Abstract
Introduction
Black Americans are disproportionately burdened by Alzheimer's disease (AD) relative to other racial groups in the United States and continue to be underrepresented in AD clinical trials. This review explores the primary barriers for participation in clinical trials among Black Americans and provides literature‐based recommendations to improve the inclusion of Black Americans in AD clinical trials.
Methods
We searched electronic databases and gray literature for articles published in the United States through January 1, 2023, ultimately identifying 26 key articles for inclusion.
Results
Barriers to participation in clinical trials for Black Americans are rooted in social determinants of health, including access to quality education and information, access to health care, economic stability, built environment, and community context. Best practices to improve the inclusion of Black Americans in clinical trials require pharmaceutical companies to adopt a multifaceted approach, investing in innovative strategies for site selection, development of local partnerships, outreach, and education.
Discussion
While multisectoral action must occur to effectively address the disproportionate burden of AD on Black Americans, the pharmaceutical industry has an important part to play in this space due to their central role in product development and clinical trials.
Keywords: Alzheimer's disease, Black Americans, barriers, best practices, clinical trials, community engagement, health equity, market access, pharmaceutical intervention, racial disparities, treatment, social determinants of health
1. INTRODUCTION
1.1. Health disparities in the United States
Long‐standing institutional structures and systems have created barriers for Black Americans to access the same quality of resources and opportunities relative to White Americans in the United States. 1 Today, while many efforts have taken place to address historical racial injustices, systems of power continue to both intentionally and unintentionally perpetuate racial inequities. This has led to a present‐day environment in which Black populations continue to face different lived experiences relative to White populations. These differences in lived experience are not subtle and pervade every aspect of life, including access to quality education, health care, living environments, and job opportunities (Figure 1). 2
FIGURE 1.

The social determinants of health, Healthy People 2030. 6
Furthermore, these racial disparities have consequences on health outcomes. The Centers for Disease Control (CDC) explains that as a result of social determinants, communities of color in the United States are at greater risk of poor health outcomes relative to White populations. 3 For example, factors such as socioeconomic stressors, environmental stressors, and discrimination, cause Black Americans to experience greater social and biological stress. This prolonged stress, also known as allostatic load, is a risk factor for many chronic conditions—such as cardiovascular disease, diabetes, obesity, depression, inflammatory and autoimmune disorders, cognitive impairment, as well as age‐related diseases—positioning Black Americans at higher risk for disease. 4 , 5 , 6
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using traditional sources as well as gray literature with the goal of identifying the key barriers and solutions to Alzheimer's disease (AD) clinical trial participation for Black Americans. These relevant citations are appropriately noted.
Interpretation: Understanding the primary barriers and solutions to AD clinical trial participation for Black Americans is critical to allow for effective and targeted intervention to advance health equity. The review shares literature‐based recommendations to guide industry in advancing equity in AD clinical trial participation for Black Americans.
Future directions: As this space is rapidly evolving, future research must be done to refine recommendations, incorporate lessons learned, and identify new opportunities for pharmaceutical companies to support communities at a local level and advance the inclusion of Black Americans in AD research.
1.2. Racial disparities in Alzheimer's disease
An estimated 10.7% (6.7 million) of Americans 65 and older have Alzheimer's disease (AD) and this number is expected to nearly triple by 2060 as the US population continues to age and as life expectancy rises. 2 However, this statistic is not consistent across racial groups in the United States. In fact, when broken down by race, this data point highlights significant racial disparities in disease burden, as 18.6% of Black Americans > 65 have AD, compared to 14% of Hispanics and just 10% of Whites > 65. 7 Literature suggests that racial inequities and social determinants of health–such as discrimination, economic stability, education access and quality, health‐care access and quality, neighborhood and built environment, and social and community context—are key forces contributing to the racial disparities in AD. 2 , 6
In the context of access to education, greater educational opportunity and attainment is associated with a reduced risk of AD. 8 , 9 , 10 , 11 , 12 , 13 , 14 However, as a result of racial disparities in access to quality education across the United States, Black Americans have lower educational attainment on average relative to White Americans. 15 Similarly, higher occupational attainment is also associated with lower risk of AD, yet because of disparities in educational attainment and racial discrimination in the workplace, Black Americans experience greater challenges in occupational attainment relative to White Americans. 11
Lower socioeconomic status is also associated with a higher risk of AD and, due to historical injustices and contemporary structural inequities, Black Americans are twice as likely as White Americans to experience poverty in the United States. 4 , 8 , 13 , 16 , 17 , 18 As lower socioeconomic status increases everyday physiological stress and consequential allostatic load, populations facing financial hardships are at higher risk of developing AD. 4 , 16 Similarly, residential segregation and “related experiences of social segregation” are also associated with higher risk of AD. 14 The adverse experiences and discrimination associated with racial segregation have put Black Americans at higher risk of developing cognitive impairment, such as AD, later in life. 16 , 19 , 20 , 21 , 22 , 23
Finally, due to social, environmental, and economic factors, there are several co‐morbidities that more commonly impact Black Americans relative to White Americans, such as hypertension and diabetes. These chronic conditions are also risk factors for AD, placing Black Americans at greater risk. 13 , 21 , 22 Future research is needed to better understand the interactions among AD and the chronic diseases that disproportionately impact Black Americans. 21
Social determinants of health not only help explain the roots of racial disparities in AD, but also how these disparities continue to be perpetuated today. 1 , 22 For example, Black Americans are twice as likely to develop AD relative to White Americans and less likely to receive appropriate care in health‐care settings or have access to novel treatments. 2 , 22 A 2020 survey conducted by the Alzheimer's Association demonstrated that half of Black Americans in the United States report experiences of racial discrimination in health‐care settings. 22 Similarly, fewer than half of Black Americans feel confident they have access to culturally competent providers, while nearly two thirds of Black Americans believe that medical research is biased against non‐White populations. 22 Therefore, not only do disparities in access to and quality of health‐care resources result in different health outcomes between Black and White populations, but factors such as mistrust of the health‐care system also perpetuate health disparities, as Black Americans are less likely to seek and receive quality care.
