Abstract
Biodiversity loss may increase the risk of infectious disease in a phenomenon known as the dilution effect. Circumstances that increase the likelihood of disease dilution are: (i) when hosts vary in their competence, and (ii) when communities disassemble predictably, such that the least competent hosts are the most likely to go extinct. Despite the central role of competence in diversity–disease theory, we lack a clear understanding of the factors underlying competence, as well as the drivers and extent of its variation. Our perspective piece encourages a mechanistic understanding of competence and a deeper consideration of its role in diversity–disease relationships. We outline current evidence, emerging questions and future directions regarding the basis of competence, its definition and measurement, the roots of its variation and its role in the community ecology of infectious disease.
Key words: Competence, dilution effect, diversity–disease, intraspecific variation
Introduction
Ongoing patterns of biodiversity loss and disease emergence have raised questions about whether and how these two phenomena are linked. In particular, proposed associations between community diversity and patterns of infectious disease have spurred research examining how parasites and pathogens (hereafter ‘parasites’) are transmitted in complex ecological communities. Theory suggests that multi-host communities can regulate parasite transmission through several pathways. First, community composition dictates the range of host species that are available to a parasite for potential infection. Second, host species densities (or biomass) determine the contact, or exposure rates, between host and parasite. Finally, the competence of each species determines the extent to which it can successfully transmit a parasite. Together, these three factors (community composition, species' densities and species' competence) provide a conceptual framework for linking diversity with the spread of infection (Fig. 1). But while community composition and species densities are well-understood metrics in disease ecology, competence is much less so, often with widely varying definitions across studies and systems. Consequently, a limited understanding of competence may impede progress in diversity–disease research.
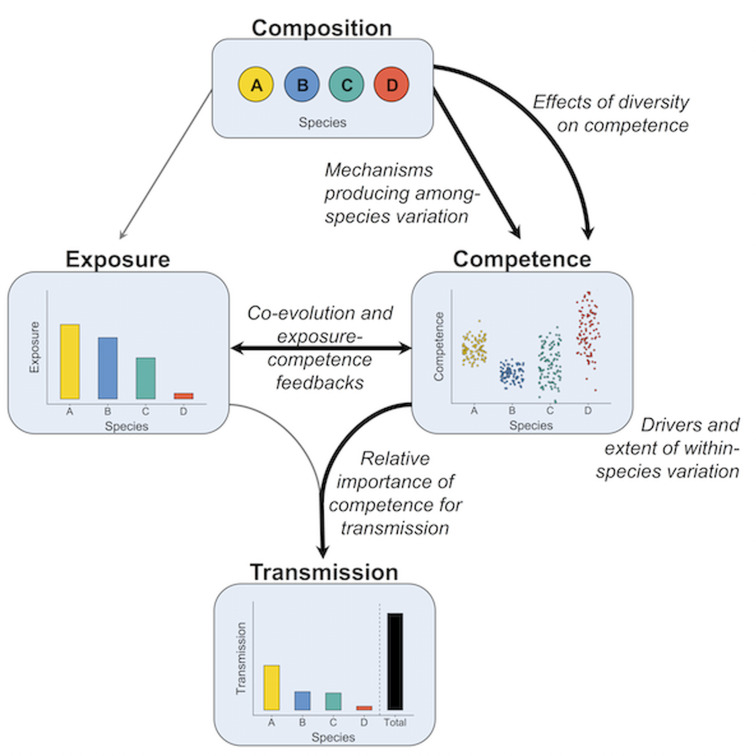
Fig. 1.
The role of competence in diversity–disease theory. Ecological communities may regulate parasite transmission through a simple pathway. Community composition (top) establishes the range of potential hosts available to a parasite. This suite of potential hosts varies in both rates of exposure (centre left) and degree of competence (centre right). Together, exposure and competence determine the degree to which each species can contribute to overall transmission. Our review raises key questions regarding competence in this pathway (bold arrows) and provides a framework to guide future attempts at answering them: What mechanisms underlie among-species variation in competence? What are the drivers and extent of within-species variation? Do species' competence values change as a function or correlate of diversity? And how does host–parasite co-evolution strengthen or decouple the relationship between exposure and competence?
Within the diversity–disease framework, the dilution effect has emerged as a prominent hypothesis through which the loss of species might result in an increase in parasite transmission (Dobson et al., 2006; Keesing et al., 2010; Ostfeld and Keesing, 2012). Disease dilution operates when the presence of additional species directly or indirectly inhibits parasite transmission, resulting in a negative relationship between species diversity and disease (Keesing et al., 2006). While there are multiple mechanisms through which dilution can occur (Keesing et al., 2006; Johnson and Thieltges, 2010), competence is a core component in mediating the effect. When low-diversity communities have higher average competence than high-diversity communities, transmission will increase as diversity declines (Ostfeld and LoGiudice, 2003; Johnson et al., 2013), assuming no concurrent changes in host density. This pattern can occur when community disassembly (loss of species) is non-random, such that the most competent hosts are also the least likely to go extinct (Joseph et al., 2013). Although the dilution effect offers a compelling direct connection between diversity and disease, the search for negative diversity–disease relationships has yielded varied findings, including strong support, contradictory evidence and sometimes non-linear relationships (Randolph and Dobson, 2012; Wood and Lafferty, 2013; Mihaljevic et al., 2014; Wood et al., 2014; Civitello et al., 2015; Huang et al., 2016; Halliday and Rohr, 2019). These conflicting results have not dampened interest in biodiversity–disease relationships by any means. Rather, they have encouraged a more mechanistic understanding of the type and strength of processes generating diversity–disease associations (Johnson et al., 2015; Halsey, 2019; Rohr et al., 2020). This mechanistic understanding will, on the one hand, require continued focus on transmission between individuals, or how host–parasite exposure rates are shaped by processes in the environment (Fig. 1, left). Equally important, on the other hand, is a careful consideration of the within-host environment, including how competence regulates transmission and whether it changes predictably with community disassembly (Fig. 1, right).
The classic mathematical models of Anderson and May (1981) emphasized the importance of competence for parasite transmission by incorporating parameters for host susceptibility to initial infection, recovery and parasite production (which we propose together comprise competence). Empirical studies have echoed this theory, demonstrating that different patterns of parasite spread can be effectively predicted by measuring these parameters. At the within-species level, genetic variation in plankton susceptibility was found to directly fuel the size of fungal epidemics in experimental mesocosms (Strauss et al., 2018), where susceptibility was measured as each genotype's prevalence of infection following controlled exposures. In the same system, intraspecific variation in host recovery (measured using dose–response curves for separate populations and time periods) was also linked with the timing of fungal epidemics in temperate lakes (Stewart Merrill, 2019). Similarly, Halliday et al. (2018) identified a key competence trait in Lolium grasses (the immune signalling hormone, salicylic acid) which, when manipulated, decreased parasite production and altered the transmission of an aggressive fungal pathogen. Hence, variation in competence, even within a species, can strongly influence parasite transmission and resulting disease.
Despite broad acknowledgement that competence is critical for transmission, this property is a missing link in diversity–disease research for several reasons. Inconsistency in the definition and quantification of competence has led to non-standard interpretations in the literature. A paucity of metrics for competence and its underlying traits (Martin et al., 2016) has also led to its empirical absence from many diversity–disease studies. In essence, competence is the product of an organism's ability to acquire, support and maintain infection to enable transmission (Huang et al., 2016), and emerges from an interaction of host and parasite traits. Owing to the technical and logistical challenges of measuring these complex properties, researchers have often relied on assumed or coarsely measured estimates of competence. So although the dilution effect proposes that parasite transmission depends on the competence of hosts (or vectors) in a system, we often lack refined competence values to test the theory. Furthermore, because competence is the outcome of multiple processes encompassing a host–parasite interaction – any of which can exhibit a broad range of variation – it is also likely that competence is a highly variable property (Gervasi et al., 2015; Martin et al., 2016). Despite this potential variation, studies of the dilution effect often attribute a species' competence to a single, static value, and within-species variation is rarely addressed. These knowledge gaps leave our empirical tests incomplete: while parasite transmission is evaluated in response to carefully measured changes in community composition and species' densities, the competence values of those same species are either unknown or treated as fixed constants. To better understand the regulatory role of competence, efforts that move beyond approximations and simplifying assumptions may be particularly valuable, as will research that determines the drivers and extent of competence variation, from the level of the host species down to that of the individual host. In addition, identifying the relative importance of competence for generating infection patterns will allow for a more synthetic understanding of how species identities shape diversity–disease relationships.
