Summary
Assisted reproductive technologies (ART) are not yet systematically available to people with sickle cell disease (SCD) or their parents. Fertility care for these groups requires addressing SCD-associated infertility risks, fertility preservation options, pregnancy possibilities and outcomes, and, when needed, infertility treatment. People with the chance of having a child with SCD may use IVF with preimplantation genetic testing to conceive an unaffected child. Also parents of children with SCD may use this technology to identify embryos to become potential future matched sibling donors for stem cell transplants. In the United States, disparities in fertility care for the SCD community is especially stark. Universal newborn screening identifies SCD and sickle cell trait, guidelines direct preconception genetic carrier screening, standard-of-care fertility preserving options exist. However, potentially transformative SCD treatments and cures are not used due to iatrogenic infertility concerns. In diversely resourced care settings, obstacles to providing fertility care to people affected by SCD persist.
In this Viewpoint, we contend that fertility care must be incorporated into the comprehensive SCD care model, supporting alignment of SCD treatment goals with reproductive life plans and delivering on the promise of individualized, high-quality care for people with SCD and their families. We consider the obligation to provide fertility care in light of the medical evidence, with acknowledgement of formidable obstacles to optimizing care, and powerful historical and ethical considerations.
Keywords: Fertility, Reproductive Health, Pregnancy, Sickle Cell Disease, Ethics, Fertility Preservation
Introduction
Survival is improving for children with SCD; a growing population of affected individuals are expected to survive into their reproductive years1. Also, innovations in fertility preservation and infertility treatment have emerged. Despite indications for fertility care, assisted reproductive technologies (ART) do not reach most people with SCD. However, people with SCD and their families have diverse indications for ART (Figure 1) including to preserve fertility, treat infertility, for in vitro fertilization with preimplantation genetic testing for monogenic disorders to select embryos without SCD, and/or for prenatal testing for SCD2. The absence of systematically provided fertility care for SCD raises concerns at the intersection of SCD-community vulnerabilities attributable to geography, race, sex, age, socioeconomic status, disease, and, for some, research participation.
Figure 1.
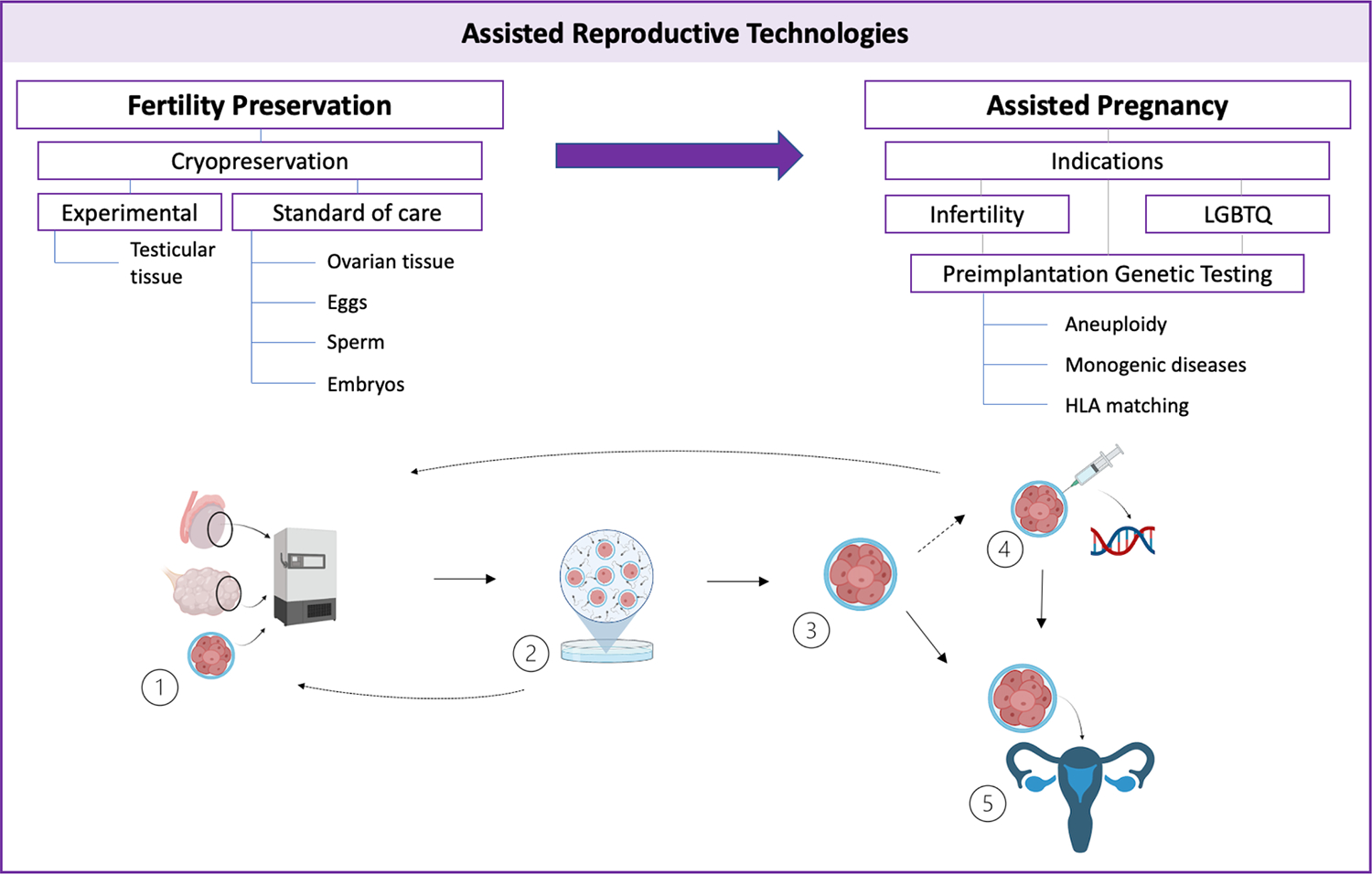
Assisted reproductive technologies (ART) use for people with sickle cell disease and their families
ART might be used for fertility preservation, infertility treatment, by LGBTQ couples, and for preimplantation genetic testing. (1) Sperm, eggs, ovarian tissue, and embryos can be cryopreserved as care standards whereas testicular tissue cryopreservation is experimental. (2) Embryos are made by in-vitro fertilization (IVF) at the time of fertility preservation or gametes can be used to generate embryos via IVF once pregnancy is desired. (3) Embryos can be stored, or (4) sampled for testing to assess aneuploidy, for monogenetic diseases such as sickle cell disease, or for haploidentical matching with sibling before (5) introduction to the uterus.
In this Viewpoint, we address existing evidence identifying infertility risks and indications for ART in SCD, barriers to providing ART care. We also consider the ethical implications of failing to provide ART care, and describe a path to advance fertility care for SCD. This framework may help propel changes in care delivery and clinical guideline development, as well as inform legal reforms. Around the world, the circumstances contributing to ART care deficiencies for people with SCD vary as national politics, history, culture, and resources contribute to the availability of SCD and fertility care3 National law varies, examples of laws governing ART use are shown in the appendix (page 2). The state of fertility care in the U.S. is a focus here because the SCD comprehensive care model is evolving and because it is unsettling that in a high resource setting fertility care is inequitably provided to some, but not all Americans. Using the U.S. as a case study clarifies obstacles common to many care contexts3 Challenges in obtaining fertility care have implications for people with SCD and their families Worldwide3
I. Indications for assisted reproductive technologies due to infertility and genetic risks
Sickle cell disease is itself a risk factor for infertility causing direct gonadal injury and, possibly, restricting opportunities to conceive due to disease-related limitations. In this sense, sickle cell disease treatments may be understood as protecting future fertility since physical and social function and overall survival are prerequisites for biological reproduction. However, critical treatments, some sickle cell disease therapies, and cures pose established and theoretical infertility risks (table 1), raising the stakes of addressing fertility concerns in routine clinical care.
