Abstract
Sphingolipids, which are components of cellular membranes and organ tissues, can be synthesized or degraded to modulate cellular responses according to environmental cues, and the balance among the different sphingolipids is important for directing immune responses, regardless of whether they originate, as intra- or extracellular immune events. Recent progress in multiomics-based analyses and methodological approaches has revealed that human health and diseases are closely related to the homeostasis of sphingolipid metabolism, and disease-specific alterations in sphingolipids and related enzymes can be prognostic markers of human disease progression. Accumulating human clinical data from genome-wide association studies and preclinical data from disease models provide support for the notion that sphingolipids are the missing pieces that supplement our understanding of immune responses and diseases in which the functions of the involved proteins and nucleotides have been established. In this review, we analyze sphingolipid-related enzymes and reported human diseases to understand the important roles of sphingolipid metabolism. We discuss the defects and alterations in sphingolipid metabolism in human disease, along with functional roles in immune cells. We also introduce several methodological approaches and provide summaries of research on sphingolipid modulators in this review that should be helpful in studying the roles of sphingolipids in preclinical studies for the investigation of experimental and molecular medicines.
Subject terms: Lipid signalling, Mechanisms of disease, Diagnostic markers
Immunology: Membrane lipids in immunity and disease
Advances in laboratory techniques and genetic databases are revealing the vital roles played by a large and complex family of lipids in immunity and disease. Sphingolipids, found in cell membranes, respond to environnmental cues to control cell signaling and metabolism. Initially named after the Sphinx because of their enigmatic nature, their complexity made detection difficult. Yoe-Sik Bae and co-workers at Sungkyunkwan University in Seoul, South Korea, have reviewed the discoveries made through methods such as mass spectrometry and integrated dataset analyses (‘multi-omics’). For example, sphingolipid levels in the blood are affected by gut bacteria, cellular levels can determine whether cells live or die, and changes in sphingolipid populations have been linked to several diseases. Furthermore, analyzing the sphingolipid content in blood could aid diagnosis, because damaged organs express unique patterns of sphingolipid products.
Introduction
The immune system is a complex network of white blood cells, organs, and secreted molecules that recognize allies/intruders, selectively excluding potential sources of damage and thereby protecting the host organism from dysfunctions known as diseases. The radar and tactical teams of the immune system need to act immediately and swiftly while fulfilling long-term needs with accurate preparedness against urgent or upcoming threats1. The innate and adaptive immune systems take part in these different tasks and provide fast and unspecific or accurate and tailored immune responses, respectively, gradually complementing mutual inadequacies with characteristics such as trained immunity and immunological memory1,2. During this process, each cell within inflamed tissues and recruited immune cells mark their state and communicate with signals for proper immune responses, expressed as surface checkpoint marker proteins and/or secreted cytokine profiles, while controlling their own signaling cascades according to the immune context1–3.
The plasma membrane and organelles (in the case of membrane-bound organelles in eukaryotic cells) are dense networks of lipids and proteins encasing a cell or within a cell, respectively, that insulate the inner spaces from the outer spaces for proper function. Lipids provide and maintain the lipid bilayer structure that separates specific areas and provides the appropriate circumstances for the cells to function due to their amphipathic nature stemming from the polarized structure of lipids with hydrophilic heads and hydrophobic tails and incorporated proteins4–6. There are three major lipids that comprise the structure of lipid bilayers. The most common lipid is glycerophospholipids (GPLs), although the composition of each lipid bilayer differs depending on cell type and distribution in tissues. GPLs are the main structural components of plasma membranes and are composed of diacylglycerol-linked hydrophobic fatty acid chains, which are usually unsaturated and centered in the membrane, and diverse hydrophilic head groups, which constitute the inner and outer surfaces of the membrane. Not restricted to the structural frame, GPLs can also act as storage anchors for signaling molecules and their precursors to modulate the functions of neighboring proteins such as receptors and transporters5. Sterols, a subgroup of steroids composed of fused rings with hydrocarbon tails and hydroxyl groups, which mainly exist as cholesterol in mammals, are incorporated into stacked lipid bilayers to sustain/stabilize the chemistry of the lipid bilayer and can be converted into biological molecules such as hormones5,6. Finally, sphingolipids, composed of usually long and saturated N-acyl hydrocarbon chains, a sphingoid base, and a head group, can recognize environmental cues and be degraded/synthesized according to cellular needs, thus playing crucial roles in signal transduction and modulation of cell metabolism4,5. Collectively, the compositional diversity of membrane lipids can act as complex codes or conditional cues of the cells, and these different patterns can decide/modulate biological functions through the interactions of lipids and proteins, such as internal/external signaling molecules and receptors. In this review, we shed light on recent progress in understanding the roles of membrane-derived lipids, especially sphingolipids, in the functions of immune cells and related diseases with clinically used/tried medicines.
Sphingolipids as “protean” signaling modulators
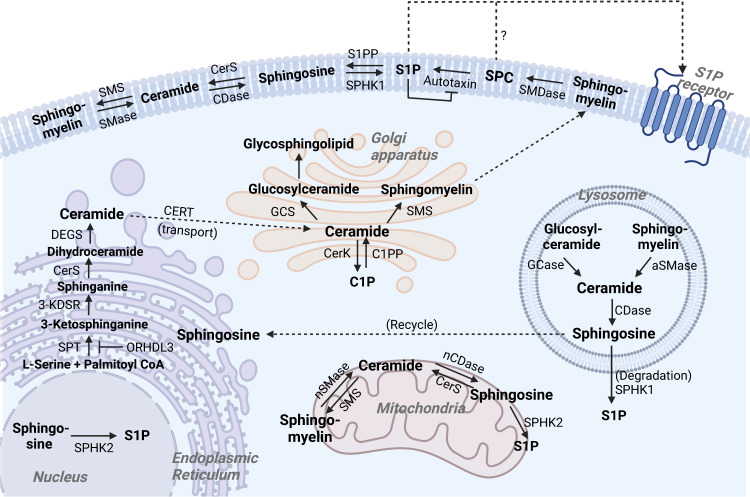
Considering the roles of sphingolipids as secondary messengers in intra- and extracellular spaces, monitoring the synthesis/breakdown of sphingolipids and sphingolipid-related enzyme activities are important clues for understanding signal transduction and the states of resting/responding cells4,7 (Fig. 1 and Table 1). Sphingomyelin, ceramides, and other lipids agglomerate to form the asymmetric but balanced lipid rafts across the cellular membrane4. Ceramides are highly concentrated and major sphingolipids in the plasma membrane and can be generated by de novo synthesis from palmitoyl-CoA and L-serine by the sequential reactions of serine palmitoyltransferase (SPT), ceramide synthase (CerS), and dihydroceramide desaturases at the cytosolic leaflet of the endoplasmic reticulum (ER). When transferred to the trans-Golgi apparatus, lysosome, and plasma membrane from the ER, ceramides can be converted to sphingomyelins by sphingomyelin synthase (encoded by the SGMS gene), and these sphingomyelins gradually increase in concentration to compose most of the sphingolipids of the plasma membrane, which respond to environmental cues as precursors of secondary signaling messengers8. Conversely, ceramides can be restored by the hydrolysis of sphingomyelins by different sphingomyelinases (SMases) in different locations4,7,8. Although ceramides themselves can act as bioactive signaling cues in a variety of physiological responses, they can be degraded into sphingosine and then transformed into sphingosine-1-phosphate (S1P) by the action of ceramidase and sphingosine kinases (SphK), respectively, or phosphorylated by ceramide kinase (CERK) to generate ceramide-1-phosphate (C1P)9–11. In a negative feedback loop, C1P can inhibit the activity of SMase and SPT, blocking the excessive accumulation of ceramides11–13. Conversely, ceramides can be recovered from C1P by C1P phosphatase and S1P and sphingosine by S1P phosphatase and CerS, referred to as the salvage pathway, diminishing the excessive production of their metabolites4,11. In an SMase-independent manner, sphingomyelin can be degraded into sphingosylphosphorylcholine (SPC) and then further transformed into S1P by the actions of sphingomyelin deacylase and autotaxin (ATX), the latter of which is an ectonucleotide pyrophosphatase (phosphodiesterase with lysophospholipase D activity), originally identified as a tumor-regulatory factor for survival and proliferation14,15. One interesting aspect of these processes is that the modulation of sphingolipid metabolism is not exclusively performed by the host. Recently, several studies have revealed and discussed that commensal or pathogenic microbiomes, especially the commensal Bacteroides, provide or hijack sphingolipids as building blocks and utilize the host machinery, thereby affecting the host’s sphingolipid content and immune responses16,17. Homeostasis among these sphingolipids, regardless of whether they originate from food, the host, or the microbiome, is important because transformed and selectively biased compositions of sphingolipids can decide the fate of cells, such as maintenance (death, survival, and proliferation), renewal, senescence, differentiation, migration/retention, immune responses, and cell metabolism, and these cells collectively make up tissues, organs, and organ systems and ultimately determine the life of the organism16–18.
Fig. 1. Synthesis and degradation of sphingolipids in cellular regions.
Sphingolipids, as components of cellular and subcellular membranes, can be synthesized by serial enzymes (de novo pathway) and can also be degraded from sphingomyelin and ceramide to smaller sphingolipids (salvage pathway) as signaling molecules according to the environmental context.
Table 1.
Molecules targeting sphingolipid metabolism and related human diseases.
| Target enzyme and protein | Gene symbol (NCBI gene ID) | Target function | Modulator(s) | Related diseases (human case studies) | |
|---|---|---|---|---|---|
| Homo sapiens | Mus musculus | ||||
| Serine palmitoyltransferase (SPT) | Convert serine + palmitoyl coenzyme A to 3-keto-dihydrosphinganine (de novo synthesis) | ||||
| SPT, long-chain base subunit 1 (SPTLC1) | SPTLC1 (10558) | Sptlc1 (268656) | Large subunit of SPT, forms the catalytic core with SPTLC2/3. | Enhancer: Fenretinide; Inhibitors: Myriocin (ISP-1), Sphingofungin B, WXP-003, SPT-IN-1, L-Cycloserine, L-Penicillamine, Imidazopyridine, Pyrazolopyridine. Lipoxamycin (G-26146). | Sensory neuropathy and motor neuron disease. Neurodegenerative disease/Alzheimer’s disease. Clear cell renal cell carcinoma. Macular telangiectasia type 2. Psoriasis. Juvenile amyotrophic lateral sclerosis. |
| SPT, long-chain base subunit 2 (SPTLC2) | SPTLC2 (9517) | Sptlc1 (20773) | Component of the large subunit of SPT as SPTLC2/3, forms the catalytic core. | Sensory neuropathy and motor neuron disease. Macular telangiectasia type 2. Congenital insensitivity to pain. Aspirin-exacerbated respiratory disease. | |
| SPT, long-chain base subunit 3 (SPTLC3) | SPTLC3 (55304) | Stplc3 (228677) | Metabolic disease. Type 2 diabetes. | ||
| SPT, small subunit A | SPTSSA (171546) | Sptssa (104725) | Either SPTSSA or SPTSSB increases the efficiency of the catalytic reaction. | – | |
| SPT, small subunit B | SPTSSB (165679) | Sptssb (66183) | – | ||
| Ormdl sphingolipid biosynthesis regulator (ORMDL) 3 | ORMDL3 (94103) | Ormdl3 (66612) | Negative regulation of de novo ceramide biosynthesis, blocking SPT activity. | – | Asthma. Atopic dermatitis. Inflammatory bowel disease (IBD, Crohn’s disease, Ulcerative colitis). Primary biliary cholangitis. Chronic obstructive pulmonary disease (COPD). Bronchiolitis. Respiratory syncytial virus infection. Aspirin-exacerbated respiratory disease. Allergic rhinitis. Ankylosing spondylitis. Atherosclerosis. |
| 3-Keto-dihydrosphinganine reductase (3-KDSR) | KDSR (2531) | Kdsr (70750) | Reduces 3-ketodihydrosphiganine to dihydrosphingosine. | – | Palmoplantar keratoderma. Thrombocytopenia. Anemia. Juvenile myelofibrosis. Erythrokeratodermia. |
| Ceramide synthase (CerS) | N-acetylate dihydrosphingosine to dihydroceramide. Each CerS prefers a different length of fatty acid chains for the sphingoid base | ||||
| CerS1 | CERS1 (10715) | Cers1 (93898) | Attaches C18 fatty acyl CoA to sphingoid base. | Inhibitors: FTY720, P053, Fumonisin B1, Australifungin. | Head and neck squamous cell carcinomas. Age-related disease. Myoclonus epilepsy. Dementia. |
| CerS2 | CERS2 (29956) | Cers2 (76893) | Located in the ER and membrane. Attaches C22-24 fatty acyl CoA to sphingoid base. Tumor metastasis suppressor. | Inhibitors: FTY720, Fumonisin B1, ST1058, ST1074, Bcl2L13, Australifungin. | Bladder cancer. Breast cancer. Tumor metastasis. Liver cancer. Ovarian cancer. Renal cancer. Rhegmatogenous retinal detachment. Multiple sclerosis (MS). |
| CerS3 | CERS3 (204219) | Cers3 (545975) | Located in ER. Attaches C26-34 fatty acyl CoA to sphingoid base. Important for epidermis to create barrier. | Inhibitors: Fumonisin B1, Australifungin. | Congenital ichthyosis. Prader-Willi syndrome. |
| CerS4 | CERS4 (79603) | Cers4 (67260) | Located in ER. Attaches C18-20 fatty acyl CoA to sphingoid base. | Inhibitors: Fumonisin B1, ST1058, ST1072, ST1074, Australifungin. | Endometrial cancer. Colon cancer. |
| CerS5 | CERS5 (91012) | Cers5 (71949) | Located in ER. Attaches C14-16 fatty acyl CoA to sphingoid base. | Inhibitor: Fumonisin B1, Australifungin. | Endometrial cancer. Colon cancer. |
| CerS6 | CERS6 (253782) | Cers6 (241447) | Located in ER. Attaches C14-16 fatty acyl CoA to sphingoid base. | Inhibitors: Fumonisin B1, Australifungin, ST1072. | Tumor metastasis. Breast cancer. Lung cancer. Ovarian cancer. Melanoma. Gastric cancer. Pancreatic cancer. Renal cancer. Leukemia. Multiple sclerosis. T2D. |
| Dihydroceramide desaturase (DEGS) | Dehydrogenate dihydroceramide to ceramide. | ||||
