Abstract
This systematic review investigated the evidence for the therapeutic potential of essential oils (EOs) against Leishmania amazonensis. We searched available scientific publications from 2005 to 2019 in the PubMed and Web of Science electronic databases, according to PRISMA statement. The search strategy utilized descriptors and free terms. The EOs effect of 35 species of plants identified in this systematic review study, 45.7% had half of the maximal inhibitory concentration (IC50) 10 < IC50 ⩽ 50 μg mL−1 and 14.3% had a 10 < IC50 μg mL−1 for promastigote forms of L. amazonensis. EOs from Cymbopogon citratus species had the lowest IC50 (1.7 μg mL−1). Among the plant species analyzed for activity against intracellular amastigote forms of L. amazonensis, 39.4% had an IC50 10 < IC50 ⩽ 50 μg mL−1, and 33.3% had an IC50 10 < IC50 μg mL−1. Aloysia gratissima EO showed the lowest IC50 (0.16 μg mL−1) for intracellular amastigotes. EOs of Chenopodium ambrosioides, Copaifera martii and Carapa guianensis, administered by the oral route, were effective in reducing parasitic load and lesion volume in L. amazonensis-infected BALB/c mice. EOs of Bixa orellana and C. ambrosioides were effective when administered intraperitoneally. Most of the studies analyzed in vitro and in vivo for the risk of bias showed moderate methodological quality. These results indicate a stimulus for the development of new phytotherapy drugs for leishmaniasis treatment.
Key words: Essential oils, Leishmania amazonensis, phytotherapy, plant oils
Introduction
Leishmaniasis is a neglected disease of high prevalence and distribution directly linked to social, environmental and climatological factors. It is endemic in 98 countries, with 350 million people at risk, and is the cause of 50 000 deaths every year worldwide (Alvar et al., 2012; World Health Organization, 2019). The majority of cutaneous leishmaniasis cases occur in Afghanistan, Algeria, Brazil, Colombia, the Islamic Republic of Iran, Pakistan, Peru, Saudi Arabia and the Syrian Arab Republic (World Health Organization, 2019).
The epidemiology of cutaneous leishmaniasis in the Americas is complex, with multiple circulating Leishmania species in the same geographical area, several reservoirs, hosts and sand fly vectors, as well as variable clinical manifestations and responses to therapy (Burza et al., 2018). The genetic complexity of the causative agents of leishmaniasis is vast, as shown by studies on the taxonomy of these trypanosomatids through molecular analysis of V7V8 SSU rRNA, Hsp70 and gGAPDH genes (Jirku et al., 2012; Espinosa et al., 2018); this may translate to the variability in clinical manifestations of the disease. American cutaneous leishmaniasis (ACL) is associated with at least 15 Leishmania species belonging to the subgenera, Viannia, Leishmania and Mundinia, allocated into the subfamily Leishmaniinae (Espinosa et al., 2018; Silveira, 2019). Leishmania braziliensis (subgenera Viannia) and Leishmania amazonensis (subgenera Leishmania) are the dominant pathogenic species in the Americas, and both are involved in cutaneous leishmaniasis which usually presents as a self-limited ulcer that heals in 3–18 months. Leishmania braziliensis is also involved in mucosal leishmaniasis, a potentially life-threatening condition that occurs in up to 10% of patients (Burza et al., 2018).
Besides, L. amazonensis is responsible for anergic diffuse cutaneous leishmaniasis (DCL), accounting for nearly 1% of all ACL cases each year in Brazil (de Lima et al., 2014; Brasil, Ministério da Saúde, 2017; Machado et al., 2019). The anergic DCL is characterized by massive dermal infiltrates, is chronic with frequent relapses, and presents with clinical, immunological, parasitological, anatomopathological and therapeutic aspects different from other forms of ACL (Costa et al., 2009). Leishmania amazonensis infection induces inhibition of the delayed type hypersensitivity of skin, low production of interferon γ (IFN-γ) and high production of interleukin-10 (IL-10) and transforming growth factor-β, leading to inefficient activation of infected macrophages and non-response to conventional treatment (Silveira et al., 1991, 1998; Silveira, 2019).
The standard treatment comprises of pentavalent antimonials, liposomal amphotericin B, pentamidines and miltefosine (Pelissari et al., 2011). These commonly used drugs are toxic, resulting in severe side-effects, such as pancreatitis, leukopenia and more importantly, cardiac arrhythmia (Machado et al., 2012). Standard drug therapy is further compounded with high costs and requires administration in a hospital environment, leading patients to discontinue treatment. Parasite resistance to antimonials has been reported, as well as disease recurrence (Sen and Chatterjee, 2011). To achieve control of leishmaniasis, the discovery of new potentially efficient and safer active compounds is necessary.
Diseases have been treated using plants since the dawn of humankind, resulting in the discovery of many bioactive substances, which are the origin for almost half of the existent drugs (Lam, 2007; Ganesan, 2008). Scientific investigations using plants are fundamental, not only for the discovery of new active substances with fewer side-effects, more efficiency and lower cost, but also to amplify knowledge on biodiversity. Much phytochemical research has been conducted employing extracts, essential oils (EOs) and their fractionated compounds. EOs are secondary substances of plants; they have a complex composition, with many compounds, which confer multiple actions to EOs. Studies have demonstrated antileishmanial activity of EOs or isolated compounds from medicinal plants on Leishmania species (de Lima et al., 2014; Islamuddin et al., 2014). Additionally, it is observed that there is selectivity in relation to the species of Leishmania on which EOs act. Thus, the EO of Nectandra hihua was 121-times more active for L. infantum than for L. amazonensis (Bosquiroli et al., 2017). The EO of Piper demeraranum was 3.8 times more active for L. amazonensis than for L. guyanensis (Moura do Carmo et al., 2012).
Considering the severity of the disease induced by L. amazonensis and the potential of EOs as a source of new bioactive drugs, we systematically investigated previous studies on in vivo and in vitro activity of EOs on this species.
Methods
Search strategy
In-depth search was conducted on PubMed and Web of Science databases to retrieve articles describing the area of interest, published between April 2005 and April 2019. PRISMA statement recommendations were followed in this systematic review (Moher et al., 2015). The descriptors or MeSH terms (Medical Subject Headings) were defined independently by five reviewers of group 1 (CELS, JO, FBPF, MPPS, TVAL, RCLS) and Three specialists (MVCL, MSTM and JJVT). The MeSH terms were divided into three blocks – block 1 (‘plant oils’, ‘volatile oils’); block 2 (‘complementary therapies’, ‘anti-infective agents’, ‘antiprotozoal agents’, ‘phytotherapy’, ‘plants medicinal’, ‘biological products’); and block 3 (‘Leishmania’, ‘leishmaniasis’), and combined to retrieve all potential published articles. The descriptors were also researched in the titles of the articles, in individual or combined pairs to increase research sensitivity.
Articles selection
Inclusion and exclusion criteria: All studies that evaluated the therapeutic effects of EOs against L. amazonensis were included. Database filters were used and abstracts were read to select studies. Only original studies published in Portuguese, English, Spanish or French languages with accessible abstracts were included. Through database filtering and abstract readings, experimental studies were selected for the systematic review. We excluded review studies, case reports, editorials, comparisons, editor comments, clinical assays, letters, news and guidelines. Articles not found through databases and those that did not meet the selection criteria were not selected.
Quality evaluation: At this stage, full-text selected articles, which constituted the articles of potential interest, were retrieved and randomly distributed to researchers in group 1. The final validation of the selected publications was conducted by consensus among three judges in group 2 (MVCL, MSTM and JJVT). The references listed in each paper were also explored for potential articles of interest not identified in the initial phase.
Data extraction
For the organization and structuring of the tables, researchers in group 1 extracted the content of interest from the papers with the support of group 2. This strategy improved the quality and precision of content extracted from each article. To reduce the risk of bias regarding the content extracted from the papers, a standardized instrument was used for the researchers. The following relevant information was extracted and inserted in the table: family, plants species, in vitro results (promastigotes, amastigotes), in vivo results and references. Researchers critically validated the information from each selected paper in pairs.
Risk of bias
The quality of the selected publications was carried out through the risk of bias in individual studies independently by two researcher specialists (JJVT and MVCL). For analysis of 23 studies exclusively in vitro, we use a checklist consisting of 15 domains, based on the CONSORT (Consolidated Standards of Reporting Trials) guidelines (Faggion, 2012). For analysis of two studies exclusively in vivo, we use the Rob (Risk of Bias) tool for animal intervention studies (Systematic Review Center for Laboratory Animal Experimentation – SYRCLE RoB tool), consisting of 12 domains (Hooijmans et al., 2014), characterized as an adjusted risk model Cochrane of the bias tool. Six studies presented experiments both in vivo and in vitro. Currently, no checklist allows evaluating in vitro and in vivo studies simultaneously. Thus, we apply the checklists separately for each design model. The analysis of the methodological quality of each publication was carried out according to the scores from zero to ten for in vivo studies and from zero to 15 for in vivo studies, where high scores allowed the high quality of publications.
Results
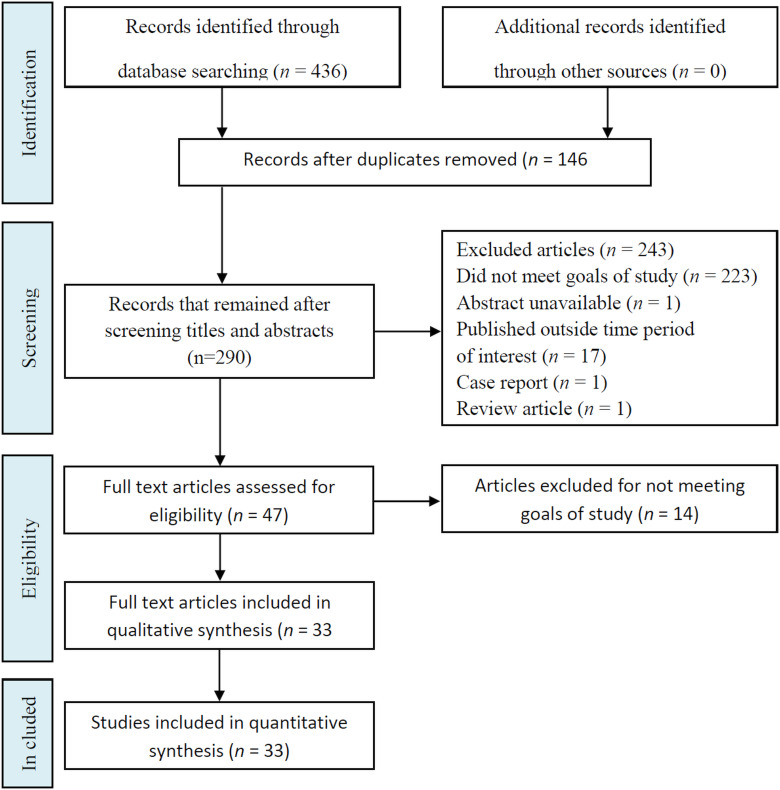
Published studies were identified in two electronic databases. During the analysis of titles and abstracts of 436 publications by the reviewers, 146 duplicated articles were excluded. Following the application of exclusion criteria, 243 studies were excluded, and 47 were selected since they corresponded to the inclusion criteria, and 14 were excluded for not meeting goals of the study (Fig. 1). The 33 confirmed primary studies were printed in PDF format, analysed thoroughly for data extraction, and were organized and structured (Table 1). This study identified 46 plants species, and all were pre-clinical and experimental. The majority (75.7%) being in vitro studies, 6.1% in vivo and 18.2% in vitro and in vivo. Several of the in vitro studies simultaneously investigated the effect of EO on promastigote (35 plant species) and amastigote (33 plant species) forms of L. amazonensis.
