Abstract
Background
This study compared the survival outcomes of abdominal radical hysterectomy (ARH) (N = 32), laparoscopic radical hysterectomy (LRH) (N = 61), robot-assisted radical hysterectomy (RRH) (N = 100) and vaginal radical hysterectomy (VRH) (N = 45) approaches for early-stage cervical cancer to identify the surgical approach that provides the best survival.
Methods
Disease-free survival (DFS) and overall survival (OS) were calculated using the Kaplan–Meier method, and survival curves were compared using the log-rank test.
Results
The volume of intraoperative blood loss was greater in the ARH group than in the LRH group, the RRH group or the VRH group [(712.50 ± 407.59) vs. (224.43 ± 191.89), (109.80 ± 92.98) and (216.67 ± 176.78) ml, respectively; P < 0.001]. Total 5-year OS was significantly different among the four groups (ARH, 96.88%; LRH, 82.45%; RRH, 94.18%; VRH, 91.49%; P = 0.015). However, no significant difference in 5-year DFS was observed among the four groups (ARH, 96.88%; LRH, 81.99%; RRH, 91.38%; VRH, 87.27%; P = 0.061).
Conclusion
This retrospective study demonstrated that ARH and RRH achieved higher 5-year OS rates than LRH for early-stage cervical cancer.
Keywords: Cervical cancer, ARH, LRH, RRH, VRH, Survival
Background
Cervical cancer (CC) is one of the most important malignant tumours threatening women’s lives and health worldwide. In 2018, there were approximately 570,000 new cases of CC worldwide, and 311,000 patients died of this disease [1, 2]. Approximately 90% of CC-related deaths occur in developing countries, where the mortality rate is estimated to be 18 times as high as in developed countries [3]. The incidence of CC is 9.9/100,000 in developed countries, and the mortality is 3.3/100,000. Meanwhile, the incidence of CC is 15.7/100,000 in developing countries, and the mortality is 8.3/100,000 [4]. Early-stage CC is usually asymptomatic and can be detected by screening on physical examination. Most outpatient patients with cervical cancer have combined contact bleeding or abnormal vaginal bleeding and/or discharge [5]. Surgery and radiation therapy are primary treatments for CC, and both treatments are thought to achieve similar survival outcomes [6]. However, patients with early-stage CC are usually treated with radical hysterectomy [7].
Laparoscopic surgery became the standard approach for radical hysterectomy in 2014 [8]. Nevertheless, in the phase III Laparoscopic Approach to Cervical Cancer (LACC) trial, minimally invasive surgery (MIS) was associated with lower disease-free survival (DFS) and overall survival (OS) rates than open surgery in patients with early-stage CC [9, 10]. Conversely, current clinical data showed that there was no significant difference in survival outcomes between MIS and open surgery for patients with cervical cancer [11]. These recent findings are contradictory to earlier guidelines, which leads to wide controversy. Therefore, in this study, we summarized the case data of CC patients in a single centre over a span of 5 years (from January 2013 to December 2017) and evaluated the survival outcomes of four different surgical approaches, namely, abdominal, laparoscopic, robot-assisted and vaginal radical hysterectomy (ARH, LRH, RRH and VRH, respectively), for early-stage CC to define the benefits of the different radical hysterectomy approaches.
Methods
Patient enrolment
Patients with early-stage cervical cancer (Stage IA2-IB2) in the Department of Obstetrics and Gynaecology of the First Medical Center of Chinese PLA General Hospital (PLAGH) from January 2013 to December 2017 were analysed. All enrolled patients were treated with radical hysterectomy and grouped according to surgical approach. The patients fully understood the advantages and disadvantages of the various surgical treatments for CC before undergoing surgery and voluntarily chose the surgical approach.
Inclusion criteria
The patients with cervical cancer were diagnosed by TCT, HPV, biopsy and/or conization. Patients had squamous cell carcinoma, adenocarcinoma or adenosquamous carcinoma of the uterine cervix. Patients were diagnosed as stage IA2 (stromal invasion, 3 to 5 mm in depth and < 7 mm in width), IB1 (tumour size of ≤ 4 cm in the greatest dimension) and IB2 (tumour size of > 4 cm in the greatest dimension) according to the 2009 FIGO (International Federation of Obstetrics and Gynaecology) staging system [12]. The patients underwent radical hysterectomy, including ARH, LRH, RRH and VRH, along with laparoscopic pelvic lymphadenectomy. Surgery, perioperative management, related clinical decision-making and postoperative follow-up were performed by the same medical team.
Exclusion criteria
Patients treated with neoadjuvant therapy (chemotherapy or radiotherapy) were excluded. We also excluded patients with higher than stage IB2 disease, those who did not undergo radical hysterectomy, those who were generally in poor condition or had severe diseases and could not tolerate anaesthesia and surgery, those who had other malignant tumours or infectious diseases that were difficult to control, those who were lost to follow-up, those who died from causes not related to CC, and those with incomplete case data.
