Abstract
Syphilis continues to be a significant public health concern worldwide. The disease is endemic in many low- and middle-income countries, and rates have risen sharply in high-income countries over the last decade. The continued prevalence of infectious and congenital syphilis worldwide highlights the need for the development of an effective syphilis vaccine to complement public health measures for syphilis control. The complex, multi-stage course of syphilis infection necessitates a holistic approach to the development of an effective vaccine, in which immunization prevents both the localized stage of infection (typified by the highly infectious chancre) and the disseminated stages of infection (typified by the secondary rash, neurosyphilis, and destructive tertiary lesions, as well as congenital syphilis). Inhibiting development of the infectious chancre would reduce transmission thus providing community-level protection, while preventing dissemination would provide individual-level protection by reducing serious sequelae and may also provide community level protection by reducing shedding during secondary syphilis. In the current study we build upon prior investigations which demonstrated that immunizations with individual, well characterized T. pallidum TprK, TprC, and Tp0751 peptides elicits partial protection against infection in the animal model. Specifically, we show here that immunization with a TprC/TprK/Tp0751 tri-antigen cocktail protects animals from progressive syphilis lesions and substantially inhibits dissemination of the infection.
Keywords: Syphilis, Treponema pallidum, Vaccine, Bacterium
1. Introduction
When penicillin became widely available after World War II, it was heralded as the key to eliminating syphilis, which had infected ~ 10 % of persons in the United States and Europe. Over the past decade, the U.S., Canada, and Europe have faced dramatically increasing cases of syphilis, primarily in men who have sex with men (MSM) and vulnerable populations [1–3]. Congenital syphilis continues to significantly impact the reproductive health of women in low- and middle-income countries, and in recent years has also increased in numbers in high-income countries [4–7]. The standard public health approach of testing, contact tracing, and treating has failed to eliminate, or even to contain, syphilis. It is increasingly clear that global eradication of syphilis will require development of an effective syphilis vaccine to complement public health elimination efforts [8–11].
Syphilis is a complex and chronic disease that is manifest in successive clinical stages that are interrupted by periods of asymptomatic infection: the infectious primary chancre (or ulcer), the disseminated rash of the secondary stage, the long period of latency when no clinical signs are present, and the serious late sequelae of tertiary syphilis including gummatous tissue destruction, serious neurological abnormalities, and cardiovascular involvement [12]. Dissemination of the bacterium is widespread, occurs early during the course of infection, and can result in invasion of the central nervous system and infection of the fetus in utero [12,13]. Further, evidence suggests oral and anal shedding of T. pallidum occurs in all stages of infection, and thus may represent a previously unrecognized source of syphilis transmission that could be targeted via delivery of a vaccine that inhibits dissemination [14–16].
The rabbit provides the ideal model for pre-clinical evaluation of vaccine candidate antigens for the following reasons. The rabbit is readily susceptible to Treponema pallidum infection and displays the classical primary, secondary, and latent stages of infection [10–12], as well as central nervous system invasion [17] and transplacental infection of the fetus [18]. The histopathology of syphilis infection in rabbits recapitulates that seen in humans, with CD4 + and CD8 + lymphocyte infiltration, a Th1-skewed immune response, and antibody recognition of the same repertoire of T. pallidum antigens. And, similar to humans, the outbred nature of the rabbit ensures a clinically relevant, broad spectrum of disease manifestations and immune responses similar to those that are experienced by humans infected with T. pallidum [10–12].
Research on T. pallidum has been, and continues to be, hampered by the inability to propagate Treponema pallidum, the causative agent of syphilis, without contamination by rabbit tissue or cells [19–21]. Identification of surface-exposed antigens, some of which are targets of critical opsonic antibodies that facilitate the ingestion and killing of the bacterium by macrophages, is difficult because of their low density in the outer membrane [22–25] and the fragility of the outer membrane during laboratory manipulations [10–12]. Nonetheless, our work over the past twenty years has identified a number of predicted T. pallidum surface antigens that, when produced as recombinant proteins in E. coli and used to immunize rabbits, can significantly attenuate the course of primary infection [26–29]. Many of these antigens belong to the 12-membered paralogous Tpr family of proteins [28–31], with additional identification of the Tp0751 protein that is central to the ability of T. pallidum to traverse tissue barriers and disseminate throughout the body of the host [27,32–34]. In the studies reported here, we describe the results of immunization with a tri-antigen cocktail comprised of peptides from two Tpr antigens (encompassing the N-terminal portion of the Subfamily I Tpr’s and the N-terminal portion of TprK), the mature, full-length version of the Tp0751 protein, and a customized adjuvant designed to elicit a robust cellular and humoral immune response. We show that this immunization regimen induces reproducible immune responses that significantly attenuate the development of infectious primary lesions and substantially inhibit T. pallidum dissemination to distant organs. Overall, this study demonstrates the promise of these T. pallidum antigens for successful syphilis vaccine development and the advantage of this two-pronged vaccination approach for achieving required individual- and community-based protection from disease.
2. Materials and Methods
2.1. Rabbits and Treponema pallidum
Outbred male New Zealand White rabbits (3.0–3.5 kg, Western Oregon Rabbitry, Philomath, OR USA for UW studies; or Charles River Laboratories, Ontario, Canada for UVic studies) with nonreactive VDRL and FTA-ABS serologies (to rule out Treponema paraluiscuniculi infection) were used for T. pallidum propagation and for immunization studies. The Nichols, Sea81-4, and Chicago strains of T. pallidum subsp. pallidum were propagated by serial rabbit passage as previously described [19]. All rabbits were fed antibiotic-free food and water, and were housed at 18–20°C. All animal studies were approved by the local institutional review boards at UW and UVic. Studies were conducted in strict accordance with standard accepted principles as set forth by the Canadian Council on Animal Care (CCAC; UVic), National Institutes of Health and the United States Department of Agriculture (UW and UVic) in facilities accredited by the American Association for the Accreditation of Laboratory Animal Care (UW and UVic) and the CCAC (UVic). Institutional biosafety approval was obtained under biosafety certificate 0055–001–008 (UW) and 13170–010 (UVic).
2.2. Recombinant antigens
Three recombinant antigens were used for immunization; two were produced at UW (Tp0897 TprK Frag 1 and Tp0117 Subfamily I N-terminal) and one at UVic (Tp0751). Constructs of recombinant proteins of Tp0897 (TprK Fragment 1;(aa 37–273) [29] and N- terminus of Tpr Subfamily I [28] Tp0117 (Tpr C Nichols template; aa 23–351), described in previous studies, were transformed and expressed in One Shot BL21 Star (DE3) pLysS Chemically Competent E. coli (Invitrogen; ThermoFisher Scientific, Waltham, MA). Inclusion bodies containing the recombinant proteins were solubilized in 6 M urea, sonicated on ice, then recombinant 6-histidine–tagged proteins were purified by nickel affinity chromatography using Ni-nitrilotriacetic acid (NTA) agarose (Qiagen, Germantown, MD) as previously described [35]. Identity of the purified peptides was confirmed by mass spectroscopy and eluted proteins were dialyzed in PBS. Purified TprK and Tpr Subfamily I peptides were refolded using Profoldin Preparative Membrane Folding Column #7 (Profoldin, Hudson MA), and evaluated by circular dichroism prior to suspension in the adjuvant for immunization, and prior to use in ELISA assays (See Supplementary Fig. S1). Soluble Tp0751 (C24-P237) was expressed and purified as previously described using affinity chromatography and size exclusion chromatography [36]. For Tp0751, the recombinant protein was placed at 4°C for 3 weeks prior to mixing with the adjuvant and performing the immunizations for the initial (Tri-antigen I) experiment conducted at UW and the immunization experiment conducted at UVic. For the subsequent experiments examining the challenge dose (Tri-antigen II) and heterologous protection (Tri-antigen III) conducted at UW, the Tp0751 protein was purified and mixed directly with the adjuvant for immunization, with no 4°C storage step. These alterations in the preparation of Tp0751 were included as a step toward optimization of the Tp0751 preparation to increase stability and enable successful recombinant protein scale-up for ultimate use in a commercial vaccine.
2.3. Adjuvants
Two custom adjuvants were tested. These adjuvants were designed to mimic the RIBI adjuvant that is no longer commercially available. RIBI Natural contains the same components as the original RIBI adjuvant: monophosphoryl lipid A [MPL] + trehalose 6,6′ -dimycolate [TDM] + cell wall skeleton from Mycobacterium phlei [CWS] [37]. RIBI Synthetic contains MPL, synthetic trehalose dicorynomycolate (TDcM (a low toxicity analogue of TDM that is considered safe for human use), and muramyl dipeptide (MDP) in hopes of inducing an equivalent immune response with less reactogenicity.
2.4. Immunization and infectious challenge
Rabbits were immunized five times, every three weeks, with 20 μg each (primary immunization) or 10 μg each (subsequent boosts) of soluble Tp0751 (C24-P237), refolded N-terminal TprK and refolded N-terminal Subfamily 1 (Tri-antigen cocktail). At UW, antigens were mixed with either of the two custom adjuvants RIBI Natural and RIBI Synthetic (PAI Life Sciences, Seattle, WA, USA), and immunizations were administered intradermally, subcutaneously and intramuscularly. At UVic, only the RIBI-Natural adjuvant was used, and the primary immunization was administered intradermally, subcutaneously and intramuscularly, while the remaining four boosts were administered subcutaneously and intramuscularly.
