Abstract
Aims:
The objective of this study was to synthesize and validate a set of compounds that selectively inhibit mPGES-1, with the potential to be developed into a novel anti-inflammatory drug.
Methods:
The synthesized compounds were characterized using 1H NMR spectroscopy and LC–MS to confirm their structure. Cellular and enzymatic assays were used to demonstrate their inhibitory activity on prostaglandin E2 production.
Results:
Docking studies revealed that compounds containing fluoro-, chloro- and methyl- groups displayed strong inhibitory activity against prostaglandin E2. The inhibitory activity of synthesized trimethyl and trifluoro was further validated using enzymatic and cell migration assays.
Conclusion:
The findings demonstrated that the synthesized compounds possess significant potential as a new generation of nonsteroidal anti-inflammatory drugs that selectively target mPGES-1 with fewer side effects.
Keywords: anti-inflammation, COX-2, COX-2-10aa-mPGES-1, COX-2-10aa-PGIS, enzymelink, microsomal prostaglandin E2 synthase-1, mPGES-1 inhibitor, prostacyclin
Nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin, Advil, Motrin, Celebrex and others, are commonly used to effectively treat inflammation, pain and fever through the inhibition of COX-1 and/or COX-2. Their anti-inflammatory effects are a result of reducing proinflammatory prostaglandin E2 (PGE2), which is synthesized by the inducible COX-2 that is coupled to inducible mPGES-1 [1–6]. In the arachidonic acid (AA) metabolism pathway, COX-1 and -2 are upstream enzymes. They are also coupled to other downstream synthases to produce a variety of prostanoids such as prostacyclin (PGI2) synthase, which produces PGI2 and is involved in vascular protection through antiplatelet aggregation and vasodilation [7–10]; thromboxane A2 (TXA2) synthase, which produces TXA2, an endogenous anti-bleeding facto; and noninducible PGE2 synthase, which produces the basal-level PGE2 involved in gastrointestinal protection [7,11].
So far, only one of the three PGE2 synthases, the inducible mPGES-1, has been identified as directly related to pathogenic inflammation and pain [11–16]. The other two PGE2-producing enzymes, mPGES-2 and cPGES, are not inducible and have less impact on the pathogenic process of inflammation [17–20]. In general, PGE2 produced by inflammatory factor-induced COX-2 coupled with the induced mPGES-1 is one of the major factors that cause acute and chronic inflammation [17–20]. The authors' group has recently identified the complex-like configuration of the upstream COX-2 and the downstream microsomal prostaglandin E synthase-1 (mPGES-1) anchored to the endoplasmic reticulum (ER) membrane [1,16]. This configuration is used to elucidate the mechanism by which AA is rapidly metabolized into proinflammatory PGE2 under pathogenic stimulation [1]. The major mechanism of the current COX-2 inhibitors used to treat inflammation and pain is the reduction of the inflammatory PGE2 production by inhibiting upstream COX-2 [4–7]. However, inhibiting COX-2 will also result in the reduction of the production of other downstream enzyme-produced prostanoids, such as PGI2, which is a major cardiovascular protector. NSAID inhibition of upstream COX could cause severe side effects, such as gastrointestinal and cardiovascular insults from overinhibition of PGI2 and basal-level PGE2 as well as excessive bleeding from overinhibition of TXA2 biosynthesis [11]. Current COX-2 inhibitors, such as Celebrex, increase heart disease risk by decreasing PGI2, which has been demonstrated in laboratories to clinical trials [21,22]. Despite this, the solution of effectively reducing inflammatory PGE2 production without reducing PGI2 through COX-2 inhibition is not available. However, mPGES-1 is a downstream enzyme of COX-2. Drugs that inhibit mPGES-1 are to unlikely affect other COX-2 downstream enzymes, such as PGI2 synthase. Therefore, an inhibitor targeting mPGES-1 will be one of the ideal drugs to reduce the unwanted side effects of the current COX-2 inhibitors, such as increased heart disease risk. The development of drugs that specifically eliminate proinflammatory PGE2 while maintaining biosynthesis of PGI2, basal PGE2 and other prostanoids has been attempted for decades [11,16,20]. Despite several compounds showing inhibitory effects on PGE2 biosynthesis and reduction of mPGES-1 activity [23–25], their specificity for mPGES-1 was poorly characterized. Therefore, specific mPGES-1 inhibitors are still not available in the current market. This study describes the chemical structures of the compounds and the methods used for the chemical modification and synthesis of 2-amino-4-nitrophenol and 2,4-diaminophenol derivatives used as anti-inflammatory drugs and mPGES-1 inhibitors. Several chemical modifications and syntheses for the derivatives are listed as examples. Their biological activities with anti-inflammatory effects and inhibitory effects on mPGES-1 activity were identified by cellular and enzymatic assays.
