Abstract
Both combined hepatocellular-cholangiocarcinoma (cHCC-CCA) and cholangiolocarcinoma are rare primary liver cancers. cHCC-CCA is believed to originate from transformed hepatocellular carcinoma or liver stem/progenitor cells. Cholangiolocarcinoma is characterized by ductular reaction-like anastomosing cords and glands resembling cholangioles or canals containing hepatocellular carcinoma components and adenocarcinoma cells. According to the 2019 revision of the World Health Organization criteria, a subtype with stem cell features as a subclassification of cHCC-CCA was abolished for lack of conclusive evidence of the stem cell origin theory. That led to the classification of cholangiolocarcinoma with hepatocytic differentiation as cHCC-CCA. Consequently, cholangiolocarcinoma without hepatocytic differentiation is classified as a subtype of small-duct cholangiocarcinoma and is assumed to originate from the bile duct. Herein, we report the first case of double primary cHCC-CCA and cholangiolocarcinoma without hepatocytic differentiation in different hepatic segments of a cirrhotic liver. We believe this case supports the validity of the new World Health Organization criteria because the pathological finding of cHCC-CCA in this case shows the transformation of hepatocellular carcinoma to cholangiocarcinoma. Furthermore, this case may demonstrate that immature ductular cell stemness and mature hepatocyte cell stemness in hepatocarcinogenesis can coexist in the same environment. The results provide valuable insights into the mechanisms of growth, differentiation, and regulation of liver cancers.
Keywords: Bile duct neoplasms, Combined hepatocellular-cholangiocarcinoma, Cholangiolocarcinoma, Cirrhosis, Liver neoplasms
Graphical abstract
Introduction
In 2020, primary liver cancer was the third leading cause of death attributable to cancer worldwide. Hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (iCCA) are the most common types of primary liver cancer, accounting for approximately 75–85% and 5–10% of cases, respectively.1 Primary malignant liver tumors arise from the major constituent liver cells, hepatocytes, biliary epithelial cells, endothelial cells, or combinations of these cells with various mesenchymal cells. Some primary liver tumors may result from the malignant transformation of hepatic progenitor cells with differentiation along two different cell lineages, as hypothesized for combined hepatocellular-cholangiocarcinoma (cHCC-CCA).2 Among primary liver cancers, cHCC-CCA and cholangiolocarcinoma (CLC) are extremely rare tumors.1 According to Japan’s 22nd National Primary Liver Cancer Follow-up Survey, cHCC-CCA and CLC account for 1.0% and 0.5% of primary liver cancers, respectively.3 In the 2019 revision of the World Health Organization (WHO) classification of tumors and the fifth edition of the Digestive System Tumors, the classification of cHCC-CCA, iCCA, and CLC have been revised.4,5
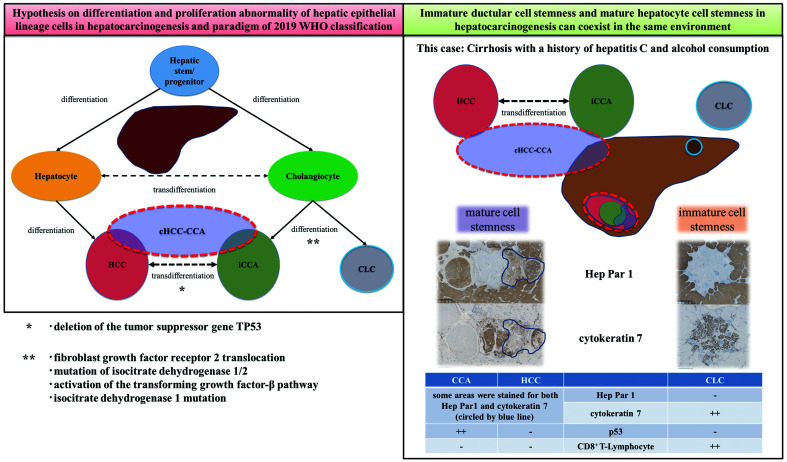
cHCC-CCA is a primary liver carcinoma with the unequivocal presence of both hepatocytic and cholangiocytic differentiation within the same tumor.4,6 The carcinogenesis mechanism of cHCC-CCA remains unclear. The fourth WHO classification refers to cHCC-CCA stem cell features based on the concept that the liver contains hepatic stem/progenitor cells that can differentiate into hepatocytes and bile duct epithelial cells even after the hepatocytes have matured.7,8 However, there is an association between a history of transarterial chemoembolization and cHCC-CCA development.9 Furthermore, it has been experimentally confirmed that hepatocytes can be transformed into cholangiocytes or progenitor cells.10,11 The cHCC-CCA subclassification based on stem/progenitor cell features was eliminated in the fifth WHO classification for lack of conclusive evidence of the stem cell origin theory.
