Abstract
Atrial fibrillation (AF) is one of the most common arrhythmias in adults, and its continued rise in the United States is complicated by the increased incidence and prevalence of several AF risk factors, such as obesity, physical inactivity, hypertension, obstructive sleep apnea, diabetes mellitus, coronary artery disease, and alcohol, tobacco, or caffeine use. Lifestyle and risk factor modification has been proposed as an additional pillar of AF therapy, added to rhythm control, rate control, and anticoagulation, to reduce AF burden and risk. Although emerging evidence largely supports the integration of lifestyle and risk factor management in clinical practice, randomized clinical trials investigating the long-term sustainability and reproducibility of these benefits remain sparse. The purpose of this review is to discuss potentially reversible risk factors on AF, share evidence for the impact on AF by modification of these risk factors, and then provide an overview of the effects of reversing or managing these risk factors on the success of various AF management strategies, such as antithrombotic, rate control, and rhythm control therapies.
Keywords: Lifestyle, risk factors, atrial fibrillation, ablation, cardioversion, rate control, rhythm control
INTRODUCTION
The prevalence of atrial fibrillation (AF) in the United States (U.S.) is projected to increase to 12.1 million cases in 2030, more than double the prevalence reported in 2010.1 Countries with high socio-demographic index have the highest burden; however, countries with middle socio-demographic index showed the largest recent increase.2 As AF incidence and prevalence are projected to continue to increase over the next several decades, AF will undoubtedly remain a large public health challenge.
Contributing to the increase in AF are its associations with older age and obesity, both risk factors for AF that are rising in the U.S. population. Besides obesity, other risk factors for AF may also be reversible, including physical inactivity, hypertension, obstructive sleep apnea (OSA), diabetes mellitus (DM), coronary artery disease (CAD), and alcohol, tobacco, or caffeine use. Several of these risk factors have been shown to reduce AF incidence as well as AF recurrence and burden.
Lifestyle and risk factor modification (LRFM) has been proposed as an additional pillar of AF therapy, added to rhythm control, rate control, and anticoagulation.3 This review will provide an overview of LRFM in AF and a comprehensive summary of the impact of reversing or managing these risk factors on the success of various AF management strategies, such as antithrombotic, rate control, and rhythm control therapies.
MECHANISMS UNDERLYING RISK FACTORS AND AF
The link between many of the proposed risk factors and AF is multifactorial. Regarding obesity, several mechanistic studies suggest that the stearic acid present in pericardial and epicardial fat can disrupt and remodel the properties of cardiac ion currents to promote arrhythmogenesis.4 Inflammatory markers associated with epicardial fat are also correlated with AF burden and severity.5,6 In addition, obesity is associated with a greater risk for hypertension and diastolic dysfunction, which increases AF susceptibility via stretch-activated left atrial channels.7,8 The risk of developing obesity and cardiovascular disease can be reduced with physical activity. Hypertension is also seen in association with obesity and is related to alterations in hemodynamics and increased ventricular afterload, causing left ventricular hypertrophy and left atrial enlargement and stiffness. Increased circulatory levels of angiotensin II, induced by hypertension, can also cause atrial fibrosis, hypertrophy, and remodeling, leading to electrophysiological changes that trigger AF development.9–11 OSA is characterized by recurrent short apneic episodes due to pharyngeal airway collapse. The intermittent hypoxia may also affect the atrial effective refractory period and increase AF susceptibility. These repetitive upper airway occlusions cause large oscillations in intrathoracic pressures, leading to short-term physiological changes, such as increased venous return, increased ventricular afterload, and, subsequently, increased left atrial pressures and enlargement. Over time, OSA can also promote prolonged systemic inflammation, a prothrombotic state, atrial fibrosis, and electrical remodeling.12,13 Although data on precise mechanistic pathways for DM remain limited, studies suggest that electrical and cardiac remodeling along with hyperglycemia-induced fibrotic change likely contribute to increased AF susceptibility.14–17
Mechanisms by which substance use in the form of alcohol, tobacco, and caffeine contributes to the development of AF have been described in the literature. Alcohol likely serves both as a trigger and propagator of AF via atrial remodeling and autonomic effects. Increased oxidative stress and autonomic function during alcohol consumption can directly impact atrial myocytes, resulting in long-term consequences such as left atrial remodeling and dilation.18,19 Smoking is associated with increased sympathetic tone, inflammation, thrombosis, endothelial dysfunction, atrial fibrosis, and oxidative stress, which are all potential factors in AF development.20 Cigarette smoke exposure has also been reported to modify the atrial electrophysiological substrate involved in arrhythmogenesis by increasing the cardiac acetylcholine activated inward rectifier potassium current (IKACh) via phosphatidylinositol 4-phosphate 5-kinase alpha (PIP5K) and ADP ribosylation factor 6 (Arf6) signaling.21 Precise mechanisms for caffeine’s impact on AF need to be elucidated. Moderate doses (<6 cups of coffee daily) of caffeine have been reported to be well-tolerated with little evidence to suggest a proarrhythmic state, whereas unusually large quantities of caffeine (>10g) have been associated with tachydysrhythmias.22
EFFECT OF LIFESTYLE OR RISK FACTOR MODIFICATION ON AF
The effect of lifestyle and risk factors on AF and the impact of modification of these risk factors on AF are summarized in Table 1. Most evidence is based on observational or retrospective studies, as few randomized studies have examined the direct effects of intentional modification of lifestyle and risk factors on AF.
TABLE 1.
Associations of modifiable risk factors and lifestyle/risk factor modification on AF incidence, burden, and progression
| Risk factors | Effect of risk factor on AF | Effect of LRFM on AF |
|---|---|---|
| Weight/obesity | ↑ | ⬇ |
| Physical inactivity | ↑ | |
| Moderate activity | ⬇ | |
| High-intensity activity | ⬇/-/⬆ | |
| SDB/OSA | ↑ | ↓/- |
| DM | ↑ | ⬇ |
| HTN | ↑ | ⬇/- |
| CAD | ↑ | NA |
| Alcohol | ↑ | ⬇ |
| Tabacco | ↑ | NA |
| Caffeine | ↓/-/↑ | - |
Note: Bolded symbols indicate at least some evidence is supported directly by randomized controlled trials.
Abbreviations: AF, atrial fibrillation; CAD, coronary artery disease; DM, diabetes mellitus; HTN, hypertension; LRFM, lifestyle and risk factor modification; NA, no data available; OSA, obstructive sleep apnea; SDB, sleep-disordered breathing.
↑, Increast AF incidence/burden/progression.
-, No effect on AF incidence/burden/progression.
↓, Decreased AF incidence/burden/progression.
Weight Management
Obesity and higher body mass index (BMI) remain strong risk factors for AF that contribute to the incidence of AF, as well as increased AF burden, including the progression from paroxysmal to persistent or permanent AF. A Mendelian randomization study suggests the relationship between obesity and AF may be causal.23 A 51-study meta-analysis showed that with each 5-unit increase in BMI, there is a corresponding increase in incident AF by 29% and 19% in cohort and case-control studies, respectively, as well as an increase in postoperative AF by 10% and post-ablation AF by 13%.24
In a series of studies from a group in Australia, Sanders and colleagues showed a benefit of weight loss on AF burden. A single-center randomized controlled trial (RCT) from this group reported that patients with symptomatic AF who underwent weight management, compared to patients who received only general lifestyle advice, experienced more significant reductions in AF burden, symptom severity scores, cumulative duration, and interventricular septal thickness.25
Also from this group, in the LEGACY (Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort) study, long-term patient outcomes were assessed over four years by the degree of weight loss: group 1 (>10%), group 2 (3 to 9%), and group 3 (<3%).26 Group 1 exhibited lower AF burden and greater arrhythmia-free survival rates. The REVERSE-AF (PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation) study, a sub-analysis of the LEGACY study, assessed the degree of weight loss and its impact on the progression of AF.27 Patients with the greatest degree of weight loss had the lowest rates of progression from paroxysmal to persistent AF and the highest rates of reversal from persistent to paroxysmal or no AF.
Physical Activity
Regular moderate aerobic exercise is effective for reducing AF burden and AF-related symptoms and improving quality of life. In addition to the effect of weight loss on AF outcome in the LEGACY study, the CARDIO-FIT (Impact of CARDIOrespiratory FITness on Arrhythmia Recurrence in Obese Individuals With Atrial Fibrillation) study explored the interaction between cardiorespiratory fitness and weight loss on rhythm control in individuals with a BMI ≥27 kg/m2 and non-permanent AF.28 Patients’ initial cardiorespiratory fitness were categorized as low (<85%), adequate (86 to 100%), or high (>100%). At the final follow-up, patients were grouped by cardiorespiratory fitness gain [≥2 metabolic equivalents (METs) vs. <2 METs]. Patients in the high cardiorespiratory fitness group experienced the greatest arrhythmia-free survival, and patients in the ≥2 METs improvement group experienced significantly reduced AF burden and symptom severity. Each unit gain in METs was associated with a 9% gain in long-term freedom from AF. Individuals with both >10% weight loss and ≥2 METs gain reported the greatest freedom from AF, suggesting that physical activity and weight loss may have a synergistic relationship.
Physical activity may mitigate some of the AF risk associated with obesity. The Women’s Health Initiative (WHI) prospective observational study of postmenopausal women over a period of 11.5 years showed that greater physical activity was correlated with lower incident AF rates and reduced the AF risk conferred by obesity.29 The HUNT-3 prospective cohort study in Norway of individuals ≥18 years of age showed that higher levels of physical activity could offset some, but not entirely, the AF risk associated with obesity.30
Exercise training in patients with AF can have favorable effects beyond AF reduction. A meta-analysis of five RCTs in AF patients found that exercise training was associated with reduced BMI and improved exercise capacity, left ventricular ejection fraction, and quality of life.31 In one study, AF patients were randomized into either a short-term aerobic and muscle-strengthening exercise program or a two-month control period.32 Those who received the short-term exercise training reported improved AF-related symptoms and health-related quality of life. A similar study randomized patients with permanent AF into either 12-week aerobic exercise training or a control group.33 Patients in the exercise training group not only reported greater quality of life, as assessed by the Minnesota Living With Heart Failure (MLHF-Q) questionnaires, but they also demonstrated greater overall exercise capacity and decreased resting pulse rate. Of note, leisure-time activities and walking were associated with decreased AF incidence, which were later supported by findings reported by a prospective study of AF-free women ≥45 years of age without AF and cardiovascular disease at enrollment34 and a population-based Swedish cohort of AF-free middle-aged and elderly women.35 These studies suggest that encouraging exercise training in patients with AF may improve not only AF burden but also symptoms and quality of life.
Research on the impact of high-intensity interval training (HIIT) on AF has yielded some conflicting reports. Non-permanent AF patients were randomized to either HIIT or to resume their regular exercise habits. Those in the HIIT group exhibited reduced AF burden and improved AF-related symptoms, peak O2, left atrial and ventricular function, lipid levels, and quality of life.36 A separate 12-week RCT showed that high-intensity physical exercise did not reduce AF burden compared to low-intensity physical exercise, though HIIT was more time-efficient.37 A randomized, prospective, longitudinal study reported greater AF occurrence in patients with concomitant hypertension and chronic kidney disease that engaged in HIIT compared to moderate exercise.38 Although there was no difference in atrial electrical activity between HIIT and yoga in a randomized study, HIIT was associated with changes in left atrial mechanical functioning and adverse remodeling of the left atrium and left ventricle.39
Although evidence suggests that moderate physical activity benefits AF reduction, extreme levels of exercise may be associated with a higher risk of AF. In a prospective study of adults ≥65 years of age, light-to-moderate-intensity exercise had lower AF incidence compared to no and high-intensity exercise, displaying a U-shaped relationship between exercise intensity and AF incidence.40 A meta-analysis of six case-control studies reported a greater risk of AF in athletes compared to non-athletes.41 A cohort study examining long-distance cross-country skiers reported a higher incidence of AF in those with faster finishing times and a high number of completed races, suggesting an exercise-dose-dependent relationship.42 Similar adverse results have been described in former professional cyclists,43 sports competitors,44 and marathon runners.45 Importantly, this level of extreme exercise capacity is not achieved by the average individual, and the 150 minutes/week of moderate-intensity or 75 minutes/week of vigorous-intensity exercise recommended for all adults by the 2018 Physical Activity Guidelines Advisory Committee do not increase the risk of AF.
