Abstract
The family Hippoboscidae is a less known group of blood-sucking flies. Deer ked are particularly important for animal health; they may act as potential vectors of disease to ungulates, and may transmit pathogens to animals and humans. The aim of this study was to investigate the presence of Trypanosoma (Megatrypanum) DNA in deer keds using molecular methods. Results prove the presence of Megatrypanum trypanosome DNA in the studied winged adult deer keds and this is the first detection of this pathogen in Lipoptena fortisetosa. In addition, this paper evidences the occurrence of L. fortisetosa in two new locations: one in the Białowieża Primeval Forest, and another in the Strzałowo Forest Inspectorate (Piska Forest), both in north-eastern Poland.
Key words: Hippoboscidae, Lipoptena cervi, Lipoptena fortisetosa, Megatrypanum, Trypanosoma
Introduction
Flies in the family Hippoboscidae (Diptera), known as ‘louse flies’ or ‘keds’ are a group of obligate parasites of mammals and birds (Rahola et al., 2011). A recent checklist of Hippoboscidae across the world retains three subfamilies (Ornithomyinae, Hippoboscinae and Lipopteninae) more than 213 species and 21 genera (Dick, 2006; Petersen, 2013). It has been shown that the two subfamilies Hippoboscinae and Lipopteninae are monophyletic groups (Petersen et al., 2007). From Europe, 30 species of Hippoboscidae are known (Petersen, 2013). In Poland, the hippoboscid fauna is relatively not well known. About 10 species are present in Poland including four which parasitize mammals: the forest fly Hippobosca equina L., 1758, the sheep ked Melophagus ovinus L., 1758 and two species of the Lipoptena – Lipoptena cervi L., 1758 and Lipoptena fortisetosa Maa, 1965 (Borowiec, 1984; Borowiec and Zatwarnicki, 1989; Kowal et al., 2016).
Worldwide, 30 Lipoptena species were recorded, five of them were described from Europe (Dick, 2006; Petersen, 2013). Lipoptena cervi and L. fortisetosa have a more northern range while the other three species have a restricted distribution in Southern Europe, including the Mediterranean islands (Petersen, 2013; Kurina et al., 2019). In Europe, the L. fortisetosa was first recorded from Czech Republic where it was initially described as a new species – L. parvula Theodore, 1967, but was later changed to L. fortisetosa by Grunin (1970) (Kurina et al., 2019).
Its appearance in Europe is probably associated with the introduction of sika and Siberian deer in 1839 (Bartoš, 2009). The species is also able to colonize the European roe deer population as a result of natural spread following contact with Siberian roe deer (Kowal et al., 2016). Lipoptena fortisetosa was reported for a few European countries, including Germany, Belarus, Moscow district in Russia, Austria, Lithuania, Moldova, Poland, the Czech Republic, Romania, Slovakia and Switzerland (Kurina et al., 2019; Oboňa et al., 2019). More recently the species has been also recorded in Italy and Estonia (Andreani et al., 2019; Kurina et al., 2019). The most important hosts of deer keds in Europe are mainly: red deer Cervus elaphus L., 1758, roe deer Capreolus capreolus (L., 1758), Eurasian elk Alces alces (L., 1758), sika deer Cervus nippon Temminck, 1838 and fallow deer Dama dama (L., 1758). In North America, the parasite is common on wapiti Cervus canadensis Erxleben, 1777 and white-tailed deer Odocoileus virginianus (Zimmermann, 1780). A wide range of animals can also be accidental hosts of L. cervi: horses, cattle, European bison, sheep, domestic dogs, red foxes, badgers and suids (Hermosilla et al., 2006; Karbowiak et al., 2014; Kowal et al., 2016). Deer keds occasionally bite also humans (Kortet et al., 2010). In Poland, while L. cervi has been observed throughout the country, L. fortisetosa has been found only in the Dolnośląskie, Małopolskie and Warmińsko-Mazurskie Voivodeships (Borowiec and Zatwarnicki, 1989; Kowal et al., 2009). Sokół and Gałęcki (2017) reported the presence of L. fortisetosa in dogs in cities in central Poland. Both L. fortisetosa and L. cervi have a specific development cycle. Upon settling on a suitable mammal host, deer keds shed their wings, remaining in a wingless form for the rest of their life. These flies are viviparous species: they generate fully-grown larvae that fall to the ground and pupate. Although the keds occur throughout the year, the winged adults only appear in high numbers from summer to early autumn (Haarløv, 1964; Borowiec, 1984).
Deer keds have an economic impact on the hunting economy. Their infestation can induce scratching and itching in free-ranging cervids, and this may impair the condition of the host following secondary bacterial infection (Dehio et al., 2004). Besides, although deer keds do not reproduce on humans and accidental hosts, people who work in forestry, or visitors, are still particularly vulnerable to deer ked infestation. As a matter of fact, deer ked bites can result in the occurrence of severe dermatitis in humans (Härkönen et al., 2009).