CLC is categorized as a small bile duct cholangiocarcinoma subtype by the fifth WHO classification, defined as a ductular configuration without mucus production accounting for >80% of the tumor.5 CLC without hepatocytic differentiation, i.e. pure biliary type, is classified as small duct-type CCA, and CLC with hepatocytic differentiation is classified as cHCC-CCA. Additionally, cHCC-CCA may have a ductular configuration without mucus production, characteristic of CLC. However, it does not occupy >80% of the tumor. Steiner and Higginson reported the first few CLC cases in 1959, which were characterized by ductular reaction-like anastomosing cords and glands resembling cholangioles or canals containing HCC components and adenocarcinoma cells. The finding led to speculation that CLC originated from the canals of Hering or cholangioles occupied by hepatic progenitor cells.12,13 The fact that there have been no reports of the simultaneous presence of cHCC-CCA, iCCA, and pure biliary type CLC casts doubt on the hypothesis that the origin cell of these tumors is a progenitor. One reason could be that an accurate clinicopathologically diagnosis has not been obtained, i.e. it has gone unnoticed and unreported. Our report describes the first case of synchronous double primary cHCC-CCA and pure biliary-type CLC in different segments derived from cirrhosis. It demonstrates that pathomorphology is still as important as molecular biology, and is highly suggestive in consideration of the hepatocarcinogenesis mechanism.
Case report
A 70 year-old man was admitted to our hospital for further examination of a liver tumor. Four years before admission, he was administered a direct-acting antiviral agent for chronic hepatitis C and achieved sustained virologic response. The patient had consulted his family physician for alcoholic cirrhosis. His family history was unremarkable. He was asymptomatic because of sobriety and was not using any medication. Physical and neurological examinations, including hepatic encephalopathy revealed no abnormalities.
Laboratory tests showed a mild decrease in hepatic reserve and total bilirubin, 0.6 (range: 0.30–1.20) mg/dL, total protein, 6.6 (range: 6.7–8.3) g/dL, albumin, 3.8 (range: 4.1–5.1) g/dL, prothrombin time, 85.0% (range: 80–120%), and international normalized ratio, 1.08 (range: 0.90–1.13). His indocyanine green retention rate at 15 min was 17.5% (reference <10%), and his platelet and white blood cell counts were 8.5×103/µL and 4,100/µL, respectively. His Child-Pugh score was 5 and classified as class A. The following serum tumor markers were within their normal range: alpha-fetoprotein, 3.2 (reference <10) ng/mL; carcinoembryonic antigen, 3.91 (reference <5) ng/mL; CA-19-9 antigen, 8.62 (reference <37) U/mL. His des-gamma-carboxy prothrombin level of 129 (range: 10–39) mAU/mL was mildly elevated. The following laboratory test results were within their normal range: aspartate aminotransferase, 19 (range: 13–30) U/L; alanine aminotransferase, 9 (range: 7–23) U/L; alkaline phosphatase, 198 (range: 106–322) IU/L; gamma-glutamyl transpeptidase, 29 (range: 13–64 U/L; creatinine, 0.68 (range: 0.65–1.07) mg/dL. The patient’s Model for End-Stage Liver Disease (MELD) score was 7. The hepatitis B virus surface antigen results were negative. The hepatitis C virus antibody results were positive, but hepatitis C virus RNA was not detected by nested reverse-transcription polymerase chain reaction.
