 4.13 | 748 | 11 | 4.38 | 498 | 7 | 5.28 | 3260 | 9 | 3.79 |
| States | ||||||||||||||||||
| Alagoas | 83 | 1 | 1.33 | 59 | 1 | 0.95 | 70 | 1 | 1.12 | 61 | 1 | 0.98 | 95 | 1 | 4.57 | 368 | 1 | 1.15 |
| Amazonas | 3 | 0 | 0.04 | 6 | 0 | 0.09 | 1 | 0 | 0.01 | 2 | 0 | 0.03 | 1 | 0 | 0.01 | 13 | 0 | 0.04 |
| Amapá | 2 | 0 | 0.15 | 1 | 0 | 0.07 | 0 | 0 | 0.00 | 1 | 0 | 0.07 | 0 | 0 | 0.00 | 4 | 0 | 0.07 |
| Bahia | 636 | 8 | 2.27 | 573 | 7 | 2.04 | 806 | 11 | 2.88 | 644 | 10 | 2.30 | 937 | 13 | 9.90 | 3596 | 10 | 2.49 |
| Ceará | 1187 | 17 | 7.02 | 1237 | 16 | 7.32 | 1152 | 14 | 6.81 | 890 | 13 | 5.26 | 1188 | 16 | 11.76 | 5654 | 15 | 6,47 |
| Distrito Federal | 32 | 0 | 0.62 | 32 | 0 | 0.62 | 19 | 0 | 0.37 | 29 | 0 | 0.56 | 26 | 0 | 0.35 | 138 | 0 | 0,67 |
| Espírito Santo | 1 | 0 | 0.01 | 10 | 0 | 0.14 | 10 | 0 | 0.14 | 5 | 0 | 0.07 | 14 | 0 | 0.13 | 40 | 0 | 0.15 |
| Goiás | 66 | 1 | 0.55 | 74 | 1 | 0.62 | 84 | 1 | 0.70 | 76 | 1 | 0.63 | 113 | 2 | 0.62 | 413 | 1 | 0.68 |
| Maranhão | 907 | 12 | 6.90 | 1029 | 13 | 7.83 | 933 | 11 | 7.10 | 1050 | 16 | 7.99 | 1213 | 16 | 17.45 | 5132 | 14 | 7.99 |
| Minas Gerais | 834 | 11 | 2.13 | 1147 | 14 | 2.93 | 1111 | 14 | 2.83 | 756 | 11 | 1.93 | 889 | 12 | 9.17 | 4737 | 13 | 2.45 |
| Mato Grosso do Sul | 475 | 6 | 9.70 | 450 | 6 | 9.19 | 490 | 7 | 10.00 | 554 | 8 | 11.31 | 312 | 4 | 4.50 | 2281 | 6 | 8.94 |
| Mato Grosso | 53 | 1 | 0.87 | 128 | 2 | 2.11 | 112 | 1 | 1.85 | 89 | 1 | 1.47 | 47 | 1 | 0.68 | 429 | 1 | 1.33 |
| Pará | 878 | 12 | 5.79 | 719 | 9 | 4.74 | 708 | 9 | 4.67 | 546 | 8 | 3.60 | 540 | 7 | 3.29 | 3391 | 9 | 4.48 |
| Paraíba | 62 | 1 | 0.82 | 62 | 1 | 0.82 | 73 | 1 | 0.97 | 81 | 1 | 1.08 | 107 | 1 | 1.55 | 385 | 1 | 1.01 |
| Pernambuco | 179 | 2 | 1.02 | 160 | 2 | 0.91 | 158 | 2 | 0.90 | 142 | 2 | 0.81 | 347 | 5 | 1.81 | 986 | 3 | 1.14 |
| Piauí | 505 | 7 | 8.10 | 466 | 6 | 7.47 | 367 | 5 | 5.88 | 403 | 6 | 6.46 | 551 | 7 | 5.40 | 2292 | 6 | 7.45 |
| Paraná | 3 | 0 | 0.01 | 4 | 0 | 0.02 | 7 | 0 | 0.03 | 5 | 0 | 0.02 | 7 | 0 | 0.09 | 26 | 0 | 0.03 |
| Rio de Janeiro | 12 | 0 | 0.04 | 4 | 0 | 0.01 | 7 | 0 | 0.02 | 14 | 0 | 0.04 | 12 | 0 | 0.24 | 49 | 0 | 0.03 |
| Rio Grande do Norte | 146 | 2 | 2.30 | 189 | 3 | 2.98 | 204 | 3 | 3.22 | 175 | 2 | 2.76 | 193 | 3 | 11.81 | 907 | 2 | 2.85 |
| Rondônia | 4 | 0 | 0.13 | 1 | 0 | 0.03 | 0 | 0 | 0.00 | 6 | 0 | 0.19 | 1 | 0 | 0.03 | 12 | 0 | 0.07 |
| Roraima | 7 | 0 | 0.78 | 8 | 0 | 0.89 | 30 | 0 | 3.33 | 30 | 0 | 3.33 | 39 | 1 | 2.17 | 114 | 0 | 3.17 |
| Rio Grande do Sul | 2 | 0 | 0.01 | 9 | 0 | 0.04 | 4 | 0 | 0.02 | 2 | 0 | 0.01 | 5 | 0 | 0.04 | 22 | 0 | 0.02 |
| Santa Catarina | 1 | 0 | 0.01 | 1 | 0 | 0.01 | 2 | 0 | 0.02 | 3 | 0 | 0.02 | 0 | 0 | 0.00 | 7 | 0 | 0.01 |
| Sergipe | 123 | 2 | 2.97 | 79 | 1 | 1.91 | 150 | 2 | 3.63 | 103 | 1 | 2.49 | 133 | 2 | 4.01 | 588 | 2 | 2.83 |
| São Paulo | 533 | 7 | 0.65 | 528 | 7 | 0.64 | 442 | 6 | 0.54 | 447 | 8 | 0.54 | 351 | 5 | 0.43 | 2301 | 6 | 0.55 |
| Tocantins | 668 | 9 | 24.14 | 907 | 11 | 32.78 | 865 | 11 | 31.26 | 624 | 9 | 22.55 | 380 | 5 | 6.45 | 3444 | 9 | 24.18 |
AAR, average annual rate per 100 000 inhabitants.
