Abstract
Background and Aims
To determine whether liver stiffness measurement (LSM) indicates liver inflammation in chronic hepatitis B (CHB) with different upper limits of normal (ULNs) for alanine aminotransferase (ALT).
Methods
We grouped 439 CHB patients using different ULNs for ALT: cohort I, ≤40 U/L (439 subjects); cohort II, ≤35/25 U/L (males/females; 330 subjects); and cohort III, ≤30/19 U/L (males/females; 231 subjects). Furthermore, 84 and 96 CHB patients with normal ALT (≤40 U/L) formed the external and prospective validation groups, respectively. We evaluated the correlation between LSM and biopsy-confirmed liver inflammation, and determined diagnostic accuracy using area under the curve (AUC). A noninvasive LSM-based model was developed using multivariate logistic regression.
Results
Fibrosis-adjusted LSM values significantly increased with increasing inflammation. The AUCs of LSM in cohorts I, II, and III were 0.799, 0.796, and 0.814, respectively, for significant inflammation (A≥2) and 0.779, 0.767, and 0.770, respectively, for severe inflammation (A=3). Cutoff LSM values in all cohorts for A≥2 and A=3 were 6.3 and 7.5 kPa, respectively. Internal, external, and prospective validations showed high diagnostic accuracy of LSM for A≥2 and A=3, and no significant differences in AUCs among the four groups. LSM and globulin independently predicted A≥2. The AUC of an LSM-globulin model for A≥2 exceeded those of globulin, ALT, and AST, but was similar to that of LSM.
Conclusions
LSM predicted liver inflammation and guided the indication of antiviral therapy for CHB in patients with normal ALT.
Keywords: Hepatitis B virus, Liver inflammation, Fibrosis, Liver stiffness measurement, Alanine aminotransferase
Graphical abstract
Introduction
Chronic hepatitis B virus (HBV) infection is a serious public health problem that affects over 240 million people worldwide,1 which can lead to hepatocellular necrosis and inflammation, liver fibrosis, cirrhosis, liver cancer, liver failure, and even death. Current guidelines for the management of chronic hepatitis B recommend that patients with normal alanine aminotransferase (ALT) levels but significant hepatic inflammation should be administered timely antiviral treatment to prevent disease progression.2–6
ALT is a sensitive diagnostic marker for liver injury,7,8 and normal ALT levels generally indicate normal liver tissue. ALT levels can also assess the need for initiating antiviral therapy for chronic hepatitis B (CHB) patients and to monitor the efficacy of this treatment. However, many studies have reported that some chronic HBV carriers with normal or near-normal ALT levels have significant hepatic necroinflammation, fibrosis, and even early cirrhosis.8–10 If the need for antiviral therapy is decided only by an elevated ALT level, some patients with normal ALT levels may miss their opportunity for treatment. In addition, the current definition of normal ALT level is controversial. The Asian Pacific Association for the Study of the Liver and the European Association for the Study of the Liver (EASL) guidelines for chronic hepatitis B recommend defining the upper limit of normal (ULN) for ALT level as 40 U/L,2,3 which is also the threshold recommended by Chinese guidelines.4 However, the American Association for the Study of Liver Diseases (AASLD) defines the ULN for ALT as 35 U/L for males and 25 U/L for females,5 and WHO guidelines define it as 30 U/L for males and 19 U/L for females.6 Liver biopsy is the gold standard for assessing hepatic necroinflammation and fibrosis, but its clinical application is often limited by its invasive nature, sampling errors, and the risk of potential complications.11 Therefore, more accurate, noninvasive diagnostic markers for the assessment of liver necroinflammation are urgently required, especially for patients with chronic HBV infection and different ULNs for ALT levels.
Liver stiffness measurement (LSM) based on transient elastography (FibroScan) is widely recommended for the noninvasive staging of hepatic fibrosis in patients with chronic hepatic diseases.2–6,12 However, our previous study found that hepatic inflammation is an independent risk factor affecting the accuracy of FibroScan evaluations of liver fibrosis staging in patients with chronic HBV infection.13 Consistent with previous studies,14,15 our study showed that LSM values may be influenced by the degree of liver necroinflammation and abnormal ALT elevation. These results prompted us to explore whether LSM values could reflect liver inflammatory activity in patients with chronic HBV infection. A recent multicenter prospective study conducted in China found that although LSM could help to determine the extent of hepatic fibrosis, an absolute decrease in LSM values during antiviral treatment was more closely correlated with improvement of liver inflammation.16 However, the results of LSM in these studies may have been affected by the severe inflammation associated with abnormal or high ALT levels. A recent study found that LSM noninvasively predicted liver inflammatory activity in chronic hepatitis B patients17 and chronic hepatitis B patients with abnormal ALT levels and indicated for antiviral therapy according to HBV-practice guidelines.2–6 Thus, that study17 has little value for guiding clinical decisions about whether to start antiviral therapy. So whether LSM also has the potential to predict liver inflammatory activity in chronic HBV infection patients with normal ALT levels has not yet been reported.
In this study, we aimed to determine the diagnostic value of LSM values as a noninvasive indicator of hepatic inflammation in patients with chronic HBV infection grouped according to different ULNs for ALT levels.
Methods
Study subjects
We retrospectively enrolled 439 patients with chronic HBV infection who underwent liver biopsy and FibroScan examination in two affiliated hospitals of Fujian Medical University (First Affiliated Hospital and The First Hospital of Quanzhou) and Xiamen Hospital of Traditional Chinese Medicine between January 2014 and January 2021. We also collected data from 84 patients with chronic HBV infection treated at Huashan Hospital affiliated to Fudan University of Shanghai between August 2017 and February 2022, who represented the external validation group. In addition, 96 patients with chronic HBV infection were prospectively enrolled in the study when they presented to the above hospitals or to Mengchao Hepatobiliary Hospital of Fujian Medical University or Xiamen Humanity Hospital for liver biopsy between August 2022 and October 2022. These patients represented our prospective validation group. Chronic HBV infection was defined as a positive test for hepatitis B surface antigen (HBsAg) for ≥6 months. All treatment-naïve patients had HBV DNA ≥500 IU/mL and never received antiviral therapy before undergoing liver biopsy. The exclusion criteria were: (1) <18 years of age, (2) body mass index (BMI) >28 kg/m2, (3) other types of hepatophilic virus infections, (4) accompanying fatty, alcoholic, drug, autoimmune, genetic, or metabolic liver diseases, (5) decompensated cirrhosis, (6) tumor, (7) severe extrahepatic disease or pregnancy, (8) abnormal ALT levels (>40 U/L), (9) poor quality liver samples, (10) accompanying hepatic steatosis (>5%) as detected using pathological examination, and (11) unreliable LSM values and incomplete data. The laboratory tests and LSMs were performed within 1 week before the liver biopsy.