| DEGS1 | DEGS1 (8560) | Degs1 (13244) | Ceramide de novo synthesis. Positive regulator of apoptosis. Located in mitochondria. | Inhibitors: Fenretinide (4-HPR); XM462, GT11 (C8-cyclopropenylceramide), Celecoxib, γ-Tocotrienol. | Hypomyelinating leukodystrophy. Intellectual disability. Epilepsy. Spastic quadriplegia. Microcephaly. Scoliosis. |
| DEGS2 | DEGS2 (123099) | Degs2 (70059) | Ceramide de novo synthesis and sphinganine metabolic process. Located in the ER and membrane. | Schizophrenia. Sudden cardiac arrest. | |
| Ceramide transfer protein (CERT) | CERT1 (10087) | Cert1 (68018) |
Transfers ceramide from the ER to the Golgi in a nonvesicle manner for sphingomyelin synthesis. Located in the cytosol and mitochondria. |
Inhibitors: E16A/(1 S,2 R)-HPCB-5, HPA-12. | Goodpasture syndrome. Alport syndrome. Intellectual disability. Alzheimer’s disease. Triple-negative breast cancer. T2D. |
| Ceramide kinase (CERK) | CERK (64781) | Cerk (223753) |
Converts ceramide to ceramide-1-phosphate (C1P). Located in mitochondria. |
Inhibitor: NVP-231 | Breast cancer. Tumor metastasis. Tumor recurrence. Cancer-drug resistance. Inherited retinal dystrophy. |
| C1P Transfer protein (CPTP) | CPTP (80772) | Cptp (79554) | Transfers C1P and sphingomyelin from the trans-Golgi to the membrane. Located in the Golgi, endosomes, nucleus, and cytosol. | – | Pancreatic cancer. Tumor metastasis. |
| Glycosylceramidase (GCase) | Hydrolyzes glucosylceramide to glucose and ceramide. | ||||
| Glycosylceramidase beta (GBA) 1 | GBA1 (2629) | Gba (14466) |
Enables glucosyltransferase and hydrolase activity. Located in the ER, lysosome, trans-Golgi. |
Inhibitor: Conduritol B epoxide. | Parkinson’s disease. Gaucher disease. Dementia with Lewy bodies. Multiple system atrophy. REM-sleep behavior disorder. Atopic dermatitis. Krabbe disease. Liver cancer. Tumor metastasis. Rheumatoid arthritis. Psoriasis vulgaris. |
| GBA2 | GBA2 (57704) | Gba2 (230101) | Located in cytosol, extrinsic membrane component of the Golgi, ER. Nonlysosomal. | Inhibitor: ACT-519276 (Sinbaglustat). | Hereditary spastic paraplegia. Autosomal-recessive cerebellar ataxia. Parkinson’s disease. Charcot-Marie-Tooth disease. Senile dementia. Melanoma. Sjögren’s syndrome. |
| Glucosylceramide synthase (GCS) | UGCG (7357) | Ugcg (22234) | Located in the Golgi and membrane. Glucosyltransferase activity. Transfers glucose to ceramide. | Inhibitors: d-threo-P4, N-butyl-deoxynojirimycin, PDMP, Genz-123346, Genz-529468, T-036, T-690, CCG-203586. | Breast cancer. Tumor metastasis. Cancer-drug resistance. Thyroid cancer. Colorectal cancer. Bladder cancer. Endometriosis. Alzheimer’s disease. Type 1 Gaucher disease. |
| Four-phosphate adapter protein 2 (FAPP2) | PLEKHA8 (84725) | Plekha8 (231999) | Regulates vesicular trafficking and transfers to trans-Golgi network and membrane. | – | Hepatitis C virus. Liver cancer. Colon cancer. T-cell acute lymphoblastic leukemia. |
| Sphingomyelin synthase (SMS) | Converts ceramide to sphingomyelin | ||||
| SMS1 | SGMS1 (259230) | Sgms1 (208449) | Localized in the Golgi membrane, majorly contributes to sphingomyelin synthesis. | Inhibitors: Malabaricone C, SAPA 1j (SMS1-IN-1), D609 | Liver cancer. Endometriosis. Systemic lupus erythematosus. Alzheimer’s disease. |
| SMS2 | SGMS2 (166929) | Sgms2 (74442) | Localized in the Golgi and plasma membrane, synthesizes sphingomyelin primarily at the cell membrane. | Inhibitors: Malabaricone C, D2/Dy105, SMS2-IN-1, 15w, SMS2-IN-Ly93. | Osteoporosis. Skeletal dysplasia. Breast cancer. Tumor metastasis. Chronic obstructive pulmonary disease (COPD). |
| SMSr | SAMD8 (142891) | Samd8 (67630) | Sphingomyelin synthase (SMS)-related protein. Localized in the cytosol and ER membrane. | – | – |
| Sphingomyelinase (SMase) | Hydrolyze sphingomyelin to form ceramide | ||||
| Acid SMase (Lysosomal/secreted) | SMPD1 (6609) | Smpd1 (20597) | Lysosomal: Located in the lysosomal region and has zinc-dependent phosphodiesterase activity that produces ceramide from sphingomyelin. | Inhibitors: Ambroxol, ARC39, Fluoxetine (Prozac), Desipramine (Norpramin), Functional Inhibitors of Acid sphingomyelinase (FIASMA). | Niemann-Pick disease. Parkinson’s disease. Depressive disorder. Sepsis. Atopic dermatitis. Allergy. Alzheimer’s disease. Alcohol dependence. Hepatitis C. Nonalcoholic fatty liver disease (NAFLD). Scleroderma. Cancer-drug resistance. Multiple myeloma. Large granular lymphocyte leukemia. Lung cancer. Colorectal cancer. Respiratory syncytial virus infection. COVID-19. Rheumatoid arthritis. |
| Secreted: Located in the extracellular region. | |||||
| Neutral SMase 1 | SMPD2 (6610) | Smpd2 (20598) | Located in the membrane. Mediates the secretion of ceramide. | Inhibitor: GW4869. | Atopic dermatitis. Large granular lymphocyte leukemia. Liver cancer. |
| Neutral SMase 2 | SMPD3 (55512) | Smpd3 (58994) | Located in the Golgi, cytoplasm and plasma membrane. Mediates the exosomal secretion of ceramide. | Inhibitors: DPTIP, Cambinol, GW4869, Altenusin. | Melanoma. Tumor metastasis. Acute myeloid leukemia. Alcohol dependence. Depressive disorder. Endometriosis. Systemic lupus erythematosus. |
| Neutral SMase 3 | SMPD4 (55627) | Smpd4 (77626) |
Located in the ER, Golgi and nuclear envelope. Mediates stress-induced cellular response. |
– | Breast cancer. Lung cancer. Colon cancer. Microcephaly. Congenital arthrogryposis. Colorectal cancer. Kidney cancer. |
| Mitochondria-associated neutral SMase | SMPD5 (392275) | Smpd5 (100503915) | Located in the ER membrane and mitochondrial membrane. | – | – |
| Neutral SMase activation associated factor | NSMAF (8439) | Nsmaf (18201) | Mediates TNF and other stress-induced cellular responses. | – | Tuberculosis. |
| Alkaline SMase | ENPP7 (339221) | Enpp7 (238011) | Expressed in the intestinal mucosal region and liver but not in other tissues. Modulates the intestinal lipid metabolic process, digests dietary sphingomyelin. Exists in membrane-bound or secreted form. | – | Colon cancer. |
| Ceramidase | Cleaves ceramide to form sphingosine. | ||||
| Acid ceramidase | ASAH1 (427) | Asah1 (11886) | Alternative splicing generates alpha and glycosylated beta subunit to form lysosomal enzyme. A heterodimer of two isoforms hydrolyzes ceramide into sphingosine. | Inhibitors: Cystatin SA, ARN14988, B-13, LCL-521. | Faber disease. Myoclonic epilepsy. Muscular dystrophy. Prostate cancer. Breast cancer. Tumor metastasis. Melanoma. Radiotherapy failure. Rectal cancer. Schizophrenia. Ovarian cancer. Head and neck cancer. Acute myeloid leukemia. Ependymoma. Large granular lymphocyte leukemia. Keloid. Atherosclerosis. Obesity. |
| Neutral ceramidase | ASAH2 (56624) | Asah2 (54447) | Located in the extracellular space, mitochondria, caveolae | Inhibitors: B-13, LCL-464. | – |
| Alkaline ceramidase 1 | ACER1 (125981) | Acer1 (171168) | Highly expressed in the epidermis. Hydrolyzes very long-chain ceramides. | Inhibitor: D-erythro-MAPP. | – |
| Alkaline ceramidase 2 | ACER2 (340484) | Acer2 (230379) | Located in the Golgi membrane. Hydrolyzes very long-chain ceramides. | Breast cancer. | |
| Alkaline ceramidase 3 | ACER3 (55331) | Acer3 (66190) | Located in the ER, Golgi membrane. | Leukodystrophy. Liver cancer. Acute myeloid leukemia. Glioma. | |
| Sphingosine kinase (SphK) | ATP-dependently phosphorylates sphingosine to sphingosine-1-phosphate (S1P). | ||||
| SphK1 | SPHK1 (8877) | Sphk1 (20698) | Located in the cytosol and plasma membrane. Involved in TNF- and NF-kB-mediated inflammatory responses. | Inhibitors: Safingol, SKI-I, SKI-II, PF-543, DMS (N,N-dimethylsphingosine). | Tumor metastasis. Breast Cancer. Gastroesophageal carcinoma. Head and neck carcinoma/Oral squamous cell carcinoma. Liver cancer. Colorectal cancer. Prostate cancer. Chemo-resistance. Tumor recurrence. Thyroid cancer. Colon cancer. Alzheimer’s disease. Autoimmune Thyroiditis. Huntington’s disease. Glioblastoma. Melanoma. Large granular lymphocyte leukemia. Acute myeloid leukemia. Immune checkpoint inhibitor-mediated antitumor efficacy. Salivary gland carcinoma. Astrocytoma. Portal vein tumor thrombus. Pancreatic cancer. Gastric cancer. Colitis-associated cancer. Bladder cancer. Nasopharyngeal carcinoma. Sacral chordoma. Uterine cervical cancer. Lung cancer. Tumor angiogenesis. Hepatitis B. Liver fibrosis. Pulmonary edema. Idiopathic pulmonary fibrosis. Spondyloarthritis. Diabetic nephropathy. T2D. Insulin resistance. Niemann-Pick disease. Sickle cell disease. |
| SphK2 | SPHK2 (56848) | Sphk2 (56632) | Located in the nucleus. Several tumor cells highly express SphK2. | Inhibitors: ABC294640, SKI-II, K-145. MP-A08, ROME (R-FTY720 methyl ether). | Tumor metastasis. Liver cancer. Gastric cancer. Glioblastoma. Lung cancer. Alzheimer’s disease. Chemo-resistance. Neuroblastoma. Breast cancer. Colorectal cancer. Large granular lymphocyte leukemia. Spondyloarthritis. Osteoarthritis. Rheumatoid arthritis. Hypertrophic scar. Alcoholic cirrhosis. |
| Spinster (SPNS) 2 | SPNS2 (124976) | Spns2 (216892) | Exports S1P to the outside of the cell membrane. | Inhibitor: SLF1081851. | Colorectal cancer. Oral squamous cell carcinoma. Lung cancer. Liver fibrosis. Chronic obstructive pulmonary disease. |
| S1P receptor (S1PR) | G protein-coupled receptor that recognize S1P and SPC. Regulation of biological processes. | ||||
| S1PR1 | S1PR1 (1901) | S1pr1 (13609) | Binds S1P with high affinity. Gαi/Gαo-, Gα12/Gα13-, and Gαq-coupled. Involved in vascular system development and maintenance. Modulates immune cell distribution and function | Modulators: FTY720 (Fingolimod), KRP-203, Syl930, Siponimod, Ponesimod, Ozanimod, AUY954, SEW2871, RP-101075, SAR247799, CYM5442, Amiselimod, Syl930, CS-0777, W061; Antagonists: VPC23019, W146, VPC44116, CL2 (chemical lead 2). | Lymphoma. Liver cancer. Tumor metastasis. Multiple sclerosis. Glioblastoma. Bladder cancer. Breast Cancer. Gastric cancer. Endometriosis. Lung cancer. Prostate cancer. Gallbladder cancer. Colorectal cancer. Urothelial carcinoma. Esophageal squamous cell carcinoma. Cancer-drug resistance. Graves’ orbitopathy. Autoimmune thyroiditis. Liver fibrosis. Asthma. Atherosclerosis. Sepsis. |
| S1PR2 | S1PR2 (9294) | S1pr2 (14739) | Gs-, Gq/G11-, and G12/G13-coupled. Negative regulation of S1PR1-mediated signaling. | Agonist: CYM5478; Antagonist: JTE-013, AB1. | Hearing impairment (Deafness). Lymphoma. Colorectal cancer. Liver fibrosis. Bladder cancer. Cholangiocarcinoma. Glioblastoma. Oral squamous cell carcinoma. Multiple sclerosis. T2D. Abdominal aortic aneurysms. Endometriosis. |
| S1PR3 | S1PR3 (1903) | S1pr3 (13610) | Gi/Go-, Gq/G11-, G12/G13-coupled | Modulator: FTY720; Antagonists: VPC23019, KRX-725-II, CAY10444, TY52156, VPC44116. | Breast Cancer. Lung cancer. Tumor metastasis. Glioblastoma. Ependymoma. Nasopharyngeal carcinoma. Renal cancer. Bladder cancer. Acute respiratory distress syndrome. Pulmonary edema. Acute lung injury. Sepsis. Multiple sclerosis. Liver fibrosis. Endometriosis. |
| S1PR4 | S1PR4 (8698) | S1pr4 (13611) | Gi/Go-, G12/G13-coupled | Modulators: Phyto-S1P, VPC23153, FTY720, KRP-203, W061, Amiselimod; Antagonists: JTE-013, CYM50367, | Breast cancer. Obstructive coronary artery disease. |
| S1PR5 | S1PR5 (53637) | S1pr5 (94226) | Gi/Go-, G12/G13-coupled | Modulators: FTY720, Siponimod, Ozanimod, W061, Amiselimod. | Large granular lymphocyte leukemia. Glioblastoma. Chronic obstructive pulmonary disease. Multiple sclerosis. |
| Sphingosine-1-phosphate phosphatase (SPP) | Dephosphorylates S1P to sphingosine | ||||
| SPP 1 | SGPP1 (81537) | Sgpp1 (20750) |
Salvage and recycling pathway for ceramide. Located in the ER and membrane. Modulates apoptotic signaling. |
– | Endometriosis. Gastric cancer. Colorectal cancer. Tumor metastasis. Schizophrenia. |
| SPP 2 | SGPP2 (130367) | Sgpp2 (433323) | Located in the ER membrane. Related to the inflammatory response. | – | Psoriasis. Endometriosis. |
| Sphingosine-1-phosphate lyase (SPL) 1 | SGPL1 (8879) | Sgpl1 (20397) |
Irreversibly degrades S1P to phosphoethanolamine and hexadecanal. Decreases the level of S1P |
Inhibitors: Tetrahydroxy-butylimidazole (THI), LX2931, LX3305. | Adrenal insufficiency. Nephrotic syndrome. Ichthyosis. Alzheimer’s disease. Huntington’s disease. Charcot-Marie-tooth neuropathy. Oral squamous cell carcinoma. Pituitary adenoma. Colorectal cancer. Colon cancer. Prostate cancer. Gastric cancer. Gastroesophageal cancer. Liver cancer. Alveolar rhabdomyosarcoma. Preeclampsia. Endometriosis. Cryptorchidism. |
| Sphingomyelin deacylase | – | – | Converts sphingomyelin to sphingosylphosphorylcholine (SPC). | – | Atopic dermatitis. |
| Autotaxin (ATX), ENPP2 | ENPP2 (5168) | Enpp2 (18606) | Converts lysophosphatidic acid (LPC) and sphingosylphosphorylcholine (SPC) to lysophosphatidic acid (LPA) and S1P, respectively. Increased in an inflammatory response. | Inhibitors: PF-8380, BBB-877, ONO-8430506, CRT0273750, HA-130, HA-155, GLPG1690, S-32826. | Breast cancer. Hepatitis C. Liver cancer. Liver cirrhosis. Primary sclerosing cholangitis. Insulin resistance. Obesity. Melanoma. Preeclampsia. Pregnancy-induced hypertension. Intrahepatic cholestasis of pregnancy. Biliary atresia. Alzheimer’s disease. Systemic sclerosis. Pancreatic cancer. Renal cancer. Thyroid cancer. Tumor metastasis. Type 1 endometrial cancer. Hematological malignancy. High-grade serous carcinoma. Prostate cancer. Esophageal cancer. Colorectal cancer. Glioblastoma. Bladder cancer. Hodgkin lymphoma. Ovarian cancer. Tumor angiogenesis. Cancer-drug resistance. Epstein‒Barr virus infection. Threatened preterm delivery. Asthma. COVID-19. Idiopathic pulmonary fibrosis. Blister. Mild cognitive impairment. Crohn’s disease. Ulcerative colitis. Posner–Schlossman syndrome. Acute myocardial infarction. Nonalcoholic fatty liver disease. Hepatitis B. Cholestasis. Cholestatic pruritus. Primary biliary cholangitis. Type 1 autoimmune pancreatitis. |
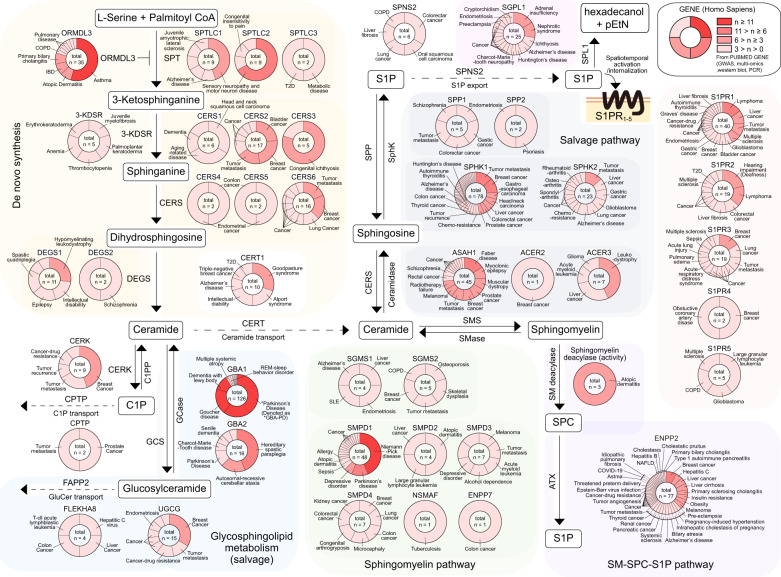
Sphingolipid-modulating enzymes and their NCBI gene ID number with their function, modulators, and reported human diseases (Analysis of disease: ~2022. Dec). Related human diseases are referenced from the NCBI GENE database (Bibliography-all citations in PubMed)21 with statistically significantly different data from GWAS, multiomics, Western blot, and PCR analysis (bold, reported articles (cases) ≥3). See also Fig. 2.