Ignored or unspecified values were not considered.
Average biennial rate per 100 000 inhabitants.
In relation to the regions and states of Brazil, the annual increase in incidence rates of VL in the period from 2006 to 2015 remained stable, without significant changes, over the years, in most variables, conferring a stable trend, without statistical significance (P > 0.05). Except for the variables that showed a decreasing trend (P < 0.05), with negative values, as in the North −67.79/100 000 inhabitants (−82.56 to −40.52; P = 0.00), in the states of Pará −47.03/100 000 inhabitants (−60.92 to −28.22; P = 0.00) and São Paulo −6.24/100 000 inhabitants (−9.30 to −3.08; P = 0.00), as well as the variables with increasing trend, with positive values (P < 0.05), such as the states of Goiás 8.89/100 000 inhabitants (0.79–17.65; P = 0.03), Paraíba 17.76/1 000 000 inhabitants (5.44–31.52; P = 0.01; P = 0.76 Paraná 0.69/100 000 inhabitants (0.14–1.25; P = 0.02) and Roraima 44.54/100 000 inhabitants (−7.62 to 126.15; P = 0.00) (Table 5).
Table 5.
Regression analysis with annual percentage of incidence rates of HVL in Brazil regions and states
| Variables | Annual incidence rates | Taxa de variação anual % (IC95%) | P* | Situação | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | ||||
| Region | |||||||||||||
| North | 8.63 | 8.80 | 9.43 | 8.47 | 7.25 | 9.37 | 6.59 | 5.92 | 4.53 | 5.41 | −67.79 (−82.56 to −40.52) | 0.00 | Descending |
| Northeast | 6.85 | 5.47 | 5.99 | 6.14 | 5.79 | 6.40 | 4.84 | 6.22 | 7.60 | 7.24 | 25.31 (−26.28 to 113.01) | 0.34 | Stable |
| Southeast | 1.61 | 1.46 | 1.76 | 1.74 | 1.66 | 1.57 | 1.36 | 1.15 | 1.20 | 1.41 | −9.01 (−20.31 to 3.90) | 0.14 | Stable |
| South | 0.03 | 0.02 | 0.02 | 0.06 | 0.05 | 0.03 | 0.04 | 0.02 | 0.04 | 0.04 | 0.23 (−0.87 to 1.34) | 0.78 | Stable |
| Midwest | 4.10 | 3.73 | 4.48 | 3.84 | 3.83 | 4.42 | 4.79 | 3.97 | 3.10 | 2.73 | −22.91 (−52.07 to 23.99) | 0.23 | Stable |
| States | |||||||||||||
| Alagoas | 1.63 | 1.03 | 0.87 | 1.03 | 1.12 | 1.12 | 1.15 | 0.80 | 1.38 | 1.67 | 3.99 (−15.71 to 28.29) | 0.68 | Stable |
| Amazonas | 0.03 | 0.06 | 0.09 | 0.09 | 0.00 | 0.03 | 0.06 | 0.00 | 0.03 | 0.00 | −1.37 (−2.99 to 0.28) | 0.10 | Stable |
| Amapá | 0.15 | 0.15 | 0.15 | 0.00 | 0.00 | 0.00 | 0.00 | 0.15 | 0.00 | 0.00 | −3.39 (−7.57 to 0.97) | 0.13 | Stable |
| Bahia | 2.78 | 1.76 | 1.47 | 2.62 | 2.91 | 2.84 | 2.22 | 2.38 | 3.74 | 2.95 | 31.52 (−7.15 to 86.29) | 0.10 | Stable |
| Ceará | 7.52 | 6.52 | 6.58 | 8.06 | 6.39 | 7.24 | 4.85 | 5.68 | 7.31 | 6.74 | −18.72 (−52.97 to 40.48) | 0.40 | Stable |
| Distrito Federal | 0.70 | 0.54 | 0.78 | 0.47 | 0.27 | 0.47 | 0.47 | 0.66 | 0.47 | 0.54 | −3.17 (−11.85 to 6.37) | 0.44 | Stable |
| Espírito Santo | 0.03 | 0.00 | 0.09 | 0.20 | 0.06 | 0.23 | 0.03 | 0.11 | 0.09 | 0.31 | 3.51 (−0.96 to 8.19) | 0.09 | Stable |
| Goiás | 0.63 | 0.47 | 0.60 | 0.63 | 0.78 | 0.62 | 0.57 | 0.70 | 0.95 | 0.93 | 8.89 (0.79 to 17.65) | 0.03 | Growing |
| Maranhão | 7.60 | 6.19 | 8.78 | 6.87 | 6.84 | 7.35 | 5.20 | 10.77 | 8.68 | 9.76 | 81.97 (−16.52 to 296.64) | 0.11 | Stable |
| Minas Gerais | 1.99 | 2.27 | 2.79 | 3.06 | 3.00 | 2.67 | 2.09 | 1.77 | 2.01 | 2.53 | 6.17 (−32.52 to 67.03) | 0.76 | Stable |