The patients were grouped using different criteria for the ULN for ALT levels: cohort I, ≤40 U/L (439 subjects); cohort II, ≤35/25 U/L (males/females; 330 subjects); and cohort III, ≤30/19 U/L (males/females; 231 subjects). A flow chart of the process of patient selection is shown in Figure 1. Written informed consent from the patients was not required due to the retrospective study design. The study was approved by the institutional review committee of Fujian Medical University, Fuzhou, Fujian Province, China.
Fig. 1. Flow chart of study subject disposition.
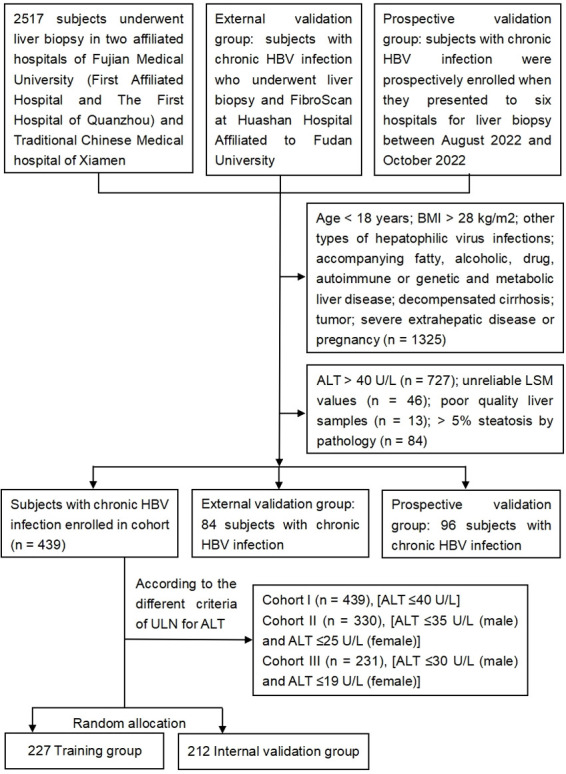
ALT, alanine aminotransferase; BMI, body mass index; HBV, hepatitis B virus; LSM, liver stiffness measurement.
Population and laboratory data
The general population data included age, sex, height, weight, alcohol consumption and/or drug use, and history of HBV infection. The following laboratory data were collected: total bilirubin (TBIL), albumin (ALB), globulin (GLOB), ALT, aspartate aminotransferase (AST), gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), HBsAg, hepatitis B envelope antigen (HBeAg), HBV DNA, platelet count (PLT), prothrombin time (PT), and alpha-fetoprotein (AFP), which were measured by drawing fasting venous blood samples.
Liver histology examination
Percutaneous liver biopsy under ultrasonic localization was performed using 16 G disposable liver puncture biopsy needles (ACUSON; Siemens, USA). Qualified liver tissue samples (i.e., length ≥15 mm and containing at least six portal veins) were fixed with formaldehyde, dehydrated, embedded in paraffin, sliced (thickness, 4–6 µm), and finally stained with Masson stain, and hematoxylin and eosin. The slides were examined by at least two experienced pathologists who were not aware of the patients’ clinical data. The METAVIR scoring system18 was used to determine the liver inflammation activity grade (A0–A3) and liver fibrosis stage (F0–F4). Liver inflammation was described as absent or mild inflammation, A0–A1; significant inflammation, ≥A2; and severe inflammation, A3. Liver fibrosis was staged as significant fibrosis, F≥2; advanced fibrosis, F≥3; and cirrhosis, F4.
Liver stiffness measurement
LSMs were performed using the M probe of the FibroScan 502 device (Echosens, France) by specially trained operators. The detection method described in the FibroScan user manual was used. The detection was continuously performed, until 10 successful tests were conducted. The LSM value was considered reliable only when rate of successful acquisitions was over 60%, and the interquartile range was less than 30%.
Statistical analysis
The SPSS v23.0 and MedCalc v19.1 software packages were used for statistical analysis. Student’s t-test was used for data with normal distribution and homogeneity of variance, and the Mann–Whitney U test was used for continuous data with skewed distribution. Univariate analysis of variance and the chi-square test were used for pairwise comparisons of continuous variables and categorical variables, respectively. Adjustments for confounding variables were performed using covariance analysis. To verify the diagnostic performance of LSM, we randomly stratified the 439 patients in this study into a training group and an internal validation group at a ratio of about 1:1, and compared the training group with the internal validation group, external validation group, and the prospective validation group, respectively. Logistic regression analysis was used to identify predictors of significant liver inflammation. The area under the receiver operating characteristic (ROC) curve (AUC) was used to evaluate diagnostic accuracy, and the DeLong test was used to compare AUCs. A two-sided p<0.05 indicated statistically significant differences.
Results
Patient characteristics
Herein, Figure 1 showed 439 enrolled patients with chronic HBV infection were divided into groups based on the different criteria for the ULN of ALT cohort I (n=439), cohort II (n=330), and cohort III (n=231). The general clinical characteristics did not significantly differ among the three cohorts, except for ALT and AST levels (all p>0.05; Table 1). Also, nearly half of the patients had significant inflammation (A≥2) and significant fibrosis (F≥2) in all three cohorts. The proportions of patients with scores of A≥2 in the cohort I, cohort II, and cohort III were 48.3% (212/439), 47.9% (158/330), and 44.2% (102/231), respectively. Similarly, the prevalence of F≥2 in the three cohorts was 43.7% (192/439), 44.8% (148/330), and 43.7% (101/231), respectively.
