Abstract
Domestic dogs can function as either paratenic or definitive hosts for the zoonotic raccoon roundworm Baylisascaris procyonis. However, factors leading to development of patent infections in dogs are under-studied. Here we compared infection dynamics of B. procyonis in dogs vs the natural raccoon host. Dogs and raccoons were inoculated 5000 or 500 B. procyonis eggs (n = 3 per dose) or were fed B. procyonis-infected laboratory mice (n = 3 per dose; mice inoculated with 1000 or 250 eggs). Fecal samples were analysed via flotation and a commercial coproantigen ELISA designed for detection of Toxocara spp. Two of 12 dogs (both received low dose larvae) developed patent infections; all 12 raccoons became infected with 10 developing patent infections. Compared with dogs, prepatent periods were shorter in raccoons and maximum egg outputs were much greater. Baylisascaris procyonis coproantigens were detectable via ELISA in all raccoons and the patently infected dogs. Finally, dogs spontaneously lost infections while all patently infected raccoons shed eggs until conclusion of the study. Our results demonstrate that dogs are clearly suboptimal hosts showing limited parasite establishment and fecundity vs raccoons. Despite the low competence, patently infected dogs still pose a risk for human exposure, emphasizing the importance of control measures.
Key words: Baylisascaris procyonis, raccoons, zoonoses, domestic dogs, Ascaridoidea, host competence
Introduction
In the face of increasing urbanization, parasites of wildlife may have increased opportunities to spill into domestic animal hosts. This is especially a concern for zoonotic parasites, as domestic hosts may act as a ‘bridge’ host and place people at risk of wildlife zoonoses that would otherwise be seldom encountered. Many helminth species are capable of producing patent infections in multiple definitive host species; however, there can be variability in many measures of host competency such as infectivity success rates, fecundity, longevity of infections and so on (Schantz et al., 1976; Johnson et al., 2003; Kapel et al., 2006; Jaleta et al., 2017). Differences in host competence may result in disparities in the relative epidemiological/ecological importance among possible definitive host species (Gervasi et al., 2015). Therefore, comparative studies on infection dynamics between unusual and/or novel hosts and natural hosts are needed to understand relative roles of these hosts in maintenance and transmission of a parasite.
Baylisascaris procyonis, the raccoon roundworm, is an example of a zoonotic parasite that can utilize more than one definitive host. The primary host is the raccoon but natural and experimental patent infections have been reported in domestic dogs (Snyder, 1983; Greve and O'Brien, 1989; Miyashita, 1993; Rudmann et al., 1996; Conboy et al., 2010; Kazacos, 2016; Yabsley and Sapp, 2017; Hazlett et al., 2018; Heller et al., 2019). Infections have been noted in other procyonids, but some of these infections may have been caused by the closely related B. potosis (Tokiwa et al., 2014). A single experimentally induced patent infection in a young Virginia opossum (Didelphis virginiana) has been reported, but further evidence for opossums as a competent definitive host species is lacking (Kazacos, 2016). This parasite has become ubiquitous across North America and is particularly common in free-ranging raccoons some regions (Upper Midwest, West Coast etc.) and now much of central/western Europe, and has been also been detected in captive populations in Japan and China (Kazacos, 2016; Heddergott et al., 2020). A wide variety of mammalian and avian species can serve as paratenic hosts and larval migration to the central nervous system [neural larva migrans (NLM)] or eyes (ocular larva migrans) can have fatal or disabling consequences. Among known human cases, few instances of full recovery have been documented; many survivors experience permanent neurologic sequalae and/or vision loss (Kazacos, 2016; Pai et al., 2007; Sircar et al., 2016).
Domestic dogs are unusual hosts for B. procyonis because they can serve as either a paratenic host and develop larva migrans with clinical disease or as a definitive host with patent intestinal infections (adult nematodes in intestine and egg shedding in feces) (Snyder, 1983; Greve and O'Brien, 1989; Miyashita, 1993; Rudmann et al., 1996; Conboy et al., 2010; Kazacos, 2016; Yabsley and Sapp, 2017; Heller et al., 2019). As dogs indiscriminately defecate instead of using defined areas (‘latrines’) like raccoons, patently infected dogs have public health significance (Page et al., 1998). Despite the public health concern, little is known about the host competence and development of patent infections in dogs. Previous studies in which dogs were experimentally inoculated with B. procyonis proved they could develop larval and adult infections, but these studies used small numbers of animals and did not quantify egg outputs (Snyder, 1983; Miyashita, 1993; Bowman et al., 2005).
In this study, our goal was to compare dogs and raccoons as definitive hosts for B. procyonis. We used two routes of exposure, oral inoculation with embryonated eggs and feeding of paratenic host tissues containing larvae, to assess the efficiency and success of these routes between the two host species, and quantified pre-patent periods and egg outputs. Also, we evaluated the ability of a commercial coproantigen ELISA (FecalDx® Antigen Test; IDEXX Laboratories, Inc.) designed to detect antigens shed by adult canine and feline ascarids (Toxocara spp.) to detect intestinal Baylisascaris infections (Elsemore et al., 2017).
Materials and methods
Animal maintenance and inoculation
Twelve 6-month-old, purpose-bred, ascarid-free beagles of mixed sexes were pair-housed and fed standard kibble with water available ad libitum. After an acclimation period of 1 week, dogs were separated into individual runs. Six dogs, chosen semi-randomly to ensure both sexes were represented in each group, were orally inoculated with either 500 or 5000 embryonated B. procyonis eggs suspended in sucrose. Eggs were derived from the anterior uteri of several adult female B. procyonis from naturally infected raccoons. Eggs were dissected from the anterior uterus, transferred to an Erlenmeyer flask with 0.5% formalin in tap water, and allowed to develop at room temperature for 4 weeks. Fully developed eggs were decorticated for 10 min in 1:1 20% sodium hypochlorite, washed 4× in phosphate buffered saline, and the number of embryonated eggs per millilitre was determined microscopically. Aliquots containing the appropriate number of eggs were stored at 4°C prior to inoculation (maximum of 45 days). Immediately before administering to dogs, aliquots were centrifuged and the egg pellet resuspended in sucrose solution for palatability.
