Abstract
Understanding of human brain development, dysfunction and neurological diseases has remained limited and challenging due to inability to recapitulate human brain-specific features in animal models. Though the anatomy and physiology of the human brain has been understood in a remarkable way using post-mortem, pathological samples of human and animal models, however, modeling of human brain development and neurological diseases remains a challenge owing to distinct complexity of human brain. In this perspective, three-dimensional (3D) brain organoids have shown a beam of light. Tremendous growth in stem cell technologies has permitted the differentiation of pluripotent stem cells under 3D culture conditions into brain organoids, which recapitulate the unique features of human brain in many ways and also offer the detailed investigation of brain development, dysfunction and neurological diseases. Their translational value has also emerged and will benefit the society once the protocols for the upscaling of brain organoids are in place. Here, we summarize new advancements in methods for generation of more complex brain organoids including vascularized and mixed lineage tissue from PSCs. How synthetic biomaterials and microfluidic technology is boosting brain organoid development, has also been highlighted. We discuss the applications of brain organoids in studying preterm birth associated brain dysfunction; viral infections mediated neuroinflammation, neurodevelopmental and neurodegenerative diseases. We also highlight the translational value of brain organoids and current challenges that the field is experiencing.
Subject terms: Neural stem cells, Developmental neurogenesis, Molecular neuroscience
Facts
Brain organoids represent complex human based in vitro tools to study brain development, dysfunction and disorders.
Human brain organoids offer better visualization of brain structure and function than two-dimensional monolayer cultures and animal models.
It is possible to study interorgan interactions, preterm and infection associated neurodevelopmental impairment in extreme details with advanced methods.
Open questions
Can human brain organoids become translational in vitro tool also where they can be employed for production of neurotransmitters such as dopamine?
To represent overall spatial and temporal cytoarchitecture of brain with its microenvironment, protocol advancement is still warranted.
Introduction
Human brain is the most complex and greatly developed organ in multicellular organisms [1]. Distinct complexity of human brain contributed by genomic changes and protracted development has made it a daunting task to understand human brain development and neurological disorders using animal models [2]. Despite this, most of our knowledge about human brain has been acquired from post-mortem, pathological samples and animal models, which do not completely recapitulate the complex human brain features and initiation of disease [3, 4]. However, recent surge in stem cell and engineering technologies have provided a boost to developing human based tools to study the human brain development and disease [5, 6].
Next efforts in stem cell technology are focused on developing methods to direct the differentiation of pluripotent stem cells (PSCs) into desired brain specific cell types. Earlier, adherent two-dimensional (2D) cell culture systems were derived from PSCs for studying neuronal development. However, 2D models lack the endogenous tissue architecture, thus poses challenges to modeling of developing brain and to study of complex cell interactions in vitro [7]. Tissue architecture and intercellular interactions are highly important for understanding neurodevelopmental disorders where multiple cell types are affected [8]. In this scenario, development of new 3D culture methods for differentiation of PSCs into 3D brain organoids has permitted the investigation of normal brain development and pathogenesis of neurological diseases [9].
Brain organoids are three-dimensional multicellular structures, derived from PSCs under specific in vitro conditions where they self-organize themselves to some extent and simulate the in vivo brain regions partly [3]. In recent years, there have been tremendous boost in technological advances to generate brain organoids that resemble specific human brain regions such as neocortex, hippocampus, hypothalamus, and midbrain [10, 11] (Table 1).
Table 1.
Different molecular cocktails to generate brain region-specific organoids.
| Sr. No. | Brain region-specific organoids | Cell type used | Intrinsic signaling or extrinsic inductive signals | Reference |
|---|---|---|---|---|
| 1 | Cerebral Cortex | Mouse ESCs, hESCs and human iPSCs |
Dkk-1 (Wnt inhibitor), LeftyA (NODAL inhibitor) and soluble BMPRIA-Fc |
[25] |
| Human H9 ES and iPSCs | Intrinsic signaling | [9] | ||
| Human ESC | IWR1e (Wnt inhibitor) and SB431542 (TGFβ inhibitor) | [19] | ||
| Human iPSC |
Dorsomorphin (BMP inhibitor) and SB-431542, FGF2 EGF, BDNF and NT3 |
[26] | ||
| Human iPSC | Recombinant mouse Noggin, FGF2, rhDkk1, EGF, BDNF, GDNF and dibutyryl-cAMP | [111] | ||
| Human iPSC | Dorsomorphine, A83-01, WNT-3A, CHIR99021, SB-431542, ascorbic acid, BDNF, GDNF, TGFβ and cAMP | [27, 28] | ||
| Human ES (H9 or H1) and iPSCs | Intrinsic signaling with CHIR 99021 | [31] | ||
| 2 | Midbrain | Human ESC lines H1 and H9 | CHIR99021, Noggin, SB-431542, SHH-C25II, FGF8, BDNF, GDNF, ascorbic acid, and db-cAMP | [30] |
| Human iPSC | LDN-193189, SB-431542, SHH, purmorphamine, FGF-8, CHIR99021, BDNF, GDNF, ascorbic acid, TGFβ and c-AMP | [27] | ||
| Human NESCs | CHIR-99021, purmorphamine, ascorbic acid, BDNF, GDNF, ascorbic acid, TGFβ and db c-AMP | [136] | ||
| 3 | Hypothalamus | Human iPSC | SB431542, LDN193189, thioglycerol, WNT3A, SHH, Purmorphamine, FGF-2 and CTNF, BDNF, GDNF, ascorbic acid and c-AMP | [27, 28] |
| 4 | Hippocampus | Human ES cells | Wnt inhibitor (IWR1e), SB431542, CHIR 99021, BMP4, chemically defined lipid concentrate | [11] |
| 5 | Anterior pituitary gland | Mouse ES cells | BMP4, SAG, DAPT, FGF10 | [137] |
| Human ESCs | Chemically defined lipid concentrate, monothioglycerol, BMP4, SAG, FGF2 | [138] | ||
| 6 | Cerebellum | Human ESCs | SB431542, FGF2, SDF1 and FGF19 | [139] |
| 7 | Spinal cord | Mouse ESCs | All-trans RA, SAG, cyclopamine | [140] |
| Mouse ESCs, Human iPSC | bFGF, CHIR99021, retinoic acid, BMP4, DAPT | [141] | ||
| 8 | Retina | Mouse ESCs, Human ESCs | IWR1-endo (Wnt inhibitor), FBS, SAG, CHIR99021 (Wnt agonist) | [142] |
| Human iPSCs | FBS, Taurine, all-trans RA | [143] | ||
| Human iPSCs | FBS, Taurine, all-trans RA | [144] | ||
| Human ESCs (H9), Human iPSCs | FBS, Taurine, insulin-like growth factor 1, all-trans RA, 9-cis retinal | [145] |
Brain organoids can be employed to study normal and abnormal developmental processes implicated in infectious diseases, genetic disorders, and cancer. In this review, we summarize new method advances for generation of brain organoids from PSCs. We discuss the applications of brain organoids in studying preterm birth and viral infections associated brain dysfunction and neurological diseases. We also summarize the translational value and the challenges that the brain organoid field is tackling.
Advancement in methods to generate brain organoids
Initial seminal work on 2D monolayer culture differentiation [12–16] and landmark studies of Sasai and Clever’s group on 3D culture have set up the platform for the development of pioneer protocols to generate brain organoids [17, 18]. In general, brain organoids can be generated using two major techniques including self-organization of hPSCs and employing external inductive signals. Sasai and Knoblich groups have pioneered the 3D brain organoid culture systems by using neural inductive molecules and the extracellular matrix (matrigel), respectively [9, 19]. Though human brain organoids emulate the essential features of fetal brain, yet, limitations of current organoid methods including interior hypoxia and cell death have paused the derivation of 3D brain structures recapitulating the late fetal developmental stages [10]. Strikingly, Gordon et al have developed a method to derive 3D human cortical organoids, which show signals of postnatal stages [20]. We further discuss advancement of methods in culturing the brain organoids (Figs. 1 and 2).
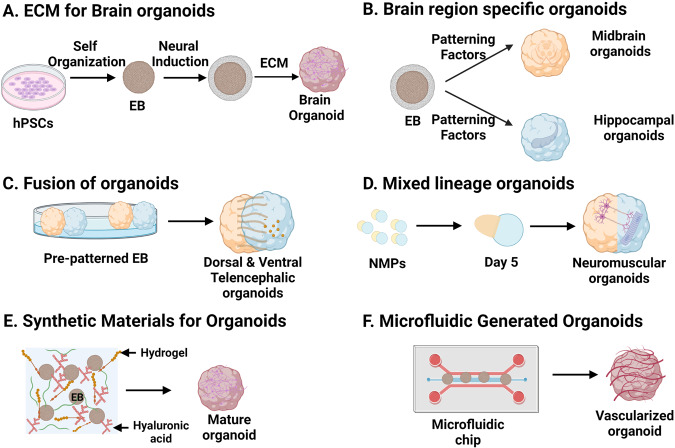
Fig. 1. Methodological advancement in brain organoid generation.
A Simple methods involving the minimal media and extracellular matrix generate self-organized whole brain organoids. B A cocktail of patterning molecules generates brain region-specific structures such as midbrain and hippocampal organoids. C Fusion of different organoids permits the study of interaction and migration between various cell types of different brain regions. D Mixed lineage organoids such as neuromuscular organoids allow the investigation of the interorgan interactions. E Synthetic materials enhance maturation of organoids. F Microfluidics develops vasculature in organoids.
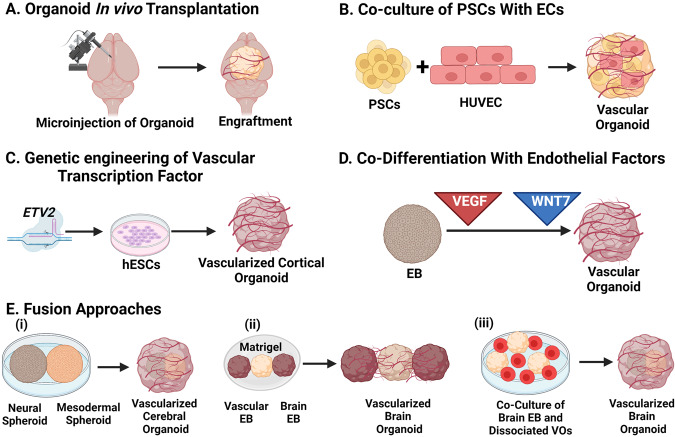
Fig. 2. Schematic of different strategies to vascularization of brain organoid.
A Microinjection of brain organoids in mice brain led to engraftment and invasion by host vasculature. B Co-culture of PSCs with endothelial cells and C genetic engineering of inducible transcription factor ETV2 in hESCs led to vascular system development in brain organoids. D EBs early fed with endothelial factors such as VEGF and later with WNT7 display vascularization along with neuronal differentiation. E Different fusion approaches led to brain organoid vascularization include fusion of (i) neural and mesodermal spheroid (ii) brain EB sandwiched with vascular EB in a Matrigel droplet (iii) brain EB and dissociated vascular organoids.
Extracellular matrix (ECM) for cerebral organoids
Lancaster et al, in their remarkable study, have exploited self-organizing and self-patterning properties of hPSCs which led to generation of cerebral organoids from hPSCs in presence of ECM [9]. This study was based on concept of neural default pathway that suggests the acquisition of default neuroectodermal fate by hPSCs in absence of external inductive signals [21]. However, Sasai group cultured hESCs in presence of external inductive signals such as TGFβ inhibitor and Wnt inhibitor for the initial patterning and later used matrigel at low concentration in presence of high (40%) O2 concentration [19].
Extracellular inductive signals for region specific organoids
Brain is a heterogeneous tissue, which is made up of several regions. Brain development is accompanied by simultaneous formation of different brain regions, which takes place by the patterning of neural tube through the concerted play of secreted morphogens/patterning factors from the organizer region [21]. Same principle has been applied by researchers to develop in vitro methods to guide the cells in the organoids to adopt a specific brain region fate by supplementing the culture media with the extracellular inductive signals or patterning factors which will either suppress or stimulate a specific developmental signaling pathway at distinct developmental stages [2].