In this review, we advocate for a more mechanistic understanding of competence and a deeper consideration of its role in diversity–disease research (see bold arrows in Fig. 1). We begin by developing a concrete definition of the term, informed by the stepwise interactions of hosts and parasites, and use this definition to decompose competence into its constituent mechanisms (Box 1). In essence, we describe competence as a parasite's within-host R0; given exposure, competence refers to the capacity of the host to transmit infection. We then discuss two competence-related criteria that increase the likelihood of disease dilution: first, that a species' competence is inversely related to its extirpation risk, and second, that competence is a robust trait (possessing limited within-species variation). Finally, building on the theme of trait robustness, we evaluate competence as a highly variable property, and consider its variation through the lens of host–parasite co-evolution. Our emerging questions and future priorities span the biological basis of competence, the roots of its variation and its role in the community ecology of infectious disease.
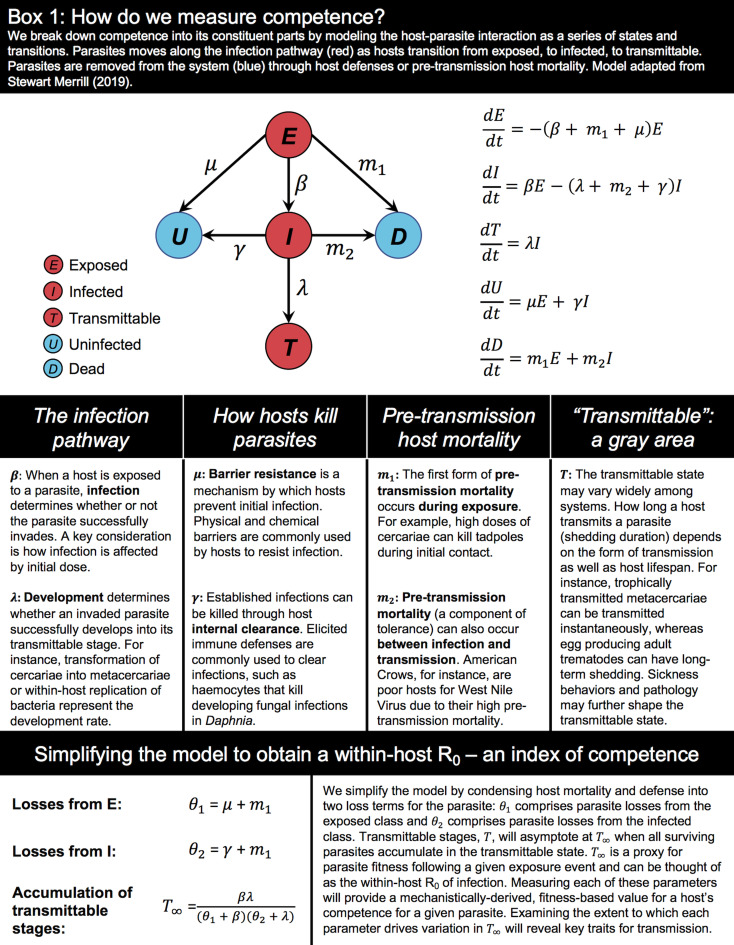
Box 1.
How do we measure competence?
Unpacking competence: towards a unifying definition and systematic quantification
What is competence, and how do we measure it? Competence is a notoriously vague term, with multiple definitions and metrics appearing throughout the diversity–disease literature (e.g. Telford and Spielman, 1993; Komar et al., 2003; Hall et al., 2009; Paaijmans et al., 2012). The first cause for this ambiguity may be an ‘endpoints’ problem. Competence is generally thought to represent the outcome of a host–parasite interaction (or how successful a parasite is within a host), but existing definitions vary in where the interaction begins, as well as where it ends. The second cause for this ambiguity may be a quantifiability problem. As ecologists, we often count discrete frequencies (i.e. individuals in a population) and measure tangible traits (i.e. body mass) – but competence is neither. Support of a parasite is the outcome of a multistep process that can be influenced by both host and parasite traits (Barron et al., 2015; Gervasi et al., 2015; Martin et al., 2016; Downs et al., 2019; Stewart Merrill et al., 2019); it can thus be hard to denote competence with a single numerical value.
Despite the sources of this ambiguity, a clear definition of competence is necessary for both isolating competence from other ecological processes and for measuring it in a currency that is comparable across systems. Here, we discuss competence as it has been historically treated, with the goal of landing on a unifying definition. To aid in our comparisons, we break the timeline of host–parasite interactions into the following steps which comprise the various definitions of competence: (i) exposure, the host is exposed to a parasite infectious stage, (ii) infection, the parasite establishes within or upon the host, (iii) development, the parasite develops, grows and/or reproduces within the host to produce the infectious stage, (iv) pre-transmission survival, the host survives long enough to enable transmission, and (v) infectivity, the infected host (as a donor host) successfully transmits infectious stages to the environment, a recipient host or a recipient vector. This timeline can be generally applied across parasite types and disease systems (Barron et al., 2015; McCallum et al., 2017), whether transmission occurs in the environment, via direct contact, via vectors or through trophic transmission. Our consideration of competence seeks to maintain this generality and identify key ingredients of competence that are translatable among systems.
Perhaps the broadest definitions of competence arise in vector-transmitted systems, where competence is an integrated measure of interactions between hosts, parasites and their vectors. For example, in early studies of tick-borne disease, rodent competence was assessed by allowing naïve ticks to feed on wild caught rodents and establishing whether or not the ticks became infected (termed ‘xenodiagnosis’; Mather et al., 1989; Telford and Spielman, 1993; Ostfeld and Keesing, 2000). This measure is effectively the product of infection prevalence and infectivity (Van Buskirk and Ostfeld, 1995) and integrates rodent exposure, infection, parasite development and pre-transmission survival in the wild, as well as the rodent's capacity to infect ticks in the laboratory. Although the ticks in the referenced studies were well-known vectors for the parasite of interest, vectors themselves have competence that, in part, determines infectivity. Vector competence has also been broadly defined as any portion of the interaction – from initial bloodmeal to infection of the subsequent host – that is governed by intrinsic factors (Beernsteen et al., 2000). The problem with these two definitions is that they blur the lines of competence. That is, when competence includes a donor host's ability to give parasites to a vector, and a recipient vector's ability to acquire those parasites, it becomes unclear where the recipient's competence begins and the donor's competence ends. Ultimately, such definitions can lead to difficulty in partitioning competence values. For instance, if infection of a vector is used to measure host competence and the vector itself has limited competence, then the host's competence will be underestimated. Competence may therefore be better measured and interpreted when it is restricted to a given host–parasite interaction or vector–parasite interaction.
At the other end of the spectrum are studies that narrowly define competence as whether or not infection occurs following exposure. This step, also often referred to as host susceptibility, neglects subsequent variation in parasite development that can be important for transmission. For instance, while multiple birds can be infected by West Nile virus, variation in their competence emerges from the magnitude and duration of their viremia (Komar et al., 2003), which can depend on both host resistance and the quality of the host as a resource. Similarly, pre-transmission survival (which can be a component of tolerance) may be an essential component of competence that is often overlooked in susceptibility assays (Box 1). American Crows exemplify the importance of pre-transmission survival: while crows are highly susceptible to West Nile virus and support high viral loads, their high pathogen-induced mortality decreases their efficacy in transmitting infection back to mosquito vectors (Komar et al., 2003; LaDeau et al., 2007; Nemeth et al., 2011).
Between these broad and narrow definitions lies a rich middle ground for defining competence. Parasitism is an intimate interaction (Combes, 2001), and we propose that competence should also be considered as an intimate property. That is, only the infection processes that are self-contained within a host–parasite interaction comprise competence. These fundamental processes are infection, parasite development and pre-transmission survival and together encompass an organism's capacity to transmit infection (Box 1). Exposure should be treated as distinct from competence, because it can be influenced by movements, behaviours and interactions of multiple players in an ecological community, as well as parasite choice (Johnson et al., 2019a) or vector selectivity (Kilpatrick et al., 2006). Likewise, infectivity (the likelihood of transmission from donor to recipient) depends on the behaviour and susceptibility of a recipient host or vector outside of the host–parasite interaction, so should not be treated as a component of competence. Competence is then strictly the capacity of a host to transmit infection. Given exposure, we can think of competence as host quality in the currency of a parasite's potential fitness, or the parasite's within-host R0 (Box 1).
