Abstract
Purpose
Immunotherapy (IO) has significantly improved outcomes in metastatic renal cell carcinoma (mRCC). Preclinical evidence suggests that responses to IO may be potentiated via immunomodulatory effects of stereotactic radiation therapy (SRT). We hypothesized that clinical outcomes from the National Cancer Database (NCDB) would demonstrate improved overall survival (OS) in patients with mRCC receiving IO + SRT versus IO alone.
Methods and Materials
Patients with mRCC receiving first-line IO ± SRT were identified from the NCDB. Conventional radiation therapy was allowed in the IO alone cohort. The primary endpoint was OS stratified by the receipt of SRT (IO + SRT vs IO alone). Secondary endpoints included OS stratified by the presence of brain metastases (BM) and timing of SRT (before or after IO). Survival was estimated using Kaplan-Meier methodology and compared via the log-rank test.
Results
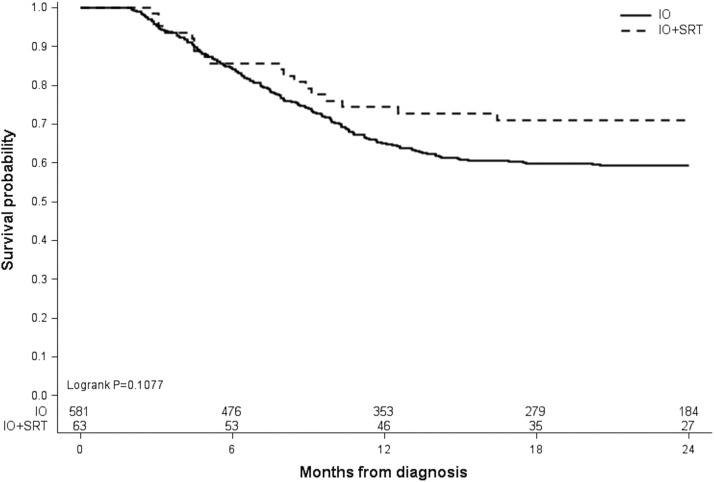
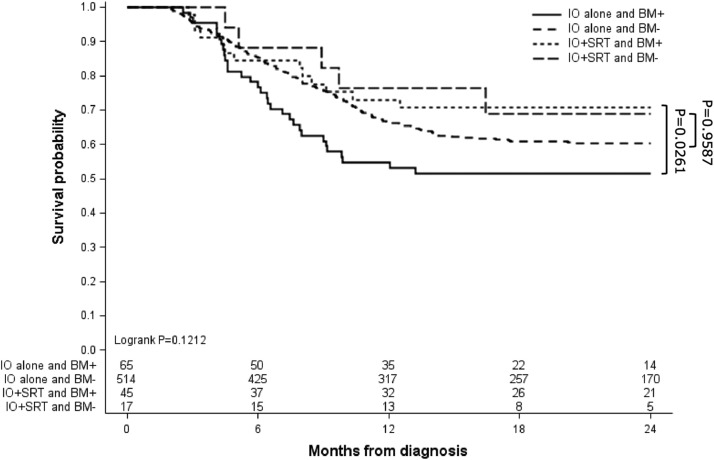
Of 644 eligible patients, 63 (9.8%) received IO + SRT, and 581 (90.2%) received IO alone. Median follow-up time was 17.7 months (range, 2-24 months). Sites treated with SRT included the brain (71.4%), lung/chest (7.9%), bones (7.9%), spine (6.3%), and other (6.3%). OS was 74.4% versus 65.0% at 1 year and 71.0% versus 59.4% at 2 years for the IO + SRT and IO alone groups, respectively, although this difference did not reach statistical significance (log-rank P = .1077). In patients with BM, however, 1-year OS (73.0% vs 54.7%) and 2-year OS (70.8% vs 51.4%) was significantly higher in those receiving IO + SRT versus IO alone, respectively (pairwise P = .0261). Timing of SRT (before or after IO) did not influence OS (log-rank P = .3185).
Conclusions
Patients with BM secondary to mRCC had prolonged OS with the addition of SRT to IO. Factors such as International mRCC Database Consortium risk stratification, oligometastatic tumor burden, SRT dose/fractionation, and utilization of doublet therapy should be considered in future analyses to better identify patients who may benefit from combined IO + SRT. Further prospective studies are warranted.