Reciprocal interference and modulation among sphingolipids are crucial for the immune context because the misguided “appearance” (migration, activation, and clonal expansion) or “exit” (ignorance, senescence, anergy, exhaustion, apoptosis, or transmigration) of immune players in the inflamed tissue stage is closely related to excessive immune responses or immune paralysis4,16,19,20. Likewise, numerous research studies that investigated human disease with genome-wide association studies and multiomics analyses of disease tissue compared to healthy control tissue (from the PubMed Gene database21) revealed that disrupted sphingolipids and their related enzymes are closely related to hereditary neuronal diseases and life-threatening diseases such as tumors and autoimmune disorders (Table 1 and Fig. 2). These results imply the importance of sphingolipid homeostasis in human health. Investigation of sphingolipids has focused on the fate of cells, namely, survival, proliferation, maintenance, or death, with altered balances among sphingolipids, and these metabolic reactions of sphingolipids are interconvertible and reciprocally communicated9–15. Ceramides and S1P are the most studied sphingolipids with respect to the functions of immune cells. Extrinsic signaling cues such as tumor necrosis factor-α (TNF-α) and lipopolysaccharide (LPS) and/or intrinsic modulators such as cytosolic kinases and transcriptional modulators (e.g., Foxp3, a master transcription factor of regulatory T (Treg) cells, can transcriptionally inhibit the expression of Sgms1, which converts ceramides to sphingomyelins) can cause ceramide accumulation, and the increased ceramide levels can selectively turn on/off intracellular machinery such as protein phosphatase 2 (PP2A), nuclear factor-κB (NF-κB), and mitogen-activated protein kinases (MAPKs)4,22–24. Moreover, enrichment of the intracellular content of ceramides can control the trafficking rate of proteins to the Golgi complex and membrane, which may cause cellular senescence25,26. Calcium-dependent activation of CERK can convert ceramide to C1P, and C1P can be secreted into extracellular regions as an auto/paracrine signaling cue or can directly act as an intracellular signaling messenger. While ceramide blocks the PI3K-Akt-mTOR pathway with PP2A activation to modulate cellular functions such as proliferation and survival, C1P enhances PI3K-mediated signaling, cell proliferation, and survival. Moreover, C1P can bind and activate cytosolic phospholipase A2, which in turn stimulates the production of arachidonic acid-derived prostaglandins (PGs), and these PGs modulate pro-/anti-inflammatory immune responses in a context-dependent manner4,25,27. The opposite roles of ceramide and S1P are also well characterized in cell death/survival and immune responses by modulation of bcl-2 and bcl-2-like protein (Bax) and the subsequent intracellular machinery for programmed cell death28, and these sphingolipid-related enzymes are closely related to the pathogenesis and development of tumors (Table 1 and Fig. 2). One significant and interesting factor in the treatment of cancer is that tumor cells can also modulate sphingolipid metabolism with ceramidase, SphK and ATX to favor their survival and maintenance while blocking the patient’s immune system29,30. The functional roles of S1P and its receptor (S1PR) in extracellular and intracellular responses have been extensively investigated, and S1PRs are closely related to human diseases such as cancer4,30 (Table 1). Understanding the kinetics of sphingolipids and related enzymes may provide insight into the current state of immune cells, and by selectively modulating these molecules (Figs. 1 and 2 and Table 1), we can develop patient-specific medicine suitable for the specific disease status. Fingolimod (FTY720, Gilenya) and its phosphorylated form (phosphorylated by SphK, p-FTY720) are clinically used as treatments to target S1PR1,3-5, but not S1PR2, for the treatment of autoimmune diseases such as multiple sclerosis (MS) by sequestering lymphocytes in lymph nodes and therefore attenuating the migration of inflammatory lymphocytes into inflamed sites31. Likewise, the modulation of sphingolipids and their related enzymes has been studied in the clinic32,33, which will be discussed in more detail in the following sections.
Fig. 2. Sphingolipid-related enzymes and reported human disease.
Alteration of sphingolipids is closely related to human disease. The numbers of reported human studies that analyzed genome-wide association studies, multiomics-based data, Western blot, and PCR comparison of disease tissue to control tissue were counted as visualized (PubMed Gene). The counted numbers are subdivided by color to facilitate understanding of the roles of sphingolipid-related enzymes in human disease.
Sphingolipids as prognostic and diagnostic indicators of disease
Several human cohort studies of diabetes, tumors, autoimmune, neuroinflammatory, and degenerative diseases, among other diseases, have revealed that alterations in sphingolipid metabolites in the blood can be detected several years before/during disease development, and these abnormalities of sphingolipid metabolism and sphingolipid-related enzymes in tissue may be closely related to the progression and pathogenesis of disease34 (Table 1 and Fig. 2). Glucosylceramidase beta 1 (GBA1) is a representative example that has been reported to be the etiological factor for Parkinson’s disease (PD), which results from heterozygous mutation of this gene, now categorized as GBA-PD35. Enhanced S1P and C1P-producing enzymes (CERK, SphK, and ATX) and activation of S1PRs in tumor tissues are closely related to poor prognostic factors that result in tumor metastasis and recurrence while attenuating chemo- and antitumor therapies, deteriorating the survival of tumor patients36–39 (Fig. 2 and Table 1). Analyses of open databases collected from a patient-derived genome atlas can also facilitate the prediction of a relationship between alterations in sphingolipid metabolism and disease progression as a prognostic approach for diseases such as tumorigenesis25,40, Alzheimer’s disease41, systemic lupus erythematosus (SLE)42, and metabolic diseases43. Prediction of disease progression in the clinic with a few drops of blood and/or minimalized biopsy followed by pretreatment with prospective patient-specific medicines may be goals for unmet medical needs to improve our medical welfare (Fig. 3). To understand the alterations in sphingolipid metabolism and related enzymes in immune responses and disease, several methodological approaches are now available for the detection/digitization and visualization of sphingolipid contents, as well as during the progression of de novo synthesis/degradation44–46. Classically, after extraction of lipids from cells and tissues with high-performance liquid chromatography (or thin-layer chromatography), electron ionization of molecules followed by tandem mass spectrometry-based sphingolipidomic analysis can provide structural and quantitative measurement of sphingolipids regarding molecular weight (mass-to-charge ratio, m/z), specific information on the structure (length of N-acyl chains, bases, and composition of head groups), and retention times. Although these techniques cannot distinguish molecules that have geometrical and optical isomers or produce similar ion products and require appropriate stable-isotype controls, the approaches present very sensitive and precise information on the molecules, including quality/purity and quantity. Therefore, researchers can determine the current composition of tissue, which has now been adapted for imaging (mass spectrometry imaging)46,47. Analysis of brain tissue from MS patients with phospho-proteomics has revealed distinct phosphorylated protein patterns and has uncovered the uncontrolled S1P-S1PR1-mediated TH17-triggered immune response that causes enhanced neuroinflammation48. Likewise, analysis of mass spectrometry-based techniques has provided links between dysregulated sphingolipid contents and defects in sphingolipid-related enzymes in the pathogeneses of pulmonary disease49, inflammatory bowel disease17, cancer30,50, and other diseases (Table 1), proving the important roles of sphingolipids in human health and disease. Analysis of the in silico retention time by a quantitative structure retention-relationship approach, comparison with a database from a mass spectral library, and/or analytic programs can facilitate the identification of sphingolipids and the interpretation of data from a target sample51. Recently, Muralidharan et al. isolated and investigated the composition of 114 different sphingolipids from 21 different murine tissues and plasma using targeted lipidomics52. According to the results, only 11 sphingolipid species are common to all tissues, which may contribute to the fundamental and essential functions of tissues, such as cell cycle regulation, while each tissue shows its own distinct distribution of sphingolipids and shows similar patterns among functionally close tissues. Since each tissue has its own specific pattern/composition of hydrocarbons in sphingolipids, analysis of blood can reveal the status of an organ that is expected to be injured or inflamed. Skin, stomach, and intestines have a tendency to have very long-chain fatty acids (C26:0, saturated 26 hydrocarbons), which may relate to barrier function with thicker membranes. Principal component analysis of tissue sphingolipids revealed that immune-related organs such as the spleen, thymus, and lymph nodes are more closely clustered, with a closer cluster between the spleen and thymus, followed by the lymph nodes, compared to the intestine, stomach, lung, brain, and adipose tissue52. However, brown adipose tissue is more similar to the lymph node, thymus, and spleen.
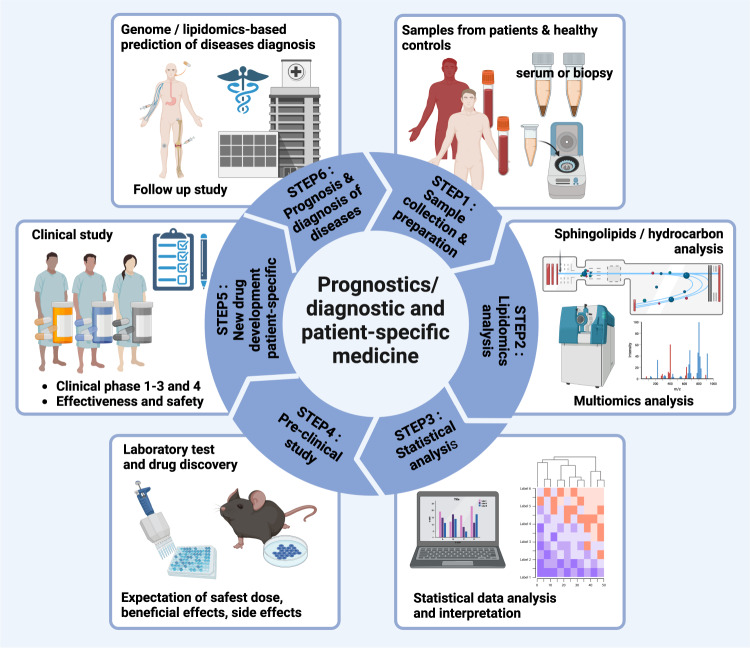
Fig. 3. Strategy for diagnosis/prognosis of diseases with sphingolipids.
Recent progress in metabolomics and multiomics analysis has enabled the investigation of sphingolipids as a marker of disease. After collection of human blood and disease tissue by biopsy, a series of procedures with mass spectrometry enable the detection of changes in sphingolipids and related molecules compared to levels in healthy volunteers. By analysis of a multiomics database, specific sphingolipids and related enzymes can be detected for human disease. Inhibition of sphingolipid enzymes in a preclinical disease model should enable investigation of the roles of sphingolipids and related enzymes.
Phylogenetically, the majority of bacterial species cannot produce sphingolipids, but Bacteroides, one of the most predominant commensal microbiomes in the intestine, can produce and provide a source of ceramides with both odd (C17:0) and even (C18:0) numbers of hydrocarbons, identical to mammalian ceramides, attenuating inflammatory responses and contributing to homeostasis of the intestinal immune system, while host-derived sphingolipids from the intestine aid the maintenance of Bacteroides species or function to control their abundance with bactericidal sphingosine17,18,53. In contrast, uncontrolled growth of pathogenic bacteria such as Pseudomonas, Staphylococcus, or Mycobacterium can disrupt the balance of the bacterial flora, and these pathogens hijack and impair host sphingolipid metabolism53. Interestingly, the spleen contains S1P with an odd number of sphingoid bases C17:1 (17-carbon with 1-unsaturated hydrocarbon chain), which usually cannot originate from mammals but can be produced by the microbiome, along with the plasma, skin, small intestine (but not the large intestine), and lungs of normal C57BL/6 mice, implying that the microbiome can affect and shape the systemic immune response52,53. Likewise, uptake of Bacteroides-derived sphingolipids (C17:0) can affect the host cell’s de novo synthesis of sphingolipids (C18:0), showing an increased frequency of odd-numbered S1P and ceramide production compared to even-numbered S1P and ceramide18. Similarly, in humans, the majority of fatty acid hydrocarbons are even-numbered, and less than 1% of fatty acids are odd-numbered in plasma. It has been reported that increased C15 and C17 are closely related to insulin resistance, and these are now accepted as biomarkers for type 2 diabetes54. The results obtained from studies that map the lipid distribution of tissues complement the tissue-specific sphingolipid atlas. Analysis of genomic approaches such as bulk mRNA and single-cell RNA sequencing analyzing lipid-related enzymes and previous reports and databases has suggested another approach for understanding tissue-specific immune (and/or nonimmune) responses in diseases. For example, mass spectrometry followed by network analysis of tumor cells after chemotherapy treatment revealed that chemotherapy of tumor cells or administration of medicine can alter sphingolipid-related enzymes such as SMase, CERK, and SphK and subsequently alter cellular sphingolipids, which may help decipher the malignancy of tumors and drug resistance38,55–57. Although these results from inflamed whole tissues may explain the unresolved aspects of the conventional immune response, information on sphingolipids from specific immune cells in normal and disease conditions, classified by the involvement of immune organs such as the spleen, thymus, lymph nodes, and bone marrow, should be investigated and discussed in more detail.
The development of traceable fluorescent molecules enables us to chase and investigate the de novo synthesis, hydrolysis, and transfer of sphingolipids with equipment for fluorescence microscopy, flow cytometry, and mass spectrometry. Incorporation of azide-functionalized, or other photoswitchable, sphingolipids followed by application of biorthogonal click chemistry, which is selective for the azide-/alkyne-modified region, with BODIPY usually conjugated as fluorescence, can reveal the alterations in the sphingolipid content and visualize the location of tagged sphingolipids58–60. Super-resolution microscopes such as expansion microscopes or atomic force microscopes can provide an alternative approach for sphingolipid imaging60,61. Quantification of sphingolipid-related mRNA and protein expression under certain conditions and subsequent gain-/loss-of-function study of a specific gene after biopsy can further strengthen the understanding of the roles of sphingolipids in normal/disease states. Techniques developed for analyzing sphingolipid metabolism, antibodies that detect sphingolipid-related enzymes and receptors, and sphingolipid-related transgenic or knockout (KO) mice in preclinical studies can be utilized as diagnostics for disease and may lead to the development of new drugs for clinical studies. Collectively, investigation of sphingolipid metabolism may supplement our understanding of the immune system in normal homeostatic and disease states, which previously could not be fully explained by nucleotides and proteins alone.
Sphingolipids as warning molecules and modulators of the innate immune response
Deviation from mutual competition or alliance of the microbiome with the host defense system leads to the direction/flow of ceramide metabolism, and the leading entity that possesses control of the host machinery determines the context of the immune response17,53. The phylum Bacteroides, containing members such as Bacteroides, Parabacteroides, Prevotella, and Porphyromonas, which compose the majority of the human gut microbiome, can process food-derived metabolites and supply a source of sphingolipids to epithelial cells, which can eventually be absorbed by the hepatic portal vein and the circulatory system18,62. With these absorbed sphingolipids, commensal bacteria can affect and govern the host metabolic system and immune response. The most differentially abundant metabolites in the stool of patients who suffer from inflammatory bowel disease, such as ulcerative colitis and Crohn’s disease, are host-produced ceramides, sphingomyelins, and sphingosines (C18:1), while sphingolipid-producing bacteria and Bacteroides-derived sphingolipids are significantly decreased. The study of SPT-sufficient or SPT-deficient Bacteroides thetaiotaomicron-adopted mice revealed the crucial role of commensal-derived sphingolipids in intestinal immune homeostasis17. Although commensal bacteria provide a source of ceramides and attenuate excessive intestinal inflammation, by crossing the barrier and subsequently activating the innate/adaptive immune system, the excessive “Renaissance” of commensal bacteria can also trigger activation of the host immune system, especially humoral immunity, with the production of antibodies and sphingosines that counteract potential sources of danger, ultimately preventing the disruption of the microflora and preparing a healthy host defense system17,63,64. In contrast, bacterial pathogens try to neutralize the host defense system and then bind to host epithelial cells and/or secrete bacterial molecules (e.g., toxins) that stimulate the accumulation of ceramides and block the production of sphingosines, facilitating and promoting bacterial colonization53,64,65. Although epithelial cells of the mucosal barrier constitute the front line of physical defense and are coated with mucins, defensins, IFN-λ, and antibodies IgA and IgM66–68, when the defense wall is damaged, epithelial cells request the reinforcement from innate/adaptive immune cells with chemokines and sphingolipid S1P69. The functional roles of S1PRs have been investigated by analyzing the spatiotemporal distribution and internalization of receptors in response to sphingolipids69,70. Here, we briefly review the recent understanding of immune cells from the aspect of sphingolipid metabolism (Fig. 4).
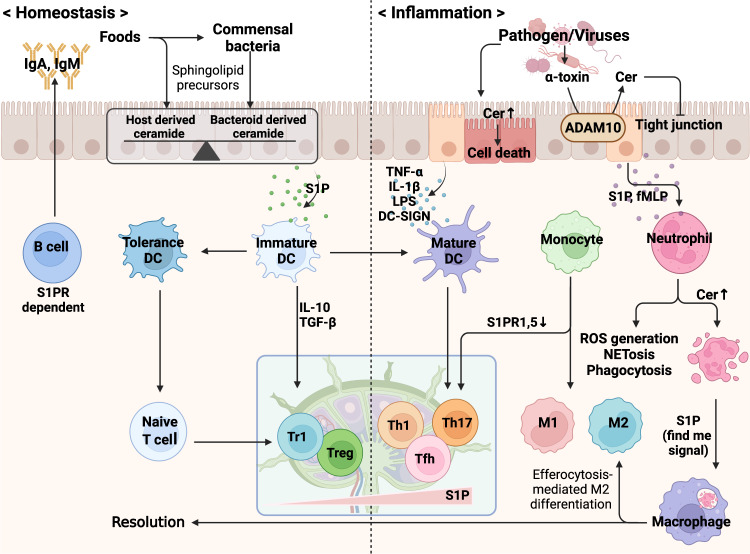
Fig. 4. Homeostasis and immunological functions of sphingolipids.