Table 1. Patient demographic and clinical characteristics.
| Cohort I (n=439) | Cohort II (n=330) | Cohort III (n=231) | p-value | |
|---|---|---|---|---|
| Age (yr) | 40.58 ± 9.91 | 40.31 ± 9.89 | 40.10 ± 10.381 | 0.895 |
| Male, n (%) | 297 (67.7) | 243 (73.6) | 173 (74.9) | 0.075 |
| BMI (kg/m2) | 22.84 ± 2.36 | 22.89 ± 2.44 | 22.68 ± 2.42 | 0.772 |
| TBIL (µmoL/L) | 14.66 ± 7.40 | 15.02± 7.59 | 15.43 ± 7.82 | 0.449 |
| ALB (g/L) | 43.77 ± 4.07 | 44.02 ± 3.87 | 44.04 ± 4.00 | 0.607 |
| GLOB (g/L) | 29.07 ± 4.97 | 28.87 ± 4.43 | 28.86 ± 4.49 | 0.785 |
| ALT (IU/L) | 26.58 ± 8.14 | 23.57 ± 6.83 | 20.90 ± 5.65 | <0.001 |
| AST (IU/L) | 24.53 ± 8.31 | 22.76 ± 7.95 | 21.10 ± 5.35 | <0.001 |
| GGT (IU/L) | 27.78 ± 18.38 | 27.03 ± 17.76 | 26.00 ± 18.40 | 0.480 |
| ALP (IU/L) | 72.47 ± 27.88 | 71.33 ± 29.39 | 71.08 ± 32.09 | 0.812 |
| PLT (109/L) | 204.82 ± 57.12 | 206.48 ± 57.33 | 202.47 ± 58.04 | 0.719 |
| HBsAg (logIU/mL) | 3.25 ± 1.15 | 3.17 ± 1.15 | 3.12 ± 1.21 | 0.330 |
| HBeAg positive (%) | 162 (36.9) | 108 (32.7) | 73 (31.6) | 0.297 |
| HBV DNA (logIU/mL) | 4.28 ± 2.17 | 3.94 ± 2.06 | 4.02 ± 2.22 | 0.077 |
| PT (s) | 12.56 ± 0.95 | 12.59 ± 0.94 | 12.66 ± 0.91 | 0.417 |
| AFP (ng/mL) | 5.01 ± 17.07 | 4.19 ± 11.38 | 4.52 ± 13.18 | 0.744 |
| LSM (kPa) | 7.55 ± 3.68 | 7.62 ± 3.59 | 7.47 ± 3.47 | 0.871 |
| Inflammationa, n (%) | 0.749 | |||
| A0–1/A2/A3 | 227 (51.7)/189 (43.1) /23 (5.2) | 172 (52.1)/145 (43.9) /13 (3.9) | 129 (55.8)/91 (39.4) /11 (4.8) | |
| A≥2 | 212 (48.3) | 158 (47.9) | 102 (44.2) | 0.568 |
| Fibrosis stage1, n (%) | 0.997 | |||
| F0–1/F2/F3/F4 | 247 (56.3)/124 (28.2) /45 (10.3)/23 (5.2) | 182 (55.2)/93 (28.2) /37 (11.2)/18(5.5) | 130 (56.3)/66 (28.6) /25 (10.8)/10 (4.3) | |
| F≥2 | 192 (43.7) | 148 (44.8) | 101 (43.7) | 0.946 |
aAccording to METAVIR system, liver inflammation activity grade ranged from 0 to 3, and liver fibrosis stage ranged from 0 to 4. ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; ALB, albumin; AFP, alpha fetoprotein; BMI, body mass index (kg/m2); GGT, gamma-glutamyl transpeptidase; GLOB, globulin; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B envelope antigen; LSM, liver stiffness measurements; PLT, platelet count; PT, prothrombin time; TBIL, total bilirubin.
Relationship between LSM values and liver inflammation in the cohort I
First, we showed ALT levels were not correlated with LSM values (r=0.065, p=0.175; Supplementary Fig. 1) in the cohort I (i.e. patients with ALT ≤ 40 U/L). Next, we analyzed the relationship of LSM values with liver fibrosis and liver inflammation activity in the cohort I. We found that LSM was significantly and positively correlated with liver fibrosis (r=0.726, p<0.001; Fig. 2A), as LSM values gradually increased with the aggravation of liver fibrosis. We also found that LSM values were positively correlated with liver inflammation activity (r=0.555, p<0.001; Fig. 2B), as LSM values were significantly higher in patients with moderate-to-severe liver inflammation activity (A≥2) than in patients with no or mild liver inflammation activity (A0–A1; p<0.001).
Fig. 2. Box plots of liver stiffness measurements (LSMs) according to liver fibrosis stage (A) and liver inflammation activity grade (B) in the cohort I.
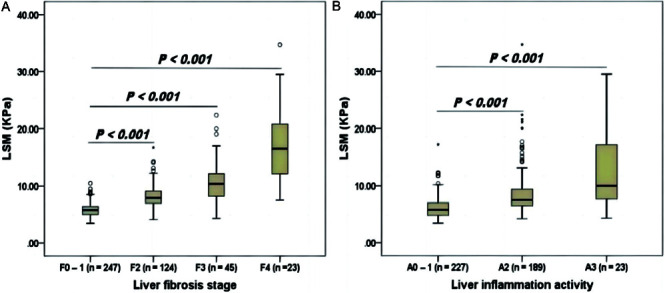
Upper and lower whiskers indicate the 75th percentile plus 1.5 interquartile range (IQR) and the 25th percentile minus 1.5 IQR, respectively. Outlier: a value greater than the 75th percentile plus 1.5 IQR.
