Abstract
Sex work occurs in many forms and sex workers of all genders have been affected by HIV epidemics worldwide. The determinants of HIV risk associated with sex work occur at several levels, including individual biological and behavioural, dyadic and network, and community and social environmental levels. Evidence indicates that effective HIV prevention packages for sex workers should include combinations of biomedical, behavioural, and structural interventions tailored to local contexts, and be led and implemented by sex worker communities. A model simulation based on the South African heterosexual epidemic suggests that condom promotion and distribution programmes in South Africa have already reduced HIV incidence in sex workers and their clients by more than 70%. Under optimistic model assumptions, oral pre-exposure prophylaxis together with test and treat programmes could further reduce HIV incidence in South African sex workers and their clients by up to 40% over a 10-year period. Combining these biomedical approaches with a prevention package, including behavioural and structural components as part of a community-driven approach, will help to reduce HIV infection in sex workers in different settings worldwide.
Introduction
The HIV epidemic continues to have a profound effect on female, male, and transgender sex workers.1–4 The median worldwide estimates show that female sex workers (FSWs) are 13·5 (95% CI 10·0–18·1) times more likely to be living with HIV than other women,3 15% of female HIV infections in 2011 were attributed to sex workers, with the highest attributable fraction in sub-Saharan African populations (17·8%).5 Substantial proportions of new infections (10–32%) occurred as a result of sex work in West African countries. In Uganda, Swaziland, and Zambia, 7–11% of new infections could be due to sex work, sex-worker clients, and clients’ regular partners.6 The UNAIDS 2015 goal of zero infections and discrimination will need effective HIV prevention strategies for those who sell or barter for sex in every region.1,4
Sex work is diverse and occurs in various contexts around the world. Although some women sell sex through formal structures such as brothels or other venues, others might work independently and solicit clients directly in public places or via cell phone or internet.7,8 Tailoring of an effective, safe HIV prevention package for FSWs to account for the contexts in which they work and the particular risks they face is needed.7
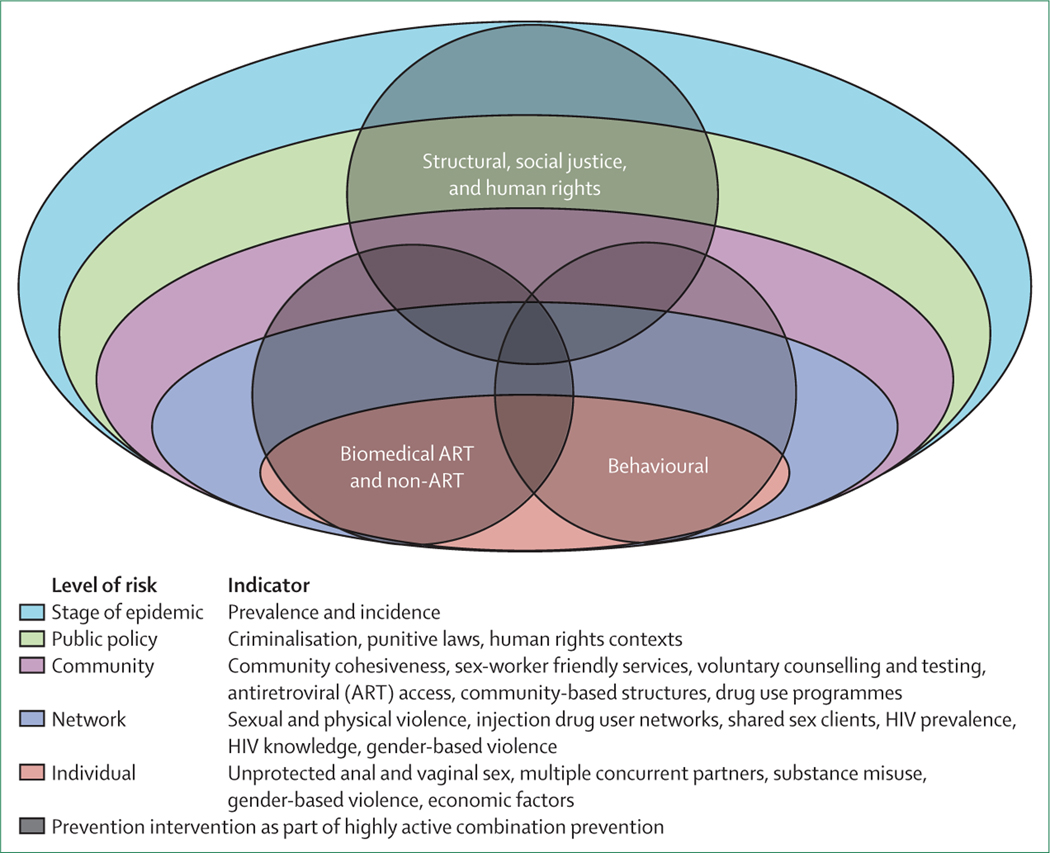
Here, we have focused on prevention interventions for FSWs and have defined sex work as exchange of sex for money or goods. Prevention options for men (Baral and colleagues9) and transgender persons who sell sex (Poteat and colleagues10) are reviewed in this Series. HIV prevention for women is a continuing challenge, and is an area where biology, physiology, gender dynamics, and behaviour have made HIV prevention research challenging, particularly in the subset of women who sell sex. We assessed interventions in three categories: biological, behavioural, and structural.11,12 Effective HIV prevention approaches for FSWs exist but have not been taken to scale or adequately resourced in most parts of the world.13 Additionally, we explored complementary strategies that can be added to a combination prevention package tailored for FSWs. An existing ecological model14 was modified to visualise multi-level domains of HIV risk for FSWs (figure 1). We present within these multi-level risks the evidence for biological, behavioural, and structural prevention interventions (table 1). In this model, we recommend that social justice principles are fully integrated into any package of combination approaches and that FSWs are meaningfully included in all aspects of programme design and implementation.11,14–16 The prevention strategies enable FSWs to exert more control over their ability to prevent HIV. In addition to reducing infections in FSWs, these strategies will positively affect networks, com munities, and country epidemics in different social, economic, and legal contexts.17 We modelled the effect of one such combination prevention package within the setting of the South African epidemic.
Figure 1:
Framework for combination prevention: levels of risk and prevention interventions
Table 1:
Possible HIV prevention interventions that are supported by direct or indirect evidence in FSWs and the risk level at which they operate
| Risk level | Type of intervention | Evidence in FSWs | |
|---|---|---|---|
|
| |||
| Community empowerment: promotion of social cohesion and capital, inclusion, and leadership skills | 2, 3, 4 | Structural | Direct |
| Advocacy and community mobilization: policy, programme, and services | 3, 4 | Structural | Direct |
| Gender-based and police violence, stigma, and discrimination | 2, 3, 4 | Structural | Direct |
| Economic strengthening, supplemental income | 1, 2 | Structural | Some |
| Rights, legal, and protection services | 1, 4 | Structural | Direct |
| Voluntary testing and linkage to services | 1 | Biomedical, behavioural, structural | Direct |
| Condoms (male, female, and condom-compatible lubricant) | 1, 2, 3, 4 | Biomedical, behavioural | Direct |
| Sex worker friendly health services, including sexual and reproductive health services | 1, 2, 3, 4 | Biomedical, structural | Direct |
| PrEP services | 1, 2, 3 | Biomedical, behavioural | Indirect |
| PEP services | 1, 2, 3 | Biomedical, behavioural | Indirect |
| HIV care, ART services, including PMTCT | 1, 2, 3, 5 | Biomedical, behavioural | Direct |
| Harm reduction in FSW-WID | 1, 2, 4 | Biomedical, structural, behavioural | Direct |
| Behaviour change by peer education and community-based counselling | 1, 2, 3 | Behavioural | Direct |
FSW=female sex worker. PrEP=pre-exposure prophylaxis. PEP=post-exposure prophylaxis. ART=antiretroviral therapy. PMTCT=prevention of mother-to-child transmission. WID=who inject drugs.
Historical perspectives
FSWs were a key affected population in the early decades of the HIV epidemic.18 HIV research with sex workers contributed to improved knowledge about host immunity in settings of recurrent infections19 and vaginal mucosal integrity during the first microbicides trials.20,21 Nonoxynol-9, a contraceptive product viewed as safe, was reported to be unsafe in sex work due to frequency of use and subsequent mucosal erosion.23
In Thailand, the 100% condom campaign was more than condom distribution: community mobilisation, education, condom availability, consistent and universal use of condoms, sexually transmitted infection (STI) tracing in clients, and follow-up in brothels.23 This programme and subsequent programmes in Cambodia and elsewhere in Asia, showed marked population-level effect s of interventions focused on safer sex practices in sex venues, including increasing condom use in sex workers and clients and reductions in other STIs in STI clinic attendees.24 Although HIV incidence was not directly measured in these programmes, ecological data suggest that they had significant effect s on the trajectories of the Thai and Cambodian HIV epidemics.25 The appropriateness and sustainability of top-down structural interventions that did not stimulate community empowerment have been restricted over time and critiqued by the sex-worker rights movement. However, eff orts to integrate the positive policy elements of these models with sex-worker participation and leadership have been successful in other settings such as in the Collective Commitment (Compromiso Colectivo) intervention in the Dominican Republic.26 Community-based combination prevention programmes in southeast Asia, Africa, and South America confirm that HIV can be controlled both within FSW networks and associated communities.27–29
The first oral pre-exposure prophylaxis (PrEP) trials in FSWs in Cambodia in 2004 and in Cameroon in 2005 were halted after participant disquiet about trial provisions.30,31 This led to a code of Good Participatory Practice Guidelines and a benchmark for community engagement in large prevention trials.32 Recent prevention efficacy trials have not specifically included or excluded FSWs and so the safety and effectiveness of these newer modalities for FSWs remains unproven.
