Neurocysticercosis (NCC) is a parasitic infection due to the larval cyst of Taenia solium. It is the most common parasitic infection of the central nervous system (CNS) worldwide and is endemic to Latin America, Asia and Africa. The most common site of infection in the CNS is the cerebral parenchyma, which accounts for 60–90% of cases. [1] The most common symptoms are headaches, seizures, and focal neurologic signs. Diagnosis of parenchymal infection is generally made by visualization of the cysts on neuroimaging. However, extra-parenchymal CNS infection involving the subarachnoid, meningeal, spinal and intraventricular spaces can also occur and may have diverse presentations with more subtle imaging findings.
Case One
A 40-year-old male presented to the emergency room of Bascom Palmer Eye Institute with blurry vision and progressively worsening headaches for six months. Past medical history was significant for treated latent tuberculosis. He was born in Guatemala and had moved to the US more than 15 years prior. On exam, his visual acuity was 20/20 in each eye. Both eyes had dense peripheral field loss to confrontation and bilateral grade 5 papilledema with hemorrhages (Fig 1a). Magnetic resonance imaging (MRI) of the brain and orbits with and without gadolinium showed prominent leptomeningeal enhancement, intraocular protrusion of the optic nerve head, and bilateral flattening of the posterior sclera (Fig. 2). The patient was immediately transferred for inpatient work-up. Acetazolamide was not initiated at this time due to concern for an infiltrative process. Lumbar puncture (LP) showed an elevated opening pressure (OP >40cm H2O), glucose <2 mg/dL, protein 108 mg/dL, and 209 WBC/uL (eosinophils 10%). Given the chronicity of the symptoms, epidemiologic factors (history of travel through desert regions) and CSF findings, he was suspected to have chronic fungal meningitis (Coccidiodes). Empiric antifungal treatment was