Materials & methods
The general method of synthesis of 2,4-diaminophenol derivatives
Polyphosphoric acid (Sigma-Aldrich) was first heated to 110°C as a reaction solvent; 0.01 mol 2-amino-4-nitrophenol (Sigma-Aldrich) and 0.015 mol corresponding benzoic acid (4-trifluoromethyl benzoic acid, Oakwood Chemical, or 4-tert-butylbenzoic acid, Acros Organics), were simultaneously added to polyphosphoric acid. Then the resultant mixture was heated to 120–180°C for 2–4 h. At the end of the reaction (confirmed by thin-layer chromatography [TLC] that there was no starting material remaining in the solution), the solution was poured into ice water and neutralized to pH 7.0 using NaHCO3. The precipitate was filtered and collected as a crude product. Then the crude midproduct was recrystallized via boiling in ethanol. The crystallized midproduct was then heated in 20 ml ethanol with SnCl2 at 70°C for 16 h. After the reaction, the mixture was cooled to room temperature and poured into ice water. Saturated NaHCO3 was used to neutralize the mixture. Then the aqueous layer was extracted twice with EtOAc (500 ml). The final combined organic layers were dried by anhydrous MgSO4 and evaporated to get the final product [26,27].
Molecular docking studies
Molecular docking studies were performed by MOE software. To identify potential inhibitors of mPGES-1, the trimeric crystal structure of mPGES-1 (Protein Data Bank ID: 4YL3 [28]) was downloaded from the Protein Data Bank and prepared by structure preparation. During the preparation, all the water molecules were removed, and hydrogen atoms were added. All the ligands were from the previously virtual screening result library [11] and performed energy minimization. The docking process was based on the general docking method of MOE.
Biological activity studies
Cell culture
Human embryonic kidney 293 cell (HEK-293) and prostate cancer (PC-3) cell lines were purchased from American Type Culture Collection (ATCC; VA, USA). HEK-293 cells and HEK-293 transfected cells were cultured in high-glucose Dulbecco's modified Eagle medium, 10% fetal bovine serum, and 1% antibiotic-antimycotic (100×) in a 100 mm cell culture dish at 37°C in a humidified 5% CO2 incubator. PC-3 cells were cultured with Kaighn's Modification of Ham's F-12 Medium containing 10% fetal bovine serum and 1% antibiotic and antimycotic in a 100 mm cell culture dish at 37°C in a humidified 5% CO2 incubator.
Construction of cDNA plasmids encoding hybrid enzymes, COX-linked Prostaglandin synthase
The cDNA sequence of the hybrid enzyme, which the C-terminus of COX-2 was linked to the N-terminus of mPGES-1 with a ten amino acids biolinker (COX-2-10aa-mPGES-1), was constructed by a polymerase chain reaction cloning approach. The resultant cDNA was subcloned into pcDNATM3.1 vectors containing a cytomegalovirus immediate-early promoter [11,16].
Establishing HEK-293 cell line expressing recombinant enzymes
The recombinant enzyme was expressed in HEK-293 cells. The cells were plated and transfected with the constructed pcDNA by the Lipofectamine 2000 method by following the manufacturer's (Invitrogen) instructions in a six-well plate. The cells were harvested for assays approximately 48 h after transfection. The enzyme expressions were determined by western blot analysis. A culture medium containing 12 μl/ml Geneticin (G418) was used to establish stable cell lines promoter [11,16].
Cancer cell PC-3 migration assay
One day before the experiment, about 8.4 × 106 PC-3 cells were cultured in six-well plates with 2 ml of growth medium. A maker line was drawn on the outside plates to identify viewing spots. The medium was prewarmed, and each treatment (DMSO, NS-398 and 2Me) was mixed with the medium. The cells were washed with phosphate-buffered saline and replaced with 1.5 ml of medium containing treatment and were observed at 0-, 15-, 24- and 39-hour points. NIS-Elements AR 3.0 (Nikon Instruments, Inc.) software was used to take images at different time points with 10× magnification and to measure gap sizes.