Cohort selection
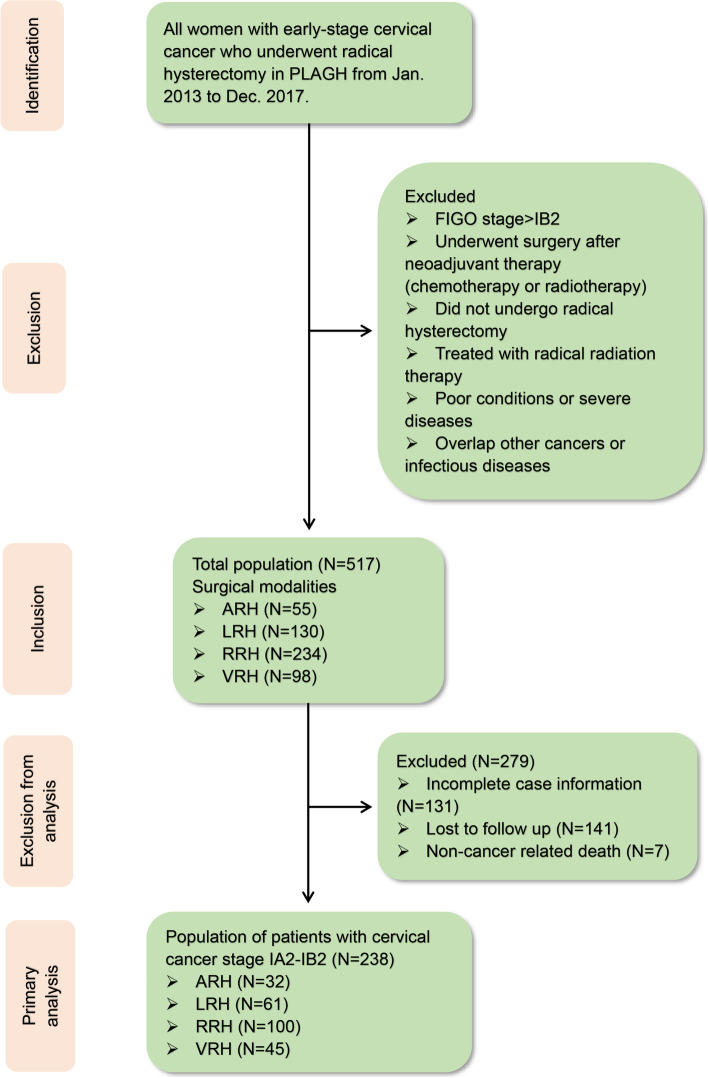
There were 517 patients diagnosed with stage IA2-IB2 CC in the First Medical Centre of PLAGH from January 2013 to December 2017. According to the above inclusion and exclusion criteria, 238 patients were enrolled (Fig. 1). Among them, 32 patients were included in the ARH group, 61 patients were included in the LRH group, 100 patients were included in the RRH group and 45 patients were included in the VRH group.
Fig. 1.
CONSORT (Consolidated Standards of Reporting Trials) diagram. Patients with early-stage cervical cancer who underwent radical surgery from January 2013 to December 2017
Measures
The general information included age, body mass index (BMI), clinical stage and pathological type. The indicators examined in the perioperative period included intraoperative bleeding volume, operation time, blood transfusion rate, postoperative exhaust time, postoperative hospital stay, number of lymph nodes resected, number of positive lymph nodes, length of removed vaginal wall, healthcare costs incurred during hospitalization (surgery, chemotherapy, other drugs, etc.) and major complications. Ultimately, we analysed DFS and OS.
Follow-up
An updated medical history and physical examination were recommended every 3 months for the first year, every 6 months for the following 2 years and then annually thereafter. The tests included routine blood tests, biochemistry, tumour biomarkers, vaginal stump TCT and HPV, chest X-ray/chest CT, pelvic and abdominal CT/MRI or gynaecological ultrasound, urinary ultrasound, ultrasound of the hepatobiliary pancreas and retroperitoneal lymph nodes, and PET-CT/MRI when the patients had suspected recurrence.
Statistical analysis
SPSS 22.0 software was used for statistical analysis. The data are presented as the mean ± SD. One-way ANOVA was used for comparisons among the four groups. A two-sided P-value < 0.05 was considered statistically significant. The DFS and OS were graphed using GraphPad Prism 7.00 and calculated using the Kaplan–Meier method, and survival curves were compared using the log-rank test.
