Abstract
Ferroptosis is a distinct type of regulated cell death characterized by iron overload and lipid peroxidation. Ferroptosis is regulated by numerous factors and controlled by several mechanisms. This cell death type has a relationship with the immune system, which may be regulated by damage-associated molecular patterns. Ferroptosis participates in the progression of autoimmune diseases, including autoimmune hepatitis, rheumatoid arthritis, systemic lupus erythematosus, inflammatory bowel disease, multiple sclerosis, Parkinson's Disease, psoriasis and insulin-dependent diabetes mellitus. The present review summarizes the role of ferroptosis in autoimmune disorders and discusses ferroptosis as a potential therapeutic target for autoimmune disease.
Keywords: ferroptosis, autoimmune disease, immune system, therapeutic target
1. Introduction
Since being named in 2012 by Dixon et al (1), ferroptosis has been probed in a wide range of pathologies and proposed as a novel therapeutic strategy for numerous diseases, including cancer (2,3), ischemia-reperfusion injury (4,5) and neurodegenerative disorder (6,7). Autoimmune disease, particularly rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), multiple sclerosis (MS), insulin-dependent diabetes mellitus (IDDM) and inflammatory bowel disease (IBD) (8,9), are heterogeneous with regard to prevalence, manifestation and pathogenesis. Accumulating evidence in recent times has shown an association of ferroptosis with the pathogenesis and development of autoimmune diseases (10-12). The present review aimed to summarize the association between ferroptosis and autoimmune disease, focusing on potential mechanisms and therapeutic strategies.
2. Overview of ferroptosis
Ferroptosis was proposed in 2012 as a distinctive type of non-apoptotic cell death (1) characterized by iron overload and lipid peroxidation. This differs from other forms of regulated cell death. The primary feature of ferroptosis is mitochondrial shrinkage, which occurs alongside an increase in mitochondrial membrane density and degeneration of mitochondrial crista but with no changes in morphology of the nucleus (1).
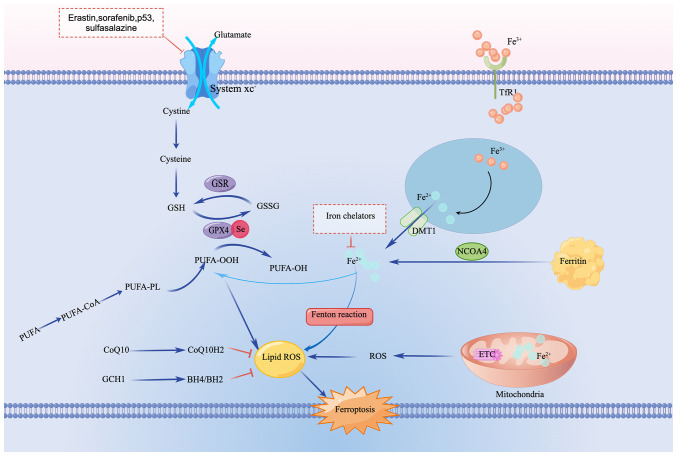
Several pathways, including the metabolism of iron, acid and lipid, have been implicated in ferroptosis (13). Excessive cytosolic Fe2+ catalyzes the Fenton reaction and activates iron-dependent metabolic enzymes, leading to production of highly reactive hydroxyl radicals and oxidized polyunsaturated fatty acids (PUFAs), which results in the promotion of the accumulation of lipid reactive oxygen species (ROS) and ferroptosis. The metabolism of amino acids, especially the system Xc/glutathione (GSH)/GSH peroxidase 4 (GPX4) axis, is key to eliminating lipid ROS, with GPX4 regarded as a key regulator of ferroptosis. Additionally, GPX4-independent pathways, such as the NADPH/ferroptosis suppressor protein 1 (FSP1)/coenzyme Q10 and the GTP cyclohydrolase-1/tetrahydrobiopterin/dihydrobiopterin axes, have also been implicated in the ferroptosis process in the past few years (14-16) (Fig. 1).
Figure 1.
Signaling pathway of ferroptosis. Ferric iron is transferred into cells by TfR1, then converted to ferrous iron and released to the cytoplasm by STEAP3 and DMT1. Elevated labile iron pool catalyzes formation of phospholipid hydroperoxides via Fenton reaction. Free cytosolic PUFAs are converted to PUFA-PLs with catalyzation by ACSL4 and LPCAT3, then PUFA-PLs are oxidized by lipoxygenase 12/15, contributing to the accumulation of phospholipid hydroperoxides. Mitochondrial dysfunction results in increased ROS production, which may also contribute to lipid peroxidation. Cystine uptake through system xc- is used for synthesis of GSH. Moreover, FSP1/CoQ10 and GCH1/BH4/BH2 are two parallel GPX4-independent pathways in suppression of ferroptosis. TfR1, transferrin receptor 1; DMT1, divalent metal transporter 1; NCOA4, nuclear receptor coactivator 4; PUFA, polyunsaturated fatty acid; ACSL4, acyl-CoA synthetase long-chain family member 4; LPCAT3, lysophosphatidylcholine acyltransferase 3; GSH, glutathione; FSP1, ferroptosis suppressor protein 1; GCH-1, guanosine triphosphate cyclohydrolase 1; BH4, tetrahydrobiopterin; BH2, dihydrobiopterin; PL, phospholipid; GSR, glutathione disulfide reductase; GSSG, glutathione oxidized; ROS, reactive oxygen species; GPX4, GSH peroxidase 4; CoQ10H2, reduced coenzyme Q10; ETC, electron transport chain.
3. Association between ferroptosis and the immune system
Two types of immunity exist in the body, innate and adaptive. The innate immune system detects invading pathogens, while the adaptive immune system promotes a specific and long-lasting protection against infection. Innate immune cells mainly include dendritic cells, macrophages and neutrophils, while the adaptive immune system generally contains T and B lymphocytes and nature killer (NK) cells (17).
In the last few years, more evidence has revealed a close association between ferroptosis and the immune system (18,19). Notably, autoimmune disease is initiated and propagated by the activation of self-antigen-reactive T cells (20), pointing to the crucial role of T cells in autoimmunity. In a study investigating ferroptosis in immunity, both antigen-specific CD8+ and CD4+ T cells failed to expand and protect infection in T cell-specific GPX4-deficient mice (TΔGpx4/ΔGpx4), whereas GPX4-deficient T cells rapidly accumulated lipid peroxides and underwent ferroptosis in vitro (21). Ferroptosis was found to be involved in immunotherapy-activated CD8+ T cells, with increased ferroptosis contributing to the anti-tumor efficacy of immunotherapy (22). Recently, the homeostasis of follicular helper T cells, a specialized subset of CD4+ T cells, was also shown to be regulated by ferroptosis (23).
Key features of ferroptosis, iron overload and lipid peroxidation, participate in immunity. Iron overload increases oxidative stress and DNA damage in T cells, leading to immune dysfunction (24), while lipid peroxidation is associated with intracellular ROS in regulatory T cells (25). Ferroptosis affects the viability of B cells, with Muri et al (26) demonstrating that GPX4 is key to the development, maintenance and responses of B1 and marginal zone B cells via suppression of ferroptosis. Moreover, the ferroptosis inducer erastin increases lipid peroxidation and promotes peripheral blood mononuclear cell proliferation and differentiation into B and NK cells (27).
In addition to its links to the adaptive immune system, ferroptosis also plays a key role in innate immunity. In tumor cells, exogenous circularly polarized magnetic field-induced ferroptosis leads to the maturation of dendritic cells (28) and in immune-competent mice, ferroptosis promotes phenotypical maturation of bone-marrow-derived dendritic cells (29). With regard to other innate immune cells, ferroptosis is associated with the infiltration of macrophages and neutrophils (30), while also regulating polarization of macrophages (31) and the recruitment of neutrophils (32).