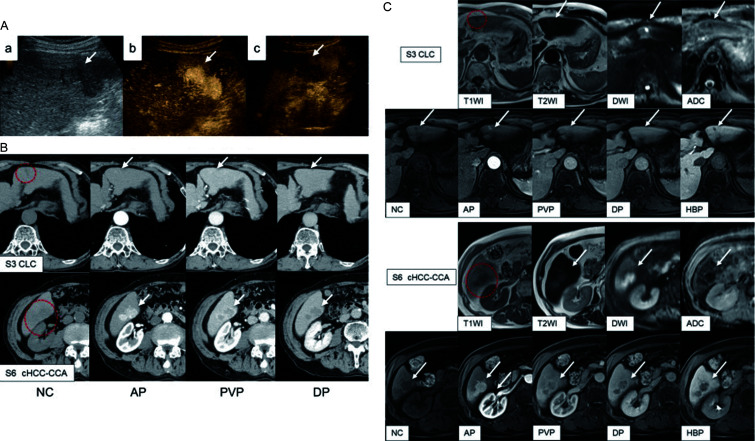
Abdominal contrast-enhanced ultrasonography revealed a 40×20 mm lobulated hypoechoic mass in liver segment 6 (S6). No tumor was detected in segment 3 (S3). Contrast-enhanced ultrasonography revealed a homogeneously hyperenhancing tumor during the arterial phase and a hypoenhancing tumor during the postvascular phase (Fig. 1A). Abdominal contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI) with gadolinium ethoxybenzyl diethylenetriamine penta-acetic acid enhancement were performed, revealing S6 tumor hypervascularity during the early phase, peritumoral enhancement during the portal venous phase, and washout during the delayed phase (Fig. 1B, C). The tumor also exhibited hypointensity during the hepatobiliary phase and hyperintensity on diffusion-weighted MRI (Fig. 1C). Additionally, the MRI revealed a small 10 mm mass in S3 with hypointensity during the hepatobiliary phase and hyperintensity on diffusion-weighted MRI. Hypervascularity was observed during the early phase of contrast-enhanced CT (Fig. 1B, C).
Fig. 1.
Imaging studies of the liver tumors. (A) Abdominal contrast-enhanced ultrasonography (CEUS) reveals the presence of a lobulated hypoechoic mass in the liver segment 6 (S6) with an approximate size of 40×20 mm (a). The segment 3 (S3) tumor could not be detected. The CEUS image reveals the tumor is homogeneous and hyperenhanced during the arterial phase (b) and hypoenhancing during the postvascular phase (c). (B) Abdominal contrast-enhanced computed tomography (CT) imaging of both tumors (red dotted circle and white arrows). The S3 tumor demonstrates slight hypoattenuation during the non-contrast (NC)-enhanced phase, partial hyperenhancement during the arterial phase (AP) up to the portal venous phase (PVP), and isoattenuation during the delayed phase (DP). The S6 tumor demonstrates hypoattenuation during the NC-enhanced phase, homogeneous hyperenhancement during the AP, peritumoral enhancement during the PVP, and washout during the DP. (C) Abdominal magnetic resonance imaging (MRI) findings of the two tumors (red dotted circle and white arrows). The S3 and S6 tumors demonstrate low signal intensity on T1-weighted MRI and sightly high intensity on T2-weighted MRI. Diffusion-weighted imaging (DWI) showed high intensity for both tumors. The apparent diffusion coefficient map created by conventional DWI showed the masses with decreased signal intensity, suggesting diffusion restriction of both tumors. These tumors showed hypointensity during the hepatobiliary phase. The S6 tumor demonstrated hyperintensity during the AP and peritumoral enhancement during the PVP. The S3 tumor demonstrated hyperintensity from the AP to the PVP.
The S6 tumor was diagnosed as a confluent multinodular-type HCC. The S3 tumor was diagnosed as a simple nodular-type HCC. A preoperative diagnosis of liver tumors was made: cT3, cN0, cM0, and stage IIIA per the TNM classification of malignant tumors. Subsequently, the patient underwent liver S3 and S6 subsegmentectomy and a cholecystectomy. As the patient had good liver reserve and performance status and this was the first HCC episode, surgery was selected because his accurate histological evaluation was considered desirable for future medical treatment.