Introduction
Immunotherapy (IO) is a first-line option for the management of metastatic renal cell carcinoma (mRCC).1,2 Historically, interleukin 2 and other immunologic agents have been successful in the management of mRCC, whereas modern trials have demonstrated improved responses with immune checkpoint inhibition (ICI) alone or in combination with tyrosine kinase inhibitors.3, 4, 5, 6, 7, 8, 9, 10 Evidence has suggested that responses to IO may be potentiated by stereotactic radiation therapy (SRT), which is a topic of growing interest due to the synergistic potential of combined modality therapy.11, 12, 13, 14, 15, 16 As opposed to conventionally fractionated radiation therapy (CFRT), SRT is a technique allowing for the delivery of highly conformal and ablative doses of radiation in typically 5 or fewer fractions. Larger doses per fraction with SRT may produce immunomodulatory effects which enhance IO responses and overcome radioresistance.17 For example, SRT is capable of promoting the expansion of tumor-antigen specific T cells and increasing T-cell effector function.15 SRT may also increase the immunogenicity of tumor cells by enhancing expression of MHC-I on the surface of tumors, making them more susceptible to T-cell-mediated killing.18 RCC has traditionally been viewed as a radioresistant tumor subtype.19 The mechanism driving resistance to CFRT implicates high expression of transcription factor hypoxia-inducible factor 1-alpha (HIF1A), which blocks endothelial cell apoptosis in the tumor microenvironment.20,21 SRT can overcome this radioresistant pathway by preventing upregulation of HIF1A and inducing a wave of endothelial apoptosis.22,23 Initiation of this apoptotic signaling cascade is not seen with CFRT and may, in contrast, explain the excellent local control (LC) rates demonstrated in RCC treated with SRT.24 Radiobiologically, cell survival experiments have also found RCC to be a low alpha/beta malignancy and thus more susceptible to higher doses per fraction as delivered with SRT.25
From a clinical standpoint, the use of SRT with standard systemic therapy options for metastatic cancers has demonstrated improved survival outcomes in several randomized trials.26, 27, 28, 29 These results have spawned significant interest in identifying populations which may benefit from the integration of SRT with systemic therapy. Interest in combined modality therapy has been particularly evident in mRCC due to the first-line use of IO agents, compelling biological rationale for combination therapy, and high rates of LC (>90%) observed for primary and metastatic RCC lesions treated with SRT.30 Recent phase 1/2 clinical trials in patients with mRCC are encouraging and have revealed excellent LC, delayed progression in those receiving combined IO + SRT, and minimal treatment-associated toxicities.31, 32, 33 Although these initial experiences are favorable, challenges remain in the selection of appropriate patients, as well as the optimal sequencing of IO and SRT. Additionally, conclusions regarding overall survival (OS) with IO + SRT are difficult to establish based on low sample sizes and lack of statistical power to demonstrate OS benefits in early clinical trials. Given this paucity of data, we conducted a retrospective review of the National Cancer Database (NCDB) to describe outcomes in patients with mRCC receiving IO + SRT versus IO alone. We hypothesized that patients who received IO + SRT would have prolonged OS compared with those receiving IO alone.
Methods and Materials
Data source
The NCDB was reviewed to identify patients who received diagnoses of mRCC from 2012 to 2017. Data were obtained from the 2017 NCDB participant user file. The NCDB is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC's NCDB and the hospitals participating in it are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Patient cohort
Eligible patients had an initial diagnosis of stage IV RCC with intact renal primary tumors treated with first-line IO and had at least 2 months of follow-up time from diagnosis. Patients who received SRT as part of their first course of treatment were included in the IO + SRT cohort. The dose/fractionation definition of SRT was as follows: ≥10 Gy in 1 fraction, ≥20 Gy in 2 fractions, ≥24 Gy in 3 fractions, or ≥25 Gy in 5 fractions. Patients who underwent conventional (non-SRT) radiation therapy courses (n = 175) were included in the IO alone cohort. Patients with incomplete data regarding demographics, therapy, tumor stage, and/or follow-up time were excluded. Patient characteristics collected included age at diagnosis, sex, race/ethnicity, Charlson-Deyo score, median income, and facility type. Disease and treatment characteristics collected included primary tumor size, tumor grade, number of involved metastatic sites, cytoreductive nephrectomy (CN) status, and details of radiation dose/fractionation. Screening criteria for study inclusion are outlined in Fig. 1.
Figure 1.
Screening criteria for study inclusion. Consolidated Standards of Reporting Trials (CONSORT) diagram describing the selection of patients with stage IV renal cell carcinoma from the National Cancer Database (NCDB) who were treated with immunotherapy (IO) alone or with combined IO and stereotactic radiation therapy (SRT).
Outcomes and statistical analysis
The primary endpoint was OS stratified by the receipt of SRT (IO + SRT vs IO alone). Survival time was calculated from the date of stage IV diagnosis to last contact or death. Events that occurred beyond 24 months were censored due to the limited expected follow-up time and low number of events to be captured beyond this time point. Secondary endpoints included OS stratified by the receipt of SRT and presence of brain metastases (BM) as well as OS stratified by the timing of IO (before or after SRT) and CN status. An additional OS analysis of all patients was performed to evaluate survival stratified by the total number of organ systems involved by metastasis (brain, bone, liver, lung, distant lymph nodes, or other sites).
Baseline characteristics were compared using χ2 tests for categorical variables based on the treatment cohort. Summary statistics were provided for dose/fractionation of radiation therapy. Survival was estimated using Kaplan-Meier methodology and compared via the log-rank test. Adjustments for multiple comparisons and pairwise post hoc analyses were performed. Analyses were performed using SAS statistical software (version 9.4; SAS Institute Inc, Cary, NC). This NCDB study was exempt from the local institutional review board.
