Abstract
The poultry red mite, Dermanyssus gallinae, is currently the most common ectoparasite affecting egg-laying hens. Since continuous culture of D. gallinae on birds is a biologically and economically costly endeavour, storage techniques for mites are urgently needed. Effects of temperature on adult and nymph survival were first studied to optimize storage conditions. Then, fecundity of D. gallinae was studied after mites were stored at optimal storage conditions. Results showed the survival rates of protonymphs (42.11%), deutonymphs (8.19%) and females (19.78%) at 5°C after 84 days were higher than those at 0, 25 and 30°C. Thereafter the fecundity and the capability of re-establishing colonies of D. gallinae were evaluated after they were stored for 40 and 80 days at 5°C. After storage, the mean number of eggs showed no statistical difference between treated (5°C for 40 or 80 days) and control groups (25°C for 7 days), while the hatching rates of eggs were in all cases above 97%. The dynamic changes of mite populations and egg numbers showed similar trends to the control group after the stored adult or nymph mites were fed on chicks. Dermanyssus gallinae can be successfully stored at 5°C for 80 days with no interference with the fecundity of mites, and the stored mites could re-establish colonies successfully. Adults and nymphs were two main stages with capability for low temperature storage. These results suggest that low temperature storage is a viable option for colony maintenance of D. gallinae under laboratory conditions.
Key words: Dermanyssus gallinae, low temperature, poultry red mite, storage
Introduction
The poultry red mite, Dermanyssus gallinae, is currently the most common ectoparasite affecting the egg-laying industry in several countries, such as Germany, France, Japan and China (Sparagano et al., 2014). Dermanyssus gallinae feeds on the blood of hosts, including poultry, budgerigars and other birds, as well as mammals, such as dogs, rats and even humans (Ramsay et al., 1975; Kowal et al., 2014). The negative effects of D. gallinae infestation on hens include stress, weight loss, reduced egg quality and production, anaemia and even mortality of the hens (Chauve, 1998). Dermanyssus gallinae has been implicated as a vector for dozens of infectious pathogens, such as Salmonella gallinarum, Erysipelothrix rhusiopathiae, Newcastle disease virus and Fowl poxvirus (Sparagano et al., 2014). Dermanyssus gallinae biting can have serious impacts on human health, including skin irritation, itching and allergic skin reactions (Rosen et al., 2010). For the development of novel control methods, laboratory rearing and long-term maintenance of populations of D. gallinae are often required.
Several effective and reliable in vivo and in vitro methods for maintaining experimental D. gallinae populations have been developed. A recently reported in vivo method presented an efficient rearing system for D. gallinae, which continuous feeds on chicks (Wang et al., 2018). However, the continuous rearing of mite colonies is often beset with problems. As D. gallinae must be regularly fed bloodmeals on birds, a major expenditure in laboratories is the rearing and maintenance of laboratory cultures of the species, in terms of high labour, material costs and time expending. Therefore, it is not surprising that, as increasing numbers of susceptible and resistant strains of D. gallinae are collected and reared, the costs of D. gallinae rearing are steadily increasing. In addition to the above operational costs, there can be biological costs. It has long been known that rearing insects for multiple generations under laboratory conditions can cause genetic drift (Mackauer, 1972; Chambers, 1977), ultimately leading to deleterious effects such as the gradual loss of fecundity or other key characteristics (Mackauer, 1972; Goto et al., 2006). It is superfluous to keep D. gallinae on birds when mites are not needed during the interval of studies, causing the problem of animal welfare too.
Alternatives to continuous culture include the use of cryopreservation and diapause for maintenance of insects. Long periods of storage can be achieved through cryopreservation, which has been successfully applied to several insect species, such as Lucilia sericata, Drosophila melanogaster and Musca domestica (Steponkus et al., 1990; Wang et al., 2000; Rajamohan et al., 2014). However, this technique must be optimized for each species primarily because of variations of biological characteristics among different insects. Diapause can also be used as a storage method but may also be difficult to implement (Hodkova and Hodek, 2004; Denlinger, 2010). There are some limitations in the use of diapause for mites, e.g. D. gallinae, due to the lack of a well characterized diapause or the inability of some species to enter diapause. Thus, alternative options for preservation are needed. To address this issue, several researchers have investigated the use of short-term low-temperature storage for some insects, such as Trichogramma, Trichogrammatoidea, Culex pipiens and Megachile rotundata (Rinehart et al., 2010; Yocum et al., 2010; Ghosh and Ballal, 2018). These studies have been conducted with varying degrees of success. Yocum and his colleagues found that 18°C for 14 days seemed to be the optimal storage temperature for short-term storage of developing bees, Megachile rotundata (Yocum et al., 2010). Rinehart reported that adult Culex pipiens can be successfully stored at reduced temperatures (Rinehart et al., 2010), showing that mating before storage, females remain reproductively viable at least for 10 weeks of storage. However, the low-temperature storage of D. gallinae has not yet been studied, which is crucially important for the maintenance of D. gallinae under laboratory conditions. Therefore, three issues: the temperature, duration of exposure and the life stage should be addressed for low-temperature storage of D. gallinae.
