Abstract
Background
Trauma to the pancreas is rare but associated with significant morbidity. Currently available management guidelines are based on low-quality evidence and data on long-term outcomes is lacking. This study aimed to evaluate clinical characteristics and patient-reported long-term outcomes for pancreatic injury.
Methods
A retrospective cohort study evaluating treatment for pancreatic injury in 11 centers across 5 European nations over >10 years was performed. Data relating to pancreatic injury and treatment were collected from hospital records. Patients reported quality of life (QoL), changes to employment and new or ongoing therapy due to index injury.
Results
In all, 165 patients were included. The majority were male (70.9%), median age was 27 years (range: 6–93) and mechanism of injury predominantly blunt (87.9%). A quarter of cases were treated conservatively; higher injury severity score (ISS) and American Association for the Surgery of Trauma (AAST) pancreatic injury scores increased the likelihood for surgical, endoscopic and/or radiologic intervention. Isolated, blunt pancreatic injury was associated with younger age and pancreatic duct involvement; this cohort appeared to benefit from non-operative management. In the long term (median follow-up 93; range 8–214 months), exocrine and endocrine pancreatic insufficiency were reported by 9.3% of respondents. Long-term analgesic use also affected 9.3% of respondents, with many reported quality of life problems (QoL) potentially attributable to side-effects of opiate therapy. Overall, impaired QoL correlated with higher ISS scores, surgical therapy and opioid analgesia on discharge.
Conclusions
Pancreatic trauma is rare but can lead to substantial short- and long-term morbidity. Near complete recovery of QoL indicators and pancreatic function can occur despite significant injury, especially in isolated, blunt pancreatic injury managed conservatively and when early weaning off opiate analgesia is achieved.
Keywords: Pancreatic trauma, Clinical course, Interdisciplinary treatment, Long-term outcomes, Quality of life
1. Background
Traumatic injury of the pancreas is rare, often associated with high morbidity and mortality and can be challenging to manage. Its incidence is around 0.3–4.7% of all abdominal traumata and it is frequently associated with injuries to other abdominal organs or major vasculature [[1], [2], [3], [4], [5]]. Mechanism of injury vary with geography; penetrating injuries (high-energy projectile or bladed weapons) predominate in the United States of America or South Africa, whereas in series from the United Kingdom, Sweden or Japan high-energy blunt trauma from motor-vehicle accidents prevails [3,[6], [7], [8], [9], [10], [11], [12]].
In hemodynamically unstable patients, assessment of pancreatic trauma is best conducted through systematic diagnostic laparotomy with exposure of the pancreato-duodenal complex. In stable patients, contrast-enhanced CT [13] is the diagnostic modality of choice. MRI with hepatobiliary contrast, endoscopic ultrasound or endoscopic retrograde cholangiopancreatography (ERCP) can play a role in the diagnosis of a major pancreatic duct disruption in the context of parenchymal injury and ERCP may support non-surgical treatment options in the form of endoscopically-placed stents [14]. Pancreatic injury identified in this way is most commonly classified by location and severity of tissue damage using the score developed by the American Association for the Surgery of Trauma (AAST) and range from minor contusions or superficial lacerations (Grade I) to disruption of the pancreatic head (Grade V) [15].
The management of patients with pancreatic trauma follows the principles of management of any patient with abdominal trauma. Due to its relative low frequency, however, specific treatment of the pancreatic injury itself is largely based on expert opinion rather than strong evidence [16,17]. For example, the World Society of Emergency Surgery (WSES) and AAST recently published guidelines on the management of duodeno-pancreatic and extrahepatic biliary trauma where 10 out of 14 recommendations on the management of pancreatic trauma were grade 2C (weak recommendation, low-quality evidence) [13]. In particular, there is controversy regarding the optimal treatment of stable patients with isolated pancreatic injury with large registry studies from the UK, Sweden and USA reaching differing conclusions [2,3,18]; these differences might be reconciled by differing injury patterns in these areas. Furthermore, advances in endoscopy have revolutionized care for pancreatic disease [19], yet its infrequent nature has meant adoption in pancreatic trauma has remained center- and clinician-dependent.
As pancreatic trauma often occurs in young adults, the potential for life-changing morbidity is significant.
In this study we sought to collate clinical characteristics, current management and patient-reported long-term outcomes for pancreatic injury in a multi-national, multi-center cohort of patients from Northern Europe. Next, we aimed to identify factors associated with better long-term outcomes following pancreatic trauma.
2. Methods
2.1. Study design and population
The checklist for reporting of observational studies by the STROBE (Strengthening the Reporting of Observational studies in Epidemiology) initiative and statement was adhered to Ref. [20].
Anticipating a low volume of cases, all patients who sustained pancreatic trauma in a period commencing from July 01, 1999 (SWE), March 01, 2001 (GER), January 01, 2007 (UK, NED and FIN), and completing on the September 30, 2017 (for all countries) were identified at 11 centers across 5 European nations (Finland, Sweden, Germany, the Netherlands and the United Kingdom). Lead centers in each country sought and received ethical approval for the study from their respective institutional ethics review boards; if required by national legislation, agreement of local ethics committees at additional participating centers was obtained (FIN: Ethics Committee Tampere University Hospital R17036; SWE: Regional Ethics Committee Lund 2017/248; NED: Ethics Committee VU University Medical Center 2017.200; GER: Ethics Committee Albert-Ludwigs-Universität Freiburg 104–17 and Ethics Committee Ludwig-Maximilians-Universität München 18–004; UK: North West – Liverpool Central Research Ethics Committee 17/NW/0626). The study was registered in the German Clinical Trials Register (DRKS00014705). The study was carried out following all guidance from the respective national research bodies in accordance with the principles set out in the Declaration of Helsinki.
Participants were identified by searching for diagnostic codes (ICD-10 S36.2, S30.1 and S39) in hospital registries and screened for inclusion. Inclusion criteria included recent trauma (within 72 h of first presentation at center), with injury of the pancreas confirmed intraoperatively, by cross-sectional or specialist imaging. While patients may have been under 18 years of age at time of injury, all were 18 years or older when they were approached for questionnaire-participation in this study.
Participants were contacted by mail and/or telephone and informed consent was obtained. Participants were asked to complete the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaires QLQ-C30 and QLQ-PAN26, validated for quality-of-life assessments in cancer and pancreatic cancer respectively, as well as a third short questionnaire designed specifically for this project, comprised of simple questions related to subjective recovery, working status, additional hospital admissions, weight loss and medication (Supplementary File 1). Official EORTC translations for the respective questionnaires were used, and the authors generated their own translations for the project-specific questionnaire, so that patients answered all questions in their native language.
2.2. Data collection
Data relating to the initial injury and treatment episodes were collected retrospectively from hospital records, exclusively by personnel with MD education background. Data relating to on-going reviews, complications of pancreatic injury and further treatments were ascertained from hospital records and patient questionnaires. Patients were asked directly about treatment they may have received relating to their injury at a different hospital/from their primary care physician.
Data collected from hospital records included demographics (age, sex, ASA-score, BMI), pre-existing medical conditions (in particular pre-existing exocrine or endocrine pancreatic disease, defined as requirement of enzyme replacement therapy or anti-diabetic medication/insulin), injury specifics (mechanism and timing of injury, injury severity score – ISS, number of organs/extremities injured), pancreatic injury (peak amylase/lipase, diagnostic modality, anatomy of injury, AAST severity score), treatment (conservative, interventional radiology, endoscopy, surgery), and treatment outcomes (mortality, length of stay, new onset endocrine/exocrine failure or regular use of analgesia). Data collected from patients directly included current quality of life, changes to employment due to injury, new or ongoing medical/surgical therapy due to injury.