Results
In total, 644 eligible patients were identified from the NCDB, consisting of 63 patients (9.8%) who received IO + SRT and 581 (90.2%) patients who received IO alone. Patient and treatment characteristics are described in Tables 1 and 2, respectively. The median follow-up time was 17.7 months (range, 2-24 months). Baseline characteristics were similar between cohorts; however, IO + SRT patients were generally younger (P = .0488), and IO alone patients had larger primary tumors (P < .0001). None of the patients undergoing SRT, compared with 32 patients (5.5%) treated with IO alone, were noted to have a Charlson-Deyo score of 3. Patients who received conventional radiation therapy were included in the IO alone cohort (n = 175), and receipt of conventional radiation therapy did not significantly affect survival within this cohort (log-rank P = .5623). Patients undergoing SRT were significantly more likely to have BM (71.4% vs 11.2%; P < .0001); however, there was no difference in the rates of metastasis to other extracranial sites (bone, liver, lung, distant lymph node, or other). There was also no difference in the total number of organ systems involved by metastasis between cohorts (P = .4826). OS for the entire cohort of patients was stratified by the number of involved organ systems (Fig. 2) and revealed a significant association between survival and number of organ systems involved by metastasis (log-rank P = .0007). Sites treated with SRT included the brain (71.4%), chest/lung (7.9%), nonspine bone (7.9%), spine (6.3%), and other (6.3%).
Table 1.
Patient characteristics
| Treatment | IO (n = 581, 90.2%) |
IO + SRT (n = 63, 9.78%) |
P value |
|---|---|---|---|
| Age at diagnosis | .0488 | ||
| <50 | 62 (10.67) | 13 (20.63) | |
| 50-59 | 172 (29.6) | 23 (36.51) | |
| 60-69 | 182 (31.33) | 16 (25.4) | |
| 70-79 | 127 (21.86) | 7 (11.11) | |
| >80 | 38 (6.54) | 4 (6.35) | |
| Sex | .2861 | ||
| Male | 415 (71.43) | 49 (77.78) | |
| Female | 166 (28.57) | 14 (22.22) | |
| Race | .1115 | ||
| White | 510 (87.78) | 54 (85.71) | |
| Black | 47 (8.09) | 3 (4.76) | |
| Other races | 24 (4.13) | 6 (9.52) | |
| Charlson-Deyo score | .1343 | ||
| 0 | 437 (75.22) | 53 (84.13) | |
| 1 | 82 (14.11) | 9 (14.29) | |
| 2 | 30 (5.16) | 1 (1.59) | |
| 3 | 32 (5.51) | 0 (0) | |
| Income | .4246 | ||
| 0-25th quartile | 87 (16.83) | 4 (8) | |
| 26th to median | 85 (16.44) | 10 (20) | |
| 51st-75th quartile | 150 (29.01) | 15 (30) | |
| >75th percentile | 195 (37.72) | 21 (42) | |
| Facility type | .066 | ||
| Community cancer program | 40 (7.1) | 3 (5) | |
| Comprehensive community cancer program | 190 (33.75) | 11 (18.33) | |
| Academic/research program | 253 (44.94) | 34 (56.67) | |
| Integrated network cancer program | 80 (14.21) | 12 (20) | |
| Primary tumor size (mm) | <.0001 | ||
| 0-40 | 4 (0.69) | 5 (7.94) | |
| 40-70 | 26 (4.48) | 7 (11.11) | |
| 70-100 | 28 (4.82) | 7 (11.11) | |
| >100 | 523 (90.02) | 44 (69.84) | |
| Tumor grade | .5725 | ||
| 1 | 3 (1.07) | 0 (0) | |
| 2 | 51 (18.21) | 4 (12.12) | |
| 3 | 121 (43.21) | 13 (39.39) | |
| 4 | 105 (37.5) | 16 (48.48) | |
| Cytoreductive nephrectomy performed | .1201 | ||
| No | 327 (56.28) | 29 (46.03) | |
| Yes | 254 (43.72) | 34 (53.97) | |
| Brain metastasis at diagnosis | <.0001 | ||
| No | 516 (88.81) | 18 (28.57) | |
| Yes | 65 (11.19) | 45 (71.43) | |
| Bone metastasis at diagnosis | .3567 | ||
| No | 314 (56.68) | 16 (48.48) | |
| Yes | 240 (43.32) | 17 (51.52) | |
| Liver metastasis at diagnosis | .8509 | ||
| No | 464 (83.6) | 28 (84.85) | |
| Yes | 91 (16.4) | 5 (15.15) | |
| Lung metastasis at diagnosis | .1543 | ||
| No | 201 (36.48) | 8 (24.24) | |
| Yes | 350 (63.52) | 25 (75.76) | |
| Distant lymph node metastasis at diagnosis | .5268 | ||
| No | 406 (73.82) | 26 (78.79) | |
| Yes | 144 (26.18) | 7 (21.21) | |
| Metastasis at other site(s) at diagnosis | .3561 | ||
| No | 422 (76.73) | 23 (69.7) | |
| Yes | 128 (23.27) | 10 (30.3) | |
| Organ systems involved by metastatic disease at diagnosis | .4826 | ||
| 1 | 300 (51.6) | 37 (58.7) | |
| 2 | 163 (28.1) | 12 (19.0) | |
| 3 | 89 (15.3) | 10 (15.9) | |
| ≥4 | 29 (5.0) | 4 (6.3) |
Abbreviations: IO = Immunotherapy; SRT = stereotactic radiation therapy.