According to the life cycle of insect vectors, Trypanosoma species are divided into two sections: Stercoraria (subgenera: Megatrypanum Hoare, 1964; Herpetosoma Doflein, 1901; Schizotrypanum Hoare, 1972) and Salivaria (subgenera: Duttonella Chalmers, 1918; Nannomonas Hoare, 1964; Trypanozoon Luhe, 1906; Pycnomonas Hoare, 1964) (Hoare, 1972). Stercorarian trypanosomes develop only in the hindgut (rectum) of the host thus allowing the metacyclic forms to leave the vector organism with the feces. The infection takes place through damaged skin or mucous membrane of the host (Hoare, 1972). During their developmental cycle, Salivarian trypanosomes enter the salivary glands of the insect vector so that it can transmit the pathogen injecting saliva into vertebrate hosts. The subgenus Megatrypanum comprises a group of large trypanosomes that infect almost all mammalian orders (Hoare, 1972). Previous studies of these parasites have typically been restricted to morphological and morphometric examinations of samples from hosts' blood and cultured forms. More recently, complex phylogenetic analyses concerning trypanosomes of the subgenus Megatrypanum revealed two main lineages of Trypanosoma theileri (TthI and TthII) and 10 genotypes associated with the host species: four genotypes from cattle, one from water buffalo, one from deer, two from duikers and one from sitatunga (Rodrigues et al., 2010a, 2010b; Garcia et al., 2011a, 2011b). The species of Megatrypanum trypanosomes described in Poland are presented in Table 1. Although Megatrypanum infections in animals are typically subclinical, some clinical cases are reported; such disease or deaths tend to be associated with stressed cattle or with the presence of concomitant infections such as bovine leucaemia virus (Matsumoto et al., 2011). Trypanosoma theileri can result in leucocytosis, neonatal death, anaemia, weight loss and a considerable drop in milking capacity (Matsumoto et al., 2011). Several neurological manifestations associated with T. wrublewskii infection, including depressive neurological signs, apathy and oedema have been observed in European bison from the Białowieża Primeval Forest (Wrublewski, 1912; Kingston et al., 1992). However, further investigation on infection with parasites of European bison did not confirm these clinical signs (Kingston et al., 1992; Karbowiak et al., 2014).
Table 1.
Trypanosoma (Megatrypanum) species and their respective hosts, described in Poland, on basis of morphological and morphometric data
| Trypanosoma species | Host | References |
|---|---|---|
| Trypanosoma wrublewskii | European bison (Bison bonasus) | Wrublewski (1912) |
| Trypanosoma theileri | Cattle (Bos taurus) | Demiaszkiewicz and Lachowicz (1991) |
| Trypanosoma stefanskii | Roe deer (Capreolus capreolus) | Kingston et al. (1992) |
| Trypanosoma cervi | Red deer (Cervus elaphus) | Wita and Kingston (1999) |
| Trypanosoma ornata | Water shrew (Neomys fodiens) | Karbowiak et al. (2005) |
Megatrypanum trypanosomes are typically transferred to the vertebrate hosts by contamination of the oral mucosa with feces or the gut contents of the infected insects such as tabanid flies (Böse et al., 1987). Hoare (1972) described that the entire life cycle of Megatrypanum trypanosomes takes place in the alimentary tract of the invertebrate host. Only a few reports have described the occurrence of Megatrypanum trypanosomes in deer keds; for example, Böse and Petersen (1991) documented the presence of trypanosomatids in the midgut and hindgut of L. cervi.
It is much more challenging to assess the effect of pathogenicity on arthropods as they tend to display higher tolerance to parasites, and thus less pronounced signs and symptoms (Lipa, 1968). Nonetheless, some studies highlighted the effect of Trypanosoma infections on the condition and survivability of the invertebrate host. Nelson (1956) reports high mortality of sheep keds, caused by obstruction of the intestine with a massive number of trypanosomes. However, Hoare (1972) did not notice any changes in the appearance and behaviour of sheep ticks in his research.
The Hippoboscidae, including deer keds, are potential vectors of a number of pathogens, including bacteria such as Bartonella spp., Anaplasma spp., Coxiella spp. and Ehrlichia spp., protozoa such as Trypanosoma (Megatrypanum) spp. and apicomplexan parasites such as Theileria spp. (Halos et al., 2004; Lee et al., 2016; Szewczyk et al., 2017).
The occurrence of Trypanosoma melophagium in sheep ked was confirmed by Martinković et al. (2012). Billeter et al. (2008) identified Bartonella melophagi in sheep ked (M. ovinus) and B. chomelii in forest flies (H. equina) in Algeria. Bartonella spp. have also been identified in deer keds collected from deer in Poland (Szewczyk et al., 2017), while the presence of Anaplasma phagocytophilum was confirmed in deer keds collected from deer in Slovakia (Víchová et al., 2011). These findings underline the potential role of these blood-sucking hippobocids in the mechanical transmission of pathogenic bacteria within the population of wild animals. They also highlight the risk of transmission of pathogens to humans and animals via the bite of infected haematophagous ectoparasites.
Our previous study reported the presence of Megatrypanum trypanosomes in some species of blood-sucking flies belonging to the Tabanidae family (Werszko et al., 2020). We hypothesize that the same trypanosomes could also be present in two species of Lipoptena: L. cervi and L. fortisetosa.