Other social factors, such as lack of health literacy and cultural norms also contribute to an environment in which AD is misunderstood in many communities. 1 , 2 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 For example, an Alzheimer's Association survey highlights that more than half of non‐White Americans believe cognitive decline and significant memory loss are a “normal part of aging.” 22 The consequences of these experiences accumulate across the life course, resulting in striking disparities in burden of disease between Black and White populations in the United States. 30 , 31 , 32
1.3. The role of clinical trials in advancing health equity in AD
While addressing these pervasive disparities in AD will require multisectoral collaboration and intervention, the pharmaceutical industry has a critical part to play in this process due to its role in the development of AD treatments. Clinical trials are a critical component of the product development process, as they create pathways to product access for populations through community engagement, disease awareness and education, and testing efficacy. 23 , 33 , 34 , 35 When patient populations and communities are excluded from the clinical trial process, they lose the opportunity to engage in both education and treatment initiatives. 22 , 23 Current data on clinical trial participation highlights striking racial disparities, revealing that Black Americans make up 13.6% of the US population, yet only 5% of clinical trial participants. 36
This disparity is even more pronounced in AD, as Black Americans are twice as likely as White Americans to be impacted by the disease yet make up just 2% of AD clinical trial participants. 8 , 37 This further perpetuates the existing racial inequities in AD, as there is a lack of data regarding the efficacy and side effects of AD treatments for racial groups underrepresented in clinical trials. 37 Comprehensive clinical trial design and market access (the ability to consistently provide individuals with the treatments they need) strategy are important to ensure AD products are accessible to the populations that are most impacted by the disease. Outreach must engage diverse populations, representative of the burden of disease, to create equitable pathways for product access.
There are few studies that explore the role of the pharmaceutical industry in addressing racial disparities in AD or successful strategies for pharmaceutical intervention. Furthermore, the current social and political climate of AD and drug development makes this topic even more significant today, as pharmaceutical companies are in critical stages of product development for AD treatments and the importance of clinical trials will only continue to grow. Herein, we explore the primary barriers impacting Black American participation in clinical trials, solutions to improve access, and the critical role the pharmaceutical industry can play to advance racial equity in AD. To conclude, we share key, literature‐based recommendations, which serve to guide the pharmaceutical industry in championing the advancement of health equity in AD through clinical trials.
Key Research Questions:
1. What are the primary barriers impacting the participation of Black Americans in AD pharmaceutical clinical trials?
2. What are effective strategies pharmaceutical companies can implement to improve access to clinical trials for Black Americans?
2. METHODS
2.1. Search strategy
This review intentionally implements a broad search strategy to allow for the inclusion of research that reflects the most current landscape of barriers and best practices to advance health equity in pharmaceutical clinical trials. To identify relevant literature, electronic databases PubMed, Scopus, and Google Scholar were searched for articles and reports published in the United States through January 1, 2023. References of identified literature were examined for relevant articles and searches were also performed to identify gray literature from reputable sources. Searches were conducted using five primary search term concepts as well as several related search terms to optimize the identification of relevant literature. Articles eligible for inclusion were those that identified barriers to participation in AD clinical trials for Black Americans and/or explored solutions for advancing equity and inclusion in clinical trial processes for AD. To meet inclusion criteria, articles had to be written in English and published in the United States. Articles published after January 1, 2023, were excluded from review. There were no exclusion criteria based on peer review, type of publication, or study design. We screened article titles and abstracts for topical relevance and omitted articles that did not meet the inclusion criteria described above.
2.2. Search terms
Alongside a public health informationist, we developed search term concepts and conducted a systematic search of our primary databases to explore current literature. Five key search term concepts were included: (1) “African Americans”[mesh] OR “African American*”[tw] OR “Black American*”[tw] OR “Afro American*”[tw]; (2) “Alzheimer disease”[mesh] OR “Alzheimer's disease*”[tw] OR “Alzheimers disease*”[tw] OR “Alzheimer disease*”[tw]; (3) “clinical trials*”[tw] OR “clinical trial*”[tw] OR “pharmaceutical*”[tw] OR “research*”[tw]; (4) “inclusion*”[tw] OR “participation*”[tw] OR “strateg*”[tw] OR “recommendations*”[tw]; and (5) “barriers*”[tw] OR “disparit*”[tw] OR “exclusion*”[tw] OR “exclude*”[tw]. Search terms were adapted to each unique database used.
3. RESULTS
3.1. Search outcomes
Our initial search of PubMed and Scopus yielded 27 unique results. After a full‐text screening of the identified literature, 10 articles were excluded from review because they did not meet the inclusion criteria. After a subsequent search of Google Scholar, reputable gray literature sources, and an examination of the references of identified literature, 9 additional articles were added for review. Table 1 provides a summary of the final 26 articles identified for inclusion.
TABLE 1.