Multicomponent and mind-body exercises, such as yoga, tai chi, and qigong, have also been proposed as potential domains for exploration. A single-center clinical trial showed that paroxysmal AF patients had improved quality of life and reduced AF burden, AF-related symptoms, heart rate, blood pressure (BP), and anxiety and depression scores after receiving yoga training.46
Sleep-disordered Breathing / Obstructive Sleep Apnea Therapy
Sleep-disordered breathing (SDB), including OSA, is often an undiagnosed risk factor among AF patients. A systematic review and meta-analysis showed that SDB is highly prevalent in AF patients, but inconsistent diagnostic tools and thresholds are often used to detect concomitant SDB in AF. Using a SDB diagnosis cutoff of apnea-hypopnea index (AHI) ≥5/h, the pooled SDB prevalence was 78%, with moderate-to-severe SDB making up 40%.47 An analysis of the ORBIT-AF (Outcomes Registry for Better Informed Treatment of AF) cohort study revealed that patients with OSA treated with continuous positive airway pressure (CPAP) were less likely to progress to more permanent AF compared to those without CPAP treatment.48 Greater benefits were observed in the younger, obese, and male patients. On the other hand, a RCT of 2,717 patients concluded that the addition of CPAP to usual care for patients with moderate-to-severe OSA and established cardiovascular disease did not significantly improve the prevention of cardiovascular events.49 Another RCT of 109 patients with paroxysmal AF and moderate-to-severe obstructive and/or central sleep apnea, who tolerated CPAP over a two-week period, failed to show a significant reduction in AF burden.50 However, the study excluded patients with LVEF <45%, BMI >40 kg/m2, or severe excessive daytime sleepiness (Epworth Sleepiness Scale score >15), arguably patient groups who may have had the most to gain from the treatment of sleep apnea. A bidirectional Mendelian randomization study found genetically predicted OSA to be causally associated with increased AF risk, suggesting that earlier OSA screening and management may have beneficial anti-arrhythmic effects.51
Diabetes Mellitus Management
DM has been associated with risk for incident AF. A large-scale study from the Veterans Health Administration Hospitals identified DM as a strong, independent risk factor for AF occurrence using a multivariate analysis.52 A large systematic review and meta-analysis of seven prospective cohort studies and four case-control studies showed that DM was associated with a 40% greater risk of AF.53 This reported association between DM and AF may be reduced after adjusting for confounding risk factors.54 A recent Swedish cohort study found that individuals with type 2 DM had an overall 35% increased risk of AF after controlling for age and sex.55
Poor glycemic control has been independently associated with an increased risk of AF,56 and certain anti-diabetic agents have been associated with lower AF risk. Serum hemoglobin A1c (HbA1c) levels have been described as a potential predictive biomarker for AF risk.57 Thiazolidinediones have been correlated with lower AF development in non-insulin-dependent patients.58 Similarly, metformin was associated with decreased AF risk in a population study of 645,710 type 2 DM patients in Taiwan.59 Pioglitazone was effective in reducing the progression rate from persistent to permanent AF in a RCT of 146 patients.60 A recent meta-analysis of 16 RCTs also concluded that sodium-glucose transport protein 2 (SGLT-2) inhibitors were associated with a 24% reduction in AF risk.61 However, no randomized trials have focused solely on glycemic control as an AF intervention.
Hypertension Management
Hypertension has been implicated as the most important contributor to AF development with the population-attributable fraction for AF being 21.6%, compared to 12.7% for BMI, 7.45% for smoking, and 8.77% for DM.62 The SPRINT (Systolic Blood Pressure Intervention Trial) RCT of intensive BP control concluded that patients with hypertension and high risk of cardiovascular disease who underwent intensive BP lowering with a target systolic BP <120 mmHg compared to a goal of <140 mmHg had a 26% reduction in AF risk.63 These results were subsequently corroborated in a recent meta-analysis.64 Uncontrolled hypertension at the upper high-normal range, systolic BP of 128–138 mmHg or diastolic BP of 80–90 mmHg, is a long-term predictor of incident AF in initially healthy middle-aged men.65 Although mineralocorticoid receptor antagonist treatment with either eplerenone or spironolactone has been shown in a meta-analysis to be associated with a decreased AF risk and recurrence66, the use of angiotensin-converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARB) have yielded mixed results in terms of primary AF prevention.67,68
Coronary Artery Disease Treatment
AF and CAD share similar risk factors69,70, suggesting that management focused on targeting common risk factors may prove beneficial. However, at this point, only beta-blockers have been associated with an antiarrhythmic effect after an acute myocardial infarction.71 Low-density lipoprotein cholesterol (LDL-C) has been long-established as a causal factor in the development of atherosclerotic cardiovascular disease, which in turn is also an independent risk factor for the development of AF. However, current studies, largely observational, have suggested that elevated levels of LDL-C and total cholesterol may be inversely associated with incident AF.72 Although the clinical significance of this “cholesterol paradox” remains unclear, hyperlipidemia should continue to be evaluated and managed as a part of a patient’s overall cardiovascular risk.73
Alcohol Reduction
Alcohol consumption has been described as having a dose-response relationship with AF development, and consuming even 1 drink/day may significantly increase AF risk.74,75 The Norwegian HUNT study demonstrated a curvilinear relationship between alcohol consumption and AF risk.76 <1 drink/day for women and <2 drinks/day for men were not associated with AF risk. There was almost no increase in AF risk up to 7 drinks/week, but >14 drinks/week was correlated with a steep increase in AF risk. Similarly, a pooled cohort study found that consumption of more than one up to 2 drinks/day was associated with a 28% increased risk of AF, and consumption of more than 4 drinks/day was associated with a 47% increased risk of AF.75
In patients with AF, AF burden and outcomes may be improved by moderation or abstinence from alcohol use. An observational study stratified patients according to alcohol consumption (abstainer-rare, light (<100 g/week), moderate (100–200 g/week), and heavy (≥200 g/week)) and found that only heavy alcohol consumption increased the risk of adverse events in AF patients, such as ischemic stroke, transient ischemic attack, systemic embolic event, or AF hospitalization.77 However, alcohol as a trigger of AF in AF patients was confirmed in a recent N-of-1 study by Marcus and colleagues.78 Furthermore, a multicenter study randomized 140 patients with a history of paroxysmal or persistent AF in sinus rhythm at the time of the study and drank ≥10 drinks/week to either abstain from alcohol or continue usual alcohol consumption.79 In the abstinence group, alcohol intake was reduced from 16.8±7.7 to 2.1±37 drinks/week, 61% completely abstained, 76% reduced intake to ≤2 drinks/week, and 86% reduced intake by >70% of baseline. In the control group, intake was slightly reduced from 16.4±6.9 to 13.2±6.5 drinks/week. Patients who followed alcohol abstinence showed reduced arrhythmia recurrence as well as lowered AF burden by 58% by six months compared to control subjects. In secondary analyses, greater weight reduction and AF symptom improvement were noted in the abstinence group compared to the control group. Patients who completely abstained had a lower risk of recurrent AF compared to patients who consumed 1–9 drinks/week or ≥10 drinks/week. These data support counseling AF patients on modifying alcohol use to improve AF burden.
Tobacco Smoking Control
A large meta-analysis showed a dose-dependent relationship between smoking and AF risk, with a stronger association in current smokers compared to former smokers.80 Tobacco smokers had a 33% higher AF risk than individuals who have never smoked.
Smoking cessation after diagnosis of AF may have beneficial consequences on AF and AF outcomes. In another study of 2,372 males with newly diagnosed AF from the Korean National Health Insurance Service database, smokers who quit after AF diagnosis and those who have never smoked had a reduced risk of cardiovascular disease and ischemic and total stroke compared to continual smokers.81 In a larger study of 97,637 patients from the Korean National Health Insurance Service database, 6.9% stopped smoking after AF diagnosis, and 14.6% continued to smoke.82 Quitters had a 30% lower risk of ischemic stroke (55% reduction in fatal stroke) and 16% reduction in all-cause death (34% reduction in death from cerebrovascular events), irrespective of oral anticoagulation status.
Caffeine Consumption
Caffeine may have differential effects on incident AF versus patients with an established history of AF. In a long-term prospective cohort study of men who participated in the Physicians’ Health Study, there was a slightly lower risk of AF among those who drank one to three cups of coffee per day; however, there was no significant increase in AF risk for consumption <1 cup/day or >3 cups/day.83 A univariate analysis of lone AF patients showed that increasing levels of coffee consumption were associated with significantly greater AF risk, with the lowest probability of spontaneous conversion observed in those consuming more than three cups of espresso per day.84 A meta-analysis of six prospective cohort studies found a very weak association between caffeine exposure and reduced AF risk, specifically an 11% reduction in AF risk for low doses (<500 mg/day) and a 16% reduction for high doses (≥500 mg/day).85 The study also described a dose-response relationship in which AF incidence decreased by six percent for every 300 mg/day increment increase in habitual caffeine intake. Although there may be a possible protective effect at low86 and elevated doses as well as habitual caffeine intake, a clear link between caffeine consumption and incident AF risk has not been established. The CRAVE (Coffee and Real-time Atrial and Ventricular Ectopy) trial recently reported at the American Heart Association Scientific Sessions in November 2021 that in healthy patients, caffeine did not increase atrial arrhythmias, though it increased premature ventricular complexes. This was concordant with other observational studies showing no increase in AF in a “primary prevention”, no prior AF population. In contrast, 25–28% of patients with AF have reported caffeine as a trigger.87,88 The I-STOP-AFib (Individualized Studies of Triggers of Paroxysmal Atrial Fibrillation) N-of-1 trial showed no significant change in AF with caffeine; however, self-selection of this trigger in the trial may have affected results.78
EFFECT OF LRFM ON AF MANAGEMENT STRATEGIES
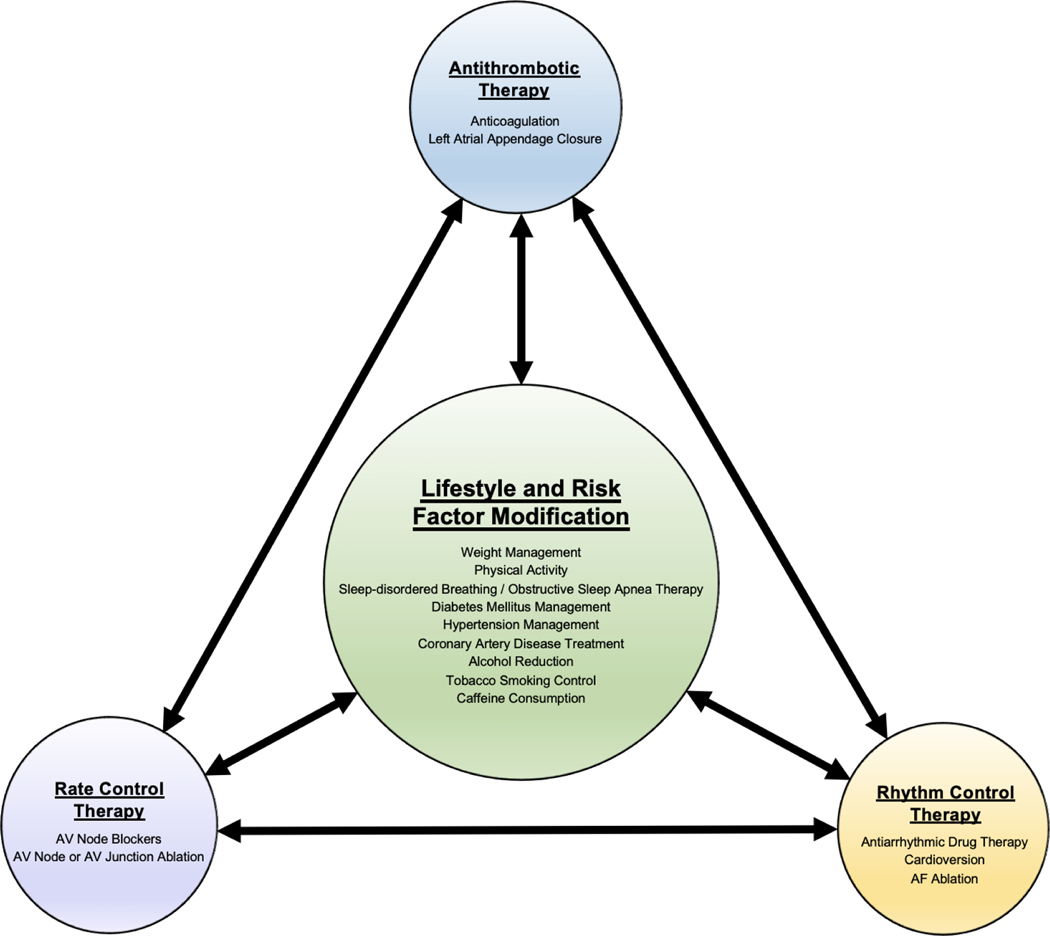
Several studies have reported associations of potentially reversible risk factors on the outcomes of AF management therapies (Figure 1). This section will review existing data (Table 2). However, randomized trials studying the impact of LRFM on AF management strategies remain sparse.