2.3. Quality of life analysis
EORTC QLQ questionnaires were evaluated according to validated EORTC instructions, combining two or more specific questions into ‘symptom’ and ‘function’ categories and scaling the result to generate a percentage. As the QLQ-PAN26 questionnaire is a relatively new addition to the EORTC collection, Cronbach's alpha was calculated as a measure of inter-question reliability for questions within categories.
Symptoms were further categorized into: (1) gastro-intestinal symptoms (GIS), (2) pain/anxiety/depression (PAD) and (3) body image/sexuality (BIS). Participants scoring greater than 20% in any sub-category from ‘nausea/vomiting’, ‘loss of appetite’, ‘digestive symptoms’, ‘altered bowel habits’, ‘constipation’, ‘diarrhea’, or ‘bloating/cramping’ were deemed to have GI symptoms. Those scoring greater than 20% in any of ‘pain’, ‘pancreatic pain’, ‘fatigue’, ‘insomnia’ or ‘anxiety/depression’ were deemed to have PAD symptoms and those scoring below 80% in any of ‘physical functioning’, ‘body image’, or ‘sexuality’ were deemed to have BIS symptoms. Participants were described as ‘minimally symptomatic’, if they scored 20% or less in GIS or PAD and more than 80% in BIS symptom sub-categories. Demographic, injury- and treatment-related data were compared between symptomatic and minimally symptomatic participants.
2.4. Statistical analysis
Continuous, non-parametric data was described using the median and inter-quartile or full ranges as indicated and categorical data using absolute numbers and percentages. Comparisons were made using Chi squared, student's T or Mann-Whitney U tests as appropriate. SPSS v24 (IBM Corp) and Prism 9.0 (GraphPad Software) were used for statistical analysis. Excel v16.43 (Microsoft) and Prism 9.0 were used to generate figures.
3. Results
3.1. Clinical characteristics of patients with pancreatic trauma
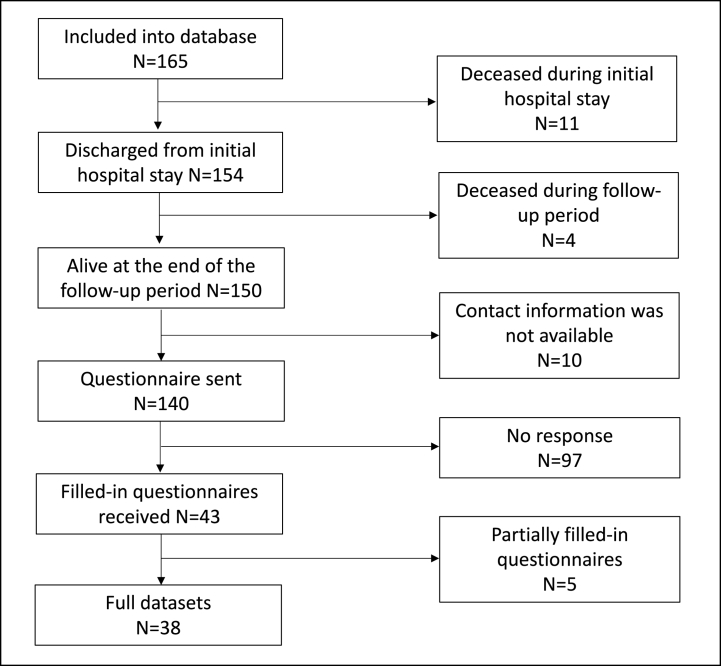
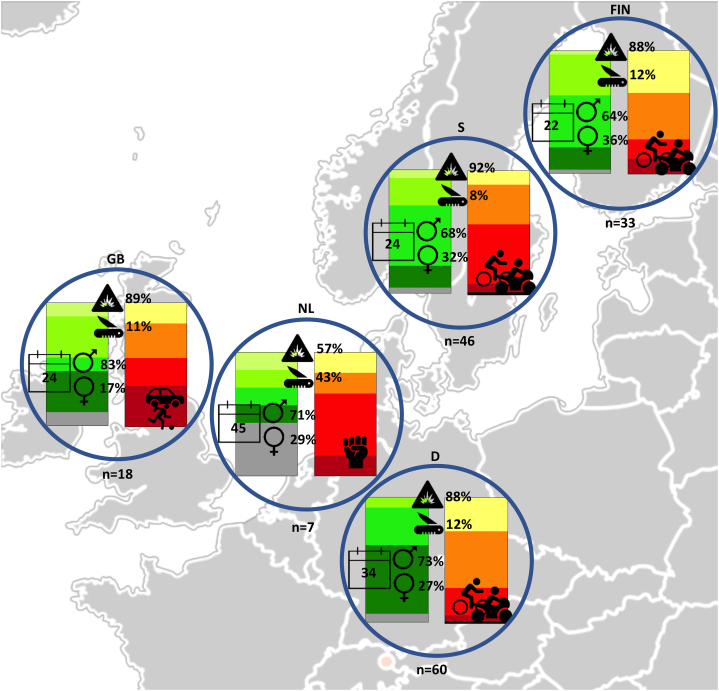
165 patients across all participating institutions met the inclusion criteria and were eligible for further analysis. The median age was 27 (range 6–93) years, and the majority of patients were male (n = 117; 70.9%). None of the patients suffered from exocrine insufficiency prior to the injury and 4/165 (2.4%) had pre-existing diabetes mellitus. Most pancreatic injury was a result of blunt abdominal trauma (n = 145; 87.9%), with mechanisms often involving high-energy motor-vehicle accidents, falls from large heights or direct blows to the abdomen (e.g. kicked by a horse, playing soccer or during martial arts contest). High injury severity scores (ISS ≥16) were seen in 123 (74.4%) of patients in our cohort, however most had only moderate pancreatic injury (AAST Grade II or III in 105 patients; 63.6%). Fig. 1 serves as a flow-chart for overview of data availability for all patients and analyses included in this study. Fig. 2 provides a visualization of demographic and injury characteristics in participating centers. Additional detailed characteristics can be found in Table 1, Table 2, Table 3.
Fig. 1.
Flow-chart visualizing data availability for all patients included in the study.
Fig. 2.
Demographics by country.
Circles display the median age (calendar), proportion of gender, proportion of blunt (explosion icon) and penetrating (knife icon) injuries as well as the leading etiology by country. Stacked bar charts represent the proportion of cases by severity of injury (Left: injury severity score (ISS): light green - > dark green - > grey = minor (≤8) - > severe (25–49) - > critical (≥50); Right: pancreatic Injury scale: yellow - > orange - > red - > dark red - > black = I - > V). FIN: Finland; S: Sweden; GB: Great Britain; NL: Netherlands; D: Germany. Icons are stock images taken from MS PowerPoint for Mac v16.44.
Table 1.