Table 2.
Treatment characteristics
| Site treated | Median dose (range) | Median number of fractions (range) |
|---|---|---|
| Brain (n = 45) | 22 Gy (10-40) | 1 (1-5) |
| Chest/lung (n = 5) | 35 Gy (25-50) | 5 (1-5) |
| Nonspine bone (n = 5) | 25 Gy (25-30) | 5 (2-5) |
| Spine (n = 4) | 25.5 Gy (18-30) | 1 (1-5) |
| Other (n = 4)* | 30 Gy (25-50) | 5 |
Includes soft tissue, abdomen, pelvis, and nonspecified sites.
Figure 2.
Overall survival of all 644 patients undergoing immunotherapy for metastatic renal cell carcinoma. Patients were stratified by the number of organ systems involved by metastatic disease.
The primary endpoint of OS was not significantly different between patients with mRCC receiving IO + SRT compared with those receiving IO alone (log-rank P = .1077; Fig. 3). It was observed, however, that 1-year survival was higher for patients receiving IO + SRT (74.4%; 95% confidence interval [CI], 61.6-83.5) compared with IO alone (65.0%; 95% CI, 60.9-68.8). Additionally, the rate of survival at 2 years remained greater in the IO + SRT group (71.0%; 95% CI, 57.9-80.6) compared with IO alone (59.4%; 95% CI, 55.1-63.3). Patient age did not significantly affect OS in IO + SRT cohort (log-rank P = .7615). When stratified for the presence of BM, OS was significantly increased in patients with BM receiving IO + SRT compared with IO alone (pairwise log-rank P = .0261; Fig. 4). The 1-year survival of patients with BM receiving IO + SRT was 73.0% (95% CI, 57.4-83.7) compared with 54.7% (95% CI, 41.8-65.9) in the IO alone cohort. This survival advantage in patients with BM was maintained at 2 years, with the IO + SRT cohort achieving 70.8% survival (95% CI, 55.0-81.9) compared with 51.4% (95% CI, 38.5-62.8) in the IO alone group. As we demonstrated that an increasing number of organ systems involved by metastasis was a negative indicator of OS, analysis of the number of organ systems involved by metastasis in patients with or without BM was then performed (Table E1). For all patients, those with BM were more likely to have a greater number of organ systems involved by mRCC (P < .0001). However, in patients who received IO + SRT, there was no difference in the number of organ systems involved by metastasis when stratified by the presence of BM (P = .3644).
Figure 3.
Overall survival of patients stratified by treatment type: immunotherapy (IO) alone or IO and stereotactic radiation therapy (SRT).
Figure 4.
Overall survival stratified by treatment type, either immunotherapy (IO) alone or IO and stereotactic radiation therapy (SRT). Patients were additionally stratified by brain metastases presence (BM+) or absence (BM–), and survival was analyzed via multiple comparisons and pairwise post hoc analysis (P value shown).
Pairwise comparisons revealed no OS differences in patients without BM who received IO + SRT versus IO alone (P = .9587) or in patients with BM who received IO + SRT versus those without BM who received IO + SRT (P = .5719). The IO + SRT cohort (n = 63) was further stratified to determine whether initiating SRT treatment first (n = 48/63; 76.2%) or IO treatment first (n = 15/63, 23.8%) affected OS (Fig. E1). No significant difference in OS was observed in patients receiving IO + SRT who began their treatment course with SRT or IO (log-rank P = .3185). Patients in the IO alone and IO + SRT cohorts were additionally stratified by CN status (Fig. E2). Receipt of CN was associated with improved OS in both the IO alone and IO + SRT cohorts (log-rank P < .0001 for both groups). Patients from the IO + SRT cohort who underwent CN had a trend toward improved OS compared with patients who underwent IO alone CN, although this was not statistically significant (log-rank P = .0690).
