Abstract
Purpose
To present the long-term results of intraoperative radiation therapy (IORT) for early breast cancer using a nondedicated linear accelerator.
Methods and Materials
The eligibility criteria were biopsy-proven invasive carcinoma, age ≥40 years, tumor size ≤3 cm, and N0M0. We excluded multifocal lesions and sentinel lymph node involvement. All patients had previously undergone breast magnetic resonance imaging. Breast-conserving surgery with margins and sentinel lymph node evaluation using frozen sections were performed in all cases. If there were no margins or involved sentinel lymph nodes, the patient was transferred from the operative suite to the linear accelerator room, where IORT was delivered (21 Gy).
Results
A total of 209 patients who were followed up for ≥1.5 years from 2004 to 2019 were included. The median age was 60.3 years (range, 40-88.6), and the mean pT was 1.3 cm (range, 0.2-4). There were 90.5% pN0 cases (7.2% of micrometastases and 1.9% of macrometastases). Ninety-seven percent of the cases were margin free. The rate of lymphovascular invasion was 10.6%. Twelve patients were negative for hormonal receptors, and 28 patients were HER2 positive. The median Ki-67 index was 29% (range, 0.1-85). Intrinsic subtype stratification was as follows: luminal A, 62.7% (n = 131); luminal B, 19.1% (n = 40); HER2 enriched 13.4% (n = 28); and triple negative, 4.8% (n = 10). Within the median follow-up of 145 months (range, 12.8-187.1), the 5-year, 10-year, and 15-year overall survival rates were 98%, 94.7%, and 88%, respectively. The 5-year, 10-year, and 15-year disease-free rates were 96.3%, 90%, and 75.6%, respectively. The 15-year local recurrence-free rate was 76%. Fifteen local recurrences (7.2%) occurred throughout the follow-up period. The mean time to local recurrence was 145 months (range, 12.8-187.1). As a first event, 3 cases of lymph node recurrence, 3 cases of distant metastasis, and 2 cancer-related deaths were recorded. Tumor size >1 cm, grade III, and lymphovascular invasion were identified as risk factors.
Conclusions
Despite approximately 7% of recurrences, we may infer that IORT may still be a reasonable option for selected cases. However, these patients require a longer follow-up as recurrences may occur after 10 years.
Introduction
Despite the efforts dedicated to awareness campaigns and screening policies, breast cancer remains among the most prevalent cancers worldwide.1 Fortunately, early diagnosis and proper treatment yield reasonable disease control rates. Generally, conservative surgery not only allows breast conservation but also conservation of lymph node chains, as the analysis of the sentinel lymph nodes avoids elective dissection of the axilla in most cases. In addition, owing to immediate oncoplastic procedures, the eventual deformity in conservative surgery is markedly less striking.
Radiation therapy (RT) is paramount in breast-conserving therapy for early breast cancer, as revealed by several studies and meta-analyses.2 In recent years, robust level 1 medical evidence has demonstrated that RT strategies can adapt to new times: conventional fractionation of 5 to 6 weeks can be replaced by moderate hypofractionation (3 weeks)3, 4, 5, 6, 7 or ultrahypofractionation (in 1 week8 or 5 weekly doses9). This strategy of shortening the treatment time through hypofractionation and the question of whether this approach should only be applied to some subgroups10 (elderly patients with early luminal tumors might not benefit from RT in addition to surgery and hormonal therapy) appropriately align with the challenging times associated with the COVID-19 pandemic.
From this point of view, accelerated partial breast irradiation (APBI) is an old strategy but remains interesting owing to the possibility of not only reducing the treatment time but also the volume of irradiation. By using different strategies, several researchers have demonstrated that in well-selected patients, equivalent oncological effectiveness is possible, with a lower level of toxicity than conventional treatments. Among APBI strategies, studies have been conducted using low- and high-dose-rate interstitial brachytherapy, balloon brachytherapy, partial 3-dimensional conformal or intensity modulated RT, intraoperative RT (IORT) using brachytherapy, electron- or kilovoltage photon-beams, and stereotactic body RT with tracking capabilities.
In 2004, our institution launched the IORT program using a nondedicated linear accelerator (ndLINAC) instead of purchasing a dedicated machine because of its costs and the possibility of expanding this approach to other centers in other developing countries. Our initial results are reported elsewhere.11, 12, 13, 14, 15 Of note, good local control, low complication rates, and good cosmetic results were achieved in well-selected patients. Additionally, some advantages of the use of ndLINAC were recognized, such as the image guidance approach (2-dimensional image taken intraoperatively for alignment between the collimator and the lead shield) and the potential use of higher electron beam energies than those available in dedicated machines.
This study aimed to reveal the long-term results of our study, with a focus on local control and survival.