Materials and methods
Fly collection and taxonomical study
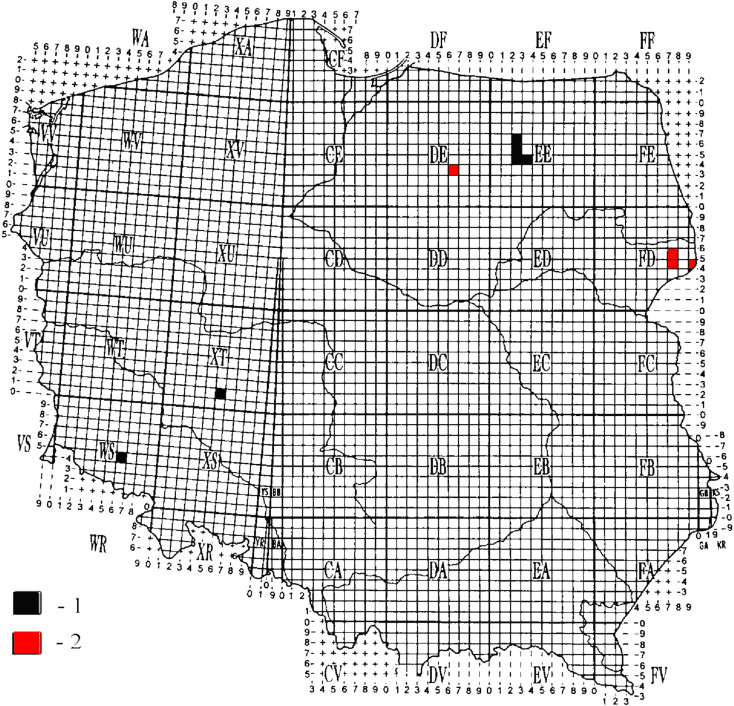
Hippoboscid specimens were collected manually from the fur of red deer during the autumn hunting seasons in the years 2018/2019, and from vegetation in autumn, using an entomological net. The flies were collected from the Strzałowo Forest Inspectorate (Piska Forest) (53°46′N, 21°27′E) and three localities in the vicinity of the Białowieża Primeval Forest (Białowieża 52°42′N, 23°52′E, Hajnówka 52°44′N, 23°35′E and Smolany Sadek 52°48′N, 23°36′E) (Fig. 1). All the locations where deer keds were collected before 2019 as well as those identified during the current study are marked on the map in Fig. 1, according to the UTM (the Universal Transverse Mercator) geographical grid. This method is commonly used for plotting the ranges of animals on a regional scale in faunistic research. Before the taxonomical identification, hippoboscid specimens were rinsed in ultrapure water (Direct-Pure® adept Ultrapure Lab Water Systems, RephiLe Bioscience, Ltd., China) and then air-dried and prepared for optical observations. Sex determination and species identification were carried out using taxonomic keys, according to Borowiec (1984) and Salvetti et al. (2020) under an OPTA-TECH microscope (Warsaw, Poland). In the current study, two dimensions were measured: the total length of the body and the largest width of the abdomen.
Fig. 1.
The documented occurrence of Lipoptena fortisetosa in Poland. (1) Localities where L. fortisetosa was collected before 2019; (2) New record-sites described during the study.
PCR and sequence analyses
DNA from each fly was extracted using a Genomic Mini AX Tissue kit (A&A Biotechnology, Gdynia, Poland), according to the manufacturer's instructions and stored at − i °C until molecular analysis. Flies were individually screened for the presence of trypanosomes using polymerase chain reaction (PCR). The following 18S rDNA oligonucleotides TrypF 150 (5′-GAA ACA CGG GAG CGG TTC CTT-3′) and TrypR 800 (5′-ACC TCA AAG CTT TCG CGT GAA G-3′) were used as previously described (Werszko et al., 2020). These primers amplified a 650 bp fragment of the 18S rRNA gene of Trypanosoma spp.
PCR reactions were conducted in a 50 μL reaction mixture containing 36 μL of deionized water, 3 μL of a 25 μm solution of MgCl2, 0.5 μL of Allegro Taq DNA polymerase (5 U μL−1) (Novazym, Poznań, Poland), 0.5 μL of dNTP-mix (10 mm), 5 μL of 10 × Taq DNA polymerase buffer (with 25 mm MgCl2), 0.5 μL of each primer (20 pmol μL−1) and 4 μL of template DNA. DNA from a Trypanosoma sp. (GenBank acc. no.: MK088728) isolated from Haematopota pluvialis (Tabanidae), was used as a positive control. As a negative control, nuclease-free water was added to the PCR mix instead of the DNA sample.
The amplification conditions include initial denaturation at 94°C for 2 min, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 55°C for 30 s, primer extension at 72°C for 30 s and final extension at 72°C for 3 min. The final phase of PCR reaction included cooling the samples to 10°C.