Discussion
In this large retrospective review of hospital-based outcomes from the NCDB, we found that there was no significant OS difference in patients with de novo mRCC receiving IO + SRT versus IO alone in the first-line setting. It was observed that rates of 1- and 2-year survival were higher in patients receiving IO + SRT (74.4% and 71.0%, respectively) compared with IO alone (65.0% and 59.4%, respectively), although these findings did not reach statistical significance (log-rank P = .1077). Patients with BM, however, were found to have significantly higher rates of survival at 1 and 2 years when treated with IO + SRT (73.0% and 70.8%, respectively) versus IO alone (54.7% and 51.4%, respectively). The timing of IO (before or after SRT) did not appear to influence OS. When stratified by the number of organ systems involved by metastasis, OS was expectedly lower in patients with an increasing number of organ systems involved by mRCC. When stratified by receipt of CN, patients who underwent CN had improved OS, consistent with prior NCDB analyses in patients with mRCC.34 Rates of OS in our cohort were comparable to reported outcomes of patients with mRCC undergoing modern IO-based systemic therapy strategies.33,35,36
The role of IO is well established in the management of mRCC, and although an underlying immunologic rationale for combining IO + SRT is apparent, it is unclear if this will translate to improved clinical outcomes. Although our retrospective analysis did not reveal a statistically significant OS benefit in unselected patients with mRCC treated with IO + SRT, we noted consistently higher OS rates at 1 and 2 years compared with those who received IO alone. Patients undergoing IO + SRT had disproportionately higher rates of BM at initial diagnosis (71.4% vs 11.2%, respectively), and despite the association of BM with poor survival in mRCC, no significant difference in OS was observed.37 Patients in each cohort otherwise had a similar number of organ systems involved by metastasis, and those without BM who received IO + SRT did not have prolonged OS compared with those with BM who received IO + SRT. In addition, patients receiving IO + SRT for BM had a similar total number of organ systems involved by mRCC compared with those receiving IO + SRT without BM, suggesting that the survival benefit in patients with BM treated with IO + SRT is not dependent on these patients having fewer organ systems involved by metastasis. Given that every patient with BM in the IO + SRT cohort underwent cranial SRT, these findings suggest that intracranial control provided by SRT may normalize survival to that of patients without BM. Moreover, in selected patients with BM, those undergoing IO + SRT were found to have significantly higher rates of OS at 1 and 2 years (by approximately 20%) compared with those undergoing IO alone with or without CFRT. These findings suggest that BM secondary to mRCC respond more favorably to SRT, which in turn may lead to prolonged OS. Our results are consistent with a large series by Wardak et al, who found that in 268 patients with mRCC BM managed with SRT, rates of LC were >90%, and over half of patients (57.5%) survived beyond 1 year.38 Generally, SRT for BM secondary to RCC is preferred (if feasible) due to the relative radioresistance to CFRT, high observed LC rates, decreased neurotoxicity compared with whole brain radiation therapy, and shorter treatment duration.30,35 Intracranial control is particularly important because IO and other systemic therapy agents may have variable CNS penetrance.39,40 High biologically effective doses delivered with SRT may not only improve LC but also potentially disrupt the immune microenvironment of BM, promote antitumor immunity, and lead to enhanced extracranial responses to systemic therapy.39,41 Although such abscopal responses are rare, several case reports have documented extracranial responses after SRT for BM, more commonly in patients treated with IO.42 Outcomes of patients in the IO + SRT cohort may also be explained by their younger age, absence of those with a Charlson-Deyo comorbidity score of >2, and smaller primary tumor size.43 Additionally, a significant confounding factor may be that patients eligible for cranial SRT had fewer BM at diagnosis and, therefore, a more favorable expected prognosis.44 To that end, however, there is no consensus on the upper limit of BM that may benefit from local treatment with SRT.45 For example, in the series by Wardak et al, patients with up to 26 BM received cranial SRT, and no survival difference was observed in those with <5 BM or ≥5 BM.38 Similarly, we found that aggressive local therapy to the primary tumor with CN was observed to have a significant survival benefit, with a trend toward improved OS if IO + SRT + CN was undertaken compared with IO + CN alone. Our results are consistent with prior literature on this topic and are again subject to significant selection bias as patients who undergo CN in mRCC tend to be younger with fewer comorbidities and with less advanced systemic disease.34
Although our results are encouraging and highlight several patient-, disease-, and treatment-related factors that may influence outcomes in mRCC, it is difficult to make definitive recommendations for IO + SRT based on limited retrospective data alone. Fortunately, 2 recently published prospective mRCC trials provide further insight into combined SRT and IO, chiefly with ICI. The RAPPORT trial was a single-arm phase 1/2 prospective trial in 30 patients with oligometastatic clear cell RCC, which evaluated SRT (20 Gy in 1 fraction) to all amenable metastatic sites followed by single-agent pembrolizumab.32 This approach yielded encouraging 1- and 2-year LC of 94% and 92%, OS of 90% and 74%, and progression-free survival of 60% and 45%, respectively. Minimal toxicity was reported, with only 13% of patients reporting grade 3 adverse events and no observed grade 4 or 5 adverse events. In contrast to the patients in our unselected mRCC cohort, those in the RAPPORT trial were strictly oligometastatic, none received SRT for BM, and none had de novo disease at time of diagnosis. The more favorable OS observed in these patients may be due to these relevant differences in disease characteristics. Similar in design, the NIVES study was a single-arm phase 2 trial which evaluated nivolumab followed by extracranial SRT in 69 patients with mRCC who failed to respond to antiangiogenic therapy.33 This trial demonstrated that combined modality therapy was well tolerated but did not find an improvement of objective response rates with SRT compared with historical controls of nivolumab alone. Patients in the NIVES study, however, were higher risk than those in the RAPPORT trial, as those with nonclear cell histology and widely metastatic disease were eligible for study inclusion. Additionally, the trial only required a single extracranial site to be amenable to SRT (30 Gy in 3 fractions) and did not mandate treatment of all metastatic sites. In terms of the sequencing of IO with SRT, pembrolizumab was started 2 to 8 days after SRT in the RAPPORT study, while nivolumab was started 7 days before SRT in the NIVES trial. In our cohort of patients undergoing IO + SRT, the timing of IO (before or after SRT) was not associated with a difference in OS. Although there is no consensus on the ideal sequencing of SRT with IO, experiments in mouse models support concomitant rather than sequential therapy for optimal priming of T-cell responses to ICI and improving survival.46 The aforementioned studies add invaluable data to better understanding combined IO + SRT in mRCC but are limited by their small sample sizes, differences in patient populations, absence of comparator arms, lack of modern doublet therapies, variability in total metastatic ablation with SRT, and nonstandardized SRT dose/fractionation. The results of other modern prospective studies combining dual ICI with SRT, such as RAD VAX RCC (ClinicalTrials.gov identifier: NCT03065179), CYTOSHRINK (ClinicalTrials.gov identifier: NCT04090710), and SAMURAI (ClinicalTrials.gov identifier: NCT05327686), will continue to provide further information regarding the value of combined modality approaches in mRCC. In fact, CYTOSHRINK aims to randomize patients to dual ICI (ipilimumab and nivolumab) with or without SRT (30-40 Gy in 5 fractions) to the primary tumor.47 This approach is particularly appealing as the presence of an arm receiving IO alone will improve the reliability of results rather than comparing outcomes to historical controls.