| Mato Grosso do Sul | 9.88 | 9.51 | 10.33 | 8.04 | 8.86 | 11.15 | 12.66 | 9.96 | 7.23 | 5.51 | −54.50 (−91.75 to 150.96) | 0.31 | Stable |
| Mato Grosso | 0.69 | 1.05 | 1.94 | 2.27 | 1.85 | 1.85 | 1.75 | 1.19 | 0.63 | 0.92 | 0.23 (−42.00 to 73.22) | 0.99 | Stable |
| Pará | 6.65 | 4.93 | 5.26 | 4.22 | 4.26 | 5.08 | 3.67 | 3.54 | 3.18 | 3.94 | −47.03 (−60.92 to −28.22) | 0.00 | Descending |
| Paraíba | 0.98 | 0.66 | 1.09 | 0.56 | 0.80 | 1.14 | 1.14 | 1.01 | 1.59 | 1.25 | 17.76 (5.44 to 31.52) | 0.01 | Growing |
| Pernambuco | 1.15 | 0.89 | 0.95 | 0.86 | 0.81 | 0.99 | 0.82 | 0.80 | 1.93 | 2.01 | 22.74 (−10.92 to 69.12) | 0.17 | Stable |
| Piauí | 7.98 | 8.21 | 9.04 | 5.90 | 5.03 | 6.73 | 6.13 | 6.80 | 9.14 | 8.53 | 69.00 (−69.0 to 268.64) | 0.90 | Stable |
| Paraná | 0.03 | 0.00 | 0.02 | 0.02 | 0.01 | 0.06 | 0.01 | 0.04 | 0.02 | 0.05 | 0.69 (0.14 to 1.25) | 0.02 | Growing |
| Rio de Janeiro | 0.06 | 0.02 | 0.00 | 0.03 | 0.01 | 0.03 | 0.03 | 0.06 | 0.03 | 0.04 | 0.46 (−0.64 to 1.58) | 0.40 | Stable |
| Rio Grande do Norte | 2.37 | 2.24 | 2.94 | 3.03 | 2.68 | 3.76 | 3.00 | 2.53 | 3.19 | 2.90 | 15.61 (−9.84 to 48.25) | 0.20 | Stable |
| Rondônia | 0.00 | 0.26 | 0.00 | 0.06 | 0.00 | 0.00 | 0.13 | 0.26 | 0.06 | 0.00 | 0.23 (−0.32 to 0.79) | 0.94 | Stable |
| Roraima | 1.11 | 0.44 | 0.44 | 1.33 | 3.55 | 3.11 | 2.22 | 4.44 | 4.00 | 4.66 | 44.54 (−7.62 to 126.15) | 0.00 | Growing |
| Rio Grande do Sul | 0.02 | 0.00 | 0.00 | 0.08 | 0.02 | 0.02 | 0.00 | 0.02 | 0.04 | 0.01 | 0.23 (−1.42 to 1.91) | 0.93 | Stable |
| Santa Catarina | 0.00 | 0.02 | 0.02 | 0.00 | 0.00 | 0.03 | 0.03 | 0.02 | 0.00 | 0.00 | 0.02 (−1.08 to 1.13) | 0.97 | Stable |
| Sergipe | 2.47 | 3.48 | 1.84 | 1.98 | 4.01 | 3.24 | 2.56 | 2.42 | 3.24 | 3.19 | 12.20 (−25.87 to 69.82) | 0.52 | Stable |
| São Paulo | 0.66 | 0.63 | 0.73 | 0.55 | 0.52 | 0.55 | 0.60 | 0.48 | 0.44 | 0.41 | −6.24 (−9.30 to −3.08) | 0.00 | Descending |
| Tocantins | 17.64 | 30.65 | 33.25 | 32.31 | 26.09 | 36.43 | 24.94 | 20.17 | 12.79 | 14.67 | −74.88 (−95.21 to 31.83) | 0.28 | Stable |
*Prais–Winsten regression (P < 0.05).
The highest rates presented, according to the classification from the highest to the lowest, referring to the total number of notified cases, were from the North (AAR of 7.35/100 000 inhabitants), Northeast (AAR of 6.20/100 000 inhabitants), Midwest (AAR of 3.79/100 000 inhabitants), Southeast (AAR of 1.50/100 000 inhabitants) and South (AAR of 0.04/100 000 inhabitants). The highest proportion of cases (64%; mean: 53; median: 63; s.d. ±5) and the highest rate of HVL over the years were from Northeast Region, between the years 2014 and 2015 (AAR of 10.83/100 000 inhabitants; mean: 6.94; 95% CI 5.40–8.47). The North Region had the second highest incidence over the years, in the period between 2008 and 2009 (AAR of 8.95/100 000 inhabitants).
In order of classification, the states that had the highest HVL rates, between 2006 and 2015, were Tocantins (AAR of 24.18/100 000 inhabitants), Mato Grosso do Sul (AAR of 8.94/100 000 inhabitants), Maranhão (AAR of 7.99/100 000 inhabitants), Piauí (AAR of 7.45/100 000 inhabitants), Ceará (AAR of 6.47/100 000 inhabitants) and Pará (AAR of 4.48/100 000 inhabitants).
