Abstract
Purpose
Endobronchial electromagnetic transponder beacons (EMT) provide real-time, precise positional data of moving lung tumors. We report results of a phase 1/2, prospective, single-arm cohort study evaluating the treatment planning effects of EMT-guided SABR for moving lung tumors.
Methods and Materials
Eligible patients were adults, Eastern Cooperative Oncology Group 0 to 2, with T1-T2N0 non-small cell lung cancer or pulmonary metastasis ≤4 cm with motion amplitude ≥5 mm. Three EMTs were endobronchially implanted using navigational bronchoscopy. Four-dimensional free-breathing computed tomography simulation scans were obtained, and end-exhalation phases were used to define the gating window internal target volume. A 3-mm expansion of gating window internal target volume defined the planning target volume (PTV). EMT-guided, respiratory-gated (RG) SABR was delivered (54 Gy/3 fractions or 48 Gy/4 fractions) using volumetric modulated arc therapy. For each RG-SABR plan, a 10-phase image-guided SABR plan was generated for dosimetric comparison. PTV/organ-at-risk (OAR) metrics were tabulated and analyzed using the Wilcoxon signed-rank pair test. Treatment outcomes were evaluated using RECIST (Response Evaluation Criteria in Solid Tumours; version 1.1).
Results
Of 41 patients screened, 17 were enrolled and 2 withdrew from the study. Median age was 73 years, with 7 women. Sixty percent had T1/T2 non-small cell lung cancer and 40% had M1 disease. Median tumor diameter was 1.9 cm with 73% of targets located peripherally. Mean respiratory tumor motion was 1.25 cm (range, 0.53-4.04 cm). Thirteen tumors were treated with EMT-guided SABR and 47% of patients received 48 Gy in 4 fractions while 53% received 54 Gy in 3 fractions. RG-SABR yielded an average PTV reduction of 46.9% (P < .005). Lung V5, V10, V20, and mean lung dose had mean relative reductions of 11.3%, 20.3%, 31.1%, and 20.3%, respectively (P < .005). Dose to OARs was significantly reduced (P < .05) except for spinal cord. At 6 months, mean radiographic tumor volume reduction was 53.5% (P < .005).
Conclusions
EMT-guided RG-SABR significantly reduced PTVs of moving lung tumors compared with image-guided SABR. EMT-guided RG-SABR should be considered for tumors with large respiratory motion amplitudes or those located in close proximity to OARs.
Introduction
The treatment of early-stage non-small cell lung cancer (NSCLC) using SABR technique was first described by Timmerman et al1 almost 2 decades ago. They reported 3-year local control rates of 90% and 40% overall survival at 5 years among patients who were medically inoperable or who preferred nonoperative management.2,3 Since that time, SABR has been widely adopted and expanded for use in the pulmonary oligometastatic4, 5, 6, 7 and oligoprogressive settings.8,9
The delivery of ablative doses of radiation to lung tumors presents several challenges because they are constantly in motion in multiple axes owing to respiratory motion and cardiac pulsations, raising the possibility of a geographic miss, particularly for tumors of the lower or middle lobes where the influence of diaphragmatic movements is the greatest. Furthermore, multiple critical organs at risk (OAR) are often in close proximity to the tumor target and must be carefully spared from the toxicity of SABR. Therefore, reliable motion control strategies are imperative to ensure both safety and effectiveness of SABR.
Various motion control strategies have been developed for lung SABR, including abdominal compression devices to reduce diaphragmatic excursion,10,11 respiratory gating using external motion amplitude surrogates,12,13 breath hold techniques,14,15 and motion encompassing techniques including internal target volumes (ITV) generated from free-breathing (FB) 4-dimensional computed tomography simulation (4D-CT) scans.16 However, techniques such as abdominal compression or breath holding may be poorly tolerated, especially among patients with limited pulmonary reserves, and require high levels of patient compliance.17 Respiratory gating by external motion amplitude surrogates is subject to potential abdominal-thoracic asynchrony, which can produce a respiratory phase lag and result in unintentional gating of the treatment during the incorrect respiratory phases, thus generating a geographic miss.18, 19, 20 Finally, tumors with large motion amplitudes can result in prohibitively large ITVs and planning target volumes (PTVs), which may encroach on nearby OARs.21 Therefore, there remains a bona fide need for the development of a well-tolerated, highly precise methodology for the effective and safe delivery of SABR for moving pulmonary tumors.
Endobronchially implanted electromagnetic transponders (EMT) are miniature encapsulated copper coils that are anchored within the small airways of the lungs by expandable nitinol anchoring legs that prevent beacon migration.22 When excited by the electromagnetic field generated by a transponder array, each beacon produces a unique response radiofrequency signal that is used to provide real-time, in vivo positional telemetry for moving lung tumors during SABR treatment with submillimeter precision.23
Real-time positional telemetry data obtained from EMTs are not subject to the same potential errors of abdominal-thoracic asynchrony because the EMTs report the position of the moving lung tumor and lung parenchyma itself instead of an external surrogate measure of lung tumor motion. Thus, endobronchially implanted EMTs can be used both in the FB or breath-held state to facilitate high-precision respiratory-gated SABR (RG-SABR) or deep-inspiration breath hold SABR (DIBH-SABR) with smaller PTV margins compared with standard image-guided SABR (IG-SABR) techniques.
EMTs have been successfully used to guide SABR treatments in other disease sites including prostate cancer and liver malignancies.24,25 Furthermore, the delivery of EMT-guided SABR for pulmonary tumors has been evaluated in several phase 1 studies,26, 27, 28, 29, 30, 31 which have found that EMT-guided SABR is feasible to deliver and is associated with minimal toxicity.
The dosimetric effect of smaller PTV margins and respiratory-gating methods afforded through the use of EMTs during pulmonary SABR is presently unknown. We report the findings of a phase 1/2 prospective cohort study that aimed to assess the effect of endobronchially implanted EMT–guided RG- or DIBH-SABR on treatment volumes and patient outcomes compared with standard IG-SABR including (1) differences in PTVs; (2) dosimetric differences to OARs; (3) toxicity profiles; and (4) treatment responses to date.
Methods and Materials
This prospective, single-arm, phase 1/2 cohort study was registered with ClinicalTrials.gov (trial identifier: NCT03322072).