Robust protocols have been developed for region specific 3D brain organoids depending on the anterior–posterior (AP) and dorso–ventral (DV) polarity of the specific region [16]. Two patterning factor cocktails guide neuroectodermal identity along AP and DV axis of neural tubes. Since, Wnt and TGFβ pose negative effects on mammalian neural differentiation [22, 23], supplementation of the Wnt and Nodal antagonists (Dkk1 and LeftyA) in serum-free floating culture of embryoid body-like aggregates (SFEB) of ES cells for first 5 days results in selective neural differentiation (∼90%). However, Wnt3a treatment during the later stages favors the pallial telencephalic fate, whereas Shh augments basal telencephalic differentiation [24]. Eiraku et al. have used Wnt, Nodal antagonists and BMPRIA-Fc in their modified SFEB with quick re-aggregation (SFEBq method), and efficiently produced polarized cortical neuroepithelia from mouse, human PSCs [19, 25]. Therefore, the first patterning factor cocktail was established, aiming at coherent neural induction and AP identity along the neural tube. Several studies have displayed efficient conversion of hPSCs to neuroectodermal identity by suppressing TGF-β and BMP signaling using the dual-SMAD inhibition approach [16, 26, 27]. Dose dependent early insulin treatment and Wnt activation have been used to generate various brain regions including midbrain, thalamic, hypothalamic and cerebellar fates [27–30]. For second patterning factor cocktail, pulse of Wnt activity at later stages can give rise to telencephalic organoids [27, 28, 31]. While hippocampal tissues can be generated by Wnt and BMP treatment [11], ventral fates of telencephalon, hypothalamus and midbrain can be derived using early SHH activation [27, 30, 32–34]. Beside these inductive signals, a few other morphogens have also been included in the differentiation media. For example, rostralisation of the cortical organoids was achieved with the treatment of FGF8 [19, 25]. While, same inductive signal can pattern different brain regions in a density gradient and temporal manner, therefore, for specific brain regions, establishing a chemically-defined patterning factor cocktail and order of treatment in a low cost and reproducible manner are the recent roadblocks [2]. Despite these challenges, brain region specific organoids are helpful in elucidating the effect of genetic mutation and treatment paradigms for a brain region specific disease [30, 35, 36]. However, brain region specific organoids lack the inter-region interactions and therefore, investigation of inter-region interactions using these methods becomes challenging. Organoid fusion approach has emerged as a possible solution to study inter-region interactions, and this technique is well discussed elsewhere [2] (Fig. 1).
Enhance the maturation of organoids
Efforts to obtain functional, mature neurons and glial cells in organoids resulted in long term culturing of organoids in 3D culture media. Long term cultivation helped in attaining calcium activity at 50 days [26, 27], spontaneous excitatory post-synaptic currents at 4 months [37] and acquisition of glial cells along with mature neurons, astrocytes, synapse, and dendritic spines at more than 6 months [38, 39]. Prolonged culturing time encourages study of mature cell types in vitro.
However, several challenges associated with prolonged culture include insufficient oxygen supply to interior part of organoids, hypoxic inner cores and cell death of the tissue. Though several approaches such as supplying increased oxygen levels in the incubator, using spinning bioreactor or gas permeable culture plates have been adopted to boost oxygen supply to the inner core, yet the maturation of healthy brain organoids were similar to mid gestation periods. Recently, Guo-Li Ming’s group has developed a method of slicing the 45-day-old neocortical organoid into sliced neocortical organoids which upon further culturing showed reduced inner hypoxia, diminished cell death, sustained neurogenesis and formation of deep and upper layer neurons over long-term cultures. The sliced organoid architecture mimics the embryonic human neocortex at third trimester of gestation [10]. Recently, Pasca and colleagues also performed long term culture of cortical spheroids and grew them up to 694 days. These spheroids displayed isoform switching in the histone deacetylase complex and NMDA receptor subunits that mark the transition from prenatal to early postnatal stages of brain development between 250 and 300 days [20].
Mixed lineage tissue organoids
Organoids not only mimic one tissue type but also recapitulate the phenotypes of two or mixed lineage tissues such as brain and retina and thus allows the investigation of interorgan interactions in vitro. Two recent papers have developed two lineage tissue organoids. Jay Gopalakrishnan’s group has explored an innovative way to develop bilaterally symmetric optic vesicles from forebrain organoids. Their method includes culturing single cell iPSCs in neural induction medium till the formation of neurospheres, followed by culturing in neurosphere medium with addition of retinol acetate. Early supplementation of retinol acetate favored early optic vesicle development in brain organoids [40]. Mina Gouti and colleagues in their seminal study used hPSC lines to generate neuromesodermal progenitors and allowed them to form 3D aggregates in neurobasal medium with several growth factors. The resultant neuromuscular organoids (NMOs) form functional neuromuscular junctions among skeletal muscle cells, spinal cord neurons, and Schwann cells simultaneously (Fig. 1) [41]. Another study differentiated iPSC lines into neuromesodermal progenitors and derived sensorimotor organoids from them. These organoids contained cells of neuronal origin, such as sensory and motor neurons, astrocytes and of mesodermal origin such as microglia, endothelial cells and skeletal muscle. They also reported physiologically active neuromuscular junctions between the motor neurons and skeletal muscle [42].
Specialized and vascularized brain organoids
Human brain organoids generated using methods discussed so far, lack vasculature; this results in restricted supply of oxygen and nutrients to the inner part of the organoids that lead to necrosis and cell death of the inner part of organoids, limiting the organoid growth [43, 44]. Absence of vasculature also impacts signaling, differentiation and maturation of brain progenitor cells [45]. Therefore, developing vasculature in organoids is important to achieve diverse, differentiated and reproducible cell repertoire (Fig. 2) [46, 47].
In this direction, first efforts were shown by Fred Gage group by transplanting human brain organoids into adult mouse brain, which provides in vivo physiological conditions to organoids and led to improved neuronal differentiation and maturation, glial cells generation, functional synapse, and blood vasculature in the grafts [48]. Next approach involves engineering of hESCs to transiently overexpress human ETS variant 2 (ETV2), leading to the emergence of ETV2-expressing cells in human cortical organoids, and finally displayed the formation of a complex vascular-like network in cortical organoids. Vasculature acquired organoids displayed numerous characteristics of blood-brain barrier (BBB) [49]. In another approach, PSCs were co-cultured with human umbilical vein endothelial cells (HUVEC) to form cortical organoid. These organoids also displayed the formation of tubular vascular system [50]. Notably, in both studies, vascular organoids exhibit functional vasculature, decreased cell death, improved maturation and synaptic connections after transplantation in mice [49, 50]. In addition, EBs cultured in VEGF rich media led to co-differentiation of vascular endothelial cells (ECs) along with neuronal cells in cerebral organoids. Addition of VEGF and WNT7a led to formation of segregated blood vessel-like structures in cerebral organoids with BBB characteristics [51]. Another alternative involves propagating neural and vascular cell types separately as spheroids followed by their fusion. Researchers have fused either neural and mesodermal spheres [52] or vascular and brain organoids [53, 54] and cultivated them for long time. Though the blood vessel formation and maturation was successful after fusion, yet blood vessel network was least responsive to pro- and anti-angiogenic conditions [52]. To avoid regional localization of blood vessels in brain organoids, Ahn et al. dissociated blood vessel organoids and co-cultured with brain organoids using neural induction media. This led to formation of long blood vessels with pericytes; however, astrocytes were not closely associated with blood vessels [55]. Beside these approaches, microfluidics technology, ECM proteins and synthetic materials have been employed to enhance the vascularization of cerebral organoids [31, 56, 57].
Functional materials in organoid induction
It has been a great challenge to recapitulate the brain ECM in 3D cultures, which led to immense usage of natural materials such as Matrigel [58]. However, its poorly defined composition, xenogenic nature, batch to batch variation and lack of reproducibility has permitted researchers to explore alternatives of matrigel [59]. Recent advances in synthetic materials have led to the development of better ECM-like materials, which are chemically defined, greatly modifiable, reproducible and xenogenic-free alternatives [59, 60]. For instance, decellularized adult porcine brain ECM has been used to grow ESC-derived brain organoids, which exhibit mature morphology on day 40 [61]. Similarly, collagen hydrogel also improves maturation of neuronal network in 3D neuronal organoids, which co-developed excitatory, inhibitory neurons and glia cells [62]. In the next leap, a composite of recombinant spider silk with human laminin 111 reduces cellular stress and intra-organoid variability and enhances neuronal maturation in cerebral organoids [63].
Researchers have employed polyethylene glycol (PEG) and generated homogeneous 3D neural tube constructs [64]. Strikingly, without addition of any neural inductive signals, Cell-Mate3D hydrogels (a mixture of hyaluronic acid (HA) and chitosan) have generated cerebral organoids in just 10-14 days [65]. In addition, HA, an important component of brain ECM, in combination with heparin causes caudalization of iPSC derived brain organoids [66]. Besides these materials, alginate either alone or in combination with HA also stimulates neural fates in 3D culture of PSCs [67]. The synthetic materials can also deliver various growth factors important for regulation of vascularization and microenvironment such as VEGF, which modulates the brain organoid development and promotes long term survival [68]. These advances in synthetic materials to mimic brain ECM help in the development of improved brain organoid models.
Microfluidics in organoid induction
Microfluidics refers to a miniaturized microfluidic cell culture device that enables organ-on-a-chip system by feeding live cells in continuously perfused chambers [69, 70]. This technology offers to control the tissue’s physicochemical microenvironment and mimics cytoarchitecture and vascular perfusion of the tissue to a great extent [56, 58]. The application of microfluidic technology in vascularization of organoids is immense [71–73]. Recently, a combination of microfluidic device polydimethylsiloxane (PDMS) chip and human brain ECM has resulted in improved neurogenesis and better electrophysiological function in 3D brain organoids [74]. The reason for improved neurogenesis could be reduction of stress as seen by Seiler et al. They observed reduced glycolytic and endoplasmic reticulum stress in cortical organoids grown on automated, PDMS fabricated microfluidic cell culture platform [75]. The microfluidic technology can also be used for co-development of cells. For instance, co-development of hPSC derived pericytes, endothelial cells and cerebral organoids in 3D printed microfluidic platform leads to the formation of organized vascular networks, which interacts with cerebral organoids and perfuse them [57]. Not only co-development but brain region specific organoids have also been generated using microfluidic chips. For instance, human iPSCs enveloped in microcapsules, generated brain region-specific organoids, which were assembled into brain assembloids using multi-layered microfluidic chip with dynamic fluid flow. These assembloids were composed of cortical, hippocampal, and thalamic organoids, which recapitulated the neural migration and interaction [76]. In addition, perfusion of brain organoids and delivery of oxygen and nutrients can be improved by maintaining active flow of media in the microfluidic chips using syringe pump, peristaltic pump and acoustofluidic minibioreactor [77–80]. Microfluidics grown brain organoids have recently been employed in toxicity studies. For instance, cannabis exposure of brain organoids on one-stop microfluidic chips led to decreased neuronal maturation, diminished neurite outgrowth and reduced spontaneous firing in organoids [81]. Micro fabricated petri dish compartment coated with PDMS was used to model the folding of human brain organoids, which displayed folds in the first week of development [82]. Thus, microfluidics combines the fields of fluid dynamics, synthetic polymer chemistry with biology to study brain structure, physiology, development and drug discovery.
Applications of brain organoids
To study neurodevelopmental impairment associated with preterm birth
Preterm birth is referred to as birth before 37 weeks of gestation period [83]. This contributes to the ~15% neonatal deaths and thus represents the single leading cause of neonatal deaths worldwide [84]. Out of survivors, ~80% very early preterm infants (birth before 28 weeks of gestation) confront varied degree of neurodevelopmental impairment into later life. After birth, preterm infant can encounter hypoxia, which can develop an acute ailment called encephalopathy of prematurity [85] that lead to impairment of complex behavioral and cognitive functions (Fig. 3) [86].