PCR products were visualized on a 1% agarose gel stained with ethidium bromide. Visualization was performed using ChemiDoc, MP Lab software (Imagine, BioRad, Hercules, USA). The resulting product was compared using the Nova 100 bp DNA Ladder Novazym (Poznań, Poland). PCR products were purified using the QIAEX II Gel extraction kit (Qiagen, Hilden, Germany) and sequenced by Genomed (Warsaw, Poland). The purified PCR products were assembled into contigs using ContigExpress, Vector NTI Advance 11.0 (Invitrogen Life Technologies, New York, USA). The obtained sequences were compared by using BLAST (BasicLocal Alignment Search Tool) with sequences available in GenBank.
Results
In total, 155 flies (118 L. cervi and 37 L. fortisetosa) were collected, including 27 adult winged deer keds (L. cervi) from vegetation caught in one single location (Smolany Sadek) through sweeping net (Table 2). In the current study L. fortisetosa was recorded in two localities in Poland: one in the Białowieża Primeval Forest, and another in the Strzałowo Forest Inspectorate (Puszcza Piska Forest), both in north-eastern Poland (Fig. 1).
Table 2.
Specimens of Lipoptena cervi and Lipoptena fortisetosa collected from different localities in Poland, analysed for Trypanosoma spp. infection (number and percentage of infection)
| Processed deer ked (species and sex) | Location | Overall | |||||
|---|---|---|---|---|---|---|---|
| Nadleśnictwo Strzałowo (Puszcza Piska Forest) | Białowieża | Hajnówka | Smolany Sadek (winged deer ked) | ||||
| L. cervi | ♀ | Analysed | 27 | 6 | 9 | 10 | 52 |
| Infected | 5 | 0 | 2 | 3 | 10 | ||
| ♂ | Analysed | 38 | 3 | 8 | 17 | 66 | |
| Infected | 7 | 2 | 3 | 2 | 14 | ||
| Total | Analysed | 65 | 9 | 17 | 27 | 118 | |
| Infected | 12 | 2 | 5 | 5 | 24 (20.33%) | ||
| L. fortisetosa | ♀ | Analysed | 16 | 8 | – | – | 24 |
| Infected | 10 | 3 | – | – | 13 | ||
| ♂ | Analysed | 11 | 2 | – | – | 13 | |
| Infected | 4 | 1 | – | – | 5 | ||
| Total | Analysed | 27 | 10 | – | – | 37 | |
| Infected | 14 | 4 | – | – | 18 (48.64%) | ||
Morphological identification
The most significant difference between the two species is the body size: L. fortisetosa is smaller than L. cervi. In both species, females are larger than males. The mean length of L. fortisetosa is 4.43 ± 0.0963 mm for females and 3.75 ± 0.0172 mm for males. In L. cervi, the mean body length is 5.90 ± 0.070 mm for females and 5.55 ± 0.050 mm for males. The mean abdomen width of L. fortisetosa is 2.31 ± 0.099 mm for females and 2.079 ± 0.022 mm for males while in L. cervi, the abdomen width is 2.86 ± 0.052 mm for females and 2.96 ± 0.050 mm for males.
The sutural pattern and the distribution of bristles in the thoracic region reveal significant morphological features to distinguish the two species. Lipoptena cervi is hairier than L. fortisetosa and the dimensions of its bristles vary, whereas all bristles in L. fortisetosa are of equal dimensions (Fig. 2).
Fig. 2.
Features on the thorax and female terminalia of Lipoptena cervi (A, B) and L. fortisetosa (C, D).
Female terminalia show other important taxonomical differences: the number of bristles on the genital opening, and the features of the pregenital sclerites and plates. Lipoptena cervi shows three pregenital aligned sclerites, with the central triangular sclerite bearing four or six bristles, and each oval external sclerites with three or four bristles. In contrast, L. fortisetosa has only one central pregenital sclerite with two long, strong bristles in the middle and one on both sides (Fig. 2).
PCR and sequence analyses
The overall positivity to Trypanosoma spp. DNA in deer keds was 27.09% (42/155). The presence of Trypanosoma spp. was detected in 24 out of 118 (20%) L. cervi. In the tested group of winged L. cervi 5 out of 27 (18.51%) were infected. The presence of trypanosomes was detected in 18 out of 37 (48.64%) L. fortisetosa. The males and females of both species of Lipoptena demonstrated similar prevalence of trypanosome infection. The positivity to trypanosome DNA among females and males L. cervi and L. fortisetosa from different locations is given in Table 2.
Four partial 18S rDNA nucleotide sequences were obtained from L. fortisetosa and three from L. cervi, including one sequence obtained from winged deer ked. Two isolates of Trypanosoma spp. obtained from L. cervi (isolates Lc99KG and Lc106KG) and one obtained from winged L. cervi (isolates Lc6SM) were identical each other, and all shared 100% similarity to T. theileri from a tse-tse fly Glossina fuscipes fuscipes Newstead, 1910 from Central Africa (KR024688) and Trypanosoma cf. cervi from white-tailed deer (Odocoileus virginianus Zimmermann, 1780) from the USA (JX178193).
