Abstract
Background
There is limited knowledge of whether cognitive-behavioral therapy (CBT) or second-generation antipsychotics (SGAs) should be recommended as the first-line treatment in individuals at clinical high risk for psychosis (CHRp).
Hypothesis
To examine whether individual treatment arms are superior to placebo and whether CBT is non-inferior to SGAs in preventing psychosis over 12 months of treatment.
Study Design
PREVENT was a blinded, 3-armed, randomized controlled trial comparing CBT to clinical management plus aripiprazole (CM + ARI) or plus placebo (CM + PLC) at 11 CHRp services. The primary outcome was transition to psychosis at 12 months. Analyses were by intention-to-treat.
Study Results
Two hundred eighty CHRp individuals were randomized: 129 in CBT, 96 in CM + ARI, and 55 in CM + PLC. In week 52, 21 patients in CBT, 19 in CM + ARI, and 7 in CM + PLC had transitioned to psychosis, with no significant differences between treatment arms (P = .342). Psychopathology and psychosocial functioning levels improved in all treatment arms, with no significant differences.
Conclusions
The analysis of the primary outcome transition to psychosis at 12 months and secondary outcomes symptoms and functioning did not demonstrate significant advantages of the active treatments over placebo. The conclusion is that within this trial, neither low-dose aripiprazole nor CBT offered additional benefits over clinical management and placebo.
Keywords: ultra-high risk, basic symptoms COGDIS, prodrome, indicated prevention, psychotherapy, second-generation antipsychotics
A state of clinical high risk for psychosis (CHRp) precedes the onset of the first psychotic episode in help-seeking young people in about two-thirds of all cases.1 The operational definition of CHRp allows the prospective identification of people with an incipient risk of transition to psychosis of up to 20% within 24 months and 22% within 36 months,2 with specific CHRp subgroups having a higher risk of developing psychosis.3 The possibility of predicting psychosis has led to numerous trials developing evidence-based programs to prevent the transition to first-episode psychoses.
While some randomized controlled trials (RCTs) in this research area have found contradicting evidence in terms of their preventive efficacy, pairwise meta-analyses showed that psychological or pooled psychological- and pharmacological-targeted interventions in CHRp are superior to standard care, resulting in an overall risk reduction of a transition to psychosis at around 50% at 12 months.4–6 However, not all RCTs,7,8 and recent meta-analyses5 have been without limitations, and network analyses9 and the latest Cochrane review10 failed to find high-quality evidence favoring any specific preventive intervention.
Thus, recommendations to utilize cognitive-behavioral therapy (CBT) as the first means of treatment lack evidence, and further trials are needed. Such trials would have to clarify whether the recommendation of psychological interventions, particularly CBT as a first-line treatment, is empirically justified or whether utilizing second-generation antipsychotics (SGAs) is more effective and should be preferred to CBT, despite their potential side effects. Only one small, single-site RCT involving head-to-head comparisons of CBT and SGAs in the CHRp population is available. This RCT found no significant differences comparing treatment arms; however, it revealed substantial symptomatic improvement in all treatment arms.8 Therefore, there is a need for further methodologically sound, large-scale studies to examine the overall and differential efficacy of CBT and SGAs in people with CHRp. Consequently, the PREVENT trial aimed to answer the following research questions for help-seeking CHRp individuals: (1) Is clinical management plus aripiprazole (CM + ARI) superior to clinical management plus placebo (CM + PLC)? (2) Is CBT more effective than CM + PLC? (3) Is CBT non-inferior to CM + ARI?
Methods
Study Design
PREVENT was a randomized, multicenter, parallel-arm RCT with 3 treatment arms provided over 52 weeks. This trial was double-blinded and placebo-controlled (CM + PLC) for the CM + ARI intervention and single-blinded for the CBT intervention. PREVENT was conducted at 11 German academic CHRp services. Three additional centers were enrolled after the study was initiated to accelerate recruitment.
Details of the study protocol can be found here: https://osf.io/vtq7d/?view_only=631fa13b1ef84d7686bf185e6d89de17
Study Participants
Inclusion criteria entailed being 18–49 years old, having a verbal IQ > 70, and a positive risk assessment to at least 1 of the following: (1) genetic risk and/or schizotypal disorder and functional decline (GRFD); (2) presence of 2 predictive basic symptoms from the “cognitive disturbances” (COGDIS) cluster11 with an at least weekly occurrence in the last 3 months; (3) at least 1 attenuated positive symptom; (4) brief limited intermittent psychotic symptoms.
The exclusion criteria were: previous psychotic episode for longer than 1 week; current or past antipsychotic treatment for longer than 4 weeks; current suicidality or dangerous behavior; current alcohol or substance dependence; organic brain disease; other medical reasons (ie, current or intended pregnancy, lactation, missing a reliable method of contraception, taking medication with anticipated interactions), and living outside of the catchment area. After receiving a complete description of the study, all participants provided written informed consent before commencing with the study.
Randomization and Masking
Participants were randomly assigned to 1 of the 3 treatment arms for 12 months (CM + ARI, CM + PLC, or CBT). Randomization was based on permuted blocks with the allocation ratio 7:5:3, CBT:(CM + ARI):(CM + PLC), stratified by center and the Montgomery–Åsberg Depression Rating Scale (MADRS, <21 vs ≥21).12 The allocation ratio resulted in the smallest number of clients in CM + PLC and was chosen to minimize participants’ risk of receiving nonspecific treatment. The independent Clinical Trials Center provided a central fax service based on prepared randomization lists generated by the Institute of Medical Statistics, Informatics, and Epidemiology, University of Cologne.
The packaging, appearance, color, and taste of the aripiprazole and placebo tablets were identical. Until the closure of the database, neither participants nor all investigators were aware of whether the participants received aripiprazole or placebo. To maintain assessor blindness, raters only had access to the anonymous patient ID. To achieve and sustain blindness regarding the outcomes, all arms were carried out independently of the assessors.
Procedures
CM was provided in addition to aripiprazole or placebo and entailed a maximum of 21 sessions. Initially, CM sessions lasted up to 60 min; the subsequent sessions lasted up to 30 min. CM was a supportive therapy, which included basic advice, symptom monitoring, and psychoeducation about at-risk symptoms and side effects. The sessions’ content was detailed in a manual by Bechdolf et al.13
The initial dose of aripiprazole was 2 mg in the first week, 2–5 mg in the second week, and 2–10 mg in the third week. After that, the maximum dosage was 15 mg. Bristol-Meyers Squibb manufactured aripiprazole and the matching placebo. Aripiprazole was chosen because of its efficacy in early-onset psychosis and its favorable side-effects profile.14
CBT included a maximum of 30 individual sessions of up to 50 min and was provided using a manual developed by Bechdolf et al.13
Supervised therapists trained in early detection and intervention of psychosis provided CM and CBT. See supplementary table 1 for details on CM and CBT. Two masked independent assessors rated session quality with a random selection of tapes to ensure CBT standards in all study centers. We used the Cognitive Therapy Scale for Psychosis (CTS-PSY) to discriminate CBT from CM.15
At 4, 8, 12, 16, 20, 28, 36, 44, and 52 weeks after baseline, blinded assessments were conducted. Antipsychotics or mood stabilizers were not allowed during the 12-month study period.