Existing prevention strategies
Existing prevention strategies include behavioural and structural approaches, and sexual and reproductive health services, including condoms, counselling, testing, and supportive linkage to care for newly diagnosed FSWs. The most effective strategies have been within community-based programmes, which have intervened on the drivers of HIV transmission in FSWs including condomless sex, STIs, gender-based violence, unsafe working environments, and poor service usage due to stigma and discrimination.2
Condom provision
Sex worker projects worldwide show the feasibility of increasing condom use to decrease STI and HIV acquisition.33–35 In Santo Domingo, Dominican Republic, condom use and rejection of condomless sex increased because of workshops and meetings with sex workers, sex establishment owners and managers, and other employees, to strengthen collective commitment to prevention, particularly in supporting sex workers to use condoms with partners. These gatherings also focused on issues of trust and intimacy in condom use negotiation between sex workers and regular paying and non-paying partners.36,37 Interventions such as motivational interviewing have improved condom use and harm reduction in FSWs who also inject drugs.38
Greater success in uptake and adoption of condoms has been reported in sex-worker programmes than any other affected population. The latest UNAIDS report states that countries’ reported condom use at last commercial sex was high and improving; 44 countries reported higher median condom use at last sex in 2012 than in 2009 (85% vs 78%).1 Cost and access to condoms, and condom carriage used as evidence of sex work by police in some settings are examples of structural barriers that can undermine an effective intervention. Provision of water-based lubricant with condoms is also recommended, although less is known about the importance of the type of lubricant. Although the evidence for the preventive effect of female condoms is scarce, some studies have shown higher acceptability of female condoms in FSWs than other women.39,40 Furthermore, improving access to and reducing cost of female condoms and lubricant could increase overall condom usage.40 Condomless sex can be more lucrative for a FSW, resulting in greater risk-taking for financial reasons. To counter this issue, the role of cash transfers for HIV prevention in sex work is also being investigated. Cash transfer could operate on at least two levels: conditional on safer sex practices as contingency management, or as a way to reduce economic vulnerability thereby encouraging behaviours with social benefits.41 In the Zomba cash-transfer trial in Malawi, adolescent girls who received transfer money were less likely to have older sexual partners and had less frequent sex, resulting in lower rates of HIV infection.42 In the RESPECT study, beneficiaries were given rewards every 4 months for remaining free of curable STIs.43 After 1 year, the study recorded a 25% drop in the incidence of STIs. A pilot study is underway to explore cash transfers in male sex workers in Mexico.44
Control of STIs
Bacterial and viral STIs can increase the efficiency of HIV transmission. Screening and treating FSWs for STIs could reduce HIV infections, although efficacy has been difficult to demonstrate.45 STI treatment might consist of active case finding and individual case management or periodic mass STI treatment (periodic presumptive treatment) regardless of diagnosis. Some empirical, uncontrolled, intervention studies in sex workers and community-based randomised controlled trials in general populations have been undertaken.45–51 Only one community-based trial (the Mwanza Trial) done in East Africa showed efficacy of individualised syndromic management of STIs against sexual transmission of HIV with a reduction of 38% in HIV incidence.47,51,52 More than 12 500 individuals in the region were recruited to this trial and it was estimated there were about 1200 sex workers or bar workers in Mwanza town at this time.52 Where the burden of STIs is high, periodic presumptive treatment of curable STIs has been effective at reducing STIs but not HIV incidence in FSWs.53,54 WHO advises only temporary use of periodic presumptive treatment51,54 and periodic presumptive treatment has a greater effect on STI control in places where other aspects of control are poor and where FSWs have little access to preventive and curative services. Screening for asymptomatic STIs in FSWs can reduce STIs,54 but in settings where resources are scarce this is often not feasible. Syndromic management to reduce STI infection in FSW networks is problematic as most STIs are asymptomatic. This situation might be changing, however, as point-of-care STI diagnostics become more available and affordable.55 Clinical trials have not confirmed that herpes simplex virus (HSV) suppressive treatment would reduce the risk of HIV acquisition in HSV2-infected, HIV-uninfected women. No protective effect of acyclovir was reported, although some benefit was seen in a subset of women who took at least 90% of their antiviral doses.56,57 These studies were undertook in general populations with no specific enrolment of sex workers56 and in women who worked in venues such as bars and cafes in Tanzania where 26–61% of enrolled women reported recent sex in exchange for money.57 Poor adherence to bi-daily pills probably contributed to results. Similarly, for individuals co-infected with HIV and HSV2, treatment with daily acyclovir to suppress HSV2 did not reduce the risk of transmission of HIV to their partners.58
HIV testing and counselling
HIV testing underlies the implementation of nearly all other prevention approaches and serostatus knowledge is needed to tailor services to individual needs. However, mandatory testing could be counter-productive and violates rights—FSWs should be able to access HIV testing and counselling with the same privacy and protection as anyone else. WHO recommends at least annual voluntary testing for sex workers. In a review of 52 low-income and middle-income countries in 2010, the median percentage of FSWs who had tested for HIV in the last 12 months and knew their test results was 49% with wide variation across countries.59 Rates of HIV testing in sex workers throughout Africa are suboptimal with only 4% of sex workers surveyed in Somalia in 2008 ever-tested.60 Similarly, in Zimbabwe in 2011, where HIV prevalence in sex workers is about 50%, half of HIV-positive FSWs were aware of their status, only 30–40% of those eligible were accessing antiretroviral therapy (ART) and fewer than a quarter of those HIV-negative reported testing in the previous 6 months.61 Indications are that testing coverage in FSWs has improved in the era of ART access.62–64 Barriers to testing in FSWs are similar to those of the general population: poor awareness of services, distance to facilities, transportation costs, opportunity costs, time constraints, and fear of a positive result with resultant discrimination and loss of income.65–68 However, additional barriers unique to FSWs include fear of authorities, linked to sex work illegality, and confidentiality concerns, particularly status disclosure to other FSWs or potential clients.69,70 Several successful interventions have increased HIV counselling and testing in sex workers.71–75 Strengthened peer support and a supportive network are associated with the willingness of FSWs to engage in testing, care, treatment initiation, and adherence.26,74,75 Even when FSWs have access to health facilities, prejudice and poor quality of care are crucial determinants of their willingness to be tested.76,77 There are studies regarding the importance of affordable, sex-worker-friendly clinics, and their ability to attract and retain FSWs.78–81
Gender-based violence interventions
Violence against sex workers is not only widespread, but is also perpetrated, legitimised, and accepted by many, including law enforcement authorities, gatekeepers, managers, clients, and intimate partners.82 It undermines HIV prevention eff orts and increases the vulnerability of sex workers to HIV transmission in several ways. Rape, forceful acceptance of condomless sex, sex with police to avoid arrest, and violence related to illicit drugs all could result in FSWs giving higher priority to their safety and survival than less immediate concerns such as HIV prevention.83 Interventions include sex-worker education on rights, community mobilisation to respond to violence and discrimination, practical warning systems in sex-work networks, sensitisation workshops with police and law enforcement authorities, and advocacy at community and policy level to promote human rights of sex workers.83 Some innovations include the sex-worker education programme devised by the Sex Workers Education and Advocacy Taskforce (SWEAT) in South Africa and the venue–level interventions identified by sex workers in Vancouver, including in-room buzzers and corridor video surveillance.84,85
Community empowerment
Meaningful involvement of sex-worker communities in the design and implementation of prevention programmes is crucial. Community empowerment reduces the vulnerability of sex workers by peer-led collective action and self-help activities including education, health services, and advocacy on issues such as violence and work conditions.86,87 Interventions in programmes such as Empower Thailand include sustained engagement with local sex workers to raise awareness about sex-worker rights, the establishment of safe spaces, the formation of collectives that define the services to be provided, and outreach and advocacy.88 Community empowerment is associated with reduction in HIV and STI prevalence and increased condom use.89 Importantly, community empowerment is feasible to implement and take to scale, is highly acceptable to FSWs, and is safe.90 Participation of FSWs—most famously illustrated by the Sonagachi Project in Northern Kolkata, India—has documented increased condom use and decreased HIV prevalence not only in FSWs but also in bridge populations.29,91 The Sonagachi Project invested substantial eff ort to define the problem of HIV prevention as a community issue and to align the short-term and long-term rewards for condom use as being in the economic best interests of all stakeholders and the sex workers.91 This programme and others show that sex-worker health outcomes can be enhanced when programmes encourage a sense of shared identity and camaraderie in sex workers, and address concerns beyond HIV and sexual health, including violence, stigma, and discrimination.27–29 Gender-responsive economic strengthening activities including vocational training, education, and micro-financing within empowerment programmes could also give FSWs control over vital economic resources, and reduce FSW vulnerability to HIV.92
Prevention taken to scale
HIV prevention interventions can be successfully taken to scale with potential to reduce HIV prevalence in FSWs. One such programme is the Avahan programme, launched in 2003 by The Bill & Melinda Gates Foundation in six Indian states. The programme aimed to reduce HIV transmission and the prevalence of STIs in vulnerable high-risk populations, notably FSWs. It promoted prevention education and services such as condom promotion, STI management, behaviour change com munication, community mobilisation, and advocacy.93 An important aspect of the Avahan programme has been its coverage, with an 80% target met within 5 years, resulting in demonstrable increases in condom uptake, and decreases in STIs and HIV.28 An example of scale-up from Africa is the Zimbabwean Sisters with a Voice programme, which is also community empowerment based, and is now present in 36 sites around the country, although studies on the effect of this programme are awaited.27 Effective scale-up needs commitment and sustained resources.13
However, it is possible that along with sustained resources, strategies that address determinants in addition to those listed above could be needed for maximum prevention effect .94,95 After the 100% Thai Condom campaign, for example, HIV prevalence levelled at about 10% in FSWs, ten times higher than the prevalence in Thai women from the general population.3
New prevention strategies
Combining the previous more established approaches with new, partially effective biomedical modalities is a potential new approach. In the last 3 years biomedical interventions that use antiretroviral drugs as prevention have become important. Antiretroviral drugs can protect uninfected individuals from acquiring infection (PrEP and post-exposure prophylaxis [PEP]), and can reduce infectiousness of infected partners (secondary prevention or treatment as prevention (TasP). Pre-exposure and post-exposure antiretroviral drugs can be provided either as oral (systemic) tablets or vaginal or rectal (topical) gels or rings known as microbicides.96 The application of antiretroviral drugs for HIV prevention to FSW populations remains to be proven.
PrEP
Seven randomised controlled trials have examined antiretroviral drugs given to HIV-negative persons for HIV prevention (table 2).97–103 In four clinical trials including women from diverse geographical and risk settings, PrEP reduced HIV acquisition by 39–75%.97–100 None specifically enrolled FSWs, however in three of the trials,97,101,103 most of the women were unmarried, up to a quarter had many partners, and between 1·9% and 12% reported transactional sex at baseline. No significant relationship between transactional sex and HIV incidence or good adherence rate was noted in the FEM-PrEP study (J Headley, FHI 360, personal communication). The only other study in which transactional sex was reported was the Global iPrEx Study102 that included men who have sex with men—although all participants had to be born male, 29 (1%) reported their present identity as female (table 2). Due to limited representation, no randomised controlled trials have specifically undertaken an efficacy sub-analysis in sex workers. Consequently, any application to sex work is based on extrapolation from a general female population, and product safety and the effect of the conditions associated with the nature of sex work (eg, frequency of sex and therefore the frequency of dosing of coitally dependent agents) on PrEP effectiveness is unknown.
Table 2:
Completed PrEP and TasP studies, and outcomes by gender and participation of sex workers (if any)
| Population (median age) | Total number (women and transgender women) | Design and intervention | Relative reduction in HIV incidence ITT | Incidence reduction in women and transgender women | Definition of sex work or any associated risk behaviours at baseline | Percentage reporting at baseline | Comments | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Partners PrEP (Kenya and Uganda) (systemic PrEP)98 | Heterosexual men and women serodiscordant (36) | 4747 (2283) | 1:1:1 Orai TDF, TVD, placebo for negative partner | TDF 67% (95% CI 44–81), TVD 75% (55–87) | TDF 68% (29–85), TVD 62% (19–82) | Sex work and transactional sex not asked; any sex with outside partner in the previous month | <10% at baseline | Sub-analysis done in highrisk women. In subgroups of women with placebo group HIV-1 incidence >5·0%, efficacy estimates 64–84%105 |
| TDF2 (Botswana) (systemic PrEP)100 | Negative heterosexual men and women (25) | 1200 (548) | 1:1 Orai TVD, placebo | 62% (22–83) | 49·4% (−21·7 to 80·8; p=0·107) | Sex work and transactional sex not asked; asked whether >1 sexual partner in the last month | <20% at baseline | No stratified analysis in specific risk groups |
| The Bangkok Tenofovir Study (Thailand) (systemic PrEP)99 | Negative PWID, men and women (31) | 2413 (489) | 1:1 Orai TDF, placebo | 49% (10–72) | 78·6% (16·8–96·7; p=0·03) | Asked whether any casual or sex work partners in the past 3 months; >1 partner in the last 3 months | Baseline 38% overall (men twice as much as women); <20% overall | No specific data on sex work in participants for stratified analysis |
| FEM-PrEP (Kenya, South Africa, Tanzania) (systemic PrEP)103 | Negative women (24) | 2120 (2120) | 1:1 Orai TVD, piacebo | No reduction | No reduction | Asked whether sex exchanged for money and gifts in the last 4 weeks | 12·6% at baseline | No significant relationship between transactional sex and HIV incidence or good adherence |
| Global iPrEx (USA, Brazil, Peru, Ecuador, Thailand, South Africa) (systemic PrEP)102 | Men and transgender women (27) | 2499 (all participants had to be born male, although 29 [1%] reported their present identity as female) | 1:1 Orai TVD, piacebo | 44% (15–63; p=0·005) | Not done | Asked whether any transactional sex in the last 6 months | 41% at baseline | Qualitative work in those who reported sex work has been undertaken and will be reported |
| VOICE (MTN 003) (Uganda, Zimbabwe, South Africa) (systemic and topical PrEP)101 | Negative women (25) | 5029 (5029) (2010 assigned to gel groups) | 1:1:1 Orai TDF, TVD, TDF gei, piacebo | No reduction | No reduction | Asked whether money, material goods, gifts, drugs, or shelter were received in exchange for vaginal or anal sex in the last year | 6·1% at baseline | 304 out of 4980 participants responded yes to this question at baseline. No separate analyses undertaken as numbers in each study group considered too small |
| CAPRISA 004 (KwaZula Natal, South Africa) (topical PrEP)97,104 |
Negative women (24) | 889 (889) | 1:1 Vaginai TDF gel coitally dependent, piacebo | 39% (6–60) | 39% (6–60) | Asked whether money ever received in exchange for sex | 1·9% at baseline | Sample too small to do separate analysis |
| HPTN 052 (USA, Brazil, Botswana, Zimbabwe, Malawi, Kenya, South Africa, India, Thailand) (TasP)105 |
Men and women serodiscordant couples (32) | 3526 (1962) | 1:1 Immediate vs delayed ART for positive partner | 96% in linked sexual transmissions |
Gender insignificant predictor of linked transmission |
Transactional sex and sex work not asked; asked whether >1 partner in the last 3 months | <5% reported at baseline | 11 out of 39 transmission events occurred outside of the enrolled dyad |
In RCTs where sex work was not asked, alternative associated risk behaviours have been listed. PrEP=pre-exposure prophylaxis. TasP=treatment as prevention. ITT=intention to treat. TDF=tenofovir. TVD=emtricitabine and tenofovir (Truvada). ART=antiretroviral therapy.