Fig. 1.
Flowchart of the different phases of articles included in systematic review.
Table 1.
Main characteristics of primary studies in vitro and in vivo with essential oils on Leishmania amazonensis
| Family | Species plants | Method of essential oils extraction | In vitro results | In vivo results | Author/year | |
|---|---|---|---|---|---|---|
| Promastigotes IC50 (μg mL−1) |
Amastigotes IC50 (μg mL−1) |
|||||
| Anacardiaceae | Myracrodruon urundeuva | The plant material was air-dried for 7 days. The hydrodistillation used a Clevenger-type apparatus (300 g, 6 h). The EO was dried over anhydrous sodium sulphate, filtered, and stored (dark flask/at 4°C) | EO: 205 | EO: 44.51; 104.52 | NR | Carvalho et al. (2017) |
| Asteraceae | Pluchea carolinensis | The fresh plant sample was collected early in the morning and manually crushed. The crude EO was extracted directly by hydrodistillation for 5 h using a Clevenger-type apparatus | EO: 24.7 | EO: 6.21 | EO (30 mg kg−1) intralesional: smaller lesion size and parasite burden | Garcia et al. (2017) |
| Achillea millefolium | The EO oil was obtained by steam distillation of the fresh leaves and flowers, employing Clevenger's apparatus | EO: 7.8 (72 h) | EO: 6.5 1 | NR | Santos et al. (2010) | |
| Matricaria chamomilla | The EO was extracted by hydrodistillation using a modified Clevenger apparatus. The fresh, chopped leaves (300 g, 2 h) were extracted in three repetitions. The EO was separated by centrifugation at 1100 g/5 min, and was stored in a glass bottle at 4°C, and protected from light | EO: 60.16 (24 h) | NR | NR | Andrade et al. (2016) | |
| Matricaria recutita | The EO was extracted by hydrodistillation (3 h) using Clevenger type apparatus. Flowers of M. recutita rinsed in running tap water then air-dried for 14 days were immersed in water and heated to boiling. EO was evaporated and collected in a condenser. The obtained EO was dried over anhydrous sodium sulfate, and stored at −4°C | EO: 10.8 (72 h) (−)-α-bisabolol: 16.0 |
(−)-α-bisabolol: 5.91 | NR | Hajaji et al. (2018) | |
| Mikania micrantha | The EO was obtained from fresh parts of the plant by hydrodistillation and stored at −18°C | NR | EO: 6.82 | NR | Houel et al. (2015) | |
| Vernonia brasiliana | The crushed fresh material (roots, leaves and flowers) was submitted to hydrodistillation for 4 h in a Clevenger-type apparatus (50 g of sample/500.0 mL water) The fraction obtained was extracted with dichloromethane. The organic layer was dried (anhydrous magnesium sulphate), and filtered |
Leaves: 213; Flower: 112; Roots: 109 | NR | NR | Martins et al. (2015) | |
| Bixaceae | Bixa orellana | The EO was obtained by hydrodistillation of the seeds dried under ventilation by using Clevenger-type equipment | NR | EO: 8.51 | EO (30 mg kg−1) for 14 days (intraperitoneal): reduction of parasite load and in the lesion size | Monzote et al. (2014a) |
| Burseraceae | Protium heptaphyllum | The EO was obtained from fresh parts of the plant by hydrodistillation and stored at −18°C | NR | EO: 34.91; 3.72 | NR | Houel et al. (2015) |
| Chenopodiaceae | Chenopodium ambrosioides | The EO of the aerial parts of the plant was obtained by distillation, under laboratory conditions, using a Clevenger apparatus | EO: 3.7 | EO: 4.61 | EO (30 mg kg−1) for 15 days (intraperitoneal route): reduction in the size of the lesion, and suppression of the number of parasites in the infected footpads | Monzote et al. (2006) |
| C. ambrosioides | The EO of the aerial parts of the plant was obtained by distillation using a Clevenger apparatus | NR | NR | EO (30 mg kg−1 day−1 intraperitoneal) prevented lesion development and decrease the parasite burden; EO (30 mg kg−1 day−1 oral route retarded the infection; EO (3% day−1 intralesional route) did not show activity |
Monzote et al. (2007) | |
| C. ambrosioides | The EO was obtained by distillation of the aerial parts of the plant using a Clevenger's apparatus | NR | NR | EO for 15 days (oral, 150 mg kg−1) was the most effective and better activity | Monzote et al. (2009) | |
| C. ambrosioides | The EO was obtained by distillation of the aerial parts of the plant using a Clevenger's apparatus | EO: 3.7; Ascaridole: 0.1; Carvacrol: 15.3; Caryophyllene: 4.9; EO + Ascaridole: 0.1 |
EO: 4.61; Ascaridole 0.31; Carvacrol 13.61; Caryophyllene 4.41 |
NR | Monzote et al. (2014a, 2014b) | |
| Fabaceae | Copaifera martii | The Copaifera sp. EO was obtained from a producer cooperative (Sena Madureira, Acre, Brazil), and tested for purity and physical and chemical characteristics | Nanocopa: 18 (24 h); 30 (48 h) | IC50 inhibition: 30 μg mL−1 50%; 90 μg mL−1 90% | Mice treated for 8 weeks: smaller lesions than those on untreated mice | Dhorm Pimentel de Moraes et al. (2018) |
| C. martii | The copaiba EO was collected from the trunk of C. martii tree | EO: 14 | NR | Oral route (100 mg kg−1 day−1) + topical route (4%, 1 mg mm−2): reduction in the size of the lesions. Topical + subcutaneous routes (100 mg kg−1 day−1): not reduction in the size of the lesion |
dos Santos et al. (2011) | |
| Vouacapoua americana | The EO was obtained from fresh parts of the plant by hydrodistillation and stored at −18°C | NR | EO: 7.22 | NR | Houel et al. (2015) | |
| Pterodon pubescens | Turbo extraction: whole fruits (30 g) were subjected to three consecutive extractions in 99.9% ethanol, and concentrated in a rotary vacuum evaporator. Maceration dynamics: cut fruit (30 g) were submitted to constant mechanical stirring for 3 consecutive days in 99.8% ethanol, and then it was concentrated on a rotary evaporator. The hexane fractions were collected (turboextraction-FHe1 and maceration-FHe2). Supercritical CO2 extraction (scCO2): the dried fruits were crushed with a knife mill (30 g) |
FHe1 (FHe, hexane fraction): 41; FHe2: 52.1; EOe3 (EOe, supercritical fluid extract): 26.3; EOe4: 26.3; EOe5: 25.9; EOe6: 28.4; EOe7: 26.4 |
FHe1: 40.71; EOe3: 33.81; N.FHe 1 (nanoemulsion, hexane extract): 2.71; N.EOe3 (nanoemulsion, supercritical extract): 1.91 |
NR | da Silva Santos et al. (2016) | |
| Lamiaceae | Tetradenia riparia | The EO was obtained of fresh leaves (60 g) by steam distillation in a Clevenger apparatus for 3 h with 600 mL of distilled water. The EO was dried with anhydrous sodium sulphate, and stored at 4°C | EO of spring: 15.47; EO of summer: 15.67; EO of autumn: 15.66; EO of winter: 13.31 | IC50 inhibition by EO at 30 ng mL−1; spring: 43.53%; summer: 32.03%; autumn: 40.54%; winter: 52.49% | Topical route (0.5% or 1%) daily for 8 weeks: reduction of the parasite load in the spleen | Cardoso et al. (2015) |
| T. riparia | The EO of T. riparia fresh leaves was obtained by hydrodistillation for 3 h using a Clevenger-type apparatus. The distilled EO was dried over anhydrous sodium sulphate and stored at −18°C | EO: 2.45; TrROY: 0.03 |
IC50 inhibition by EO at 30 μg mL−1: 65%; IC50 inhibition by TrROY at 10 μg mL−1: 48% |
NR | Demarchi et al. (2015) | |
| Ocimum canum | The EO was extracted by hydrodistillation using a Clevenger system. 100 g of dry leaves diluted in water (1:10) were boiled to 100°C for 3 h. The EO was dried with anhydrous sodium sulphate | EO: 17.4 | EO: 13.11 | NR | da Silva et al. (2018) | |
| Origanum vulgare | The aerial parts were dried for 48 h and sprayed in an electric knife mill. The EO was extracted by hydrodistillation using a Clevenger apparatus. The plant diluted in water (1:10) were boiled to 100°C for 3 h. The EO was dried with anhydrous sodium sulphate | EO: 405.5 | NR | NR | Teles et al. (2019) | |
| Ocimum gratissimum | Fresh leaves from the plant were cut into pieces and subjected to steam distillation. Then, it was extracted with petroleum ether, which was removed carefully, and the EO was obtained | EO: 135; Eugenol: 80 |
EO: 1002 | NR | Ueda-Nakamura et al. (2006) | |
| Lauraceae | Nectandraamazonum | Fresh material (leaves) from N. amazonum was milled and EO was extracted by hydrodistillation in a Clevenger apparatus for 5 h, and dried with anhydrous sodium sulphate | NR | EO: 22.11 | NR | Bosquiroli et al. (2017) |
| N. gardneri | Fresh material (stem bark) from N. gardneri was milled and EO was extracted by hydrodistillation in a Clevenger apparatus for 5 h, and dried with anhydrous sodium sulphate | NR | EO: 2.11 | NR | ||
| N. hihua | Fresh material (stem bark) from N. hihua was milled and EO was extracted by hydrodistillation in a Clevenger apparatus for 5 h, and dried with anhydrous sodium sulphate | NR | EO: 24.21 | NR | ||
| N. megapotamica | Fresh material (stem bark) from N. megapotamica was milled and EO (NMEO1) was extracted by hydrodistillation in a Clevenger apparatus for 5 h, and dried with anhydrous sodium sulphate | NR | EO: 19.01 | NR | ||
| N. megapotamica | Fresh material (leaves) from N. megapotamica was milled and EO (NMEO2) was extracted by hydrodistillation in a Clevenger apparatus for 5 h, and dried with anhydrous sodium sulphate | NR | EO: 21.31 | NR | ||
| Cinnamomum zeylanicum | The leaves were dried for 48 h and sprayed in an electric knife mill. The EO was extracted by hydrodistillation using a Clevenger apparatus. The plant diluted in water (1:10) were boiled to 100°C for 3 h. The EO was dried with anhydrous sodium sulphate | EO: >500 | NR | NR | Teles et al. (2019) | |
| Cryptocarya aschersoniana | Fresh leaves of C. aschersoniana (300 g) were subjected to hydrodistillation for 2 h in a Clevenger-type apparatus and collected manually. The extraction procedure was done in triplicate | EO: 4.46 | NR | NR | Andrade et al. (2018) | |
| Meliaceae | Carapa guianensis | The C. guianensis (andiroba) EO was obtained from a producer cooperative (Cruzeiro do Sul Acre, Brazil), and tested for purity and physical and chemical characteristics | EO: 590 (24 h); EO: 260 (48 h) | IC50 inhibition by EO at 300 μg mL−1: 96% | EO for 8 weeks (100-160 mg kg−1, oral route): amastigotes were less abundant in lesions of treated-mice | Dhorm Pimentel de Moraes et al. (2018) |
| C. guianensis and 5 fractions | The process of obtaining C. guianensis seed oil was an artisanal manner. The fruit was cooked, the andiroba nuts removed, and macerated to obtain the oil |
EO: did not exhibit leishmanicidal activity; Limonoid-rich fractions: LF3: 10.53; LF4: 25.30; LF5: 56.90 |
Limonoid-rich fractions: LF3: 271; LF4: 78.421; LF5: 352.21 | NR | Oliveira et al. (2018) | |
| Myrtaceae | Syzygium cumini | Large leaves of S. cumini were collected from a mature tree in the flowering stage. Plant material was air-dried for 7 days, cut into small pieces and the EO extracted by hydrodistillation using a Clevenger-type apparatus (300 g, 3 h). The EO was dried (anh. Na2SO4), and centrifuged | EO: 60 | NR | NR | Dias et al. (2013) |