Both host and parasite traits can lead to deviations from the infection pathway and thereby reduce competence (Box 1, transitions to U and D). Host resistance, which ultimately serves to reduce the burden of infection, can remove parasites at the point of exposure (Box 1; μ or ‘barrier resistance’) or lead to host recovery from infection (Box 1; γ or ‘internal clearance’). Death of a host can likewise nullify a parasite's transmission, whether mortality occurs in response to high host vulnerability or high parasite pathogenicity (Box 1). Although tolerance is not relegated to one specific pathway in the infection process, it can broadly permeate host–parasite interactions, thereby shaping competence (Baucom and de Roode, 2011; Adelman and Hawley, 2017; Burgan et al., 2019). Host tolerance is a strategy by which hosts limit the fitness costs from parasites (Råberg et al., 2007) and is generally thought to be inversely related to both resistance and mortality (Rohr et al., 2010). By allowing parasites to progress to their transmittable forms, tolerance should hence promote competence.
Growing from a functional definition of competence is the need to quantify competence more systematically. For many diversity–disease relationships, species' differences in competence have not been measured or have been assumed based on prior estimates, due to technical and logistic barriers that limit measurement (Kilpatrick et al., 2006; Allan et al., 2009; Clay et al., 2009; Gottdenker et al., 2012). For others, competence has been inferred from the prevalence of infection in natural populations (Telfer et al., 2005; Carver et al., 2011). Infection prevalence as a proxy is especially problematic because it conflates competence with exposure. That is, a given species may be highly suitable for infection but experience only limited parasite exposure; in this case, low infection prevalence would incorrectly designate the species as a low competence host. While estimates of host competence from the field are highly desirable, it is essential that field studies adequately control for variation in parasite exposure. In addition, infection prevalence does not provide information on the number of parasite infective stages produced from an infection. By neglecting the magnitude of parasite production (and for continuously-transmitted parasites, the duration of parasite production), prevalence is a poor approximation of an organism's capacity to transmit infection.
Quantifying exposure has been historically challenging, but parasite and disease ecologists across several systems have developed creative solutions. For environmentally transmitted parasites, eDNA has been used to quantify the density of parasite propagules where they co-occur with hosts (Huver et al., 2015; Hall et al., 2016). Similar technological innovations include using host DNA from vector bloodmeals to establish host–vector contact rates (Alcaide et al., 2009) as well as the use of radio tracking and radio frequency identification to measure contact among hosts (Adelman et al., 2015; Manlove et al., 2017). Basic natural history observations can also be leveraged to quantify per-capita exposure. For instance, Stewart Merrill (2019) counted the number of infectious fungal spores contacting the site of infection in wild-caught Daphnia hosts and assessed the prevalence of established infections within the same populations. Resistant (and thereby non-competent) populations could then be identified as those that faced high levels of contact with fungal spores but experienced low or zero prevalence of transmittable infections. Simple empirical and quantitative methods that disentangle infection from exposure may be a valuable first step towards describing natural levels of competence in the field, while the second step will entail also quantifying the magnitude of parasite production.
Competence is a key variable in diversity–disease theory, and it is thus important to obtain reliable estimates of competence that encompass infection, parasite development and pre-transmission survival. Importantly, each of these fundamental processes can be dependent on the magnitude of exposure or on time. For instance, pre-transmission mortality may increase as a function of infection intensity [e.g. chytridiomycosis in frogs (Stockwell et al., 2010), white nose syndrome in bats (Langwig et al., 2017)], and parasites may be immunologically cleared with increasing time since infection (trematode metacercariae in frogs; LaFonte and Johnson, 2013). Dose-dependence and temporal dynamics of a host–parasite interaction should thus be considered when quantifying competence. Obtaining these refined values will require a strong empirical foundation. To that end, controlled infection assays, with high replication, can be especially powerful tools. Through extending studies of competence to include multiple host genotypes, populations, time periods and environmental conditions, we can estimate average competence values for particular species, and quantify the range of within-species variation (Box 2). In addition, we can begin to explore how the components of competence correlate with one another and determine which components may serve as proxies or simplifications of competence.
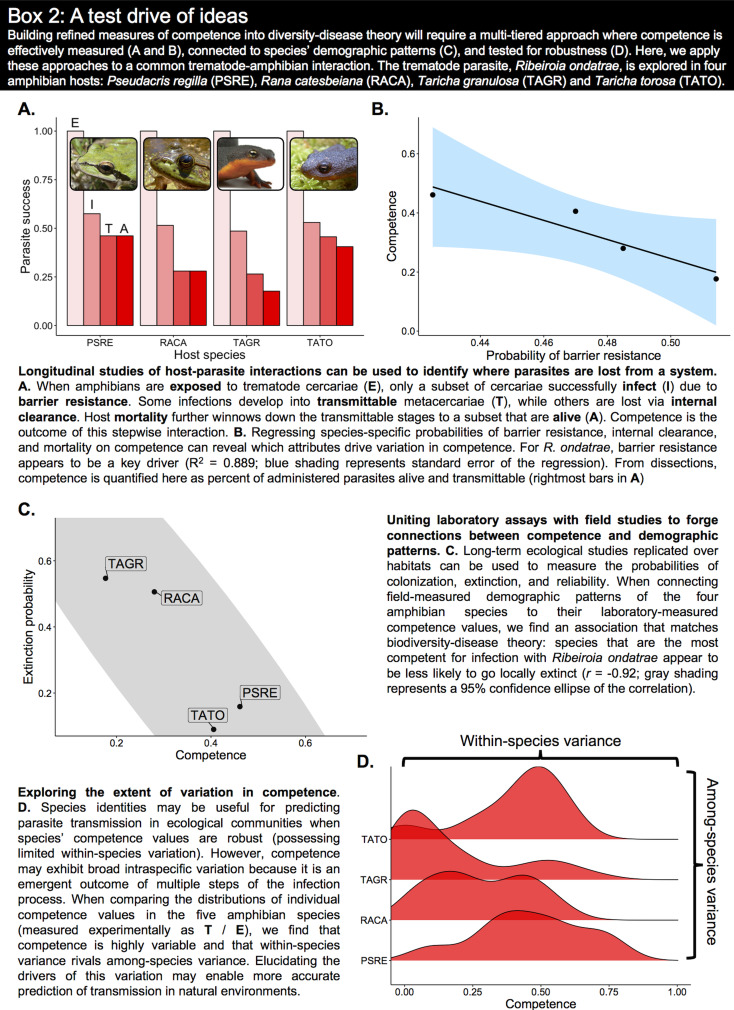
Box 2.
A test drive of ideas
How to measure competence will vary from system to system, but there are some essential ingredients that can guide our attempts. First and foremost, any attempt to measure competence should focus on ultimate parasite output given exposure (the ratio between transmittable parasites and parasites exposed, or T/E in Box 1). In an experimental setting, controlled exposures will provide both of these pieces of information. For parasites that replicate within the host, such as viruses, fungi and bacteria, this ratio will exceed one (unless the host recovered or died from infection). In Daphnia-Metschnikowia interactions, for example, the number of fungal spores produced per inoculated fungal spore can range from zero to the tens of thousands, providing ample variation for making comparisons. For parasites that do not undergo within-host replication, such as many flatworms, this ratio will scale from zero to one. For example, competent amphibian hosts may allow up to 100% of administered trematode cercariae (E) to form metacercariae that are infective to bird predators (T), whereas in entirely non-competent amphibians, this value will be 0% (Box 2). When a parasite is transmitted in one discrete event, as with parasitoids and trophically-transmitted parasites, the ratio of transmittable parasites to parasites exposed may be a valuable representation of competence.