At UW, 3 weeks following the 5th immunization, 8 rabbits immunized using RIBI-Natural adjuvant, 8 rabbits immunized using RIBI-Synthetic adjuvant, and 8 naïve control rabbits were challenged intradermally at 10 sites on their shaved backs with 105 T. pallidum (Nichols strain) per site. During the subsequent monitoring period, one rabbit each from the unimmunized and RIBI Synthetic groups died from causes unrelated to vaccine administration (as assessed by necropsy); for the remaining monitoring period there were 7 evaluable rabbits in each of those groups. Lesions developing at challenge sites were monitored daily for diameter and height of induration. Lesion volume was determined as described at https://www.sangakoo.com/en/unit/the-spherical-dome-surface-area-and-volume. During the observation period, lesions that progressed to ulceration were noted. At day 2 post-challenge (pc), 2 lesions on each rabbit were biopsied (4 mm punch), minced, and homogenized in 400 μl TRIzol (Invitrogen), then quick frozen using dry ice and stored until extraction for determination of cytokine mRNA. At Day 19 pc, material was aspirated from each of the remaining 8 lesions per rabbit for (1) darkfield microscopic determination of T. pallidum and (2) suspension in lysis buffer (10 mM Tris [pH 8 0], 0 1 M EDTA, and 0 5% SDS) for T. pallidum quantitation by qPCR. On day 21 pc, biopsies were taken from two additional lesions. Each biopsy was minced, then divided and placed into 400 μl lysis buffer for T. pallidum quantitation or 400 μl TRIzol for measurement of cytokine mRNA.
In two subsequent experiments at UW, rabbits were immunized, challenged, and monitored as described above (using the RIBI Natural adjuvant) except that: 1) two challenge doses (105 and 103 Nichols strain T. pallidum per site) were tested in one study; and 2) groups of immunized and unimmunized control rabbits were challenged at 105/site with heterologous T. pallidum Chicago or Sea 81–4 strains and an “adjuvant only” control group was included.
At UVic, 3 weeks following the final immunization, 8 immunized and 4 naïve control rabbits were challenged intradermally at ten sites on their shaved backs with 106 T. pallidum (Nichols strain) per site, and monitored as described above. During the monitoring period one immunized and challenged rabbit was removed from the study due to development of a fever, and subsequent administration of the anti-inflammatory drug Metacam. Lesion aspirates were taken from both control and immunized animals 10 days pc, pooled, and examined by dark-field microscopy for the presence of motile T. pallidum.
2.5. T. pallidum quantitation by qPCR
At UW, DNA was isolated from lesion aspirates using the QIAamp DNA mini-kit (QIAGEN, Inc., Chatsworth, CA). Punch biopsies (4 mm) were minced and homogenized in lysis buffer and incubated with 50 μl proteinase K (20 mg/ml; Qiagen, Valencia, CA, USA) overnight at 56 °C. Next, 450 μl of the QIAamp AL buffer were added and incubation was continued for another 10 min at 70 °C. DNA extraction was performed according to the manufacturer’s protocol. DNA was eluted from the columns with 200 μl molecular grade water.
To determine the number of treponemes present in each sample, a real time quantitation of the Tp0574 (tpN47) single copy gene was performed in a ViiA7 Real Time PCR System (Applied Biosystems; ThermoFisher Scientific, Waltham, MA) using the PowerUp SYBR Green Master Mix. The forward primer sequence: 5′ -CAA GTA CGA GGG GAA CAT CG- 3′ and the reverse primer sequence 5′ -TGA TCG CTG ACA AGC TTA GG-3′ were used to amplify a portion of the single-copy gene tp0574 (encoding TpN47) in the sample. Serial 10-fold dilutions were prepared from a stock of linearized plasmid stock containing 6 X 106 copies of the T47 gene/μl of extracted DNA, ultimately reaching a theoretical concentration of 6 X 10−1 copies/μl, with 3 μl extracted DNA used per reaction. Construction of a plasmid control for quantitative PCR has been described in detail by Giacani et al. [38].
2.6. Cytokine analysis
RNA from lesion biopsies was isolated using the Trizol Reagent (Invitrogen; ThermoFisher Scientific, Waltham, MA) following the manufacturer’s instructions. RNA was treated with 2 units of Turbo DNase Free (Ambion; ThermoFisher Scientific) to remove any contaminating genomic DNA according to the manufacturer’s specifications. cDNA synthesis was performed using SuperScript III reverse transcriptase (Invitrogen; ThermoFisher Scientific) as recommended by the manufacturer using random hexamers. Quantitative real-time PCR was performed on cDNA to assess abundance of cytokine mRNA levels of γ-IFN, IL-2 and IL-4 using the primers and control plasmid (cytokine plasmid 1) as previously described [39]. An additional plasmid was similarly constructed, containing portions of the IL-12/IL-23p40, IL-17A, IL-17F, IL-22, and TGF-β gene sequences (cytokine plasmid 2), using the primers described by Schnupf et al. [40]. These plasmids were used to derive standard curves for these gene targets. The original concentrations were 3.2×109 copies/μl for cytokine plasmid 1 and 4.8×109 copies/μl for cytokine plasmid 2. with 3 μl extracted DNA used per reaction Serial 10-fold dilutions were prepared from sub-stocks of the linearized plasmids containing 3.2 or 4.8 ×106 copies of the cytokine gene/μl, ultimately reaching a theoretical concentration of 3.2 or 4.8 ×10−1 copies/μl, with 3 μl extracted DNA used per reaction. Cytokine message quantification reactions in test samples were performed in 5 replicates of a cDNA (diluted 1:5) on a ViiA 7 system (Applied Biosystems; ThermoFisher Scientific, Waltham, MA) using Power Sybr Green Master Mix. A melting curve analysis was performed after the amplification phase to exclude non-specific amplification or formation of primer dimers.
2.7. Dissemination of T. pallidum
Following the observation period (day 42 pc), the UW rabbits were euthanized and their popliteal lymph nodes were harvested for the Rabbit Infectivity Test (RIT) [41] to determine the presence and relative quantity of viable T. pallidum in these distant sites, as a measure of dissemination of the bacteria. The nodes were minced on stainless steel mesh in 10 % normal rabbit serum (NRS) in 0.9 % NaCl (10 % serum saline) and injected intratesticularly into one testis of naïve seronegative rabbits. The recipient animals were monitored daily for visual signs of infection (orchitis) for up to three months or until orchitis appeared. Semi-quantitation of viable T. pallidum in the injected sample was determined by length of time to development of orchitis, using historical [42] and laboratory data for the Nichols strain. At UVic, challenged rabbits were euthanized on day 14 pc (to assess the level of protection against early dissemination) and the RIT was performed as described above, except that blood was collected from the recipient rabbits every two weeks for serological testing, and the rabbits were euthanized on day 90 post lymph node transfer.
2.8. Antibody titers
Blood was collected from rabbits prior to each immunization and prior to infectious challenge for determination of serum antibody titers to each of the recombinant antigens. ELISA assays, using 15 pmol of the recombinant protein (soluble Tp0751, refolded N-terminal TprK, or refolded N-terminal Subfamily 1) were performed using 96-well polystyrene plates as previously described [29] except that the primary antibody was incubated at 4°C overnight (UW) or 1 h at room temperature (UVic) and the secondary antibodies used at UW were alkaline phosphatase goat anti-rabbit IgG (H + L; Sigma, St. Louis MO, USA) or alkaline phosphatase goat anti-rabbit IgM (μ chain; Sigma, St. Louis MO, USA), while at UVic the secondary antibody used was horseradish peroxidase goat anti-rabbit IgG (H + L; Sigma-Aldrich Canada Ltd). Refolded antigens were tested by circular dichroism before being coated on the plates. Adsorbed pre-challenge sera were diluted 1/20,000 initially, then serial twofold dilutions were tested in the IgG assay; for IgM, the initial dilution was 1/20 followed by twofold dilutions. The maximum dilution tested was 1/5,120,000 for IgG and 1/5120 for IgM. Final IgG and IgM titers are determined as the highest dilution yielding 2X the optical density of the preimmune serum sample for that rabbit, and values for each rabbit serum are reported as mean +/− SE of triplicate wells.
3. Results
Four separate immunization and challenge studies were performed: three at the University of Washington (UW), and one at University of Victoria (UVic) (see Fig. 1). The first UW study (Tri I) and the UVic study will be described initially, followed by the subsequent studies performed at UW that examined the effects of different infectious challenge doses (Tri II) and the level of protection against challenge with heterologous strains (Tri III).
Fig. 1. Protection experiment pipeline.

Shown is a schematic for the immunization and challenge experiments (left) and subsequent rabbit infectivity test analysis (right), for testing the protective capacity of the tri-antigen cocktail vaccine. Unique aspects of the experiments conducted at the different locations are indicated by different colored boxes: University of Washington, purple boxes; University of Victoria, red boxes.
3.1. Overall experimental design
The tri-antigen cocktail comprised the N-terminal portion of the Subfamily I Tpr’s, the N-terminal portion of TprK, and the mature, full-length version of Tp0751. We examined the predicted amino acid sequences for relevant regions of these proteins in all available sequences at the time that these studies began. We found the following levels of conservation: Tp0751 (AA 24–237) was 100 % identical in all 11 sequences available; N-term Sub I (Tp0117, AA 23–351) was 100 % identical in 24 of 29 strains and > 97 % identical in the remaining 5 strains; N-term TprK (Tp0897, AA 37–273) was 100 % identical in the non-V regions in 9 of 12 strains, and 99.5 % identical in the remaining 3 strains. The three antigens were mixed with one of two custom adjuvants, here called RIBI Natural and RIBI Synthetic (see Methods for description). The studies performed at UW used either both custom adjuvants independently (Tri I) or only the RIBI Natural adjuvant (Tri II and III), while the UVic study used only the RIBI Natural adjuvant. In Tri I at UW, after receiving 5 immunizations with the tri-antigen cocktail in either of the two adjuvants, groups of rabbits were challenged intradermally with 105 Nichols strain T. pallidum at each of ten sites on their clipped backs; a group of unimmunized control rabbits was also challenged (Fig. 1). In Tri I, all groups initially had 8 rabbits, but one rabbit from the unimmunized and RIBI Synthetic groups died of unrelated causes during the study. All rabbits were observed daily for development and progression of skin lesions (chancres). Treponema pallidum load was determined in lesion aspirates and biopsies, and biopsies were examined for cytokine mRNA. Pre-challenge serum IgG and IgM antibody titers were determined separately for each antigen. At UVic, a group of 8 rabbits was immunized with the same tri-antigen cocktail in the RIBI Natural adjuvant, with a similar primary immunization as performed at UW and a different boost regimen (no intradermal immunizations performed at UVic for the boosts, see Methods; Fig. 1). At UVic, this immunized rabbit group, as well as a group of four unimmunized control rabbits, were challenged with 106 Nichols strain T. pallidum at each of 10 sites on their shaved back. For both locations, at the end of the observation period, the relative number of T. pallidum disseminating to distant sites was determined by the rabbit infectivity test (RIT) (see Methods and Fig. 1).