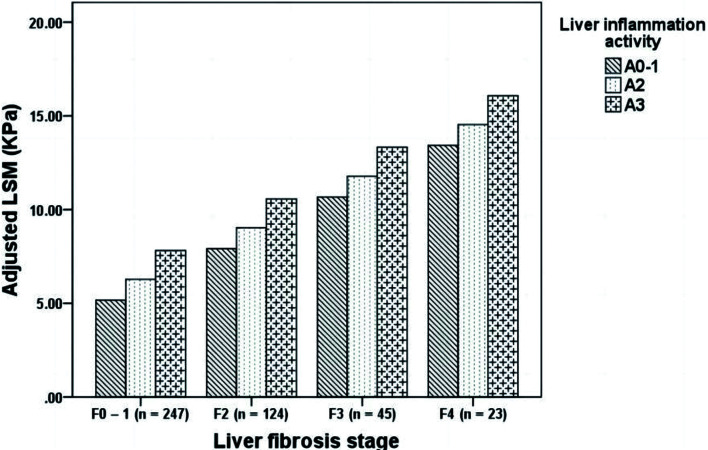
As in previous studies,19,20 liver inflammation was significantly associated with liver fibrosis in this study (r=0.458, p<0.001). So we then performed covariance analysis to exclude liver fibrosis as a co-response variable for LSM value elevation in the cohort I. After taking liver fibrosis as a covariable, we found that the adjusted LSM values still increased with aggravation of the liver inflammation activity (Supplementary Table 1). The difference in the adjusted mean LSM values among patients with different liver inflammation activity grades was still statistically significant (F=15.584, p<0.001).
To confirm that the relationship between LSM values and liver inflammation was independent of liver fibrosis, we grouped the patients in the cohort I with the same liver fibrosis stage according to their liver inflammation activity, and compared the adjusted LSM values. The adjusted LSM values still gradually increased with aggravation of liver inflammation in each subgroup with the same liver fibrosis stage, and the differences among the different subgroups were statistically significant (p<0.001; Fig. 3). These results suggested that the relationship between LSM values and liver inflammation was independent of liver fibrosis, and that LSM may reflect liver inflammation.
Fig. 3. Comparison of adjusted liver stiffness measurement (LSM) values in cohort I of subjects with different liver inflammation activities at the same liver fibrosis stage.
Predictive performance of LSM for significant and severe liver inflammation in the three cohorts with different ULNs for ALT
We assessed the diagnostic accuracy of LSM for predicting significant liver inflammation (A≥2) and severe liver inflammation (A=3) in the three cohorts with different ULNs for ALT (Table 2). The AUCs of LSM for these predictions in the cohort I, cohort II, and cohort III were 0.799, 0.796, and 0.814, respectively, in the case of A≥2 and 0.779, 0.767, and 0.770, respectively, in the case of A=3. There were no statistical differences in the AUCs for predicting A≥2 and A=3 among the three cohorts (all p>0.05). The cutoff LSM values for the prediction of A≥2 and A=3 in all cohorts were 6.3 kPa and 7.5 kPa, respectively.
Table 2. Diagnostic accuracy of liver stiffness measurement for predicting A≥2 and A=3 in patient cohorts with different upper limits of normal for alanine aminotransferase.
| Inflammation activity | AUC (95%CI) | Cutoff value | Sensitivity/specificity (%) | PPV/NPV (%) |
|---|---|---|---|---|
| Cohort I | ||||
| A≥2 | 0.799 (0.758–0.836) | 6.3 | 78.70/68.91 | 70.82/77.17 |
| A=3 | 0.779 (0.737–0.817) | 7.5 | 82.61/68.51 | 72.40/79.76 |
| Cohort II | ||||
| A≥2 | 0.796 (0.748–0.838) | 6.3 | 81.65/65.12 | 70.07/78.02 |
| A=3 | 0.767 (0.717–0.811) | 7.5 | 84.62/66.56 | 71.68/88.23 |
| Cohort III | ||||
| A≥2 | 0.814 (0.757–0.862) | 6.3 | 85.29/68.22 | 72.85/82.26 |
| A=3 | 0.770 (0.710–0.823) | 7.5 | 81.82/69.09 | 72.58/79.17 |
AUC, area under the receiver operating characteristic curve; LSM, liver stiffness measurement; NPV, negative predictive value; PPV, positive predictive value.
We analyzed the cutoff values of LSM for significant and severe inflammation in subgroups based on fibrosis stage (Supplementary Table 2). When the cutoff values of LSM for the prediction of A≥2 and A=3 were 6.3 and 7.5 kPa, respectively, the accuracy and sensitivity of LSM were higher in each fibrosis stage subgroup, but the specificity was lower in patients with significant fibrosis, which may be due to the influence of fibrosis on the assessment of LSM.
Validations of LSM’s predictive value for significant and severe inflammation
To further verify the predictive value of LSM for liver inflammation, 439 patients with chronic HBV infection were randomly divided into a training group (n=227) and an internal validation group (n=212) at about 1:1. An additional 84 patients with chronic HBV infection and normal ALT levels from Huashan Hospital were enrolled as an external validation group, and 96 patients from six hospitals were prospectively recruited as a prospective validation group (Fig. 1). The characteristics of the patients in these four groups are displayed in Supplementary Table 3.
Next, the optimal cut-offs of LSM for A≥2 and A=3 determined in the training group were confirmed in the three validation groups (Table 3). The diagnostic accuracy of LSM was high for the segregation of liver inflammation grades. In the training group, the AUCs of LSM for A≥2 and A=3 were 0.803 and 0.765, respectively, which did not significantly differ from the corresponding AUCs in the internal, external, and prospective validation groups (0.796 and 0.793, 0.800 and 0.937, and 0.877 and 0.940, respectively; all p>0.05). Using cutoff LSM values of 6.5 kPa and 7.3 kPa, we found that the capacity to predict significant and severe liver inflammation, respectively, was excellent in the three validation groups (Table 3). However, severe liver fibrosis may be present in patients with CHB, which may lead to false-positive LSM values.