Patient population
Eligible patients were adults (≥18 years) with surgically inoperable, biopsy-proven or suspected malignancies located in the right middle lobe, lingula, or lower lobes of the lungs, including (1) American Joint Committee on Cancer (seventh edition) stage I or II NSCLC or (2) pulmonary oligometastatic or oligoprogressive tumors. Upper lobe tumors were eligible if the tumor motion amplitude was ≥5 mm (verified by fluoroscopy or 4D-CT scan). Maximal tumor diameter was ≤4 cm. Confirmation of malignancy by biopsy was encouraged; however, a positron emission tomography–positive tumor demonstrating growth on serial CT scans was acceptable in lieu of biopsy. Participants required forced expiratory volume in 1 second (FEV1) of ≥0.8 L and diffusing capacity for carbon monoxide (DLCO) of ≥35% predicted, Zubrod performance status of 0 to 2, life expectancy of ≥6 months, and ability to tolerate bronchoscopy and SABR. Patients were excluded in the following circumstances: target tumor in contact with the proximal bronchial tree; history of SABR to the same lesion; uncontrolled systemic disease; connective tissue disease; autoimmune disorder; active pulmonary infection; requirement for supplemental oxygen at rest; or inability to lie supine for ≥30 minutes.
EMT implantation procedures
A high-resolution, noncontrast CT scan of the chest with 1.25-mm slice thickness was performed and exported to the SuperDimension electromagnetic navigational bronchoscopy system (Medtronic). Before implantation, a thoracic surgeon preselected 3 EMT implantation locations that were positioned within 3 cm of the target tumor in the small airways of the lung (2-3 mm diameter), and the endobronchial routes to navigate to the preselected sites were mapped. Under general anesthesia or conscious sedation, 3 anchored EMTs (Calypso Beacons; Varian Medical Systems) were implanted endobronchially by a trained thoracic surgeon using the SuperDimension electromagnetic navigational bronchoscopy system (Medtronic). The locations of EMT implants were verified by fluoroscopy directly after each EMT placement.
Radiation therapy
CT simulation scan
One week after EMT implantation, patients underwent a FB 4D-CT simulation scan (Siemens SOMATOM Definition Edge; Siemens) in the supine position with arms abducted above their heads, body immobilized with a Vac-Lok bag (CIVCO Radiotherapy), and knee rest in either the FB state or in a prone position with arms down for a DIBH-CT simulation scan. The respiratory-gating system for scanners (Varian Medical Systems) was used to create the respiratory wave form for generation of the 10-respiratory phase correlated CT data sets for the 4D-CT simulation scan. Coregistration of pretreatment positron emission tomography scans was encouraged to aid contouring.
Treatment planning
Treatment planning was performed using the Eclipse (version 15.6) treatment planning system (Varian Medical Systems). For the first 2 patients (phase 1 of the study), the gross target volume (GTV) was delineated in all 10 respiratory-phase correlated CT data sets, the ITV was generated using the accumulate structure function of all 10 GTVs (Boolean merge) in Eclipse, and the PTV consisted of the ITV plus a 5-mm isometric expansion. For the remainder of the study (phase 2 of the study), the GTV was contoured at end-exhalation (CT50 data set) plus several respiratory phases before and after CT50 (typically CT30-CT70, when the tumor is minimally displaced from end-exhalation). The GTVs contoured on CT30-70 were Boolean merged to generate a respiratory gating window ITV. The PTV consisted of a 3-mm isometric expansion around the gating window ITV. For patients treated with prone DIBH, 2 simulation scans were obtained: (1) prone DIBH-CT simulation scan and (2) supine FB 4D-CT simulation scan (for backup). For prone DIBH treatment, the ITV was defined as the GTV from the breath hold CT simulation scan, and the PTV was generated using a 3-mm isometric expansion of the ITV. A 3-mm isometric expansion of the EMTs was generated to determine the treatment threshold. Dose prescriptions were 54 Gy in 3 fractions for tumors located ≥1 cm away from the chest wall or 48 Gy in 4 fractions for tumors located <1 cm from the chest wall or other critical OAR. Dose coverage specifications for treatment planning were as follows: (1) 99% of the ITV covered by a minimum of 110% of the prescription dose; (2) 95% of the PTV covered by the prescription dose; and (3) 99% of the PTV covered by a minimum of 90% of the prescription dose. Dose hotspots >105% were confined within the PTV. Treatment plans were optimized using the Varian Acuros dosimetric algorithm with inhomogeneity correction and volumetric modulated arc therapy delivery using 2 coplanar 240° arcs, with a 10 MV flattening filter free photon beam and maximum dose-rate of 2400 MU/min. OAR constraints were derived from the Radiation Therapy Oncology Group 0618 protocol by Timmerman et al32 and American Association of Physicists in Medicine Task Group 101.33
Treatment delivery
SABR treatments were delivered using a Varian Edge linear accelerator with a 6°-of-freedom couch. EMT beacon localization telemetry was used to measure the intertransponder distance (ITD) difference (defined as the difference of distance between any 2 EMTs measured at planning and before each treatment fraction), and the geometric residual (GR; defined as root mean squared error of intertransponder distance) was calculated. A GR < 0.2 cm was required to proceed with EMT-guided RG-SABR. Daily setup and verification cone beam CT scans were performed daily to verify beacon positions pre- and posttreatment and to assess for the possibility of beacon migration. EMT positional data were captured in real-time during each fraction to ensure data fidelity throughout the entire setup and treatment procedure and to allow personnel to gain familiarity with the operation of the EMT system. For the first 2 patients (phase 1), the beam was not gated on/off using EMT telemetry and patients were treated FB. For phase 2, patients were FB and the beam was gated on/off automatically using the EMT telemetry. For 1 patient, who was simulated and treated with a prone DIBH technique because of tumor location, audio coaching was used to provide feedback to the patient when EMT telemetry confirmed they were in a proper breath hold.
Analysis
Baseline patient (age, sex, pulmonary function, performance status, comorbidities), disease (tumor location, tumor volumes, pathology, stage, maximum tumor motion amplitude), and treatment (radiation therapy [RT] dose/fractionation) variables were tabulated for descriptive purposes.
Study endpoints
For each targeted tumor, 2 SABR treatment plans were generated: (1) an EMT-guided RG- or DIBH-SABR plan and (2) a standard IG-SABR plan.
The primary study endpoint was percentage change in PTVs when comparing the EMT-guided RG- (or DIBH)-SABR technique to standard IG-SABR. Percentage change in PTV was calculated as
Secondary endpoints included percentage change in OAR metrics, including mean lung dose (MLD), V5, V10, V20, and D2cm, calculated as
Dosimetric data were extracted from the RG- and DIBH-SABR and IG-SABR plans for each patient, including PTV, MLD, lung V5, V10, V20, D2cm, and dose to OARs of interest (chest wall, esophagus, heart, ribs, and spinal canal). For RG- or DIBH-SABR, ITD difference, GR, gantry tracking time, and beam-on durations were extracted from the Calypso system for each patient per fraction. RG- or DIBH-SABR and IG-SABR metrics were tabulated and compared using the nonparametric Wilcoxon sign-rank pair test, and P values <.05 were considered statistically significant differences.