Outcomes
The primary outcome transition to psychosis at 12 months was defined as one or more of the 5 positive scales of the Structured Interview for Prodromal Symptoms (SIPS/SOPS), rated with a score of 6 and lasting longer than 7 days.
The secondary outcomes were time to transition, psychopathological symptoms, psychosocial functioning, safety, and side effects (supplementary table 2). Adherence was measured as the number of sessions the patient attended in CBT or CM relative to the possible total number of sessions. The number of possible sessions was determined by time-on-study.
Data Analysis
The expected transition risk for PREVENT was based on McGlashan16 (38% for CM + PLC and 16% for CM + ARI and CBT, respectively). We assumed that a 50% reduction in transition rates favoring the active arms should be considered clinically meaningful. Thus, the difference in the transition rates taken for the active and control arms (22%) divided by 2 is the non-inferior margin (11%). Given these assumptions, we calculated a total sample size of N = 380 to establish active treatments’ efficacy and account for 10% follow-up losses. Under these conditions, each related 2-sided test with individual level α = 0.05 will have a power of at least 90%. So the combined power for demonstrating superiority for the active treatments of the trial would be about 80%. Following the intention-to-treat principle, all randomized subjects were included in the analysis. The prespecified per-protocol analysis comprised all patients who attended at least two-thirds (67%) of the treatment sessions during the individual follow-up time; the criterion for the number of sessions was chosen post hoc and judged as clinically meaningful.
To answer the 3 research questions (1) Is CM plus Aripiprazole more effective than CM plus placebo? (2) Is CBT more effective than CM plus Placebo? (3) Is CBT not less effective than CM plus Aripiprazole?) the primary outcome, “transition to psychosis,” was analyzed following a closed testing procedure using (unstratified) -test, starting with the overall test (at a 2-sided significance level of 5%) and followed by pairwise comparisons, each at a local significance level of 5% (2-sided). For the statistical analysis of secondary outcomes and calculation of percentages of transitions, refer to supplementary table 2.
To control for informative (ie, non-ignorable) loss to follow-up, potentially biasing the pre-planned analysis “time to transition,” we introduced the unregistered exploratory post hoc analysis “time-on-study” (ie, time to censoring or time to drop-out). This analysis followed the same test procedure described for analyzing the primary outcome. No adjustments for multiple testing were made in this analysis.
Note that the proportional hazards assumption was violated for the distributions of time-to-event and time-on-study. Thus no hazard ratios are reported.
The quality of psychopathological assessments (intraclass correlations) was calculated according to Fleiss17 using a gold standard rating on the absence or presence of at-risk symptoms) after training masked assessors in the use of interviews.
All calculations were done using SPSS Statistics 27 (IBM Corp., Armonk, NY, USA).
An independent data monitoring and safety committee monitored the incidence rates of serious adverse events (SAEs) for the study duration.
The study was registered as ISRCTN 02658871; EudraCT (2007-001573-28).
Results
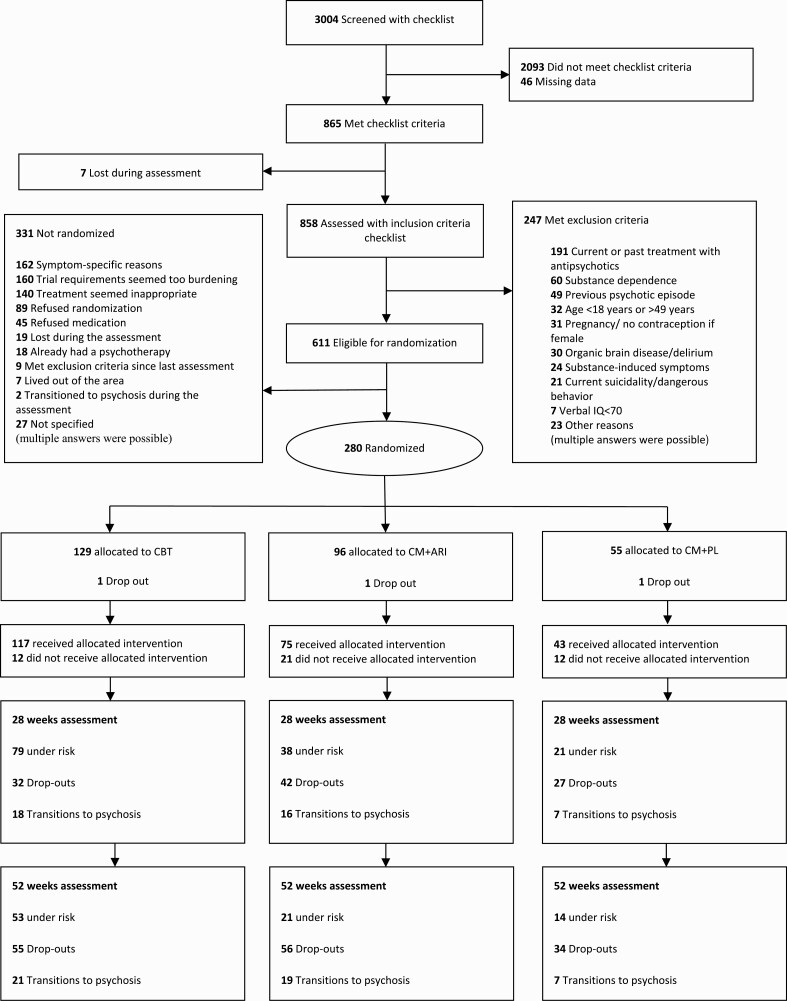
From April, 1 2008 to September 30, 2013, 280 participants were randomized. Two-thirds of the sample were male, and the mean age was 24.4 (SD = 5.1) years. Approximately 50% of the participants were students or trainees, and almost 95% lived independently. The most frequent at-risk states were attenuated positive symptoms (62%) and basic symptom COGDIS criteria (23%). Risk symptoms were reported for up to 1 year in 48%, up to 5 years in 40%, and up to 10 years in 12% of cases. Patients were mainly referred by mental health professionals (about 76%) or self-referrals (19.5%). The available Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) assessments (n = 203) showed that 132 participants (77%) already fulfilled the criteria of at least 1 Axis I diagnosis (table 1; supplementary table 3), with no significant differences between the treatment groups. The 2 most commonly used substances were alcohol and cannabis. There was no difference in substance use between groups (as indicated in the supplementary table 3).