Overview of articles examined.
| Author and year | Article title | Type of source | Study design | Findings |
|---|---|---|---|---|
| Alzheimer's Association, 2002 | African American's and Alzheimer's disease: a silent epidemic | Report | NA | Black Americans are underrepresented in AD clinical trials and community outreach efforts must be redesigned for inclusion. |
| Alzheimer's Association, 2008 | Serving African American families: home and community‐based services for people with dementia and their caregivers. | Toolkit | NA | Recommended ways to support Black populations impacted by AD include providing culturally sensitive educational materials on dementia and caregiving, increasing awareness of AD through community outreach, facilitate connections to resources and services, develop dementia‐competent services to provide support to caregivers. |
| Alzheimer's Association, 2021 | 2021 Alzheimer's disease facts and figures, special report: race, ethnicity and Alzheimer's in America | Annual report | NA | Differences in lived experience and clinical trial design explain how Black populations are underrepresented in AD clinical trials. |
| Babulal et al., 2020 | Perspectives on ethnic and racial disparities in Alzheimer's disease and related dementias: Update and areas of immediate need | Qualitative research | Review | Recommendations to increase inclusion of Black Americans = in AD clinical trials include developing trainings for practitioners and researchers to improve cultural competence, creating educational materials for communities, and incorporating the impact of social determinants of health into AD trial design. |
| Ballard et al., 2013 | Challenges and opportunities: recruitment and retention of African Americans for Alzheimer disease research: lessons learned. | Qualitative research | Community research participation model | The transparency, accountability, and behavior of the trial researchers is critical in determining Black American participation in AD clinical trials. |
| Baquet et al., 2008 | Clinical trials: the art of enrollment | Qualitative research | Literature review | Key barriers to participation include SES, distance to trial site, awareness, trust, fear, cultural competency, and costs. Strategy to improve diversity in clinical trials must include increasing research on barriers to participation in each community context, developing diverse teams, developing educational materials, creating sustainable trial infrastructure in community settings. |
| Clark et al., 2019 | Increasing diversity in clinical trials: overcoming critical barriers | Qualitative research | Literature review | Solutions for increasing diversity in clinical trials include building trust, increasing communication, and developing a common understanding of goals, growing awareness of clinical trials, revisioning the role of the study coordinator, ensuring proper resources. A multi‐stakeholder approach is critical. |
| Coakley et al., 2011 | Dialogues on diversifying clinical trials: successful strategies for engaging women and minorities in clinical trials | Report | NA | Increasing diversity in clinical trials requires strategy related to the inclusion of more female and minority physicians, improved transparency, greater cultural sensitivity in outreach, increased education and outreach, intentional community engagement, and an increased use of technology. |
| Cocroft et al., 2020 | Racially diverse participant registries to facilitate the recruitment of African Americans into presymptomatic Alzheimer's disease studies | Qualitative research | Systematic review | Five key lessons regarding the creation of diverse registries include “(1) cultural sensitivity (2) building trust relationships (3) inclusive access to research (4) ongoing connection with research participants (5) allocating resources to meet the costs.” |
| Denny et al., 2018 | Perspective on the “African American participation in Alzheimer disease research: Effective strategies” | Qualitative research | Workshop and systematic review | Key learnings and recommendations to increase Black American participation in AD research include building awareness, increasing evidence‐based recruitment strategies, practicing transparency, building diverse teams, training on cultural competency, developing long‐term community relationships and partnerships, offering incentives. |
| Esiaka et al., 2022 | A Mini‐Review of Strategies for Recruiting Older African Americans to Alzheimer's Disease | Qualitative research | Mini‐review | Strategies to advance participation of Black American populations in AD clinical trials include community outreach, education, at home access, and partnership with local organizations. |
| Gilmore‐Bykovskyi et al., 2019 | Recruitment and retention of underrepresented populations in Alzheimer's disease research: A systematic review. | Qualitative research | Systematic review | Attitudes, barriers/facilitators, education, trust, and religiosity are key factors influencing Black American participation in AD clinical trials. |
| Graham et al., 2018 | Best strategies to recruit and enroll elderly Blacks into clinical and biomedical research | Quantitative and qualitative research | Retrospective study and community outreach | Health fairs, educational materials and advertisements tailored to communities’ unique health interests and needs, and personal referrals were the most successful means of recruiting Black populations to AD clinical trials. |
| Hughson et al., 2016 | A review of approaches to improve participation of culturally and linguistically diverse populations in clinical trials | Qualitative research | Systematic review | Significant barriers to participation in clinical trials for diverse populations include mistrust, communication and language, culture, SES, time, and transportation. Suggested strategies to address these barriers include community outreach and relationship building, improved communication tactics, greater cultural sensitivity, improving physical access to trials, and educating companies on common barriers and pitfalls to inclusion. |
| Indorewalla et al., 2021 | Modifiable barriers for recruitment and retention of older adults participants from underrepresented minorities in Alzheimer's disease research | Qualitative research | Narrative review | Barriers to participation in AD clinical trials for Black populations include mistrust, lack of awareness, the need for a study partner, and participant burden. |
| Langbaum et al., 2022 | Recommendations to address key recruitment challenges of Alzheimer's disease clinical trials | Qualitative research | Advisory panel | The panel identified 27 solutions to improve current AD clinical trial processes, including community outreach, virtual clinical trials, and educational campaigns. |
| Lincoln et al., 2018 | Fundamental causes of barriers to participation in Alzheimer's clinical research among African Americans | Qualitative research | Focus group analysis | Factors that influence beliefs and participation in clinical trials among Black American populations include (1) lived experience and discrimination, (2) cultural trauma, (3) cultural norms, (4) a lack of cultural competency in recruitment methods. Recommended ways to increase diversity in clinical trials include transparency, culturally appropriate community outreach and education, and addressing mistrust of research processes. |