But what about when parasites possess continuous transmission? When hosts continually shed infective stages, be they virions in circulation or helminth eggs exiting with feces, the competence ratio should also be multiplied by the average lifespan of the infection. The duration of this patent infection period will be influenced by host recovery, natural parasite mortality and host mortality (Komar et al., 2003). Although measuring ultimate competence from interactions is important for making biological comparisons, the crux of our perspective piece is that we should also determine its proximate causes. To that end, the model we provide in Box 1 can be applied to longitudinal data (hosts experimentally exposed in the lab, or sentinel hosts naturally exposed in the field) to determine which components of the interaction are bottlenecks or amplifiers of parasite production (see also Supplementary Materials). By collecting infection state data from hosts sampled through time, transition rates and associated probabilities for the infection process can be estimated (Stewart Merrill, 2019). Critical components of competence can then serve as guides for identifying biomarkers for competence (Gervasi et al., 2017; Burgan et al., 2018), as well as the molecular and physiological mechanisms that comprise competence.
Sources and scales of competence variation: evaluating criteria for the dilution effect
The dilution effect is expected to operate when two competence-related criteria are met: first, when species' competence values are inversely correlated with their local extirpation risk, and second, when competence is robust (possessing less within-species variation than among-species variation). For biodiversity loss to result in communities with higher average competence, competent species must persist during community disassembly (Young et al., 2013; Johnson et al., 2015). For species identities to be a primary predictor of parasite transmission, each species' competence value should be robust and possess limited variation. But how often are these two circumstances met? Although life history theory provides a framework for linking competence to demographic patterns, empirical evidence supporting this link is rarely studied. Moreover, the growing discovery of super-spreading individuals (or hosts that contribute disproportionately to transmission) suggests that species may generally exhibit broad intraspecific variation in competence (Woolhouse et al., 1997; Lloyd-Smith et al., 2005; Vazquez-Prokopec et al., 2016; Martin et al., 2019). Moving diversity–disease theory forward will thus involve testing these two key questions and assessing the sources and extent of competence variation across biological scales.
Is competence inversely correlated with local extinction risk?
Among-species variation in competence is thought to emerge from species life history strategies. Life history theory suggests that species fall on a spectrum from slow to fast pace of life, which is characterized by differential investment in current reproduction or maintenance and long-term survival (Stearns, 1992; Montiglio et al., 2018). Species that invest heavily in current reproduction tend to be short-lived, small-bodied and reproduce frequently. Conversely, species that invest in future reproduction (maintenance and survival) are more long-lived, large-bodied and slow reproducing. In theory, these reproductive strategies correspond with both the competence values and demographic parameters that are central to diversity–disease theory (Joseph et al., 2013). Species that maximize reproduction may be environmentally persistent and resilient to extirpation (Cardillo et al., 2008, but see also Isaac and Cowlishaw, 2004), but may also be more suitable for parasitic infections because their high investment in reproduction trades off against immune defences that might combat infection (Lee, 2006; Cronin et al., 2010; Previtali et al., 2012). Hence, life history strategy, extirpation risk and competence come together in a triad that is (theoretically) linked by trade-offs. Establishing the validity of each proposed trade-off is important for predicting whether average community competence will or will not change as a function of species' extirpations and community disassembly.
There is some work in support of competence–life history associations (reviewed in Joseph et al., 2013). For instance, Han et al. (2015) used machine-learning to identify life history traits associated with reservoir status in rodents and found that fast-pace-of-life rodents, which begin reproduction early in life and reproduce frequently, were more likely to serve as reservoirs for zoonotic pathogens. Although this study did not explicitly measure competence, it provides broad evidence for the idea that life history traits are linked with an organism's capacity to support infection. Similarly, Johnson et al. (2012) found that amphibian species with higher rates of development (a fast pace-of-life trait) were the most competent, being more likely to experience trematode infection, as well as trematode-induced malformations that increase the likelihood of trophic transmission. Among avian hosts, competence (assessed using xenodiagnosis) for Lyme disease, West Nile encephalitis and Eastern Equine encephalitis was also partially explained by host body size and clutch size (Huang et al., 2013), which can covary with extinction risk. To date, a direct link between competence and extirpation risk remains a largely understudied endeavour (Young et al., 2013; Huang et al., 2016). There has been work demonstrating that natural community disassembly is non-random and that competent hosts are the most likely to persist in low richness communities (LaCroix et al., 2013; Johnson et al., 2019b). While such studies make use of static gradients in community composition, future research should work towards capturing the species' dynamics that lead to community disassembly. Ground-truthing the extirpation–competence relationship can be accomplished by measuring species' competence values alongside their natural population trends, such as colonization rates, local extinction rates, densities and turnover (Box 2).
How robust a trait is competence?
Phenotypic variation within a species can approach that of trait variation among species (Messier et al., 2010; Bolnick et al., 2011; Violle et al., 2012) and such broad intraspecific variation can affect the outcome of species interactions (Des Roches et al., 2018). When competence exhibits similar variation, species identities alone (i.e. their average competence values) will become less useful as predictors of infection pattern and process. Consequently, an important challenge in diversity–disease research will be to reconcile intraspecific variation in competence with the trait robustness assumption that often dominates community ecology.
Within-species variation in competence emerges at several biological scales (VanderWaal and Ezenwa, 2016). Populations of a given species can vary in average competence over space and time due to local environmental factors, seasonal variation and local adaptation (Ostfeld and Keesing, 2000; Kilpatrick et al., 2010; Altizer et al., 2006; Becker et al., 2019; Mordecai et al., 2019). Moving down a scale, individual variation in body size (Stewart Merrill et al., 2019), sex (Zuk and McKean, 1996) and developmental stage (Johnson et al., 2011; Merrill et al., 2019) can further shape a host's capacity to support infection, as can the individual's somatic state, owing to resource availability (Cressler et al., 2014) or stress levels (Gervasi et al., 2017). Central questions arising from these observations are how fixed or static species' competence values are, and to what degree within-species variation rivals among-species variation (i.e. how robust species' values are). Partitioning variation in competence into both biological and environmental sources will help identify which biological scales (e.g. environment, species, population, age class, individual) play strong roles in regulating transmission.
There are multiple ways by which within-species variation in competence might influence the direction or magnitude of diversity–disease relationships (Fig. 2). If host species exhibit high overlap in their competence distributions (as demonstrated in Box 2D), then their roles as competent or diluter hosts may be reversed under different environmental contexts (Fig. 2B: Broad within-species variation). This possibility could lead to null patterns between diversity and disease because community competence does not predictably change with species losses or gains. If the environmental drivers of biodiversity loss, like urbanization, habitat degradation or pollution, also influence competence (Martin et al., 2010; Messina et al., 2018), then the competence of a particular species may vary depending on whether it is observed in an intact or disassembled community. Likewise, the loss of biodiversity itself may shape the competence of persisting species, because the physiological underpinnings of competence can be affected by species interactions (de Roode et al., 2011; Martin et al., 2010; Adamo et al., 2016). One mechanism that might mediate either of these pathways is chronic stress, which has immunosuppressive effects (Råberg et al., 1998; Adamo, 2017) that could increase competence (LaFonte and Johnson, 2013; Gervasi et al., 2017). When persisting species' competence increases as a direct response (or as an indirect correlate) of biodiversity loss, a negative biodiversity–disease correlation can emerge (Fig. 2C: Competence negatively covaries with diversity). The converse is also true: if persisting species become less competent with biodiversity loss (e.g. Young et al., 2013), reductions in diversity may be associated with less transmission (Fig. 2D: Competence positively covaries with diversity). These potential outcomes highlight the need to move beyond diversity–disease correlations alone and investigate competence as a mechanism mediating their shape. Mathematical models represent a valuable tool for exploring the potential consequences of differing competence distributions, as well as exploring the relative contribution of competence to diversity–disease relationships (Luis et al., 2018).
Fig. 2.
Within-species variation in competence may influence or obscure diversity–disease relationships. (A) The dilution effect can occur when within-species variation in competence is limited compared to among-species variation. Here, competence is a robust trait such that each species' competence value is roughly equivalent across communities. When communities are nested and competence is inversely related to extirpation risk (two of the requirements for dilution; Johnson et al., 2015), average community competence will decrease with richness, yielding strong potential for a negative diversity–disease relationship. But when within-species variation in competence is broad, there are several possibilities for the shape of diversity–disease relationships. (B) If within-species variation is idiosyncratic (species competence values do not fall along a similar gradient), then species may reverse roles depending on environmental context, being more competent in some communities and less competent in others. Even when communities are nested, the random values of each species can lead to a null relationship between community richness and average competence; leading to diversity–disease ambiguity. If within-species variation falls along a predictable gradient (C and D), shaped by either community-level processes or environmental correlates of biodiversity, then there is potential for either a positive diversity–disease relationship or a negative one, depending on how competence correlates with richness. [See also Gervasi et al. (2015) for a helpful depiction of how within-species variation can scale up to affect transmission at the community level.]