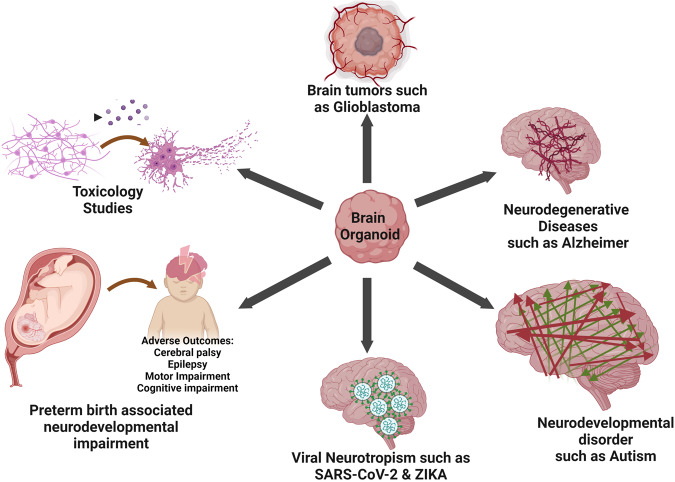
Fig. 3. Applications of brain organoids.
Brain organoids can be used to study preterm birth-associated neurodevelopmental impairment, viral neurotropism such as SARS-CoV-2 and Zika, Neurodevelopmental and neurodegenerative disorders, brain tumors such as glioblastoma and for toxicology studies.
Brain organoids permit the modeling of these complex functions and umbilical cord tissue and cord blood can be used to generate iPSC derived brain organoids [87]. The molecular mechanisms underlying hypoxia mediated neurodevelopmental impairments in very early preterm infants are not understood well. Recently, Pasca et al have delineated the effect of oxygen deprivation on corticogenesis using human brain-region-specific organoids. Oxygen deprivation reduces the population of cortical intermediate progenitors, which contribute immensely to the human cerebral cortex expansion. Notably, this reduction was associated with the unfolded protein response (UPR) pathway as addition of a small-molecule inhibitor of the UPR pathway rescues the decrease in intermediate progenitors following hypoxia [85].
To study viral infection mechanism/neurotropism
Infectious diseases are caused by pathogens, which regularly emerge and spread with pandemic or epidemic potential and shatter human life. The recent outbreak by novel human coronavirus SARS-CoV-2 from China and Zika virus in America has emerged as pandemic and epidemic, respectively. They devastated human species by causing COVID-19 and microcephaly, respectively.
Detection of SARS-CoV-2 and ZIKV RNA in some patient’s brains and a suspected association between the ZIKV outbreak and congenital microcephaly in newborns has shown the neurotropism and neurotoxic effects of these viruses. There is no direct experimental evidence, which concludes the neurotoxic and birth defects of SARS-CoV-2 and ZIKV, respectively and therefore, has led to employing 3D brain models to reveal viral infection mechanisms [88, 89]. Recently, a few studies have validated that SARS-CoV-2 infects cortical neurons in brain organoids, iPSC-derived hNPCs, neurospheres, brain spheroids, astrocytes and microglia [90–94] and caused metabolic alterations, microglia mediated synapse engulfment and neuronal cell death [58, 60]. Contradictory, other studies suggest the sparse or rare infection of neurons, astrocytes and microglia by SARS-CoV-2 [95, 96]. However, choroid plexus organoids show robust infection of SARS-CoV-2 followed by cell death and compromised barrier integrity. Abundance of SARS-CoV-2 entry receptors in choroid plexus cells may lead to the dramatic infection of choroid plexus organoids [96]. Similarly, cerebral organoids have also advanced our understanding of infection mechanisms of ZIKV. ZIKV efficiently replicates in brain organoids and diminishes their growth [97]. ZIKV selectively infects NPCs and forebrain organoids and causes reduced proliferation of NPCs followed by enormous cell death, reduced neuronal volume and organoid size [27, 66, 67]. In organoids, ZIKV activates innate immune receptor TLR3 and depletes NPCs [98]. Mechanistically, in forebrain organoids, NS2A protein of ZIKV diminishes proliferation of radial glial cells and leads to adherens junction (AJ) complex deficits [99]. Recently, ZIKV infection in brain organoids led to accumulation of Aβ plaques, increased p-Tau expression and expedites AD pathology [100].
Further, the disparity between different studies about the cell type specific infection of these viruses could be because of usage of different viral strains such as wild type, spike-pseudotypisized viral vector, delta and omicron isolates [93, 101, 102]. Besides these mechanistic studies, brain organoids have also been employed to reveal strain specific neurotropism of ZIKV and for therapeutic development [103, 104]. For instance, SARS-CoV-2 infection in brain organoids can be prevented by halting ACE2 receptor with antibodies or by culturing organoids with cerebrospinal fluid from a COVID-19 patient [105]. Recently, Sofosbuvir, a FDA approved antiviral (anti-hepatitis C) prevented SARS-CoV-2 replication, decreased neuronal death and rescued synaptic integrity in infected cortical organoids [92]. Notably, Sofosbuvir also protected human NPCs and 3D neurospheres from ZIKV mediated cell death [106]. Strikingly, brain organoid defects elicited by ZIKV were rescued by the type I interferons and RNAi enhancer enoxacin [97, 107]. Together, these studies have highlighted the use of human brain organoid models in revealing neurotropism and delineating the molecular mechanisms underlying viral infections of brain, and also in screening potential therapeutic candidates.
To understand mechanisms underlying neurological diseases
Neurological diseases include neurodevelopmental and neurodegenerative diseases and mechanistic understanding and drug development of neurological diseases has hampered due to inaccessibility of patient brain tissues at disease initiation stage. This has led to development of 3D brain organoids, which can recapitulate the complexity, physiology and pathology of the human brain. We describe this section using specific examples of neurodevelopmental and neurodegenerative disorders (Fig. 3), while most of these diseases have been mentioned in Table 2.
Table 2.
Disease modeling using 3D human brain organoids and challenges.
| Neurological disease | Challenge | Brain region-specific organoids | Growth factors/ECM used | Cellular phenotype/phenomenon | Reference |
|---|---|---|---|---|---|
| Microcephaly-related disease conditions | |||||
| Microcephaly | Mouse models do not mimic the severity of Microcephaly because of smaller brain and limited expansion | Cerebral organoids | Y-27632, N2 supplement, Heparin, Matrigel, B27 supplement without vitamin A, Insulin | Progenitor cells with decreased radial glial cells show premature neural differentiation | [9] |
| Microcephaly patients with Aspm mutations possess extremely reduced brain size, which is quite difficult to mimic in animal models | Cortical organoids | endo-IWR1, LDN-193189, SB431542, heparin, BDNF, GDNF, cAMP, matrigel | Patient-derived cortical organoids displayed proliferation deficient progenitors, less mature neurons and abnormal cortical lamination, neuronal dysfunction | [37] | |
| Lissencephaly (Miller Dieker Syndrome) | Absence of outer radial glial cells in developing rodent cortex leads to milder phenotypes in Pafah1b1+/− mice as compared to human patients | Cerebral organoids | Y-27632, WNT inhibitor, TGF-β inhibitor, FBS, Matrigel and heparin | Defective cell migration, massive apoptosis of founder neuroepithelial stem cells, Defective mitosis in outer radial glia | [146] |
| Neurodevelopmental disorders | |||||
| Bipolar disorder | Mechanistic understanding of the Bipolar Disorder is unclear due to the scarcity of causal genes displaying robust effect sizes | Cerebral organoids with dorsal forebrain identity | STEMdiff Cerebral Organoid Kit & maturation kit with BDNF | Cerebral organoids derived from diseased and control iPSCs were not apparently different. Bipolar cerebral organoids show precise defects in response to stimulation and depolarization | [147] |
| Bipolar disorder/schizophrenia | Underlying cellular mechanisms for the pathogenesis of Bipolar disorder are not clear due to heterogeneity and technical constraints on cellular complexity of the brain. | Cerebral organoids | SB431542, LDN-193189, Y-27632, Matrigel | Accelerated neuronal differentiation, increased GABAergic specification, decreased cell proliferation following reduced Wnt signaling | [148] |
| Developmental and epileptic encephalopathies (DEE) | DEE is a heterogeneous disorder associated with intractable seizures, abnormal brain development, and functional abnormalities. Every patient has distinct genetic background and brain development, which cannot be modeled in a rodent model | Cerebral organoids | bFGF, Y-27632, Matrigel, Insulin, CHIR-99021, vitamin C | Defective DNA damage response, activation of Wnt pathway, abnormal cortical differentiation with disproportionate glutamatergic and GABAergic neurons and increased astrogenesis | [149] |
| Timothy syndrome | In vitro modeling of migration of interneurons and their functional integration into human cortex during fetal development remains a challenge | Forebrain spheroids containing cortical (hCS) and subpallium spheroids (hSS) | hCS: dorsomorphin, SB-431542, FGF2, EGF, BDNF, NT-3 hSS: FGF2, EGF, IWP-2, SAG, NT-3, allopregnanolone, retinoic acid, BDNF | Increased saltation frequency led to defective migration of interneurons | [32] |
| Tuberous sclerosis complex (TSC) | Highly heterogeneous developmental disorder, cortical tubers are not formed in rodent models | Cortical spheroid | Dorsomorphin, SB431542, EGF, FGF, BDNF, NT-3 | Hypertrophic NPCs, enlarged and dysmorphic neuronal cells with enhanced glia production | [150] |
| Angelman syndrome (AS) | AS mice models show synaptic dysfunction and impaired plasticity; however, mechanisms in AS patients remain unclear | Cortical organoids | Dorsomorphin, SB431542, B27 without vitamin A, EGF, FGF-basic, BDNF, GDNF, NT3, db-cAMP | Hyper-excitability and synchronous firing, UBE3A via degradation of calcium- and voltage-dependent big potassium (BK) channels inhibits neuronal hyper-excitability | [151] |
| Rett Syndrome | Non-availability of embryonic or perinatal post-mortem tissues from Rett Syndrome patients | Cerebral organoids | Y-27632, SB431542, dorsomorphin, Matrigel, B27 without vitamin A, B27 with vitamin A | increased ventricular area, reduced radial thickness and massive increase of neural progenitor cells followed by abnormal migration of neurons and aberrant neurogenesis | [152] |
| Lack of studies on influence of MeCP2 mutation on human interneuron development and function | Human medial ganglionic eminence (MGE) and cortical organoids | LDN-193189, SB-431542, XAV-939, Y-27632, hMGEO: B27 supplement without vitamin A, recombinant SHH, purmorphamine hCO: B27 supplement with vitamin A, BDNF, ascorbic acid, cAMP | BET inhibitor, JQ1, improves the functional deficits of RTT interneurons in brain organoids by showing area-scale synchronization of calcium surges and rescuing the dysregulated transcriptome | [153] | |
| RTT phenotypes are widespread and various kind of mutations present the disease phenotypes differently | Dorsal and ventral forebrain organoids | Dorsal Forebrain: Dorsomorphine, A83-01, CHIR99021, SB-431542, Heparin Ventral: SB-431542, LDN-193189, IWP2, SAG and Heparin. BDNF, GDNF, dibutyryl cAMP and ascorbic acid | Premature development of the deep-cortical layer with less proliferating neural progenitor/proliferative cells along with the impairments of interneuron’s migration, dysfunctional RTT neurons, defects in production of medial ganglionic eminence (MGE) progenitors in ventral organoids | [154] | |
| Genetic mutation of MeCp2 gene impairs neurodevelopment, still therapeutic treatment for this syndrome is not available | Cortical organoids | SB431542, Dorsomorphin, Y-27632, FGF2, EGF, BDNF, GDNF, NT-3, ascorbic acid and dibutyryl-cAMP | In MECP2‐KO cortical organoids, two lead compounds rescued synaptic pathways and neural network function | [155] | |
| Down syndrome | Technically inaccessible pathological samples, presence of asynchronous and heterogeneous disease phenotypes in in vitro culture | Cerebral organoids | N2 supplement, Matrigel | Diminished neurogenesis, reduced proliferation and decreased expression of cortical neuronal markers in layer II and IV in the subcortical regions | [156] |
| Vague knowledge of role of Olig genes in GABAergic neuron generation and inconsistencies in recapitulating DS-related genotype–phenotype relationships and contradictory results from human samples | Ventral forebrain organoid | SB431542, Noggin, Matrigel, Laminin, B27-RA, FGF2, hLIF, CHIR99021, Y-27632, SHH, purmorphamine, BDNF, GDNF, dibutyryl-cyclic AMP and ascorbic acid | Overproduction of OLIG2+ Progenitors, disproportionate interneuron production, recognition memory disrupted in neuronal chimeric mice | [157] | |