started with fluconazole. However, extended CSF/serum/imaging studies, including CT Chest, CSF β-D-glucan, meningoencephalitis panel, CSF and blood coccidioides antibodies, histoplasma urine antigen, CSF mycobacterium polymerase chain reaction (PCR) and culture, and cryptococcal CSF antigens were all within normal limits. CSF testing for neurocysticercosis was not obtained. After being hospitalized for 14 days, he was discharged in stable condition but did not return for follow-up with ophthalmology.
Figure 1.
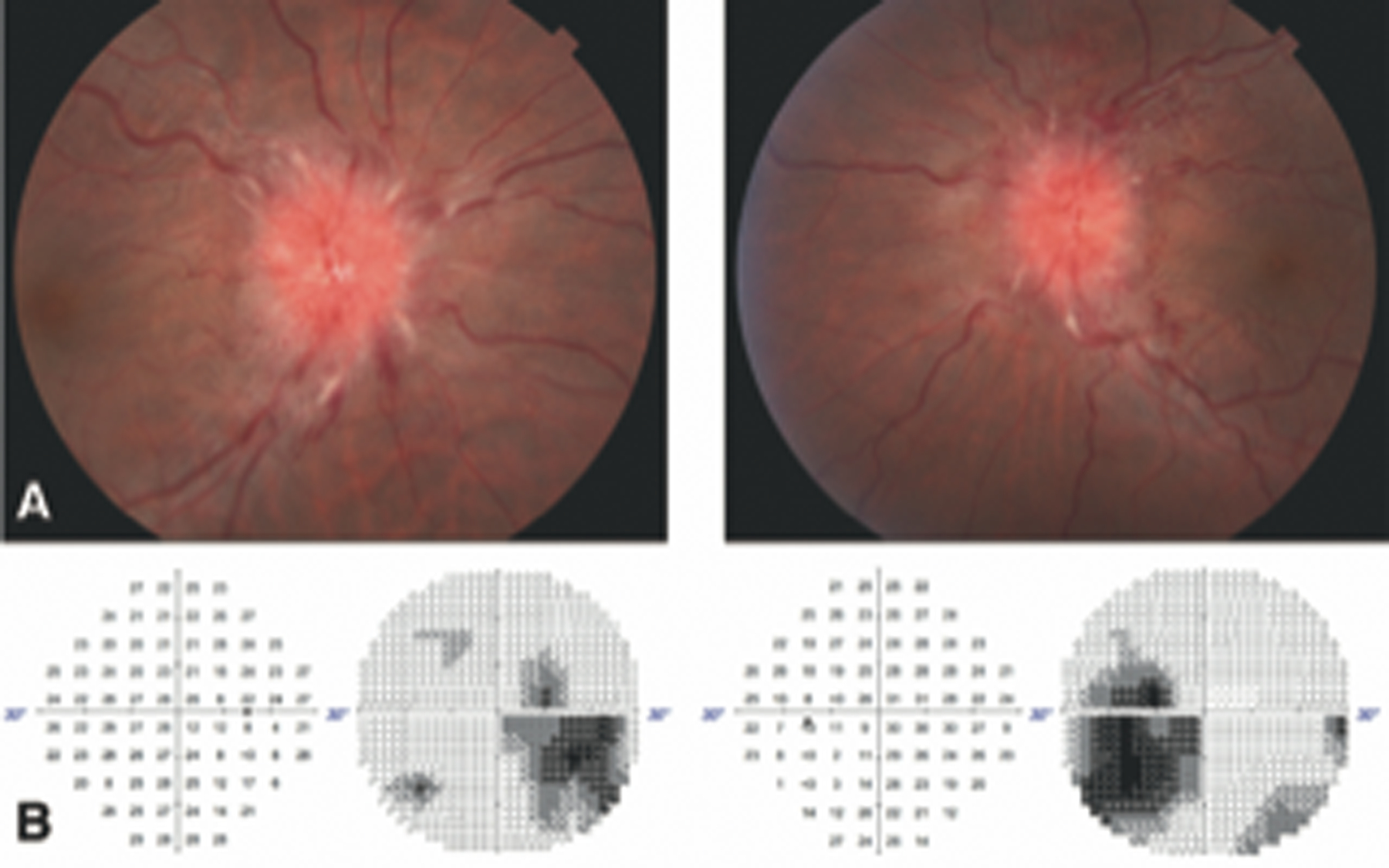
Case 1: Fundus photo taken at initial presentation showing grade V papilledema with surrounding Paton lines and disc hemorrhage in both eyes (A). Humphrey visual field (30–2) taken 1 month after presentation showing significant inferior temporal field loss with enlarged blind spots in both eyes (B).
Figure 2.
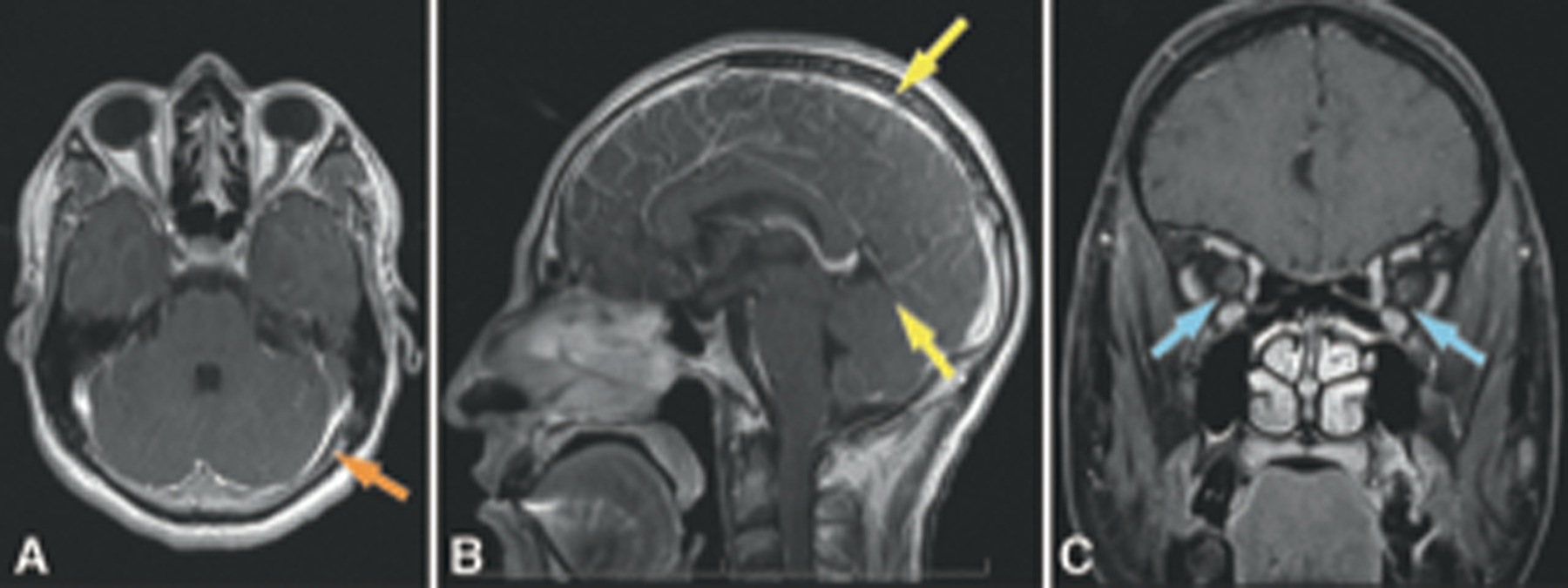
Case 1: MRI brain and orbits with and without gadolinium performed at initial presentation showing leptomeningeal enhancement (A: Axial with orange arrow, B: Sagittal with yellow arrows), and perioptic nerve sheath enhancement (C, blue arrows).
He presented to our emergency room again one month later due to worsening vision of both eyes and new onset left hemibody numbness. His visual acuity had decreased to 20/40 in the right eye and remained 20/20 in the left eye with progression of visual field loss. Papilledema remained grade 5 but with worsening obscuration of major vessels. Neurological exam was notable for decreased hemibody sensation at the T9–10 level. Acetazolamide extended release (ER) 500mg twice daily was started immediately and he was admitted to Univeristy of Miami for further management. A repeat brain MRI with and without gadolinium revealed worsened pachymeningitis. Thoracic and lumbar spine MRI with and without gadolinium showed mildly enhancing mid-right anterolateral spinal cord lesions and enhancement of