A strong dose–response relationship between adherence to PrEP pill-taking or gel-use and HIV protection was shown (table 3). No HIV protection was reported in the two trials in which adherence to PrEP was lowest.101,103 By contrast, in the Partners PrEP (discordant couples) and iPrEx (MSM) studies, case-control analyses suggested that those using PrEP consistently had greater than 90% reduction in HIV risk.98,102 The Partners PrEP study team undertook an analysis in higher risk subgroups within the Partners PrEP Study, including groups of higher risk women. High risk was defined by criteria including viral load of partner, unprotected sex, and younger age. In these subgroups, PrEP had consistently higher efficacy for HIV-1 protection.106,107
Table 3:
Completed PrEP (oral and topical) studies and protection estimates relative to adherence
| Drug detection in blood and vaginal samples from non-seroconverters | HIV protection estimate as related to high adherence | |
|---|---|---|
|
| ||
| Partners PrEP98 (systemic PrEP) | 81% | 86% (TDF), 90% (TVD), in patients with detectable levels |
| TDF2100 (systemic PrEP) | 79% | 78% excluding follow-up when patients had no PrEP refills |
| FEM-PrEP103 (systemic PrEP) | 35–38% at a single visit, 26% at two consecutive visits | Too low to assess efficacy |
| Global iPrEx102 (systemic PrEP) | 51% | 92% (95% CI 40–99) (TVD) |
| The Bangkok Tenofovir Study99 (Thailand) (systemic PrEP) | 66% | 74% in participants with detectable drug levels |
| VOICE101 (systemic and topical PrEP) | <30% of samples; about 50% of women had no detectable levels | Too low to assess efficacy |
| CAPRISA 00497 (topical PrEP) | >1000 ng/mL TDF in vaginal fluid protective | 54% in the high adherers, >80% of sex acts covered with gel use |
PrEP=pre-exposure prophylaxis. TDF=tenofovir. TVD=emtricitabine and tenofovir (Truvada).
The most recent clinical trial of systemic PrEP included drug users in Thailand99 and had 2413 participants (about 20% were female). Participants were asked to report whether they had sexual intercourse with people other than their live-in partner including casual or sex work. This behaviour was reported in 38% of the participants (fewer women than men) at baseline (table 2). The HIV incidence reduction of 49% for those on PrEP is important because FSWs who inject drugs are often the most vulnerable (and marginalised) subgroup of FSWs.108 HIV prevalence in women engaging in both injection drug use and sex work is higher than in the general FSW population.109,110 Women had the best adherence in this study. Combination prevention packages, including harm reduction strategies and PrEP for FSWs who inject drugs, are promising.111
In studies where adherence was greatest, the positive findings support the biological effectiveness of PrEP for preventing HIV acquisition,96 but the trials with negative results suggest that PrEP was an unacceptable or unfeasible mode of prevention for some women. The reasons for this are unknown but some of the reported adherence barriers might be relevant to FSWs, including absence of support from family and partners. Whereas the possible role of low-risk perception by women might seem less relevant to FSWs, it is well known that intimate partners could present an unanticipated risk to FSWs, with data to show that FSWs are less likely to use condoms consistently with intimate partners.112,113 Acceptability studies (of hypothetical prevention products) have been done in FSWs and have shown favourable outcomes, but have also raised some concerns from sex workers, including STI risk, privacy, and cost.114,115
Topical vaginal gel applied during sexual intercourse in the CAPRISA 004 study was protective and levels of protection correlated with adherence.97 About 20 (1·9% of all trial participants) were self-reported sex workers in this study (table 2), too small a number for subgroup analyses. Coital application could suit women having intermittent sexual intercourse better than the more regular encounters that occur in sex work. Coital application might be an appealing dosing strategy for sex work, because the gel can also lubricate. The maximum frequency of application that would be safe in this setting is still unknown. Host biological factors could alter the activity of topical biomedical interventions; an analysis of HIV risk in women in CAPRISA 004 showed that despite adequate levels of vaginal tenofovir, women with higher systemic or mucosal immune activation, such as might occur with STIs, were more likely to become infected with HIV than women without evidence of activation.116,117 Studies in Kenyan sex workers have shown that resistance to HIV infection could be attributed to a balance of immune quiescence and a focused innate antiviral response.118
Additionally, complex questions regarding adherence and dual-protection remain. Demonstration projects that can assess the real-world effect of PrEP in sex workers beyond clinical trial settings are needed. This might include addressing concerns surrounding uptake, such as cost and side-effect s, adherence barriers such as detention and reluctance to carry pills that could be stigmatising, and combining PrEP usage with condoms or other behavioural measures. PrEP is probably an important addition to HIV prevention in transgendered FSWs in which the HIV transmission probability per sexual transaction is very high. Although PrEP as a user-controlled method might provide personal protection against HIV, STIs and unwanted pregnancy for FSWs remain a risk, especially if there is no option for condoms. The implications for other STIs and unintended pregnancy due to condom migration should be guarded against with the ancillary provision of information and sexual and reproductive health services. PrEP could be a potent additional choice for some FSWs, but not all. The challenge is to find ways that FSWs can identify suitability for themselves. In all clinical trials, condom usage increased and STI diagnoses decreased during the study, suggesting that PrEP could work synergistically with other prevention modalities;96 however, public awareness of PrEP could lead to increased demand for condomless commercial sex. PrEP should be part of a combination prevention package that is voluntary and includes condom promotion.119 As PrEP is introduced in sex-worker populations, community engagement, further behavioural and social science research, and careful programme monitoring and assessment will be needed.119 Important research areas are listed in table 4. WHO and Centers for Disease Control and Prevention (CDC) have offered early guidance and have called for demonstration projects including all key affected populations.120–122 A variety of open-label and demonstration projects in women are on-going or imminent and a confirmatory vaginal gel study is underway at present.123 Zimbabwe has approved a PrEP demonstration project in FSWs, SAPPH-Ire, which commenced in 2014 and will be nested within the already well-established Sisters with a Voice Program (table 5).
Table 4:
Factors associated with sex work that affect the effectiveness of PrEP
| Sex-work-related factors that could affect effectiveness of PrEP and use (systemic and topical) | |
|---|---|
|
| |
| Biomedical | |
| Host factors | Systemic or mucosal immune activation |
| Frequency of sex | Daily product vs intermittent product use |
| Type of sex | Trauma, erosions; willingness to use a gel for added lubrication |
| Concurrent STIs | Erosions, ulcers, immune activation |
| Behavioural | |
| Adherence to pill taking and product use | Willingness to carry pills and products; difficulty taking daily pills and products |
| Adherence to programme | Willingness to be frequently tested and attend services regularly |
| Condom use | Less consistent use |
| Risk perception | Motivation to use daily product |
| Alcohol and drug misuse | Effect on adherence |
| Structural | |
| Detention | Inability to adhere to pills or programme |
| Access to product and pills | Acceptable access points |
| Cost | Access to free product and willingness to pay |
| Intimate partners | Transmitted resistance; consistent use |
| Client-related factors | Transmitted resistance; pressure for condomless sex |
| Manager-related factors | Pressure for condomless sex; mandatory use and denied use |
PrEP=pre-exposure prophylaxis. STI=sexually transmitted infection.
Table 5:
Trials in progress and planned, and demonstration PrEP (oral and topical) projects in women and female sex workers
| Population | Design, product, and follow-up duration | Location | Timeline | |
|---|---|---|---|---|
|
| ||||
| Clinical trials | ||||
| FACTS 001 | 2600 heterosexual women | Placebo RCT, 1% TDF gel, BAT 24 | South Africa | Enrolling, 2015 |
| ASPIRE | Heterosexual women | Placebo RCT, dapivirine vaginal ring | Zimbabawe, Malawi, Uganda, South Africa | Fully enrolled, 2015 |
| RING study | Heterosexual women | Placebo RCT, dapivirine vaginal ring | South Africa | Enrolling, 2016 |
| FACTS 002 | 100 young women (aged 16–17) | Safety and acceptability, 1% TDF vaginal gel, BAT 24 | South Africa | Under review |
| CHAMPS-SA PLUSPILLS PrEP | 150 young men and women (aged 15–19) | Open label TVD oral | South Africa | Under review |
| Follow-on and demonstration studies | ||||
| Partners PrEP (post-placebo phase) | 4747 heterosexual HIV serodiscordant couples | Randomised daily oral TDF vs TVD (unblended), follow-up 12 months | Kenya, Uganda | Fully enrolled |
| CDC 494/TDF2 open-label extension | 1219 heterosexual men and women | Open label TVD, 12 months follow-up | Botswana | Enrolling, results 2014 |
| Partners Demonstration Project | 1000 HIV serodiscordant couples | Open label, daily TVD oral as bridge to treatment in infected partner, follow-up 24 months | Kenya, Uganda | Enrolling, results 2014–15 |
| CAPRISA 008 | ·· | Open label, 1% TDF vaginal gel, BAT 24 | South Africa | Results 2015 |
| SAPPH-Ire FSW RCT | 2800 FSWs | Open label, oral daily TVD | Zimbabwe | Enrolling |
| TAPS: Expanded use of ART for treatment and prevention for female sex workers in South Africa | 400 FSWs for PrEP, 300 FSWs for ART | Open label, PrEP for negative FSWs and immediate ART for FSWs living with HIV | South Africa | Enrolling |
RCT=randomised controlled trial. TVD=emtricitabine and tenofovir (Truvada). TDF=tenofovir. PrEP=pre-exposure prophylaxis. BAT 24=one dose before sex, one dose after sex, but no more than two doses in 24 h. FSW=female sex worker.
PEP
PEP is most commonly used for needle-stick incidents and, increasingly, for sexual assault. Non-occupational PEP for sexual prevention has not been scaled up worldwide. Reasons for this include user reluctance (the need to access care within 72 h and continue treatment for 28 days, and the side-effects), and inadequate services (the need for testing and scarcity of PEP starter packs on demand).124 PEP is probably not scalable, practical, or sustainable as a sole intervention for sex workers, although it has a role in sexual assault and other episodes of unanticipated condomless sex. In a study from Kenya, PEP was well accepted by urban FSWs with greater than 10% requesting PEP at least once during the year after its introduction. However, PEP use was not associated with reduced HIV acquisition in this study.125
Earlier treatment
Earlier treatment of HIV-positive FSWs can improve clinical outcomes and reduce transmission of HIV to their HIV-negative sexual partners, including clients.126,127 HPTN 052, a randomised controlled trial in serodiscordant couples, showed a 96% reduction in HIV transmission from HIV-positive individuals, treated earlier and virally suppressed, when compared with those in whom treatment was deferred.105 Importantly, 11 (28%) of 39 infections occurred as a result of relationships outside of the treatment dyad. This study did not enrol sex workers nor enquire about transactional sex, however these data suggest that encouraging HIV-positive sex workers to voluntarily access effective, comprehensive HIV services will improve personal health prognosis and might protect clients from acquiring HIV infection from sex workers (table 2).105,127–131 Reduced HIV transmission could have indirect prevention benefits within sex-worker networks. Available information on ART coverage, retention, adherence, and viral suppression in FSWs is restricted to only a few research settings in sub-Saharan Africa, North America, and Asia. These data suggest that FSWs can attain high levels of adherence and viral suppression, at least in the short term and in research settings. Some adherence concerns have been raised.132 Information on long-term outcomes and retention pre-ART are particularly sparse.133 FSWs might delay or be denied access to health care for reasons of stigma, cost, and victimisation, which can hinder adequate treatment outcomes, antenatal care, prevention of vertical HIV transmission during pregnancy,134 and the prevention of continuing transmission to clients. HIV services, including ART, that are acceptable, effective, and accessible for all FSWs have well documented individual and public health benefits.51,135,136
Modelling HIV prevention strategies: network level effect
The interventions described here have proven or plausible potential to protect the individual FSW, but the effect of these interventions at a network or community level depends on the local epidemic and setting.17 To assess the probable effect of some of these newer HIV prevention strategies for FSWs, we developed a mathematical model applied to South Africa. South Africa has a severe HIV epidemic that is generalised and driven mostly by heterosexual sex. Our objectives were to gauge the extent to which commercial sex drives heterosexual HIV transmission; the effect of past changes in condom use on HIV incidence in FSWs and their clients; and the potential future effect of promoting oral or topical PrEP, and earlier ART to FSWs in South Africa.