Statistical considerations
Sample size and power calculations were performed using the STATA version 12 software package (College Station, TX). Zhao et al,34 who carried out dosimetric analyses of lung tumors undergoing SABR lung treatments with similar characteristics (T1-T2N0 NSCLC) to those that were eligible for this study, found a median PTV of 62.9 cm3 (standard deviation 43.6 cm3). Presuming similar sized tumor volumes as Zhao et al, a sample size of 28 tumors would yield a power of 0.99 to detect a 40% reduction in the mean PTV between standard IG-SABR based treatment compared with EMT-guided RG-SABR plans for the same tumor, with an α of 0.05 and using a 1-sided test for statistical significance. A reduced sample size of 13 tumors (keeping all other parameters the same) would yield a power of 0.89.
Ethical considerations
This trial was conducted with prior written approvals from the University of Manitoba Biomedical Research Ethics Board (Approval: B2017:48) and the CancerCare Manitoba Research Resource Impact Committee (Approval: 2017-014). Written informed consent was obtained from all patients before initiation of any trial-related procedures. All patient care in this trial was in accordance with the principles of human medical research in the Declaration of Helsinki and the Canadian Tri-Council Policy Statement for ethical conduct for human research trials (version 2.0).
Patient follow-up
Patients were followed with serial clinical assessments and chest CT scans every 6 months for 3 years. Target tumor dimensions were recorded at each CT scan, treatment response was graded according to RECIST (Response Evaluation Criteria in Solid Tumours; version 1.1), and the percentage change in maximum tumor dimensions was calculated. Pulmonary function tests (PFTs) were done at 6, 18, and 36 months posttreatment. History and physical examinations were done at each visit with administration of the European Organisation for Research and Treatment of Cancer QLQ-C30 and QLQ-LC13 questionnaires. Toxicity profiles were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5. Because of the COVID-19 pandemic lockdowns, some patient follow-up investigations were mandatorily canceled in accordance with public health directives for follow-up investigations in the period of March 2020 to July 2021.
Results
Between December 2017 and December 2021, 41 patients were assessed for eligibility. Because of concerns regarding patient safety and human and material (including personal protective equipment) resource shortages during the COVID-19 pandemic, trial accrual was temporarily halted by order of the Biomedical Research Ethics Board from March 2020 to July 2021. Sixteen patients (39.0%) did not meet eligibility criteria and 8 (19.5%) opted to forgo trial participation, leaving 17 (41.5%) eligible patients who consented to trial participation. One patient withdrew consent and 1 had disease progression before EMT implantation procedures. EMTs were successfully implanted in 15 patients. No significant beacon migration was noted between the time of EMT implantation to the start of SABR or between the first fraction of SABR until completion of the final fraction of SABR. One patient had a tracking failure of the EMTs because of the distance between the array to the beacons of just greater than 20 cm in 2 of the beacons. These 2 beacons did not migrate after their successful implantation, but the distance from the beacons to the array was larger than planned because of anterior protuberance of the abdomen, which was inferior to the lowest slices of the CT chest used to plan the navigational bronchoscopy. The increased abdominal standoff distance was discovered at the time of setting up for the first fraction of SABR. The patient was offered to be resimulated in the prone position for EMT-guided SABR; however, the patient did not want to wait for a repeat CT simulation scan and treatment planning processes and instead opted to have immediate treatment using the backup IG-SABR plan. Two patients were treated in the phase 1 feasibility component of the trial, and 12 patients were treated with EMT-guided RG- or DIBH-SABR in phase 2 of the trial. One patient in phase 2 had 2 target tumors treated simultaneously by RG-SABR using the same set of 3 EMTs. One patient was treated with EMT-guided DIBH-SABR in the prone position. Thus, a total of 13 target lung tumors were treated with EMT-guided RG- or DIBH-SABR (see Fig. 1 for CONSORT diagram). The study was closed to accrual in December 2021 in accordance with the recommendation by the trial monitoring committee, which concluded that enough patients had been accrued to make a judgment on the primary endpoint.
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) diagram.
Patient and tumor characteristics
Baseline patient characteristics are summarized in Table 1. The study cohort consisted of 8 men and 7 women, with a median age of 73 years (22-89). Seven patients had T1N0M0 NSCLC (53%), 2 patients had T2N0M0 NSCLC (13%), and 6 patients had pulmonary oligometastatic disease (40%). Four patients (27%) had central lesions (ie, located within 2 cm of the proximal bronchial tree) as per the Timmerman et al35 definition and 11 had peripheral lesions (73%). Four tumors were located in the upper lobes (25%), and 12 tumors were located in the lower lobes (75%). Maximum tumor dimension at baseline was 2.1 cm (0.7-3.8 cm). Mean pre-SABR FEV1 was 2.0 L (range, 0.8-3.45) predicted. Pre-SABR DLCO was 84% (52%-123%) predicted.
Table 1.
Patient, disease, and treatment characteristics
| Variable | No. | % | |
|---|---|---|---|
| Sex | Male | 8 | 53 |
| Female | 7 | 47 | |
| Median age | 73 (22-89) | ||
| Stage | T1N0M0 | 7 | 47 |
| T2N0M0 | 2 | 13 | |
| Oligometastatic | 6 | 40 | |
| Central/peripheral | Central | 4 | 27 |
| Peripheral | 11 | 73 | |
| Location | Upper lobes | 4* | 25 |
| Lower lobes | 12* | 75 | |
| Dose/fractionation | 48 Gy/4 fractions | 7 | 47 |
| 54 Gy/3 fractions | 8 | 53 | |
| Median tumor dimension (cm) | 2.1 (0.7–3.8) | ||
| Mean pre-SABR FEV1 (%) | 74 (33–118) | ||
| Mean pre-SABR DLCO (%) | 84 (52–123) | ||
| Mean tumor motion (cm) | 1.2 (0.5–4.0) | ||
| Mean track time (min) | 19.9 (8.6–46) | ||
| Mean beam-on time (min) | 2.8 (1.6–6.7) | ||
| Mean intertransponder distance difference (cm) | 0.087 (0–0.27) | ||
| Mean geometric residual (cm) | 0.057 (0–0.12) | ||
Abbreviations: DLCO = diffusing capacity for carbon monoxide; FEV1 = forced expiratory volume in 1 second; SABR = stereotactic ablative radiotherapy.
One patient had 2 lesions in the lower lobes, with 16 total tumors from 15 patients.
