Abstract
Congenital hyperinsulinism (HI), a beta cell disorder most commonly caused by inactivating mutations of beta cell KATP channels, results in dysregulated insulin secretion and persistent hypoglycemia. Children with KATP-HI are unresponsive to diazoxide, the only FDA-approved drug for HI, and utility of octreotide, the second-line therapy, is limited because of poor efficacy, desensitization, and somatostatin receptor type 2 (SST2)-mediated side effects. Selective targeting of SST5, an SST receptor associated with potent insulin secretion suppression, presents a new avenue for HI therapy. Here, we determined that CRN02481, a highly selective nonpeptide SST5 agonist, significantly decreased basal and amino acid–stimulated insulin secretion in both Sur1−/− (a model for KATP-HI) and wild-type mouse islets. Oral administration of CRN02481 significantly increased fasting glucose and prevented fasting hypoglycemia compared to vehicle in Sur1−/− mice. During a glucose tolerance test, CRN02481 significantly increased glucose excursion in both WT and Sur1−/− mice compared to the control. CRN02481 also reduced glucose- and tolbutamide-stimulated insulin secretion from healthy, control human islets similar to the effects observed with SS14 and peptide somatostatin analogs. Moreover, CRN02481 significantly decreased glucose- and amino acid-stimulated insulin secretion in islets from two infants with KATP-HI and one with Beckwith-Weideman Syndrome-HI. Taken together, these data demonstrate that a potent and selective SST5 agonist effectively prevents fasting hypoglycemia and suppresses insulin secretion not only in a KATP-HI mouse model but also in healthy human islets and islets from HI patients.
Keywords: congenital hyperinsulinism, hypoglycemia, beta cells, insulin secretion, somatostatin receptor
Congenital hyperinsulinism (HI) is a beta cell disorder that results in dysregulated insulin secretion and persistent hypoglycemia. HI is the most common cause of persistent hypoglycemia in neonates, infants, and children, and it is associated with a high risk of serious complications such as seizures, developmental and behavioral problems, and intellectual disabilities (1, 2). Up to 14 genetic loci have been associated with HI; however, the most common causes are inactivating mutations in the genes encoding the ATP-sensitive potassium channel (KATP-HI), accounting for up to 57% of cases followed at our Center located in Philadelphia, Pennsylvania (3), which is consistent with the experience of other HI Centers (4, 5). Currently, available treatments for KATP-HI are extremely limited. The only FDA-approved drug for the treatment of HI is the KATP channel activator, diazoxide. Consequently, due to the mutations that alter the functionality or expression of KATP channels resulting in their HI, children with KATP-HI are typically unresponsive to this therapy. Octreotide, a somatostatin analogue, is used as a second-line therapy. However, due to limited efficacy, tachyphylaxis, and side effects, such as gastrointestinal discomfort, thrombosis, and necrotizing enterocolitis, its use is limited, particularly in neonates (6, 7). Unfortunately, due to the dearth of therapies and the limitations of available treatments, most infants with KATP-HI require a pancreatectomy for intractable hypoglycemia. Infants with the focal form of the disease, which account for approximately 50% of cases, can often be cured by resection of the lesion by an experienced surgical team, however, for cases with diffuse disease, pancreatectomy is only palliative with 50% of patients requiring additional therapy for persistent hypoglycemia (8, 9). As a result, there is a dire need for new and better therapies for infants with KATP-HI.
Somatostatin analogs (SSAs) are an attractive option for HI treatment due to their ability to effectively inhibit insulin secretion. Somatostatin achieves its inhibitory effects on pancreatic hormone secretion through interaction with somatostatin receptors (SSTs), which belong to the superfamily of G protein–coupled receptors (GPCR) (10, 11). The expression of SSTs in the mammalian pancreas has been an area of significant interest. RT-PCR analysis of the rodent pancreas has confirmed gene expression of SST1, 2, 3, and 5 but not SST4 (12). In addition, immunostaining of the rodent pancreas has identified the expression of SST2 on alpha cells, while beta and delta cells express both SST2 and SST5 (13, 14, 15, 16, 17, 18). The use of knockout mouse models and receptor-specific analogs established that SST2 modulates the suppression of both insulin and glucagon, whereas SST5 principally regulates insulin release (19, 20, 21, 22). Both pancreatic alpha and beta cells in humans exhibit high expression of SST2, while beta cells additionally express SST1 and 5 (10, 13, 23, 24, 25, 26). In contrast to rodent models, SST2 activation in human islets inhibits glucagon secretion. and SST1, 2, and 5 receptors participate in the regulation of insulin secretion (10, 27, 28, 29). This unique network of somatostatin receptor expression and regulation of glucose homeostasis provides potential targets for receptor-specific strategies for the treatment of HI. Octreotide, used off-label in the treatment of HI, has an affinity for SST2; thus, some of its observed lack of effectiveness may be explained by a weaker affinity for beta-cell specific SSTs and potentially counterproductive on-target effects such as glucagon suppression. Moreover, the most significant side effects associated with octreotide can be attributed to interaction with and activation of SST2 (30). It is our hypothesis that a therapy specifically targeting SST5 would inhibit insulin secretion without the detrimental side effects seen with SST2 activation. In addition to having suboptimal efficacy/side effect profiles, octreotide and other somatostatin analogs used off-label in the treatment of HI are all injectable peptides that require subcutaneous (octreotide, lanreotide) or intramuscular (octreotide long-acting release) administration. Thus, a therapy that could be dosed orally would greatly increase the ease of treatment and remove the possibility of injection site pain and reactions. Crinetics Pharmaceuticals launched a medicinal chemistry campaign aimed to discover selective nonpeptide SST5 agonists as a new approach to managing hyperinsulinemic hypoglycemia. Those efforts lead to the discovery of CRN02481 (also known as compound 28), a very specific and potent SST5 agonist with good drug-like properties (31). Here we describe the evaluation of CRN02481 on insulin secretion particularly in the context of HI. After treatment of Sur1−/− (also known as Abcc8−/−) mice, a model of KATP-HI due to the lack of expression of the Sulfonylurea Receptor 1 subunit of the KATP channels, with CRN02481 we observed a significant decrease of insulin secretion both in vitro during perifusion of isolated islets and in vivo, rescuing fasting hypoglycemia. Importantly, CRN02481 treatment resulted in a significant decrease in stimulated insulin secretion in both healthy and control human islets and in pancreatic islets isolated from three different patients with HI. Taken together, these results illustrate the potential of SST5-specific therapies like CRN02481 for the treatment of HI.
Results
Selectivity of the nonpeptide SST5 agonist CRN02481 for human and mouse SST5 receptor
Chinese Hamster Ovary (CHO) cells expressing each of the human or mouse SST subtypes receptors were treated with NKH477 to increase the production of intracellular cAMP. Upon SSTR activation by the specific agonist (SS14, Octreotide, Lanreotide, Pasireotide or CRN02481), cAMP levels decreased in a concentration-dependent manner and potencies were calculated and expressed as EC50s (Table 1). As expected, the native ligand SS14 was non-selective at the five human somatostatin receptors while octreotide exhibits considerable potency at hSST2 (EC50 = 0.06 nM), but exhibiting less potency for hSST5 (EC50 = 2.7 nM) and hSST3 (EC50 = 5.2 nM). Lanreotide shows weaker activity at hSST5 (EC50 = 16 nM) and hSST3 (EC50 = 200 nM) making it more specific at hSST2 (EC50 = 0.1 nM) than octreotide. By comparison, pasireotide is an extremely potent SST5 (EC50 = 0.081 nM) agonist that still retains subnanomolar activity at both SST2 (EC50 = 0.59 nM) and SST3 (EC50 = 0.78 nM) (Table 1).
Table 1.
Potency of somatostatin, octreotide, lanreotide, pasireotide and CRN02481 at human and mouse somatostatin receptors
| Receptor | SS14 |
Octreotide |
Lanreotide |
Pasireotide |
CRN02481 |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human |
Mouse |
Human |
Mouse |
Human |
Mouse |
Human |
Mouse |
Human |
Mouse |
|||||||||||
| pEC50 ± SEM | EC50 (nM) | pEC50 ± SEM | EC50 (nM) | pEC50 ± SEM | EC50 (nM) | pEC50 ± SEM | EC50 (nM) | pEC50 ± SEM | EC50 (nM) | pEC50 ± SEM | EC50 (nM) | pEC50 ± SEM | EC50 (nM) | pEC50 ± SEM | EC50 (nM) | pEC50 ± SEM | EC50 (nM) | pEC50 ± SEM | EC50 (nM) | |
| SST1 | 9.0 ± 0.1 | 1.0 | ND | ND | <5.0 | >10,000 | ND | ND | <5.0 | >10,000 | ND | ND | 6.7 ± 0.2 | 180 | ND | ND | <6.0 | >1000 | ND | ND |
| SST2 | 9.9 ± 0.1 | 0.13 | 10 ± 0.5 | 0.03 | 10 ± 0.1 | 0.06 | 11 ± 0.0 | 0.02 | 10 ± 0.0 | 0.1 | 10.6 | 0.03 | 9.2 ± 0.1 | 0.59 | 9.1 ± 0.1 | 0.88 | 6.4 ± 0.0 | 440 | 6.5 ± 0.1 | 320 |
| SST3 | 9.8 ± 0.0 | 0.16 | 9.8 ± 0.1 | 0.15 | 8.3 ± 0.1 | 5.2 | ND | ND | 6.7 ± 0.1 | 200 | ND | ND | 9.1 ± 0.1 | 0.78 | ND | ND | 7.4 ± 0.1 | 39 | 8.0 ± 0.1 | 10 |
| SST4 | 9.7 ± 0.0 | 0.20 | ND | ND | 6.4 ± 0.1 | 450 | ND | ND | 6.1 ± 0.1 | 820 | ND | ND | <5.1 | >8100 | ND | ND | 8.2 ± 0.1 | 5.7 | ND | ND |
| SST5 | 10 ± 0.1 | 0.06 | 10 ± 0.1 | 0.07 | 8.6 ± 0.1 | 2.7 | 12 ± 0.1 | 0.002 | 7.8 ± 0.1 | 16 | ND | ND | 10.1 ± 0.1 | 0.081 | 10.5 | 0.032 | 9.4 ± 0.1 | 0.37 | 10 ± 0.1 | 0.04 |
ND: Not determined. pEC50 and SEM given with two significant figures.