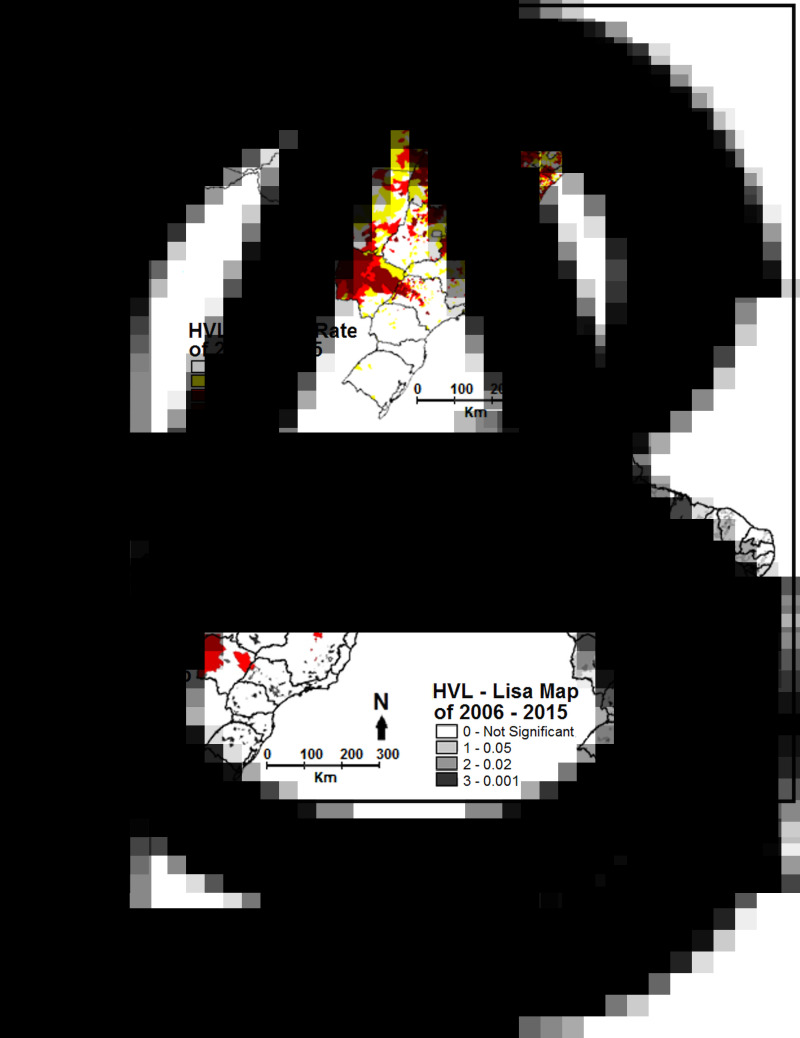
The thematic maps showed the presence of municipalities and/or clusters statistically significant (P < 0.05), with high HVL rates, in the Northeast, North, Midwest and Southeast Regions (Fig. 1A). A higher concentration of LVH rates forming clusters was found in the Northeast Region, covering the nine northeastern states. In the North Region, there was a concentration of clusters throughout the state of Tocantins and the southeast of the state of Pará. In the Midwest Region, the rate clusters encompassed practically the entire state of Mato Grosso do Sul and the central and southern part of the state of Mato Grosso. The global Moran's I was 0.46 (P < 0.01), indicating similarity between neighbouring municipalities.
Fig. 1.
HVL data by municipalities of residence in Brazil, between the years 2006 to 2015. (A) Crude rate distribution per 100 000 inhabitants; (B) Moran map; (C) LISA map.
Discussion
This study provides an in-depth view of the HVL in Brazil, characterizing spatial and temporal patterns of its occurrence, in the period. Spatial clusters of HVL were presented in this study. Despite the slight decrease in the number of cases reported nationally in recent years, HVL has expanded geographically to other regions. This information is worrying and follow different pattern, related to regions, as sex, age group and skin colour, exposing a problem for the public health (Nascimento et al., 2011; Martins-Melo et al., 2014a; Brasil, 2015; Druzian et al., 2015; Herrador et al., 2015; Lane, 2016).
HVL is expanding geographically in Brazil. The epidemiological profile of this disease has been modified in developing countries (Herrador et al., 2015), due to its expansion from rural to urban areas (Nascimento et al., 2011; Albuquerque et al., 2014; Brasil, 2015; Druzian et al., 2015).
The higher prevalence found in male individuals may be related to socioeconomic, behavioural and environmental factors (Martins-Melo et al., 2014b). The literature indicates that the disease affects both sexes, but men are described as the most susceptible (Lane, 2016). Occurrence was higher in the age group between 1 and 4 years old, with higher incidence between those who were under 1 year, possibly because children are more susceptible to morbidity and mortality, probably due to the greater contact with animals, the cycle of home/peridomestic transmission and by vectors, as well as nutritional and immune deficiencies (Martins-Melo et al., 2014a; Guimarães et al., 2015).
The HVL prevalence was higher between those with mixed-race – and most of the Brazilian population considers themselves as with mixed-race. The higher incidence was found between the indigenous people. Indigenous populations are more susceptible to HVL, probably due because of the gold mining activities, the increased immigration from endemic areas and the visits to family members taking dogs contaminated by Leishmania or contaminated in the place visited (Guimarães et al., 2015; Silva and Abud, 2016).
The method for diagnosing HVL used with the greatest number of positive cases was the indirect immunofluorescence test. This test is more effective than the parasitological test, since it is based on the antibody response (World Health Organization., 2010; Dupnik et al., 2011;Souza et al., 2012; Cota et al., 2013, 2014; Albuquerque et al., 2014; Druzian et al., 2015; Távora et al., 2015).
According to DATASUS, out of the HVL patients, 93% were considered cured and 7% died, with prevalent numbers in the Northeast Region (49.74%) and in the state of Minas Gerais (18.87%). HVL is a potentially lethal disease if not treated and diagnosed early (Martins-Melo et al., 2014a).
Concerning the type of entry, 96% were new cases and 4% were recurrence. The mortality rates are often higher in immunocompromised individuals and in recurrences (Gomes et al., 2012; Fontoura et al., 2018b).
Using geoprocessing techniques, we analysed the distribution of the occurrence of HVL, and the detection of statistically significant spatial clusters. The HVL distribution is heterogeneous, but, with the different techniques for spatial analysis, it is possible to identify areas with greater and lower needs for interventions, where control measures, when targeted, become more effective (Martins-Melo et al., 2014a; Silva and Abud, 2016).
The national AAR of HVL was 1.95 per 100 000 inhabitants. When analysing the spatial distribution of HVL rates, we found that HVL was recorded in all Brazilian regions – with clusters prevalent in the Northeast Region. However, the highest rate was in the North Region (rate 7.04/100 000 inhabitants), specifically in the state of Tocantins (rate 21.65/100 000 inhabitants). The HVL notified cases have increased and expanded to other areas in the state of Tocantins (Fontoura et al., 2016). Irregular land occupation causes environmental imbalance.
A study carried out in Tocantins State evaluated the correlation of the HVL incidence rate with environmental and climate variables. These rates increase as night temperature increases, as well as air humidity and precipitation (Reis et al., 2019). Temperature increase is associated with phlebotomine density increase, contributing to the occurrence of higher contact between vector and host and favouring the disease spread (Galati et al., 2015), as well as its activity (Rivas et al., 2014). Consequently, there is higher parasitic load in the vector due to the increase in the number of times blood repast occurs (Serafim et al., 2018) and Straw mosquito infectivity.