the pia around the lumbar spinal cord and conus medullaris (Fig. 3A). Repeat LP showed OP of 44cm H2O and CSF analysis showed glucose <2 mg/dL, protein 124 mg/dL, and 67 WBC/uL. Due to the high risk of permanent vision loss, a ventriculoperitoneal (VP) shunt was placed 9 days after the admission. The patient reported improved vision, and acetazolamide ER was discontinued one day after VP shunt placement. Extended CSF studies were unrevealing, including flow cytometry analysis, VDRL, mycobacterium PCR, Lyme antibodies, and cultures for acid-fast bacilli and fungi. Antifungal treatment was continued.
Figure 3.

Case 1: 3A: MRI thoracic spine with and without gadolinium performed 1 month after presentation showing abnormal increased T2 signal without expansion in the mid-right anterolateral cord (yellow arrow). 3B: MRI lumbar spine performed 3 months after presentation showing diffuse leptomeningeal enhancement coating the distal thoracic cord and cauda equina (dotted line), as well as intrathecal arachnoid loculations within the lumbar thecal sac (yellow arrow).
Two months later he presented to the University of Miami Hospital emergency room with abdominal pain, urinary retention, and difficulty ambulating. MRI thoracic spine with and without gadolinium showed diffuse leptomeningeal enhancement with worsening fluid collections, rim enhancing lesions, and cord compression (Fig 3B). Biopsy of the spinal lesions was performed via laminectomy. Histopathology showed intramedullary neurocysticercosis (NCC) (Fig. 4). He was started on a 3-month course of albendazole, praziquantel, and prednisone, but discontinued the treatment after 6 weeks on his own. Despite this, he continued to have significant improvement in his neurological and visual symptoms. On follow-up 3 months after his initial presentation, his visual acuity was 20/20 in both eyes with significantly improved visual fields and complete resolution of optic disc edema.