Table 1. Putative infertility risks associated with sickle cell disease and its treatments and cures.
Few infertility risks are universal features of SCD, underscoring the need for individualized care that considers age, sex, genotype, disease complications and measures of treatment dose, duration and adherence. Uncertainties related to infertility risks can be incorporated into information sharing about disease complications and treatment benefits.
| People with ovaries | People with testicles | |
|---|---|---|
| Untreated sickle cell disease | Ovarian reserve decline is accelerated in adulthood4,5,6; some adolescents and young adults have diminished ovarian reserve6,9,10 | Hypogonadism7,8 |
| Sperm abnormalities7,8 | ||
| Pregnancy is high-risk for maternal and fetal morbidity & mortality2 | Priapism, & erectile dysfunction7,8 | |
| Disease modifying therapies | Hydroxyurea: associated with diminished ovarian reserve6,9,10; concern for early embryonal developmental changes, teratogenesis2 | Hydroxyurea: toxic to sperm (reversibility suggested), outstanding questions about long-term effects to spermatogonial pool11 |
| Red cell transfusions: ovarian follicle iron deposition possible12, pituitary iron overload uncommon, chelators are teratogenic | Transfusions: testicular and pituitary iron deposition possible13 | |
| L-glutamine, crizanlizumab, voxelotor: no data | L-glutamine, crizanlizumab, voxelotor: no data | |
| Curative/HSCT preparative regimens | Alkylating Agents: gonadal toxicity and infertility14 | Alkylating Agents: gonadal toxicity and infertility14 |
| Total Body Irradiation: Reduced ovarian reserve, infertility, uterine damage reducing future blastocyst implantation14 | Total Body Irradiation: shielding spares the testicles14 | |
Infertility risks & fertility preservation for people with testicles
SCD itself is a well-recognized infertility risk for people with testicles, who we will refer to men and boys in this publicaton.15 The testes and penis are damaged by haemolysis, hypoxic-ischemic injury, and priapism which can result in low testosterone, semen abnormalities and erectile dysfunction7,8 The majority (91%) of adult men with SCD have at least one abnormal value on semen analysis8 Hydroxyurea may further reduce sperm counts, sometimes leading to frank azoospermia (complete absence of sperm).14 Hydroxyurea’s toxic effects on sperm seems to be reversible and limited studies indicate that the spermatogonial pool is untouched by therapy. However larger, systematic studies in men of reproductive age who have received life-long hydroxyurea treatment are needed to draw firm conclusions11 Chronic red cell transfusion may prevent gonadal damage associated with SCD pathophysiology; a possibility raised by studies in younger male patients, aged 14 to 16 years with SCD.13 However, transfusional iron overload may cause testicular iron deposition and exposure to reactive oxygen species that can damage sperm and impair spermatogenesis13 While the effects of iron overload on male fertility is addressed in people without SCD, the effects of chronic iron overload on testicular function and spermatogenesis in men with SCD is inadequately studied. Curative therapies require exposure to gonadotoxic preparative regimens posing significant and potentially irreversible infertility risks as alkylating agents are universally used in SCD protocols and are classified as high-risk for future infertility.14,16
Fertility preservation as a care standard exists for postpubescent boys and men by use of sperm cryopreservation. For prepubescent boys, testicular tissue cryopreservation remains experimental. To successfully bank sperm, men may need to discontinue hydroxyurea to improve sperm recovery.14 Repeating semen analysis approximately three months after stopping hydroxyurea might allow adequate recovery, but the precise time course of recovery is not established14 and pre-hydroxyurea treatment semen analysis is not usually available for comparison. Pre-treatment semen analysis might help identify SCD-attributable semen abnormalities, helping to identify when baseline is reached. In France, post-pubertal males are conservatively offered the opportunity to sperm bank before initiating hydroxyurea.15 This practice is not yet widespread. Outside of the pre- haematopoietic stem-cell transplantation (HSCT) setting, there are no universally accepted approaches to offering fertility preservation for SCD.
Infertility risks & fertility preservation for people with ovaries
When discussing infertility risks and preservation for people with ovaries, we will refer to people in this group as women and girls. Women with SCD have a narrower reproductive window than unaffected women: adults with SCD studied in the USA, UK and Nigeria demonstrate an accelerated age-associated decline in ovarian reserve, suggesting that biological infertility occurs earlier than in unaffected women and leading these investigators to conclude that counselling is indicated for women with SCD4–6,9,17 Some adolescent and young women, aged 12 to 30 years, with sickle cell anaemia (haemoglobin SS and haemoglobin Sβ0) have diminished ovarian reserve, which is a risk factor for premature ovarian insufficiency, infertility, poor in vitro fertilization outcomes and an indication for offering fertility preservation.6,9,10 Whether disease severity or SCD treatment is the primary driver of this finding is an area of ongoing research. SCD therapies may help avoid ovarian injury associated with SCD pathophysiology. However, hydroxyurea and chronic transfusion therapy also pose potential infertility risks. In three studies, hydroxyurea use among post-pubertal adolescents and women with sickle cell anaemia was associated with diminished ovarian reserve and the number to harm (cause diminished ovarian reserve) was 1.9 – 4.1.9 Additional studies are needed to establish whether hydroxyurea is a proxy for disease severity or a cause of diminished ovarian reserve18 Transfusion may help protect ovarian reserve, which is suggested by one study of 26 young adult women aged 19 to 30 years with sickle cell anaemia in which no subject receiving chronic red cell transfusions (N=8) had diminished ovarian reserve.9 However, iron overload associated with chronic transfusions poses at least a theoretical infertility risk as ovarian iron deposition and follicular damage is possible and might impair fertility12 Finally, HSCT is an indication for fertility preservation, as the universal use of alkylating agents threatens female fertility. Also, when total body irradiation is required, female reproductive organs cannot be shielded.14,16
Fertility preserving options exist as care standards for prepubescent and postpubescent people using ovarian tissue cryopreservation and oocyte or embryo cryopreservation. Both have been reported in girls and women with SCD with normal ovarian follicle density in ovarian tissue of girls with sickle cell anaemia before HSCT.18,19 As expanding opportunities for fertility preservation arise, addressing the potential risks associated with these interventions is important. Both ovarian tissue and oocyte harvest require anaesthesia which can cause SCD complications.18 Furthermore, controlled ovarian hyperstimulation for oocyte harvest can cause ovarian hyperstimulation syndrome, venous thromboembolism and SCD complications18. Thus, women with SCD undergoing fertility preservation require close clinical monitoring. Whether and when to discontinue hydroxyurea before fertility preservation for women is not established29 and is challenging as SCD complications can occur during therapy hiatus.