Sequences of Trypanosoma spp. obtained from two L. fortisetosa (isolates Lf4KG and Lf15KG) were identical to each other and showed 100% similarity to T. theileri from European bison Bison bonasus from Poland (KF765799). These isolates also showed 100% identity with T. theileri from cattle from Poland (KF924257). In addition, they also shared a high identity (100%) with trypanosomes isolated in the USA and Brazil (JX853185, JX178162, JX178185, JX178188 and AY773679) and water buffalo Bubalus bubalis (L., 1758) from Brazil (AY773674). One nucleotide sequence from L. fortisetosa (isolate Lf10KG) infected with Trypanosoma spp. was 100% identical to T. melophagium from sheep ked (M. ovinus) from Croatia and the UK (HQ664912 and FN666409). One sequence derived from L. fortisetosa (isolate 1LfHka) infected with Trypanosoma spp. demonstrated high similarity (99.6%) to T. theileri from sitatunga Tragelaphus spekii (Sclater, 1863) from Cameroon (FM202489) and Trypanosoma sp. from horse fly Hybomitra tarandina (L., 1758) from Russia (MK156791). The derived sequences of Trypanosoma spp. were submitted to the GenBank database under the accession numbers: MT394044, MT393974, MT393977, MT393982, MT393983, MT393984 and MT393991.
Discussion
First records of L. fortisetosa in Poland date back to the 1980s (Borowiec and Zatwarnicki, 1989). However, due to its dynamic spread observed in recent years, it can be considered an invasive species in the country; furthermore, owing to the possibility that it could be introduced to other European countries with its host, it could also be considered as an alien invasive species (Kowal et al., 2016). In addition, the presence of these blood-sucking flies may play a significant role in the circulation and maintenance of vector-borne pathogens; they may also demonstrate significant vector competence for infectious agents, despite not being well recognized. Indeed, as the deer keds shed their wings when they find a suitable host and settle for the rest of their lives, the possibility of transmitting a pathogen from one host to another is believed relatively low. Nevertheless, deer keds are recognized as an important group of haematophagous insects for both veterinary parasitology and medical reasons since they have been proved able to transmit Bartonella spp., Borrelia spp. and Trypanosoma (Megatrypanum) spp. (Böse and Petersen, 1991; Dehio et al., 2001; Vichová et al., 2011; Szewczyk et al., 2017), they are recognized as an important group of haematophagous insects for both veterinary parasitology and medical reasons. It is important to note that most part of the studies performed so far have concerned L. cervi (Halos et al., 2004), while much less is known about the competence of L. fortisetosa as a pathogen vector, and less data have been acquired on trypanosomes in other Lipoptena species.
In the current study, the overall prevalence of infection with Trypanosoma spp. was 27.09% (42/155) among deer keds. The highest positivity was reported in the case of L. fortisetosa (48.64%, 18/37). The presence of Trypanosoma spp. was detected in 24 of the 118 (20%) L. cervi. Including the tested group of winged L. cervi, five out of 27 (18.51%) were infected.
Böse and Petersen (1991) report the identification of Megatrypanum trypanosomes in the midgut and hindgut of 9/37 L. cervi collected from deer. High levels of infection with other pathogens have also been observed in L. cervi; the presence of Bartonella spp. was observed in 75.12% of L. cervi collected from deer in Poland (Szewczyk et al., 2017). Víchová et al. (2011) detected A. phagocytophilum infection in only two out of 19 tested deer keds collected from deer in Slovakia. Pathogens present in L. fortisetosa have not been well studied. Lee et al. (2016) report the presence of Coxiella spp., Theileria luwenshuni and Theileria ovis in L. fortisetosa in inland regions of South Korea.
The studies mentioned above were based on wingless flies collected from hosts. In this case it was not known whether the fly was infected or the pathogen was present only in the host blood withdrawn from the vertebrate. We provide the first molecular evidence for Trypanosoma spp. DNA in five out of 27 investigated winged deer keds (L. cervi) collected from vegetation. The winged flies had not fed since they left the pupae; therefore, we suppose the only explanation for the presence of trypanosome DNA is transovarial transmission. The present findings support the hypothesis of a transstadial transmission of Trypanosoma spp. in these species of Lipoptena, but the topic remains open at this stage of research. Korhonen et al. (2015) report the presence of Bartonella DNA in L. cervi pupae and winged adults, thus supporting the potential of deer ked for vector competence of Bartonella spp. and indicating their transstadial transmission; their findings also demonstrate that adult winged deer keds do harbour bartonellae. Víchová et al. (2011) found winged deer keds to be negative for the presence of A. phagocytophilum, indicating that they do not serve as competent vectors of this pathogen. Similarly, all L. cervi collected in a field study by de la Fuente et al. (2005) were found to be negative for the presence of A. phagocytophilum and A. marginale. In contrast, Bartonella spp. was found to be present in pools of winged unfed deer keds (Dehio et al., 2004; Duodu et al., 2013) in midgut bacterial aggregates of Bartonella schoenbuchensis. It is therefore possible that deer keds support the replication of the pathogen and serve as potential biological vectors (Dehio et al., 2004).