Temperature is one of the key factors influencing activity and vitality of D. gallinae (Harrison, 1963). Nordenfors and Tucci found that temperature could affect oviposition, molting, development, viability and life cycle parameters of D. gallinae (Nordenfors et al., 1999; Tucci et al., 2008). Importantly, information on survivability at low temperature can provide useful information for the understanding of population dynamics during storage of D. gallinae. Specifically, Nordenfors reported that females survived for up to 9 months at 5°C with LT50 values of 58 days. However, D. gallinae are not tolerant of freezing, as females survived for 10 min at −20°C but all died after 20 min (Nordenfors et al., 1999). It has reported that, increased cold and extended time yielded higher mortality for bed bugs, Cimex lectularius, reaching 100% mortality rate in adults after 1 h at either −16 or −18°C (Benoit et al., 2009). Although nymphs are predominant in the D. gallinae population (Wang et al., 2018), the survival rates of engorged protonymphs and deutonymphs at low temperature were still unknown, a very essential parameter to study the storage conditions of D. gallinae. What's more, water balance and starvation are reported as two of the main factors determining the ability of ectoparasite to survive off host, e.g. ticks (Needham and Teel, 1991). Rosendale found dehydration reduced protein and lipid availability, and ticks exposed to dehydration would have decreased longevity through reduced energetic reserves (Rosendale et al., 2017). Besides, there are differences in dehydration resistance between life stages of bed bug, Cimex hemipterus, and high temperature increases water loss of C. lectularius and C. hemipterus, leading to a significant reduction in the length of survival under dehydrating conditions (Benoit et al., 2007; 2009; How and Lee, 2010). Specifically, C. hemipterus survived longest under the interaction of low temperature (20°C) and high relative humidity (RH) (75–100%), and starved C. hemipterus started to die after losing 35–45% of their body weights (How and Lee, 2010). Ticks with high fat reserves survived longer than starved ticks with low lipid levels when they were exposed to low temperature (Herrmann and Gern, 2013). Coudron also reported that there was a clear effect of food source on egg and nymph survival, adult survival and fecundity and egg viability following cold storage of Perillus bioculatus (Coudron et al., 2009). The availability of food during cold storage improved survival and reproduction of Podisus maculiventris (Thorpe and Aldrich, 2004). As reported, the cuticular permeability and water loss increased exponentially across increasing temperatures, and higher temperatures lead to increased energy consumption (Van Es et al., 1998). However, the effects of the availability of food on the survival of D. gallinae were still unknown.
The present study was conducted to establish a low-temperature storage method for D. gallinae. Initially, we evaluated the effect of four temperatures (30, 25, 5 and 0°C) on the survival of females, deutonymphs and protonymphs to optimize storage conditions. Then, a colony of D. gallinae was stored at the optimized storage conditions. After storage, we tested the fecundity of stored females, and evaluated whether stored nymphs or adults could establish colonies, as well as the changes in the proportions of D. gallinae populations at different life stages. Finally, we evaluated the effect of low temperature on the body weight of D. gallinae to explore the possible relationship between availability of food and survival of the mites.
Materials and methods
Dermanyssus gallinae used in the experiments
The D. gallinae colony used in these investigations was originally collected from a layer farm in the Pinggu District of Beijing in China. The mites were maintained at 30°C, 70% RH with a photoperiod of 12:12 h light:dark (L:D), and were continuously fed on chicks.
Effect of temperatures on survival of D. gallinae
To evaluate the effect of storage temperatures on survival and duration of exposure of D. gallinae at different life stages, the experiments were carried out following a previously described method (Nordenfors et al., 1999). Engorged females, deutonymphs and protonymphs were collected from colonies, and placed individually in 96-well enzyme-linked immunosorbent assay (ELISA) plates. Each plate contained 96 mites and there were four ELISA plates for each life stage, e.g. 384 mites in total at each stage. The openings were sealed with Parafilm M (SPI Supplies, Westchester, US), which helped retain moisture and allowed air exchange but prevented the escape of mites. Plates containing engorged protonymphs, deutonymphs or females were exposed to four different temperatures: the optimum temperature for D. gallinae (30°C), the room temperature (25°C) and two low temperatures (0 and 5°C). In this experiment, we did not take humidity into account according to the results of Nordenfors (Nordenfors et al., 1999). Refrigerators were used for 0 and 5°C, and incubators for 25 and 30°C.
The total period for the tests was 12 weeks, and mites were observed weekly. At each observation time, mites were observed using a stereomicroscope (SteREO-Discovery.V12, ZEISS Microscopy). The number of dead mites were recorded, and survival rates of engorged females, deutonymphs and protonymphs were calculated using the formula: Survival rate (%) = (Number of surviving mites/Total number of mites in the ELISA plate) × 100. Based on these results, 5°C was chosen as optimal storage temperature for duration of storage (e.g. 40 or 80 days).
Low-temperature storage of D. gallinae
In order to ensure that low-temperature storage: 5°C for 40 or 80 days of D. gallinae was practicable, we monitored the changes in the proportions of mite populations at different life stages during storage, and tested the fecundity of stored females, evaluated whether stored nymphs or adults could establish colonies again. In details, three traps containing large quantities of mites with a mix of life stages were randomly removed from the cages, placed in three conical flasks, which were allocated to three experimental groups. In each trap, the numbers of mites at different life stages were different which were observed using a stereomicroscope prior to the start of the experiment, but the proportions of mites at different life stages in the three traps were similar (Wang et al., 2018). The flasks were then sealed with parafilm (Ningbo dengyue new material technology co., ltd, China). In group I, the flask was maintained at 25°C for 7 days as the control group; in group II, the flask was kept at 5°C for 40 days; in group III, the flask was kept at 5°C for 80 days. After exposure, all mites in the flask of each group were transferred to 12-cm diameter Petri dishes, respectively. In each group, the number of live mites at different life stages was recorded by observing them using a stereomicroscope. The stored mites in group I, II and III were used in the following two experiments.