Figure 1: Synergy of LRFM and Traditional AF Management Strategies.
AF management should follow an integrated approach, utilizing lifestyle and risk factor modifications in conjunction with antithrombotic, rate control, and/or rhythm control therapies. AF = atrial fibrillation. AV = atrioventricular.
TABLE 2.
Effect of modifiable risk factors and lifesyle/risk factor modification on AF management strategies
| AF management strategy | Weight/obesity | Phiyslcal inactivity | SDB/OSA | DM | HTN | CAD | Alcohol | Tobacco | Caffeine | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
||||||||||
| RF | LHFM | RF | LRFM | RF | LRFM | RF | LRFM | RF | LRFM | RF | LRFM | RF | LRFM | RF | LRFM | RF | LRFM | |
| Antithrombotic therapy | ||||||||||||||||||
| Anticoagulation | -/⬆ | NA | NA | NA | NA | NA | - | NA | ↓ | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| LAAC | NA | NA | NA | NA | NA | NA | ↓/- | NA | NA | NA | NA | NA | NA | NA | ↓ | NA | NA | NA |
| Rate control | NA | NA | NA | ↑ | - | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Rhythm control | ||||||||||||||||||
| AAD therapy | ↓/- | NA | NA | NA | ↓ | NA | NA | NA | NA | - | NA | NA | NA | NA | NA | NA | NA | NA |
| cardioversion | ↓ | ↑ | NA | ↑ | ↓ | - | ↓ | - | ↓ | -/⬆ | NA | ↓/↑ | - | NA | ↓/- | NA | ↓ | NA |
| AF ablation | ↓ | -/↑ | ↓ | NA | ↓ | -/↑ | ↓ | ↑ | ↓ | -/⬆ | NA | ↑ | ↓/- | ↑ | ↓/- | ↑ | NA | NA |
Note: Bolded symbols indicate at least some evidence is supported directly by randomized controlled trials.
Abbreviations: AAD, antiarrhythmic drug; AF, atrial fibrillation; CAD, coronary artery disease; DM, diabetes mellitus; HTN, hypertension; LAAC, left atrial appendage; closure; LRFM, lifestyle and risk factor modification; NA, no data available; OSA, obstructive sleep apnea; RF, risk factor; SDB, sleep-disordered breathing.
↑, Higher success of AF management strategy.
—, No effect on AF management strategy.
↓. Lower success of AF management strategy.
Antithrombotic Therapy
Anticoagulation
The impact of obesity on the efficacy of anticoagulation using non-vitamin K antagonist oral anticoagulants (NOACs) has been a concern, as levels are not monitored, in contrast to INR monitoring used for warfarin.89,90 However, four NOAC versus warfarin trials with BMI stratification data demonstrated preserved efficacy and similar bleeding risk of NOACs versus warfarin in AF patients with obesity.91 Overall, NOACs can be efficacious and safe for AF patients with a BMI ≤50 kg/m2. Interestingly, some clinical trials have observed an “obesity paradox” in which higher BMI was associated with better overall outcomes, such as reduced stroke risk, in patients treated with anticoagulant therapy.92,93 However, the weight classes of enrolled participants in these trials were not equally distributed, resulting in biased data comparisons. Overall, studies remain limited for patients experiencing severe obesity. A meta-analysis of RCTs suggested no difference in NOAC effects between different BMI classes among AF patients; however, patient data was limited in the class III obesity category, and the overall findings and trends were not significant and/or conclusive.94
Although some subanalyses of phase III clinical studies95, registries, and small-sample prospective trials96 have reported comparable efficacy and safety of NOACs in patients with or without DM, the original studies were not explicitly designed to examine the effect of DM on clinical outcomes in AF patients treated with NOACs versus warfarin. The impact of DM management on AF anticoagulation outcomes and drug interactions between NOACs and common antidiabetic agents remain areas in need of further study.97
The effect of high BP on anticoagulation-associated intracranial hemorrhage or hemorrhagic expansion is variably reported.98–100 For AF patients, higher BP has been associated with an increased risk of thromboembolic complications. A cross-sectional, longitudinal analysis of data from the SPORTIF (Stroke Prevention using an ORal Thrombin Inhibitor in AF) III and V trials reported increased rates of stroke and systemic embolic events with higher BP levels, especially for individuals with systolic BP levels ≥140 mmHg.101
Patients with concomitant CAD and AF may require triple antithrombotic therapy (dual antiplatelet plus oral anticoagulation) around the time of acute coronary syndromes and/or percutaneous coronary intervention (PCI). However, such regimens are associated with a higher risk of bleeding. Current consensus recommendations include: minimization of the use of triple therapy to short durations (e.g., 30 days) due to the increased risk of bleeding; when an antiplatelet is needed along with an anticoagulant, clopidogrel is most often recommended; when aspirin is used, limit dosing to <100 mg/day; use of a proton pump inhibitor when dual antithrombotic agents are used; for PCI in AF patients, a NOAC is preferred over warfarin and use, e.g. oral anticoagulation with a P2Y12 antiplatelet agent for the first six to 12 months (or aspirin for the last six months if stable ischemic disease) with change to anticoagulation monotherapy after one year.102,103 Recommendations may be subject to change as additional studies in this area are reported.
Left Atrial Appendage Closure
Although AF patients with DM are at higher risk for bleeding and thromboembolism, outcomes after left atrial appendage closure (LAAC) appear comparable to those in non-diabetics.104,105 However, a recent multicenter study of 277 patients with LAAC and prior gastrointestinal bleeding reported DM as a risk factor for higher mortality after LAAC.106 Smoking was identified as an independent predictor of device-associated thrombus after LAAC with one device.107 The impact of modification of lifestyle or risk factors on LAAC outcomes has not been studied.
Rate Control Therapy
Except for better rate control with moderate physical activity, other lifestyle or AF risk factors do not appear to significantly affect pharmacological rate control therapies using AV node blockers or nonpharmacologic approaches with AV node or AV junction ablation.
A prospective pilot study of 10 permanent AF patients observed a significant decrease in ventricular rate with regular moderate physical activity.108 A retrospective study noted that the presence and severity of OSA had no correlation with inadequate heart rate control, suggesting that successful rate control in AF patients can be achieved regardless of whether the patient has OSA.109 An association of obesity and/or type 2 DM with cardiac fibrosis that affects the conduction system has been theorized to predispose patients to bradyarrhythmias and heart block following the prescription of AV nodal-blocking drugs.110 However, we found no data to support a clinically significant hypersensitivity to these drugs in obese or diabetic patients with AF.
Rhythm Control Therapy
In an international, blinded RCT, investigators assigned patients with early AF (diagnosed ≤1 year before enrollment) to either rhythm control (treatment with either antiarrhythmic drugs or AF ablation) or usual care (management of AF-related symptoms).111 Those receiving early rhythm control therapy had a lower risk of adverse cardiovascular outcomes than the usual care group; however, there were no differences in symptoms and left ventricular function at two years. Although the percentage of primary safety outcome events (composite of death, stroke, or serious adverse events related to rhythm control therapy) did not differ between the two groups, the rate of serious adverse events related to rhythm control therapy was 4.9% compared to 1.4% among those assigned to usual care. In this section, the impact of LRFM on various rhythm control therapies, such as antiarrhythmic drugs, cardioversion, and AF ablation, is discussed.
Antiarrhythmic Drug Therapy
Obesity is an established risk factor for AF, but the precise mechanisms of how it affects antiarrhythmic drug response remain unclear. An observational cohort study found that patients with obesity were less likely to respond to class I antiarrhythmic drugs compared to patients without obesity. However, both groups responded similarly to a potassium channel blocker.112 Patients with more severe OSA were more likely to be non-responders to antiarrhythmic drug therapy for AF than those with milder disease.113 Although some retrospective analyses have associated renin-angiotensin system (RAS) inhibitors with the prevention of AF development in patients with a history of chronic heart failure (CHF) or left ventricular hypertrophy (LVH),114–117 a retrospective analysis of the CTAF (Canadian Trial of Atrial Fibrillation) randomized multicenter study, which enrolled few CHF or LVH patients and excluded patients with severely symptomatic CHF, did not observe additional benefit against AF recurrence with the inclusion of RAS blockers to antiarrhythmic agents.118 However, there are no randomized studies specifically addressing LRFM effects on antiarrhythmic drug (AAD) therapy outcomes.
Cardioversion
Various observational studies have identified risk factors associated with AF recurrence versus success after cardioversion. Higher direct current cardioversion (DCCV) failure has been observed in persistent AF patients with obesity. An observational substudy within a RCT studying manual pressure augmentation noted that patients with higher BMIs were more likely to fail shocks.119 It was hypothesized that the lower success rate may be due to higher transthoracic impedance, longer interelectrode distance, and decreased transthoracic current flow secondary to current dissipation. However, success rates improved among patients with obesity when incorporating the use of paddles with manual pressure augmentation and escalating energy outputs to 360J.
The RACE 3 (Routine vs. Aggressive risk factor driven upstream rhythm Control for prevention of Early atrial fibrillation in heart failure) trial randomized patients with early persistent AF and mild-to-moderate heart failure (HF) who received electrical cardioversion to either conventional therapy or targeted therapy of underlying conditions.120 The targeted therapy group included four therapies: (i) mineralocorticoid receptor antagonists (MRAs), (ii) statins, (iii) angiotensin-converting enzyme inhibitors and/or receptor blockers, and (iv) cardiac rehabilitation including physical activity, dietary restrictions, and counseling. Greater improvement in sinus rhythm maintenance was observed in the targeted therapy group compared to the conventional therapy group. However, conclusions regarding specific interventions are limited since four interventions were started simultaneously in the targeted therapy group.
Higher AF recurrence after cardioversion was noted in patients with untreated OSA compared to patients without a polysomnographic diagnosis of OSA.121 AF recurrence at 12 months was 82% in the untreated OSA group (n=27), 42% in the treated OSA group (n=12), and 53% in the control group (n=79). Of the patients without any OSA therapy, a greater nocturnal fall in oxygen saturation was associated with higher AF recurrence rates. However, a RCT of adults who underwent successful direct current cardioversion did not find a difference in AF recurrence between the groups that were treated with positive airway pressure versus usual care.122
Diabetes mellitus has been a long-established independent risk factor for AF recurrence following successful DCCV, and it has been associated with a 4.6 times higher risk of AF recurrence compared to patients without DM.123,124 Type 2 DM, higher HbA1c levels, digoxin treatment, statin use, left atrial size, and LV ejection fraction have all been identified as independent risk factors for either immediate DCCV failure or AF relapse.125 Although pioglitazone has been associated with positive effects following catheter ablation126, a prospective randomized trial did not find an impact on AF recurrence after electrical cardioversion.127
The impact of hypertension management on cardioversion success and AF recurrence varies across observational studies and RCTs. In a post-hoc retrospective analysis, pretreatment with ACEi was observed to improve acute cardioversion but was not associated with reduced AF recurrence or improved sinus rhythm maintenance.128 A separate retrospective review found that patients who had a lower BP immediately prior to the cardioversion were more likely to achieve sinus rhythm.129 These results are similar to those reported in a RCT of persistent AF patients, in which those treated with amiodarone plus irbesartan before cardioversion had reduced AF recurrence rates compared to those with amiodarone alone.130 Another RCT of persistent AF patients also found that the addition of enalapril to amiodarone decreased arrhythmia recurrence and improved long-term sinus rhythm maintenance.131 However, a RCT with 171 persistent AF patients did not find that treatment with candesartan for three to six weeks before and six months after cardioversion impacted AF recurrence rates.132 Similar results were seen in a multicenter RCT that enrolled 1,442 patients with a prior history of at least underlying cardiovascular disease, DM, or left atrial enlargement and had a successful cardioversion for AF in the previous two weeks. Patients assigned to receive valsartan rather than a placebo did not have a reduction in AF recurrence.133
In one study of 116 patients with lone AF cardioverted within 48 hours of arrhythmia onset, higher ingestion of coffee (>3 cups/day), but not alcohol nor smoking habits, was associated with a reduced probability of spontaneous conversion.84 A prospective study later reported smoking to be an independent risk factor for atrial arrhythmia recurrence after cardioversion in women and was associated with increased mortality risk in men.134
In summary, randomized controlled studies have shown that targeted therapy of underlying conditions in AF patients and HF effectively improved weight, BMI, lipid profile, and HF and led to greater sinus rhythm maintenance, whereas OSA therapy and DM management did not affect AF recurrence post-cardioversion. Other retrospective or observational reviews have noted an association between obesity and decreased cardioversion success and between higher caffeine use and lower spontaneous AF conversion. Mixed results and conclusions have been reported for hypertension management, coronary artery disease treatment, and smoking cessation.