| ASD | Phenotypic heterogeneity and absence of behavioral phenotypes of ASD in rodent models | Telencephalic organoids | B27 supplement without vitamin A, N2 supplement, 2-mercaptoethanol, Y-27632, FGF2, Noggin, rhDkk1, EGF, ascorbic acid, BDNF, GDNF and dibutyryl-cAMP | Elevated neuronal maturation and synaptic overgrowth, Massive inhibitory synapses in ASD-derived neurons, balance between the number of excitatory and inhibitory neurons in ASD-organoids was disturbed | [111] |
| Heterogeneous population of neurons expressing forebrain, midbrain, and hindbrain markers was present in monolayer neuronal culture system | Cerebral organoid | DKK-1, BMPRIA-Fc, SB431542, N2 supplement, laminin, and fibronectin, B27 and l-glutamine | Radial glia progenitor cells, GABAergic and glutamatergic neurons were present in cerebral organoids | [114] | |
| To integrate the finding that DNA methylation patterns of GAD1 are dysregulated during development from patient’s post-mortem brain and rodent models | Cerebral organoids | Y-27632, Matrigel | Early development follows a diverse methylation patterns in GAD1 in ASD cerebral organoids | [158] | |
| Lack of understanding of cell lineages and molecular pathways implicated in telencephalic development in ASD | single neural rosettes derived telencephalic organoids | N2 Supplement, Heparin, B27 Supplement with vitamin A, Dorsomorphin, SB431542, EGF, FGF, BDNF, GDNF and NT-3 | Smaller size of SHANK3-/-organoids, decreased population of neurons with smaller nuclei sizes, decreased number of excitatory synaptic puncta | [159] | |
| ASD | Mechanisms underlying valproic acid contribution to accelerating ASD risk in human are not known | Forebrain organoids | SB431542, LDN193189, Y27632, Heparin, CHIR99021, WNT-3A, Matrigel, insulin, Forskolin, Ascorbic Acid, BDNF, GDNF | Disturbed synaptic transmission in VPA treated organoids, differentially expressed genes enriched in calcium, and potassium signaling pathways, oxytocin signaling, synaptic transmission, and neural development | [160] |
| How interactions among gene–environment increases ASD risk are unknown | Brain organoids | FGF, EGF, GDNF, BDNF | Exposure of CHD8 +/− organoids with organophosphate pesticide resulted in disturbed Neurite outgrowth and imbalance of excitatory/inhibitory neurotransmitters | [161] | |
| Neurodegenerative diseases | |||||
| Alzheimer disease | Two-dimensional in vitro models poorly demonstrate the aggregation of extracellular protein involved in Alzheimer disease | Neural organoids | Y-27632, SB431532, IWRe1, Dorsomorphin, heparin Matrigel, B27 supplement | Amyloid beta, tau pathology and endosome abnormality is mimicked in fAD organoids, which were responsive to drug treatment | [162] |
| Investigation of AD was hindered in absence of non-invasive method for harvesting brain tissue from living patients | Cerebral organoids | Y27632, Matrigel | Aβ deposits and Tau phosphorylation was observed in cerebral organoids | [163] | |
| AD organoids should mimic the genetic background of patients and the functional features of AD brain | Cerebral organoid | Y-27632, Matrigel | AD organoids displayed a small size, increased Aβ42/Aβ40 ratio, disrupted calcium homeostasis, and enhanced neuronal activity | [164] | |
| 5-Hydroxymethylcytosine contribution to AD pathology has not been determined | Forebrain organoids | FGF, Matrigel, Dorsomorphin, A-83, Heparin CHIR99021,SB-431542, B27, GDNF, BDNF, TFGβ, cAMP | Forebrain organoids mimicked cellular and molecular phenotypes of AD brain. More 5hmC peaks in EBs compared to mature organoids and modulated in intragenic regions | [165] | |
| Parkinson | 2D models do not mimic the neuron- glia interaction in a spatially organized cellular architecture | Midbrain-like organoids | SB-431542, LDN-193189, CHIR99021, SAG, Y-27632, ascorbic acid, Matrigel | Midbrain organoids comprised of dopaminergic neurons, which secrete dopamine. In patient organoids, number and complexity of mDANs was decreased | [36] |
| Immaturity and heterogeneity of Midbrain organoids (MO) architecture and less efficient protocol | Midbrain organoids | CHIR99021, Noggin, SB431542, Dorsomorphin, A83-01, LDN, FGF8, SAG, Matrigel | MOs comprised of homogenous distribution of dopaminergic neurons, astrocytes and oligodendrocytes | [126] | |
| Mutation in DNAJC6 is associated with early-onset Parkinson’s disease, but role of DNAJC6 in PD pathogenesis was unknown | Midbrain-Like organoids (MLOs) | B27, SB431542, Noggin,CHIR99021, Y27632, purmophamine, SHH-C25II, FGF8, BDNF, GDNF, Matrigel, ascorbic acid | MLOs display death of dopamine neuron, aggregation of α-synuclein, dysfunction of mitochondria and lysosomes | [166] | |
| Frontotemporal dementia | Role of p25/Cdk5 in tauopathy using organoid model was not known | Cerebral organoids | SB431532, IWRe1,Dorsomorphin, heparin, FBS, matrigel | Tau phosphorylation was reduced and expression of synaptophysin increased in cerebral organoids derived isogenic iPSC lines | [167] |
| Zika virus | Inefficient organoid differentiation protocols lead to enormous variability in brain organoids | Cortical organoids and ventral telencephalic organoids | Y-27632, 40% O2, B27 without vitamin A, Growth Factor Reduced Matrigel, heparin, Leukemia Inhibitory Factor | Functional neurons display network-like activities in organoids, Increased progenitor apoptosis leads to smaller organoids size, efficacy of different drug tested | [168] |
| Schizophrenia | Functional role of interaction between DISC1 and Ndel1/Nde1 in brain development is difficult to delineate | Forebrain organoids | Dorsomorphine, A83-01, Matrigel, Insulin, WNT-3A, CHIR99021, SB-431542 | Cell-cycle defects in radial glial cells in forebrain organoids resulted in reduced proliferation of neural stem cells in the ventricular zone and disturbed neurogenesis | [169] |
| Prenatal hypoxic injury | Technical challenge in accessing human fetal brain tissues and inappropriate animal models have led to poor understanding of effects of hypoxia on progenitor steady state and developmental progression during early human brain development | Cerebral organoids | Matrigel, N2 supplement, B27 supplement without vitamin A, insulin | Immense apoptosis in cerebral organoids followed by greater loss of outer radial glia progenitors and differentiating neuroblasts/immature neurons. During hypoxia, NSCs shifted to symmetric division and replete their population | [170] |
| Tuberous sclerosis/periventricular heterotopia | Partial recapitulation of human cortex features by mouse models have led to non-suitability of these models for understanding the mechanisms of neuronal heterotopia | Cerebral spheroids | Y-27632, N2 supplement, Heparin, Matrigel, B27 supplement without vitamin A, Insulin | Defective morphology of neural progenitor cells and altered neuronal migration in a subset of neurons | [171] |
| Retinitis pigmentosa | Retinal organoids | Matrigel, G418 and/or Ataluren (PTC124) | Massive cell death of rod photoreceptor at day 150 followed by thinning of the outer nuclear layer by day 180 of culture in patient-derived organoids | [172] | |
| Brain cancers | |||||
| Brain tumor including glioblastoma and central nervous system primitive neuroectodermal tumor | Modeling of brain tumors is limited owing to genetic heterogeneity and non-suitability of animal models | Neoplastic cerebral organoid | bFGF, Y-27632, Matrigel, vitamin A | By using gene editing techniques and generating gain or loss of function phenotypes for several genes, tumorigenesis was established in cerebral organoids | [173] |
| Glioblastoma | Inability to recapitulate the cellular and mutational diversity of parental tumors in in vitro models and longer development time of these models. | Glioblastoma organoids (GBOs) | Patient Glioblastoma Tissue, N2 supplement, B27 supplement w/o vitamin A, insulin | Cellular composition of parental tumors recapitulated in GBOs as they display enormous heterogeneity in cell identity and morphology | [95] |
| Limited donor availability for patient-derived xenograft (PDX) models and findings from mouse genetic models are not translatable to human clinical trials | Cerebral organoids | Y-27632, Heparin, N2 supplement, 1X B27 supplement w/o vitamin A, insulin, Matrigel | Invasive phenotype of genetically modified cells inside the organoid caused massive destruction of surrounding organoid structures, and display tumor pathology upon transplantation in mice | [174] | |
| Limited GBM models due to the lack of a normal human microenvironment and the inability to recapitulate the GBM biology | Cerebral organoid glioma | Y-27632, Heparin, N2 supplement, 1X B27 supplement w/o vitamin A, insulin, Matrigel | regional proliferation followed by massive invasion accompanied by formation of tumor microtubes and phenocopy patient glioblastoma | [175] | |
Autism spectrum disorder (ASD)
ASD is a disorder of abnormal brain development, which results in language impairment, diminished social interaction and stereotypic behaviors in children [108, 109]. Immense genetic and phenotypic heterogeneity pose challenges to study ASD in animal and cellular models. However, cerebral and forebrain organoids derived from ASD patients display the clinical phenotype of ASD by showing an enlarged cortical plate thickness [110]. Telencephalic organoids derived from patient iPSCs demonstrate an enhanced cell cycle, increased expression of FOXG1 gene, which causes exaggerated production of GABAergic inhibitory neurons, highlighting an uneven GABA/glutamate neuronal population in patients [111]. Recently, cortical organoids derived from ASD patients harboring mutations in three risk genes SUV420H1, ARID1B and CHD8 also display asynchronous development of GABAergic neurons and excitatory projection neurons [112]. Dysregulated proliferation and alternative splicing causes enhanced production of inhibitory and delayed generation of excitatory neurons [113]. Moreover, telencephalic organoids generated from fetal iPSCs revealed the enrichment of ASD de novo mutations in functional enhancers, which disturb the binding sites of homeodomain transcription factors such as NR4A2, Hes1, NFIX, and Sox3 [43]. In addition, patient brain organoids also display activation of different gene networks and pathways. For instance, telencephalic organoids derived from CHD8 (autism candidate gene) heterozygous KO iPSC lines highlight the Wnt/β-catenin signaling and axonal guidance as the top significant affected pathways [114]. Patient forebrain organoids harboring a homozygous mutation in CNTNAP2 gene, display an enhanced organoid volume caused by an increased proliferation of neural progenitor cells. CRISPR-Cas9 mediated repair of CNTNAP2 mutation in organoids led to reversal of cortical overgrowth phenotypes and partially rescued transcriptional profiles [115]. Patient cortical organoids harboring 16p11.2 copy number variation (CNV) display altered ratio of neurons to neural progenitors with depletion of neural progenitors in case of deletion [76]. Transcriptomic and proteomic profiling of these organoids revealed neuron migration, actin cytoskeleton and Wnt signaling as dysregulated pathways. RhoA inhibition in cortical organoids rescued neuron migration defects but not the neurite length [116]. In addition, Orgo-Seq of patient cerebral organoids identified immature neurons and intermediate progenitor cells as crucial cells for 16p11.2 deletions [117].
Alzheimer’s disease (AD)
AD is the primary neurodegenerative disease, associated with cognitive decline and behavioral deterioration resulting into dementia [118]. Early onset, familial AD was first modeled in cerebral organoids by mutating the APP and PSEN1 genes in the human neural progenitor cells and differentiating them in 3D cultures. These organoids display robust extracellular deposition of amyloid-β peptides (Aβ) and neurofibrillary tangles (NFT) [119]. Since then, patient cerebral organoids have been generated harboring mutations in PSEN1, APOE4 genes and recapitulating pathogenic features of AD [120, 121]. Transcriptomic analysis of patient cerebral organoids elucidates the upregulation of stress granules and dysregulated RNA metabolism [121].
