Abstract
Purpose
This study's objective was to report cancer control and toxicity outcomes after proton radiation therapy (RT) in testicular seminoma and to compare secondary malignancy (SMN) risks with photon-based treatment alternatives.
Methods and Materials
Consecutive patients with stage I-IIB testicular seminoma treated with proton RT at a single institution were retrospectively analyzed. Kaplan-Meier estimates for disease-free and overall survival were computed. Toxicities were scored using Common Terminology Criteria for Adverse Events version 5.0. Photon comparison plans, including 3-dimensional conformal RT (3D-CRT) and intensity modulated RT (IMRT)/volumetric arc therapy (VMAT), were created for each patient. Dosimetric parameters and SMN risk predictions for different in-field organs-at-risk were compared between the techniques. Excess absolute SMN risks were estimated with organ equivalent dose modeling.
Results
Twenty-four patients were included (median age, 38.5 years). The majority of patients had stage II disease (IIA, 12 [50.0%]; IIB, 11 [45.8%]; IA, 1 [4.2%]). Seven (29.2%) and 17 (70.8%) patients had de novo and recurrent disease, respectively (de novo/recurrent: IA, 1/0; IIA, 4/8; IIB, 2/9). Most acute toxicities were mild (grade 1 [G1], 79.2%; G2, 12.5%) with G1 nausea being most common (70.8%). No serious events (G3-5) occurred. With a median follow-up time of 3 years (interquartile range, 2.1-3.6 years), 3-year disease-free and overall survival rates were 90.9% (95% confidence interval, 68.1%-97.6%) and 100% (95% confidence interval, 100%-100%), respectively. There were no documented late toxicities in the follow-up period, including worsening serial creatinine levels suggestive of early nephrotoxicity. Proton RT had significant reductions in mean organ-at-risk doses to the kidneys, stomach, colon, liver, bladder, and body compared with both 3D-CRT and IMRT/VMAT. Proton RT had significantly lower SMN risk predictions compared with 3D-CRT and IMRT/VMAT.
Conclusions
Cancer control and toxicity outcomes using proton RT in stage I-IIB testicular seminoma are consistent with existing photon-based RT literature. However, proton RT may be associated with significantly lower SMN risks.
Introduction
Primary testicular seminoma is an overall infrequent diagnosis but is the most common solid malignancy diagnosed in adolescent and young adult males with rising incidences worldwide.1 Compared with other cancers, testicular seminoma is highly curable with current standard-of-care treatment, including radical inguinal orchiectomy with or without adjuvant chemotherapy or radiation therapy (RT). Given the young age of onset and favorable long-term outcomes, there is heightened emphasis on reducing short- and long-term treatment morbidities. Throughout the years, survivorship has been improved through optimal selection for surveillance and by de-intensifying adjuvant treatment, either by using chemotherapy regimens with lower toxicities or de-escalating RT (ie, field size shrinkage and dose reduction).2, 3, 4, 5, 6
Proton RT has the possibility to further improve the therapeutic ratio in early stage testicular seminoma.7 Current United States (US) national guidelines recommend using the photon-based 3-dimensional conformal RT (3D-CRT) approach with parallel-opposed anterior-posterior/posterior-anterior (AP/PA) fields. With 3D-CRT, ionizing radiation must traverse the entire abdominopelvic region to treat posteriorly located at-risk or clinically involved para-aortic or iliac lymph node chains. Thus, large volumes of uninvolved tissue and organs (kidneys, colon, stomach, and liver) become inadvertently exposed to the full prescribed radiation dose, raising concern for long-term side effects such as gastrointestinal (GI) tract disorders, chronic nephrotoxicity, and especially radiation-induced secondary malignancies (SMNs), a leading cause of morbidity and mortality in this population. Intensity modulated RT (IMRT) and volumetric arc therapy (VMAT) are more modern photon planning and delivery approaches, but US national guidelines currently recommend against their use in testicular seminoma. IMRT/VMAT reduces the volume of tissue receiving full prescription dose; however, a larger volume is exposed to lower doses (ie, “low-dose bath”), potentially creating even greater SMN risks than 3D-CRT.8 Protons are another RT strategy. Due to its physical properties, proton RT to typical testicular seminoma targets can minimize the volume of normal, non-target tissue receiving the full prescribed dose without the low-dose bath.9 Despite these dosimetric advantages, there is little evidence confirming adequate outcomes using proton RT in a real-world setting. This study aims to validate cancer control and toxicity profiles following proton RT for testicular seminoma and seeks to confirm that clinically delivered plans provide favorable SMN risks.
Methods and Materials
Design and study population
This study was a retrospective case series conducted at a single institution with approval provided by its institutional review board (Hospital of the University of Pennsylvania). All consecutive patients with de novo or recurrent stage I-II primary testicular, pure seminoma treated with proton RT from 2010 to 2021 were included. Disease was staged using the American Joint Committee on Cancer eighth edition staging system. Exclusion criteria included non-testicular primary site (eg, primary mediastinal), non-pure seminoma histology (eg, non-seminoma germ cell tumor or other), distant metastasis, or RT treatment using a photon technique. Two additional photon comparison treatment plans (3D-CRT and IMRT/VMAT) were generated for each patient to compare organ-at-risk (OAR) dosimetry and SMN risk profiles. Supplementary Materials provide in-depth discussion of RT treatment planning and delivery details (Appendix E1).