Although the goal of this retrospective analysis was to evaluate OS in patients receiving IO + SRT, other endpoints (outside of the scope of this retrospective review), such as the decision to change the systemic therapy strategy and quality of life, should also be considered in future studies. For example, in patients with oligometastatic RCC, LC with SRT may allow patients with indolent progression to delay initiating systemic therapy and maintain their quality of life.48 In patients with more advanced RCC, LC of a dominant area of progression with SRT may also prevent symptomatic complications and/or delay changing to potentially more toxic next-line systemic therapy. In a recently published prospective phase 2 study by Cheung et al, 37 patients with mRCC underwent SRT to oligoprogressive sites while on tyrosine kinase inhibitor therapy.49 With this approach, LC was 93%, no grade 3 or greater toxicities were noted, and approximately half of patients (53%) did not have to change their systemic therapy strategy 1 year after SRT. The financial toxicity of starting or changing to next-line systemic therapy cannot be understated, as SRT may be less costly depending on the type and length of systemic therapy, especially when considering that the total costs associated with modern IO regimens in the United States are routinely in the hundreds of thousands of dollars.50
There are several limitations to this study, chiefly its retrospective nature, which introduces selection bias and limits the ability to draw reliable conclusions on the causal effect of SRT on clinical outcomes. Although NCDB data are collected from CoC-accredited hospitals, data entry is often incomplete or inconsistent and may lead to errors in data interpretation. For example, oligometastatic tumor burden (total number of metastases) is not reliably captured in the NCDB and may significantly bias outcomes in a metastatic patient population receiving local therapy with SRS. However, we did note that there was no statistically significant difference in the number of organ systems involved by metastasis between the IO alone and IO + SRT cohorts (P = .4826), possibly limiting this confounding effect on OS analyses. Other limitations include the relatively small sample size of patients undergoing IO + SRT (n = 63/644, 9.8%), heterogeneous patient population, and lack of indication for SRT. Additionally, the NCDB does not contain specifics regarding International mRCC Database Consortium risk categorization, radiation therapy delivery or related toxicity, IO mechanism of action, or subsequent courses of therapy after progression. In addition, the patients in our study received diagnoses from 2012 to 2017 and may not be generalizable to those diagnosed later, when more modern IO regimens would be commonly used. For example, in the period of our analysis, patients could have received a variety of IO agents, including IL-2, IFN-ɑ, ICI, or a combination of these therapies. We propose that future prospective studies account for these limitations to better understand which patients would derive the greatest benefit from IO + SRT.
Conclusion
In this retrospective NCDB analysis of patients with mRCC undergoing first-line IO, SRT in conjunction with IO did not improve OS compared with IO alone. Although survival was generally more favorable in those undergoing combined modality therapy, this finding did not reach statistical significance. In patients with BM, however, IO + SRT led to significantly improved rates of OS at 1 and 2 years. The results of this study, paired with the encouraging prospective outcomes of recent clinical trials, demonstrate the need for further randomized prospective studies investigating the potential advantages of IO + SRT in mRCC. We propose that patients with BM be included in future studies and that factors such as International mRCC Database Consortium risk stratification, oligometastatic tumor burden, multisite SRT, and the use of doublet therapy be considered in further analyses. Biomarker and immunologic assays may provide further insight into the interplay between IO + SRT and help guide the selection of patients who may benefit from combined therapy.
Footnotes
Sources of support: This project was supported by the Washington University Institute of Clinical and Translational Sciences, which is, in part, supported by the National Institutes of Health/National Center for Advancing Translational Sciences Clinical and Translational Science Awards (grant #UL1 TR002345).
Disclosures: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.adro.2023.101238.
Appendix. Supplementary materials
References
- 1.Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:706–720. doi: 10.1093/annonc/mdz056. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network. National clinical guidelines in oncology - Kidney cancer. 2022. Version 4.2022. https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf. Accessed January 5, 2022.