Treatment characteristics
Treatment characteristics are summarized in Table 1. Seven patients were treated with 48 Gy in 4 fractions (47%) and 8 with 54 Gy in 3 fractions (53%). Mean PTV for IG-SABR plans was 40.1 cc (range, 14.7-112.5) and 22.1 cc (range, 7.9-56.5) for RG-SABR. The average maximum tumor motion amplitude was 1.2 cm (range, 0.5-4). Mean tracking time (including setup and treatment) per fraction was 19.9 minutes (range, 8.6-46) with mean beam-on time of 2.8 minutes (range, 1.7-6.7) per fraction. Mean ITD difference per fraction was 0.087 mm (range, 0-0.27) with mean GR per fraction of 0.057 cm (range, 0-0.12). In 4 patients, 1 of the 3 implanted beacons needed to be disabled because of tracking failure (all of which were in patients in the supine position with the posterior-most beacon located just beyond the 20-cm range of the Calypso array).
Dosimetric and treatment planning outcomes
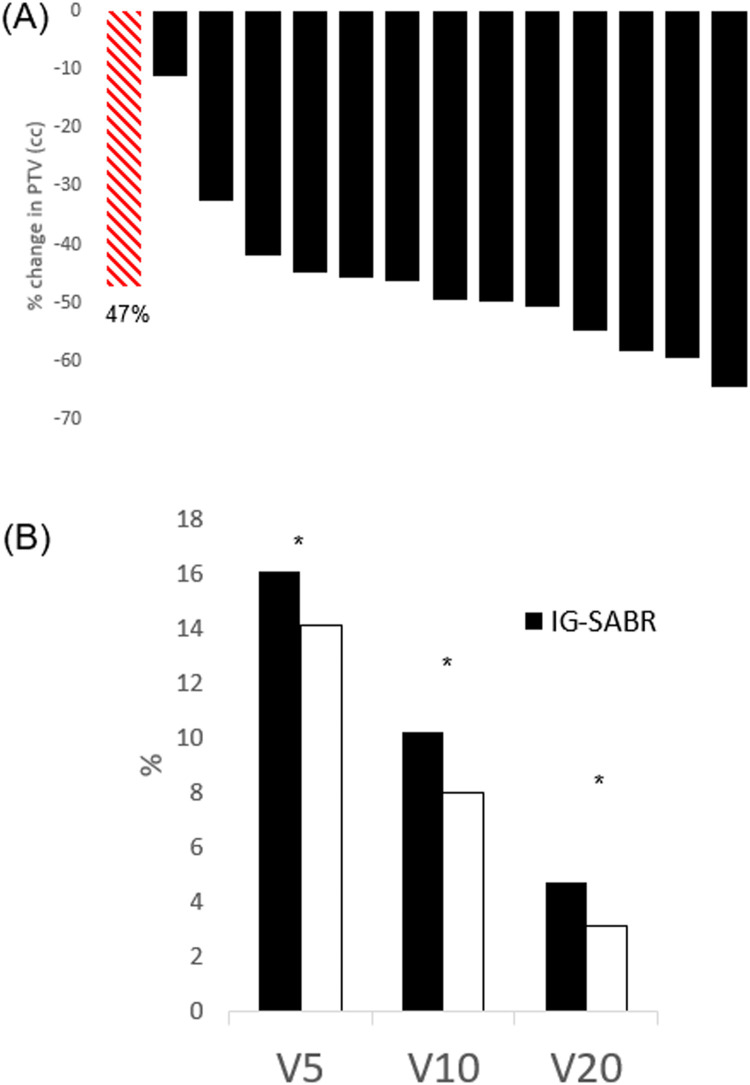
The PTVs of EMT-guided RG- (or DIBH)-SABR plans were significantly reduced compared with PTVs of the same tumors when planned using IG-SABR techniques (FB 4D-CT simulation scan plus 5-mm IG-PTV margins). Compared with IG-SABR, we observed that EMT-guided SABR plans yielded mean %PTV and %ITV reductions of 47.2% (range, 11%-65%; P < .005) and 36.4% (6.0%-65.5%; P < .005) respectively (Fig. 2A, Table 2).
Figure 2.
Dosimetric outcomes: (A) waterfall plot of percentage planning target volume reduction comparing EMT beacon guided, respiratory-gated, SABR with standard image-guided SABR. Red striped bar represents the mean reduction of 47.2%, P < .005. (B) Lung dose metric(V5Gy, V10Gy, V20Gy) comparison of standard image-guided SABR (black columns) with EMT beacon guided, respiratory-gated, SABR. *P < .005.
Table 2.
Lung dosimetric outcomes
| IG-SABR (mean) | RG-SABR (mean) | Absolute Reduction | Relative Reduction (%) | p- value | |
|---|---|---|---|---|---|
| MLD (Gy) | 3.8 (2.4–6.1) | 3.1 (2.0–5.1) | 0.7 (0.3–1.3) | 20.2 (3.8–34.2) | <.005 |
| V5 (%) | 16.1 (8.9–27.3) | 14.1 (8.5–19.7) | 2.0 (–0.3 to 3.4) | 11.6 (–3.0 to 20.1) | <.005 |
| V10 (%) | 10.2 (5.2–14.5) | 8.0 (4.6–15.2) | 2.2 (0.57–4.2) | 20.3 (9.0–36.2) | <.005 |
| V20 (%) | 4.7 (2.2–8.7) | 3.1 (1.6–6.0) | 1.6 (0.4–2.9) | 31.1 (19.6–48.8) | <.005 |
| D2cm (GY) | 30.9 (24.2–39.2) | 26.1 (21.1–32.8) | 4.8 (1.4–14.2) | 15.5 (4.2–36.3) | <.005 |
| ITV (cc) | 15.4 (3.8–54.4) | 9.0 (2.7–29.8) | 6.4 (0.4–24.6) | 36.4 (6.0–65.5) | <.005 |
| PTV (cc) | 40.1 (14.7–112.5) | 21.6 (7.9–56.5) | 18.5 (6.6–43.4) | 47.2 (11.1–64.5) | <.005 |
Abbreviations: IG = image guided; ITV = internal target volume; MLD = mean lung dose; PTV = planning target volume; RG = respiratory gated; SABR = stereotactic ablative radiotherapy; V5 (%) = percentage volume of lungs receiving 5 Gy; V10 (%) = percentage volume of lungs receiving 10 Gy; V20 (%) = percentage volume of lungs receiving 20 Gy.