| S. cumini | The S. cumini EO was extracted of leaves by conventional steam hydrodistillation | EO: 60 α-Pinene: 19.7 |
EO: 38.11; EO: 43.92; α-Pinene: 15.61; 16.12 |
NR | Rodrigues et al. (2015) | |
| Piperaceae | Piper rivinoides | The EOs of Piper sp. were obtained from fresh leaves (600 g) by hydrodistillation in a Clevenger apparatus for 4 h with 600 mL of water. The EOs were collected, centrifuged at 5000 rpm/2 min, and stored at −4oC | EO: 10.9 | EO: >2002 | NR | Bernuci et al. (2016) |
| P. mosenii | EO: 17.4 | EO: >2002 | NR | |||
| P. cernuum | EO: 27.1 | EO: >2002 | NR | |||
| P. diospyrifolium | EO: 13.5 | EO: 76.12 | NR | |||
| P. arboretum | EO: 15.2 | EO: >2002 | NR | |||
| P. aduncum | EO: 25.9 | EO: 32.22 | NR | |||
| P. gaudichaudianum | EO: 93.5 | NR | NR | |||
| P. xylosteoides | EO: >100 | NR | NR | |||
| P. mikanianum | EO: >100 | NR | NR | |||
| P. cubeba | The P. cubeba EO was obtained by steam distillation was purchased from Suraj Bala Exports | EO: 326.5 | NR | NR | Esperandim et al. (2013) | |
| P. claussenianum | The EOs were separately extracted from leaves and inflorescences of fresh and air-dried material. Each fresh and dried sample (about 100 g) was extracted by hydrodistillation for 2 h in a Clevenger-type apparatus. The EOs were dried over anhydrous sodium sulphate and stored at 4°C | EO leaves: 30.4; EO inflorescens: 1.328 |
NR | NR | Marques et al. (2010) | |
| P. demeraranum | The leaves of P. demeraranum (600 g) were dried at room temperature, ground, and submitted to hydrodistillation (4 h) using a modified Clevenger-type apparatus. The EO was collected, and stored in glass flasks at −4°C. | EO: 86 | EO: 781 | NR | Moura do Carmo et al. (2012) | |
| P. duckei | The EO of P. duckei leaves were dried at room temperature, ground, and submitted to hydrodistillation (4 h) using a modified Clevenger-type apparatus. After the end of each distillation, the EO was stored in glass flasks at −4°C | EO: 46 | EO: 421 | NR | Moura do Carmo et al. (2012) | |
| P. hispidum | The EO was obtained from fresh parts of the plant by hydrodistillation and stored at −18°C | NR | EO: 4.71; EO: 3.42 |
NR | Houel et al. (2015) | |
| Plantaginaceae | Otacanthus azureus | The EO was obtained from fresh parts of the plant by hydrodistillation and stored at −18°C. | NR | EO: 16.11; EO: 0.72 |
NR | Houel et al. (2015) |
| Poaceae | Cymbopogon citratus | The EO was obtained from fresh parts of the plant by hydrodistillation and stored at −18°C | NR | EO: 5.32 | NR | Houel et al. (2015) |
| C. citratus | The EO was obtained by steam distillation of fresh leaves from the plant, employing Clevenger's apparatus, and was stored at −20°C | EO: 1.7 Citral: 8.0 |
EO: 3.21; Citral: 25.01 |
NR | Santin et al. (2009) | |
| Rubiaceae | Mitracarpus frigidus | The EO was obtained from dried aerial parts of M. frigidus by hydrodistillation (3 h) using a Clevenger type apparatus. The EO was stored at 4°C | EO: 89.7 | NR | NR | Fabri et al., 2012 |
| Scrophulariaceae | Achetaria guianensis | The EO was obtained from fresh parts of the plant by hydrodistillation and stored at −18°C | NR | EO: 6.32 | NR | Houel et al. (2015) |
| Verbenaceae | Lippia sidoides | The fresh aerial parts of L. sidoides (100 g) were submitted to hydrodistillation in a Clevenger-type apparatus for 3 h. The EO were dried over anhydrous sodium sulphate and stored at −20°C | EO: 44.38; Thymol: 19.47 |
EO: 34.41 | NR | de Medeiros et al. (2011) |
| Aloysia gratissima | Fresh aerial parts (leaves and branches, 1020 g) of A. gratissima were hydrodistilled (2 L water) in a modified Clevenger apparatus (6 h). The volatile EO was stored in sealed amber ampoules at −20°C | EO: 25 (48 h); 14 (72 h) | EO: 0.161; Guaiacol: 0.011 |
NR | Garcia et al. (2018) | |
| Zingiberaceae | Curcuma longa | The leaves were dried for 48 h and sprayed in an electric knife mill. The EO was extracted by hydrodistillation using a Clevenger apparatus. The plant diluted in water (1:10) were boiled to 100°C for 3 h. The EO was dried with anhydrous sodium sulphate | EO: 308.4 | EO: 63.31 | NR | Teles et al. (2019) |
EO, essential oil; IC50, concentration of the EO that reduced the survival of Leishmania parasites by 50% compared with untreated parasites; CC50, cytotoxicity to cells 50%; 1IC50, obtained with intracellular amastigote on peritoneal macrophages of Balb/c mouse infected with Leishmania; 2IC50, obtained with axenic Leishmania amastigote; NO, nitric oxide; NR, not reported.
Among the EOs of 35 plant species identified in this systematic review study, 45.7% had a 10 < IC50 ⩽ 50 μg mL−1 and 14.3% had a 10 < IC50 μg mL−1 for promastigote forms of L. amazonensis (Supplementary File S1 and S1a). Cymbopogon citratus species had the lowest IC50 (1.7 μg mL−1). However, we identified bioactive agents from ten plant species with IC50 values higher than 100 μg mL−1 for Leishmania promastigotes. However, 33 species of plants investigated for activity against intracellular amastigote forms of L. amazonensis, 39.4% had a 10< IC50 ⩽ 50 μg mL−1 and 33.3% had a 10 < IC50 μg mL−1. The EO of Aloysia gratissima showed the lowest IC50 (0.16 μg mL−1) for intracellular amastigotes (Garcia et al., 2018).
The study of Leishmania infection in animal models has been described in eight articles, including the study of antileishmanial activity of EOs of six plant species, Pluchea carolinensis (Garcia et al., 2017), Bixa orellana (Monzote et al., 2014a), C. martii (dos Santos et al., 2011; Dhorm Pimentel de Moraes et al., 2018), Tetradenia riparia (Cardoso et al., 2015), C. guianensis (Dhorm Pimentel de Moraes et al., 2018) and C. ambrosioides (Monzote et al., 2006, 2007, 2009). Of the 33 publications selected, eight used the in vivo model, and the same study used between one and three routes of administration. Among the EOs of these species, EOs of C. ambrosioides (Monzote et al., 2009), C. martii (dos Santos et al., 2011; Dhorm Pimentel de Moraes et al., 2018) and C. guianensis (Dhorm Pimentel de Moraes et al., 2018), when administered by the oral route, were effective in reducing parasitic load and lesion volume in L. amazonensis-infected BALB/c mice. EOs of B. orellana (Monzote et al., 2014a) and C. ambrosioides (Monzote et al., 2006) were effective when administered intraperitoneally. EO of T. riparia, when given topically, did not alter the volume of lesions, but reduced the parasitic load on the spleen and lymph nodes of L. amazonensis-infected BALB/c mice.
Risk of bias assessment
The quality assessments of 33 selected publications were mentioned in Supplementary Tables S2–S5. The score of in vitro studies ranged from 5 to 9 out of a total of 15 points (Supplementary File S2 and S3). All in vitro studies performed the domains for structured abstract, scientific background, and rationale, objectives and/or hypotheses, and intervention of each group. No studies reported allocation sequence generation, allocation concealment mechanism, implementation, outcomes and estimation. Only one publication (Monzote et al., 2014a, 2014b) applied the blinding domain. The articles with the in vivo experimental model had scores ranging from 3 to 7, among ten domains (Supplementary File S4 and S5). All publications developed the domains baseline characteristics, selective outcome reporting, other sources of bias. The allocation sequence generation domain was applied in most publications. The domain blinding of personnel and participants, and random outcome assessment was performed in only one study (dos Santos et al., 2011).
Discussion
Natural plant products are essential sources for drug innovation, and the selection and validation of extracts or molecules with relevant pharmacological action requires strategies for choosing effective bioproducts (Clardy and Walsh, 2004; Cos et al., 2006). In this systematic review, we retrieved studies on EOs of plants that specifically act against L. amazonensis, a principal causative agent in cutaneous leishmaniasis. Various screening approaches are available to identify the primary pharmacological actions of natural products on Leishmania. Promastigote forms of Leishmania multiply in defined culture media, and thus, the determination of IC50 of a bioproduct is widely employed among studies. Another interesting bioassay method evaluates the concentration capable of killing 50% of amastigote forms (IC50) in peritoneal macrophages of BALB/c mice infected with Leishmania (Cos et al., 2006). Several articles also address the mechanisms of action of these bioproducts by ultrastructural analysis using transmission electron microscope (TEM), investigation of cell death induction and studies on microbicidal metabolites of macrophages, such as nitric oxide (NO) and various cytokines that modulate the immune response. Measurement of the 50% toxicity concentration of bioproducts on murine cells or cell lines (CC50), and the action on erythrocytes allows an assessment of toxicity the bioproducts may be. These studies usually precede animal model trials in which mice are infected, develop the disease and are subsequently treated using various protocols. Each test should contain at least one reference drug to ascertain test performance and proper interpretation of the screening results. Relevant and selective activity is related to IC50-values below 100 μg mL−1 for extracts and below 25 μm for pure compounds (Cos et al., 2006). In this review, we studied the EOs of plant species from the following families:
Anacardiaceae family
Myracrodruon urundeuva: M. urundeuva is native to South America and is used in traditional medical practices in Brazil for the treatment of mycoses, candidiasis, bacterial infections and as an anti-inflammatory agent (Pereira et al., 2014). The EO of this species (MuEO) contains monoterpene and sesquiterpene hydrocarbons, the main constituent being β-myrcene (42.46%), followed by α-myrcene (37.23%) and caryophyllene (4.28%). β-myrcene showed anti-L. amazonensis activity, as previously reported (Carvalho et al., 2017; Machado et al., 2012), preferably against the intracellular amastigote forms, which are involved in the development of the clinical manifestations of leishmaniasis. In optical microscopy, it was observed that MuEO-induced morphological changes in promastigotes, showing cells with round or spherical shapes, as well as the presence of cell debris (typical of cell lysis), suggesting leishmanicidal activity (Carvalho et al., 2017). MuEO decreased macrophage viability only at high concentrations (>200 μg mL−1). However, cytotoxicity against erythrocytes was low, with no alteration on lysosomal activity or NO production in macrophages, which indicates probable lack of direct involvement in immunomodulatory mechanisms (Carvalho et al., 2017).