Determination of PGE2 inhibitory effects using HPLC-scintillation analyzer
The effect of compounds that inhibited PGE2 production was determined by HPLC. Different concentrations of the compounds (1 μM, 10 μM, 100 μM and 1 mM) were added to HEK-COX-2-10aa-mPGES-1 cells for 10 min in a total volume of 225 μl (25 μl of the lysates + 200 μl of 2.5 mM glutathione in phosphate-buffered saline). Afterward, (14C)-AA, which was purchased from Aersham Pharmacia Biotech (NJ, USA), was added as the substrate and incubated for 5 min. The reaction was then terminated by adding 200 μl of Buffer A. After centrifugation at 13,000 r.p.m. for 10 min, the supernatant was loaded onto a reverse phase C18 column (Varian Microsorb-MV 100–5, 4.6 mm × 250 mm). Samples were run with Buffer A (1.0 ml/min) with a gradient from 35% to 100% of acetonitrile for 40 min. The full metabolite profile was obtained by a flow scintillation analyzer (Packard 150TR, 16).
NMR spectroscopy
1H NMR spectra were recorded on a 600 MHz spectrometer at room temperature. 1H NMR was measured in parts per million (p.p.m., δ) relative to the signal of tetramethylsilane (0.0 p.p.m.). Data for 1H NMR were reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad), coupling constant J (Hz) and integration.
Determination of the molecular weights of the synthetic compounds using LC–MS
The synthetic compound was directly injected into the Waters Micromass LC–MS/MS system by an autosampler. The compounds were first separated by the HPLC C18 column (for positive mode) or polymer column (for negative mode [6]) and then automatically injected into the mass detector equipped with ESI or atmospheric pressure chemical ionization source in a negative or positive mode.
Results
Identification of 4-(fluoro-, difluoro- or trifluoro-methyl)-benzoic acid-modified derivatives of 2,4-diaminophenol derivatives inhibiting mPGES-1 by docking study using a high-resolution crystal 3D structure of the native trimer of human mPGES-1
In a prior study, the authors discovered that one of the 2,4-diaminophenol derivatives – compound 10, with a core structure of benzoxazoles (Figure 3A) – specifically binds to mPGES-1 while preserving the production of PGI2 [11]. Based on this, the authors hypothesized that other, similar derivatives may also function as mPGES-1 inhibitors. To validate this hypothesis, they designed a set of derivatives modified by 4-(fluoro- [1F-], difluoro- [2F-] or trifluoro- [3F-] methyl)-benzoic acid (Figure 1A) and docked them into the substrate-binding pocket of human mPGES-1, which was represented by a high-resolution trimer crystal structure, using MOE software (Figure 1B, left panel). They then visualized the interactions of each bound derivative with the substrate-binding pocket of the mPGES-1 trimer (Figure 1B, right panels). The results of the docking study for the three compounds were compared and are summarized in Table 1. It was found that the binding affinities of the compounds followed the order of 1F > 2F > 3F, but the scores did not show significant differences. Based on these findings, the 3F derivative was selected for further chemical synthesis and biological activity characterization.
Figure 3. . Chemical synthesis pathway of 3F.

(A) Basic structure of 4-diaminophenol with R- representing the various functional groups. (B) Chemical synthesis of trifluoro (3F) derivatives. The purification procedures are described in the method section; the yield of midproduct 3F-NO2 is 87%; and the final yield of 3F is 52.3% (Rf: 2-amino-4-nitrophenol: 0.8; 4-trifluoromethyl benzoic acid: 0.89; 3F-NO2: 0.79; 3F: 0.48; solvent: EA: Hexanes [V:V] = 3:1).
3F: Trifluoro; PPA: Polyphosphoric acid.
Figure 1. . Binding of fluoro (1F), difluoro (2F) and trifluoro (3F) derivatives to mPGES-1 by docking study using the crystal 3D structure of the native trimer form of human mPGES-1 (Protein Data Bank ID: 4YL3).

(A) Chemical structure of 1F, 2F and 3F derivatives. (B) Binding of 1F, 2F and 3F into the pocket of the trimer of mPGES-1.
1F: Fluoro; 2F: Difluoro; 3F: Trifluoro; ER: Endoplasmic reticulum; GSH: Glutathione.
Table 1. . Comparison of the scores for the docking of the derivatives with the mPGES-1 trimer.