We have presented competence as both complex and (potentially) variable. Integrating this complexity with that of ecological communities may make diversity–disease relationships seem intractable. However, we view competence as an exciting opportunity to delve into complexity, so that we may extract generality. With a clearer understanding of the biological basis of competence, we can make informed predictions about its relationship to species' life history traits and species' demographic patterns. If the traits or processes underlying competence consistently co-vary (explored in Vazquez-Prokopec et al., 2016), or if one trait in particular drives variation in competence (Box 2), then there are additional opportunities for simplifying competence and identifying proxies that can be tracked in natural systems (Gervasi et al., 2017; Burgan et al., 2018). With such proxies, we can begin considering competence as a functional trait (McGill et al., 2006) and predict how it may be generally distributed within ecological communities, and how the shape of the competence distribution might change under community disassembly. Ultimately, the balance of organismal process with ecological pattern may provide insight into when and where the dilution effect operates.
Competence as an emergent property of host–parasite interactions
Antagonistic co-evolution can strengthen or decouple links between a species' exposure in the environment and its competence for infection. For parasites, selection may favour traits that reinforce positive exposure–competence associations. The opposite is true for hosts: if infection is costly, selection may favour traits that produce negative exposure–competence associations. This fluctuating selection may ultimately produce variation in competence over space and time (Thompson, 2005). Furthermore, the evolutionary push and pull between host and parasite means that competence is not just a host trait but is an emergent property of their interaction.
We can envision evolution at play when the dilution effect operates on environmentally-transmitted parasites. In this scenario, a parasite is broadly exposed to an assemblage of host species, some of which are competent for transmission and others of which are less so. This is thought to be the case for the fungal parasite, Metschnikowia bicuspidata, whose abundant aquatic fungal spores are indiscriminately consumed by multiple plankton species that vary in their competence (Hall et al., 2009). Broad parasite exposure among multiple host species may impart two selection pressures on the players in the system. First, the parasite may face pressure to adapt to its low competence hosts. Alternatively, high competence hosts may face pressure to avoid, resist or tolerate infection.
There is evidence for antagonistic co-evolution playing out in both hosts and parasites. Weinstein et al. (2018) found that the rodent species experiencing the highest pathogenicity to raccoon roundworms also tended to exhibit the strongest avoidance behaviours at spatial hot spots of transmission. Likewise, host resistance has been observed to increase in response to elevated parasite exposure through time (amphibian responses to chytrid fungus; Voyles et al., 2018) and over space (stickleback occurring in sympatry with helminths; Kalbe and Kurtz, 2006). On the parasite's side, Ebert (1994) demonstrated that the ability of microsporidians to infect and reproduce within Daphnia decreases as a function of geographic distance between host genotype and parasite genotype. An added dimension to parasite adaptation is parasite choice (Johnson et al., 2019a), which may evolve to strengthen or relax host–parasite encounter rates beyond those expected by density- and frequency-dependent processes alone. As hosts vary in frequency and quality over the landscape, their importance for parasite transmission may also vary, and the potential for local adaptation may make competence an evolutionary dynamic property. Further understanding the factors that produce negative or positive co-variation in exposure and competence is important for identifying key hosts in transmission (Box 3; Hawley et al., 2011; Ezenwa et al., 2016).
Host–parasite co-evolution counters the view that competence is shaped by life history strategy. That is, while the co-evolutionary viewpoint considers competence a unique outcome of the pressures and constraints of a particular host–parasite interaction, the life history viewpoint considers competence a more general host property. It is therefore important to divide competence into its host vs parasite sources, asking: How common is it for a single host species to be generally competent for multiple parasite species? How common is it for a single parasite to perform well in multiple host species? And how much of observable variation in competence is unique to a given host–parasite interaction? Answering these questions and relating them to parasite transmission strategy and host life history may provide clarity in how competence is distributed among diverse host–parasite interactions.
Conclusion
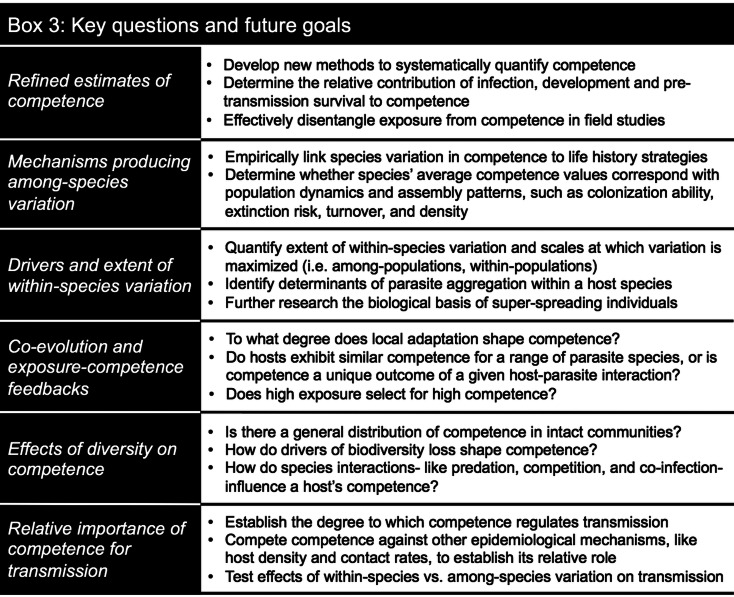
Competence may be critical for understanding disease in a changing world. However, attempts to define this property have yet to adequately capture its complexity. While research on diversity and disease acknowledges that species presences and densities are dynamic through space and time, the competence values of those same species are often assumed to be static and invariable. Embracing the dynamic nature of competence may elevate our understanding of how parasite transmission responds to changes in biodiversity and may resolve controversy on the mechanisms generating diversity–disease relationships. This endeavour will require careful empirical work, and research at the laboratory-field interface will be especially important for identifying the mechanisms of competence and connecting them to ecological processes. Advancing our understanding of competence may take several approaches, such as quantitative tools to better measure it, eco-immunological theory to understand the forces shaping it and mechanistic studies that evaluate its consequences for transmission (Box 3). Ultimately, a more rigorous framing of competence will bridge within-host processes to the community ecology of infectious disease.
Acknowledgements
The authors thank Matthew Bitters, Brendan Hobart, Travis McDevitt-Galles, Loren Merrill, Wynne Moss and Zachary Phillips for their feedback on early drafts of the manuscript. Additionally, Andrew Dean, Andrew Fenton and Zoi Rapti provided helpful ideas on the quantification of competence.
Financial support
Tara Stewart Merrill is a Simons Foundation fellow of the Life Sciences Research Foundation. This material is based upon work supported by the David and Lucile Packard Foundation, the National Science Foundation (DEB 1754171) and the Burroughs Welcome Fund Travel Grant. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Conflict of interest
The authors declare no conflicts of interest.
Ethical standards
Not applicable.
Glossary
|
Barrier resistance: a host trait (or set of traits) that prevents a parasite from infecting at the point of exposure by creating a barrier to infection. Biodiversity-disease relationship: any quantitative relationship between a metric for biodiversity (e.g. species richness) and a metric for disease (e.g. prevalence). Competence: the capacity of a host to transmit infection given exposure to a parasite. Exposure: contact between a host and parasite (or infected vector) in the environment. Development: the within-host maturity of a parasite to its transmittable state, whether by morphological development or replication. Dilution effect: a phenomenon where high diversity communities have lower average competence than low diversity communities, producing a negative biodiversity–disease relationship. Extirpation risk: the likelihood that a species will be lost from an ecological community during community disassembly. Host resistance: a general strategy in which hosts limit infection by parasites. Host tolerance: a general strategy in which hosts permit parasitic infection while reducing the fitness costs associated with infection. Infection: a parasite established on/within its host Infectivity: the likelihood that a host (or vector) will successfully transmit a parasite to another host (or vector). In other words, the successful transfer of a parasite from donor to recipient. Internal clearance: a host trait (or set of traits) that removes a parasite after the parasite has already infected the host. Pre-transmission survival: the likelihood that a host survives infection to enable transmission. Susceptibility: the probability with which a host becomes infected following exposure to a parasite. Trait robustness: a trait is robust when its within-species variation is lower than its among-species variation. |
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182020000943.