Table 1.
Baseline Characteristics for the Overall Sample and the 3 Treatment Arms
| CBT (n = 129) | CM + ARI (n = 96) | CM + PLC (n = 55) | |
|---|---|---|---|
| Age (years) | 24.2 (5.4) | 24.2 (5.0) | 24.9 (5.4) |
| Male (%)/Female (%) | 78 (60.5)/51 (39.5) | 71 (74.0)/25 (26.0) | 33 (60.0)/22 (40.0) |
| Grown-up in German-speaking countries | 118 (91.5%) | 85 (89.5%) | 53 (96.4%) |
| Employment status | |||
| Full/part-time | 29 (22.5%) | 14 (16.8%) | 9 (16.3%) |
| Student/training | 63 (48.9%) | 49 (51.6%) | 26 (47.3%) |
| Unemployed/other | 30 (23.2%) | 22 (25.3%) | 18 (32.7%) |
| Livelihood | 37 (28.7%) | 22 (23.4%) | 14 (25.5%) |
| own | 68 (52.7%) | 47 (50.0%) | 26 (47.3%) |
| other | |||
| Living | |||
| Independent | 72 (55.8%) | 60 (62.5%) | 33 (60.0%) |
| Parents, family/supervision | 58 (44.9%) | 35 (36.4%) | 23 (41.8%) |
| Entry criteria | |||
| BLIPS | 6 (4.7%) | 6 (6.3%) | 4 (7.3%) |
| APS | 101 (78.3%) | 62 (64.6%) | 35 (63.6%) |
| BS-COGDIS | 73 (56.6%) | 55 (57.3%) | 24 (43.6.9%) |
| GRFD | 18 (14.0%) | 11 (11.5%) | 15 (27.3%) |
| SIPS | |||
| Positive | 7.4 (4.0) | 6.4 (4.0) | 7.7 (5.3) |
| Negative | 10.7 (5.7) | 9.8 (5.8) | 11.1 (5.7) |
| Disorganization | 3.5 (2.3) | 3.8 (2.6) | 3.6 (2.9) |
| General | 8.0 (3.5) | 7.4 (3.8) | 8.4 (3.5) |
| COGDIS | 12.3 (10.7) | 10.6 (8.4) | 12.3 (10.7) |
| SOFAS | 52.5 (12.8) | 53.2 (12.8) | 51.8 (12.9) |
| MADRS | 20.1 (7.7) | 19.2 (8.0) | 20.8 (7.8) |
We do not report all categories; therefore, the values do not add up to 100%.
Note: APS, attenuated positive symptoms; ARI, aripiprazole; BLIPS, brief limited intermittent psychotic symptoms; BS-COGDIS, basic symptoms of the “cognitive disturbances” cluster; CBT, cognitive-behavioral therapy; CM, clinical management; COGDIS, Predictive basic symptoms cluster “cognitive disturbances”; GRFD, genetic risk, and functional decline; PLC, placebo; MADRS, Montgomery-Åsberg Depression Rating Scale; SIPS, Structured Interview for Prodromal Symptoms and its companion Scale of Prodromal Symptoms (SOPS); SOFAS, Social and Occupational Functioning Assessment Scale.
Primary Outcome “Transition to Psychosis”
At 12 months, 21 of 129 patients allocated to CBT, 19 of 96 patients in CM + ARI, and 7 of 55 patients in CM + PLC had transitioned to psychosis (see figure 1 for CONSORT diagram). There was no significant difference in the overall test ( (2) = 1.29 test, P = .524, 2-sided) or any pairwise post hoc exploratory comparisons. CBT was non-inferior to CM + ARI regarding the pre-fixed margin of 11% ( (2) = 1.22, P = .260). The transition rates at 6 months were 18 patients in CBT, 16 in CM + ARI, and 7 in CM + PL.
Fig. 1.
CONSORT diagram of participants flow in the PREVENT trial.
Secondary Outcome Measures
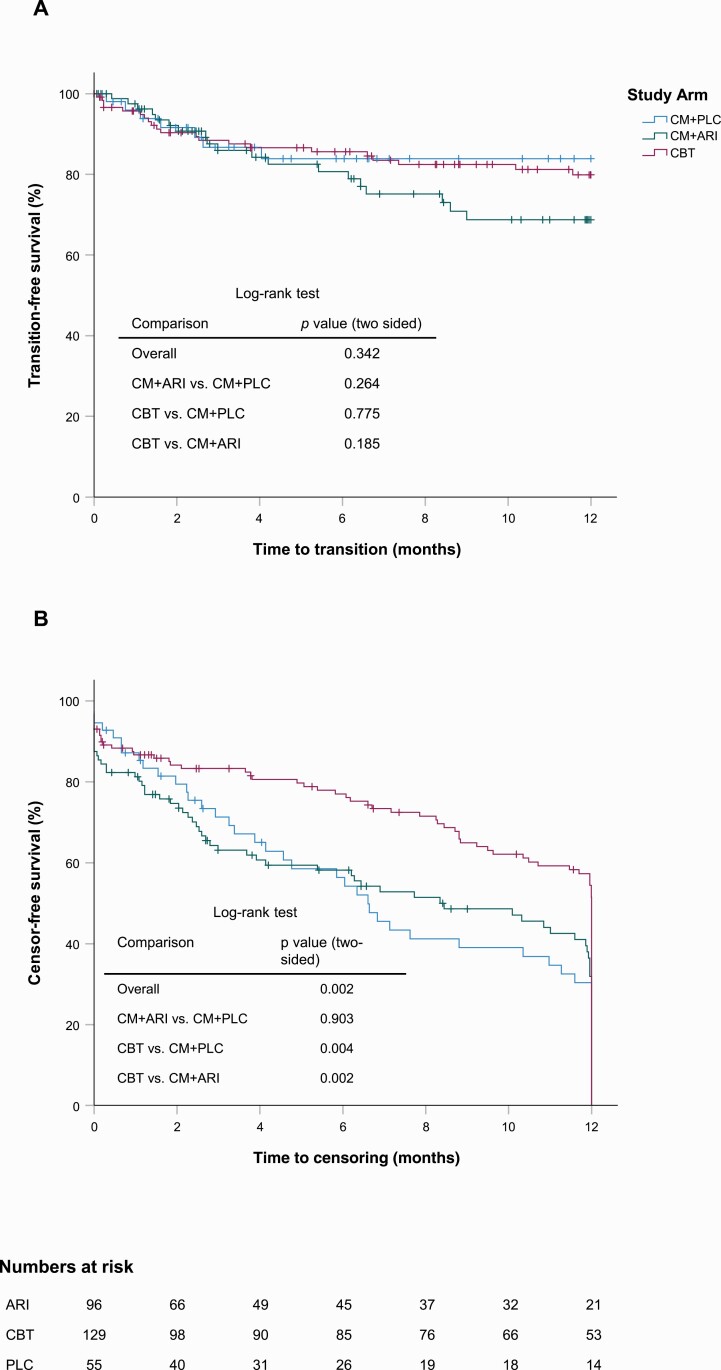
The cumulative transition rate at 12 months was 20.1% in CBT (95% CI = 12.3–28.0), 31.3% in CM + ARI (95% CI = 19.2–43.3), and 16.1% in CM + PLC (95% CI = 5.0–27.2). There was no significant difference in the overall test (log-rank test, P = .342, 2-sided) or any of the pairwise post hoc exploratory comparisons (ie, CM + ARI vs CM + PLC [P = .264], CBT vs CM + PLC [P = .775], CBT vs CM + ARI [P = .185]). Overall, 22.5% (95% CI = 17.0–28.8) of all participants transitioned to psychosis within 52 weeks (see figure 2A for the Kaplan–Meier estimates).