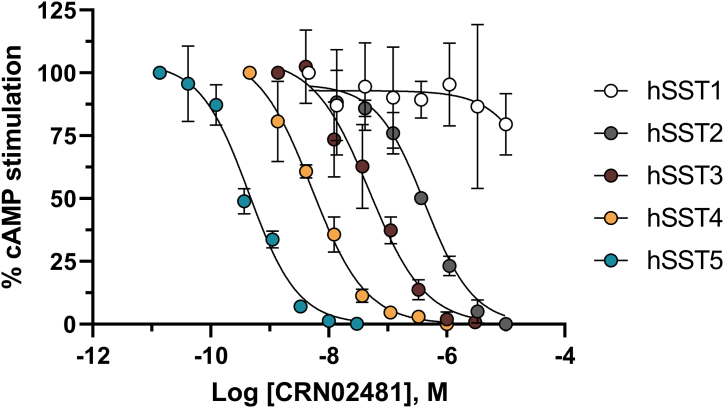
In contrast to SS14 peptide analogs, CRN02481 was designed to be very potent and selective for the human SST5 receptor (31). It shows 15-, 100-, 1200-, and >2700-fold less activity at the SST4, SST3, SST2, and SST1 receptors, respectively (Fig. 1 and Table 1 and (31)). CRN02481 exhibits a similar selectivity profile at the murine SST receptors, activating the mouse SST5 receptor with high potency (EC50 = 0.041 nM) and possessing decreased activity at mouse SST2 (EC50 = 320 nM) and SST3 receptors (EC50 = 10 nM).
Figure 1.
Selectivity of the nonpeptide somatostatin receptor 5 (SST5) agonist CRN02481 for human SST5 receptor. Inhibition of stimulated cAMP production by CRN02481 action at the human SST receptors was evaluated in CHO-K1 cells expressing each of the human SST receptors. Concentration–response curves shown are from individual representative experiments. All points represent the mean ± SD of either triplicate or quadruplicate readings.
In addition to its selectivity within the SST receptor family, CRN02481 also showed little cross-reactivity over a panel of 64 human GPCRs. Even at a concentration of 10 μM, inhibition >70% was only observed at two receptors (dopamine transporter and adrenergic beta 2) (Table S1).
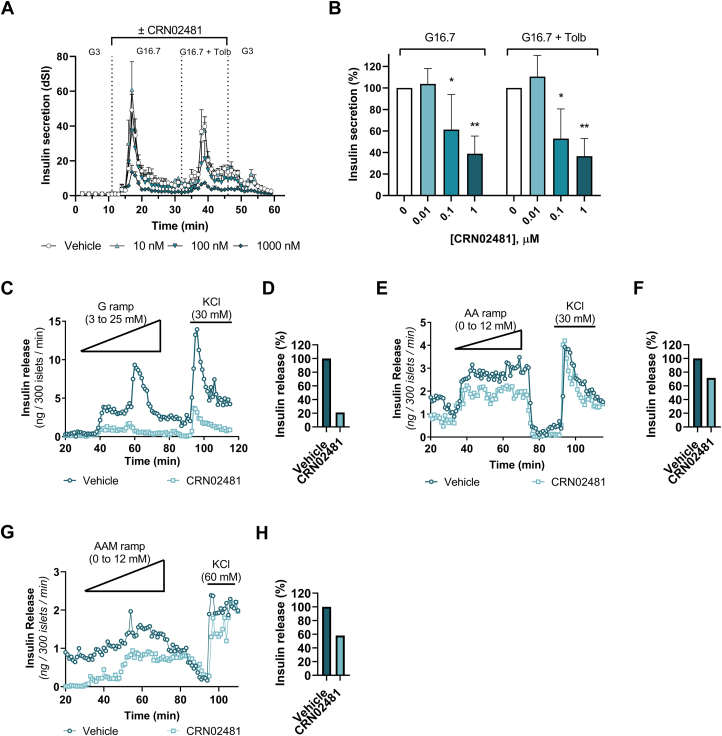
CRN02481 decreases insulin secretion from primary mouse-isolated islets
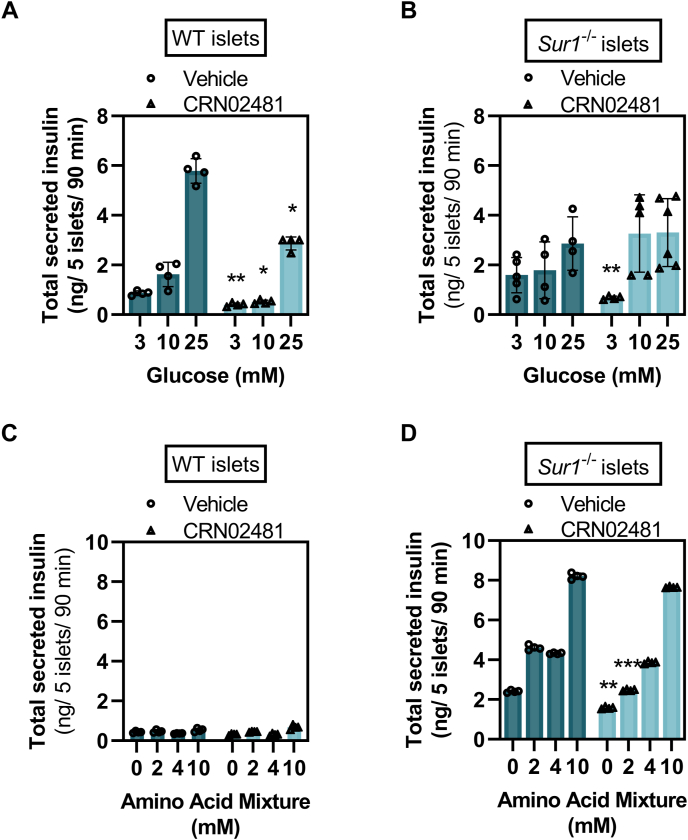
In static batch incubation of pancreatic islets isolated from wild-type (WT) mice and the hyperinsulinism mouse model Sur1−/− mice CRN02481 significantly decreased basal levels of insulin secretion (3 mM glucose) compared to vehicle control (Fig. 2, A and B).
Figure 2.
CRN02481 decreases insulin secretion from primary isolated islets. Secreted insulin from batch incubation of (A) WT or (B) Sur1−/− primary isolated pancreatic islets stimulated with denoted glucose concentrations and treated with either vehicle control or CRN02481 (500 nM). For WT, CRN02481 treatment is compared to vehicle control: 3 mM glucose (F(1, 42) = 8.57, p = 0.005); 10 mM glucose (F(1, 38) = 4.53, p = 0.04); 25 mM glucose (F(1, 46) = 4.68, p = 0.04). For Sur1−/−, CRN02481 is compared to vehicle control: 3 mM glucose (F(1, 29) = 25.16, p = 2.42 × 10−5); 10 mM glucose (F(1, 34) = 19.45, p = 0.002); 25 mM glucose (F(1, 38) = 1.09, p = 0.30). Secreted insulin from batch incubation of (C) WT or (D) Sur1−/− primary isolated islets stimulated with denoted physiological amino acid mixture (AAM) concentrations and treated with either vehicle control or CRN02481 (500 nM). For WT, CRN02481 treatment is compared to Vehicle control: 0 mM AAM (F(1, 38) = 1.39, p = 0.24); 2 mM AAM (F(1, 34) = 0.03, p = 0.86); 4 mM AAM (F(1, 38) = 0.30, p = 0.58); 10 mM AAM (F(1, 34) = 0.98, p = 0.33). For Sur1−/−, CRN02481 is compared to Vehicle control: 0 mM AAM (F(1, 38) = 7.16, p =0.01); 2 mM AAM (F(1,38) = 15.63, p = 0.0003); 4 mM AAM (F(1, 38) = 2.96, p = 0.09), 10 mM AAM (F(1, 38) = 0.63, p = 0.43). p-values calculated with a one-way ANOVA Test. (Statistically significant comparison to Vehicle control: ∗p ≤ 0.05; ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001). Data represent mean ± SD compared to vehicle control. n = 4 to 5. WT, wild type.
As we have previously described (32), Sur1−/− islets only minimally increase insulin secretion in response to high glucose (25 mM); however, they respond to stimulation with a physiological amino acid mixture (AAM) mimicking the phenotype observed in individuals with KATP-HI who exhibit impaired glucose-stimulated insulin secretion (33) and protein-induced hypoglycemia (34, 35). As expected, WT islets did not secrete insulin in response to AAM, while Sur1−/− islets demonstrated significantly increased insulin secretion in response to AAM (Fig. 2, C and D). In WT islets, CRN02481 inhibited glucose-stimulated insulin secretion at all three glucose concentrations (3, 10, and 25 mM) (Fig. 2A). Treatment with CRN02481 significantly decreased insulin secretion both basally and in response to AAM in Sur1−/− islets to roughly basal levels when stimulated with 2 mM AAM compared to vehicle control (Fig. 2D). These results indicate that treatment with the selective nonpeptide SST5 agonist CRN02481 significantly decreases both basal and fuel-stimulated insulin secretion in primary isolated islets from the hyperinsulinism mouse model Sur1−/− mice.
