Abstract
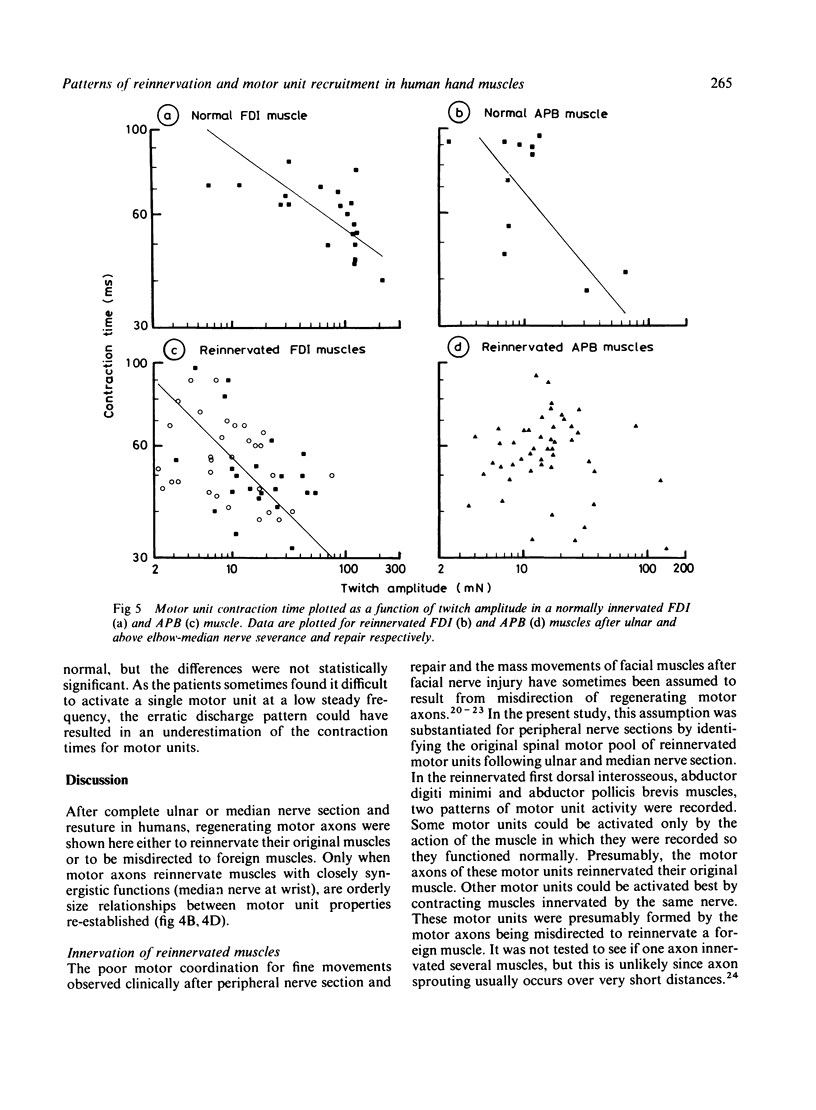
Following complete ulnar or above-elbow median nerve sections, there was no significant correlation between motor unit size (twitch amplitude) and recruitment threshold, as assessed by spike triggered averaging. This absence of orderly recruitment was attributed to misdirection of motor axons during regeneration. Following median nerve section at wrist level, where the reinnervated muscles have more synergistic actions, orderly recruitment by size appeared to be re-established. Thus, the size principle of motor unit recruitment can be re-established after nerve section in humans, if motor axons innervate their original muscles or ones with closely synergistic functions.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreassen S., Bar-On E. Estimation of motor unit twitches. IEEE Trans Biomed Eng. 1983 Nov;30(11):742–748. doi: 10.1109/tbme.1983.325189. [DOI] [PubMed] [Google Scholar]
- Ariano M. A., Armstrong R. B., Edgerton V. R. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973 Jan;21(1):51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Brinkman C., Porter R., Norman J. Plasticity of motor behavior in monkeys with crossed forelimb nerves. Science. 1983 Apr 22;220(4595):438–440. doi: 10.1126/science.6836289. [DOI] [PubMed] [Google Scholar]
- Brushart T. M., Mesulam M. M. Alteration in connections between muscle and anterior horn motoneurons after peripheral nerve repair. Science. 1980 May 9;208(4444):603–605. doi: 10.1126/science.7367884. [DOI] [PubMed] [Google Scholar]
- Calancie B., Bawa P. Limitations of the spike-triggered averaging technique. Muscle Nerve. 1986 Jan;9(1):78–83. doi: 10.1002/mus.880090113. [DOI] [PubMed] [Google Scholar]
- Clough J. F., Kernell D., Phillips C. G. The distribution of monosynaptic excitation from the pyramidal tract and from primary spindle afferents to motoneurones of the baboon's hand and forearm. J Physiol. 1968 Sep;198(1):145–166. doi: 10.1113/jphysiol.1968.sp008598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandall W. F., Goldberg S. J., Wilson J. S., McClung J. R. Muscle units divided among retractor bulbi muscle slips and between the lateral rectus and retractor bulbi muscles in cat. Exp Neurol. 1981 Feb;71(2):251–260. doi: 10.1016/0014-4886(81)90086-8. [DOI] [PubMed] [Google Scholar]
- Dubowitz V. Pathology of experimentally re-innervated skeletal muscle. J Neurol Neurosurg Psychiatry. 1967 Apr;30(2):99–110. doi: 10.1136/jnnp.30.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESSLEN E. Electromyographic findings on two types of misdirection of regenerating axons. Electroencephalogr Clin Neurophysiol. 1960 Aug;12:738–741. doi: 10.1016/0013-4694(60)90120-6. [DOI] [PubMed] [Google Scholar]