Methods and Materials
We established a single-institution, prospective, phase 2 cohort in May 2004, which comprised patients with early invasive no special type breast carcinoma who fulfilled the study's eligibility criteria. The local ethics committee approved the research protocol, and each patient signed an informed consent form before inclusion in the study. The inclusion criteria, surgery issues, and radiation aspects are described elsewhere.11 Of note, eligible patients were older than 40 years, had lesions <3 cm, had clinically negative lymph nodes, had histologically confirmed invasive no special breast carcinoma, and had undergone breast magnetic resonance imaging (MRI) to rule out multifocality and multicentricity. Suspected cases were referred for histologic confirmation of new tumor foci.
During surgery, after sentinel lymph node and margin evaluation,16 the shielding disc was placed beneath the target parenchyma and above the muscle. The shielding discs consisted of 3 joined 3-mm layers made of lead (face down), aluminum (middle), and silicon (face up) to avoid backscattered electron absorption effects in the breast parenchyma after disc interaction. Breast parenchyma was approximated over the disc. Subsequently, the patient was transferred to the ndLINAC room under general anesthesia. Patient transport followed the recommendations of the hospital infection control committee. IORT was delivered from the collimator surface to the bottom of the shield according to the thickness of the breast parenchyma to be treated. A single dose of 21 Gy was then administered. A portal film or an electronic portal imaging device scan (both 2-dimensional images) was used to ensure alignment between the collimator and shield. If the alignment was unsatisfactory, appropriate corrections were made by the radiation oncologist. Briefly, these corrections involved changing the position of the shield to the collimator, which was performed carefully to maintain the target breast parenchyma in the desired position. Another image was then captured, and this procedure was repeated until the alignment was considered appropriate. After treatment completion, surgery was performed in the operating room. According to the definitive pathologic results, a multidisciplinary team evaluated the need for adjuvant systemic therapies.
Statistical method
The following variables were analyzed: age on the procedure date; clinical and pathologic staging; definitive histology; systemic treatments (frequency and types); follow-up time; cumulative incidence of local, locoregional, and distant recurrences at the last follow-up; and death rates. In addition, data on the outcomes of patients who experienced cancer-related events were collected. Descriptive and frequency analyses were conducted by calculating the means, standard deviations, medians, and interquartile ranges. The Kolmogorov-Smirnov test was used to verify the normal distribution of the numerical variables.
Survival estimates were calculated using the Kaplan-Meier method with the log-rank test for comparison. All outcomes were considered from the date of surgery. Overall survival was defined as the time to death by any cause, disease-free survival until the detection of the first recurrence, local disease-free survival until recurrence in the treated breast, and locoregional-free survival regarding lymph node recurrences. Univariate and multivariate analyses were performed, and odds ratios were calculated using logistic regression. Statistical analyses were performed using Statistical Package for the Social Sciences software version 20. For statistical formalism purposes, a significance P-value of 5% and 95% confidence interval were selected.
Results
Patient characteristics
A total of 209 patients were included, and the follow-up period was ≥1.5 years. The median age was 60.3 years (range, 40-89). Table 1 shows the patient characteristics.
Table 1.
Patient characteristics
| Characteristic | N | % | |
|---|---|---|---|
| Age, y | 40-49 | 46 | 22.0 |
| 50-59 | 56 | 26.8 | |
| 60-69 | 76 | 36.4 | |
| 70-79 | 26 | 12.4 | |
| ≥80 | 5 | 2.4 | |
| cT | Nonpalpable tumor | 141 | 67.5 |
| ≤2 cm | 58 | 27.7 | |
| 2.1-3 cm | 10 | 4.8 | |
| pT | ≤1 cm | 57 | 27.3 |
| 1.1-2 cm | 117 | 56.0 | |
| 2.1-3 cm | 33 | 15.8 | |
| >3 cm | 2 | 0.9 | |
| pN | Negative | 190 | 90.9 |
| Micrometastasis | 15 | 7.2 | |
| Macrometastasis | 4 | 1.9 | |
| Margin status after frozen sections | Initially free | 128 | 61.2 |
| Free after 1 ampliation | 62 | 29.7 | |
| Free after 2 ampliations | 15 | 7.2 | |
| Free after 3 ampliations | 3 | 1.4 | |
| Free after 5 ampliations | 1 | 0.5 | |
| Margin status at definitive pathology | Free | 206 | 98.6 |
| Positive | 3 | 1.4 | |
| Definitive histology | DCIS | 2 | 1 |
| NOS carcinoma | 196 | 93.8 | |
| Invasive lobular carcinoma | 1 | 0.5 | |
| Mucinous carcinoma | 7 | 3.3 | |
| Tubular carcinoma | 1 | 0.5 | |
| Papillary-type carcinoma | 1 | 0.5 | |
| Neuroendocrine carcinoma | 1 | 0.5 | |
| Histologic grade | I | 32 | 15.3 |
| II | 113 | 54.1 | |
| III | 64 | 30.6 | |
| Nuclear grade | I | 16 | 7.7 |
| II | 109 | 52.2 | |
| III | 84 | 40.1 | |
| Lymphovascular invasion | Negative | 187 | 89.5 |
| Positive | 22 | 10.5 | |
| Ki-67 status | ≤14% | 86 | 41.1 |
| >14% | 62 | 29.7 | |
| Unavailable data | 61 | 29.2 | |
| Estrogen receptor status | Positive | 196 | 93.8 |
| Negative | 13 | 6.2 | |
| Progesterone receptor status | Positive | 193 | 92.3 |
| Negative | 16 | 7.7 | |
| HER2 status | Positive (FISH included) | 28 | 13.4 |
| Negative | 181 | 86.6 | |
| Intrinsic subtypes | Luminal A | 131 | 62.7 |
| Luminal B | 40 | 19.1 | |
| Luminal HER2 | 25 | 12.0 | |
| Pure HER2 | 3 | 1.4 | |
| TNBC | 10 | 4.8 | |
Abbreviations: cT = clinical tumor size; DCIS = ductal carcinoma in situ; FISH = fluorescence in situ hybridization; HER = human epidermal growth factor receptor; NOS = nonspecial type; pN = pathologic lymph node status; pT = pathologic tumor size; TNBC = triple-negative breast cancer.