Variable and outcome definitions
Electronic medical records were reviewed to collect variables of interest including baseline demographic information, disease-related factors, proton treatment details, survival outcomes, and toxic effects. Disease-free survival (DFS) and overall survival (OS) were defined as the time from RT start to date of relapse or death, respectively. Patients with their last time point as their last oncologic clinic follow-up date were censored from all survival analyzes. Acute (<6 months) and late (≥6 months) RT-related toxic effects were graded using Common Terminology Criteria for Adverse Events version 5.0. Nephrotoxicity was evaluated by trending any available serial serum creatinine (Cr) levels (n = 23) and assessing for new or worsening hypertension. New onset hypertension post-RT was defined as elevated systolic or diastolic blood pressures ≥130/80 mm Hg on 2 consecutive clinical follow-up appointments or new anti-hypertensive medication start. In those patients with pre-existing hypertension, worsening hypertension was defined as uncontrollable blood pressure or management requiring additional anti-hypertensives. Available complete blood counts were also evaluated to assess post-RT bone marrow toxic effects (n = 13). Any patients who subsequently underwent chemotherapy for relapse were excluded from the complete blood count analysis. Radiation-induced SMN risks were estimated using the organ equivalent dose (OED, Gy) concept to calculate excess absolute risks (per 104 person-years [PY]). This methodology was developed and previously described in detail by Schneider et al.10,11 Refer to Supplementary Materials for details on SMN risk calculations for this study (Appendix E2).
Statistical analysis
Baseline characteristics were summarized using descriptive statistics (categorical variables: medians, ranges, and interquartile ranges [IQRs]; continuous variables: frequency counts and proportions). Kaplan-Meier curves were constructed for survival outcomes. Dosimetric and SMN comparison data were summarized with means and associated standard errors of mean. Statistical comparisons were done using matched Friedman and analysis of variance tests with multiple comparisons corrections. In figures and text, adjusted P values and significance levels are denoted as either non-significant (ns; P > 0.05), P < .05(*), P < .01(**), P < .001(***), or P < .0001(****). All statistical analyzes were done using GraphPad Prism 9.3 (GraphPad Software Inc, San Diego, CA).
Results
Study population
Twenty-four patients were included with a median age of 38.5 years (range, 22.0-57.0 years; IQR, 30.5-44.0 years) and a median follow-up time of 3.0 years (range, 0.0-7.6 years; IQR, 2.1-3.6 years) (Table 1). Seven (29.2%) and 17 (70.8%) patients had de novo and recurrent disease, respectively. All recurrent patients opted for active surveillance rather than adjuvant therapy following orchiectomy for prior stage I disease. Median time from orchiectomy to recurrence was 13.2 months (range, 3.0-53.7 months; IQR, 6.3-16.9 months). Of the entire study population, most were stage II (IIA: n = 12 [50.0%], de novo = 4, recurrent = 8; IIB: n = 11 [45.8%], de novo = 2, recurrent = 9). One patient (4.2%) had de novo stage IA disease. Median primary tumor size was 4.0 cm (range, 1.5-8.0 cm; IQR, 2.6-5.5 cm; 1 [4.2%] unknown pTX), and lymphovascular space invasion was noted in 7 patients (29.2%). Sixteen patients (66.7%) had only 1 enlarged pathologic node on computed tomography abdominopelvic imaging. Two or 3 positive nodes were seen in 5 (20.8%) and 2 (8.3%) patients, respectively. Of those with positive nodes (cN1-2), the median largest node size was 1.9 cm (range, 1.2-4.2 cm; IQR, 1.6-2.3 cm).
Table 1.