- Fraunfelder F. T., Meyer S. M. Ocular toxicity of antineoplastic agents. Ophthalmology. 1983 Jan;90(1):1–3. doi: 10.1016/s0161-6420(83)34600-5. [DOI] [PubMed] [Google Scholar]
- Gillespie M. J., Gordon T., Murphy P. R. Reinnervation of the lateral gastrocnemius and soleus muscles in the rat by their common nerve. J Physiol. 1986 Mar;372:485–500. doi: 10.1113/jphysiol.1986.sp016021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon T., Stein R. B. Reorganization of motor-unit properties in reinnervated muscles of the cat. J Neurophysiol. 1982 Nov;48(5):1175–1190. doi: 10.1152/jn.1982.48.5.1175. [DOI] [PubMed] [Google Scholar]
- Gordon T., Stein R. B., Thomas C. K. Innervation and function of hind-limb muscles in the cat after cross-union of the tibial and peroneal nerves. J Physiol. 1986 May;374:429–441. doi: 10.1113/jphysiol.1986.sp016089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory J. E., Luff A. R., Proske U. Muscle receptors in the cross-reinnervated soleus muscle of the cat. J Physiol. 1982 Oct;331:367–383. doi: 10.1113/jphysiol.1982.sp014377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENNEMAN E., SOMJEN G., CARPENTER D. O. FUNCTIONAL SIGNIFICANCE OF CELL SIZE IN SPINAL MOTONEURONS. J Neurophysiol. 1965 May;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Hallin R. G., Wiesenfeld Z., Lindblom U. Neurophysiological studies on patients with sutured median nerves: faulty sensory localization after nerve regeneration and its physiological correlates. Exp Neurol. 1981 Jul;73(1):90–106. doi: 10.1016/0014-4886(81)90047-9. [DOI] [PubMed] [Google Scholar]
- Karpati G., Engel W. K. "Type grouping" in skeletal muscles after experimental reinnervation. Neurology. 1968 May;18(5):447–455. doi: 10.1212/wnl.18.5.447. [DOI] [PubMed] [Google Scholar]
- Kimura J., Rodnitzky R. L., Okawara S. H. Electrophysiologic analysis of aberrant regeneration after facial nerve paralysis. Neurology. 1975 Oct;25(10):989–993. doi: 10.1212/wnl.25.10.989. [DOI] [PubMed] [Google Scholar]
- Kugelberg E., Edström L., Abbruzzese M. Mapping of motor units in experimentally reinnervated rat muscle. Interpretation of histochemical and atrophic fibre patterns in neurogenic lesions. J Neurol Neurosurg Psychiatry. 1970 Jun;33(3):319–329. doi: 10.1136/jnnp.33.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAWRENCE D. G., KUYPERS H. G. PYRAMIDAL AND NON-PYRAMIDAL PATHWAYS IN MONKEYS: ANATOMICAL AND FUNCTIONAL CORRELATION. Science. 1965 May 14;148(3672):973–975. doi: 10.1126/science.148.3672.973. [DOI] [PubMed] [Google Scholar]