AF Ablation
The impact of risk factor modification on AF ablation outcomes has been studied mostly via retrospective and prospective observational studies. Very few RCTs regarding this topic have been reported in the literature.
In retrospective studies from Cleveland Clinic, patients with morbid obesity undergoing AF ablation were compared to a control group with BMI <30 kg/m2 matched by age, sex, ejection fraction, AF type, HF presence and type, and left atrial volume index.135 Greater arrhythmia recurrence was observed in patients with morbid obesity compared to the control group. Within the morbid obesity group, lower arrhythmia recurrence was seen among those with other risk factor modifications: sleep apnea screening and treatment, BP control, glycemic control, and weight loss ≥5%. A greater number of met risk factor modification goals was correlated with lower arrhythmia recurrence, reduced need for repeat ablation or direct-current cardioversion, and fewer arrhythmia-related hospitalizations. AF recurrence was retrospectively assessed in patients with morbid obesity (BMI ≥40 kg/m2 or BMI ≥35 kg/m2 with obesity-related complications) who received bariatric surgery either before or after ablation.136 Patients who had pre-ablation bariatric surgery experienced lower rates of AF recurrence.
In the ARREST (Aggressive Risk Factor Reduction Study for Atrial Fibrillation) prospective, observational cohort study from Australia, patients with a BMI >27 kg/m2 and at least one cardiac risk factor who underwent AF ablation were offered aggressive risk factor management consisting of BP control, weight management, lipid management, glycemic control, sleep-disordered breathing management, and smoking and alcohol counseling.137 Compared to the control group, patients receiving risk factor management showed decreased AF frequency, duration, symptoms, and symptom severity at follow-up. Single-procedure drug-unassisted and multiple-procedure arrhythmia-free survival were significantly better in the risk factor management group. However, these findings may be limited by selection bias and potential confounders.
The SORT-AF (Supervised Obesity Reduction Trial for AF Ablation Patients) study was a prospective RCT of symptomatic AF patients with a BMI between 30 and 40 kg/m2.138 All patients underwent AF ablation and were randomized to either weight reduction or usual care. AF ablation was effective at significantly reducing AF burden in patients with obesity; however, interestingly, there was no significant difference in AF burden reduction 3- and 12-months post-ablation between the weight-reduction or usual care groups. Contrary to previous landmark studies, including the LEGACY and CARDIO-FIT studies, that included various AF therapy strategies, the SORT-AF study focused solely on weight loss in the context of AF ablation. In contrast to the ARREST-AF observational study, where AF ablation patients were grouped into risk factor management versus control depending on the patient’s personal decision and motivation, SORT-AF randomized all eligible patients into a structured weight reduction program. Despite the rate of non-compliance and differences in motivation in the weight reduction program, the mean weight reduction of four percent of initial body weight was comparable to other studies and provided a more realistic scenario. However, the weight loss may have been too little to significantly impact AF burden, as evidenced by the LEGACY study, which reported the greatest effect in patients who lost >10% of their body weight. BMI reduction and increased exercise capacity were associated with reduced AF recurrence in persistent compared to paroxysmal AF patients, which may be related to the association of obesity with more advanced atrial substrate.
In patients enrolled in the DISCERN AF (Discerning Symptomatic and Asymptomatic Episodes Pre and Post Radiofrequency Ablation of Atrial Fibrillation) study, daily activity level was inversely associated with daily AF burden post-ablation.139 However, a causal relationship cannot be established since decreased AF burden may permit patients to pursue more daily physical activities.
A nurse-led risk factor modification program to improve weight loss and OSA care among AF patients undergoing catheter ablation was conducted.140 Nurses provided in-person consultations and monthly telephone calls to patients for up to one year. Enrolled patients demonstrated significant weight loss (4.7% ± 5.3% versus 0.3% ± 4.4%) and improved OSA care compared to patients who declined enrollment in the program; however, outcomes in terms of arrhythmia control (0–6 self-terminating recurrences with ≤1 cardioversion for nonparoxysmal AF) and freedom from arrhythmias (no recurrences on or off antiarrhythmic drugs) were similar at one year. Similar to the SORT-AF study, the failure to show a benefit of weight loss on AF ablation outcomes may be due to an inadequate degree of weight loss (4.7% compared to the 10% weight loss showing effects in the LEGACY study). Also, this program was part of a quality improvement initiative with voluntary participation and was not a research study. Thus, the authors stated that not all enrolled patients were treated to the original management strategy and/or completed the necessary follow-up, which limited the ability to determine if participation in the program was directly responsible for the findings reported.140
OSA has been associated with greater AF recurrence following ablation in AF patients. A meta-analysis of eight studies (one RCT and seven prospective cohort studies) on OSA showed that patients treated with CPAP had a 44% decreased risk of AF, and among those who underwent catheter ablation, AF recurred in 18% of those who used CPAP and 37% in those who did not.141 Other meta-analyses of observational studies have highlighted similar correlations between OSA, CPAP treatment, and AF recurrence risk after catheter ablation.142–144 However, these findings are inconsistent with a recent RCT where 83 patients with paroxysmal AF after pulmonary vein isolation (PVI) were assigned to either CPAP treatment or standard care. PVI considerably decreased AF burden in both groups with no between-group difference. Despite a reduction in AHI from 26.7 ± 14 events/hour to 1.7 ± 1.3 events/hour at follow-up in the CPAP group, there was no further reduction in the risk of AF recurrence after ablation with CPAP treatment.145
Worse outcomes and greater AF recurrence rates have been observed in patients with DM undergoing ablation. An observational study of seven high-volume European centers found that arrhythmia relapses were more common in diabetic patients 12 months after AF ablation.146 Additionally, a higher rate of atrial arrhythmia relapse was observed in patients with persistent AF compared to those with paroxysmal AF. A prospective observational cohort study of paroxysmal AF patients with type 2 DM showed that the use of pioglitazone before undergoing catheter ablation increased the probability of achieving sinus rhythm after a single ablation and decreased the necessity for repeat ablation.126 A retrospective observational cohort study found that worsening and higher 12-month pre-ablation HbA1c levels were associated with greater AF recurrence rates after ablation.147 Thirty-two point four percent of patients with HbA1c <7% at the time of ablation developed recurrent AF compared to 68.75% of the patients with HbA1c >9%. Treatment of diabetic patients with metformin was also independently associated with a significant decrease in the recurrence of atrial arrhythmias after AF catheter ablation.148
Management of hypertension prior to undergoing ablation has been associated with mixed results. Early prospective and retrospective studies did not observe a decreased AF recurrence rate following ablation in patients pretreated with ACEi or ARBs.149,150 A RCT showed that aggressive BP lowering before AF catheter ablation did not affect arrhythmia recurrence compared to individuals who received standard BP treatment.151 However, other works have highlighted how the autonomic nervous system may contribute to the development and progression of AF. In one study, 86 patients were randomized to either PVI or PVI with renal artery denervation (RDN) and followed over 12 months. The addition of RDN to PVI was associated with decreased AF recurrence, AF burden, and BP.152 The ERADICATE-AF (Evaluate Renal Denervation in Addition to Catheter Ablation to Eliminate Atrial Fibrillation) trial also examined whether adding RDN to PVI could improve freedom from AF, atrial flutter, or atrial tachycardia at 12 months. Three hundred and two patients with paroxysmal AF were randomized into either PVI or PVI with RDN. The mean reduction in baseline systolic BP was three mmHg in the PVI-only group compared to 16 mmHg in the RDN with PVI group. RDN with PVI was associated with a significantly increased likelihood of freedom from AF at 12 months, and patients undergoing RDN developed AF about three months later compared to the PVI-only group. The number of composite fatal and nonfatal major adverse cardiac events was equal in the two groups. The investigators noted the findings of the study are limited by the lack of a formal sham-control RDN.153 These studies suggest that RDN may be a useful adjunct along with PVI for slowing AF progression, especially in patients with resistant hypertension, or at least may provide some proof of concept that BP control can contribute to AF reduction.
Observational, but no randomized studies, have been published exploring the relationship between alcohol consumption and the success of ablation in AF patients. An observational study of paroxysmal AF patients noted that although the rate of AF recurrence was lower after the initial catheter ablation in those who did not consume alcohol, there was no difference in outcome after the final catheter ablation between those who did and did not consume alcohol.154 Interestingly, the frequency, rather than the volume, of alcohol consumption was associated with AF recurrence after the initial catheter ablation. A retrospective analysis of both paroxysmal and persistent AF patients did not find moderate or heavy alcohol consumption to be significantly predictive of early or late AF recurrence in patients following PVI.155 In a single-center, observational study, ethyl glucuronide in hair (hEtG) was used as a marker for long-term alcohol consumption. The study reported that male AF patients with hEtG ≥7pg/mg, indicative of repeated alcohol consumption, experienced higher rates of re-ablation compared to patients with hEtG <7pg/mg.156
The association of smoking with AF ablation outcomes has been studied observationally, but little data on the effects of smoking cessation are available. A study recruited 59 patients with drug-refractory AF to examine the impact of cigarette smoking on AF recurrence after PVI. The smoking group consisted of both former and current smokers. Smokers tended to have larger pulmonary vein diameters and left atrial volume and were at higher risk for AF recurrence after PVI than nonsmokers.157 Another study comparing 201 patients with persistent AF showed no difference in long-term ablation outcomes between smokers and nonsmokers. However, compared to nonsmokers, smokers had a higher incidence of nonpulmonary vein (NPV) triggers, which was associated with increased recurrence and worse outcomes following catheter ablation.158 No data are available on the effects of caffeine on post-ablation outcomes in AF patients.
In summary, retrospective and prospective observational studies have reported poorer outcomes after AF ablation with greater BMI values, less daily physical activity, OSA without CPAP treatment, DM and higher HbA1c levels, and hypertension. Mixed effects have been observed for alcohol and tobacco, and no congruent evidence currently exists for weight management, OSA treatment, hypertension management, alcohol reduction, or tobacco cessation to decrease AF recurrence or burden after AF ablation. More prospective, randomized studies of various LRFM are needed to establish conclusive recommendations.
CONCLUSIONS
Managing reversible risk factors is a strategy with immense potential to impact the care and treatment success of AF patients, especially relating to AF burden and recurrence. Most reports discussing AF and its management within the context of weight/obesity, physical inactivity, SDB/OSA, DM, hypertension, CAD, and alcohol, tobacco, and caffeine use are observational studies. Thus, more randomized controlled studies are required to delineate the effects of combining LRFM with traditional AF management strategies, such as antithrombotic, rate control, and rhythm control therapies.
Sources of Funding:
MKC: National Institutes of Health grants R01 HL 090620, R01 HL 111314, R01 HL158071, P01HL158502; American Heart Association Atrial Fibrillation Strategically Focused Research Network grant 18SFRN34110067, 18SFRN34170013; the NIH National Center for Research Resources for Case Western Reserve University and Cleveland Clinic Clinical and Translational Science Award UL1-RR024989, the Cleveland Clinic Department of Cardiovascular Medicine philanthropy research funds, and the Tomsich Atrial Fibrillation Research Fund. LY: Case Western Reserve University School of Medicine Dean’s Research Fellowship award.
Footnotes
Disclosures: none.