Results
Baseline comparison of the four groups according to different radical hysterectomy approaches
We identified 517 patients who underwent radical hysterectomy for early-stage CC during the inclusion period. Of these, 238 patients (40.03%) were selected for primary analysis (Fig. 1). The majority of the patients had stage IB1 disease (89.92%). The baseline characteristics are summarized in Table 1. Thirty-one patients (96.88%) in the ARH group had stage IB1 disease, of whom 26 patients (81.25%) had tumour histology indicating squamous cell carcinoma, and 16 patients (50.00%) received postoperative adjuvant therapy. Fifty-two patients (85.25%) with stage IB1 disease were included in the LRH group, of whom 49 patients (80.33%) had squamous cell carcinoma according to tumour histology, and 33 patients (54.10%) received postoperative adjuvant therapy. The RRH group included 91 (91.00%) stage IB1 patients, of whom 89 patients (89.00%) had squamous cell carcinoma according to tumour histology, and 55 patients (55.00%) received postoperative adjuvant therapy. The VRH group included 40 patients (88.89%) with stage IB1 disease, of whom 40 (88.89%) had squamous cell carcinoma according to tumour histology, and 22 patients (48.89%) received postoperative adjuvant therapy. However, there were no significant differences in age, BMI, FIGO stage, histology or postoperative adjuvant therapy among the four groups (P > 0.05).
Table 1.
The baseline characteristics of patients with early-stage cervical cancer
| Variables | ARH group | LRH group | RRH group | VRH group | P value |
|---|---|---|---|---|---|
| No. of patients | 32 | 61 | 100 | 45 | |
| Age (years old) | 50.13 ± 8.89 | 48.97 ± 8.59 | 48.64 ± 9.89 | 46.04 ± 8.16 | 0.220 |
| BMI (kg/m2) | 25.42 ± 3.24 | 24.06 ± 2.81 | 24.12 ± 3.50 | 24.15 ± 3.23 | 0.210 |
| FIGO stage | 0.701 | ||||
| IA2 | 0 (0.00%) | 5 (8.20%) | 5 (5.00%) | 3 (6.67%) | |
| IB1 | 31 (96.88%) | 52 (85.24%) | 91 (91.00%) | 40 (88.89%) | |
| IB2 | 1 (3.12%) | 4 (6.56%) | 4 (4.00%) | 2 (4.44%) | |
| Histology | 0.444 | ||||
| Squamous cell carcinoma | 26 (81.25%) | 49 (80.33%) | 89 (89.00%) | 40 (88.89%) | |
| Adenocarcinoma | 6 (18.75%) | 11 (18.03%) | 11 (11.00%) | 4 (8.89%) | |
| Adenosquamous carcinoma | 0 (0.00%) | 1 (1.64%) | 0 (0.00%) | 1 (2.22%) | |
| Postoperative adjuvant therapy | 16 (50.00%) | 33 (54.10%) | 55 (55.00%) | 22 (48.89%) | 0.894 |
BMI Body mass index in kg/cm2, FIGO International Federation for Gynaecology and Obstetrics
Comparison of perioperative indices among the four groups
Then, we compared the perioperative indicators among the four groups. Our data showed that there was no significant difference in postoperative hospital stays, number of removed lymph nodes, number of positive lymph nodes and resected length of the vagina in the four groups (P > 0.05), while the differences in mean surgery time, intraoperative blood loss, postoperative exhaust time and healthcare costs were significantly different (P < 0.05); these data are summarized in Table 2.
Table 2.
Characteristics of the perioperative periods in four groups
| Variables | ARH group | LRH group | RRH group | VRH group | P value |
|---|---|---|---|---|---|
| No. of patients | 32 | 61 | 100 | 45 | |
| Mean surgery time (min) | 182.31 ± 55.75 | 184.34 ± 35.31 | 212.32 ± 57.13 | 139.11 ± 36.54 | < 0.001 |
| Intraoperative blood loss (ml) | 712.50 ± 407.59 | 224.43 ± 191.89 | 109.80 ± 92.98 | 216.67 ± 176.78 | < 0.001 |
| Postoperative hospital stays (day) | 13.88 ± 4.41 | 12.26 ± 6.39 | 11.70 ± 4.84 | 11.51 + 3.29 | 0.150 |
| Postoperative exhaust time (day) | 2.28 ± 0.77 | 1.85 ± 0.70 | 2.11 ± 0.62 | 1.96 ± 0.60 | 0.013 |
| Healthcare cost (× 104, CNY) | 4.54 ± 1.21 | 3.73 ± 1.41 | 6.66 ± 1.32 | 3.12 ± 1.09 | < 0.001 |
| No. of lymph nodes | 22.91 ± 9.74 | 23.59 ± 9.22 | 23.69 ± 10.17 | 21.87 ± 8.64 | 0.739 |
| No. of positive lymph nodes | 0.03 ± 0.18 | 0.21 ± 1.54 | 0.37 ± 1.35 | 0.13 ± 0.46 | 0.467 |
| Resected length of the vagina (cm) | 1.83 ± 0.83 | 1.89 ± 0.70 | 1.61 ± 0.68 | 1.72 ± 0.74 | 0.094 |
Additionally, the mean surgery time in the ARH group was the shortest among the four different groups, whereas RRH had the longest. The ARH group had the largest intraoperative blood loss volume among the four different groups, while the RRH group had the least intraoperative blood loss. However, the postoperative exhaust time in the LRH group was the shortest while that in the ARH group was the longest. Among the four groups, the healthcare costs were the highest in the RRH group.