| Name | S score | Root mean square deviation |
|---|---|---|
| 1F | -4.9685 | 1.8743 |
| 2F | -4.9529 | 1.4980 |
| 3F | -4.7437 | 0.9538 |
| 1Me | -5.0828 | 0.6300 |
| 2Me | -5.1173 | 1.1271 |
| 3Me | -5.3499 | 1.6791 |
| 1CI | 207.7725 | 3.6457 |
| 2CI | 1356.0842 | 2.9810 |
| 3CI | 315.2350 | 3.0853 |
| 1Phenyl | 741.1112 | 3.6780 |
| 2Phenyl | 480.7134 | 3.2397 |
| 3Phenyl | 82.2087 | 6.8950 |
Identification of 4-(methyl-, dimethyl- or trimethyl)-benzoic acid-modified derivatives of 2,4-diaminophenol inhibiting mPGES-1 by docking study using a high-resolution crystal 3D structure of the native trimer of human mPGES-1
The second group of compounds, consisting of 4-(methyl- [1Me-], dimethyl- [2Me-] or trimethyl- [3Me-])-benzoic acid modifications of 2,4-diaminophenol (as shown in Figure 2A), was designed and docked into the mPGES-1 trimer-binding pocket. All three Me derivatives binding to the human mPGES-1 trimer pocket were identified (Figure 2B, left panel). The bound structures of the three Me derivatives that interacted with the substrate-binding pocket of the mPGES-1 trimer were also individually demonstrated (as shown in Figure 2B, right panels). The docking results showed that the binding affinities to the mPGES-1 of the three Me derivatives were significantly higher than those of all the 1F, 2F and 3F derivatives. The detailed scores are compared and shown in Table 1. The binding affinities are ranked as 3Me > 2Me > 1Me. As a result, the 3Me derivative was selected for chemical synthesis and pharmacological tests. The authors expected both groups of the compounds, the F and Me derivatives, to have the potential to be excellent mPGES-1 inhibitors in experimental tests.
Figure 2. . Binding of methyl (1Me), dimethyl (2Me) and trimethyl (3Me) derivatives to mPGES-1 by docking study using the crystal 3D structure of the native trimer form of human mPGES-1 as described in the method section.

(A) Chemical structure of 1Me, 2Me and 3Me derivatives. (B) Binding of 1Me, 2Me and 3Me into the pocket of the trimer of mPGES-1.
1Me: Methyl; 2Me: Dimethyl; 3Me: Trimethyl; ER: Endoplasmic reticulum; GSH: Glutathione.
Chemical synthesis & purification of the trifluoro derivative as a model for all fluoro derivatives
From the docking study, it was found that 1F, 2F and 3F derivatives have similar binding affinities for the mPGES-1 pocket. Here, the chemical synthesis of the 3F derivative is described as an example. The method can be applied to the other 1F and 2F syntheses. The method for the synthesis of the 3F derivative started from 2-amino-4-nitrophenol, a low-cost compound. The chemical synthesis was performed by the addition of 4-(trifluoromethyl)benzoic acid to 2-amino-4-nitrophenol in a two-step reaction (Figure 3). TLC plates were used to monitor the reactions (Rf = 0.48). The synthesized crude compound was simply purified by two steps: extraction and normal phase HPLC purification.
Identification of synthesized 3F-2,4-diaminophenol derivative structures
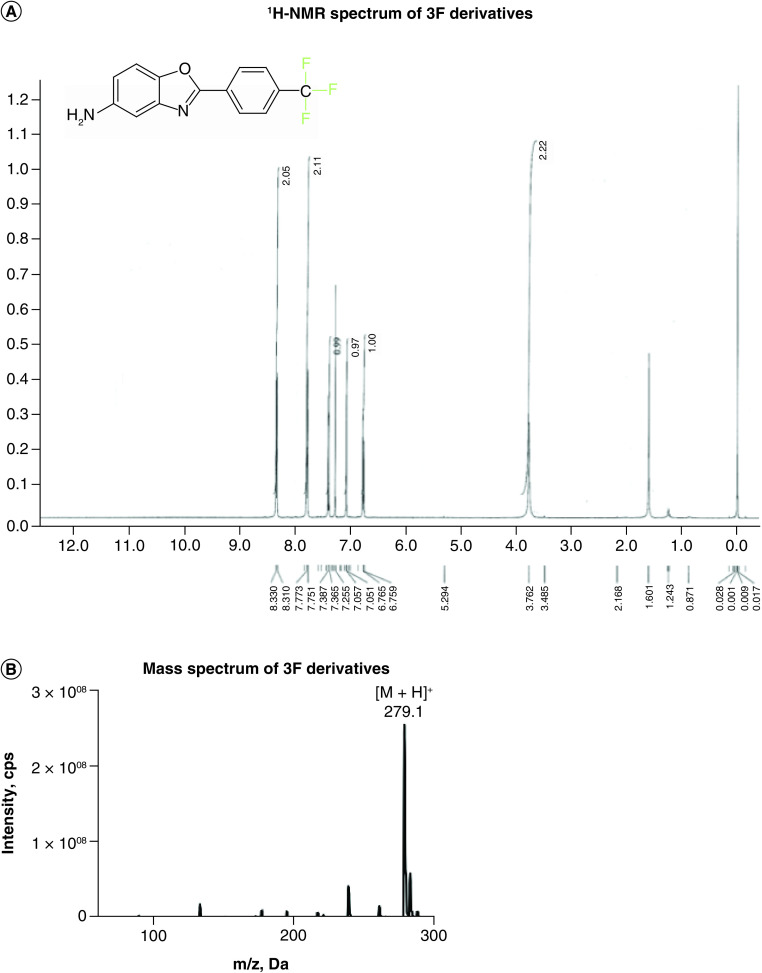
The structure of the 3F derivative was confirmed through 1H NMR spectroscopy, as indicated by the full assignments shown in Figure 4. The spectra confirmed the chemical structure of the 3F derivative, as depicted in both Figure 1 & Figure 4. One step further, the molecular weight for the synthesized compound, 2-[4-(trifluoromethyl)phenyl]-1,3-benzoxazole-5-amine, was confirmed by LC–MS with a correct mass 279.1 (MH+).