4. Damage-associated molecular patterns (DAMPs)
Although the pathology of autoimmune disease is complex, it is hypothesized that inflammation serves a key role in autoimmunity (33). DAMPs, endogenous molecules released by damaged tissue or dying cells, have been proved to be detrimental in inflammatory response and lead to the development of inflammatory disorders (34,35). Autoimmune diseases, such as SLE (36) and IBD (37), are among the inflammatory disorders initiated by DAMPs. In the host, DAMPs either activate innate immune cells, leading to release of various cytokines and chemokines and activation of adaptive immune responses, or stimulate adaptive immune cells directly (35).
As a key part of regulated cell death, ferroptosis can stimulate the release of DAMPs. Adenosine triphosphate (ATP) and high mobility group box 1 (HMGB1), two well-characterized DAMPs, are released along during ferroptosis in murine fibrosarcoma MCA205 or glioma GL261(29) and p53 R273H-expressing non-small cell lung cancer cells (38). Cotreatment with erastin and celastrol initiates expression of heat shock proteins (HSPs) (39). Using the immunoprecipitation assay, an interaction between HSP90 and GPX4 has been demonstrated in a model of acute kidney injury (AKI) (40). Another DAMP, calreticulin, also participates in ferroptosis. In the head and neck squamous cell carcinoma, Zhao et al (41) found ferroptosis reverses immunosuppressive microenvironments by releasing calreticulin and HMGB1, while Van Loenhout et al (42) demonstrated that auranofin and plasma-treated PBS mixture-induced ferroptosis led to a significant increase in calreticulin, ATP and HMGB1. The aforementioned reports point to a close link between ferroptosis and DAMPs, which may partly explain the mechanism of ferroptosis-mediated autoimmunity. Autoimmune diseases are also associated with cytokines and chemokines. In a mouse AKI model, ferrostatin-1 was shown to prevent upregulation of IL-33(43). Additionally, liproxstatin-1 alleviates radiation-induced lung fibrosis via the downregulation of TGF-β1(44). Ferroptosis, therefore, could have an intimate relationship with autoimmune disease.
5. Ferroptosis and autoimmune disease
Autoimmune diseases are complicated and characterized by the development of specific autoantibodies and the presence of autoreactive T cells, leading to the impairment of sustained immune responses and organs (45). Recent studies have highlighted the association between ferroptosis and autoimmune disease (Table I).
Table I.
Role of ferroptosis in autoimmune disease.
| Disease | effect Ferroptosis | (Refs.) |
|---|---|---|
| Autoimmune hepatitis | Promote | (47,48) |
| Rheumatoid arthritis | Inconsistent | (51-54) |
| Systemic lupus erythematosus | Promoting | (60) |
| Inflammatory bowel disease | Promoting | (63-65) |
| Multiple sclerosis | Promoting | (70,71) |
| Parkinson's disease | Promoting | (76-79) |
| Psoriasis | Promoting | (81,82) |
| Insulin-dependent diabetes mellitus | Promoting | (88) |
6. Ferroptosis and autoimmune hepatitis (AIH)
AIH is an immune-mediated inflammatory liver disorder characterized by histological abnormality, as well as elevated aspartate aminotransferase, alanine aminotransferase and total IgG and the presence of autoantibodies (46).
In concanavalin A (ConA)-induced hepatitis, redox-active iron accumulation and malondialdehyde (MDA) are detected in the hepatic tissues of mice. Moreover, the expression of GPX4 and system xc- is markedly decreased in the liver of ConA-treated mice and is accompanied by the downregulation of caveolin-1(47). In LO2 hepatocyte cell line, the overexpression of caveolin-1 results in the upregulation of the expression of system xc-, suggesting that cavelolin-1 protects against ConA-induced AIH by inhibiting ferroptosis. In another study, levels of cyclooxygenase2 and acyl-coenzyme A synthase long-chain family member 4 (ACSL4) were shown to be upregulated in the liver tissue of S100-induced AIH model mice, while the levels of GPX4 and ferritin heavy chain 1 (FTH1) are downregulated (48). In addition, GPX4 knockdown via adeno-associated virus injection aggravates severity of S100-induced AIH. The aforementioned studies suggested that ferroptosis is a possible mediator of AIH.
7. Ferroptosis and RA
RA is the most common autoimmune inflammatory arthritis in adults and is characterized by chronic destructive synovitis and multisystem disorder (49,50).
In the rheumatoid synovium and synovial fluid of patients with RA, the levels of iron accumulation and lipid peroxidation increase, while in a collagen-induced arthritis (CIA) mouse model, selectively targeting fibroblasts in vivo to induce ferroptosis attenuates arthritis progression, indicating that ferroptosis inducers serve as candidates for RA treatment (51). Sulfasalazine, a U.S. Food and Drug Administration-approved RA drug, is an effective inducer of ferroptosis (52). As shown by Ling et al (53), the expression of ACSL4 declines, while the expression of FTH1, GPX4, and cystine/glutamate antiporter solute carrier family 7 member 11 are increased in the RA synovium of CIA model mouse compared with healthy control (53). Luo and Zhang (54) showed that in a lipopolysaccharide-induced synovitis cell model, MDA levels are increased, whereas GPX4 levels are decreased, representing an increase in ferroptosis in human synoviocytes; inhibition of ferroptosis may be a new therapeutic strategy for synovitis (54). Further studies are required to identify the exact role of ferroptosis in RA.
8. Ferroptosis and SLE
SLE is a multisystem autoimmune disease characterized by formation of autoantibodies, deposition of immune complexes and inflammation that primarily presents in women of reproductive age. The pathogenetic mechanisms of SLE are complex and this disorder is prone to relapse and remissions, leading to considerable morbidity and mortality (55,56).
Previous studies have examined the association between iron metabolism and SLE (57-59). Li et al (60) investigated the direct association between ferroptosis and SLE; the study demonstrated ferroptosis of neutrophils in lupus-prone mice and patients with SLE and hypothesized that neutrophil ferroptosis as an essential driver of neutropenia in SLE and treatment using specific ferroptosis inhibitors may ameliorate SLE severity and symptoms (60).
9. Ferroptosis and IBD
IBD, including Crohn's disease (CD) and ulcerative colitis (UC), is a complex chronic inflammation disorder that arises due to dysregulated immune response (61). Smoking, diet, lifestyle and behavior, as well as gut microbiota, are all key contributors to disease pathogenesis (62).
Mayr et al (63) found GPX4 activity is impaired and lipid peroxidation is augmented in small intestinal epithelial cells (IECs) of patients with CD. They also found that PUFA exposure induces lipid peroxidation and cytokine production by GPX4 small-interfering RNA IECs, while the genetic deletion of ACSL4 abrogates PUFA-induced cytokine production, suggesting that inflammatory cytokine production in IECs of patients with CD is driven by ferroptotic mechanisms (63). Xu et al (64) demonstrated significantly downregulated GPX4 and notably upregulated ACSL4 expression in the colonic biopsy specimens of patients with CD. In addition, MDA content and prostaglandin-endoperoxide synthase 2 (PTGS2) levels are higher in colon samples from mice with trinitrobenzene sulfonic acid-induced colitis, pointing to a close association between ferroptosis and CD (64).