The city of Araguaia, in the North of the state, has one of the highest rates of HVL (Silva, 2016). In addition to intense urban expansion, deforestation without proper planning and inadequate infrastructure shows climate and environmental factors favourable to the vector development (Reis et al., 2019). Besides climate and environmental factors, others have been associated with subjects, including socioeconomic status, low immunity, and nutritional condition (Toledo et al., 2017).
The VL is a disease associated with poverty that also perpetuates it (Lane, 2016). The high number of cases in the Northeast Region reflects the socio-environmental conditions that favour the spread of HVL (Martins-Melo et al., 2014b; Silva et al., 2015).
According to the Moran maps of HVL, the Northeast Region, part of the North Region and part of the Midwest Region are among the regions with the greatest need for intervention (in red, which corresponds to high-high). The LISA map indicated the statistically significant clusters (P = 0.001). Thus, it is possible to observe the importance of TerraView software for spatial analysis studies, showing regions with greater and lower intervention needs (Campi and Nascimento, 2014).
Poor living conditions in the community favour the proliferation of diseases (Diro et al., 2014; Castelo Branco et al., 2016). It is necessary to develop the correction of precarious infrastructure questions and inadequate packaging waste, and to improve HDI indicators (Brasil, 2014; Ursine et al., 2016). The lack of basic sanitation and the breeding of animals around the home favour human and canine infections by attracting Lutzomyia longipalpis (Lane, 2016).
From the moment one invests to improve the poor living conditions of a population, the spread of disease must be minimized. The number of HVL cases increases in regions with conditions conducive to the development of sandflies, especially in the peridomicile (Nascimento et al., 2011; Albuquerque et al., 2014; Brasil, 2015; Druzian et al., 2015). In a place where rigorous control measures were adopted, with the reorganization of the home, construction of suitable places for animal shelters away from the residence, improvement of the sanitary facilities, proper packaging waste, pruning of trees, it was possible to drastically reduce the number of sandflies around 90% (Machado et al., 2016). Ignorance in relation to VL control measures, both for the population and health professionals, added to the lack of infrastructure for early diagnosis and treatment in health services, has contributed to the expansion of VL in Brazil (Lane, 2016).
The HVL has changed its epidemiological profile and increased its morbidity and mortality. This fact requires urgent attention from epidemiological surveillance agencies, aiming at preventive and interventional measures, such as combating the vector and breeding sites, mainly with investments from the agencies responsible for correcting deficiencies in infrastructure of basic sanitation (adequate waste disposal and sewage), especially in communities with poor living conditions (Ursine et al., 2016). The HVL has been spread to other areas, but it has also maintained the old outbreaks, indicating the inefficiency of current control measures.
There are still many obstacles to control HVL, with enormous challenges (Araújo et al., 2012; Menon et al., 2016; Silva and Abud, 2016). All patients with characteristic signs and symptoms of HVL in endemic areas should be investigated, aiming at early diagnosis and treatment (Alexandrino-de-Oliveira et al., 2010; Martins-Melo et al., 2014b; Brasil, 2015), in addition to making compulsory notification (World Health Organization., 2010; Brasil, 2014; Albuquerque et al., 2014), so that databases of institutions such as the World Health Organization and the Pan American Health Organization were fed (Araújo et al., 2012; Das et al., 2014; Albuquerque et al., 2014). However, despite the adoption of preventive measures, intending to interrupt the transmission cycle, such as early treatment of positive human cases, chemical control of vectors, and elimination of infected domestic reservoirs, there has been an increase in the HVL impulse in national public health (Machado et al., 2016). The adaptive characteristics of the L. longipalpis vector hinder the epidemiological control (Van Griensven et al., 2014; Castelo Branco et al., 2016).
Secondary data are subject to limitations due to possible inconsistencies in information and/or underreporting, despite significant progress in the quality and the coverage of information in recent years (Martins-Melo et al., 2014b; Cardim et al., 2015). In addition to underreporting, there are ignored or blank items, which should have been filled out or reported correctly, limiting the robustness of the data. As the canine data are incomplete for many municipalities, with little information about areas research, universe of dogs and type of survey (sample or census), the occurrence values can be biased and/or inconclusive.
Conclusion
Our study offered the detection and analysis of clusters of HVL rates and the occurrence, as well as pointed out the sites with greater and lower need for intervention. Based on the Prais–Winsten estimation, we found a stabilization of HVL in the average annual rates per 100 000 inhabitants. Mortality rates have remained stable in the last 5 years, and there has been a slight drop in the average annual rates of new cases in the last 9 years, suggesting that, in some way, interventional actions have an effect on reducing or maintaining cases of VL in different epidemiological contexts, despite the many obstacles to the control of this disease. It is expected that these findings will be useful for planning disease surveillance and control actions in the country.
Financial support
This work was supported by the Foundation for Research and Scientific and Technological Development of Maranhão – FAPEMA (PAEDT, concession number 02290/15; UNIVERSAL, concession number 01015/17). Dr Ana Lucia Abreu-Silva is a research productivity fellow of National Scientific and Technological Development Council (Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq) grant number 309885/2017-5.
Ethical standards
This study was approved by the ethics committee of the Universidade Federal do Maranhão (UFMA), under protocol: 1.073.550 and CAAE: 41557314.5.0000.5087.
Conflict of interest
The authors declare no conflict of interest.