Overall, 14 deaths occurred during the study period, 6 of which were related to breast cancer. Notably, 57% of all deaths occurred before the 10-year follow-up. Twenty-three other cancer-related events occurred. Among them, 15 local recurrences and 3 cases of lymph node metastasis were the first breast cancer-related events. Most local or locoregional recurrences (75%) occurred after the 10-year follow-up.
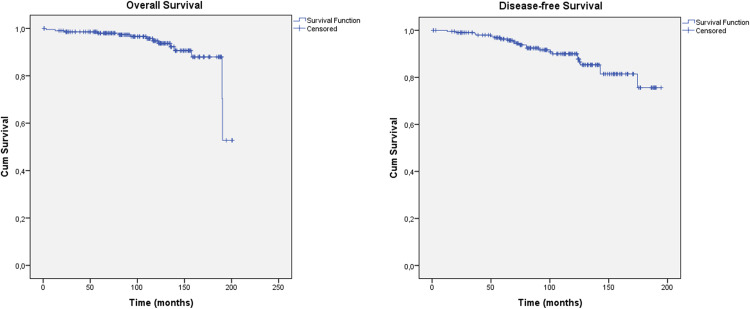
Figure 1 illustrates the overall survival and disease-free survival outcomes. The 5-year, 10-year, and 15-year overall survival rates were 98.0%, 94.7%, and 88.0%, respectively. Based on univariate analysis, a tumor size of ≤1 cm (pT1a or b) was associated with better survival (P = .026). The 5-year, 10-year, and 15-year disease-free rates were 96.3%, 90.0%, and 75.6%, respectively. Larger tumors >1 cm or >2 cm (pT1 vs pT2) were associated with worse outcomes (P = .009 and P = .027, respectively), such as histologic grade 3 (P = .020).
Figure 1.
Overall survival and disease-free survival.
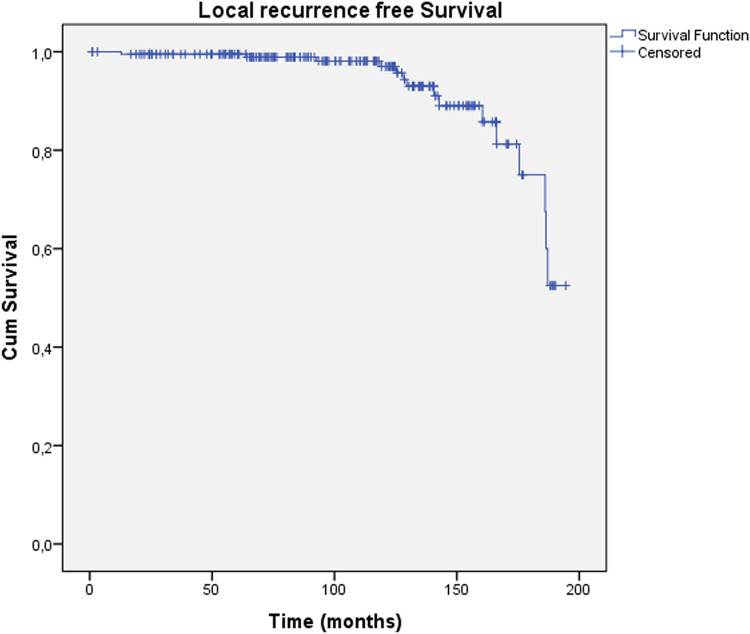
Figure 2 shows the actuarial local recurrence-free survival. Fifteen local recurrences (7.2%) (as the first event) occurred throughout the follow-up period. The median time to local recurrence was 140.7 months (range, 12.8-187.1). None of the variables were related to local or locoregional recurrence. However, lymph vascular invasion was marginally significant in both groups (P = .075). Only 3 cases of lymph node metastasis as the first breast cancer-related event were recorded.