Baseline characteristics of patients with pure testicular seminoma treated with proton radiation therapy (RT)
| Baseline characteristic | n (%) or median (range, IQR) | |
|---|---|---|
| Total number of patients | N = 24 | |
| Follow-up time (y) | 3.0 (0.0-7.6, 2.1-3.6) | |
| Demographic information | ||
| Age (y), median (range, IQR) | 38.5 (22.0-57.0, 30.5-44.0) | |
| Race/Ethnic identity | ||
| White or Caucasian | 23 (95.8%) | |
| Other | 1 (4.2%) | |
| Hypertension | ||
| No | 16 (66.7%) | |
| Yes | 8 (33.3%) | |
| Hyperlipidemia | ||
| No | 20 (83.3%) | |
| Yes | 4 (16.7%) | |
| Diabetes mellitus | ||
| No | 23 (95.8%) | |
| Yes | 1 (4.2%) | |
| CAD | ||
| No | 23 (95.8%) | |
| Yes | 1 (4.2%) | |
| Number of comorbidities* | ||
| 0 | 15 (62.5%) | |
| 1 | 6 (25.0%) | |
| 2+ | 3 (12.5%) | |
| Smoking status | ||
| Never | 16 (66.7%) | |
| Former | 4 (16.7%) | |
| Current | 4 (16.7%) | |
| Disease-related factors | ||
| Disease status | ||
| De novo | 7 (29.2%) | |
| Recurrent | 17 (70.8%) | |
| Time from orchiectomy to recurrence (mo)†, median (range, IQR) | 13.2 (3.0-53.7, 6.3-16.9) | |
| Testicle laterality | ||
| Right | 14 (58.3%) | |
| Left | 9 (37.5%) | |
| Unknown | 1 (4.2%) | |
| Pathologic T stage | ||
| pTX | 1 (4.2%) | |
| pT1a | 3 (12.5%) | |
| pT1b | 12 (50.0%) | |
| pT2 | 8 (33.3%) | |
| Clinical N stage | ||
| cN0 | 1 (4.2%) | |
| cN1 | 12 (50.0%) | |
| cN2 | 11 (45.8%) | |
| Serum markers | ||
| SX | 12 (50.0%) | |
| S0 | 10 (41.7%) | |
| S1 | 2 (8.3%) | |
| Overall TNM stage (all cM0) [#de novo/recurrent] | ||
| IA | 1 (4.2%) [1/0] | |
| IB | 0 (0.0%) [0/0] | |
| IIA | 12 (50.0%) [4/8] | |
| IIB | 11 (45.8%) [2/9] | |
| Primary tumor size (cm), median (range, IQR) | 4.0 (1.5-8.0, 2.6-5.5) | |
| Unknown | 1 (4.2%) | |
| Number of positive nodes | ||
| 0 | 1 (4.2%) | |
| 1 | 16 (66.7%) | |
| 2 | 5 (20.8%) | |
| 3 | 2 (8.3%) | |
| Largest node size (cm)‡, median (range, IQR) | 1.9 (1.2-4.2, 1.6-2.3) | |
| LVSI | ||
| No | 16 (66.7%) | |
| Yes | 7 (29.2%) | |
| Unknown | 1 (4.2%) | |
| Proton RT treatment details | ||
| Prescription dose(s) | ||
| Adjuvant setting | ||
| 20 Gy/10 fx | 1 (4.2%) | |
| Definitive setting | ||
| 25 Gy/20 fx, CD: 10 Gy/5 fx | 1 (4.2%) | |
| 25.5 Gy/17 fx, CD: 10 Gy/5 fx | 22 (91.7%) | |
| Proton technique | ||
| Passive scattering | 3 (12.5%) | |
| Uniform scanning | 2 (8.3%) | |
| Pencil-beam scanning | 19 (79.2%) | |
| RT field type | ||
| Para-aortic only | 2 (8.3%) | |
| Modified dog-leg | 22 (91.7%) | |
| GTVn size (cc)‡ | 5.9 (2.6-42.0, 4.5-8.6) | |
| CTV size (cc)§ | 660.8 (318.8-984.2, 507.1-758.7) | |
| PTV size (cc)§ | 1082.2 (571.0-1452.3, 905.0-1257.8) | |
Abbreviations: CAD = coronary artery disease; CD = cone down (all sequential); CTV = clinical target volume; fx = fractions; GTVn = gross tumor volume of nodes; IQR = interquartile range; LVSI = lymphovascular space invasion; PTV = planning target volume; TNM = tumor, node, and metastasis staging.
Composite number of comorbidities including hypertension, hyperlipidemia, diabetes mellitus, and CAD.
†Only calculated for those with recurrent disease (n = 17/24, 70.8%).
‡Only calculated for those with cN1-2 (n = 23/24, 95.8%).
Combined initial elective nodal volume and boost volumes.
Regarding proton RT, majority were treated with definitive doses either with 25.5 Gy in 17 fractions (1.5 Gy/fraction, n = 22 [91.7%]) or less commonly with 25 Gy in 20 fractions (1.25 Gy/fraction, n = 1 [4.2%]) to the initial at-risk nodal regions followed by sequential boost to any pathologic nodes (10 Gy/5 fractions, 2 Gy/fraction). The patient with stage IA disease opted for adjuvant RT instead of surveillance after orchiectomy and received 20 Gy in 10 fractions (2 Gy/fraction) in May 2015. The majority were treated using a pencil-beam scanning technique (n = 19 [79.2%]) followed by passive scattering (n = 3 [12.5%]) and uniform scanning (n = 2 [8.3%]). Fields were designed to target the para-aortic strip only and modified dog-leg nodal volumes in 2 (8.3%) and 22 (91.7%) patients, respectively. Apart from the patient with stage IA disease who opted for adjuvant RT, the other patient who had para-aortic–only irradiation was a unique stage IIB case with de novo presentation of a biopsy-proven seminomatous left para-aortic lymph node (2.2 cm, cN2) with no clinically discernible testicular primary (cT0).
Proton RT toxic effects
Acute proton RT–related toxicities were limited to G1 (n = 19 [79.2%]) and G2 (n = 3 [12.5%]) events (Table 2). Two patients (8.3%) developed no acute toxicities. Nausea (n = 17 [70.8%]; all G1), fatigue (n = 10 [41.7%]; all G1), and dermatitis (n = 6 [25%]; 83.3% G1, 16.7% G2) were commonly experienced acute toxicities. Other GI toxicities included anorexia (n = 6 [12.5%]; all G1), dyspepsia (n = 4 [16.7%]; 50.0% G1, 50.0% G2), and diarrhea (n = 4 [16.7%]; all G1). Genitourinary toxicities were least common (urinary frequency: n = 2 [8.3%]; all G1) or never reported (ie, dysuria).