- 3.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380:1116–1127. doi: 10.1056/NEJMoa1816714. [DOI] [PubMed] [Google Scholar]
- 4.Powles T, Plimack ER, Soulières D, et al. Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): Extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol. 2020;21:1563–1573. doi: 10.1016/S1470-2045(20)30436-8. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021;384:829–841. doi: 10.1056/NEJMoa2026982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med. 2021;384:1289–1300. doi: 10.1056/NEJMoa2035716. [DOI] [PubMed] [Google Scholar]
- 8.Motzer RJ, Escudier B, George S, et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: Updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer. 2020;126:4156–4167. doi: 10.1002/cncr.33033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee CH, Shah AY, Rasco D, et al. Lenvatinib plus pembrolizumab in patients with either treatment-naive or previously treated metastatic renal cell carcinoma (Study 111/KEYNOTE-146): A phase 1b/2 study. Lancet Oncol. 2021;22:946–958. doi: 10.1016/S1470-2045(21)00241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDermott DF, Lee JL, Bjarnason GA, et al. Open-label, single-arm phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced clear cell renal cell carcinoma. J Clin Oncol. 2021;39:1020–1028. doi: 10.1200/JCO.20.02363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng J, Lalani AK, Swaminath A. Cytoreductive stereotactic body radiotherapy (SBRT) and combination SBRT with immune checkpoint inhibitors in metastatic renal cell carcinoma. Can Urol Assoc J. 2021;15:281–286. doi: 10.5489/cuaj.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ukleja J, Kusaka E, Miyamoto DT. Immunotherapy combined with radiation therapy for genitourinary malignancies. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.663852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buttigliero C, Allis S, Tucci M, et al. Role of radiotherapy in improving activity of immune-modulating drugs in advanced renal cancer: Biological rationale and clinical evidences. Cancer Treat Rev. 2018;69:215–223. doi: 10.1016/j.ctrv.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Gupta A, Probst HC, Vuong V, et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol. 2012;189:558–566. doi: 10.4049/jimmunol.1200563. [DOI] [PubMed] [Google Scholar]
- 15.Sharabi AB, Nirschl CJ, Kochel CM, et al. Stereotactic radiation therapy augments antigen-specific PD-1-mediated antitumor immune responses via cross-presentation of tumor antigen. Cancer Immunol Res. 2015;3:345–355. doi: 10.1158/2326-6066.CIR-14-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marciscano AE, Haimovitz-Friedman A, Lee P, et al. Immunomodulatory effects of stereotactic body radiation therapy: Preclinical insights and clinical opportunities. Int J Radiat Oncol Biol Phys. 2021;110:35–52. doi: 10.1016/j.ijrobp.2019.02.046. [DOI] [PubMed] [Google Scholar]
- 18.Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–1271. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deschavanne PJ, Fertil B. A review of human cell radiosensitivity in vitro. Int J Radiat Oncol Biol Phys. 1996;34:251–266. doi: 10.1016/0360-3016(95)02029-2. [DOI] [PubMed] [Google Scholar]
- 20.Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8:89–91. doi: 10.1016/j.ccr.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Stinauer MA, Kavanagh BD, Schefter TE, et al. Stereotactic body radiation therapy for melanoma and renal cell carcinoma: Impact of single fraction equivalent dose on local control. Radiat Oncol. 2011;6:34. doi: 10.1186/1748-717X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 23.De Meerleer G, Khoo V, Escudier B, et al. Radiotherapy for renal-cell carcinoma. Lancet Oncol. 2014;15:e170–e177. doi: 10.1016/S1470-2045(13)70569-2. [DOI] [PubMed] [Google Scholar]
- 24.Siva S, Louie AV, Warner A, et al. Pooled analysis of stereotactic ablative radiotherapy for primary renal cell carcinoma: A report from the International Radiosurgery Oncology Consortium for Kidney (IROCK) Cancer. 2018;124:934–942. doi: 10.1002/cncr.31156. [DOI] [PubMed] [Google Scholar]
- 25.Ning S, Trisler K, Wessels BW, et al. Radiobiologic studies of radioimmunotherapy and external beam radiotherapy in vitro and in vivo in human renal cell carcinoma xenografts. Cancer. 1997;80:2519–2528. doi: 10.1002/(sici)1097-0142(19971215)80:12+<2519::aid-cncr26>3.3.co;2-t. [DOI] [PubMed] [Google Scholar]
- 26.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: Long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol. 2020;38:2830–2838. doi: 10.1200/JCO.20.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez DR, Tang C, Zhang J, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: Long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019;37:1558–1565. doi: 10.1200/JCO.19.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iyengar P, Wardak Z, Gerber DE, et al. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: A phase 2 randomized clinical trial. JAMA Oncol. 2018;4 doi: 10.1001/jamaoncol.2017.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang XS, Bai YF, Verma V, et al. Randomized trial of first-line tyrosine kinase inhibitor with or without radiotherapy for synchronous oligometastatic EGFR-mutated NSCLC [e-pub ahead of print]. J Natl Cancer Inst. doi:10.1093/jnci/djac015, accessed May 5, 2022.