Figure 2.
Local recurrence-free survival.
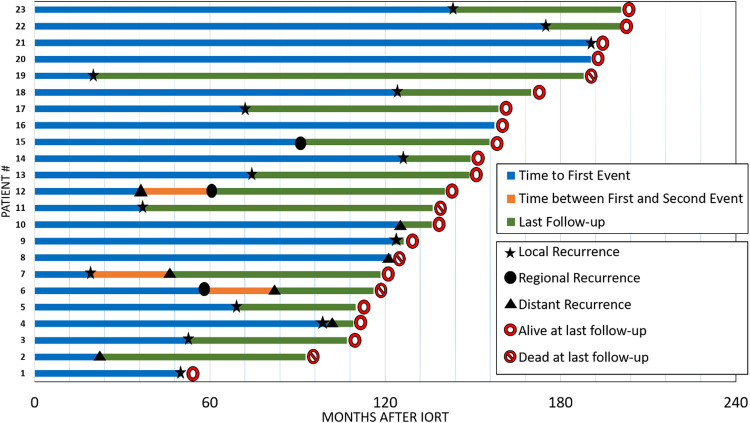
Figure 3 illustrates the follow-up of all patients who had breast cancer-related events. Of note, some cases had >1 event after the first event but were still followed up. The treatments administered to these patients varied, but in general, patients with local and regional recurrence underwent mastectomy with or without axillary lymphadenectomy, whereas those with metastatic disease were administered systemic therapies according to the tumor subtype and the patient's current condition. Of the mastectomy cases, 1 had lymph nodes in the pathologic specimen, which led to further postmastectomy external beam RT, including the chest wall and lymph node sites.
Figure 3.
Outcomes of patients with breast cancer events.
Table 2 shows the exploratory analysis results of cases of local recurrence as a function of grouping by subtype. Notably, a predominance of Luminal A and B cases was found among patients with local recurrence, mainly because of the initial selection of treatment cases. Of the Luminal A and B cases, 2.88% and 2.4%, respectively, had local recurrence within the entire sample. Univariate analysis was performed to correlate the studied variables with local recurrence, overall survival, and disease-free survival (Table E1). A pathologic tumor size >1 cm and histologic grade 3 were associated with worse overall and disease-free survival (Table E1). The presence of lymphovascular invasion was related to local recurrence; however, the difference was not statistically significant (P = .074; Table E1). The calculated odds ratios, according to clinically significant variables, revealed that tumor size, histologic grade, and lymphovascular invasion are risk factors for these patients (Table 3).
Table 2.
Exploratory analysis of local recurrence outcome
| Parameter | Local recurrence cases | Total cases | % from total cases | % from total sample |
|---|---|---|---|---|
| Luminal A | 6 | 130 | 4.62 | 2.88 |
| Luminal B | 5 | 40 | 12.50 | 2.40 |
| Triple positive | 3 | 25 | 12.00 | 1.44 |
| HER2 enriched | 0 | 3 | 0 | 0 |
| Triple negative | 1 | 10 | 10.00 | 0.48 |
Abbreviations: HER2 = human epidermal growth factor receptor type 2.
Table 3.
Significant risk factors associated respectively with the survivals’ outcomes (variables that entered the equation were age, tumor size, histologic grade, margins status, lymphovascular invasion, and molecular subtypes)
| 95% confidence interval |
||||
|---|---|---|---|---|
| Survival type | Odds ratio | Lower | Upper | P |
| Overall survival | ||||
| pT > 1 cm | 7.7 | 0.9 | 62.3 | 0.056 |
| Disease-free survival | ||||
| pT > 1 cm | 4.1 | 1.3 | 12.6 | 0.015 |
| Histologic grade 3 | 2.5 | 1.1 | 5.8 | 0.032 |
| Local disease-free survival | ||||
| LVI positive | 4.8 | 1.3 | 17.4 | 0.016 |
| Histologic grade 3 | 3.3 | 1.0 | 10.5 | 0.045 |
Abbreviations: LVI = lymphovascular invasion; pT = pathologic tumor size.
Discussion
This study sought to reveal the long-term results of our single institutional series involving well-selected patients, 100% of whom underwent local staging with breast MRI to rule out multicentricity and multifocality. This fact can be considered unprecedented when our data are compared with those of other studies. In a previous publication,11 the unprecedented use of portal films or electronic portal imaging devices in all patients was assessed to optimize the alignment between the collimator and protective lead shields placed beneath the breast parenchyma. By combining these facts with the treatment experience obtained using ndLINACs, higher electron beam energies than those available in dedicated/exclusive machines could be employed.