For inoculation of dogs with B. procyonis larvae, outbred non-Swiss albino mice (Envigo Laboratories Inc.) were orally inoculated with either 250 or 1000 B. procyonis eggs and euthanized via CO2 asphyxiation with cervical dislocation at the onset of clinical disease [7–9 days post-inoculation (DPI)]. Immediately following euthanasia, carcasses were skinned, and the feet, tail and large bones were removed. The brain, eyes, masseter muscles and tongue were removed from the skull and combined with the rest of the carcass. These tissues were coarsely minced, mixed with a small amount of canned dog food, and immediately fed to six dogs. Each dog was fed one carcass, with the exception of one dog (DL-1) who was fed four mice inoculated with 250 eggs (for a total of ~1000 larvae).
As noted in results, not all dogs became infected so to further explore susceptibility, dogs that did not become patently infected were randomized to new groups and re-inoculated. Doses were either 100 larvae, 250 larvae, or 500 eggs and inoculations were as described above. Sampling was as described below for the original inoculation.
Twelve male, subadult (~12-week old), captive-bred raccoons were group housed during quarantine but then transferred to individual housing before being inoculated using the same conditions and methods as described for the dogs. Oral inoculations of raccoons with eggs were facilitated by mild sedation using intramuscular injection of ketamine (5 mg kg−1). Raccoon fecal samples were tested upon arrival using centrifugal floatation and the coproantigen ELISA described below to ensure animals were negative prior to inoculation.
Sample collection and parasite detection
During quarantine, fecal samples were collected from each dog and group of raccoons. After inoculation, fecal samples were collected from each individual animal run daily for three days and then weekly until 21 DPI, at which time daily sampling was initiated again to ensure that the first day of patency was detected. After the onset of patency, fecal samples were collected 2–3 times a week for each animal. Due to variation in feces production, fecal samples were not available for every animal each day of sampling. Samples were stored at −20°C prior to processing.
For detection of eggs, 2–3 g of feces were subjected to standard centrifugal fecal flotation procedures using Sheather's sucrose flotation solution (specific gravity = 1.27) (Hendrix and Robinson, 2016). The number of eggs per gram of feces (EPG) was determined via the modified Wisconsin technique, using the precise sample weight. Samples with very high egg burdens were diluted, and EPG calculated accordingly.
Three to five grams of feces were submitted to IDEXX Laboratories, Inc. (Westbrook, Maine, USA) for ascarid coproantigen detection using a commercially available proprietary ELISA (FecalDx® Antigen Test). This assay has been validated for the detection of Toxocara canis and T. cati in domestic dogs and cats, respectively (Elsemore et al., 2017). Results were reported in optical density (OD650) values; samples with OD650 ⩾ 0.100 were considered positive. The maximum OD650 value for this assay is 4.000. Prior to this study, this assay was used to test a small pilot set of raccoon feces from five B. procyonis necropsy-positive raccoons to confirm reactivity to B. procyonis antigens.
Animal monitoring and disposition
After inoculations, dogs and raccoons were monitored at least twice a day for 20 DPI for evidence of neurological involvement (e.g. behavioural abnormalities). After 20 DPI, animals were monitored daily.
Selected raccoons were treated after a minimum of 60 days post-patency (DPP) with ivermectin (1 mg kg−1) mixed into food. Fecal samples were collected for 3–4 days post-treatment to ensure that animals became antigen and egg negative. Nematodes that were expelled were collected and counted. Animals that did not become negative on both assays were re-treated and resampled. After treatment, raccoons were sedated via intramuscular injection of xylazine (0.5 mg kg−1) and ketamine (5 mg kg−1) and euthanized via intracardiac injection of sodium pentobarbital (1 mL/4 kg). The small intestine of raccoons was removed, dissected and examined for nematodes. Two raccoons were not treated but were euthanized and necropsied to collect live worms for another study; three more were transferred to a different study following treatment so were not euthanized. After dogs were consistently negative for B. procyonis eggs and antigen, they were transferred to another experiment; this precluded necropsy and/or anthelmintic treatment.
Statistical analysis
Differences in the onset of antigen positivity and patency were analysed via ANOVA followed by Tukey's Honestly Significant Difference to identify which groups differed significantly. Statistical analysis was conducted using R statistical software version 3.1.2 (R Core Team, 2014).
Results
Patency and egg output
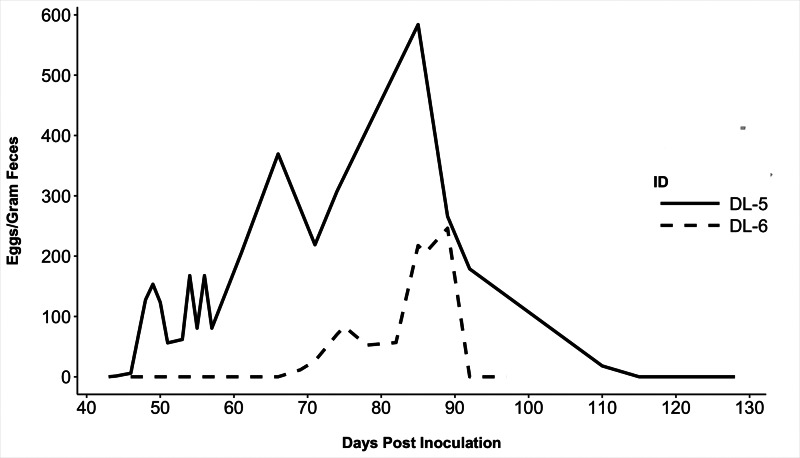
Only 2 of 12 dogs (1 male, 1 female) became patent following the original inoculation, both in the low-dose larval group. B. procyonis eggs were first detected in the feces of dog DL-5 at 44 DPI and DL-6 at 69 DPI (Table 1). At peak shedding, DL-5 shed 584 EPG and DL-6 shed 218 EPG (Fig. 1). These two dogs ceased shedding eggs after 66 and 20 DPP, respectively.