Ferroptosis has also been investigated in UC, with Xu et al (65) reporting its involvement in IEC death in UC (65). The aforementioned study found several ferroptosis-associated genes to be remarkably down- or upregulated in human colonic biopsy samples from patients with UC, while PTGS2 is elevated and GPX4 diminished in colonic IECs from experimental colitis mice. Preventing ferroptosis through inhibiting the Nrf2/heme oxygenase-1 signaling pathway may be a valuable approach to inhibit progression of UC in dextran sulfate sodium (DSS)-induced experimental colitis mice (66,67). The aforementioned findings suggested that ferroptosis is involved in IBD and could serve as a therapeutic target.
10. Ferroptosis and MS
MS is considered an autoimmune disorder of the central nervous system that is characterized by inflammation, demyelination and degeneration (68,69). mRNA levels of GPX4 in the brain of patients with MS are decreased, while the levels of GPX4 mRNA and protein are decreased and lipid peroxidation is enhanced in an experimental autoimmune encephalomyelitis mouse model (70). Jhelum et al (71) investigated the underlying mechanism of cuprizone (CZ), a copper chelator, used to induce oligodendrocyte (OL) cell loss and demyelination, revealing that CZ treatment resulted in an increase in mRNA expression of nuclear receptor coactivator 4, transferrin receptor 1 and PTGS2, as well as lipid peroxidation, and a decrease in the expression of GPX4 and system xc- in the brain tissue of experimental mice. Additionally, the CZ-induced loss of OL and demyelination was prevented by ferrostatin-1(71). These results indicated that ferroptosis is a potential therapeutic target for MS.
11. Ferroptosis and Parkinson's disease (PD)
PD is one of the most common types of neurodegenerative disorder (72) and has also been proposed as an autoimmune disease (73). Numerous studies have examined the association between ferroptosis and PD (7,74,75).
In 2016, Do Van et al (7) reported the role of ferroptosis in PD for the first time, finding ferroptosis components in PD neuropathology. Moreover, the aforementioned study found dopaminergic neuronal loss is inhibited by ferroptosis-specific inhibitors ferostatin-1 and liproxstatin-1 and that modulation of the ferroptotic signaling cascade is a possible target for drug candidates for PD. Ferroptosis occurs in the pathology of PD and they share several hallmarks, including iron overload, lipid peroxidation and decreased GSH levels (76-78). Recently, Zuo et al (79) demonstrated that paraquat, a neurotoxin that increases the risk of PD, significantly induces iron accumulation in the cytoplasm and mitochondria of SH-SY5Y human neuroblastoma cells via the ferritinophagy pathway; however, ferritinophagy-mediated ferroptosis is significantly ameliorated by ferrostatin-1, pointing to the inhibition of ferroptosis as a potential new strategy for the prevention of neurotoxicity or PD (79). Reagents targeting ferroptosis could be used in the treatment of PD in the future.
12. Ferroptosis and psoriasis
Psoriasis is a chronic immune-mediated inflammatory skin disease characterized by hyperproliferation of keratinocytes and excessive infiltration of immune cells. Currently, it is considered a systemic disease associated with metabolic, arthritic and cardiovascular comorbidities (80).
A previous study showed a significant reduction in GPX4 and elevation in Nrf2 downstream targets in psoriatic skin lesions compared with samples from healthy patients (81). Additionally, the mRNA levels of ACSL4, PTGS2 and TFR are much higher in psoriasis lesions than in healthy controls. Furthermore, in an imiquimod (IMQ)-induced mouse model of psoriasis, immunohistochemical analysis uncovered notably increased ACSL4 levels and markedly decreased GPX4 levels in the basal epidermal layer and ferrostatin-1 treatment attenuated IMQ-induced psoriasis-like dermatitis (82). Ferroptosis is, therefore, a potential physiological mechanism for eliminating inflammatory response in psoriasis.
13. Ferroptosis and IDDM
IDDM is a chronic disorder stemming from autoimmune damage of pancreatic β cells (83). While ferroptosis is involved in cell death of the myocardium and renal tubules during diabetes (84-87), the role of ferroptosis in the death of β cells is unknown. In 2018, Bruni et al reported massive ferroptosis in pancreatic islets isolated from IDDM patients, whereas the transplantable number of islet equivalents increased following addition of ferrostatin (88). Along with evidence that ferroptosis is induced in rat pancreatic β cells after exposure to tert-butyl hydroperoxide (89), ferroptosis can be considered a possible mode of β cell destruction. However, more studies are required to determine the link between ferroptosis and β cell death.
14. Ferroptosis as a therapeutic target for autoimmune disease
As aforementioned, there is an association between ferroptosis and autoimmune disease. Therefore, targeting ferroptosis is a promising therapeutic option for autoimmune disease. Ferroptosis can primarily be inhibited by iron chelators and lipophilic antioxidants (1). The present review summarizes anti-ferroptosis agents and their potential benefits in the treatment of autoimmune disorder (Table II).
Table II.
Therapeutic options for autoimmune disease.
| Reagent | Mechanism | Disease | (Refs.) |
|---|---|---|---|
| Deferoxamine | Iron chelation | RA, PD | (90,94) |
| Deferiprone | Iron chelation | MS, PD, IBD | (67,95,96) |
| Ferrostatin-1 | Peroxidation inhibition | AIH, IBD, PD | (7,48,64) |
| Liproxstatin-1 | Peroxidation inhibition | IBD, PD | (7,67) |
| Selenium | Peroxidation inhibition | MS, IBD, PD, psoriasis | (102-104,107) |
| N-acetylcysteine | Peroxidation inhibition | RA, SLE | (108,109) |
| Polyphenol | Peroxidation inhibition | RA, IBD, IDDM, PD | (111-116) |
| α-tocopherol | Peroxidation inhibition | RA, MS, PD | (100-102) |
RA, rheumatoid arthritis; PD, Parkinson's disease; MS, multiple sclerosis; IBD, inflammatory bowel disease; SLE, systemic lupus erythematosus; IDDM, insulin-dependent diabetes mellitus; AIH, autoimmune hepatitis.
15. Iron chelators
Deferoxamine (DFO) has been investigated in the treatment of several types of autoimmune disease. In patients with RA, DFO prevents synovial injury (90) and improves anemia (91). A pilot study showed that patients with MS tolerate a short course of DFO therapy relatively well (92), however, no effect on disease progression has been noted (93). In addition to its effect against RA and MS, DFO has been found to ameliorate motor defects and pathology in a PD rat model (94). Deferiprone (DFP), another iron chelator, suppresses disease activity in a mouse model of MS (95). Additionally, DFP reportedly improves motor performance of patients with PD in a phase II clinical trial (96). DFP can ameliorate DSS-induced UC in a mouse model by suppressing ferroptosis (67). The aforementioned studies indicate that iron chelators are promising therapeutic options for autoimmune disease. However, larger clinal trials are needed to determine the value of iron chelators in the therapy of autoimmune disease.
16. Lipophilic antioxidants
Ferrostatin-1 and liproxstatin-1 are well-known inhibitors of ferroptosis. Numerous studies have investigated these ferroptosis inhibitors in autoimmune diseases, including AIH, IBD and PD (48,97). To the best of our knowledge, however, examinations have yet to be conducted in models other than experimental mouse models. Hence, clinical trials must be performed to explore their roles in patients.
Vitamin E is a key lipid soluble antioxidant that can suppress ferroptosis by inhibiting 15-lipoxygenase (98). Reports show that supplementation with vitamin E relieves joint pain in patients with RA (99). In patients with SLE, vitamin E is said to suppress autoantibody production. Moreover, vitamin E improves functional capacity and gait parameters in patients with relapsing-remitting MS (100) and improves clinical signs and metabolic status in patients with PD (101). Furthermore, supplementation with vitamin E is a feasible option for the management of patients with severe forms of psoriasis as it decreases the markers of oxidative stress (102). Vitamin E treatment, therefore, may be a therapeutic option for autoimmune diseases.