References
- 1.Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. Oct 15 2013;112(8):1142–7. doi: 10.1016/j.amjcard.2013.05.063 [DOI] [PubMed] [Google Scholar]
- 2.Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: An increasing epidemic and public health challenge. Int J Stroke. Feb 2021;16(2):217–221. doi: 10.1177/1747493019897870 [DOI] [PubMed] [Google Scholar]
- 3.Chung MK, Eckhardt LL, Chen LY, et al. Lifestyle and Risk Factor Modification for Reduction of Atrial Fibrillation: A Scientific Statement From the American Heart Association. Circulation. Apr 21 2020;141(16):e750–e772. doi: 10.1161/CIR.0000000000000748 [DOI] [PubMed] [Google Scholar]
- 4.O’Connell RP, Musa H, Gomez MS, et al. Free Fatty Acid Effects on the Atrial Myocardium: Membrane Ionic Currents Are Remodeled by the Disruption of T-Tubular Architecture. PLoS One. 2015;10(8):e0133052. doi: 10.1371/journal.pone.0133052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batal O, Schoenhagen P, Shao M, et al. Left atrial epicardial adiposity and atrial fibrillation. Circ Arrhythm Electrophysiol. Jun 2010;3(3):230–6. doi: 10.1161/CIRCEP.110.957241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yorgun H, Canpolat U, Aytemir K, et al. Association of epicardial and peri-atrial adiposity with the presence and severity of non-valvular atrial fibrillation. Int J Cardiovasc Imaging. Mar 2015;31(3):649–57. doi: 10.1007/s10554-014-0579-5 [DOI] [PubMed] [Google Scholar]
- 7.Munger TM, Dong YX, Masaki M, et al. Electrophysiological and hemodynamic characteristics associated with obesity in patients with atrial fibrillation. J Am Coll Cardiol. Aug 28 2012;60(9):851–60. doi: 10.1016/j.jacc.2012.03.042 [DOI] [PubMed] [Google Scholar]
- 8.Stritzke J, Markus MR, Duderstadt S, et al. The aging process of the heart: obesity is the main risk factor for left atrial enlargement during aging the MONICA/KORA (monitoring of trends and determinations in cardiovascular disease/cooperative research in the region of Augsburg) study. J Am Coll Cardiol. Nov 17 2009;54(21):1982–9. doi: 10.1016/j.jacc.2009.07.034 [DOI] [PubMed] [Google Scholar]
- 9.Healey JS, Connolly SJ. Atrial fibrillation: hypertension as a causative agent, risk factor for complications, and potential therapeutic target. Am J Cardiol. May 22 2003;91(10A):9G–14G. doi: 10.1016/s0002-9149(03)00227-3 [DOI] [PubMed] [Google Scholar]
- 10.Zankov DP, Omatsu-Kanbe M, Isono T, et al. Angiotensin II potentiates the slow component of delayed rectifier K+ current via the AT1 receptor in guinea pig atrial myocytes. Circulation. Mar 14 2006;113(10):1278–86. doi: 10.1161/CIRCULATIONAHA.104.530592 [DOI] [PubMed] [Google Scholar]
- 11.Boldt A, Wetzel U, Weigl J, et al. Expression of angiotensin II receptors in human left and right atrial tissue in atrial fibrillation with and without underlying mitral valve disease. J Am Coll Cardiol. Nov 19 2003;42(10):1785–92. doi: 10.1016/j.jacc.2003.07.014 [DOI] [PubMed] [Google Scholar]
- 12.Dimitri H, Ng M, Brooks AG, et al. Atrial remodeling in obstructive sleep apnea: implications for atrial fibrillation. Heart Rhythm. Mar 2012;9(3):321–7. doi: 10.1016/j.hrthm.2011.10.017 [DOI] [PubMed] [Google Scholar]
- 13.Linz D, McEvoy RD, Cowie MR, et al. Associations of Obstructive Sleep Apnea With Atrial Fibrillation and Continuous Positive Airway Pressure Treatment: A Review. JAMA Cardiol. Jun 1 2018;3(6):532–540. doi: 10.1001/jamacardio.2018.0095 [DOI] [PubMed] [Google Scholar]
- 14.Devereux RB, Roman MJ, Paranicas M, et al. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. May 16 2000;101(19):2271–6. doi: 10.1161/01.cir.101.19.2271 [DOI] [PubMed] [Google Scholar]
- 15.Zhang Q, Liu T, Ng CY, Li G. Diabetes mellitus and atrial remodeling: mechanisms and potential upstream therapies. Cardiovasc Ther. Oct 2014;32(5):233–41. doi: 10.1111/1755-5922.12089 [DOI] [PubMed] [Google Scholar]
- 16.Watanabe M, Yokoshiki H, Mitsuyama H, Mizukami K, Ono T, Tsutsui H. Conduction and refractory disorders in the diabetic atrium. Am J Physiol Heart Circ Physiol. Jul 2012;303(1):H86–95. doi: 10.1152/ajpheart.00010.2012 [DOI] [PubMed] [Google Scholar]
- 17.Kato T, Yamashita T, Sekiguchi A, et al. AGEs-RAGE system mediates atrial structural remodeling in the diabetic rat. J Cardiovasc Electrophysiol. Apr 2008;19(4):415–20. doi: 10.1111/j.1540-8167.2007.01037.x [DOI] [PubMed] [Google Scholar]
- 18.Mandyam MC, Vedantham V, Scheinman MM, et al. Alcohol and vagal tone as triggers for paroxysmal atrial fibrillation. Am J Cardiol. Aug 1 2012;110(3):364–8. doi: 10.1016/j.amjcard.2012.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McManus DD, Yin X, Gladstone R, et al. Alcohol Consumption, Left Atrial Diameter, and Atrial Fibrillation. J Am Heart Assoc. Sep 14 2016;5(9)doi: 10.1161/JAHA.116.004060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. May 19 2004;43(10):1731–7. doi: 10.1016/j.jacc.2003.12.047 [DOI] [PubMed] [Google Scholar]
- 21.Chidipi B, Chang M, Abou-Assali O, et al. The Arf6/PIP5K pathway activates IKACh in cigarette smoke mediated atrial fibrillation. Cell Signal. Sep 20 2022;100:110475. doi: 10.1016/j.cellsig.2022.110475 [DOI] [PubMed] [Google Scholar]
- 22.Fabrizio C, Desiderio M, Coyne RF. Electrocardiogram Abnormalities of Caffeine Overdose. Circ Arrhythm Electrophysiol. Jul 2016;9(7)doi: 10.1161/CIRCEP.115.003088 [DOI] [PubMed] [Google Scholar]
- 23.Chatterjee NA, Giulianini F, Geelhoed B, et al. Genetic Obesity and the Risk of Atrial Fibrillation: Causal Estimates from Mendelian Randomization. Circulation. Feb 21 2017;135(8):741–754. doi: 10.1161/CIRCULATIONAHA.116.024921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong CX, Sullivan T, Sun MT, et al. Obesity and the Risk of Incident, Post-Operative, and Post-Ablation Atrial Fibrillation: A Meta-Analysis of 626,603 Individuals in 51 Studies. JACC Clin Electrophysiol. Jun 2015;1(3):139–152. doi: 10.1016/j.jacep.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 25.Abed HS, Wittert GA, Leong DP, et al. Effect of weight reduction and cardiometabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial. JAMA. Nov 20 2013;310(19):2050–60. doi: 10.1001/jama.2013.280521 [DOI] [PubMed] [Google Scholar]
- 26.Pathak RK, Middeldorp ME, Meredith M, et al. Long-Term Effect of Goal-Directed Weight Management in an Atrial Fibrillation Cohort: A Long-Term Follow-Up Study (LEGACY). J Am Coll Cardiol. May 26 2015;65(20):2159–69. doi: 10.1016/j.jacc.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 27.Middeldorp ME, Pathak RK, Meredith M, et al. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: the REVERSE-AF study. Europace. Dec 1 2018;20(12):1929–1935. doi: 10.1093/europace/euy117 [DOI] [PubMed] [Google Scholar]
- 28.Pathak RK, Elliott A, Middeldorp ME, et al. Impact of CARDIOrespiratory FITness on Arrhythmia Recurrence in Obese Individuals With Atrial Fibrillation: The CARDIO-FIT Study. J Am Coll Cardiol. Sep 1 2015;66(9):985–96. doi: 10.1016/j.jacc.2015.06.488 [DOI] [PubMed] [Google Scholar]
- 29.Azarbal F, Stefanick ML, Salmoirago-Blotcher E, et al. Obesity, physical activity, and their interaction in incident atrial fibrillation in postmenopausal women. J Am Heart Assoc. Aug 20 2014;3(4)doi: 10.1161/JAHA.114.001127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garnvik LE, Malmo V, Janszky I, Wisloff U, Loennechen JP, Nes BM. Physical activity modifies the risk of atrial fibrillation in obese individuals: The HUNT3 study. Eur J Prev Cardiol. Oct 2018;25(15):1646–1652. doi: 10.1177/2047487318784365 [DOI] [PubMed] [Google Scholar]
- 31.Kato M, Kubo A, Nihei F, Ogano M, Takagi H. Effects of exercise training on exercise capacity, cardiac function, BMI, and quality of life in patients with atrial fibrillation: a meta-analysis of randomized-controlled trials. Int J Rehabil Res. Sep 2017;40(3):193–201. doi: 10.1097/MRR.0000000000000232 [DOI] [PubMed] [Google Scholar]
- 32.Hegbom F, Stavem K, Sire S, Heldal M, Orning OM, Gjesdal K. Effects of short-term exercise training on symptoms and quality of life in patients with chronic atrial fibrillation. Int J Cardiol. Mar 2 2007;116(1):86–92. doi: 10.1016/j.ijcard.2006.03.034 [DOI] [PubMed] [Google Scholar]
- 33.Osbak PS, Mourier M, Kjaer A, Henriksen JH, Kofoed KF, Jensen GB. A randomized study of the effects of exercise training on patients with atrial fibrillation. Am Heart J. Dec 2011;162(6):1080–7. doi: 10.1016/j.ahj.2011.09.013 [DOI] [PubMed] [Google Scholar]
- 34.Everett BM, Conen D, Buring JE, Moorthy MV, Lee IM, Albert CM. Physical activity and the risk of incident atrial fibrillation in women. Circ Cardiovasc Qual Outcomes. May 2011;4(3):321–7. doi: 10.1161/CIRCOUTCOMES.110.951442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drca N, Wolk A, Jensen-Urstad M, Larsson SC. Physical activity is associated with a reduced risk of atrial fibrillation in middle-aged and elderly women. Heart. Oct 2015;101(20):1627–30. doi: 10.1136/heartjnl-2014-307145 [DOI] [PubMed] [Google Scholar]
- 36.Malmo V, Nes BM, Amundsen BH, et al. Aerobic Interval Training Reduces the Burden of Atrial Fibrillation in the Short Term: A Randomized Trial. Circulation. Feb 2 2016;133(5):466–73. doi: 10.1161/CIRCULATIONAHA.115.018220 [DOI] [PubMed] [Google Scholar]
- 37.Skielboe AK, Bandholm TQ, Hakmann S, Mourier M, Kallemose T, Dixen U. Cardiovascular exercise and burden of arrhythmia in patients with atrial fibrillation - A randomized controlled trial. PLoS One. 2017;12(2):e0170060. doi: 10.1371/journal.pone.0170060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiuchi MG, Chen S, Hoye NA. The effects of different physical activities on atrial fibrillation in patients with hypertension and chronic kidney disease. Kidney Res Clin Pract. Sep 2017;36(3):264–273. doi: 10.23876/j.krcp.2017.36.3.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Opondo MA, Aiad N, Cain MA, et al. Does High-Intensity Endurance Training Increase the Risk of Atrial Fibrillation? A Longitudinal Study of Left Atrial Structure and Function. Circ Arrhythm Electrophysiol. May 2018;11(5):e005598. doi: 10.1161/CIRCEP.117.005598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mozaffarian D, Furberg CD, Psaty BM, Siscovick D. Physical activity and incidence of atrial fibrillation in older adults: the cardiovascular health study. Circulation. Aug 19 2008;118(8):800–7. doi: 10.1161/CIRCULATIONAHA.108.785626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdulla J, Nielsen JR. Is the risk of atrial fibrillation higher in athletes than in the general population? A systematic review and meta-analysis. Europace. Sep 2009;11(9):1156–9. doi: 10.1093/europace/eup197 [DOI] [PubMed] [Google Scholar]