Trypanosomes of the subgenus Megatrypanum do not show high specificity for the insect as a host, and the same species of trypanosomes may infect different flies (Böse et al., 1987). The absence of A. phagocytophilum in winged deer keds collected from vegetation and the presence of Bartonella pathogens may indicate that these pathogens demonstrate host specificity for the vector. Until now, deer keds have not been shown to transmit any infectious agents to humans. The causes underlying the variation of prevalence and intensity of blood parasites are poorly known (Sol et al., 2000). The prevalence of microparasite infection in an insect can arise both from factors intrinsic to the host, such as genotype resistance, biochemical immunity processes, behaviour or state of health and from extrinsic factors, such as differences in exposure to vectors (Sol et al., 2000).
All isolates (except to isolate 1LfHka) obtained in this study were identical to the Trypanosoma spp. sequences in the GenBank database. Moreover, isolates Lf4KG and Lf15KG were identical to those of T. theileri isolates obtained previously from cattle and European bison inhabiting the same area.
It seems there is a growing range of arthropods that might serve as potential vectors for transmissible pathogens. The blood-sucking flies, including insects from the genus Lipoptena, are important potential vectors that can disseminate a variety of pathogens in natural foci of transmission disease.
In conclusion, the current study examines the presence of Megatrypanum trypanosomes using molecular methods in L. cervi and L. fortisetosa (Hippoboscidae). It describes the first detection of Trypanosoma spp. in L. fortisetosa, and first recorded presence of trypanosomes in an unfed winged L. cervi from vegetation. The study also identifies two new localities where L. fortisetosa was recorded in Poland: one in the Białowieża Primeval Forest, and another in the Strzałowo Forest Inspectorate (Puszcza Piska Forest), both in north-eastern Poland.
Acknowledgements
The authors would like to express their gratitude to Marek Bogdaszewski, head of the Witold Stefański Institute of Parasitology of the Polish Academy of Sciences (Deer farm in Kosewo Górne), for enabling the samples to be collected from red deer. We are grateful to Zbigniew Ciepluch, Forest Inspector of the Strzałowo Forest Inspectorate for enabling sample collection.
Financial support
This study was supported by the MINIATURA 2 grant no. 2018/02/X/NZ8/00037 Research Project, funded by the National Science Centre, Poland.
Conflict of interest
The authors declare no conflicts of interest.
Ethical standards
Not applicable.
References
- Andreani A, Sacchetti P and Belcari A (2019) Comparative morphology of the deer keds Lipoptena fortisetosa first recorded from Italy. Medical and Veterinary Entomology 33, 140–153. [DOI] [PubMed] [Google Scholar]
- Bartoš, L (2009) Chapter 39. Sika deer in continental Europe. In McCullough DR, Takatsuki S and Kaji K (eds), Sika Deer: Biology and Management of Native and Introduced Populations. Springer, pp. 573–594. doi: 10.1007/978-4-431-09429-6_39. [DOI] [Google Scholar]
- Billeter SA, Levy MG, Chomel BB and Breitschwerdt EB (2008) Vector transmission of Bartonella species with emphasis on the potential for tick transmission. Medical and Veterinary Entomology 22, 1–15. [DOI] [PubMed] [Google Scholar]
- Borowiec L (1984) Wpleszczowate – Hippoboscidae. Klucze do oznaczania owadów Polski Cz. 28, z. 21. Wydawnictwo PWN, Warszawa.
- Borowiec L and Zatwarnicki T (1989) Lipoptena fortisetosa Maa, 1965 (Diptera, Hippoboscidae), nowy gatunek dla fauny Polski. Przegląd Zoologiczny 33, 579–582. [Google Scholar]
- Böse R and Petersen K (1991) Lipoptena cervi (Diptera), a potential vector of Megatrypanum trypanosomes of deer (Cervidae). Parasitology Research 77, 723–725. [DOI] [PubMed] [Google Scholar]
- Böse R, Friedhoff KT and Olbrich S (1987) Transmission of Trypanosoma theileri to cattle by Tabanidae. Parasitology Research 73, 421–424. [DOI] [PubMed] [Google Scholar]
- Dehio C, Lanz C, Pohl R, Behrens P, Bermond D, Piemont Y, Pelz K and Sander A (2001) Bartonella Schoenbuchii sp. nov., isolated from the blood of wild roe deer. International Journal Systematic Evolutionary Microbiology 51, 1557–1565. [DOI] [PubMed] [Google Scholar]
- Dehio C, Sauder U and Hiestand R (2004) Isolation of Bartonella schoenbuchensis from Lipoptena cervi, a blood-sucking arthropod causing deer ked dermatitis. Journal of Clinical Microbiology 42, 5320–5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Fuente J, Naranjo V, Ruiz-Fons F, Höfle U, Fernández De Mera IG, Villanúa D, Almazán C, Torina A, Caracappa S, Kocan KM and Gortázar C (2005) Potential vertebrate reservoir hosts and invertebrate vectors of Anaplasma marginale and A. phagocytophilum In Central Spain. Vector Borne and Zoonotic Diseases 5, 390–401. [DOI] [PubMed] [Google Scholar]