Comparison of survival outcomes among four different surgical approaches
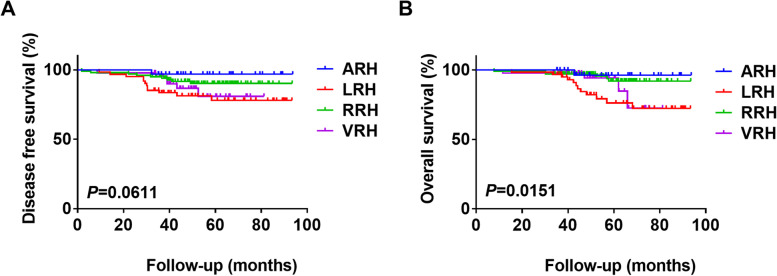
The mean follow-up time of all patients was 57 months (range 43 to 69 months), the interquartile range was 43 months to 68 months, the median follow-up time was 55 months (4.49 years), the 5-year DFS rate was 89.00% (95% CI 88.21–89.81%) and the 5-year OS rate was 91.13% (95% CI 90.07–92.20%). The five-year DFS rate was 96.88% in the ARH group, 81.99% (95% CI 83.21–86.56%) in the LRH group, 91.38% (95% CI 93.03–94.48%) in the RRH group and 87.27% (95% CI 87.85–91.47%) in the VRH group. The overall DFS curves of the four groups were compared using the log-rank test and showed no significant differences (P = 0.061) (Fig. 2A).
Fig. 2.
A Disease-free survival (DFS). B Overall survival (OS)
Furthermore, the 5-year OS rate was 96.88% (95% CI 96.38–97.37%) the ARH group, 82.45% (95% CI 87.17–92.00%) the LRH group, 94.18% (95% CI 96.95–97.23%) the RRH group and 91.49% (95% CI 95.42–96.74%) the VRH group. The overall OS curves of the four groups were compared using the log-rank test and showed statistically significant differences (P = 0.015) (Fig. 2B).
Discussion
This study analysed the clinical data of patients with early-stage CC treated by four different surgical approaches (ARH, LRH, RRH and VRH) in a single centre over 5 years, and we noted no significant differences in DFS among the four groups. However, LRH was associated with shorter OS than ARH or RRH. Therefore, this study showed that not all the survival outcome indicators of MIS were inferior to those of ARH. Furthermore, the intraoperative blood loss and postoperative exhaust time of the three MIS procedures were better than those of ARH. The intraoperative blood loss was lowest in the RRH group, but this group had the highest healthcare costs.
This study is the first retrospective analysis to simultaneously compare the clinical characteristics and survival outcomes of ARH, LRH, RRH and VRH performed by the same medical team in a single centre. Based on our study results, we demonstrated that both ARH and RRH achieved higher 5-year OS than LRH for early-stage CC. Thus, we do not think that robotic surgery is unsafe as standard laparoscopic surgery. Nevertheless, the weakness of this study is the relatively small sample size of each group. We collected 517 patients to analyse the oncological outcomes of different radical hysterectomy approaches. However, based on the inclusive and exclusive criteria, we excluded approximately almost half of the whole data, which cannot allow us to draw definitive conclusions on the survival outcomes of different surgical approaches.
The standard approach for radical hysterectomy is the open abdominal approach. According to the guidelines, radical hysterectomy can be performed via open surgery and MIS. MIS has emerged as one of the preferred approaches for treating gynaecological malignancies [13, 14]. However, recent retrospective reviews and prospective observational studies demonstrated that MIS was associated with lower DFS and OS than open surgery in CC patients. The exact reason why MIS correlates with worse DFS and OS is still unknown. However, there are several potential explanations. These theories are as follows: (I) lower radically using MIS [15], (II) a lack of expertise in minimally invasive hysterectomy compared to open radical hysterectomy [16], (III) an increased risk of developing intraabdominal metastasis due to CO2 [17, 18], and (IV) tumour dissemination at the time of colpotomy [19]. Controversially, robot-assisted MIS obtained similar oncologic outcomes to open surgery. Therefore, the clinical advantages of robot-assisted MIS for the treatment of CC remain to be confirmed.