click here to view supplementary material
References
- Adamo SA (2017) The stress response and immune system share, borrow, and reconfigure their physiological network elements: evidence from the insects. Hormones and Behavior 88, 25–30. [DOI] [PubMed] [Google Scholar]
- Adamo SA, Easy RH, Kovalko I, MacDonald J, McKeen A, Swanburg T, Turnbull KF and Reeve C (2016) Predator stress-induced immunosuppression: trade-off, immune redistribution or immune reconfiguration? Journal of Experimental Biology 220, 868–875. [DOI] [PubMed] [Google Scholar]
- Adelman JS and Hawley DM (2017) Tolerance of infection: a role for animal behavior, potential immune mechanisms, and consequences for parasite transmission. Hormones and Behavior 88, 79–86. [DOI] [PubMed] [Google Scholar]
- Adelman JS, Moyers SC, Faine DR and Hawley DM (2015) Feeder use predicts both acquisition and transmission of a contagious pathogen in a North American songbird. Proceedings of the Royal Society B: Biological Sciences 282, 20151429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcaide M, Rico C, Ruiz S, Soriguer R, Muñoz J and Figuerola J (2009) Disentangling vector-borne transmission network: a universal DNA barcoding method to identify vertebrate hosts from arthropod bloodmeals. PLoS ONE 4, e7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan BF, Langerhans RB, Ryberg WA, Landesman WJ, Griffin NW, Katz RS, Oberle BJ, Schutzenhofer MR, Smyth KN, de St Maurice A, Clark L, Crooks KR, Hernandez DE, McLean RG, Ostfeld RS and Chase JM (2009) Ecological correlates of risk and incidence of West Nile virus in the United States. Oecologia 158, 699–708. [DOI] [PubMed] [Google Scholar]
- Altizer S, Dobson A, Hosseini P, Hudson P, Pascual M and Rohani P (2006) Seasonality and the dynamics of infectious diseases. Ecology Letters 9, 467–484. [DOI] [PubMed] [Google Scholar]
- Anderson RM and May RM (1981) The population dynamics of microparasites and their invertebrate hosts. Philosophical Transactions of the Royal Society B: Biological Sciences 291, 451–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron DG, Gervasi SS, Pruitt JN and Martin LB (2015) Behavioral competence: how host behaviors can interact to influence parasite transmission risk. Behavioral Sciences 6, 35–40. [Google Scholar]
- Baucom RS and de Roode JC (2011) Ecological immunology and tolerance in plants and animals. Functional Ecology 25, 18–28. [Google Scholar]
- Becker DJ, Albery GF, Kessler MK, Lunn TJ, Falvo CA, Czirják GA, Martin LB and Plowright RK (2019) Macroimmunology: the drivers and consequences of spatial patterns in wildlife immune defence. Journal of Animal Ecology 89, 972–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beernsten BT, James AA and Christensen BM (2000) Genetics of mosquito vector competence. Microbiology and Molecular Biology Reviews 64, 115–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolnick DI, Amarasekare P, Araújo MS, Bürger R, Levine JM, Novak M, Rudolf VHW, Schreiber SJ, Urban MC and Vasseur D (2011) Why intraspecific trait variation matters in community ecology. Trends in Ecology and Evolution 26, 183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgan SC, Gervasi SS and Martin LB (2018) Parasite tolerance and host competence in avian host defense to West Nile virus. EcoHealth 15, 360–371. [DOI] [PubMed] [Google Scholar]
- Burgan SC, Gervasi SS, Johnson LR and Martin LB (2019) How individual variation in host tolerance affects competence to transmit parasites. Physiological and Biochemical Zoology 92, 49–57. [DOI] [PubMed] [Google Scholar]
- Cardillo M, Mace GM, Gittleman JL, Jones KE, Bielby J and Purvis A (2008) The predictability of extinction: biological and external correlates of decline in mammals. Proceedings of the Royal Society B: Biological Sciences 275, 1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver S, Kuenzi A, Bagamian KH, Mills JN, Rollin PE, Zanto SN and Douglass R (2011) A temporal dilution effect: hantavirus infection in deer mice and the intermittent presence of voles in Montana. Oecologia 166, 713–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitello DJ, Cohen J, Fatima H, Halstead NT, Liriano J, McMahon TA, Ortega CN, Sauer EL, Sehgal T, Young S and Rohr JR (2015) Biodiversity inhibits parasites: broad evidence for the dilution effect. Proceedings of the National Academy of Sciences 28, 8667–8671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay CA, Lehmer EM, St. Jeor S and Dearing MD (2009) Sin Nombre Virus and rodent species diversity: a test of the dilution and amplification hypotheses. PLoS ONE 4, e6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combes C (2001) Parasitism: The Ecology and Evolution of Intimate Interactions. Chicago, IL: The University of Chicago Press. [Google Scholar]
- Cressler CE, Nelson WA, Day T and McCauley E (2014) Disentangling the interaction among host resources, the immune system and pathogens. Ecology Letters 17, 284–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin JP, Welsh ME, Dekkers MG, Abercrombie ST and Mitchell CE (2010) Host physiological phenotype explains pathogen reservoir potential. Ecology Letters 13, 1221–1232. [DOI] [PubMed] [Google Scholar]
- de Roode JC, Rarick RM, Mongue AJ, Gerardo NM and Hunter MD (2011) Aphids indirectly increase virulence and transmission potential of a monarch butterfly parasite by reducing defensive chemistry of a shared food plant. Ecology Letters 14, 453–461. [DOI] [PubMed] [Google Scholar]
- Des Roches S, Post DM, Turley NE, Bailey JK, Hendry AP, Kinnison MT, Schweitzer JA and Palkovacs EP (2018) The ecological importance of intraspecific variation. Nature Ecology and Evolution 2, 57–64. [DOI] [PubMed] [Google Scholar]
- Dobson A, Cattadori I, Holt RD, Ostfeld RS, Keesing F, Krichbaum K, Rohr JR, Perkins SE and Hudson PJ (2006) Sacred cows and sympathetic squirrels: the importance of biological diversity to human health. PLoS Medicine 3, e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs CJ, Schoenle LA, Han BA, Harrison JF and Martin LB (2019) Scaling of host competence. Trends in Parasitology 35, 182–192. [DOI] [PubMed] [Google Scholar]
- Ebert D (1994) Virulence and local adaptation of a horizontally transmitted parasite. Science (New York, N.Y.) 265, 1084–1086. [DOI] [PubMed] [Google Scholar]
- Ezenwa VO, Archie EA, Craft ME, Hawley DM, Martin LB, Moore J and White L (2016) Host-behaviour-parasite feedback: an essential link between animal behaviour and disease ecology. Proceedings of the Royal Society B: Biological Sciences 283, 20153078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasi SS, Civitello DJ, Klivitis HJ and Martin LB (2015) The context of host competence: a role for plasticity in host-parasite dynamics. Trends in Parasitology 31, 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervasi SS, Burgan SC, Hofmeister E, Unnasch TR and Martin LB (2017) Stress hormones predict a host superspreader phenotype in the West Nile virus system. Proceedings of the Royal Society B: Biological Sciences 284, 20171090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottdenker NL, Chaves LF, Calzada JE, Saldaña A and Carroll CR (2012) Host life history strategy, species diversity, and habitat influence Trypanosoma cruzi vector infection in changing landscapes. PLoS Neglected Tropical Diseases 6, e1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SR, Becker CR, Simons JL, Duffy MA, Tessier AJ and Cáceres CE (2009) Friendly competition: evidence for a dilution effect among competitors in a planktonic host-parasite system. Ecology 90, 791–801. [DOI] [PubMed] [Google Scholar]
- Hall EM, Crespi EJ, Goldberg CS and Brunner JL (2016) Evaluating environmental DNA-based quantification of ranavirus infection in wood frog populations. Molecular Ecology Resources 16, 423–433. [DOI] [PubMed] [Google Scholar]
- Halliday FW and Rohr JR (2019) Measuring the shape of the biodiversity-disease relationship across systems reveals new findings and key gaps. Nature Communications 10, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday FW, Umbanhowar J and Mitchell CE (2018) A host immune hormone modifies parasite species interactions and epidemics: insights from a field manipulation. Proceedings of the Royal Society B: Biological Sciences 285, 20182075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halsey S (2019) Defuse the dilution effect debate. Nature Ecology & Evolution 3, 145–146. [DOI] [PubMed] [Google Scholar]