Assisted Reproductive Technologies as an Intergenerational Concern
Opportunities to use ART are important not only for people with SCD, but also for their families. The parents of children with SCD and adults with SCD worry about having an affected child. Even in high resource settings, many do not know that IVF with preimplantation genetic testing exists.20–22,24,25 Interest in this technology may in part be explained by the reality that having a child with SCD may use as much as 34% of a family’s income26 and some parents choose not to have additional children after the birth of a child with SCD. In a single report of 60 couples in the U.K. who used IVF with preimplantation genetic testing to avoid SCD in offspring, the cumulative livebirth rate was 54% per cycle and 63% per couple; this rate might have been higher had couples elected used their maximum allowable IVF cycles.24 The availability of preimplantation genetic testing may affect additional family building choices as testing may be done select embryos without SCD that are also human leukocyte antigen matched to an affected child. Such an embryo might ultimately become a matched, unaffected sibling donor without SCD for HSCT. The low number of embryos are identified for transfer with this approach and even when successful, other obstacles and ethical considerations may limit uptake.27
Clearly, standard of care fertility-preserving interventions are relevant to contemporary SCD care. Clinicians, patients, and families may now compare established and theorized infertility risks associated with untreated SCD to disease-specific treatments and cures.14 People with SCD and their families require counselling about ART opportunities, even as studies defining pregnancy outcomes following fertility preservation for people with SCD are limited in number and confined to the post-HSCT setting.14 Counselling can incorporate existing evidence and evidence gaps which, even under the best circumstances, is complicated. However, for most people with SCD, significant roadblocks prevent opportunities to receive ideal clinical care.
II. Barriers to ART Education, Counselling, Referral, and Care
The chance to use ART to preserve fertility or build a family is stymied by layered barriers to care including that experts are lacking, wait-times are long and financial systems to address care costs are incomplete or non-existent.
Fertility care is limited by the lack of comprehensive SCD care for affected individuals in many countries. Inadequate or absent SCD care compromises opportunities to provide disease-specific fertility care.1 However even comprehensive SCD centres with access to fertility experts lack standard approaches to providing fertility care. SCD guidelines identify infertility risks in SCD, but do not contain recommendations for the timing, personnel, or content of fertility counselling.28,29 The absence of reccomendations might partly reflect evidence gaps at the time guidelines were written.2 Now emerging evidence can inform practice. For example, investigators in Nigeria, the U.S., and U.K. have concluded that the low ovarian reserve in women with SCD compared to unaffected women presents an indication to provide fertility counselling, consider monitoring of ovarian reserve and to share fertility preservation opportunities.5,9,10 The conservative approaches that direct oncofertility care provision may be applied to SCD.9,30–32 Growing recognition of HSCT-associated infertility risks presents another, indicated opportunity to perform fertility assessments. After HSCT, follow-up clinics are needed to address whether fertility preservation opportunities remain or whether infertility treatments are indicated.31,33 The prospects for successful fertility preservation in this setting is low, putting the onus for care in the pretransplant setting.34
Some clinicians worry that addressing uncertainties about the effects of hydroxyurea on fertility will deter treatment acceptance35,36 and some limit HSCT referrals due to infertility concerns.37 Fertility risks are, for some patients and families, a reason for treatment refusal.6 In the U.S., infertility risks are reportedly addressed during routine hydroxyurea counselling at a centre with high levels of treatment uptake.13,38 Many clinicians feel challenged to integrate fertility care into standard SCD care. Haematologists are not trained to provide ART counselling and may lack confidence about how to counsel about a topic that is sensitive and private, requires tailored information sharing, acknowledgment of uncertainty, attention to emerging evidence, and awareness of patients’ health literacy.22,39 Individualized fertility counselling attends to patients’ values and preferences and includes information about required evaluation procedures (such as masturbation or transvaginal ultrasound). The need to address acute disease complications or difficulties with medication adherence may override fertility discussions.40–42 However, addressing fertility risks and opportunities for fertility preservation might help align SCD treatment with patient’s goals and might even help increase use of SCD treatments and cures. This possibility, which might improve SCD treatment uptake, deserves consideration as life expectancy for people with SCD is stagnant and adult morbidity is considerable.43
Even with counselling and referral for care, the costs associated with ART care in much of the world starkly restricts access to care as insurance either does not exist or does not provide sufficient coverage for most people16, even in countries with a publicly funded health sector.31 Fertility preservation for girls and women is especially expensive, requiring costly medications, resource-intensive clinical monitoring, and sedated oocyte retrieval or laparoscopic surgery to harvest ovarian tissue for cryopreservation. Even with partial insurance coverage, people may be unable to afford out-of-pocket IVF costs, which average $12,000 (£ 9,745) per cycle.44 In the U.S., 19 states have some legislation governing fertility coverage: IVF coverage is required in 13 states and fertility preservation coverage in 11 states (some states require both). However, government employees and most publicly insured people are excluded from these mandates which also vary in allowed treatments and qualification for coverage. Unsurprisingly, there are persistent disparities in access to care45 and even when coverage is mandated, Black and Hispanic women disproportionately report facing barriers to receiving fertility care.46
People with SCD may be further excluded from using ART by virtue of their insurance and care indication. People with SCD in low-income and middle-income countries mostly rely upon private clinics that are inaccessible to most people. This reliance is also true in the U.S. where 60% of people with SCD are publicly insured and only two states include fertility preservation coverage for publicly insured people45 and where all government employees are denied coverage for ART care. In some places, restricted ART access for people with SCD is partly a consequence of policies that only incrementally expand access to fertility care. In the U.K., policies enable ART access, but priority is given to those with a higher chance of treatment success.47 People with SCD can be denied ART if they have diminished ovarian reserve or may not qualify for fertility preservation before gonadotoxic therapies because they are not yet infertile.16,31,47 IVF with preimplantation genetic testing use is also restricted in many places (Appendix, page 2). In the U.K., people with the genetic potential to have a child with SCD receive coverage through the National Health Service (NHS) for three cycles of IVF with preimplantation genetic testing.24 However, in many places where SCD is common, prenatal diagnosis with the possibility for continuing or terminating a pregnancy is used and IVF with prenatal genetic testing is largely inaccessible.48
SCD-related barriers to providing fertility care rest on a foundation of global reproductive healthcare inequality. Comprehensive fertility care for people with SCD and their families is needed even as abortion is restricted in the U.S., despite movement toward liberalizing abortion access across the globe.49 Abortion restrictions jeopardize ART care because IVF involves embryo creation and storage. Also, because beyond outlawing abortion, some state laws give embryos citizenship rights that are equivalent to or supersede the rights of pregnant people.50 Often therapeutic abortion, not IVF preimplantation genetic testing, is used to end pregnancies affected by SCD, and is the most readily available option for most people. There is some indication that, given the opportunity, couples would prefer IVF with preimplantation genetic testing to therapeutic abortion; when these options are not shared or are unavailable, couples are denied comprehensive reproductive choices.51
III. Ethical Considerations
The failure to provide equitable access to ART services for people who are at risk for having a child with SCD requires ethical appraisal since existing evidence and technological advances have thus far not inspired coordinated action to reach affected families in most countries.
Denying reproductive autonomy to people with SCD through inadequate preconception genetic testing and counselling, insurmountable personal costs for care, or inadequate specialists risks recapitulating coercive reproductive injustice. In the U.S., histories of rape, forced pregnancy and state-sanctioned, non-consensual sterilization disproportionately affected Black communities who are more likely to be affected by SCD in the U.S.52 SCD history is entwined with this history. In the 1970s, Linus Pauling infamously endorsed eugenic approaches to reducing the incidence of SCD, while the poorly named Sickle Cell Control Act53 heightened fears that sickle cell testing could lead to reproductive coercion, forced sterilization, unequal reproductive care, and inadequate genetic counselling.54–56 Hemoglobinopathy testing is compulsory in parts of the Middle East and Africa and is used to either discourage or legally prohibit marriage between couples who may have an affected child.57 Progressive advances in fertility care and genetic testing require the SCD community to reckon with how to ensure that non-coercive, patient-centered fertility care is offered to people with SCD and their families to make SCD and fertility treatment choices in alignment with their preferences and values. Although ART may not be widely available for most people with SCD, counselling that respects autonomy can be offered. Ideally, this counselling addresses what is known about infertility risks, genetic inheritance, and existing family building options to support reproductive healthcare decisions.