Figure 4.

Case 1: Section of the intradural extramedullary lesion obtained after L2–L3 lumbar laminectomy showing cysticercal cysts on haemtoxylin and eosin stain (red arrows). Magnification 20X.
Case Two
A 40-year-old woman presented with worsening chronic headache that started 4 years prior. She endorsed new onset left-sided face and cheek numbness. Past medical history was unremarkable. She was born in Guatemala and moved the the US more than 20 years prior. MRI brain revealed left lentiform nucleus lacunar infarct. Her stroke workup was unrevealing and she was treated symptomatically for headache. She subsequently had transient vision loss and double vision for which she was not evaluated.
One year later, the patient returned with worsening headache and blurry vision. Visual acuity was 20/20 in both eyes, Humphrey visual field testing showed non-specific misses, and exam revealed bilateral grade I papilledema (Fig 5 D–G). MRI brain and orbits with and without gadolinium was unrevealing. MRA head and neck and MRV head were also unrevealing. Lumbar puncture had opening pressure of 35cm H2O with normal protein, glucose 31 mg/dL (serum 81mg/dL), 177 WBC/uL (35% lymphocytes, 43% eosinophils) and 62 RBC/uL. She then developed extremity paresthesias and diffuse arthralgias. Additional work-up to rule out hyper-eosinophilic syndrome and lymphoma were unrevealing. Repeat LP had negative cytology and positive cysticercosis IgG, though this was thought to represent prior infection given the lack of imaging findings. Given the lack of other diagnosis, she was treated with albendazole 600mg orally twice daily, praziquantel 1200mg orally three times a day, dexamethasone 5mg three times a day, and acetazolamide 500mg twice daily.
Figure 5.

Case 2: MRI brain and orbits with and without gadolinium performed 3 months after initial presentation. T1 pre-contrast (A) and post-contrast (B) images showing cysts (yellow arrows) within the subarachnoid space. MRI FIESTA sequence (C) showing multiple additional cysts in grape-like clusters within the chiasmatic cisterns (yellow asterisks). Optic nerve appearance by Optos widefield imaging (D upper) and horizontal OCT B-scan (D lower) at presentation, before VP shunt (E) and after VP shunt (F). Left column is right eye images and right column is left eye images. Humphrey visual field (30–2) taken at the time of diagnosis.
Over the next three months her symptoms did not improve. Vision declined and papilledema worsened. Repeat LP’s showed persistently elevated opening pressure and eosinophilia. The dose of acetazolamide was increased to 1000mg twice daily. Metagenomic next generation sequencing (mNGS) of the CSF confirmed infection by T. solium. Repeat MRI brain with and without gadolinium revealed diffuse leptomeningeal enhancement and extensive grape-like clusters in the chiasmatic cistern, consistent with a diagnosis of racemose neurocysticercosis (Fig. 5A–C). A VP shunt was placed with resolution of papilledema and improvement in vision. The patient was continued on albendazole and praziquantel, and anti-inflammatory therapy was escalated with oral prednisone 26mg and later etanercept 50mg weekly as a corticosteroid-sparing agent (this is an unlabeled use of etanercept). Acetazolamide was maintained at 1000mg twice daily until completion of corticosteroid and anti-parasitic treatment, and was limited to this dose due to side effects. The patient continues to have chronic headaches (severity 1–3/10) that are being managed symptomatically. Follow up MRIs have shown stable subarachnoid cysts.