Fig. 2.
A) Kaplan-Meier plots of the outcome time to transition to psychosis. B) Reverse Kaplan-Meier plot of the censoring pattern.
CBT was non-inferior to CM + ARI regarding the prefixed non-inferiority margin of 11%. The observed difference in the cumulative transition rate at 12 months was −14.6% (95% CI = −29.7–0.5).
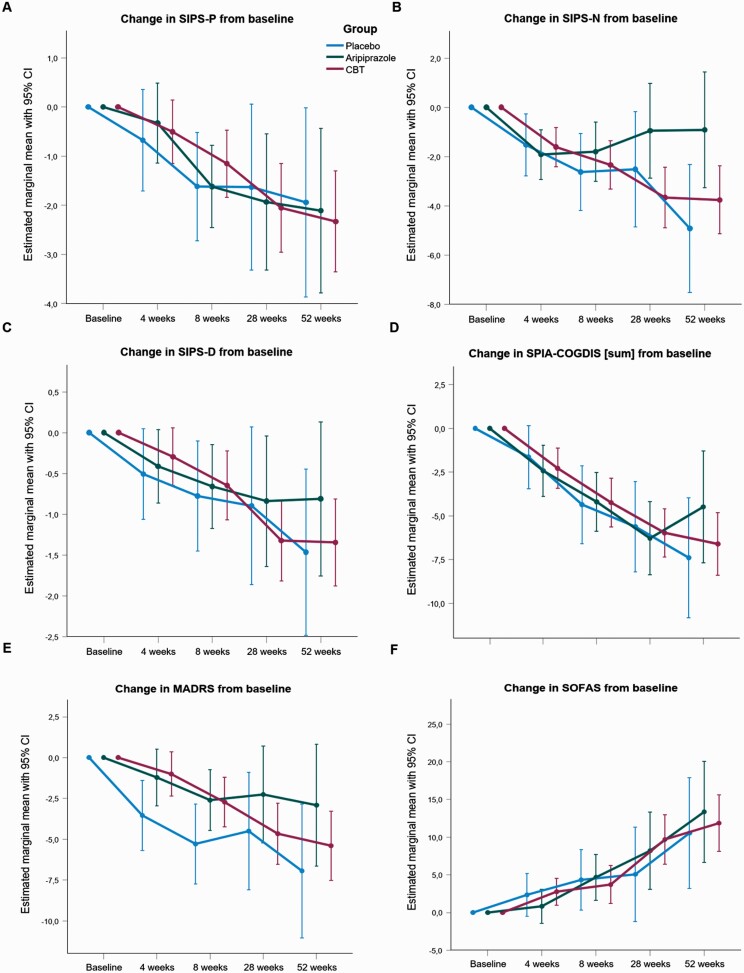
No statistically significant difference between the treatment arms indicated by significant treatment × time interactions at weeks 4, 8, 28, and 52 (figure 3 and supplementary table 5) were seen for SIPS-positive, SIPS-negative, SIPS-disorganization, SIPS-general, COGDIS, MADRS, and the Social and Occupational Functioning Assessment Scale (SOFAS). However, change from baseline in symptoms, and psychosocial functioning as measured by the SIPS-positive, SIPS-disorganization, COGDIS, MADRS, and SOFAS was significant in all treatment arms.
Fig. 3.
Linear mixed models fixed effects (change from baseline ) plots A) of (attenuated) positive symptoms (SIPS-P), B) negative symptoms (SIPS-N), C) disorganized symptoms (SIPS-D), D) predictive basic symptoms' cognitive disturbances' cluster (COGDIS), E) Montgomery-Åsberg Depression Rating Scale (MADRS), F) Social and Occupational Functioning Assessment Scale (SOFAS).
Per-protocol Analysis
In the per-protocol analysis, 16 of 101 patients allocated to CBT, 19 of 78 patients in CM + ARI, and 7 of 42 patients in the CM + PLC had transitioned to psychosis at week 52. The overall test was not significant ( [2] = 2.25, P = .323). In the exploratory pairwise comparisons, CBT was not superior to CM + PLC (P = .140), yet patients in CBT fared better than those in CM + ARI (P = .002). CM + ARI showed no advantage compared to CM + PLC (P = .165).
Safety, Adverse Events, and Side-Effects
During the trial and 30 days posttrial, 18 SAEs were reported, affecting 7 patients in the CM + ARI and 9 in CBT. Five non-psychiatric SAEs were reported in CBT. All SAEs were rated as unlikely or not related to the intervention. Four non-psychiatric SAEs were reported in CM + ARI, 2 of which were assessed as probably/likely being related to aripiprazole. Three psychiatric SAEs were reported in CBT (suicidal ideations), and all were rated as unlikely to be related to the intervention. Finally, 6 psychiatric SAEs were reported in CM + ARI, 1 of which was rated as probably/likely related to aripiprazole. No SAEs were reported in CM + PLC. A detailed list of SAEs and ratings can be found in supplementary table 7.