Table 1.
Routes of inoculation and days post-inoculation (DPI) on which a positive antigen result was first obtained and when patency was observed in experimental dogs
| Dog ID | Route of exposure | Re-exposure route | DPI of first antigen positive sample – DPI of last antigen positive sample | DPI of first patent sample – DPI of last patent sample | Difference between antigen positivity and patency (days) |
|---|---|---|---|---|---|
| DE-1 | 5000 eggs | 100 L3 | NA | NA | NA |
| DE-2 | 5000 eggs | 250 L3 | NA | NA | NA |
| DE-3 | 5000 eggs | 500 eggs | NA | NA | NA |
| DE-4 | 500 eggs | 100 L3 | NA | NA | NA |
| DE-5 | 500 eggs | 250 L3 | NA | NA | NA |
| DE-6 | 500 eggs | 500 eggs | NA | NA | NA |
| DL-1 | 1000 L3a | 100 L3 | 73b | NA | NA |
| DL-2 | 1000 L3 | 250 L3 | NA | NA | NA |
| DL-3 | 1000 L3 | 500 eggs | NA | NA | NA |
| DL-4 | 250 L3 | 500 eggs | NA | NA | NA |
| DL-5 | 250 L3 | ND | 38 −115 | 44–110 | 6 |
| DL-6 | 250 L3 | ND | 44–104 | 69–89 | 25 |
aFed L3 larvae in tissue of infected mouse fed 1000 or 250 eggs.
bA single antigen-positive result; all other time points were negative.
Fig. 1.
Baylisascaris procyonis eggs/gram feces for the two dogs that developed patent infections. Both dogs spontaneously cleared infections without treatment (solid line = dog DL-5; dashed line = dog DL-6).
The 10 dogs that did not become patent were randomized and re-inoculated (Table 1). These doses were chosen based on success of the prior low larval dose, and to maintain one egg-inoculated group for comparison. None of the re-inoculated dogs became patent or antigen positive by 85 DPI.
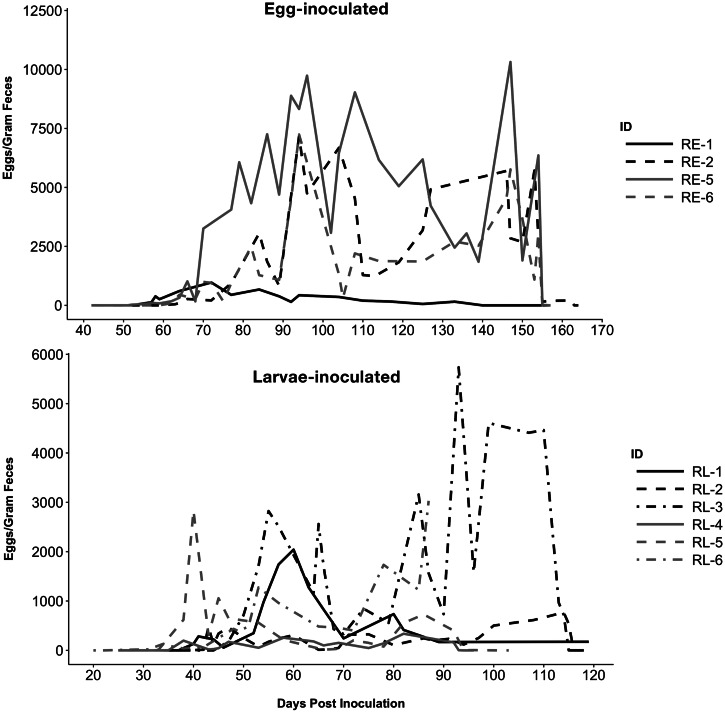
Ten of 12 raccoons became patent (Table 2). Egg shedding generally followed a pattern of a steep increase within the first week of patency, and then large fluctuations which generally followed observed worm expulsion events as noted below. The two raccoons that did not become patent were RE-3 (5000 eggs) and RE-4 (500 eggs). RE-3 intermittently shed unfertilized, empty eggs and expelled one small female nematode measuring 6 cm after treatment. RE-4 never became patent and was euthanized on 94 DPI for unrelated reasons. Two male worms measuring 5.5 cm were recovered from the small intestine. Overall, prepatent periods were significantly shorter (p = 0.0005) in larvae-inoculated raccoons (avg. 37 DPI) vs egg inoculated (avg. 58 DPI). Analysis by dose/route group revealed that prepatency in the low larval groups was significantly shorter than both the low and high egg groups (p = 0.010, p = 0.005). Prepatency in the high-dose larval group was also significantly shorter than the high-dose egg group (p = 0.024). No other significant differences in patency among dose/route groups were found.
Table 2.