References
- Albuquerque LCPD, Mendonça IR, Cardoso PN, Baldaçara LR, Borges MRMM, da Borges JC and da Pranchevicius MCS (2014) HIV/AIDS-related visceral leishmaniasis: a clinical and epidemiological description of visceral leishmaniasis in northern Brazil. Revista da Sociedade Brasileira de Medicina Tropical 47, 38–46. [DOI] [PubMed] [Google Scholar]
- Alexandrino-de-Oliveira P, Santos-Oliveira JR, Dorval MEC, das Da-Costa FCB, Pereira GROL, da Cunha RV, Paniago AMM and Da-Cruz AM (2010) HIV/AIDS-associated visceral leishmaniasis in patients from an endemic area in Central-west Brazil. Memórias do Instituto Oswaldo Cruz 105, 692–697. [DOI] [PubMed] [Google Scholar]
- Araújo VEM, Morais MHF, Reis IA, Rabello A and Carneiro M (2012) Early clinical manifestations associated with death from visceral leishmaniasis. PLoS Neglected Tropical Diseases 6, e1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo VEM, Pinheiro LC, de Almeida MCM, de Menezes FC, Morais MHF, Reis IA, Assunção RM and Carneiro M (2013) Relative risk of visceral leishmaniasis in Brazil: a spatial analysis in urban area. PLoS Neglected Tropical Diseases 7, e2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda RMF, Cardoso DT, Teixeira-Neto RG, Barbosa DS, Ferraz RK, Morais MHF, Belo VS and da Silva ES (2019) Space-time analysis of the incidence of human visceral leishmaniasis (VL) and prevalence of canine VL in a municipality of southeastern Brazil: identification of priority areas for surveillance and control. Acta Tropica 197, 105052. [DOI] [PubMed] [Google Scholar]
- Barbosa DS and Werneck GL (2011) Spatial distribution and definition of priority areas for surveillance of visceral leishmaniasis in São Luís, Maranhão, Brazil, 1999–2007. FIOCRUZ – Fundação Oswaldo Cruz Escola Nacional de Saúde Pública Sergio Arouca Programa de Pós-graduação Epidemiologia em Saúde Pública, 1, 30–61. [Google Scholar]
- Barbosa DS, Belo VS, Rangel MES and Werneck GL (2014) Spatial analysis for identification of priority areas for surveillance and control in a visceral leishmaniasis endemic area in Brazil. Acta Tropica 131, 56–62. [DOI] [PubMed] [Google Scholar]
- Brasil MS (2014) Manual de vigilância e controle da leishmaniose visceral, 1a. ed. Brasil: Brasília-Distrito FederalMinistério da Saúde, S. de V. em S. D. de V. and Transmissíveis. Ministério da Saúde, Brasília-Distrito Federal. [Google Scholar]
- Brasil MS (2015) Manual de recomendações para diagnóstico, tratamento e acompanhamento de pacientes com a coinfecção Leishmania-HIV, 1a. ed. Brasil: Ministério da Saúde, S. de V. em S. D. de V. and Transmissíveis. Ministério da Saúde, Brasília-Distrito Federal. [Google Scholar]
- Brasil MS (2017a) Sistemas de Informação de Agravos de Notificação (SINAN). Leishmaniose visceral: Casos confirmados de 2001 a 2006.
- Brasil MS (2017b) Sistemas de Informação de Agravos de Notificação (SINAN), leishmaniose visceral: Casos confirmados de 2007 a 2015. Brasil. Ministério da Saúde (MS).
- Brilhante AF, Melchior LAK, Nunes VLB, de Cardoso CO and Galati EAB (2017) Epidemiological aspects of American cutaneous leishmaniasis (ACL) in an endemic area of forest extractivist culture in western Brazilian Amazonia. Revista do Instituto de Medicina Tropical de São Paulo 59, 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campi SFS and Nascimento LFC (2014) Spatial distribution of C-sections within the state of São Paulo. Revista da Associação Médica Brasileira 60, 419–423. [Google Scholar]
- Cardim MFM, Vieira CP and Chiaravalloti-Neto F (2015) Spatial and spatiotemporal occurrence of human visceral leishmaniasis in Adamantina, State of São Paulo, Brazil. Revista da Sociedade Brasileira de Medicina Tropical 48, 716–723. [DOI] [PubMed] [Google Scholar]
- Carvalho RM and Nascimento LF (2014). Space-time description of dengue outbreaks in Cruzeiro, Sao Paulo, in 2006 and 2011. Revista da Associação Médica Brasileira (1992) 60, 565–570. [DOI] [PubMed] [Google Scholar]
- Carvalho FLN, Riboldi EDO, Bello GL, Ramos RR, Barcellos RB, Gehlen M, Halon ML, Romão PRT, Dallegrave E and Rossetti MLR (2018) Canine visceral leishmaniasis diagnosis: a comparative performance of serological and molecular tests in symptomatic and asymptomatic dogs. Epidemiology and Infection 146, 571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelo Branco PV, Soares R-EP, de Jesus LCL, Moreira VR, Alves HJ, de Castro Belfort MR, Silva VLM and Ferreira Pereira SR (2016) The antileishmanial drug miltefosine (Impavido®) causes oxidation of DNA bases, apoptosis, and necrosis in mammalian cells. Mutation Research/Genetic Toxicology and Environmental Mutagenesis 806, 34–39. [DOI] [PubMed] [Google Scholar]
- Costa DL, Rocha RL, Carvalho RMA, Lima-Neto AS, Harhay MO, Costa CHN, Barral-Neto M and Barral AP (2013) Serum cytokines associated with severity and complications of kala-azar. Pathogens and Global Health 107, 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa JS, Dos Santos-Júnior FM, Moreira RS and Góes MADO (2019) Tendência temporal da sífilis congênita em Sergipe, Brasil, 2006–2017. Revista de Saúde Coletiva da UEFS 9, 8. [Google Scholar]