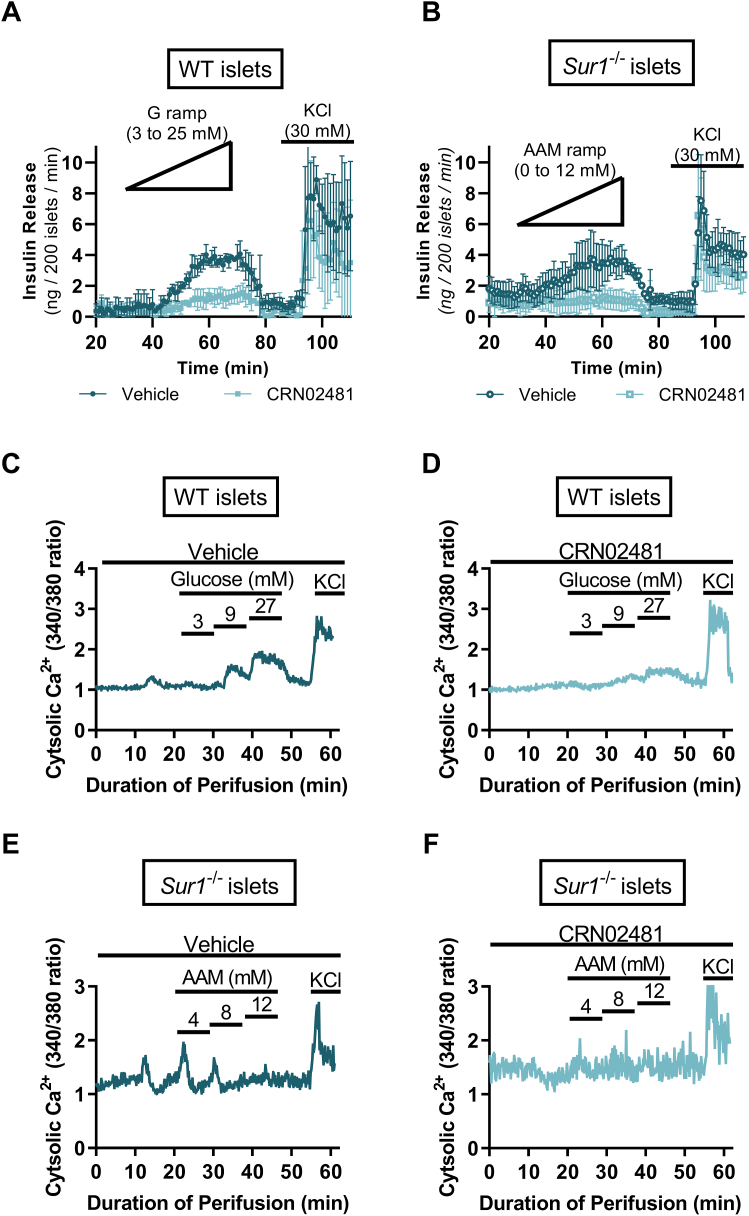
CRN02481 significantly abrogates insulin secretion and reduced cytosolic calcium in response to secretagogues
We also assessed the effect of CRN02481 on insulin secretion kinetics through the perifusion of isolated islets. Treatment with CRN02481 significantly reduced insulin secretion in WT islets in response to a glucose ramp (0–25 mM) compared to vehicle control (2-way ANOVA, p < 0.0001) (Fig. 3A). Similarly, CRN02481 treatment of Sur1−/− islets resulted in significantly reduced insulin secretion to nearly basal levels in response to an AAM ramp (0–12 mM) compared to vehicle control (2-way ANOVA, p < 0.0001) (Fig. 3B). To further evaluate the effect of CRN02481 on insulin secretion we measured cytosolic calcium, [Ca2+]c, of islets isolated from Sur1−/− mice during perifusion. The increases in [Ca2+]c in response to glucose were completely abrogated in the WT islets with the treatment of CRN02481 (Fig. 3, C and D). Likewise, in response to AAM, the flux of [Ca2+]c was also decreased in Sur1−/− islets (Fig. 3, E and F). These data demonstrate a notable suppression of insulin secretion in both WT and Sur1−/− primary isolated islets in vitro in response to treatment with CRN02481.
Figure 3.
CRN02481 significantly abrogates insulin secretion and calcium flux in response to glucose and amino acid stimulus. Perifusion of primary isolated islets to assess insulin release in (A) WT islets stimulated with glucose ramp (0–25 mM) and KCl (30 mM) or (B) Sur1−/− islets stimulated with amino acid (AA) ramp (0–12 mM) and KCl (30 mM). Two-way ANOVA with Tukey’s multiple comparisons revealed that insulin release is significantly decreased with CRN02481 (500 nM) treatment for both WT (F(1, 384) = 94.75, p < 0.0001) and Sur1−/− (F(1,384) = 221.2, p < 0.0001) islets when compared to vehicle. (n = 3). Intracellular Ca2+ measurement of primary isolated islets for assessment of calcium signaling in WT islets treated with glucose steps (3 mM, 9 mM, 27 mM), KCl (30 mM), and (C) vehicle or (D) 500 nM CRN02481. Sur1−/− islets treated with amino acid steps (4 mM, 8 mM, and 12 mM), KCl (30 mM), and (E) vehicle or (F) 500 nM CRN02481. Two-way ANOVA with Tukey’s multiple comparisons demonstrated that treatment with CRN02481 resulted in significantly decreased intracellular Ca2+ signaling in both WT (F(1, 3660) = 56.60, p < 0.0001) and Sur1−/− islets (F(1, 3660) = 799.0, p < 0.0001) compared to vehicle (n = 3–6). Data represent mean ± SD compared. WT, wild type.
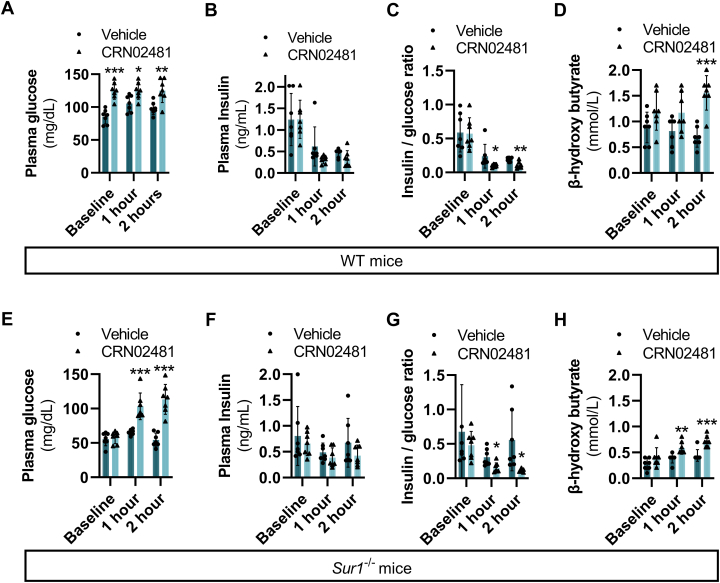
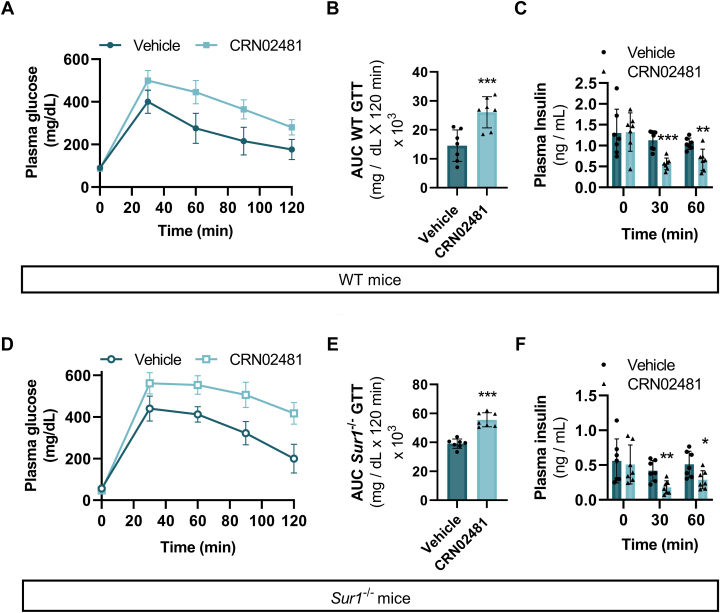
Treatment with CRN02481 increases plasma glucose and decreases insulin secretion in both WT and Sur1−/− mice
With the effect of CRN02481 on insulin secretion evident in isolated islets, we assessed the response in vivo. Fasting plasma glucose significantly increased at 1 and 2 h after dosing with CRN02481 in WT mice compared to vehicle-treated mice (Fig. 4A). Although absolute plasma insulin levels did not show a significant decrease (Fig. 4B), the ratio of insulin to glucose at 1 and 2 h was significantly decreased compared to control (Fig. 4C). Fasting plasma β-hydroxybutyrate concentration was also significantly increased at 2 h after CRN02481 treatment compared to controls in WT mice (Fig. 4D), supportive of insulin suppression. Interestingly, CRN02481 treatment of Sur1−/− mice demonstrated effects similar to that seen in WT mice. In Sur1−/− mice we observed a significant increase in fasting plasma glucose concentration, with no significant decrease in absolute plasma insulin concentration and a significant decrease in the insulin-to-glucose ratio (Fig. 4, E–G). Sur1−/− mice also demonstrated a significant increase in fasting plasma β-hydroxybutyrate at 1 and 2 h after CRN02481 treatment compared to vehicle-treated mice (Fig. 4H).
Figure 4.
Treatment with the SST5 agonist, CRN02481, after fasting increases plasma glucose and decreases insulin secretion in both WT and Sur1−/−KATP-HI. Fasting evaluation after overnight fast (16 h) at baseline (0 h), 1 h, or 2 h post-treatment with either vehicle or CRN02481 (30 mg/kg) of WT mice assessing (A) plasma glucose [Baseline: (F(1, 12) = 38.46, p = 4.57 × 10-5); 1 h (F(1, 12) = 8.04, p = 0.02); 2 h (F(1, 12) = 12.11, p = 0.005)], (B) plasma insulin [Baseline (F(1, 12) = 0.0004, p = 0.98); 1 h (F(1, 12) = 3.14, p = 0.10; 2 h (F(1, 12) = 2.76, p = 0.12)], (C) insulin/glucose ratio [Baseline (F(1, 12) = 0.01, p = 0.91); 1 h (F(1, 12) = 4.50, p = 0.05); 2 h (F(1, 12) = 14.79, p = 0.002)], and (D) β-hydroxy butyrate [Baseline (F(1, 12) = 3.08, p = 0.10); 1 h (F(1, 12) = 3.818, p = 0.07); 2 h (F(1, 12) = 33.64, p = 8.47 × 10-5)]. Fasting evaluation after overnight fast (16 h) at baseline (0 h), 1 h, or 2 h post-treatment with either vehicle or CRN02481 (30 mg/kg) of Sur1−/− mice assessing (E) plasma glucose [Baseline (F(1, 12) = 0.18, p = 0.67; 1 h (F(1, 12) = 25.87, p = 0.0002); 2 h (F(1, 12) = 42.59, p = 2.83 × 10-5)], (F) plasma insulin [Baseline (F(1, 12) = 0.40, p = 0.54); 1 h (F(1, 12) = 1.09, p = 0.31); 2 h (F(1, 12) = 1.67, p = 0.221)], (G) insulin/glucose ratio [Baseline (F(1, 12) = 0.54, p = 0.48); 1 h (F(1, 12) = 8.36, p = 0.01); 2 h (F(1, 12) = 6.94, p = 0.02)], and (H) β-hydroxybutyrate [Baseline (F(1, 12) = 2.35, p = 0.15); 1 h (F(1, 12) = 12.25, p = 0.004); 2 h (F(1, 12) = 21.23, p = 0.0006)]. (n = 7) p-values calculated with one-way ANOVA Test compared to vehicle control (∗∗∗p < 0.001; ∗∗p < 0.01; ∗p < 0.05). Data represent mean ± SD.