Routes of inoculation and days post-inoculation (DPI) on which a positive antigen result was first obtained and when patency was observed in experimental raccoons
| Raccoon ID | Dose/route of exposure | DPI of first antigen positive sample (DPI) | DPI of first patent sample | Difference (days) | No. worms recovered after treatment or necropsy | Size range of recovered worms (cm) |
|---|---|---|---|---|---|---|
| RE-1 | 5000 eggs | 52 | 57 | 5 | NDd | NA |
| RE-2 | 5000 eggs | 40 | 63 | 23 | 0 | NA |
| RE-3 | 5000 eggs | 59 | NA | NA | 2c | 6–14 |
| RE-4 | 500 eggs | 39b | NA | NA | 2c,d | 6–6.0 |
| RE-5 | 500 eggs | 35 | 50 | 15 | 18 | 8–17 |
| RE-6 | 500 eggs | 39 | 62 | 23 | 12 | 6–19 |
| RL-1 | 1000 L3a | 19 | 37 | 18 | NDd | NA |
| RL-2 | 1000 L3 | 13 | 39 | 26 | 15 | 5–13 |
| RL-3 | 1000 L3 | 18 | 47 | 29 | 14 | 4–16 |
| RL-4 | 250 L3 | 20 | 35 | 15 | 6 | 6–13 |
| RL-5 | 250 L3 | 21 | 33 | 12 | 11 | 12–18 |
| RL-6 | 250 L3 | 14 | 31 | 17 | NDd | NA |
aFed L3 larvae in tissue of infected mouse fed 1000 or 250 eggs.
bIntermittent positive results recoded on 39, 44 and 82 DPI; all other time points negative.
cNon-patent, single-sex infection.
dNot treated; animals were euthanized for unrelated reasons or transferred to other studies.
Eight raccoons were treated (RE-2, RE-3, RE-5, RE-6, RL-2, RL-3, RL-4, RL-5) and all but three of these became antigen- and egg-negative within 4 days of treatment (Figs. 2 and 3). Raccoons RE-2, RE-5 and RL-5 failed to become egg- and antigen-negative in the 4-day timeframe, although RE-5 was egg-negative at 4 days post-treatment. Re-treatment of these raccoons 1 week later was successful. Upon final disposition and necropsy, RE-1, RE-2, RE-3 and RE-5 were negative for any B. procyonis adults; other treated raccoons were transferred to other experiments following demonstration of egg- and antigen-negativity. The number of worms passed ranged from 6 to 18 among all treated raccoons. However, during the study, most raccoons spontaneously passed developing and mature worms at various points. Most spontaneous passages occurred within the first 20 DPP. Worms expelled were usually of variable sizes, with average size increasing with the length of infection (data not shown). While most expulsion events involved only single to a few worms, raccoons RE-5 and RL-2 passed large numbers (43 and ~60, respectively) between 50 and 55 DPI.
Fig. 2.
Baylisascaris procyonis eggs/gram feces for patently infected raccoons inoculated with eggs (top) or larvae (bottom). Black lines indicate animals in the high-dose groups (5000 eggs or 1000 L3 larvae) and grey lines in the low-dose groups (500 eggs or 250 L3 larvae). Treatment occurred on 153 DPI for selected egg-inoculated raccoons (RE-2, RE-3, RE-5, RE-6); among larvae-inoculated raccoons, treatment was administered on 114 DPI for RL-2 and RL-3, and on 92 DPI for RL-4 and RL-5.
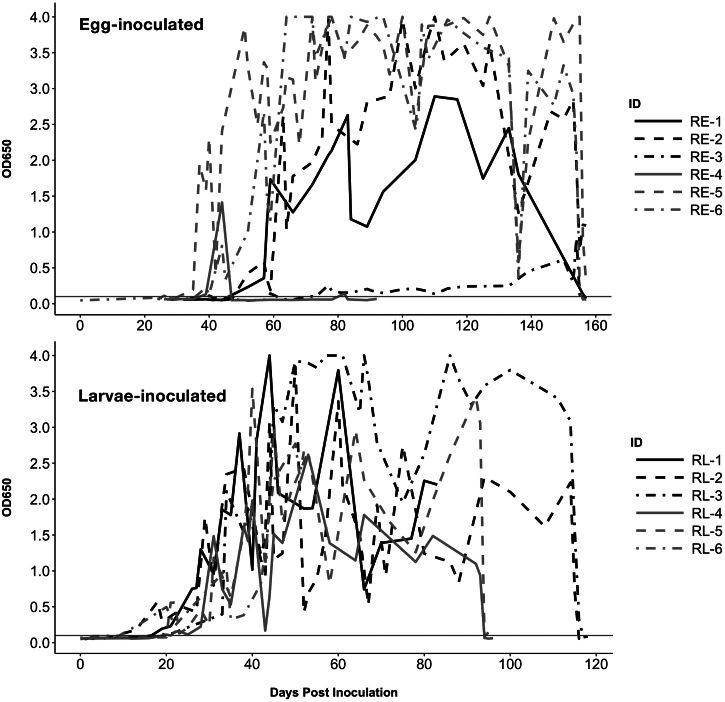
Fig. 3.
ELISA coproantigen values for raccoons inoculated with eggs (top) or larvae (bottom). Black lines indicate animals in the high-dose groups (5000 eggs or 1000 L3 larvae) and grey lines in the low-dose groups (500 eggs or 250 L3 larvae). Horizontal line indicates positive cutoff value (OD650 ⩾ 0.100); the maximum assay value is OD650 = 4.000. Treatment occurred on 153 DPI for all raccoons in A, 114 DPI for RL-2 and RL-3, and 92 DPI for RL-4 and RL-5.