People with SCD and their families should receive equitable access to the benefits, not just the risks, afforded by medical advances in testing. Fertility care is a benefit of hemoglobinopathy testing but testing that might help people make reproductive choices instead leads to harm. There are longstanding fears that sickle cell testing could be weaponized against intended beneficiaries.54,58 These decade old reports validate concerns. In 2021, New York Times’ reporters59 published evidence that medical examiners attributed the deaths of dozens of men in police custody to sickle cell trait carriage, even though this attribution is not medically plausible. Families that might benefit from testing are also not receiving care: Another New York Times story60 identified that in the U.S., preconception counselling is not reaching couples with genetic potential to have a child with SCD, despite a recommendation to universally offer preconception screening from the American College of Obstetricians and Gynecologists. Meanwhile, newborn screening programs identify children in need of life-saving interventions. As these programs are increasingly implemented in high prevalence, low-resource settings, essential resources are urgently needed to implement life-saving care.61 These testing programs also identify carriers of hemoglobinopathy traits, this information also needs to be available and understood to those who are tested. In these diverse care settings, there is a need to ensure that the people who bear the risks of hemoglobinopathy disease and trait testing also benefit from knowing the results.57
Fertility care in SCD systems must account for the reality that girls and women are at particular risk for harm.2,62 Women are more likely than men to report that infertility is a source of extreme pain or stres31 and less likely to be referred for fertility preservation63 even though clinicians believe women care more about reproduction and want to discuss it more than men.64 In general, women are under-counselled about infertility risks associated with medical treatment and, this is also a racial disparity that may reach people with SCD. In the U.S., people with SCD are mostly Black and Black women especially experience delays in receiving infertility care46 and have poorer IVF outcomes than white women, an inadequately explained finding that is not attributable to biologically mediated difference, and that needs further investigation.65 Unfortunately, ART registries are not structured to collect information that could address SCD-specific knowledge gaps.2 Women provide most of the care for children with SCD and report mental disorders, stigma, relational compromise, and economic jeopardy; some may also have grief associated with the death of another child with SCD.66 Fertility care for SCD must honour personal choices to use, or not, fertility preservation, therapeutic abortion, or IVF with preimplantation genetic testing for SCD and help spotlight the ways that improving outcomes for people with SCD is intimately tied to women’s health and well-being.
The need to address fertility for children with SCD is, to some degree, contingent up treatment choices, and raises additional ethical concerns. People under the age of full legal responsibility should participate in medical decision-making to their maximum ability, which is a challenging dictum in practice given the complexities of SCD care. Fertility preservation allows minors whose future fertility is threatened to retain future reproductive decision-making rights.67 When decisions are made for children, the general ethical approach is to support their right to an open future. This right helps ensure that as many decisions as possible are preserved for minors to make once they attain age of majority or maturity.68 Offering this care also requires accounting for uncertainty as the precise risks for infertility from untreated SCD or its therapies are not established.14 The implications of future infertility for survivors of childhood SCD are incompletely considered. Infertility confers grave social and economic consequences that are disproportionately experienced by women. In countries where SCD is most prevalent and ART resources are slim, this reality needs focused consideration, especially as childhood survival improves, hydroxyurea use starts in childhood, and curative intervention expands to these low resource settings.69,70
Finally, vulnerability arises when people with SCD are also research participants. Fertility preservation is available as part of some SCD curative trials.14 This option eliminates a barrier to pursuing cure, but also raises the possibility that access to fertility care will motivate trial participation. Also, fertility preservation provided on a research basis may only cover upfront costs, and a finite number of years of gamete storage, but the youngest research participants may require two decades of gamete storage before individuals reach the age of majority. Ongoing storage fees may become an unaccounted for, indefinite expense (US $300–500 or £240–400 per year). If cryopreserved gametes are ultimately discarded due to financial constraints, then this perceived benefit of research participation may become meaningless. The need for fertility care on a research basis may become more pronounced as studies of therapeutics and cures expands to low resource societies.
IV. Advancing Fertility Care in Sickle Cell Disease
Ensuring that all people with SCD have access to comprehensive and expert SCD care is necessary to ensure that infertility risks, opportunities for fertility preservation and the existence of IVF with preimplantation genetic testing are incorporated into SCD care (Panel).71 We have highlighted structural components of building access to ART care for people with SCD (Figure 2). This multidisciplinary care will include haematologists, and reproductive healthcare experts (i.e., paediatric endocrinology, genetic counsellors, urology, reproductive endocrinology/infertility, and maternal foetal medicine). Addressing fertility care will help create referral pathways, clinical education opportunities, solutions for individual concerns, and opportunities for care system delivery optimization and resource allocation.
Panel.
Opportunities to address fertility during routine sickle cell disease care
| • During new evaluations unless post-menopause |
| • At puberty onset or at delayed puberty diagnosis |
| • Initiation or change in SCD treatment approach including pursuit of curative therapy |
| • Initiation of hormonal contraception |
| • Screening for and treating priapism |
| • Transition from pediatric care; integration into adult care |
| • Annual preconception counseling and with family planning discussions |
| • Parents of children with SCD who can be offered referral to genetic counseling and discussion of comprehensive reproductive options (including, but not limited to IVF +PGT-M) |
Figure 2.
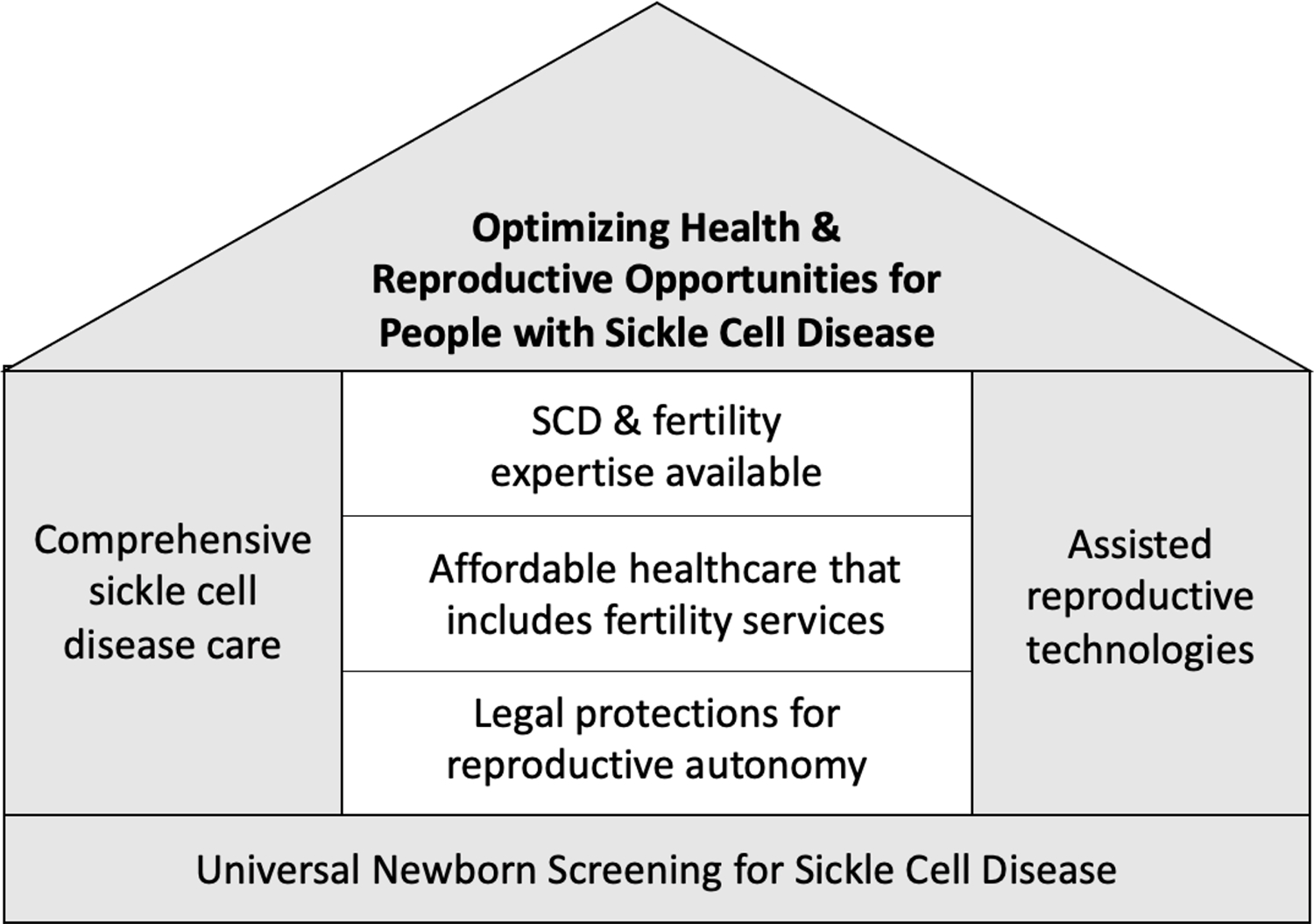
Optimizing health and reproductive opportunities for people with sickle cell disease