Figure 4. . Structure determination of 2,4-diaminophenol trifluoro (3F) derivative.
(A)1H NMR of 3F taken in CDCl3 at 600 MHz. 3.76 (1H, s), 6.73–6.76 (1H, dd, J = 8.4 and 2.4 Hz), 7.05 (1H, d, J = 2.4 Hz), 7.36–7.38 (1H, d, J = 8.8 Hz), 7.75–7.77 (2H, d, J = 8.8 Hz), 8.31–8.33 (2H, d, J = 8 Hz). (B) LC–MS spectrum of 2,4-diaminophenol 3F derivative. MH+ 279.1.
3F: Trifluoro.
Chemical synthesis & structure identification of 3Me-2,4-diaminophenol derivatives
The docking study has significantly increased understanding of the high binding affinities of the 1Me, 2Me and 3Me derivatives to mPGES-1 within a similar range. The synthesis of the 3Me derivative was used as an example and was carried out starting from 2-amino-4-nitrophenol. The steps of the reaction are shown in Figure 5A, and TLC analysis was used to monitor the reactions (Rf = 0.46). The synthesized 3Me compound was purified through chemical extraction and HPLC purification. The structure of the 3Me derivative was verified through 1H NMR spectroscopy, and the correct chemical structure was confirmed by the full assignments for the 1H NMR spectra (Figure 5B). The structure of 3Me was further confirmed by 13C NMR spectrum (Figure 5C).
Figure 5. . Chemical synthesis and structure determination of 2,4-diaminophenol trimethyl (3Me) derivatives.

(A) Chemical reaction steps; the final yield of 3Me is 47.8%. (B)1H NMR of 3Me in CDCl3 at 600 MHz. 1H NMR: δ 1.28 (9H, s), 6.56 (1H, dd, J = 8.4, 1.8 Hz), 7.27–7.44 (3H, 7.33 [ddd, J = 8.0, 1.3, 0.4 Hz], 7.39 [ddd, J = 1.8, 0.5 Hz]), 7.63 (1H, dd, J = 8.4, 0.5 Hz), 7.94 (2H, ddd, J = 8.0, 1.6, 0.4 Hz). (C)13C NMR of 3Me taken in CDCl3 at 600 MHz. 13C NMR: δ 31.131 (3C, s), 35.029 (1C, s), 110.708 (1C, s), 125.829–125.936 (2C, 125.829 [s], 125.936 [s]), 125.936 (2C, s), 127.313–127.393 (3C, 127.313 [s], 127.393 [s]), 143.166 (1C, s), 145.521 (1C, s), 154.914 (1C, s), 163.802 (1C, s). (Rf: 2-amino-4-nitrophenol: 0.81; 4-tert-butylbenzoic acid: 0.87; 3Me-NO2: 0.71; 3Me: 0.46; solvent: EA: Hexanes [V:V] = 3:1).
3Me: Trimethyl; PPA: Polyphosphoric acid.
Determination of the biological activity of synthesized derivatives targeting mPGES-1-catalyzed PGE2 biosynthesis
Effects of synthesized representative derivatives on inhibition of mPGES-1 activity
The inhibitory effect on PGE2 production by mPGES-1 was determined using previously developed HEK-293 cells expressing the engineered enzymelink, COX-2-10aa-mPGES-1. All three compounds – 3F, 2Me and 3Me benzoic acid-modified 2,4-diaminophenol – were able to inhibit inflammatory PGE2 production with very similar dose-response concentrations of the COX-2 inhibitor (Figure 6). This suggests that the backbone structure, 2,4-diaminophenol derivatives with the modifications at the position 1 -OH group and position 2 -NH2 group using benzoic acid, is a key structure in generating the inhibitors targeting mPGES-1.