The model (described in the appendix) stratifies the population by age, sex, marital status, male circumcision status, and sexual risk behaviour. HIV-infected adults not on ART were divided into four CD4 groups further stratified by knowledge of HIV status. The probability of HIV transmission per sex act depended on the HIV disease stage of the infected partner, the sex and circumcision status of the uninfected partner, and the type of relationship (sex-worker client, short-term non-marital, or long-term marital). Rates of HIV transmission also depended on levels of condom usage, which were assumed to depend on the type of relationship. Rates of condom use were assumed to have increased over time, partly due to condom promotion programmes and partly due to reductions in unprotected sex after HIV diagnosis. The model was fitted to age-specific HIV prevalence data from South African antenatal and household surveys, and recorded mortality data.
The change in HIV incidence in FSWs and their clients over the period from mid-2015 to mid-2025 was assessed if new HIV prevention strategies were promoted to FSWs, alone or in combination. HIV prevention programmes include oral PrEP, topical PrEP (microbicides), and early ART together with 6-monthly HIV screening (a TasP strategy, in which all HIV-diagnosed sex workers were offered ART regardless of their CD4 count). Because of the uncertainty regarding rates of uptake, effectiveness, and risk compensation for the different prevention methods, an uncertainty analysis was done to assess the range of possible results. The distributions chosen to represent the uncertainty around each parameter are summarised in table 6; 1000 parameter combinations were randomly sampled from these distributions using Latin hypercube sampling.153 Assumed male rates of sex-worker contact and female rates of retirement from sex work were also included in the uncertainty analysis.
Table 6:
Commercial sex assumptions
| Mean | SD* | |
|---|---|---|
|
| ||
| Proportion of men who visit sex workers | 35% | ·· |
| Scaling factor for male rate of visiting sex workers137†‡ | 3·50 | 1·50 |
| Relative rate of visiting sex workers in married men138 | 0·25 | ·· |
| Annual number of clients per sex worker139–144 | 750 | ·· |
| Annual rate of retirement from sex work141,142 | 0·33 | 0·10 |
| Annual rate of PrEP uptake in sex worker114 | 0·30 | 0·10 |
| Average PrEP effectiveness98,100,101,103 | 40% | 24% |
| Reduction in condom use in women using PrEP145 | 10% | 10% |
| Annual rate of microbicide uptake in sex workers97,146,147 | 0·30 | 0·10 |
| Average microbicide effectiveness97,101 | 25% | 13% |
| Reduction in condom use in women using microbicides145 | 10% | 10% |
| Average time spent on PrEP and microbicides (years)141 | 5 | ·· |
| ART uptake in women with CD4 >350 cells/μL148 | 60% | 16% |
| Reduction in infectiousness after ART initiation105,149–152 | 80% | 12% |
SDs are shown only for those parameters that are included in the uncertainty analysis. Gamma priors are used to represent uncertainty around all parameters, except for those that are formatted as percentages (uncertainty is represented using beta prior distributions).
Further details in the appendix.
Based on fitting model to sex worker population size estimates.137 PrEP=pre-exposure prophylaxis. ART=antiretroviral therapy.
When fitted to South African data sources, our model suggested that in 1990 HIV transmission between FSWs and their clients accounted for 11% of heterosexual transmission in South Africa (IQR 8–14%). By 2010, this proportion had declined to 6% (IQR 5–8%). This was because transmission in high-risk groups accounted for a lower fraction of total transmission as the epidemic became more generalised, and condom use in FSWs and their clients increased more than in other relationship types.154 Increases in condom use accounted for a 65% reduction in the HIV incidence rate in clients in 2010 and a 76% reduction in HIV incidence rates in FSWs. Further details regarding the proportion of heterosexual transmission attributable to commercial sex and the effect of past increases in condom use are described in the appendix.
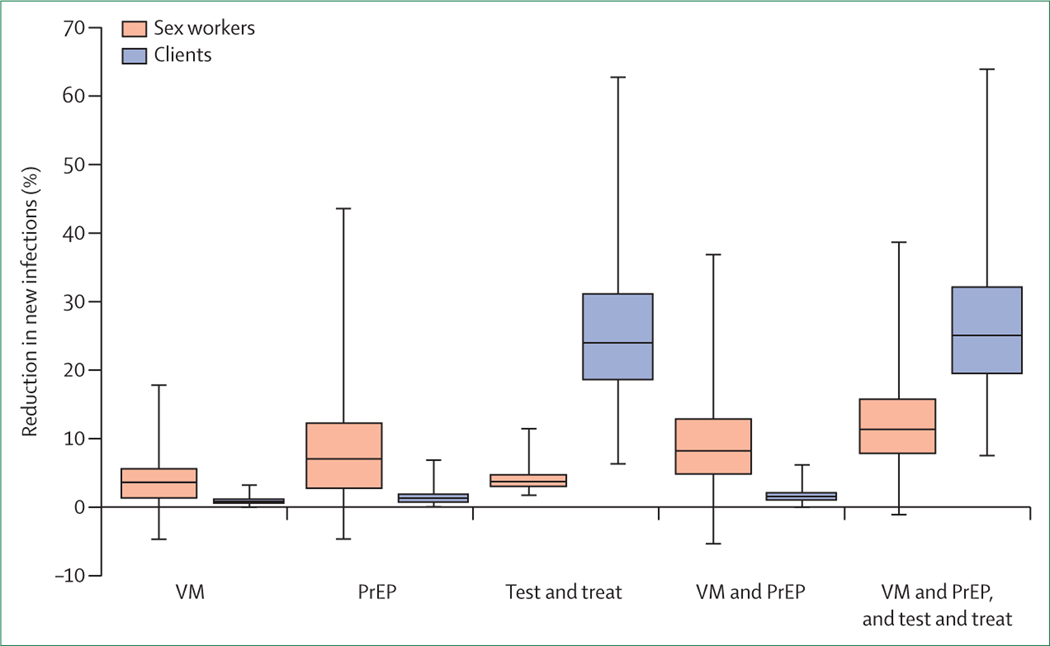
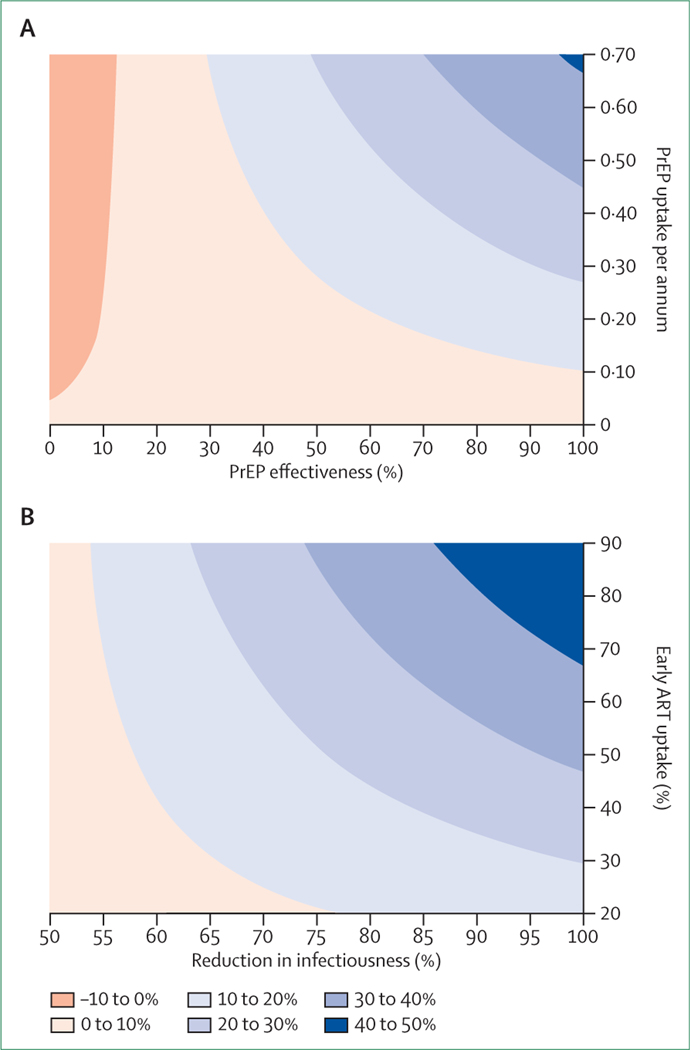
Oral and topical PrEP alone would have only a modest effect on HIV incidence in FSWs (figure 2). However, substantial variation occurs in the range of possible outcomes, with the assumed annual rate of PrEP uptake and PrEP effectiveness being the most important determinants of the percentage reduction in sex-worker HIV incidence over the 2015–25 period (figure 3A). Under pessimistic assumptions, if effectiveness was low (<10%) and sex workers using PrEP reduced their condom use by more than 10%, the net effect on HIV incidence in FSWs could be negative, although there would still be a positive effect on HIV incidence in clients if women using PrEP were tested regularly, because earlier diagnosis would reduce transmission potential. Under very optimistic assumptions (effectiveness >95% and rate of PrEP uptake >0·65 per person-year), PrEP could reduce HIV incidence in South African sex workers by 40% or more over the 2015–25 period (figure 3A). Further uncertainty analysis is presented in the appendix.
Figure 2: Percentage reduction in new HIV infections in sex workers and clients from 2015 to 2025.
Percentage reductions in clients are calculated only for infections acquired during commercial sex (excluding infections acquired from non-commercial sex partners). Box-and-whisker plots represent minimum, 25th percentile, median, 75th percentile, and maximum values from the 1000 parameter values in the uncertainty analysis. PrEP=pre-exposure prophylaxis. VM=vaginal microbicide.
Figure 3: Effect of uptake and efficacy on percentage reduction in new HIV infections from 2015 to 2025.
(A) Reduction in incidence in sex workers if only PrEP is provided to sex workers.
(B) Reduction in incidence in clients if test and treat interventions are introduced in sex workers. Percentage reductions in clients are calculated only for infections acquired during commercial sex (excluding infections acquired from non-commercial sex partners). Contour plots represent the expected reductions in new HIV infections at different uptake and efficacy levels. PrEP=pre-exposure prophylaxis.
A TasP strategy in FSWs would have a moderate effect on HIV incidence rates in clients (but little effect in FSWs). It would reduce incidence in South African clients by 23% (IQR 19–28%) over the 2015–25 period (figure 2), which would provide an indirect prevention benefit to FSWs. The estimated effect was particularly sensitive to the assumed proportion of FSWs who chose early ART after diagnosis (before meeting standard eligibility criteria) and the assumed reduction in infectiousness after ART initiation (figure 3B). One reason why this intervention does not have a more substantial effect is that rates of HIV testing and ART initiation in South Africa are already high (the modelled proportion of HIV-positive sex workers receiving ART in 2015 is 60% before the introduction of the TasP strategy); if we assumed that no prevention or treatment strategies were available in South Africa before the TasP strategy, the predicted effect would be a 54% reduction in HIV incidence in clients (IQR 50–58%), with reductions as large as 70% if rates of early ART uptake and virological suppression were high.
Combining a TasP strategy with the provision of oral and topical PrEP, the model estimates a 25% reduction in HIV incidence in clients (IQR 19–32%) and an 11% reduction in HIV incidence in sex workers (IQR 7–15%) (figure 2). Further discussion of the model results and limitations are included in the appendix.