In our previous publication, we reported toxicity and esthetic results. The median time of appearance of late toxicity was of 8 months. Fibrosis was observed in 21 patients, whereas fat necrosis was seen in 15 cases. At the 1-year evaluation, 92.7% had a score of good or excellent.13 We believe that this study provided a background to support IORT as an option in selected patients, even if some findings from recent years are considered, and may have shed light on the role of APBI modalities in breast cancer treatment:
-
1.
With the onset of the COVID-19 pandemic, there has been increased interest in therapeutic strategies focusing on treatment de-escalation, shortening treatment time, and reducing the burden on health services. In the last 2 years, we saw the publication the findings of the FAST trial9 and the FAST-FORWARD trial,8 which have undisputedly validated ultrahypofractionated whole-breast RT for most patients.
-
2.
During the pandemic, the results of long-term studies, namely the TARGIT17 and ELIOT18 trials, which revealed the advantages and disadvantages of using IORT, were published. In the ELIOT study, local recurrence rates were significantly higher in patients treated with IORT, whereas in the TARGIT study, the differences in breast recurrence-free survival were similar among study arms.
-
3.
Other studies exploring different forms of APBI have been conducted over the last 5 years, such as the NSABP B-39/RTOG 0413,19 RAPID,20 Florence,20 Hungarian Brachytherapy,21 and IMPORT LOW22 trials. Overall, these studies revealed the equivalence in local control outcomes and some advantages in terms of toxicity and treatment time.
-
4.
Studies validating the omission of RT after breast-conserving surgery in selected patients (generally postmenopausal patients with tumors in the early stages and positive hormone receptors) have been published in recent years, such as the PRIME-2 trial.23
Based on the information collected, and the conclusions of the main meta-analyses that compared APBI with whole-breast RT,24, 25, 26, 27, 28 which suggested higher recurrence rates in APBI cases, with no difference in overall survival, and the caution regarding the inclusion of IORT as a safer modality of APBI in the main treatment guidelines,29, 30, 31, 32, 33, 34 the following statements highlight relevant information regarding IORT that should be known by a daily practice clinician:
-
1)
IORT competes with other forms of APBI. According to the data, recurrences with IORT are higher than those with other forms of APBI, and the logistics and cost of treatment are more complex with IORT than with other forms of APBI (especially external beam APBI).
-
2)
APBI competes with radiation therapy omission. Although nonirradiated patients will have more recurrences, they will achieve the same survival as irradiated patients.
-
3)
APBI competes with whole-breast RT ultrahypofractionation. The logistics of ultrahypofractionation are the same, if not better, than those of APBI, and the toxicity rates are equivalent. Furthermore, 1 to 5 APBI fractions do not differ from 5 whole-breast RT fractions in terms of treatment time.
Of note, the randomized studies involving APBI, especially IORT, did not uniformly follow the patient selection criteria. Many of the studies selected their inclusion criteria before the publication of the main guidelines. Furthermore, currently known criteria were not considered for patient selection (eg, grouping by intrinsic subtypes), in which interpretation of the main data are dependent on retrospective assessments involving independent predictors. Of note, subset analysis of patients enrolled in the ELIOT trial serves as an example.35 Luminal A cases (grouped in the retrospective analysis) had a local recurrence rate of 1.5% versus 4.4% for the entire IORT arm (in the 2014 publication).36 In our study, 62% of cases were Luminal A cases, of which 2.8% had local recurrence at any time during follow-up (also one-third of the entire sample). The remaining subtypes were poorly represented in our sample to draw further conclusions; however, in larger studies (such as ELIOT and TARGIT trials), even if poorly represented, triple-negative and HER2-positive patients might have contaminated the recurrence endpoints if a separate analysis was not conducted in the original publications.
Regarding patient selection, although contemporary guidelines suggest caution when considering IORT in the same manner as other forms of APBI, when the IORT cases were retrospectively analyzed and grouping is performed based on the American Society for Radiation Oncology and European Society of Radiotherapy and Oncology suitability criteria, the local recurrence rates can be very interesting, as revealed by Horst et al.15 Among >3200 patients in 11 trials (including our study), the American Society for Radiation Oncology “suitable” and European Society of Radiotherapy and Oncology “good” patients treated with IORT had local recurrence rates of 0.66% and 0.61%, respectively. These recurrence rates, despite no significant gains in survival, are interesting compared with those observed in the PRIME-2 trial at the 10-year follow-up to assess local recurrence: 9.8% versus 0.9% for nonirradiated and irradiated patients, respectively.24 Further, recurrence did not occur in patients >70 years, in addition to the 2.8% failures in luminal A patients. Any RT modality should be considered for patients included in the main studies that explore the role of RT omission, as there is no guarantee that a hypothetical patient undergoing conservative surgery will tolerate hormone therapy. Further, after 5 years, the patient may still experience a relapse, undergo another operation, and receive new hormonal treatment. Preliminary data suggest that RT is more cost-effective than hormone therapy.37
Differences in outcomes between the forms of APBI may be due to uncertainties across seminal studies. Table E2 shows some parameters that may represent uncertainties in the interpretation of results, specifically when the different forms of treatment are compared. If the statistical methodology is radically considered to validate a study, only the external beam APBI studies and the Hungarian study can be confirmed to have a satisfactory statistical design Table E2. Nonetheless, contemporary studies involving APBI have revealed interesting rates of local control without impairing patient survival, regardless of their drawbacks. As a result, IORT is continuously performed in selected patients at our institution, despite the lack of a randomized study to validate our approach in the performance of IORT (with ndLINAC). Our selection criteria involved the performance of breast MRI in addition to the criteria recommended by the guidelines. Additionally, owing to the use of ndLINAC, IORT was initiated for breast cancer cases in 2004 without major investments in equipment. Of note, this strategy can be easily replicated in other centers.