Several new methods have also been developed to model the late onset AD. Recently, BBB leakage in AD was mimicked in a remarkable way by exposing the brain organoids to human serum. In this condition, key hallmarks of AD such as enhanced levels of Aβ peptides, p-tau, and synaptic loss were observed in the brain organoids [122]. In addition, herpes simplex virus (HSV-1) infection of cerebral organoids resulted in the onset of AD features without the addition of any exogenous modulators of AD [123]. Co-culture of neuron, astrocytes and microglia in a 3D microfluidic chamber recapitulated AD features and specifically microglia causes neuronal damage by secreting TNF-α and nitric oxide via IFN-γ and TLR4 mediated mechanisms [124].
Parkinson’s disease (PD)
PD is another most common neurodegenerative disorder, which exhibits tremors, drooping posture, muscle rigidity, walking difficulty and cognitive decline in aging population [125].
Recently, Kwak et al. have developed midbrain-like organoids, which contain the dopaminergic neurons, astrocytes and oligodendrocytes and display great homogeneity and maturity. In addition, astrocytes present in the organoids break down 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a representative dopaminergic neurotoxin, therefore, facilitates in vitro modeling of PD [126]. Several other conditions were also standardized for midbrain organoid generation such as the cell number, the timing of the embedding and the maturation protocol [27, 36, 127, 128]. Midbrain-like organoids generated from PD patients harboring the LRRK2-G2019S mutation revealed the increased expression of FOXA2, a transcription factor necessary for production of dopaminergic neurons, thus indicate a neurodevelopmental defect in midbrain dopaminergic neurons [36]. Isogenic midbrain organoids generated with a LRRK2-G2019S mutation also mimicked the hallmarks of PD. Gene expression profiling of 3D organoids harboring LRRK2-G2019S mutation revealed thiol-oxidoreductase (TXNIP) as one of the upregulated genes and inhibition of TXNIP rescued PD pathology in organoids [35].
Therefore, these results suggest that brain organoids not only reveal causes and pathological mechanisms of neurological diseases, but can also be employed for drug screening and to elucidate the effect of genetic, epigenetic and environmental stressors.
Translational value for therapeutic platform
Regionally patterned brain organoids can be helpful in secreting neurotransmitters such as dopamine, acetylcholine, GABA, whose production goes awry in neurodegenerative diseases. For this overwhelming approach, one needs the well-established globally standardized protocols for organoid establishment and quality control. If these protocols demonstrate enormous robustness, reproducibility, scaling up, and turns into cheaper technology than bacteria, then the day is not far from establishing the organoid technology for cheaper production of neurotransmitters. This will have immense application for Parkinson’s patients who will be able to receive dopamine secreted by a human system instead of bacterial system.
Current challenges
Brain organoids offer unprecedented opportunity to study development, evolution and neurological diseases; however there are a number of challenges to be overcome. One of the major limitation includes reproducibility with inter batch variations [129]. The batch effect is prominent in un-guided organoids due to variations in morphology, differentiation efficiency, and cellular compositions [38, 130]. In addition, use of different PSC lines results in variable organoid size, morphology and also compromises the reproducibility of brain organoids. Therefore, appropriate stringent controls with comparable age, genetic background and gender are essential while comparing the size of the patients derived organoids to reveal the pathological phenotype of the disease. Another limitation of the current protocols includes the limited supply of nutrients and oxygen to the core of the organoid, followed by necrosis, restricted maturation and even absence of cell types from organoids. To overcome this limitation, though several efforts have developed vasculature in organoids, yet protocols need to be refined [49, 131]. Current protocols are also unable to generate full six-layered laminar cytoarchitecture of neocortex and fully mature glial cells in organoids.
Future perspective and conclusion
3D brain organoid field has held immense potential in bringing the in vivo tissue architecture to a dish and thus has presented innumerable opportunities for disease modeling, transplantation, drug discovery and toxicology [129]. 3D brain organoids show outstanding possibilities in comparison to 2D neural rosettes in harboring organized tissue architecture by displaying unique layers of neural progenitors and appropriate migration of differentiating neurons. Multidisciplinary approaches have led to the introduction of bioreactors, microfluidic and robotic devices, 3D printers to boost the drug screening and toxicology studies in brain organoids [79, 132]. Several protocols have emerged that allow the derivation of brain region specific organoids which mimic the specific region of the brain and thus permit the study of biological mechanisms underlying brain development and dysfunction in a spatial and temporal manner. However, protocol refinement is still needed that allows the generation of all six layers of cortical neurons in cortical organoids and all the regions of hippocampus in hippocampal organoids [133]. In addition, engineered bio-scaffolds can further help in improving the spatio-temporal migration of neurons in organoid cortical plate [134]. Heterogeneity and variability among different samples and within the same protocol is yet a robust challenge. While efforts have been initiated to tackle this challenge, it warrants future investigations on exploring exogenous patterning molecules, mini-bioreactors and mini-scaffolds to enhance homogeneity [19, 26, 27, 31, 44].
Another major area in the organoid field that requires much attention is the limited maturation of the organoids due to inefficient supply of nutrients and oxygen to the core of the organoids, which has restricted organoids to model the late fetal stages. To achieve this goal, approaches must be established that can help in developing vasculature and mature non-neuronal cell types inside the organoids. Though several approaches have been adopted including co-culture of endothelial cells, microfluidic perfusion, ectopic expression of ETV2 in brain organoids and transplanting brain organoids in rodents to accomplish in vivo vascularization, bioengineered substances such as hydrogels and nanoparticles [48, 49, 132, 135], yet, multidisciplinary integrated approaches are warranted to achieve the advanced maturation and survival of organoids. Generation of non-neuronal cell types such as astrocytes, oligodendrocytes and microglia in brain organoids further help in studying the neuron-glia interaction. Long-term culturing promotes the maturation of neurons along with the differentiation of glial cells in the organoids [20]. In addition, the field requires novel strategies to model the key hallmarks of aging to study age related neurodegenerative diseases.
In brief, integration of bioengineering, microfluidics and automation permit further technological advancement in 3D brain organoid field, which will unravel novel disease mechanisms and help in diagnostic and therapeutic development of neurological diseases.
Acknowledgements
The author greatly acknowledges the SERB Research Scientist fellowship award (SB/SRS/2020-21/45/LS) by the Science and Engineering Research Board, Department of Science and Technology (DST), Government of India. The funding agency had no role in writing of manuscript or in the decision to submit the article for publication. The author also acknowledges Translational Health Science and Technology Institute (THSTI), Faridabad and Amity University, Noida for infrastructure support. The author apologizes to authors whose articles are not cited due to space limitations. The figures were created with BioRender.com.
Funding
This work was funded by the Science and Engineering Research Board, Govt. of India.
Competing interests
The author declares no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20:327–48. doi: 10.1007/s11065-010-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sidhaye J, Knoblich JA. Brain organoids: an ensemble of bioassays to investigate human neurodevelopment and disease. Cell Death Differ. 2021;28:52–67. doi: 10.1038/s41418-020-0566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Lullo E, Kriegstein AR. The use of brain organoids to investigate neural development and disease. Nat Rev Neurosci. 2017;18:573–84. doi: 10.1038/nrn.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams JW, Cugola FR, Muotri AR. Brain organoids as tools for modeling human neurodevelopmental disorders. Physiology. 2019;34:365–75. doi: 10.1152/physiol.00005.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Kelava I, Lancaster MA. Stem cell models of human brain development. Cell Stem Cell. 2016;18:736–48. doi: 10.1016/j.stem.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Quadrato G, Brown J, Arlotta P. The promises and challenges of human brain organoids as models of neuropsychiatric disease. Nat Med. 2016;22:1220–8. doi: 10.1038/nm.4214. [DOI] [PubMed] [Google Scholar]
- 9.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–9. doi: 10.1038/nature12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian X, Su Y, Adam CD, Deutschmann AU, Pather SR, Goldberg EM, et al. Sliced human cortical organoids for modeling distinct cortical layer formation. Cell Stem Cell. 2020;26:766–81.e9. doi: 10.1016/j.stem.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakaguchi H, Kadoshima T, Soen M, Narii N, Ishida Y, Ohgushi M, et al. Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat Commun. 2015;6:8896. doi: 10.1038/ncomms9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003;21:183–6. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- 13.Zhang SC, Wernig M, Duncan ID, Brüstle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–33. doi: 10.1038/nbt1201-1129. [DOI] [PubMed] [Google Scholar]
- 14.Shi Y, Kirwan P, Smith J, Robinson HPC, Livesey FJ. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci. 2012;15:477–86.S1. doi: 10.1038/nn.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaspard N, Bouschet T, Hourez R, Dimidschstein J, Naeije G, van den Ameele J, et al. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–7. doi: 10.1038/nature07287. [DOI] [PubMed] [Google Scholar]
- 16.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–80. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–5. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 18.Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011;472:51–6. doi: 10.1038/nature09941. [DOI] [PubMed] [Google Scholar]
- 19.Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando S, Eiraku M, et al. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc Natl Acad Sci USA. 2013;110:20284–9. doi: 10.1073/pnas.1315710110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon A, Yoon SJ, Tran SS, Makinson CD, Park JY, Andersen J, et al. Long-term maturation of human cortical organoids matches key early postnatal transitions. Nat Neurosci. 2021;24:331–42. doi: 10.1038/s41593-021-00802-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muguruma K, Sasai Y. In vitro recapitulation of neural development using embryonic stem cells: from neurogenesis to histogenesis. Dev Growth Differ. 2012;54:349–57. doi: 10.1111/j.1440-169X.2012.01329.x. [DOI] [PubMed] [Google Scholar]
- 22.Aubert J, Dunstan H, Chambers I, Smith A. Functional gene screening in embryonic stem cells implicates Wnt antagonism in neural differentiation. Nat Biotechnol. 2002;20:1240–5. doi: 10.1038/nbt763. [DOI] [PubMed] [Google Scholar]
- 23.Parisi S, D’Andrea D, Lago CT, Adamson ED, Persico MG, Minchiotti G. Nodal-dependent Cripto signaling promotes cardiomyogenesis and redirects the neural fate of embryonic stem cells. J Cell Biol. 2003;163:303–14. doi: 10.1083/jcb.200303010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watanabe K, Kamiya D, Nishiyama A, Katayama T, Nozaki S, Kawasaki H, et al. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci. 2005;8:288–96. doi: 10.1038/nn1402. [DOI] [PubMed] [Google Scholar]