Sphingolipids can be derived from the host and food metabolites and can be modulated by interactions with commensal bacteria, maintaining immune tolerance. Infection with pathogenic bacteria can block and hijack host sphingolipid metabolism, activating immune cascades. Pathogens can stimulate epithelial cell death and enhance the contents of ceramides that facilitate the breakdown of the barrier. Innate immune cells such as neutrophils and macrophages detect inflammatory cues and migrate into inflamed sites. Increased intracellular and extracellular sphingolipids guide the direction of the immune response, and pathogens can attenuate the host immune response, while antigen-presenting cells activate the adaptive immune system. S1P, as a ‘find me’ signal from apoptotic cells, induces resolution with an efferocytic process.
Neutrophils, the most abundant and crucial defenders of the innate immune system, patrol the bloodstream, and when they detect danger signals with pattern recognition receptors, they migrate into inflamed sites and do their jobs in a context-dependent manner19. The decision for eternal sleep (apoptosis) or not (NETosis, necrosis, and ferroptosis) after their role is complete is crucial because a neutrophil corpse that is left behind can act as a “dying message” to other immune cells that can follow the intention of the deceased cell. Not surprisingly, from the cradle (granulopoiesis) to the grave, sphingolipids can tremendously affect a neutrophil’s short life19. When human CD34+ hematopoietic stem/progenitor cells are activated in vitro, the TNF-α-triggered neutral SMase (nSMase)-ceramide axis can shape the hematopoietic system to favor myelopoiesis but not erythropoiesis, while S1P can restore erythroid differentiation, switching the direction of GATA, PU.1, and the autophagy-related machinery71. S1P and fMLP secreted from inflamed epithelial cells and tissues can attract the migration of neutrophils, and the intracellular ceramide content of neutrophils can be increased when TNF-α and fMLP are encountered72,73. Increased ceramide can modulate the function of neutrophils in terms of cell migration, the generation of reactive oxygen species, neutrophil extracellular trap formation, and bactericidal activities74–77, and increased sphingomyelin in neutrophils may be related to neutrophil infiltration and phagocytic activity78. Likewise, CerS2,6 modulates the migration of neutrophils, and CerS6 KO exacerbates the development of experimental autoimmune encephalomyelitis (EAE) with enhanced infiltration of neutrophils, while CERS2-deficient mice showed delayed development of EAE with decreased chemokine receptors (CCR1, CXCR1, and CXCR2)79,80, implying that the length of the carbon chain attached to the sphingoid base may differentially affect neutrophil function. One of the most interesting aspects of neutrophil-mediated phagocytosis is that this engulfment can increase the expression of SMase and subsequent ceramide production, and administration of exogenous ceramide can inhibit neutrophil functions such as phagocytosis and degranulation, while C1P can enhance phagocytosis77,81,82. Upon exposure to immune contexts such as fMLP, SphK activity can be increased in neutrophils, and the S1P that is subsequently produced can enhance/sustain neutrophil function in an autocrine or paracrine manner. Likewise, SphK1 deficiency in neutrophils results in diminished pathogen-killing activity, and administration of exogenous S1P rescues the function of SphK KO neutrophils. The accumulation of ceramide (de novo, C16- and C24-ceramide) can turn on programmed cell death in neutrophils83, and the apoptotic body can initiate resolution with the efferocytosis process, educating immune-suppressive innate/adaptive cells19. However, exposure to S1P, as well as IL-8 and HMGB1, can induce the expression of anti-apoptotic Bcl-x in neutrophils, and prolonged exposure to these inflammatory cues can induce other forms of neutrophil death, such as necrosis or ferroptosis, followed by “frustrated” efferocytosis, which are closely related to chronic inflammatory diseases19.
During/after immune responses, neutrophils can request reinforcement from other immune cells, such as monocytes, macrophages, and dendritic cells (DCs), to inflamed tissues19. The spatiotemporal action/activation of ceramide, C1P, S1P-S1PR, and related surface receptors such as CD69 can guide the distribution of these innate immune cells and orchestrate the overall immune response, while these cells can be a source of sphingolipids such as S1P4,16,73,84. During immune responses, exposure to TNF-α activates CERK/SphK and stimulates the phosphorylation of ceramide/sphingosine, resulting in the production of the proinflammatory cytokines IL-1β and CCL285,86. During the progression of murine MS, activated inflammatory monocytes (CD11b+CCR2+Ly6ChighLy6Glow) can migrate to and temporally reside in lymph nodes with expression of CD69, which retains inflammatory monocytes in the lymph node by internalization of S1PR1,5, and these monocytes prime and educate lymphocytes by supplying S1P and inflammatory cytokines for the development of TH17 and follicular helper T cells84. The function and phenotype of monocytes are heterogeneous and plastic in immune responses, and monocytes can differentiate into proinflammatory (M1) or immune-regulatory (M2) phenotype macrophages or DCs, which have distinct roles87. S1P-S1PR1 mediates the trafficking and migration of monocytes and macrophages88, and S1P can block the apoptosis of macrophages via PI3K-Akt signaling while skewing phenotype switching toward M289. S1PR2 can oppose S1PR1-mediated migration and differentiation of macrophages, with S1P-S1PR2 altering macrophage phenotypes to M1 and suppressing phagocytosis but not bactericidal capacity89–92. TNF-α activates CERK and stimulates C1P production, which can modulate the induction of a proinflammatory phenotype in macrophages, and secreted C1P directly inhibits TNF-α converting enzyme (TACE/ADAM17, the active form of which resides within the cholesterol-rich membrane region) and therefore calms down the LPS-induced production of mature TNF-α, cutting off the excessive cycle of inflammation85,93. One of the main immunological roles of monocytes and macrophages is “to eat”, i.e., phagocytosis, which is the clearance of pathogens, foreign molecules, cellular debris, and apoptotic bodies94. The synthesis of sphingolipids, but not glycosphingolipids, is required for the phagocytosis of specific pathogens such as Mycobacterium tuberculosis, thereby modulating actin dynamics and the formation of the phagocytic cup during engulfment and internalization95. Analysis of phagocytosis with quantitative lipidomics during the process of phagosomal maturation revealed that early phagosomes have enhanced activity of neutral ceramidase compared to CerS under neutral (pH 7.0) conditions, resulting in decreased ceramide levels. However, ceramides and glucosylceramides with long hydrocarbon chains (16:0, 18:0, 20:0, 22:0, and 24:0 but not 12:0 and 14:0) are gradually enriched on mature phagosomes of Raw264.7 cells, a murine macrophage cell line, with enhanced glucosylceramide synthase (GCS) activity, while sphingosines and the expression/activity of CerS2 are decreased96. During phagosomal maturation, the levels of sphingomyelin and C1P are comparable in early and late phagosomes, but activation of CERK and C1P can facilitate Fc receptor-mediated phagocytosis, collectively implying the delicate roles of sphingolipids in macrophage function82,96. Although some bacteria and hosts have evolved to live together by symbiosis, other bacteria called pathogens have evolved to exploit and hijack host metabolites for their own purposes53,62. The sphingolipid metabolism pathway is not exempt from being hijacked, and some pathogens cause the disruption of host sphingolipid metabolism to neutralize and counteract the host defense system53,62. Different pathogens utilize different strategies; fungi synthesize their own sphingolipids and specific enzymes for synthesis and can use their own and host sphingolipids. Most pathogenic bacteria and viruses act as “parasites” of host sphingolipid metabolism, thereby disturbing the host defense system16,53,65. Neisseria gonorrhoeae, the major cause of meningitis and septicemia, Pseudomonas aeruginosa, which is an opportunistic pathogen and the cause of cystic fibrosis and sepsis, and Staphylococcus aureus can stimulate the acid SMase (aSMase) of epithelial cells and fibroblasts, facilitating invasion through a sphingomyelin-decreased and ceramide-enriched platform97–99 and evading the innate immune defense system100–102. The enrichment of ceramides in host cells can induce cell death and thereby destroy the barriers formed by cells. Even worse, several kinds of toxins produced by pathogens (e.g., the α-toxin produced by S. aureus) can bind to surface metalloproteinases (e.g., ADAM10) of the host and induce the release of acid ceramidase-mediated ceramide outside of the inflamed walls, which eventually leads to degradation of tight junctions99. Moreover, the produced chemokines and S1P recruit innate/adaptive immune cells, and recruited macrophages can wisely utilize the SMase/ceramidase strategy of the pathogen to rearrange its cytoskeleton, facilitating the process of phagocytosis96,103.
A series of fierce battles end with dead immune and tissue cells, and upon completing their duties, some immune cells, such as neutrophils, accept their programmed death, apoptosis, in the immune context to herald the end of the war and a new era of regeneration19,104. Phagocytes can trace CX3CL1, ATP, LPC, and S1P as “find me” signals from apoptotic bodies, and apoptotic body-derived S1P can turn on the anti-apoptotic system of macrophages and stimulate the production of anti-inflammatory IL-10 to decrease inflammatory responses104. Macrophages check the state of the target to determine whether it has exposed phosphatidylserine on the cell surface to distinguish dead targets from live targets, which express CD47, CD31, and CD24 as “don’t eat-me” signals on the outer membrane. During the process of efferocytosis, phagocytes recognize phosphatidylserine with surface receptors such as TIM1, 4 and LDL-receptor-related protein 1, and other receptors such as MERTK and scavenger receptor (CD36) can facilitate the uptake of dead cells and debris104. CD36 is a major scavenger receptor for oxidized LDL (oxLDL), and in turn, oxLDL enhances CD36 expression by lipid peroxidation with peroxisome proliferator-activated receptor (PPAR)-γ, and oxLDL can also maintain macrophage survival and enhance proliferation105,106. The PPARγ agonists thiazolidinediones can upregulate S1PR1107, and silencing of aSMase can enhance the transcription of PPARγ-coactivator-1 α (PGC1-α)108, which can repress foam cell formation and atherosclerosis109. On the other hand, soluble SMase secreted by macrophages and endothelial cells and the subsequently increased ceramide levels are closely related to aggregated atherosclerotic lesions, and ceramides can reduce CD36 expression and thereby decrease oxLDL uptake by monocytes and macrophages110,111. The administration of bacterial SMase to macrophages can cause intracellular trapping of CD36, resulting in a significant reduction in CD36 on monocytes and macrophages110. Increased ceramide levels via de novo ceramide synthesis can inhibit the efferocytic function of resident alveolar macrophages112, while de novo synthesis of ceramides for inflammasome-mediated inflammation is dispensable113.
During phagocytosis, macrophages can load some lysosomal particles to the major histocompatibility complex (MHC) and present it as a molecular pattern to lymphocytes, which recognize and initiate the adaptive immune response. Furthermore, macrophages do not present efferocytic particles, if not infected, on the MHC and thereby context-dependently turn on/off the link to the adaptive immune response19. DC is another type of potent and professional antigen-presenting cell that directs/maintains the immune context-dependent activation and differentiation of lymphocytes114. Before exposure to pathogens, DCs reside in peripheral tissue in the immature state, waiting for their designation. S1P can promote the migration of human immature DCs but not LPS-stimulated mature DCs115, and selective recruitment of immature DCs to inflamed peripheral tissues by S1P implies that S1P favors the role of immature DCs in peripheral tissues in certain immune contexts114. Immature DCs can migrate into inflamed sites and then capture and process antigens, thus proceeding to their irreversible maturation process26,114. Inflammatory cues such as TNF-α, IL-1β, and LPS and ligation of DC-SIGN and CD40 can stimulate SMase, subsequently leading to the accumulation of intracellular ceramides during DC differentiation, and ceramides are required for pathogen uptake and phagosome formation26,116. Moreover, increased intracellular ceramide interferes with vesicle trafficking of newly synthesized or phagocytosed MHC epitopes to be loaded on the MHC, therefore limiting the variation and loading of presented epitopes26,117. During maturation, DCs reduce their capacity for antigen uptake and processing while fully optimizing their antigen presentation ability with enhanced expression of MHC and costimulatory surface molecules such as CD80/86, CD83, CD40, and chemokine receptor CCR7 (which leads cells to T-cell zones of secondary lymphoid organs according to gradients of CCL19/21 through lymphatic vessels), and once migrated to the T-cell zones of secondary lymphoid organs, mature DCs efficiently transmit the processed molecules on MHC for epitope-specific lymphocytes to prime/initiate the adaptive immune response114,118. Programmed cell death, or apoptosis, of DCs is a physiologically required phenomenon for the elimination of end-stage matured cells. Endogenous ceramides can suppress Akt- and NF-κB-mediated signaling as well as Bcl-x when limited by extracellular cues such as an absence of serum and endogenous and exogenous S1P119–121. Inhibition or deficiency of ceramidase or SphK can sensitize DCs to ceramide-induced cell death, and tumor cells such as B16 melanoma release C16/24 ceramides and stimulate ceramide-induced apoptosis of DCs, attenuating DC-mediated lymphocyte activation120–122. Collectively, the results imply that the extracellular and/or intracellular contents of sphingolipids and their plasticity in innate immune cells are closely related to the direction of current and subsequent immune responses. Recently, analysis of plasma metabolomics has shown that after anti-tuberculosis BCG vaccination, the levels of sphingolipids such as S1P, sphingomyelin, N-acylsphingosine, and glucosylceramide are significantly changed, and the trained immunity response may be closely associated with these altered sphingolipid metabolites123, implicating the involvement of sphingolipids in trained immunity.
Functional roles of sphingolipids in lymphocytes
The functional roles of sphingolipids, especially ceramides and S1P, have been well investigated in terms of T-lymphocyte distribution and activation69,118. The differentiation of each lymphocyte in inflammatory circumstances and in homeostasis are also well characterized with regard to the understanding of immune disease pathogenesis7,118. The functional roles of S1P have been characterized with its receptors S1PR1-5, and S1P-S1PR regulate many aspects of immune cell function, such as the migration and maintenance of lymphocytes. S1PRs are differentially expressed in lymphocytes; S1PR1 and S1PR4 are mainly expressed by T cells, while B cells express S1PR1-4, but S1PR3 is not expressed in human B cells. S1PRs are not necessarily expressed simultaneously and are differentially expressed during diverse stages of cell activation and maturation7,69,118. The best studied S1PR is S1PR1, with a special focus on lymphocyte migration out of the thymus and secondary lymphoid organs into the blood and inflamed sites, with counteraction of the chemokine receptor CCR7. Spontaneous activation of S1PR1 in the lymph node and spleen is required to sustain and maintain naive lymphocytes in normal homeostasis, supplying necessary substances for lymphocytes such as IL-2124. S1PR1 is the representative receptor that modulates immune cell trafficking and development, stimulating the PI3K-Akt-mTOR and Stat3 pathways, while S1PR2 can counteract S1PR1-mediated activities118,125. Sphingolipid-mediated responses are not stereotypically fixed in one direction of cellular function. For example, by signaling through the differentially expressed S1PRs that can be internalized and/or altered by extracellular and intracellular signaling, on immune cells such as immune-activator conventional T (Tconv) and Treg cells, S1P can selectively and properly modulate the migration, proliferation, differentiation, and pro-/anti-inflammatory function of immune cells via different S1PRs according to environmental cues. Notably, excessive S1PR1 blocks Treg development and function via the Akt/mTOR-mediated pathway and modulates Treg distribution in the body by selective activation with CCR7, directing time-dependent modulation of early-time immune activation of Tconv cells followed by the immune-regulatory response of Tregs and affecting development of resident memory T cells that migrate to the lymph nodes126,127. Likewise, excessive S1PR1 activation, which activates the PI3K-Akt- and p-Stat3-mediated TH1 and TH17 responses, and enhanced expression of S1PR1 have been reported in autoimmune patients with MS48,128. S1P can enhance TNF-induced expression of the receptor activator of nuclear factor kB ligand and in turn aggravate the pathogenesis of inflammatory bone disease129,130. Due to its similar structure to other sphingolipids, especially S1P, the signaling pathways of SPC have been investigated with regard to S1PRs, and the study of the roles of SPC has focused on vascular/cardiovascular disease and tumors131. Studies of fingolimod, a novel functional antagonist for S1PR1,3-5, have revealed and supported important roles of S1P-mediated signaling in the modulation of immune disease. In an unphosphorylated or phosphorylated form mediated by SphK, fingolimod can impair the ability of lymphocytes and induce the internalization and terminal degradation of S1PR1, a crucial receptor for lymphocyte maintenance, proliferation, and function as well as distribution in the host body132. Ozanimod is a newly FDA-approved medicine for MS that selectively antagonizes S1PR1,5, and several S1PR modulators have been developed for the selective inhibition of S1PR133. Likewise, modulation of sphingolipids and their related enzymes has been studied in the clinic. Inhibition of tumor sphingolipid metabolism with fingolimod or fenretinide facilitates the apoptosis of tumors while blocking the cell cycle and survival of cancer cells134. Although SPC and S1P can share the same S1PRs, SPC and S1P oppositely regulate the activity and expression of ATX (Enpp2), which is positioned between SPC and S1P in the SPC-ATX-S1P axis, and as such, the choice of SPC and S1P in S1PR signaling may be another modulator of cellular responses similar to LPC and LPA14,15,131. In a normal immune system response, S1PRs are differentially internalized or enhanced with context-dependent activation of lymphocytes, according to environmental cues. Ceramide is another important player in lymphocyte activation. Of note, T-cell receptor (TCR)-induced ceramides can serve as a negative regulator of TCR signaling and can modulate the activation, survival, and proliferation of lymphocytes with IL-2 secretion and the distribution and signaling of TCR as nanoclusters135–137. Likewise, coreceptor CD28 engagement can enhance SMase-mediated ceramide generation with PI3K-Akt-mTOR signaling that promotes lymphocyte activation and proliferation138 and modulates the transport of IL-2139, collectively implying the crucial roles of sphingolipids in mediating TCR signaling. Likewise, functional roles of SMase in TH1, TH17, and Treg cells were reported140. With an increase in SMase activity and increased ceramide production, ceramides enhance TH1/TH17 differentiation via phosphorylation of JNK and PI3K-Akt-mTOR140,141, while CD28-mediated activation of SMase acts as a negative regulator of Treg function and differentiation, dampening the induction and stability of the Treg-specific transcription factor Foxp3142. However, intermediate TCR stimulation triggered by an adequate amount of antigen can induce FOXP3 expression, and Tregs contain high levels of ceramides for PP2A activity that modulate IL-2Rβ signaling pathways and sustain Treg stability by trapping its modulatory protein SET22,143, implying that the proper level of ceramides and the modulation of related intracellular enzymes and molecules are important for lymphocyte fate. Differentially expressed PP2A in Treg and Tconv cells (with high expression of SET in the resting state) results in the high sensitivity to IL-2 of Tregs, which cannot express IL-2, and therefore facilitates prior activation of Tregs compared to NKT, CD8, and CD4 Tconv144.