Newborn screening programs are essential for identifying and treating children with SCD and create recognized opportunities for counselling to address family building for affected families. Hemoglobinopathy trait carriers around the world might wish to use prenatal diagnosis, IVF with preimplantation genetic testing or therapeutic abortion, or not. They need neutral, patient-centered, primary care clinicians and informed community health workers for information to appraise reproductive risks and review reproductive choices.56 Healthcare providers involved in pre-conception and post-conception counselling – obstetricians and gynaecologists, general practitioners, nurses, and community health workers – must be involved in hemoglobinopathy testing, counselling, and ensuring that, when indicated, comprehensive reproductive options are addressed.22 Developing the personnel and reproductive healthcare infrastructure to provide fertility counselling will allow systems to respond when care access expands or technology advances, for example, as IVF with preimplantation genetic testing becomes more widely available or as non-invasive prenatal diagnostic testing for SCD becomes possible.72
While developing counselling infrastructure is a step towards providing fertility care, other considerations are needed in the paediatric and adult SCD care settings (Appendix, page 1).15 Paediatric oncofertility specialists honour the need for dyadic care and consider patients’ developmental needs during the provision of fertility care, incorporating future reproductive concerns into opportunities to optimize SCD treatment and care during childhood. Fertility preservation indications are expanding for SCD, and may be reviewed with attention to risks, benefits, uncertainties, and barriers to available interventions.14
In adult SCD care, infertility risks are included in family planning discussions.73 This approach helps individualize counselling and helps determine assessments and referrals.14 Where semen analysis and ovarian reserve testing is available, opportunities for testing can be shared. Patient preference helps dictate assessments since outside of the pre-transplant setting, there is not yet a clear approach to universal screening. Thus, semen analysis and ovarian reserve testing (or referral for testing) may be offered through a shared decision-making process that clarifies the potential benefits and limitations of testing. Testing can help affected individuals decide whether to pursue fertility preservation or timing of pregnancies. In acknowledgement of infertility risks, adults with SCD can be monitored during attempts to conceive and conservative referrals for infertility care made for couples who have been trying to conceive for longer than six months. Global efforts to expand IVF access are relevant for the SCD community and even when not yet available, supportive care can be offered to families experiencing infertility-related stressors. Poor access to ART should not deter counselling. For some young adults with SCD, simply knowing that IVF with preimplantation genetic testing exists alleviates anxiety.22 Even when ART care is not a publicly available, these discussions might shape employment choices (towards those who provide better fertility benefits), financial priorities and family building plans.
An invigorated care model alone will not fill evidence gaps or surmount cost-related barriers to care. International, federal, state, and non-governmental organizations will need to devote resources to optimize care. We have adressed actions needed to advance fertility care in the U.S., a high-resource country with draconian reproductive healthcare laws, where action to address inequality and affirm the value of the lives and families of people with SCD is urgently needed (Table 2). Ultimately, these overarching needs must be widely addressed to advance fertility care for SCD:
Table 2.
Approaches to address fertility care for haemoglobinopathy trait carriers and people with sickle cell disease in the USA require coordinated actions by institutions and organisations
| Actions to address, clinical care, research and access gaps | ||
|---|---|---|
| Screening for hemoglobinopathy traits | CDC, USPTF, ACOG, ASH, AAP | Universal pre-conception genetic counseling |
| Opportunity for hemoglobinopathy testing | ||
| Clinical Care | NHLBI/NIH, ASH, ACOG, ASRM, AAP, ASPHO, SMFM, NASCC | Comprehensive reproductive care guidelines |
| Systematic approach to fertility and preconception counseling, evaluations, referrals | ||
| Research | CDC ART Surveillance & Research Team, SART | Create ART Surveillance Systems to identify SCD-related use & define outcomes for IVF+PGT in couples without infertility diagnosis |
| NIH (NHLBI, NICHD, ORWH), ASH, ASRM, AUA | Create RFAs for high-quality clinical fertility research in SCD | |
| Policy Changes | CMS, HRSA, State and Federal Lawmakers | Fund comprehensive SCD care |
| Fund or mandate coverage for patients anticipating iatrogenic infertility | ||
| Comprehensive, non-coercive genetic counseling and ART coverage for people with SCD and hemoglobin carrier couples | ||
ACOG: American College of Obstetrics and Gynecology, ASH: American Society of Hematology, ASPHO: American Society of Pediatric Hematology & Oncology, ASRM: American Society of Reproductive Medicine, ART: Artificial Reproductive Technology, AUA: American Urological Association, CDC: Centers for Disease Control and Prevention, CMS: Centers for Medicaid and Medicare, HRSA: Health Resources and Services Administration, IVF++PGT: In Vitro Fertilization + Preimplantation Genetic Testing, NICHD: National Institute of Child Health and Human Development, NIH: National Institutes of Health, NHLBI: National Heart, Lung and Blood Institute, ORWH: Office for Research in Women’s Health, NASCC=National Alliance for Sickle Cell Centers, RFA: Research Funding Announcement; SART: Society for Assisted Reproductive Technologies, SMFM, Society for Maternal and Fetal Medicine, USPTF: United States Preventive Task Force
Advancing fertility care
Guidelines that standardize fertility care indications will galvanize equitable care delivery by use of medical criteria to dictate care. Care standards will inform content, timing, and approach to fertility care and form a foundation for measuring quality and developing research. Like most SCD care standards, regional or national recommendations are necessary as all guidelines are informed by the best available scientific evidence and with sensitivity to available resources. Focused research funding will address outstanding questions about fertility in SCD (panel 2). Definitive studies about hydroxyurea’s effects on ovarian follicles and the spermatogonial pool is especially critical as a growing population of young children with SCD empirically take chronic hydroxyurea.14 Patient centered optimizing fertility care delivery in SCD are also necessary in low-resource, high prevalence countries as well as in high resource settings.