Table 2.
Acute proton radiation therapy (RT) toxicities in pure testicular seminoma
| Acute proton RT toxicities* | n (%) | |
|---|---|---|
| Highest grade | ||
| None | 2 (8.3%) | |
| G1 | 19 (79.2%) | |
| G2 | 3 (12.5%) | |
| G3-5 | 0 (0.0%) | |
| Fatigue | ||
| Any grade | 10 (41.7%) | |
| G1 | 10 (100.0%) | |
| G2 | 0 (0.0%) | |
| Dermatitis | ||
| Any grade | 6 (25.0%) | |
| G1 | 5 (83.3%) | |
| G2 | 1 (16.7%) | |
| Anorexia | ||
| Any grade | 3 (12.5%) | |
| G1 | 3 (100.0%) | |
| G2 | 0 (0.0%) | |
| Dyspepsia | ||
| Any grade | 4 (16.7%) | |
| G1 | 2 (50.0%) | |
| G2 | 2 (50.0%) | |
| Nausea | ||
| Any grade | 17 (70.8%) | |
| G1 | 17 (100.0%) | |
| G2 | 0 (0%) | |
| Diarrhea | ||
| Any grade | 4 (16.7%) | |
| G1 | 4 (100.0%) | |
| G2 | 0 (0.0%) | |
| Dysuria | ||
| Any grade | 0 (0.0%) | |
| G1 | 0 (0.0%) | |
| G2 | 0 (0.0%) | |
| Urinary frequency | ||
| Any grade | 2 (8.3%) | |
| G1 | 2 (100.0%) | |
| G2 | 0 (0.0%) | |
Acute toxicities graded using Common Terminology Criteria for Adverse Events version 5.0. Abbreviations: G1-5 = grade 1 to 5.
There were no significant changes in serum Cr levels compared with baseline up to 5 years post-RT (Fig. E1). One patient (4.2%) was noted to have either new onset or worsening hypertension. For bone marrow toxic effects, blood levels remained within normal limits post-RT up to 5 years. There were significant declines at either 2 or 3 years post-RT, but these blood levels returned to baseline afterward (Fig. E2). Otherwise, there were no other late proton RT–related toxic effects, including SMN, GI toxicities, and cardiovascular events.
Survival outcomes
The only patient with stage I disease was immediately lost to follow-up after completing adjuvant proton RT. In the remaining group of all stage II patients (n = 23), 2 experienced relapses resulting in a 3-year DFS of 90.9% (95% confidence interval [CI], 68.1%-97.6%; Fig. 1). Both relapses were out-of-field failures in patients treated for recurrent stage IIB disease. One patient relapsed with a supraclavicular lymph node at 0.3 years post-RT, whereas the other developed a contralateral internal iliac lymph node and paravertebral mass in the lower thoracic spine at 0.7 years post-RT. Both patients had subsequent salvage with a platinum-based chemotherapy regimens (etoposide-cisplatin and etoposide-ifosfamide-cisplatin × 4 cycles, respectively). Thus, 3-year DFS estimates for patients with stage IIA and IIB disease were 100% (95% CI, 100%-100%) and 79.5% (95% CI, 39.3%-94.5%), respectively. No deaths occurred in the study period, leading to a 3-year OS of 100% (95% CI, 100%-100%; Fig. 1).
Figure 1.
Disease-free survival (DFS) and overall survival (OS) in patients with testicular seminoma (n = 23) treated with proton radiation therapy (RT). Abbreviations: CI = confidence interval; EP = etoposide-cisplatin; LN = lymph node; rIIB = recurrent stage IIB; VIP = etoposide-ifosfamide-cisplatin.
Dosimetric analysis
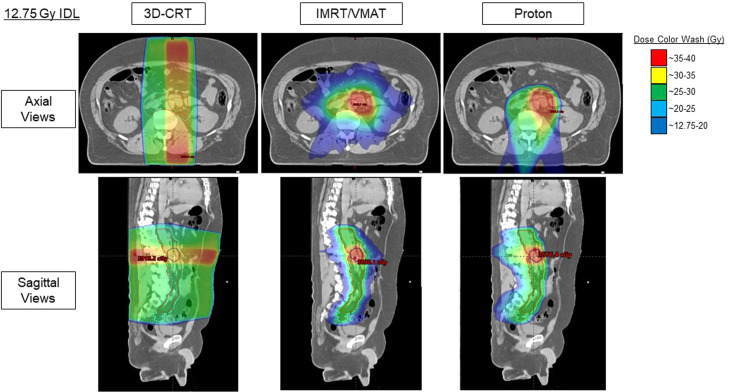
As all patients were treated with proton RT, photon comparison plans were generated to assess dosimetric differences and estimate SMN incidences. Figure 2 shows representative 3D dose distributions on a patient's planning computed tomography scan, and Fig. E3 shows a representative cumulative dose-volume histogram. As viewed, proton RT's dose distribution has better conformality with more sparing of nearby tissues. IMRT/VMAT is also conformal in the high-dose region but delivers higher integral doses (ie, low-dose bath) to surrounding tissue compared with 3D-CRT and proton RT.
Figure 2.