3.2. Delayed type hypersensitivity developed at challenge sites in immunized rabbits by 48 h post challenge (UW Tri I & UVic)
Each intradermal challenge site was monitored daily post challenge for clinical evidence of a delayed-type hypersensitivity (DTH) response (i.e., erythema and induration). At 48 h post-challenge (pc), clinical DTH was present in all immunized groups, but was absent in the unimmunized controls (Fig. 2a). Because interferon-γ (IFNγ) is characteristic of DTH, at UW biopsies of two challenge sites per rabbit were taken at 48 h pc and examined by qPCR for IFNγ message, which was found to be present in both immunized groups, but not in the unimmunized controls (Fig. 2b).
Fig 2.

Immunization with the tri-antigen cocktail induces both a delayed type hypersensitivity (Panel a) and an interferon-γ (IFN-γ, Panel b) response upon challenge with virulent T. pallidum. a. Following immunization, rabbits were challenged intradermally on their backs at 10 sites per rabbit. The challenge doses at UW and UVic were 105 and 106 T. pallidum Nichols strain per site, respectively. Forty-eight hours post-challenge, both immunized groups (RIBI Natural, orange, n = 8 rabbits UW, n = 7 UVic; RIBI Synthetic, purple, n = 7 rabbits UW) had evidence of clinical DTH (erythema and induration) at the majority (Fig. 2a left panel) or all (Fig. 2a right panel) of the challenge sites, while no erythema/induration was seen at any site in the unimmunized control rabbits (blue; n = 7 rabbits UW, n = 4 UVic). b. At UW, biopsies of two challenge sites per rabbit were examined by qPCR for interferon-γ message (n = 16 lesions for RIBI Natural, and 14 lesions each for RIBI Synthetic and Unimmunized). IFNγ was detected in the immunized, but not the unimmunized control, rabbits. Results are presented as mean +/− SEM. An unpaired t test with Welch’s correction (Fig. 2a, left panel), a Chi-squared test (Fig. 2a, right panel), and an unpaired t test (Fig. 2b) were used to analyze the differences between the immunized and unimmunized groups.
3.3. Immunization with the tri-antigen cocktail significantly reduces challenge lesion volume, local bacterial load, and progression to ulceration (UW Tri I & UVic)
At both locations, diameters and height of lesions were measured daily. At UW, after resolution of the DTH, lesion volumes in the control unimmunized rabbits were larger than in either immunized group for most of the 30-day observation period (Fig. 3a, left panel), until the expected resolution of the chancres in all groups. The proportion of total lesion sites that progressed to ulceration by days 19 and 30 post-challenge (Fig. 3b, left panel) was significantly lower in rabbits immunized using the RIBI Natural adjuvant, compared to the unimmunized controls. Of note, nearly 100 % of rabbits in the unimmunized group ultimately developed some ulcerative lesions, compared to 37.5 % of rabbits (3 of 8) in the RIBI Natural group. There was no significant difference in mean proportion of lesions progressing to ulceration between the RIBI Synthetic and unimmunized groups, and 62.5 % of rabbits (5 of 8) in the RIBI Synthetic group had some ulcerative lesions. Similarly, at UVic, immunization with the tri-antigen cocktail led to a significantly lower lesion volume (Fig. 3a, right panel) compared to the lesions arising in the unimmunized animals through day 14 post-challenge. Further, the UVic tri-antigen-immunized rabbit group had a significantly lower proportion of lesions that progressed to ulceration (Fig. 3b, right panel), with ulceration of 38.6 % of the lesions in the immunized rabbit group compared to 100 % of lesion ulceration in the unimmunized rabbit group.
Fig. 3.

Immunization with the tri-antigen cocktail decreases both lesion volume (Panel a) and ulceration (Panel b). a. At both UW and UVic, immunization with the tri-antigen cocktail decreased lesion volume compared to unimmunized control rabbits, regardless of the Nichols challenge dose (RIBI Natural-immunized rabbits, orange circles, n = 8 UW, n = 7 UVic; RIBI Synthetic-immunized rabbits, purple squares, n = 7 UW; unimmunized control rabbits, blue triangles, n = 7 UW, n = 4 UVic). Lesion volume was greater in immunized vs control rabbits on days 2 and 5 (Fig. 3a left panel, P < 0.0001) due to clinical DTH, while lesion volume was smaller in immunized vs control rabbits on days 10–25 (105 dose, Fig. 3a left panel, P < 0.0001) and days 7–14 (106 dose, Fig. 3a right panel, P = 0.03) due to attenuation of chancre development in immunized rabbits. b. Lesions in immunized rabbits challenged with either 106 or 105 T. pallidum per site exhibited less ulceration. At days 14 (106 dose, Fig. 3b right panel), 19 and 30 (105 dose, Fig. 3b left panel), the proportion of lesions ulcerating was significantly lower in RIBI Natural-immunized animals compared to unimmunized control rabbits (P = 0.009 [d.14], P = 0.001 [d.19] and P = 0.0003 [d.30]). At both time points (days 19 and 30, Fig. 3b left panel), there was a non-significant trend toward a lower proportion of lesions ulcerating in RIBI Synthetic-immunized animals, compared to unimmunized animals (P = 0.11 and P = 0.17, respectively). Data shown are mean +/− SEM. Comparisons were conducted by unpaired t test (Fig. 3a and Fig. 3b left panels), unpaired t test with Welch’s correction (Fig. 3b right panel), and two-way ANOVA (Fig. 3a right panel).
3.4. T. pallidum burden in challenge sites is lower in immunized rabbits (UW Tri I & UVic)
Needle aspirates were taken from each lesion on post-challenge days 19 (UW) and 10 (UVic) and examined by darkfield microscopy (DF) for presence (Fig. 4a) and number (Fig. 4b) of T. pallidum. At both locations, the number of lesions containing microscopically detectable T. pallidum was significantly lower in immunized animals compared to the unimmunized animals. At UW, all lesions (100 %) in unimmunized rabbits were DF+, compared to 37.5 % in rabbits immunized using the RIBI Natural adjuvant, and 67.4 % in the RIBI Synthetic group (Fig. 4a, left panel). Similarly, at UVic, only 34.3 % of the lesions in rabbits immunized with the tri-antigen cocktail had T. pallidum detectable by darkfield microscopy, compared to 92.5 % of the lesions in unimmunized rabbits (Fig. 4a, right panel). At UW, lesion aspirates from both immunized groups had significantly lower total (all lesions from each rabbit) numbers of T. pallidum detectable in 100 microscope fields, compared to lesion aspirates from the unimmunized controls (Fig. 4b, left panel). At UW, lesion aspirates were also analyzed by qPCR to determine the number of copies of the single copy gene tp0574, as an indication of T. pallidum numbers (Fig. 4b, right panel). The number of copies of tp0574 was significantly lower in the RIBI Natural-immunized group than in unimmunized animals, but there was no significant difference between the RIBI Synthetic and unimmunized groups. Note that at UW, by both darkfield microscopy and qPCR, there was one outlier rabbit in the animals immunized with the RIBI Natural adjuvant which had a larger number of T. pallidum detectable than the others in the group.
Fig. 4.

Presence of T. pallidum in lesion aspirates at Days 10 and 19 post challenge (pc). Presence of T. pallidum Nichols strain were determined in aspirates of 8 to 10 lesions per rabbit from each individual rabbit. a. At days 10 (106 dose, Fig. 4a right panel) and 19 (105 dose, Fig. 4a left panel) pc, lesions were examined for the presence of viable T. pallidum using darkfield microscopy. All immunized groups had significantly lower T. pallidum burden in lesions compared to the unimmunized group, regardless of the challenge dose (P = 0.001 and P = 0.04 for Natural and Synthetic, respectively, compared to unimmunized, Fig. 4a left panel and P = 0.005, Fig. 4a right panel). b. Total number of T. pallidum seen in darkfield microscopic examination of 100 fields per lesion aspirate and the number of copies of tp0574 (single copy gene) in pooled lesion aspirates of 8 lesions per rabbit for unimmunized (blue, n = 7 rabbits); RIBI Natural (orange, n = 8 rabbits); and RIBI Synthetic (purple, n = 7 rabbits) groups. The number of T. pallidum seen by darkfield microscopy was significantly lower in both immunized groups than in unimmunized controls, (P = 0.001 and P = 0.004 for Natural and Synthetic, respectively, compared to unimmunized, Fig. 4b left panel). The RIBI-Natural group had fewer T. pallidum detected by qPCR than the unimmunized rabbits (P = 0.008, Fig. 4b right panel). The T. pallidum burden in the RIBI-Synthetic group was not significantly different from the unimmunized rabbits as measured by qPCR. Results are presented as mean and 95 % CI. Unpaired t test (Fig. 4a right panel and Fig. 4b) and an unpaired t test with Welch’s correction (Fig. 4a left panel) were used to analyze the differences between the immunized and unimmunized groups; P < 0.05 was considered to be a significant difference.