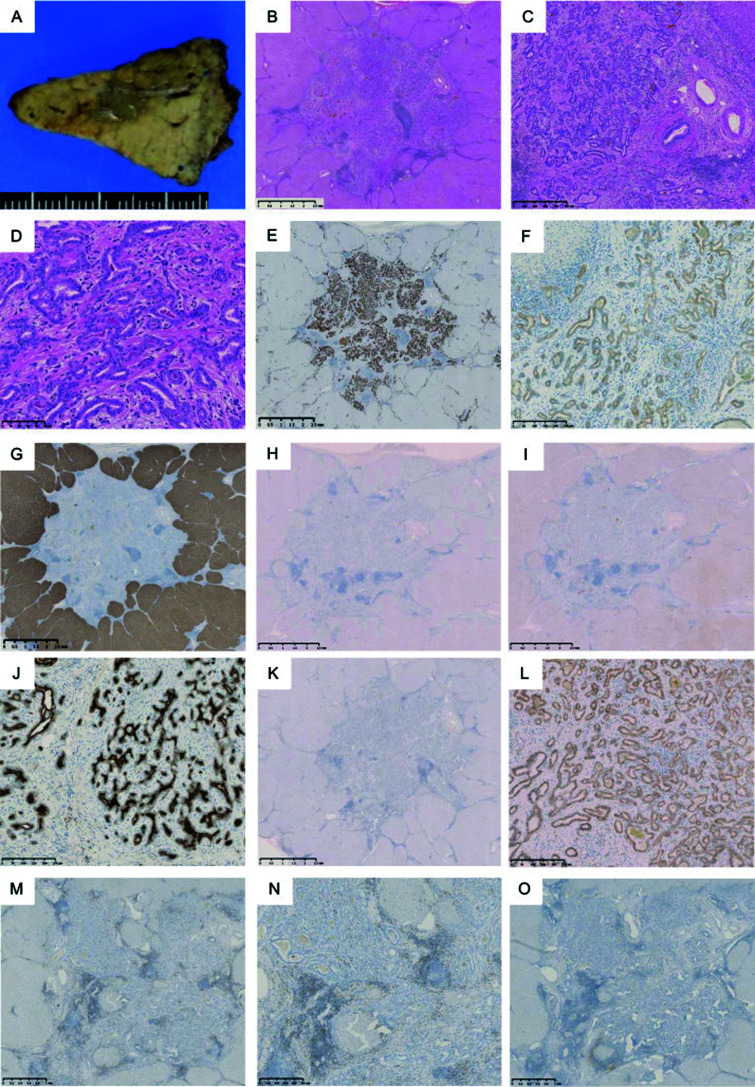
Macroscopically, the resected S3 tumor specimen was a light-green 11×6×7 mm nodule. The S6 tumor was mixed off-white and green 38×23×33 mm nodule (Figs. 2A and 3A). Microscopically, pathological examination revealed the S3 tumor was a pure biliary-type CLC, while the S6 tumor was cHCC-CCA. No cancerous emboli traces were found in the veins or lymph vessels of either tumor. The hepatic tissue adjacent to the tumors was cirrhotic (Figs. 2 and 3). The S3 tumor appeared as cells with round to oval nuclei that proliferated in a small gland anastomosing pattern without mucus production, mimicking ductular reactions with a background of abundant fibrous stroma (Fig. 2B–D). That tumor was immunohistologically positive for cytokeratin (CK) 7 and CK19 (Fig. 2E and F). It was negative for hepatocyte markers, including hepatocyte paraffin 1 (Hep Par 1), glypican 3, and CD10 (Fig. 2G–I). Epithelial membrane antigen staining results of the glandular lumen were positive (Fig. 2J). Additionally, p53-immunopositive cells and nuclear β-catenin expression were not observed (Fig. 2K, L).
Fig. 2.
Macroscopic and histological findings of the liver segment 3 (S3) tumor. The S3 tumor was a light-green 11×6×7 mm nodule (A). Histopathological examination revealed neoplastic small bile ductules (cholangiocytes) arranged in a staghorn-like configuration similar to bile ductular proliferation (hematoxylin and eosin staining) (B: magnification ×20; C: magnification ×100). The S3 tumor appeared as cells with round to oval nuclei that proliferated in an anastomosing pattern of small glands without mucus production, mimicking ductular reactions with a background of abundant fibrous stroma (D: magnification ×200). This tumor was immunohistologically positive for cytokeratin (CK) 7 (E: magnification ×20) and CK19 (F: magnification ×200) and negative for hepatocyte markers, such as hepatocyte paraffin 1 (Hep Par 1) (G: magnification ×20), glypican 3 (H: magnification ×20), and CD10 (I: magnification ×20). Epithelial membrane antigen was positivity at the glandular lumen (J: magnification ×200). Neither p53-immunopositive cells (K: magnification ×20) nor nuclear expression of β-catenin was observed (L: magnification ×100). Infiltrating CD8-positive T-lymphocyte were observed (M: magnification ×20) (N: magnification ×40) and CD4-positive T-lymphocytes were not observed (O: magnification ×20).