Table 3. Diagnostic performance of LSM for predicting significant and severe inflammation in the internal, external, and prospective validation groups.
| AUC (95%CI) | Cutoff values | Accuracy (%) | Sensitivity/ specificity (%) | PPV/NPV (%) | |
|---|---|---|---|---|---|
| Training group (n=227) | |||||
| A≥2 | 0.803 (0.746–0.853) | 6.3 | 72.25 | 79.63/65.55 | 67.72/78.00 |
| A=3 | 0.765 (0.704–0.819) | 7.5 | 63.00 | 84.62/61.68 | 11.83/98.51 |
| Internal validation group (n=212) | |||||
| A≥2 | 0.796 (0.735–0.848) | 6.3 | 73.58 | 84.62/62.96 | 68.75/80.95 |
| A=3 | 0.793 (0.732–0.845) | 7.5 | 69.81 | 80.00/69.31 | 11.43/98.59 |
| External validation group (n=84) | |||||
| A≥2 | 0.800 (0.669–0.880) | 6.3 | 78.57 | 70.27/85.11 | 72.22/83.33 |
| A=3 | 0.937 (0.862–0.978) | 7.5 | 78.57 | 100/77.22 | 21.74/100 |
| Prospective validation group (n=96) | |||||
| A≥2 | 0.877 (0.790–0.937) | 6.3 | 83.33 | 97.62/72.22 | 73.21/97.50 |
| A=3 | 0.937 (0.862–0.978) | 7.5 | 78.13 | 100/76.40 | 25.00/100 |
AUC, area under the receiver operating characteristic curve; LSM, liver stiffness measurement; NPV, negative predictive value; PPV, positive predictive value.
Factors related to significant liver inflammation in the cohort I
The 439 subjects in the cohort I were subdivided according to the grade of liver inflammation activity into a significant inflammation group (A≥2, n=212) and a no significant inflammation group (A<2, n=227). Univariate analysis (Table 4) revealed that only LSM, BMI, GLOB, ALT, and AST values were significantly associated with significant liver inflammation (p<0.05); no statistical differences in the other variables were found between the two subgroups. Further multivariate analysis found that only LSM (odds ratio=1.62, p<0.001) and GLOB values (OR=1.14, p=0.002) were independent predictors of significant liver inflammation (Table 4). By incorporating LSM and GLOB values into a multiple regression model, we developed a noninvasive model named LG to predict significant liver inflammation (A≥2) in the cohort I: logit (P)=−7.229 + 1.631 × LSM (kPa) + 1.137 × GLOB (g/L).
Table 4. Univariate and multivariate analysis of the relationship between noninvasive indexes and significant liver inflammation in cohort I.
| Variables | Univariate analyses |
Multivariate analyses |
||
|---|---|---|---|---|
| OR (95%CI) | p-value | OR (95%CI) | p-value | |
| Age (yr) | 0.99 (0.97–1.01) | 0.648 | ||
| Male (%) | 1.26 (0.85–1.89) | 0.255 | ||
| BMI (kg/m2) | 1.17 (1.05–1.31) | 0.008 | 1.15 (1.00–1.32) | 0.056 |
| TBIL (µmoL/L) | 0.99 (0.96–1.01) | 0.376 | ||
| ALB (g/L) | 1.00 (0.95–1.05) | 0.918 | ||
| GLOB (g/L) | 1.16 (1.10–1.22) | < 0.001 | 1.14 (1.05–1.24) | 0.002 |
| ALT (IU/L) | 1.03 (1.00–1.05) | 0.036 | 0.99 (0.95–1.04) | 0.705 |
| AST (IU/L) | 1.06 (1.03–1.10) | < 0.001 | 1.03 (0.98–1.08) | 0.236 |
| GGT (IU/L) | 1.00 (0.99–1.01) | 0.708 | ||
| ALP (IU/L) | 1.01 (1.00–1.02) | 0.060 | ||
| PLT (109/L) | 1.00 (1.00–1.01) | 0.978 | ||
| HBsAg (logIU/mL) | 1.11 (0.94–1.31) | 0.210 | ||
| HBeAg positive (%) | 1.16 (0.79–1.71) | 0.456 | ||
| HBV DNA (logIU/mL) | 1.01 (0.93–1.10) | 0.818 | ||
| PT (s) | 0.99 (0.81–1.21) | 0.941 | ||
| AFP (ng/mL) | 1.00 (0.98–1.01) | 0.517 | ||
| LSM (kPa) | 1.66 (1.47–1.88) | < 0.001 | 1.62 (1.36–1.92) | < 0.001 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; ALB, albumin; AFP, alpha fetoprotein; BMI, body mass index (kg/m2); GGT, gamma-glutamyl transpeptidase; GLOB, globulin; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen; HBeAg, hepatitis B envelope antigen; LSM, liver stiffness measurements; PLT, platelet count; PT, prothrombin time; TBIL, total bilirubin.
Comparison of diagnostic accuracy of the LG model with other noninvasive markers of significant liver inflammation
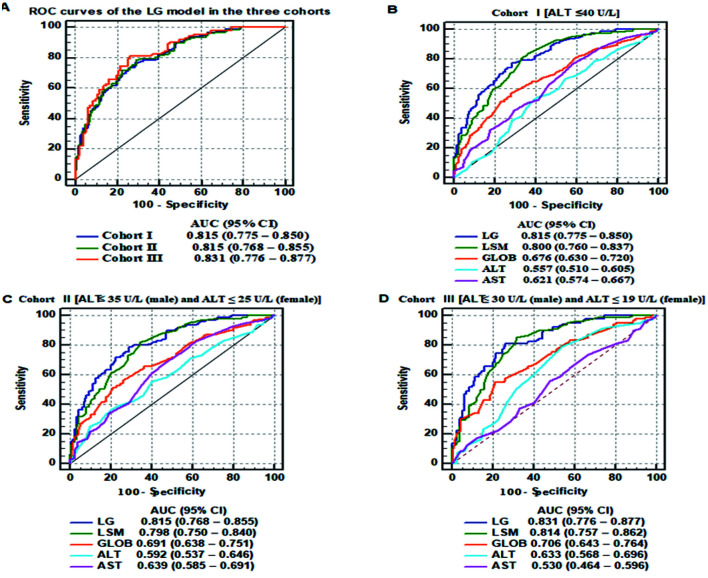
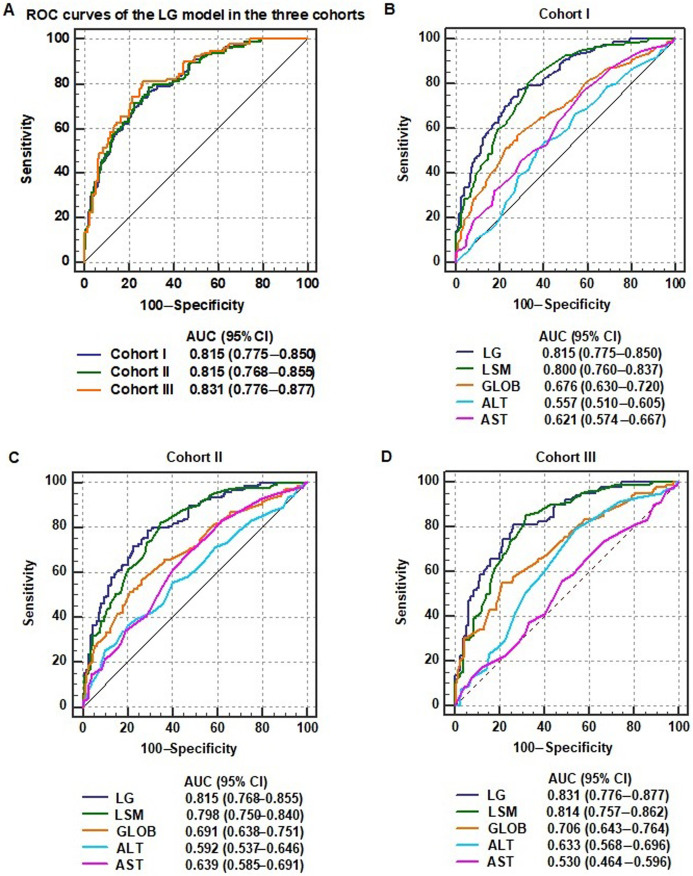
We analyzed the diagnostic accuracy of the LG model for significant liver inflammation in the three cohorts. The AUCs of the LG model for predicting A≥2 in the cohort I, cohort II, and cohort III were 0.815, 0.815, and 0.831, respectively. There were no significant differences in the AUCs for predicting A≥2 among the three cohorts (all p>0.05; Fig. 4A).
Fig. 4. (A–D) Receiver operating characteristic (ROC) curves of noninvasive markers, including liver stiffness measurement (LSM), globulin (GLOB), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) values and the LSM and GLOB (LG) model, for the diagnosis of significant liver inflammation in patients with chronic hepatitis B virus (HBV) infection grouped according different upper limits of normal for ALT.