3.5. Immunization with both adjuvants resulted in development of robust IgG and IgM antibody titers (UW Tri I & UVic)
Antigen-specific antibody titers in pre-challenge sera were determined by ELISA assays using all three purified recombinant protein antigens (UW) or only Tp0751 (UVic); results are shown in Fig. 5a (UW) and Fig. 5b (UVic). High IgG titers (group means ranging from 1/80,000 to 1/2,560,000) were seen against all three antigens (Fig. 5a, left panel and Fig. 5b), although titers against the Tpr Subfamily I antigen were lower in both adjuvant groups than for the other antigens (Fig. 5a, left panel). As expected, IgM titers were significantly lower than IgG (maximum of 1/640; Fig. 5a, right panel). At UW, except for the IgG titer to Tp0751, there were no significant differences observed in titers between the adjuvant groups.
Fig. 5. Antibody titers in pre-challenge sera.

ELISA assay was used to determine IgG (Fig. 5a left panel and 5b) and IgM (Fig. 5a right panel) titers in pre-challenge sera. At UVic, IgG titers were determined only for the Tp0751 antigen. Values shown are mean +/− SEM of the reciprocal dilutions yielding ODs 2-fold higher than the pre-immune serum for that rabbit. Note that the Y-axis for IgG is given in thousands (e.g. 5120 K = 5,120,000) while the IgM titer is the actual dilution. Except for the IgG titers against Tp0751 (P = 0.0001), there were no significant differences in pre-challenge titers between the two adjuvant groups. Titers were compared using unpaired t test; P < 0.05 was considered to be significant. Different secondary antibodies and a longer primary antibody incubation were used at UW compared to UVic, which could explain the different apparent IgG titer for the Tp0751 antigen.
3.6. Cytokine response at challenge sites 48 h post challenge indicates a Th1/Th17 response in immunized rabbits (UW Tri I)
Biopsies of two challenge sites per rabbit were examined for quantitation of message for ten cytokines. As shown in Fig. 6, immunized rabbits had levels of message for the pro-inflammatory Th-1 cytokines IL-2, interferon-γ (IFNγ; also shown in Fig. 2b above), and TNFα that were significantly higher compared to unimmunized control rabbits. Robust IL-17A & IL-17F levels were also seen in biopsies from immunized, but not unimmunized, rabbits. In contrast, levels of the anti-inflammatory (Th2) cytokines IL-10 and TGF-β were significantly higher at challenge sites in unimmunized rabbits, compared to either immunized group. There were no significant differences in any cytokine message at 48 h between the RIBI Natural and the RIBI Synthetic groups.
Fig 6. Cytokine message in 48-hour biopsies demonstrates a Th1/Th17 immune response was generated by the tri-antigen immunization.
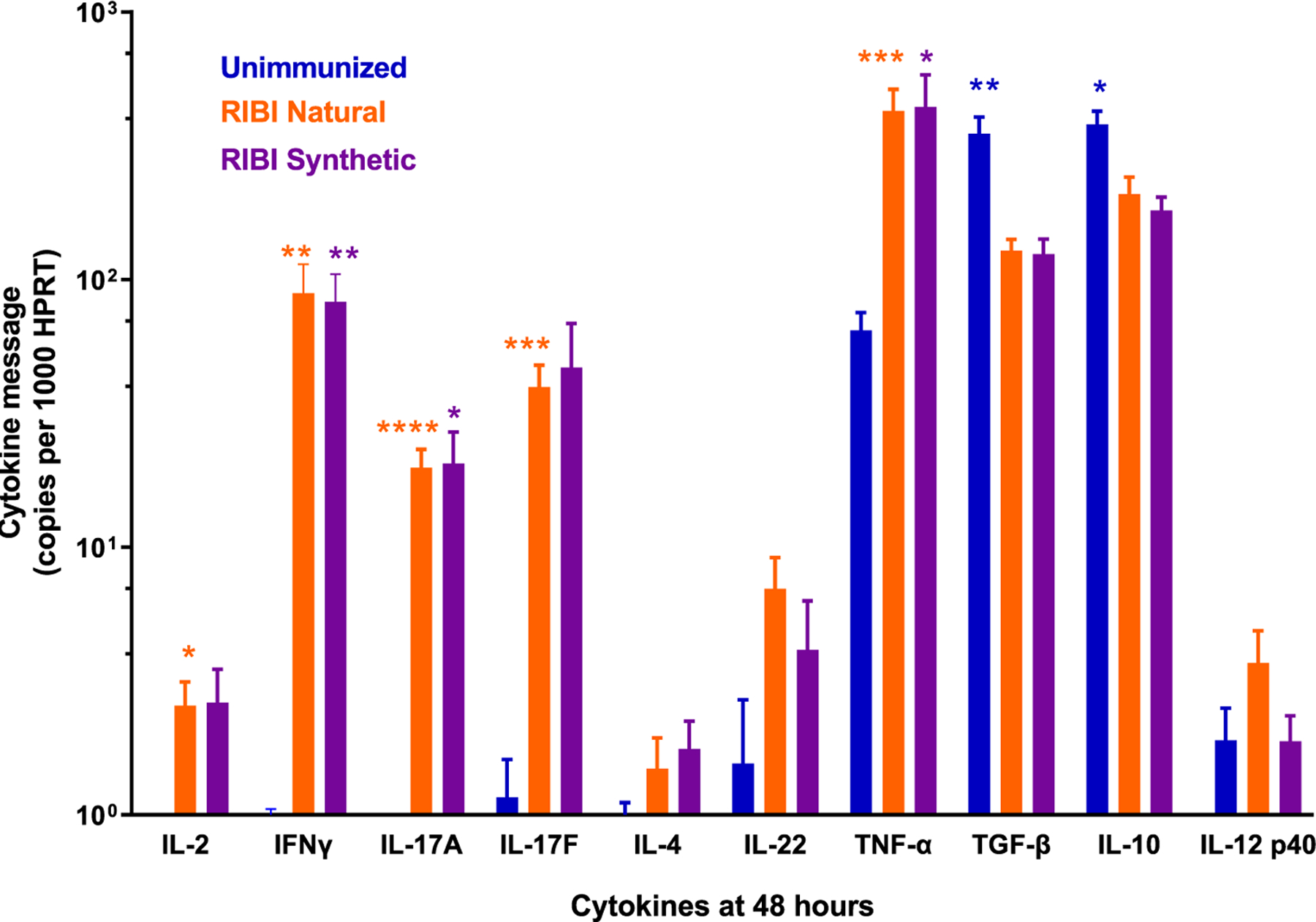
Erythema and induration were observed at all challenge sites at 48 h, consistent with a delayed type hypersensitivity (DTH) response. The cytokines generally associated with this response are those seen in Th1-skewed profiles. In biopsies of the 48-hr 105 T. pallidum Nichols strain challenge sites, IL-2, Interferon-γ (IFN γ), and TNFα message levels were significantly higher in both immunized groups compared to controls, indicating a Th1 response. IL-17 A and IL-17F levels were also significantly higher in immunized versus control rabbits, consistent with a Th17 profile. In contrast, levels of the anti-inflammatory cytokines TGF-β and IL-10 were significantly higher in the unimmunized vs the immunized rabbits at 48 h post challenge. No differences in cytokine levels were seen between the two immunized groups. Data shown are mean +/− SEM values from 14 to 16 biopsies per group, and comparisons were analyzed using an unpaired t test with Holm-Sidak adjustment for multiple comparisons. P < 0.05 was considered to be a significant difference: (* <0.05; ** <0.02; *** <0.005; ****<0.0001).
3.7. Inhibition of T. pallidum dissemination (UW Tri I & UVic)
The presence of viable T. pallidum in lymph nodes (LN) was determined by the rabbit infectivity test (RIT), with time to development of orchitis being a surrogate measure of number of viable treponemes in the transferred LN material. With the RIT, a longer time to orchitis indicates lower numbers of treponemes in the transferred material and, correspondingly, greater inhibition of treponemal dissemination through immunization. LN transfer was conducted on days 42 (UW) and 14 (UVic) post-challenge. At both locations, immunized rabbits had reduced numbers of treponemes in their popliteal LN (Fig. 7a and 7c). At UW, the mean +/− SEM time to orchitis for RIT recipients from unimmunized rabbits was 23.7 +/− 0.4 days (n = 7), compared to 27 +/−0.7 days for the RIBI Natural (n = 8; P = 0.002) and 24.4 +/− 0.7 days for RIBI Synthetic (n = 7; n.s.) recipients (Fig. 7a). At UVic, two unimmunized rabbits and one tri-antigen-immunized RIBI Natural rabbit did not develop orchitis but seroconverted at day 42 post-LN transfer, while one rabbit from the tri-antigen-immunized RIBI Natural group did not seroconvert or develop orchitis by day 90 post-LN transfer. For the animals that did develop orchitis at UVic, the mean +/− SEM time to orchitis for RIT recipients from unimmunized rabbits was 28 +/− 1 day (n = 2), compared to 31 +/− 0.89 days (n = 5) for the tri-antigen cocktail-immunized recipients (Fig. 7c). Because the length of time to development of orchitis is inversely related to the inoculum, we were able to estimate the number of viable T. pallidum present in the inoculated LN material at both locations (Fig. 7b and 7d). At UW, unimmunized rabbits had an estimated mean of 1472 T. pallidum in their LN, compared to 183 T. pallidum in the RIBI Natural group and 915 T. pallidum in the RIBI Synthetic group. This represents an 88 % and 38 % reduction in measurable dissemination, respectively, in these immunized rabbit groups compared to unimmunized control rabbits (Fig. 7b). At UVic, unimmunized rabbits had an estimated mean of 98 T. pallidum in their LN, compared to an estimated mean of 15 T. pallidum in the LN of immunized animals, representing an estimated 85 % reduction in T. pallidum dissemination to the LN in the immunized rabbit group compared to the unimmunized control rabbit group (Fig. 7d). The lower absolute number of T. pallidum disseminating to the LN in the experiment conducted at UVic in comparison to the experiment conducted at UW, for both the unimmunized and immunized groups, likely corresponds to the earlier day of LN transfer (day 14 at UVic in comparison to day 42 at UW).