Since researchers reported the first case of laparoscopic radical cervical cancer [20], laparoscopic surgery and robotic surgery have been widely used in the treatment of CC patients and reported in many relevant clinical studies [21–23]. Most studies focused on perioperative conditions such as intraoperative blood loss, postoperative hospital stay, postoperative exhaust time and survival outcomes. A previous retrospective analysis showed that neither the laparoscopic approach nor the robot-assisted laparoscopic approach reduced the patients’ 5-year progression-free survival (PFS) and OS rates compared with the abdominal approach [24, 25]. Patients undergoing laparoscopy are at higher risk of developing intrapelvic recurrences and peritoneal carcinomatosis [26]. The LACC trial results showed that the 4.5-year PFS and 3-year OS rates in the MIS group were significantly lower than those in the ARH group, and the recurrence rate in early-stage CC patients who underwent MIS (84.4% laparoscopic and 15.6% robotic surgery) was approximately four times that in patients who underwent ARH [27]. Likewise, the SUCCOR study showed that MIS (78.5% laparoscopic and 21.5% robotic surgery) was associated with a lower OS rate than open surgery [28]. Nevertheless, the adoption of open surgery did not correlate with an increase in complication rates in those two analyses [27, 29]. In contrast, clinical data from the Memorial Sloan Kettering Cancer Centre showed that there was no significant difference in survival outcomes between MIS (10% laparoscopic and 90% robotic surgery) and open surgery for patients with CC, while the complication rates of MIS were significantly lower [11]. The adoption of robotic MIS does not seem to compromise survival when compared with open surgery [30]. The proportion of robotic surgery in MIS may be the key factor influencing the outcomes in these findings. Based on these results, compared with open surgery, MIS datasets comprising a low percentage of robotic surgery might lead to lower survival, whereas MIS datasets in which the majority of procedures are robotic surgery might lead to a lack of difference. Therefore, it is necessary to divide MIS into laparoscopic and robotic groups for comparisons with open surgery, as in our study. Moreover, conization may also affect the outcomes. Previous conization in patients undergoing radical hysterectomy was associated with improved DFS and OS compared with that in patients who did not undergo conization [31, 32]. At present, the factors that are most important for prognosis in CC are staging, tumour size, lymph node involvement, depth of stromal invasion and type of LVSI [33, 34]; additionally, the surgeon’s experience [35], the tumour free distance [36], the type of lymphadenectomy [37, 38] and the MIS approach should be adopted in selected cases [39, 40].
Currently, an international multicentre randomized controlled trial (Robot-Assisted Approach to Cervical Cancer, NCT03719547) evaluating the efficacy of robotic surgery and open surgery is underway in China [41]. In addition, although there is limited research on vaginal surgery for CC, it is still one of the surgical treatment options for patients with early-stage CC. A systematic review and meta-analysis showed that laparo-assisted vaginal radical hysterectomy did not appear to affect DFS and OS in early-stage CC patients, comparing with the open approach group of the LACC trial [42]. Nevertheless, MIS and vaginal surgery showed the highest recurrence rate compared to ARH for patients with early-stage CC regarding fertility-sparing treatments in tumours > 2 cm in size [43]. Thus, more studies will be needed to compare the surgical approaches of ARH, LRH, RRH and VRH in the early-stage CC.
In this study, we analysed the clinical data of patients with early-stage CC who underwent ARH, LRH, RRH and VRH performed by the same medical team in a single centre over 5 years and compared the perioperative indicators and survival outcomes. To date, this study is the newest analysis comparing the four different surgical approaches for patients with early-stage CC.
Conclusions
In this retrospective study, we demonstrated that there was no significant difference in the mean age, BMI, FIGO stage, histology or postoperative adjuvant therapy among the ARH, LRH, RRH and VRH groups. The total 5-year OS curves were significantly different among the four groups. Additionally, the 5-year OS rates in the ARH and RRH groups were better than those in the LRH group. The survival outcomes of ARH and RRH were similar for patients with early-stage CC.
Acknowledgements
Not applicable.
Abbreviations
- ARH
Abdominal radical hysterectomy
- BMI
Body mass index
- CC
Cervical cancer
- CONSORT
Consolidated Standards of Reporting Trials
- DFS
Disease-free survival
- FIGO
International Federation of Obstetrics and Gynaecology
- LACC
Laparoscopic approach to cervical cancer
- LRH
Laparoscopic radical hysterectomy
- MIS
Minimally invasive surgery
- OS
Overall survival
- PFS
Progression-free survival
- RRH
Robot-assisted radical hysterectomy
- VRH
Vaginal radical hysterectomy
Authors’ contributions
YM and WF were involved in the study conception and design. NZ, CG, JX and QT were participated in data collection and analysis. XJ and WY drafted and approved this article. LL revised the article critically. All authors have read and approved the manuscript for publication.
Funding
This work was supported by the Chinese PLA military family planning project [grant number 19JSZ15].
Availability of data and materials
Data are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki. The research protocol and procedures were reviewed and approved by the Ethics Committee of the Chinese PLA General Hospital (Approval No. of Ethics Committee: S2022-268–01). Written consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nina Zhang, Xiangshu Jin and Wen Yang contributed equally to this work.
Contributor Information
Wensheng Fan, Email: fanws99@163.com.