Significant relative reductions were also observed in the mean V5 (–11.6% [–20.1% to –3.0%]; P < .005), V10 (–20.3% [–36.2% to –9.0%]; P < .005), V20 (–31.1% [–48.8% to –19.6%]; P < .005), D2cm (–15.5% [–36.3% to –4.2%]; P < .005), and MLD (–20.2% [–34.2% to –3.8%]; P < .005) of the bilateral lungs with RG- (or DIBH)-SABR treatment plans compared with IG-SABR treatment plans (Table 2, Fig. 2B). For IG-SABR plans, the absolute mean MLD, V5, V10, V20, and D2cm were 3.8 Gy (2.4-6.1 Gy), 16.1% (8.9%-27.3%), 10.2% (5.2%-14.5%), 4.7% (2.2%-8.7%), and 3.1 Gy (2.4-3.9 Gy), respectively. In RG- (or DIBH)-SABR, the absolute mean MLD, V5, V10, V20, and D2cm were 3.1 Gy (2.0-5.1 Gy), 14.1% (8.5%-19.7%), 7.9% (4.6%-15.2%), 3.1% (1.6%-6.0%), and 2.6 Gy (2.1-3.3 Gy), respectively (Table 2). For other thoracic OARs, significant reductions in maximum point dose (Dmax) were observed in chest wall (–8.4% [–29.2% to 6.0%]; P < .005), esophagus (–10.3% [–37.0% to 14.3%]; P < .025), heart (–18.3% [–55.9% to 3.8%]; P < .005), and ribs (–4.9% [–12.7% to 11.2%]; P < .025). There was no significant sparing of the spinal cord Dmax (1.7% [–25.7% to 31.1%]; P = .86). Dose-volume analyses for these OARs showed a greater effect on the relative dose change in chest wall (–26.6% [–100.0% to 0.02%]; P < .005), esophagus (–24.1% [–74.5% to 29.8%]; P < .005), heart (–17.8% [–60.8% to 23.1%]; P < .005), and ribs (–13.9% [–100% to 8.8%]; P < .025) (Table E1).
Case illustrations
Case 1: Left lower lobe tumor located in close proximity to the heart
Prone DIBH-SABR technique was used in a patient with metastatic colorectal carcinoma with a solitary pulmonary metastasis in the right lower lobe located posteriolateral to the heart. When simulated using an FB 4D-CT scan and planned using the standard IG SABR for a dose of 48 Gy in 4 fractions, the PTV margin extended into the heart with heart Dmax of 6182 cGy (Fig. 3A). When CT simulation was repeated in prone DIBH, the distance between the heart and the target tumor increased such that when using a 3-mm EMT-based PTV margin, the PTV no longer overlapped with the heart and the heart Dmax was reduced to 2523 cGy (59.2% reduction). This patient successfully received EMT-guided DIBH-SABR and did not suffer any grade 2+ acute/late toxicities (Fig. 3A).
Figure 3.
Illustrative cases. (A) Sixty-two-year-old male with a solitary pulmonary metastasis from colorectal cancer. Top: standard IG-SABR was not safe/feasible to deliver because of PTV (orange contour) was overlapping with heart (pink contour) and heart maximum dose of 6182 cGy. Bottom: Prone deep-inspiration breath hold SABR using Calypso EMT guidance with maximum heart dose of 2523 cGy. The heart (pink contours) and PTV (orange contours) no longer overlap. (B) Twenty-two-year-old female with metastatic synovial sarcoma with 2 pulmonary metastases in close proximity. Both tumors were treated concurrently using 1 set of EMT beacons. Red contours: standard IG-SABR PTVs. Blue contours: EMT guided, respiratory-gated, SABR PTVs. PTVs were reduced by 50.6% (anterior tumor) and 54.7% (posterior tumor), respectively, using EMT-guided respiratory-gated SABR as compared with IG-SABR. Abbreviations: EMT = electromagnetic transponders; IG = image guided; PTV = planning target volume.
Case 2: Two right lower lobe tumors treated concurrently with EMT-guided RG-SABR with 1 set of beacons
Although EMTs are typically used for the treatment of a single pulmonary tumor target, it is possible to treat more than 1 tumor target with a single set of 3 EMTs if the targets are located in relative proximity to one another. A 22-year-old woman with metastatic synovial sarcoma presented with 2 pulmonary oligometastatic lesions of the right lower lobe in close proximity to the diaphragm with large respiratory motion amplitude of 4.0 cm (Fig. 3B). To spare the maximum amount of normal lung from RT dose, the decision was made to use EMT-guided RG-SABR for both tumor targets simultaneously. This treatment was successfully delivered to a dose of 54 Gy in 3 fractions without complications. Compared with FB IG-SABR, PTVs were reduced by 50.6% for the anterior tumor and 54.7% for the tumor located more posteriorly in the right lower lobe, with a corresponding 29.9% reduction in lung V20 combined (Fig. 3B).
Treatment-related toxicity
No grade 2+ acute or late treatment-related toxicities were observed during or after EMT implantation. No postimplant complications such as pneumothorax, pulmonary hemorrhage, pneumonia, bronchitis, or expectoration of EMTs occurred. No grade 2+ SABR-related treatment toxicities were observed during treatment or follow-up. Eight patients reported no treatment-related toxicities whatsoever. Six patients reported grade 1 toxicities during follow-up including: fatigue (2), cough (1), dyspnea (1), chest wall pain (1), and radiation pneumonitis (1).
Tumor outcomes
The database was frozen on October 15, 2022, with a median follow-up of 18 months (6-48), and no local treatment failures were observed. Three patients had regional and distant disease progression: 1 at 6 weeks and 2 at 6 months after treatment. One had regional progression of NSCLC, while the other 2 had systemic progression of nonpulmonary primary malignancies. In terms of best treatment response, 5 (33%) patients had complete response on serial imaging, 7 (47%) patients had partial response, and 3 (20%) had stable disease (Table E2). The progression-free survival at 12 and 24 months was 79%, and the median progression-free survival was not reached (Fig. E1B).
All patients completed scans at 6-month follow-up, and 9 patients were able to complete PFTs (access to PFTs was limited during the COVID-19 lockdowns). Average maximum tumor dimension at 6 months was 1.3 cm (0.7-3.4 cm), compared with the baseline of 2.1 cm (0.7-3.8 cm). Mean percentage reduction of tumor volume was 53% at 6 months compared with baseline measurements (Fig. E1A; P < .005). Of the 9 patients who completed PFTs at 6 months, mean FEV1 change was not statistically significant at –8.1% (–27% to 15%; P > .1). There was a significant change in DLCO of –17.6% (–41.7% to 15.9%; P < .025).
Discussion
This study found that EMT-guided SABR is highly precise both during localization and treatment delivery. During localization, EMTs provided highly accurate positional telemetry with a mean ITD difference of 0.087 cm (0-0.27 cm) and GR of 0.057 cm, which are consistent with values reported in the literature.26,28,30 Although a 5-mm PTV margin was used in the feasibility studies of EMTs by others,26, 27, 28, 29, 30, 31 we found that with the degree of precision afforded by the real-time positional telemetry from the EMTs, a smaller PTV margin of 3 mm was justified.12,24 The 3-mm PTV expansion margin employed in this study is the smallest in vivo PTV margin among the published lung SABR literature.