Baseline characteristics of conservatively versus non-conservatively treated patients.
| Conservative (n = 40) | Non-conservative (n = 122) | P-value | |
|---|---|---|---|
| Age y, median (range) | 31 (7–89) | 25 (6–93) | 0.830§ |
| Sex | |||
| Male | 25 (62.5%) | 90 (73.8%) | 0.173† |
| Female | 15 (37.5%) | 32 (26.2%) | |
| Endocrine insufficiency | |||
| Type I DM | 0 (0%) | 0 (0%) | 0.307† |
| Type II DM | 2 (5%) | 2 (1.6%) | |
| None | 38 (95%) | 117 (95.9%) | |
| Unknown | 0 (0%) | 3 (2.5%) | |
| ASA-score | |||
| I | 24 (60.0%) | 76 (62.3%) | 0.352† |
| II | 12 (30.0%) | 21 (17.2%) | |
| III | 3 (7.5%) | 11 (9.0%) | |
| IV | 1 (2.5%) | 6 (4.9%) | |
| V | 0 (0.0%) | 5 (4.1%) | |
| Unknown | 0 (0.0%) | 3 (2.5%) | |
| Injury mechanism | |||
| Blunt | 39 (97.5%) | 104 (85.2%) | 0.037†* |
| Penetrating | 1 (2.5%) | 18 (14.8%) | |
| ISS severity score | |||
| Minor (≤8) | 3 (7.5%) | 4 (3.3%) | 0.001†* |
| Moderate (9–15) | 17 (42.5%) | 15 (12.3%) | |
| Serious (16–24) | 12 (30%) | 44 (36.1%) | |
| Severe (25–49) | 5 (12.5%) | 49 (40.2%) | |
| Critical (50–74) | 2 (5.0%) | 5 (4.1%) | |
| Maximum (75) | 1 (2.5%) | 4 (3.3%) | |
| Unknown | 0 (0.0%) | 1 (0.8%) | |
| Pancreatic injury scale | |||
| I | 17 (42.5%) | 19 (15.6%) | <0.001†* |
| II | 19 (47.5%) | 38 (31.1%) | |
| III | 2 (5%) | 44 (36.1%) | |
| IV | 2 (5%) | 15 (12.3%) | |
| V | 0 (0%) | 2 (1.6%) | |
| Unknown | 0 (0%) | 4 (3.3%) | |
| Anatomic location | |||
| Head | 5 (12.5%) | 9 (7.4%) | 0.313† |
| Uncinate process | 3 (7.5%) | 3 (2.5%) | |
| Neck | 2 (5.0%) | 5 (4.1%) | |
| Body | 4 (10.0%) | 21 (17.2%) | |
| Tail | 7 (17.5%) | 38 (31.1%) | |
| Head and body | 6 (15.0%) | 20 (16.4%) | |
| Body and tail | 5 (12.5%) | 13 (10.7%) | |
| Unknown | 8 (20%) | 13 (10.7%) | |
* Indicates significant P-value, § Independent sample T-test, † Chi Square test.
Table 2.
Demographics and outcomes of patients with isolated trauma to the pancreas versus polytrauma including the pancreas.
| Isolated Pancreas Trauma (n = 47) | Polytrauma (n = 101) | Injury pattern data unavailable (n = 17) | P-value | |
|---|---|---|---|---|
| Age y, median (IQR) | 20 (15) | 32 (29) | 59 (21) | <0.001& |
| Sex | ||||
| Male | 32 (68.1%) | 73 (72.3%) | 12 (70.6%) | 0.698† |
| Female | 15 (31.9%) | 28 (27.7%) | 5 (29.4%) | |
| ASA | ||||
| I | 39 (83.0%) | 57 (56.4%) | 5 (29.4%) | 0.010†* |
| II | 4 (8.5%) | 23 (22.8%) | 7 (41.2%) | |
| III | 3 (6.4%) | 9 (8.9%) | 2 (11.8%) | |
| IV | 0 | 7 (6.9%) | 0 | |
| V | 0 | 5 (5.0%) | 0 | |
| Unknown | 1 (2.1%) | 0 | 3 (17.6%) | |
| Mechanism | ||||
| Blunt | 45 (95.7%) | 87 (86.1%) | 13 (76.5%) | 0.080†* |
| Penetrating | 2 (4.3%) | 14 (13.9%) | 3 (17.6%) | |
| Unknown | 0 | 0 | 1 | |
| Etiology | ||||
| Assault | 9 (19.1%) | 14 (13.9%) | 3 (18.8%) | 0.028†* |
| Bike/motorbike | 16 (34.0%) | 27 (26.7%) | 4 (25.0%) | |
| Car accident | 3 (6.4%) | 22 (21.8%) | 4 (25.0%) | |
| Pedestrian accident | 0 | 3 (3.0%) | 0 | |
| Sport trauma | 13 (27.7%) | 12 (11.9%) | 2 (12.5%) | |
| Fall from height | 5 (10.6%) | 11 (10.9) | 2 (12.5%) | |
| Suicide attempt | 0 | 7 (6.9%) | 0 | |
| Other/unknown | 1 (2.1%) | 5 (5.0%) | 1 (6.3%) | |
| Injury Severity Score (ISS) (median/IQR) | 18 (7) | 26 (16) | 27 (16) | <0.001&* |
| Referral to center | ||||
| Yes | 33 (70.2%) | 39 (38.6%) | 8 (47.1%) | <0.001†* |
| No | 10 (21.3%) | 60 (59.4%) | 8 (47.1%) | |
| Unknown | 4 (8.5%) | 2 (2%) | 1 (5.9%) | |
| Peak serum amylase (mean factor of upper limit of normal/SEM) | 15.2 (2.55) | 5.63 (0.729) | 6.47 (3.27) | 0.001§* |
| Pancreatic injury scale | ||||
| I | 7 (14.9%) | 26 (25.7%) | 3 (17.6%) | 0.161† |
| II | 13 (27.7%) | 37 (36.6%) | 9 (52.9%) | |
| III | 19 (40.4%) | 24 (23.8%) | 3 (17.6%) | |
| IV | 7 (14.9%) | 10 (9.9%) | 0 | |
| V | 1 (2.1%) | 1 (1.0%) | 0 | |
| Unknown | 0 | 3 (3.0%) | 2 (11.8%) | |
| Anatomic location of pancreatic injury | ||||
| Head | 4 (8.5%) | 10 (9.9%) | 1 (5.9%) | 0.551† |
| Uncinate process | 2 (4.3%) | 4 (4.0%) | 0 | |
| Neck | 2 (4.3%) | 5 (5.0%) | 0 | |
| Body | 7 (14.9%) | 16 (15.8%) | 2 (11.8%) | |
| Tail | 8 (17%) | 33 (32.7%) | 4 (23.5%) | |
| Head and body | 11 (23.4%) | 14 (13.9%) | 1 (5.9%) | |
| Body and tail | 7 (14.9) | 11 (10.9%) | 0 | |
| Unknown | 6 (12.8%) | 8 (7.9%) | 9 (52.9%) | |
| Pancreatic duct involvement | ||||
| No | 19 (40.4%) | 61 (62.2%) | 11 (68.8%) | 0.030†* |
| Suspected | 9 (19.1%) | 16 (16.3%) | 1 (6.3%) | |
| Confirmed | 19 (40.4%) | 21 (21.4%) | 4 (25%) | |
| Treatment | ||||
| Conservative | 13 (28.3%) | 22 (21.8%) | 5 (33.3%) | 0.392† |
| Non-conservative | 33 (71.7%) | 79 (78.2%) | 10 (66.7%) | |
| Number of interventions (median/IQR) | 2 (2) | 1 (2) | 2 (3) | 0.681& |
| Length of hospital stay d, median (IQR) | 18 (22) | 19 (26) | 22 (18) | 0.655& |
| Length of critical care stay d, median (IQR) | 2 (8) | 5 (12) | 8 (27) | 0.045&* |
| Opioid analgesia on discharge | ||||
| Yes | 11 (26.8%) | 27 (31.8%) | 5 (29.4%) | 0.572† |
| No | 30 (73.2%) | 58 (68.2%) | 12 (70.6%) | |
| Endocrine insufficiency on discharge | ||||
| Yes | 1 (2.1%) | 6 (6.6%) | 13 (81.3%) | 0.004†* |
| No | 40 (85.1%) | 85 (93.4%) | 1 (6.3%) | |
| Unknown | 6 (12.8%) | 0 | 2 (12.5%) | |
| Exocrine insufficiency on discharge | ||||
| Yes | 5 (10.6%) | 10 (11%) | 0 | 0.216† |
| No | 37 (78.7%) | 78 (85.7%) | 14 (87.5) | |
| Unknown | 5 (10.6%) | 3 (3.3%) | 2 (12.5%) | |
Comparisons are between the two known groups only, using the & Mann-Whitney U Test, § Independent sample T-test, † Chi Square test. * Indicates significant P-value.