Yuanguang Meng, Email: meng6512@vip.sina.com.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Olawaiye AB, Baker TP, Washington MK, Mutch DG. The new (Version 9) American Joint Committee on Cancer tumor, node, metastasis staging for cervical cancer. CA Cancer J Clin. 2021;71:287–298. doi: 10.3322/caac.21663. [DOI] [PubMed] [Google Scholar]
- 3.Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019;393:169–182. doi: 10.1016/S0140-6736(18)32470-X. [DOI] [PubMed] [Google Scholar]
- 4.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 5.Arbyn M, Weiderpass E, Bruni L, de Sanjose S, Saraiya M, Ferlay J, Bray F. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8:e191–e203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noguchi T, Zaitsu M, Oki I, Haruyama Y, Nishida K, Uchiyama K, Sairenchi T, Kobashi G. Recent increasing incidence of early-stage cervical cancers of the squamous cell carcinoma subtype among young women. Int J Environ Res Public Health 2020;17(20):7401. [DOI] [PMC free article] [PubMed]
- 7.Liontos M, Kyriazoglou A, Dimitriadis I, Dimopoulos MA, Bamias A. Systemic therapy in cervical cancer: 30 years in review. Crit Rev Oncol Hematol. 2019;137:9–17. doi: 10.1016/j.critrevonc.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Basaran D, Leitao MM., Jr The landmark series: minimally invasive surgery for cervical cancer. Ann Surg Oncol. 2021;28:204–211. doi: 10.1245/s10434-020-09265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, Buda A, Yan X, Shuzhong Y, Chetty N, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895–1904. doi: 10.1056/NEJMoa1806395. [DOI] [PubMed] [Google Scholar]
- 10.Melamed A, Margul DJ, Chen L, Keating NL, Del Carmen MG, Yang J, Seagle BL, Alexander A, Barber EL, Rice LW, et al. Survival after minimally invasive radical hysterectomy for early-stage cervical cancer. N Engl J Med. 2018;379:1905–1914. doi: 10.1056/NEJMoa1804923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brandt B, Sioulas V, Basaran D, Kuhn T, LaVigne K, Gardner GJ, Sonoda Y, Chi DS, Long Roche KC, Mueller JJ, et al. Minimally invasive surgery versus laparotomy for radical hysterectomy in the management of early-stage cervical cancer: Survival outcomes. Gynecol Oncol. 2020;156:591–597. doi: 10.1016/j.ygyno.2019.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, Barakat R, Pearl ML, Sharma SK. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol. 2012;30:695–700. doi: 10.1200/JCO.2011.38.8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogani G, Borghi C, Leone Roberti Maggiore U, Ditto A, Signorelli M, Martinelli F, Chiappa V, Lopez C, Sabatucci I, Scaffa C, et al. Minimally invasive surgical staging in early-stage ovarian carcinoma: a systematic review and meta-analysis. J Minim Invasive Gynecol. 2017;24:552–562. doi: 10.1016/j.jmig.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Lovell DY, Naumann RW. The benefits of laparoscopic radical hysterectomy for cervical cancer: res ipsa loquitur? J Minim Invasive Gynecol. 2022;29:805–806. doi: 10.1016/j.jmig.2022.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Leitao MM, Jr, Zhou QC, Brandt B, Iasonos A, Sioulas V, Lavigne Mager K, Shahin M, Bruce S, Black DR, Kay CG, et al. The MEMORY Study: MulticentEr study of Minimally invasive surgery versus Open Radical hYsterectomy in the management of early-stage cervical cancer: survival outcomes. Gynecol Oncol. 2022;166:417–424. doi: 10.1016/j.ygyno.2022.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondo E, Yoshida K, Kubo-Kaneda M, Nii M, Okamoto K, Magawa S, Nimua R, Okumura A, Okugawa T, Yamawaki T, et al. Does vaginal cuff creation and avoidance of a uterine manipulator improve the prognosis of total laparoscopic radical hysterectomy for early cervical cancer? A retrospective multicenter study. Cancers (Basel). 2022;14(18):4389. [DOI] [PMC free article] [PubMed]
- 18.Lin F, Pan L, Li L, Li D, Mo L. Effects of a simulated CO2 pneumoperitoneum environment on the proliferation, apoptosis, and metastasis of cervical cancer cells in vitro. Med Sci Monit. 2014;20:2497–2503. doi: 10.12659/MSM.891179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bogani G, Di Donato V, Scambia G, Raspagliesi F, Chiantera V, Sozzi G, Golia D'Auge T, Muzii L, Benedetti Panici P, D'Oria O, et al. Radical hysterectomy for early stage cervical cancer. Int J Environ Res Public Health. 2022;19(18):11641. [DOI] [PMC free article] [PubMed]