Upon challenge with a glucose tolerance test (IPGTT), we observed significantly increased plasma glucose and decreased plasma insulin levels in WT mice after treatment with CRN02481 compared to vehicle-treated mice (Fig. 5, A–C). Similarly, a significant increase in plasma glucose and a significant decrease in plasma insulin was observed in Sur1−/− mice after glucose challenge compared to vehicle-treated mice (Fig. 5, D–F). These results demonstrate that treatment with CRN02481 significantly decreases insulin secretion and abrogates fasting hypoglycemia in the Sur1−/− hyperinsulinism mouse model.
Figure 5.
SST5 agonist, CRN02481, increases plasma glucose and decreases insulin secretion in both WT and Sur1−/−KATP-HI mouse models after glucose challenge.A, glucose tolerance test (GTT) in WT mice treated with vehicle or CRN02481 (30 mg/kg) by gavage followed by I.P. injection of 2 g/kg glucose. Two-way ANOVA with Tukey’s multiple comparisons: (F(1, 60) = 80.45, p < 0.0001). (n = 7) (B) Area under the curve (AUC) calculation for GTT of WT mice. One way ANOVA: (F(1, 12) = 15.98, p = 0.002). C, plasma insulin levels of WT mice during 0, 30, and 60 min time points during GTT. One-way ANOVA: 0 min (F(1, 12) = 0.005, p = 0.94); 30 min (F(1, 12) = 30.88, p = 0.0001); 60 min (F(1, 12) = 12.43, p = 0.004). D, GTT in Sur1−/− mice treated with vehicle or CRN02481 (30 mg/kg) by gavage followed by I.P. injection of 2 g/kg glucose. Two-way ANOVA with Tukey’s multiple comparisons: (F(1, 60) = 124.3, p <0.0001) (n = 7). E, AUC calculated for Sur1−/− mice GTT. One-way ANOVA: (F(1, 12) = 56.67, p = 6.97 × 10-6). F, plasma insulin levels of Sur1−/− mice during 0, 30, and 60 min time points during GTT. One-way ANOVA: 0 min (F(1, 12) = 0.105, p = 0.75); 30 min (F(1, 12) = 13.16, p = 0.003); 60 min (F(1, 12) = 6.67, p = 0.02). (n = 7) (Comparison to vehicle control: ∗∗∗p < 0.001; ∗∗p <0.01; p < 0.05). Data represent mean ± SD. WT, wild type.
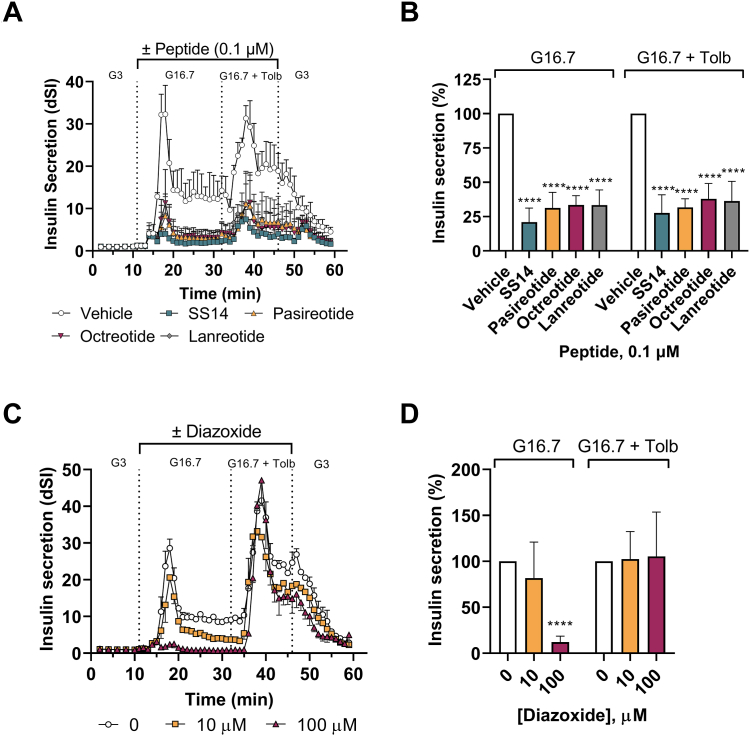
Effect of somatostatin, somatostatin peptide analogs, and diazoxide on glucose- and tolbutamide-stimulated insulin secretion in isolated healthy human islets
To have a better comparison between a specific SST5 nonpeptide like CRN02481, somatostatin, SSAs, and diazoxide, we evaluated the effect of these molecules in glucose- and tolbutamide-stimulated insulin secretion in healthy human islets from several donors (Table 2). Tolbutamide is a sulfonylurea that blocks the KATP channel resulting in insulin secretion while diazoxide is a KATP channel opener that causes the opposing effect and suppresses insulin secretion. As expected, SS14 and analogs (octreotide, lanreotide, and pasireotide) suppressed insulin secretion in human islets stimulated with high glucose (16.7 mM) alone or high glucose (16.7 mM) with tolbutamide (0.1 μM). SS14 produced the strongest effect, suppressing ∼80% insulin in both conditions. The peptide analogs had similar effects among them, suppressing insulin ∼65 to 70% (Fig. 6, A and B). These results confirm the few reports demonstrating the inhibitory effect of SS14 in static assays in human islets (28, 36) and octreotide in perfused human pancreas (29). These data also show the activity of lanreotide and pasireotide in the dynamic secretion of insulin in isolated human islets for the first time. Because the three analogs each possess mixed pharmacology at the SST family of receptors (Table 1), it is difficult to deconvolute the contribution of each receptor to the anti-secretory activity observed. However, we have shown that specific and potent small molecules targeting SST3 and SST4 receptors (EC50 = < 1 nM) have no effect on glucose- and tolbutamide-stimulated insulin secretion in healthy human islets (37). Copy of the poster can be found here: https://crinetics.com/pipeline/crn04777-oral-sst5-agonist-congenital-hyperinsulinism-congenital-hi/). Therefore the anti-secretory activity of these peptide agonists is due to their activation of SST2 and SST5 receptors. The ability of SS14 and analogs to suppress tolbutamide-stimulated insulin secretion is also shown here for the first time and confirms these peptides act downstream of the closure of the KATP channel. Diazoxide suppressed glucose-stimulated insulin secretion at concentrations 1000-fold higher than SS14 and importantly, did not suppress tolbutamide-stimulated insulin secretion (Fig. 6, C and D), mimicking its inability to suppress insulin secretion in children with KATP- HI.
Table 2.
Donor information
| Prodo ID | Gender | Ethnicity | Age (y) | BMI | HbA1C (%) | Causeofdeath | Islet index | Islet purityby count (%) | Isletviability | Islet sterilitygram stain | Isletsused infigure |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HP-20066-01 | Female | Caucasian | 60 | 25.8 | 5.1 | Stroke | 0.64 | 90 | 95 | Negative | 6D |
| HP-20191-01 | Female | Caucasian | 39 | 22.1 | 5.6 | Head trauma | 0.70 | 95 | 95 | Negative | 6C and 6D |
| HP-20262-01 | Male | Asian | 67 | 24.4 | 5.8 | Stroke | 0.54 | 90–95 | 95 | Negative | 6B |
| HP-20281-01 | Male | Caucasian | 55 | 25.6 | 4.9 | Stroke | 1.14 | 90 | 95 | Negative | 6B |
| HP-20317-01 | Male | Hispanic | 58 | 26.8 | 4.9 | Stroke | 0.67 | 90 | 95 | Negative | 6B |
| HP-20341-01 | Male | Hispanic | 35 | 18.1 | 5.3 | Stabbing | 1.00 | 90 | 95 | Negative | 6B |
| HP-21100-01 | Male | African American | 66 | 34.3 | 5.5 | Head trauma | 0.85 | 90 | 95 | Negative | 6D |
| HP-21141-01 | Male | Caucasian | 55 | 21.3 | 5.6 | Head trauma | 0.65 | 90 | 95 | Negative | 6A, 6B, 6D and 7B |
| HP-21155-01 | Male | Hispanic | 18 | 20.4 | 5.4 | Head trauma | 1.00 | 85–90 | 95 | Negative | 6D and 7B |
| HP-21167-01 | Male | Caucasian | 46 | 28.1 | 5.0 | Stroke | 0.83 | 85 | 95 | Negative | 6D, 7A and 7B |
| HP-21203-01 | Male | Hispanic | 25 | 26.5 | 5.8 | Head trauma | 1.50 | 95 | 95 | Negative | 7B |
| HP-21189-01 | Male | Hispanic | 36 | 26.3 | 5.4 | Head trauma | 1.10 | 90 | 95 | Negative | 6B |
Figure 6.