The treatment arms differed in the side effects measured by Extrapyramidal Symptom Rating Scale (ESRS) sum-scores at 8 weeks (U = 1423, P = .008), 18 weeks (U = 1624.5, P = .027), and 52 weeks (U = 185.0, P = .001). There were more extrapyramidal symptoms in CM + ARI than in CBT at 8 weeks (U = 1321.0, P = .009) and 52 weeks (U = 185.0, P = .001). The sub-score parkinsonism of the ESRS differed between the treatment arms at 8 weeks (U = 1420.0, P = .007), 18 weeks (U = 1660.5, P = .047), and 52 weeks (U = 185.0, P = .001). More akathisia was rated in CM + ARI than in CBT at 8 (U = 1407.5, P = .023) and 40 weeks (U = 461.0, P = .008). At 4 weeks, patients reported more tardive dyskinesia in CM + ARI than in CBT (U = 2044.5, P = .021).
There was more parkinsonism in CM + ARI than in CM + PLC at 8 weeks (U = 488.0, P = .030) and 52 weeks (U = 51.0, P = .037).
The treatment arms differed in the assessors’ global rating of interference by side effects with the patient’s daily performance (UKU) at 4 weeks (U = 1752.5, P = .020), 8 weeks (U = 1095.0, P < .001), and 18 weeks (U = 1486.5, P = .004).
Compared to CM + PL, CBT had no more side effects as measured by the ESRS or UKU.
Patients’ Preferences
Most patients (57.2%) would have preferred to be randomized to CBT, 16.7% preferred CM + ARI, 0.7% chose CM + PLC, and 25.4% stated that they had no preference regarding allocation (χ2[2] = 187.5, P < .0001). The conclusion is that within
Therapy Dose
The median (range) number of attended sessions was 18 (0–41) for CBT and 7 (0–30) for CM + ARI and CM + PLC. It was calculated from the overall pill count that the median dose of aripiprazole was 3.8 mg per day (range = 0.32–24), taken for an average of 164 (SD = 129) days.
Adherence
Of the patients randomized to CM + ARI or CM + PLC, 13% presented with an adherence rate of less than 50%, 8.7% with an adherence rate of 50%–67%, and 78.3% with an adherence rate of more than 67%. In CBT, 12.5% of patients had an adherence rate of less than 50%, 8.9% had an adherence rate of 50%–67%, and 78.6% had an adherence rate of more than 67%. Adherence rates did not differ between study arms (P = .983).
Concomitant Medication
PREVENT study psychiatrists prescribed antidepressants for 30% of patients in CBT, 40% in CM + ARI, and 35% in CM + PLC (P = .390). Anxiolytics were prescribed for 3.6% of the patients in CBT, 2.9% in CM + ARI, and 2.5% in CM + PLC (P = .990).
Quality of CBT
Audiotaped treatments of 119 sessions assessed the quality of CBT, confirming that specific cognitive-behavioral techniques were applied in CBT (n = 87, CTS-PSY mean [SD] = 20.4 [2.6]) and that CBT techniques were absent in CM (n = 32, CTS-PSY mean [SD] = 6.7 [1.9]; t[73.9] = 30.54, P < .001). CBT quality was rated good, as indicated by a mean global rating of 43.9 (SD = 4.8).15
Inter-Rater Reliability
The intraclass correlations of the masked assessor’s ratings ranged from good (0.69) to excellent (0.98).17
Time-on-Study
The distributions of the exploratory post hoc analysis time-on-study differed significantly in the 3 arms (2-sided log-rank test, P = .002; mean ± SE time-on-study for CM + PLC 7.9 ± 0.7, CM + ARI 8.9 ± 0.5 and CBT 10.0 ± 0.3 months). The cumulative drop-outs at 6 months were 32 of 129 patients in CBT, 42 of 96 in CM + ARI, and 27 of 55 in CM + PLC, and at 12 months, the rates were 55 in CBT, 56 in CM + ARI, and 34 in CM + PLC (see figure 2B for the Kaplan–Meier estimates). Compared to CBT, there were more premature terminations in CM + ARI (P = .002) and CM + PLC (P = .004).
Of participants in CM + PLC and CM + ARI, 25% dropped out of the trial because they did not want to take antipsychotics, 10% felt that the treatment was inappropriate, 6% had side effects, 6% met exclusion criteria, 5% did not participate in therapy or ratings, 15% gave other reasons, and 11% withdrew their consent. In CBT, 16% met exclusion criteria (mainly due to treatment outside the study), 9% felt that the treatment was too burdensome, for 7% treatment was inappropriate, 7% felt that due to remission, they needed no more treatment, 5% did not participate in treatment or ratings, 5% gave other reasons, and 23% withdrew their consent (multiple responses were possible). Data on the reasons for drop-out from the study were available in 70% of the cases.
Discussion
The PREVENT trial was the first multicenter trial to compare the effects of CBT, SGAs, CM, and placebo on preventing transition to psychosis.
Analysis of the primary outcome revealed no significant difference between the three treatment arms (CBT vs CM + ARI vs CM + PLC) in patients transitioning to psychosis. Exploratory pairwise comparisons showed that both active arms were not superior to CM + PL (research questions 1 and 2), and CBT was non-inferior to CM + ARI in preventing a transition to psychosis. Thus, research questions 1 (Is CM + ARI superior to CM + PLC?) and 2 (Is CBT superior to CM + PLC?) have to be answered negatively. Research question 3 (Is CBT non-inferior to CM + ARI?) has to be answered positively, meaning that CBT is non-inferior to CM + ARI.
The results that neither CBT nor CM + ARI is more effective than CM + PL align with findings published in the meta-analysis by Davies et al.,9 who states a “lack of evidence to favor specific preventive interventions in psychosis,” as well as with conclusions drawn in the Cochrane review by Kuharic et al.10 which states that “no firm conclusions can be made” regarding preventive interventions in psychosis. Therefore, the rationale of most meta-analyses and guidelines,4–6,18 which suggest the preference of utilizing CBT as the first-line treatment in patients at CHRp, remains derived from a pragmatical rather than evidence-based notion. Of note is that some of the secondary outcomes and exploratory analyses support the idea of utilizing CBT as the first-line treatment for people with CHRp: Participants preferred to be randomized to CBT, and patients in the “pill arms” left the trial earlier. Most patients discontinued CM + ARI or CM + PL because they did not want to take antipsychotics, considered the treatment inappropriate, or experienced side effects. Overall, these findings and the results from the side-effects ratings add to the evidence that CBT is more acceptable and tolerable in people with CHRp than low-dose aripiprazole. Nevertheless, besides the AEs and SAEs, the side effects of psychotherapy were not assessed by a specific scale.19
In line with the results of the primary outcomes, analysis of the secondary outcomes, such as psychopathology and psychosocial functioning, showed no significant differences between the treatment arms. The secondary outcomes analyses revealed that all patients improved over time regardless of the allocated treatment arm. This improvement in all treatment arms suggests that CM may have acted as an effective active treatment, which is not inferior to aripiprazole and CBT.