- 20.Nezhat C, Nezhat F, Burrell MO, Benigno B, Welander CE. Laparoscopic radical hysterectomy with paraaortic and pelvic node dissection. Am J Obstet Gynecol. 1994;170:699. doi: 10.1016/S0002-9378(94)70251-9. [DOI] [PubMed] [Google Scholar]
- 21.Nam JH, Kim JH, Kim DY, Kim MK, Yoo HJ, Kim YM, Kim YT, Mok JE. Comparative study of laparoscopico-vaginal radical hysterectomy and abdominal radical hysterectomy in patients with early cervical cancer. Gynecol Oncol. 2004;92:277–283. doi: 10.1016/j.ygyno.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez J, Rauh-Hain JA, Saenz J, Isla DO, Rendon Pereira GJ, Odetto D, Martinelli F, Villoslada V, Zapardiel I, Trujillo LM, et al. Oncological outcomes of laparoscopic radical hysterectomy versus radical abdominal hysterectomy in patients with early-stage cervical cancer: a multicenter analysis. Int J Gynecol Cancer. 2021;31:504–511. doi: 10.1136/ijgc-2020-002086. [DOI] [PubMed] [Google Scholar]
- 23.Lee EJ, Kang H, Kim DH. A comparative study of laparoscopic radical hysterectomy with radical abdominal hysterectomy for early-stage cervical cancer: a long-term follow-up study. Eur J Obstet Gynecol Reprod Biol. 2011;156:83–86. doi: 10.1016/j.ejogrb.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 24.Cao T, Feng Y, Huang Q, Wan T, Liu J. Prognostic and safety roles in laparoscopic versus abdominal radical hysterectomy in cervical cancer: a meta-analysis. J Laparoendosc Adv Surg Tech A. 2015;25:990–998. doi: 10.1089/lap.2015.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang YZ, Deng L, Xu HC, Zhang Y, Liang ZQ. Laparoscopy versus laparotomy for the management of early stage cervical cancer. BMC Cancer. 2015;15:928. doi: 10.1186/s12885-015-1818-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bogani G, Ghezzi F, Chiva L, Gisone B, Pinelli C, Dell'Acqua A, Casarin J, Ditto A, Raspagliesi F. Patterns of recurrence after laparoscopic versus open abdominal radical hysterectomy in patients with cervical cancer: a propensity-matched analysis. Int J Gynecol Cancer. 2020;30:987–992. doi: 10.1136/ijgc-2020-001381. [DOI] [PubMed] [Google Scholar]
- 27.Frumovitz M, Obermair A, Coleman RL, Pareja R, Lopez A, Ribero R, Isla D, Rendon G, Bernardini MQ, Buda A, et al. Quality of life in patients with cervical cancer after open versus minimally invasive radical hysterectomy (LACC): a secondary outcome of a multicentre, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 2020;21:851–860. doi: 10.1016/S1470-2045(20)30081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiva L, Zanagnolo V, Querleu D, Martin-Calvo N, Arevalo-Serrano J, Capilna ME, Fagotti A, Kucukmetin A, Mom C, Chakalova G, et al. SUCCOR study: an international European cohort observational study comparing minimally invasive surgery versus open abdominal radical hysterectomy in patients with stage IB1 cervical cancer. Int J Gynecol Cancer. 2020;30:1269–1277. doi: 10.1136/ijgc-2020-001506. [DOI] [PubMed] [Google Scholar]
- 29.Bogani G, Donato VD, Scambia G, Landoni F, Ghezzi F, Muzii L, Panici PB, Raspagliesi F. investigator of the Italian Gynecological Cancer Study G: Practice patterns and 90-day treatment-related morbidity in early-stage cervical cancer. Gynecol Oncol. 2022;166:561–566. doi: 10.1016/j.ygyno.2022.07.022. [DOI] [PubMed] [Google Scholar]
- 30.Jensen PT, Schnack TH, Froding LP, Bjorn SF, Lajer H, Markauskas A, Jochumsen KM, Fuglsang K, Dinesen J, Sogaard CH, et al. Survival after a nationwide adoption of robotic minimally invasive surgery for early-stage cervical cancer - A population-based study. Eur J Cancer. 2020;128:47–56. doi: 10.1016/j.ejca.2019.12.020. [DOI] [PubMed] [Google Scholar]
- 31.Manzour N, Chiva L, Chacon E, Martin-Calvo N, Boria F, Minguez JA, Alcazar JL, Group SS SUCCOR Risk: design and validation of a recurrence prediction index for early-stage cervical cancer. Ann Surg Oncol. 2022;29:4819–4829. doi: 10.1245/s10434-022-11671-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chacon E, Manzour N, Zanagnolo V, Querleu D, Nunez-Cordoba JM, Martin-Calvo N, Capilna ME, Fagotti A, Kucukmetin A, Mom C, et al. SUCCOR cone study: conization before radical hysterectomy. Int J Gynecol Cancer. 2022;32:117–124. doi: 10.1136/ijgc-2021-002544. [DOI] [PubMed] [Google Scholar]