Panel 2:
Research questions regarding fertility, infertility risk and Assisted Reproductive Technology in sickle cell disease
| • Define effects of sickle cell disease pathophysiology and treatments on oocyte quality, blastocyst development and miscarriage |
| • Establish infertility rates and risks in people with sickle cell disease; include all genotypes |
| • Define gonadoprotection or gonadotoxicity of hydroxyurea, chronic transfusion, and other sickle cell disease therapies in chronically exposed people with attention to dose and duration of treatment |
| • Establish ideal timing of fertility preserving interventions and study protocols to optimize outcomes and minimize complications |
| • Identify which sickle cell disease treatments need to be discontinued, and for what time period, before gamete cryopreservation |
| • Define pregnancy outcomes when cryopreserved gametes are used |
| • Measure barriers to fertility preservation |
Policies are needed that minimize out of pocket expenses and enable fertility preserving care for patients with current or anticipated iatrogenic infertility and those interested in IVF with preimplantation genetic testing.46 In the U.S., advocacy organizations help bridge fertility care gaps in cancer by negotiating lower costs for fertility preservation with fertility centres and pharmaceutical companies and through direct grants to individuals.31 These approaches can also support people with SCD.31 For publicly insured people in the U.S. to access fertility care, the Centers for Medicaid and Medicare must act. The NHS funds fertility treatment up to age 40 years and fertility preservation on medical grounds and needs to make SCD an indication for care in anticipation of infertility risks. In low-resource settings, expanding the SCD and fertility care workforces will be essential to gain access to ART care.
The ability to have biological children, if one desires, is an essential aspect of freedom and pursuing happiness. Integrating ART into care for people with SCD with recognized fertility risks is no extravagance; the action upholds a fundamental human right. SCD care limitations are rooted in hundreds of years of racial healthcare transgressions and inequalities, and over a half-century of failures to fund SCD research, registries, and care. We should expect more. Investments in SCD treatment and care along with robust healthcare policies are needed to ensure that people with SCD and their families receive equitable, indicated fertility care.
Supplementary Material
Acknowledgements:
Thanks to Jaanvi Mahesh and Nora Khalil for research assistance for this paper and to Dr. Kirsten Williams for support with realizing Figure 1 which was created with Biorender.com.
Abbreviations
- ART
Assisted reproductive technologies
- HSCT
Hematopoietic Stem Cell Transplant
- HLA
Human leukocyte antigen
- IVF
In Vitro Fertilization
- SCA
Sickle cell anaemia
- SCD
Sickle cell disease
Footnotes
Conflict of Interest: LHP declares grant funding from NIH/NHLBI K23HL146841 and U01 HL156620-01, the American Society of Hematology, Doris Duke Charitable Foundation Grant #2020147, and the Mellon Foundation, consulting fees from Global Blood Therapeutics and Novo Nordisk, support for meeting attendance from the American Society of Hematology and the Hemostasis and Thrombosis Research Society, serves on the CRESCENT DSMB and is an advisor to the Sickle Cell Reproductive Health Education Directive, serves in a leadership role at the Foundation for Women and Girls with Blood Disorders’ Sickle Cell Disease Learning Action Network and on the American Society of Hematology’s Maternal Health Working Group. AN declares grant funding from Global Blood Therapeutics, consulting fees from Global Blood Therapeutics, Bluebird Bio, Novartis and Dispersol, received honoraria from the American Society of Pediatric Hematology, support for meeting attendance from the Foundation for Women and Girls with Blood Disorders (FWGBD), serves on DSMB for Editas Medicine, the PIVOT Trial and the PUSH UP Trail, and serves in a leadership role at the Foundation for Women and Girls with Blood Disorders’ Sickle Cell Disease Learning Action Network. SL declares grant funding from PCORI, HRSA, NIH and CHRC MD, consulting fees from Novartis, Pfizer, Bluebird Bio, Novo Nordisk, and Magenta, participates in DSMBs for OSMB SPARCO (NIH) and on an ad hoc basis for ASBMT, serves the Vice President of the National Alliance for Sickle Cell Centers, and holds stock in Pfzier and Teva. TW declares consulting fees from Agios Pharmaceuticals, Novo Nordisk, Fulcrum, Global Blood Therapeutics and Bluebird Bio, support for travel provided by Agios Pharmaceuticals. ADM declares honoraria from PhenX and support from the Wharton Foundation. LRM, EON, MSC have no conflicts of interest to declare.
References
- 1.Mburu J, Odame I Sickle cell disease: Reducing the global disease burden. Int J Lab Hematol 2019;41(S1):82–8. Doi: 10.1111/ijlh.13023. [DOI] [PubMed] [Google Scholar]
- 2.Pecker LH., Sharma D, Nero A, et al. Knowledge gaps in reproductive and sexual health in girls and women with sickle cell disease. Brit J Haematol 2021;194(6):970–9. Doi: 10.1111/bjh.17658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vayena E, Rowe PJ., Peterson HB. Assisted reproductive technology in developing countries: why should we care? Fertil Steril 2002;78(1):13–5. Doi: 10.1016/s0015-0282(02)03177-1. [DOI] [PubMed] [Google Scholar]
- 4.Kopeika J, Oyewo A, Punnialingam S, et al. Ovarian reserve in women with sickle cell disease. PLOS ONE 2019;14(2):e0213024. Doi: 10.1371/journal.pone.0213024.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garba SR., Makwe CC., Osunkalu VO., et al. Ovarian reserve in Nigerian women with sickle cell anaemia: a cross- sectional study. J Ovarian Res 2021;14(1):174. Doi: 10.1186/s13048-021-00927-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pecker LH., Hussain S., Christianson MS., et al. Hydroxycarbamide exposure and ovarian reserve in women with sickle cell disease in the Multicenter Study of Hydroxycarbamide. Brit J Haematol 2020;191(5):880–7. Doi: 10.1111/bjh.16976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Idris IM., Galadanci JA., Abba A., et al. Cross Sectional Survey of Priapism and Sexual Dysfunction in 353 Men with Sickle Cell Disease. Blood 2019;134(Supplement_1):2302–2302. Doi: 10.1182/blood-2019-128825. [DOI] [Google Scholar]
- 8.Musicki B, Burnett AL. Testosterone Deficiency in Sickle Cell Disease: Recognition and Remediation. Front Endocrinol 2022;13:892184. Doi: 10.3389/fendo.2022.892184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pecker LH., Hussain S., Mahesh J., et al. Diminished ovarian reserve in young women with sickle cell anemia. Blood 2022;139(7):1111–5. Doi: 10.1182/blood.2021012756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elchuri SV., Williamson RS., Brown RC., et al. The effects of hydroxyurea and bone marrow transplant on Anti-Müllerian hormone (AMH) levels in females with sickle cell anemia. Blood Cells, Molecules, and Diseases 2015;55(1):56–61. Doi: 10.1016/j.bcmd.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Joseph L, Jean C, Manceau S, et al. Effect of hydroxyurea exposure before puberty on sperm parameters in males with sickle cell disease. Blood 2021;137(6):826–9. Doi: 10.1182/blood.2020006270. [DOI] [PubMed] [Google Scholar]