Although our results suggest low local recurrence rates at follow-up (LR = 7.2% over 15 years), we did not compare our rates with those of the main studies as we performed a single-arm trial (without a control arm), grouping patients at a “low speed” (just >200 patients in 15 years). However, comparable results were observed in terms of local control and toxicities in addition to the experience gained with ndLINAC treatment. When the recurrence rates from historical studies of external beam RT are considered, the rates were found to be comparable to those of our study. For example, a meta-analysis by the Oxford group revealed that at 15 years of follow-up, RT reduced the rates of local recurrence in well-selected patients to approximately 15% compared with approximately 7% observed in our study, which also had a 15-year follow-up period.38
Cost was not considered in this study. Specifically on cost, it is important to note that breast cancer is among the most prevalent malignancies, which means it is 1 of the most. costly oncologic treatments. Thus, it is worth thinking of cost effectiveness along different RT types and fractionations. Deshmukh et al have shown through a prospective trial that hypofractionated RT was better than conventionally fractionated RT or IORT.39 However, they considered dedicated equipment for such comparisons, which makes IORT through ndLINAC advantageous. On the other hand, Eisavi et al reported their results of systematic review based on current evidence, showing that IORT was associated with lower costs compared with external beam RT.40
Our study had several highlights, including the long-term follow-up, capability and the low rate of events to detect higher risk factors, such as tumor size >1 cm, histologic grade 3, and lymphovascular invasion. The presence of these features may aid decision making regarding APBI. Furthermore, patients with later recurrences could be identified during follow-up (>10 years). Even when located in the same initially affected quadrant, these recurrences have a differential diagnosis of a second primary tumor. Although this hypothesis should be refuted for these patients, they should not be exempt from continued long-term posttherapy follow-up.
Table E3 shows a simple comparison between APBI and other forms of RT for breast cancer. Despite more complex logistics, IORT can be advantageous even when strong competitors, such as whole-breast RT in ultrahypofractionation, are available, as an even faster and less toxic treatment than whole-breast RT and other forms of APBI, would be administered. In a review, Offersen et al suggested that the breast-parenchyma target volume of IORT is the smallest among the APBI modalities, and IORT spares the skin and chest wall, unlike other forms of APBI.41
In the near future, adequate selection criteria, such as oncogenomic panels, will be available for more intensive treatment of patients or treatments that could be excessive may be discarded. New forms of treatment may be employed in daily practice, such as stereotactic body RT in the breast cancer treatment scenario, whether as part of neoadjuvant, adjuvant, or definitive strategies. Because of the advent of the Internet, telemedicine practices, and the use of artificial intelligence, information is being disseminated and interpreted by clinical practitioners at an ever-increasing speed. Accordingly, the main selection criteria chosen by scientists may be modified earlier than 10 or 15 years for application in the relevant studies.
Currently, we are pleased with the results observed in our patients treated with IORT, as nearly half a century of medical evidence is available on this topic. The choice of IORT for use in our institution was strategic, and the results led to the generation of new knowledge. We believe that APBI can still be used in clinical practice, especially if decisions are supported by multidisciplinary teams.
Conclusions
Our long-term results support the use of IORT with ndLINACs in terms of local control and survival outcomes. Some of the analyzed predictive factors were found to influence our results, such as tumor size, histologic grade, and lymphovascular invasion, despite the low event rate. Despite the trend of treatment de-escalation, IORT for breast cancer is still applicable for selected patients.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.adro.2023.101233.