- Cota GF, de Sousa MR, de Freitas Nogueira BM, Gomes LI, Oliveira E, Assis TSM, de Mendonça ALP, Pinto BF, Saliba JW and Rabello A (2013) Comparison of parasitological, serological, and molecular tests for visceral leishmaniasis in HIV-infected patients: a cross-sectional delayed-type study. The American Journal of Tropical Medicine and Hygiene 89, 570–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota GF, de Sousa MR, de Mendonça ALP, Patrocinio A, Assunção LS, de Faria SR and Rabello A (2014) Leishmania-HIV co-infection: clinical presentation and outcomes in an urban area in Brazil. PLoS Neglected Tropical Diseases 8, e2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Halder A, Rabidas VN, Mandal A and Das P (2014) Specific noninvasive detection of Leishmania donovani in desquamated buccal cell swab samples from human visceral Leishmaniasis-HIV coinfected patients. Journal of Clinical Microbiology 52, 1238–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diro E, Lynen L, Mohammed R, Boelaert M, Hailu A and van Griensven J (2014) High parasitological failure rate of visceral leishmaniasis to sodium stibogluconate among HIV co-infected adults in Ethiopia. PLoS Neglected Tropical Diseases 8, e2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druzian AF, de Souza AS, de Campos DN, Croda J, Higa MG, Dorval MEC, Pompilio MA, de Oliveira PA and Paniago AMM (2015) Risk factors for death from visceral leishmaniasis in an urban area of Brazil. PLoS neglected tropical diseases 9, e0003982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte-Cunha M, Souza-Santos R, Matos HJ and Oliveira ML (2012) Epidemiological aspects of leprosy: a spatial approach. Cadernos de Saude Publica 28, 1143–1155. [DOI] [PubMed] [Google Scholar]
- Dupnik KM, Nascimento EL, Rodrigues-neto JF, Keesen T, Duarte I and Jeronimo SMB (2011) New challenges in the epidemiology and treatment of visceral leishmaniasis in periurban areas. Drug Development Research 72, 451–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falavina LP, Lentsck MH and de Mathias TAF (2019) Tendência e distribuição espacial de doenças infecciosas em gestantes no estado do Paraná-Brasil. Revista Latino-Americana de Enfermagem 27, 1–10. doi: 10.1590/1518-8345.2838.3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontoura IG, Fontoura VM and Nascimento LFC (2016) Análise espacial da ocorrência de leishmaniose visceral no estado do Tocantins, Brasil. Ambiente e Agua – An Interdisciplinary Journal of Applied Science 11, 1088. [Google Scholar]
- Fontoura VM, Graepp-Fontoura I, Santos FS, Santos Neto M, de Tavares HSA, Bezerra MOL, de Feitosa MO, Neves AF, de Morais JCM and Nascimento LFC (2018a) Socio-environmental factors and diarrheal diseases in under five-year old children in the state of Tocantins, Brazil. PLoS ONE 13, e0196702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontoura IG, Barbosa DS, de Andrade Paes AM, Santos FS, Neto MS, Fontoura VM, Lopes Costa JM and Abreu Silva AL (2018b) Epidemiological, clinical and laboratory aspects of human visceral leishmaniasis (HVL) associated with human immunodeficiency virus (HIV) coinfection: a systematic review – CORRIGENDUM. Parasitology 145, 1819–1819. [DOI] [PubMed] [Google Scholar]
- Galati EAB, de Camara TNL, Natal D and Chiaravalloti-neto F (2015) Mudanças climáticas e saúde urbana. Revista Universidade de São Paulo – USP 107, 79–90. [Google Scholar]
- Gomes MLS, Romero GAS and Werneck GL (2012) Coinfecção leishmaniose visceral e Aids no Brasil, 2001 a 2010. Dissertação (Mestrado) – Escola Nacional de Saúde Pública Sergio Arouca 105.
- Guimarães AGF, Alves GBM, de Pessoa AM and da Junior NJS (2015) Spatial analysis of visceral leishmaniasis in the municipality of Rondonópolis, in the Brazilian State of Mato Grosso, from 2003 to 2012: human, canine and vector distribution in areas of disease transmission. Revista da Sociedade Brasileira de Medicina Tropical 48, 291–300. [DOI] [PubMed] [Google Scholar]
- Herrador Z, Gherasim A, Jimenez BC, Granados M, San Martín JV and Aparicio P (2015) Epidemiological changes in leishmaniasis in Spain according to hospitalization-based records, 1997–2011: raising awareness towards leishmaniasis in non-HIV patients. PLoS Neglected Tropical Diseases 9, e0003594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBGE (2017a) Instituto Brasileiro de Geografia e Estatística. Divisão regional do Brasil em regiões geográficas imediatas e regiões geográficas intermediárias. Brasil, Ministério da Saúde.
- IBGE (2017b) Instituto Brasileiro de Geografia e Estatística. Banco de tabelas - SIDRA. Instituto Brasileiro de Geografia e Estatística.
- IBGE (2020) Instituto Brasileiro de Geografia e Estatística. Sistema IBGE de Recuperação Automática - SIDRA. Brasil, Ministério da Saúde.
- INPE, Instituto de Pesquisas Espaciais and (2015) AULA 8 – Operações de Análise Espacial. In Inpe. São José dos Campos-São Paulo, pp. 1–53. http://www.dpi.inpe.br/DPI/ . [Google Scholar]
- Karagiannis-Voules D-A, Scholte RGC, Guimarães LH, Utzinger J and Vounatsou P (2013) Bayesian geostatistical modeling of leishmaniasis incidence in Brazil. PLoS Neglected Tropical Diseases 7, e2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane VFM (2016) Análise Epidemiológica Da Leishmaniose Visceral Humana No Brasil: Contribuição Às Politicas De Controle. Tese (Doutorado) – Universidade de Brasilia 158.