Figure 6. . Dose-response curves for the effects of the synthesized derivatives on mPGES-1-synthesized inflammatory prostaglandin E2 (PGE2), compared with COX-2 inhibitor.
HEK-293 cells expressing inflammatory PGE-producing enzymelink, COX-2-10aa-MPGES-1, described previously [11], were treated with increasing concentrations (1 μM, 3 μM and 10 μM, of the derivatives. Arachidonic acid (0.5 μM) was added to initiate the biosynthesis of PGE2 through COX-2 and mPGES-1 catalysis [11]. The produced PGE2 was detected by ELISA(11). COX-2 inhibitor NS-398 was used as the positive control. An unrelated chemical compound, #6, was used as a negative control.
2Me: Dimethyl; 3F: Trifluoro; 3Me: Trimethyl; PGE2: Prostaglandin E2.
Determination of the anti-inflammatory activity of derivatives using cancer cell migration assay
Inflammation is a factor that stimulates cell migration and is associated with a wide range of ailments, including cancer [29]. Inflammatory PGE2 produced by mPGES-1 has been reported to be directly associated with cancer cell migration and development [13,30]. Here, cellular migration of the pancreatic cancer cell line PC-3 is used as a model to show the anti-inflammatory effects of the derivatives. A gap of the cultured PC-3 cells was created first (Figure 7, day 0). Then, the different concentrations of the derivatives or positive control, a COX-2 inhibitor, were added to the cells. After 39 h of culture, the gaps were measured (Figure 7A) and quantified (Figure 7B). All three derivatives – 2Me, 3Me and 3F benzoic acid-modified 2,4-diaminophenol derivatives – showed very effective inhibition of cancer cell migration within the gaps, similar to that of the positive control, a COX-2 inhibitor, NS-398. DMSO was used as the negative control.
Figure 7. . Anti-inflammatory and anticancer activities in cell cultural conditions.

PC-3 migration assay treated with the lead compound. (A) The representative images of the PC-3 migration at 0 h (left) and 39 h (right) with DMSO, NS-398 and dimethyl. (B) The time-course gap size of the PC-3 migration. The gap size was obtained using NIS-Elements software (NIKON); n = 6. (C) Quantitative and significant analyses.
2Me: Dimethyl; 3F: Trifluoro; 3Me: Trimethyl.
Discussion
The side effects of COX-2 inhibitors, which include increased risk of heart disease [3–7], have limited their uses. Thus, there has been an increased focus on determining a replacement with anti-inflammatory effects similar to those of NSAIDs without the negative cardiovascular side effects. Since inducible mPGES-1 was identified as a key downstream enzyme that is coupled to inducible COX-2 [11–16] to produce the inflammatory PGE2, it has become possible to replace COX-2 inhibitors with an mPGES-1 inhibitor, which is unlikely to have any negative impacts on patients with heart disease. However, an effective mPGES-1 inhibitor is not available in the market yet. The method to make 2,4-diaminophenol derivatives that specifically inhibit mPGES-1 described here has immense potential to develop into a novel anti-inflammatory drug that targets mPGES-1.
The study has shown that the 1-3F and 1-3Me derivatives from 2,4-diaminophenol effectively bind to the pocket of mPGES-1, but not the COX-2 and prostacyclin synthase (PGIS). This finding provides a basis to consider the synthesis of compounds that exert their anti-inflammatory effects by reducing inflammatory PGE2 synthesis using mPGES-1 inhibition. COX-2 and PGIS, which are needed to produce the vascular protector PGI2, are not affected when mPGES-1 is targeted. The 3D structural models used in this method can be extended to other methods for use in the identification of other compounds that regulate prostanoid synthesis. This method has also shown that other functional groups, such as the 1-3 Cl and phenyl group, may also impact the inhibition of mPGES-1. The IC50 value reported in this study was obtained using a cellular-based assay, which required higher concentrations of the compounds compared with the IC50 value obtained using the enzyme-based assay. It is anticipated that the final concentration of the compounds within the cellular environment will be much lower than the added concentration required for the assay. The study's primary objective was to evaluate the specificity of the designed compounds in inhibiting mPGES-1 in a cellular environment. Future studies will focus on optimizing the IC50 values of these compounds using pure enzyme assays.