The conclusion regarding CM being an effective treatment may also explain why our findings showed no superior effects when comparing CM + ARI vs CM + PLC or CBT vs CM + PLC. Considering that CM may have been an active and effective treatment, it is highly likely that CM affected the primary outcome. The greater the suspected effect of CM on transition rates, the more difficult it is to demonstrate the preventive superiority of ARI or CBT. Manualized and supervised CM was offered in specialized academic CHRp services following a high-quality standard, which can be expected to have preventive effects when utilized alone.
However, there are also alternative explanations as to why CBT and ARI were not more effective compared to placebo regarding the transition to psychosis. One possible answer may be that methodological limitations might have contributed to the nonsignificant results:
Not all planned participants could be recruited. Rather than 380 participants, we could only randomize 280 participants. The 12 months transition rate of 22.9% was much lower than the initially projected rate of 38% for the CM + PLC arm. The lower-than-expected recruitment and transition rates might have contributed to the inability of the study to detect significant differences between the treatment arms. However, no significant differences between the treatment arms in the well-powered symptomatic and functional outcomes were found.
Another possible explanation why CBT and ARI were not more effective compared to placebo is that approximately half of the baseline sample were students or trainees, suggesting a high level of functioning. This high functional level may have contributed to an overall good outcome, leading to a ceiling effect masking the treatment effects. A similar observation was that positive symptom severity of the PREVENT baseline sample was at the lower bound of the previously reported samples defined by SIPS criteria alone.20 The overall low positive symptoms of our sample might have limited the range of potential improvement in the efficacy analyses, and the results may not generalize to groups with higher baseline positive symptoms.
The prescribed amount of aripiprazole (median 3.8 mg/day) was about one-third to one-quarter of the recommended dose for full-blown psychosis. Olanzapine and risperidone have been described in similarly low dosages (Olanzapine: between 5 and 15 mg/day;16 Risperidone: up to 2 mg/day or 1.3 mg on average/day)8 in earlier RCTs in CHRp. Nevertheless, it remains unclear whether higher doses of aripiprazole would have led to fewer transitions to psychosis. The average doses of aripiprazole were recorded by pill counts rather than blood levels of antipsychotics, which could be considered a methodological limitation.
Whether the amount of an average of 18 CBT sessions is sufficient for the treatment of CHRp cannot yet be stated with certainty; however, evidence from routine settings suggests it is adequate.21 Initially, 30 sessions were planned for CBT and 20 for CM. On average, participants in the CBT arm received 11 more sessions than in CM. The equivalent effectiveness of CBT and CM occurred despite this difference.
As a final point, we observed a potentially important difference in the exploratory post hoc analysis time-on-study. Participants in the drug/placebo arms left the study earlier than in CBT. Thus, there might be informative (ie, non-ignorable) loss to follow-up, and the planned primary analysis results are at high risk of bias. These differences in the drop-outs between the treatment arms may also have contributed to the result.
All of the possible reasons mentioned above may have contributed to the fact that CM + ARI and CBT were not superior to CM + PLC in preventing transition to psychosis. However, we speculate that the most plausible explanation for our findings is attributed to the unexpectedly high efficacy of CM in at least a part of the CHRp population.
Our results suggest that adding low-dose aripiprazole to CM or offering CBT does not reduce the transition risk to psychosis more effectively than CM and placebo.8,16,22 These results corroborate findings from the other 3-arm trial, as well as findings from 2 other SGA trials in the field.8,16 In PREVENT, there were substantial improvements in all 3 treatment arms regarding attenuated positive and negative symptoms, basic symptoms from the COGDIS cluster, psychosocial functioning, and depression. These results again support the hypothesis that the high standard of CM provided by specialized CHRp services may, on its own, be an effective treatment for a subsample of the CHRp population.23–25 Future trials investigating the prevention of psychosis should assess the efficacy of CM as a sole treatment entity compared with other treatment approaches.
The PREVENT trial indicated that CM is as effective as CBT. However, the results of our previous RCT showed that a multimodal integrated psychological intervention is superior to CM.26 To understand whether people with CHRp benefit best from CM or if an integrated psychotherapeutic treatment is a superior option, we suggest that future trials adopt a component research design to detect active therapeutic components by comparing CM to CBT and integrated psychological interventions.27
Supplementary Material
Acknowledgments
Andreas Bechdolf has been a consultant and/or advisor to or has received honoraria or grants from Janssen, Johnson & Johnson, LB Pharmaceuticals, Lundbeck, Otsuka, Pfizer and Recordati Pharma. Walter de Millas has received honoraria for lecture and consulting fees from Otsuka. Jürgen Gallinat has received speaker fees from Lundbeck, Janssen-Cilag, Lilly and Otsuka, and research funding from the German Federal Ministry of Education and Research and the German Science Foundation. Alkomiet Hasan has received speaker fees from Janssen, Otsuka and Lundbeck and is also a member of their advisory boards, and he was editor of the WFSBP and DGPPN S3 guidelines on schizophrenia. Georg Juckel has received honoraria from Janssen, Servier, Astra-Zeneca and Trommsdorf. Anne. Karow has been a consultant and/or advisor to or has received honoraria from AstraZeneca, Bristol-Myers Squibb, Lilly Deutschland, Janssen Cilag, Lundbeck, Otsuka Pharma and Roche Deutschland Holding. Martin Lambert has received honoraria for lectures from Otsuka Pharma and Janssen Cilag, consultation from Janssen Cilag as well as grants from the German Research Foundation, the Federal Ministry of Research and Education and the Innovation Fund. Andreas Meyer-Lindenberg received consultancy fees from Boehringer Ingelheim, Elsevier, Brainsway, Lundbeck International Neuroscience Foundation, Sumitomo Dainippon Pharma, Academic Medical Centre of the University of Amsterdam, Synapsis Foundation-Alzheimer Research Switzerland, IBS Centre for Synaptic Brain Dysfunction, Blueprint Partnership, University of Cambridge, German Centre for Neurodegenative Diseases, University of Zürich, L.E.K. Consulting, ICARE Schizophrenia and Science Advances; and fees for lectures/interviews from the Lundbeck International Foundation, Paul-Martini-Foundation, Lilly Germany, Atheneum, Fama Public Relations, Institut d'investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Jansen-Cilag, Hertie Foundation, Bodelschwingh-Clinic, Pfizer, Atheneum, University of Freiburg, Schizophrenia Academy, Hong Kong Society of Biological Psychiatry, Fama Public Relations, Spanish Society of Psychiatry, Reunions I Ciencia S.L. and Brain Centre Rudolf Magnus UMC Utrecht; and grants from Prix Roger de Spolberch, 2016, and the Lilly Neuroscience Clinical Research Award. Thomas Wobrock was a member of the Advisory Board of Janssen Cilag and Otsuka/Lundbeck, has accepted paid speaking engagements for Alpine Biomed, AstraZeneca, Bristol Myers Squibb, Eli Lilly, I3G, Glaxo-Smith-Kline, Janssen Cilag, Novartis, Lundbeck, Otsuka, Roche, Sanofi-Aventis and Pfizer, has received a research grant from AstraZeneca, Cerbomed, I3G and AOK (health insurance company) and received research support from the German Research Foundation and the Federal Ministry of Education and Research. Mathias Zink received a grant from Servier and speaker fees and travel support from Lundbeck, Otsuka, Ferrer, Trommsdorff and Janssen. All other authors have declared that there are no conflicts of interest in relation to the subject of this study. Please see the supplement for further acknowledgments.