Appendix. Supplementary materials
References
- 1.Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Darby S, McGale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yarnold J, Ashton A, Bliss J, et al. Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: Long-term results of a randomised trial. Radiother Oncol. 2005;75:9–17. doi: 10.1016/j.radonc.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 5.Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 6.Smith BD, Bellon JR, Blitzblau R, et al. Radiation therapy for the whole breast: Executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol. 2018;8:145–152. doi: 10.1016/j.prro.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Offersen B, Nielsen HM, Jacobsen EH, et al. Hypo- versus normofractionated radiation of early breast cancer in the randomized DBCG HYPO trial. Radiother Oncol. 2018;127:S312. [Google Scholar]
- 8.Brunt AM, Haviland JS, Wheatley DA, et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020;395:1613–1626. doi: 10.1016/S0140-6736(20)30932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunt AM, Haviland JS, Sydenham M, et al. Ten-year results of FAST: A randomized controlled trial of 5-fraction whole-breast radiotherapy for early breast cancer. J Clin Oncol. 2020;38:3261–3272. doi: 10.1200/JCO.19.02750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunkler IH, Williams JL, Jack WJL, et al. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): A randomised controlled trial. Lancet Oncol. 2015;16:266–273. doi: 10.1016/S1470-2045(14)71221-5. [DOI] [PubMed] [Google Scholar]
- 11.Hanna SA, de Barros ACSD, de Andrade FEM, et al. Intraoperative radiation therapy in early breast cancer using a linear accelerator outside of the operative suite: An “image-guided” approach. Int J Radiat Oncol Biol Phys. 2014;89:1015–1023. doi: 10.1016/j.ijrobp.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 12.Barros AC, Hanna SA, Carvalho HA, et al. Intraoperative full-dose of partial breast irradiation with electrons delivered by standard linear accelerators for early breast cancer. Int J Breast Cancer. 2014;2014:568136. doi: 10.1155/2014/568136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanna SA, Carvalho HA, Andrade FEM, Bevilacqua JLB, Piato JRM, Docema MF, Barros ASCD. Adjuvant intra-operative electron-beam therapy for early invasive breast carcinoma using non-dedicated linear accelerator: Toxicity, efficacy, and aesthetic satisfaction. Cancer Res 2013;73:P5-14-10.
- 14.Hanna SA, Martella E, Carvalho HA, da Silva JLF, de Barros ASCD. Prospective phase II trial with intra-operative radiotherapy in initial breast cancer using nondedicated linear accelerator. J Clin Oncol 2011;29: (suppl; abstr e11560)
- 15.Horst KC, Maluta S, Vicini F, Hanna SA, Philippson C. Optimal patient selection for electron intraoperative radiotherapy (IORT) as sole treatment for breast cancer. J Clin Oncol 2016;34:(suppl; abstr 1059)
- 16.Barros A, Pinotti M, Ricci MD, Nisida AC, Pinotti JA. Immediate effects of intraoperative evaluation of surgical margins over the treatment of early infiltrating breast carcinoma. Tumori. 2003;89:42–45. doi: 10.1177/030089160308900109. [DOI] [PubMed] [Google Scholar]
- 17.Vaidya JS, Bulsara M, Baum M, et al. Long term survival and local control outcomes from single dose targeted intraoperative radiotherapy during lumpectomy (TARGIT-IORT) for early breast cancer: TARGIT-A randomised clinical trial. BMJ. 2020;370:m2836. doi: 10.1136/bmj.m2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orecchia R, Veronesi U, Maisonneuve P, et al. Intraoperative irradiation for early breast cancer (ELIOT): Long-term recurrence and survival outcomes from a single-centre, randomised, phase 3 equivalence trial. Lancet Oncol. 2021;22:597–608. doi: 10.1016/S1470-2045(21)00080-2. [DOI] [PubMed] [Google Scholar]
- 19.Vicini FA, Cecchini RS, White JR, et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: A randomised, phase 3, equivalence trial. Lancet. 2019;394:2155–2164. doi: 10.1016/S0140-6736(19)32514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whelan TJ, Julian JA, Berrang TS, et al. External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node-negative breast cancer (RAPID): A randomised controlled trial. Lancet. 2019;394:2165–2172. doi: 10.1016/S0140-6736(19)32515-2. [DOI] [PubMed] [Google Scholar]
- 21.Polgár C, Ott OJ, Hildebrandt G, et al. Late side-effects and cosmetic results of accelerated partial breast irradiation with interstitial brachytherapy versus whole-breast irradiation after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: 5-year results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:259–268. doi: 10.1016/S1470-2045(17)30011-6. [DOI] [PubMed] [Google Scholar]
- 22.Coles CE, Griffin CL, Kirby AM, et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet. 2017;390:1048–1060. doi: 10.1016/S0140-6736(17)31145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunkler IH, Williams LJ, Jack WJL, Cameron D, Dixon JM. PRIME 2 randomised trial (Postoperative Radiotherapy In Minimum-risk Elderly): Wide local excision and adjuvant hormonal therapy +/- whole breast irradiation in women. =/>65 years with early invasive breast cancer: 10 year results. Paper presented at: 45th San Antonio Breast Cancer Symposium. December 8-11; San Antonio, TX; 2020. [Google Scholar]