Next, we compared the diagnostic accuracy of LG model with other noninvasive markers for A≥2 in the three cohorts. In the cohort I, the AUC of the LG model (0.815, 95% confidence interval: 0.775–0.850) for predicting A≥2 was significantly higher than those of GLOB (0.676, 95% CI: 0.630–0.720; p<0.001), ALT (0.557, 95% CI: 0.510–0.605; p<0.001), and AST (0.621, 95% CI: 0.574–0.667; p<0.001), but equal to that of LSM (0.800, 95% CI: 0.760–0.837; p=0.206; Fig. 4B and Supplementary Table 4). The cutoff point, sensitivity, and specificity of the LG model in the cohort I were 0.409, 76.89%, and 71.37%, respectively. Similarly, the AUCs of the LG model for predicting A≥2 in cohort II and cohort III were significantly higher than those of GLOB, ALT, and AST (all p<0.05); however, there was no significant difference between the AUCs of the LG model and the LSM values (Fig. 4C, D).The results indicate that LSM and the prediction model containing LSM were more specific than other clinical indicators for predicting significant liver inflammation in patients with chronic HBV infection and normal ALT levels, despite the criteria used for the ULN of ALT levels.
Discussion
Serum ALT is one of the most common biochemical indexes used to assess hepatic histological inflammation.7 It is generally accepted that high ALT levels are a marker of hepatic necroinflammation, and that chronic HBV carriers with normal ALT levels have no or only mild hepatic inflammation, and are not sensitive to antiviral drugs. Such patients are thought not to need antiviral therapy. However, many studies have found that some chronic HBV carriers with persistently normal or slightly abnormal ALT levels have severe hepatic necroinflammation and fibrosis.8–10 In this study, nearly half of the patients in each of the three cohorts with different ULNs for ALT had significant liver inflammation (A≥2), yielding prevalence rates of 44.2–48.3%. According to the guidelines for chronic hepatitis B management,2–6 antiviral therapy should be started for these patients. Therefore, relying only on the ALT level to determine whether to start antiviral therapy is limiting. It is necessary to conduct further examination or find other meaningful indicators to assess hepatic inflammation in patients with chronic HBV infection and different ULNs for ALT.
Recently, transient elastography (FibroScan) has become widely accepted for hepatic fibrosis staging.12,21 FibroScan is a noninvasive method that scans a volume of liver tissue approximately 100 times that examined using liver biopsy; thus, this scan has a low probability of being affected by sampling errors. The reproducibility of this scan is good both within and between observer groups. According to the Chinese consensus on the clinical application of transient elastography for detecting liver fibrosis,22 in patients with chronic HBV infection and normal ALT levels, the thresholds for the exclusion and diagnosis for advanced liver fibrosis are LSM <6.0 kPa and LSM ≥9.0 kPa, respectively. Therefore, according to the Chinese chronic hepatitis B guidelines,4 patients with LSM ≥9.0 kPa are considered to have significant liver fibrosis, and should be given antiviral treatment; for patients with LSM values between 6.0 and 9.0 kPa, if a decision cannot be made based on the clinical findings, a liver biopsy should be considered. Meanwhile, clinical indicators that can serve as alternatives for liver biopsy are urgently needed to further evaluate treatment regimens. Recently, some studies have found that a rapid decline of LSM values in the early stage of primary antiviral therapy in patients with chronic HBV infection is closely related to the improvement of hepatic inflammation.16,23 studies have shown that LSM reflects liver necroinflammation in patients with chronic liver disease.17,24,25 However, these studies have little value for guiding clinical decisions about the start of antiviral treatment, as the included subjects were chronic hepatitis B patients with abnormal ALT levels. Therefore, we explored whether LSM also has potential applications in predicting liver necroinflammation in patients with chronic HBV infection and normal ALT levels, as determined using different ULNs. To confirm this hypothesis, we first showed normal ALT levels did not affect LSM values in the cohort I, which had ALT levels of ≤40 U/L (r=0.065, p=0.175). Next, we analyzed the relationship between LSM values and liver inflammation in the cohort I. As expected, LSM was associated with not only liver fibrosis but also liver inflammation, and LSM values increased with the aggravation of liver necroinflammation. However, as the progression of hepatic fibrosis is often accompanied by active liver necroinflammation, the gradual increase in LSM values with increasing liver inflammation may be attributable to its direct positive correlation with liver fibrosis. Therefore, to further verify the relationship between LSM values and liver inflammation, we performed covariance analysis using liver fibrosis as a covariable; we divided patients at each stage of liver fibrosis into subgroups based on liver inflammation activity, and then compared the adjusted LSM values between the subgroups. The results showed that at each hepatic fibrosis stage, LSM values significantly differed among subgroups with different liver inflammation activities (F=15.584, p<0.001), indicating that at a given liver fibrosis stage, LSM values still increased with aggravation of liver inflammation activity.