The two experiments were conducted using an in vivo feeding system of D. gallinae (Wang et al., 2018). Five-day-old chicks were used as the host of D. gallinae, who were given water and food ad libitum. The chicks were housed under a protocol approved by the Laboratory Animal Institute of China Agricultural University within Beijing Animal Use and Care Association (Approval No.: CAU20180408-4). The feeding device was placed in the incubator at 30°C, RH 70% and light intensity of 3600 lux for a 12:12 h L:D photoperiod.
Fecundity of stored females
The first experiment was conducted to study the effect of low-temperature storage on the fecundity of stored females by assessing oviposition and hatchability of eggs. In this experiment, 9 chicks were equally assigned to three cages. Two hundred and twenty adult female mites were picked out from each group (e.g. group I, II and III), and introduced to the cages. The mites were allowed to feed on chicks for 12 h. Thereafter, engorged females (n = 50) from each cage were collected and placed individually in 96-well ELISA plates. The number of eggs per female was recorded daily for 7 days, and the development of eggs was monitored using a stereomicroscope. The oviposition and hatchability were calculated. The experiments were repeated three times.
The dynamic changes of mites and eggs after chicks were challenged with stored mites
To investigate whether the mites after storage could establish colonies in the laboratory again, a second experiment was conducted. In this experiment, 54 chicks and 18 cages were used, with three chicks per cage. Two hundred and twenty adults or nymphs were picked out from each group, and transferred to the cage, resulting in two cages in each group (one having 220 adults, and the other with 220 nymphs). There were three replicates. The mites were allowed to feed on chicks continuously, and the population dynamics of D. gallinae was recorded weekly for 4 weeks following a previously described method (Wang et al., 2018).
The changes in mass and appearance of D. gallinae at low temperature
To explore the potential relationship between availability of food and survival of the mites, the effect of low temperature on the mite mass was evaluated by measuring body weight changes of females while simultaneously photographing them over time at 5 and 25°C. The females starved for 7 days were weighed, and then fed on chicks. The engorged mites were collected, weighed and transferred into two 50-mL centrifuge tubes and set as two experimental groups. Each centrifuge tube contained 500 engorged females, and two tubes were kept in the dark at 5 and 25°C with 70% RH, respectively. The mites were observed daily for 1 week. During each observation, females (n = 100) at each temperature were weighed using Cubis® Analytical Balance (Sartorius Lab Instruments GmbH & Co. KG, Goettingen, Germany), and photographed using a stereomicroscope (scale bar of 200 μm). The experiments were repeated three times. After observation, mites were transferred back to the centrifuge tube.
Statistical analysis
The survival curves were estimated using the Kaplan–Meier method and compared with the log-rank test. The dynamic changes of mites and eggs were performed using a two-way analysis of variance (ANOVA), and the mean number of eggs were performed using a one-way analysis of variance (ANOVA). Duncan's multiple range test was used to compare the differences. The Student's t-test was performed to compare the body weights. The criterion for statistical significance was set at P < 0.05. SPSS version 24.0 was used for all statistical analyses.
Results
Effect of temperatures on survival of D. gallinae
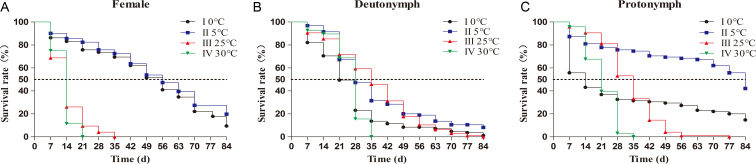
The survival rates of mites are illustrated in Fig. 1. We found that females (A), deutonymphs (B) and protonymphs (C) exhibited increased longevity with decreased storage temperatures from 30 to 5°C. For females (A), the survival rates of mites kept at 0 and 5°C after 84 days were 9.48 and 19.78%, respectively, while they were decreased to 0% after 21 days at 30°C and 35 days at 25°C. LT50 values for females at 0 and 5°C (both of 56 days) were higher than for the mites at 25 and 30°C (both of 14 days). For deutonymphs (B) and protonymphs (C), the survival rates at 30°C both decreased to 0% after 35 days. This decreased longevity indicated that 30°C was not suitable for mite storage. In addition, the survival rates of deutonymphs and protonymphs kept at 25°C decreased to 0% after 84 and 77 days, respectively. It was noted that the LT50 value for protonymphs at 5°C (84 days) was remarkably higher than for the mites at 0°C (14 days), 25°C (35 days) and 30°C (21 days). In summary, kept at 5°C, the survival rates of females (19.78%), deutonymphs (8.19%) and protonymphs (42.11%) after 84 days were the highest. These results demonstrated that the longevity of females, deutonymphs and protonymphs dramatically increased by storage at low temperature of 5°C, and therefore, 5°C was considered as optimum storage temperature. The duration of storage was determined as 40 or 80 days, preliminary. Moreover, females, deutonymphs and protonymphs were all chosen as stages of mites for low-temperature storage.