- 12.Ni Z, Li Y, Song D, et al. Iron-overloaded follicular fluid increases the risk of endometriosis-related infertility by triggering granulosa cell ferroptosis and oocyte dysmaturity. Cell Death Dis 2022;13(7):579. Doi: 10.1038/s41419-022-05037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabrielsen JS., Lamb DJ., Lipshultz LI. Iron and a Man’s Reproductive Health: The Good, the Bad, and the Ugly. Curr Urol Rep 2018;19(8):60. Doi: 10.1007/s11934-018-0808-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nickel RS., Maher JY., Hsieh MH., et al. Fertility after Curative Therapy for Sickle Cell Disease: A Comprehensive Review to Guide Care. J Clin Medicine 2022;11(9):2318. Doi: 10.3390/jcm11092318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Montalembert M, Voskaridou E, Oevermann L, et al. Real-Life experience with hydroxyurea in patients with sickle cell disease: Results from the prospective ESCORT-HU cohort study. Am J Hematol. 2021;96(10):1223–1231. doi: 10.1002/ajh.26286 [DOI] [PubMed] [Google Scholar]
- 16.Mishkin AD., Mapara MY., Reshef R. Iatrogenic Infertility After Curative Stem Cell Transplantation in Patients with Sickle Cell Disease. Annals of Internal Medicine 2018. Doi: 10.7326/m18-0185. [DOI] [PubMed] [Google Scholar]
- 17.Queiroz AM., Lobo CL de C., Ballas SK. Menopause in Brazilian women with sickle cell anemia with and without hydroxyurea therapy. Hematology Transfus Cell Ther 2020. Doi: 10.1016/j.htct.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pecker LH., Maher JY., Law JY., et al. Risks associated with fertility preservation for women with sickle cell anemia. Fertility and Sterility 2018;110(4):720–31. Doi: 10.1016/j.fertnstert.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 19.Mamsen LS., Kristensen SG., Pors SE., et al. Consequences of β-Thalassemia or Sickle Cell Disease for Ovarian Follicle Number and Morphology in Girls Who Had Ovarian Tissue Cryopreserved. Front Endocrinol 2021;11:593718. Doi: 10.3389/fendo.2020.593718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schultz CL., Tchume-Johnson T., Jackson T., et al. Reproductive intentions in mothers of young children with sickle cell disease. Pediatr Blood Cancer 2020;67(5):e28227. Doi: 10.1002/pbc.28227. [DOI] [PubMed] [Google Scholar]
- 21.Attia M, Kripalani S, Darbari I, et al. Parents of Children with Sickle Cell Disease Are Interested in Preimplantation Genetic Testing. J Pediatrics 2020;223:178–182.e2. Doi: 10.1016/j.jpeds.2020.04.027. [DOI] [PubMed] [Google Scholar]
- 22.Early ML., Strodel RJ., Lake IV., et al. Acceptable, hopeful, and useful: development and mixed-method evaluation of an educational tool about reproductive options for people with sickle cell disease or trait. J Assist Reprod Gen 2021:1–11. Doi: 10.1007/s10815-021-02358-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wonkam A, Vries J de., Royal CD., et al. Would you terminate a pregnancy affected by sickle cell disease? Analysis of views of patients in Cameroon. Journal of Medical Ethics 2014;40(9):615–20. Doi: 10.1136/medethics-2013-101392. [DOI] [PubMed] [Google Scholar]
- 24.Vali S, Mukhtar S, Nandi A, et al. Cumulative outcome of pre-implantation genetic diagnosis for sickle cell disease: a 5-year review. British Journal of Haematology 2020;52:828. Doi: 10.1111/bjh.16930. [DOI] [PubMed] [Google Scholar]
- 25.Oyewo A, Salubi-Udu J, Khalaf Y, et al. Preimplantation genetic diagnosis for the prevention of sickle cell disease: current trends and barriers to uptake in a London teaching hospital. Human Fertility (Cambridge, England) 2009;12(3):153–9. Doi: 10.1080/14647270903037751. [DOI] [PubMed] [Google Scholar]
- 26.Simpson S Sickle cell disease: a new era. Lancet Haematol 2019;6(8):e393–4. Doi: 10.1016/s2352-3026(19)30111-5. [DOI] [PubMed] [Google Scholar]
- 27.Kahraman S, Beyazyurek C, Yesilipek MA., et al. Successful haematopoietic stem cell transplantation in 44 children from healthy siblings conceived after preimplantation HLA matching. Reprod Biomed Online 2014;29(3):340–51. Doi: 10.1016/j.rbmo.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Yawn BP., John-Sowah J. Management of Sickle Cell Disease: Recommendations from the 2014 Expert Panel Report. American Family Physician 2015;92(12):1069–76. [PubMed] [Google Scholar]
- 29.Association CH. Consensus Statement on the Care of Patients with Sickle Cell Disease in Canada. Version 2.0 2015. [Google Scholar]
- 30.Quinn CT., Ware RE. Reproductive equity: preserve the reserve. Blood 2022;139(7):963–5. Doi: 10.1182/blood.2021015021. [DOI] [PubMed] [Google Scholar]
- 31.Mishkin AD., Mapara MY., Barhaghi M., et al. Fertility Concerns and Access to Care for Stem Cell Transplantation Candidates with Sickle Cell Disease. Biology of Blood and Marrow Transplantation 2020;26(8):e192–7. Doi: 10.1016/j.bbmt.2020.03.025. [DOI] [PubMed] [Google Scholar]
- 32.Kesse-Adu R, Cartwright R, Thomas N, et al. Feritility and Attitudes to Fertility in a Cohort of Women with Sickle Cell disease In South East London. Blood 2013;122(21):4686–4686. Doi: 10.1182/blood.v122.21.4686.4686. [DOI] [Google Scholar]
- 33.Loren AW., Senapati S. Fertility preservation in patients with hematologic malignancies and recipients of hematopoietic cell transplants. Blood 2019;134(9):746–60. Doi: 10.1182/blood.2018846790. [DOI] [PubMed] [Google Scholar]
- 34.Hyman JH., Tulandi T. Fertility Preservation Options After Gonadotoxic Chemotherapy. Clin Medicine Insights Reproductive Heal 2013;7:61–9. Doi: 10.4137/cmrh.s10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bakshi N, Sinha CB., Ross D., et al. Proponent or collaborative: Physician perspectives and approaches to disease modifying therapies in sickle cell disease. PLOS ONE 2017;12(7):e0178413. Doi: 10.1371/journal.pone.0178413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brandow AM., Panepinto JA. Hydroxyurea use in sickle cell disease: the battle with low prescription rates, poor patient compliance and fears of toxicities. Expert Review of Hematology 2010;3(3):255–60. Doi: 10.1586/ehm.10.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meier ER., Abraham AA., Ngwube A., et al. Hematopoietic stem cell transplant referral patterns for children with sickle cell disease vary among pediatric hematologist/oncologists’ practice focus: A Sickle Cell Transplant Advocacy and Research Alliance (STAR) study. Pediatr Blood Cancer 2021;68(3):e28861. Doi: 10.1002/pbc.28861. [DOI] [PubMed] [Google Scholar]
- 38.Karkoska K, Todd K, Niss O, et al. Implementation of near-universal hydroxyurea uptake among children with sickle cell anemia: A single-center experience. Pediatr Blood Cancer 2021;68(6):e29008. Doi: 10.1002/pbc.29008. [DOI] [PubMed] [Google Scholar]
- 39.Early ML., Kumar P., Marcell AV., et al. Literacy assessment of preimplantation genetic patient education materials exceed national reading levels. J Assist Reprod Gen 2020;37(8):1913–22. Doi: 10.1007/s10815-020-01837-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Power-Hays A, Patterson A, Sobota A Household material hardships impact emergency department reliance in pediatric patients with sickle cell disease. Pediatr Blood Cancer 2020;67(10):e28587. Doi: 10.1002/pbc.28587. [DOI] [PubMed] [Google Scholar]