Table 3.
Characteristics of patients with and without long-term symptoms.
| Pain/Anxiety/Depression | GI Symptoms | Body Image/Sexuality | Minimally Symptomatic | P-value | |
|---|---|---|---|---|---|
| Total number | 12/38 (31.6%) | 14/38 (36.8%) | 12/38 (31.6%) | 18/38 (47.4%) | |
| Age y, median (IQR) | 22 (17) | 22 (12) | 39 (28) | 21 (31) | 0.409& |
| Sex | |||||
| Male | 9/23 (39.1%) | 8/23 (34.8%) | 6/23 (26.1%) | 11/23 (47.8%) | 0.604& |
| Female | 3/15 (20%) | 6/15 (40%) | 6/15 (40%) | 7/15 (46.7%) | |
| Time since injury mo., median (IQR) | 130 (92) | 104 (105) | 130 (85) | 101 (73) | 0.438& |
| ISS median (IQR) | 22 (15) | 21 (14) | 29 (14) | 18 (7) | 0.014&* |
| Pancreatic injury scale | |||||
| I | 1/6 | 1/6 | 1/6 | 5/6 | 0.419& |
| II | 6/15 | 4/15 | 8/15 | 5/15 | |
| III | 4/14 | 7/14 | 3/14 | 7/14 | |
| IV | 1/2 | 2/2 | 0/2 | 0/2 | |
| V | 0/0 | 0/0 | 0/0 | 0/0 | |
| Pancreatic duct involvement | 8/20 | 10/20 | 5/20 | 8/20 | 0.620† |
| Treatment | |||||
| Operative | 9/25 | 13/25 | 10/25 | 8/25 | 0.016†* |
| Non-operative | 3/13 | 1/13 | 2/13 | 10/13 | |
| Length of hospital stay d, median (IQR) | 15 (41) | 20 (19) | 18 (25) | 17 (22) | 0.290& |
| Length of critical care stay d, median (IQR) | 4 (29) | 5 (16) | 5 (19) | 4 (9) | 0.406& |
| Regular opioid analgesia on discharge | 5/15 | 8/15 | 8/15 | 2/15 | 0.006†* |
As patients may fall into several of the three symptom categories, comparisons are between ‘symptomatic’ and ‘minimally symptomatic’ groups; * Indicates significant P-value; & Mann-Whitney U Test, † Chi Square test.
3.2. Injury and treatment characteristics of conservatively and non-conservatively managed patients
40 patients (24.2%) were managed conservatively. Of the remaining 122 patients who received interventions such as endoscopy, surgery and/or interventional radiology procedures, 106 (89.3%) underwent surgery, including 43 (35.2%) instances of pancreatic resection (partial or total). The remaining operations were tube drainage or other, non-pancreatic abdominal surgeries. The median number of interventions was 1, with up to 8 interventions reported in some patients. Overall inpatient mortality was 6.7% (Supplementary Table 4 + 5).
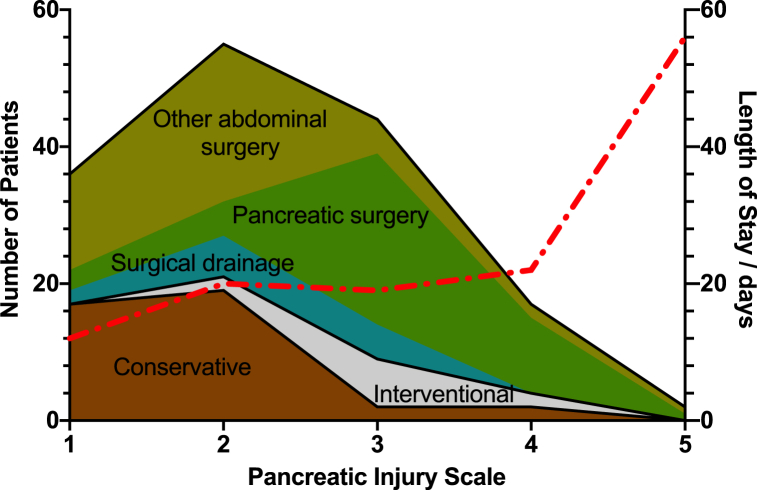
Table 1 summarizes the characteristics of patients treated conservatively and non-conservatively (additional details in Supplementary Table 5): there was no statistically significant difference in age, gender or ASA. While blunt trauma was the predominant mechanism of injury, penetrating trauma was notably almost exclusively managed operatively (17/18; 94.4%; p = 0.037), often due to associated hollow viscus or vascular injuries. Conservatively managed patients had lower ISS, with the modal category being ‘moderate – ISS 9–15’, compared to ‘severe – ISS 25–29’ for patients treated non-conservatively (p = 0.001). Pancreatic injury scale values were also lower in those treated conservatively, with 36 (90%) in categories I or II of the AAST pancreatic injury scale, compared to 57 (46.7%) of those managed non-conservatively (p < 0.001, see also Fig. 3). There was no difference in the anatomical distribution of pancreatic injury between the two groups (p = 0.313). The median length of hospital-stay correlated positively with pancreatic injury scale values, although this correlation did not reach statistical significance (Fig. 3; Spearman r = 0.90, p = 0.083).
Fig. 3.
Management strategies according to pancreatic injury severity.
Stacked area chart displaying the management strategies of the number of patients (left Y-axis) according to the pancreatic injury scale (X-axis), where brown = conservative management, grey = non-surgical intervention and green = surgery (shades of green indicate type of surgery). Median length of hospital stay according to pancreatic injury scale is overlayed as dashed red line and measured on the right Y-axis.
62.3% of patients were discharged on regular analgesia, often involving both weak and strong opioids (pre-injury: 1.2%) and there was evidence of exocrine and endocrine insufficiency in 9.7% and 5.2% of patients respectively (pre-injury: 0% and 2.4%). This improved somewhat to 12% taking regular analgesia, 4% of exocrine and 4.6% of endocrine insufficiency after a median 6 months of follow-up of 121 (73.3%) patients (Supplementary Table 4).