Suppressive effect of SS14, peptide analogs and diazoxide on insulin secretion in islets from healthy donors. Isolated healthy human islets (Prodo Labs, CA) were loaded in a perifusion system (Biorep, FL) and treated over time with 3 mM glucose (G3), 16.7 mM glucose (G16.7), and 16.7 mM glucose + 100 μM tolbutamide (G16.7 + Tolb) in the presence or absence of SS14 and analog peptides, pasireotide, octreotide and lanreotide at 0.1 μM (A and B) and diazoxide at 10 μM and 100 μM (C and D). Insulin secretion was quantified using an ELISA assay (Mercodia, Uppsala, Sweden) and the dynamic stimulation index (dSI) was calculated as stimulated insulin levels/basal insulin levels. (A and C) Mean dSI ± SD (n = 2 technical replicates) from one representative donor. (B and D) Percent insulin secretion was calculated in each condition and compared to the vehicle group which was set to 100%. One-way ANOVA analysis with Dunnett’s multiple comparisons of peptide (B): G16.7 (F(4, 16) = 38.76, p < 0.0001 for all peptide comparisons to vehicle) and G16.7 + Tolb (F(4, 15) = 24.32, p < 0.0001 for all comparisons to vehicle); diazoxide (D): G16.7 (F(2, 12) = 45.27, p = 0.25 for 10 μM, p < 0.0001 for 100 μM compared to vehicle) and G16.7 + Tolb (F(2, 12) = 0.039, p = 0.99 for 10 μM and p = 0.94 for 100 μM compared to vehicle). Data represent mean ± SD from 4 to 6 independent donors in (B) and 3 to 6 independent donors in (D). (Comparison to Vehicle control: ∗∗∗∗, p ≤ 0.0001).
Insulin secretion in isolated pancreatic islets from healthy human donors and human patients with HI is significantly suppressed by treatment with CRN02481
With significant downregulation of insulin secretion evident in the mouse models, and the effect of the peptides and diazoxide as controls and comparators, we evaluated the effect of CRN02481 on glucose- and tolbutamide-stimulated insulin secretion in isolated human islets from four donors (Table 2). Perifusion with either vehicle or increasing concentrations of CRN02481 (10 nM, 100 nM, or 1000 nM) was performed on isolated healthy, control human islets accompanied by stimulation with high glucose alone or high glucose with tolbutamide. CRN02481 significantly reduced both high glucose- and tolbutamide-stimulated insulin secretion in a concentration-dependent manner with the maximum effect at 1000 nM (Fig. 7, A and B). These results indicate that activation of SST5 by CRN02481 resulted in the expected anti-secretory effect on insulin in healthy human islets and more importantly showed that the compound worked downstream of the KATP channel having the ability to also suppress tolbutamide-stimulated insulin secretion, in contrast to diazoxide (Fig. 6, C and D). We were also able to assess the effect of CRN02481 in islets from patients with HI. Perifusion was performed on pancreatic islets isolated from tissue collected after the pancreatectomy of three patients with HI (Table 3). The first patient had hyperinsulinism associated with Beckwith-Wiedemann Syndrome due to uniparental paternal isodisomy for chromosome region 11p(UPD-BWS) (38). Distinct from KATP-HI, BWS-HI patients exhibit an exaggerated insulin secretion in response to glucose leading to hypoglycemia (39). Treatment with CRN02481 significantly decreased insulin secretion during perifusion with a glucose ramp (3–25 mM) compared to vehicle control (Fig. 7, C and D). Genetic testing of the second patient revealed two heterozygous recessive mutations in ABCC8, which resulted in diazoxide-unresponsive diffuse KATP-HI. Similar to islets from Sur1−/− mice, KATP-HI islets only minimally increase insulin secretion in response to high glucose (25 mM) but do respond to stimulation with a physiological amino acid mixture (33, 34). Perifusion of these pancreatic islets exhibited a significant decrease in basal insulin secretion (min 20–30) and in response to an AAM ramp (0–12 mM) with CRN02481 treatment compared to vehicle control (Fig. 7, E and F). The third patient was diagnosed with diffuse KATP-HI caused by a mutation of ABCC8 (c.3577delG) and deletion of ABCC8 exon 20 and 29. Similar to the second patient, perifusion results showed that CRN02481 significantly decreased basal insulin secretion (min 20–30) and in response to the AAM ramp compared to vehicle control (Fig. 7, G and H). Further analysis revealed no change in glucagon secretion with the treatment of CRN02481 in islets from these three HI patients (Fig. S1). Remarkably, CRN02481 treatment of human islets from patients with hyperinsulinism demonstrated a significant decrease in insulin secretion and illustrates the feasibility of SST5-specific therapies for the treatment of HI.
Figure 7.
CRN02481 significantly curtails insulin secretion in pancreatic islets from human healthy donors and patients with HI. Isolated human healthy, control islets (Prodo Labs, CA) were loaded in a perifusion system (Biorep, FL) and treated over time with 3 mM glucose (G3), 16.7 mM glucose (G16.7), and 16.7 mM glucose + 100 μM tolbutamide (G16.7 + Tolb) in the presence of absence of increasing concentrations of CRN02481 as described in material and methods. Insulin secretion was quantified using an ELISA assay (Mercodia, Uppsala, Sweden) and the dynamic stimulation index (dSI) was calculated as stimulated insulin levels/basal insulin levels. (A) Mean dSI ± SD (n = 2 technical replicates) from one representative donor. B, percent insulin secretion was calculated in each condition and compared to the vehicle group which was set to 100%. One-way ANOVA analysis with Dunnett’s multiple comparisons: G16.7 (F(3, 12) = 10.21, p = 0.98 for 0.01 μM, p = 0.04 for 0.1 μM, and p = 0.002 for 1 μM compared to vehicle); G16.7 + Tolb (F(3, 12) = 14.49, p = 0.77 for 0.01 μM, p = 0.01 for 0.1 μM, and p = 0.001 for 1 μM compared to vehicle). Data represent mean ± SD from 4 independent donors compared to vehicle. C, Perifusion of primary isolated BWS-HI human islets to assess insulin release with treatment of CRN02481 (500 nM) in response to glucose ramp (0–25 mM) and KCl (Two-way ANOVA with Tukey’s: (F(1, 95) = 146.3, p < 0.0001). D, percent insulin release for BWS-HI (Patient #1) human islet perifusion. E, Perifusion of primary isolated KATP-HI human islets (Patient #2) to assess insulin release with treatment of CRN02481 (500 nM) in response to AAM ramp (0–12 mM) and KCl (Two-way ANOVA with Tukey’s: (F(1, 95) = 119.4, p < 0.0001). F, percent insulin release for KATP-HI Patient #2 perifusion. G, Perifusion of primary isolated KATP-HI human islets (Patient #3) (Two-way ANOVA with Tukey’s: (F(1, 95) = 163.5, p < 0.0001). H, percent insulin release for KATP-HI perifusion (Patient #3). (Compared to vehicle: ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001). Data represent mean ± SD compared to vehicle.
Table 3.
Clinical characteristics of HI islets donors
| Subject characteristics | HI patient #1 | HI patient #2 | HI patient #3 |
|---|---|---|---|
| Gender | Male | Male | Male |
| Ethnicity | Caucasian | Black | Caucasian |
| Age (y) | 0.18 | 0.16 | 0.1 |
| Weight-for-length | 68 percentile | 82 percentile | 78 percentile |
| HbA1C | 4.8 | 4.7 | ND |
Discussion
The only FDA-approved treatment for congenital hyperinsulinism is diazoxide, a potent KATP channel opener. Unfortunately, diazoxide is ineffective for the treatment of the most common and severe form of hyperinsulinism, KATP-HI. Therefore, the discovery of a therapy that bypasses the canonical KATP channel mechanism is critical for the treatment of KATP-HI and other diazoxide-unresponsive cases, as well as represents a potential alternative to diazoxide treatment. The pulsatile synchronicity shared between insulin and somatostatin presents a unique regulatory relationship and presents an opportunity to harness the inhibitory regulation of somatostatin on insulin secretion. Importantly, somatostatin receptor signaling effects occur late in the insulin secretion pathway and are downstream of known HI-causing mutations, therefore a potent somatostatin agonist should be effective against all forms of HI.
Previous studies have identified the ability of somatostatin to decrease the amplitude of insulin pulses while having no effect on the pulse cycles (40, 41). Additionally, somatostatin abrogates insulin secretion by binding to and activating SST receptors and inhibitory signaling action on adenylate cyclase followed by a decrease in cAMP or activation of calcineurin (42, 43, 44). Therefore, somatostatin is an attractive potential therapy for HI due to its insulin secretion inhibitory abilities involving a mechanism distinct from the KATP channel. Unfortunately, the use of SS14 is severely hampered by a short half-life in circulation. To counter the limiting short half-life of endogenous somatostatin, stabilized somatostatin peptide analogs such as octreotide and lanreotide were generated. These peptides primarily target SST2 with subnanomolar activity, but still have additional potency at the SST5 and SST3 receptors (Table 1). Both octreotide and lanreotide are limited by potentially harmful side effects linked to potent SST2 affinity, such as glucagon suppression and the observation of necrotizing enterocolitis, as well as a requirement for parenteral administration (6, 7). Pasireotide is a second-generation synthetic polypeptide analog of somatostatin, which boasts a 40-fold higher affinity for SST5 than octreotide but maintains a comparable affinity for SST2 and also targets the SST3 receptor (Table 1 and (45)). Interestingly, pasireotide causes less inhibition of glucagon secretion through SST2 than first-generation somatostatin analogs but does not completely prevent it (46). A single case report describes the successful use of pasireotide to control persistent hypoglycemia of an adult with hyperinsulinemic hypoglycemia (47). However, a more recent case report using pasireotide for the treatment of a severe KATP-HI case indicates that while no side effects were observed, both short- and long-acting pasireotide injections were insufficient to maintain glycemic control (48).
In this study, pure lines of WT (C57BL/6J) and Sur1−/− male mice were used for experiments. While we acknowledge these limitations, it is important to note that in vitro and in vivo data in this study including perifusion, fasting plasma glucose, plasma insulin, glucose tolerance, and insulin-to-glucose ratios, were comparable to that we have seen previously using littermate controls (32, 49). The Sur1−/− mouse model of KATP-HI exhibits a fasting hypoglycemia phenotype similar to the human phenotype (50), moreover, the islet phenotype demonstrates the same characteristics as human islets isolated from infants with hyperinsulinism due to inactivating KATP channel mutations: high cytosolic calcium concentration and altered fuel-stimulated insulin secretion with lack of response to stimulation with glucose and increased amino-acid stimulated insulin secretion (32, 51). The altered fuel-stimulated insulin secretion explains the in vivo phenotype of impaired glucose tolerance and protein-induced hypoglycemia (33, 34). Importantly, we have used the Sur1−/− mouse model for proof-of-concept studies for the development of other therapeutic approaches (49, 52, 53), which have been successfully translated to studies in affected patients (54, 55). Only male mice were used for this study, but we do not expect to see a different response in female Sur1−/− mice since they also exhibit the same phenotype as male mice (fasting hypoglycemia, glucose intolerance). An SST5 agonist that has moved forward into clinical studies has addressed this limitation (56). Sur1−/− mice and WT mice received CRN02481 orally at a dose of 30 mg/kg based on a short-term dose-finding study where the effect of different doses on fasting glucose was evaluated (57).