- 25.Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–32. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Paşca AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N, et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods. 2015;12:671–8. doi: 10.1038/nmeth.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 2016;165:1238–54. doi: 10.1016/j.cell.2016.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qian X, Jacob F, Song MM, Nguyen HN, Song H, Ming GL. Generation of human brain region-specific organoids using a miniaturized spinning bioreactor. Nat Protoc. 2018;13:565–80. doi: 10.1038/nprot.2017.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiang Y, Tanaka Y, Cakir B, Patterson B, Kim KY, Sun P, et al. hESC-derived thalamic organoids form reciprocal projections when fused with cortical organoids. Cell Stem Cell. 2019;24:487–97.e7. doi: 10.1016/j.stem.2018.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jo J, Xiao Y, Sun AX, Cukuroglu E, Tran HD, Göke J, et al. Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell. 2016;19:248–57. doi: 10.1016/j.stem.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lancaster MA, Corsini NS, Wolfinger S, Gustafson EH, Phillips AW, Burkard TR, et al. Guided self-organization and cortical plate formation in human brain organoids. Nat Biotechnol. 2017;35:659–66. doi: 10.1038/nbt.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N, et al. Assembly of functionally integrated human forebrain spheroids. Nature. 2017;545:54–9. doi: 10.1038/nature22330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bagley JA, Reumann D, Bian S, Lévi-Strauss J, Knoblich JA. Fused cerebral organoids model interactions between brain regions. Nat Methods. 2017;14:743–51. doi: 10.1038/nmeth.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiang Y, Tanaka Y, Patterson B, Kang YJ, Govindaiah G, Roselaar N, et al. Fusion of regionally specified hPSC-derived organoids models human brain development and interneuron migration. Cell Stem Cell. 2017;21:383–98.e7. doi: 10.1016/j.stem.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim H, Park HJ, Choi H, Chang Y, Park H, Shin J, et al. Modeling G2019S-LRRK2 sporadic Parkinson’s disease in 3D midbrain organoids. Stem Cell Rep. 2019;12:518–31. doi: 10.1016/j.stemcr.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smits LM, Reinhardt L, Reinhardt P, Glatza M, Monzel AS, Stanslowsky N, et al. Modeling Parkinson’s disease in midbrain-like organoids. NPJ Parkinsons Dis. 2019;5:5. doi: 10.1038/s41531-019-0078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li R, Sun L, Fang A, Li P, Wu Q, Wang X. Recapitulating cortical development with organoid culture in vitro and modeling abnormal spindle-like (ASPM related primary) microcephaly disease. Protein Cell. 2017;8:823–33. doi: 10.1007/s13238-017-0479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quadrato G, Nguyen T, Macosko EZ, Sherwood JL, Min Yang S, Berger DR, et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature. 2017;545:48–53. doi: 10.1038/nature22047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renner M, Lancaster MA, Bian S, Choi H, Ku T, Peer A, et al. Self-organized developmental patterning and differentiation in cerebral organoids. EMBO J. 2017;36:1316–29. doi: 10.15252/embj.201694700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gabriel E, Albanna W, Pasquini G, Ramani A, Josipovic N, Mariappan A, et al. Human brain organoids assemble functionally integrated bilateral optic vesicles. Cell Stem Cell. 2021;28:1740–57.e8. doi: 10.1016/j.stem.2021.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Faustino Martins JM, Fischer C, Urzi A, Vidal R, Kunz S, Ruffault PL, et al. Self-organizing 3D human trunk neuromuscular organoids. Cell Stem Cell. 2020;26:172–86.e6. doi: 10.1016/j.stem.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Pereira JD, DuBreuil DM, Devlin AC, Held A, Sapir Y, Berezovski E, et al. Human sensorimotor organoids derived from healthy and amyotrophic lateral sclerosis stem cells form neuromuscular junctions. Nat Commun. 2021;12:4744. doi: 10.1038/s41467-021-24776-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amiri A, Coppola G, Scuderi S, Wu F, Roychowdhury T, Liu F, et al. Transcriptome and epigenome landscape of human cortical development modeled in organoids. Science. 2018;362:eaat6720. doi: 10.1126/science.aat6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pollen AA, Bhaduri A, Andrews MG, Nowakowski TJ, Meyerson OS, Mostajo-Radji MA, et al. Establishing cerebral organoids as models of human-specific brain evolution. Cell. 2019;176:743–56.e17. doi: 10.1016/j.cell.2019.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–40. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 46.Khakipoor S, Crouch EE, Mayer S. Human organoids to model the developing human neocortex in health and disease. Brain Res. 2020;1742:146803. doi: 10.1016/j.brainres.2020.146803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LaMontagne E, Muotri AR, Engler AJ. Recent advancements and future requirements in vascularization of cortical organoids. Front Bioeng Biotechnol. 2022;10:1048731. doi: 10.3389/fbioe.2022.1048731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mansour AA, Gonçalves JT, Bloyd CW, Li H, Fernandes S, Quang D, et al. An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol. 2018;36:432–41. doi: 10.1038/nbt.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cakir B, Xiang Y, Tanaka Y, Kural MH, Parent M, Kang YJ, et al. Engineering of human brain organoids with a functional vascular-like system. Nat Methods. 2019;16:1169–75. doi: 10.1038/s41592-019-0586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi Y, Sun L, Wang M, Liu J, Zhong S, Li R, et al. Vascularized human cortical organoids (vOrganoids) model cortical development in vivo. PLoS Biol. 2020;18:e3000705. doi: 10.1371/journal.pbio.3000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ham O, Jin YB, Kim J, Lee MO. Blood vessel formation in cerebral organoids formed from human embryonic stem cells. Biochem Biophys Res Commun. 2020;521:84–90. doi: 10.1016/j.bbrc.2019.10.079. [DOI] [PubMed] [Google Scholar]
- 52.Wörsdörfer P, Dalda N, Kern A, Krüger S, Wagner N, Kwok CK, et al. Generation of complex human organoid models including vascular networks by incorporation of mesodermal progenitor cells. Sci Rep. 2019;9:15663. doi: 10.1038/s41598-019-52204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun XY, Ju XC, Li Y, Zeng PM, Wu J, Zhou YY, et al. Generation of vascularized brain organoids to study neurovascular interactions. Elife. 2022;11:e76707. doi: 10.7554/eLife.76707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kook MG, Lee SE, Shin N, Kong D, Kim DH, Kim MS, et al. Generation of cortical brain organoid with vascularization by assembling with vascular spheroid. Int J Stem Cells. 2022;15:85–94. doi: 10.15283/ijsc21157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahn Y, An JH, Yang HJ, Lee DG, Kim J, Koh H, et al. Human blood vessel organoids penetrate human cerebral organoids and form a vessel-like system. Cells. 2021;10:2036. doi: 10.3390/cells10082036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saorin G, Caligiuri I, Rizzolio F. Microfluidic organoids-on-a-chip: the future of human models. Semin Cell Dev Biol. 2023;144:41–54. doi: 10.1016/j.semcdb.2022.10.001. [DOI] [PubMed] [Google Scholar]
- 57.Salmon I, Grebenyuk S, Abdel Fattah AR, Rustandi G, Pilkington T, Verfaillie C, et al. Engineering neurovascular organoids with 3D printed microfluidic chips. Lab Chip. 2022;22:1615–29. doi: 10.1039/D1LC00535A. [DOI] [PubMed] [Google Scholar]
- 58.Passaro AP, Stice SL. Electrophysiological analysis of brain organoids: current approaches and advancements. Front Neurosci. 2020;14:622137. doi: 10.3389/fnins.2020.622137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aisenbrey EA, Murphy WL. Synthetic alternatives to Matrigel. Nat Rev Mater. 2020;5:539–51. doi: 10.1038/s41578-020-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kratochvil MJ, Seymour AJ, Li TL, Paşca SP, Kuo CJ, Heilshorn SC. Engineered materials for organoid systems. Nat Rev Mater. 2019;4:606–22. doi: 10.1038/s41578-019-0129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simsa R, Rothenbücher T, Gürbüz H, Ghosheh N, Emneus J, Jenndahl L, et al. Brain organoid formation on decellularized porcine brain ECM hydrogels. PLoS ONE. 2021;16:e0245685. doi: 10.1371/journal.pone.0245685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zafeiriou MP, Bao G, Hudson J, Halder R, Blenkle A, Schreiber MK, et al. Developmental GABA polarity switch and neuronal plasticity in bioengineered neuronal organoids. Nat Commun. 2020;11:3791. doi: 10.1038/s41467-020-17521-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sozzi E, Kajtez J, Bruzelius A, Wesseler MF, Nilsson F, Birtele M, et al. Silk scaffolding drives self-assembly of functional and mature human brain organoids. Front Cell Dev Biol. 2022;10:1023279. doi: 10.3389/fcell.2022.1023279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ranga A, Girgin M, Meinhardt A, Eberle D, Caiazzo M, Tanaka EM, et al. Neural tube morphogenesis in synthetic 3D microenvironments. Proc Natl Acad Sci USA. 2016;113:E6831–9. doi: 10.1073/pnas.1603529113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lindborg BA, Brekke JH, Vegoe AL, Ulrich CB, Haider KT, Subramaniam S, et al. Rapid induction of cerebral organoids from human induced pluripotent stem cells using a chemically defined hydrogel and defined cell culture medium. Stem Cells Transl Med. 2016;5:970–9. doi: 10.5966/sctm.2015-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bejoy J, Wang Z, Bijonowski B, Yang M, Ma T, Sang QX, et al. Differential effects of heparin and hyaluronic acid on neural patterning of human induced pluripotent stem cells. ACS Biomater Sci Eng. 2018;4:4354–66. doi: 10.1021/acsbiomaterials.8b01142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bozza A, Coates EE, Incitti T, Ferlin KM, Messina A, Menna E, et al. Neural differentiation of pluripotent cells in 3D alginate-based cultures. Biomaterials. 2014;35:4636–45. doi: 10.1016/j.biomaterials.2014.02.039. [DOI] [PubMed] [Google Scholar]
- 68.Karal-Yılmaz O, Serhatlı M, Baysal K, Baysal BM. Preparation and in vitro characterization of vascular endothelial growth factor (VEGF)-loaded poly(D,L-lactic-co-glycolic acid) microspheres using a double emulsion/solvent evaporation technique. J Microencapsul. 2011;28:46–54. doi: 10.3109/02652048.2010.523795. [DOI] [PubMed] [Google Scholar]
- 69.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32:760–72. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 70.Zhu Y, Wang L, Yu H, Yin F, Wang Y, Liu H, et al. In situ generation of human brain organoids on a micropillar array. Lab Chip. 2017;17:2941–50. doi: 10.1039/C7LC00682A. [DOI] [PubMed] [Google Scholar]
- 71.Matsui TK, Tsuru Y, Hasegawa K, Kuwako KI. Vascularization of human brain organoids. Stem Cells. 2021;39:1017–24. doi: 10.1002/stem.3368. [DOI] [PubMed] [Google Scholar]
- 72.Ingber DE. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat Rev Genet. 2022;23:467–91. doi: 10.1038/s41576-022-00466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aazmi A, Zhou H, Lv W, Yu M, Xu X, Yang H, et al. Vascularizing the brain in vitro. iScience. 2022;25:104110. doi: 10.1016/j.isci.2022.104110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cho AN, Jin Y, An Y, Kim J, Choi YS, Lee JS, et al. Microfluidic device with brain extracellular matrix promotes structural and functional maturation of human brain organoids. Nat Commun. 2021;12:4730. doi: 10.1038/s41467-021-24775-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seiler ST, Mantalas GL, Selberg J, Cordero S, Torres-Montoya S, Baudin PV, et al. Modular automated microfluidic cell culture platform reduces glycolytic stress in cerebral cortex organoids. Sci Rep. 2022;12:20173. doi: 10.1038/s41598-022-20096-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu Y, Zhang X, Sun L, Wang Y, Zhao Y. Engineering human brain assembloids by microfluidics. Adv Mater. 2023;35:e2210083. doi: 10.1002/adma.202210083. [DOI] [PubMed] [Google Scholar]
- 77.Wang Y, Wang L, Zhu Y, Qin J. Human brain organoid-on-a-chip to model prenatal nicotine exposure. Lab Chip. 2018;18:851–60. doi: 10.1039/C7LC01084B. [DOI] [PubMed] [Google Scholar]