Representative 3-dimensional (3D) dose distributions between 3D conformal radiation therapy (3D-CRT), intensity modulated radiation therapy (IMRT)/volumetric arc therapy (VMAT), and proton radiation therapy (set at 12.75 Gy isodose line [IDL]).
For a more objective assessment, mean and maximum dose values to different OARs (kidneys, stomach, colon, liver, bladder, spinal cord, and encompassed body contour) were compared between 3D-CRT, IMRT/VMAT, and proton RT plans (Fig. 3). As shown, proton RT plans were overall superior and had significantly lower mean and doses received by 50% of volume (D50%) to many OARs compared with 3D-CRT and IMRT/VMAT. For instance, mean body doses were lowest with proton RT (2.6 Gy) compared with 3D-CRT (4.8 Gy***) and IMRT/VMAT (5.3 Gy****). Only 2 D50% and mean values did not meet statistical significance or favor proton RT: ipsilateral kidney D50% and spinal cord mean compared with 3D-CRT and IMRT/VMAT, respectively. Although the absolute ipsilateral kidney D50% dose was lower in proton versus 3D-CRT, the difference did not reach statistical significance (2.5 Gy vs 4.5 Gyns). However, proton RT had significantly lower mean ipsilateral kidney doses compared with 3D-CRT (6.1 Gy vs 8.2 Gy**). Furthermore, proton RT had significantly better sparing (significantly lower mean and D50%) of the contralateral and combined kidneys compared with either photon modality. The second parameter was the spinal cord mean with IMRT/VMAT having significantly lower values (8.5 Gy) compared with proton RT (12.9 Gy**) as well as 3D-CRT (17.3 Gy****).
Figure 3.
Quantitative dosimetric comparisons between 3-dimensional conformal radiation therapy (3D-CRT), intensity modulated radiation therapy (IMRT)/volumetric arc therapy (VMAT), and proton radiation therapy. Friedman with Dunn's multiple comparisons tests used for statistical comparisons. P value significance levels denoted as either non-significant (ns; P > 0.05), P < .05(*), P < .01(**), P < .001(***), or P < .0001(****). Abbreviations: D50% = dose received by 50% of volume; max = maximum; SEM = standard error of mean.
Notable maximum dose differences included higher stomach doses in IMRT/VMAT plans (26.3 Gy) compared with 3D-CRT (19.6 Gy***) and proton RT (18.7 Gy****). However, maximum spinal cord values were improved with IMRT/VMAT (17.2 Gy) compared with 3D-CRT (33.6 Gy***) and proton RT (25.7 Gy**). Compared with 3D-CRT, proton RT had significantly lower liver (25.5 Gy vs 27.3 Gy**), spinal cord (25.7 Gy vs 33.6 Gy**), and body (36.3 Gy vs 37.9 Gy****) maximum doses but higher colon doses (37.2 Gy vs 35.6 Gy*). There were no differences in maximum kidney doses.
When comparing the 2 photon techniques, 3D-CRT had significantly lower mean doses to most OARs compared with IMRT/VMAT (ipsilateral kidney: 8.2 Gy vs 11.0 Gy**; contralateral kidney: 4.7 Gy vs 10.1 Gy***; bilateral kidneys: 6.5 Gy vs 10.5 Gy**; liver: 3.4 Gy vs 5.2 Gy**; bladder: 5.4 Gy vs 7.9 Gy**; body: 4.8 Gy vs 5.3 Gy*). Mean stomach (8.1 Gy vs 6.5 Gyns) and colon (10.2 Gy vs 12.4 Gyns) doses were not significantly different. IMRT/VMAT had better spinal cord sparing compared with 3D-CRT as previously mentioned.
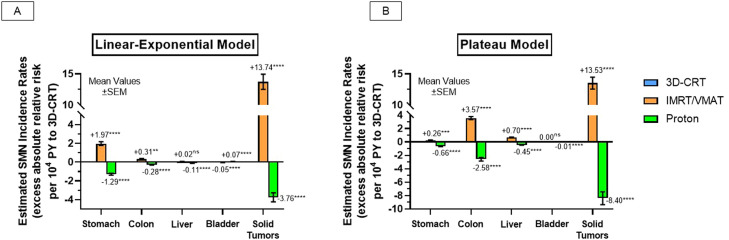
Radiation-induced SMN incidence comparisons
Figure 4 shows the radiation-induced SMN comparison results between the 3 RT techniques. Compared with 3D-CRT, proton RT significantly reduced predicted absolute SMN incidence rates in multiple OARs using 2 dose-response models (linear-exponential and plateau estimates, respectively, per 104 PY—stomach: –1.29****, –0.66****; colon: –0.28****, –2.58****; liver: –0.11****, –0.45****; solid tumors: –3.76****, –8.40****). There was a discrepancy between the models with bladder SMN estimates, with the linear-exponential model predicting modestly higher rates with proton RT (+0.07/104 PY****) but lower with the plateau model (–0.01/104 PY****). Generally, IMRT/VMAT had significantly higher SMN estimates compared with 3D-CRT (linear-exponential and plateau estimates, respectively, per 104 PY—stomach: +1.97****, +0.26***; colon: +0.31**, +3.57****; liver: +0.02ns, +0.70****; bladder: –0.05****, 0.00ns; solid tumors: +13.74****, +13.53****). Table E2 and E2 shows estimated OEDs and excess absolute risks.