Fig. 1.
Survival rates of engorged females (A), deutonymphs (B) and protonymphs (C) after continuous incubation at different temperatures. Engorged females, deutonymphs and protonymphs were placed individually in ELISA plates. Each plate contained 96 mites and there were four ELISA plates for each life stage, e.g. 384 mites in total at each stage. Plates were kept at 0, 5, 25 and 30°C, and mites were observed weekly for 12 weeks. Survival rates of females, deutonymphs and protonymphs were recorded and calculated as follow: Survival rate (%) = (Number of surviving mites/Total number of mites in ELISA plate) × 100.
Proportions of different stages mites
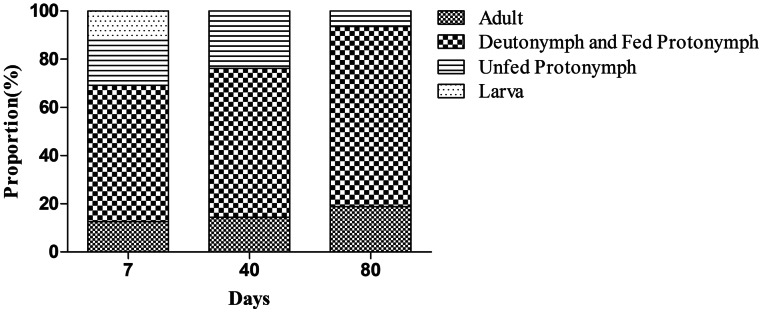
Instead of picking out the mites at different stages one by one, it would be more convenient that large quantities of mites with a mix of life stages were exposed to the low-temperature for storage. Because fed protonymphs and fed deutonymphs were indistinguishable and could not be precisely identified after storage, they were allocated as one group; the unfed protonymphs, which could be accurately identified by their appearance (white with 8 legs), were allocated as another group. The changes in the proportions of different life stage mites (adults, deutonymphs and fed protonymphs, unfed protonymphs and larva) are presented in Fig. 2. The proportion of adults, and deutonymphs and fed protonymphs after being kept at 25°C for 7 days was 12.6 and 56.5%, respectively. These proportions were 14.4 and 61.8% after being kept at 5°C for 40 days, and were 18.8 and 74.8% if kept at 5°C for 80 days. We observed that the proportion of larvae was 12.2% after being kept at 25°C for 7 days, and were 0% when kept at 5°C for 40 days, indicating that the larvae either died or developed into the next stage. The proportion of unfed protonymphs was 18.7% after 7 days at 25°C, was 23.9% after 40 days at 5°C and was 6.4% after 80 days at 5°C, suggesting that unfed protonymphs were not proper for low-temperature storage. These results showed the proportion of deutonymphs and fed protonymphs were highest during low temperature storage, indicating they were predominant in the population. It was proved again that adults and nymphs were the two main stages of mites that could be stored successfully at low temperature.
Fig. 2.
Proportions of D. gallinae at different life stages after low-temperature storage. Traps containing mites were exposed to different temperatures: 25°C for 7 days as control (group I), 5°C for 40 days (group II) and 5°C for 80 days (group III). After exposure, the number of living mites in the traps at different stages was counted under a stereomicroscope and the percentage of mites at different stages was calculated. When counting, all deutonymphs and fed protonymphs together were classified as a single subgroup, and the unfed protonymphs were classified as a separate subgroup. The experiments were repeated three times.
Fecundity of stored females
The effect of low-temperature storage on the fertility of D. gallinae was evaluated by assessing the oviposition of females and hatchability of eggs, and the results are shown in Table 1. After exposure to 25°C for 7 days (group I), 5°C for 40 days (group II) and 5°C for 80 days (group III), females were allowed to feed on chicks. The mean number of eggs per female in group I was 3.63 ± 1.26, which was slightly lower than those in group II (4.20 ± 1.23) and group III (3.69 ± 1.38), although there was no statistical difference (P > 0.05). Additionally, the hatchability of eggs was 97.99, 97.87 and 97.92% in the three groups, respectively, indicating high egg viability. The results of oviposition and hatchability indicated that the storage at low temperature of 5°C did not interfere with the fecundity of stored females, and therefore we did not evaluate the effect of low-temperature storage on the oviposition and hatchability of subsequent generations.
Table 1.
Oviposition of D. gallinae and egg hatchability after low-temperature storage
| Group | Mean number of eggs per female (mean ± s.d.) | Hatchability (%) |
|---|---|---|
| I (25°C for 7 days) | 3.63 ± 1.26 | 97.99 |
| II (5°C for 40 days) | 4.20 ± 1.23 | 97.87 |
| III (5°C for 80 days) | 3.69 ± 1.38 | 97.92 |
Notes. Mites were exposed to different experimental conditions: 25°C for 7 days as control (group I), 5°C for 40 days (group II) and 5°C for 80 days (group III). After exposure, female mites from each group were allowed to feed on chicks for 12 h. Then, 50 engorged females from each group were collected and placed individually in ELISA plates. Egg numbers and egg development were recorded daily for 7 days. The oviposition and hatchability were calculated. The experiments were repeated three times.