Sphingolipids in cytotoxic lymphocytes are now being looked at with interest to understand the modulation of immune responses against viruses or tumors145,146. Encountering a virus can trigger activation of SphK1/2, which modulates clonal activation of CD8 T cells to control viral clearance and persistence. Although SphK1 and SphK2 share the same enzymatic function of S1P generation, SphK1 mainly localizes to the cytosol and plasma membrane, while SphK2 resides in the nucleus and cytoplasm. In an interesting viral response, viruses hijack host SphK1/2 to replicate themselves while suppressing host defense systems145. Inhibition of SphK restores and enhances the function of CD8 and CD4 T cells, but SphK deficiency results in excessive lymphocyte numbers and ultimately host death145. Likewise, tumor cells activate SphK and ATX to modulate sphingolipid metabolism, favoring an immunosuppressive response in the tumor microenvironment29,146,147. Cancer cells utilize the sphingolipid contents to relieve hypoxia- and energy-induced stresses and enhance their survival, promoting autophagy, proliferation, and migration and triggering angiogenesis. Likewise, the functions of ceramide nanoliposomes as antitumor agents are being investigated for the treatment of cancer32,148. Collectively, these results imply that sphingolipids can act as immune context molecules presented by viruses and tumors to deceive cytotoxic lymphocytes, and altered sphingolipid metabolism is required for evasion of the host immune system, resulting in exhaustion and anergy of CD8 T cells.
S1PR1 and S1PR2 regulate B cells in the germinal center reaction and development of memory B cells and plasma cells149,150. Aged S1pr2-deficient mice develop diffuse large B-cell lymphoma with increased germinal center B cells and spontaneous germinal center formation. Under homeostatic conditions, S1PR2 antagonizes the activation of Akt and prosurvival signals, which can be activated by other S1PRs, while S1PR1 directs the exit of follicular B cells from the marginal zone to the blood151. Collectively, competition to control sphingolipid metabolism among the host, commensal bacteria, and pathogens can decide the direction of the immune response, and therefore, understanding the alterations in sphingolipid signaling in the host immune system and subsequent effective treatment of medicine can facilitate the prevention and treatment of human disease.
Disrupted homeostasis of sphingolipids in disease
As discussed above, abnormalities in sphingolipid metabolism and sphingolipid-related enzymes are closely related to disease progression (Fig. 2), and these alterations in sphingolipid-mediated signaling in immune players can contribute to the pathogenesis of disease. Considering the crucial roles of sphingolipids in the fate of immune and nonimmune cells as well as inflammagen-like pathogens and tumors, understanding sphingolipid dynamics in the disease-specific immune response may be helpful for controlling human disease. Here, we briefly discuss some studies of how altered sphingolipid metabolism worsens health problems.
Cancer
Sphingolipids and sphingolipid-related enzymes are strongly related to the development and malignancy of human cancer, and ceramide and S1P have been implicated in tumor immunology30,134. With enhanced SphK and ATX and downregulated S1P lyase in tumors, S1P can promote Stat3- and Akt-mediated tumor cell growth with upregulation of Bcl-2/Bcl-xL while resisting p53-mediated apoptosis and stimulating a vicious cycle of tumorigenesis152–156. Likewise, downregulation of CerS promotes the development of tumors with prolonged inflammation and dysregulated ER stress157,158, and the acidic tumor microenvironment can activate aSMase and induce metalloproteinase-9, which promotes metastasis and immune evasion of tumors159,160. Modulation of nSMase2 can enhance the antitumor response to anti-PD-1 therapy161, but tumor-infiltrating Tregs can reduce the expression of endothelial nSMase2, preventing lymphocyte migration;162 moreover, enriched S1P and LPA in the tumor microenvironment can reprogram the antitumor activity of infiltrated lymphocytes29,163. The results suggest that targeted sphingolipid modulation in tumor patients can provide a strategy for cancer therapy and can promote the survival of tumor patients151.
Inflammatory disease
Alterations in ceramide and C1P levels can tune the direction of the inflammatory cascade by modulating the TNF-α-mediated response164. Deficiency of CerS2 enhances the activity of TACE, thereby worsening LPS-mediated septic shock165, while CERK modulates the production of TNF-α by inhibiting TACE93,166. Likewise, Cerk-deficient mice showed an impaired immune response to Streptococcus pneumoniae with neutropenia, and acid ceramidase loss in myeloid cells attenuated the intestinal recruitment of neutrophils167,168. Increased activity of SMase is related to host cell death and organ failure in severe septic patients169, and aSMase can paralyze the immune response against Pseudomonas aeruginosa, inducing macrophage apoptosis with redox signaling100. TNF-α can induce aSMase and subsequent Bid-mediated caspase-3/-9 activation, which can induce cellular apoptosis;170 however, aSMase is required for early host defense against Listeria monocytogenes171 and modulates LPS-palmitic acid-amplified inflammatory signaling in macrophages172. Inhibition of SphK1 attenuates the symptoms of polymicrobial sepsis173, and deficiency of SGPL1 enhances the proinflammatory response while impairing neutrophil trafficking174, implying the differential roles of sphingolipids in the host defense system. Modulation of sphingolipid-related enzymes has also been implicated in intestinal immune homeostasis. Deficiency of CerS6 or CERK aggravates the pathogenesis of experimental colitis175,176, and deficiency of alkaline SMase also enhances dextran sulfate sodium-induced colitis in mice with ATX upregulation, the last of which can enhance the TH17 and B-cell response in the colon177–179.
Autoimmune disease
FTY720, an FDA-approved S1PR modulator for treating MS, provides proof that the selective modulation of sphingolipid metabolism and its signaling pathway can cure patients with autoimmune disease31. Although the differences in the carbon chains in sphingolipids such as CerS2 and CerS6 may affect the progression of autoimmune disease through neutrophil trafficking79,80, targeting sphingolipid-related enzymes and sphingolipid receptors is a good strategy for the treatment of autoimmune disease4. Knockdown of Cerk ameliorates MS-like behavior and cuprizone-induced demyelination180, and genetic deletion of ATX in CD11b+ myeloid cells and deficiency of aSMase attenuated the severity of EAE181,182. In the progression of SLE, SMPD1 can enhance BCR signaling in B lymphocytes183, and the dysfunction of SMPD3 can enhance the inflammatory response of macrophages and B lymphocytes184. Pharmacological inhibition of aSMase reduces joint swelling and the production of inflammatory cytokines in antigen-induced arthritis185, and increased ATX expression in synovial fibroblasts mediates the pathogenesis of autoimmune arthritis186. Likewise, CerS and SMase modulate the lymphocyte allogenic response and impact graft-versus-host disease187,188, collectively implying that the inhibition of CERK, SMase, or ATX can be good targets against autoimmune disease.
Neuronal disease
The most extensively investigated sphingolipid in neuronal disease is Parkinson’s disease- and Goucher disease-associated GBA135. GBA1 modulates the ratio of α-synuclein tetramer-monomer, preventing the accumulation of lipid-rich aggregates and preserving subsequent motor and cognitive function189. Haplodeficiency or homozygous deficiency of GBA1 can impair cellular bioenergetics associated with mitochondrial dysfunction and defects in mitophagy190. TH1- and TH17-mediated immune responses are increased, and complement-derived glucosylceramide accumulates in tissue191,192. It has been reported that aSMase-deficient mice show a broad range of abnormalities in the central nervous system193, and aSMase can modulate autophagic processes in Alzheimer’s disease by modulating lysosomal biogenesis194. Neuronal SphK1 can acetylate COX2195, which can metabolically produce PGs and mediate the reciprocal activation of amyloid-β and IL-1β in Alzheimer’s inflamed sites196, and neuronal-specific deficiency of S1P lyase can aggravate the calcium-dependent hyperphosphorylation of Tau protein and elevate abnormal histone3/4 acetylation pathogenesis and abnormal histone acetylation197. The S1P transporter Spns2 is also closely related to LPS- and amyloid-β-induced neuronal inflammation198, and the inhibition of SMS1 can be a good approach to controlling Alzheimer’s disease by promoting lysosomal degradation of BACE1199. From the early stage of Huntington’s disease, biopsies of patients show increased SGPL1 and decreased SphK1 in postmortem brain tissue200, and SphK1 stimulation can exert neuroprotective effects in a mouse model of Huntington’s disease201.
Respiratory disease
Impaired sphingolipid synthesis and/or increased ceramide can induce airway hyperreactivity and allergic response202,203. ORMDL3, as a negative regulator of de novo sphingolipid biosynthesis, attenuates antigen- and FcεRI-stimulated mast cell activation by modulating autophagy activation204,205, and CD4-specific CerS2 null mice are protected from ovalbumin-induced asthma206. Likewise, an accumulation of ceramide can cause pulmonary inflammation and mediate lung fibrosis207, and deficiency of aSMase and sufficiency of acid ceramidase can attenuate lung inflammation and fibrosis208,209, suggesting that this may be a therapeutic target against respiratory diseases such as chronic obstructive pulmonary disease, asthma, and idiopathic pulmonary fibrosis.
Metabolic disorder
Ceramide and ceramide derivatives are emerging modulators of metabolic fitness. Hypothalamic neurons of the central nervous system can regulate body weight and energy homeostasis by interacting with the leptin receptor by modulating the expression of GCS210. Ceramide is reported to induce vascular dysfunction in diet-induced obesity by dephosphorylation of the eNOS/Akt/Hsp90 signaling complex with PP2A, and inhibition of ceramide synthesis can enhance the insulin response211,212. Likewise, inhibition of ORMDL3 impairs adipocyte thermogenesis and induces insulin resistance213, and CERT is involved in muscle insulin resistance214, while knockdown of Cerk can improve glucose intolerance by attenuating MCP-1/CCR2-mediated inflammation215. Alterations in sphingolipid metabolism also modulate the fate of adipocytes and hepatocytes. Defective expression of DEGS1 impairs adipocyte differentiation, and ATX can suppress brown adipose differentiation while enhancing the expression of adipose tissue and impairing the insulin response in diet-induced obesity216–218.
Abnormal accumulation of unfolded protein, which induces ER stress, can upregulate de novo sphingolipid synthesis, and ceramide can induce hepatic insulin resistance by suppressing PPARγ2219,220. Hepatocyte-specific CerS2 deficiency enhances insulin sensitivity and attenuates diet-induced hepatic steatosis221, and overexpression of aSMase in the liver can improve hepatic glucose and lipid metabolism through activation of Akt, GSK3, and AMPK222, suggesting the crucial roles of sphingolipids in the metabolic response. The functional role of sphingolipids is also implicated in metabolic liver disease. The expression and activity of SphK1 are significantly increased in fibrotic livers compared to normal livers, and SphK1 can promote liver fibrosis by modulating collagen deposition and α-SMA223. Notably, hepatocyte-secreted ATX can aggravate nonalcoholic fatty liver disease by autocrine inhibition of the PPARα/FGF21 axis224. On the other hand, inhibition of aSMase can prevent the progression of the early stage of nonalcoholic steatohepatitis225, collectively suggesting that sphingolipid-related enzymes can be therapeutic targets against metabolic disease.
Concluding remarks
Studies on the functional roles of sphingolipids and alterations in sphingolipid contents have been limited due to the limited techniques for the detection of sphingolipids and methods for extraction. Recent progress in lipidomics of the plasma and organ tissue has revealed that changes in sphingolipid metabolites and related small molecules are closely related to human diseases (Table 1 and Fig. 2). This finding implies that analysis of sphingolipid patterns with a few drops of blood and tissue biopsy can be used as diagnostic and prognostic tools (Fig. 3). Each organ that performs its normal functions is composed of distinct sphingolipid hydrocarbons, suggesting that increased peaks of unique sphingolipid hydrocarbons52 imply a damaged organ or inflamed tissue, facilitating the diagnosis of human disease. Homeostasis among subsets of sphingolipids, such as ceramides, sphingosines, S1P, C1P, and SPC, is important for the maintenance, progression, and regulation of the immune response7,118. We analyzed reported sphingolipid-related human diseases from the PubMed Gene database in Table 1 and visualized the information with Fig. 2 to facilitate understanding of human diseases from the aspect of sphingolipid metabolism. The small counts of numbers in Fig. 2 among the diagrams imply that further extensive human research is required for the diagnosis of human disease. Therefore, we hope that future extensive studies on sphingolipid-mediated signaling in the immune system will result in an improvement in human health.
Acknowledgements
This study was supported by the Basic Science Research Program Planning through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (NRF-2021R1A2C3011228, NRF-2020M3A9D3038435, and NRF-2017R1A5A1014560) and by the Korea Drug Development Fund funded by the Ministry of Science and ICT, Ministry of Trade, Industry, and Energy, and Ministry of Health and Welfare (HN22C0255, Republic of Korea). The figures were created with BioRender.com.