3.3. Characteristics of patients with isolated pancreatic injury versus polytrauma
47 patients with isolated pancreatic trauma were compared to 101 with pancreatic injury as part of polytrauma, defined as injury to multiple organ systems (see Supplementary Table 3; data on clearly defined injury patterns could not be recovered in retrospect for the remaining 17 patients). The findings are summarized in Table 2. Patients with isolated pancreatic trauma were younger (median age 20 versus 32 years; p < 0.001) and fitter (83% versus 56.4% ASA 1; p = 0.01) than those involved in polytrauma. There was no statistically significant difference in the sex distribution across groups. There were very few (n = 2; 4.3%) cases of penetrating trauma in the isolated pancreatic injury group, compared to 14 (13.9%; p = 0.08) in the polytrauma group, although this did not reach statistical significance. There was, however, a higher incidence of low-velocity sports and bike injuries in the isolated pancreatic injury group. Although the distribution of AAST pancreatic injury categories was similar, patients with isolated pancreatic injury had much higher levels of serum amylase at presentation (15.2, compared to 5.63 times upper limit of normal; p = 0.001) which correlated with higher rates of pancreatic ductal injury (suspected or confirmed in 59.5% versus 37.7%; p = 0.030). Although a true comparison in imaging modality was not possible (as many cases were imaged with either MRI or CT, but not both), pancreatic ductal injury was identified more frequently by MRI than CT, although this difference was not statistically significant (OR 2.93; 95% CI 0.362–23.8; p = 0.293).
Treatment characteristics, median number of interventions and median length of hospital stay was similar across both groups, but patients with isolated pancreatic injury had a shorter critical care stay (median 2 versus 5 days; p = 0.045). There were no statistically significant differences in either opioid use (26.8% vs 31.8%; p = 0.572) or exocrine deficiency (10.6% vs 11%; p = 0.216) on discharge between patients with isolated pancreatic injury or pancreatic injury as part of polytrauma, however the rate of endocrine dysfunction was lower in the isolated pancreatic injury cohort (2.1% vs 6.6%; p = 0.004) and associated with resection of the pancreatic body/tail.
3.4. Long-term outcomes following pancreatic trauma
Of the 165 patients with pancreatic trauma, 25 were either deceased or no contact details were available for them or their next of kin. The remaining 140 patients were all sent quality of life and follow-up questionnaires, which were returned by 43 (30.7%) participants (38 completed the full set of questionnaires). There were no statistically significant differences in demographics, pattern/severity of injury or treatment in those who completed the questionnaires to those who did not (Supplementary Table 6).
The median time from injury to completion of the questionnaire was 93 (range 8–214) months. 27 (62.8%) participants stated they had completely recovered from their injury. 34 (87.2% when excluding retirement due to age) were in work and in 50% of the cases this was the same employment to before the injury. Only in one (5.9%) participant was the injury responsible for a change in employment. 15 (34.9%) of respondents were discharged from hospital with new medication following their injury (analgesia in 14 (93.3%) of those instances), reducing to 4 patients (26.7%; or 9.3% of all respondents) still taking pain medication relating to the injury at time of long-term follow-up. There were 4 (9.3%) cases of current endocrine insufficiency and 4 (9.3%) cases of ongoing pancreatic enzyme replacement therapy. Most respondents (n = 26; 60.5%) had not lost weight following the injury, but those that had, reported a median weight loss of 9 kg (Supplementary Table 7).
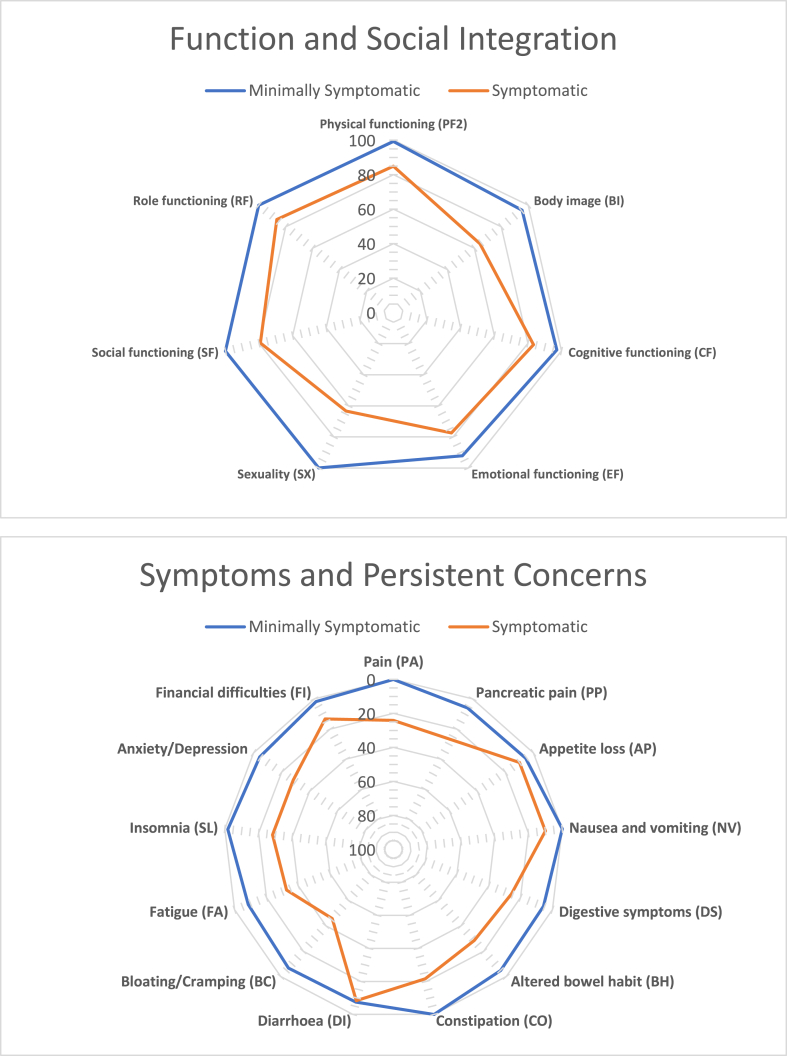
Eighteen out of 38 (47.4%) respondents reported only mild or no impairment to their quality of life. Symptoms relating to either the gastro-intestinal tract (GIS), feelings of pain/anxiety/depression (PAD) or reduced body image/sexual dysfunction (BIS) were reported by the remaining 20 respondents. We used a cut-off score of 20% for each of those symptom categories to discern mildly/minimally symptomatic and (relevantly) symptomatic resondents (see methods). Some patients reported concerns in more than one QoL area (Fig. 4). Table 3 summarizes the characteristics of patients with and without long-term symptoms. There were no significant differences in age or sex of symptomatic and non-symptomatic responders. Patients reporting impairment in the BIS category had a higher injury severity score than those with minimal symptoms (29 versus 18; p = 0.014). Pancreatic injury scale or pancreatic duct involvement did not significantly contribute to long-term symptoms. A higher proportion of patients in the symptomatic group had surgery for their initial injury (17/25, 68%) and most treated without surgery had no long-term symptoms (10/13, 76.9%; p = 0.016). Hospital and critical care stay did not correlate with long-term symptoms, however regular opioid use did. Interestingly, more respondents discharged on regular opioid analgesia reported ongoing problems with GIS (8/15) and BIS (8/15) than problems with PAD (5/15), with an odds ratio of 8.89 (95% CI 1.51–44.7; p = 0.007). Only 2/15 (13.3%) of respondents discharged on opioids following their injury reported no significant long-term symptoms. Similarly, those with long-term persistent symptoms (including non-pain related) were more likely to report taking regular opioid analgesia than those without (odds ratio 7.85, 95% CI 0.937–94.5; p = 0.043).