- Luff A. R., Webb S. N. Electromyographic activity in the cross-reinnervated soleus muscle of unrestrained cats. J Physiol. 1985 Aug;365:13–28. doi: 10.1113/jphysiol.1985.sp015756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackel R., Kunesch E., Waldhör F., Struppler A. Reinnervation of mechanoreceptors in the human glabrous skin following peripheral nerve repair. Brain Res. 1983 May 23;268(1):49–65. doi: 10.1016/0006-8993(83)90389-x. [DOI] [PubMed] [Google Scholar]
- Milner-Brown H. S., Stein R. B., Lee R. G. Pattern of recruiting human motor units in neuropathies and motor neurone disease. J Neurol Neurosurg Psychiatry. 1974 Jun;37(6):665–669. doi: 10.1136/jnnp.37.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner-Brown H. S., Stein R. B., Yemm R. The contractile properties of human motor units during voluntary isometric contractions. J Physiol. 1973 Jan;228(2):285–306. doi: 10.1113/jphysiol.1973.sp010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner-Brown H. S., Stein R. B., Yemm R. The orderly recruitment of human motor units during voluntary isometric contractions. J Physiol. 1973 Apr;230(2):359–370. doi: 10.1113/jphysiol.1973.sp010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan M. J., Pinter M. J., Dum R. P., Burke R. E. Kinesiological studies of self- and cross-reinnervated FDL and soleus muscles in freely moving cats. J Neurophysiol. 1985 Oct;54(4):852–866. doi: 10.1152/jn.1985.54.4.852. [DOI] [PubMed] [Google Scholar]
- Stein R. B., French A. S., Mannard A., Yemm R. New methods for analysing motor function in man and animals. Brain Res. 1972 May 12;40(1):187–192. doi: 10.1016/0006-8993(72)90126-6. [DOI] [PubMed] [Google Scholar]
- Sumner B. E., Sutherland F. I. Quantitative electron microscopy on the injured hypoglossal nucleus in the rat. J Neurocytol. 1973 Sep;2(3):315–328. doi: 10.1007/BF01104033. [DOI] [PubMed] [Google Scholar]
- Thomas C. K., Ross B. H., Stein R. B. Motor-unit recruitment in human first dorsal interosseous muscle for static contractions in three different directions. J Neurophysiol. 1986 May;55(5):1017–1029. doi: 10.1152/jn.1986.55.5.1017. [DOI] [PubMed] [Google Scholar]
- Tsukahara N., Fujito Y., Oda Y., Maeda J. Formation of functional synapses in the adult cat red nucleus from the cerebrum following cross-innervating of forelimb flexor and extensor nerves. I. Appearance of new synaptic potentials. Exp Brain Res. 1982;45(1-2):1–12. doi: 10.1007/BF00235757. [DOI] [PubMed] [Google Scholar]