Author contributions
Conceptualization: M.L. and Y.B.; writing–original draft preparation: M.L., S.Y.L., and Y.B.; review and editing: Y.B. and M.L.; visualization (figures): M.L. and S.Y.L.; visualization (tables): M.L.; project administration and funding acquisition: Y.B.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marshall JS, Warrington R, Watson W, Kim HL. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018;14:49. doi: 10.1186/s13223-018-0278-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ochando J, Mulder WJM, Madsen JC, Netea MG, Duivenvoorden R. Trained immunity—basic concepts and contributions to immunopathology. Nat. Rev. Nephrol. 2023;19:23–37. doi: 10.1038/s41581-022-00633-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp. Mol. Med. 2018;50:1–11. doi: 10.1038/s12276-018-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hannun YA, Obeid LM. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018;19:175–191. doi: 10.1038/nrm.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merrill AH., Jr Sphingolipid and glycosphingolipid metabolic pathways in the era of sphingolipidomics. Chem. Rev. 2011;111:6387–6422. doi: 10.1021/cr2002917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo J, Yang H, Song B-L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020;21:225–245. doi: 10.1038/s41580-019-0190-7. [DOI] [PubMed] [Google Scholar]
- 7.Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510:58–67. doi: 10.1038/nature13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milhas D, Clarke CJ, Hannun YA. Sphingomyelin metabolism at the plasma membrane: implications for bioactive sphingolipids. FEBS Lett. 2010;584:1887–1894. doi: 10.1016/j.febslet.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bornancin F. Ceramide kinase: the first decade. Cell. Signal. 2011;23:999–1008. doi: 10.1016/j.cellsig.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Diaz Escarcega R, McCullough LD, Tsvetkov AS. The functional role of sphingosine kinase 2. Front. Mol. Biosci. 2021;8:683767. doi: 10.3389/fmolb.2021.683767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hait NC, Maiti A. The role of sphingosine-1-phosphate and ceramide-1-phosphate in inflammation and cancer. Mediators Inflamm. 2017;2017:4806541. doi: 10.1155/2017/4806541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gómez-Muñoz A, Kong JY, Salh B, Steinbrecher UP. Ceramide-1-phosphate blocks apoptosis through inhibition of acid sphingomyelinase in macrophages. J. Lipid Res. 2004;45:99–105. doi: 10.1194/jlr.M300158-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Granado MH, Gangoiti P, Ouro A, Arana L, Gómez-Muñoz A. Ceramide 1-phosphate inhibits serine palmitoyltransferase and blocks apoptosis in alveolar macrophages. Biochim. Biophys. Acta. 2009;1791:263–272. doi: 10.1016/j.bbalip.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 14.Clair T, et al. Autotaxin hydrolyzes sphingosylphosphorylcholine to produce the regulator of migration, sphingosine-1-phosphate. Cancer Res. 2003;63:5446–5453. [PubMed] [Google Scholar]
- 15.van Meeteren LA, et al. Inhibition of autotaxin by lysophosphatidic acid and sphingosine 1-phosphate. J. Biol. Chem. 2005;280:21155–21161. doi: 10.1074/jbc.M413183200. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, et al. Functions of sphingolipids in pathogenesis during host–pathogen interactions. Front. Microbiol. 2021;12:701041. doi: 10.3389/fmicb.2021.701041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown EM, et al. Bacteroides-derived sphingolipids are critical for maintaining intestinal homeostasis and symbiosis. Cell Host Microbe. 2019;25:668–680.e667. doi: 10.1016/j.chom.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson EL, et al. Sphingolipids produced by gut bacteria enter host metabolic pathways impacting ceramide levels. Nat. Commun. 2020;11:2471. doi: 10.1038/s41467-020-16274-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee M, Lee SY, Bae YS. Emerging roles of neutrophils in immune homeostasis. BMB Rep. 2022;55:473–480. doi: 10.5483/BMBRep.2022.55.10.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ElTanbouly MA, Noelle RJ. Rethinking peripheral T cell tolerance: checkpoints across a T cell’s journey. Nat. Rev. Immunol. 2021;21:257–267. doi: 10.1038/s41577-020-00454-2. [DOI] [PubMed] [Google Scholar]
- 21.Brown GR, et al. Gene: a gene-centered information resource at NCBI. Nucleic Acids Res. 2015;43:D36–D42. doi: 10.1093/nar/gku1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Apostolidis SA, et al. Phosphatase PP2A is requisite for the function of regulatory T cells. Nat. Immunol. 2016;17:556–564. doi: 10.1038/ni.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacKichan ML, DeFranco AL. Role of ceramide in lipopolysaccharide (LPS)-induced signaling. LPS increases ceramide rather than acting as a structural homolog. J. Biol. Chem. 1999;274:1767–1775. doi: 10.1074/jbc.274.3.1767. [DOI] [PubMed] [Google Scholar]
- 24.Chen CL, et al. Ceramide induces p38 MAPK and JNK activation through a mechanism involving a thioredoxin-interacting protein-mediated pathway. Blood. 2008;111:4365–4374. doi: 10.1182/blood-2007-08-106336. [DOI] [PubMed] [Google Scholar]
- 25.Trayssac M, Hannun YA, Obeid LM. Role of sphingolipids in senescence: implication in aging and age-related diseases. J. Clin. Invest. 2018;128:2702–2712. doi: 10.1172/JCI97949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sallusto F, Nicolò C, De Maria R, Corinti S, Testi R. Ceramide inhibits antigen uptake and presentation by dendritic cells. J. Exp. Med. 1996;184:2411–2416. doi: 10.1084/jem.184.6.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoeferlin LA, Wijesinghe DS, Chalfant CE. The role of ceramide-1-phosphate in biological functions. Handb. Exp. Pharmacol. 2013;215:153–166. doi: 10.1007/978-3-7091-1368-4_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueda N. Ceramide-induced apoptosis in renal tubular cells: a role of mitochondria and sphingosine-1-phoshate. Int. J. Mol. Sci. 2015;16:5076–5124. doi: 10.3390/ijms16035076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matas-Rico E, et al. Autotaxin impedes anti-tumor immunity by suppressing chemotaxis and tumor infiltration of CD8(+) T cells. Cell Rep. 2021;37:110013. doi: 10.1016/j.celrep.2021.110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogretmen B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer. 2018;18:33–50. doi: 10.1038/nrc.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barry B, Erwin AA, Stevens J, Tornatore C. Fingolimod rebound: a review of the clinical experience and management considerations. Neurol. Ther. 2019;8:241–250. doi: 10.1007/s40120-019-00160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw J, et al. Novel sphingolipid-based cancer therapeutics in the personalized medicine era. Adv. Cancer Res. 2018;140:327–366. doi: 10.1016/bs.acr.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canals D, Perry DM, Jenkins RW, Hannun YA. Drug targeting of sphingolipid metabolism: sphingomyelinases and ceramidases. Br. J. Pharm. 2011;163:694–712. doi: 10.1111/j.1476-5381.2011.01279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wigger L, et al. Plasma dihydroceramides are diabetes susceptibility biomarker candidates in mice and humans. Cell Rep. 2017;18:2269–2279. doi: 10.1016/j.celrep.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 35.Do J, McKinney C, Sharma P, Sidransky E. Glucocerebrosidase and its relevance to Parkinson disease. Mol. Neurodegener. 2019;14:36. doi: 10.1186/s13024-019-0336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gril B, et al. Reactive astrocytic S1P3 signaling modulates the blood-tumor barrier in brain metastases. Nat. Commun. 2018;9:2705. doi: 10.1038/s41467-018-05030-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song S, et al. S1PR1 predicts patient survival and promotes chemotherapy drug resistance in gastric cancer cells through STAT3 constitutive activation. EBioMedicine. 2018;37:168–176. doi: 10.1016/j.ebiom.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matula K, et al. Regulation of cellular sphingosine-1-phosphate by sphingosine kinase 1 and sphingosine-1-phopshate lyase determines chemotherapy resistance in gastroesophageal cancer. BMC Cancer. 2015;15:762. doi: 10.1186/s12885-015-1718-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang S, Liang Y, Chang W, Hu B, Zhang Y. Triple negative breast cancer depends on sphingosine kinase 1 (SphK1)/sphingosine-1-phosphate (S1P)/sphingosine 1-phosphate receptor 3 (S1PR3)/notch signaling for metastasis. Med. Sci. Moni. Basic Res. 2018;24:1912–1923. doi: 10.12659/MSM.905833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Y, Liu B, Chen Y, Xing Y, Zhang Y. Multi-Omics prognostic signatures based on lipid metabolism for colorectal cancer. Front. Cell Dev. Biol. 2022;9:811957. doi: 10.3389/fcell.2021.811957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baloni P, et al. Multi-Omic analyses characterize the ceramide/sphingomyelin pathway as a therapeutic target in Alzheimer’s disease. Commun. Biol. 2022;5:1074. doi: 10.1038/s42003-022-04011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang X, et al. Multi-platform omics analysis reveals molecular signatures for pathogenesis and activity of systemic lupus erythematosus. Front. Immunol. 2022;13:833699. doi: 10.3389/fimmu.2022.833699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu C, Jia W. Multi-omics profiling: the way toward precision medicine in metabolic diseases. J. Mol. Cell Biol. 2021;13:576–593. doi: 10.1093/jmcb/mjab051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson CH, Ivanisevic J, Siuzdak G. Metabolomics: beyond biomarkers and towards mechanisms. Nat. Rev. Mol. Cell Biol. 2016;17:451–459. doi: 10.1038/nrm.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aderemi AV, Ayeleso AO, Oyedapo OO, Mukwevho E. Metabolomics: a scoping review of its role as a tool for disease biomarker discovery in selected non-communicable diseases. Metabolites. 2021;11:418. doi: 10.3390/metabo11070418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wigger D, Gulbins E, Kleuser B, Schumacher F. Monitoring the sphingolipid de novo synthesis by stable-isotope labeling and liquid chromatography-mass spectrometry. Front. Cell Dev. Biol. 2019;7:210. doi: 10.3389/fcell.2019.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scherer M, Leuthäuser-Jaschinski K, Ecker J, Schmitz G, Liebisch G. A rapid and quantitative LC-MS/MS method to profile sphingolipids. J. Lipid Res. 2010;51:2001–2011. doi: 10.1194/jlr.D005322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garris CS, et al. Defective sphingosine 1-phosphate receptor 1 (S1P1) phosphorylation exacerbates TH17-mediated autoimmune neuroinflammation. Nat. Immunol. 2013;14:1166–1172. doi: 10.1038/ni.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ghidoni R, Caretti A, Signorelli P. Role of sphingolipids in the pathobiology of lung inflammation. Mediators Inflamm. 2015;2015:487508. doi: 10.1155/2015/487508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nagahashi M, et al. High levels of sphingolipids in human breast cancer. J. Surg. Res. 2016;204:435–444. doi: 10.1016/j.jss.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsugawa H, et al. Comprehensive identification of sphingolipid species by in silico retention time and tandem mass spectral library. J. Cheminformatics. 2017;9:19. doi: 10.1186/s13321-017-0205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muralidharan S, et al. A reference map of sphingolipids in murine tissues. Cell Rep. 2021;35:109250. doi: 10.1016/j.celrep.2021.109250. [DOI] [PubMed] [Google Scholar]
- 53.Wang J, et al. Functions of sphingolipids in pathogenesis during host-pathogen interactions. Front. Microbiol. 2021;12:701041. doi: 10.3389/fmicb.2021.701041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang L, et al. Circulating saturated fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Nutrients. 2019;11:998. doi: 10.3390/nu11050998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu S, et al. Ceramide kinase mediates intrinsic resistance and inferior response to chemotherapy in triple‐negative breast cancer by upregulating Ras/ERK and PI3K/Akt pathways. Cancer Cell Int. 2021;21:42. doi: 10.1186/s12935-020-01735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhadwal P, Randhawa V, Vaiphei K, Dahiya D, Agnihotri N. Clinical relevance of CERK and SPHK1 in breast cancer and their association with metastasis and drug resistance. Sci. Rep. 2022;12:18239. doi: 10.1038/s41598-022-20976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jacobi J, et al. Targeting acid sphingomyelinase with anti-angiogenic chemotherapy. Cell. Signal. 2017;29:52–61. doi: 10.1016/j.cellsig.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garrido M, Abad JL, Fabriàs G, Casas J, Delgado A. Azide-tagged sphingolipids: new tools for metabolic flux analysis. Chembiochem. 2015;16:641–650. doi: 10.1002/cbic.201402649. [DOI] [PubMed] [Google Scholar]
- 59.Fink J, et al. Azidosphinganine enables metabolic labeling and detection of sphingolipid de novo synthesis. Org. Biomol. Chem. 2021;19:2203–2212. doi: 10.1039/D0OB02592E. [DOI] [PubMed] [Google Scholar]
- 60.Götz R, et al. Nanoscale imaging of bacterial infections by sphingolipid expansion microscopy. Nat. Commun. 2020;11:6173. doi: 10.1038/s41467-020-19897-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang W, Chan YH, Kwon S, Tandukar J, Gao R. Nanoscale fluorescence imaging of biological ultrastructure via molecular anchoring and physical expansion. Nano Converg. 2022;9:30. doi: 10.1186/s40580-022-00318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rohrhofer J, Zwirzitz B, Selberherr E, Untersmayr E. The impact of dietary sphingolipids on intestinal microbiota and gastrointestinal immune homeostasis. Front. Immunol. 2021;12:635704. doi: 10.3389/fimmu.2021.635704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Verhaegh R, Becker KA, Edwards MJ, Gulbins E. Sphingosine kills bacteria by binding to cardiolipin. J. Biol. Chem. 2020;295:7686–7696. doi: 10.1074/jbc.RA119.012325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu Y, Liu Y, Gulbins E, Grassmé H. The anti-infectious role of sphingosine in microbial diseases. Cells. 2021;10:1105. doi: 10.3390/cells10051105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chiricozzi E, et al. Sphingolipids role in the regulation of inflammatory response: from leukocyte biology to bacterial infection. J. Leukoc. Biol. 2018;103:445–456. doi: 10.1002/JLB.3MR0717-269R. [DOI] [PubMed] [Google Scholar]
- 66.Chen K, Magri G, Grasset EK, Cerutti A. Rethinking mucosal antibody responses: IgM, IgG and IgD join IgA. Nat. Rev. Immunol. 2020;20:427–441. doi: 10.1038/s41577-019-0261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu D, Lu W. Defensins: a double-edged sword in host immunity. Front. Immunol. 2020;11:764. doi: 10.3389/fimmu.2020.00764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Linden SK, Sutton P, Karlsson NG, Korolik V, McGuckin MA. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008;1:183–197. doi: 10.1038/mi.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baeyens AAL, Schwab SR. Finding a way out: S1P signaling and immune cell migration. Annu. Rev. Immunol. 2020;38:759–784. doi: 10.1146/annurev-immunol-081519-083952. [DOI] [PubMed] [Google Scholar]
- 70.Bryan AM, Del Poeta M. Sphingosine-1-phosphate receptors and innate immunity. Cell. Immunol. 2018;20:e12836. doi: 10.1111/cmi.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Orsini M, et al. Sphingolipid-mediated inflammatory signaling leading to autophagy inhibition converts erythropoiesis to myelopoiesis in human hematopoietic stem/progenitor cells. Cell Death Differ. 2019;26:1796–1812. doi: 10.1038/s41418-018-0245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakamura T, et al. Ceramide regulates oxidant release in adherent human neutrophils. J. Biol. Chem. 1994;269:18384–18389. doi: 10.1016/S0021-9258(17)32319-0. [DOI] [PubMed] [Google Scholar]
- 73.Zhao X, et al. Neutrophil recruitment mediated by sphingosine 1-phosphate (S1P)/S1P receptors during chronic liver injury. Cell. Immunol. 2021;359:104243. doi: 10.1016/j.cellimm.2020.104243. [DOI] [PubMed] [Google Scholar]
- 74.Karandashova S, Kummarapurugu AB, Zheng S, Chalfant CE, Voynow JA. Neutrophil elastase increases airway ceramide levels via upregulation of serine palmitoyltransferase. Am. J. Physiol. Lung Cell Mol. Physiol. 2018;314:L206–l214. doi: 10.1152/ajplung.00322.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Izawa K, et al. Disrupting ceramide-CD300f interaction prevents septic peritonitis by stimulating neutrophil recruitment. Sci. Rep. 2017;7:4298. doi: 10.1038/s41598-017-04647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meher AK, et al. Novel role of IL (Interleukin)-1β in neutrophil extracellular trap formation and abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 2018;38:843–853. doi: 10.1161/ATVBAHA.117.309897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mansfield PJ, Hinkovska-Galcheva V, Carey SS, Shayman JA, Boxer LA. Regulation of polymorphonuclear leukocyte degranulation and oxidant production by ceramide through inhibition of phospholipase D. Blood. 2002;99:1434–1441. doi: 10.1182/blood.V99.4.1434. [DOI] [PubMed] [Google Scholar]