| Lines et al., 2014 | Racial and ethnic disparities among individuals with Alzheimer's disease in the United States: a literature review | Qualitative research | Literature review | Potential contributors to racial disparities in AD include genetics, comorbidities, poverty, education level, culture, discrimination. Highlight importance of equal provision of health services to advance equity. |
|
National Institute for Aging, 2020 |
NIA: strategic directions for research 2020‐2025 | Report | NA | Causes of health disparities are multidimensional, and therefore require multifaceted solutions to effectively address them. Including diverse populations in research will require new strategy such as the implementation of community based participatory research methods. |
| Robinson et al., 2020 | Framework for creating storytelling materials to promote African American/Black adult enrollment in research on Alzheimer's disease and related disorders | Quantitative and qualitative research | Semi‐structured interviews | Culturally relevant communication and educational materials are critical in the recruitment of Black populations to AD clinical trials. |
| Shaw et al., 2020 | Recruitment of older African Americans in Alzheimer's disease clinical trials using a community education approach | Qualitative research | Community engaged research approach | Community based recruitment methods increase AD knowledge, clinical trial interest, as well as recruitment into “observational and lifestyle” AD clinical trials. |
| Shin et al., 2016 | Underrepresentation of African Americans in Alzheimer's Trials: A Call for Affirmative Action | Article | NA | Recommendations for increasing inclusion of African American populations in AD clinical trials include developing relationships with community‐based organizations and dedicating budget specifically to the recruitment of minority populations. |
| U.S. Food and Drug Administration, 2022 | Diversity plans to improve enrollment of participants from underrepresented racial and ethnic populations in clinical trials: draft guidance for industry | Guidance document | NA | To increase diversity in clinical trials companies must design strategy in a way that accounts for factors such as site location and access, community engagement, and ways to streamline processes to ease the burden of participation for patients. |
| Weiner et al., 2007 | Alzheimer's disease demonstration grants to states program: cross‐state report on initiatives targeting limited English‐speaking populations and African American communities | Report | NA | There are several areas critical to better engaging diverse populations in AD research: (1) Building trust in communities by investing in relationships and partnering with community organizations, (2) building greater awareness and understanding of AD, (3) using culturally appropriate language and strategies, and (4) understanding the role of family in different contexts. |
| Woods‐Burnham et al., 2021 | The role of diverse populations in us clinical trials | Commentary | NA | Increasing diversity in clinical trials requires more diversity among physician teams, community partnerships, greater education and awareness, cultural competency and diversity trainings for clinical trial staff, and greater consideration around cost and transportation. |
| Zhou et al., 2017 | African Americans are less likely to enroll in preclinical Alzheimer's disease clinical trials. | Quantitative and qualitative research | Secondary analysis of interviews (a mixed‐methods experimental design) | Black American populations called attention to the importance of factors such as study risks, the requirement of a study partner, study procedures, and study location as strong influences on participation. |
Abbreviations: AD, Alzheimer's disease; NA, not applicable; SES, socioeconomic status.
3.2. Barriers to clinical trial participation for Black Americans
Barriers to pharmaceutical clinical trial participation are multifaceted and rooted in the differences in lived experience between Black and White populations (Figure 2). The literature demonstrates Black Americans hold greater mistrust in the health‐care system relative to White Americans due to both historical injustices in scientific research and present‐day discrimination in health‐care settings. 1 , 2 , 22 , 23 As a result, they are also less likely to feel comfortable participating in the clinical trial process. 1 , 2 , 22 , 23 Furthermore, due to racial disparities in access to quality information and education, Black Americans experience lower levels of awareness and understanding of AD relative to White populations. 1 , 25 , 38 Cultural norms and stigmas also influence participation. The Alzheimer's Association explains that not only is the concern for developing AD lower among Black Americans relative to White Americans, but approximately one in five Black Americans report that they would “feel insulted if a doctor suggested a cognitive assessment.” 22
FIGURE 2.

Top barriers to clinical trial participation for Black Americans.
Physical barriers to participation also exist. As a result of structural inequities, Black Americans are twice as likely to experience poverty relative to White Americans. 2 , 22 This disparity in socioeconomic status not only impacts economic stability, but also housing environment and community context, transportation options, and the amount of spare time one might have in the day to participate in a clinical trial. 39 Together, these factors inhibit Black Americans from participating in clinical trials. 1 , 2 , 22 , 23 , 34 , 38
3.3. Improving access to clinical trials for Black Americans
The included literature highlights a series of tangible best practices to improve equity in clinical trials and market access for Black Americans (Figure 3). First and foremost, far before a clinical trial begins, pharmaceutical companies must begin building sustainable, long‐term relationships in communities. 33 , 34 , 38 , 40 , 41 , 42 , 43 , 44 This is done for the purpose of demonstrating investment in community well‐being as well as gaining a stronger understanding of community needs before initiating a research process. Research suggests developing relationships through organizations central to the community (included examples: community centers, churches, barber shops, and schools) are successful methods to begin engagement. 33 , 34 , 45 , 46 Second, site selection is also critical, as it makes clinical trials more visible and accessible to diverse populations. 38 , 39 , 41 , 47 Not only do sites need to be in locations that are physically accessible to diverse populations, but they also must be in locations that are trusted by the community. 38 , 48 , 49 However, research shows that developing a site in a diverse area cannot be viewed as a stand‐alone solution, and subsequent actions must occur to make the engagement process successful. 50 , 51
FIGURE 3.
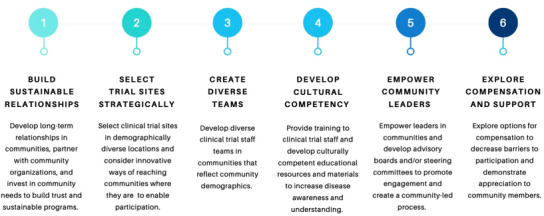
Best practices to improve clinical trial participation for Black Americans .