- 78.Wang Y, Wang L, Guo Y, Zhu Y, Qin J. Engineering stem cell-derived 3D brain organoids in a perfusable organ-on-a-chip system. RSC Adv. 2018;8:1677–85. doi: 10.1039/C7RA11714K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khan I, Prabhakar A, Delepine C, Tsang H, Pham V, Sur M. A low-cost 3D printed microfluidic bioreactor and imaging chamber for live-organoid imaging. Biomicrofluidics. 2021;15:024105. doi: 10.1063/5.0041027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cai H, Ao Z, Wu Z, Song S, Mackie K, Guo F. Intelligent acoustofluidics enabled mini-bioreactors for human brain organoids. Lab Chip. 2021;21:2194–205. doi: 10.1039/D1LC00145K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ao Z, Cai H, Havert DJ, Wu Z, Gong Z, Beggs JM, et al. One-stop microfluidic assembly of human brain organoids to model prenatal cannabis exposure. Anal Chem. 2020;92:4630–8. doi: 10.1021/acs.analchem.0c00205. [DOI] [PubMed] [Google Scholar]
- 82.Karzbrun E, Kshirsagar A, Cohen SR, Hanna JH, Reiner O. Human brain organoids on a chip reveal the physics of folding. Nat Phys. 2018;14:515–22. doi: 10.1038/s41567-018-0046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bhatnagar S, Majumder PP, Salunke DM. Interdisciplinary Group for Advanced Research on Birth Outcomes—DBT India Initiative (GARBH-Ini). A pregnancy cohort to study multidimensional correlates of preterm birth in India: study design, implementation, and baseline characteristics of the participants. Am J Epidemiol. 2019;188:621–31. doi: 10.1093/aje/kwy284. [DOI] [PubMed] [Google Scholar]
- 84.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–40. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 85.Pașca AM, Park JY, Shin HW, Qi Q, Revah O, Krasnoff R, et al. Human 3D cellular model of hypoxic brain injury of prematurity. Nat Med. 2019;25:784–91. doi: 10.1038/s41591-019-0436-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hodyl NA, Aboustate N, Bianco-Miotto T, Roberts CT, Clifton VL, Stark MJ. Child neurodevelopmental outcomes following preterm and term birth: what can the placenta tell us? Placenta. 2017;57:79–86. doi: 10.1016/j.placenta.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 87.Harbuzariu A, Pitts S, Cespedes JC, Harp KO, Nti A, Shaw AP, et al. Modelling heme-mediated brain injury associated with cerebral malaria in human brain cortical organoids. Sci Rep. 2019;9:19162. doi: 10.1038/s41598-019-55631-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martines RB, Bhatnagar J, Keating MK, Silva-Flannery L, Muehlenbachs A, Gary J, et al. Notes from the field: evidence of Zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses—Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:159–60. doi: 10.15585/mmwr.mm6506e1. [DOI] [PubMed] [Google Scholar]
- 89.Tang H, Hammack C, Ogden SC, Wen Z, Qian X, Li Y, et al. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell. 2016;18:587–90. doi: 10.1016/j.stem.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang BZ, Chu H, Han S, Shuai H, Deng J, Hu YF, et al. SARS-CoV-2 infects human neural progenitor cells and brain organoids. Cell Res. 2020;30:928–31. doi: 10.1038/s41422-020-0390-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bullen CK, Hogberg HT, Bahadirli-Talbott A, Bishai WR, Hartung T, Keuthan C, et al. Infectability of human BrainSphere neurons suggests neurotropism of SARS-CoV-2. ALTEX. 2020;37:665–71. doi: 10.14573/altex.2006111. [DOI] [PubMed] [Google Scholar]
- 92.Mesci P, de Souza JS, Martin-Sancho L, Macia A, Saleh A, Yin X, et al. SARS-CoV-2 infects human brain organoids causing cell death and loss of synapses that can be rescued by treatment with Sofosbuvir. PLoS Biol. 2022;20:e3001845. doi: 10.1371/journal.pbio.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kong W, Montano M, Corley MJ, Helmy E, Kobayashi H, Kinisu M, et al. Neuropilin-1 mediates SARS-CoV-2 infection of astrocytes in brain organoids, inducing inflammation leading to dysfunction and death of neurons. mBio. 2022;13:e0230822. doi: 10.1128/mbio.02308-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Samudyata, Oliveira AO, Malwade S, Rufino de Sousa N, Goparaju SK, Gracias J, et al. SARS-CoV-2 promotes microglial synapse elimination in human brain organoids. Mol Psychiatry. 2022;27:3939–50. doi: 10.1038/s41380-022-01786-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jacob F, Pather SR, Huang WK, Zhang F, Wong SZH, Zhou H, et al. Human pluripotent stem cell-derived neural cells and brain organoids reveal SARS-CoV-2 neurotropism predominates in choroid plexus epithelium. Cell Stem Cell. 2020;27:937–50.e9. doi: 10.1016/j.stem.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pellegrini L, Albecka A, Mallery DL, Kellner MJ, Paul D, Carter AP, et al. SARS-CoV-2 infects the brain choroid plexus and disrupts the blood-CSF barrier in human brain organoids. Cell Stem Cell. 2020;27:951–61.e5. doi: 10.1016/j.stem.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Krenn V, Bosone C, Burkard TR, Spanier J, Kalinke U, Calistri A, et al. Organoid modeling of Zika and herpes simplex virus 1 infections reveals virus-specific responses leading to microcephaly. Cell Stem Cell. 2021;28:1362–79.e7. doi: 10.1016/j.stem.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dang J, Tiwari SK, Lichinchi G, Qin Y, Patil VS, Eroshkin AM, et al. Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell. 2016;19:258–65. doi: 10.1016/j.stem.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yoon KJ, Song G, Qian X, Pan J, Xu D, Rho HS, et al. Zika-virus-encoded NS2A disrupts mammalian cortical neurogenesis by degrading adherens junction proteins. Cell Stem Cell. 2017;21:349–358.e6. doi: 10.1016/j.stem.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee SE, Choi H, Shin N, Kong D, Kim NG, Kim HY, et al. Zika virus infection accelerates Alzheimer’s disease phenotypes in brain organoids. Cell Death Discov. 2022;8:153. doi: 10.1038/s41420-022-00958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ramani A, Müller L, Ostermann PN, Gabriel E, Abida-Islam P, Müller-Schiffmann A, et al. SARS-CoV-2 targets neurons of 3D human brain organoids. EMBO J. 2020;39:e106230. doi: 10.15252/embj.2020106230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yi SA, Nam KH, Yun J, Gim D, Joe D, Kim YH, et al. Infection of brain organoids and 2D cortical neurons with SARS-CoV-2 pseudovirus. Viruses. 2020;12:1004. doi: 10.3390/v12091004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JLM, Guimarães KP, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534:267–71. doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu M, Lee EM, Wen Z, Cheng Y, Huang WK, Qian X, et al. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nat Med. 2016;22:1101–7. doi: 10.1038/nm.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Song E, Zhang C, Israelow B, Lu-Culligan A, Prado AV, Skriabine S, et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med. 2021;218:e20202135. doi: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mesci P, Macia A, Moore SM, Shiryaev SA, Pinto A, Huang CT, et al. Author correction: Blocking Zika virus vertical transmission. Sci Rep. 2018;8:8794. doi: 10.1038/s41598-018-26959-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Xu YP, Qiu Y, Zhang B, Chen G, Chen Q, Wang M, et al. Zika virus infection induces RNAi-mediated antiviral immunity in human neural progenitors and brain organoids. Cell Res. 2019;29:265–73. doi: 10.1038/s41422-019-0152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saxena R, Babadi M, Namvarhaghighi H, Roullet FI. Role of environmental factors and epigenetics in autism spectrum disorders. Prog Mol Biol Transl Sci. 2020;173:35–60. doi: 10.1016/bs.pmbts.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 109.Marchetto MC, Belinson H, Tian Y, Freitas BC, Fu C, Vadodaria K, et al. Altered proliferation and networks in neural cells derived from idiopathic autistic individuals. Mol Psychiatry. 2017;22:820–35. doi: 10.1038/mp.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schafer ST, Paquola ACM, Stern S, Gosselin D, Ku M, Pena M, et al. Pathological priming causes developmental gene network heterochronicity in autistic subject-derived neurons. Nat Neurosci. 2019;22:243–55. doi: 10.1038/s41593-018-0295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mariani J, Coppola G, Zhang P, Abyzov A, Provini L, Tomasini L, et al. FOXG1-dependent dysregulation of GABA/glutamate neuron differentiation in autism spectrum disorders. Cell. 2015;162:375–90. doi: 10.1016/j.cell.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Paulsen B, Velasco S, Kedaigle AJ, Pigoni M, Quadrato G, Deo AJ, et al. Autism genes converge on asynchronous development of shared neuron classes. Nature. 2022;602:268–73. doi: 10.1038/s41586-021-04358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Villa CE, Cheroni C, Dotter CP, López-Tóbon A, Oliveira B, Sacco R, et al. CHD8 haploinsufficiency links autism to transient alterations in excitatory and inhibitory trajectories. Cell Rep. 2022;39:110615. doi: 10.1016/j.celrep.2022.110615. [DOI] [PubMed] [Google Scholar]
- 114.Wang P, Mokhtari R, Pedrosa E, Kirschenbaum M, Bayrak C, Zheng D, et al. CRISPR/Cas9-mediated heterozygous knockout of the autism gene CHD8 and characterization of its transcriptional networks in cerebral organoids derived from iPS cells. Mol Autism. 2017;8:11. doi: 10.1186/s13229-017-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.de Jong JO, Llapashtica C, Genestine M, Strauss K, Provenzano F, Sun Y, et al. Cortical overgrowth in a preclinical forebrain organoid model of CNTNAP2-associated autism spectrum disorder. Nat Commun. 2021;12:4087. doi: 10.1038/s41467-021-24358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Urresti J, Zhang P, Moran-Losada P, Yu NK, Negraes PD, Trujillo CA, et al. Cortical organoids model early brain development disrupted by 16p11.2 copy number variants in autism. Mol Psychiatry. 2021;26:7560–80. doi: 10.1038/s41380-021-01243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lim ET, Chan Y, Dawes P, Guo X, Erdin S, Tai DJC, et al. Orgo-Seq integrates single-cell and bulk transcriptomic data to identify cell type specific-driver genes associated with autism spectrum disorder. Nat Commun. 2022;13:3243. doi: 10.1038/s41467-022-30968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Sci Transl Med. 2011;3:77sr1. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Choi SH, Kim YH, Hebisch M, Sliwinski C, Lee S, D’Avanzo C, et al. A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature. 2014;515:274–8. doi: 10.1038/nature13800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gonzalez C, Armijo E, Bravo-Alegria J, Becerra-Calixto A, Mays CE, Soto C. Modeling amyloid beta and tau pathology in human cerebral organoids. Mol Psychiatry. 2018;23:2363–74. doi: 10.1038/s41380-018-0229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhao J, Fu Y, Yamazaki Y, Ren Y, Davis MD, Liu CC, et al. Author correction: APOE4 exacerbates synapse loss and neurodegeneration in Alzheimer’s disease patient iPSC-derived cerebral organoids. Nat Commun. 2021;12:2707. doi: 10.1038/s41467-021-23081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen X, Sun G, Tian E, Zhang M, Davtyan H, Beach TG, et al. Modeling sporadic Alzheimer’s disease in human brain organoids under serum exposure. Adv Sci. 2021;8:e2101462. doi: 10.1002/advs.202101462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cairns DM, Rouleau N, Parker RN, Walsh KG, Gehrke L, Kaplan DL. A 3D human brain-like tissue model of herpes-induced Alzheimer’s disease. Sci Adv. 2020;6:eaay8828. doi: 10.1126/sciadv.aay8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Park J, Wetzel I, Marriott I, Dréau D, D’Avanzo C, Kim DY, et al. A 3D human triculture system modeling neurodegeneration and neuroinflammation in Alzheimer’s disease. Nat Neurosci. 2018;21:941–51. doi: 10.1038/s41593-018-0175-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Galet B, Cheval H, Ravassard P. Patient-derived midbrain organoids to explore the molecular basis of Parkinson’s disease. Front Neurol. 2020;11:1005. doi: 10.3389/fneur.2020.01005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kwak TH, Kang JH, Hali S, Kim J, Kim KP, Park C, et al. Generation of homogeneous midbrain organoids with in vivo-like cellular composition facilitates neurotoxin-based Parkinson’s disease modeling. Stem Cells. 2020;38:727–40. doi: 10.1002/stem.3163. [DOI] [PubMed] [Google Scholar]
- 127.Nickels SL, Modamio J, Mendes-Pinheiro B, Monzel AS, Betsou F, Schwamborn JC. Reproducible generation of human midbrain organoids for in vitro modeling of Parkinson’s disease. Stem Cell Res. 2020;46:101870. doi: 10.1016/j.scr.2020.101870. [DOI] [PubMed] [Google Scholar]