Fig. 4.
Patient-reported symptom and function indicators.
Radio charts of positive (Top panel: function; social integration) and negative (bottom panel: persistent symptoms/concerns) indicators of quality of life using the EORTCs QLQ-C30 and PAN-26 questionnaires. Minimally symptomatic (n = 18) patients are denoted by the blue line, symptomatic (n = 20) patients by the red line. Please note the inverse scale bar so that values closer to the center consistently indicate worse function or greater symptoms.
4. Discussion
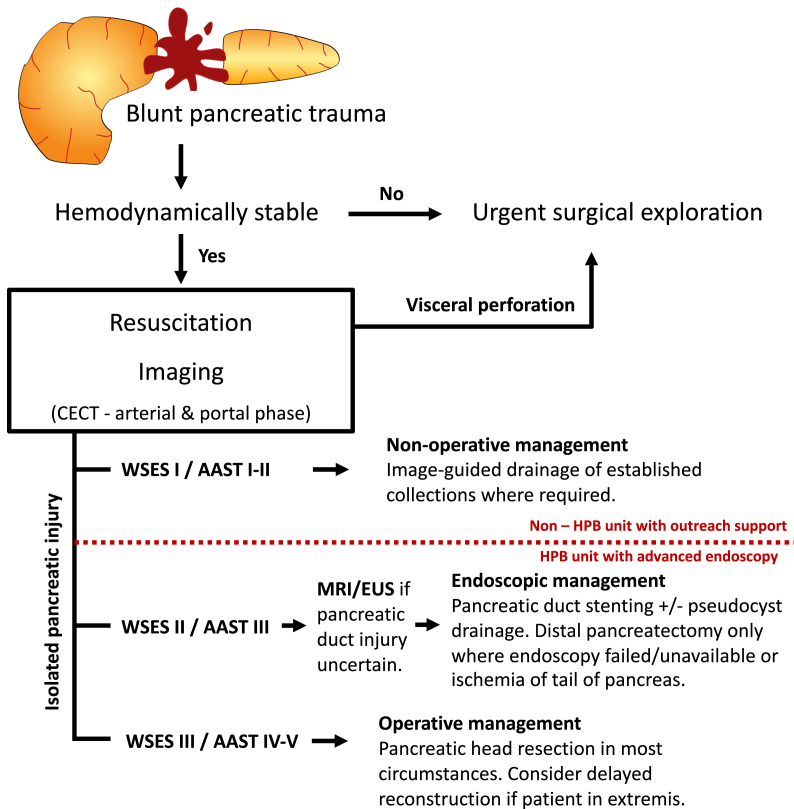
In this first multi-center, multi-national cohort across Northern Europe we confirm that injury to the pancreas is rare and can be associated with substantial short-term morbidity. In addition, we here report considerable patient and physician-reported long-term morbidity, but at the same time identify a significant proportion of patients to make a near complete recovery. Identification of this latter sub-group is of particular significance as affected patients are generally young adults and their injuries may have maximum impact on their education, professional and personal lives. Based on observed patterns of injury and treatment we highlight potential risk factors for worse long-term outcomes. Leaning on and complementing available guidelines, we also propose an algorithm (Fig. 5) dedicated to the management of blunt isolated pancreatic trauma.
Fig. 5.
Management flow-chart for blunt pancreatic trauma.
Proposed flow chart for the investigation and management of blunt pancreatic trauma. Abbreviations: CECT – contrast-enhanced computerized tomography; MRI – magnetic resonance imaging; EUS – endoscopic ultrasound; WSES – world society of emergency surgery; AAST – American Association for the Surgery for Trauma; HPB – hepatopancreatobiliary.
Patients who sustained pancreatic trauma in Northern Europe during the early 21st century form a relatively homogeneous group of predominantly young adult males, with little or no co-morbidity. 87.9% of pancreatic injuries in our cohort were the result of blunt trauma, either from direct blows to the abdomen (sports injuries sustained during games of soccer, martial arts or horse-riding or the classical ‘handle-bar’ injury), or as part of a multi-system polytrauma from road-traffic accidents. This is in stark contrast to series reported from the USA or South Africa [[6], [7], [8],21], where the proportions of blunt and penetrating injuries are reversed. As a result, we saw a large proportion of isolated pancreatic trauma without associated visceral or major vascular injury which is not usually seen with penetrating injuries where the blade/projectile crosses the abdominal cavity and/or retroperitoneum to cause pancreatic injury. Our sub-group was younger, fitter and had lower injury severity scores compared to those with multi-visceral injury. These patients were also more likely to have pancreatic duct disruption (ie AAST III), had a much higher serum amylase on admission (median 15× upper limit of normal) and had favorable short and long-term outcome, which appeared to correlate with non-operative management, with percutaneous/endoscopic drainage of fluid collections and/or placement of pancreatic duct stents. New onset diabetes in this sub-group only occurred after distal pancreatectomy. Furthermore, long-term adverse outcomes were associated with surgical management of the initial injury. Pancreatic injury AAST Grade III is the most debated clinical scenario concerning surgical vs. conservative/endoscopic management. In this context, while certainly only retrospective and of low evidence-level quality, our data is supportive of a non-surgical management where possible: for patients with low-velocity, blunt pancreatic injury with (proximal) duct disruption and without uncontrolled haemorrhage, hollow visceral perforation or other indication for immediate surgery.
33–53% of patients in our cohort were referred to a specialist pancreatic center for further management with the exception of the UK, where all but 1 patient had been referred to a specialist center. Although HPB care is centralized in the UK, this resulted from selection bias as only specialist pancreatic referral centers took part in the identification of patients. Inclusion of a large proportion of patients from specialist centers may bias our patient cohort towards the more severe end of the spectrum as low-grade injuries may have been managed in a non-specialist secondary care setting. On the other hand, optimal care with regards to availability of advanced endoscopy, interventional radiology and experience in pancreatic surgery is available only in specialist or tertiary centers. As almost no patients with minor parenchymal injury (AAST I) required specialist treatment and all made a full recovery; we propose this group could safely be managed in non-HPB, non-tertiary centers with remote specialist input/imaging review. AAST II graded injuries could be managed in non-HPB units but are likely to require specialist input for specific procedures (e.g.: percutaneous/endoscopic drainage) and/or management of associated injuries. We propose any patient with a pancreatic injury grade of III or greater should be managed in a specialist HPB unit with experience in, and access to advanced HPB endoscopy and surgery.