- 30.Zaorsky NG, Lehrer EJ, Kothari G, et al. Stereotactic ablative radiation therapy for oligometastatic renal cell carcinoma (SABR ORCA): A meta-analysis of 28 studies. Eur Urol Oncol. 2019;2:515–523. doi: 10.1016/j.euo.2019.05.007. [DOI] [PubMed] [Google Scholar]
- 31.Correa RJM, Louie AV, Zaorsky NG, et al. The emerging role of stereotactic ablative radiotherapy for primary renal cell carcinoma: A systematic review and meta-analysis. Eur Urol Focus. 2019;5:958–969. doi: 10.1016/j.euf.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Siva S, Bressel M, Wood ST, et al. Stereotactic radiotherapy and short-course pembrolizumab for oligometastatic renal cell carcinoma: The RAPPORT trial. Eur Urol. 2022;81:364–372. doi: 10.1016/j.eururo.2021.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Masini C, Iotti C, De Giorgi U, et al. Nivolumab in combination with stereotactic body radiotherapy in pretreated patients with metastatic renal cell carcinoma. Results of the phase II NIVES study. Eur Urol. 2022;81:274–282. doi: 10.1016/j.eururo.2021.09.016. [DOI] [PubMed] [Google Scholar]
- 34.Hanna N, Sun M, Meyer CP, et al. Survival analyses of patients with metastatic renal cancer treated with targeted therapy with or without cytoreductive nephrectomy: A National Cancer Data Base study. J Clin Oncol. 2016;34:3267–3275. doi: 10.1200/JCO.2016.66.7931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rathmell WK, Rumble RB, Van Veldhuizen PJ, et al. Management of metastatic clear cell renal cell carcinoma: ASCO guideline. J Clin Oncol. 2022;40:2957–2995. doi: 10.1200/JCO.22.00868. [DOI] [PubMed] [Google Scholar]
- 36.Nguyen EK, Lalani AKA, Ghosh S, et al. Outcomes of radiation therapy plus immunotherapy in metastatic renal cell carcinoma: Results from the Canadian Kidney Cancer Information System. Adv Radiat Oncol. 2022;7 doi: 10.1016/j.adro.2022.100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vickers MM, Al-Harbi H, Choueiri TK, et al. Prognostic factors of survival for patients with metastatic renal cell carcinoma with brain metastases treated with targeted therapy: Results from the International Metastatic Renal Cell Carcinoma Database Consortium. Clin Genitourin Cancer. 2013;11:311–315. doi: 10.1016/j.clgc.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 38.Wardak Z, Christie A, Bowman A, et al. Stereotactic radiosurgery for multiple brain metastases from renal-cell carcinoma. Clin Genitourin Cancer. 2019;17:e273–e280. doi: 10.1016/j.clgc.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berghoff AS, Venur VA, Preusser M, et al. Immune checkpoint inhibitors in brain metastases: From biology to treatment. Am Soc Clin Oncol Educ Book. 2016;35:e116–e122. doi: 10.1200/EDBK_100005. [DOI] [PubMed] [Google Scholar]
- 40.Rubino S, Oliver DE, Tran ND, et al. Improving brain metastases outcomes through therapeutic synergy between stereotactic radiosurgery and targeted cancer therapies. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.854402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng L, Liang H, Burnette B, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gui C, Kleinberg LR, Lim M, et al. Extracranial abscopal responses after radiation therapy for intracranial metastases: A review of the clinical literature and commentary on mechanism. Cureus. 2019;11:e4207. doi: 10.7759/cureus.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volpe A, Patard JJ. Prognostic factors in renal cell carcinoma. World J Urol. 2010;28:319–327. doi: 10.1007/s00345-010-0540-8. [DOI] [PubMed] [Google Scholar]
- 44.Ali MA, Hirshman BR, Wilson B, et al. Survival patterns of 5750 stereotactic radiosurgery-treated patients with brain metastasis as a function of the number of lesions. World Neurosurg. 2017;107:944–951. doi: 10.1016/j.wneu.2017.07.062. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogelbaum MA, Brown PD, Messersmith H, et al. Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol. 2022;40:492–516. doi: 10.1200/JCO.21.02314. [DOI] [PubMed] [Google Scholar]
- 46.Dovedi SJ, Adlard AL, Lipowska-Bhalla G, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014;74:5458–5468. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 47.Lalani A-KA, Swaminath A, Pond GR, et al. 2023. Phase II trial of cytoreductive stereotactic hypofractionated radiotherapy with combination ipilimumab/nivolumab for metastatic kidney cancer (cytoshrink) Paper presented at: ASCO GU Cancers Symposium. February 16-18, 2023; San Fransisco, CA. [Google Scholar]
- 48.Tang C, Msaouel P, Hara K, et al. Definitive radiotherapy in lieu of systemic therapy for oligometastatic renal cell carcinoma: A single-arm, single-centre, feasibility, phase 2 trial. Lancet Oncol. 2021;22:1732–1739. doi: 10.1016/S1470-2045(21)00528-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheung P, Patel S, North SA, et al. Stereotactic radiotherapy for oligoprogression in metastatic renal cell cancer patients receiving tyrosine kinase inhibitor therapy: A phase 2 prospective multicenter study. Eur Urol. 2021;80:693–700. doi: 10.1016/j.eururo.2021.07.026. [DOI] [PubMed] [Google Scholar]
- 50.Shay R, Nicklawsky A, Gao D, et al. A cost-effectiveness analysis of nivolumab plus ipilimumab versus pembrolizumab plus axitinib and versus avelumab plus axitinib in first-line treatment of advanced renal cell carcinoma. Clin Genitourin Cancer. 2021;19 doi: 10.1016/j.clgc.2021.01.009. 370-370.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.