The dynamic changes of mites and eggs after chicks were challenged with stored mites
To investigate whether stored mites could establish colonies again, stored adults or nymphs were introduced to chicks. The dynamic changes of mites are presented in Fig. 3A. All curves exhibited a remarkable increase of the D. gallinae population in 4 weeks, confirming that D. gallinae populations were successfully established after introducing stored nymphs or adults to the system. Specifically, mite populations in three groups – Adult-7d, Adult-40d and Adult-80d – were significantly higher than those in the other three groups – Nymph-7d, Nymph-40d and Nymph-80d – at 7, 14, 21 and 28 days (P < 0.05). However, there was no statistical difference in results of mite numbers among the three storage conditions: 25°C for 7 days, 5°C for 40 days and 5°C for 80 days at 7, 14 and 21 days. Mite populations in the Nymph-80d group were significantly lower than the other two Nymph groups (P < 0.05).
Fig. 3.
Total number of mites (A) and eggs (B) in the rearing system after challenging stored mites. Traps containing mites were exposed to different temperatures: 25°C for 7 days as control (group I), 5°C for 40 days (group II) and 5°C for 80 days (group III). After exposure, 220 adults or nymphs from each group were transferred to the cage, resulting in two cages in each group (one having 220 adults, and the other with 220 nymphs). There were three replicates. The total number of mites and eggs in the rearing system were counted at 7, 14, 21 and 28 days post-challenge. All values are shown as mean ± s.d. from three independent experiments.
The dynamic changes of eggs are shown in Fig. 3B. We observed that the variation in the number of eggs was similar to that of mites. The egg numbers in three groups of adults were significantly higher than those in the other three groups of nymphs at 7, 14, 21 and 28 days (P < 0.05). For both mites and eggs, population size in the three adult groups was higher than those in the three nymph groups. This indicated that adults had a faster reproduction rate than nymphs. This might result from that nymphs need a development period to adults before multiplication, and thus nymphs would need longer time than adults to reach equal numbers of mites. These results revealed that after exposure to low-temperature storage, adults and nymphs could establish colonies, and that the reproduction rate of D. gallinae was not affected by low-temperature storage.
The changes in mass and appearance of D. gallinae at low temperature
The changes in body weight (A) and micrographs of females under a stereomicroscope (B) at 5 and 25°C are shown in Fig. 4. The body weights of females at 5°C were significantly higher than those at 25°C from 1–7 days (P < 0.05) (Fig. 4A). Specifically, at 25°C, the mean body weight of females was 253 ± 16 μg on day 0, and decreased to 74 ± 5 μg on day 3, with minor changes afterwards. However, at 5°C, the mean body weight of females decreased slowly over a period of 7 days, e.g. from 250 ± 11 to 234 ± 10 μg. Notably, micrographs showed that the females took longer time to digest the blood at 5°C, maintaining the reddish colour until the final observation period (day 7), indicating that the blood was digested slowly, while the mites kept at 25°C turned greyish, indicating that nearly all blood was digested.
Fig. 4.
Body weights (A) and micrographs of females under a stereomicroscope (B) at different temperatures. Engorged females were continuously kept in the dark at 5 and 25°C. Mites were observed daily for 7 days. For each observation, a random sample (n = 100) of females was weighed and photographed using a stereomicroscope at the same magnification. The experiments were repeated three times.
Discussion
The objective of this study was to develop a low-temperature storage method and assess the fecundity of D. gallinae after low-temperature storage. The effect of low temperatures on organisms is the result of a complex interaction between the thermal history of the individual organism, developmental stage, temperature and duration of exposure (Rajamohan and Sinclair, 2008). To establish a low-temperature storage method for D. gallinae, we believe three crucial issues need to be systematically and properly addressed: temperature, duration of exposure and stage selection of D. gallinae. Therefore, we evaluated the effect of low temperatures on the survival rates of females, deutonymphs and protonymphs to choose stages of mites and optimize the storage conditions. After this initial evaluation, D. gallinae was exposed to the optimal low temperature storage conditions, namely temperature and duration. After storage, we tested the fecundity of females, and evaluated whether nymphs or adults could establish colonies, as well as the changes in the proportions of D. gallinae at different life stages during storage.
Temperature is one of the most important environmental factors, influencing nearly every aspect of the life of mites and ticks, e.g. fecundity, longevity and egg viability (Davey, 1988; Ganjisaffar et al., 2011). Therefore, knowledge of how particular developmental stages will respond to low temperature can be useful for the understanding of their population dynamics. Specifically, we evaluated the effects of various temperatures (0, 5, 25 and 30°C) on the survival of mites at different developmental stages, including females, deutonymphs and protonymphs. Among the four temperatures tested, the survival rates of mites at three stages (females, deutonymphs and protonymphs) after storage for 84 days at 5°C were the highest, while unfed protonymphs were untenable. For example, the survival rate of females kept at 5°C for 84 days (19.78%) was higher than that at 0°C (10.42%), 25°C (0%) and 30°C (0%); and the survival rate of protonymphs kept at 5°C for 84 days (42.11%) was highest in those at 0°C (14.74%), 25°C (0%) and 30°C (0%). Therefore, two crucial factors for the storage method for D. gallinae: the temperature of 5°C, and the duration of 40 and 80 days, had been determined.