Figure 4.
Radiation-induced secondary malignancy (SMN) risk-prediction analysis between 3-dimensional conformal radiation therapy (3D-CRT), intensity modulated radiation therapy (IMRT)/volumetric arc therapy (VMAT), and proton radiation therapy using (A) linear-exponential modeling and (B) plateau modeling. Analysis of variance with Dunnett's multiple comparisons used for statistical comparisons. P value significance levels denoted as either non-significant (ns; P > 0.05), P < .05(*), P < .01(**), P < .001(***), or P < .0001(****). Abbreviations: PY = person-years; SEM = standard error of mean.
Discussion
Similar to craniospinal irradiation in pediatric cancers, RT clinical target volumes in testicular seminoma represent an attractive case for the dosimetric advantages of proton RT.12 First, patients with testicular seminoma are relatively young with favorable expected cancer outcomes and prolonged life expectancies. Second, the target in seminoma is relatively large and posteriorly located. These 2 features mean both the high-dose conformality and low-dose bath are important with the former to reduce acute and late tissue effects and the latter to reduce long-term SMN risks. IMRT/VMAT typically improves high-dose conformality compared with 3D-CRT but at the expense of low-dose spill. Proton RT generates plans that are both more conformal at high and low doses compared with 3D-CRT.
Compared with other cancers, testicular seminoma is uncommon, so a prospective trial comparing proton RT to 3D-CRT in a controlled setting would be unfeasible. Thus, the oncologic community must rely on peer-reviewed, retrospective studies to assess the safety and efficacy of proton RT in this disease setting. Here, patients with majority stage II disease (95.8%) undergoing definitive proton RT experienced favorable 3-year DFS rates of 90.9% (95% CI, 68.1%-97.6%). This estimate is comparable to prospective multicenter data using AP/PA 3D-CRT in 94 patients with stage II disease who had long-term DFS rates of 88.9% to 95.3%.3 Furthermore, there were no documented serious late proton RT–related toxic effects in this cohort, including SMN, GI toxicities, and cardiovascular events. Proton RT also preserved renal function with no significant serum Cr changes, likely attributed to the kidney sparing afforded by proton RT (bilateral kidney D50% and mean doses of 1.0 Gy and 4.7 Gy, respectively). One patient (4.2%) was noted to develop either new or worsening hypertension, but it is difficult to ascertain whether this was RT related versus an unrelated cause. Although these late toxicity data are reassuring, much longer follow-up is needed to better establish long-term risks attributed to proton RT.
Acute toxicities in this cohort were acceptable with all being mild and self-limiting (highest G1: 79.2%, G2: 12.5%, no serious G3-5 events). Two patients (8.3%) were symptom free during proton treatment. The most common acute toxicity was G1 nausea (70.8%), which is consistent with 3D-CRT prospective data.3,5 In a recent retrospective comparison study between proton RT (n = 11) and 3D-CRT (n = 44), most acute toxicities (fatigue, nausea/vomiting, and dysuria) were not significantly different between the 2 modalities.13 However, proton RT was found to have significantly lower diarrhea and higher dermatitis rates. These adverse skin reactions can be attributed to the high skin entrance dose when generating proton's spread-out Bragg peak and are more commonly associated with older proton delivery techniques, like passive scattering. The low proportions of passive scattering cases in this series precluded meaningful statistical comparisons with more modern proton techniques, like pencil-beam scanning. However, 2 of the 3 acute G2 events (dermatitis and dyspepsia) were in patients treated with a passive scattering technique.
This is the first testicular seminoma study examining dosimetric and predicted SMN incidence rates between 3 modern RT techniques (3D-CRT, IMRT/VMAT, and proton RT) using proton plans that were used clinically and with all proton delivery uncertainties taken into account. Although all patients underwent proton RT, photon plans were created for each patient, which allows for pair matching in statistical analyzes and for more accurate dose distribution comparisons specific to that patient's unique anatomy. As demonstrated, proton RT had superior dosimetry compared with both 3D-CRT and IMRT/VMAT with significant reductions in multiple OAR mean doses. These findings are consistent with prior dosimetric studies in testicular seminoma comparing proton RT to 3D-CRT alone.13, 14, 15, 16 Between the 2 photon modalities, 3D-CRT plans had lower mean doses to multiple in-field OARs, except for the stomach and spinal cord. These results are consistent with a previous study comparing 3D-CRT and IMRT/VMAT dose distributions.17 Although IMRT/VMAT had improved spinal cord sparing, the maximum doses with proton RT and 3D-CRT are both acceptable and are highly unlikely to cause spinal cord injury. Thus, taken together with the findings associating IMRT/VMAT with higher SMN risks, this study provides further validation that 3D-CRT should remain the preferred photon technique in testicular seminoma, especially when proton RT is unavailable.