Fig. 7. Rabbit infectivity test (RIT) and number of viable T. pallidum in popliteal lymph nodes post challenge.

RIT was used to determine the approximate number of viable T. pallidum Nichols strain in the popliteal lymph nodes (LN) of challenged rabbits, as time to development of orchitis following injection of LN suspensions into naïve recipient rabbits is inversely related to the number of viable T. pallidum inoculated. a. Time (days) to orchitis for the unimmunized control, and tri-antigen-immunized RIBI Natural and RIBI Synthetic groups. Means and 95 % CI are shown. The RIBI Natural group had a significantly longer time to orchitis, indicating fewer treponemes in their LN, compared to both the unimmunized and RIBI Synthetic groups. This demonstrates a significant reduction in T. pallidum dissemination in the RIBI Natural-immunized rabbits. Comparisons were analyzed using an unpaired t test. P < 0.05 was considered to be a significant difference. b. Time to orchitis with the Nichols strain of T. pallidum is inversely correlated with the number of infectious T. pallidum inoculated. Using historical [42] and contemporary unpublished data from our laboratory, we plotted the time to orchitis (days) for inocula of 200, 500, 100,000 and 25,000,000 T. pallidum Nichols strain, and used the resulting best-fit line (Y = −0.2718x + 9.6013) to estimate the number of infectious treponemes present in the LN from the unimmunized and immunized rabbits. Immunized rabbits had 88 % (RIBI Natural) and 38 % (RIBI Synthetic) reductions in the number of T. pallidum disseminating to the popliteal LN, compare to unimmunized rabbits. c. In the experiment conducted at UVic, for RIT animals that did develop orchitis, the RIBI Natural group had a longer time to orchitis compared to the unimmunized group, indicating fewer treponemes in their LN, although the trend did not achieve statistical significance, perhaps due to the small sample size in the unimmunized group. Mean +/− SEM are shown, and comparisons were analyzed using an unpaired t test. In this experiment, RIT animals receiving LN from two unimmunized rabbits and one tri-antigen-immunized RIBI Natural rabbit did not develop orchitis but seroconverted at day 42 post-LN transfer and were euthanized, while one RIT rabbit receiving LN from the tri-antigen-immunized RIBI Natural group failed to seroconvert or develop orchitis by day 90 post-LN transfer. d. Estimation of the number of infectious treponemes present in the LN from the unimmunized and immunized rabbits that did develop orchitis using the best-fit line (Fig. 7b). Immunized rabbits had an approximately 85 % reduction in the number of T. pallidum disseminating to the popliteal LN compared to unimmunized rabbits; an approximation is provided as the time to orchitis for the immunized rabbits fell just outside of the best-fit line.
3.8. Effect of challenge dose on level of protection (UW Tri II)
Two additional experiments using only the RIBI Natural adjuvant were conducted independently at UW. In the first of these experiments (Tri II), we examined the role of challenge dose on level of protection induced by the vaccine. Immunization induced robust pre-challenge IgG and IgM antibody titers in all animals prior to challenge (Fig. 8). Following immunization, groups of 4 immunized rabbits were challenged intradermally with either 103 or 105 Nichols T. pallidum per site. Following challenge, a DTH response was reproducibly observed at 48 h, but only for the immunized rabbits receiving the 105challenge dose; no DTH was seen with the lower challenge dose (Fig. 9a). Significant attenuation of lesion volume was observed at both challenge doses for rabbits immunized with the tri-antigen cocktail (Fig. 9b), and reduced numbers of T. pallidum were observed in lesion aspirates collected from immunized rabbits at both challenge doses, compared to the unimmunized controls (Fig. 9c). In contrast, reduction of lesion ulceration was observed for tri-antigen cocktail-immunized animals at only the 105challenge dose (Fig. 9d). These data confirm that immunization with the tri-antigen cocktail provides reproducible protection from lesion development, but that a superior level of protection was not shown with a reduced challenge dose. In fact, DTH and reduced lesion ulceration were seen only following the higher-dose challenge.
Fig. 8. Antibody titers in pre-challenge sera.
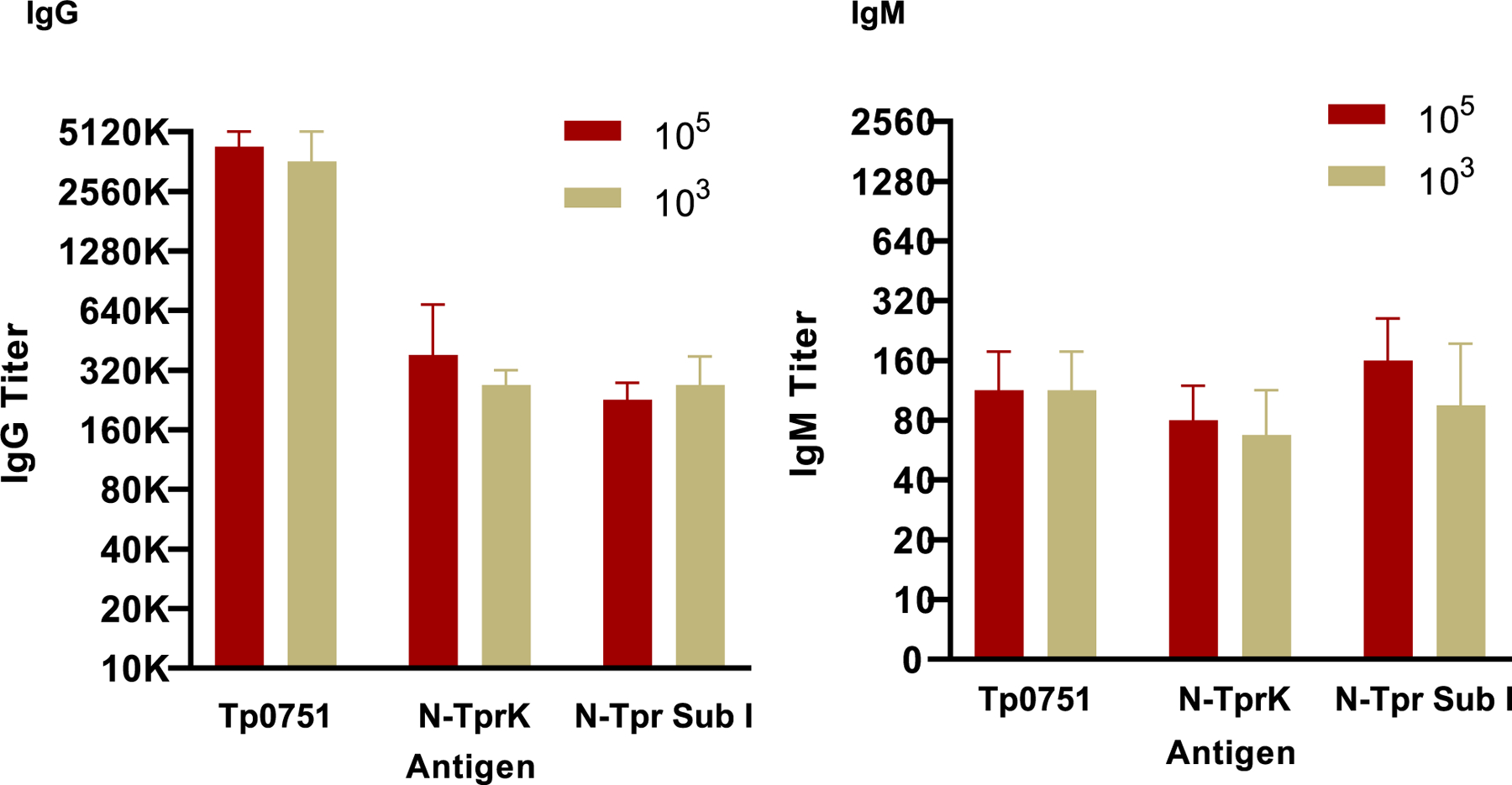
Pre-challenge IgG and IgM antibody titers were determined at UW for experiment Tri II as described for Fig. 5. There were no significant differences in pre-challenge titer between the 105 and 103 challenge groups.
Fig. 9. Lesion attenuation in immunized animals challenged with different doses.

Following immunization, rabbits were challenged intradermally on their backs at 10 sites per rabbit with 105 T. pallidum Nichols strain per site (n = 4) or 103 T. pallidum per site (n = 4). At 48 h, evidence of DTH (erythema and induration) was recorded. Unimmunized rabbits showed no evidence of DTH at 48 h. While immunized rabbits challenged with 105 T. pallidum per site developed DTH at nearly all sites by 48 h, rabbits challenged with 103 T. pallidum per site failed to show evidence of DTH at 48 h. The lesions in immunized rabbits were significantly smaller (Fig. 9b) and had fewer T. pallidum detected by qPCR (Fig. 9c) than in unimmunized rabbits, regardless of the challenge dose. Inset in Fig. 9b shows early lesion volumes in rabbits challenged with 103 T. pallidum per site; immunized rabbits demonstrated DTH (erythema and induration) at Day 10 post challenge following the lower dose challenge. Lesions in immunized rabbits challenged with 105 T. pallidum per site had a lower proportion of lesions ulcerating, compared to unimmunized rabbits. In contrast, high proportions of lesions progressed to ulceration in rabbits challenged with 103 T. pallidum per site, regardless of immunization status (Fig. 9d). Data reflect the proportion of challenge sites ulcerating by the end of the observation period: day 41 for 105 dose, and day 48 for 103 dose. Data are shown as mean +/− SEM (Fig. 9 a, b and d) and mean and 95 % CI (Fig. 9c). An unpaired t test was used to analyze the difference between the immunized and unimmunized groups in panels a, c, and d; two-way ANOVA was used in panel b. P < 0.05 was considered to be a significant difference.