Coproantigen dynamics
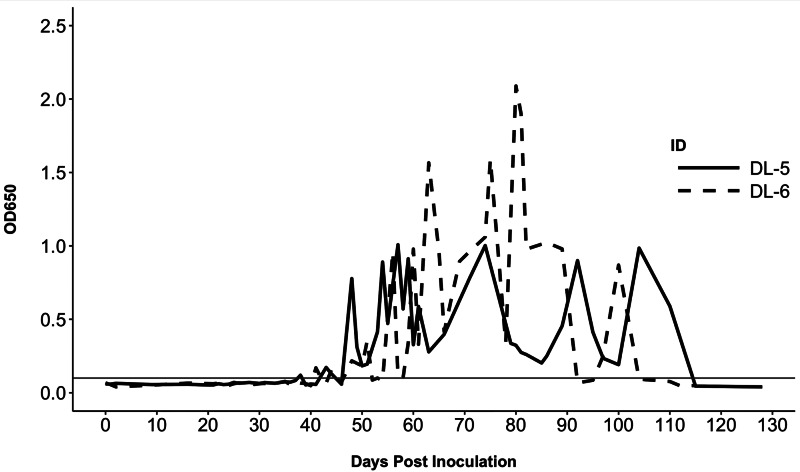
Both dogs that became patent after the first exposure became antigen positive prior to egg shedding, on 38 DPI (dog DL-5) and 41 DPI (DL-6) (Fig. 4). Following the cessation of egg shedding in DL-6, antigen values fell to borderline levels, fluctuating above and below the cutoff threshold (OD650 ⩾ 0.100) over the span of 10 days, and became consistently negative by 104 DPI. Dog DL-5 was antigen negative on 115 DPI and was consistently negative until the conclusion of the study (Fig. 4). A single sample (73 DPI) from DL-1 yielded a positive result, but all subsequent samples were negative. This dog was housed next to antigen-positive DL-6 and contamination of the sample could have occurred. None of the dogs inoculated with eggs became antigen positive.
Fig. 4.
ELISA coproantigen values for two dogs that developed patent infections (solid line = Dog DL-5; dashed line = Dog DL-6). Horizontal line indicates positive cutoff value (OD650 ⩾ 0.100); the maximum assay value is OD650 = 4.000.
All but one raccoon became antigen positive at variable time points and OD650 values were considerably higher than the two antigen positive dogs (Fig. 3). The onset of antigen positivity was significantly earlier (p = 0.0005) for larvae-inoculated raccoons (avg. 17.5 DPI) vs egg inoculated raccoons (avg. 42.5 DPI). RE-4 had sporadic positive results on 39 DPI (OD650 = 0.132), 44 DPI (OD650 = 0.132) and 82 DPI (OD650 = 0.141) and was negative at all other time points tested; as noted above this raccoon was only infected with two male worms.
Discussion
Our data demonstrate that raccoons, the natural host of B. procyonis, are superior hosts for the parasite as they develop infections more often after exposure, have higher worm burdens and higher egg output compared with domestic dogs. Although all 12 exposed raccoons became infected, only 2 of 12 dogs inoculated became patently infected during the first exposure and none of the remaining 10 became infected after re-exposure. In addition, both patent dogs self-cleared the infection whereas none of the raccoons lost infections. For one dog (DL-6), egg shedding ceased abruptly after only 20 days. After that, it had a borderline coproantigen OD650 for another 2 weeks, possibly indicating a single worm or a single sex of worms that lingered for some time after being cleared. The other dog (DL-5) passed eggs for a longer period, but EPG levels remained low and decreased after the peak observed at 41 DPI after which time a number of empty, unfertilized eggs were passed on its final egg-positive fecal exam (66 DPP). Five days later, it became antigen negative. This pattern suggests male worms may have senesced early during the infection and that the remaining females were exhausting spermatozoa stores and senesced shortly thereafter. Similarly, one of the raccoons (RL-5) began passing a mixture of unfertilized and fertilized eggs around 60 DPP and only female worms were recovered following treatment (suggesting males were expelled/lost previously). Throughout the study, egg counts in raccoons were frequently an order of magnitude greater than those observed in the two dogs, even after large numbers of worms were spontaneously purged, and all but one of the raccoons that developed patent infections remained positive throughout the course of the study. Among the raccoons inoculated with 250 larvae, peak EPG values were 338–10 500 compared to 584 and 218 EPG in the patent dogs which had received the same dose.
Consistent with previous studies on dogs and raccoons, dogs which were inoculated with eggs did not establish patent B. procyonis infections, and raccoons inoculated via feeding of larvae in mice tissues became antigen positive and patent much sooner than egg-inoculated raccoons (Dubey, 1982; Miyashita, 1993). A previous inoculation of three 8-week old puppies with eggs was successful in establishing a patent infection only in the puppy receiving the most eggs (30 000), whereas three of four puppies that were fed mice infected with larvae became patent (Miyashita, 1993). However, one study on dogs did observe severe NLM and VLM in adult dogs inoculated with very high numbers of eggs (10 000–200 000) (Snyder, 1983). Collectively, these data suggest infection likelihood is influenced by age and route of exposure for dogs, similar to results observed with raccoons, which generally only become infected via ingestion of infected paratenic hosts as adults (Kazacos, 2016). Age resistance to patent infection with the related T. canis is also well-documented. In dogs over 3–5 weeks of age, the vast majority, if not all larvae undergo somatic migration and become arrested in tissues (commonly kidney, liver, heart and brain), never reaching the gastrointestinal tract for further maturation in a phenomenon termed ‘age resistance’ (Sprent, 1958; Greve, 1971). This may be the case with dogs inoculated with B. procyonis eggs, with migrating larvae becoming arrested or killed in somatic tissue instead of reaching the small intestine. We did not observe any clinical or behavioural abnormalities in dogs inoculated with eggs in our study that would confirm active larva migrans, although associated signs may be mild or absent in some infections. This has been observed previously in other paratenic hosts, including experimentally infected rodents and people occupationally exposed to low levels of B. procyonis eggs (Sapp et al., 2016a, 2016b).