Next, we evaluated the performance of LSM in the prediction of liver inflammation. All the AUCs of LSM for A≥2 and A=3 in the cohort I, cohort II, and cohort III were >0.76, and there were no significant differences in these AUCs among the three cohorts. These results showed that LSM was equally valuable in diagnosing liver inflammation in patients with chronic HBV infection and normal ALT levels, despite the criteria used for the ULN for ALT. The cutoff LSM values for A≥2 and A=3 in all cohorts were 6.3 kPa and 7.5 kPa, respectively. Furthermore, we analyzed the cutoff values of LSM for A≥2 and A=3 in subgroups based on fibrosis stage. The results showed that LSM had higher accuracy and sensitivity in each fibrosis stage, but lower specificity in patients with significant fibrosis, which may be due to the influence of high fibrosis on the predictive value of LSM. In other words, patients with LSM values >6.3 kPa were considered to have significant liver inflammation; for such patients, we recommend that clinicians consider performing a liver biopsy for pathological diagnosis or combine this result with other clinical indicators to comprehensively evaluate the need for further treatment. In contrast, patients with LSM ≥7.5 kPa are considered to have severe liver inflammation, and we recommend that antiviral therapy be immediately started for such patients. Patients with LSM values <6.3 kPa need no further examination, and require only regular review. The cutoff LSM value for A≥2 in this study (6.3 kPa) was lower than that required for the diagnosis of significant liver fibrosis according to the Chinese consensus on the clinical application of transient elastography for the detection of liver fibrosis.22 Therefore, the LSM value of 6.3 kPa was more significant for starting antiviral therapy in patients with chronic HBV infection, despite the ULN for ALT. Moreover, the internal, external, and prospective validations showed that the diagnostic accuracy of LSM for predicting A≥2 and A=3 was high, and that there were no significant differences in AUCs among the four groups.
Finally, we further analyzed the factors related to significant liver inflammation in the cohort I. Logistic regression analysis revealed that LSM and globulin (GLOB) values were independent predictors of A≥2. Consistent with this, some studies have found that globulin may be clinically useful for predicting liver inflammation in patients with chronic liver disease.26,27 Hence, we used the combination of LSM and GLOB values to establish a diagnostic model named LG. Comparative analysis of the AUCs showed that the LG model had a better diagnostic value than commonly used clinical markers of liver inflammation. The AUCs of the LG model in the three cohorts with different ULNs for ALT were all significantly higher than the AUCs of GLOB, ALT, and AST levels, which are usually considered related to liver inflammation. However, the AUCs of the LG model were similar to those of LSM. Several studies have recommended the revision of the ULN for ALT levels;8,28,29 however, our study showed that LSM had the same diagnostic value for liver inflammation even when different criteria were applied for the ULN for ALT levels.
Several limitations of our study should be acknowledged. First, it was a multicenter, cross-sectional study, but the number of patients with severe liver inflammation (A=3) was relatively small, which may have led to selection bias. Second, the number of patients with HBV DNA below the lower limit of detection was insufficient in this study, and hence, the study population could not represent the overall population of patients with chronic HBV infection and normal ALT levels. Thus, further validation of our results in these patients is still needed.
Our results show that LSM values were indicative of liver inflammation, with high accuracy in predicting significant liver inflammation. The dual features of LSM may help clinicians in deciding whether to start antiviral therapy rather than simply setting a different cutoff value to distinguish liver inflammation and liver fibrosis stage. Despite the criteria used for the ALT ULN, patients with chronic HBV infection should be initially screened with LSM evaluation based on FibroScan, which will help to determine whether further examination and treatment are needed.
Supporting information
ALT, alanine aminotransferase; LSM, liver stiffness measurement.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AUC
area under the receiver operating characteristic curve
- CI
confidence interval
- GLOB
globulin
- HBV
hepatitis B virus
- HBsAg
hepatitis B surface antigen
- HBeAg
hepatitis B envelope antigen
- LSM
liver stiffness measurement
- NPV
negative predictive value
- OR
odds ratio
- PPV
positive predictive value
- SD
standard deviation
- SE
standard error
- TBIL
total bilirubin
- ULN
upper limit of normal
Ethical statement
Written informed consent from the patients was not required due to the retrospective study design. The study was approved by the institutional review committee of Fujian Medical University, Fuzhou, Fujian Province, China.
Data sharing statement
All data are available upon request.