Discussion
Extra-parenchymal neurocysticercosis is a rare manifestation of NCC involving the subarachnoid, meningeal, and intraventricular spaces. It has a slow onset and prolonged incubation time. Diagnosis can be challenging due to a variable clinical presentation with initially subtle and non-diagnostic neuroimaging findings. Both of the patients in this report presented with chronic headache, decreased vision, papilledema, and CSF eosinophilia. However, diagnosis was challenging and MRI imaging of the brain and orbits were initially unremarkable. A high clinical suspicion is necessary to recognize this occult disease. Diagnosis is often further complicated by the extremely long incubation period of racemose NCC; the median time from exposure to onset is 22.2 years. [2]
Spinal EPNCC, as seen in our first case, is very rare, accounting for 1% to 5% of all NCC cases. [1] Involvement of the spine can be intra- or extra-medullary, and is thought to occur via ventriculoependymal migration from the basal cisterns. [3] Cysts may be located at any spinal level and can cause paresthesias, paraparesis, hemiparesis, and motor ataxia. [3] Patients with isolated spinal EPNCC may present with blurred vision as their primary complaint. [4] A constellation of other symptoms such as headache, nausea, vomiting, and findings of bilateral papilledema, visual field defects, with normal MRI of the brain may lead to the incorrect diagnosis of idiopathic intracranial hypertension If LP with CSF analysis is not performed. [4]
Subarachnoid EPNCC, or racemose NCC, as illustrated in our second case, is a particularly aggressive variant with relatively higher morbidity and mortality due to its tendency to cause obstructive hydrocephalus. [5] In subarachnoid EPNCC, structurally aberrant, slow-growing T. solium larvae lacking scolices invade and expand within the brain cisterns. [1, 2] Basal subarachnoid NCC may also incite a robust inflammatory response leading to arachnoiditis, vasculitis, nerve entrapment, and stroke. [6] Indeed, vision loss in these cases may result from a combination of intracranial hypertension as well as inflammation of the optic nerve and chiasm when encased by inflammatory exudate. [1] Rarely, cysts lodged within the cerebral aqueduct and cisternal spaces can cause bilateral fourth nerve palsy, facial myokymia, upbeat nystagmus, periodic alternating nystagmus, and rhythmic oculopalatal myoclonus. [7]
MRI imaging for the diagnosis of extra-parenchymal NCC can be challenging. Cyst fluid is very similar to CSF in density, which may impede identification of racemose cysts where scolices are not present. [8] [9] However, MRI sequences like FIESTA (Fast Imaging Employing Steady-State Acquisition) may be particularly helpful in delineating the cyst wall and membranes. Additional diagnostic approaches include CSF/serum electroimmunotransfer blot (EITB) assay for the detection of cysticercoid IgG antibody [10], and quantitative PCR or metagenomic next-generation sequencing (mNGS)-based pathogen analysis of CSF [11]. Of note, though the EITB assay has a sensitivity of 86%, it does not differentiate between active and prior infection. [12] Although multiple case series have been published demonstrating the efficacy of mNGS in the diagnosis of NCC, [13, 14] its diagnostic performance compared to other assays (such as EITB) remains unclear. This could be an area of future research. Notwithstanding, the unbiased sequencing of all microbial DNA fragments within the CSF has been particularly useful in cases of chronic meningitis of unknown etiology. [15]
Medical therapy is most commonly employed, but there remains no standard of treatment, even in the most recent guidelines from the Infectious Diseases Society of America [16]. Recent reports suggest combined surgical and medical approaches may have the best outcome [17], as in the case of our patients, who are stable with only mild residual deficits. Both albendazole and praziquantel are cysticidal agents commonly used for NCC, and can be used together, as illustrated in these cases. A prolonged course may be required for subarachnoid and spinal EPNCC. [1] Additionally, corticosteroid therapy should be considered prior to initiation of anti-helminthic agents in cases of EPNCC to minimize inflammatory response. Corticosteroid-sparing agents such as methotrexate and etanercept have also been employed for this purpose. [18, 19] As demonstrated in the second case, EPNCC can continue to progress while on antiparasitic treatment. Due to slow and incomplete response to treatment, directed therapy for high intracranial pressure (ICP) and hydrocephalus may be necessary. In both cases, acetazolamide was not sufficient and surgical management was effectively instituted. Secondary complications such as seizures and headaches may also require targeted therapy.