- 41.Smeltzer MP., Howell KE., Treadwell M., et al. Identifying barriers to evidence-based care for sickle cell disease: results from the Sickle Cell Disease Implementation Consortium cross-sectional survey of healthcare providers in the USA. Bmj Open 2021;11(11):e050880. Doi: 10.1136/bmjopen-2021-050880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pecker LH., Silver EJ., Roth M., et al. Pediatric Hematologists Report Infrequent Prognosis Discussions in the Routine Care of Children with Sickle Cell Disease. Journal of Health Care for the Poor and Underserved 2020;31(1):398–423. Doi: 10.1353/hpu.2020.0030. [DOI] [PubMed] [Google Scholar]
- 43.DeBaun MR., Ghafuri DL., Rodeghier M., et al. Decreased median survival of adults with sickle cell disease after adjusting for left truncation bias: a pooled analysis. Blood 2019;133(6):615–7. Doi: 10.1182/blood-2018-10-880575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jain T, Grainger DA., Ball GD., et al. 30 years of data: impact of the United States in vitro fertilization data registry on advancing fertility care. Fertil Steril 2019;111(3):477–88. Doi: 10.1016/j.fertnstert.2018.11.015. [DOI] [PubMed] [Google Scholar]
- 45.Ortega REF., Yoeun SW., Mesina O., et al. Assessment of Health Insurance Benefit Mandates for Fertility Preservation Among 11 US States. Jama Heal Forum 2021;2(12):e214309. Doi: 10.1001/jamahealthforum.2021.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Galic I, Negris O, Warren C, et al. Disparities in access to fertility care: who’s in and who’s out. F S Reports 2021;2(1):109–17. Doi: 10.1016/j.xfre.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Latif S, Silva SMD., Davies M., et al. Fertility preservation provision in the NHS: a national assessment of care policies. Hum Fertility Camb Engl 2022:1–6. Doi: 10.1080/14647273.2022.2045519. [DOI] [PubMed] [Google Scholar]
- 48.Kilbride MK. In vitro fertilisation with preimplantation genetic testing: the need for expanded insurance coverage. J Med Ethics 2021;47(12):e40–e40. Doi: 10.1136/medethics-2019-105879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haematology TL. The dystopia of enforced childbirth. Lancet Haematol 2022;9(7):e461. Doi: 10.1016/s2352-3026(22)00181-8. [DOI] [PubMed] [Google Scholar]
- 50.Feinberg EC., Kawwass JF., Cedars MI. Roe v Wade and the Threat to Fertility Care. Obstetrics Gynecol 2022;Publish Ahead of Print. Doi: 10.1097/aog.0000000000004928. [DOI] [PubMed] [Google Scholar]
- 51.Wonkam A, Hurst S A Call for Policy Action in Sub-Saharan Africa to Rethink Diagnostics for Pregnancy Affected by Sickle Cell Disease: Differential Views of Medical Doctors, Parents and Adult Patients Predict Value Conflicts in Cameroon. Omics J Integr Biology 2014;18(7):472–80. Doi: 10.1089/omi.2013.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Owens DC. Listening to Black women saves Black lives. Lancet 2021;397(10276):788–9. Doi: 10.1016/s0140-6736(21)00367-6. [DOI] [PubMed] [Google Scholar]
- 53.Wailoo K, Pemberton SG. The troubled dream of genetic medicine. Johns Hopkins Univ Pr; 2006. [Google Scholar]
- 54.Whitten CF. Sickle-Cell Programming — An Imperiled Promise. New Engl J Medicine 1973;288(6):318–9. Doi: 10.1056/nejm197302082880612. [DOI] [PubMed] [Google Scholar]
- 55.Scott RB., Castro O. Screening for Sickle Cell Hemoglobinopathies. Jama 1979;241(11):1145–7. Doi: 10.1001/jama.1979.03290370049028. [DOI] [PubMed] [Google Scholar]
- 56.Addressing Sickle Cell Disease: A Strategic Plan and Blueprint for Action. Washington, DC: The National Academies Press; 2020. [PubMed] [Google Scholar]
- 57.Pecker LH., Naik RP. The current state of sickle-cell trait: implications for reproductive and genetic counseling. Blood 2018:blood-2018-06-848705. Doi: 10.1182/blood-2018-06-848705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bowman JE. Genetic screening programs and public policy. Phylon 1960 1977;38(2):117–42. [PubMed] [Google Scholar]
- 59.LaForgia M How Sickle Cell Trait in Black People Can Give the Police Cover” New York Times, May 15, 2021 [Google Scholar]
- 60.Kolata G The Math is Brutally Simple, but Not Widely Taught. New York Times, Dec 28, 2021 [Google Scholar]
- 61.Zhou AE., Travassos MA. Bringing Sickle-Cell Treatments to Children in Sub-Saharan Africa. New Engl J Med 2022;387(6):488–91. Doi: 10.1056/nejmp2201763. [DOI] [PubMed] [Google Scholar]
- 62.Eichelberger KY., Doll K, Ekpo GE., et al. Black Lives Matter: Claiming a Space for Evidence-Based Outrage in Obstetrics and Gynecology. Am J Public Health 2016;106(10):1771–2. Doi: 10.2105/ajph.2016.303313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anderson RA., Weddell A., Spoudeas HA., et al. Do doctors discuss fertility issues before they treat young patients with cancer? Hum Reprod 2008;23(10):2246–51. Doi: 10.1093/humrep/den252. [DOI] [PubMed] [Google Scholar]
- 64.Quinn GP., Vadaparampil ST., Gwede CK., et al. Discussion of fertility preservation with newly diagnosed patients: oncologists’ views. J Cancer Surviv 2007;1(2):146–55. Doi: 10.1007/s11764-007-0019-9. [DOI] [PubMed] [Google Scholar]
- 65.Humphries LA., Chang O., Humm K., et al. Influence of race and ethnicity on in vitro fertilization outcomes: systematic review. American Journal of Obstetrics and Gynecology 2016;214(2):212.e1–212.e17. Doi: 10.1016/j.ajog.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 66.Marsh VM., Kamuya DM., Molyneux SS. ‘All her children are born that way’: gendered experiences of stigma in families affected by sickle cell disorder in rural Kenya. Ethnic Health 2011;16(4–5):343–59. Doi: 10.1080/13557858.2010.541903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klipstein S, Fallat ME., Savelli S., et al. Fertility Preservation for Pediatric and Adolescent Patients with Cancer: Medical and Ethical Considerations. Pediatrics 2020;145(3):e20193994. Doi: 10.1542/peds.2019-3994. [DOI] [PubMed] [Google Scholar]
- 68.Quinn GP., Stearsman DK., Campo-Engelstein L., et al. Preserving the Right to Future Children: An Ethical Case Analysis. The American Journal of Bioethics 2012;12(6):38–43. Doi: 10.1080/15265161.2012.673688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ofosu-Budu D, Hanninen V Living as an infertile woman: the case of southern and northern Ghana. Reprod Health 2020;17(1):69. Doi: 10.1186/s12978-020-00920-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hollos M, Whitehouse B Women in limbo: Life course consequences of infertility in a Nigerian community. Hum Fertil 2014;17(3):188–91. Doi: 10.3109/14647273.2014.936052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kanter J, Smith WR., Desai PC., et al. Building access to care in adult sickle cell disease: defining models of care, essential components, and economic aspects. Blood Adv 2020;4(16):3804–13. Doi: 10.1182/bloodadvances.2020001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Campen J, Silcock L, Yau S, et al. A novel non-invasive prenatal sickle cell disease test for all at-risk pregnancies. Brit J Haematol 2020;190(1):119–24. Doi: 10.1111/bjh.16529. [DOI] [PubMed] [Google Scholar]
- 73.Oteng-Ntim E, Pavord S, Howard R, et al. Management of sickle cell disease in pregnancy. A British Society for Haematology Guideline. Brit J Haematol 2021;194(6):980–95. Doi: 10.1111/bjh.17671. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