Patients with testicular cancer are at higher risks for developing treatment-related SMNs, which are a leading cause of mortality in this patient population.18,19 In a large population-based study of 40,576 patients with testicular cancer (both pure seminoma and non-seminoma), treatment with chemotherapy alone, RT alone, or both significantly resulted in increased relative risks of developing solid cancers of 1.8 (95% CI, 1.3-2.5), 2.0 (95% CI, 1.9-2.2), and 2.9 (95% CI, 1.9-4.2), respectively.20 These findings warrant investigation of SMN risk mitigation strategies. From an RT standpoint, SMN risk reduction has been expected to be accomplished with decreasing dose and field size.21
As shown here, RT modality choice has the opportunity to further modify SMN risks. Compared with the recommended AP/PA 3D-CRT approach, proton RT was predicted to significantly reduce the SMN incidences in multiple OARs, which is in agreement with prior studies.14,15 Surprisingly, there was 1 discrepancy in the 2 SMN prediction models with the linear-exponential calculation associating proton RT with a modestly increased risk of bladder tumors. In contrast, the plateau model predicted significantly lower bladder SMNs, which is directionally more consistent with other examined OARs. This discrepancy likely lies with the varying assumptions of both models. In contrast to the plateau model, the linear-exponential model neglects full repair of irradiated cells, so the irradiated tissue volume in the lower dose range becomes more dominant in the estimate as mutated cell sterilization occurs at higher doses. The bladder is the most anteriorly oriented structure of examined OARs, so the worsening range uncertainty and lateral penumbra of protons with increasing depth likely exposes more bladder volume to very low doses, especially when trying to adequately treat pelvic nodal chains of the modified dog-leg volume. As one would expect, this is less of an issue when treating para-aortic only fields as pelvic nodes are omitted, which results in negligible bladder doses with proton RT. Smaller proton series of patients with stage I disease support this claim as adjuvant proton RT to the para-aortic region had lower predicted bladder SMN risks compared with 3D-CRT using linear-exponential modeling.14,15 Likewise, calculated bladder OEDs in the 2 para-aortic cases in this cohort were less dissimilar between the techniques. In any case, considering the other findings, this stand-alone discrepancy between the models should not negate proton RT's dosimetric advantages and comparatively higher SMN risk reductions to other OARs as described here.
There are limitations to the present study. Foremost, despite being the largest known proton experience to date, absolute patient numbers are small, so the CIs around cancer control and toxicity estimates are wider than what can be achieved in more common diseases. As the need for RT for testicular seminoma is relatively infrequent, even at a large referral center, the challenge of small numbers will remain until there is substantially greater utilization of proton RT for testicular seminoma, which will enable aggregation across institutions. Second, the study is retrospective, raising potential for selection bias. A particular risk with the retrospective dosimetric comparison is it may have a priori excluded patients with unfavorable proton dosimetry as they would have been treated using an alternative delivery strategy. However, this proton cohort reflects all patients with testicular seminoma treated with RT at this institution over the study period, except for 1 patient whose insurance denied proton coverage. Third, the SMN risk estimates are based on models rather than actual outcomes. Since a comparison of actual SMN outcomes would require many decades of follow-up, this study attempted to increase the robustness of the analysis by comparing SMN risk predictions with 2 separate models. The fact that both models resulted in essentially the same overall conclusion is reassuring. Although this study is the first SMN risk comparison primarily of patients with stage II testicular seminoma (who typically receive higher RT doses to larger volumes than stage I), the results are also consistent with previous SMN risk analyzes in cohorts with stage I.14,15
Conclusions
Given the young age and high cure rates of patients with testicular seminoma, there is a strong emphasis on reducing short- and long-term treatment morbidities. Amongst those considered for RT, proton RT can potentially improve the therapeutic ratio by minimizing toxic effects without compromising disease control. The present study supports this notion as proton RT was associated with cancer control and toxicity outcomes consistent with existing photon literature. Furthermore, clinical proton plans are possibly associated with lowered SMN risk estimates using 2 distinct SMN risk models. Current US national guidelines recommend 3D-CRT as the preferred RT technique for testicular seminoma. This study and other prior reports suggest that, when available, proton RT should be preferred over 3D-CRT.13, 14, 15
Disclosures
John P. Christodouleas reports employee status at Elekta Inc., grant funding from Merck & Co. Inc. unrelated to this work, and royalties from Wolters Kluwer unrelated to this work. No other disclosures were reported.
Acknowledgments
We thank Russell Maxwell for contributions to data acquisition and curation, statistical analysis, results interpretation, drafting the manuscript, and manuscript review and editing. We thank Yushi Chang for contributions to photon comparison planning, and manuscript review and editing. We thank Christina Paul for contributions to photon comparison planning and manuscript review and editing. We thank David J. Vaughn for contributions to conceptualization and design and manuscript review and editing. We thank John P. Christodouleas for contributions to conceptualization and design, supervision, results interpretation, and manuscript review and editing.
Footnotes
Sources of support: This work had no specific funding.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.adro.2023.101259.