Acetazolamide has been used for the treatment of secondary intracranial hypertension in select patients. In a rat model of hemorrhagic stroke, acetazolamide was shown to increase intracranial compliance and to mitigate ICP spikes, though this did not lead to improved functional outcomes. [20] In a patient with compromised venous outflow from the superior sagittal sinus after gunshot injury, acetazolamide in combination with surgical decompression was successfully utilized to improve flow through the venous system. [21] Likewise, lumbar puncture and acetazolamide were the initial treatments for dural venous sinus compression caused by meningioma in a case series of 16 patients. [22] 5 patients with normal visual acuity and mild visual field change were successfully managed with acetazolamide alone. In a single case report, acetazolamide resolved papilledema secondary to primary leptomeningeal lymphoma mimicking idiopathic intracranial hypertension, even before the initiation of chemotherapeutic agents. [23] However, use of acetazolamide in patients with secondary intracranial hypertension (and thus a separate primary disease process) may require careful monitoring of electrolytes, as patients can develop severe hyperchloremic acidosis. [24] A randomized control trial of acetazolamide for the treatment of secondary intracranial hypertension due to cryptococcal meningitis was prematurely terminated for this reason. [24] In retrospect, the patient in our first case may have benefited from initiation of acetazolamide therapy immediately after presentation to the emergency room.
In conclusion, clinicians should consider extraparenchymal NCC in patients with chronic meningitis who have spent significant time in endemic countries. Immunologic testing and metagenomic next generation sequencing should be considered in cases with a high clinical suspicion. Early biopsy may be necessary in cases where laboratory work-up remains negative.
Funding Statement:
NIH P30 026877 (Stanford), NIH Center Grant P30 EY014801 (University of Miami), Unrestricted grant from Research to Prevent Blindness (Stanford & University of Miami)
Footnotes
Conflict of Interest Statement: The authors do not have any conflicts of interest.
References
- 1.Mahale RR, Mehta A, Rangasetty S: Extraparenchymal (Racemose) Neurocysticercosis and Its Multitude Manifestations: A Comprehensive Review. J Clin Neurol 2015, 11(3):203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nash TE, O’Connell EM, Hammoud DA, Wetzler L, Ware JM, Mahanty S: Natural History of Treated Subarachnoid Neurocysticercosis. Am J Trop Med Hyg 2020, 102(1):78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hackius M, Pangalu A, Semmler A: Neurological picture. Isolated spinal neurocysticercosis. J Neurol Neurosurg Psychiatry 2015, 86(2):234–235. [DOI] [PubMed] [Google Scholar]
- 4.Seo JH, Seo HJ, Kim SW, Shin H: Isolated spinal neurocysticercosis : unusual ocular presentation mimicking pseudotumor cerebri. J Korean Neurosurg Soc 2011, 49(5):296–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia HH, Gonzalez AE, Evans CA, Gilman RH, Cysticercosis Working Group in P: Taenia solium cysticercosis. Lancet 2003, 362(9383):547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callacondo D, Garcia HH, Gonzales I, Escalante D, Nash TE, Cysticercosis Working Group in P: High frequency of spinal involvement in patients with basal subarachnoid neurocysticercosis. Neurology 2012, 78(18):1394–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keane JR: Cysticercosis: unusual neuro-ophthalmologic signs. J Clin Neuroophthalmol 1993, 13(3):194–199. [PubMed] [Google Scholar]
- 8.Karegowda LH, Shenoy PM, Prakashini K, Karur G: A rare case of racemose neurocysticercosis of the posterior fossa. BMJ Case Rep 2014, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carrillo Mezo R, Lara Garcia J, Arroyo M, Fleury A: Relevance of 3D magnetic resonance imaging sequences in diagnosing basal subarachnoid neurocysticercosis. Acta Trop 2015, 152:60–65. [DOI] [PubMed] [Google Scholar]