Fluctuations in egg shedding by raccoons were commonly observed following purge events in which many adult nematodes were spontaneously expelled. Although the egg outputs of some animals appeared to stabilize after 30 DPP, this varied by individual and events of expulsion. In naturally infected raccoons, extremely high EPG values (up to 225 000) are commonly observed, but none of our raccoons reached levels above 10 500 EPG (Snyder and Fitzgerald, 1987; Kazacos, 2016). It is possible that multiple exposures over time are required to build infection intensity to such a level required for these high EPG counts, or that the subadult, captive-bred raccoons in this trial do not shed eggs to the degree typical of naturally-infected juvenile raccoons from highly endemic areas. A trial involving experimental infection of wild-caught raccoons found a higher mean EPG (~15 000 EPG), although the number of larvae those raccoons received was not provided (Reed et al., 2012). Further, the final intensity among our raccoons following treatment was relatively low (maximum of 18 worms recovered), which supports the notion that repeated exposure is required for very high intensity, high output infections common in the wild. A single dose of ivermectin via the oral route was generally successful in clearing infections; however, two doses were required in three of the raccoons. This is similar to the observation in previous trials that multiple doses of milbemycin oxime were necessary to clear higher worm burdens in naturally infected dogs (Bowman et al., 2005).
Among patently infected raccoons, those in low-dose groups tended to become patent and/or antigen positive slightly sooner than those in the respective high dose groups receiving the same exposure (e.g. low dose larvae patency was achieved between 31 and 35 DPI; high dose larvae between 37 and 47 DPI). Also, prepatency in the low larval groups was significantly shorter than both the low and high egg groups (p = 0.010, p = 0.005). While further in-depth statistical interpretation of these trends is difficult due to the low numbers within these groups, it could point to low doses being more efficient in establishing infection. It is worth nothing that although two dogs in our study became patently infected, these two dogs were also in a low dose larval group. In ascarid-naïve dogs inoculated with varying doses of T. canis eggs, a similar effect occurred in response to dose as none of the dogs inoculated with a high dose of eggs (10 000) developed patent infections (Dubey, 1978). It is unknown why lower doses appear more successful, although it may be related to crowding stress on developing larvae and perhaps a strong non-specific immune reaction triggered by a large inoculum (compared to a smaller inoculum in which larvae may be able to evade host immunity more effectively). It is possible that a ‘carrying capacity’ is reached following a large inoculum, after which growth may be negatively impacted by crowding stress, leading to delays in reproductive onset (i.e. patency). One previous study did not find evidence of crowding on egg output in naturally infected raccoons, although that does not rule out crowding impacts on rate of development (Weinstein, 2016).
Another possible question regarding susceptibility and infection burden is the sex of the host, for example, male skunks appear to be more frequently and more heavily infected with B. columnaris than females (Wirsing et al., 2007). We were restricted to using male raccoons in this trial, which precluded observing sex-based differences, and the two dogs that became patent were a male and a female. More work will be required to assess if B. procyonis truly exhibits host sex bias in establishment of infections.
Our data confirm that a commercial roundworm coproantigen ELISA that was developed and validated for detection of Toxocara spp. antigens in domestic dogs and cats effectively cross-reacts with B. procyonis antigens. This may be explained because many somatic and excretory-secretory antigens are conserved among Ascaris, Toxocara and Baylisascaris spp. (Boyce et al., 1988; Wang et al., 2008; Dangoudoubiyam and Kazacos, 2009; Xie et al., 2013). This suggests that this assay would be useful for rapid and high-throughput diagnosis of ascarid infections in dogs and possibly other hosts, although the cross-reaction between the ascarid antigens complicates epidemiologic studies when species present is important. However, a significant advantage of this assay is the detection of luminal infections that are in the pre-patent period. In the original validation of this assay for Toxocara, dogs inoculated with T. canis eggs became antigen-positive by 31 DPI but did not became patent until 38–41 DPI (Elsemore et al., 2017). In our study, we found much more variation in the time between the first antigen positive and patency, ranging from 5 to 29 days, but infected animals were consistently antigen positive before they became patent. This difference could be due to differences in the growth and development of B. procyonis vs Toxocara; the marked size variation among spontaneously expelled B. procyonis adults suggests growth rates of individual worms may vary widely in the same host following a single exposure. Importantly, assay characteristics (e.g. differences between the binding efficiency of Toxocara spp. and B. procyonis antigen to the monoclonal capture antibody) could lead to species-level differences in detection thresholds. However, sensitivity seems high for Baylisascaris because in one raccoon that was only intermittently antigen positive, only two small (~5.5 cm) male worms were recovered. This assay also confirmed treatment success as antigen levels dropped below the detectable threshold rapidly (within 24 h) of worm expulsion. Further studies are necessary to fully quantify the performance characteristics (i.e. sensitivity, specificity, predictive values) of this coproantigen ELISA for the detection of different Baylisascaris spp. infections in definitive hosts.
In summary, patent infections with B. procyonis were difficult to establish in dog hosts by either route suggesting they are not an ideal host for the parasite. However, those dogs that do develop patent intestinal infections represent a risk for egg shedding in a domestic environment. This apparent low level of adaptation to dogs may could indicate a recent host-switching event if the growing raccoon populations in urban/suburban areas have allowed greater opportunities for exposure of domestic dogs to B. procyonis. Our work suggests that for dogs over 6 months of age, consumption of low numbers of larvae in paratenic host tissue is the most likely route leading to intestinal infection. While this could partially explain the rarity of patent infections in dogs, it seems unlikely that this route is the sole cause of all reported ‘patent’ B. procyonis infections in dogs. Coprophagy is very common among pet dogs, and not all dogs have access to prey or the drive to hunt. For example, 8% of dogs with egg-positive fecal exams from 2013 to 2018 were toy group breeds, which seem unlikely to be exposed through consuming paratenic hosts (Yabsley and Sapp, 2017). Further, the presence of Eimeria spp., a group of coccidian parasites that commonly infects raccoons but does not infect dogs, in 15% of canine fecal samples indicated that coprophagy was frequent (Yabsley and Sapp, 2017). Regardless of the reason B. procyonis eggs are passed in the feces (intestinal patent infections vs coprophagy), dog feces can represent a risk of infection (i.e. if spuriously passed eggs become larvated/infectious in a domestic environment). As mentioned previously, the strong age susceptibility patterns shown by other ascarids means that trials on young puppies may be warranted to assess the possibility that coprophagy/consumption of B. procyonis eggs may lead to patency only in juveniles. Finally, to the best of our knowledge, no intestinal Baylisascaris spp. infections have been detected in wild canids. This is despite the great potential for exposure via the predation of infected paratenic hosts and extensive parasitological studies on coyotes, foxes and so on from areas where B. procyonis is common in raccoons (Yabsley and Sapp, unpublished data). Further investigation of the host, parasite and epidemiological factors leading to the establishment of intestinal B. procyonis infections in canids is still warranted, owing to the public health risk of pet dogs as definitive hosts for this high-consequence zoonosis.