For drug discovery, one of the key factors is that the potential drug can be synthesized easily at a low cost. The methods described here for the synthesis of 2,4-diaminophenol derivatives are relatively simple and effective with high yields. The initial compound, 2,4-diaminophenol, is a popular and low-cost compound suitable for large-scale synthesis and cost-effective production. The chemicals used for the addition of the 1-3F and 1-3Me functional groups are also commercially available and easily obtained.
It should be indicated that other mPGES-1 inhibitors have been tested [20,24,25]. However, the compounds in this study are more specific to mPGES-1. Noncrossed inhibition for PGIS and COX-2 was also tested. These compounds are more attractive candidates for the development of a new generation of anti-inflammatory drugs that can replace COX-2 inhibitors or be powerful alternatives that reduce the risk of heart disease for those who currently use the common COX-2 inhibitors, such as Celecoxib.
Conclusion
There are several advantages of the current findings. The chemical structures of the active compounds specifically inhibit mPGES-1, but not COX and PGI2 synthase, have been confirmed by our group. Due to the relatively simple chemical structures of the active compounds, they can be synthesized within less steps and low costs. The methods used to make the final active compounds of the 2,4-diaminophenol derivatives require only two-step reactions, which can be performed in any chemical lab. Finally, due to only two-step reactions being involved, the purification and characterization steps are also minimized, which saves production costs.
Summary points.
In this study, a set of compounds specifically inhibiting inducible inflammatory mPGES-1, discovered from a cross-screening using enzymelinks COX-2-10aa-mPGES-1 and COX-2-10aa-PGIS as targets, were further optimized by a computational approach.
The computational platform, MOE, integrated computer-aided molecular simulation, crystal structural-based optimization of the docking active site and analysis of the interaction forces of induced-fit docking between the crystal structural mPGES-1 and the 3D structures of the compounds.
The platform-designed compounds 3F and 3Me were synthesized and purified. The methods used to synthesize and purify the first two group derivatives were described. The final compounds were characterized by 1H NMR spectroscopy and LC–MS.
The typical compounds inhibiting PGE2 biosynthesis targeted mPGES-1 activity were confirmed by cellular and enzymatic assays. The study demonstrated that the compounds tested have great potential to be developed into a new generation of nonsteroidal anti-inflammatory drugs with fewer side effects by specifically targeting downstream mPGES-1.
Footnotes
Financial & competing interests disclosure
This work was supported by NIH grants (RO1 HL56712 and HL79389 for KH Ruan) and American Heart Association grants (10GRNT4470042 and 14GRNT20380687 for KH Ruan). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Akasaka H, So SP, Ruan KH. Relationship of the topological distances and activities between mPGES-1 and COX-2 versus COX-1: implications of the different post-translational endoplasmic reticulum organizations of COX-1 and COX-2. Biochemistry 54(23), 3707–3715 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Rainsford KD. Anti-inflammatory drugs in the 21st century. Subcell. Biochem. 42, 3–27 (2007). [DOI] [PubMed] [Google Scholar]
- 3.Zarghi A, Arfaei S. Selective COX-2 inhibitors: a review of their structure–activity relationships. Iran. J. Pharm. Res. 10(4), 655–683 (2011). [PMC free article] [PubMed] [Google Scholar]
- 4.Chakraborti AK, Garg SK, Kumar R, Motiwala HF, Jadhavar PS. Progress in COX-2 inhibitors: a journey so far. Curr. Med. Chem. 17(15), 1563–1593 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Blain H, Jouzeau JY, Netter P, Jeandel C. Non-steroidal anti-inflammatory agents with selective inhibitory activity on cyclooxygenase-2. Interest and future prospects. Rev. Med. Intern. 21(11), 978–988 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Gunter BR, Butler KA, Wallace RL, Smith SM, Harirforoosh S. Non-steroidal anti-inflammatory drug-induced cardiovascular adverse events: a meta-analysis. J. Clin. Pharm. Ther. 42(1), 27–38 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. Toxicol. 38, 97–120 (1998). [DOI] [PubMed] [Google Scholar]
- 8.Ruan KH. Advance in understanding the biosynthesis of prostacyclin and thromboxane A2 in the endoplasmic reticulum membrane via the cyclooxygenase pathway. Mini. Rev. Med. Chem. 4(6), 639–647 (2004). [DOI] [PubMed] [Google Scholar]
- 9.Ruan KH, Deng H, So SP. Engineering of a protein with cyclooxygenase and prostacyclin synthase activities that converts arachidonic acid to prostacyclin. Biochemistry 45(47), 14003–14011 (2006). [DOI] [PubMed] [Google Scholar]