Selenium, an essential trace element with antioxidant properties, has been assessed as a potential treatment for autoimmune diseases. Supplementation with selenium relieves inflammatory reaction in patients with MS (103), IBD (104) and psoriasis (102). However, it has shown no significant clinical benefit against RA (105,106). In preclinical studies, selenium decreases loss of dopamine and slows the progression of neurodegeneration during PD (107).
N-acetylcysteine, a pharmaceutical drug with an anti-ferroptosis property, has been investigated in the treatment of RA and SLE. In patients with RA, the oral administration of N-acetylcysteine relieves severity of joint pain and improves physical performance (108). In patients with SLE, N-acetylcysteine inhibits lupus disease activity (109).
Polyphenols are natural antioxidants that prevent ferroptosis owing to their ROS scavenging property (110). Resveratrol, a well-studied polyphenol, decreases disease activity score assessment for 28 joints in patients with RA (111), decreases the clinical colitis activity index score and improves quality of life in patients with UC (112,113) and exerts antidiabetic and antioxidant effects in patients with IDDM (114). Other polyphenols, such as pomegranate juice could alleviate disease activity of patients with RA (115), and licorice could improve symptoms in patients with PD (116).
Although the aforementioned experiments posited lipophilic antioxidants as having potential role in the therapy of autoimmune disease, larger and more in-depth studies are required to determine the exact impact of various polyphenols in the treatment of autoimmune disorder.
17. Conclusion
The present review summarized research on ferroptosis in autoimmune disorders and discussed ferroptosis as a promising therapeutic target. Although autoimmune diseases are heterogeneous in manifestation, there are commonalities between these disorders with respect to ferroptosis. Among these key commonalities is inflammation (117,118). As an important part of regulated cell death, ferroptosis stimulates release of DAMPs and inflammatory cytokines, leading to activation of immune response and eventually promoting the development of autoimmune disease.
Recently, the role of ferroptosis in autoimmune diseases has been reviewed. Fan et al (11) highlighted crosstalk between ferroptosis and different immune cells and discussed the role of ferroptosis in autoimmune disease and Lai et al (10) also discussed how ferroptosis contributes to the pathogenesis of SLE, RA and IBD. However, the autoimmune diseases included in the aforementioned reviews are relatively limited and did not summarize the association between ferroptosis and autoimmune response. Hence, the present review is more comprehensive and may provide more information about the association between ferroptosis and autoimmune disease.
Even though recent evaluations have investigated ferroptosis in autoimmune disorders, the association between this cell death type and autoimmune diseases is relatively undeveloped. Therefore, more studies are required to determine the association between ferroptosis and autoimmune disease, including Graves' disease, Hashimoto thyroiditis, coeliac disease, Addison disease and autoimmune myocarditis and polyendocrine syndrome type 2. Additionally, although numerous ferroptosis-related reagents have been investigated in the treatment of various autoimmune diseases, the reported efficacy pertains mainly to basic studies, patient sample sizes and follow-up periods were relatively limited. Hence, larger clinical trials must be performed to highlight and confirm the treatment values of ferroptosis-associated regents.
In summary, ferroptosis plays a critical role in the pathogenesis of autoimmune diseases and is a promising therapeutic target for autoimmune diseases.
Acknowledgements
Figures were produced by Figdraw (www.figdraw.com), we express our gratitude.
Funding Statement
Funding: The present study was supported by the Zhejiang Provincial Science Foundation of China (grant no. LGF21H020006), the Pioneer Innovation Team of Jiaxing Institute of Atherosclerotic Diseases (grant no. XFCX-DMYH), Program of the First Hospital of Jiaxing (grant no. 2021-YA-001), the Jiaxing Key Laboratory of Arteriosclerotic Diseases (grant no. 2020-dmzdsys) and Jiaxing Key Laboratory of Virus-mediated Infectious Diseases (grant no. 2021-bdzdsys).
Availability of data and materials
Not applicable.
Authors' contributions
LS and XW drafted the manuscript. CZ edited the manuscript. YC designed the study and revised the manuscript. All authors have read and approved the final manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez SW, Sviderskiy VO, Terzi EM, Papagiannakopoulos T, Moreira AL, Adams S, Sabatini DM, Birsoy K, Possemato R. NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature. 2017;551:639–643. doi: 10.1038/nature24637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JY, Nam M, Son HY, Hyun K, Jang SY, Kim JW, Kim MW, Jung Y, Jang E, Yoon SJ, et al. Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc Natl Acad Sci USA. 2020;117:32433–32442. doi: 10.1073/pnas.2006828117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedmann Angeli JP, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang X, Wang H, Han D, Xie E, Yang X, Wei J, Gu S, Gao F, Zhu N, Yin X, et al. Ferroptosis as a target for protection against cardiomyopathy. Natl Acad Sci USA. 2019;116:2672–2680. doi: 10.1073/pnas.1821022116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park MW, Cha HW, Kim J, Kim JH, Yang H, Yoon S, Boonpraman N, Yi SS, Yoo ID, Moon JS. NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer's diseases. Redox Biol. 2021;41(101947) doi: 10.1016/j.redox.2021.101947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Do Van B, Gouel F, Jonneaux A, Timmerman K, Gelé P, Pétrault M, Bastide M, Laloux C, Moreau C, Bordet R, et al. Ferroptosis, a newly characterized form of cell death in Parkinson's disease that is regulated by PKC. Neurobiol Dis. 2016;94:169–178. doi: 10.1016/j.nbd.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Hayter SM, Cook MC. Updated assessment of the prevalence, spectrum and case definition of autoimmune disease. Autoimmun Rev. 2012;11:754–765. doi: 10.1016/j.autrev.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Cho JH, Gregersen PK. Genomics and the multifactorial nature of human autoimmune disease. N Engl J Med. 2011;365:1612–1623. doi: 10.1056/NEJMra1100030. [DOI] [PubMed] [Google Scholar]