Asteraceae family
Achillea millefolium: A. millefolium, which is native to Europe, North America and South Australia, is a herbaceous plant with a perennial life cycle and is popularly referred to as ‘thousand leaves’. EO from the leaves and flowers of A. millefolium was active against L. amazonensis with a promising IC50 of 6.5 μg mL−1 and CC50 of 72.0 μg mL−1 (Santos et al., 2010). In scanning electron microscopy (SEM), the parasites revealed alterations of shape and size, and in TEM, ultrastructural changes in the flagellar membrane, abnormal membrane structures, rupture of the plasma membrane, atypical vacuoles, myelin-like figures and vesicles that resembled autophagic vacuoles were observed (Santos et al., 2010). Achillea millefolium extracts have also been reported to possess anti-inflammatory activity (Tadic et al., 2017), which may be related to its content of phenolic compounds, more specifically, dicaffeoylquinic acids, luteolin, apigenin and its glycosides. Additionally, Villalva et al. (2019) reported the inhibitory effect of A. millefolium fractions on IL-1β, IL-6 and tumour necrosis factor-α (TNF-α) secretion.
Matricaria chamomilla: The gas chromatography-mass spectrometry (GC-MS) analyses for M. chamomilla EO identified the main constituents as β-farnesene (52.73%), bisabolol oxide (12.09%), α-farnesene (10.34%) and α-bisabolo (9.83%) (Andrade et al., 2016). This same study compared the IC50/24 h (60.16 μg mL−1) for promastigote forms of L. amazonensis, and cytotoxicity against L6 cells (CC50/24 h 173.04 μg mL−1) using the selectivity index (173.04 μg mL−1), and it was considered moderately active (50 < IC50 ⩽ 150 μg mL−1) (Andrade et al., 2016).
Matricaria recutita: M. recutita is native to northern Europe and grows wild in Central European countries, Eastern Europe, western Asia, the Mediterranean region of North Africa and the Americas. Chamomile is one of the plants most cited for medicinal purposes in qualitative studies, for adult or paediatric use (Brasil, Ministério da Saúde, 2015). It has uses mentioned in pharmacopoeias, ethnobotanical studies, folk medicine, complementary and alternative medicine. A bio-guided study conducted by Hajaji et al. (2018) identified the mechanism involved in Tunisian chamomile EO leishmanicidal action. The IC50 values for L. amazonensis promastigote were low and ranging from 10.8 μg mL−1 for the EO to 16.0 μg mL−1 for (−)-α-bisabolol. The CC50 on macrophages J774.A1 was 31.9 μg mL−1, and the selectivity index was 5.5. The (−)-α-bisabolol was able to activate a programmed cell death process. This compound induced phosphatidylserine externalization and membrane damage, decrease the mitochondrial membrane potential and total ATP levels in the promastigote of L. amazonensis (Hajaji et al., 2018). Matricaria recutita and its active compound ( − )-α-bisabolol can be a natural potential alternative to the available drugs.
Mikania micrantha: M. micrantha is native to Central and South America and is considered as an invasive weed, growing in cattle fields. Laurella et al. (2017) isolated and identified four sesquiterpene lactones of the germacranolide type from M. micrantha organic extracts: mikanolide, dihydromikanolide and deoxymikanolide scandenolide. They observed that mikanolide and deoxymikanolide showed significant activity against L. braziliensis promastigotes, while dihydromikanolide displayed moderate activity. Houel et al. (2015) used a strategy to discover bioactive natural products based on bioinspiration, which allows the transposition of these desirable properties to a corresponding research field. These authors evaluated the antileishmanial properties of selected anti-dermatophytic plants, looking for a match with antileishmanial activity. Mikania micrantha EO showed weak to non-existent activity against selected dermatophytic filamentous fungi but achieved action against L. amazonensis axenic amastigotes, while presenting low cytotoxicity to VERO cells and BALB/c mice peritoneal macrophages (Houel et al., 2015).
Pluchea carolinensis: This plant has a broad native distribution, from Mexico and Central America to South America. Garcia et al. (2017) described that the EO from aerial parts of P. carolinensis contained at least 44 compounds, the main component being selin-11-en-4α-ol. This EO inhibited the growth of promastigote and amastigote forms of L. amazonensis, while its cytotoxicity was 5-fold higher for peritoneal macrophages than that for the parasites. In an experimental model of infection, BALB/c mice treated with five doses of the EO (30 mg kg−1) by intralesional route presented reduced lesion size and parasite burden. This potential EO may target specific molecules or pathways in the amastigote form or induce defence mechanisms in the macrophages that contributed to the antileishmanial activity.
Vernonia brasiliana: V. brasiliana is a bush tree observed in the Brazilian savannah, also known as ‘assa-peixe’. The EO from flowers, roots and leaves of V. brasiliana did not display cytotoxicity against Vero (ATCC CCL 81) and RAW 264.7 cell lines, but they showed inhibitory activity against T. cruzi (Martins et al., 2015). The only study about V. brasiliana, by Martins et al. (2015) showed that (a) in the EOs obtained by hydrodistillation, the major components found in the flowers were (E)-hex-2-enal (4.0%), hexan-1-ol (4.2%), (Z)-hex- 2-en-1-ol (6.3%) and palmitic acid (8.3%); (b) in the roots, the major components were α-isocomene (15.4%), α-gurjunene (9.6%), β-isocomene (10.3%), transcaryophyllene (10.4%) and palmitic acid (5.3%); (c) the major components of EOs in the leaves were trans-caryophyllene (8.7%), germacrene-D (10.2%) and caryophyllene oxide (4.5%).
Bixaceae family
Bixa orellana: B. orellana, also known as the ‘anatto’ or ‘achiote’ plant, is a small perennial tree, native of South and Central American forests (Lopes et al., 2012). Monzote et al. (2014a) analysed the EO of the seed of B. orellana by gas chromatography-mass spectrometry analysis and reported ishwarane (18.6%), geranylgeraniol (9.1%), and bicyclogermacrene (8.4%) as the major components. This EO was active against L. amazonensis and controlled the progression of cutaneous disease in BALB/c mice; at the same time, the CC50 was 7-fold higher for host cells when compared with that for the parasites (Monzote et al., 2014a). The EO of B. orellana seeds has a complex chemical composition, as its activity could be attributed to geranylgeraniol, which induced mitochondrial alteration, abnormal chromatin condensation in the nucleus, and an increase in superoxide anion production ultimately leading to apoptosis-like cell death (Lopes et al., 2012).
Burseraceae family
Protium heptaphyllum: P. heptaphyllum, commonly called ‘almecegueira’, is known to produce an amorphous resin which is obtained from the stem; its constituents are compounds such as α- and β-amyrin, taraxastan-3-oxo-20-ol, and sitostenonein (Nogueira et al., 2019). Houel et al. (2015) examined whether the antidermatophytic activity of the EO may be an indicator for the discovery of active natural products against L. amazonensis. Since P. heptaphyllum exhibited a good anti-dermatophytic activity against filamentous fungi and axenic amastigotes (IC50 3.7 μg mL−1), it indicates a correspondence between both the activities. P. heptaphyllum is rich in the aromatic monocyclic monoterpene, p-cymene [1-methyl-4-(1-methyl ethyl) benzene], that is the biological precursor of carvacrol (de Cassia da Silveira et al., 2017). The p-cymene was also shown to diminish NO production in murine macrophages incubated with lipopolysaccharide (de Santana et al., 2015).
Chenopodiaceae family
Chenopodium ambrosioides: C. ambrosioides is an aromatic and medicinal plant found in the tropics and several regions of America and Africa (Cruz et al., 2007). Monzote et al. (2006) observed an intense inhibitory action of the EO against promastigote and amastigote forms of L. amazonensis, with IC50 values of 3.7 and 4.6 μg mL−1, respectively. Additionally, they noted that BALB/c mice infected and treated with the EO (30 mg kg−1) for 15 days by intraperitoneal route, presented with a reduction in the size of the lesions and suppression of the number of parasites in the infected footpads when compared to the control animals. Monzote et al. (2007) confirmed these results; intraperitoneal treatment reduced parasite burden, oral administration delayed infection, and the intralesional route was not valid. Monzote et al. (2009) compared the antileishmanial effect of C. ambrosioides EO in different doses administered by oral route in BALB/c mice and the conventional drugs, glucantime, amphotericin B and pentamidine, all administered for 15 days. The EO in a 150 mg kg−1 dose exhibited better antileishmanial activity and no macroscopic toxic effects. Interestingly, a study demonstrated synergism between C. ambrosioides EO with pentamidine against L. amazonensis promastigotes (Monzote et al., 2007). The high-resolution gas chromatography-mass spectrometry (HRGC-MS) analysis of this EO showed that its main components were carvacrol (62.36%) and ascaridole (22.54%). Chenopodium ambrosioides EO, combined with ascaridole compound, exhibited potent antileishmanial activity against promastigote and amastigote forms. Exploration of its mechanism of action suggests a breakdown of mitochondrial membrane potential and a modification of redox indices (Monzote et al., 2014b).
Fabaceae family
Copaifera martii: Copaifera are trees found in Latin America and West Africa; they live for about 400 years, reaching between 25 and 40 m in height. The origin of the name seems to have come from the native language of the Tupi Indians ‘cupa-yba’ which means ‘depot tree’. Santos et al. (2008) studied eight different kinds of Brazilian Copaifera oils for antileishmanial activity and observed activity against promastigote forms of L. amazonensis (IC50 5–22 μg mL−1). Oral administration with C. martii oil caused a significant reduction in average lesion size in L. amazonensis infection (dos Santos et al., 2011). Morphological and ultrastructural analyses demonstrated notable changes in parasite cells treated with this oil. The main ultrastructural effect was mitochondrial swelling. Copaifera martii EO led to an increase in plasma membrane permeability and depolarization in the mitochondrial membrane potential in parasite cells. Development of C. martii oil formulation as a nanoemulsion in a delivery system led to a reduction in L. amazonensis infection levels in macrophage cultures and ultrastructural analyses by SEM revealed that exposure to nanoemulsions induced change in parasite cell shape to oval and retracted flagellae. The treatment of L. amazonensis-infected BALB/c mice with nanoemulsions showed significant beneficial effects on lesion size, parasite burden and lesional histopathology (Dhorm Pimentel de Moraes et al., 2018).