Third, clinical trial staff teams must reflect the demographics of the populations being served. 40 , 41 , 42 , 43 , 44 , 47 Failure to build diverse clinical trial staff teams erodes trust and decreases participation of non‐White participants in clinical trials. 42 , 44 Fourth, clinical trial teams must engage in cultural competency trainings to better understand the unique contexts of the communities being served. 33 , 38 , 42 , 52 , 53 , 54 Teams should also develop culturally competent educational materials for communities that fit the specific context of each population and address the questions, concerns, or information gaps that may exist. 33 , 34 , 38 , 40 , 42 , 43 , 44 , 47 , 54 , 55 These materials should be easily accessible in trusted community settings, outside of health‐care facilities. 51 Fifth, community leaders and influencers are key partners. Gaining the buy‐in, support, and participation of these community members is critical in building awareness, acceptance, and maximum participation. 33 , 34 , 38 , 40 , 43 , 52 Finally, as community members are sacrificing valuable time and resources to participate, they should be supported and compensated throughout the process. 34 , 41 , 56 , 57 This can occur in the form of gift cards, meals, transportation, or other incentives that may help ease the burden of participation. 34 , 39 , 41 , 42 , 44 , 47
4. DISCUSSION
4.1. The current climate of product development for AD
For decades pharmaceutical companies have competed to win the race for approval of the first ever disease modifying AD treatment. 58 In June 2021, Biogen became the first pharmaceutical company to gain Food and Drug Administration (FDA) approval for a disease modifying therapy with the release of their product, Aduhelm (aducanumab). 59 While this was celebrated as a major milestone in AD research and treatment, many experts expressed skepticism regarding the true potential impact of the drug due to high costs, unknown efficacy, and a non‐representative clinical trial population. 60 After intense backlash, Aduhelm's initial list cost of $56,000 per patient per year was reduced to a list price of $28,000 per year; however, this price tag remained prohibitively high for most of the US population. 61 To further complicate this environment, the US government announced in April 2022 that Medicare would only cover costs of Aduhelm for clinical trial participants, limiting access to the product. 62 This decision yielded even greater inequities in product access due to Biogen's phase 3 clinical trial practices, as zero participants identified as Black out of > 3,000 participants, across 348 sites, in 20 countries. 63 Thus, as a result of clinical trial processes as well as national policies around cost and coverage, the product became virtually inaccessible to Black Americans, further reinforcing disparities in AD.
However, as a result of growing concern around the lack of diversity in clinical trials, Biogen promised to increase racial diversity for ENVISION phase 4 clinical trials stating that 18% of participants would be from Black and Latino populations. 64 — 65 In April 2022, the Center for Medicare and Medicaid Services (CMS) also announced a new National Coverage Determination (NCD) for monoclonal antibodies directed against amyloid for treatment of AD. Among other criteria, this NCD stated that, to obtain coverage, a study must include a racially diverse population “representative of the national population with MCI [mild cognitive impairment] due to AD or mild AD dementia.” 66 This shift in policy marked a significant milestone prompting pharmaceutical companies to prioritize racial diversity in clinical trials to a degree never seen before.
However, many companies have not yet developed the proper infrastructure or tools to sustainably engage with racially diverse communities and successfully overcome the barriers to participation in clinical trials. In April 2022, the FDA issued guidance to support companies through this change, recommending new tactics for site location and access, community engagement, and easing the burden of participation. 41 While this guidance serves as a helpful starting point, a change of this magnitude requires a fundamental shift in the way pharmaceutical companies implement clinical trials, develop market access strategy, and interact with communities moving forward.
4.2. Recommendations to advance the inclusion Black Americans in AD clinical trials
The literature stresses that as industry demonstrates a continued investment in the well‐being of populations, they will become increasingly valued and trusted members of the community. Experts explain that the most impactful step industry can take to begin improving equity in AD is to become more engaged members of diverse communities by listening to and supporting community needs. 33 , 38 , 39 , 40 , 47 , 50 , 52 , 55 In current efforts to engage diverse populations in AD clinical trials, this element is still largely missing, as it requires a long‐term investment of time and resources in communities. However, increased diversity in clinical trials cannot be achieved without the prioritization of sustainable mechanisms for inclusion.
We recommend several concrete steps to improve clinical trial strategy and advance equity in AD. Research in the field of oncology demonstrates that a “multipronged” approach results in improved inclusion of Black Americans in clinical trials, highlighting the importance of implementing the below recommendations together. 67
4.2.1. Fostering sustainable relationships in communities
As a component of a larger strategy to build sustainable relationships in communities, pharmaceutical companies can work to gain a strong understanding of community context and needs through increased community‐based participatory research methods. 34 , 39 , 48 , 50 Pharmaceutical companies can also demonstrate their long‐term commitment to communities by establishing a consistent presence in the community, facilitating connections to resources, and supporting community programs and organizations. Partnering with community organizations and patient advocacy groups can not only help companies build key relationships in communities but can allow companies to gain a stronger understanding of community needs. To support community needs and engage with community members impacted by AD, companies might also consider providing support programs and services for the informal caregivers of AD patients. 38 , 48
4.2.2. Prioritizing community‐led processes
Pharmaceutical companies should aspire to implement human‐centered design tactics, such as including community members and leaders in the clinical trial design process to develop targeted and effective solutions for inclusive clinical trial engagement. To create a community‐led clinical trial process and promote community engagement, pharmaceutical companies can also aim to build advisory boards and steering committees comprised of diverse stakeholders and community members. 46 , 52 This allows companies to incorporate the perspectives of the communities they aim to serve in all aspects of the clinical trial process.