- 10.Lai B, Wu CH, Wu CY, Luo SF, Lai JH. Ferroptosis and autoimmune diseases. Front Immunol. 2022;13(916664) doi: 10.3389/fimmu.2022.916664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan J, Jiang T, He D. Emerging insights into the role of ferroptosis in the pathogenesis of autoimmune diseases. Front Immunol. 2023;14(1120519) doi: 10.3389/fimmu.2023.1120519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohl K, Rauen T, Tenbrock K. Dysregulated neutrophilic cell death in SLE: A spotlight on ferroptosis. Signal Transduct Target Ther. 2021;6(392) doi: 10.1038/s41392-021-00804-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu H, Chen Y, Jing L, Zhai C, Shen L. The link between ferroptosis and cardiovascular diseases: A novel target for treatment. Front Cardiovasc Med. 2021;8(710963) doi: 10.3389/fcvm.2021.710963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kraft VAN, Bezjian CT, Pfeiffer S, Ringelstetter L, Müller C, Zandkarimi F, Merl-Pham J, Bao X, Anastasov N, Kössl J, et al. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent Sci. 2020;6:41–53. doi: 10.1021/acscentsci.9b01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius E, Scheel CH, et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 17.Iwasaki A, Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Wang Y, Guo L, Gao W, Tang TL, Yan M. Interaction between macrophages and ferroptosis. Cell Death Dis. 2022;13(355) doi: 10.1038/s41419-022-04775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stockwell BR, Jiang X. A physiological function for ferroptosis in tumor suppression by the immune system. Cell Metab. 2019;30:14–15. doi: 10.1016/j.cmet.2019.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akirav EM, Ruddle NH, Herold KC. The role of AIRE in human autoimmune disease. Nat Rev Endocrinol. 2011;7:25–33. doi: 10.1038/nrendo.2010.200. [DOI] [PubMed] [Google Scholar]
- 21.Matsushita M, Freigang S, Schneider C, Conrad M, Bornkamm GW, Kopf M. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J Exp Med. 2015;212:555–568. doi: 10.1084/jem.20140857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W, Green M, Choi JE, Gijón M, Kennedy PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569:270–274. doi: 10.1038/s41586-019-1170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao Y, Chen Z, Zhang H, Chen C, Zeng M, Yunis J, Wei Y, Wan Y, Wang N, Zhou M, et al. Selenium-GPX4 axis protects follicular helper T cells from ferroptosis. Nat Immunol. 2021;22:1127–1139. doi: 10.1038/s41590-021-00996-0. [DOI] [PubMed] [Google Scholar]
- 24.Shaw J, Chakraborty A, Nag A, Chattopadyay A, Dasgupta AK, Bhattacharyya M. Intracellular iron overload leading to DNA damage of lymphocytes and immune dysfunction in thalassemia major patients. Eur J Haematol. 2017;99:399–408. doi: 10.1111/ejh.12936. [DOI] [PubMed] [Google Scholar]
- 25.Anupam K, Kaushal J, Prabhakar N, Bhatnagar A. Effect of redox status of peripheral blood on immune signature of circulating regulatory and cytotoxic T cells in streptozotocin induced rodent model of type I diabetes. Immunobiology. 2018;223:586–597. doi: 10.1016/j.imbio.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Muri J, Thut H, Bornkamm GW, Kopf M. B1 and marginal zone B cells but not follicular B2 cells require Gpx4 to prevent lipid peroxidation and ferroptosis. Cell Rep. 2019;29:2731–2744.e4. doi: 10.1016/j.celrep.2019.10.070. [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Xie N, Gao W, Kang R, Tang D. The ferroptosis inducer erastin promotes proliferation and differentiation in human peripheral blood mononuclear cells. Biochem Biophys Res Commun. 2018;503:1689–1695. doi: 10.1016/j.bbrc.2018.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu B, Choi B, Li W, Kim DH. Magnetic field boosted ferroptosis-like cell death and responsive MRI using hybrid vesicles for cancer immunotherapy. Nat Commun. 2020;11(3637) doi: 10.1038/s41467-020-17380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Efimova I, Catanzaro E, Van der Meeren L, Turubanova VD, Hammad H, Mishchenko TA, Vedunova MV, Fimognari C, Bachert C, Coppieters F, et al. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J Immunother Cancer. 2020;8(e001369) doi: 10.1136/jitc-2020-001369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Z, Qiu X, Yan Y, Liang Q, Cai Y, Peng B, Xu Z, Xia F. Evaluation of ferroptosis-related gene AKR1C1 as a novel biomarker associated with the immune microenvironment and prognosis in breast cancer. Int J Gen Med. 2021;14:6189–6200. doi: 10.2147/IJGM.S329031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai E, Han L, Liu J, Xie Y, Kroemer G, Klionsky DJ, Zeh HJ, Kang R, Wang J, Tang D. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. 2020;16:2069–2083. doi: 10.1080/15548627.2020.1714209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li W, Feng G, Gauthier JM, Lokshina I, Higashikubo R, Evans S, Liu X, Hassan A, Tanaka S, Cicka M, et al. Ferroptotic cell death and TLR4/Trif signaling initiate neutrophil recruitment after heart transplantation. J Clin Invest. 2019;129:2293–2304. doi: 10.1172/JCI126428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall SW, Cooke A. Autoimmunity and inflammation: Murine models and translational studies. Mamm Genome. 2011;22:377–389. doi: 10.1007/s00335-011-9338-2. [DOI] [PubMed] [Google Scholar]
- 34.Roh JS, Sohn DH. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. 2018;18(e27) doi: 10.4110/in.2018.18.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20:95–112. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- 36.Schaper F, Westra J, Bijl M. Recent developments in the role of high-mobility group box 1 in systemic lupus erythematosus. Mol Med. 2014;20:72–79. doi: 10.2119/molmed.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boyapati RK, Dorward DA, Tamborska A, Kalla R, Ventham NT, Doherty MK, Whitfield PD, Gray M, Loane J, Rossi AG, et al. Mitochondrial DNA is a pro-inflammatory damage-associated molecular pattern released during active IBD. Inflamm Bowel Dis. 2018;24:2113–2122. doi: 10.1093/ibd/izy095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Freire Boullosa L, Van Loenhout J, Flieswasser T, De Waele J, Hermans C, Lambrechts H, Cuypers B, Laukens K, Bartholomeus E, Siozopoulou V, et al. Auranofin reveals therapeutic anticancer potential by triggering distinct molecular cell death mechanisms and innate immunity in mutant p53 non-small cell lung cancer. Redox Biol. 2021;42(101949) doi: 10.1016/j.redox.2021.101949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu M, Fan Y, Li D, Han B, Meng Y, Chen F, Liu T, Song Z, Han Y, Huang L, et al. Ferroptosis inducer erastin sensitizes NSCLC cells to celastrol through activation of the ROS-mitochondrial fission-mitophagy axis. Mol Oncol. 2021;15:2084–2105. doi: 10.1002/1878-0261.12936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen C, Wang D, Yu Y, Zhao T, Min N, Wu Y, Kang L, Zhao Y, Du L, Zhang M, et al. Legumain promotes tubular ferroptosis by facilitating chaperone-mediated autophagy of GPX4 in AKI. Cell Death Dis. 2021;12(65) doi: 10.1038/s41419-020-03362-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao YY, Lian JX, Lan Z, Zou KL, Wang WM, Yu GT. Ferroptosis promotes anti-tumor immune response by inducing immunogenic exposure in HNSCC. Oral Dis. 2023;29:933–941. doi: 10.1111/odi.14077. [DOI] [PubMed] [Google Scholar]