Combination prevention for FSWs: five intervention levels
Scale-up of potential interventions to mitigate HIV acquisition and transmission by FSWs includes factors other than the hierarchy of scientific evidence. Acceptability in the FSW community, cost, logistics, and potential side-effect s are additional factors.155,156
The design of an FSW-tailored HIV prevention package needs an approach that recognises all levels of risk, and consists of biomedical, behavioural, and structural interventions (figure 1). The epidemic context (risk level 5) in which the sex work occurs is an important determinant of HIV risk, and the importance of sex-worker-focused interventions depends on this context.17 In South Africa we estimated that between 6% and 11% of adult HIV transmission is attributable to sex work, but in other regions where the HIV epidemic is more concentrated, FSW-specific interventions might be more important. For example, other models suggest that providing a topical gel to FSWs would reduce HIV incidence in the general population by only 9% in the South African context, compared with 48% in Benin157 where sex work is estimated to account for more than half of HIV infections in men.158 Previous modelling has shown that FSW interventions probably have less effect in mature epidemics than in early-stage epidemics.159,160 The effect of promoting PrEP to high-risk groups is highly dependent on sexual mixing patterns in the population and levels of heterogeneity in HIV risk.161 Our simulations suggest that TasP interventions could have less effect in settings where access to HIV testing and ART is already high.2 Other modelling studies suggest that a high background level of ART coverage will probably increase the cost per HIV infection prevented by PrEP.162 This implies that the benefit of promoting new prevention methods to FSWs is dependent on pre-existing levels of access to HIV prevention and treatment. Knowing the local epidemic and thus tailoring the response to it, is a fundamental step advocated by UNAIDS and increasingly adopted by national programmes.17 The need for continuing epidemiological monitoring and specific FSW surveillance in each country is essential.158
Any HIV prevention package must consider environmental or policy factors (risk level 4) that define the conditions in which sex is bought and sold.16 These factors include the capacity of FSWs to choose and use products to protect against STIs, unintended pregnancy, HIV, and other infections. Other contextual factors include the criminalisation of sex work, and policies that govern the conduct of sex work, which define the ability of FSWs to access safe work places and confidential services. Local laws and policy, and cultural factors affect the levels of discrimination associated with accessing HIV services or selling sex while living with HIV. The contrast between a legalised indoors environment (where women can access appropriate occupational health services and are safer from violence) and the illegal street-based environment (where women experience constant violence and have high rates of drug use and health problems) is stark when considering what interventions could operate at risk level 4. These factors can be subject to rapid change in any one setting. Improved working conditions, reduction in police brutality, and empowerment of FSWs have been described because of policy reform and decriminalisation of commercial sex in New Zealand and are well described in this Series.2,163,164
Community-based services and community advocacy, engagement, and mobilisation of the sex work community are essential (risk level 3). In conjunction with strong civil society and peer initiatives, these can reduce the stigma, discrimination, and marginalisation of FSWs, which are themselves determinants of risk. Participatory programmes that have behavioural and structural effect s such as those seen in Sonagachi, Avahan, and other community-based programmes are examples.27–29,165 Our model suggests that condom distribution and HIV communication programmes have already had a substantial effect on HIV transmission between FSWs and their clients in South Africa. These programmes in combination are estimated to have reduced HIV incidence in FSWs by 76% and clients by 65% in 2010. Similar success could have been achieved in other regions where levels of condom use are already high. A model-based assessment of the Avahan programme in southern India suggests that since it began, increases in condom use have reduced new HIV infections by 48–67%,165 and similar reductions have been estimated when modelling the effect of Project SIDA in Benin, which has promoted condoms and STI screening in FSWs.158 In 2007, the median proportion of FSWs who reported condom use with their last client was high in all regions,166 suggesting that existing interventions in other regions might already have had an important effect on HIV incidence in FSWs and their clients, although this effect cannot be quantified in some settings due to scarce data on trends in condom use and HIV prevalence.
Network level factors (risk level 2) operate within social, sexual, and injection networks, and are poorly understood in the context of FSWs. Modelling studies have suggested that in some settings, prevention programmes that reach regular clients and managers could be important in reducing HIV incidence in FSWs, particularly when the average time spent in commercial sex is short.167 Additionally, effective interventions at this level are particularly relevant to STIs and needle and syringe safety. Compounding factors that apply at the community and network level include ethnic origin, migration, citizenship status, literacy, economic security, marital status, drug use, social capital, and education—all factors strongly associated with HIV acquisition in FSWs.
We have already described a number of biomedical and behavioural interventions that reduce HIV risk at the individual level (risk level 1). Biomedical interventions under development such as longer-acting vaginal rings, long-acting injectable PrEP, and products that combine antiretroviral agents, contraceptives, or other anti-STI medications could facilitate adherence and enhance the prevention package available to FSWs in the future. Rectal microbicides could be of importance in sex work associated with anal sex. A source of uncertainty when considering the potential effect of oral and topical PrEP is the probable extent of risk compensation. Increased unprotected sex is less likely to attenuate the protective value of PrEP when individuals recognise their risk, want to use PrEP, and are motivated to be adherent.168 Data from the Partners PrEP trial show that after unblinding, individuals receiving PrEP were marginally more likely to have unprotected sex with individuals other than their study partner.145 Some modelling studies have also raised this concern.169–171 High-quality social and behavioural preparedness research is needed to track trends in condom use, and incidence of STIs and unwanted pregnancies.172
Tailored combination prevention for FSWs should take into account the type of sex work. Some of the modalities might be easier to implement in specific settings (eg, 100% condom promotion initially had an effect in establishment-based FSWs). Reaching the poorest and most marginalised sex workers (eg, those who work on the street or at truck stops) still presents formidable challenges for the future.
Conclusions
Reducing HIV transmissions associated with sex work by making sex work safer both for the workers themselves and their clients are important components in achieving prevention services for all. This review gives evidence of an impressive array of already existing prevention modalities that can be combined and applied to reduce risk of HIV acquisition in FSW populations worldwide. New biomedical technologies, including topical and oral antiretroviral-based PrEP and earlier antiretroviral TasP, must be additive to, and not replacements for, more established prevention modalities.12,16 We also emphasise the paucity of information on the effectiveness in FSWs, particularly of the newer modalities. The Sonagachi29 and Empower Thailand173 programmes have shown the importance of community-led initiatives to ensure increasing resources are directed at a transformative change in behaviour. These include individual interventions such as condom use, and structural interventions such as law reform, protective policing, and comprehensive and voluntary services.164 High levels of coverage and usage of services, and quality and sustainability, are critical to maximise the effect .13,164,174–176 Inadequate financing for FSW HIV prevention programming is a crucial reason why HIV prevention coverage remains so low. Notwithstanding sex workers’ disproportionate risk of acquiring HIV, prevention programmes for sex workers account for a meagre share of HIV prevention funding worldwide.13,164 In most regions, national governments have allocated few national resources to prevent HIV in sex workers, with international donors funding most of the HIV prevention eff orts for this group.13 Our model simulations suggest that condom promotion and distribution programmes in South Africa have already reduced HIV incidence in FSWs and their clients by more than 70%. Expansion of voluntary, effective early treatment together with PrEP could further reduce HIV incidence in South African FSWs and their clients. Careful, consultative addition of these approaches in tandem to a tailored prevention package for sex workers that recognises and supports safe workplaces and respectful communities will go far in eliminating HIV infections, eradicating discrimination, and ending AIDS deaths.
Supplementary Material
Key messages.
Effective HIV prevention approaches for female sex workers exist but have not been taken to scale or adequately resourced in most parts of the world.
Prevention interventions should integrate principles of social justice and meaningfully include sex workers in programme design and implementation.
Existing and effective prevention interventions include condom promotion, sexually transmitted infection prevention and treatment, HIV counselling and testing, gender-based violence prevention, and economic and community empowerment.
Stigma and criminalisation form barriers to such interventions and a less punitive more enabling legal and medical environment is required.
Modelling suggests that condom promotion may have already reduced incidence in sex workers and their clients by up to 70% in South Africa. Additional biomedical interventions such as pre-exposure prophylaxis or treatment as prevention could further reduce this by 40%.
Both topical and oral pre-exposure prophylaxis have been proven to reduce HIV incidence in high-risk men and women. However, its effectiveness in sex workers has yet to be determined.
Earlier initiation of antiretroviral therapy, with the requisite access to services is likely to benefit the health of sex workers and reduce HIV incidence in their clients and other sexual partners.
New biomedical technologies must be additive to, and not replacements for, more established prevention modalities. Interventions that combine behavioural, biological, and structural factors have the potential to have the greatest effect on the health of sex workers, their clients, and the wider population.
Search strategy and selection criteria.
The literature review focused on HIV prevention programmes and interventions, and in particular those that focused on the female sex worker (FSW) population. This review included observational studies, randomised controlled trials, and consensus papers or programme reports from organisations, when they were peer reviewed. We undertook a targeted web-based search of reported literature from select sites including WHO and the Joint UN Programme on HIV AIDS (UNAIDS) to retrieve information regarding new policy guidelines on FSWs and the latest evidence regarding HIV prevention. Data from systematic reviews of HIV prevention interventions both in the female population and general population were included as often data and programmes specifically addressing FSWs were scarce. The review was restricted to articles and documents published in English since 1990, with a particular emphasis on newer publications beginning in 2000. PubMed and Google Scholar were searched in addition to hand searching the bibliographies of selected peer-reviewed articles. Key words for the search criteria included “HIV prevention”, “Female SWs”, “IDU”, “PREP”, “peer support programmes”, “PEP” and “STI treatment”, “ART”, “community participation”, “condom use”, “biomedical HIV prevention”. Abstracts of retrieved articles were read and if they were pertinent to the research question, full texts were then retrieved. Due to the dearth of information specifically related to HIV prevention in the FSW population, inclusion criteria were broad to ensure a comprehensive understanding of HIV interventions available, even if they had not necessarily been tested in the specific population in question. As the fields of HIV prevention interventions are rapidly changing, with studies underway, the review was updated several times. Of the 2350 papers identified in the search, 69 were included that gave a broad range of interventions that have an effect on HIV prevention, either in the FSW population or general population. Those that did not directly measure HIV prevalence or incidence reduction but were reported to have a reduction on other factors (eg, STI treatment) known to be linked to HIV reduction were also included.
Acknowledgments
L-GB is supported in part by National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), UCT CTU grant 2UM1AI069519-08. LJ received support from NIH (1R01AI094586-01) and Hasso Plattner Foundation. SH received support from NIAID, Microbicide Trials Network (1UM1AI068633). WC received support from USAID Preventive Technologies Agreement (GHO-A-00-09-00016-00) and NIAID, NIH HIV Prevention Trials Network (1U01A1068619-01). This article and The Lancet Series on HIV and sex workers was supported by grants to the Center for Public Health and Human Rights at Johns Hopkins Bloomberg School of Public Health from The Bill & Melinda Gates Foundation and from The United Nations Family Planning Association (UNFPA).
Footnotes
Declaration of interests
We declare no competing interests.
Contributor Information
Linda-Gail Bekker, The Desmond Tutu HIV Centre University of Cape Town, Republic of South Africa.
Leigh Johnson, Centre for Infectious Disease Epidemiology and Research University of Cape Town, Republic of South Africa.
Frances Cowan, Research Department of Infection and Population Health, University College London, London, UK; Centre for Sexual Health and HIV/AIDS Research Health and HIV/AIDS Research (CeSHHAR) Zimbabwe, Harare, Zimbabwe.
Cheryl Overs, Michael Kirby Centre for Public Health and Human Rights, Melbourne, Australia.
Donela Besada, The Desmond Tutu HIV Foundation, Cape Town, Republic of South Africa.
Sharon Hillier, Unversity of Pittsburgh Department of Obstetrics, Gynecology and Reproductive Sciences, Pittsburgh, PA, USA.
Willard Cates, Jr, FHI 360, Durham, NC, USA.