4.2.3. Building diverse teams and cultural competency
Pharmaceutical companies can provide training to employees with the goal of building teams in the field that better understand the barriers to participation experienced by diverse populations. 44 , 52 , 53 Furthermore, staff in the field should be demographically representative of the target population. 36 , 42 , 52 One way pharmaceutical companies can achieve this is by striving to include community members themselves on the clinical trial teams. 36 , 42 , 52 This may be an important consideration when it comes to improving access for underrepresented populations within Black communities, such as Black men. 68 Finally, as educational materials are developed for clinical trial sites, it is important that they are rooted in the specific context of a community and tailored to how and where community members prefer to receive information. 38 , 39 , 40 , 42 , 49 , 51 , 54
4.2.4. Innovating access to clinical trial sites
Pharmaceutical companies must consider how to make the clinical trial process most accessible in the context of each unique community setting. 36 , 41 , 42 , 44 , 50 This could mean increasing site locations to various trusted community settings, devising more flexible site hours, creating mobile sites, offering transportation benefits, or leveraging technology for increased at‐home efficiency. 45 , 48 , 57 This also includes considering creative methods for compensation and support, such as offering financial benefits, meals, daycare, or technology to support the clinical trial process. 41 , 42 , 56
5. CONCLUSION
This review explores the role of clinical trials in advancing health equity in AD for Black Americans. The literature demonstrates that, due to differences in lived experience accumulated across the life course, not only are Black Americans twice as likely as White Americans to be impacted by AD, but they are also less likely to have access to AD clinical trials. This review brings to light important best practices to reduce barriers to clinical trial participation and advance equity in AD for Black Americans. There is great urgency to prioritize health equity in AD today, as pharmaceutical companies are currently in critical phases of product development for AD treatments. By developing more equitable practices for diversity and inclusion in clinical trials, the pharmaceutical industry can play a key role in the advancement of health equity in AD for Black Americans.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest. Author disclosures are available in the supporting information.
CONSENT STATEMENT
Consent was not necessary.
Supporting information
supplementary information
ACKNOWLEDGMENTS
The authors would like to extend their gratitude to the teams and individuals that supported the research process, including Mary Quiceno, Tanesha Tyler‐Carr, Greg White, Jochen Fleischmann, Joyce Balls‐Berry, David Henley, Allitia DiBernardo, Donna Hesson, Paul Herman, David Savold, and Jared A. Hanson. Dr. Thorpe was supported by NIA: P30AG059298, K02AG059140, and NIMHD U54MD000214.
Savold J, Cole M, Thorpe RJ. Barriers and solutions to Alzheimer's disease clinical trial participation for Black Americans. Alzheimer's Dement. 2023;9:e12402. 10.1002/trc2.12402
REFERENCES
- 1. African American's and Alzheimer's disease: a silent epidemic . Alzheimer's Association; 2002.
- 2. Lines LM, Sherif N, Weiner JM. Racial and ethnic disparities among individuals with Alzheimer's disease in the United States: a literature review; 2014 10.3768/rtipress.2014.RR.0024.1412 [DOI]
- 3. Racism and health. Centers for Disease Control and Prevention. Accessed April 27, 2022. https://www.cdc.gov/healthequity/racism‐disparities/index.html
- 4. Yaffe K, Falvey C, Harris TB, et al. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ. 2013;347:f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fact sheet: health disparities and stress. APA. Accessed April 27, 2022. https://www.apa.org/topics/racism‐bias‐discrimination/health‐disparities‐stress
- 6. Social determinants of health: healthy people 2030. Health.gov. Accessed April 27, 2022. https://health.gov/healthypeople/priority‐areas/social‐determinants‐health
- 7. The Alzheimer's disease crisis – by the numbers. Us Against Alzheimer's. Accessed April 27, 2022. https://www.usagainstalzheimers.org/learn/alzheimers‐crisis
- 8. Peterson RL, Fain MJ, Butler A, Ehiri JE, Carvajal SC. The role of social and behavioral risk factors in explaining racial disparities in age‐related cognitive impairment: a structured narrative review. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2020;27(2):173‐96. [DOI] [PubMed] [Google Scholar]
- 9. Gupta VK, Winter M, Cabral H, et al. Disparities in age‐associated cognitive decline between African‐American and Caucasian populations: the roles of health literacy and education. J Am Geriatr Soc. 2016;64(8):1716‐23. [DOI] [PubMed] [Google Scholar]
- 10. Kukull WA, Higdon R, Bowen JD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59(11):1737‐46. [DOI] [PubMed] [Google Scholar]
- 11. Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R, Influence of education and occupation on the incidence of Alzheimer's disease. JAMA. 1994;271(13):1004‐10. [PubMed] [Google Scholar]
- 12. Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1‐2):125‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen C, Zissimopoulos JM. Racial and ethnic differences in trends in dementia prevalence and risk factors in the United States. Alzheimers Dement Transl Res Clin Interv. 2018;4:510‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev. 2008;18(3):223‐54. [DOI] [PubMed] [Google Scholar]
- 15. Ethnic and racial disparities in education. APA. Accessed April 27, 2022. https://www.apa.org/ed/resources/racial‐disparities