Selective nonpeptide agonists of somatostatin receptors are the next evolution of somatostatin-related treatment for HI, which allows for more specific SST receptor selectivity and, importantly, the ability to be dosed orally. Our characterization of CRN02481, an SST5 selective nonpeptide agonist, discovered after a medicinal chemistry campaign aimed to find SST5 agonists (31), has clearly demonstrated its ability to significantly decrease insulin secretion similar to SS14 and its analogs and to be more potent and effective than diazoxide in suppressing tolbutamide-stimulated insulin secretion in healthy human islets. CRN02481 also prevents fasting hypoglycemia in an in vivo model of KATP-HI and more importantly it exhibited anti-secretory effects on insulin in islets from HI patients. The high selectivity of CRN02481 for SST5 reduces the likelihood of having unspecific actions or an effect on glucagon secretion and other potential side effects of SST2 activation. Our own unpublished data indicates that another very specific SST5 agonist, similar to CRN02481 and equally potent, does not have any effect on the dynamic secretion of glucagon in response to arginine in isolated healthy human islets from eight independent donors. These results show the clear potential of SST5 selective agonists to be developed as a future therapy for patients with diazoxide unresponsive KATP-HI as well as have broad potential for other patients with HI.
Experimental procedures
CRN02481 compound details
CRN02481, 3-{4-[(3S)-3-aminopyrrolidin-1-yl]-5-(4-methyl-1H-1,3-benzodiazol-2-yl)pyridin-3-yl-5-fluorobenzonitrile was discovered, synthesized, and characterized as described in detail in (31). It possesses good stability when incubated with both human or rat liver microsome preparations, and exhibits good oral bioavailability in preclinical species, exhibiting a 32% oral bioavailability in rats and a 55% oral bioavailability in dogs (58).
Inhibition of stimulated cAMP production by CRN02481 action at the human and mouse SST receptors
SST subtypes are Gi/o-coupled GPCRs that inhibit adenylyl cyclase (AC) activity, resulting in a decrease of intracellular cyclic AMP (cAMP) upon activation by the agonist. We used homogeneous time-resolved fluorescence technology (HRTF cAMP kit, Cisbio) to evaluate agonist potency at various SSTR subtypes by measuring intracellular cAMP concentrations.
CHO cells stably expressing one of the human or mouse SSTR subtypes were grown and treated as described in detail in Zhao et al (31). In brief, cells cultured for 4 days in a 96-well plate format were treated with NKH477, a soluble forskolin analog that induces the production of cAMP, plus several concentrations of SS14, Octreotide, Lanreotide, Pasireotide or CRN02481, in assay buffer for 20 min at 37 °C. The final concentration of agonists ranges from 0 to 10000 nM. cAMP levels were measured after lysis following the instructions provided by Cisbio. The time-resolved fluorescent signal was read in a multi-plate reader and cAMP concentrations calculated by regression to a standard curve. Agonists potency (EC50) was determined by plotting agonist concentration vs cAMP levels and using standard curve-fitting methods. All data manipulations were performed using GraphPad Prism v6 or v7 (GraphPad, San Diego. CA)
Animal studies
WT and Sur1−/− mice studies were conducted at the Children’s Hospital of Philadelphia and approved by the Institutional Animal Care and Use Committee (IACUC).
The generation and genotyping of Sur1−/− mice were previously described (59). Mice are maintained in our mouse colony in a C57Bl/6 genetic background. Male, 8 to 10 weeks old, Sur1−/− mice were generated via Sur1−/− male x Sur1−/− female crosses and used in all experiments. Mice were maintained on a 12:12-h light-dark cycle and were fed a standard rodent chow diet (LabDiet, Picolab rodent diet 5053).
WT C57BL/6J male mice were acquired from Jackson laboratories, (Bar Harbor, ME) at 8 to 10 weeks of age. Mice were allowed to acclimate to a 12:12-h light-dark cycle, housing humidity and temperature for at least 72 h prior to initiation of the study. Mice were group-housed and maintained on a standard rodent diet (LabDiet, Picolab rodent diet 5053). All animals were provided free access to drinking water.
Fasting evaluation
Sur1−/− mice and WT mice received CRN02481 orally at a dose of 30 mg/kg or an equal volume of vehicle (25 mM Citrate Buffer, pH 3) after an overnight fast (16–18 h). This dose was selected based on a short-term dose-finding study where the effect of different doses on fasting glucose was evaluated (57). A copy of the poster can be found here: https://crinetics.com/pipeline/crn04777-oral-sst5-agonist-congenital-hyperinsulinism-congenital-hi/). Plasma glucose and β-hydroxybutyrate levels (BHB) were assessed at baseline (0 h), 1, and 2 h after treatment by a portable glucose meter (Stat Strip Xpress Glu, Nova biomedical) and Ketone meter (Nova Max plus, Nova biomedical). Blood was obtained from a tail nick. Blood was collected for measurement of plasma insulin levels using Mouse Ultrasensitive Insulin ELISA kit (Catalog # 80-Insmsu-E10, Alpco, RRID:AB_2792981).
Glucose tolerance test
Sur1−/− mice and WT mice received CRN02481 orally at a dose of 30 mg/Kg or an equal volume of vehicle (25 mM Citrate Buffer, pH 3) after an overnight fast (16 h). One hour after administration of CRN02481 or vehicle, dextrose (2 g/kg) was given by i.p. injection. Plasma glucose concentrations were measured at baseline and every 30 min for 2 h. Blood was also obtained at baseline (0 h), 30 min, and 60 min for measurement of plasma insulin by ELISA (Catalog # 80-Insmsu-E10, Alpco).
Pancreatic islet batch incubations, perifusions, and intracellular calcium imaging
Human HI islets were isolated from fresh pancreata from surgical specimens from neonates procured through an institutional review board–approved protocol at the Congenital Hyperinsulinism Center at the Children’s Hospital of Philadelphia (Table 3). The pancreas was injected with collagenase (Sigma-Aldrich) and digested. Islets were handpicked under microscopy and cultured in RPMI-1640 medium containing 5 mM glucose for 2 to 3 days prior to perifusion studies. Sur1−/− and WT mouse (8–16 weeks old) islets were digested (Collagenase P, Roche), isolated, and then cultured for 72 h in RPMI 1640 (GIBCO 11879) medium supplemented with 10 mM glucose, 10% fetal bovine serum, 10% glutamine, and 10% penicillin/streptomycin at 37 °C in a 5% CO2, 95% air-humidified incubator.
For static incubations, 4 to 5 replicates with 5 islets each were treated with CRN02481 (100 nM) or vehicle (DMSO) and glucose or physiological amino acid mix (AAM) for 1.5 h and then the supernatant was collected to assess secreted insulin by Homogeneous Time Resolved Fluorescence (HTRF) (Catalog # 62IN1PEH, CisBio, RRID:AB2890910). The physiological mixture of 20 amino acids was previously described (51). For perifusion, 300 cultured (mouse or human) islets were loaded onto a nylon filter in a chamber and perifused with Krebs-Ringer bicarbonate buffer (KRBB) (115 mmol/liter NaCl, 24 mmol/liter NaHCO3, 5 mmol/liter KCl, 1 mmol/liter MgCl2, 2.5 mmol/liter CaCl2, 10 mm HEPES, pH 7.4) with 0.25% bovine serum albumin at a flow rate of 1 ml/min. During perifusion islets were treated with vehicle (DMSO) or CRN02481 (500 nM) or vehicle at time 0 and then stimulated with a glucose ramp (3–25 mM) or AAM ramp (0–12 mM) for 40 min followed by a 20 min wash in KRBB and finally KCl (30 mM) (20 min). Fractions were collected at a rate of 1 ml/min and were assessed for secreted insulin or glucagon (Catalog # 62CGLPEG, CisBio, RRID:AB_2936335) by HTRF. For intracellular Ca2+ measurement, islets were pre-incubated with the Fura 2 fluorescent probe, treated with CRN02481 (500 nM) or vehicle, and then exposed to increasing concentrations of glucose or AAM. Measurement of intracellular Ca2+ was calculated as the ratio of the excitation of Fura 2 at 334 and 390 nm.
Human pancreatic islets, obtained from Prodo Labs, (Aliso Viejo. CA) were cultured in PIM(S) media supplemented with PIM(ABS), PIM(3X), and PIM(G) (Prodo Labs) following the manufacturer indications. Perifusion experiments were performed using the PERI5 perifusion system (Biorep Technologies) with islets from several independent healthy donors (Table 2). Briefly, human islets were equilibrated with low glucose (3 mM) for 74 min, followed by high glucose (16.7 mM) for 21 min, and high glucose (16.7 mM) plus tolbutamide (100 μM) for another 14 min in the presence or absence of increasing concentrations of CRN02481 (10 nM, 100 nM, and 1000 nM). Perifusion ended with low glucose (3 mM) for 14 min. Fractions were collected at 4 °C at a rate of 100 μl/min and insulin was measured using a commercially available ELISA kit (Catalog # 10–1132–01, Mercodia, RRID:AB_2783838). The dynamic stimulation index was calculated by dividing the insulin concentration in response to any given stimulus at any time by the average basal insulin level which was calculated as the mean insulin levels during the last 10 min of the equilibration period. The area under the curve (AUC) during CRN02481 treatments was calculated and compared to the average AUC of the vehicle group which was set at 100%. All data manipulations were performed using GraphPad Prism v8 or v9 (GraphPad, San Diego, CA, RRID:SCR_002798).
Statistics
Statistical analyses were performed on Excel or GraphPad Prism software v8 or v9. Results are presented as mean ± standard deviation (SD). The level of significance was set at p < 0.05. For multiple measurements, data were analyzed using 2-way ANOVA Repeated Measures, Tukey’s multiple comparison test. Single time endpoints data were analyzed using a one-way ANOVA Test. Differences were considered significant at p < 0.05.