Persistent use of opiate analgesia was associated with 8 times greater odds of reporting long-term impairment to quality of life. While arguably this could be a result of the underlying significant injury, it is worth noting that many of the quality of life impairments relate to potential side-effects of opiate analgesia (i.e. constipation, nausea, bloating/cramping and insomnia) as opposed to persistent pain or functional impairment [22]. There is mounting evidence that a liberal approach to opioid prescription following surgery contributes to increased opioid use over longer periods of time [23,24] and efforts are underway to curtail opioid use and prescription across the US and Europe. While no causation can be determined from our dataset, the strong correlation between long-term opioid use and long-term (non-pain) symptoms would indicate the need to find alternative modes of pain control following pancreatic trauma. In light of the association with polytrauma, a multi-modal approach combining analgesia, physiotherapy and psychotherapy is likely to achieve optimal outcomes [25].
While our international, multicentric, long-term outcome study represents the largest of its kind from Northern Europe to date, its observational nature means data demonstrate association, not causation. The infrequent nature of pancreatic trauma, however, hampers prospective interventional trials. By identifying and reviewing every case of pancreatic trauma we ensured accuracy beyond what could be achieved through registry studies and inclusion of the QoL cohort allowed us to incorporate long-term follow-up in a way that is largely lacking in the literature. Although we had hoped for higher turn-around numbers, our questionnaire response rate of around 1/3 is in keeping with studies of health care satisfaction [26,27] and the demography of responders appears similar to non-responders (Supplementary Table 6) limiting participation bias. Nevertheless, response and recall bias cannot be excluded from our study, as the validated EORTC questionnaires have been dedicatedly developed for cancer patients and our own long-term questionnaire (Supplementary File 1) has not been validated. Data on exocrine or endocrine failure in our study is reliant on usage of pancreatic exocrine replacement therapy or diabetic medication rather than objective assessment of exocrine function. This represents a real-world compromise in data interpretation, although may under-represent the frequency of pancreatic insufficiency. Delayed deterioration of pancreatic function has been reported following surgery for chronic pancreatitis [28]. However, as pancreatic endocrine function would be expected to deteriorate over a >10 year period in any adult population, one needs to urge caution when interpreting these data. Lastly, treatment options have changed over the duration of the study. Advances in endoscopic ultrasound in particular over this time period mean that treatment options available to patients later in the cohort may have not been available to those early on.
5. Conclusion
Pancreatic trauma remains a rare, but significant injury. As it affects predominantly young adults it can lead to life-long morbidity with relevant cost implications for the individual and health-care systems alike. The nature of pancreas-associated complications and changes in available therapies over recent years, many of which are highly specialist and of limited availability, mean that data relating to pancreatic trauma is insufficiently captured in current national and international registries. Through careful collation of data across 5 European countries, we identified a sub-group of patients (isolated, blunt pancreatic injury) who likely benefit from less invasive therapy. We were also able to identify opiate analgesia as being associated with lower long-term quality of life indicators, especially those not related to pain. In order to be able to offer the best, personalized therapeutic approach to patients with this significant pathology, we propose the creation of an international, pancreas-specific trauma registry to register cases and their management, follow patients and track outcomes, and provide information and guidance to affected individuals, their family and treating physicians in the form of evidence-driven guidelines for management and follow-up.
Ethics approval and consent to participate
Lead centers in each country sought and received ethical approval for the study from their respective institutional ethics review boards; if required by national legislation, agreement of local ethics committees at additional participating centers was obtained (FIN: Ethics Committee Tampere University Hospital R17036; SWE: Regional Ethics Committee Lund 2017/248; NED: Ethics Committee VU University Medical Center 2017.200; GER: Ethics Committee Albert-Ludwigs-Universität Freiburg 104–17 and Ethics Committee Ludwig-Maximilians-Universität München 18–004; UK: North West – Liverpool Central Research Ethics Committee 17/NW/0626). The study was registered in the German Clinical Trials Register (DRKS00014705).
Funding
No specific funding has been received for this study.
Authors' contributions
Conceptualization: Meijer, Vaalavuo, Regnér, Ruess, Szatmary.
Data curation: Meijer, Vaalavuo, Regnér, Ruess, Szatmary, Sallinen, Lemma, Arnelo, Valente, Westermark, An, Moir, Irwin, Sutton, Biesel, Hopt, Fichtner-Feigl, Wittel, Weniger, Karle, Bloemers, Charnley.
Formal analysis: Meijer, Vaalavuo, Regnér, Ruess, Szatmary.
Funding acquisition: Not applicable.
Investigation: Meijer, Vaalavuo, Regnér, Ruess, Szatmary, Sallinen, Lemma, Arnelo, Valente, Westermark, An, Moir, Irwin, Sutton, Biesel, Hopt, Fichtner-Feigl, Wittel, Weniger, Karle, Bloemers, Charnley.
Methodology: Meijer, Vaalavuo, Regnér, Ruess, Szatmary.
Project administration: Meijer, Vaalavuo, Regnér, Ruess, Szatmary.
Visualization: Meijer, Vaalavuo, Regnér, Ruess, Szatmary.
Writing – original draft: Meijer, Vaalavuo, Regnér, Ruess, Szatmary.
Writing – review & editing: Sallinen, Lemma, Arnelo, Valente, Westermark, An, Moir, Irwin, Sutton, Biesel, Hopt, Fichtner-Feigl, Wittel, Weniger, Karle, Bloemers, Charnley.
Data availability statement
All data included in article and supplemental material are referenced in the article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank all individuals that contributed to this study. This effort was part of Pancreas 2000 (www.pancreas2000.org) – a post-graduate educational program supported by the European Pancreatic Club (EPC) and United European Gastroenterology (UEG) that prepares young gastroenterologists, surgeons, radiologists, and other physicians for a specialization in Pancreatology. We acknowledge support by the Open Access Publication Fund of the University of Freiburg.
Footnotes
on behalf of the PANIC (PANcreas Injury Cohort) Study Group within the Pancreas2000 network.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e17436.
Contributor Information
Dietrich A. Ruess, Email: dietrich.ruess@uniklinik-freiburg.de.
Peter Szatmary, Email: szatmary@liverpool.ac.uk.