Vouacapoua americana: V. americana exhibits slow growth and has potential economic value, occurring in small subpopulations in the French Guiana, Guyana, Peru, Suriname and the Brazilian states of Amapá, Pará, Amazonas and Maranhão. Its wood is widely used in construction and shipbuilding. Moreover, the species grows in areas that undergo strong anthropization and the decline of habitat quality is constant (Brasil, Ministério da Saúde, 2017). Vouacapoua americana EO shows activity against Microsporum gypseum, M. canis and Trichophyton mentagrophytes, important dermatophytic fungi. On axenic amastigote forms of L. amazonensis, the IC50 of the EO was 7.2, with no cytotoxicity in BALB/c mice peritoneal macrophages and VERO cells (Houel et al., 2015).
Pterodon pubescens: The components extraction from P. pubescens by ‘Supercritical CO2 extraction’ (scCO2), which does not use organic solvents and is environmentally sustainable, detected high geranylgeraniol and 14,15-epoxy-geranylgeraniol content (da Silva Santos et al., 2016). This extract had high inhibitory activity against intracellular amastigotes of L. amazonensis (IC50 1.9 μg mL−1), and the effect was likely due to the high geranylgeraniol derivative content of the fluid, which resulted in superoxide anion production, leading to parasite death (Lopes et al., 2012; da Silva Santos et al., 2016).
Lamiaceae family
Tetradenia riparia: T. riparia is native to South Africa, where it is one of the most aromatic and popular medicinal plants (Gazim et al., 2014). The EO from T. riparia is a complex mixture of terpenoids, including monoterpenes, sesquiterpenes and diterpenes, the most representative class (Gazim et al., 2010, 2014). The most considerable amount of EO in T. riparia was found during winter, and the oil content decreased significantly in spring (Gazim et al., 2010). In this context, the EOs of T. riparia obtained in spring, summer, autumn and winter showed similar activity against L. amazonensis. However, BALB/c mice infected with L. amazonensis and treated topically did not present with a reduction in lesion size, but parasite load in the spleen decreased significantly (Cardoso et al., 2015). Demarchi et al. (2015) observed that the EO of T. riparia is more effective in promoting the death of promastigote and amastigote forms of L. amazonensis than an isolated diterpene EO, 6,7-dehydroroyleanone. Additionally, increased inducible nitric oxide synthase (iNOS) mRNA expression or nitrite production by macrophages infected with L. amazonensis did not occur after 24 h, suggesting that this EO does not act on parasites through this important elimination pathway. The EO and diterpene derived from T. riparia probably promoted the death of Leishmania parasites through mitochondrial metabolism pathways, a mechanism of cell death that resembles apoptosis. TEM showed that the EO was able to modify the promastigote ultrastructures, suggesting autophagy demonstrated as chromatin condensation, blebbing, membranous profiles and nuclear fragmentation (Demarchi et al., 2015). However, T. riparia EO can modulate an immune response. Demarchi et al. (2016) reported that IFN-γ production was inhibited in infected macrophages, and the EO blocked this inhibition. Besides, the EO inhibited some of the most critical cytokines necessary for the establishment of infection, including granulocyte-macrophage colony-stimulating factor, IL-4, IL-10 and TNF-α. The diterpene 6,7-dehydroroyleanone induced a decrease in IL-4 levels and an increase in IL-12 (Terron-Monich et al., 2019). Thus, the EO of T. riparia is an agent that can stimulate a protective immune response against intracellular pathogens and inhibit or suppress pathological immune reactions.
Ocimum canum: Ocimum species comprise medicinal plants used in traditional medicine for the treatment of microbial diseases, helminthic diseases, inflammation, cardiac diseases, hepatic diseases and metabolic diseases, among many others. Uritu et al. (2018) and da Silva et al. (2018) identified the chemical constituents of O. canum, assessed by gas chromatography-mass spectrometry analyses: thymol (42.15%), p-cymene (21.17%) and γ-terpinene (19.81%) were the major compounds. Antiprotozoal activity of the EO against L. amazonensis promastigotes and intracellular amastigotes were high (IC50 17.4 and 13.1 μg mL−1, respectively), with low cytotoxicity against BALB/c peritoneal macrophages (CC50 315.3 μg mL−1). The EO of O. canum induced ultrastructural alterations promastigotes of L. amazonensis, such as the appearance of autophagosome-like structures, discontinuity of nuclear membrane and exocytic activity by the flagellar pocket, and this exacerbated autophagic response can result in parasite cell death (da Silva et al., 2018).
Ocimum gratissimum: O. gratissimum is widely found in several geographical regions in South America and Africa and is used as a medicinal plant with analgesic activity. It contains several proanthocyanidins, which have shown significant antioxidant activity, and tannins, saponins, steroids, alkaloids, terpenoids, flavonoids, phenols and cardiac glycosides (Igbinosa et al., 2013). The eugenol-rich EO of O. gratissimum inhibited L. amazonensis growth, in promastigote and amastigote forms, with an IC50 of 135 and 100 μg mL−1, respectively. IC50 of eugenol was 80 μg mL−1 (Ueda-Nakamura et al., 2006). According to these authors, the EO showed no cytotoxic effects against mammalian cells. Leishmania amazonensis exposed to the EO underwent considerable ultrastructural alterations, as observed by TEM: the appearance of two or more nuclei or flagella suggesting interference in cell division, internal mitochondrial membrane considerably altered with an increased number of crystals, and the mitochondrial matrix appearing less electron-dense in some amastigotes. NO production by the infected macrophages was increased.
Origanum vulgare: Origanum is a genus of herbaceous perennials and shrubs native to Europe, North Africa and much of temperate Asia. The plants have strong aromatic leaves and abundant tubular flowers with long-lasting coloured bracts (Uritu et al., 2018). The people from old Egypt used Origanum to disinfect and preserve food (Prerna and Vasudeva, 2015). Teles et al. (2019) identified 20 compounds in O. vulgare EO and the main compounds were cis-p-menth-2-en-1-ol (33.8%) and linalyl acetate (13.9%). However, antiprotozoal activity on promastigotes was low (IC50 405.5 μg mL−1). According to Sanchez-Suarez et al. (2013) O. vulgare EO of the Colombian species exhibited activity against promastigotes of L. panamensis.
Lauraceae family
Nectandra amazonum, N. gardneri, N. hihua and N. megapotamica: EOs extracted by hydrodistillation from stem bark/leaves of N. amazonum, N. gardneri and N. hihua presented sesquiterpene compounds as the major constituents, while phenylpropanoids were predominant in the EO extracted from N. megapotamica (Bosquiroli et al., 2017). EOs of N. gardneri and N. megapotamica induced a significant increase in NO production by infected macrophages, which may mediate the intracellular death of L. amazonensis (Bosquiroli et al., 2017).
Cinnamomum zeylanicum: C. zeylanicum, commonly called cinnamon, is used as a seasoning in cooking and is cultivated mainly in countries like India, Sri Lanka and China. Extracts, EOs and cinnamon isolates have applications in food, cosmetics and pesticides due to antimicrobial, antioxidant and antifungal properties. Teles et al. (2019) identified 15 compounds in C. zeylanicum EO, and the major one was cinnamic aldehyde (46.3%). Its anti-promastigote activity was not considered significant (IC50 >500 μg mL−1).
Cryptocarya aschersoniana: The EO of C. aschersoniana obtained by hydrodistillation presented monoterpene hydrocarbons (48.8%), limonene (42.3%), linalool (9.7%) and nerolidol (8.6%) as predominant constituents. Its EO had high activity against L. amazonensis promastigote forms (IC50 4.46 μg mL−1); however, it also demonstrated relatively high cytotoxicity against mouse peritoneal macrophages (CC50 7.71 μg mL−1) (Andrade et al., 2018).
Meliaceae family
Carapa guianensis: C. guianensis is the Amazon's traditional phytotherapeutic, and its EO is used for its anti-microbial and anti-inflammatory properties for skin diseases. The C. guianensis seed EO did not exhibit antileishmanial activity; however, three limonoid-rich EO fractions demonstrated activity against intracellular amastigotes of L. amazonensis, which was attributed to the compounds 11β-hydroxygedunin and 6α and 11β-diacetoxygedunin (Oliveira et al., 2018). Dhorm Pimentel de Moraes et al. (2018) reported the development of nanoemulsions as a delivery system for C. guianensis EO (nanoandi), with toxic activity against promastigotes of Leishmania species. Ultrastructural analyses by SEM revealed that exposure to nanoemulsion induced changes in the oval cell shape and flagellae retraction. Interestingly, the C. guianensis EO nanoemulsions were effective orally in BALB/c mice infected with L. amazonensis (Dhorm Pimentel de Moraes et al., 2018).
Myrtaceae family
Syzygium cumini: S. cumini is a large tree, popularly known as ‘black plum’, ‘jambolan’, ‘jamum’ or ‘java plum’. The chemical composition and biological potential of the EO extracted from S. cumini leaves revealed a high abundance of monoterpenes (87.12%), and the major components were α-pinene (31.85%), (Z)-β-ocimene (28.98%) and (E)-β-ocimene (11.7%) (Dias et al., 2013). This EO showed significant activity against promastigote forms of L. amazonensis (IC50 60 μg mL−1). The α-pinene has activity against L. amazonensis promastigote forms (IC50 19.7 μg mL−1), axenic forms (IC50 15.6 μg mL−1) and intracellular amastigotes (IC50 16.1 μg mL−1) and was more effective than the EO from S. cumini against axenic and intracellular amastigotes (Rodrigues et al., 2015). The EO and α-pinene antileishmanial effects were mediated by immunomodulatory activity, as evidenced by an increase in both phagocytic and lysosomal activities and elevated NO levels induced by these components of S. cumini (Rodrigues et al., 2013).
Piperaceae family
Piper rivinoides, P. mosenii , P. cernuum, P. arboretum, P. aduncum, P. gaudichaudianum, P. xylosteoides, P. mikanianum and P. diospyrifolium: Bernuci et al. (2016) evaluated the activity of EOs of nine Piper species on L. amazonensis promastigotes and found IC50 <30 μg mL−1 for EOs of P. rivinoides, P. mosenii , P. cernuum, P. arboretum and P. aduncum. However, the EOs of P. gaudichaudianum, P. xylosteoides and P. mikanianum showed IC50 >100 μg mL−1. Piper diospyrifolium and P. aduncum alone showed activity against intracellular amastigotes. Analyses of EOs obtained from inflorescences and leaves fresh or dried of P. claussenianum identified sesquiterpenes as the main constituents in the leaves, with a predominance of (E)-nerolidol (up to 83%), while in the EO of inflorescences, monoterpenes were the majority, with predominantly linalool (>50%) (Marques et al., 2010). Interestingly, only the EO from fresh leaves of P. claussenianum, which was rich in (E)-nerolidol, inhibited the L. amazonensis growth.
Piper demeraranum, P. duckei and P. hispidum: P. demeraranum and P. duckei EOs exhibited biological activity against promastigote and amastigote forms of L. amazonensis, P. duckei EO being the most active (Moura do Carmo et al., 2012). The main constituents found in P. demeraranum EO were limonene (19.3%) and β-elemene (33.1%), and in P. duckei EO, the major components were germacrene D (14.7%) and trans-caryophyllene (27.1%) (Moura do Carmo et al., 2012). The species P. hispidum was identified by Houel et al. (2015) as the most promising Piper EO (IC50 4.7 μg mL−1), and the most abundant compounds found in this EO were sesquiterpenes, notably curzerene and furanodiene.