- 42.Van Loenhout J, Freire Boullosa L, Quatannens D, De Waele J, Merlin C, Lambrechts H, Lau HW, Hermans C, Lin A, Lardon F, et al. Auranofin and cold atmospheric plasma synergize to trigger distinct cell death mechanisms and immunogenic responses in glioblastoma. Cells. 2021;10(2936) doi: 10.3390/cells10112936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin-Sanchez D, Ruiz-Andres O, Poveda J, Carrasco S, Cannata-Ortiz P, Sanchez-Niño MD, Ruiz Ortega M, Egido J, Linkermann A, Ortiz A, Sanz AB. Ferroptosis, but not necroptosis, is important in nephrotoxic folic acid-induced AKI. J Am Soc Nephrol. 2017;28:218–229. doi: 10.1681/ASN.2015121376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X, Duan L, Yuan S, Zhuang X, Qiao T, He J. Ferroptosis inhibitor alleviates radiation-induced lung fibrosis (RILF) via down-regulation of TGF-β1. J Inflamm (Lond) 2019;16(11) doi: 10.1186/s12950-019-0216-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhernakova A, Withoff S, Wijmenga C. Clinical implications of shared genetics and pathogenesis in autoimmune diseases. Nat Rev Endocrinol. 2013;9:646–659. doi: 10.1038/nrendo.2013.161. [DOI] [PubMed] [Google Scholar]
- 46.Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, Vierling JM, Alsawas M, Murad MH, Czaja AJ. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 Practice guidance and guidelines from the American association for the study of liver diseases. Hepatology. 2020;72:671–722. doi: 10.1002/hep.31065. [DOI] [PubMed] [Google Scholar]
- 47.Deng G, Li Y, Ma S, Gao Z, Zeng T, Chen L, Ye H, Yang M, Shi H, Yao X, et al. Caveolin-1 dictates ferroptosis in the execution of acute immune-mediated hepatic damage by attenuating nitrogen stress. Free Radic Biol Med. 2020;148:151–161. doi: 10.1016/j.freeradbiomed.2019.12.026. [DOI] [PubMed] [Google Scholar]
- 48.Zhu L, Chen D, Zhu Y, Pan T, Xia D, Cai T, Lin H, Lin J, Jin X, Wu F, et al. GPX4-regulated ferroptosis mediates S100-induced experimental autoimmune hepatitis associated with the Nrf2/HO-1 signaling pathway. Oxid Med Cell Longev. 2021;2021(6551069) doi: 10.1155/2021/6551069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, Vaysbrot E, McNaughton C, Osani M, Shmerling RH, et al. 2015 American college of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1–26. doi: 10.1002/acr.22783. [DOI] [PubMed] [Google Scholar]
- 50.Fraenkel L, Bathon JM, England BR, St Clair EW, Arayssi T, Carandang K, Deane KD, Genovese M, Huston KK, Kerr G, et al. 2021 American college of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2021;73:1108–1123. doi: 10.1002/acr.24596. [DOI] [PubMed] [Google Scholar]
- 51.Wu J, Feng Z, Chen L, Li Y, Bian H, Geng J, Zheng ZH, Fu X, Pei Z, Qin Y, et al. TNF antagonist sensitizes synovial fibroblasts to ferroptotic cell death in collagen-induced arthritis mouse models. Nat Commun. 2022;13(676) doi: 10.1038/s41467-021-27948-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Floros KV, Cai J, Jacob S, Kurupi R, Fairchild CK, Shende M, Coon CM, Powell KM, Belvin BR, Hu B, et al. MYCN-amplified neuroblastoma is addicted to iron and vulnerable to inhibition of the system Xc-/glutathione axis. Cancer Res. 2021;81:1896–1908. doi: 10.1158/0008-5472.CAN-20-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ling H, Li M, Yang C, Sun S, Zhang W, Zhao L, Xu N, Zhang J, Shen Y, Zhang X, et al. Glycine increased ferroptosis via SAM-mediated GPX4 promoter methylation in rheumatoid arthritis. Rheumatology (Oxford) 2022;61:4521–4534. doi: 10.1093/rheumatology/keac069. [DOI] [PubMed] [Google Scholar]
- 54.Luo H, Zhang R. Icariin enhances cell survival in lipopolysaccharide-induced synoviocytes by suppressing ferroptosis via the Xc-/GPX4 axis. Exp Ther Med. 2021;21(72) doi: 10.3892/etm.2020.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gordon C, Amissah-Arthur MB, Gayed M, Brown S, Bruce IN, D'Cruz D, Empson B, Griffiths B, Jayne D, Khamashta M, et al. The British Society for Rheumatology guideline for the management of systemic lupus erythematosus in adults. Rheumatology (Oxford) 2018;57:e1–e45. doi: 10.1093/rheumatology/kex286. [DOI] [PubMed] [Google Scholar]
- 56.Tsokos GC, Lo MS, Costa Reis P, Sullivan KE. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat Rev Rheumatol. 2016;12:716–730. doi: 10.1038/nrrheum.2016.186. [DOI] [PubMed] [Google Scholar]
- 57.Indrakanti DL, Alvarado A, Zhang X, Birmingham DJ, Hinton A, Rovin BH. The interleukin-6-hepcidin-hemoglobin circuit in systemic lupus erythematosus flares. Lupus. 2017;26:200–203. doi: 10.1177/0961203316659153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen W, Li W, Zhang Z, Tang X, Wu S, Yao G, Li K, Wang D, Xu Y, Feng R, et al. Lipocalin-2 exacerbates lupus nephritis by promoting Th1 cell differentiation. J Am Soc Nephrol. 2020;31:2263–2277. doi: 10.1681/ASN.2019090937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scindia Y, Wlazlo E, Ghias E, Cechova S, Loi V, Leeds J, Ledesma J, Helen C, Swaminathan S. Modulation of iron homeostasis with hepcidin ameliorates spontaneous murine lupus nephritis. Kidney Int. 2020;98:100–115. doi: 10.1016/j.kint.2020.01.025. [DOI] [PubMed] [Google Scholar]
- 60.Li P, Jiang M, Li K, Li H, Zhou Y, Xiao X, Xu Y, Krishfield S, Lipsky PE, Tsokos GC, Zhang X. Glutathione peroxidase 4-regulated neutrophil ferroptosis induces systemic autoimmunity. Nat Immunol. 2021;22:1107–1117. doi: 10.1038/s41590-021-00993-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12:205–217. doi: 10.1038/nrgastro.2015.34. [DOI] [PubMed] [Google Scholar]
- 62.Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res. 2019;2019(7247238) doi: 10.1155/2019/7247238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mayr L, Grabherr F, Schwärzler J, Reitmeier I, Sommer F, Gehmacher T, Niederreiter L, He GW, Ruder B, Kunz KTR, et al. Dietary lipids fuel GPX4-restricted enteritis resembling Crohn's disease. Nat Commun. 2020;11(1775) doi: 10.1038/s41467-020-15646-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu J, Liu S, Cui Z, Wang X, Ning T, Wang T, Zhang N, Xie S, Min L, Zhang S, et al. Ferrostatin-1 alleviated TNBS induced colitis via the inhibition of ferroptosis. Biochem Biophys Res Commun. 2021;573:48–54. doi: 10.1016/j.bbrc.2021.08.018. [DOI] [PubMed] [Google Scholar]
- 65.Xu M, Tao J, Yang Y, Tan S, Liu H, Jiang J, Zheng F, Wu B. Ferroptosis involves in intestinal epithelial cell death in ulcerative colitis. Cell Death Dis. 2020;11(86) doi: 10.1038/s41419-020-2299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen Y, Wang J, Li J, Zhu J, Wang R, Xi Q, Wu H, Shi T, Chen W. Astragalus polysaccharide prevents ferroptosis in a murine model of experimental colitis and human Caco-2 cells via inhibiting NRF2/HO-1 pathway. Eur J Pharmacol. 2021;911(174518) doi: 10.1016/j.ejphar.2021.174518. [DOI] [PubMed] [Google Scholar]
- 67.Chen Y, Zhang P, Chen W, Chen G. Ferroptosis mediated DSS-induced ulcerative colitis associated with Nrf2/HO-1 signaling pathway. Immunol Lett. 2020;225:9–15. doi: 10.1016/j.imlet.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 68.Montalban X, Gold R, Thompson AJ, Otero-Romero S, Amato MP, Chandraratna D, Clanet M, Comi G, Derfuss T, Fazekas F, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Eur J Neurol. 2018;25:215–237. doi: 10.1177/1352458517751049. [DOI] [PubMed] [Google Scholar]