References
- 1.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–1555. doi: 10.1016/s0140-6736(15)61412-x. [DOI] [PubMed] [Google Scholar]
- 2.Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 4.Hou J, Wang G, Wang F, Cheng J, Ren H, Zhuang H, et al. Guideline of Prevention and Treatment for Chronic Hepatitis B (2015 Update) J Clin Transl Hepatol. 2017;5(4):297–318. doi: 10.14218/JCTH.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- 7.Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC, Public Policy Committee of the American Association for the Study of Liver D Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47(4):1363–1370. doi: 10.1002/hep.22109. [DOI] [PubMed] [Google Scholar]
- 8.Duan M, Chi X, Xiao H, Liu X, Zhuang H. High-normal alanine aminotransferase is an indicator for liver histopathology in HBeAg-negative chronic hepatitis B. Hepatol Int. 2021;15(2):318–327. doi: 10.1007/s12072-021-10153-2. [DOI] [PubMed] [Google Scholar]
- 9.Lai M, Hyatt BJ, Nasser I, Curry M, Afdhal NH. The clinical significance of persistently normal ALT in chronic hepatitis B infection. J Hepatol. 2007;47(6):760–767. doi: 10.1016/j.jhep.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Chao DT, Lim JK, Ayoub WS, Nguyen LH, Nguyen MH. Systematic review with meta-analysis: the proportion of chronic hepatitis B patients with normal alanine transaminase </= 40 IU/L and significant hepatic fibrosis. Aliment Pharmacol Ther. 2014;39(4):349–358. doi: 10.1111/apt.12590. [DOI] [PubMed] [Google Scholar]
- 11.Ilic I, Milovanovic T. The risk-benefit assessment of liver biopsy in times of noninvasive screening for liver fibrosis. J Hepatol. 2020;73(3):701–702. doi: 10.1016/j.jhep.2020.05.017. [DOI] [PubMed] [Google Scholar]
- 12.European Association for Study of L, Asociacion Latinoamericana para el Estudio del H EASL-ALEH Clinical Practice Guidelines: Noninvasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237–264. doi: 10.1016/j.jhep.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Huang LL, Yu XP, Li JL, Lin HM, Kang NL, Jiang JJ, et al. Effect of liver inflammation on accuracy of FibroScan device in assessing liver fibrosis stage in patients with chronic hepatitis B virus infection. World J Gastroenterol. 2021;27(7):641–653. doi: 10.3748/wjg.v27.i7.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye J, Wang W, Feng S, Huang Y, Liao X, Kuang M, et al. Precise fibrosis staging with shear wave elastography in chronic hepatitis B depends on liver inflammation and steatosis. Hepatol Int. 2020;14(2):190–201. doi: 10.1007/s12072-020-10017-1. [DOI] [PubMed] [Google Scholar]
- 15.Zeng J, Zheng J, Jin JY, Mao YJ, Guo HY, Lu MD, et al. Shear wave elastography for liver fibrosis in chronic hepatitis B: Adapting the cut-offs to alanine aminotransferase levels improves accuracy. Eur Radiol. 2019;29(2):857–865. doi: 10.1007/s00330-018-5621-x. [DOI] [PubMed] [Google Scholar]
- 16.Dong XQ, Wu Z, Li J, Wang GQ, Zhao H, China Hep BRFARG. Declining in liver stiffness cannot indicate fibrosis regression in patients with chronic hepatitis B: A 78-week prospective study. J Gastroenterol Hepatol. 2019;34(4):755–763. doi: 10.1111/jgh.14498. [DOI] [PubMed] [Google Scholar]
- 17.Jiang K, Zhang L, Li J, Hu H, Huang Q, Qiu T, et al. Diagnostic efficacy of FibroScan for liver inflammation in patients with chronic hepatitis B: a single-center study with 1185 liver biopsies as controls. BMC Gastroenterol. 2022;22(1):37. doi: 10.1186/s12876-022-02108-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24(2):289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 19.Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology. 2015;61(3):1066–1079. doi: 10.1002/hep.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Czaja AJ. Hepatic inflammation and progressive liver fibrosis in chronic liver disease. World J Gastroenterol. 2014;20(10):2515–2532. doi: 10.3748/wjg.v20.i10.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barr RG, Wilson SR, Rubens D, Garcia-Tsao G, Ferraioli G. Update to the Society of Radiologists in Ultrasound Liver Elastography Consensus Statement. Radiology. 2020;296(2):263–274. doi: 10.1148/radiol.2020192437. [DOI] [PubMed] [Google Scholar]
- 22.Chinese Foundation for Hepatitis P, Control, Chinese Society of Infectious D, Chinese Society of Hepatology CMA, Liver Disease Committee of Chinese Research Hospital A Consensus on clinical application of transient elastography detecting liver fibrosis: a 2018 update. Zhonghua Gan Zang Bing Za Zhi. 2019;27(3):182–191. doi: 10.3760/cma.j.issn.1007-3418.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Ji D, Chen Y, Shang Q, Liu H, Tan L, Wang J, et al. Unreliable Estimation of Fibrosis Regression During Treatment by Liver Stiffness Measurement in Patients With Chronic Hepatitis B. Am J Gastroenterol. 2021;116(8):1676–1685. doi: 10.14309/ajg.0000000000001239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L, Zhu M, Cao L, Yao M, Lu Y, Wen X, et al. Liver Stiffness Measurement Can Reflect the Active Liver Necroinflammation in Population with Chronic Liver Disease: A Real-world Evidence Study. J Clin Transl Hepatol. 2019;7(4):313–321. doi: 10.14218/JCTH.2019.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin M, Glaser KJ, Manduca A, Mounajjed T, Malhi H, Simonetto DA, et al. Distinguishing between Hepatic Inflammation and Fibrosis with MR Elastography. Radiology. 2017;284(3):694–705. doi: 10.1148/radiol.2017160622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Xu H, Qu L, Wang X, Wu R, Gao X, et al. Red blood cell distribution width and globulin, noninvasive indicators of fibrosis and inflammation in chronic hepatitis patients. Eur J Gastroenterol Hepatol. 2016;28(9):997–1002. doi: 10.1097/MEG.0000000000000662. [DOI] [PubMed] [Google Scholar]
- 27.Liu XD, Wu JL, Liang J, Zhang T, Sheng QS. Globulin-platelet model predicts minimal fibrosis and cirrhosis in chronic hepatitis B virus infected patients. World J Gastroenterol. 2012;18(22):2784–2792. doi: 10.3748/wjg.v18.i22.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137(1):1–10. doi: 10.7326/0003-4819-137-1-200207020-00006. [DOI] [PubMed] [Google Scholar]
- 29.Lee JK, Shim JH, Lee HC, Lee SH, Kim KM, Lim YS, et al. Estimation of the healthy upper limits for serum alanine aminotransferase in Asian populations with normal liver histology. Hepatology. 2010;51(5):1577–1583. doi: 10.1002/hep.23505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ALT, alanine aminotransferase; LSM, liver stiffness measurement.