The third crucial issue was the selection of the developmental stages of mites. It had been reported that no further egg development occurred at 5°C (Nordenfors et al., 1999), and therefore the mite populations would not increase. While monitoring the proportion of mites at different life stages, we found that after being exposed to 5°C for 40 and 80 days the eggs shrank, dried and had no further development, and the proportion of larvae decreased to 0%, either because they died or because they developed into the next stage. These results reveal that the eggs and larvae are not adequate for low-temperature storage. In addition, after being exposed to 5°C for 40 and 80 days, the proportions of unfed protonymphs continuously decreased, and were much lower than those of deutonymphs and the fed protonymphs, indicating that low-temperature storage is not suitable for unfed protonymphs. Surprisingly, when kept at 5°C, three stages mites including females (19.79%), deutonymphs (9.38%) and protonymphs (42.71%) survived for at least 84 days without access to food. Taking into account the survival rates, duration of exposure and the proportion of mites at each developmental stage, we found that adults and nymphs were the two main stages for the successful low temperature preservation of mites.
The fecundity of stored D. gallinae was assessed from two experiments after being kept at 5°C for 40 and 80 days: oviposition of stored females and the dynamic changes of mites and eggs. In our study, the mean number of eggs produced by females which had been kept at 5°C for 40 and 80 days didn't decrease, with no significant difference from those in control groups. Moreover, the hatchability of eggs in all groups was above 97%, indicating high egg viability. Therefore, we did not evaluate the effect of storage on the fecundity of subsequent generations, e.g. F1 and F2 generations. Nevertheless, the consequences of a low-temperature stress may be long lasting, appearing in the progeny of the individuals subjected to the stress, and the transgenerational issues about the fecundity of some species exposed to cold temperature have been reported. Rinehart reported the cold storage of Culex pipiens in the absence of diapause, and assessed the fecundity of stored females (Rinehart et al., 2010). He found that egg raft sizes did not fully recover in the F1 generation after females were treated at 6°C, which showed a 21% reduction in egg raft size as compared with controls, but then the egg production returned to normal levels by the F2 generation. Storing adult mymarid wasps Gonatocerus ashmeadi Girault at 10°C for >20 days also induces a decrease in their F1 generation's fecundity and longevity, and skews the sex ratio toward males. Therefore, delayed effects of low-temperature stress on the fecundity of subsequent generations of D. gallinae need to be explored further. These present results demonstrate that low-temperature storage did not interfere with the fecundity of stored females of D. gallinae. Additionally, evolution of the mite and egg populations over time in the rearing system showed similar patterns in both the treated and control groups, after chicks were challenged with stored adults or nymphs, with an exception for the subset of nymph-80d, indicating that after low-temperature storage, mite populations could be re-established. In a word, our results suggest that D. gallinae can be successfully stored at the low temperature of 5°C for at least 80 days.
The results of this study clearly indicate a variable response of D. gallinae by stage to continuous exposure to the same temperature. The variation among the same developmental stages of D. gallinae might be related to the availability of food. Both pre-storage and post-storage nutrition improved survival and reproduction of Chrysoperla carnea following cold storage (Chang et al., 1995). Coudron reported that nutrient quality influenced the response of Perillus bioculatus to cold storage at different developmental stages (Coudron et al., 2009). As shown in Figs. 1 and S1, there is a difference between protonymphs and unfed protonymphs in the survival period when they were kept at the same temperature. Engorged protonymphs could survive for up to 77 days at 25°C and up to 35 days at 30°C. However, the unfed protonymphs, who developed from the eggs laid by females, survived only for up to 14 days at 25°C and up to 28 days at 30°C. This difference in the survival period between engorged protonymphs and unfed protonymphs, both at the same stage, was likely caused by the availability of food. These results demonstrate that pre-storage nutrition improved longevity for D. gallinae when continuously exposed to a specific temperature.
Moreover, a variable response of D. gallinae at the same life stage continuously exposed to different temperatures was also observed in this study. For example, engorged females could survive for at least 84 days at 5°C with a LT50 value of 56 days, but only for up to 35 days at 25°C with a LT50 value of 14 days. Interestingly, we found that the body weight of females decreased slowly (e.g. from 250 ± 11 μg to 234 ± 10 μg) over a period of 7 days at 5°C. While, it decreased quickly at 25°C from 253 ± 16 μg on day 0 to 74 ± 5 μg on day 3, indicating that the weight loss of females at 5°C was much lower than that at 25°C in 7 days. It should be pointed out that a number of physiological parameters would affect the weight of D. gallinae, e.g. reduced transpiration at low temperature, reduced functioning of the Malpighian tubules resulting in reduced water excretion, reduced metabolism resulting in the accumulation of nutrients and waste, reduction in egg development and egg laying resulting in accumulation and retention of egg material internally etc. (Nordenfors et al., 1999; Pritchard et al., 2015; Rosendale et al., 2017). Of course, slower blood digestion could have a strong relationship to the less loss of body mass at low temperature, which could have been obviously observed in micrographs in Fig. 4B. Therefore, the retained water, food (blood) and energy of females at low-temperature might make a contribution to prolonged longevity of D. gallinae. It has reported that starvation and hydric stress are two of the most critical factors limiting tick survival; similarly, for D. gallinae, understanding these two factors and their interaction are important for predicting mite longevity and population dynamics, which needs to be further studied.