- Demiaszkiewicz AW and Lachowicz J (1991) Trypanosoma theileri Laveran, 1902 – pasożytem bydła w Polsce. Medycyna Weterynaryjna 47, 112–113. [Google Scholar]
- Dick CW (2006). Checklist of World Hippoboscidae (Diptera: Hippoboscoidea), Chicago: Department of Zoology, Field Museum of Natural History, pp. 1–7. [Google Scholar]
- Duodu S, Madslien K, Hjelm E, Molin Y, Paziewska-Harris A, Harris PD, Colquhoun DJ and Ytrehus B (2013) Bartonella infections in deer keds (Lipoptena cervi) and moose (Alces alces) in Norway. Applied and Environmental Microbiology 79, 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia H, Kamyingkird K, Rodrigues AC, Jittapalapong S, Teixeira MMG and Desquesnes M (2011a) High genetic diversity in field isolates of Trypanosoma theileri assessed by analysis of cathepsin L-like sequences disclosed multiple and new genotypes infecting cattle in Thailand. Veterinary Parasitology 17, 363–367. [DOI] [PubMed] [Google Scholar]
- Garcia HA, Rodrigues AC, Martinković F, Minervino AH, Campaner M, Nunes VL, Paiva F, Hamilton PB and Teixeira MM (2011b) Multilocus phylogeographical analysis of Trypanosoma (Megatrypanum) genotypes from sympatric cattle and water buffalo populations supports evolutionary host constraint and close phylogenetic relationships with genotypes found in other ruminants. International Journal for Parasitology 41, 1385–1396. [DOI] [PubMed] [Google Scholar]
- Haarløv N (1964) Life cycle and distribution pattern of Lipoptena cervi (L.) (Dipt, Hippobosc.) on Danish deer. Oikos 15, 93–129. [Google Scholar]
- Halos L, Jamal T, Maillard R, Girard B, Guillot J, Chomel B, Vayssier-Taussat M and Boulouis HJ (2004) Role of Hippoboscidae flies as potential vectors of Bartonella spp. infecting wild and domestic ruminants. Applied and Environmental Microbiology 70, 6302–6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härkönen S, Laine M, Vornanen M and Renuala T (2009) Deer ked (Lipoptena cervi) dermatitis in humans an increasing nuisance in Finland. Alces 45, 73–79. [Google Scholar]
- Hermosilla C, Pantchev N, Bachmann R and Bauer C (2006) Lipoptena cervi (deer ked) in two naturally infested dogs. Veterinary Research 159, 286–288. [DOI] [PubMed] [Google Scholar]
- Hoare CA (1972) The Trypanosomes of Mammals. Oxford and Edinburgh: Blackwell Scientific Publications. [Google Scholar]
- Karbowiak G, Wita I and Rychlik L (2005) Trypanosoma (Megatrypanum) ornata sp. n., a parasite of the Eurasian water shrew Neomys fodiens (Pennant, 1771). Acta Protozoologica 44, 363–367. [Google Scholar]
- Karbowiak G, Demiaszkiewicz AW, Pyziel AM, Wita I, Moskwa B, Werszko J, Bień J, Goździk K, Lachowicz J and Cabaj W (2014) The parasitic fauna of the European bison (Bison bonasus) (Linnaeus, 1758) and their impact on the conservation. Part 1. The summarising list of parasites noted. Acta Parasitologica 59, 363–371. [DOI] [PubMed] [Google Scholar]
- Kingston N, Bobek B, Perzanowski K, Wita I and Maki L (1992) Description of Trypanosoma (Megatrypanum) stefanskii sp. n. from roe deer (Capreolus capreolus) in Poland. Journal of the Helminthological Society of Washington 59, 89–95. [Google Scholar]
- Korhonen EM, Pérez Vera C, Pulliainen AT, Sironen T, Aaltonen K, Kortet R, Härkönen L, Härkönen S, Paakkonen T, Nieminen P, Mustonen AM, Ylönen H and Vapalahti O (2015) Molecular detection of Bartonella spp. in deer ked pupae, adult keds and moose blood in Finland. Epidemiology and infection 143, 578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortet R, Harkonen L, Hokkanen P, Harkonen S, Kaitala A, Kaunisto S, Laaksonen S, Kekalainen J and Ylonen H (2010) Experiments on the ectoparasitic deer ked that often attacks humans; preferences for body parts, colour and temperature. Bulletin of Entomological Research 100, 279–285. [DOI] [PubMed] [Google Scholar]
- Kowal J, Nosal P, Rościszewska M and Matysek M (2009) New records of Lipoptena fortisetosa Maa, 1965 (Diptera: Hippoboscidae) in Poland. Dipteron 25, 27–29. [Google Scholar]
- Kowal J, Nosal P, Kornaś S, Wajdzik M, Matysek M and Basiaga M (2016) Różnorodność i znaczenie muchówek z rodziny narzępikowatych – pasożytów jeleniowatych. Medycyna Weterynaryjna 72, 745–749. [Google Scholar]