- 16. Zhang Z, Hayward MD, Yu YL. Life course pathways to racial disparities in cognitive impairment among older Americans. J Health Soc Behav. 2016;57(2):184‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Steenland K, Goldstein FC, Levey A, Wharton W. A meta‐analysis of Alzheimer's disease incidence and prevalence comparing African‐Americans and Caucasians. J Alzheimers Dis JAD. 2016;50(1):71‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Galvin JE, Chrisphonte S, Chang LC. Medical and social determinants of brain health and dementia in a multicultural community cohort of older adults. J Alzheimers Dis JAD. 2021;84(4):1563‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zuelsdorff M, Okonkwo OC, Norton D, et al. Stressful life events and racial disparities in cognition among middle‐aged and older adults. J Alzheimers Dis JAD. 2020;73(2):671‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Trammell AR, McDaniel DJ, Obideen M, et al. Perceived stress is associated with Alzheimer's disease cerebrospinal fluid biomarkers in African Americans with mild cognitive impairment. J Alzheimers Dis JAD. 2020;77(2):843‐53. [DOI] [PubMed] [Google Scholar]
- 21. Dilworth‐Anderson R, Hendrie HC, Manly JJ, Khachaturian AS, Fazio S. Diagnosis and assessment of Alzheimer's disease in diverse populations. Alzheimers Dement. 2008;4(4):305‐9. [DOI] [PubMed] [Google Scholar]
- 22. 2021 Alzheimer's disease facts and figures. Alzheimer's Association;2021. https://www.alz.org/media/documents/alzheimers‐facts‐and‐figures.pdf [DOI] [PubMed]
- 23. Lincoln KD, Chow T, Gaines BF, Fitzgerald T. Fundamental causes of barriers to participation in Alzheimer's clinical research among African Americans. Ethn Health. 2021;26(4):585‐99. [DOI] [PubMed] [Google Scholar]
- 24. Gupta VK, Winter M, Cabral H, et al. Disparities in age‐associated cognitive decline between African‐American and Caucasian populations: the roles of health literacy and education. J Am Geriatr Soc. 2016;64(8):1716‐23. [DOI] [PubMed] [Google Scholar]
- 25. Dilworth‐Anderson P, Hendrie HC, Manly JJ, Khachaturian AS, Fazio S. Diagnosis and assessment of Alzheimer's disease in diverse populations. Alzheimers Dement. 2008;4(4):305‐9. [DOI] [PubMed] [Google Scholar]
- 26. Glover CM, CoCroft S, James BD, Barnes LL. Perceptions of risk factors for Alzheimer disease among community‐dwelling, nondemented older African Americans. Alzheimer Dis Assoc Disord. 2019;33(3):254‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chin AL, Negash S, Hamilton R. Diversity and disparity in dementia: the impact of ethnoracial differences in Alzheimer disease. Alzheimer Dis Assoc Disord. 2011;25(3):187‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roberts JS, Connell CM, Cisewski D, Hipps YG, Demissie S, Green RC. Differences between African Americans and Whites in their perceptions of Alzheimer disease. Alzheimer Dis Assoc Disord. 2003;17(1):19‐26. [DOI] [PubMed] [Google Scholar]
- 29. Connell CM, Roberts JS, McLaughlin SJ, Akinleye D. Racial differences in knowledge and beliefs about Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23(2):110‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev. 2008;18(3):223‐54. [DOI] [PubMed] [Google Scholar]
- 31. Trammell AR, McDaniel DJ, Obideen M, et al. Perceived stress is associated with Alzheimer's disease cerebrospinal fluid biomarkers in African Americans with mild cognitive impairment. J Alzheimers Dis JAD. 2020;77(2):843‐53. [DOI] [PubMed] [Google Scholar]
- 32. Barnes LL, Bennett DA. Alzheimer's disease in African Americans: risk factors and challenges for the future. Health Aff Proj Hope. 2014;33(4):580‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cocroft S, Welsh‐Bohmer KA, Plassman BL, et al. Racially diverse participant registries to facilitate the recruitment of African Americans into presymptomatic Alzheimer's disease studies. Alzheimers Dement J Alzheimers Assoc. 2020;16(8):1107‐14. [DOI] [PubMed] [Google Scholar]
- 34. Shaw AR, Perales‐Puchalt J, Moore T, et al. Recruitment of older African Americans in Alzheimer's disease clinical trials using a community education approach; 2021. https://www.medrxiv.org/content/10.1101/2020.07.16.20155556v2 [DOI] [PMC free article] [PubMed]
- 35. Franzen S, Smith JE, van den Berg E, et al. Diversity in Alzheimer's disease drug trials: the importance of eligibility criteria. Alzheimers Dement. 2022;18(4):810‐823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Clinical trials seek to fix their lack of racial mix. AAMC. Accessed April 27, 2022. https://www.aamc.org/news‐insights/clinical‐trials‐seek‐fix‐their‐lack‐racial‐mix
- 37. Langreth R, Campbell M. Alzheimer's trials exclude black patients at ‘astonishing’ rate. Bloomberg. Accessed April 27, 2022. https://www.bloomberg.com/news/articles/2022‐04‐19/drug‐trials‐are‐more‐likely‐to‐admit‐white‐people#xj4y7vzkg
- 38. Serving African American families: home and community‐based services for people with dementia and their caregivers. Alzheimer's Association. Accessed April 27, 2022. https://www.alz.org/national/documents/aoagrant_kts_aa.pdf
- 39. Baquet CR, Henderson K, Commiskey P, Morrow JN. Clinical trials: the art of enrollment. Semin Oncol Nurs. 2008;24(4):262‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weiner J, Mitchell N, Alzheimer's disease demonstration grants to states program: cross‐state report on initiatives targeting limited English‐speaking populations and African American communities. 2007. https://www.alz.org/national/documents/aoagrant_cs_crossstate_07.pdf
- 41. Diversity plans to improve enrollment of participants from underrepresented racial and ethnic populations in clinical trials: draft guidance for industry. U.S. Food and Drug Administration. Accessed April 27, 2022. https://www.fda.gov/regulatory‐information/search‐fda‐guidance‐documents/diversity‐plans‐improve‐enrollment‐participants‐underrepresented‐racial‐and‐ethnic‐populations
- 42. Coakley M, Fadiran EO, Parrish LJ, Griffith RA, Weiss E, Carter C. Dialogues on diversifying clinical trials: successful strategies for engaging women and minorities in clinical trials. J Womens Health. 2012;21(7):713‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hughson J, Woodward‐Kron R, Parker A, et al. A review of approaches to improve participation of culturally and linguistically diverse populations in clinical trials. Trials. 2016;17(1):263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Woods‐Burnham L, Johnson JR, Hooker SE, Bedell FW, Dorff TB, Kittles RA. The role of diverse populations in us clinical trials. Med. 2021;2(1):21‐4. [DOI] [PubMed] [Google Scholar]