- 128.Ha J, Kang JS, Lee M, Baek A, Kim S, Chung SK, et al. Simplified brain organoids for rapid and robust modeling of brain disease. Front Cell Dev Biol. 2020;8:594090. doi: 10.3389/fcell.2020.594090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chiaradia I, Lancaster MA. Brain organoids for the study of human neurobiology at the interface of in vitro and in vivo. Nat Neurosci. 2020;23:1496–508. doi: 10.1038/s41593-020-00730-3. [DOI] [PubMed] [Google Scholar]
- 130.Tanaka Y, Cakir B, Xiang Y, Sullivan GJ, Park IH. Synthetic analyses of single-cell transcriptomes from multiple brain organoids and fetal brain. Cell Rep. 2020;30:1682–9.e3. doi: 10.1016/j.celrep.2020.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pham MT, Pollock KM, Rose MD, Cary WA, Stewart HR, Zhou P, et al. Generation of human vascularized brain organoids. Neuroreport. 2018;29:588–93. doi: 10.1097/WNR.0000000000001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Brassard JA, Lutolf MP. Engineering stem cell self-organization to build better organoids. Cell Stem Cell. 2019;24:860–76. doi: 10.1016/j.stem.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 133.Mansour AA, Schafer ST, Gage FH. Cellular complexity in brain organoids: current progress and unsolved issues. Semin Cell Dev Biol. 2021;111:32–9. doi: 10.1016/j.semcdb.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 134.Siegenthaler JA, Ashique AM, Zarbalis K, Patterson KP, Hecht JH, Kane MA, et al. Retinoic acid from the meninges regulates cortical neuron generation. Cell. 2009;139:597–609. doi: 10.1016/j.cell.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Takebe T, Enomura M, Yoshizawa E, Kimura M, Koike H, Ueno Y, et al. Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation. Cell Stem Cell. 2015;16:556–65. doi: 10.1016/j.stem.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 136.Monzel AS, Smits LM, Hemmer K, Hachi S, Moreno EL, van Wuellen T, et al. Derivation of human midbrain-specific organoids from neuroepithelial stem cells. Stem Cell Rep. 2017;8:1144–54. doi: 10.1016/j.stemcr.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Suga H, Kadoshima T, Minaguchi M, Ohgushi M, Soen M, Nakano T, et al. Self-formation of functional adenohypophysis in three-dimensional culture. Nature. 2011;480:57–62. doi: 10.1038/nature10637. [DOI] [PubMed] [Google Scholar]
- 138.Ozone C, Suga H, Eiraku M, Kadoshima T, Yonemura S, Takata N, et al. Functional anterior pituitary generated in self-organizing culture of human embryonic stem cells. Nat Commun. 2016;7:10351. doi: 10.1038/ncomms10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Muguruma K, Nishiyama A, Kawakami H, Hashimoto K, Sasai Y. Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 2015;10:537–50. doi: 10.1016/j.celrep.2014.12.051. [DOI] [PubMed] [Google Scholar]
- 140.Meinhardt A, Eberle D, Tazaki A, Ranga A, Niesche M, Wilsch-Bräuninger M, et al. 3D reconstitution of the patterned neural tube from embryonic stem cells. Stem Cell Rep. 2014;3:987–99. doi: 10.1016/j.stemcr.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Duval N, Vaslin C, Barata TC, Frarma Y, Contremoulins V, Baudin X, et al. BMP4 patterns Smad activity and generates stereotyped cell fate organization in spinal organoids. Development. 2019;146:dev175430. doi: 10.1242/dev.175430. [DOI] [PubMed] [Google Scholar]
- 142.Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10:771–85. doi: 10.1016/j.stem.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 143.Zhong X, Gutierrez C, Xue T, Hampton C, Vergara MN, Cao LH, et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun. 2014;5:4047. doi: 10.1038/ncomms5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cowan CS, Renner M, De Gennaro M, Gross-Scherf B, Goldblum D, Hou Y, et al. Cell types of the human retina and its organoids at single-cell resolution. Cell. 2020;182:1623–40.e34. doi: 10.1016/j.cell.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kaya KD, Chen HY, Brooks MJ, Kelley RA, Shimada H, Nagashima K, et al. Transcriptome-based molecular staging of human stem cell-derived retinal organoids uncovers accelerated photoreceptor differentiation by 9-cis retinal. Mol Vis. 2019;25:663–78. [PMC free article] [PubMed] [Google Scholar]
- 146.Bershteyn M, Nowakowski TJ, Pollen AA, Di Lullo E, Nene A, Wynshaw-Boris A, et al. Human iPSC-derived cerebral organoids model cellular features of lissencephaly and reveal prolonged mitosis of outer radial glia. Cell Stem Cell. 2017;20:435–49.e4. doi: 10.1016/j.stem.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kathuria A, Lopez-Lengowski K, Vater M, McPhie D, Cohen BM, Karmacharya R. Transcriptome analysis and functional characterization of cerebral organoids in bipolar disorder. Genome Med. 2020;12:34. doi: 10.1186/s13073-020-00733-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sawada T, Chater TE, Sasagawa Y, Yoshimura M, Fujimori-Tonou N, Tanaka K, et al. Developmental excitation-inhibition imbalance underlying psychoses revealed by single-cell analyses of discordant twins-derived cerebral organoids. Mol Psychiatry. 2020;25:2695–711. doi: 10.1038/s41380-020-0844-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Steinberg DJ, Repudi S, Saleem A, Kustanovich I, Viukov S, Abudiab B, et al. Modeling genetic epileptic encephalopathies using brain organoids. EMBO Mol Med. 2021;13:e13610. doi: 10.15252/emmm.202013610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Blair JD, Hockemeyer D, Bateup HS. Genetically engineered human cortical spheroid models of tuberous sclerosis. Nat Med. 2018;24:1568–78. doi: 10.1038/s41591-018-0139-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sun AX, Yuan Q, Fukuda M, Yu W, Yan H, Lim GGY, et al. Potassium channel dysfunction in human neuronal models of Angelman syndrome. Science. 2019;366:1486–92. doi: 10.1126/science.aav5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mellios N, Feldman DA, Sheridan SD, Ip JPK, Kwok S, Amoah SK, et al. MeCP2-regulated miRNAs control early human neurogenesis through differential effects on ERK and AKT signaling. Mol Psychiatry. 2018;23:1051–65. doi: 10.1038/mp.2017.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Xiang Y, Tanaka Y, Patterson B, Hwang SM, Hysolli E, Cakir B, et al. Dysregulation of BRD4 function underlies the functional abnormalities of MeCP2 mutant neurons. Mol Cell. 2020;79:84–98.e9. doi: 10.1016/j.molcel.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gomes AR, Fernandes TG, Vaz SH, Silva TP, Bekman EP, Xapelli S, et al. Modeling Rett Syndrome with human patient-specific forebrain organoids. Front Cell Dev Biol. 2020;8:610427. doi: 10.3389/fcell.2020.610427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Trujillo CA, Adams JW, Negraes PD, Carromeu C, Tejwani L, Acab A, et al. Pharmacological reversal of synaptic and network pathology in human MECP2-KO neurons and cortical organoids. EMBO Mol Med. 2021;13:e12523. doi: 10.15252/emmm.202012523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tang XY, Xu L, Wang J, Hong Y, Wang Y, Zhu Q, et al. DSCAM/PAK1 pathway suppression reverses neurogenesis deficits in iPSC-derived cerebral organoids from patients with Down syndrome. J Clin Investig. 2021;131:e135763. doi: 10.1172/JCI135763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xu R, Brawner AT, Li S, Liu JJ, Kim H, Xue H, et al. OLIG2 drives abnormal neurodevelopmental phenotypes in human iPSC-based organoid and chimeric mouse models of Down Syndrome. Cell Stem Cell. 2019;24:908–26.e8. doi: 10.1016/j.stem.2019.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pearson G, Song C, Hohmann S, Prokhorova T, Sheldrick-Michel TM, Knöpfel T. DNA methylation profiles of GAD1 in human cerebral organoids of autism indicate disrupted epigenetic regulation during early development. Int J Mol Sci. 2022;23:9188. doi: 10.3390/ijms23169188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang Y, Chiola S, Yang G, Russell C, Armstrong CJ, Wu Y, et al. Modeling human telencephalic development and autism-associated SHANK3 deficiency using organoids generated from single neural rosettes. Nat Commun. 2022;13:5688. doi: 10.1038/s41467-022-33364-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Meng Q, Zhang W, Wang X, Jiao C, Xu S, Liu C, et al. Human forebrain organoids reveal connections between valproic acid exposure and autism risk. Transl Psychiatry. 2022;12:130. doi: 10.1038/s41398-022-01898-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Modafferi S, Zhong X, Kleensang A, Murata Y, Fagiani F, Pamies D, et al. Gene-environment interactions in developmental neurotoxicity: a case study of synergy between chlorpyrifos and CHD8 knockout in human BrainSpheres. Environ Health Perspect. 2021;129:77001. doi: 10.1289/EHP8580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Raja WK, Mungenast AE, Lin YT, Ko T, Abdurrob F, Seo J, et al. Self-organizing 3D human neural tissue derived from induced pluripotent stem cells recapitulate Alzheimer’s disease phenotypes. PLoS ONE. 2016;11:e0161969. doi: 10.1371/journal.pone.0161969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hernández D, Rooney LA, Daniszewski M, Gulluyan L, Liang HH, Cook AL, et al. Culture variabilities of human iPSC-derived cerebral organoids are a major issue for the modelling of phenotypes observed in Alzheimer’s disease. Stem Cell Rev Rep. 2022;18:718–31. doi: 10.1007/s12015-021-10147-5. [DOI] [PubMed] [Google Scholar]
- 164.Yin J, VanDongen AM. Enhanced neuronal activity and asynchronous calcium transients revealed in a 3D organoid model of Alzheimer’s disease. ACS Biomater Sci Eng. 2021;7:254–64. doi: 10.1021/acsbiomaterials.0c01583. [DOI] [PubMed] [Google Scholar]
- 165.Kuehner JN, Chen J, Bruggeman EC, Wang F, Li Y, Xu C, et al. 5-Hydroxymethylcytosine is dynamically regulated during forebrain organoid development and aberrantly altered in Alzheimer’s disease. Cell Rep. 2021;35:109042. doi: 10.1016/j.celrep.2021.109042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wulansari N, Darsono WHW, Woo HJ, Chang MY, Kim J, Bae EJ, et al. Neurodevelopmental defects and neurodegenerative phenotypes in human brain organoids carrying Parkinson’s disease-linked DNAJC6 mutations. Sci Adv. 2021;7:eabb1540. doi: 10.1126/sciadv.abb1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Seo J, Kritskiy O, Watson LA, Barker SJ, Dey D, Raja WK, et al. Inhibition of p25/Cdk5 attenuates tauopathy in mouse and iPSC models of frontotemporal dementia. J Neurosci. 2017;37:9917–24. doi: 10.1523/JNEUROSCI.0621-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Watanabe M, Buth JE, Vishlaghi N, de la Torre-Ubieta L, Taxidis J, Khakh BS, et al. Self-organized cerebral organoids with human-specific features predict effective drugs to combat Zika virus infection. Cell Rep. 2017;21:517–32. doi: 10.1016/j.celrep.2017.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ye F, Kang E, Yu C, Qian X, Jacob F, Yu C, et al. DISC1 regulates neurogenesis via modulating kinetochore attachment of Ndel1/Nde1 during mitosis. Neuron. 2017;96:1041–54.e5. doi: 10.1016/j.neuron.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Daviaud N, Chevalier C, Friedel RH, Zou H. Distinct vulnerability and resilience of human neuroprogenitor subtypes in cerebral organoid model of prenatal hypoxic injury. Front Cell Neurosci. 2019;13:336. doi: 10.3389/fncel.2019.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Klaus J, Kanton S, Kyrousi C, Ayo-Martin AC, Di Giaimo R, Riesenberg S, et al. Altered neuronal migratory trajectories in human cerebral organoids derived from individuals with neuronal heterotopia. Nat Med. 2019;25:561–8. doi: 10.1038/s41591-019-0371-0. [DOI] [PubMed] [Google Scholar]
- 172.Lane A, Jovanovic K, Shortall C, Ottaviani D, Panes AB, Schwarz N, et al. Modeling and rescue of RP2 retinitis pigmentosa using iPSC-derived retinal organoids. Stem Cell Rep. 2020;15:67–79. doi: 10.1016/j.stemcr.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bian S, Repic M, Guo Z, Kavirayani A, Burkard T, Bagley JA, et al. Author correction: Genetically engineered cerebral organoids model brain tumor formation. Nat Methods. 2018;15:748. doi: 10.1038/s41592-018-0118-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ogawa J, Pao GM, Shokhirev MN, Verma IM. Glioblastoma model using human cerebral organoids. Cell Rep. 2018;23:1220–9. doi: 10.1016/j.celrep.2018.03.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Linkous A, Balamatsias D, Snuderl M, Edwards L, Miyaguchi K, Milner T, et al. Modeling patient-derived glioblastoma with cerebral organoids. Cell Rep. 2019;26:3203–11.e5. doi: 10.1016/j.celrep.2019.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]