- 42.Andersen K, Farahmand B, Ahlbom A, et al. Risk of arrhythmias in 52 755 long-distance cross-country skiers: a cohort study. Eur Heart J. Dec 2013;34(47):3624–31. doi: 10.1093/eurheartj/eht188 [DOI] [PubMed] [Google Scholar]
- 43.Baldesberger S, Bauersfeld U, Candinas R, et al. Sinus node disease and arrhythmias in the long-term follow-up of former professional cyclists. Eur Heart J. Jan 2008;29(1):71–8. doi: 10.1093/eurheartj/ehm555 [DOI] [PubMed] [Google Scholar]
- 44.Elosua R, Arquer A, Mont L, et al. Sport practice and the risk of lone atrial fibrillation: a case-control study. Int J Cardiol. Apr 14 2006;108(3):332–7. doi: 10.1016/j.ijcard.2005.05.020 [DOI] [PubMed] [Google Scholar]
- 45.Molina L, Mont L, Marrugat J, et al. Long-term endurance sport practice increases the incidence of lone atrial fibrillation in men: a follow-up study. Europace. May 2008;10(5):618–23. doi: 10.1093/europace/eun071 [DOI] [PubMed] [Google Scholar]
- 46.Lakkireddy D, Atkins D, Pillarisetti J, et al. Effect of yoga on arrhythmia burden, anxiety, depression, and quality of life in paroxysmal atrial fibrillation: the YOGA My Heart Study. J Am Coll Cardiol. Mar 19 2013;61(11):1177–82. doi: 10.1016/j.jacc.2012.11.060 [DOI] [PubMed] [Google Scholar]
- 47.Kadhim K, Middeldorp ME, Elliott AD, et al. Prevalence and Assessment of Sleep-Disordered Breathing in Patients With Atrial Fibrillation: A Systematic Review and Meta-analysis. Can J Cardiol. Nov 2021;37(11):1846–1856. doi: 10.1016/j.cjca.2021.09.026 [DOI] [PubMed] [Google Scholar]
- 48.Holmqvist F, Guan N, Zhu Z, et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation-Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J. May 2015;169(5):647–654 e2. doi: 10.1016/j.ahj.2014.12.024 [DOI] [PubMed] [Google Scholar]
- 49.McEvoy RD, Antic NA, Heeley E, et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N Engl J Med. Sep 8 2016;375(10):919–31. doi: 10.1056/NEJMoa1606599 [DOI] [PubMed] [Google Scholar]
- 50.Traaen GM, Aakeroy L, Hunt TE, et al. Effect of Continuous Positive Airway Pressure on Arrhythmia in Atrial Fibrillation and Sleep Apnea: A Randomized Controlled Trial. Am J Respir Crit Care Med. Sep 1 2021;204(5):573–582. doi: 10.1164/rccm.202011-4133OC [DOI] [PubMed] [Google Scholar]
- 51.Chen L, Sun X, He Y, Lu Y, Zheng L. Obstructive sleep apnea and atrial fibrillation: insights from a bidirectional Mendelian randomization study. BMC Med Genomics. Feb 16 2022;15(1):28. doi: 10.1186/s12920-022-01180-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Movahed MR, Hashemzadeh M, Jamal MM. Diabetes mellitus is a strong, independent risk for atrial fibrillation and flutter in addition to other cardiovascular disease. Int J Cardiol. Dec 7 2005;105(3):315–8. doi: 10.1016/j.ijcard.2005.02.050 [DOI] [PubMed] [Google Scholar]
- 53.Huxley RR, Filion KB, Konety S, Alonso A. Meta-analysis of cohort and case-control studies of type 2 diabetes mellitus and risk of atrial fibrillation. Am J Cardiol. Jul 1 2011;108(1):56–62. doi: 10.1016/j.amjcard.2011.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bohne LJ, Johnson D, Rose RA, Wilton SB, Gillis AM. The Association Between Diabetes Mellitus and Atrial Fibrillation: Clinical and Mechanistic Insights. Front Physiol. 2019;10:135. doi: 10.3389/fphys.2019.00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seyed Ahmadi S, Svensson AM, Pivodic A, Rosengren A, Lind M. Risk of atrial fibrillation in persons with type 2 diabetes and the excess risk in relation to glycaemic control and renal function: a Swedish cohort study. Cardiovasc Diabetol. Jan 18 2020;19(1):9. doi: 10.1186/s12933-019-0983-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huxley RR, Alonso A, Lopez FL, et al. Type 2 diabetes, glucose homeostasis and incident atrial fibrillation: the Atherosclerosis Risk in Communities study. Heart. Jan 2012;98(2):133–8. doi: 10.1136/heartjnl-2011-300503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qi W, Zhang N, Korantzopoulos P, et al. Serum glycated hemoglobin level as a predictor of atrial fibrillation: A systematic review with meta-analysis and meta-regression. PLoS One. 2017;12(3):e0170955. doi: 10.1371/journal.pone.0170955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chao TF, Leu HB, Huang CC, et al. Thiazolidinediones can prevent new onset atrial fibrillation in patients with non-insulin dependent diabetes. Int J Cardiol. Apr 19 2012;156(2):199–202. doi: 10.1016/j.ijcard.2011.08.081 [DOI] [PubMed] [Google Scholar]
- 59.Chang SH, Wu LS, Chiou MJ, et al. Association of metformin with lower atrial fibrillation risk among patients with type 2 diabetes mellitus: a population-based dynamic cohort and in vitro studies. Cardiovasc Diabetol. Aug 10 2014;13:123. doi: 10.1186/s12933-014-0123-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu B, Wang J, Wang G. Beneficial effects of pioglitazone on retardation of persistent atrial fibrillation progression in diabetes mellitus patients. Int Heart J. 2014;55(6):499–505. doi: 10.1536/ihj.14-107 [DOI] [PubMed] [Google Scholar]
- 61.Li WJ, Chen XQ, Xu LL, Li YQ, Luo BH. SGLT2 inhibitors and atrial fibrillation in type 2 diabetes: a systematic review with meta-analysis of 16 randomized controlled trials. Cardiovasc Diabetol. Aug 26 2020;19(1):130. doi: 10.1186/s12933-020-01105-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huxley RR, Lopez FL, Folsom AR, et al. Absolute and attributable risks of atrial fibrillation in relation to optimal and borderline risk factors: the Atherosclerosis Risk in Communities (ARIC) study. Circulation. Apr 12 2011;123(14):1501–8. doi: 10.1161/CIRCULATIONAHA.110.009035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soliman EZ, Rahman AF, Zhang ZM, et al. Effect of Intensive Blood Pressure Lowering on the Risk of Atrial Fibrillation. Hypertension. Jun 2020;75(6):1491–1496. doi: 10.1161/HYPERTENSIONAHA.120.14766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang W, Wang JG. Prevention of Atrial Fibrillation by Intensive Antihypertensive Treatment. Hypertension. Jun 2020;75(6):1414–1416. doi: 10.1161/HYPERTENSIONAHA.120.14856 [DOI] [PubMed] [Google Scholar]
- 65.Grundvold I, Skretteberg PT, Liestol K, et al. Upper normal blood pressures predict incident atrial fibrillation in healthy middle-aged men: a 35-year follow-up study. Hypertension. Feb 2012;59(2):198–204. doi: 10.1161/HYPERTENSIONAHA.111.179713 [DOI] [PubMed] [Google Scholar]
- 66.Neefs J, van den Berg NW, Limpens J, et al. Aldosterone Pathway Blockade to Prevent Atrial Fibrillation: A Systematic Review and Meta-Analysis. Int J Cardiol. Mar 15 2017;231:155–161. doi: 10.1016/j.ijcard.2016.12.029 [DOI] [PubMed] [Google Scholar]
- 67.Schneider MP, Hua TA, Bohm M, Wachtell K, Kjeldsen SE, Schmieder RE. Prevention of atrial fibrillation by Renin-Angiotensin system inhibition a meta-analysis. J Am Coll Cardiol. May 25 2010;55(21):2299–307. doi: 10.1016/j.jacc.2010.01.043 [DOI] [PubMed] [Google Scholar]
- 68.Anand K, Mooss AN, Hee TT, Mohiuddin SM. Meta-analysis: inhibition of renin-angiotensin system prevents new-onset atrial fibrillation. Am Heart J. Aug 2006;152(2):217–22. doi: 10.1016/j.ahj.2006.01.007 [DOI] [PubMed] [Google Scholar]
- 69.Bayturan O, Puri R, Tuzcu EM, et al. Atrial fibrillation, progression of coronary atherosclerosis and myocardial infarction. Eur J Prev Cardiol. Mar 2017;24(4):373–381. doi: 10.1177/2047487316679265 [DOI] [PubMed] [Google Scholar]
- 70.Ruddox V, Sandven I, Munkhaugen J, Skattebu J, Edvardsen T, Otterstad JE. Atrial fibrillation and the risk for myocardial infarction, all-cause mortality and heart failure: A systematic review and meta-analysis. Eur J Prev Cardiol. Sep 2017;24(14):1555–1566. doi: 10.1177/2047487317715769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McMurray J, Kober L, Robertson M, et al. Antiarrhythmic effect of carvedilol after acute myocardial infarction: results of the Carvedilol Post-Infarct Survival Control in Left Ventricular Dysfunction (CAPRICORN) trial. J Am Coll Cardiol. Feb 15 2005;45(4):525–30. doi: 10.1016/j.jacc.2004.09.076 [DOI] [PubMed] [Google Scholar]
- 72.Ding WY, Protty MB, Davies IG, Lip GYH. Relationship between lipoproteins, thrombosis, and atrial fibrillation. Cardiovasc Res. Feb 21 2022;118(3):716–731. doi: 10.1093/cvr/cvab017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sagris D, Harrison SL, Lip GYH. Lipids and atrial fibrillation: New insights into a paradox. PLoS Med. Aug 2022;19(8):e1004067. doi: 10.1371/journal.pmed.1004067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose-response meta-analysis. J Am Coll Cardiol. Jul 22 2014;64(3):281–9. doi: 10.1016/j.jacc.2014.03.048 [DOI] [PubMed] [Google Scholar]
- 75.Csengeri D, Sprunker NA, Di Castelnuovo A, et al. Alcohol consumption, cardiac biomarkers, and risk of atrial fibrillation and adverse outcomes. Eur Heart J. Mar 21 2021;42(12):1170–1177. doi: 10.1093/eurheartj/ehaa953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gemes K, Malmo V, Laugsand LE, et al. Does Moderate Drinking Increase the Risk of Atrial Fibrillation? The Norwegian HUNT (Nord-Trondelag Health) Study. J Am Heart Assoc. Oct 20 2017;6(10)doi: 10.1161/JAHA.117.007094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lim C, Kim TH, Yu HT, et al. Effect of alcohol consumption on the risk of adverse events in atrial fibrillation: from the COmparison study of Drugs for symptom control and complication prEvention of Atrial Fibrillation (CODE-AF) registry. Europace. Apr 6 2021;23(4):548–556. doi: 10.1093/europace/euaa340 [DOI] [PubMed] [Google Scholar]
- 78.Marcus GM, Modrow MF, Schmid CH, et al. Individualized Studies of Triggers of Paroxysmal Atrial Fibrillation: The I-STOP-AFib Randomized Clinical Trial. JAMA Cardiol. Nov 14 2021;doi: 10.1001/jamacardio.2021.5010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Voskoboinik A, Kalman JM, De Silva A, et al. Alcohol Abstinence in Drinkers with Atrial Fibrillation. N Engl J Med. Jan 2 2020;382(1):20–28. doi: 10.1056/NEJMoa1817591 [DOI] [PubMed] [Google Scholar]
- 80.Aune D, Schlesinger S, Norat T, Riboli E. Tobacco smoking and the risk of atrial fibrillation: A systematic review and meta-analysis of prospective studies. Eur J Prev Cardiol. Sep 2018;25(13):1437–1451. doi: 10.1177/2047487318780435 [DOI] [PubMed] [Google Scholar]