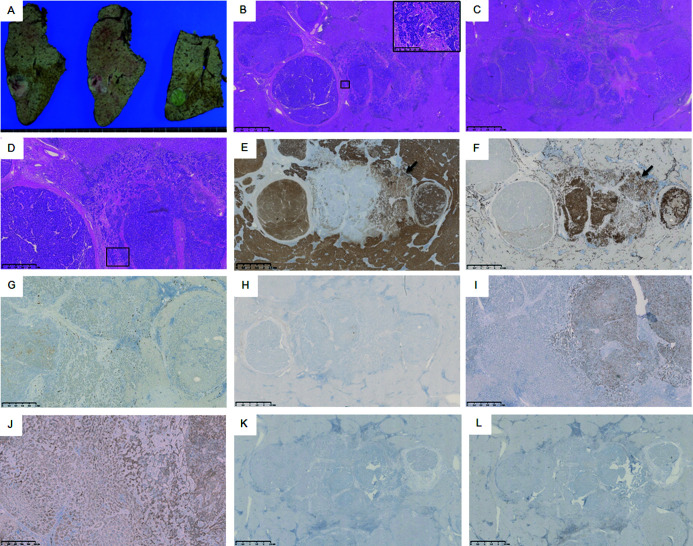
Fig. 3.
Macroscopic and histological findings of the liver segment 6 (S6) tumor. The S6 tumor appeared as an off-white and green 38 x 23 x 33 mm. nodule (A). The tumor consisted of both moderately differentiated hepatocellular carcinoma and moderately differentiated cholangiocarcinoma (hematoxylin and eosin staining B: magnification ×20; C: magnification ×20 of consecutive sections; D: magnification ×50). No cancerous emboli traces were found in the vein and lymph vessel of both tumors. The hepatic tissue adjacent to the tumors was cirrhotic. Hepatocellular carcinoma (HCC) with a thick trabecular appearance (lateral) and cholangiocarcinoma (CCA) with glandular structures were embedded in the desmoplastic stroma (central) and intimately interdigitated at the transitional region (B, C, and D). These CCA cells showed mucus production (box in B; magnification ×200). Immunohistochemistry revealed that the HCC component was positive for hepatocyte markers, such as hepatocyte paraffin 1 (Hep Par 1) (E: magnification ×20). The CCA component was positive for cytokeratin (CK) 7 (F: magnification ×20), partially positive for CK19 (G: magnification ×50), and negative for Hep Par1 (E: magnification ×20) and epithelial membrane antigen (H: magnification ×20). p53-immunopositive cells were observed in only the CCA component (I: magnification ×50). Nuclear expression of β-catenin was not observed (J: magnification ×100). Neither CD8-positive T-lymphocyte (K: magnification ×20) CD4-positive T-lymphocytes were not observed (L: magnification ×20).
The S6 tumor consisted of both moderately differentiated HCC and moderately differentiated cholangiocarcinoma. HCC with a thick trabecular appearance (lateral) and CCA with glandular structures embedded in the desmoplastic stroma (central) were intimately interdigitated in the transitional region (Fig. 3B–D). The CCA cells showed mucus production (box in Fig. 3B). Immunohistochemistry revealed the HCC component was positive for hepatocyte markers, such as Hep Par 1 (Fig. 3E). The CCA component was positive for CK7, partially positive for CK19, and negative for Hep Par 1 (Fig. 3E–G). Additionally, p53-immunopositive cells were observed in only the CCA component. The nuclear expression of β-catenin was not observed (Fig. 3I, J). The S3 tumor was diagnosed as pure biliary-type CLC without hepatocytic differentiation. The S6 tumor was diagnosed as cHCC-CCA without a CLC component. Based on the histological findings, the patient’s liver tumors were diagnosed as synchronous double primary liver cancer. No recurrence was observed in the patient 2 years postoperatively. This case is the first report of concurrent cHCC-CCA and CLC in different liver segments.