- 33.Queiroz ACM, Fabri V, Mantoan H, Sanches SM, Guimaraes APG, Ribeiro ARG. da Nogueira Silveira Lima JP, Chen MJ, Baiocchi G, da Costa A: Risk factors for pelvic and distant recurrence in locally advanced cervical cancer. Eur J Obstet Gynecol Reprod Biol. 2019;235:6–12. doi: 10.1016/j.ejogrb.2019.01.028. [DOI] [PubMed] [Google Scholar]
- 34.Ronsini C, Anchora LP, Restaino S, Fedele C, Arciuolo D, Teodorico E, Bizzarri N, Zannoni GF, Ferrandina G, Scambia G, Fanfani F. The role of semiquantitative evaluation of lympho-vascular space invasion in early stage cervical cancer patients. Gynecol Oncol. 2021;162:299–307. doi: 10.1016/j.ygyno.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Pedone Anchora L, Bizzarri N, Gallotta V, Chiantera V, Fanfani F, Fagotti A, Cosentino F, Vizzielli G, Carbone V, Ferrandina G, Scambia G. Impact of surgeon learning curve in minimally invasive radical hysterectomy on early stage cervical cancer patient survival. Facts Views Vis Obgyn. 2021;13:231–239. doi: 10.52054/FVVO.13.3.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bizzarri N, Pedone Anchora L, Zannoni GF, Carbone V, Bruno M, Fedele C, Gallotta V, Chiantera V, Avesani G, Gui B, et al. Validation of tumour-free distance as novel prognostic marker in early-stage cervical cancer: a retrospective, single-centre, cohort study. Br J Cancer. 2021;125:561–568. doi: 10.1038/s41416-021-01384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ronsini C, De Franciscis P, Carotenuto RM, Pasanisi F, Cobellis L, Colacurci N. The oncological implication of sentinel lymph node in early cervical cancer: a meta-analysis of oncological outcomes and type of recurrences. Medicina (Kaunas). 2022;58(11):1539. [DOI] [PMC free article] [PubMed]
- 38.Mathevet P, Lecuru F, Uzan C, Boutitie F, Magaud L, Guyon F, Querleu D, Fourchotte V, Baron M, Bats AS. Senticol g: Sentinel lymph node biopsy and morbidity outcomes in early cervical cancer: results of a multicentre randomised trial (SENTICOL-2) Eur J Cancer. 2021;148:307–315. doi: 10.1016/j.ejca.2021.02.009. [DOI] [PubMed] [Google Scholar]
- 39.Bizzarri N, Pedone Anchora L, Kucukmetin A, Ratnavelu N, Korompelis P, Carbone V, Fedele C, Bruno M, Vizzielli G, Gallotta V, et al. Protective role of conization before radical hysterectomy in early-stage cervical cancer: a propensity-score matching study. Ann Surg Oncol. 2021;28:3585–3594. doi: 10.1245/s10434-021-09695-4. [DOI] [PubMed] [Google Scholar]
- 40.Pedone Anchora L, Turco LC, Bizzarri N, Capozzi VA, Lombisani A, Chiantera V, De Felice F, Gallotta V, Cosentino F, Fagotti A, et al. How to select early-stage cervical cancer patients still suitable for laparoscopic radical hysterectomy: a propensity-matched study. Ann Surg Oncol. 2020;27:1947–1955. doi: 10.1245/s10434-019-08162-5. [DOI] [PubMed] [Google Scholar]
- 41.Falconer H, Palsdottir K, Stalberg K, Dahm-Kahler P, Ottander U, Lundin ES, Wijk L, Kimmig R, Jensen PT, Zahl Eriksson AG, et al. Robot-assisted approach to cervical cancer (RACC): an international multi-center, open-label randomized controlled trial. Int J Gynecol Cancer. 2019;29:1072–1076. doi: 10.1136/ijgc-2019-000558. [DOI] [PubMed] [Google Scholar]
- 42.Ronsini C, Kohler C, De Franciscis P, La Verde M, Mosca L, Solazzo MC, Colacurci N. Laparo-assisted vaginal radical hysterectomy as a safe option for minimal invasive surgery in early stage cervical cancer: a systematic review and meta-analysis. Gynecol Oncol. 2022;166:188–195. doi: 10.1016/j.ygyno.2022.04.010. [DOI] [PubMed] [Google Scholar]
- 43.Ronsini C, Solazzo MC, Bizzarri N, Ambrosio D, La Verde M, Torella M, Carotenuto RM, Cobellis L, Colacurci N, De Franciscis P. Fertility-sparing treatment for early-stage cervical cancer >/= 2 cm: a problem with a thousand nuances-a systematic review of oncological outcomes. Ann Surg Oncol. 2022;29:8346–8358. doi: 10.1245/s10434-022-12436-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the corresponding author upon reasonable request.