Nonanchored, gold seed pulmonary fiducial markers implanted into lung parenchyma via transthoracic or endobronchial route are associated with a significant rate of seed migration (as high as 19%) along needle tracks or vascular/ventilatory pressure gradients.36,37 Coiled fiducial markers (nonanchored) are also associated with significant rates of marker migration. Casutt et al38 reported 16 fiducial coil migrations in their lung SABR study within a cohort of 207 patients. Among the 16 migrated coils, 13 coils were missing on CT simulation scans while the remainder were discovered in other unexpected pulmonary segments. By contrast, during this study, we did not observe any significant migration of EMTs after implantation based on pretreatment cone beam CT scans for each patient and serial follow-up CT scans (data not shown), highlighting the value of the miniature anchoring legs of the Calypso EMTs, which held the EMTs firmly in place in the endobronchial lumen of 2- to 3-mm diameter airways of the lungs. McDonald et al39 retrospectively investigated 17 patients treated with anchored EMT-guided SABR and found 68% of EMTs were within 5 mm of the locations at treatment planning on post-SABR imaging at a median of 2.5 years. Of those that migrated ≥5 mm, all were attributed to peritumor fibrosis,39 which altered the conformation of the lung parenchyma during follow-up.
Conventional pulmonary IG-SABR treatments using motion encompassing ITV techniques in the lower lobes of the lungs have a propensity for large PTVs relative to the size of the primary target, especially among tumors with large motion amplitudes located close to the diaphragm. The use of EMTs in this study reduced PTVs by 2 key avenues. Firstly, the real-time target location and motion telemetry allows for accurate, automated gating of the linear accelerator such that SABR is delivered only during the gating window, and thus a smaller ITV is generated because it did not have to include other phases of the respiratory cycle when the tumor is moving the most. Secondly, the high precision positional telemetry minimized uncertainty of the target location such that a smaller expansion on ITV (3 mm) could be used to generate the PTV. In this prospective cohort study assessing the effect of EMTs on PTVs for lung tumor SABR treatments, we found that the use of these 2 aforementioned strategies afforded by the EMTs led to a mean 47.2% reduction in PTVs compared with IG-SABR. These findings bolster the findings of a prospective clinical trial by Booth et al,40 who demonstrated PTV reduction with EMT-guided RG-SABR of 26%. In their study, a single end-exhalation phase was designated as the gated window with a PTV margin of 5 mm. They reported a decrease in MLD from 2.9 to 2.6 Gy (9.3% reduction). In the present study, the 47.2% PTV reduction and average MLD decrease from 3.8 to 3.1 Gy (20.2% relative reduction) could potentially be attributed to the use of the combination of a respiratory gating window and a smaller PTV margin of 3 mm. The risk of radiation pneumonitis (RP) has been correlated with MLD and V20.41, 42, 43 For example, Barriger et al41 reviewed 251 patients and found significant differences in incidence of grade 2 to 4 RP with the thresholds of MLD of 4 Gy and V20 of 4%. The present study showed RG-SABR achieved reduction of the mean V20 to 3.1% from 4.7% compared with IG-SABR plans (Table 2). Thus, it is conceivable that for some patients, EMT-guided RG-SABR may effectively reduce the risk of grade 2+ RP.
Although we observed a statistically significant reduction of dose to OARs including the chest wall, ribs, esophagus, and heart, we note that mean doses in both IG-SABR and EMT-guided SABR plans were below the dose constraints outlined by American Association of Physicists in Medicine Task Group 101 report.44 This is likely because of the heterogeneity of tumor locations and their varied proximities to OARs. The benefits of EMT-guided SABR are of heightened importance for treatment of a tumor/PTV that is located in close proximity to an OAR, especially if PTV overlaps or dose fall-off regions would be expected to result in acute or late treatment-related toxicity. Thus, with the use of implanted EMTs, careful selection of patients who may appreciably benefit from their use is key. An example of such a patient treated on this study had a left lower lobe tumor that was located in close proximity to the heart (Fig. 3A), for which standard IG-SABR would have been expected to cause considerable harm to the heart. Without EMTs, cases such as this would be managed with nonablative dose-per-fraction hypofractionated RT to meet OAR constraints, which would likely deprive the patient of the higher biologically effective dose of SABR. Therefore, EMTs can be considered, especially in cases when SABR would not otherwise be feasible to deliver.
In this study, EMT implantation procedures were safe and well-tolerated by all patients with no procedure-related complications observed, consistent with the experience reported in prior studies.26, 27, 28, 29, 30, 31 A 7-day interval between EMT implantation and CT simulation scans was employed in this study to allow for any postimplant settling of the EMTs before the simulation scans. However, there was no definitive evidence, albeit difficult to quantify (since comparisons of postimplant fluoroscopic images compared with the CT simulation scan were inherently qualitative in nature), of any postimplant EMT migration seen in our cohort. Thus, it is possible that a 7-day time interval between EMT implant and CT simulation scan may actually be too lengthy. Compared with forced shallow breathing methods (eg, abdominal compression or coached breathing), respiratory gating of an FB patient required essentially no additional special effort or compliance from the patient compared with standard FB IG-SABR, and as such our technique was well tolerated by all who underwent treatment.
The primary limitation of this study is its sample size, which is smaller than the originally planned sample size of 28. This study was closed early, largely as a result of significant challenges to accrual. Of the 41 patients who were screened eligible, only 17 (41.5%) patients were enrolled. This was likely a result of a few factors, including (1) protocol related barriers; (2) COVID-19 pandemic–related barriers; and (3) patient preference. Thirty-nine percent of the candidates screened did not meet eligibility criteria because of medical comorbidities, poor general/pulmonary function, and inadmissible tumor characteristics, and a higher patient referral rate may overcome these protocol-related barriers. However, our accrual was suspended for over a year because of COVID-19 lockdowns, leading to missed opportunities to recruit potential candidates. In addition, about one-fifth of patients declined to participate despite meeting eligibility requirements. This may relate to the fact that EMT implantation is often done under general anesthesia, which may not appeal to patients who wished to avoid operative-style procedures in the first place. In the pandemic era, COVID-19 risk-averse patients may also be weary of the additional in-person appointments at the hospital and tests required by the protocol because of fears associated with iatrogenic COVID-19 infection, especially given the high rate of mortality associated with patients with comorbidities, which made them inoperable to begin with. Furthermore, an inverse relationship between study enrollment rates and COVID-19 waves has been reported in the literature.45 Exacerbation of socioeconomic inequity and financial burden related to the pandemic has also been speculated as a factor.45, 46, 47 Whether these higher-than-normal, risk-averse behaviors and social-financial factors were responsible, in part, for our lower-than-expected accrual pace is difficult to objectively determine. Finally, given our study's modest sample size, our study was not powered to determine, on a population level, which patients would serve to benefit the most from our approach. However, with further use of this approach off trial, we intend to quantify the benefits of EMT-guided and gated SABR in a larger population and provide guidance as to which specific patients, from an OAR dosimetric point of view, benefit the most from their use.