- 81.Choi S, Chang J, Kim K, et al. Association of smoking cessation after atrial fibrillation diagnosis on the risk of cardiovascular disease: a cohort study of South Korean men. BMC Public Health. Feb 3 2020;20(1):168. doi: 10.1186/s12889-020-8275-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lee SR, Choi EK, Jung JH, Han KD, Oh S, Lip GYH. Smoking Cessation after Diagnosis of New-Onset Atrial Fibrillation and the Risk of Stroke and Death. J Clin Med. May 21 2021;10(11)doi: 10.3390/jcm10112238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bodar V, Chen J, Gaziano JM, Albert C, Djousse L. Coffee Consumption and Risk of Atrial Fibrillation in the Physicians’ Health Study. J Am Heart Assoc. Aug 6 2019;8(15):e011346. doi: 10.1161/JAHA.118.011346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mattioli AV, Bonatti S, Zennaro M, Mattioli G. The relationship between personality, socio-economic factors, acute life stress and the development, spontaneous conversion and recurrences of acute lone atrial fibrillation. Europace. May 2005;7(3):211–20. doi: 10.1016/j.eupc.2004.02.006 [DOI] [PubMed] [Google Scholar]
- 85.Cheng M, Hu Z, Lu X, Huang J, Gu D. Caffeine intake and atrial fibrillation incidence: dose response meta-analysis of prospective cohort studies. Can J Cardiol. Apr 2014;30(4):448–54. doi: 10.1016/j.cjca.2013.12.026 [DOI] [PubMed] [Google Scholar]
- 86.Caldeira D, Martins C, Alves LB, Pereira H, Ferreira JJ, Costa J. Caffeine does not increase the risk of atrial fibrillation: a systematic review and meta-analysis of observational studies. Heart. Oct 2013;99(19):1383–9. doi: 10.1136/heartjnl-2013-303950 [DOI] [PubMed] [Google Scholar]
- 87.Groh CA, Faulkner M, Getabecha S, et al. Patient-reported triggers of paroxysmal atrial fibrillation. Heart Rhythm. Jul 2019;16(7):996–1002. doi: 10.1016/j.hrthm.2019.01.027 [DOI] [PubMed] [Google Scholar]
- 88.Hansson A, Madsen-Hardig B, Olsson SB. Arrhythmia-provoking factors and symptoms at the onset of paroxysmal atrial fibrillation: a study based on interviews with 100 patients seeking hospital assistance. BMC Cardiovasc Disord. Aug 3 2004;4:13. doi: 10.1186/1471-2261-4-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martin KA, Beyer-Westendorf J, Davidson BL, Huisman MV, Sandset PM, Moll S. Use of direct oral anticoagulants in patients with obesity for treatment and prevention of venous thromboembolism: Updated communication from the ISTH SSC Subcommittee on Control of Anticoagulation. J Thromb Haemost. Aug 2021;19(8):1874–1882. doi: 10.1111/jth.15358 [DOI] [PubMed] [Google Scholar]
- 90.Sebaaly J, Kelley D. Direct Oral Anticoagulants in Obesity: An Updated Literature Review. Ann Pharmacother. Nov 2020;54(11):1144–1158. doi: 10.1177/1060028020923584 [DOI] [PubMed] [Google Scholar]
- 91.Wang SY, Giugliano RP. Non-Vitamin K Antagonist Oral Anticoagulant for Atrial Fibrillation in Obese Patients. Am J Cardiol. Jul 15 2020;127:176–183. doi: 10.1016/j.amjcard.2020.04.016 [DOI] [PubMed] [Google Scholar]
- 92.Balla SR, Cyr DD, Lokhnygina Y, et al. Relation of Risk of Stroke in Patients With Atrial Fibrillation to Body Mass Index (from Patients Treated With Rivaroxaban and Warfarin in the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation Trial). Am J Cardiol. Jun 15 2017;119(12):1989–1996. doi: 10.1016/j.amjcard.2017.03.028 [DOI] [PubMed] [Google Scholar]
- 93.Sandhu RK, Ezekowitz J, Andersson U, et al. The ‘obesity paradox’ in atrial fibrillation: observations from the ARISTOTLE (Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation) trial. Eur Heart J. Oct 7 2016;37(38):2869–2878. doi: 10.1093/eurheartj/ehw124 [DOI] [PubMed] [Google Scholar]
- 94.Shaikh F, Wynne R, Castelino RL, Inglis SC, Ferguson C. Effectiveness of Direct Oral Anticoagulants in Obese Adults With Atrial Fibrillation: A Systematic Review of Systematic Reviews and Meta-Analysis. Front Cardiovasc Med. 2021;8:732828. doi: 10.3389/fcvm.2021.732828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Scheen AJ, Sprynger M, Lancellotti P. [Anticoagulation of diabetic patients with nonvalvular atrial fibrillation]. Rev Med Liege. Feb 2021;76(2):93–97. Anticoagulation des patients diabetiques avec fibrillation auriculaire non valvulaire. [PubMed] [Google Scholar]
- 96.Samos M, Bolek T, Stanciakova L, et al. Does type 2 diabetes affect the on-treatment levels of direct oral anticoagulants in patients with atrial fibrillation? Diabetes Res Clin Pract. Jan 2018;135:172–177. doi: 10.1016/j.diabres.2017.11.024 [DOI] [PubMed] [Google Scholar]
- 97.Pridavkova D, Samos M, Bolek T, et al. Type 2 Diabetes, Atrial Fibrillation, and Direct Oral Anticoagulation. J Diabetes Res. 2019;2019:5158308. doi: 10.1155/2019/5158308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Suda S, Aoki J, Shimoyama T, et al. Characteristics of Acute Spontaneous Intracerebral Hemorrhage in Patients Receiving Oral Anticoagulants. J Stroke Cerebrovasc Dis. Apr 2019;28(4):1007–1014. doi: 10.1016/j.jstrokecerebrovasdis.2018.12.013 [DOI] [PubMed] [Google Scholar]
- 99.Schlunk F, Chang Y, Ayres A, et al. Blood pressure burden and outcome in warfarin-related intracerebral hemorrhage. Int J Stroke. Oct 2016;11(8):898–909. doi: 10.1177/1747493016658300 [DOI] [PubMed] [Google Scholar]
- 100.Kuramatsu JB, Gerner ST, Schellinger PD, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA. Feb 24 2015;313(8):824–36. doi: 10.1001/jama.2015.0846 [DOI] [PubMed] [Google Scholar]
- 101.Lip GY, Frison L, Grind M, Invetigators S. Effect of hypertension on anticoagulated patients with atrial fibrillation. Eur Heart J. Mar 2007;28(6):752–9. doi: 10.1093/eurheartj/ehl504 [DOI] [PubMed] [Google Scholar]
- 102.Lemesle G. Aspirin on Top of Anticoagulation in Patients With Concomitant Stable Coronary Artery Disease and Atrial Fibrillation. Circulation. Jan 29 2019;139(5):617–619. doi: 10.1161/CIRCULATIONAHA.118.037440 [DOI] [PubMed] [Google Scholar]
- 103.Kumbhani DJ, Cannon CP, Beavers CJ, et al. 2020 ACC Expert Consensus Decision Pathway for Anticoagulant and Antiplatelet Therapy in Patients With Atrial Fibrillation or Venous Thromboembolism Undergoing Percutaneous Coronary Intervention or With Atherosclerotic Cardiovascular Disease: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. Feb 9 2021;77(5):629–658. doi: 10.1016/j.jacc.2020.09.011 [DOI] [PubMed] [Google Scholar]
- 104.Litwinowicz R, Bartus M, Ceranowicz P, et al. Left atrial appendage occlusion for stroke prevention in diabetes mellitus patients with atrial fibrillation: Long-term results. J Diabetes. Jan 2019;11(1):75–82. doi: 10.1111/1753-0407.12824 [DOI] [PubMed] [Google Scholar]
- 105.Azizy O, Rammos C, Lehmann N, Rassaf T, Kalsch H. Percutaneous closure of the left atrial appendage in patients with diabetes mellitus. Diab Vasc Dis Res. Sep 2017;14(5):407–414. doi: 10.1177/1479164117712176 [DOI] [PubMed] [Google Scholar]
- 106.Faroux L, Cruz-Gonzalez I, Arzamendi D, et al. Incidence, predictors, and clinical impact of bleeding recurrence in patients with prior gastrointestinal bleeding undergoing LAAC. Pacing Clin Electrophysiol. Jul 2021;44(7):1216–1223. doi: 10.1111/pace.14293 [DOI] [PubMed] [Google Scholar]
- 107.Saw J, Tzikas A, Shakir S, et al. Incidence and Clinical Impact of Device-Associated Thrombus and Peri-Device Leak Following Left Atrial Appendage Closure With the Amplatzer Cardiac Plug. JACC Cardiovasc Interv. Feb 27 2017;10(4):391–399. doi: 10.1016/j.jcin.2016.11.029 [DOI] [PubMed] [Google Scholar]
- 108.Plisiene J, Blumberg A, Haager G, et al. Moderate physical exercise: a simplified approach for ventricular rate control in older patients with atrial fibrillation. Clin Res Cardiol. Nov 2008;97(11):820–6. doi: 10.1007/s00392-008-0692-3 [DOI] [PubMed] [Google Scholar]
- 109.Konecny T, Brady PA, Park JY, et al. Usefulness of Heart Rate Control in Atrial Fibrillation Patients With Obstructive Sleep Apnea. Am J Cardiol. Nov 1 2018;122(9):1482–1488. doi: 10.1016/j.amjcard.2018.07.030 [DOI] [PubMed] [Google Scholar]
- 110.Packer M. Heightened risk of intensive rate control in patients with atrial fibrillation who are obese or have type 2 diabetes: A critical review and re-evaluation. J Cardiovasc Electrophysiol. Dec 2019;30(12):3020–3024. doi: 10.1111/jce.14236 [DOI] [PubMed] [Google Scholar]
- 111.Kirchhof P, Camm AJ, Goette A, et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N Engl J Med. Oct 1 2020;383(14):1305–1316. doi: 10.1056/NEJMoa2019422 [DOI] [PubMed] [Google Scholar]
- 112.Ornelas-Loredo A, Kany S, Abraham V, et al. Association Between Obesity-Mediated Atrial Fibrillation and Therapy With Sodium Channel Blocker Antiarrhythmic Drugs. JAMA Cardiol. Jan 1 2020;5(1):57–64. doi: 10.1001/jamacardio.2019.4513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Monahan K, Brewster J, Wang L, et al. Relation of the severity of obstructive sleep apnea in response to anti-arrhythmic drugs in patients with atrial fibrillation or atrial flutter. Am J Cardiol. Aug 1 2012;110(3):369–72. doi: 10.1016/j.amjcard.2012.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ducharme A, Swedberg K, Pfeffer MA, et al. Prevention of atrial fibrillation in patients with symptomatic chronic heart failure by candesartan in the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Am Heart J. Jul 2006;152(1):86–92. [PubMed] [Google Scholar]
- 115.Vermes E, Tardif JC, Bourassa MG, et al. Enalapril decreases the incidence of atrial fibrillation in patients with left ventricular dysfunction: insight from the Studies Of Left Ventricular Dysfunction (SOLVD) trials. Circulation. Jun 17 2003;107(23):2926–31. doi: 10.1161/01.CIR.0000072793.81076.D4 [DOI] [PubMed] [Google Scholar]
- 116.Wachtell K, Lehto M, Gerdts E, et al. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention For End Point Reduction in Hypertension (LIFE) study. J Am Coll Cardiol. Mar 1 2005;45(5):712–9. doi: 10.1016/j.jacc.2004.10.068 [DOI] [PubMed] [Google Scholar]
- 117.Maggioni AP, Latini R, Carson PE, et al. Valsartan reduces the incidence of atrial fibrillation in patients with heart failure: results from the Valsartan Heart Failure Trial (Val-HeFT). Am Heart J. Mar 2005;149(3):548–57. doi: 10.1016/j.ahj.2004.09.033 [DOI] [PubMed] [Google Scholar]
- 118.Palardy M, Ducharme A, Nattel S, et al. Absence of protective effect of renin-angiotensin system inhibitors on atrial fibrillation development: insights from the Canadian Trial of Atrial Fibrillation (CTAF). Can J Cardiol. Sep 2008;24(9):709–13. doi: 10.1016/s0828-282x(08)70670-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Voskoboinik A, Moskovitch J, Plunkett G, et al. Cardioversion of atrial fibrillation in obese patients: Results from the Cardioversion-BMI randomized controlled trial. J Cardiovasc Electrophysiol. Feb 2019;30(2):155–161. doi: 10.1111/jce.13786 [DOI] [PubMed] [Google Scholar]
- 120.Rienstra M, Hobbelt AH, Alings M, et al. Targeted therapy of underlying conditions improves sinus rhythm maintenance in patients with persistent atrial fibrillation: results of the RACE 3 trial. Eur Heart J. Aug 21 2018;39(32):2987–2996. doi: 10.1093/eurheartj/ehx739 [DOI] [PubMed] [Google Scholar]
- 121.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. May 27 2003;107(20):2589–94. doi: 10.1161/01.CIR.0000068337.25994.21 [DOI] [PubMed] [Google Scholar]