- 10.Ruan KH, So SP, Cervantes V, Wu H, Wijaya C, Jentzen RR. An active triple-catalytic hybrid enzyme engineered by linking cyclo-oxygenase isoform-1 to prostacyclin synthase that can constantly biosynthesize prostacyclin, the vascular protector. FEBS J. 275(23), 5820–5829 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruan DT, Tang N, Akasaka H, Lu R, Ruan KH. Engineering ‘enzymelink’ for screening lead compounds to inhibit mPGES-1 while maintaining prostacyclin synthase activity. Future Med. Chem. 13(13), 1091–1103 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murakami M, Naraba H, Tanioka T et al. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J. Biol. Chem. 275(42), 32783–32792 (2000). [DOI] [PubMed] [Google Scholar]
- 13.Kamei D, Murakami M, Nakatani Y, Ishikawa Y, Ishii T, Kudo I. Potential role of microsomal prostaglandin E synthase-1 in tumorigenesis. J. Biol. Chem. 278(21), 19396–19405 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Nakanishi M, Gokhale V, Meuillet EJ, Rosenberg DW. mPGES-1 as a target for cancer suppression: a comprehensive invited review “Phospholipase A2 and lipid mediators”. Biochimie 92(6), 660–664 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakanishi M, Montrose DC, Clark P et al. Genetic deletion of mPGES-1 suppresses intestinal tumorigenesis. Cancer Res. 68(9), 3251–3259 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Ruan KH, Cervantes V, So SP. Engineering of a novel hybrid enzyme: an anti-inflammatory drug target with triple catalytic activities directly converting arachidonic acid into the inflammatory prostaglandin E2. Protein Eng. Des. Sel. 22(12), 733–740 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murakami M, Nakatani Y, Tanioka T, Kudo I. Prostaglandin E synthase. Prostaglandins Other Lipid Mediat. 68–69, 383–399 (2002). [DOI] [PubMed] [Google Scholar]
- 18.Gudis K, Tatsuguchi A, Wada K et al. Microsomal prostaglandin E synthase (mPGES)-1, mPGES-2 and cytosolic PGES expression in human gastritis and gastric ulcer tissue. Lab. Invest. 85(2), 225–236 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Mattila S, Tuominen H, Koivukangas J, Stenbäck F. The terminal prostaglandin synthases mPGES-1, mPGES-2, and cPGES are all overexpressed in human gliomas. Neuropathology 29(2), 156–165 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Samuelsson B, Morgenstern R, Jakobsson PJ. Membrane prostaglandin E synthase-1: a novel therapeutic target. Pharmacol. Rev. 59(3), 207–224 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Patrono C. Cardiovascular effects of cyclooxygenase-2 inhibitors: a mechanistic and clinical perspective. Br. J. Clin. Pharmacol. 82(4), 957–964 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fries S, Grosser T. The cardiovascular pharmacology of COX-2 inhibition. Hematology Am. Soc. Hematol. Educ. Program 1, 445–451 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Ding K, Zhou Z, Hou S et al. Structure-based discovery of mPGES-1 inhibitors suitable for preclinical testing in wild-type mice as a new generation of anti-inflammatory drugs. Sci. Rep. 8(1), 5205 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lauro G, Cantone V, Potenza M et al. Discovery of 3-hydroxy-3-pyrrolin-2-one-based mPGES-1 inhibitors using a multi-step virtual screening protocol. Medchemcomm 9(12), 2028–2036 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou S, Zhou Z, Ding K et al. DREAM-in-CDM approach and identification of a new generation of anti-inflammatory drugs targeting mPGES-1. Sci. Rep. 10(1), 10187 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chancellor DR, Davies KE, De Moor O et al. Discovery of 2-arylbenzoxazoles as upregulators of utrophin production for the treatment of Duchenne muscular dystrophy. J. Med. Chem. 54(9), 3241–3250 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Karatas E, Foto E, Ertan-Bolelli T et al. Discovery of 5-(or 6)-benzoxazoles and oxazolo[4,5-b]pyridines as novel candidate antitumor agents targeting hTopo IIα. Bioorg. Chem. 112, 104913 (2021). [DOI] [PubMed] [Google Scholar]
- 28.Luz JG, Antonysamy S, Kuklish SL et al. Crystal structures of mPGES-1 inhibitor complexes form a basis for the rational design of potent analgesic and anti-inflammatory therapeutics. J. Med. Chem. 58(11), 4727–4737 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Liggett JL, Zhang X, Eling TE, Baek SJ. Anti-tumor activity of non-steroidal anti-inflammatory drugs: cyclooxygenase-independent targets. Cancer Lett. 346(2), 217–224 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ricciotti E, Fitzgerald GA. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 31(5), 986–1000 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]