Conclusion
EMT-guided RG-SABR with 3-mm PTV margins reduced the mean PTV by 47% compared with standard IG-SABR. Careful selection of patients with moving lung tumors with larger motion amplitudes or those that are located in close proximity of thoracic OARs maximizes the therapeutic utility of EMTs for SABR.
Acknowledgments
We thank our patient volunteers and their families for participating in this study. We thank Darlene Courtney for radiation treatment planning work included in the analysis. We thank Kathryn Dyck, Kathi Klapp, Rose Woloshyn, and Lori Walker from the Clinical Trials Unit for their diligent work in support of the operations of this clinical trial, including patient accrual and follow-up. We thank Elizabeth Lylyk for the regulatory and ethical compliance work for this study. We thank Dr China-Li Hillman for arranging thin-slice CT scans of the chest for purposes of navigational bronchoscopy for patients in this study.
Footnotes
Sources of support: Research operating grant funds were provided via an institutional research operating grant from Varian Medical Systems to CancerCare Manitoba. In-kind donation of electromagnetic beacons used in this study were provided by Varian Medical Systems.
Disclosures: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data sharing is available upon reasonable request to the corresponding author.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.adro.2023.101243.
Appendix. Supplementary materials
References
- 1.Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation: Results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest. 2003;124:1946–1955. doi: 10.1378/chest.124.5.1946. [DOI] [PubMed] [Google Scholar]
- 2.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timmerman RD, Hu C, Michalski JM, et al. Long-term results of stereotactic body radiation therapy in medically inoperable stage I non–small cell lung cancer. JAMA Oncol. 2018;4:1287–1288. doi: 10.1001/jamaoncol.2018.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milano MT, Katz AW, Schell MC, Philip A, Okunieff P. Descriptive analysis of oligometastatic lesions treated with curative-intent stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:1516–1522. doi: 10.1016/j.ijrobp.2008.03.044. [DOI] [PubMed] [Google Scholar]
- 5.Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol. 2009;27:1579–1584. doi: 10.1200/JCO.2008.19.6386. [DOI] [PubMed] [Google Scholar]
- 6.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): A randomised, phase 2, open-label trial. Lancet. 2019;393:2051–2058. doi: 10.1016/S0140-6736(18)32487-5. [DOI] [PubMed] [Google Scholar]
- 7.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: Long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol. 2020;38:2830–2838. doi: 10.1200/JCO.20.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol. 2012;7:1807–1814. doi: 10.1097/JTO.0b013e3182745948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gan GN, Weickhardt AJ, Scheier B, et al. Stereotactic radiation therapy can safely and durably control sites of extra-central nervous system oligoprogressive disease in anaplastic lymphoma kinase-positive lung cancer patients receiving crizotinib. Int J Radiat Oncol Biol Phys. 2014;88:892–898. doi: 10.1016/j.ijrobp.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heinzerling JH, Anderson JF, Papiez L, et al. Four-dimensional computed tomography scan analysis of tumor and organ motion at varying levels of abdominal compression during stereotactic treatment of lung and liver. Int J Radiat Oncol Biol Phys. 2008;70:1571–1578. doi: 10.1016/j.ijrobp.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 11.Bouilhol G, Ayadi M, Rit S, et al. Is abdominal compression useful in lung stereotactic body radiation therapy? A 4DCT and dosimetric lobe-dependent study. Physica Medica. 2013;29:333–340. doi: 10.1016/j.ejmp.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 12.Guckenberger M, Krieger T, Richter A, et al. Potential of image-guidance, gating and real-time tracking to improve accuracy in pulmonary stereotactic body radiotherapy. Radiother Oncol. 2009;91:288–295. doi: 10.1016/j.radonc.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Jang SS, Huh GJ, Park SY, Yang PS, Cho EY. The impact of respiratory gating on lung dosimetry in stereotactic body radiotherapy for lung cancer. Phys Med. 2014;30:682–689. doi: 10.1016/j.ejmp.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Berson AM, Emery R, Rodriguez L, et al. Clinical experience using respiratory gated radiation therapy: Comparison of free-breathing and breath-hold techniques. Int J Radiat Oncol Biol Phys. 2004;60:419–426. doi: 10.1016/j.ijrobp.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 15.Lee S, Zheng Y, Podder T, et al. Tumor localization accuracy for high-precision radiotherapy during active breath-hold. Radiother Oncol. 2019;137:145–152. doi: 10.1016/j.radonc.2019.04.036. [DOI] [PubMed] [Google Scholar]
- 16.Underberg RWM, Lagerwaard FJ, Cuijpers JP, Slotman BJ, van Sörnsen de Koste JR, Senan S. Four-dimensional CT scans for treatment planning in stereotactic radiotherapy for stage I lung cancer. Int J Radiat Oncol Biol Phys. 2004;60:1283–1290. doi: 10.1016/j.ijrobp.2004.07.665. [DOI] [PubMed] [Google Scholar]
- 17.Wu VWC, Ng APL, Cheung EKW. Intrafractional motion management in external beam radiotherapy. J Xray Sci Technol. 2019;27:1071–1086. doi: 10.3233/XST-180472. [DOI] [PubMed] [Google Scholar]
- 18.Cano Porras D, Lunardi AC, Marques da Silva CCB, et al. Comparison between the phase angle and phase shift parameters to assess thoracoabdominal asynchrony in COPD patients. J Appl Physiol. 2017;122:1106–1113. doi: 10.1152/japplphysiol.00508.2016. [DOI] [PubMed] [Google Scholar]
- 19.Kogan D, Jain A, Kimbro S, Gutierrez G, Jain V. Respiratory inductance plethysmography improved diagnostic sensitivity and specificity of obstructive sleep apnea. Respir Care. 2016;61:1033–1037. doi: 10.4187/respcare.04436. [DOI] [PubMed] [Google Scholar]
- 20.Yorke E, Rosenzweig KE, Wagman R, Mageras GS. Interfractional anatomic variation in patients treated with respiration-gated radiotherapy. J Appl Clin Med Phys. 2005;6:19–32. doi: 10.1120/jacmp.v6i2.2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J, Wu Q, Zhao B, et al. To gate or not to gate - dosimetric evaluation comparing gated versus ITV-based methodologies in stereotactic ablative body radiotherapy (SABR) treatment of lung cancer. Radiat Oncol. 2016;11:125. doi: 10.1186/s13014-016-0699-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nader DA. Safety of endobronchial implantation of electromagnetic fiducials for real-time tracking of lung tumors during radiotherapy. J Thorac Oncol. 2013;8 S171-S171. [Google Scholar]