Abbreviations
- AAST
American Association for the Surgery of Trauma
- ASA-score
American Society of Anesthesiology-score
- BIS
Body image/sexuality
- BMI
Body Mass Index
- CT
Computed Tomography
- EORTC
European Organization for Research and Treatment of Cancer
- ERCP
Endoscopic retrograde cholangiopancreatography
- FIN
Finland
- GER
Germany
- GI
Gastro-intestinal
- GIS
Gastro-intestinal symptoms
- HPB
Hepatopancreaticobiliary
- ISS
Injury Severity Score
- MRI
Magnetic resonance imaging
- NED
The Netherlands
- PAD
Pain/anxiety/depression
- QLQ
Quality of Life Questionnaires
- QoL
Quality of Life
- STROBE
Strengthening the Reporting of Observational studies in Epidemiology
- SWE
Sweden
- UK
United Kingdom
- USA
United States of America
- WSES
World Society of Emergency Surgery
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Rozich N.S., Morris K.T., Garwe T., Sarwar Z., Landmann A., Siems C.B., et al. Blame it on the injury: trauma is a risk factor for pancreatic fistula following distal pancreatectomy compared with elective resection. J. Trauma Acute Care Surg. 2019;87:1289–1300. doi: 10.1097/TA.0000000000002495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siboni S., Kwon E., Benjamin E., Inaba K., Demetriades D. Isolated blunt pancreatic trauma: a benign injury? J. Trauma Acute Care Surg. 2016;81:855–859. doi: 10.1097/TA.0000000000001224. [DOI] [PubMed] [Google Scholar]
- 3.O'Reilly D.A., Bouamra O., Kausar A., Malde D.J., Dickson E.J., Lecky F. The epidemiology of and outcome from pancreatoduodenal trauma in the UK, 1989-2013. Ann. R. Coll. Surg. Engl. 2015;97:125–130. doi: 10.1308/003588414X14055925060712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ragulin-Coyne E., Witkowski E.R., Chau Z., Wemple D., Ng S.C., Santry H.P., et al. National trends in pancreaticoduodenal trauma: interventions and outcomes. HPB (Oxford) 2014;16:275–281. doi: 10.1111/hpb.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Recinos G., DuBose J.J., Teixeira P.G., Inaba K., Demetriades D. Local complications following pancreatic trauma. Injury. 2009;40:516–520. doi: 10.1016/j.injury.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 6.Vasquez J.C., Coimbra R., Hoyt D.B., Fortlage D. Management of penetrating pancreatic trauma: an 11-year experience of a level-1 trauma center. Injury. 2001;32:753–759. doi: 10.1016/s0020-1383(01)00099-7. [DOI] [PubMed] [Google Scholar]
- 7.Krige J.E., Jonas E., Thomson S.R., Kotze U.K., Setshedi M., Navsaria P.H., et al. Resection of complex pancreatic injuries: benchmarking postoperative complications using the accordion classification. World J. Gastrointest. Surg. 2017;9:82–91. doi: 10.4240/wjgs.v9.i3.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krige J.E., Kotze U.K., Nicol A.J., Navsaria P.H. Isolated pancreatic injuries: an analysis of 49 consecutive patients treated at a level 1 trauma centre. J. Vis. Surg. 2015;152:349–355. doi: 10.1016/j.jviscsurg.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Krige J.E., Kotze U.K., Nicol A.J., Navsaria P.H. Morbidity and mortality after distal pancreatectomy for trauma: a critical appraisal of 107 consecutive patients undergoing resection at a level 1 trauma centre. Injury. 2014;45:1401–1408. doi: 10.1016/j.injury.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 10.Scollay J.M., Yip V.S., Garden O.J., Parks R.W. A population-based study of pancreatic trauma in scotland. World J. Surg. 2006;30:2136–2141. doi: 10.1007/s00268-006-0039-z. [DOI] [PubMed] [Google Scholar]
- 11.Antonacci N., Di Saverio S., Ciaroni V., Biscardi A., Giugni A., Cancellieri F., et al. Prognosis and treatment of pancreaticoduodenal traumatic injuries: which factors are predictors of outcome? J Hepatobiliary Pancreat Sci. 2011;18:195–201. doi: 10.1007/s00534-010-0329-6. [DOI] [PubMed] [Google Scholar]
- 12.Morita T., Takasu O., Sakamoto T., Mori S., Nakamura A., Nabeta M., et al. Long-term outcomes of pancreatic function following pancreatic trauma. Kurume Med. J. 2017;63:53–60. doi: 10.2739/kurumemedj.MS00001. [DOI] [PubMed] [Google Scholar]
- 13.Coccolini F., Kobayashi L., Kluger Y., Moore E.E., Ansaloni L., Biffl W., et al. Duodeno-pancreatic and extrahepatic biliary tree trauma: wses-aast guidelines. World J. Emerg. Surg. 2019;14:56. doi: 10.1186/s13017-019-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim S., Kim J.W., Jung P.Y., Kwon H.Y., Shim H., Jang J.Y., et al. Diagnostic and therapeutic role of endoscopic retrograde pancreatography in the management of traumatic pancreatic duct injury patients: single center experience for 34 years. Int. J. Surg. 2017;42:152–157. doi: 10.1016/j.ijsu.2017.03.054. [DOI] [PubMed] [Google Scholar]
- 15.Moore E.E., Cogbill T.H., Malangoni M.A., Jurkovich G.J., Champion H.R., Gennarelli T.A., et al. Organ injury scaling, ii: pancreas, duodenum, small bowel, colon, and rectum. J. Trauma. 1990;30:1427–1429. [PubMed] [Google Scholar]
- 16.Girard E., Abba J., Cristiano N., Siebert M., Barbois S., Letoublon C., et al. Management of splenic and pancreatic trauma. J. Vis. Surg. 2016;153:45–60. doi: 10.1016/j.jviscsurg.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Iacono C., Zicari M., Conci S., Valdegamberi A., De Angelis M., Pedrazzani C., et al. Management of pancreatic trauma: a pancreatic surgeon's point of view. Pancreatology. 2016;16:302–308. doi: 10.1016/j.pan.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Mohseni S., Holzmacher J., Sjolin G., Ahl R., Sarani B. Outcomes after resection versus non-resection management of penetrating grade iii and iv pancreatic injury: a trauma quality improvement (tqip) databank analysis. Injury. 2018;49:27–32. doi: 10.1016/j.injury.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 19.Vozzo C.F., Sanaka M.R. Endoscopic management of pancreaticobiliary disease. Surg. Clin. 2020;100:1151–1168. doi: 10.1016/j.suc.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 20.von Elm E., Altman D.G., Egger M., Pocock S.J., Gotzsche P.C., Vandenbroucke J.P., et al. The strengthening the reporting of observational studies in epidemiology (strobe) statement: guidelines for reporting observational studies. Int. J. Surg. 2014;12:1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 21.Mansfield N., Inaba K., Berg R., Beale E., Benjamin E., Lam L., et al. Early pancreatic dysfunction after resection in trauma: an 18-year report from a level i trauma center. J. Trauma Acute Care Surg. 2017;82:528–533. doi: 10.1097/TA.0000000000001327. [DOI] [PubMed] [Google Scholar]
- 22.Benyamin R., Trescot A.M., Datta S., Buenaventura R., Adlaka R., Sehgal N., et al. Opioid complications and side effects. Pain Physician. 2008;11:S105–S120. [PubMed] [Google Scholar]
- 23.Howard R., Fry B., Gunaseelan V., Lee J., Waljee J., Brummett C., et al. Association of opioid prescribing with opioid consumption after surgery in Michigan. JAMA Surg. 2019;154 doi: 10.1001/jamasurg.2018.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scully R.E., Schoenfeld A.J., Jiang W., Lipsitz S., Chaudhary M.A., Learn P.A., et al. Defining optimal length of opioid pain medication prescription after common surgical procedures. JAMA Surg. 2018;153:37–43. doi: 10.1001/jamasurg.2017.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manchikanti L., Singh V., Kaye A.D., Hirsch J.A. Lessons for better pain management in the future: learning from the past. Pain Ther. 2020;9:373–391. doi: 10.1007/s40122-020-00170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robb K.A., Gatting L., Wardle J. What impact do questionnaire length and monetary incentives have on mailed health psychology survey response? Br. J. Health Psychol. 2017;22:671–685. doi: 10.1111/bjhp.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Todd A.L., Porter M., Williamson J.L., Patterson J.A., Roberts C.L. Pre-notification letter type and response rate to a postal survey among women who have recently given birth. BMC Med. Res. Methodol. 2015;15:104. doi: 10.1186/s12874-015-0097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jalleh R.P., Williamson R.C. Pancreatic exocrine and endocrine function after operations for chronic pancreatitis. Ann. Surg. 1992;216:656–662. doi: 10.1097/00000658-199212000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data included in article and supplemental material are referenced in the article.