Appendix. Supplementary materials
References
- 1.Cheng L, Albers P, Berney DM, et al. Testicular cancer. Nat Rev Dis Primers. 2018;4:29. doi: 10.1038/s41572-018-0029-0. [DOI] [PubMed] [Google Scholar]
- 2.Fosså SD, Horwich A, Russell JM, et al. Optimal planning target volume for stage I testicular seminoma: A Medical Research Council randomized trial. J Clin Oncol. 1999;17:1146–1154. doi: 10.1200/JCO.1999.17.4.1146. [DOI] [PubMed] [Google Scholar]
- 3.Classen J, Schmidberger H, Meisner C, et al. Radiotherapy for stages IIA/B testicular seminoma: Final report of a prospective multicenter clinical trial. J Clin Oncol. 2003;21:1101–1106. doi: 10.1200/JCO.2003.06.065. [DOI] [PubMed] [Google Scholar]
- 4.Classen J, Schmidberger H, Meisner C, et al. Para-aortic irradiation for stage I testicular seminoma: Results of a prospective study in 675 patients. Br J Cancer. 2004;90:2305–2311. doi: 10.1038/sj.bjc.6601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones WG, Fosså SD, Mead GM, et al. Randomized trial of 30 versus 20 Gy in the adjuvant treatment of stage I testicular seminoma: A report on Medical Research Council Trial TE18, European Organisation for the Research and Treatment of Cancer Trial 30942 (ISRCTN18525328) J Clin Oncol. 2005;23:1200–1208. doi: 10.1200/JCO.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Oliver RT, Mason MD, Mead GM, et al. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: A randomised trial. Lancet. 2005;366:293–300. doi: 10.1016/S0140-6736(05)66984-X. [DOI] [PubMed] [Google Scholar]
- 7.Hoppe BS, Mamalui-Hunter M, Mendenhall NP, Li Z, Indelicato DJ. Improving the therapeutic ratio by using proton therapy in patients with stage I or II seminoma. Am J Clin Oncol. 2013;36:31–37. doi: 10.1097/COC.0b013e3182354b9e. [DOI] [PubMed] [Google Scholar]
- 8.Hall EJ, Wuu CS. Radiation-induced second cancers: The impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys. 2003;56:83–88. doi: 10.1016/s0360-3016(03)00073-7. [DOI] [PubMed] [Google Scholar]
- 9.LaRiviere MJ, Santos PMG, Hill-Kayser CE, Metz JM. Proton therapy. Hematol Oncol Clin North Am. 2019;33:989–1009. doi: 10.1016/j.hoc.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Schneider U, Zwahlen D, Ross D, Kaser-Hotz B. Estimation of radiation-induced cancer from three-dimensional dose distributions: Concept of organ equivalent dose. Int J Radiat Oncol Biol Phys. 2005;61:1510–1515. doi: 10.1016/j.ijrobp.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 11.Schneider U, Kaser-Hotz B. Radiation risk estimates after radiotherapy: Application of the organ equivalent dose concept to plateau dose-response relationships. Radiat Environ Biophys. 2005;44:235–239. doi: 10.1007/s00411-005-0016-1. [DOI] [PubMed] [Google Scholar]
- 12.Ho ESQ, Barrett SA, Mullaney LM. A review of dosimetric and toxicity modeling of proton versus photon craniospinal irradiation for pediatrics medulloblastoma. Acta Oncol. 2017;56:1031–1042. doi: 10.1080/0284186X.2017.1324207. [DOI] [PubMed] [Google Scholar]
- 13.Pasalic D, Prajapati S, Ludmir EB, et al. Outcomes and toxicities of proton and photon radiation therapy for testicular seminoma. Int J Part Ther. 2020;7:11–20. doi: 10.14338/IJPT-20-00018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simone CB, 2nd, Kramer K, O'Meara WP, et al. Predicted rates of secondary malignancies from proton versus photon radiation therapy for stage I seminoma. Int J Radiat Oncol Biol Phys. 2012;82:242–249. doi: 10.1016/j.ijrobp.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Efstathiou JA, Paly JJ, Lu HM, et al. Adjuvant radiation therapy for early stage seminoma: Proton versus photon planning comparison and modeling of second cancer risk. Radiother Oncol. 2012;103:12–17. doi: 10.1016/j.radonc.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Choo R, Kazemba B, Choo CS, Lester SC, Whitaker T. Proton therapy for stage IIA-B seminoma: A new standard of care for treating retroperitoneal nodes. Int J Part Ther. 2018;5:50–57. doi: 10.14338/IJPT-18-00001.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zilli T, Boudreau C, Doucet R, et al. Bone marrow-sparing intensity-modulated radiation therapy for stage I seminoma. Acta Oncol. 2011;50:555–562. doi: 10.3109/0284186X.2011.564650. [DOI] [PubMed] [Google Scholar]
- 18.Fosså SD, Aass N, Harvei S, Tretli S. Increased mortality rates in young and middle-aged patients with malignant germ cell tumours. Br J Cancer. 2004;90:607–612. doi: 10.1038/sj.bjc.6601558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zagars GK, Ballo MT, Lee AK, Strom SS. Mortality after cure of testicular seminoma. J Clin Oncol. 2004;22:640–647. doi: 10.1200/JCO.2004.05.205. [DOI] [PubMed] [Google Scholar]
- 20.Travis LB, Fosså SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: Focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354–1365. doi: 10.1093/jnci/dji278. [DOI] [PubMed] [Google Scholar]
- 21.Zwahlen DR, Martin JM, Millar JL, Schneider U. Effect of radiotherapy volume and dose on secondary cancer risk in stage I testicular seminoma. Int J Radiat Oncol Biol Phys. 2008;70:853–858. doi: 10.1016/j.ijrobp.2007.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.