- 24.Kong L, Cheng J, Ding X, et al. Efficacy and safety of accelerated partial breast irradiation after breast-conserving surgery: A meta-analysis of published comparative studies. Breast J. 2014;20:116–124. doi: 10.1111/tbj.12226. [DOI] [PubMed] [Google Scholar]
- 25.Valachis A, Mauri D, Polyzos NP, Mavroudis D, Georgoulias V, Casazza G. Partial breast irradiation or whole breast radiotherapy for early breast cancer: A meta-analysis of randomized controlled trials. Breast J. 2010;16:245–251. doi: 10.1111/j.1524-4741.2010.00905.x. [DOI] [PubMed] [Google Scholar]
- 26.Hickey BE, Lehman M. Partial breast irradiation versus whole breast radiotherapy for early breast cancer. Cochrane Database Syst Rev. 2021;8 doi: 10.1002/14651858.CD007077.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu G, Dong Z, Huang B, et al. Efficacy and safety of accelerated partial breast irradiation: A meta-analysis of published randomized studies. Oncotarget. 2017;8 doi: 10.18632/oncotarget.19225. 59,581-59,591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marta GN, Macedo CR, de Andrade Carvalho H, Hanna SA, da Silva JL, Riera R. Accelerated partial irradiation for breast cancer: Systematic review and meta-analysis of 8653 women in eight randomized trials. Radiother Oncol. 2015;114:42–49. doi: 10.1016/j.radonc.2014.11.014. Erratum for: Radiother Oncol. 2015;115:436-437. [DOI] [PubMed] [Google Scholar]
- 29.Correa C, Harris EE, Leonardi MC, et al. Accelerated Partial Breast Irradiation: Executive summary for the update of an ASTRO evidence-based consensus statement. Pract Radiat Oncol. 2017;7:73–79. doi: 10.1016/j.prro.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Polgár C, Van Limbergen E, Pötter R, et al. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: Recommendations of the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009) Radiother Oncol. 2010;94:264–273. doi: 10.1016/j.radonc.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 31.Shah C, Vicini F, Shaitelman SF, et al. The American Brachytherapy Society consensus statement for accelerated partial-breast irradiation. Brachytherapy. 2018;17:154–170. doi: 10.1016/j.brachy.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 32.The American Society of Breast Surgeons. Consensus statement for accelerated partial breast irradiation. Available at: https://www.breastsurgeons.org/docs/statements/Consensus-Statement-for-Accelerated-Partial-Breast-Irradiation.pdf/. Accessed September 2021.
- 33.Tom MC, Joshi N, Vicini F, et al. The American Brachytherapy Society consensus statement on intraoperative radiation therapy. Brachytherapy. 2019;18:242–257. doi: 10.1016/j.brachy.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 34.Harness JK, Davies K, Via C, et al. Meta-analysis of local invasive breast cancer recurrence after electron intraoperative radiotherapy. Ann Surg Oncol. 2018;25:137–147. doi: 10.1245/s10434-017-6130-x. [DOI] [PubMed] [Google Scholar]
- 35.Leonardi MC, Maisonneuve P, Mastropasqua MG, et al. How do the ASTRO consensus statement guidelines for the application of accelerated partial breast irradiation fit intraoperative radiotherapy? A retrospective analysis of patients treated at the European Institute of Oncology. Int J Radiat Oncol Biol Phys. 2012;83:806–813. doi: 10.1016/j.ijrobp.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): A randomised controlled equivalence trial. Lancet Oncol. 2013;14:1269–1277. doi: 10.1016/S1470-2045(13)70497-2. [DOI] [PubMed] [Google Scholar]
- 37.Ward MC, Vicini F, Chadha M, et al. Radiation therapy without hormone therapy for women age 70 or above with low-risk early breast cancer: A microsimulation. Int J Radiat Oncol Biol Phys. 2019;105:296–306. doi: 10.1016/j.ijrobp.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 38.Early Breast Cancer Trialists’ Collaborative Group. Darby S, McGale P, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deshmukh AA, Shirvani SM, Lal L, et al. Cost-effectiveness analysis comparing conventional, hypofractionated, and intraoperative radiotherapy for early-stage breast cancer. J Natl Cancer Inst. 2017;109:djx068. doi: 10.1093/jnci/djx068. [DOI] [PubMed] [Google Scholar]
- 40.Eisavi M, Rezapour A, Alipour V, et al. Cost-effectiveness analysis of intraoperative radiation therapy versus external beam radiation therapy for the adjuvant treatment of early breast cancer: A systematic review. Med J Islam Repub Iran. 2020;34:167. doi: 10.47176/mjiri.34.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Offersen BV, Overgaard M, Kroman N, Overgaard J. Accelerated partial breast irradiation as part of breast conserving therapy of early breast carcinoma: A systematic review. Radiother Oncol. 2009;90:1–13. doi: 10.1016/j.radonc.2008.08.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.