References
- 1.UNAIDS. Global HIV/AIDS Report. Geneva: United Nations, 2013. [Google Scholar]
- 2.Shannon K, Strathdee SA, Goldenberg SM, et al. Global epidemiology of HIV among female sex workers: influence of structural determinants. Lancet 2014; published online July 22. 10.1016/S0140-6736(14)60931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baral S, Beyrer C, Muessig K, et al. Burden of HIV among female sex workers in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12: 538–49. [DOI] [PubMed] [Google Scholar]
- 4.Kerrigan D, Wirtz A, Baral S, et al. The global HIV epidemics among sex workers. Washington DC: The World Bank, 2012. [Google Scholar]
- 5.Prüss-Ustün A, Wolf J, Driscoll T, Degenhardt L, Neira M, Calleja JMG. HIV due to female sex work: regional and global estimates. PloS One 2013; 8: e63476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gouws E, Cuchi P. Focusing the HIV response through estimating the major modes of HIV transmission: a multi-country analysis. Sex Transm Infect 2012; 88 (suppl 2): i76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harcourt C, Donovan B. The many faces of sex work. Sex Transm Infect 2005; 81: 201–06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buzdugan R, Halli SS, Cowan FM. The female sex work typology in India in the context of HIV/AIDS. Trop Med Int Health 2009; 14: 673–87. [DOI] [PubMed] [Google Scholar]
- 9.Baral SD, Friedman MR, Geibel S, et al. Male sex workers: practices, contexts, and vulnerabilities for HIV acquisition and transmission. Lancet 2014; published online July 22. 10.1016/S0140-6736(14)60801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poteat T, Wirtz AL, Radix A, et al. HIV risk and preventive interventions in transgender women sex workers. Lancet 2014; published online July 22. 10.1016/S0140-6736(14)60833-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vandenbruaene M King Kennard Holmes—Chair of the Department of Global Health of the University of Washington. Lancet Infect Dis 2007; 7: 516–20. [DOI] [PubMed] [Google Scholar]
- 12.Coates TJ, Richter L, Caceres C. Behavioural strategies to reduce HIV transmission: how to make them work better. Lancet 2008; 372: 669–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.UNAIDS, World Bank. The global economic crisis: prevention and treatment programmes: vulnerabilities and impact. Geneva: World Bank, UNAIDS, 2009. [Google Scholar]
- 14.Baral S, Logie CH, Grosso A, Wirtz AL, Beyrer C. Modifi ed social ecological model: a tool to guide the assessment of the risks and risk contexts of HIV epidemics. BMC Public Health 2013; 13: 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerrigan D, Kennedy CE, Morgan-Thomas R, et al. A community empowerment approach to the HIV response among sex workers: effectiveness, challenges, and considerations for implementation and scale-up. Lancet 2014; published online July 22. 10.1016/S0140-6736(14)60973-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.International Pathfinder. Combination prevention of HIV: a technical guide to working with key affected populations. Watertown, MA: Pathfinder International, 2004. [Google Scholar]
- 17.Jones A, Cremin I, Abdullah F, et al. Transformation of HIV from pandemic to low-endemic levels: a public health approach to combination prevention. Lancet 2014; published online April 14. 10.1016/S0140-6736(13)62230-8 [DOI] [PubMed] [Google Scholar]
- 18.Illife J The African AIDS epidemic: a history. Athens, OH: Ohio University Press, 2006. [Google Scholar]
- 19.Kulkarni PS, Butera ST, Duerr AC. Resistance to HIV-1 infection: lessons learned from studies of highly exposed persistently seronegative (HEPS) individuals. AIDS Rev 2003; 5: 87–103. [PubMed] [Google Scholar]
- 20.Abdool Karim SS, Laga M, Stein Z, Rustomjee R, Abdool Karim Q. Phase 1 trial of nonoxynol-9 fi lm among sex workers in South Africa. AIDS 1999; 13: 1511–15. [DOI] [PubMed] [Google Scholar]
- 21.Morar NS, Ramjee G, Abdool Karim SS. Vaginal insertion and douching practices among sex workers at truck stops in KwaZulu-Natal. S Afr Med J 1998; 88: 470. [PubMed] [Google Scholar]
- 22.Van Damme L, Ramjee G, Alary M, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet 2002; 360: 971–77. [DOI] [PubMed] [Google Scholar]
- 23.Rojanapithayakorn W, Hanenberg R. The 100% condom program in Thailand. AIDS 1996; 10: 1–7. [DOI] [PubMed] [Google Scholar]
- 24.Ainsworth M, Beyrer C, Soucat A. AIDS and public policy: the lessons and challenges of ‘success’ in Thailand. Health Policy 2003; 64: 13–37. [DOI] [PubMed] [Google Scholar]
- 25.Jana S, Rojanapithayakorn W, Steen R. Harm reduction for sex workers. Lancet 2006; 367: 814. [DOI] [PubMed] [Google Scholar]
- 26.Kerrigan D, Barrington C, Sweat M, et al. Environmental-structural interventions to reduce HIV/STI risk among female sex workers in the Dominican Republic. Am J Public Health 2006; 96: 120–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sibongile Mtetwa FMC. “Sisters”—the first four years of the Zimbabwe National Sex Work Programme. Sisters with a Voice. http://www.unicef.org/zimbabwe/ZIM_resources_nationalsexworkprogram.pdf (accessed April 20, 2014). [Google Scholar]
- 28.Ng M, Gakidou E, Levin-Rector A, Khera A, Murray CJL, Dandona L. Assessment of population-level effect of Avahan, an HIV-prevention initiative in India. Lancet 2011; 378: 1643–52. [DOI] [PubMed] [Google Scholar]
- 29.Ghose T, Swendeman DT, George SM. The role of brothels in reducing HIV risk in Sonagachi, India. Qual Health Res 2011; 21: 587–600. [DOI] [PubMed] [Google Scholar]
- 30.Mills E, Rachlis B, Wu P, Wong E, Wilson K, Singh S. Media reporting of tenofovir trials in Cambodia and Cameroon. BMC Int Health Hum Rights 2005; 5: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh JA, Mills EJ. The abandoned trials of pre-exposure prophylaxis for HIV: what went wrong? PLoS Med 2005; 2: e234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.UNAIDS A. Good participatory practice. Guidelines for biomedical HIV prevention trials. Geneva: UNAIDS, AVAC, 2007. [Google Scholar]
- 33.Shannon K, Csete J. Violence, condom negotiation, and HIV/STI risk among sex workers. JAMA 2010; 304: 573–74. [DOI] [PubMed] [Google Scholar]
- 34.Weller SC. A meta-analysis of condom effectiveness in reducing sexually transmitted HIV. Soc Sci Med 1993; 36: 1635–44. [DOI] [PubMed] [Google Scholar]
- 35.Hanenberg RS, Sokal DC, Rojanapithayakorn W, Kunasol P. Impact of Thailand’s HIV-control programme as indicated by the decline of sexually transmitted diseases. Lancet 1994; 344: 243–45. [DOI] [PubMed] [Google Scholar]
- 36.Kerrigan D, Moreno L, Rosario S, Sweat M. Adapting the 100% Thai Condom Campaign: developing a culturally appropriate model for the Dominican Republic. 2001. Culture Health Sex 2001; 3: 221–40. [Google Scholar]
- 37.Population Council. Community approaches and government policy reduce HIV risk in the Dominican Republic. Horisons http://www.popcouncil.net/pdfs/horizons/drcmntygvtplcysum.pdf (accessed April 20, 2014). [Google Scholar]
- 38.Strathdee SA, Abramovitz D, Lozada R, et al. Reductions in HIV/STI incidence and sharing of injection equipment among female sex workers who inject drugs: results from a randomized controlled trial. PLoS One 2013; 8: e65812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Witte SS, El-Bassel N, Wada T, Gray O, Wallace J. Acceptability of female condom use among women exchanging street sex in New York City. Int J STD AIDS 1999; 10: 162–68. [DOI] [PubMed] [Google Scholar]
- 40.French PP, Latka M, Gollub EL, Rogers C, Hoover DR, Stein ZA. Use-effectiveness of the female versus male condom in preventing sexually transmitted disease in women. Sex Transm Dis 2003; 30: 433–39. [DOI] [PubMed] [Google Scholar]
- 41.Heise L, Lutz B, Ranganthan M, Watts C. Cash Transfers for HIV Prevention: considering their potential. J Int AIDS Soc 2013; 16: 18615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baird S, Garfein R, McIntosh C, Ozler B. Effect of cash transfer program for schooling on prevalence of HIV and Herpes Simplex type 2 in Malawi: a cluster randomised trial. Lancet 2012; 379: 1320–29. [DOI] [PubMed] [Google Scholar]
- 43.deWalque D, Dow WH, Nathan R, et al. Incentivising safe sex: a randomised trial of conditional cash transfers for HIV and sexually transmitted infection prevention in rural Tanzania. BMJ Open 2012; 2: e000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galárraga O, Sosa-Rubí S, Infante C, Gertler P, Bertozzi SM. Willingness-to-accept reductions in HIV risks: conditional economic incentives in Mexico. Eur J Health Econ 1014; 15: 41–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manhart LE, Holmes KK. Randomized controlled trials of individual-level, population-level, and multilevel interventions for preventing sexually transmitted infections: what has worked? J Infect Dis 2005; 191 (suppl 1): S7–24. [DOI] [PubMed] [Google Scholar]
- 46.Alary M, Mukenge-Tshibaka L, Bernier F, et al. Decline in the prevalence of HIV and sexually transmitted diseases among female sex workers in Cotonou, Benin, 1993–1999. AIDS 2002; 16: 463–70. [DOI] [PubMed] [Google Scholar]
- 47.Grosskurth H, Gray R, Hayes R, Mabey D, Wawer M. Control of sexually transmitted diseases for HIV-1 prevention: understanding the implications of the Mwanza and Rakai trials. Lancet 2000; 355: 1981–87. [DOI] [PubMed] [Google Scholar]
- 48.Kaul R, Kimani J, Nagelkerke NJ, et al. Monthly antibiotic chemoprophylaxis and incidence of sexually transmitted infections and HIV-1 infection in Kenyan sex workers: a randomized controlled trial. JAMA 2004; 291: 2555–62. [DOI] [PubMed] [Google Scholar]
- 49.Labbé AC, Dzokoto A, Khonde N, Pépin J, Meda H, Asamoah-Adu C. A randomized placebo-controlled trial of routine monthly antibiotics against gonococcal and chlamydial infections among female sex workers in Ghana and Bénin: intention-to-treat analysis. In: 15th Biennial Congress of the International Society for Sexually Transmitted Diseases Research (ISSTDR); Ottawa, Canada; 2003: 27–30. [Google Scholar]
- 50.Laga M, Alary M, Behets F, et al. Condom promotion, sexually transmitted diseases treatment, and declining incidence of HIV-1 infection in female Zairian sex workers. Lancet 1994; 344: 246–48. [DOI] [PubMed] [Google Scholar]
- 51.Shahmanesh M, Patel V, Mabey D, Cowan F. Effectiveness of interventions for the prevention of HIV and other sexually transmitted infections in female sex workers in resource poor setting: a systematic review. Trop Med Int Health 2008; 13: 659–79. [DOI] [PubMed] [Google Scholar]
- 52.Grosskurth H, Mosah F, Todd J, et al. A community trial of the impact of improved sexually transmitted disease treatment on the HIV epidemic in rural Tanzania: 2. Baseline survey results. AIDS 1995; 9: 923–34. [DOI] [PubMed] [Google Scholar]
- 53.Steen R, Chersich M, Gerbase A, et al. Periodic presumptive treatment of curable sexually transmitted infections among sex workers: a systematic review. AIDS 2012; 26: 437–45. [DOI] [PubMed] [Google Scholar]
- 54.World Health Organization. Prevention and treatment of HIV and other sexually transmitted infections for SWs in low- and middle-income countries: recommendations for a public health approach. Geneva: World Health Organization, UNAIDS, 2012. [PubMed] [Google Scholar]
- 55.Tucker JD, Bien CH, Peeling RW. Point-of-care testing for sexually transmitted infections: recent advances and implications for disease control. Curr Opin Infect Dis 2013; 26: 73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Celum C, Wald A, Hughes J, et al. Effect of acyclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 371: 2109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Watson-Jones D, Weiss HA, Rusizoka M, et al. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N Engl J Med 2008; 358: 1560–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lingappa JR, Baeten JM, Wald A, et al. Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: a randomised placebo-controlled trial. Lancet 2010; 375: 824–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.World Health Organization. Global HIV/AIDS response: epidemic update and health sector progress towards universal access: progress report 2011. Geneva: World Health Organization, 2011. [Google Scholar]
- 60.Kriitmaa K, Testa A, Osman M, et al. HIV prevalence and characteristics of sex work among female sex workers in Hargeisa, Somaliland, Somalia. AIDS 2010; 24 (suppl 2): S61–67. [DOI] [PubMed] [Google Scholar]
- 61.Cowan FM, Mtetwa S, Davey C, et al. Engagement with HIV prevention treatment and care among female sex workers in Zimbabwe: a respondent driven sampling survey. PLoS One 2013; 8: e77080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marum E, Taegtmeyer M, Parekh B, et al. “What took you so long?” The impact of PEPFAR on the expansion of HIV testing and counseling services in Africa. J Acquir Immune Defic Syndr 2012: 60 (suppl 3): S63–69. [DOI] [PubMed] [Google Scholar]
- 63.UNAIDS. Global report on the global AIDS epidemic 2012. Geneva: UNAIDS, 2012. [Google Scholar]
- 64.USAID. DHS comparative reports no. 30. Demographic patterns of HIV testing uptake in sub-Saharan Africa. 2013. http://dhsprogram.com/pubs/pdf/CR30/CR30.pdf (accessed June 18, 2014).