Data availability
All the data resulting from these studies are contained within the manuscript or available upon request to corresponding author: Diva D. De Leon at deleon@chop.edu.
Supporting information
This article contains supporting information.
Conflict of interest
P. A., E. R. B. and S. F. B work for Crinetic Pharmaceuticals. D. D. D. L. has received consulting fees from Crinetics Pharmaceuticals, Zealand Pharma, Hanmi Pharmaceutical, Eiger Biopharmaceuticals, and Heptares Therapeutics. D. D. D. L. has received research funding from Tiburio Therapeutics, and Twist Pharma for studies not included in this manuscript.
Acknowledgments
We thank Nicolai Doliba and Andrea Rozo for their support and expertise in completing the calcium imaging experiments.
Author contributions
C. J. investigation, formal analysis, and writing – original manuscript; J. C. investigation, writing – review and editing; P. A. investigation, writing – review and editing; E. R. B. formal analysis, investigation, writing – review and editing; S. F. B. formal analysis, writing – review and editing; D. D. D. L investigation, writing – review and editing.
Funding and additional information
This work was supported in part by University of Pennsylvania Diabetes Research Center NIH grant P30-DK-19525. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Reviewed by members of the JBC Editorial Board. Edited by Mike Shipston
Supporting information
References
- 1.Lord K., Radcliffe J., Gallagher P.R., Adzick N.S., Stanley C.A., De Leon D.D. High risk of Diabetes and neurobehavioral deficits in individuals with surgically treated hyperinsulinism. J. Clin. Endocrinol. Metab. 2015;100:4133–4139. doi: 10.1210/jc.2015-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avatapalle H.B., Banerjee I., Shah S., Pryce M., Nicholson J., Rigby L., et al. Abnormal neurodevelopmental outcomes are common in children with transient congenital hyperinsulinism. Front. Endocrinol. (Lausanne) 2013;4:60. doi: 10.3389/fendo.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Snider K.E., Becker S., Boyajian L., Shyng S.L., MacMullen C., Hughes N., et al. Genotype and phenotype correlations in 417 children with congenital hyperinsulinism. J. Clin. Endocrinol. Metab. 2013;98:E355–363. doi: 10.1210/jc.2012-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kapoor R.R., Flanagan S.E., Arya V.B., Shield J.P., Ellard S., Hussain K. Clinical and molecular characterisation of 300 patients with congenital hyperinsulinism. Eur. J. Endocrinol. 2013;168:557–564. doi: 10.1530/EJE-12-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saint-Martin C., Arnoux J.B., de Lonlay P., Bellanne-Chantelot C. KATP channel mutations in congenital hyperinsulinism. Semin. Pediatr. Surg. 2011;20:18–22. doi: 10.1053/j.sempedsurg.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Laje P., Halaby L., Adzick N.S., Stanley C.A. Necrotizing enterocolitis in neonates receiving octreotide for the management of congenital hyperinsulinism. Pediatr. Diabetes. 2010;11:142–147. doi: 10.1111/j.1399-5448.2009.00547.x. [DOI] [PubMed] [Google Scholar]
- 7.McMahon A.W., Wharton G.T., Thornton P., De Leon D.D. Octreotide use and safety in infants with hyperinsulinism. Pharmacoepidemiol. Drug Saf. 2017;26:26–31. doi: 10.1002/pds.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adzick N.S., De Leon D.D., States L.J., Lord K., Bhatti T.R., Becker S.A., et al. Surgical treatment of congenital hyperinsulinism: results from 500 pancreatectomies in neonates and children. J. Pediatr. Surg. 2019;54:27–32. doi: 10.1016/j.jpedsurg.2018.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lord K., Dzata E., Snider K.E., Gallagher P.R., De Leon D.D. Clinical presentation and management of children with diffuse and focal hyperinsulinism: a review of 223 cases. J. Clin. Endocrinol. Metab. 2013;98:E1786–1789. doi: 10.1210/jc.2013-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gahete M.D., Cordoba-Chacon J., Duran-Prado M., Malagon M.M., Martinez-Fuentes A.J., Gracia-Navarro F., et al. Somatostatin and its receptors from fish to mammals. Ann. N. Y Acad. Sci. 2010;1200:43–52. doi: 10.1111/j.1749-6632.2010.05511.x. [DOI] [PubMed] [Google Scholar]
- 11.Patel Y.C. Molecular pharmacology of somatostatin receptor subtypes. J. Endocrinol. Invest. 1997;20:348–367. doi: 10.1007/BF03350317. [DOI] [PubMed] [Google Scholar]
- 12.Raulf F., Perez J., Hoyer D., Bruns C. Differential expression of five somatostatin receptor subtypes, SSTR1-5, in the CNS and peripheral tissue. Digestion. 1994;55:46–53. doi: 10.1159/000201201. [DOI] [PubMed] [Google Scholar]
- 13.Strowski M.Z., Blake A.D. Function and expression of somatostatin receptors of the endocrine pancreas. Mol. Cell Endocrinol. 2008;286:169–179. doi: 10.1016/j.mce.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Ludvigsen E., Olsson R., Stridsberg M., Janson E.T., Sandler S. Expression and distribution of somatostatin receptor subtypes in the pancreatic islets of mice and rats. J. Histochem. Cytochem. 2004;52:391–400. doi: 10.1177/002215540405200310. [DOI] [PubMed] [Google Scholar]
- 15.Mitra S.W., Mezey E., Hunyady B., Chamberlain L., Hayes E., Foor F., et al. Colocalization of somatostatin receptor sst5 and insulin in rat pancreatic beta-cells. Endocrinology. 1999;140:3790–3796. doi: 10.1210/endo.140.8.6937. [DOI] [PubMed] [Google Scholar]
- 16.Dournaud P., Gu Y.Z., Schonbrunn A., Mazella J., Tannenbaum G.S., Beaudet A. Localization of the somatostatin receptor SST2A in rat brain using a specific anti-peptide antibody. J. Neurosci. 1996;16:4468–4478. doi: 10.1523/JNEUROSCI.16-14-04468.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kimura N., Schindler M., Kasai N., Kimura I. Immunohistochemical localization of somatostatin receptor type 2A in rat and human tissues. Endocr. J. 2001;48:95–102. doi: 10.1507/endocrj.48.95. [DOI] [PubMed] [Google Scholar]
- 18.Schindler M., Sellers L.A., Humphrey P.P., Emson P.C. Immunohistochemical localization of the somatostatin SST2(A) receptor in the rat brain and spinal cord. Neuroscience. 1997;76:225–240. doi: 10.1016/s0306-4522(96)00388-0. [DOI] [PubMed] [Google Scholar]
- 19.Rossowski W.J., Coy D.H. Specific inhibition of rat pancreatic insulin or glucagon release by receptor-selective somatostatin analogs. Biochem. Biophys. Res. Commun. 1994;205:341–346. doi: 10.1006/bbrc.1994.2670. [DOI] [PubMed] [Google Scholar]
- 20.Fagan S.P., Azizzadeh A., Moldovan S., Ray M.K., Adrian T.E., Ding X., et al. Insulin secretion is inhibited by subtype five somatostatin receptor in the mouse. Surgery. 1998;124:254–258. [PubMed] [Google Scholar]
- 21.Strowski M.Z., Parmar R.M., Blake A.D., Schaeffer J.M. Somatostatin inhibits insulin and glucagon secretion via two receptors subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology. 2000;141:111–117. doi: 10.1210/endo.141.1.7263. [DOI] [PubMed] [Google Scholar]
- 22.Strowski M.Z., Kohler M., Chen H.Y., Trumbauer M.E., Li Z., Szalkowski D., et al. Somatostatin receptor subtype 5 regulates insulin secretion and glucose homeostasis. Mol. Endocrinol. 2003;17:93–106. doi: 10.1210/me.2001-0035. [DOI] [PubMed] [Google Scholar]
- 23.Kumar U., Sasi R., Suresh S., Patel A., Thangaraju M., Metrakos P., et al. Subtype-selective expression of the five somatostatin receptors (hSSTR1-5) in human pancreatic islet cells: a quantitative double-label immunohistochemical analysis. Diabetes. 1999;48:77–85. doi: 10.2337/diabetes.48.1.77. [DOI] [PubMed] [Google Scholar]
- 24.Berk S.C., Rohrer S.P., Degrado S.J., Birzin E.T., Mosley R.T., Hutchins S.M., et al. A combinatorial approach toward the discovery of non-peptide, subtype-selective somatostatin receptor ligands. J. Comb. Chem. 1999;1:388–396. doi: 10.1021/cc990017h. [DOI] [PubMed] [Google Scholar]
- 25.Rohrer S.P., Birzin E.T., Mosley R.T., Berk S.C., Hutchins S.M., Shen D.M., et al. Rapid identification of subtype-selective agonists of the somatostatin receptor through combinatorial chemistry. Science. 1998;282:737–740. doi: 10.1126/science.282.5389.737. [DOI] [PubMed] [Google Scholar]
- 26.Rohrer S.P., Schaeffer J.M. Identification and characterization of subtype selective somatostatin receptor agonists. J. Physiol. Paris. 2000;94:211–215. doi: 10.1016/s0928-4257(00)00215-1. [DOI] [PubMed] [Google Scholar]
- 27.Singh V., Grotzinger C., Nowak K.W., Zacharias S., Goncz E., Pless G., et al. Somatostatin receptor subtype-2-deficient mice with diet-induced obesity have hyperglycemia, nonfasting hyperglucagonemia, and decreased hepatic glycogen deposition. Endocrinology. 2007;148:3887–3899. doi: 10.1210/en.2006-1659. [DOI] [PubMed] [Google Scholar]
- 28.Zambre Y., Ling Z., Chen M.C., Hou X., Woon C.W., Culler M., et al. Inhibition of human pancreatic islet insulin release by receptor-selective somatostatin analogs directed to somatostatin receptor subtype 5. Biochem. Pharmacol. 1999;57:1159–1164. doi: 10.1016/s0006-2952(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 29.Brunicardi F.C., Atiya A., Moldovan S., Lee T.C., Fagan S.P., Kleinman R.M., et al. Activation of somatostatin receptor subtype 2 inhibits insulin secretion in the isolated perfused human pancreas. Pancreas. 2003;27:e84–89. doi: 10.1097/00006676-200311000-00019. [DOI] [PubMed] [Google Scholar]