Contributor Information
Andreas Bechdolf, Department of Psychiatry, Psychotherapy, and Psychosomatics, Vivantes Klinikum Am Urban and Vivantes Klinikum im Friedrichshain, Berlin, Germany; Department of Psychiatry and Psychotherapy CCM, Charité - Universitätsmedizin Berlin, Berlin, Germany; Department of Psychiatry and Psychotherapy, Faculty of Medicine and University Hospital Cologne, Cologne, Germany.
Hendrik Müller, Department of Psychiatry and Psychotherapy, Faculty of Medicine and University Hospital Cologne, Cologne, Germany.
Martin Hellmich, Department of Psychiatry and Psychotherapy, Faculty of Medicine and University Hospital Cologne, Cologne, Germany; Institute of Medical Statistics and Computational Biology, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany.
Walter de Millas, Department of Psychiatry and Psychotherapy, Charité Universitätsmedizin Berlin Campus Mitte, Berlin, Germany.
Peter Falkai, Department of Psychiatry and Psychotherapy, University Hospital, LMU Munich, Munich, Germany.
Wolfgang Gaebel, Department of Psychiatry and Psychotherapy, Medical Faculty, Heinrich-Heine-University, Dusseldorf, Germany.
Jürgen Gallinat, Department of Psychiatry and Psychotherapy, Charité Universitätsmedizin Berlin Campus Mitte, Berlin, Germany.
Alkomiet Hasan, Department of Psychiatry and Psychotherapy and Psychosomatics Medical Faculty, University of Augsburg, Augsburg, Germany.
Andreas Heinz, Department of Psychiatry and Psychotherapy, Charité Universitätsmedizin Berlin Campus Mitte, Berlin, Germany.
Birgit Janssen, Department of Psychiatry and Psychotherapy, Medical Faculty, Heinrich-Heine-University, Dusseldorf, Germany.
Georg Juckel, Department of Psychiatry, Psychotherapy, and Preventive Medicine, Ruhr University Bochum, Bochum, Germany.
Anne Karow, Department of Psychiatry and Psychotherapy, Centre for Psychosocial Medicine, University Medical Centre Hamburg-Eppendorf, Hamburg, Germany.
Seza Krüger-Özgürdal, Department of Psychiatry, Psychotherapy, and Preventive Medicine, Ruhr University Bochum, Bochum, Germany.
Martin Lambert, Department of Psychiatry and Psychotherapy, Centre for Psychosocial Medicine, University Medical Centre Hamburg-Eppendorf, Hamburg, Germany.
Wolfgang Maier, Department of Psychiatry and Psychotherapy, Rhineland Friedrich Wilhelms University of Bonn, Bonn, Germany.
Andreas Meyer-Lindenberg, Central Institute of Mental Health, Medical Faculty Mannheim, University of Heidelberg, Germany.
Verena Pützfeld, Department of Psychiatry and Psychotherapy, Faculty of Medicine and University Hospital Cologne, Cologne, Germany.
Franziska Rausch, Central Institute of Mental Health, Medical Faculty Mannheim, University of Heidelberg, Germany.
Frank Schneider, Department of Psychiatry and Psychotherapy, Medical Faculty, Heinrich-Heine-University, Dusseldorf, Germany; Department of Psychiatry, Psychotherapy, and Psychosomatics, RWTH Aachen, Aachen, Germany.
Hartmut Stützer, Institute of Medical Statistics and Computational Biology, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany.
Thomas Wobrock, Department of Psychiatry and Psychotherapy, Georg-August-University Göttingen, Göttingen, Germany; Centre of Mental Health, County Hospitals Darmstadt-Dieburg, Groß-Umstadt, Germany.
Michael Wagner, Department of Psychiatry and Psychotherapy, Rhineland Friedrich Wilhelms University of Bonn, Bonn, Germany; Department of Neurodegenerative Diseases and Geriatric Psychiatry, Rhineland Friedrich Wilhelms University of Bonn, Bonn, Germany.
Mathias Zink, Central Institute of Mental Health, Medical Faculty Mannheim, University of Heidelberg, Germany; District Hospital for Psychiatry, Psychotherapy, and Psychosomatics, Ansbach, Germany.
Joachim Klosterkötter, Department of Psychiatry and Psychotherapy, Faculty of Medicine and University Hospital Cologne, Cologne, Germany.
Funding
The German Research Foundation funded this study (DFG, grant KL 970/7-1). Bristol-Myers Squibb provided aripiprazole and the matching placebo but played no role in the study design, data collection, analysis, interpretation, or report writing. The corresponding author had full access to all the data in the study and was responsible for submitting it for publication.
Statement of Ethics
The trial was conducted ethically following the World Medical Association Declaration of Helsinki.
Consent to Participate Statement
All participants provided written informed consent before any research activity.
Study Approval Statement
The trial protocol was reviewed and approved by the Ethics Committee of the Medical Faculty of the University of Cologne (Approval number 07-158). The primary responsibility laid with the ethics committee in Cologne. Institutional ethics boards reviewed and approved the protocol at the trial sites.
Authors’ Contributions
A.B. and H.S. developed the design of the study; H.S. and M.H. undertook statistical analysis of the primary and secondary outcomes; and H.M. and A.B. wrote the first draft of the manuscript with input from M.H. and H.S. All authors were involved in recruiting and treating the patients, source data capture, interpretation of the findings and contributed ideas and revising the paper.
Data Availability
The data supporting this study’s findings are not publicly available due to ongoing research interests but are available from Andreas Bechdolf (A.B.) upon reasonable request.