Acknowledgements
The authors acknowledge the animal resources staff of the UGA College of Veterinary Medicine for the husbandry of the study animals and IDEXX Laboratories staff for coproantigen testing.
Financial support
This work was partially funded by the National Center for Veterinary Parasitology, the American Society of Parasitologists (Willis A. Reid Graduate Research Award), and IDEXX Laboratories, Inc. Additional support was provided by the wildlife management agencies of the Southeastern Cooperative Wildlife Disease Study member states through the Federal Aid to Wildlife Restoration Act (50 Stat.917) and by a U.S. Department of the Interior Cooperative Agreement. SGHS was partially funded by The University of Georgia.
Ethics statement
All procedures involving dogs, raccoons and mice were reviewed and approved by the University of Georgia's IACUC committee (A2016 10-009).
Conflict of interest
D. Elsemore and R. Hanna are employed by IDEXX Laboratories, Inc.
References
- Bowman DD, Ulrich MA, Gregory DE, Neumann NR, Legg W and Stansfield D (2005) Treatment of Baylisascaris Procyonis infections in dogs with milbemycin oxime. Veterinary Parasitology 129, 285–290. [DOI] [PubMed] [Google Scholar]
- Boyce WM, Branstetter BA and Kazacos KR (1988) Comparative analysis of larval excretory-secretory antigens of Baylisascaris procyonis. Toxocara canis and Ascaris suum by Western blotting and enzyme immunoassay. International Journal of Parasitology 18, 109–113. [DOI] [PubMed] [Google Scholar]
- Conboy G, Stewart TA and Taylor A (2010) Baylisascaris procyonis infection in raccoons and dogs on Prince Edward Island. Proceedings of the American Association of Veterinary Parasitologists, Canada, 55, p. 56.
- Dangoudoubiyam S and Kazacos KR (2009) Differentiation of larva migrans caused by Baylisascaris Procyonis and Toxocara species by western blotting. Clinical Vaccine Immunology 16, 1563–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP (1978) Patent Toxocara canis infection in ascarid-naive dogs. Journal of Parasitology 64, 1021–1023. [PubMed] [Google Scholar]
- Dubey JP (1982) Baylisascaris Procyonis and eimerian infections in raccoons. Journal of the American Veterinary Association 181, 1292–1294. [PubMed] [Google Scholar]
- Elsemore DA, Geng J, Cote J, Hanna R, Lucio-Forster A and Bowman DD (2017) Enzyme-linked immunosorbent assays for coproantigen detection of Ancylostoma caninum and Toxocara canis in dogs and Toxocara cati in cats. Journal of Veterinary Diagnostic Investigation 29, 645–653. [DOI] [PubMed] [Google Scholar]
- Gervasi SS, Civitello DJ, Kilvitis HJ and Martin LB (2015) The context of host competence: a role for plasticity in host–parasite dynamics. Trends in Parasitology 31, 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve JH (1971) Age resistance to Toxocara canis in ascarid-free dogs. American Journal of Veterinary Research 32, 1185–1192. [PubMed] [Google Scholar]
- Greve JH and O'Brien S (1989) Adult Baylisascaris Infections in two dogs. Companion Animal Practice 19, 41–43. [Google Scholar]
- Hazlett M, Cai HY, Sparling S and You Q (2018) Neurologic Baylisascaris procyonis infection in a young dog. Canadian Veterinary Journal 59, 1325. [PMC free article] [PubMed] [Google Scholar]
- Heddergott M, Steinbach P, Schwarz S, Anheyer-Behmenburg HE, Sutor A, Schliephake A, Jeschke D, Striese M, Müller F, Meyer-Kayser E, Stubbe M, Osten-Sachen N, Krüger S, Gaede W, Runge M, Hoffmann L, Ansorge H, Conraths FJ and Frantz AC (2020) Geographic distribution of raccoon roundworm, Baylisascaris Procyonis, Germany and Luxembourg. Emerging Infectious Diseases 26, 821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller HB, Arnold S and Dreyfus JL (2019) Baylisascaris procyonis central nervous system infection in a four-month-old gordon setter dog. Journal of the American Animal Hospital Association 55, e553–e501. [DOI] [PubMed] [Google Scholar]
- Hendrix CM and Robinson E (2016) Diagnostic Parasitology for Veterinary Technicians, 4th ed. Maryland Heights, Missouri: Elsevier Health Sciences. [Google Scholar]
- Jaleta TG, Rödelsperger C and Streit A (2017) Parasitological and transcriptomic comparison of Strongyloides ratti infections in natural and in suboptimal permissive hosts. Experimental Parasitology 180, 112–118. [DOI] [PubMed] [Google Scholar]
- Johnson M, Mackintosh C, Labes R, Taylor M and Wharton D (2003) Dictyocaulus species: cross infection between cattle and red deer. New Zealand Veterinary Journal 51, 93–98. [DOI] [PubMed] [Google Scholar]
- Kapel CM, Torgerson P, Thompson R and Deplazes P (2006) Reproductive potential of Echinococcus multilocularis in experimentally infected foxes, dogs, raccoon dogs and cats. International Journal for Parasitology 36, 79–86. [DOI] [PubMed] [Google Scholar]