- 69.Hemmer B, Archelos JJ, Hartung HP. New concepts in the immunopathogenesis of multiple sclerosis. Nat Rev Neurosci. 2002;3:291–301. doi: 10.1038/nrn784. [DOI] [PubMed] [Google Scholar]
- 70.Hu CL, Nydes M, Shanley KL, Morales Pantoja IE, Howard TA, Bizzozero OA. Reduced expression of the ferroptosis inhibitor glutathione peroxidase-4 in multiple sclerosis and experimental autoimmune encephalomyelitis. J Neurochem. 2019;148:426–439. doi: 10.1111/jnc.14604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jhelum P, Santos-Nogueira E, Teo W, Haumont A, Lenoël I, Stys PK, David S. Ferroptosis mediates cuprizone-induced loss of oligodendrocytes and demyelination. J Neurosci. 2020;40:9327–9341. doi: 10.1523/JNEUROSCI.1749-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stewart DA. NICE guideline for Parkinson's disease. Age Ageing. 2007;36:240–242. doi: 10.1093/ageing/afm040. [DOI] [PubMed] [Google Scholar]
- 73.Sulzer D, Alcalay RN, Garretti F, Cote L, Kanter E, Agin-Liebes J, Liong C, McMurtrey C, Hildebrand WH, Mao X, et al. T cells from patients with Parkinson's disease recognize α-synuclein peptides. Nature. 2017;546:656–661. doi: 10.1038/nature22815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thapa K, Khan H, Kanojia N, Singh TG, Kaur A, Kaur G. Therapeutic insights on ferroptosis in Parkinson's disease. Eur J Pharmacol. 2022;930(175133) doi: 10.1016/j.ejphar.2022.175133. [DOI] [PubMed] [Google Scholar]
- 75.Zhang P, Chen L, Zhao Q, Du X, Bi M, Li Y, Jiao Q, Jiang H. Ferroptosis was more initial in cell death caused by iron overload and its underlying mechanism in Parkinson's disease. Free Radic Biol Med. 2020;152:227–234. doi: 10.1016/j.freeradbiomed.2020.03.015. [DOI] [PubMed] [Google Scholar]
- 76.Dexter DT, Wells FR, Lees AJ, Agid F, Agid Y, Jenner P, Marsden CD. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson's disease. J Neurochem. 1989;52:1830–1836. doi: 10.1111/j.1471-4159.1989.tb07264.x. [DOI] [PubMed] [Google Scholar]
- 77.Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, Jenner P, Marsden CD. Basal lipid peroxidation in substantia nigra is increased in Parkinson's disease. J Neurochem. 1989;52:381–389. doi: 10.1111/j.1471-4159.1989.tb09133.x. [DOI] [PubMed] [Google Scholar]
- 78.Sofic E, Lange KW, Jellinger K, Riederer P. Reduced and oxidized glutathione in the substantia nigra of patients with Parkinson's disease. Neurosci Lett. 1992;142:128–130. doi: 10.1016/0304-3940(92)90355-b. [DOI] [PubMed] [Google Scholar]
- 79.Zuo Y, Xie J, Li X, Li Y, Thirupathi A, Zhang J, Yu P, Gao G, Chang Y, Shi Z. Ferritinophagy-mediated ferroptosis involved in paraquat-induced neurotoxicity of dopaminergic neurons: Implication for neurotoxicity in PD. Oxid Med Cell Longev. 2021;2021(9961628) doi: 10.1155/2021/9961628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Parisi R, Iskandar IYK, Kontopantelis E, Augustin M, Griffiths CEM, Ashcroft DM. National, regional, and worldwide epidemiology of psoriasis: Systematic analysis and modelling study. BMJ. 2020;369(m1590) doi: 10.1136/bmj.m1590. Global Psoriasis Atlas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arbiser JL, Bonner MY, Ward N, Elsey J, Rao S. Selenium unmasks protective iron armor: A possible defense against cutaneous inflammation and cancer. Biochim Biophys Acta Gen Subj. 2018;1862:2518–2527. doi: 10.1016/j.bbagen.2018.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shou Y, Yang L, Yang Y, Xu J. Inhibition of keratinocyte ferroptosis suppresses psoriatic inflammation. Cell Death Dis. 2021;12(1009) doi: 10.1038/s41419-021-04284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Atkinson MA, Maclaren NK. The pathogenesis of insulin-dependent diabetes mellitus. N Engl J Med. 1994;331:1428–1436. doi: 10.1056/NEJM199411243312107. [DOI] [PubMed] [Google Scholar]
- 84.Zang H, Wu W, Qi L, Tan W, Nagarkatti P, Nagarkatti M, Wang X, Cui T. Autophagy inhibition enables Nrf2 to exaggerate the progression of diabetic cardiomyopathy in mice. Diabetes. 2020;69:2720–2734. doi: 10.2337/db19-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ni T, Huang X, Pan S, Lu Z. Inhibition of the long non-coding RNA ZFAS1 attenuates ferroptosis by sponging miR-150-5p and activates CCND2 against diabetic cardiomyopathy. J Cell Mol Med. 2021;25:9995–10007. doi: 10.1111/jcmm.16890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li S, Zheng L, Zhang J, Liu X, Wu Z. Inhibition of ferroptosis by up-regulating Nrf2 delayed the progression of diabetic nephropathy. Free Radic Biol Med. 2021;162:435–449. doi: 10.1016/j.freeradbiomed.2020.10.323. [DOI] [PubMed] [Google Scholar]
- 87.Kim S, Kang SW, Joo J, Han SH, Shin H, Nam BY, Park J, Yoo TH, Kim G, Lee P, Park JT. Characterization of ferroptosis in kidney tubular cell death under diabetic conditions. Cell Death Dis. 2021;12(160) doi: 10.1038/s41419-021-03452-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bruni A, Bornstein S, Linkermann A, Shapiro AMJ. Regulated cell death seen through the lens of islet transplantation. Cell Transplant. 2018;27:890–901. doi: 10.1177/0963689718766323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Krümmel B, Plötz T, Jörns A, Lenzen S, Mehmeti I. The central role of glutathione peroxidase 4 in the regulation of ferroptosis and its implications for pro-inflammatory cytokine-mediated beta-cell death. Biochim Biophys Acta Mol Basis Dis. 2021;1867(166114) doi: 10.1016/j.bbadis.2021.166114. [DOI] [PubMed] [Google Scholar]
- 90.Fudman EJ, Till GO, Fox IH. Deferoxamine induced decreases of lipid peroxides in rheumatoid arthritis. J Rheumatol. 1987;14:686–691. [PubMed] [Google Scholar]
- 91.Salvarani C, Baricchi R, Lasagni D, Boiardi L, Piccinini R, Brunati C, Macchioni P, Portioli I. Effects of desferrioxamine therapy on chronic disease anemia associated with rheumatoid arthritis. Rheumatol Int. 1996;16:45–48. doi: 10.1007/BF01816434. [DOI] [PubMed] [Google Scholar]
- 92.Lynch SG, Peters K, LeVine SM. Desferrioxamine in chronic progressive multiple sclerosis: A pilot study. Mult Scler. 1996;2:157–160. doi: 10.1177/135245859600200306. [DOI] [PubMed] [Google Scholar]
- 93.Lynch SG, Fonseca T, LeVine SM. A multiple course trial of desferrioxamine in chronic progressive multiple sclerosis. Cell Mol Biol (Noisy-le-grand) 2000;46:865–869. [PubMed] [Google Scholar]