The EOs are complex mixtures of compounds, which might possess several activities, that act on multiple targets, and cause death of the parasite by various mechanisms (Salehi et al., 2019). The ultrastructural alterations induced by EOs of Piper species on L. amazonensis were mitochondrial swelling, intense exocytic activity in the flagellar pocket, and vacuoles in the cytoplasm, suggesting the depletion of ergosterol and alteration of the physical properties of the membranes of the parasites (Vendrametto et al., 2010). Additionally, nerolidol from P. Aduncum may lead to parasite death by reduction in cell size, loss of mitochondrial membrane potential, phosphatidylserine exposure and DNA degradation (Ceole et al., 2017).
Plantaginaceae family
Otacanthus azureus: Houel et al. (2015) described a high in vitro activity (IC50 0.7 μg mL−1) for O. Azureus EO, and the value was similar to that of the reference compound, amphotericin B (0.3 μg mL−1). This EO was composed of sesquiterpenes, with the main component being β-copaen-4-α-ol (23%), alongside α-humulene (10.6%), α-copaene (8.8%), myrtenal (5.6%), viridiflorol (5.1%) and trans-pinocarveol (4.3%). Interestingly, none of the main components of O. azureus EO have been identified as antileishmanial agents.
Poaceae family
Cymbopogon citratus: C. citratus is originally from India, possesses aromatic leaves, and is known as a source of ethnomedicines (Puatanachokchai et al., 2002). Santin et al. (2009), through the analysis of a chromatogram obtained by gas chromatography coupled with mass spectrometry, identified the monoterpene, citral, a mixture of the stereoisomers, geranial (42.2%), neral (36.3%) and β-myrcene (13.2%) as the main compound in C. citratus EO. In the same study, these authors suggested the activity of the EO on promastigotes, axenic amastigote and intracellular amastigote forms of L. amazonensis may be explained by the synergistic effects of various compounds of the EO. The promastigote forms of L. amazonensis treated with C. citratus EO presented notable morphological and ultrastructural alterations by light microscopy, SEM and TEM (Santin et al., 2009). Houel et al. (2015) identified C. citratus EO as a potent antidermatophytic agent, and with high antileishmanial activity. However, its EO was considered quite toxic and therefore, is not useful as a medicine.
Rubiaceae family
Mitracarpus frigidus: Chemical analysis of the M. frigidus EO obtained by hydrodistillation of the aerial parts of the plant resulted in the identification of 12 known compounds, linalool (29.29%) and eugenol acetate (15.85%) were the major constituents, followed by 5-hydroxy-isobornyl isobutyrate (8.41%), 5-methyl-1-undecene (7.69%) and methyl salicylate (6.55%) (Fabri et al., 2012). This EO exhibited a potent antifungal effect against Cryptoccocus neoformans, Candida albicans and an expressive activity against L. amazonensis promastigote forms. The antioxidant activity of the EO investigated through 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging was significant (IC50 of 38 μg mL−1) (Fabri et al., 2012).
Scrophulariaceae family
Achetaria guianensis: Houel et al. (2015) examined whether the antidermatophytic activity of EOs can be used as an indicator for the discovery of active natural products against L. amazonensis. They examined seven EOs that exhibited potential antimicrobial obtained from fragrant plant species from French Guiana. Achetaria guianensis (EO of leaves and stems) was one of the species studied, but the EO did not show activity against dermatophytic filamentous fungi. The activity of the EO on axenic amastigote forms of L. amazonensis was high (6.3 μg mL−1), and cytotoxicity expressed as median toxic dose (TD50) on BALB/c mice peritoneal macrophages and VERO cells were 32.5 and 30.7 μg mL−1, respectively (Houel et al., 2015).
Verbenaceae family
Lippia sidoides: L. sidoides, popularly known as ‘alecrim pimento’, is grown mainly in Northeast Brazil. de Medeiros et al. (2011) analysed the chemical composition of L. sidoides EO through GC/MS and found the oxygenated monoterpene thymol to be its principal constituent (78.4%). Monoterpene hydrocarbons such as ρ-cymene (6.3%) and other compounds were detected in smaller amounts. Study of the EO from L. sidoides and its major compound thymol on L. amazonensis showed significant activity against promastigote forms. However, thymol showed toxicity against peritoneal macrophages and low selectivity against the promastigotes, when compared with the crude EO. However, no cytotoxic effect was observed in macrophages treated with the crude EO. Incubation of L. amazonensis-infected macrophages with L. sidoides EO showed a remarkable reduction in amastigote survival within the macrophages. Significant morphological alterations, such as the accumulation of large lipid droplets in the cytoplasm, disrupted membrane and wrinkled cells, were usually seen in parasites treated with the EO (de Medeiros et al., 2011).
Aloysia gratissima: A. gratissima is known as ‘Brazil lavender’ and is commonly used in Brazilian folk medicine for the treatment of digestive and respiratory diseases. The only study on A. gratissima identified in this review was conducted by Garcia et al. (2018). These authors detected in A. gratissima the monoterpene, 1,8-cineole (17.6%) and the sesquiterpene alcohol, guaiol (10.5%), along with other guaiol isomers (azulene-types structures, 7.3%), hydrocarbons of the germacrene-type sesquiterpenes (>17%), trans-caryophyllene and its oxide (7%) in the EO of leaves. The results showed that the EO killed promastigotes and intracellular amastigotes of L. amazonensis at an IC50 of 25 and 0.16 μg mL−1, respectively. The EO of A. gratissima was safe for macrophages with up to 100 μg mL−1, as evaluated by dehydrogenase activity, membrane integrity and phagocytic capacity of macrophages, and did not induce NO in resting macrophages and inhibited the production of NO in lipopolysaccharide-stimulated macrophages. Ultrastructural analysis suggested that the EO and guaiol act directly on parasites, affecting the kinetoplast, mitochondrial matrix and plasma membrane of promastigotes (Garcia et al., 2018).
Zingiberaceae family
Curcuma longa: Study on C. longa EO revealed 17 compounds, turmerone (55.43%), β-turmerone (12.02%) and γ-curcumene (6.96%) being the major compounds. Curcuma longa EO has potent antipromastigote activity, which showed a 4.87-fold lower IC50 value for intracellular amastigotes, when compared to the IC50 for promastigotes (Teles et al., 2019). According to these authors, there was a reduction of infection in BALB/c mice. Curcuma longa EO inhibited NO production in peritoneal macrophages, suggesting that there may be other possible mechanisms involved in the intracellular anti-amastigote activity of C. longa EO (Teles et al., 2019).
Strengths and limitations of the study
We conducted an extensive search in two databases, which was checked and validated by the researchers in this study, to ensure greater accuracy of the findings. The MeSH terms have been tirelessly revised to provide higher sensitivity to research. Our limitations were the search in only two databases, the setting of English, Portuguese, Spanish and French and a limited search period. Despite the vast heterogeneity of the substances identified in the selected studies, we detected a limited number of publications for each EO, which made it difficult to expand the discussion of the findings. It is important to comment that many of the compounds identified in this review study are potential therapeutic targets for the treatment of L. amazonensis infection, as they have low toxicity against host cells and potent antileishmanial action. The risk of bias in the selected in vivo and in vitro studies was moderate. It is essential to highlight that no study reported allocation sequence generation, allocation concealment mechanism, implementation, outcomes and estimation. Another critical point was that only one study applied the domain blinding of personnel and participants, and the random outcome assessment was performed. These flaws tend to increase the risk of bias in publications of an experimental nature.
Conclusion and future directions
The systematic review of 33 experimental assays showed that EOs and other investigated products possess significantly variable antileishmanial activity against promastigote and amastigote forms of L. amazonensis. The in vivo studies identified that the route of administration of the EO was important, and the intraperitoneal route is more effective. The discovery of new leishmanicidal bioactive agents is eminent in the face of increased incidence of leishmaniasis and current treatment difficulties. Plants and their secondary compounds, such as EOs, are a promising source for the discovery of new active agents and formulation of prototypes for potential drugs, as revealed by the selected 23 primary studies, which also reported the accurate safety margins of the bioactive agents. Many researchers have been investigating and tracking bioactive agents of new plants, being of paramount relevance for the advancement of these studies to the phase of controlled clinical assays.
Acknowledgements
We thank Editage for the quality and clarity in the English language review.
Financial support
This study was supported by the CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brasil), and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brasil).
Conflict interest
The authors declare that there is no conflict of interest.
Ethical standards
Not applicable.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182020001304.
click here to view supplementary material
References
- Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, Cano J, Jannin J and den Boer M (2012) Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 7, e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade MA, Azevedo CD, Motta FN, Dos Santos ML, Silva CL, de Santana JM and Bastos IMD (2016) Essential oils: in vitro activity against Leishmania amazonensis, cytotoxicity and chemical composition. BMC Complementary and Alternative Medicine 16, 444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade PM, Melo DC, Alcoba AET, Ferreira Junior WG, Pagotti MC, Magalhaes LG, Santos T, Crotti AEM, Alves CCF and Miranda MLD (2018) Chemical composition and evaluation of antileishmanial and cytotoxic activities of the essential oil from leaves of Cryptocarya aschersoniana Mez. (Lauraceae Juss.). Anais da Academia Brasileira de Ciencias 90, 2671–2678. [DOI] [PubMed] [Google Scholar]
- Bernuci KZ, Iwanaga CC, Fernadez-Andrade CM, Lorenzetti FB, Torres-Santos EC, Faioes VD, Goncalves JE, do Amaral W, Deschamps C, Scodro RB, Cardoso RF, Baldin VP and Cortez DA (2016) Evaluation of chemical composition and antileishmanial and antituberculosis activities of essential oils of Piper species. Molecules 21, 1698. doi: 10.3390/molecules21121698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosquiroli LSS, Dos Santos Ferreira AC, Farias KS, da Costa EC, Matos MFC, Kadri MCT, Rizk YS, Alves FM, Perdomo RT, Carollo CA and Pinto de Arruda CC (2017) In vitro Antileishmania activity of sesquiterpene-rich essential oils from Nectandra species. Pharmaceutical Biology 55, 2285–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil, Ministério da Saúde e Anvisa (2015) Monografia da espécie Matricaria chamomilla L. (= Chamomilla recutita (L.) Rauschert, camomila). https://portalarquivos2.saude.gov.br/images/pdf/2017/setembro/11/Monografia-Camomila.pdf (Accessed 08 June 2020).
- Brasil, Ministério da Saúde (2017). Manual de Vigilância da Leishmaniose Tegumentar Americana. Secretaria de Vigilância em Saúde. 2. Ed. http://bvsms.saude.gov.br/bvs/publicacoes/manual_vigilancia_leishmaniose_tegumentar.pdf (Accessed 23 November 2019).