- Han BA, Schmidt JP, Bowden SE and Drake JM (2015) Rodent reservoirs of future zoonotic diseases. Proceedings of the National Academy of Sciences 112, 7039–7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley DM, Etienne RS, Ezenwa VO and Jolles AE (2011) Does animal behavior underlie covariation between host exposure to infectious agents and susceptibility to infection? Implications for disease dynamics. Integrative and Comparative Biology 51, 528–539. [DOI] [PubMed] [Google Scholar]
- Huang ZYX, de Boer WF, van Langevelde F, Olson V, Blackburn TM and Prins HHT (2013) Species’ life-history traits explain interspecific variation in reservoir competence: a possible mechanism underlying the dilution effect. PLoS ONE 8, e54341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZYX, Langevelde FV, Estrada-Pena A, Suzán G and De Boer WF (2016) The diversity-disease relationship: evidence for and criticisms of the dilution effect. Parasitology 143, 1075–1086. [DOI] [PubMed] [Google Scholar]
- Huver JR, Koprivnikar J, Johnson PTJ and Whyard S (2015) Development and application of an eDNA method to detect and quantify a pathogenic parasite in aquatic ecosystems. Ecological Applications 25, 991–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac NJ and Cowlishaw G (2004) How species respond to multiple extinction threats. Proceedings of the Royal Society B: Biological Sciences 271, 1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PTJ and Thieltges DW (2010) Diversity, decoys and the dilution effect: how ecological communities affect disease risk. Journal of Experimental Biology 213, 961–970. [DOI] [PubMed] [Google Scholar]
- Johnson PTJ, Kellermanns E and Bowerman J (2011) Critical windows of disease risk: amphibian pathology driven by developmental changes in host resistance and tolerance. Functional Ecology 25, 726–734. [Google Scholar]
- Johnson PTJ, Rohr JR, Hoverman JT, Kellermanns E, Bowerman J and Lunde KB (2012) Living fast and dying of infection: host life history drives interspecific variation in infection and disease risk. Ecology Letters 15, 235–242. [DOI] [PubMed] [Google Scholar]
- Johnson PTJ, Preston DL, Hoverman JT and Richgels KLD (2013) Biodiversity reduces disease through predictable changes in host community competence. Nature 494, 230–234. [DOI] [PubMed] [Google Scholar]
- Johnson PTJ, Ostfeld RS and Keesing F (2015) Frontiers in research on biodiversity and disease. Ecology Letters 18, 1119–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PTJ, Calhoun DM, Riepe TB and Koprivnikar J (2019a) Chance or choice? Understanding parasite selection and infection in multi-host communities. International Journal for Parasitology 49, 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PTJ, Calhoun DM, Riepe T, McDevitt-Galles T and Koprivnikar J (2019b) Community disassembly and disease: realistic – but not randomized – biodiversity losses enhance parasite transmission. Proceedings of the Royal Society B: Biological Sciences 286, 20190260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph MB, Mihaljevic JR, Orlofske SA and Paull SA (2013) Does life history mediate changing disease risk when communities disassemble? Ecology Letters 16, 1405–1412. [DOI] [PubMed] [Google Scholar]
- Kalbe M and Kurtz J (2006) Local differences in immunocompetence reflect resistance of sticklebacks against the eye fluke Diplostomum pseudopathaceum. Parasitology 132, 105–116. [DOI] [PubMed] [Google Scholar]
- Keesing F, Holt RD and Ostfeld RS (2006) Effects of species diversity on disease risk. Ecology Letters 9, 485–498. [DOI] [PubMed] [Google Scholar]
- Keesing F, Belden LK, Daszak P, Dobson A, Harvell CD, Holt RD, Hudson P, Jolles A, Jones KE, Mitchell CE, Myers SS, Bogich T and Ostfeld RS (2010) Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468, 647–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Daszak P, Jones MJ, Marra PP and Kramer LD (2006) Host heterogeneity dominates West Nile virus transmission. Proceedings of the Royal Society B: Biology Sciences 273, 2327–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick AM, Fonseca DM, Ebel GD, Reddy MR and Kramer LD (2010) Spatial and temporal variation in vector competence of Culex pipiens and Cx. restuans mosquitoes for West Nile virus. The American Journal of Tropical Medicine and Hygiene 83, 607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar N, Langevin S, Hinten S, Nemeth NM, Edwards E, Hettler DL, Davis BS, Bowen RA and Bunning ML (2003) Experimental infection of North American birds with the New York 1999 strain of West Nile Virus. Emerging Infectious Diseases 9, 311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCroix C, Jolles A, Seabloom EW, Power AG, Mitchell CE and Borer ET (2013) Non-random biodiversity loss underlies predictable increases in viral disease prevalence. Journal of the Royal Society Interface 11, 20130947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaDeau SL, Kilpatrick AM and Marra PP (2007) West Nile virus emergence and large-scale declines of North American bird populations. Nature 447, 710–713. [DOI] [PubMed] [Google Scholar]
- LaFonte BE and Johnson PTJ (2013) Experimental infection dynamics: using immunosuppression and in vivo parasite tracking to understand resistance in an amphibian-trematode system. Journal of Experimental Biology 216, 3700–3708. [DOI] [PubMed] [Google Scholar]
- Langwig KE, Frick WF, Hoyt JR, Parise KL, Drees KP, Kunz TH, Foster JT and Kilpatrick AM (2017) Drivers of variation in species impacts for a multi-host fungal disease of bats. Philosophical Transactions of the Royal Society B: Biological Sciences 371, 20150456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KA (2006) Linking immune defense and life history at the levels of the individual and the species. Integrative and Comparative Biology 46, 1000–1015. [DOI] [PubMed] [Google Scholar]
- Lloyd-Smith JO, Schreiber SJ, Kopp PE and Getz WM (2005) Superspreading and the effect of individual variation on disease emergence. Nature 438, 355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis AD, Kuenzi AJ and Mills JN (2018) Species diversity concurrently dilutes and amplifies transmission in a zoonotic host-pathogen system through competing mechanisms. Proceedings of the National Academy of Sciences of the USA 115, 7979–7984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manlove KR, Cassirer EF, Plowright RK, Cross PC and Hudson PJ (2017) Contact and contagion: probability of transmission given contact varies with demographic state in bighorn sheep. Journal of Animal Ecology 86, 908–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LB, Hopkins WA, Mydlarz LD and Rohr JR (2010) The effects of anthropogenic global changes on immune functions and disease resistance. Annals of the New York Academy of Sciences 1195, 129–148. [DOI] [PubMed] [Google Scholar]
- Martin LB, Burgan SC, Adelman JS and Gervasi SS (2016) Host competence: an organismal trait to integrate immunology and epidemiology. Integrative and Comparative Biology 56, 1225–1237. [DOI] [PubMed] [Google Scholar]
- Martin LB, Addison BA, Bean AGD, Buchanan KL, Crino OL, Eastwood JR, Flies AS, Hamede R, Hill GE, Klaasen M, Koch RE, Martens JM, Napolitano C, Narayan EJ, Peacock L, Peel AJ, Peters A, Raven N, Risely A, Roast MJ, Rollins LA, Ruiz-Aravena M, Selechnik D, Stokes HS, Ujvari B and Grogan LF (2019) Extreme competence: keystone hosts of infections. Trends in Ecology and Evolution 34, 303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather TM, Wilson ML, Moore SI, Ribeiro JMC and Spielman A (1989) Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi). American Journal of Epidemiology 130, 143–150. [DOI] [PubMed] [Google Scholar]