3.9. Protection against heterologous strain challenge (UW Tri III)
To determine the ability of the tri-antigen cocktail to protect against heterologous strain challenge, groups of rabbits (8 per group) were immunized with the tri-antigen cocktail using the RIBI Natural adjuvant and tested for protection against infectious challenge with the Nichols, Chicago, and Sea 81–4 T. pallidum strains, in comparison to unimmunized control groups challenged with each of the strains. One group of four rabbits received adjuvant alone and were challenged with the Nichols strain; no significant differences were seen in lesion measurements or dissemination between the unimmunized and adjuvant only rabbits, demonstrating that the adjuvant itself did not affect the challenge outcome in the absence of the tri-antigen immunogens (data not shown). For rabbits challenged with the Chicago and Sea 81–4 strains, lesions that developed at the challenge sites (105 Tp/site) in immunized animals were significantly smaller at ~ 3–4 weeks post-challenge than in unimmunized rabbits; this difference was seen following challenge with either strain (Fig. 10a). In contrast to the immunized rabbits challenged with the Sea 81–4 strain, the Chicago lesions in immunized rabbits increased rapidly in size after day 26 pc, reaching volumes similar to those seen in the unimmunized controls: the Sea 81–4 lesions remained significantly smaller in immunized rabbits than in controls throughout the observation period. To assess T. pallidum burden, qPCR was performed on aspirates collected from lesions of animals in the Chicago group on day 22 post-challenge, and on biopsies collected from challenge sites of animals in the Sea 81–4 group on day 25 post-challenge. In both cases the T. pallidum burden at the challenge sites was significantly lower in the immunized rabbits compared to the unimmunized rabbits (Fig. 10b). To examine dissemination of the Chicago strain in immunized vs unimmunized rabbits, RIT was performed as described above. Animals receiving LN from immunized rabbits challenged with the Chicago strain had a slight delay in mean time to orchitis, although the group results were not statistically significant (Fig. 10c, left panel). Because we knew that the Sea 81–4 strain does not cause large lesions or development of orchitis, we could not use “time to orchitis” as an indicator of treponemal dissemination or assess T. pallidum burden from lesion aspirates. Instead, qPCR was performed on biopsies of challenge sites and in testes from the LN recipient rabbits, extracted into a defined volume of saline, to assess the relative amount of T. pallidum dissemination in unimmunized control and immunized rabbit groups challenged with Sea 81–4. This analysis showed there was a statistically significant difference in the level of T. pallidum dissemination in the immunized, compared to the unimmunized, rabbits, although it should be noted the absolute number of tp47 copies detected in the Sea 81–4-challenged rabbits was very low (Fig. 10c, right panel).
Fig. 10. Heterologous protection of the tri-antigen cocktail (UW).

a. Immunization with the tri-antigen cocktail resulted in decreased lesion volume in rabbits challenged with the heterologous strains T. pallidum subsp. pallidum Chicago (Fig. 10a, left panel) and T. pallidum subsp. pallidum Sea 81–4 (Fig. 10a, right panel). Means +/− SEM are shown. Differences between immunized and unimmunized animals in each group were analyzed by an unpaired t test with Welch’s correction at each time point. P < 0.05 was considered to be significant and is shown by *. b. Number of copies of tp0574 (single copy gene) in pooled (by rabbit) lesion aspirates for unimmunized and immunized rabbits challenged with T. pallidum subsp. pallidum Chicago (Fig. 10b, left panel) and pooled (by rabbit) biopsies for animal groups challenged with T. pallidum subsp. pallidum Sea 81–4 (Fig. 10b, right panel). Lesion aspirates were taken on day 22 (Chicago) and biopsies were collected on day 25 (Sea 81–4) post-challenge; timing of the sampling was determined by the appearance of the lesions (large but not yet ulcerating) in the unimmunized animals for each strain c. Time (days) to orchitis or qPCR quantitation for the RIT rabbits receiving LN from the unimmunized control and tri-antigen-immunized rabbit groups challenged with T. pallidum subsp. pallidum Chicago (Fig. 10c, left panel) or T. pallidum subsp. pallidum Sea 81–4 (Fig. 10c, right panel). Animals receiving LN from immunized rabbits exhibited a slight trend towards delayed orchitis, although statistical significance was not achieved. For the Sea 81–4 challenged rabbits, because the Sea 81–4 strain produces virtually no lesions or orchitis following infection, time to development of orchitis in recipient animals could not be determined, nor could lesion aspirates be collected to assess T. pallidum burden. Instead dissemination in the Sea 81–4 RIT recipient animals was determined by extracting the testes from RIT recipient rabbits into a defined volume of saline, followed by qPCR to determine the relative amount of T. pallidum dissemination in the challenged rabbits. There was a significant difference between the immunized and unimmunized groups, although the absolute tp0574 copies were very low. Data shown are mean and 95 % CI and differences between immunized and unimmunized animals in each group were analyzed by the Mann-Whitney test. P < 0.05 was considered significant.
4. Discussion
The ultimate goal of a syphilis vaccine is to completely prevent infection by any strain of T. pallidum. Despite many attempts to devise a syphilis vaccine [43,44], to date there is only one report, in the rabbit model, of complete protection against the homologous strain of T. pallidum, with a vaccine requiring 60 intravenous doses of γ-irradiated T. pallidum (total dose 3.71 × 109 organisms/rabbit) over a 37-week period [45]. This is obviously not a viable vaccination schedule for administration in humans, and another drawback is that the γ-irradiated T. pallidum vaccine did not induce protection against a heterologous strain of T. pallidum [45]. Subsequent efforts in that laboratory to reduce the number of doses or total bacteria, or to use stored frozen irradiated bacteria, failed to provide protection. Thus, development of a vaccine against T. pallidum infection is a challenging endeavour. Further, because of the complex course of syphilis infection, an effective vaccine must inhibit transmission (to protect the public) as well as dissemination (to protect the individual). Transmission most commonly occurs via the ulcerative primary chancre or early mucosal or skin lesions [46,47], so preventing the development of an ulcerative chancre in an individual could potentially reduce transmission to sex partners, even in the absence of protecting the individual. However, protecting against dissemination of T. pallidum from the site of infection would protect that individual from development of the subsequent stages of syphilis, including neurosyphilis, and would protect against congenital syphilis. Our tri-antigen vaccine cocktail has been designed with both goals in mind—prevention of ulcerative chancres and dissemination.
The current study was designed to test, for the first time, the combined protective capacity of a tri-antigen vaccine cocktail consisting of refolded peptides from multiple Tpr antigens and the mature, full-length version of the Tp0751 protein, combined with a custom-produced adjuvant designed to elicit robust Th1 cellular and humoral immune responses. This study also tested the reproducibility of the candidate vaccine by performing multiple independent experiments, at two different locations (UW and UVic), as well as the efficacy of the vaccine to protect against two different T. pallidum challenge doses and three different T. pallidum strains. There were several differences between the experiments conducted between the two locations, a feature that increases the rigor of our findings: the UVic study used the same antigen preparations and the RIBI Natural adjuvant as the UW Tri I study, but the outbred rabbits were from a different source; the immunization protocol did not include intradermal boosts; the challenge dose was 106 T. pallidum per site, rather than 105 as used at UW; and initiation of the RIT was conducted 14 days following T. pallidum challenge, rather than 42 days after challenge (see Fig. 1). Despite these differences, the findings of DTH responses at 48 h (Fig. 2 and Fig. 9), significant attenuation of lesion volume and progression (Fig. 3 and Fig. 9), reduced numbers of T. pallidum in lesions (Fig. 4, Fig. 9, and Fig. 10), robust pre-challenge IgG antibody titers (Fig. 5, Fig. 8, and Fig. 9), and reduced dissemination of T. pallidum (Fig. 7 and Fig. 10) were consistently seen in immunized rabbits in these studies. These data confirm the reproducibility of the results from this study, specifically significant attenuation of lesion size and progression and reduced dissemination of T. pallidum to distant tissues in rabbits immunized with the tri-antigen antigen cocktail.
Previous immunization and protection experiments analyzed the protection afforded by immunization with the individual Tp0751, N-term Sub I and N-term TprK antigens (called Fragment 1 in the earlier publication) [26–29]. These results showed partial protection against T. pallidum dissemination (Tp0751 immunization) and lesion development (N-term Tpr SubI/TprK immunization). It should be noted that immunization with Tp0751 alone did not protect against lesion development to the extent observed via immunization with the tri-antigen cocktail, and animals immunized with N-term SubI/TprK were not tested for reduction of dissemination. It should also be noted that the current studies used refolded Tpr antigens for the first time and examined additional outcomes compared to the earlier publications. Specifically, these additional outcome measures include measurement of volume of lesions, quantitative PCR analysis of lesion aspirates, quantitation of mRNA for interferon-γ in lesion biopsies, determination of pre-challenge IgM titers in immunized rabbits, determination of the relative number of T. pallidum disseminating to lymph nodes post-challenge, and protection against challenge with different strains. Therefore, the contributions of individual antigens to the protection achieved with the tri-antigen cocktail is difficult to assess. Further, due to the expense, workload, and complexity of the conducted rabbit immunization and challenge experiments, we could not do a side-by-side comparison of the protection achieved with individual antigens vs the tri-antigen cocktail.
The attenuation of lesion progression induced by our tri-antigen vaccine is the result of careful study concerning the type of immune response that is needed to limit T. pallidum proliferation at the challenge site. Based on our earlier studies, we hypothesized that a DTH response is responsible, along with opsonic antibody, for the clearance of T. pallidum from healing primary infections, with T lymphocytes producing IFNγ to activate macrophages for phagocytosis and killing of opsonized T. pallidum [48,49]. In further studies, we observed that the appearance of clinical DTH at challenge sites correlated with subsequent attenuation of lesion progression [28,29]. In the studies reported here, that hypothesis was reinforced, with immunized groups developing DTH 48 h following challenge with 105 or 106 T. pallidum per site and demonstrating significant attenuation of lesion progression, compared to the unimmunized rabbits that failed to develop DTH.