Discussion
Along with other solid tumors, liver tumors are classified based by their gross appearance and histopathology. However, with the recent development of molecular biological methods, the molecular profile of gene expression has revealed details of tumor origin, leading to the discovery of new treatment options. One of the technologies that have been clinically applied is gene panel testing.14 The fifth WHO classification subcategorizes iCCA into small and large duct types because both types were found to have distinct differences in their clinicopathological and molecular profiles.5,15 Small-duct type iCCA is frequently associated with fibroblast growth factor receptor 2 translocation and the mutation of isocitrate dehydrogenase 1/2, an inhibitor of hepatocytic differentiation and gatekeeper of iCCA generation. Additionally, it is characterized by small ductular structures with a mucin-poor cuboidal cell lining.16
Moeini et al.17 investigated the molecular profile of CLC cases that were defined without hepatocytic differentiation (pure biliary-type CLC) during an integrated genomic analysis consisting of comprehensive molecular characterization. The analysis included histological characterization, whole-genome expression profiling, single-nucleotide polymorphism array, and whole-exome sequencing. According to their results, CLC as characterized by chromosomal stability, transforming growth factor-β pathway activation, and expression of genes related to inflammation and immunity. It is described as a molecular entity indicating a bile duct origin different from cHCC-CCA.17 Additionally, an isocitrate dehydrogenase 1 mutation was detected in CLC, which is common to small duct-type iCCAs. Therefore, CLC has a genomic profile similar that of iCCA, but CLC with hepatocytic differentiation has a genomic profile similar to that of cHCC-CCA.17 In the fifth WHO classification, pure biliary-type CLC was classified as a subtype of small bile duct cholangiocarcinoma. As mentioned above, because hepatic progenitor cells have the potential to differentiate into hepatocytes or cholangiocytes, CLC is thought to proliferate into HCC or iCCA. Additionally, because there are few cases of CLC coexisting with HCC or iCCA, which often show CLC in the HCC structure, CLC with hepatocytic differentiation is thought to be derived from HCC.18
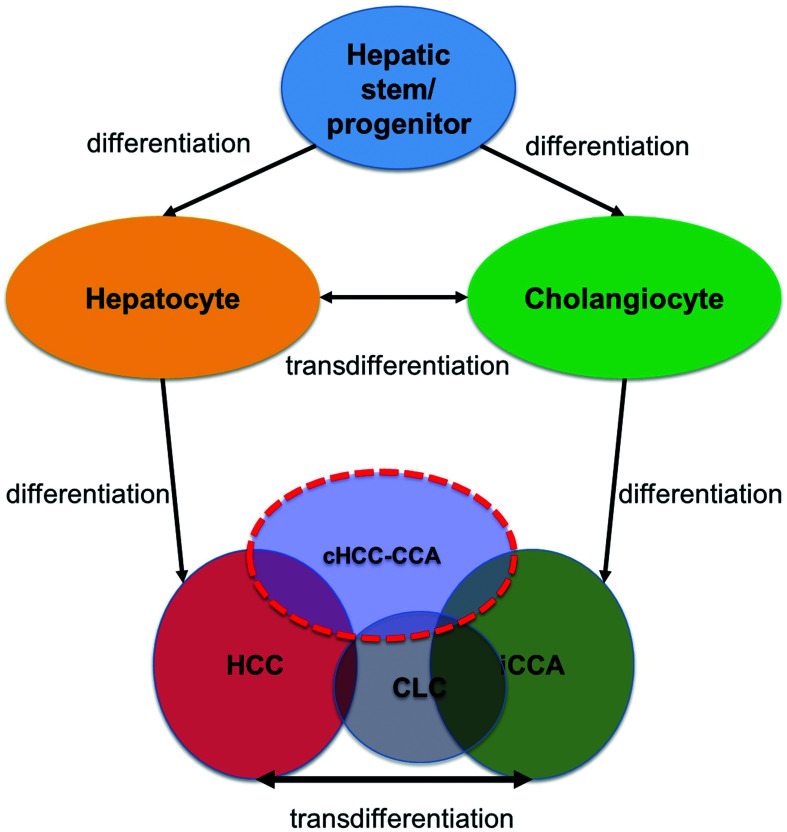
The hypothesis on differentiation and proliferation abnormality of hepatic epithelial lineage cells in hepatocarcinogenesis and paradigm of the fifth WHO classification can thus be summarized as shown in Figure 4. Based on the molecular profiles and the lack of conclusive evidence for the stem cell origin theory, the subtype with stem cell features as a cHCC-CCA subclassification was abolished in the fifth WHO classification. Furthermore, a theory of cHCC-CCA development considers that iCCA characteristics are acquired as HCC progresses.7 However, the hypothesis that hepatocarcinogenesis starts from liver stem cells that develop with both HCC and iCCA characteristics from an early stage has not been completely denied, and further research is warranted.
Fig. 4.