- Kurina O, Kirik H, Õunap H and Õunap E (2019) The northernmost record of a blood-sucking ectoparasite, Lipoptena fortisetosa Maa (Diptera: Hippoboscidae), in Estonia. Biodiversity Data Journal 7, e47857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-H, Kim K-T, Kwon O-D, Ock Y, Kim T, Choi D and Kwak D (2016) Novel detection of Coxiella spp., Theileria luwenshuni, and T. ovis endosymbionts in deer keds (Lipoptena fortisetosa). PLoS ONE 11, e0156727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipa JJ (1968) Układ pasożyt-żywiciel u pierwotniaków pasożytujących w stawonogach I kręgowcach. Kosmos 17, 41–55. [Google Scholar]
- Martinković F, Matanović K, Rodrigues AC, Garcia HA and Teixeira MMG (2012) Trypanosoma (Megatrypanum) melophagium in the sheep ked Melophagus ovinus from organic farms in Croatia: phylogenetic inferences support restriction to sheep and sheep keds and close relationship with trypanosomes from other ruminant species. The Journal of Eukaryotic Microbiology 59, 134–144. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Sato A, Hozumi M, Onishi H, Kabeya M, Sugawara M and Takaishi H (2011) A case of a Japanese black cow developing trypanosomosis together with enzootic bovine leucosis. Journal of the Japan Veterinary Medical Association 64, 941–945. [Google Scholar]
- Nelson WA (1956) Mortality in the sheep ked, Melophagus ovinus (L.) caused by Trypanosoma melophagium. Nature 178, 750. [DOI] [PubMed] [Google Scholar]
- Oboňa J, Sychra O, Greš S, Heřman P, Manko P, Roháček J, Šestáková A, Šlapák J and Hromada M (2019) A revised annotated checklist of louse flies (Diptera, Hippoboscidae) from Slovakia. Zoo Keys 862, 129–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen FT (2013) Fauna Europaea: hippoboscidae. In Beuk P and Pape T (eds), Fauna Europaea: Diptera, Brachycera. Fauna Europaea. 2.6. https://fauna-eu.org.
- Petersen FT, Meier R, Kutty SN and Wiegmann BM (2007) The phylogeny and evolution of host choice in the Hippoboscoidea (Diptera) as reconstructed using four molecular markers. Molecular Phylogenetics and Evolution 45, 111–122. [DOI] [PubMed] [Google Scholar]
- Rahola, N, Goodman, SM and Robert, V (2011) The Hippoboscidae (Insecta: Diptera) from Madagascar, with new records from the ‘Parc National de Midongy Befotaka’. Parasite 18, 127–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues AC, Garcia HA, Batista JS, Minervino AH, Góes-Cavalcante G, Maia da Silva F, Ferreira RC, Campaner M, Paiva F and Teixeira MMG (2010a) Characterization of spliced leader genes of Trypanosoma (Megatrypanum) theileri: phylogeographical analysis of Brazilian isolates from cattle supports spatial clustering of genotypes and parity with ribosomal markers. Parasitology 137, 111–122. [DOI] [PubMed] [Google Scholar]
- Rodrigues AC, Garcia HA, Ortiz PA, Cortez AP, Martinkovic F, Paiva F, Batista JS, Minervino AH, Campaner M, Pral EM, Alfieri SC and Teixeira MMG (2010b) Cysteine proteases of Trypanosoma (Megatrypanum) theileri: cathepsin L-like gene sequences as targets for phylogenetic analysis, genotyping diagnosis. Parasitology International 59, 318–325. [DOI] [PubMed] [Google Scholar]
- Salvetti M, Bianchi A, Marangi M, Barlaam A, Giacomelli S, Bertoletti I, Roy L and Giangaspero A (2020) Deer keds on wild ungulates in northern Italy, with a taxonomic key for the identification of Lipoptena spp. of Europe. Medical and Veterinary Entomology 34, 74–85. [DOI] [PubMed] [Google Scholar]
- Sokół R and Gałęcki R (2017) Prevalence of keds on city dogs in central Poland. Medical and Veterinary Entomology 31, 114–116. [DOI] [PubMed] [Google Scholar]
- Sol D, Jovani R and Torres J (2000) Geographical variation in blood parasites in feral pigeons: the role of vectors. Ecography 23, 307–314. [Google Scholar]
- Szewczyk T, Werszko J, Steiner-Bogdaszewska Ż, Laskowski Z and Karbowiak G (2017) Molecular detection of Bartonella spp. in deer ked (Lipoptena cervi) in Poland. Parasites and Vectors 10, 487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Víchová B, Majláthová V, Nováková M, Majláth I, Čurlík J, Bona M and Peťko B (2011) PCR detection of re-emerging tick-borne pathogen, Anaplasma phagocytophilum, in deer ked (Lipoptena cervi) a blood-sucking ectoparasite of cervids. Biologia 66, 1082. [Google Scholar]
- Werszko J, Szewczyk T, Steiner-Bogdaszewska Ż, Wróblewski P, Karbowiak G and Laskowski Z (2020) Molecular detection of Megatrypanum trypanosomes in tabanid flies. Medical and Veterinary Entomology 34, 69–73. [DOI] [PubMed] [Google Scholar]
- Wita I and Kingston N (1999) Trypanosoma cervi in red deer, Cervus elaphus, in Poland. Acta Parasitologica 44, 93–98. [Google Scholar]
- Wrublewski KJ (1912) Die Trypanosomose (Schlafkrankheit) der Wisente. Zeitschrift fűr Infektionskrankheiten, parasitäre Krankheiten und Hygiene der Haustiere 12, 376–384. [Google Scholar]