The type of immune response that provides a DTH cellular infiltration is a Th1-skewed cellular response, which is seen in syphilitic lesions. A number of histopathological studies of chancres have shown CD4 + and CD8 + T lymphocyte infiltration and local production of IFNγ for macrophage activation; this response is seen in active and resolving primary syphilis in both rabbits [39,50] and humans [51,52]. In addition to CD4 + and CD8 + lymphocytes, studies of human syphilis have identified infiltrating Th17 cells in secondary lesions [53]. In our study, we detected abundant message not only for IFNγ, but also for IL-17A and IL-17F in 48-hour biopsies of challenge sites from immunized, but not unimmunized, rabbits. Th17 cells are thought to play a role in responding to bacterial infections, but their specific function is not yet clear, although they may produce IFNγ. Our finding of IL-17 in rabbits further demonstrates the highly similar cytokine milieu of chancres in humans and rabbits, confirming the appropriateness of the rabbit as a model for pre-clinical studies of syphilis.
For these studies, we sought to induce a Th1 response by immunization in hopes that the early local response at the challenge site would successfully clear the treponemes so that chancres would not develop. To this end, we used a custom adjuvant. Our early studies with individual antigens [26,28–30] employed the RIBI (MPL + TDM + CWS) adjuvant, with promising results in terms of attenuation of lesions. Because that adjuvant is no longer commercially available, the principal authors conducted a three-year evaluation of fourteen commercial and custom adjuvants, seeking to reproduce the results they had obtained with the RIBI adjuvant. From this work, we determined that we needed both TLR-2 and TLR-4 agonists in our adjuvant. Ultimately, we obtained a custom adjuvant (PAI Life Sciences, Seattle) that was produced according to the original RIBI protocol; this was the RIBI Natural adjuvant used in this study. In an attempt to make an equivalent adjuvant, but with less reactogenicity for potential human use, the RIBI Synthetic adjuvant was developed. After seeing reduced efficacy of the RIBI Synthetic immunization, we conducted all subsequent studies using the RIBI Natural adjuvant. Indeed, immunization with our tri-antigen cocktail in the RIBI Natural adjuvant successfully induced a Th1 immune response: high levels of IFNγ message were detected in biopsies of 48-hr challenge sites. Although total clearance of the bacteria did not occur and lesions did appear, our immunized rabbits had significantly reduced treponemal burden and lack of ulceration, compared to the unimmunized groups, consistent with our hypothesis that a Th1-skewed immune response provides a measure of protection against syphilis.
Also critical for the development of a syphilis vaccine is the ability of the vaccine to protect against disseminated T. pallidum infection. In the current study, the capacity of the tri-antigen vaccine cocktail to prevent against disseminated infection was investigated, following challenge, by transferring popliteal lymph nodes from immunized and unimmunized rabbits to naïve rabbits, and assessing infection development via monitoring for orchitis (both locations) and seroconversion (UVic). At both locations, lymph nodes from tri-antigen vaccine cocktail-immunized rabbits contained fewer viable T. pallidum, as demonstrated by delayed development of orchitis in recipient naïve animals, with comparable reductions in T. pallidum burden at both locations (88 % at UW and 85 % at UVic). Although statistical significance was not achieved in the experiment conducted at UVic, perhaps due to the small number of unimmunized rabbits tested or the earlier date of collection of the popliteal lymph nodes post-challenge (day 14 at UVic compared to day 42 at UW), a similar trend towards delayed onset of orchitis was observed. Significantly, one RIT animal receiving lymph nodes from a tri-antigen cocktail-immunized rabbit at UVic did not develop orchitis or seroconvert, even up to 90 days after injection, indicating a lack of T. pallidum dissemination to the lymph nodes in the immunized animal. Overall, these results demonstrate that immunization of rabbits with the tri-antigen vaccine cocktail reduced dissemination of T. pallidum within the host, but also indicate that further optimization will be required to attain a vaccine formulation that can achieve consistent and complete protection against treponemal dissemination in an immunized individual.
Based upon prior investigations, the Tp0751 protein included in the tri-antigen vaccine cocktail is presumed to be responsible for inhibiting treponemal dissemination. These published investigations include demonstration of reduced treponemal dissemination following immunization [27], and reports from two independent laboratories linking Tp0751 protein to roles in host cell adhesion and cell–cell junctional disruption, functions that would contribute to treponemal dissemination [32–34,36,54,55]. Another report by Luthra et al. [56] investigating the protective capacity of Tp0751 in the rabbit animal model found that 75 % (3/4) of recipient rabbits receiving lymph nodes from Tp0751-immunized rabbits did not develop orchitis or seroconvert by day 90 post-lymph node transfer, compared to 50 % (1/2) of recipient rabbits receiving lymph nodes from control sham-immunized animals. The authors of this study attributed this difference in dissemination to rabbit-to-rabbit variability, which is possibly a factor, however their data (despite the small number of controls) certainly do not rule out a role for immunization with Tp0751 in reducing dissemination. The fact the authors immunized with a protein containing aa 25–237 (rather than 24–237 as used by us) is not insignificant, as our unpublished studies have shown that the inclusion of aa 24 is important in the induction of appropriate immune responses. Thus, their data are consistent with our prior experimental results [27] and those of the current study, and collectively demonstrate that Tp0751 immunization plays a role in inhibition of T. pallidum dissemination in the host.
We recognize that the challenge dose that we routinely use (105 at UW or 106 at UVic) is quite high compared to that expected in a natural infection. Thus, in the Tri II study, we investigated the capacity of the tri-antigen vaccine cocktail to protect against 105 and 103 T. pallidum challenge doses. While significant reduction of both lesion volume and treponemal burden was observed in tri-antigen cocktail-immunized animals at both challenge doses compared to unimmunized controls, DTH was not observed in the tri-antigen-immunized animals challenged with 103 T. pallidum, nor was there a difference in lesion ulceration observed between tri-antigen cocktail-immunized animals and control unimmunized animals at this lower challenge dose. The immunized rabbits challenged with 103 T. pallidum did, however, show measurable erythema and induration (clinical DTH) at day 10 pc (Fig. 9b, inset), while none was seen in unimmunized control animals at that same time. By day 10, the treponemes in these animals would have undergone ~ 7 generations, and the bacterial load following a 103 challenge would be ~ 105. The clinical DTH persisted in the immunized rabbits and the volume of the lesions in immunized animals was larger than in unimmunized rabbits through day 15, by which time the control rabbits had begun to develop typical chancres; at subsequent time points, the mean lesion volumes between immunized and unimmunized rabbits diverged widely as the control lesion volumes enlarged rapidly. These data support the hypothesis that an early DTH-type infiltration is responsible for limiting the number of T. pallidum in lesions, and further support our earlier hypothesis that there may be a “critical antigenic mass” [57] needed to trigger cellular infiltration of the challenge site. Interestingly, TGF-β and IL-10 message levels were significantly higher in 48-hour biopsies from unimmunized rabbits compared to the immunized groups (Fig. 6). Both of these cytokines are known to have anti-inflammatory functions and, in unimmunized animals, their higher levels in early responses to T. pallidum challenge may contribute to the continued survival of the treponemes in the chancre until an acquired Th-1 response is induced by infection. A similar mechanism may be occurring in the immunized rabbits that are challenged with 103 T. pallidum in that, at 48 h, there was an abundance of message for TGFb in challenge site biopsies from both immunized and unimmunized rabbits, while no IFNγ message was detectable (data not shown). It appears that a higher antigen concentration is needed to induce a DTH response in immunized rabbits, and perhaps the lack of a robust early local infection contributed to the greater severity in the low-dose challenge rabbits. The implications of this for vaccine development are not clear.
A successful syphilis vaccine must be broadly protective against many T. pallidum strains, so we investigated the efficacy of the tri-antigen vaccine cocktail for protecting against infectious challenge with two heterologous T. pallidum strains, Chicago and Sea 81–4. The results showed a significant reduction of both lesion volume and treponemal burden in tri-antigen cocktail-immunized animals challenged with both T. pallidum strains compared to unimmunized controls. It should be noted that significant differences in lesion volume for the Chicago-challenged rabbits were transient, with the volumes between immunized and unimmunized rabbits overlapping after day 26. The reason for the inability of the vaccine response to limit Chicago growth, as was seen with the Nichols and the Sea 81–4 strains, is unknown. One notable difference between Chicago and the Nichols strain is the rapid rate at which Chicago’s tprK gene undergoes sequence variation due to segmental gene conversion [58], compared to the Nichols strain. One of the antigens used in our vaccine cocktail is the N-terminal portion of TprK, containing 3 of the 7 variable regions of TprK. While V1 and V2 are only modestly variable, the V3 sequence varies readily. It is possible that the variability of V3 in the challenge organisms, compared to the original TprK immunogen, permits the escape from binding of opsonic antibody and thus, escape from opsonophagocytic killing.
RIT animals receiving lymph nodes from tri-antigen cocktail-immunized animals challenged with the T. pallidum Chicago strain showed a trend toward delayed orchitis compared to animals receiving lymph nodes from control unimmunized rabbits, although statistical significance was not achieved. Due to the inability of Sea 81–4-to result in orchitis (even during routine intratesticular passage in rabbits), treponemal burden in RIT recipient rabbits was directly measured. In this case, the tri-antigen cocktail-immunized Sea 81–4-challenged animals exhibited significantly reduced T. pallidum dissemination compared to unimmunized controls. It should be noted that these heterologous protection experiments used a version of Tp0751 that had not undergone natural processing to generate lower molecular mass fragments corresponding to the structural lipocalin domain found within the mature protein [54,59], a phenomenon that may contribute to the enhanced inhibition of dissemination observed in the earlier Nichols-challenged rabbits in UW Tri I and the UVic studies.