- 122.Caples SM, Mansukhani MP, Friedman PA, Somers VK. The impact of continuous positive airway pressure treatment on the recurrence of atrial fibrillation post cardioversion: A randomized controlled trial. Int J Cardiol. Mar 1 2019;278:133–136. doi: 10.1016/j.ijcard.2018.11.100 [DOI] [PubMed] [Google Scholar]
- 123.Soran H, Younis N, Currie P, Silas J, Jones IR, Gill G. Influence of diabetes on the maintenance of sinus rhythm after a successful direct current cardioversion in patients with atrial fibrillation. QJM. Mar 2008;101(3):181–7. doi: 10.1093/qjmed/hcm123 [DOI] [PubMed] [Google Scholar]
- 124.Potpara T, Marinkovic-Eric J, Grujic M, Radojkovic-Cirovic B, Vujisic-Tesic B, Petrovic M. [Effect of diabetes mellitus in recovery and maintenance of sinus rhythm in patients with persistent atrial fibrillation]. Srp Arh Celok Lek. May-Jun 2002;130(5–6):189–92. Uticaj dijabetesa melitusa na uspostavljanje i odrzavanje sinusnog ritma kod bolesnika s perzistentnom fibrilacijom pretkomora. doi: 10.2298/sarh0206189p [DOI] [PubMed] [Google Scholar]
- 125.Soran H, Banerjee M, Mohamad JB, et al. Risk Factors for Failure of Direct Current Cardioversion in Patients with Type 2 Diabetes Mellitus and Atrial Fibrillation. Biomed Res Int. 2018;2018:5936180. doi: 10.1155/2018/5936180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gu J, Liu X, Wang X, et al. Beneficial effect of pioglitazone on the outcome of catheter ablation in patients with paroxysmal atrial fibrillation and type 2 diabetes mellitus. Europace. Sep 2011;13(9):1256–61. doi: 10.1093/europace/eur131 [DOI] [PubMed] [Google Scholar]
- 127.Gu J, Hu W, Song ZP, Liu X, Zhang DD. PPARgamma agonist use and recurrence of atrial fibrillation after successful electrical cardioversion. Hellenic J Cardiol. Sep - Oct 2017;58(5):387–390. doi: 10.1016/j.hjc.2017.01.025 [DOI] [PubMed] [Google Scholar]
- 128.Van Noord T, Crijns HJ, van den Berg MP, Van Veldhuisen DJ, Van Gelder IC. Pretreatment with ACE inhibitors improves acute outcome of electrical cardioversion in patients with persistent atrial fibrillation. BMC Cardiovasc Disord. Jan 24 2005;5(1):3. doi: 10.1186/1471-2261-5-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kaluzay J, Ferencik M, Mardiakova K, Remisova S. Blood pressure prior to cardioversion predicts a conversion to sinus rhythm in patients with atrial fibrillation. Bratisl Lek Listy. 2008;109(3):116–20. [PubMed] [Google Scholar]
- 130.Madrid AH, Bueno MG, Rebollo JM, et al. Use of irbesartan to maintain sinus rhythm in patients with long-lasting persistent atrial fibrillation: a prospective and randomized study. Circulation. Jul 16 2002;106(3):331–6. doi: 10.1161/01.cir.0000022665.18619.83 [DOI] [PubMed] [Google Scholar]
- 131.Ueng KC, Tsai TP, Yu WC, et al. Use of enalapril to facilitate sinus rhythm maintenance after external cardioversion of long-standing persistent atrial fibrillation. Results of a prospective and controlled study. Eur Heart J. Dec 2003;24(23):2090–8. doi: 10.1016/j.ehj.2003.08.014 [DOI] [PubMed] [Google Scholar]
- 132.Tveit A, Grundvold I, Olufsen M, et al. Candesartan in the prevention of relapsing atrial fibrillation. Int J Cardiol. Aug 9 2007;120(1):85–91. doi: 10.1016/j.ijcard.2006.08.086 [DOI] [PubMed] [Google Scholar]
- 133.Investigators G-A, Disertori M, Latini R, et al. Valsartan for prevention of recurrent atrial fibrillation. N Engl J Med. Apr 16 2009;360(16):1606–17. doi: 10.1056/NEJMoa0805710 [DOI] [PubMed] [Google Scholar]
- 134.Kinoshita M, Herges RM, Hodge DO, et al. Role of smoking in the recurrence of atrial arrhythmias after cardioversion. Am J Cardiol. Sep 1 2009;104(5):678–82. doi: 10.1016/j.amjcard.2009.04.041 [DOI] [PubMed] [Google Scholar]
- 135.Donnellan E, Wazni OM, Kanj M, et al. Impact of risk-factor modification on arrhythmia recurrence among morbidly obese patients undergoing atrial fibrillation ablation. J Cardiovasc Electrophysiol. Aug 2020;31(8):1979–1986. doi: 10.1111/jce.14607 [DOI] [PubMed] [Google Scholar]
- 136.Donnellan E, Wazni OM, Kanj M, et al. Association between pre-ablation bariatric surgery and atrial fibrillation recurrence in morbidly obese patients undergoing atrial fibrillation ablation. Europace. Oct 1 2019;21(10):1476–1483. doi: 10.1093/europace/euz183 [DOI] [PubMed] [Google Scholar]
- 137.Pathak RK, Middeldorp ME, Lau DH, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol. Dec 2 2014;64(21):2222–31. doi: 10.1016/j.jacc.2014.09.028 [DOI] [PubMed] [Google Scholar]
- 138.Gessler N, Willems S, Steven D, et al. Supervised Obesity Reduction Trial for AF ablation patients: results from the SORT-AF trial. Europace. Oct 9 2021;23(10):1548–1558. doi: 10.1093/europace/euab122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Proietti R, Birnie D, Ziegler PD, Wells GA, Verma A. Postablation Atrial Fibrillation Burden and Patient Activity Level: Insights From the DISCERN AF Study. J Am Heart Assoc. Dec 4 2018;7(23):e010256. doi: 10.1161/JAHA.118.010256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yaeger A, Keenan BT, Cash NR, et al. Impact of a nurse-led limited risk factor modification program on arrhythmia outcomes in patients with atrial fibrillation undergoing catheter ablation. J Cardiovasc Electrophysiol. Feb 2020;31(2):423–431. doi: 10.1111/jce.14336 [DOI] [PubMed] [Google Scholar]
- 141.Qureshi WT, Nasir UB, Alqalyoobi S, et al. Meta-Analysis of Continuous Positive Airway Pressure as a Therapy of Atrial Fibrillation in Obstructive Sleep Apnea. Am J Cardiol. Dec 1 2015;116(11):1767–73. doi: 10.1016/j.amjcard.2015.08.046 [DOI] [PubMed] [Google Scholar]
- 142.Li L, Wang ZW, Li J, et al. Efficacy of catheter ablation of atrial fibrillation in patients with obstructive sleep apnoea with and without continuous positive airway pressure treatment: a meta-analysis of observational studies. Europace. Sep 2014;16(9):1309–14. doi: 10.1093/europace/euu066 [DOI] [PubMed] [Google Scholar]
- 143.Congrete S, Bintvihok M, Thongprayoon C, et al. Effect of obstructive sleep apnea and its treatment of atrial fibrillation recurrence after radiofrequency catheter ablation: A meta-analysis. J Evid Based Med. Aug 2018;11(3):145–151. doi: 10.1111/jebm.12313 [DOI] [PubMed] [Google Scholar]
- 144.Shukla A, Aizer A, Holmes D, et al. Effect of Obstructive Sleep Apnea Treatment on Atrial Fibrillation Recurrence: A Meta-Analysis. JACC Clin Electrophysiol. Mar - Apr 2015;1(1–2):41–51. doi: 10.1016/j.jacep.2015.02.014 [DOI] [PubMed] [Google Scholar]
- 145.Hunt TE, Traaen GM, Aakeroy L, et al. Effect of continuous positive airway pressure therapy on recurrence of atrial fibrillation after pulmonary vein isolation in patients with obstructive sleep apnea: A randomized controlled trial. Heart Rhythm. Sep 2022;19(9):1433–1441. doi: 10.1016/j.hrthm.2022.06.016 [DOI] [PubMed] [Google Scholar]
- 146.Creta A, Providencia R, Adragao P, et al. Impact of Type-2 Diabetes Mellitus on the Outcomes of Catheter Ablation of Atrial Fibrillation (European Observational Multicentre Study). Am J Cardiol. Mar 15 2020;125(6):901–906. doi: 10.1016/j.amjcard.2019.12.037 [DOI] [PubMed] [Google Scholar]
- 147.Donnellan E, Aagaard P, Kanj M, et al. Association Between Pre-Ablation Glycemic Control and Outcomes Among Patients With Diabetes Undergoing Atrial Fibrillation Ablation. JACC Clin Electrophysiol. Aug 2019;5(8):897–903. doi: 10.1016/j.jacep.2019.05.018 [DOI] [PubMed] [Google Scholar]
- 148.Deshmukh A, Ghannam M, Liang J, et al. Effect of metformin on outcomes of catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. May 2021;32(5):1232–1239. doi: 10.1111/jce.14954 [DOI] [PubMed] [Google Scholar]
- 149.Rosen BD, Akoum N, Burgon N, Vergara G, Marrouche N, Bader F. Renin Angiotenin Blocker Pre-treatment and Recurrence After Pulmonary Vein Isolation in Patients with Paroxysmal and Persistent Atrial Fibrillation. J Atr Fibrillation. Oct-Nov 2013;6(3):898. doi: 10.4022/jafib.898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tayebjee MH, Creta A, Moder S, et al. Impact of angiotensin-converting enzyme-inhibitors and angiotensin receptor blockers on long-term outcome of catheter ablation for atrial fibrillation. Europace. Nov 2010;12(11):1537–42. doi: 10.1093/europace/euq284 [DOI] [PubMed] [Google Scholar]
- 151.Parkash R, Wells GA, Sapp JL, et al. Effect of Aggressive Blood Pressure Control on the Recurrence of Atrial Fibrillation After Catheter Ablation: A Randomized, Open-Label Clinical Trial (SMAC-AF [Substrate Modification With Aggressive Blood Pressure Control]). Circulation. May 9 2017;135(19):1788–1798. doi: 10.1161/CIRCULATIONAHA.116.026230 [DOI] [PubMed] [Google Scholar]
- 152.Romanov A, Pokushalov E, Ponomarev D, et al. Pulmonary vein isolation with concomitant renal artery denervation is associated with reduction in both arterial blood pressure and atrial fibrillation burden: Data from implantable cardiac monitor. Cardiovasc Ther. Aug 2017;35(4)doi: 10.1111/1755-5922.12264 [DOI] [PubMed] [Google Scholar]
- 153.Steinberg JS, Shabanov V, Ponomarev D, et al. Effect of Renal Denervation and Catheter Ablation vs Catheter Ablation Alone on Atrial Fibrillation Recurrence Among Patients With Paroxysmal Atrial Fibrillation and Hypertension: The ERADICATE-AF Randomized Clinical Trial. JAMA. Jan 21 2020;323(3):248–255. doi: 10.1001/jama.2019.21187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Takigawa M, Takahashi A, Kuwahara T, et al. Impact of Alcohol Consumption on the Outcome of Catheter Ablation in Patients With Paroxysmal Atrial Fibrillation. J Am Heart Assoc. Nov 28 2016;5(12)doi: 10.1161/JAHA.116.004149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Barham WY, Sauer WH, Fleeman B, et al. Impact of Alcohol Consumption on Atrial Fibrillation Outcomes Following Pulmonary Vein Isolation. J Atr Fibrillation. Dec 2016;9(4):1505. doi: 10.4022/jafib.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Barmano N, Charitakis E, Kronstrand R, et al. The association between alcohol consumption, cardiac biomarkers, left atrial size and re-ablation in patients with atrial fibrillation referred for catheter ablation. PLoS One. 2019;14(4):e0215121. doi: 10.1371/journal.pone.0215121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fukamizu S, Sakurada H, Takano M, et al. Effect of Cigarette Smoking on the Risk of Atrial Fibrillation Recurrence after Pulmonary Vein Isolation. Journal of Arrhythmia. 2010;26(1):21–29. [Google Scholar]
- 158.Cheng WH, Lo LW, Lin YJ, et al. Cigarette smoking causes a worse long-term outcome in persistent atrial fibrillation following catheter ablation. J Cardiovasc Electrophysiol. May 2018;29(5):699–706. doi: 10.1111/jce.13451 [DOI] [PubMed] [Google Scholar]