- Kazacos KR (2016) Baylisascaris Larva Migrans–Circular 1412. Reston, Virginia: US Geological Survey. [Google Scholar]
- Miyashita M (1993) Prevalence of Baylisascaris Procyonis in raccoons in Japan and experimental infections of the worm to laboratory animals. Seikatsu eisei (Journal of the Urban Living Health Association 37, 137–151. [Google Scholar]
- Page LK, Swihart RK and Kazacos KR (1998) Raccoon latrine structure and its potential role in transmission of Baylisascaris Procyonis to vertebrates. The American Midland Naturalist 140, 180–185. [Google Scholar]
- Pai PJ, Blackburn BG, Kazacos KR, Warrier RP and Bégué RE (2007) Full recovery from Baylisascaris procyonis eosinophilic meningitis. Emerging Infectious Diseases 13, 982–931. [DOI] [PubMed] [Google Scholar]
- R Core Team (2014) R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Reed C, Henke se and Kresta AE (2012) Frequency of deposition and location of Baylisascaris Procyonis eggs in raccoon feces. Journal of Wildlife Diseases 48, 190–194. [DOI] [PubMed] [Google Scholar]
- Rudmann DG, Kazacos KR, Storandt ST, Harris DL and Janovitz EB (1996) Baylisascaris Procyonis larva migrans in a puppy: a case report and update for the veterinarian. Journal of the American Animal Hospital Association 32, 73–76. [DOI] [PubMed] [Google Scholar]
- Sapp SGH, Rascoe LN, Wilkins PP, Handali S, Gray EB, Eberhard M, Woodhall DM, Montgomery SP, Bailey KL and Lankau EW (2016a) Baylisascaris Procyonis roundworm seroprevalence among wildlife rehabilitators, United States and Canada, 2012–2015. Emerging Infectious Diseases 22, 2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapp SGH, Weinstein SB, McMahan CS and Yabsley MJ (2016b) Variable infection dynamics in four Peromyscus species following experimental inoculation with Baylisascaris procyonis. Journal of Parasitology 102, 538–544. [DOI] [PubMed] [Google Scholar]
- Schantz P, Colli C, Cruz-Reyes A and Prezioso U (1976) Sylvatic echinococcosis in Argentina. II. Susceptibility of wild carnivores to Echinococcus granulosus (Batsch, 1786) and host-induced morphological variation. Tropenmedizin und Parasitologie 27, 70–78. [PubMed] [Google Scholar]
- Sircar AD, Abanyie F, Blumberg D, Chin-Hong P, Coulter KS, Cunningham D, Huskins WC, Langelier C, Reid M, Scott BJ, Shirley DA, Babik JM, Belova A, Sapp SGH, McAuliffe I, Rivera HN, Yabsley MJ and Montgomery SP (2016) Raccoon roundworm infection associated with central nervous system disease and ocular disease – six states, 2013–2015. Morbidity and Mortality Report Weekly 65, 930–933. [DOI] [PubMed] [Google Scholar]
- Snyder DE (1983) The prevalence, cross-transmissibility to domestic animals and adult structure of Baylisascaris procyonis (Nematoda) from Illinois raccoons (Procyon lotor). Doctoral Dissertation. University of Illinois at Urbana-Champaign. [Google Scholar]
- Snyder DE and Fitzgerald PR (1987) Contaminative potential, egg prevalence, and intensity of baylisascaris procyonis-infected raccoons (Procyon Lotor) from Illinois, with a comparison to worm intensity. Proceedings of the Helminthological Society of Washington 54, 141–145. [Google Scholar]
- Sprent JF (1958) Observations on the development of Toxocara canis (Werner, 1782) in the dog. Parasitology 48, 184–209. [DOI] [PubMed] [Google Scholar]
- Tokiwa T, Nakamura S, Taira K and Une YJP (2014) Baylisascaris Potosis n. sp., a new ascarid nematode isolated from captive kinkajou, Potos flavus, from the Cooperative Republic of Guyana. Parasitology International 63, 591–596. [DOI] [PubMed] [Google Scholar]
- Wang T, He G, Yang G, Fei Y, Zhang Z, Wang C, Yang Z, Lan J, Luo L and Liu L (2008) Cloning, expression and evaluation of the efficacy of a recombinant Baylisascaris schroederi Bs-Ag3 antigen in mice. Vaccine 26, 6919–6924. [DOI] [PubMed] [Google Scholar]
- Weinstein SB (2016) Baylisascaris Procyonis demography and egg production in a California raccoon population. Journal of Parasitology 102, 622–628. [DOI] [PubMed] [Google Scholar]
- Wirsing AJ, Azevedo FC, Larivière S and Murray DL (2007) Patterns of gastrointestinal parasitism among five sympatric prairie carnivores: are males reservoirs? Journal of Parasitology 93, 504–510. [DOI] [PubMed] [Google Scholar]
- Xie Y, Chen S, Yan Y, Zhang Z, Li D, Yu H, Wang C, Nong X, Zhou X, Gu X, Wang S, Peng X and Yang G (2013) Potential of recombinant inorganic pyrophosphatase antigen as a new vaccine candidate against Baylisascaris schroederi in mice. Veterinary Research 44, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabsley MJ and Sapp SGH (2017) Prevalence of Baylisascaris In domestic dog coprological examinations in the United States, 2013–2016. Veterinary Parasitology: Regional Studies and Reports 9, 65–69. [DOI] [PubMed] [Google Scholar]