- Machado CJS, Silva EG and Vilani RM (2016) O uso de um instrumento de política de saúde pública controverso: a eutanásia de cães contaminados por leishmaniose no Brasil. Saúde e Sociedade 25, 247–258. [Google Scholar]
- Martins-Melo FR, Lima MDS, Ramos AN, Alencar CH and Heukelbach J (2014a) Mortality and case fatality due to visceral leishmaniasis in Brazil: a nationwide analysis of epidemiology, trends and spatial patterns. PLoS ONE 9, e93770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins-Melo FR, da Lima MS, Alencar CH, Ramos AN and Heukelbach J (2014b) Epidemiological patterns of mortality due to visceral leishmaniasis and HIV/AIDS co-infection in Brazil, 2000–2011. Transactions of the Royal Society of Tropical Medicine and Hygiene 108, 338–347. [DOI] [PubMed] [Google Scholar]
- Mehrjou A, Hosseini R and Nadjar Araabi B (2016) Improved Bayesian information criterion for mixture model selection. Pattern Recognition Letters 69, 22–27. [Google Scholar]
- Menon SS, Rossi R, Nshimyumukiza L and Zinszer K (2016) Decentralized control of human visceral leishmaniasis in endemic urban areas of Brazil: a literature review. Tropical Medicine and Health 44, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento ET, Moura MLN, Queiroz JW, Barroso AW, Araujo AF, Rego EF, Wilson ME, Pearson RD and Jeronimo SM (2011) The emergence of concurrent HIV-1/AIDS and visceral leishmaniasis in Northeast Brazil. Transactions of the Royal Society of Tropical Medicine and Hygiene 105, 298–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira WK, de França GVA, Carmo EH, Duncan BB, de Souza Kuchenbecker R and Schmidt MI (2017) Infection-related microcephaly after the 2015 and 2016 Zika virus outbreaks in Brazil: a surveillance-based analysis. The Lancet 23, 861–870. doi: 10.1016/S0140-6736(17)31368-5 [DOI] [PubMed] [Google Scholar]
- Otranto D and Dantas-Torres F (2013) The prevention of canine leishmaniasis and its impact on public health. Trends in Parasitology 29, 339–345. [DOI] [PubMed] [Google Scholar]
- Reis LL, da Balieiro AAS, Fonseca FR and Gonçalves MJF (2019) Leishmaniose visceral e sua relação com fatores climáticos e ambientais no Estado do Tocantins, Brasil, 2007 a 2014. Cadernos de Saúde Pública 35, 1–14. [DOI] [PubMed] [Google Scholar]
- Rivas GB, de Souza NA, Peixoto AA and Bruno RV (2014) Effects of temperature and photoperiod on daily activity rhythms of Lutzomyia longipalpis (Diptera: Psychodidae). Parasites & Vectors 7, 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues NCP, Lino VTS, Daumas RP, de Andrade MKN, O'Dwyer G, Monteiro DLM, Gerardi A, Fernandes GHBV, Ramos JAS, Ferreira CEG and da Leite IC (2016) Temporal and spatial evolution of dengue incidence in Brazil, 2001–2012. PLoS ONE 11, e0165945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos Neto M, Da Silva FBG, Sodré MB, Yamamura M, Santos FS, de Costa ACPJ, de Serra MAAO, de Gordon ASA, Pascoal LM, Bezerra JM, dos Santos LH, de Andrade HLP, Fontoura IG, Pieri FM and Arcêncio RA (2017) Deaths by tuberculosis in a priority city for disease control in the Brazilian Northeast: sociodemographic-operational characteristics and vulnerable territories. International Archives of Medicine 10, 1–12. [Google Scholar]
- Serafim TD, Coutinho-Abreu IV, Oliveira F, Meneses C, Kamhawi S and Valenzuela JG (2018) Sequential blood meals promote Leishmania replication and reverse metacyclogenesis augmenting vector infectivity. Nature Microbiology 3, 548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva RA (2016) Urbanização pela Migração em Araguaína – TO. Caminhos de Geografia 17, 1–15. [Google Scholar]
- Silva CEDF and Abud AKDS (2016) Anaerobic biodigestion of sugarcane vinasse under mesophilic conditions using manure as inoculum. Ambiente e Agua – An Interdisciplinary Journal of Applied Science 11, 763. [Google Scholar]
- Silva TAM, Gomes LI, Oliveira E, Coura-Vital W, de Silva LA, Pais FS-M, Ker HG, Reis AB, Rabello A and Carneiro M (2015) Genetic homogeneity among Leishmania (Leishmania) infantum isolates from dog and human samples in Belo Horizonte Metropolitan Area (BHMA), Minas Gerais, Brazil. Parasites & Vectors 8, 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza GF, Biscione F, Greco DB and Rabello A (2012) Slow clinical improvement after treatment initiation in Leishmania/HIV coinfected patients. Revista da Sociedade Brasileira de Medicina Tropical 45, 147–150. [DOI] [PubMed] [Google Scholar]
- Távora LGF, Nogueira MB and Gomes ST (2015) Visceral Leishmaniasis/HIV co-infection in northeast Brazil: evaluation of outcome. The Brazilian journal of infectious diseases : an official publication of the Brazilian Society of Infectious Diseases 19, 651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo CRS, de Almeida AS, de Chaves SAM, Sabroza PC, Toledo LM and Caldas JP (2017) Vulnerability to the transmission of human visceral leishmaniasis in a Brazilian urban area. Revista de Saúde Pública 51, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursine RL, Dias JVL, Morais HA and Pires HHR (2016) Human and canine visceral leishmaniasis in an emerging focus in Araçuaí, Minas Gerais: spatial distribution and socio-environmental factors. Memórias do Instituto Oswaldo Cruz 111, 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Griensven J, Diro E, Lopez-Velez R, Ritmeijer K, Boelaert M, Zijlstra EE, Hailu A and Lynen L (2014) A screen-and-treat strategy targeting visceral leishmaniasis in HIV-infected individuals in endemic East African countries: the way forward? PLoS Neglected Tropical Diseases 8, e3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation (2009) International Symposium on advances in visceral leishmaniasis therapy – Statement on the outcome of the meeting, Madrid, 18–19 June. International Symposium on advances in visceral leishmaniasis therapy – Statement on the outcome of the meeting, Madrid, 18–19 June 4–8.
- World Health Organization (2010) Control of the leishmaniases: report of a meeting of the WHO Expert Committee on the Control of Leishmaniases. Geneva, Switzerland.
- World Health Organization (2015) Visceral leishmaniasis: control strategies and epidemiological situation update in East Africa. Geneva, Switzerland.