- 23.Franz AM, Schmitt D, Seitel A, et al. Standardized accuracy assessment of the calypso wireless transponder tracking system. Phys Med Biol. 2014;59:6797–6810. doi: 10.1088/0031-9155/59/22/6797. [DOI] [PubMed] [Google Scholar]
- 24.Sandler HM, Liu PY, Dunn RL, et al. Reduction in patient-reported acute morbidity in prostate cancer patients treated with 81-Gy intensity-modulated radiotherapy using reduced planning target volume margins and electromagnetic tracking: Assessing the impact of margin reduction study. Urology. 2010;75:1004–1008. doi: 10.1016/j.urology.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Worm ES, Høyer M, Hansen R, et al. A prospective cohort study of gated stereotactic liver radiation therapy using continuous internal electromagnetic motion monitoring. Int J Radiat Oncol Biol Phys. 2018;101:366–375. doi: 10.1016/j.ijrobp.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 26.Harris W, Yorke E, Li H, et al. Can bronchoscopically implanted anchored electromagnetic transponders be used to monitor tumor position and lung inflation during deep inspiration breath-hold lung radiotherapy? Med Phys. 2022;49:2621–2630. doi: 10.1002/mp.15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaccard M, Champion A, Dubouloz A, et al. Clinical experience with lung-specific electromagnetic transponders for real-time tumor tracking in lung stereotactic body radiotherapy. Phys Imaging Radiat Oncol. 2019;12:30–37. doi: 10.1016/j.phro.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarkar V, Szegedi M, Paxton A, et al. Preliminary clinical experience with Calypso anchored beacons for tumor tracking in lung SBRT. Med Phys. 2020;47:4407–4415. doi: 10.1002/mp.14300. [DOI] [PubMed] [Google Scholar]
- 29.Boggs DH, Popple R, McDonald A, et al. Electromagnetic transponder based tracking and gating in the radiotherapeutic treatment of thoracic malignancies. Pract Radiat Oncol. 2019;9:456–464. doi: 10.1016/j.prro.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 30.Schmitt D, Nill S, Roeder F, Gompelmann D, Herth F, Oelfke U. Motion monitoring during a course of lung radiotherapy with anchored electromagnetic transponders: Quantification of inter- and intrafraction motion and variability of relative transponder positions. Strahlenther Onkol. 2017;193:840–847. doi: 10.1007/s00066-017-1183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dobelbower MC, Popple RA, Minnich DJ, et al. Anchored transponder guided lung radiation therapy. Pract Radiat Oncol. 2020;10:e37–e44. doi: 10.1016/j.prro.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Timmerman RD, Paulus R, Pass HI, et al. Stereotactic body radiation therapy for operable early-stage lung cancer: Findings from the NRG Oncology RTOG 0618 trial. JAMA Oncol. 2018;4:1263–1266. doi: 10.1001/jamaoncol.2018.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma CM, Coffey CW, DeWerd LA, et al. AAPM protocol for 40–300 kV x-ray beam dosimetry in radiotherapy and radiobiology. Med Phys. 2001;28:868–893. doi: 10.1118/1.1374247. [DOI] [PubMed] [Google Scholar]
- 34.Zhao B, Yang Y, Li T, Li X, Heron DE, Huq MS. Image-guided respiratory-gated lung stereotactic body radiotherapy: Which target definition is optimal? Med Phys. 2009;36:2248–2257. doi: 10.1118/1.3129161. [DOI] [PubMed] [Google Scholar]
- 35.Timmerman R, Mcgarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 36.Bhagat N, Fidelman N, Durack JC, et al. Complications associated with the percutaneous insertion of fiducial markers in the thorax. Cardiovasc Intervent Radiol. 2010;33:1186–1191. doi: 10.1007/s00270-010-9949-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anantham D, Feller-Kopman D, Shanmugham LN, et al. Electromagnetic navigation bronchoscopy-guided fiducial placement for robotic stereotactic radiosurgery of lung tumors: A feasibility study. Chest. 2007;132:930–935. doi: 10.1378/chest.07-0522. [DOI] [PubMed] [Google Scholar]
- 38.Casutt A, Noirez L, Bernasconi M, et al. Endobronchial coil spring fiducial markers for CyberKnife® stereotactic body radiation therapy. Respirology. 2021;26:469–476. doi: 10.1111/resp.14006. [DOI] [PubMed] [Google Scholar]
- 39.McDonald AM, Colvin T, Boggs DH, et al. Longitudinal assessment of anchored transponder migration following lung stereotactic body radiation therapy. J Appl Clin Med Phys. 2019;20:17–22. doi: 10.1002/acm2.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Booth J, Caillet V, Briggs A, et al. MLC tracking for lung SABR is feasible, efficient and delivers high-precision target dose and lower normal tissue dose. Radiother Oncol. 2021;155:131–137. doi: 10.1016/j.radonc.2020.10.036. [DOI] [PubMed] [Google Scholar]
- 41.Barriger RB, Forquer JA, Brabham JG, et al. A dose-volume analysis of radiation pneumonitis in non-small cell lung cancer patients treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys. 2012;82:457–462. doi: 10.1016/j.ijrobp.2010.08.056. [DOI] [PubMed] [Google Scholar]
- 42.Lu C, Lei Z, Wu H, Lu H. Evaluating risk factors of radiation pneumonitis after stereotactic body radiation therapy in lung tumor: Meta-analysis of 9 observational studies. PLoS One. 2018;13 doi: 10.1371/journal.pone.0208637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao J, Yorke ED, Li L, et al. Simple factors associated with radiation-induced lung toxicity after stereotactic body radiation therapy of the thorax: A pooled analysis of 88 studies. Int J Radiat Oncol Biol Phys. 2016;95:1357–1366. doi: 10.1016/j.ijrobp.2016.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med Phys. 2010;37:4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 45.Unger JM, Xiao H, LeBlanc M, Hershman DL, Blanke CD. Cancer clinical trial participation at the 1-year anniversary of the outbreak of the COVID-19 pandemic. JAMA Network Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.18433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De B, Kaiser KW, Ludmir EB, et al. Radiotherapy clinical trial enrollment during the COVID-19 pandemic. Acta Oncologica. 2021;60:312–315. doi: 10.1080/0284186X.2020.1865564. [DOI] [PubMed] [Google Scholar]
- 47.Nipp RD, Hong K, Paskett ED. Overcoming barriers to clinical trial enrollment. Am Soc Clin Oncol Educ Book. 2019;39:105–114. doi: 10.1200/EDBK_243729. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.