- Burza S, Croft SL and Boelaert M (2018) Leishmaniasis. Lancet (London, England) 392, 951–970. [DOI] [PubMed] [Google Scholar]
- Cardoso BM, de Mello TF, Lopes SN, Demarchi IG, Lera DS, Pedroso RB, Cortez DA, Gazim ZC, Aristides SM, Silveira TG and Lonardoni MV (2015) Antileishmanial activity of the essential oil from Tetradenia riparia obtained in different seasons. Memorias do Instituto Oswaldo Cruz 110, 1024–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho CE, Sobrinho-Junior EP, Brito LM, Nicolau LA, Carvalho TP, Moura AK, Rodrigues KA, Carneiro SM, Arcanjo DD, Cito AM and Carvalho FA (2017) Anti-leishmania activity of essential oil of Myracrodruon urundeuva (Engl.) Fr. All.: composition, cytotoxity and possible mechanisms of action. Experimental Parasitology 175, 59–67. [DOI] [PubMed] [Google Scholar]
- Ceole LF, Cardoso MDG and Soares MJ (2017) Nerolidol, the main constituent of Piper aduncum essential oil, has anti-Leishmania braziliensis activity. Parasitology 144, 1179–1190. [DOI] [PubMed] [Google Scholar]
- Clardy J and Walsh C (2004) Lessons from natural molecules. Nature 432, 829–837. [DOI] [PubMed] [Google Scholar]
- Cos P, Vlietinck AJ, Berghe DV and Maes L (2006) Anti-infective potential of natural products: how to develop a stronger in Vitro ‘proof-of-concept’. Journal of Ethnopharmacology 106, 290–302. [DOI] [PubMed] [Google Scholar]
- Costa JML, Uthant AA, CostaMl EAN, Bezerril AC and Barral A (2009) Diffuse cutaneous leishmaniasis (DCL) in Brazil after 60 years of your first description. Gazeta Médica da Bahia 79, 19–24. [Google Scholar]
- Cruz GV, Pereira PV, Patricio FJ, Costa GC, Sousa SM, Frazao JB, Aragao-Filho WC, Maciel MC, Silva LA, Amaral FM, Barroqueiro ES, Guerra RN and Nascimento FR (2007) Increase of cellular recruitment, phagocytosis ability and nitric oxide production induced by hydroalcoholic extract from Chenopodium ambrosioides leaves. Journal of Ethnopharmacology 111, 148–154. [DOI] [PubMed] [Google Scholar]
- da Silva VD, Almeida-Souza F, Teles AM, Neto PA, Mondego-Oliveira R, Mendes Filho NE, Taniwaki NN, Abreu-Silva AL, da Silva Calabrese K and Mouchrek Filho VE (2018) Chemical composition of Ocimum canum Sims. Essential oil and the antimicrobial, antiprotozoal and ultrastructural alterations it induces in Leishmania amazonensis promastigotes. Industrial Crops and Products 119, 201–208. [Google Scholar]
- da Silva Santos E, Garcia FP, Outuki PM, Hoscheid J, Nunes de Goes PR, Cardozo-Filho L, Nakamura CV and Carvalho Cardoso ML (2016) Optimization of extraction method and evaluation of antileishmanial activity of oil and nanoemulsions of Pterodon pubescens benth. fruit extracts. Experimental Parasitology 170, 252–260. [DOI] [PubMed] [Google Scholar]
- de Cassia da Silveira ESR, Lima TC, da Nobrega FR, de Brito AEM and de Sousa DP (2017) Analgesic-like activity of essential oil constituents: an update. International Journal of Molecular Sciences 18, 2352. doi: 10.3390/ijms18122392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima SC, Teixeira MJ, Lopes JE Jr, de Morais SM, Torres AF, Braga MA, Rodrigues RO, Santiago GM, Martins AC and Nagao-Dias AT (2014) In vitro and in vivo leishmanicidal activity of Astronium fraxinifolium (Schott) and Plectranthus amboinicus (Lour.) Spreng against Leishmania (Viannia) braziliensis. Biomed Research International 2014, 848293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarchi IG, Thomazella MV, de Souza Terron M, Lopes L, Gazim ZC, Cortez DA, Donatti L, Aristides SM, Silveira TG and Lonardoni MV (2015) Antileishmanial activity of essential oil and 6,7-dehydroroyleanone isolated from Tetradenia riparia. Experimental Parasitology 157, 128–137. [DOI] [PubMed] [Google Scholar]
- Demarchi IG, Terron Mde S, Thomazella MV, Mota CA, Gazim ZC, Cortez DA, Aristides SM, Silveira TG and Lonardoni MV (2016) Antileishmanial and immunomodulatory effects of the essential oil from Tetradenia riparia (Hochstetter) Codd. Parasite Immunology 38, 64–77. [DOI] [PubMed] [Google Scholar]
- de Medeiros M, da Silva AC, Cito AM, Borges AR, de Lima SG, Lopes JA and Figueiredo RC (2011) In vitro antileishmanial activity and cytotoxicity of essential oil from Lippia sidoides Cham. Parasitology International 60, 237–241. [DOI] [PubMed] [Google Scholar]
- de Santana MF, Guimarães AG, Chaves DO, Silva JC, Bonjardim LR, de Lucca Júnior W, Ferro JN, Barreto Ede O, dos Santos FE, Soares MB, Villarreal CF, Quintans Jde S and Quintans-Júnior LJ (2015) The anti-hyperalgesic and anti-inflammatory profiles of p-cymene: evidence for the involvement of opioid system and cytokines. Pharmaceutical Biology 53, 1583–1590. [DOI] [PubMed] [Google Scholar]
- Dhorm Pimentel de Moraes AR, Tavares GD, Soares Rocha FJ, de Paula E and Giorgio S (2018) Effects of nanoemulsions prepared with essential oils of copaiba- and andiroba against Leishmania Infantum and Leishmania amazonensis infections. Experimental Parasitology 187, 12–21. [DOI] [PubMed] [Google Scholar]
- Dias CN, Rodrigues KA, Carvalho FA, Carneiro SM, Maia JG, Andrade EH and Moraes DF (2013) Molluscicidal and leishmanicidal activity of the leaf essential oil of Syzygium cumini (L.) SKEELS from Brazil. Chemistry and Biodiversity 10, 1133–1141. [DOI] [PubMed] [Google Scholar]
- dos Santos AO, Costa MA, Ueda-Nakamura T, Dias-Filho BP, da Veiga-Junior VF, de Souza Lima MM and Nakamura CV (2011) Leishmania amazonensis: effects of oral treatment with copaiba oil in mice. Experimental Parasitology 129, 145–151. [DOI] [PubMed] [Google Scholar]
- Esperandim VR, Silva Ferreira D, Sousa Rezende KC, Magalhães LG, Souza JM, Pauletti PM, Januário AH, Laurentz RS, Bastos JK, Símaro GV, Cunha WR and Andrade e Silva ML (2013) In vitro antiparasitic activity and chemical composition of the essential oil obtained from the fruits of Piper cubeba. Planta Medica 79, 1653–1655. [DOI] [PubMed] [Google Scholar]
- Espinosa OA, Serrano MG, Camargo EP, Teixeira MMG and Shaw JJ (2018) An appraisal of the taxonomy and nomenclature of trypanosomatids presently classified as Leishmania and Endotrypanum. Parasitology 145, 430–442. [DOI] [PubMed] [Google Scholar]
- Fabri RL, Coimbra ES, Almeida AC, Siqueira EP, Alves TM, Zani CL and Scio E (2012) Essential oil of Mitracarpus frigidus as a potent source of bioactive compounds. Anais da Academia Brasileira de Ciencias 84, 1073–1080. [DOI] [PubMed] [Google Scholar]
- Faggion CM Jr (2012). Guidelines for reporting pre-clinical in vitro studies on dental materials. The Journal of Evidence-based Dental Practice 12, 182–189. [DOI] [PubMed] [Google Scholar]
- Ganesan A (2008) The impact of natural products upon modern drug discovery. Current Opinion in Chemical Biology 12, 306–317. [DOI] [PubMed] [Google Scholar]
- Garcia M, Scull R, Satyal P, Setzer WN and Monzote L (2017) Chemical characterization, antileishmanial activity, and cytotoxicity effects of the essential oil from leaves of Pluchea carolinensis (Jacq.) G. Don. (Asteraceae). Phytotherapy Research 31, 1419–1426. [DOI] [PubMed] [Google Scholar]
- Garcia MCF, Soares DC, Santana RC, Saraiva EM, Siani AC, Ramos MFS, Danelli M, Souto-Padron TC and Pinto-da-Silva LH (2018) The in vitro antileishmanial activity of essential oil from Aloysia gratissima and guaiol, its major sesquiterpene against Leishmania amazonensis. Parasitology 145, 1219–1227. [DOI] [PubMed] [Google Scholar]
- Gazim ZC, Amorim AC, Hovell AM, Rezende CM, Nascimento IA, Ferreira GA and Cortez DA (2010) Seasonal variation, chemical composition, and analgesic and antimicrobial activities of the essential oil from leaves of Tetradenia riparia (Hochst.) Codd in southern Brazil. Molecules 15, 5509–5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazim ZC, Rodrigues F, Amorin AC, de Rezende CM, Sokovic M, Tesevic V, Vuckovic I, Krstic G, Cortez LE, Colauto NB, Linde GA and Cortez DA (2014) New natural diterpene-type abietane from Tetradenia riparia essential oil with cytotoxic and antioxidant activities. Molecules 19, 514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajaji S, Sifaoui I, López-Arencibia A, Reyes-Batlle M, Jiménez IA, Bazzocchi IL, Valladares B, Akkari H, Lorenzo-Morales J and Piñero JE (2018) Leishmanicidal activity of α-bisabolol from Tunisian chamomile essential oil. Parasitology Research 117, 2855–2867. [DOI] [PubMed] [Google Scholar]
- Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M and Langendam MW (2014) SYRCLE's risk of bias tool for animal studies. BMC Medical Research Methodology 14, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houel E, Gonzalez G, Bessiere JM, Odonne G, Eparvier V, Deharo E and Stien D (2015) Therapeutic switching: from antidermatophytic essential oils to new leishmanicidal products. Memorias do Instituto Oswaldo Cruz 110, 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igbinosa EO, Uzunuigbe EO, Igbinosa IH, Odjadjare EE, Igiehon NO and Emuedo OA (2013) In vitro assessment of antioxidant, phytochemical and nutritional properties of extracts from the leaves of Ocimum gratissimum (Linn). African Journal of Traditional, Complementary, and Alternative Medicines 10, 292–298. [PMC free article] [PubMed] [Google Scholar]
- Islamuddin M, Sahal D and Afrin F (2014) Apoptosis-like death in Leishmania donovani promastigotes induced by eugenol-rich oil of Syzygium aromaticum. Journal of Medical Microbiology 63, 74–85. [DOI] [PubMed] [Google Scholar]
- Jirku M, Yurchenko VY, Lukes J and Maslov DA (2012) New species of insect trypanosomatids from Costa Rica and the proposal for a new subfamily within the Trypanosomatidae. Journal of Eukaryotic Microbiology 59, 537–547. [DOI] [PubMed] [Google Scholar]
- Lam KS (2007) New aspects of natural products in drug discovery. Trends in Microbiology 15, 279–289. [DOI] [PubMed] [Google Scholar]
- Laurella LC, Cerny N, Bivona AE, Sanchez Alberti A, Giberti G, Malchiodi EL, Martino VS, Catalan CA, Alonso MR, Cazorla SI and Sulsen VP (2017) Assessment of sesquiterpene lactones isolated from Mikania plants species for their potential efficacy against Trypanosoma cruzi and Leishmania sp. PLoS Neglected Tropical Diseases 11, e0005929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes MV, Desoti VC, Caleare Ade O, Ueda-Nakamura T, Silva SO and Nakamura CV (2012) Mitochondria superoxide anion production contributes to geranylgeraniol-induced death in Leishmania amazonensis. Evidence-Based Complementary and Alternative Medicine: ECAM 2012, 298320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado M, Pires P, Dinis AM, Santos-Rosa M, Alves V, Salgueiro L, Cavaleiro C and Sousa MC (2012) Monoterpenic aldehydes as potential anti-leishmania agents: activity of Cymbopogon citratus and citral on L. infantum, L. tropica and L. major. Experimental Parasitology 130, 223–231. [DOI] [PubMed] [Google Scholar]