References
- 1. Häfner H, Maurer K, Löffler W, et al. The ABC Schizophrenia Study: a preliminary overview of the results. Soc Psychiatry Psychiatr Epidemiol. 1998;33(8):380–386. [DOI] [PubMed] [Google Scholar]
- 2. Fusar-Poli P, Salazar de Pablo G, Correll CU, et al. Prevention of psychosis: advances in detection, prognosis, and intervention. JAMA Psychiatry. 2020;77(7):755–765. [DOI] [PubMed] [Google Scholar]
- 3. Fusar-Poli P, Cappucciati M, Borgwardt S, et al. Heterogeneity of psychosis risk within individuals at clinical high risk: a meta-analytical stratification. JAMA Psychiatry. 2016;73(2):113–120. [DOI] [PubMed] [Google Scholar]
- 4. Mei C, van der Gaag M, Nelson B, et al. Preventive interventions for individuals at ultra high risk for psychosis: an updated and extended meta-analysis. Clin Psychol Rev. 2021;86:102005. [DOI] [PubMed] [Google Scholar]
- 5. Zheng Y, Xu T, Zhu Y, et al. Cognitive behavioral therapy for prodromal stage of psychosis—outcomes for transition, functioning, distress, and quality of life: a aystematic review and meta-analysis. Schizophr Bull. 2022;48(1):8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmidt SJ, Schultze-Lutter F, Schimmelmann BG, et al. EPA guidance on the early intervention in clinical high risk states of psychoses. Eur Psychiatry. 2015;30(3):388–404. [DOI] [PubMed] [Google Scholar]
- 7. Stain HJ, Bucci S, Baker AL, et al. A randomised controlled trial of cognitive behaviour therapy versus non-directive reflective listening for young people at ultra high risk of developing psychosis: the detection and evaluation of psychological therapy (DEPTh) trial. Schizophr Res. 2016;176(2-3):212–219. [DOI] [PubMed] [Google Scholar]
- 8. McGorry P, Nelson B, Phillips L, et al. Randomized controlled trial of interventions for young people at ultra-high risk of psychosis: twelve-month outcome. J Clin Psychiatry. 2012;74(4):10030. [DOI] [PubMed] [Google Scholar]
- 9. Davies C, Cipriani A, Ioannidis JPA, et al. Lack of evidence to favor specific preventive interventions in psychosis: a network meta-analysis. World Psychiatry. 2018;17(2):196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuharic DB, Kekin I, Hew J, Kuzman MR, Puljak L.. Interventions for prodromal stage of psychosis. Cochrane Database Syst Rev. 2019;(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schultze-Lutter F, Michel C, Schmidt S, et al. EPA guidance on the early detection of clinical high risk states of psychoses. Eur Psychiatry. 2015;30(3):405–416. [DOI] [PubMed] [Google Scholar]
- 12. Fusar-Poli P, Frascarelli M, Valmaggia L, et al. Antidepressant, antipsychotic and psychological interventions in subjects at high clinical risk for psychosis: OASIS 6-year naturalistic study. Psychol Med. 2015;45(6):1327–1339. [DOI] [PubMed] [Google Scholar]
- 13. Bechdolf A. Kognitive Verhaltenstherapie bei Personen mit erhöhtem Psychoserisiko: ein Behandlungsmanual. Bern: Huber; 2010. [Google Scholar]
- 14. Findling RL, Robb A, Nyilas M, et al. A multiple-center, randomized, double-blind, placebo-controlled study of oral aripiprazole for treatment of adolescents with schizophrenia. Am J Psychiatry. 2008;165(11):1432–1441. [DOI] [PubMed] [Google Scholar]
- 15. Haddock G, Devane S, Bradshaw T, et al. An investigation into the psychometric properties of the cognitive therapy scale for psychosis (CTS-Psy). Behav Cogn Psychother. 2001;29(02):221–233. [Google Scholar]
- 16. McGlashan TH, Zipursky RB, Perkins D, et al. Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am J Psychiatry. 2006;163(5):790–799. [DOI] [PubMed] [Google Scholar]
- 17. Fleiss JL. Statistical Methods for Rates and Proportions. New York-London-Sydney-Toronto: Wiley; 1981. [Google Scholar]
- 18. National Institute for Health and Clinical Excellence (NICE). Psychosis and Schizophrenia in Adults: The NICE Guideline on Treatment and Management. London: National Collaborating Centre for Mental Health; 2014. [Google Scholar]
- 19. Klatte R, Strauss B, Flückiger C, Färber F, Rosendahl J.. Defining and assessing adverse events and harmful effects in psychotherapy study protocols: a systematic review. Psychotherapy. 2022. [DOI] [PubMed] [Google Scholar]
- 20. Woods SW, Walsh BC, Powers AR, McGlashan TH.. Reliability, validity, epidemiology, and cultural variation of the Structured Interview for Psychosis-risk Syndromes (SIPS) and the Scale Of Psychosis-risk Symptoms (SOPS). In: Handbook of Attenuated Psychosis Syndrome Across Cultures: Springer; 2019:85–113. [Google Scholar]
- 21. Robinson L, Delgadillo J, Kellett S.. The dose-response effect in routinely delivered psychological therapies: a systematic review. Psychother Res. 2020;30(1):79–96. [DOI] [PubMed] [Google Scholar]
- 22. McGorry PD, Yung AR, Phillips LJ, et al. Randomized controlled trial of interventions designed to reduce the risk of progression to first-episode psychosis in a clinical sample with subthreshold symptoms. Arch Gen Psychiatry. 2002;59(10):921–928. [DOI] [PubMed] [Google Scholar]
- 23. McGorry PD, Nelson B, Markulev C, et al. Effect of ω-3 polyunsaturated fatty acids in young People at ultrahigh risk for psychotic disorders: the NEURAPRO randomized clinical trial. JAMA Psychiatry. 2016;74(1):19–27. [DOI] [PubMed] [Google Scholar]
- 24. Morrison AP, French P, Stewart SL, et al. Early detection and intervention evaluation for people at risk of psychosis: multisite randomised controlled trial. BMJ. 2012;344:e2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hartmann JA, McGorry PD, Schmidt SJ, et al. Opening the black box of cognitive-behavioural case management in clients with ultra-high risk for psychosis. Psychother Psychosom. 2017;86(5):292–299. [DOI] [PubMed] [Google Scholar]
- 26. Bechdolf A, Wagner M, Ruhrmann S, et al. Preventing progression to first-episode psychosis in early initial prodromal states. Br J Psychiatry. 2012;200(1):22–29. [DOI] [PubMed] [Google Scholar]
- 27. Pozza A, Domenichetti S, Dèttore D.. Cognitive behavioural therapy for help-seeking adolescents and young adults with at-risk-mental state: effects on subclinical positive symptoms. Early Interv Psychiatry. 2021;15(3):513–524. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this study’s findings are not publicly available due to ongoing research interests but are available from Andreas Bechdolf (A.B.) upon reasonable request.