Overall, results of this study demonstrate that D. gallinae can be successfully stored at the low temperature of 5°C for at least 80 days. Besides the capability in maintaining D. gallinae at low-temperature for a period, the low-temperature storage method also has other advantages such as simplicity, small space requirements, convenience for manipulation and low costs, which can facilitate its widespread use in laboratories. For example, the various traps containing D. gallinae, e.g. corrugated paper, the AVIVET trap and the monitoring trap (Nordenfors et al., 1999; Lammers et al., 2017; Wang et al., 2018), could be placed in a vented container kept in refrigerator, and when needed in the experiment, the mites could be introduced to chicks for reproduction immediately. Although only a single colony was included in the present study, in the later studies the low-temperature storage method has been applied on the preservation of other colonies in our laboratory, e.g. the susceptible and resistant strains of D. gallinae, which confirmed the general application of low-temperature storage of D. gallinae. Therefore, low-temperature storage is a useful and reliable method to maintain D. gallinae strains under laboratory conditions.
Conclusions
Our findings demonstrate that D. gallinae can be successfully stored at the low temperature of 5°C for at least 80 days. Adults and nymphs were the two main stages of mites that could be stored successfully. Storage of D. gallinae at low temperatures did not interfere with the fecundity of mites in terms of oviposition and hatchability, and the stored mites could re-establish the colonies successfully. These results suggest that low temperature storage is a viable option for the colony maintenance of D. gallinae under laboratory conditions.
Ethical standards
Five-day-old chicks were used as the host of D. gallinae, who were given water and food ad libitum. The chicks were housed under a protocol approved by the Laboratory Animal Institute of China Agricultural University within Beijing Animal Use and Care Association (Approval No.: CAU20180408-4).
Declarations of interest
None.
Financial support
This work was supported by the ‘National Natural Science Foundation of China’ (Grant No. 31873008) and the ‘National Key Research and Development Program of China’ (Grant No. 2017YFD0501200 and 2018YFD0502305).
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182020000463.
click here to view supplementary material
References
- Benoit JB, Del Grosso NA, Yoder JA and Denlinger DL (2007) Resistance to dehydration between bouts of blood feeding in the bed bug, Cimex lectularius, is enhanced by water conservation, aggregation, and quiescence. American Journal of Tropical Medicine and Hygiene 76, 987–993. [PubMed] [Google Scholar]
- Benoit JB, Lopez-Martinez G, Teets NM, Phillips SA and Denlinger DL (2009) Responses of the bed bug, Cimex lectularius, to temperature extremes and dehydration: levels of tolerance, rapid cold hardening and expression of heat shock proteins. Medical & Veterinary Entomology 23, 418–425. [DOI] [PubMed] [Google Scholar]
- Chambers DL (1977) Quality control in mass rearing. Annual Review of Entomology 22, 289–308. [Google Scholar]
- Chang YF, Tauber MJ and Tauber CA (1995) Storage of the mass-produced predator Chrysoperla carnea (Neuroptera: Chrysopidae): influence of photoperiod, temperature, and diet. Environmental Entomology 24, 1365–1374. [Google Scholar]
- Chauve C (1998) The poultry red mite Dermanyssus gallinae (De Geer, 1778): current situation and future prospects for control. Veterinary Parasitology 79, 239–245. [DOI] [PubMed] [Google Scholar]
- Coudron TA, Popham HJR and Ellersieck MR (2009) Influence of diet on cold storage of the predator Perillus bioculatus (F.). Biocontrol 54, 773–783. [Google Scholar]
- Davey RB (1988) Effect of temperature on the ovipositional biology and egg viability of the cattle tick Boophilus annulatus (Acari: Ixodidae). Experimental & Applied Acarology 5, 1–14. [DOI] [PubMed] [Google Scholar]
- Denlinger DL (2010) Why study diapause? Entomological Research 38, 1–9. [Google Scholar]
- Ganjisaffar F, Fathipour Y and Kamali K (2011) Temperature-dependent development and life table parameters of Typhlodromus bagdasarjani (Phytoseiidae) fed on two-spotted spider mite. Experimental and Applied Acarology 55, 259–272. [DOI] [PubMed] [Google Scholar]
- Ghosh E and Ballal CR (2018) Short-term storage of the egg parasitoids. Trichogramma and Trichogrammatoidea. Egyptian Journal of Biological Pest Control 28, 34. [Google Scholar]
- Goto SG, Han B and Denlinger DL (2006) A nondiapausing variant of the flesh fly, Sarcophaga bullata, that shows arrhythmic adult eclosion and elevated expression of two circadian clock genes, period and timeless. Journal of Insect Physiology 52, 1213–1218. [DOI] [PubMed] [Google Scholar]
- Harrison IR (1963) Population studies on the poultry red mite Dermanyssus gallinae (Deg. Bulletin of Entomological Research 53, 657–664. [Google Scholar]