- 78.Qureshi A, et al. Role of sphingomyelin synthase in controlling the antimicrobial activity of neutrophils against Cryptococcus neoformans. PLoS ONE. 2011;5:e15587. doi: 10.1371/journal.pone.0015587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eberle M, et al. Exacerbation of experimental autoimmune encephalomyelitis in ceramide synthase 6 knockout mice is associated with enhanced activation/migration of neutrophils. Immunol. Cell Biol. 2015;93:825–836. doi: 10.1038/icb.2015.47. [DOI] [PubMed] [Google Scholar]
- 80.Barthelmes J, et al. Lack of ceramide synthase 2 suppresses the development of experimental autoimmune encephalomyelitis by impairing the migratory capacity of neutrophils. Brain Behav. Immun. 2015;46:280–292. doi: 10.1016/j.bbi.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 81.Hinkovska-Galcheva VT, et al. The formation of ceramide-1-phosphate during neutrophil phagocytosis and its role in liposome fusion *. J. Biol. Chem. 1998;273:33203–33209. doi: 10.1074/jbc.273.50.33203. [DOI] [PubMed] [Google Scholar]
- 82.Hinkovska-Galcheva V, et al. Ceramide 1-phosphate, a mediator of phagocytosis. J. Biol. Chem. 2005;280:26612–26621. doi: 10.1074/jbc.M501359200. [DOI] [PubMed] [Google Scholar]
- 83.Seumois G, et al. De novo C16- and C24-ceramide generation contributes to spontaneous neutrophil apoptosis. J. Leukoc. Biol. 2007;81:1477–1486. doi: 10.1189/jlb.0806529. [DOI] [PubMed] [Google Scholar]
- 84.Baeyens A, et al. Monocyte-derived S1P in the lymph node regulates immune responses. Nature. 2021;592:290–295. doi: 10.1038/s41586-021-03227-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Al-Rashed F, et al. Ceramide kinase regulates TNF-α-induced immune responses in human monocytic cells. Sci. Rep. 2021;11:8259. doi: 10.1038/s41598-021-87795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Syed SN, Weigert A, Brüne B. Sphingosine kinases are involved in macrophage NLRP3 inflammasome transcriptional induction. Int. J. Mol. Sci. 2020;21:4733. doi: 10.3390/ijms21134733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Coillard A, Segura E. In vivo differentiation of human monocytes. Front. Immunol. 2019;10:1907. doi: 10.3389/fimmu.2019.01907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weichand B, et al. Apoptotic cells enhance sphingosine-1-phosphate receptor 1 dependent macrophage migration. Eur. J. Immunol. 2013;43:3306–3313. doi: 10.1002/eji.201343441. [DOI] [PubMed] [Google Scholar]
- 89.Weigert A, Olesch C, Brüne B. Sphingosine-1-phosphate and macrophage biology-how the sphinx tames the big eater. Front. Immunol. 2019;10:1706. doi: 10.3389/fimmu.2019.01706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang J, et al. Sphingosine 1-phosphate (S1P)/S1P receptor2/3 axis promotes inflammatory M1 polarization of bone marrow-derived monocyte/macrophage via G(α)i/o/PI3K/JNK pathway. Cell. Physiol. Biochem. 2018;49:1677–1693. doi: 10.1159/000493611. [DOI] [PubMed] [Google Scholar]
- 91.Hou J, et al. Sphingosine 1-phosphate receptor 2 signaling suppresses macrophage phagocytosis and impairs host defense against sepsis. Anesthesiology. 2015;123:409–422. doi: 10.1097/ALN.0000000000000725. [DOI] [PubMed] [Google Scholar]
- 92.Hou L, et al. Macrophage sphingosine 1-phosphate receptor 2 blockade attenuates liver inflammation and fibrogenesis triggered by NLRP3 inflammasome. Front. Immunol. 2020;11:1149. doi: 10.3389/fimmu.2020.01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lamour NF, et al. Ceramide kinase regulates the production of tumor necrosis factor α (TNFα) via inhibition of TNFα-converting enzyme. J. Biol. Chem. 2011;286:42808–42817. doi: 10.1074/jbc.M111.310169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Uribe-Querol E, Rosales C. Phagocytosis: our current understanding of a universal biological process. Front. Immunol. 2020;11:1066. doi: 10.3389/fimmu.2020.01066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Niekamp P, et al. Sphingomyelin biosynthesis is essential for phagocytic signaling during Mycobacterium tuberculosis host cell entry. mBio. 2021;12:e03141–20. doi: 10.1128/mBio.03141-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mehendale N, Mallik R, Kamat SS. Mapping sphingolipid metabolism pathways during phagosomal maturation. ACS Chem. Biol. 2021;16:2757–2765. doi: 10.1021/acschembio.1c00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Grassmé H, et al. Acidic sphingomyelinase mediates entry of N. gonorrhoeae into nonphagocytic cells. Cell. 1997;91:605–615. doi: 10.1016/S0092-8674(00)80448-1. [DOI] [PubMed] [Google Scholar]
- 98.Yu H, et al. Defective acid sphingomyelinase pathway with Pseudomonas aeruginosa infection in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 2009;41:367–375. doi: 10.1165/rcmb.2008-0295OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Krones D, et al. Staphylococcus aureus α-toxin induces acid sphingomyelinase release from a human endothelial cell line. Front. Microbiol. 2021;12:694489. doi: 10.3389/fmicb.2021.694489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang Y, Li X, Carpinteiro A, Gulbins E. Acid sphingomyelinase amplifies redox signaling in Pseudomonas aeruginosa-induced macrophage apoptosis. J. Immunol. 2008;181:4247–4254. doi: 10.4049/jimmunol.181.6.4247. [DOI] [PubMed] [Google Scholar]
- 101.Managò A, et al. Pseudomonas aeruginosa pyocyanin induces neutrophil death via mitochondrial reactive oxygen species and mitochondrial acid sphingomyelinase. Antioxid. Redox Signal. 2015;22:1097–1110. doi: 10.1089/ars.2014.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tafesse FG, et al. Disruption of sphingolipid biosynthesis blocks phagocytosis of Candida albicans. PLoS Pathog. 2015;11:e1005188. doi: 10.1371/journal.ppat.1005188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lang J, et al. Acid ceramidase of macrophages traps herpes simplex virus in multivesicular bodies and protects from severe disease. Nat. Commun. 2020;11:1338. doi: 10.1038/s41467-020-15072-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Doran AC, Yurdagul A, Tabas I. Efferocytosis in health and disease. Nat. Rev. Immunol. 2020;20:254–267. doi: 10.1038/s41577-019-0240-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Feng J, et al. Induction of CD36 expression by oxidized LDL and IL-4 by a common signaling pathway dependent on protein kinase C and PPAR-gamma. J. Lipid Res. 2000;41:688–696. doi: 10.1016/S0022-2275(20)32377-4. [DOI] [PubMed] [Google Scholar]
- 106.Hundal RS, et al. Oxidized low density lipoprotein inhibits macrophage apoptosis through activation of the PI 3-kinase/PKB pathway. J. Lipid Res. 2001;42:1483–1491. doi: 10.1016/S0022-2275(20)30282-0. [DOI] [PubMed] [Google Scholar]
- 107.Koch A, et al. PPARγ agonists upregulate sphingosine 1-phosphate (S1P) receptor 1 expression, which in turn reduces S1P-induced [Ca(2+)]i increases in renal mesangial cells. Biochim Biophys. Acta. 2013;1831:1634–1643. doi: 10.1016/j.bbalip.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 108.Coazzoli M, et al. Acid sphingomyelinase downregulation enhances mitochondrial fusion and promotes oxidative metabolism in a mouse model of melanoma. Cells. 2020;9:848. doi: 10.3390/cells9040848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McCarthy C, et al. Macrophage PPAR gamma Co-activator-1 alpha participates in repressing foam cell formation and atherosclerosis in response to conjugated linoleic acid. EMBO Mol. Med. 2013;5:1443–1457. doi: 10.1002/emmm.201302587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Luan Y, Griffiths HR. Ceramides reduce CD36 cell surface expression and oxidised LDL uptake by monocytes and macrophages. Arch. Biochem. Biophys. 2006;450:89–99. doi: 10.1016/j.abb.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 111.Marathe S, Kuriakose G, Williams KJ, Tabas I. Sphingomyelinase, an enzyme implicated in atherogenesis, is present in atherosclerotic lesions and binds to specific components of the subendothelial extracellular matrix. Arterioscler. Thromb. Vasc. Biol. 1999;19:2648–2658. doi: 10.1161/01.ATV.19.11.2648. [DOI] [PubMed] [Google Scholar]
- 112.Petrusca DN, et al. Sphingolipid-mediated inhibition of apoptotic cell clearance by alveolar macrophages. J. Biol. Chem. 2010;285:40322–40332. doi: 10.1074/jbc.M110.137604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Camell CD, et al. Macrophage-specific de novo synthesis of ceramide is dispensable for inflammasome-driven inflammation and insulin resistance in obesity. J. Biol. Chem. 2015;290:29402–29413. doi: 10.1074/jbc.M115.680199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu J, Zhang X, Cheng Y, Cao X. Dendritic cell migration in inflammation and immunity. Cell. Mol. Immunol. 2021;18:2461–2471. doi: 10.1038/s41423-021-00726-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Idzko M, et al. Sphingosine 1-phosphate induces chemotaxis of immature and modulates cytokine-release in mature human dendritic cells for emergence of Th2 immune responses. FASEB J. 2002;16:625–627. doi: 10.1096/fj.01-0625fje. [DOI] [PubMed] [Google Scholar]
- 116.Avota E, Gulbins E, Schneider-Schaulies S. DC-SIGN mediated sphingomyelinase-activation and ceramide generation is essential for enhancement of viral uptake in dendritic cells. PLoS Pathog. 2011;7:e1001290. doi: 10.1371/journal.ppat.1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhao D, et al. Inhibition of acid ceramidase regulates MHC class II antigen presentation and suppression of autoimmune arthritis. Cytokine. 2020;135:155219. doi: 10.1016/j.cyto.2020.155219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hartel JC, Merz N, Grösch S. How sphingolipids affect T cells in the resolution of inflammation. Front. Pharmacol. 2022;13:1002915. doi: 10.3389/fphar.2022.1002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Franchi L, Malisan F, Tomassini B, Testi R. Ceramide catabolism critically controls survival of human dendritic cells. J. Leukoc. Biol. 2006;79:166–172. doi: 10.1189/jlb.1004601. [DOI] [PubMed] [Google Scholar]
- 120.Eigenbrod S, Derwand R, Jakl V, Endres S, Eigler A. Sphingosine kinase and sphingosine-1-phosphate regulate migration, endocytosis and apoptosis of dendritic cells. Immunol. Invest. 2006;35:149–165. doi: 10.1080/08820130600616490. [DOI] [PubMed] [Google Scholar]
- 121.Schwiebs A, et al. Activation-induced cell death of dendritic cells is dependent on sphingosine kinase 1. Front. Pharmacol. 2016;7:94. doi: 10.3389/fphar.2016.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kanto T, Kalinski P, Hunter OC, Lotze MT, Amoscato AA. Ceramide mediates tumor-induced dendritic cell apoptosis. J. Immunol. 2001;167:3773–3784. doi: 10.4049/jimmunol.167.7.3773. [DOI] [PubMed] [Google Scholar]
- 123.Koeken VACM, et al. Plasma metabolome predicts trained immunity responses after antituberculosis BCG vaccination. PLoS Biol. 2022;20:e3001765. doi: 10.1371/journal.pbio.3001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mendoza A, et al. Lymphatic endothelial S1P promotes mitochondrial function and survival in naive T cells. Nature. 2017;546:158–161. doi: 10.1038/nature22352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xiong Y, et al. CD4 T cell sphingosine 1-phosphate receptor (S1PR)1 and S1PR4 and endothelial S1PR2 regulate afferent lymphatic migration. Sci. Immunol. 2019;4:eaav1263. doi: 10.1126/sciimmunol.aav1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu G, et al. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat. Immunol. 2009;10:769–777. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nat. Immunol. 2010;11:1047–1056. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brana C, et al. Immunohistochemical detection of sphingosine-1-phosphate receptor 1 and 5 in human multiple sclerosis lesions. Neuropathol. Appl. Neurobiol. 2014;40:564–578. doi: 10.1111/nan.12048. [DOI] [PubMed] [Google Scholar]
- 129.Xiao L, et al. SPHK1-S1PR1-RANKL axis regulates the interactions between macrophages and BMSCs in inflammatory bone loss. J. Bone Miner. Res. 2018;33:1090–1104. doi: 10.1002/jbmr.3396. [DOI] [PubMed] [Google Scholar]
- 130.Ryu J, et al. Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EMBO J. 2006;25:5840–5851. doi: 10.1038/sj.emboj.7601430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Contos JJ, Ishii I, Chun J. Lysophosphatidic acid receptors. Mol. Pharmacol. 2000;58:1188–1196. doi: 10.1124/mol.58.6.1188. [DOI] [PubMed] [Google Scholar]
- 132.Brinkmann V, et al. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 2010;9:883–897. doi: 10.1038/nrd3248. [DOI] [PubMed] [Google Scholar]
- 133.Sun Y, et al. Ozanimod for treatment of relapsing-remitting multiple sclerosis in adults: a systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 2020;11:589146. doi: 10.3389/fphar.2020.589146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Companioni O, Mir C, Garcia-Mayea Y, LLeonart ME. Targeting sphingolipids for cancer therapy. Front. Oncol. 2021;11:745092. doi: 10.3389/fonc.2021.745092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Martín-Leal A, et al. CCR5 deficiency impairs CD4(+) T-cell memory responses and antigenic sensitivity through increased ceramide synthesis. EMBO J. 2020;39:e104749. doi: 10.15252/embj.2020104749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tonnetti L, Verí MC, Bonvini E, D’Adamio L. A role for neutral sphingomyelinase-mediated ceramide production in T cell receptor-induced apoptosis and mitogen-activated protein kinase-mediated signal transduction. J. Exp. Med. 1999;189:1581–1589. doi: 10.1084/jem.189.10.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bai A, Kokkotou E, Zheng Y, Robson SC. Role of acid sphingomyelinase bioactivity in human CD4+ T-cell activation and immune responses. Cell Death Dis. 2015;6:e1828–e1828. doi: 10.1038/cddis.2015.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chan G, Ochi A. Sphingomyelin-ceramide turnover in CD28 costimulatory signaling. Eur. J. Immunol. 1995;25:1999–2004. doi: 10.1002/eji.1830250730. [DOI] [PubMed] [Google Scholar]
- 139.Stoffel B, Bauer P, Nix M, Deres K, Stoffel W. Ceramide-independent CD28 and TCR signaling but reduced IL-2 secretion in T cells of acid sphingomyelinase-deficient mice. Eur. J. Immunol. 1998;28:874–880. doi: 10.1002/(SICI)1521-4141(199803)28:03<874::AID-IMMU874>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 140.Bai A, et al. CD39 and CD161 modulate Th17 responses in Crohn’s disease. J. Immunol. 2014;193:3366–3377. doi: 10.4049/jimmunol.1400346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hose M, et al. T cell-specific overexpression of acid sphingomyelinase results in elevated T cell activation and reduced parasitemia during Plasmodium yoelii infection. Front. Immunol. 2019;10:1225. doi: 10.3389/fimmu.2019.01225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hollmann C, et al. Inhibition of acid sphingomyelinase allows for selective targeting of CD4+ conventional versus Foxp3+ regulatory T cells. J. Immunol. 2016;197:3130–3141. doi: 10.4049/jimmunol.1600691. [DOI] [PubMed] [Google Scholar]
- 143.Sharabi A, et al. PP2A enables IL-2 signaling by preserving IL-2Rβ chain expression during Treg development. JCI Insight. 2019;5:e126294. doi: 10.1172/jci.insight.126294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tang Q. Therapeutic window of interleukin-2 for autoimmune diseases. Diabetes. 2015;64:1912–1913. doi: 10.2337/db15-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Studstill CJ, et al. Sphingosine kinase 2 restricts T cell immunopathology but permits viral persistence. J. Clin. Investig. 2020;130:6523–6538. doi: 10.1172/JCI125297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lau P, et al. Sphingosine kinase 1 promotes tumor immune evasion by regulating the MTA3-PD-L1 axis. Cell. Mol. Immunol. 2022;19:1153–1167. doi: 10.1038/s41423-022-00911-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Peyruchaud O, Saier L, Leblanc R. Autotaxin implication in cancer metastasis and autoimunne disorders: functional implication of binding autotaxin to the cell surface. Cancers. 2019;12:105. doi: 10.3390/cancers12010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kester M, et al. Preclinical development of a C6-ceramide NanoLiposome, a novel sphingolipid therapeutic. Biol. Chem. 2015;396:737–747. doi: 10.1515/hsz-2015-0129. [DOI] [PubMed] [Google Scholar]
- 149.Sic H, et al. Sphingosine-1-phosphate receptors control B-cell migration through signaling components associated with primary immunodeficiencies, chronic lymphocytic leukemia, and multiple sclerosis. J. Allergy Clin. Immunol. 2014;134:420–428.e415. doi: 10.1016/j.jaci.2014.01.037. [DOI] [PubMed] [Google Scholar]
- 150.Blaho VA, Hla T. An update on the biology of sphingosine 1-phosphate receptors. J. Lipid Res. 2014;55:1596–1608. doi: 10.1194/jlr.R046300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Green JA, Cyster JG. S1PR2 links germinal center confinement and growth regulation. Immunol. Rev. 2012;247:36–51. doi: 10.1111/j.1600-065X.2012.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Colié S, et al. Disruption of sphingosine 1-phosphate lyase confers resistance to chemotherapy and promotes oncogenesis through Bcl-2/Bcl-xL upregulation. Cancer Res. 2009;69:9346–9353. doi: 10.1158/0008-5472.CAN-09-2198. [DOI] [PubMed] [Google Scholar]