- 45. Langbaum JB, Zissimopoulos J, Au R, et al. Recommendations to address key recruitment challenges of Alzheimer's disease clinical trials. [published online ahead of print, 2022 Aug 10] Alzheimers Dement. 2022. 10.1002/alz.12737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shin J, Doraiswamy PM. Underrepresentation of African‐Americans in Alzheimer's trials: a call for affirmative action. Front Aging Neurosci. 2016;3(8):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Clark LT, Watkins L, Piña IL, et al. Increasing diversity in clinical trials: overcoming critical barriers. Curr Probl Cardiol. 2019;44(5):148‐72. [DOI] [PubMed] [Google Scholar]
- 48. Esiaka D, Yarborough CC, Fausto BA, Gluck MA. A mini‐review of strategies for recruiting older African Americans to Alzheimer's disease research [published online ahead of print, 2022 Sep 18]. Community Health Equity Res Policy. 2022;272684×221118493. 10.1177/0272684x221118493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gilmore‐Bykovskyi AL, Jin Y, Gleason C, et al. Recruitment and retention of underrepresented populations in Alzheimer's disease research: a systematic review. Alzheimers Dement (N Y). 2019;19(5):751‐770. Erratum in: Alzheimers Dement (N Y). 2022;6(1):e12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. NIA: strategic directions for research 2020‐2025. National Institute for Aging. Accessed April 27, 2022. https://www.nia.nih.gov/sites/default/files/2020‐05/nia‐strategic‐directions‐2020‐2025.pdf
- 51. Graham LA, Ngwa J, Ntekim O, et al. Best strategies to recruit and enroll elderly Blacks into clinical and biomedical research. Clin Interv Aging. 2017;22(13):43‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Denny A, Streitz M, Stock K, et al. Perspective on the “African American participation in Alzheimer disease research: effective strategies” workshop, 2018. Alzheimers Dement. 2020;16(12):1734‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ballard EL, Gwyther LP, Edmonds HL. Challenges and opportunities: recruitment and retention of African Americans for Alzheimer disease research: lessons learned. Alzheimer Dis Assoc Disord. 2010;24(0):S19‐23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Robinson RAS, Williams IC, Cameron JL, et al. Framework for creating storytelling materials to promote African American/Black adult enrollment in research on Alzheimer's disease and related disorders. Alzheimers Dement. 2020; 6(1), e12076. 10.1002/trc2.12076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Babulal GM, Quiroz YT, Albensi BC, et al. Perspectives on ethnic and racial disparities in Alzheimer's disease and related dementias: update and areas of immediate need. Alzheimers Dement. 2019;15(2):292‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Indorewalla KK, O'Connor MK, Budson AE, DiTerlizzi CG, Jackson J. Modifiable barriers for recruitment and retention of older adults participants from underrepresented minorities in Alzheimer's disease research. J Alzheimers Dis. 2021;80(3):927‐940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhou Y, Elashoff D, Kremen S, Teng E, Karlawish J, Grill JD. African Americans are less likely to enroll in preclinical Alzheimer's disease clinical trials. Alzheimers Dement (N Y). 2016;3(1):57‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cummings J, Lee G, Nahed P, Kambar MEZN, Zhong K, Fonseca J, Taghva K. Alzheimer's disease drug development pipeline: 2022. Alzheimers Dement (N Y). 2022;8(1):e12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Commissioner of FDA grants accelerated approval for Alzheimer's drug. FDA. Accessed April 27, 2022. https://www.fda.gov/news‐events/press‐announcements/fda‐grants‐accelerated‐approval‐alzheimers‐drug
- 60. Hamilton J. Cost and controversy are limiting use of new Alzheimer's drug. NPR. Accessed April 27, 2022. https://www.npr.org/sections/health‐shots/2021/11/08/1052833252/cost‐and‐controversy‐are‐limiting‐use‐of‐new‐alzheimers‐drug
- 61. Belluck P. Medicare proposes to sharply limit coverage of the Alzheimer's drug Aduhelm. NYT. Accessed April 27, 2022. https://www.nytimes.com/2022/01/11/health/aduhelm‐medicare‐alzheimers.html
- 62. Reed T. Medicare's limits on Aduhelm coverage sparks questions about future drugs. Axios Accessed April 27, 2022. https://www.axios.com/medicares‐limits‐on‐aduhelm‐coverage‐sparks‐questions‐about‐future‐drugs‐9e7e99b9‐a4aa‐4b08‐95be‐7a227f2d2dc6.html
- 63. Haeberlein S. EMERGE and ENGAGE topline results: two Phase 3 studies to evaluate aducanumab in patients with early Alzheimer's disease. Biogen. https://investors.biogen.com/static‐files/8e58afa4‐ba37‐4250‐9a78‐2ecfb63b1dcb
- 64. Under pressure, Biogen outlines ambitious diversity goals for Aduhelm study — but the Medicare fight is still on. Endpoints News. Accessed April 27, 2022. https://endpts.com/biogen‐announces‐new‐diversity‐benchmarks‐in‐confirmatory‐aduhelm‐trial‐while‐continuing‐to‐fight‐medicare‐decision/
- 65. Update on the phase 4 envision confirmatory study of Aduhelm. Biogen. Accessed April 27, 2022. https://investors.biogen.com/news‐releases/news‐release‐details/update‐phase‐4‐envision‐confirmatory‐study‐aduhelmr
- 66. Medicare coverage policy for monoclonal antibodies directed against amyloid for the treatment of Alzheimer's disease. CMS. Accessed April 27, 2022. https://www.cms.gov/newsroom/fact‐sheets/medicare‐coverage‐policy‐monoclonal‐antibodies‐directed‐against‐amyloid‐treatment‐alzheimers‐disease
- 67. Arring NM, Aduse‐Poku L, Jiagge E, et al. A scoping review of strategies to increase black enrollment and retention in cancer clinical trials. JCO Oncol Pract. 2022;18(9):614‐632. [DOI] [PubMed] [Google Scholar]
- 68. Tobin CST, Gutiérrez Á, Erving CL, Norris KC, Thorpe RJ. When resilience becomes risk: a latent class analysis of psychosocial resources and allostatic load among African American men. Am J Men's Health. 2022;16(3). 10.1177/15579883221104272 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary information