- Machado GU, Prates FV and Machado PRL (2019) Disseminated leishmaniasis: clinical, pathogenic, and therapeutic aspects. Anais Brasileiros De Dermatologia 94, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques AM, Barreto AL, Batista EM, Curvelo JA, Velozo LS, Moreira Dde L, Guimaraes EF, Soares RM and Kaplan MA (2010) Chemistry and biological activity of essential oils from Piper claussenianum (Piperaceae). Natural Product Communications 5, 1837–1840. [PubMed] [Google Scholar]
- Martins MM, de Aquino FJT, de Oliveira A, do Nascimento EA, Chang R, Borges MS, de Melo GB, da Silva CV, Machado FC and de Morais SAL (2015) Chemical composition, antimicrobial and antiprotozoal activity of essential oils from Vernonia brasiliana (Less) Druce (Asteraceae). Journal of Essential Oil Bearing Plants 18, 561–569. [Google Scholar]
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P and Stewart LA (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews 4, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzote L, Montalvo AM, Almanonni S, Scull R, Miranda M and Abreu J (2006) Activity of the essential oil from Chenopodium ambrosioides grown in Cuba against Leishmania amazonensis. Chemotherapy 52, 130–136. [DOI] [PubMed] [Google Scholar]
- Monzote L, Montalvo AM, Scull R, Miranda M and Abreu J (2007) Activity, toxicity and analysis of resistance of essential oil from Chenopodium ambrosioides after intraperitoneal, oral and intralesional administration in BALB/c mice infected with Leishmania amazonensis: a preliminary study. Biomedicine & Pharmacotherapy 61, 148–153. [DOI] [PubMed] [Google Scholar]
- Monzote L, Garcia M, Montalvo AM, Linares R and Scull R (2009) Effect of oral treatment with the essential oil from Chenopodium ambrosioides against cutaneous leishmaniasis in BALB/c mice, caused by Leishmania amazonensis. Forschende Komplementarmedizin (2006) 16, 334–338. [DOI] [PubMed] [Google Scholar]
- Monzote L, Garcia M, Scull R, Cuellar A and Setzer WN (2014a) Antileishmanial activity of the essential oil from Bixa orellana. Phytotherapy Research 28, 753–758. [DOI] [PubMed] [Google Scholar]
- Monzote L, Pastor J, Scull R and Gille L (2014b) Antileishmanial activity of essential oil from Chenopodium ambrosioides and its main components against experimental cutaneous leishmaniasis in BALB/c mice. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology 21, 1048–1052. [DOI] [PubMed] [Google Scholar]
- Moura do Carmo DF, Amaral AC, Machado GM, Leon LL and Silva JR (2012) Chemical and biological analyses of the essential oils and main constituents of Piper species. Molecules 17, 1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira AO, Oliveira YIS, Adjafre BL, de Moraes MEA and Aragao GF (2019) Pharmacological effects of the isomeric mixture of alpha and beta amyrin from Protium heptaphyllum: a literature review. Fundamental & Clinical Pharmacology 33, 4–12. [DOI] [PubMed] [Google Scholar]
- Oliveira I, Moragas Tellis CJ, Chagas M, Behrens MD, Calabrese KDS, Abreu-Silva AL and Almeida-Souza F (2018) Carapa guianensis Aublet (Andiroba) seed oil: chemical composition and antileishmanial activity of limonoid-rich fractions. Biomed Research International 2018, 5032816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelissari DM, Cechinel MP, de Sousa-Gomes ML and Ferreira de Lima Junior FE (2011) Treatment of visceral leishmaniasis and American cutaneous leishmaniasis in Brazil. Epidemiol Serv Saúde 20, 107–110. [Google Scholar]
- Pereira PS, Barros LM, Brito AM, Duarte AE and Maia AJ (2014) Uso da Myracroduon urundeuva Allemão (aroeira do sertão) pelos agricultores no tratamento de doenças. Revista Cubana de Plantas Medicinales 19, 51–60. [Google Scholar]
- Prerna P and Vasudeva N (2015) Origanum majorana L.–Phyto-pharmacological review. Indian Journal of Natural Products and Resources 6, 261–267. [Google Scholar]
- Puatanachokchai R, Kishida H, Denda A, Murata N, Konishi Y, Vinitketkumnuen U and Nakae D (2002) Inhibitory effects of lemon grass (Cymbopogon citratus, Stapf) extract on the early phase of hepatocarcinogenesis after initiation with diethylnitrosamine in male Fischer 344 rats. Cancer Letters 183, 9–15. [DOI] [PubMed] [Google Scholar]
- Rodrigues IA, Azevedo MM, Chaves FC, Bizzo HR, Corte-Real S, Alviano DS, Alviano CS, Rosa MS and Vermelho AB (2013) In vitro cytocidal effects of the essential oil from Croton cajucara (red sacaca) and its major constituent 7-hydroxycalamenene against Leishmania chagasi. BMC Complementary and Alternative Medicine 13, 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues KA, Amorim LV, Dias CN, Moraes DF, Carneiro SM and Carvalho FA (2015) Syzygium cumini (L.) Skeels essential oil and its major constituent alpha-pinene exhibit anti-Leishmania activity through immunomodulation in vitro. Journal of Ethnopharmacology 160, 32–40. [DOI] [PubMed] [Google Scholar]
- Salehi B, Zakaria ZA, Gyawali R, Ibrahim SA, Rajkovic J, Shinwari ZK, Khan T, Sharifi-Rad J, Ozleyen A, Turkdonmez E, Valussi M, Tumer TB, Monzote Fidalgo L, Martorell M and Setzer WN (2019) Piper species: a comprehensive review on their phytochemistry, biological activities and applications. Molecules 24, 1364. doi: 10.3390/molecules24071364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Suarez J, Riveros I and Delgado G (2013) Evaluation of the leishmanicidal and cytotoxic potential of essential oils derived from ten Colombian plants. Iranian Journal of Parasitology 8, 129–136. [PMC free article] [PubMed] [Google Scholar]
- Santin MR, dos Santos AO, Nakamura CV, Dias Filho BP, Ferreira IC and Ueda-Nakamura T (2009) In vitro activity of the essential oil of Cymbopogon citratus and its major component (citral) on Leishmania amazonensis. Parasitology Research 105, 1489–1496. [DOI] [PubMed] [Google Scholar]
- Santos AO, Ueda-Nakamura T, Dias Filho BP, Veiga Junior VF, Pinto AC and Nakamura CV (2008) Effect of Brazilian copaiba oils on Leishmania amazonensis. Journal of Ethnopharmacology 120, 204–208. [DOI] [PubMed] [Google Scholar]
- Santos AO, Santin AC, Yamaguchi MU, Cortez LE, Ueda-Nakamura T, Dias-Filho BP and Nakamura CV (2010) Antileishmanial activity of an essential oil from the leaves and flowers of Achillea millefolium. Annals of Tropical Medicine & Parasitology 104, 475–483. [DOI] [PubMed] [Google Scholar]
- Sen R and Chatterjee M (2011) Plant derived therapeutics for the treatment of Leishmaniasis. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology 18, 1056–1069. [DOI] [PubMed] [Google Scholar]
- Silveira FT (2019) What makes mucosal and anergic diffuse cutaneous leishmaniases so clinically and immunopathogically different? A review in Brazil. Transactions of the Royal Society of Tropical Medicine and Hygiene 113, 505–516. doi: 10.1093/trstmh/trz037 [DOI] [PubMed] [Google Scholar]
- Silveira FT, Lainson R, Shaw JJ, De Souza AA, Ishikawa EA and Braga RR (1991) Cutaneous leishmaniasis due to Leishmania (Leishmania) amazonensis in Amazonian Brazil, and the significance of a negative Montenegro skin-test in human infections. Transactions of the Royal Society of Tropical Medicine and Hygiene 85, 735–738. [DOI] [PubMed] [Google Scholar]
- Silveira FT, Blackwell JM, Ishikawa EA, Braga R, Shaw JJ, Quinnell RJ, Soong L, Kima P, McMahon-Pratt D, Black GF and Shaw MA (1998) T cell responses to crude and defined leishmanial antigens in patients from the lower Amazon region of Brazil infected with different species of Leishmania of the subgenera Leishmania and Viannia. Parasite Immunology 20, 19–26. [DOI] [PubMed] [Google Scholar]
- Tadic V, Arsic I, Zvezdanovic J, Zugic A, Cvetkovic D and Pavkov S (2017) The estimation of the traditionally used yarrow (Achillea millefolium L. Asteraceae) oil extracts with anti-inflammatory potential in topical application. Journal of Ethnopharmacology 199, 138–148. [DOI] [PubMed] [Google Scholar]
- Teles AM, Rosa T, Mouchrek AN, Abreu-Silva AL, Calabrese KDS and Almeida-Souza F (2019) Cinnamomum zeylanicum, Origanum vulgare, and Curcuma longa essential oils: chemical composition, antimicrobial and antileishmanial activity. Evidence-Based Complementary and Alternative Medicine: ECAM 2019, 2421695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terron-Monich MS, Demarchi IG, da Silva PRF, Ramos-Milare A, Gazim ZC, Silveira TGV and Lonardoni MVC (2019) 6,7-Dehydroroyleanone Diterpene derived from Tetradenia riparia essential oil modulates IL-4/IL-12 release by macrophages that are infected with Leishmania amazonensis. Parasitology Research 118, 369–376. [DOI] [PubMed] [Google Scholar]
- Ueda-Nakamura T, Mendonca-Filho RR, Morgado-Diaz JA, Korehisa Maza P, Prado Dias Filho B, Aparicio Garcia Cortez D, Alviano DS, Rosa Mdo S, Lopes AH, Alviano CS and Nakamura CV (2006) Antileishmanial activity of Eugenol-rich essential oil from Ocimum gratissimum. Parasitology International 55, 99–105. [DOI] [PubMed] [Google Scholar]
- Uritu CM, Mihai CT, Stanciu GD, Dodi G, Alexa-Stratulat T, Luca A, Leon-Constantin MM, Stefanescu R, Bild V, Melnic S and Tamba BI (2018) Medicinal plants of the family Lamiaceae in pain therapy: a review. Pain Research & Management 2018, 7801543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrametto MC, Santos AO, Nakamura CV, Dias Filho BP, Cortez DA and Ueda-Nakamura T (2010) Evaluation of antileishmanial activity of eupomatenoid-5, a compound isolated from leaves of Piper regnellii var. pallescens. Parasitology International 59, 154–158. [DOI] [PubMed] [Google Scholar]
- Villalva M, Jaime L, Villanueva-Bermejo D, Lara B, Fornari T, Reglero G and Santoyo S (2019) Supercritical anti-solvent fractionation for improving antioxidant and anti-inflammatory activities of an Achillea millefolium L. extract. Food Research International 115, 128–134. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2019) Leishmaniasis: Epidemiological Report of the Americas. http://iris.paho.org/xmlui/bitstream/handle/123456789/50505/Leishreport2019_eng.pdf?ua=1 (Accessed 10 December 2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182020001304.
click here to view supplementary material