- Herrmann C and Gern L (2013) Survival of Ixodes ricinus (Acari: Ixodidae) nymphs under cold conditions is negatively influenced by frequent temperature variations. Ticks and Tick-borne Diseases 4, 445–451. [DOI] [PubMed] [Google Scholar]
- Hodkova M and Hodek I (2004) Photoperiod diapause and cold-hardiness. European Journal of Entomology 101, 445–458. [Google Scholar]
- How YF and Lee C-Y (2010) Effects of temperature and humidity on the survival and water loss of Cimex hemipterus (Hemiptera: Cimicidae). Journal of Medical Entomology 47, 987–995. [DOI] [PubMed] [Google Scholar]
- Kowal J, Nosal P, Niedziółka R and Kornaś S (2014) Presence of blood-sucking mesostigmatic mites in rodents and birds kept in pet stores in the Cracow area, Poland. Annals of Tropical Medicine and Parasitology 60, 61–64. [PubMed] [Google Scholar]
- Lammers GA, Bronneberg RGG, Vernooij JCM and Stegeman JA (2017) Experimental validation of the AVIVET trap, a tool to quantitatively monitor the dynamics of Dermanyssus gallinae populations in laying hens. Poultry Science 96, 1563–1572. [DOI] [PubMed] [Google Scholar]
- Mackauer M (1972) Genetic aspects of insect production. Entomophaga 17, 27–48. [Google Scholar]
- Needham GR and Teel PD (1991) Off-host physiological ecology of ixodid ticks. Annual Review of Entomology 36, 659–681. [DOI] [PubMed] [Google Scholar]
- Nordenfors H, Höglund J and Uggla A (1999) Effects of temperature and humidity on oviposition, molting, and longevity of Dermanyssus gallinae (Acari: Dermanyssidae). Journal of Medical Entomology 36, 68–72. [DOI] [PubMed] [Google Scholar]
- Pritchard J, Kuster T, Sparagano O and Tomley F (2015) Understanding the biology and control of the poultry red mite Dermanyssus gallinae: a review. Avian Pathology 44, 143–153. [DOI] [PubMed] [Google Scholar]
- Rajamohan A and Sinclair BJ (2008) Short-term hardening effects on survival of acute and chronic cold exposure by Drosophila melanogaster larvae. Journal of Insect Physiology 54, 708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamohan A, Rinehart JP and Leopold RA (2014) Cryopreservation of embryos of Lucilia sericata (Diptera: Calliphoridae). Journal of Medical Entomology 51, 360–367. [DOI] [PubMed] [Google Scholar]
- Ramsay GW, Mason PC and Hunter AC (1975) Chicken mite (Dermanyssus gallinae) infesting a dog. New Zealand Veterinary Journal 23, 155–155. [DOI] [PubMed] [Google Scholar]
- Rinehart JP, Yocum GD, Leopold RA and Robich RM (2010) Cold storage of Culex pipiens in the absence of diapause. Journal of Medical Entomology 47, 1071. [DOI] [PubMed] [Google Scholar]
- Rosen S, Yeruham I and Braverman Y (2010) Dermatitis in humans associated with the mites Pyemotes tritici, Dermanyssus gallinae. Ornithonyssus bacoti and Androlaelaps casalis in Israel. Medical & Veterinary Entomology 16, 442–444. [DOI] [PubMed] [Google Scholar]
- Rosendale AJ, Dunlevy ME, Fieler AM, Farrow DW, Davies B and Benoit JB (2017) Dehydration and starvation yield energetic consequences that affect survival of the American dog tick. Journal of Insect Physiology 101, 39–46. [DOI] [PubMed] [Google Scholar]
- Sparagano OAE, George DR, Harrington DWJ and Giangaspero A (2014) Significance and control of the poultry Red Mite, Dermanyssus gallinae. Annual Review of Entomology 59, 447–466. [DOI] [PubMed] [Google Scholar]
- Steponkus PL, Myers SP, Lynch DV, Gardner L, Bronshteyn V, Leibo SP, Rall WF, Pitt RE, Lin TT and Maclntyre RJ (1990) Cryopreservation of Drosophila melanogaster embryos. Nature 26, 575–576. [DOI] [PubMed] [Google Scholar]
- Thorpe KW and Aldrich JR (2004) Conditions for short-term storage of field-collected spined soldier bug, Podisus maculiventris (Say) (Heteroptera: Pentatomidae), adults prior to augmentative release. Journal of Entomological Science 39, 483–489. [Google Scholar]
- Tucci EC, Prado AP and Araújo RP (2008) Development of Dermanyssus gallinae (Acari: Dermanyssidae) at different temperatures. Veterinary Parasitology 155, 127–132. [DOI] [PubMed] [Google Scholar]
- Van Es RP, Hillerton JE and Gettinby G (1998) Lipid consumption in Ixodes ricinus (Acari: Ixodidae): temperature and potential longevity. Bulletin of Entomological Research 88, 567. [Google Scholar]
- Wang WB, Leopold RA, Nelson DR and Freeman TP (2000) Cryopreservation of Musca domestica (Diptera: Muscidae) embryos. Cryobiology 41, 153–166. [DOI] [PubMed] [Google Scholar]
- Wang C, Ma Y, Yu H, Xu J, Cai J and Pan B (2018) An efficient rearing system rapidly producing large quantities of poultry red mites, Dermanyssus gallinae (Acari: Dermanyssidae), under laboratory conditions. Veterinary Parasitology 258, 38–45. [DOI] [PubMed] [Google Scholar]
- Yocum GD, Rinehart JP, West M and Kemp WP (2010) Interrupted incubation and short-term storage of the alfalfa pollinator Megachile rotundata (Hymenoptera: Megachilidae): a potential tool for synchronizing bees with bloom. Journal of Economic Entomology 103, 234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0031182020000463.
click here to view supplementary material