- 153.Furuya H, et al. Sphingosine kinase 1 expression enhances colon tumor growth. J. Transl. Med. 2017;15:120. doi: 10.1186/s12967-017-1220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Oskouian B, et al. Sphingosine-1-phosphate lyase potentiates apoptosis via p53- and p38-dependent pathways and is down-regulated in colon cancer. Proc. Natl Acad. Sci. USA. 2006;103:17384–17389. doi: 10.1073/pnas.0600050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Magkrioti C, et al. The autotaxin-lysophosphatidic acid axis promotes lung carcinogenesis. Cancer Res. 2018;78:3634–3644. doi: 10.1158/0008-5472.CAN-17-3797. [DOI] [PubMed] [Google Scholar]
- 156.Benesch MG, et al. Tumor-induced inflammation in mammary adipose tissue stimulates a vicious cycle of autotaxin expression and breast cancer progression. FASEB J. 2015;29:3990–4000. doi: 10.1096/fj.15-274480. [DOI] [PubMed] [Google Scholar]
- 157.Chen W, et al. Downregulation of ceramide synthase 1 promotes oral cancer through endoplasmic reticulum stress. Int. J. Oral. Sci. 2021;13:10. doi: 10.1038/s41368-021-00118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.El-Hindi K, et al. T-cell-specific CerS4 depletion prolonged inflammation and enhanced tumor burden in the AOM/DSS-induced CAC model. Int. J. Mol. Sci. 2022;23:1866. doi: 10.3390/ijms23031866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kachler K, et al. Enhanced acid sphingomyelinase activity drives immune evasion and tumor growth in non-small cell lung carcinoma. Cancer Res. 2017;77:5963–5976. doi: 10.1158/0008-5472.CAN-16-3313. [DOI] [PubMed] [Google Scholar]
- 160.Kato Y, et al. Acidic extracellular pH increases calcium influx-triggered phospholipase D activity along with acidic sphingomyelinase activation to induce matrix metalloproteinase-9 expression in mouse metastatic melanoma. FEBS J. 2007;274:3171–3183. doi: 10.1111/j.1742-4658.2007.05848.x. [DOI] [PubMed] [Google Scholar]
- 161.Montfort A, et al. Neutral sphingomyelinase 2 heightens anti-melanoma immune responses and anti-PD-1 therapy efficacy. Cancer Immunol. Res. 2021;9:568–582. doi: 10.1158/2326-6066.CIR-20-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Akeus P, et al. Regulatory T cells reduce endothelial neutral sphingomyelinase 2 to prevent T-cell migration into tumors. Eur. J. Immunol. 2021;51:2317–2329. doi: 10.1002/eji.202149208. [DOI] [PubMed] [Google Scholar]
- 163.Chakraborty P, et al. Pro-survival lipid sphingosine-1-phosphate metabolically programs T cells to limit anti-tumor activity. Cell Rep. 2019;28:1879–1893.e1877. doi: 10.1016/j.celrep.2019.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Józefowski S, et al. Ceramide and ceramide 1-phosphate are negative regulators of TNF-α production induced by lipopolysaccharide. J. Immunol. 2010;185:6960–6973. doi: 10.4049/jimmunol.0902926. [DOI] [PubMed] [Google Scholar]
- 165.Ali M, Saroha A, Pewzner-Jung Y, Futerman AH. LPS-mediated septic shock is augmented in ceramide synthase 2 null mice due to elevated activity of TNFα-converting enzyme. FEBS Lett. 2015;589:2213–2217. doi: 10.1016/j.febslet.2015.06.045. [DOI] [PubMed] [Google Scholar]
- 166.Tanaka A, et al. Effects of ceramide kinase knockout on lipopolysaccharide-treated sepsis-model mice: changes in serum cytokine/chemokine levels and increased lethality. J. Pharmacol. Sci. 2022;150:1–8. doi: 10.1016/j.jphs.2022.05.006. [DOI] [PubMed] [Google Scholar]
- 167.Espaillat MP, et al. Loss of acid ceramidase in myeloid cells suppresses intestinal neutrophil recruitment. FASEB J. 2018;32:2339–2353. doi: 10.1096/fj.201700585R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Graf C, et al. Neutropenia with impaired immune response to Streptococcus pneumoniae in ceramide kinase-deficient mice. J. Immunol. 2008;180:3457–3466. doi: 10.4049/jimmunol.180.5.3457. [DOI] [PubMed] [Google Scholar]
- 169.Claus RA, et al. Role of increased sphingomyelinase activity in apoptosis and organ failure of patients with severe sepsis. FASEB J. 2005;19:1719–1721. doi: 10.1096/fj.04-2842fje. [DOI] [PubMed] [Google Scholar]
- 170.Heinrich M, et al. Cathepsin D links TNF-induced acid sphingomyelinase to Bid-mediated caspase-9 and -3 activation. Cell Death Differ. 2004;11:550–563. doi: 10.1038/sj.cdd.4401382. [DOI] [PubMed] [Google Scholar]
- 171.Utermöhlen O, Karow U, Löhler J, Krönke M. Severe impairment in early host defense against Listeria monocytogenes in mice deficient in acid sphingomyelinase. J. Immunol. 2003;170:2621–2628. doi: 10.4049/jimmunol.170.5.2621. [DOI] [PubMed] [Google Scholar]
- 172.Jin J, et al. Acid sphingomyelinase plays a key role in palmitic acid-amplified inflammatory signaling triggered by lipopolysaccharide at low concentrations in macrophages. Am. J. Physiol. Endocrinol. Metab. 2013;305:E853–E867. doi: 10.1152/ajpendo.00251.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ugwu FN, Ho J. Preclinical evidence of sphingosine kinase 1 inhibition in alleviation of intestinal epithelial injury in polymicrobial sepsis. Inflamm. Res. 2019;68:723–726. doi: 10.1007/s00011-019-01255-7. [DOI] [PubMed] [Google Scholar]
- 174.Allende ML, et al. Sphingosine-1-phosphate lyase deficiency produces a pro-inflammatory response while impairing neutrophil trafficking. J. Biol. Chem. 2011;286:7348–7358. doi: 10.1074/jbc.M110.171819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Helke K, et al. Ceramide synthase 6 deficiency enhances inflammation in the DSS model of colitis. Sci. Rep. 2018;8:1627. doi: 10.1038/s41598-018-20102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Suzuki S, Tanaka A, Nakamura H, Murayama T. Knockout of ceramide kinase aggravates pathological and lethal responses in mice with experimental colitis. Biol. Pharm. Bull. 2018;41:797–805. doi: 10.1248/bpb.b18-00051. [DOI] [PubMed] [Google Scholar]
- 177.Zhang P, et al. Deficiency of alkaline SMase enhances dextran sulfate sodium-induced colitis in mice with upregulation of autotaxin. J. Lipid Res. 2018;59:1841–1850. doi: 10.1194/jlr.M084285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dong YL, et al. Autotaxin-lysophosphatidic acid axis blockade improves inflammation by regulating Th17 cell differentiation in DSS-induced chronic colitis mice. Inflammation. 2019;42:1530–1541. doi: 10.1007/s10753-019-01015-z. [DOI] [PubMed] [Google Scholar]
- 179.Lin S, et al. Autotaxin determines colitis severity in mice and is secreted by B cells in the colon. FASEB J. 2019;33:3623–3635. doi: 10.1096/fj.201801415RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tanaka A, et al. Ceramide kinase knockout ameliorates multiple sclerosis-like behaviors and demyelination in cuprizone-treated mice. Life Sci. 2022;296:120446. doi: 10.1016/j.lfs.2022.120446. [DOI] [PubMed] [Google Scholar]
- 181.Ninou I, et al. Genetic deletion of autotaxin from CD11b+ cells decreases the severity of experimental autoimmune encephalomyelitis. PLoS ONE. 2020;15:e0226050. doi: 10.1371/journal.pone.0226050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Becker KA, et al. Blockade of experimental multiple sclerosis by inhibition of the acid sphingomyelinase/ceramide system. Neurosignals. 2017;25:88–97. doi: 10.1159/000484621. [DOI] [PubMed] [Google Scholar]
- 183.Wang C, et al. Sphingomyelin synthase 1 enhances BCR signaling to promote lupus-like autoimmune response. EBioMedicine. 2019;45:578–587. doi: 10.1016/j.ebiom.2019.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Liu F, et al. TLR-induced SMPD3 defects enhance inflammatory response of B cell and macrophage in the pathogenesis of SLE. Scand. J. Immunol. 2017;86:377–388. doi: 10.1111/sji.12611. [DOI] [PubMed] [Google Scholar]
- 185.Beckmann N, et al. Regulation of arthritis severity by the acid sphingomyelinase. Cell. Physiol. Biochem. 2017;43:1460–1471. doi: 10.1159/000481968. [DOI] [PubMed] [Google Scholar]
- 186.Nikitopoulou I, et al. Autotaxin expression from synovial fibroblasts is essential for the pathogenesis of modeled arthritis. J. Exp. Med. 2012;209:925–933. doi: 10.1084/jem.20112012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sofi MH, et al. Ceramide synthesis regulates T cell activity and GVHD development. JCI Insight. 2017;2:e91701. doi: 10.1172/jci.insight.91701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Herz J, et al. Acid sphingomyelinase is a key regulator of cytotoxic granule secretion by primary T lymphocytes. Nat. Immunol. 2009;10:761–768. doi: 10.1038/ni.1757. [DOI] [PubMed] [Google Scholar]
- 189.Glajch KE, et al. Wild-type GBA1 increases the α-synuclein tetramer-monomer ratio, reduces lipid-rich aggregates, and attenuates motor and cognitive deficits in mice. Proc. Natl Acad. Sci. USA. 2021;118:e2103425118. doi: 10.1073/pnas.2103425118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Li H, et al. Mitochondrial dysfunction and mitophagy defect triggered by heterozygous GBA mutations. Autophagy. 2019;15:113–130. doi: 10.1080/15548627.2018.1509818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pandey MK, et al. Complement drives glucosylceramide accumulation and tissue inflammation in Gaucher disease. Nature. 2017;543:108–112. doi: 10.1038/nature21368. [DOI] [PubMed] [Google Scholar]
- 192.Pandey MK, Rani R, Zhang W, Setchell K, Grabowski GA. Immunological cell type characterization and Th1-Th17 cytokine production in a mouse model of Gaucher disease. Mol. Genet. Metab. 2012;106:310–322. doi: 10.1016/j.ymgme.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sikora J, et al. Acid ceramidase deficiency in mice results in a broad range of central nervous system abnormalities. Am. J. Pathol. 2017;187:864–883. doi: 10.1016/j.ajpath.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lee JK, et al. Acid sphingomyelinase modulates the autophagic process by controlling lysosomal biogenesis in Alzheimer’s disease. J. Exp. Med. 2014;211:1551–1570. doi: 10.1084/jem.20132451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lee JY, et al. Neuronal SphK1 acetylates COX2 and contributes to pathogenesis in a model of Alzheimer’s Disease. Nat. Commun. 2018;9:1479. doi: 10.1038/s41467-018-03674-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang P, et al. Aggravation of Alzheimer’s disease due to the COX-2-mediated reciprocal regulation of IL-1β and Aβ between glial and neuron cells. Aging Cell. 2014;13:605–615. doi: 10.1111/acel.12209. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 197.Alam S, Piazzesi A, Abd El Fatah M, Raucamp M, van Echten-Deckert G. Neurodegeneration caused by S1P-lyase deficiency involves calcium-dependent tau pathology and abnormal histone acetylation. Cells. 2020;9:2189. doi: 10.3390/cells9102189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhong L, et al. Lipid transporter Spns2 promotes microglia pro-inflammatory activation in response to amyloid-beta peptide. Glia. 2019;67:498–511. doi: 10.1002/glia.23558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lu MH, et al. Inhibition of sphingomyelin synthase 1 ameliorates alzheimer-like pathology in APP/PS1 transgenic mice through promoting lysosomal degradation of BACE1. Exp. Neurol. 2019;311:67–79. doi: 10.1016/j.expneurol.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 200.Di Pardo A, et al. Defective sphingosine-1-phosphate metabolism is a druggable target in Huntington’s disease. Sci. Rep. 2017;7:5280. doi: 10.1038/s41598-017-05709-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Di Pardo A, et al. Stimulation of sphingosine kinase 1 (SPHK1) is beneficial in a Huntington’s disease pre-clinical model. Front. Mol. Neurosci. 2019;12:100. doi: 10.3389/fnmol.2019.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Worgall TS, et al. Impaired sphingolipid synthesis in the respiratory tract induces airway hyperreactivity. Sci. Transl. Med. 2013;5:186ra167. doi: 10.1126/scitranslmed.3005765. [DOI] [PubMed] [Google Scholar]
- 203.Oyeniran C, et al. Aberrant ORM (yeast)-like protein isoform 3 (ORMDL3) expression dysregulates ceramide homeostasis in cells and ceramide exacerbates allergic asthma in mice. J. Allergy Clin. Immunol. 2015;136:1035–1046.e1036. doi: 10.1016/j.jaci.2015.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Li J, et al. ORMDL3 functions as a negative regulator of antigen-mediated mast cell activation via an ATF6-UPR-autophagy-dependent pathway. Front. Immunol. 2021;12:604974. doi: 10.3389/fimmu.2021.604974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bugajev V, et al. Negative regulatory roles of ORMDL3 in the FcεRI-triggered expression of proinflammatory mediators and chemotactic response in murine mast cells. Cell. Mol. Life Sci. 2016;73:1265–1285. doi: 10.1007/s00018-015-2047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Shin SH, et al. Ceramide synthase 2 null mice are protected from ovalbumin-induced asthma with higher T cell receptor signal strength in CD4+ T cells. Int. J. Mol. Sci. 2021;22:2713. doi: 10.3390/ijms22052713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ziobro R, Henry B, Edwards MJ, Lentsch AB, Gulbins E. Ceramide mediates lung fibrosis in cystic fibrosis. Biochem. Biophys. Res. Commun. 2013;434:705–709. doi: 10.1016/j.bbrc.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dhami R, He X, Schuchman EH. Acid sphingomyelinase deficiency attenuates bleomycin-induced lung inflammation and fibrosis in mice. Cell. Physiol. Biochem. 2010;26:749–760. doi: 10.1159/000322342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Becker KA, et al. Acid ceramidase rescues cystic fibrosis mice from pulmonary infections. Infect. Immun. 2021;89:e00677–20. doi: 10.1128/IAI.00677-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nordström V, et al. Neuronal expression of glucosylceramide synthase in central nervous system regulates body weight and energy homeostasis. PLoS Biol. 2013;11:e1001506. doi: 10.1371/journal.pbio.1001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhang QJ, et al. Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes. 2012;61:1848–1859. doi: 10.2337/db11-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Holland WL, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 213.Song Y, et al. Ablation of ORMDL3 impairs adipose tissue thermogenesis and insulin sensitivity by increasing ceramide generation. Mol. Metab. 2022;56:101423. doi: 10.1016/j.molmet.2021.101423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Bandet CL, et al. Ceramide transporter CERT is involved in muscle insulin signaling defects under lipotoxic conditions. Diabetes. 2018;67:1258–1271. doi: 10.2337/db17-0901. [DOI] [PubMed] [Google Scholar]
- 215.Mitsutake S, et al. Ceramide kinase deficiency improves diet-induced obesity and insulin resistance. FEBS Lett. 2012;586:1300–1305. doi: 10.1016/j.febslet.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 216.Barbarroja N, et al. Increased dihydroceramide/ceramide ratio mediated by defective expression of degs1 impairs adipocyte differentiation and function. Diabetes. 2015;64:1180–1192. doi: 10.2337/db14-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nishimura S, et al. ENPP2 contributes to adipose tissue expansion and insulin resistance in diet-induced obesity. Diabetes. 2014;63:4154–4164. doi: 10.2337/db13-1694. [DOI] [PubMed] [Google Scholar]
- 218.Federico L, et al. Autotaxin and its product lysophosphatidic acid suppress brown adipose differentiation and promote diet-induced obesity in mice. Mol. Endocrinol. 2012;26:786–797. doi: 10.1210/me.2011-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kim GT, et al. Upregulation of the serine palmitoyltransferase subunit SPTLC2 by endoplasmic reticulum stress inhibits the hepatic insulin response. Exp. Mol. Med. 2022;54:573–584. doi: 10.1038/s12276-022-00766-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Li Y, et al. Sphingomyelin synthase 2 activity and liver steatosis: an effect of ceramide-mediated peroxisome proliferator-activated receptor γ2 suppression. Arterioscler. Thromb. Vasc. Biol. 2013;33:1513–1520. doi: 10.1161/ATVBAHA.113.301498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Fan S, et al. Hepatocyte-specific deletion of LASS2 protects against diet-induced hepatic steatosis and insulin resistance. Free Radic. Biol. Med. 2018;120:330–341. doi: 10.1016/j.freeradbiomed.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 222.Osawa Y, et al. Acid sphingomyelinase regulates glucose and lipid metabolism in hepatocytes through AKT activation and AMP-activated protein kinase suppression. FASEB J. 2011;25:1133–1144. doi: 10.1096/fj.10-168351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lan T, et al. Sphingosine kinase 1 promotes liver fibrosis by preventing miR-19b-3p-mediated inhibition of CCR2. Hepatology. 2018;68:1070–1086. doi: 10.1002/hep.29885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Qiu H, et al. Hepatocyte-secreted autotaxin exacerbates nonalcoholic fatty liver disease through autocrine inhibition of the PPARα/FGF21 axis. Cell. Mol. Gastroenterol. Hepatol. 2022;14:1003–1023. doi: 10.1016/j.jcmgh.2022.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Fucho R, et al. ASMase regulates autophagy and lysosomal membrane permeabilization and its inhibition prevents early stage non-alcoholic steatohepatitis. J. Hepatol. 2014;61:1126–1134. doi: 10.1016/j.jhep.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]