- 10.Arroyo G, Rodriguez S, Lescano AG, Alroy KA, Bustos JA, Santivanez S, Gonzales I, Saavedra H, Pretell EJ, Gonzalez AE et al. : Antibody Banding Patterns of the Enzyme-Linked Immunoelectrotransfer Blot and Brain Imaging Findings in Patients With Neurocysticercosis. Clin Infect Dis 2018, 66(2):282–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Connell EM, Harrison S, Dahlstrom E, Nash T, Nutman TB: A Novel, Highly Sensitive Quantitative Polymerase Chain Reaction Assay for the Diagnosis of Subarachnoid and Ventricular Neurocysticercosis and for Assessing Responses to Treatment. Clin Infect Dis 2020, 70(9):1875–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Proano-Narvaez JV, Meza-Lucas A, Mata-Ruiz O, Garcia-Jeronimo RC, Correa D: Laboratory diagnosis of human neurocysticercosis: double-blind comparison of enzyme-linked immunosorbent assay and electroimmunotransfer blot assay. J Clin Microbiol 2002, 40(6):2115–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan S, Qiao X, Liu L, Wu H, Zhou J, Sun R, Chen Q, Huang Y, Mao C, Yuan J et al. : Next-Generation Sequencing of Cerebrospinal Fluid for the Diagnosis of Neurocysticercosis. Front Neurol 2018, 9:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fei X, Li C, Zhang Y, Zhang H, Liu X, Ji X, Shi Y, Liu N, Wu M, Du F et al. : Next-generation sequencing of cerebrospinal fluid for the diagnosis of neurocysticercosis. Clin Neurol Neurosurg 2020, 193:105752. [DOI] [PubMed] [Google Scholar]
- 15.Wilson MR, O’Donovan BD, Gelfand JM, Sample HA, Chow FC, Betjemann JP, Shah MP, Richie MB, Gorman MP, Hajj-Ali RA et al. : Chronic Meningitis Investigated via Metagenomic Next-Generation Sequencing. JAMA Neurol 2018, 75(8):947–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White AC Jr., Coyle CM, Rajshekhar V, Singh G, Hauser WA, Mohanty A, Garcia HH, Nash TE: Diagnosis and Treatment of Neurocysticercosis: 2017 Clinical Practice Guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Am J Trop Med Hyg 2018, 98(4):945–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi B, Ge P, Yang H, Bi C, Li Y: Spinal intramedullary cysticercosis: a case report and literature review. Int J Med Sci 2011, 8(5):420–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitre E, Talaat KR, Sperling MR, Nash TE: Methotrexate as a corticosteroid-sparing agent in complicated neurocysticercosis. Clin Infect Dis 2007, 44(4):549–553. [DOI] [PubMed] [Google Scholar]
- 19.Nash TE, Ware JM, Coyle CM, Mahanty S: Etanercept to Control Inflammation in the Treatment of Complicated Neurocysticercosis. Am J Trop Med Hyg 2019, 100(3):609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williamson MR, Wilkinson CM, Dietrich K, Colbourne F: Acetazolamide Mitigates Intracranial Pressure Spikes Without Affecting Functional Outcome After Experimental Hemorrhagic Stroke. Transl Stroke Res 2019, 10(4):428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birk DM, Tobin MK, Moss HE, Feinstein E, Charbel FT, Alaraj A: Improvement in venous outflow following superior sagittal sinus decompression after a gunshot wound to the head: case report. J Neurosurg 2015, 123(1):81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hartmann A, Latting MW, Lee MS, Moster ML, Saindane AM, Newman NJ, Biousse V: Papilloedema from Dural Venous Sinus Compression by Meningiomas. Neuroophthalmology 2019, 43(3):171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takagi M, Oku H, Kida T, Akioka T, Ikeda T: Case of Primary Leptomeningeal Lymphoma Presenting with Papilloedema and Characteristics of Pseudotumor Syndrome. Neuroophthalmology 2017, 41(3):149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newton PN, Thai le H, Tip NQ, Short JM, Chierakul W, Rajanuwong A, Pitisuttithum P, Chasombat S, Phonrat B, Maek ANW et al. : A randomized, double-blind, placebo-controlled trial of acetazolamide for the treatment of elevated intracranial pressure in cryptococcal meningitis. Clin Infect Dis 2002, 35(6):769–772. [DOI] [PubMed] [Google Scholar]