- 65.Guest PP BJ, Janyam S, Phuengsamran D. Survey of sexual and reproductive health of sex workers in Thaliand. Bangkok, Thailand: Insitute for Population and Social Research, 2007. [Google Scholar]
- 66.Hong Y, Zhang C, Li X, et al. HIV testing behaviors among female sex workers in Southwest China. AIDS Behav 2012; 16: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ngo AD, Ratliff EA, McCurdy SA, Ross MW, Markham C, Pham HT. Health-seeking behaviour for sexually transmitted infections and HIV testing among female sex workers in Vietnam. AIDS Care 2007; 19: 878–87. [DOI] [PubMed] [Google Scholar]
- 68.Wang Y, Li B, Zheng J, et al. Factors related to female sex workers’ willingness to utilize VCT service: a qualitative study in Jinan city, northern China. AIDS Behav 2009; 13: 866–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Munoz J, Adedimeji A, Alawode O. ‘They bring AIDS to us and say we give it to them’: socio-structural context of female sex workers’ vulnerability to HIV infection in Ibadan, Nigeria. SAHARA J 2010; 7: 52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scorgie F, Nakato D, Akoth DO, et al. I expect to be abused and I have fear: sex workers experiences of human rights violations and barriers to accessing health care in four African countries. Johannesburg, South Africa: Sex Workers Alliance, 2011. [Google Scholar]
- 71.Hargreaves J, Mtetwa S, Dirawo J, et al. HIV incidence, testing and treatment among female sex workers accessing outreach clinics in Zimbabwe, 2009–13: analysis of programme data. 17th ICASA Conference; Cape Town, South Africa; Nov 8–12, 2013. [Google Scholar]
- 72.Luchters S, Chersich M, Rinyiru A, et al. Impact of five years of peer-mediated interventions on sexual behavior and sexually transmitted infections among female sex workers in Mombasa, Kenya. BMC Public Health 2008; 8: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rou K, Sullivan SG, Liu P, Wu Z. Scaling up prevention programmes to reduce the sexual transmission of HIV in China. Int J Epidemiol 2010; 39 (suppl 2): ii38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hong Y, Fang X, Li X, Liu Y, Li M. Environmental support and HIV prevention behaviors among female sex workers in China. Sex Transm Dis 2008; 35: 662–67. [DOI] [PubMed] [Google Scholar]
- 75.Lippman SA, Donini A, Diaz J, Chinaglia M, Reingold A, Kerrigan D. Social-environmental factors and protective sexual behavior among sex workers: the Encontros intervention in Brazil. Am J Public Health 2010; 100 (suppl 1): S216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brown B, Duby Z, Bekker LG. Sex workers: an introductory manual for health care workers in South Africa. Cape Town, South Africa: Desmond Tutu HIV Foundation, 2012. [Google Scholar]
- 77.Pleaner M, Motloung T, Richter M, Jankelowitz L. RHRU support for sex workers. A resource pack for health care providers. 2009. http://www.tlhethiopia.org/index.php/resources/cat_view/6-researches?start=5 (accessed June 11, 2014). [Google Scholar]
- 78.Basu I, Jana S, Rotheram-Borus MJ, et al. HIV prevention among sex workers in India. J Acquired Immune Def Syndr 2004; 36: 845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ngugi EN BE, Jackson DJ. Interventions for commercial SWs and their clients. In: Gibney L, DiClemente RJ, Vermund SH, eds. Preventing HIV in developing countries: biomedical and behavioral approaches. New York: Plenum Press, 1999: 205–29. [Google Scholar]
- 80.Chersich M, Luchters S, Ntaganira I, et al. Priority interventions to reduce HIV transmission in sex work settings in sub-Saharan Africa and delivery of these services. J Int AIDS Society 2013; 16: 17980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scambler G, Paoli F. Health work, female sex workers and HIV/AIDS: Global and local dimensions of stigma and deviance as barriers to effective interventions. Soc Sci Med 2008; 66: 1848–62. [DOI] [PubMed] [Google Scholar]
- 82.Human Rights Watch. Fanning the flames. How human rights abuses are fueling the AIDS epidemic in Karzakhstan New York, USA: Human Rights Watch, 2003. [Google Scholar]
- 83.WHO. Violence against women and HIV/AIDS: critical Intersections—violence against sex workers and HIV prevention. Geneva: World Health Organization Department of Gender, Women and Health, 2005. [Google Scholar]
- 84.SWEAT. Work wise: sex worker handbook on human rights, health and violence. Cape Town, South Africa: Sex Worker Education and Advocacy Taskforce, 2004. [Google Scholar]
- 85.Shannon K, Kerr T, Alinott S, Chettiar J. Shoveller J, Tyndall M Social and structural violence and power relations in mitigating HIV risk in drug using women in survival sex work. Soc Sci Med 2008; 66: e921. [DOI] [PubMed] [Google Scholar]
- 86.Simoni JM, Nelson KM, Franks JC, Yard SS, Lehavot K. Are peer interventions for HIV efficacious? A systematic review. AIDS Behav 2011; 15: 1589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Overs C, Loff B. The tide cannot be turned without us: sex workers and the global response to HIV. J Int AIDS Soc 2013; 16: 18459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wirtz AL, Pretorius C, Beyrer C, et al. Epidemic impacts of a community empowerment intervention for HIV prevention among female sex workers in generalized and concentrated epidemics. PLoS One 2014; 9: e88047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kerrigan DL, Fonner VA, Stromdahl S, Kennedy CE. Community empowerment among female sex workers is an effective HIV prevention intervention: a systematic review of the peer-reviewed evidence from low- and middle-income countries. AIDS Behav 2013; 17: 1926–40. [DOI] [PubMed] [Google Scholar]
- 90.Parker RG, Easton D, Klein CH. Structural barriers and facilitators in HIV prevention: a review of international research. AIDS 2000; 14: S22–32. [DOI] [PubMed] [Google Scholar]
- 91.Jana S, Basu I, Rotheram-Borus MJ, Newman PA. The Sonagachi Project: a sustainable community intervention program. AIDS Educat Prev 2004; 16: 405–14. [DOI] [PubMed] [Google Scholar]
- 92.Cornman H Microfi nance, HIV, and women’s empowerment. Arlington, VA: USAID, AIDSTAR-One, Task Order; 1, 2012. [Google Scholar]
- 93.Verma R, Shekhar A, Khobragade S, et al. Scale-up and coverage of Avahan: a large-scale HIV-prevention programme among female sex workers and men who have sex with men in four Indian states. Sex Transm Infect 2010; 86 (suppl 1): i76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.WHO. Preventing HIV among sex workers in sub-Saharan Africa. A literature review. Geneva: World Health Organization, 2011. [Google Scholar]
- 95.Chersich MF, Luchters S, Ntaganira I, et al. Priority interventions to reduce HIV transmission in sex work settings in sub-Saharan Africa and delivery of these services. J Int AIDS Soc 2013; 16: 17980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baeten JM, Haberer JE, Liu AY, Sista N. Preexposure prophylaxis for HIV prevention: where have we been and where are we going? J Acquir Immune Defic Syndr 2013; 63 (suppl 2): S122–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abdool Karim Q, Abdool Karim SS, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010; 329: 1168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med 2012; 367: 399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2013; 381: 2083–90. [DOI] [PubMed] [Google Scholar]
- 100.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012; 367: 423–34. [DOI] [PubMed] [Google Scholar]
- 101.Marrazzo J, Ramjee G, Nair G, et al. Pre-exposure prophylaxis for HIV in women: daily oral tenofovir, oral tenofovir-emtricitabine, or vaginal tenofovir gel in the VOICE Study (MTN 003). 20th Conference on Retroviruses and Opportunistic Infections; Atlanta, GA, USA; Mar 3–6, 2013. Abstr #26LB. [Google Scholar]
- 102.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363: 2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med 2012; 367: 411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abdool Karim Q, Kharsany A, Frohlich J, et al. Recruitment of high risk women for HIV prevention trials: baseline HIV prevalence and sexual behavior in the CAPRISA 004 tenofovir gel trial. Trials 2011; 12: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365: 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Murnane PM, Celum C, Mugo N, et al. Efficacy of preexposure prophylaxis for HIV-1 prevention among high-risk heterosexuals: subgroup analyses from a randomized trial. AIDS 2013; 27: 2155–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kahle EM, Hughes JP, Lingappa JR, et al. An empiric risk scoring tool for identifying high-risk heterosexual HIV-1-serodiscordant couples for targeted HIV-1 prevention. J Acquir Immune Defic Syndr 2013; 62: 339–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lowndes CM, Alary M, Platt L. Injection drug use, commercial sex work, and the HIV/STI epidemic in the Russian Federation. Sex Transm Dis 2003; 30: 46–48. [DOI] [PubMed] [Google Scholar]
- 109.Folch C, Sanclemente C, Esteve A, Martro E, Molinos S, Casabona J. Social characteristics, risk behaviours and diff erences in the prevalence of HIV/sexually transmitted infections between Spanish and immigrant female sex workers in Catalonia, Spain. Med Clin 2009; 132: 385–88 (in Spanish). [Google Scholar]
- 110.Mamaev T Results of HIV sentinel epidemiologic surveillance among SWs in Osh City of Kyrgyz Rep. Mikrobiol Epidemiol Immunobiol 2007; 3: 72–74. [PubMed] [Google Scholar]
- 111.Strathdee SA, Hallett TB, Bobrova N, et al. HIV and risk environment for injecting drug users: the past, present, and future. Lancet 2010; 376: 268–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yam EA, Mnisi Z, Sithole B, et al. Association between condom use and use of other contraceptive methods among female sex workers in Swaziland: a relationship-level analysis of condom and contraceptive use. Sex Transm Dis 2013; 40: 406–12. [DOI] [PubMed] [Google Scholar]
- 113.Ulibarri M Strathdee S; Lozada R, Magis-Rodriguez C, et al. Intimate partner violence among female sex workers in two Mexico–U.S. Border cities: Partner characteristics and HIV risk behaviors as correlates of abuse. Psychol Trauma 2010; 2: 318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Eisengerich A, Wheelock A, Gomez G, et al. Attitudes and acceptance of oral and parenteral HIV PrEP among potential user groups: a multinational study. PLoS One 2012; 7: e28238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weeks MR, Mosack KE, Abbott M, et al. Microbicide acceptability among High -risk urban US women: experiences and preceptions of sexually transmitted HIV prevention. Sex Transm Dis 2004; 31: 682–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Naranbhai V, Karim SSA, Altfeld M, et al. Innate immune activation enhances HIV acquisition in women, diminishing the effectiveness of tenofovir microbicide gel. J Infect Dis 2012; 206: 993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Roberts L, Passmore J-A, Williamson C, et al. Genital tract inflammation in women participating in the CAPRISA TFV microbicide trial who became infected with HIV: a mechanism for breakthrough infection? 18th Conference on Retroviruses and Opportunistic Infections; Feb 27–Mar 2, 2011; Boston, MA, USA. Poster abstract 991. http://retroconference.org/2011/Abstracts/41472.htm (accessed June 16, 2014). [Google Scholar]
- 118.Yao XD, Omange RW, Henrick BM, et al. Acting locally: innate mucosal immunity in resistance to HIV-1 infection in Kenyan commercial sex workers. Mucosal Immunol 2014; 7: 268–79. [DOI] [PubMed] [Google Scholar]
- 119.US Women and PREP Working Group Position Statement (press release). http://sisterlove.org/wp-contents/uploads/uploads/2013/03/working-group-on-us-women-and-prep-statement.pdf (accessed April 20, 2014).