Hypothesis of the differentiation and proliferation abnormality of hepatic epithelial lineage cells in hepatocarcinogenesis and paradigm of the 2019 revision of the World Health Organization (WHO) classification of tumors. cHCC-CCA, combined hepatocellular-cholangiocarcinoma. CLC, cholangiolocarcinoma; HCC, hepatocellular carcinoma; iCCA, intrahepatic cholangiocarcinoma.
In this case, the S3 tumor was diagnosed as a pure biliary-type CLC. A ductular configuration was not seen in the S6 tumor (cHCC-CCA). Moreover, findings that cause suspicion of intrahepatic metastasis include the presence of portal vein tumor embolization or a cancerous lesion in the largest carcinoma vicinity that is clearly smaller and of similar or less differentiated histology. However, none of those were present in this case. In addition, there were no p53-immunopositive cells and CD8-positive T-lymphocytes as seen in the CCA component of cHCC-CCA. Thus, these two types of tumors developed as individual primary tumors.
In this case, the HCC component had a typical fibrous capsule, indicating that some time had passed since the HCC occurred. In the iCCA component, some areas were stained for both Hep Par1 and CK7 (black arrows in Fig. 3E and F). The histological findings may indicate the transdifferentiation of HCC into CCA. Additionally, p53-immunopositive cells were observed in only the CCA component, and the nuclear expression of β-catenin was not observed in this case. Deletion of the tumor suppressor gene TP53 has been reported to induce differentiation of hepatocytes into the bile duct epithelium in a mouse liver tumor model.19 In this case, cHCC-CCA may have been established via the transformation of mature hepatocytes.
Recently, it has been reported that the liver microenvironment may define the phenotype of liver tumors.20 The background liver immunological potential in the present case was unclear. Nonetheless, the immune microenvironment was investigated for the presence of tumor-infiltrating lymphocytes in cHCC-CCA and CLC, and tumor-infiltrating CD8+ lymphocytes were observed to be prominent in CLC. However, no significant infiltration of CD8+ lymphocytes was seen in the cHCC-CCA, including the CCA component (Figs. 2M and 3L). Hence, further studies are warranted to determine if there are differences in the development of immune escape in tumors of the same bile duct origin and if there is a relationship between tumor transdifferentiation and immune escape mechanisms. In conclusion, the origin of hepatocarcinogenesis in this case was the existence of immature and mature cell stemness in the same background. Therefore, we must strive for accurate pathological diagnosis and believe that these two rare liver cancers with different molecular profiles occurred simultaneously, indicating the necessity for further research on background liver pathology in carcinogenesis.
Acknowledgments
The authors thank Dr. Jiro Watanabe († March 2021; Department of Laboratory and Pathology, National Hospital Organization, Fukuyama Medical Center) for helpful discussions.
Abbreviations
- CCA
cholangiocarcinoma
- CK
cytokeratin
- CT
contrast-enhanced computed tomography
- cHCC-CCA
combined hepatocellular-cholangiocarcinoma
- CLC
cholangiolocarcinoma
- HCC
hepatocellular carcinoma
- iCCA
intrahepatic cholangiocarcinoma
- MRI
magnetic resonance imaging
- WHO
World Health Organization
Ethical statement
The study was performed following the ethical standards of the institutions and the Declaration of Helsinki as revised in 2013. Written informed consent was obtained from the patient for publication of this case report.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Wang A, Wu L, Lin J, Han L, Bian J, Wu Y, et al. Whole-exome sequencing reveals the origin and evolution of hepato-cholangiocarcinoma. Nat Commun. 2018;9(1):894. doi: 10.1038/s41467-018-03276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kudo M, Izumi N, Kokudo N, Sakamoto M, Shiina S, Takayama T, et al. Report of the 22nd nationwide follow-up Survey of Primary Liver Cancer in Japan (2012-2013) Hepatol Res. 2022;52(1):5–66. doi: 10.1111/hepr.13675. [DOI] [PubMed] [Google Scholar]