- McCallum H, Fenton A, Hudson PJ, Lee B, Levick B, Norman R, Perkins SE, Viney M, Wilson AJ and Lello J (2017) Breaking beta: deconstructing the parasite transmission function. Philosophical Transactions of the Royal Society of London B: Biological Sciences 372, 20160084. doi: 10.1098/rstb.2016/0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill BJ, Enquist BJ, Weiher E and Westoby M (2006) Rebuilding community ecology from functional traits. Trends in Ecology and Evolution 21, 178–185. [DOI] [PubMed] [Google Scholar]
- Merrill L, Stewart Merrill TE, Barger AM and Benson TJ (2019) Avian health across the landscape: nestling immunity covaries with changing landcover. Integrative and Comparative Biology 59, 1150–1164. [DOI] [PubMed] [Google Scholar]
- Messier J, McGill BJ and Lechowicz MJ (2010) How do traits vary across ecological scales? A case for trait-based ecology. Ecology Letters 13, 838–848. [DOI] [PubMed] [Google Scholar]
- Messina S, Edwards DP, Eens M and Constantini D (2018) Physiological and immunological responses of birds and mammals to forest degradation: a meta-analysis. Biological Conservation 224, 223–229. [Google Scholar]
- Mihaljevic JR, Joseph MB, Orlofske SA and Paull SH (2014) The scaling of host density with richness affects the direction, shape, and detectability of diversity-disease relationships. PLoS ONE 9, e97812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montiglio P-O, Dammhahn M, Messier GD and Réale DJF (2018) The pace-of-life syndrome revisited: the role of ecological conditions and natural history on the slow-fast continuum. Behavioral Ecology and Sociobiology 72, 116. [Google Scholar]
- Mordecai EA, Caldwell JM, Grossman MK, Lippi CA, Johnson LR, Neira M, Rohr JR, Ryan SJ, Savage V, Shocket MS, Sippy R, Stewart Ibarra AM, Thomas MB and Villena O (2019) Thermal biology of mosquito-borne disease. Ecology Letters 22, 1690–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth N, Thomsen B, Spraker T, Benson J, Bosco-Lauth A, Oesterle P, Bright J, Muth J, Gidlewski T, Campbell T and Bowen R (2011) Clinical and pathologic responses of American crows (Corvus brachyrhynchos) and fish crows (C. ossifragus) to experimental West Nile virus infection. Veterinary Pathology 48, 1061–1074. [DOI] [PubMed] [Google Scholar]
- Ostfeld RS and Keesing F (2000) The function of biodiversity in the ecology of vector-borne zoonotic diseases. Canadian Journal of Zoology 78, 2061–2078. [Google Scholar]
- Ostfeld RS and Keesing F (2012) Effects of host diversity on infectious disease. Annual Review of Ecology Evolution and Systematics 43, 157–182. [Google Scholar]
- Ostfeld RS and LoGiudice K (2003) Community disassembly, biodiversity loss, and the erosion of an ecosystem service. Ecology 84, 1421–1427. [Google Scholar]
- Paaijmans K, Blanford S, Chan B and Thomas MB (2012) Warmer temperatures reduce the vectorial capacity of malaria mosquitoes. Biology Letters 8, 465–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previtali MA, Ostfeld RS, Keesing F, Jolles AE, Hanselmann R and Martin LB (2012) Relationship between pace of life and immune responses in wild rodents. Oikos 121, 1483–1492. [Google Scholar]
- Råberg L, Grahn M, Hasselquist D and Svensson E (1998) On the adaptive significance of stress-induced immunosuppression. Proceedings of the Royal Society B: Biological Sciences 265, 1637–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Råberg L, Sim D and Read AF (2007) Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science (New York, N.Y.) 318, 812–814. [DOI] [PubMed] [Google Scholar]
- Randolph SE and Dobson AD (2012) Pangloss revisited: a critique of the dilution effect and the biodiversity-buffers-disease paradigm. Parasitology 139, 847–863. [DOI] [PubMed] [Google Scholar]
- Rohr JR, Raffel TR and Hall CA (2010) Developmental variation in resistance and tolerance in a multi-host-parasite system. Functional Ecology 24, 1110–1121. [Google Scholar]
- Rohr JR, Civitello DJ, Halliday FW, Hudson PJ, Lafferty KD, Wood CL and Mordecai EA (2020) Towards common ground in the biodiversity-disease debate. Nature Ecology and Evolution 4, 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns SC (1992) The Evolution of Life Histories. London, UK: Oxford University Press. [Google Scholar]
- Stewart Merrill TE (2019) Variable Immunity and Its Consequences for Parasite Dynamics (PhD thesis). University of Illinois at Urbana-Champaign, Urbana, Illinois. [Google Scholar]
- Stewart Merrill TE, Hall SR, Merrill L and Cáceres CE (2019) Variation in immune defense shapes disease outcomes in laboratory and wild Daphnia. Integrative and Comparative Biology 59, 1203–1219. [DOI] [PubMed] [Google Scholar]
- Stockwell MP, Clulow J and Mahony MJ (2010) Host species determines whether infection load increases beyond disease-causing thresholds following exposure to the amphibian chytrid fungus. Animal Conservation 13, 62–71. [Google Scholar]
- Strauss AT, Bowling AM, Duffy MA, Cáceres CE and Hall SR (2018) Linking host traits, interactions with competitors and disease: mechanistic foundations for disease dilution. Functional Ecology 32, 1271–1279. [Google Scholar]
- Telfer S, Brown KJ, Sekules R, Begon M, Hayden T and Birtles R (2005) Disruption of a host-parasite system following the introduction of an exotic host species. Parasitology 130, 661–668. [DOI] [PubMed] [Google Scholar]
- Telford SR and Spielman A (1993) Reservoir competence of white-footed mice for Babesia microti. Journal of Entomology 30, 223–227. [DOI] [PubMed] [Google Scholar]
- Thompson JN (2005) Coevolution: the geographic mosaic of coevolutionary arms races. Current Biology 15, R992–R994. [DOI] [PubMed] [Google Scholar]
- Van Buskirk J and Ostfeld RS (1995) Controlling Lyme disease by modifying the density and species composition of tick hosts. Ecological Applications 5, 1133–1140. [Google Scholar]
- VanderWaal KL and Ezenwa VO (2016) Heterogeneity in pathogen transmission: mechanisms and methodology. Functional Ecology 30, 1606–1622. [Google Scholar]
- Vazquez-Prokopec GM, Perkins TA, Waller LA, Lloyd AL, Reiner AC Jr, Scott TW and Kitron U (2016) Coupled heterogeneities and their impact on parasite transmission and control. Trends in Parasitology 32, 356–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violle C, Enquist BJ, McGill BJ, Jiang L, Albert CH, Hulshof C, Jung V and Messier J (2012) The return of the variance: intraspecific variability in community ecology. Trends in Ecology and Evolution 27, 244–252. [DOI] [PubMed] [Google Scholar]
- Voyles J, Woodhams DC, Saenz V, Byrne AQ, Perez R, Rios-Sotlea G, Ryan MJ, Bletz MC, Sobell FA, McLetchie S, Reinert L, Rosenblum EB, Rollins-Smith LA, Ibáñez R, Ray JM, Griffith EJ, Ross H and Richards-Zawacki CL (2018) Shifts in disease dynamics in a tropical assemblage are not due to pathogen attenuation. Science (New York, N.Y.) 359, 1517–1519. [DOI] [PubMed] [Google Scholar]
- Weinstein SB, Moura CW, Mendez JF and Lafferty KD (2018) Fear of feces? Tradeoffs between disease risk and foraging drive animal activity around raccoon latrines. Oikos 127, 927–934. [Google Scholar]
- Wood CL and Lafferty KD (2013) Biodiversity and disease: a synthesis of ecological perspectives on Lyme disease transmission. Trends in Ecology and Evolution 28, 239–247. [DOI] [PubMed] [Google Scholar]
- Wood CL, Lafferty KD, DeLeo G, Young HS, Hudson PJ and Kuris AM (2014) Does biodiversity protect humans against infectious disease? Ecology 95, 817–832. [DOI] [PubMed] [Google Scholar]
- Woolhouse MEJ, Dye C, Etard J-F, Smith T, Charlwood JD, Garnett GP, Hagan P, Hii JLK, Ndhlovu PD, Quinnell RJ, Watts CH, Chandiwana SK and Anderson RM (1997) Heterogeneities in the transmission of infectious agents: Implications for the design of control programs. Proceedings of the National Academy of Sciences of the United States of America 94, 338–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young H, Griffin RH, Wood CL and Nunn CL (2013) Does habitat disturbance increase infectious disease risk for primates? Ecology Letters 16, 656–663. [DOI] [PubMed] [Google Scholar]
- Zuk M and McKean KA (1996) Sex differences in parasite infections: patterns and processes. International Journal for Parasitology 26, 1009–1023. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182020000943.
click here to view supplementary material