- 94.Febbraro F, Andersen KJ, Sanchez-Guajardo V, Tentillier N, Romero-Ramos M. Chronic intranasal deferoxamine ameliorates motor defects and pathology in the α-synuclein rAAV Parkinson's model. Exp Neurol. 2013;247:45–58. doi: 10.1016/j.expneurol.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 95.Sweeney ME, Slusser JG, Lynch SG, Benedict SH, Garcia SL, Rues L, LeVine SM. Deferiprone modulates in vitro responses by peripheral blood T cells from control and relapsing-remitting multiple sclerosis subjects. Int Immunopharmacol. 2011;11:1796–1801. doi: 10.1016/j.intimp.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Devos D, Moreau C, Devedjian JC, Kluza J, Petrault M, Laloux C, Jonneaux A, Ryckewaert G, Garçon G, Rouaix N, et al. Targeting chelatable iron as a therapeutic modality in Parkinson's disease. Antioxid Redox Signal. 2014;21:195–210. doi: 10.1089/ars.2013.5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang B, Hou S, Huang S, Li H, Li Y. doi: 10.2174/1566524022666220525144630. Ferroptosis inhibitor regulates the disease progression of systematic lupus erythematosus mice model through Th1/Th2 ratio. Curr Mol Med: May 25, 2022 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 98.Hinman A, Holst CR, Latham JC, Bruegger JJ, Ulas G, McCusker KP, Amagata A, Davis D, Hoff KG, Kahn-Kirby AH, et al. Vitamin E hydroquinone is an endogenous regulator of ferroptosis via redox control of 15-lipoxygenase. PLoS One. 2018;13(e0201369) doi: 10.1371/journal.pone.0201369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Edmonds SE, Winyard PG, Guo R, Kidd B, Merry P, Langrish-Smith A, Hansen C, Ramm S, Blake DR. Putative analgesic activity of repeated oral doses of vitamin E in the treatment of rheumatoid arthritis. Results of a prospective placebo controlled double blind trial. Ann Rheum Dis. 1997;56:649–655. doi: 10.1136/ard.56.11.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Aristotelous P, Stefanakis M, Pantzaris M, Pattichis CS, Calder PC, Patrikios IS, Sakkas GK, Giannaki CD. The effects of specific omega-3 and omega-6 polyunsaturated fatty acids and antioxidant vitamins on gait and functional capacity parameters in patients with relapsing-remitting multiple sclerosis. Nutrients. 2021;13(3661) doi: 10.3390/nu13103661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Taghizadeh M, Tamtaji OR, Dadgostar E, Daneshvar Kakhaki R, Bahmani F, Abolhassani J, Aarabi MH, Kouchaki E, Memarzadeh MR, Asemi Z. The effects of omega-3 fatty acids and vitamin E co-supplementation on clinical and metabolic status in patients with Parkinson's disease: A randomized, double-blind, placebo-controlled trial. Neurochem Int. 2017;108:183–189. doi: 10.1016/j.neuint.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 102.Kharaeva Z, Gostova E, De Luca C, Raskovic D, Korkina L. Clinical and biochemical effects of coenzyme Q(10), vitamin E, and selenium supplementation to psoriasis patients. Nutrition. 2009;25:295–302. doi: 10.1016/j.nut.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 103.Odinak MM, Bisaga GN, Zarubina IV. New approaches to antioxidant therapy in multiple sclerosis. Zh Nevrol Psikhiatr Im S S Korsakova. 2002;(Suppl):S72–S75. (In Russian) [PubMed] [Google Scholar]
- 104.Miroliaee AE, Esmaily H, Vaziri-Bami A, Baeeri M, Shahverdi AR, Abdollahi M. Amelioration of experimental colitis by a novel nanoselenium-silymarin mixture. Toxicol Mech Methods. 2011;21:200–208. doi: 10.3109/15376516.2010.547887. [DOI] [PubMed] [Google Scholar]
- 105.Peretz A, Siderova V, Nève J. Selenium supplementation in rheumatoid arthritis investigated in a double blind, placebo-controlled trial. Scand J Rheumatol. 2001;30:208–212. doi: 10.1080/030097401316909549. [DOI] [PubMed] [Google Scholar]
- 106.Tarp U, Overvad K, Thorling EB, Graudal H, Hansen JC. Selenium treatment in rheumatoid arthritis. Scand J Rheumatol. 1985;14:364–368. doi: 10.1111/j.1600-0773.1986.tb02784.x. [DOI] [PubMed] [Google Scholar]
- 107.Zafar KS, Siddiqui A, Sayeed I, Ahmad M, Salim S, Islam F. Dose-dependent protective effect of selenium in rat model of Parkinson's disease: Neurobehavioral and neurochemical evidences. J Neurochem. 2003;84:438–446. doi: 10.1046/j.1471-4159.2003.01531.x. [DOI] [PubMed] [Google Scholar]
- 108.Batooei M, Tahamoli-Roudsari A, Basiri Z, Yasrebifar F, Shahdoust M, Eshraghi A, Mehrpooya M, Ataei S. Evaluating the effect of oral N-acetylcysteine as an adjuvant treatment on clinical outcomes of patients with rheumatoid arthritis: A randomized, double blind clinical trial. Rev Recent Clin Trials. 2018;13:132–138. doi: 10.2174/1574887113666180307151937. [DOI] [PubMed] [Google Scholar]
- 109.Lai ZW, Hanczko R, Bonilla E, Caza TN, Clair B, Bartos A, Miklossy G, Jimah J, Doherty E, Tily H, et al. N-acetylcysteine reduces disease activity by blocking mammalian target of rapamycin in T cells from systemic lupus erythematosus patients: A randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2012;64:2937–2946. doi: 10.1002/art.34502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zheng K, Dong Y, Yang R, Liang Y, Wu H, He Z. Regulation of ferroptosis by bioactive phytochemicals: Implications for medical nutritional therapy. Pharmacol Res. 2021;168(105580) doi: 10.1016/j.phrs.2021.105580. [DOI] [PubMed] [Google Scholar]
- 111.Khojah HM, Ahmed S, Abdel-Rahman MS, Elhakeim EH. Resveratrol as an effective adjuvant therapy in the management of rheumatoid arthritis: A clinical study. Clin Rheumatol. 2018;37:2035–2042. doi: 10.1007/s10067-018-4080-8. [DOI] [PubMed] [Google Scholar]
- 112.Samsami-Kor M, Daryani NE, Asl PR, Hekmatdoost A. Anti-inflammatory effects of resveratrol in patients with ulcerative colitis: A randomized, double-blind, placebo-controlled pilot study. Arch Med Res. 2015;46:280–285. doi: 10.1016/j.arcmed.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 113.Samsamikor M, Daryani NE, Asl PR, Hekmatdoost A. Resveratrol supplementation and oxidative/anti-oxidative status in patients with ulcerative colitis: A randomized, double-blind, placebo-controlled pilot study. Arch Med Res. 2016;47:304–309. doi: 10.1016/j.arcmed.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 114.Movahed A, Raj P, Nabipour I, Mahmoodi M, Ostovar A, Kalantarhormozi M, Netticadan T. Efficacy and safety of resveratrol in type 1 diabetes patients: A two-month preliminary exploratory trial. Nutrients. 2020;12(161) doi: 10.3390/nu12010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ghavipour M, Sotoudeh G, Tavakoli E, Mowla K, Hasanzadeh J, Mazloom Z. Pomegranate extract alleviates disease activity and some blood biomarkers of inflammation and oxidative stress in rheumatoid arthritis patients. Eur J Clin Nutr. 2017;71:92–96. doi: 10.1038/ejcn.2016.151. [DOI] [PubMed] [Google Scholar]
- 116.Petramfar P, Hajari F, Yousefi G, Azadi S, Hamedi A. Efficacy of oral administration of licorice as an adjunct therapy on improving the symptoms of patients with Parkinson's disease, A randomized double blinded clinical trial. J Ethnopharmacol. 2020;247(112226) doi: 10.1016/j.jep.2019.112226. [DOI] [PubMed] [Google Scholar]
- 117.Lin D, Zhang M, Luo C, Wei P, Cui K, Chen Z. Targeting ferroptosis attenuates inflammation, fibrosis, and mast cell activation in chronic prostatitis. J Immunol Res. 2022;2022(6833867) doi: 10.1155/2022/6833867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhao T, Yang Q, Xi Y, Xie Z, Shen J, Li Z, Li Z, Qin D. Ferroptosis in rheumatoid arthritis: A potential therapeutic strategy. Front Immunol. 2022;13(779585) doi: 10.3389/fimmu.2022.779585. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.