- 4.Sempoux C, Kakar S, Kondo F, Schirmacher P. WHO classification of tumours: Digestive system tumours. Lyon: International Agency for Research on Cancer; 2019. Combined hepatocellular-cholangiocarcinoma and undifferentiated primary liver carcinoma. [Google Scholar]
- 5.Nakanuma YKD, Komuta M, Zen Y. WHO classification of tumours: Digestive system tumours, 5th ed. Lyon: International Agency for Research on Cancer; 2019. Intrahepatic cholangiocarcinoma. [Google Scholar]
- 6.Brunt E, Aishima S, Clavien PA, Fowler K, Goodman Z, Gores G, et al. cHCC-CCA: Consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentiation. Hepatology. 2018;68(1):113–126. doi: 10.1002/hep.29789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasaki M, Sato Y, Nakanuma Y. Mutational landscape of combined hepatocellular carcinoma and cholangiocarcinoma, and its clinicopathological significance. Histopathology. 2017;70(3):423–434. doi: 10.1111/his.13084. [DOI] [PubMed] [Google Scholar]
- 8.Theise ND NO, Park YN, et al. WHO classification of tumors: Digestive system tumors. Lyon: International Agency for Research on Cancer; 2010. Combined hepatocellular-cholangiocarcinoma. [Google Scholar]
- 9.Zen C, Zen Y, Mitry RR, Corbeil D, Karbanova J, O’Grady J, et al. Mixed phenotype hepatocellular carcinoma after transarterial chemoembolization and liver transplantation. Liver Transpl. 2011;17(8):943–954. doi: 10.1002/lt.22314. [DOI] [PubMed] [Google Scholar]
- 10.Nishikawa Y, Doi Y, Watanabe H, Tokairin T, Omori Y, Su M, et al. Transdifferentiation of mature rat hepatocytes into bile duct-like cells in vitro. Am J Pathol. 2005;166(4):1077–1088. doi: 10.1016/s0002-9440(10)62328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Wong PP, Sjeklocha L, Steer CJ, Sahin MB. Mature hepatocytes exhibit unexpected plasticity by direct dedifferentiation into liver progenitor cells in culture. Hepatology. 2012;55(2):563–574. doi: 10.1002/hep.24712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steiner PE, Higginson J. Cholangiolocellular carcinoma of the liver. Cancer. 1959;12(4):753–759. doi: 10.1002/1097-0142(195907/08)12:4<753::aid-cncr2820120420>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 13.Komuta M, Spee B, Vander Borght S, De Vos R, Verslype C, Aerts R, et al. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology. 2008;47(5):1544–1556. doi: 10.1002/hep.22238. [DOI] [PubMed] [Google Scholar]
- 14.Prasad V, Fojo T, Brada M. Precision oncology: origins, optimism, and potential. Lancet Oncol. 2016;17(2):e81–e86. doi: 10.1016/s1470-2045(15)00620-8. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi A, Misumi K, Shibahara J, Arita J, Sakamoto Y, Hasegawa K, et al. Distinct Clinicopathologic and Genetic Features of 2 Histologic Subtypes of Intrahepatic Cholangiocarcinoma. Am J Surg Pathol. 2016;40(8):1021–1030. doi: 10.1097/pas.0000000000000670. [DOI] [PubMed] [Google Scholar]
- 16.Lowery MA, Ptashkin R, Jordan E, Berger MF, Zehir A, Capanu M, et al. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin Cancer Res. 2018;24(17):4154–4161. doi: 10.1158/1078-0432.Ccr-18-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moeini A, Sia D, Zhang Z, Camprecios G, Stueck A, Dong H, et al. Mixed hepatocellular cholangiocarcinoma tumors: Cholangiolocellular carcinoma is a distinct molecular entity. J Hepatol. 2017;66(5):952–961. doi: 10.1016/j.jhep.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Kondo F, Fukusato T. Pathogenesis of cholangiolocellular carcinoma: Possibility of an interlobular duct origin. Intern Med. 2015;54(14):1685–1694. doi: 10.2169/internalmedicine.54.3540. [DOI] [PubMed] [Google Scholar]
- 19.Katz SF, Lechel A, Obenauf AC, Begus-Nahrmann Y, Kraus JM, Hoffmann EM, et al. Disruption of Trp53 in livers of mice induces formation of carcinomas with bilineal differentiation. Gastroenterology. 2012;142(5):1229–1239.e1223. doi: 10.1053/j.gastro.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 20.Seehawer M, Heinzmann F, D’Artista L, Harbig J, Roux PF, Hoenicke L, et al. Necroptosis microenvironment directs lineage commitment in liver cancer. Nature. 2018;562(7725):69–75. doi: 10.1038/s41586-018-0519-y. [DOI] [PMC free article] [PubMed] [Google Scholar]