- 120.CDC. Preexposure prophylaxis for the prevention of HIV infecton in the United States—2014. A clinical practice guideline. Atlanta, GA: CDC, 2014. [Google Scholar]
- 121.WHO. Guidance on pre-exposure oral prophylaxis (PrEP) for serodiscordant couples, men and transgender women who have sex with men at high risk of HIV: recommendations for use in the context of demonstration projects. Geneva: World Health Organization, 2012. http://apps.who.int/iris/bitstream/10665/75188/1/9789241503884_eng.pdf?ua=1 (accessed June 10, 2014). [PubMed] [Google Scholar]
- 122.WHO. PrEP demonstration projects. A framework for country level procotol development. April 2013. Geneva: World Health Organization. http://apps.who.int/iris/bitstream/10665/112799/1/9789241507172_eng.pdf?ua=1 (accessed June 10, 2014). [Google Scholar]
- 123.FACTS Consortium. FACTS 001 study design. http://www.facts-consortium.co.za/?page_id=83 (accessed April 20, 2014).
- 124.Gostin LO, Lazzarini Z, Alexander D, Brandt AM, Mayer KH, Silverman DC. HIV testing, counseling, and prophylaxis after sexual assault. JAMA 1994; 271: 1436–44. [PubMed] [Google Scholar]
- 125.Izulla P, McKinnon LR, Munyao J, et al. HIV postexposure prophylaxis in an urban population of female sex workers in Nairobi, Kenya. J Acquir Immune Defic Syndr 2013; 62: 220–25. [DOI] [PubMed] [Google Scholar]
- 126.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med 2000; 342: 921–29. [DOI] [PubMed] [Google Scholar]
- 127.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet 2001; 357: 1149–53. [DOI] [PubMed] [Google Scholar]
- 128.Braunstein SL, Umulisa MM, Veldhuijzen NJ, et al. HIV diagnosis, linkage to HIV care, and HIV risk behaviors among newly diagnosed HIV-positive female sex workers in Kigali, Rwanda. J Acquir Immune Defic Syndr 2011; 57: e70–76. [DOI] [PubMed] [Google Scholar]
- 129.Kayigamba FR, Bakker MI, Fikse H, Mugisha V, Asiimwe A, Schim van der Loeff MF. Patient enrolment into HIV care and treatment within 90 days of HIV diagnosis in eight Rwandan health facilities: a review of facility-based registers. PLoS One 2012; 7: e36792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.McClelland RS, Graham SM, Richardson BA, et al. Treatment with antiretroviral therapy is not associated with increased sexual risk behavior in Kenyan female sex workers. AIDS 2010; 24: 891–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shannon K, Bright V, Duddy J, Tyndall MW. Access and utilization of HIV treatment and services among women sex workers in Vancouver’s Downtown Eastside. J Urban Health 2005; 82: 488–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Diabaté S, Zannou DM, Geraldo N, et al. Antiretroviral therapy among HIV-1 infected female sex workers in Benin: a comparative study with patients from the general population. World J AIDS 2011; 1: 94. [Google Scholar]
- 133.Huet C, Ouedraogo A, Konaté I, et al. Long term virological, immunological and mortality outcomes in a cohort of HIV-infected female sex workers treated with highly active antiretroviral therapy in Africa. BMC Public Health 2011; 11: 700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dickinson C, Attawell K, Druce N. Progress on scaling up integrated services for sexual and reproductive health and HIV. Bull World Health Organ 2009; 87: 846–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eaton JW, Menzies NA, Stover J, et al. Health benefits, costs, and cost-effectiveness of earlier eligibility for adult antiretroviral therapy and expanded treatment coverage: a combined analysis of 12 mathematical models. Lancet Glob Health 2014; 2: e23–34. [DOI] [PubMed] [Google Scholar]
- 136.Scheibe A DF, Shannon K. HIV Prevention among female sex workers in Africa. SAHARA J 2012; 9: 67–172. [DOI] [PubMed] [Google Scholar]
- 137.Sex Workers Education and Advocacy Taskforce. Sex workers in South Africa: a rapid population size estimation study. http://www.sanac.org.za/publications/reports/cat_view/7-publications/9-reports (accessed June 10, 2014).
- 138.Leclerc PM, Garenne M. Clients of commercial sex workers in Zambia: prevalence, frequency and risk factors. Open Demography J 2008; 1: 1–10. [Google Scholar]
- 139.Varga CA. The condom conundrum: barriers to condom use among commercial sex workers in Durban, South Africa. Afr J Reprod Health 1997; 1: 74–88. [PubMed] [Google Scholar]
- 140.Abdool Karim QA, Abdool Karim SS, Soldan K, Zondi M. Reducing the risk of HIV infection among South African sex workers: socioeconomic and gender barriers. Am J Public Health 1995; 85: 1521–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ramjee G, Abdool Karim SS, Sturm AW. Sexually transmitted infections among sex workers in KwaZulu-Natal, South Africa. Sex Transm Dis 1998; 25: 346–49. [DOI] [PubMed] [Google Scholar]
- 142.Dunkle KL, Beksinska ME, Rees VH, et al. Risk factors for HIV infection among sex workers in Johannesburg, South Africa. Int J STD AIDS 2005; 16: 256–61. [DOI] [PubMed] [Google Scholar]
- 143.van Loggerenberg F, Mlisana K, Williamson C, et al. Establishing a cohort at high risk of HIV infection in South Africa: challenges and experiences of the CAPRISA 002 acute infection study. PLoS One 2008; 3: e1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Delva W, Richter M, De Koker P, Chersich M, Temmerman M. Sex work during the 2010 FIFA World Cup: results from a three-wave cross-sectional survey. PLoS One 2011; 6: e28363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mugwanya KK, Donnell D, Celum C, et al. Sexual behaviour of heterosexual men and women receiving antiretroviral pre-exposure prophylaxis for HIV prevention: a longitudinal analysis. Lancet Infect Dis 2013; 13: 1021–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nel AM, Mitchnick LB, Risha P, Muungo LT, Norick PM. Acceptability of vaginal fi lm, soft-gel capsule, and tablet as potential microbicide delivery methods among African women. J Womens Health 2011; 20: 1207–14. [DOI] [PubMed] [Google Scholar]
- 147.van der Straten A, Montgomery ET, Cheng H, et al. High acceptability of a vaginal ring intended as a microbicide delivery method for HIV prevention in African women. AIDS Behav 2012; 16: 1775–86. [DOI] [PubMed] [Google Scholar]
- 148.Heffron R, Ngure K, Mugo N, et al. Willingness of Kenyan HIV-1 serodiscordant couples to use antiretroviral-based HIV-1 prevention strategies. J Acquir Immune Defic Syndr 2012; 61: 116–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet 2010; 375: 2092–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Attia S, Egger M, Müller M. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS 2009; 23: 1397–404. [DOI] [PubMed] [Google Scholar]
- 151.Jia Z, Ruan Y, Li Q, et al. Antiretroviral therapy to prevent HIV transmission in serodiscordant couples in China (2003–11): a national observational cohort study. Lancet 2013; 382: 1195–203. [DOI] [PubMed] [Google Scholar]
- 152.Birungi J, Wang H, Ngolobe MH, et al. Lack of effectiveness of antiretroviral therapy (ART) as an HIV prevention tool for serodiscordant couples in a rural ART program without viral load monitoring in Uganda. 19th International AIDS Conference; July 22–27, 2012; Washington DC, USA. Abstr TUAC0103. [Google Scholar]
- 153.Blower SM, Dowlatabadi H. Sensitivity and uncertainty analysis of complex models of disease transmission: an HIV model, as an example. Int Stat Rev 1994; 62: 229–43. [Google Scholar]
- 154.Johnson L THEMBISA version 1.0: A model for evaluating the impact of HIV/AIDS in South Africa. 2014. http://webdav.uct.ac.za/depts/epi/publications/documents/THEMBISA%20version%201.0.pdf (accessed June 10, 2014).
- 155.Padian NS, Buve A, Balkus J, Serwadda D, Cates W Jr. Biomedical interventions to prevent HIV infection: evidence, challenges, and way forward. Lancet 2008; 372: 585–99. [DOI] [PubMed] [Google Scholar]
- 156.Padian NS, McCoy SI, Balkus JE, Wasserheit JN. Weighing the gold in the gold standard: challenges in HIV prevention research. AIDS 2010; 24: 621–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vickerman P, Watts C, Delany S, Alary M, Rees H, Heise L. The importance of context: model prjections on how microbicide impact could be affected by the underlying epidemiologic and behavioral situation in two African settings. Sex Transm Dis 2006; 33: 397–405. [DOI] [PubMed] [Google Scholar]
- 158.Lowndes CM, Alary M, Belleau M et al. West Africa HIV/AIDS epidemiology and response synthesis. Washington, DC: World Bank, 2008. http://siteresources.worldbank.org/INTHIVAIDS/Resources/375798-1132695455908/WestAfricaSynthesisNov26.pdf. [Google Scholar]
- 159.Boily MC, Lowndes C, Alary M. The impact of HIV epidemic phases on the effectiveness of core group interventions: insights from mathematical models. Sex Transm Infect 2002; 78 (suppl 1): i78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hallett TB, Garnett GP, Mupamberiyi Z, Gregson S. Measuring effectiveness in community randomized trials of HIV prevention. Int J Epidemiol 2008; 37: 77–87. [DOI] [PubMed] [Google Scholar]
- 161.Gomez GB BA, Case KK, Wheelock A, Vassall A, Hankins C. The cost and impact of scaling up pre-exposure prophylaxis for HIV prevention: a systematic review of cost-effectiveness modelling studies. PLoS Med 2013; 10: e1001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pretorius C, Stover J, Bollinger L, Bacaër N, Williams B. Evaluating the cost-effectiveness of pre-exposure prophylaxis (PrEP) and its impact on HIV-1 transmission in South Africa. PLoS One 2010; 5: e13646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Harcourt C, O’Connor J, Egger S, et al. The decriminalisation of prostitution is associated with better coverage of health promotion programs for sex workers. Aust N Z J Public Health 2010; 34: 482–86. [DOI] [PubMed] [Google Scholar]
- 164.Beyrer C, Crago A-L, Bekker L-G, et al. An action agenda for HIV and sex workers. Lancet 2014; published online July 22. 10.1016/S0140-6736(14)60933-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Boily MC, Pickles M, Lowndes CM, et al. Positive impact of a large-scale HIV prevention programme among female sex workers and clients in South India. AIDS 2013; 27: 1449–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.UNAIDS. Report on the global AIDS epidemic: 2010. http://www.unaids.org/documents/20101123_GlobalReport_em.pdf (accessed June 11, 2014).
- 167.Watts C, Zimmerman C, Foss AM, Hossain M, Cox A, Vickerman P. Remodelling core group theory: the role of sustaining populations in HIV transmission. Sex Transm Infect 2010; 86 (suppl 3): iii85–92. [DOI] [PubMed] [Google Scholar]
- 168.Underhill K, Operario D, Skeer M, Mimiaga M, Mayer K. Packaging PrEP to prevent HIV: an integrated framework to plan for pre-exposure prophylaxis implementation in clinical practice. J Acquir Immune Defic Syndr 2010; 55: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Foss AM, Vickerman PT, Heise L, Watts CH. Shifts in condom use following microbicide introduction: should we be concerned? AIDS 2003; 17: 1227–37. [DOI] [PubMed] [Google Scholar]
- 170.Karmon E, Potts M, Getz WM. Microbicides and HIV: help or hindrance? J Acquir Immune Defic Syndr 2003; 34: 71–75. [DOI] [PubMed] [Google Scholar]
- 171.Vissers DCJ, Voeten HACM, Nagelkerke NJD, Habbema, de Vlas SJ The impact of pre-exposure prophylaxis (PrEP) on HIV epidemics in Africa and India: a simulation study. PLoS One 2008; 3: e2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Michael M, Rosengarten M. Innovation and biomedicine: ethics, evidence and expectation in HIV. London: Palgrave Macmillan, 2013. [Google Scholar]
- 173.Empower Thailand. http://www.empowerfoundation.org/index_en.html (accessed June 10, 2014).
- 174.UNAIDS. At risk and neglected: four key populations—report on the Global AIDS Epidemic. Geneva: UNAIDS, 2006. [Google Scholar]
- 175.UNFPA. HIV and sex work: preventing HIV risk and vulnerability: media fact sheet. New York: UNFPA, 2010. [Google Scholar]
- 176.Pickles M, Boily M-C, Vickerman P, et al. Assessment of the population-level effectiveness of the Avahan HIV-prevention programme in South India: a preplanned, causal-pathway-based modelling analysis. Lancet Glob Health 2013; 1: e289–99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.