- 30.Cejvan K., Coy D.H., Efendic S. Intra-islet somatostatin regulates glucagon release via type 2 somatostatin receptors in rats. Diabetes. 2003;52:1176–1181. doi: 10.2337/diabetes.52.5.1176. [DOI] [PubMed] [Google Scholar]
- 31.Zhao J., Wang S., Hee Kim S., Han S., Rico-Bautista E., Sturchler E., et al. Discovery of 4-(3-aminopyrrolidinyl)-3-aryl-5-(benzimidazol-2-yl)-pyridines as potent and selective SST5 agonists for the treatment of congenital hyperinsulinism. Bioorg. Med. Chem. Lett. 2022;71 doi: 10.1016/j.bmcl.2022.128807. [DOI] [PubMed] [Google Scholar]
- 32.Li C., Buettger C., Kwagh J., Matter A., Daikhin Y., Nissim I.B., et al. A signaling role of glutamine in insulin secretion. J. Biol. Chem. 2004;279:13393–13401. doi: 10.1074/jbc.M311502200. [DOI] [PubMed] [Google Scholar]
- 33.Grimberg A., Ferry R.J., Jr., Kelly A., Koo-McCoy S., Polonsky K., Glaser B., et al. Dysregulation of insulin secretion in children with congenital hyperinsulinism due to sulfonylurea receptor mutations. Diabetes. 2001;50:322–328. doi: 10.2337/diabetes.50.2.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fourtner S.H., Stanley C.A., Kelly A. Protein-sensitive hypoglycemia without leucine sensitivity in hyperinsulinism caused by K(ATP) channel mutations. J. Pediatr. 2006;149:47–52. doi: 10.1016/j.jpeds.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 35.Hsu B.Y., Kelly A., Thornton P.S., Greenberg C.R., Dilling L.A., Stanley C.A. Protein-sensitive and fasting hypoglycemia in children with the hyperinsulinism/hyperammonemia syndrome. J. Pediatr. 2001;138:383–389. doi: 10.1067/mpd.2001.111818. [DOI] [PubMed] [Google Scholar]
- 36.Singh V., Brendel M.D., Zacharias S., Mergler S., Jahr H., Wiedenmann B., et al. Characterization of somatostatin receptor subtype-specific regulation of insulin and glucagon secretion: an in vitro study on isolated human pancreatic islets. J. Clin. Endocrinol. Metab. 2007;92:673–680. doi: 10.1210/jc.2006-1578. [DOI] [PubMed] [Google Scholar]
- 37.Elizabeth Rico P., Zhao J., Chen M., Kusnetzow A.K., Zhu Y.F., Betz S.F. Selective somatostatin 5 (SST5) and somatostatin 2 (SST2) nonpeptide agonists potently suppress glucose- and tolbutamide-stimulated dynamic insulin secretion from isolated human islets. J. Endocr. Soc. 2021;5:A325. [Google Scholar]
- 38.Kalish J.M., Boodhansingh K.E., Bhatti T.R., Ganguly A., Conlin L.K., Becker S.A., et al. Congenital hyperinsulinism in children with paternal 11p uniparental isodisomy and Beckwith-Wiedemann syndrome. J. Med. Genet. 2016;53:53–61. doi: 10.1136/jmedgenet-2015-103394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munns C.F., Batch J.A. Hyperinsulinism and beckwith-wiedemann syndrome. Arch. Dis. Child. Fetal. Neonatal. Ed. 2001;84:F67–69. doi: 10.1136/fn.84.1.F67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hellman B., Salehi A., Gylfe E., Dansk H., Grapengiesser E. Glucose generates coincident insulin and somatostatin pulses and antisynchronous glucagon pulses from human pancreatic islets. Endocrinology. 2009;150:5334–5340. doi: 10.1210/en.2009-0600. [DOI] [PubMed] [Google Scholar]
- 41.Porksen N., Munn S.R., Steers J.L., Veldhuis J.D., Butler P.C. Effects of somatostatin on pulsatile insulin secretion: elective inhibition of insulin burst mass. Am. J. Physiol. 1996;270:E1043–1049. doi: 10.1152/ajpendo.1996.270.6.E1043. [DOI] [PubMed] [Google Scholar]
- 42.Heisler S., Reisine T.D., Hook V.Y., Axelrod J. Somatostatin inhibits multireceptor stimulation of cyclic AMP formation and corticotropin secretion in mouse pituitary tumor cells. Proc. Natl. Acad. Sci. U. S. A. 1982;79:6502–6506. doi: 10.1073/pnas.79.21.6502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jakobs K.H., Aktories K., Schultz G. A nucleotide regulatory site for somatostatin inhibition of adenylate cyclase in S49 lymphoma cells. Nature. 1983;303:177–178. doi: 10.1038/303177a0. [DOI] [PubMed] [Google Scholar]
- 44.Renstrom E., Ding W.G., Bokvist K., Rorsman P. Neurotransmitter-induced inhibition of exocytosis in insulin-secreting beta cells by activation of calcineurin. Neuron. 1996;17:513–522. doi: 10.1016/s0896-6273(00)80183-x. [DOI] [PubMed] [Google Scholar]
- 45.Hofland L.J., van der Hoek J., Feelders R., van Aken M.O., van Koetsveld P.M., Waaijers M., et al. The multi-ligand somatostatin analogue SOM230 inhibits ACTH secretion by cultured human corticotroph adenomas via somatostatin receptor type 5. Eur. J. Endocrinol. 2005;152:645–654. doi: 10.1530/eje.1.01876. [DOI] [PubMed] [Google Scholar]
- 46.de Heide L.J., Laskewitz A.J., Apers J.A. Treatment of severe postRYGB hyperinsulinemic hypoglycemia with pasireotide: a comparison with octreotide on insulin, glucagon, and GLP-1. Surg. Obes. Relat. Dis. 2014;10:e31–33. doi: 10.1016/j.soard.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 47.Schwetz V., Horvath K., Kump P., Lackner C., Perren A., Forrer F., et al. Successful medical treatment of adult nesidioblastosis with pasireotide over 3 Years: a case report. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000003272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mooij C.F., Tacke C.E., van Albada M.E., Barthlen W., Bikker H., Mohnike K., et al. First report on the use of pasireotide in a case of severe congenital hyperinsulinism due to a homozygous ABCC8 mutation. Ann Pediatr. Endocrinol. Metab. 2021;26:278–283. doi: 10.6065/apem.2142010.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Leon D.D., Li C., Delson M.I., Matschinsky F.M., Stanley C.A., Stoffers D.A. Exendin-(9-39) corrects fasting hypoglycemia in SUR-1-/- mice by lowering cAMP in pancreatic beta-cells and inhibiting insulin secretion. J. Biol. Chem. 2008;283:25786–25793. doi: 10.1074/jbc.M804372200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Leon D.D., Stanley C.A. Determination of insulin for the diagnosis of hyperinsulinemic hypoglycemia. Best Pract. Res. Clin. Endocrinol. Metab. 2013;27:763–769. doi: 10.1016/j.beem.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li C., Ackermann A.M., Boodhansingh K.E., Bhatti T.R., Liu C., Schug J., et al. Functional and metabolomic consequences of KATP channel inactivation in human islets. Diabetes. 2017;66:1901–1913. doi: 10.2337/db17-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel P., Charles L., Corbin J., Goldfine I.D., Johnson K., Rubin P., et al. A unique allosteric insulin receptor monoclonal antibody that prevents hypoglycemia in the SUR-1(-/-) mouse model of KATP hyperinsulinism. MAbs. 2018;10:796–802. doi: 10.1080/19420862.2018.1457599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soundarapandian M.M., Juliana C.A., Chai J., Haslett P.A., Fitzgerald K., De Leon D.D. Activation of Protein Kinase A (PKA) signaling mitigates congenital hyperinsulinism associated hypoglycemia in the Sur1-/- mouse model. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calabria A.C., Li C., Gallagher P.R., Stanley C.A., De Leon D.D. GLP-1 receptor antagonist exendin-(9-39) elevates fasting blood glucose levels in congenital hyperinsulinism owing to inactivating mutations in the ATP-sensitive K+ channel. Diabetes. 2012;61:2585–2591. doi: 10.2337/db12-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stefanovski D., Vajravelu M.E., Givler S., De Leon D.D. Exendin-(9-39) effects on glucose and insulin in children with congenital hyperinsulinism during fasting and during a meal and a protein challenge. Diabetes Care. 2022;45:1381–1390. doi: 10.2337/dc21-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferrara-Cook C.T., Luo R., Torre E.D.l., Wang Y., Betz S.F., Madan A., et al. ENDO2022; Atlanta, GA: 2022. ABSTRACT PSUN304) CRN04777, an Oral, Nonpeptide SST5-Selective Somatostatin Agonist Dose Dependently Suppresses Basal and Stimulated Insulin Secretion. [Google Scholar]
- 57.Emmanuel Sturchler P., Fowler M., Athanacio J., Kredel T., Johns M., Zhao J., et al. SAT-169 selective nonpeptide somatostatin subtype 5 (sst5) agonists suppress glucose- and sulfonylurea-induced insulin secretion in rats. J. Endocr. Soc. 2019;3 doi: 10.1210/js.2019-SAT-169. [DOI] [Google Scholar]
- 58.Markison S K.A., Shin R., Sturchler A., Zhao J., Zhu Y.F., Struthers S. The Endocrine Society's 100th Annual Meeting and Expo; Chicago, IL: 2018. Selective Somatostatin Subtype 5 (Sst5) Agonists for the Treatment of Hyperinsulinism: Orally-Bioavailable Small Molecules Suppress Insulin and Rescue Glyburide-Induced Hypoglycemia. ABSTRACT; pp. i463–i464. : 39, i463–i464. [Google Scholar]
- 59.Shiota C., Larsson O., Shelton K.D., Shiota M., Efanov A.M., Hoy M., et al. Sulfonylurea receptor type 1 knock-out mice have intact feeding-stimulated insulin secretion despite marked impairment in their response to glucose. J. Biol. Chem. 2002;277:37176–37183. doi: 10.1074/jbc.M206757200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data resulting from these studies are contained within the manuscript or available upon request to corresponding author: Diva D. De Leon at deleon@chop.edu.