The strengths of the studies reported here include the obvious attenuation of lesion progression and reduction of treponemal dissemination conferred by immunization, the reproducibility of the results at different locations and using rabbits from different sources, and the sensitivity to detect differences in immunity induced by even slightly different adjuvants. The studies also confirm the essentially identical cellular infiltration and cytokine milieu in rabbits as is seen in natural infection of humans, validating the model. Further, unlike the many studies of other infections that are conducted in inbred mice, rabbits are outbred, as are humans, and show the same, sometimes vexing, variability in responses to immunization and to infection, reinforcing the need for having large samples sizes in treatment and control groups.
The major weakness of our studies is that complete protection against infection was not achieved. Although reproducible and significant attenuation in lesion development and progression, as well as reduction in the number of treponemes disseminating in the host, were seen in our studies, all of the animals were infected by the challenge organisms. This raises the question of whether sterile protection can actually be achieved. The Miller experiment mentioned earlier [45] is seen as the gold standard level of protection. However, that study has never been reproduced, even in the Miller lab. The inability of even long-standing, treated or untreated, syphilis infection to confer protection against reinfection by heterologous strains suggests that a successful vaccine will need to be more effective than nature itself. Improvements in the level of protection are likely achievable with different antigen combinations, different antigen formulations, and improved adjuvants. Certainly, the mRNA technology that has been used so successfully with SARS Co-V2 and other viruses will be explored with bacterial pathogens if single (or a few) truly protective antigens can be identified for those more complex organisms. Given the possibility that sterile protection against syphilis infection may not be achievable, an important question is whether a syphilis vaccine that is partly effective in preventing lesion ulceration or dissemination would be acceptable and sufficient to reduce the global syphilis burden.
Supplementary Material
Acknowledgements
The following persons are thanked for their valuable assistance in the conduct of this work: at UVic, Ayman Haimour, Ethan Schovanek, Sean Waugh, Bradley Bachmeier, and Darcy Sutherland; at UW, Fartun Sheygo, Austin Haynes, and Ken Tapia (for statistical assistance). We thank the veterinarians and animal care staff at both institutions; without their dedication, particularly during the initial COVID-19 outbreak, this work would not have been possible.
Research Funding
This work has been supported primarily by R01 AI123196 (SAL + CEC, multiple PIs) from the National Institutes of Health. The early and ongoing investigations of the antigens used in this study were supported by R01AI42143, R01AI18988, P01AI34616, and AI63940 to SAL; this study was also supported by R37AI051334 and U19AI144133 from NIAID and SCI-178732 from CIHR to CEC. Initial analysis of adjuvants was supported by Life Sciences Discovery Fund 08–12 from the State of Washington to SAL & CEC.
Footnotes
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Caroline Cameron has patent issued to University of Victoria.
Ethics declaration
Rabbits used in these studies were provided care according to the Guide for the Care and Use of Laboratory Animals. All procedures performed on animals at the University of Washington were approved by the University of Washington Institutional Animal Care and Use Committee (IACUC; protocol number 2090–08; principal investigator, SAL.). All procedures performed on animals at the University of Victoria were approved by the University of Victoria Animal Care Committee (ACC; protocol number 2016–033; principal investigator, CEC).
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.11.002.
Data availability
Data will be made available on request.
References
- [1].O’Byrne P, MacPherson P. Syphilis. BMJ 2019;365:. 10.1136/bmj.l4159l4159. [DOI] [PMC free article] [PubMed]
- [2].Choudhri Y, Miller J, Sandhu J, Leon A, Aho J. Infectious and congenital syphilis in Canada, 2010–2015. Can Commun Dis Rep 2018;44(2):43–8. 10.14745/ccdr.v44i02a02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Spiteri G, Unemo M, Mardh O, Amato-Gauci AJ. The resurgence of syphilis in high-income countries in the 2000s: a focus on Europe. Epidemiol Infect 2019;147:e143. 10.1017/S0950268819000281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Arrieta AC, Singh J. Congenital Syphilis. N Engl J Med 2019;381(22):2157. 10.1056/NEJMicm1904420. [DOI] [PubMed] [Google Scholar]
- [5].Lancet (ed). Congenital syphilis in the USA. Lancet 2018;392(10154):1168. 10.1016/S0140-6736(18)32360-2. [DOI] [PubMed] [Google Scholar]
- [6].Korenromp EL, Rowley J, Alonso M, Mello MB, Wijesooriya NS, Mahiané SG, et al. Global burden of maternal and congenital syphilis and associated adverse birth outcomes-Estimates for 2016 and progress since 2012. PLoS ONE 2019;14(2):e0211720. 10.1371/journal.pone.0211720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nelson R Congenital syphilis and other STIs rise in the USA. Lancet Infect Dis 2018;18(11):1186–2117. 10.1016/S1473-3099(18)30618-2. [DOI] [PubMed] [Google Scholar]
- [8].Peeling RW, Mabey D, Kamb ML, Chen XS, Radolf JD, Benzaken AS. Syphilis. Nat Rev Dis Primers 2017;3:17073. 10.1038/nrdp.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lithgow KV, Cameron CE. Vaccine development for syphilis. Expert Rev Vaccines 2017;16(1):37–44. 10.1080/14760584.2016.1203262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cameron CE, Lukehart SA. Current status of syphilis vaccine development: need, challenges, prospects. Vaccine 2014;32(14):1602–9. 10.1016/j.vaccine.2013.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cameron CE. Syphilis vaccine development: requirements, challenges and opportunities. Sex Transm Dis 2018;45(9S):S17–9. 10.1097/OLQ.0000000000000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].LaFond RE, Lukehart SA. Biological basis for syphilis. Clin Microbiol Rev 2006;19(1):29–49. 10.1128/CMR.19.1.29-49.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Lukehart SA, Hook EW, Baker-Zander SA, Collier AC, Critchlow CW, Handsfield HH. Invasion of the central nervous system by Treponema pallidum: implications for diagnosis and treatment. Ann Intern Med 1988;109 (11):855–62. 10.7326/0003-4819-109-11-855. [DOI] [PubMed] [Google Scholar]
- [14].Towns JM, Leslie DE, Denham I, Wigan R, Azzato F, Williamson DA, et al. Treponema pallidum detection in lesion and non-lesion sites in men who have sex with men with early syphilis: a prospective, cross-sectional study. Lancet Infect Dis 2021;21(9):1324–31. 10.1016/S1473-3099(20)30838-0. [DOI] [PubMed] [Google Scholar]
- [15].Wang C, Hu Z, Zheng X, Ye M, Liao C, Shang M, et al. A new specimen for syphilis diagnosis: evidence by high loads of Treponema pallidum DNA in saliva. Clin Infect Dis 2021;73(9):e3250–8. 10.1093/cid/ciaa1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Golden M, O’Donnell M, Lukehart S, Swenson P, Hovey P, Godornes C, et al. Treponema pallidum nucleic acid amplification testing to augment syphilis screening among men who have sex with men. J Clin Microbiol 2019;57(8). 10.1128/JCM.00572-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tantalo LC, Lukehart SA, Marra CM. Treponema pallidum strain-specific differences in neuroinvasion and clinical phenotype in a rabbit model. J Infect Dis 2005;191(1):75–80. 10.1086/426510. [DOI] [PubMed] [Google Scholar]
- [18].Fitzgerald TJ. Experimental congenital syphilis in rabbits. Can J Microbiol 1985;31(9):757–62. 10.1139/m85-142. [DOI] [PubMed] [Google Scholar]
- [19].Lukehart SA, Marra CM. Isolation and laboratory maintenance of Treponema pallidum Curr Protocols Microbiol 2007;Chapter 12(Unit 12A.1). 10.1002/9780471729259.mc12a01s7. [DOI] [PubMed] [Google Scholar]
- [20].Edmondson DG, Hu B, Norris SJ. Long-term in vitro culture of the syphilis spirochete Treponema pallidum subsp. pallidum. mBio 2018;9(3): e01153–18. 10.1128/mBio.01153-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Edmondson DG, Norris SJ. In Vitro Cultivation of the Syphilis Spirochete Treponema pallidum. Curr Protoc 2021;1(2):e44. 10.1002/cpz1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Izard J, Renken C, Hsieh C-E, Desrosiers DC, Dunham-Ems S, La Vake C, et al. Cryo-electron tomography elucidates the molecular architecture of Treponema pallidum, the syphilis spirochete. J Bacteriol 2009;191(24):7566–80. 10.1128/JB.01031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu J, Howell JK, Bradley SD, Zheng Y, Zhou ZH, Norris SJ. Cellular Architecture of Treponema pallidum: Novel Flagellum, Periplasmic Cone, and Cell Envelope as Revealed by Cryo Electron Tomography. J Mol Biol 2010;403(4):546–61. 10.1016/j.jmb.2010.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Radolf JD, Norgard MV, Schulz WW. Outer membrane ultrastructure explains the limited antigenicity of virulent Treponema pallidum. Proc Natl Acad Sci U S A 1989;86(6):2051–5. 10.1073/pnas.86.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Walker EM, Zampighi GA, Blanco DR, Miller JN, Lovett MA. Demonstration of rare protein in the outer membrane of Treponema pallidum subsp. pallidum by freeze-fracture analysis. J Bacteriol 1989;171(9):5005–11. 10.1128/jb.171.9.5005-5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cameron CE, Lukehart SA, Castro C, Molini B, Godornes C, Van Voorhis WC. Opsonic potential, protective capacity, and sequence conservation of the Treponema pallidum subspecies pallidum Tp92. J Infect Dis 2000;181 (4):1401–13. 10.1086/315399. [DOI] [PubMed] [Google Scholar]
- [27].Lithgow KV, Hof R, Wetherell C, Phillips D, Houston S, Cameron CE. A defined syphilis vaccine candidate inhibits dissemination of Treponema pallidum subspecies pallidum. Nat Commun 2017:8. 10.1038/ncomms14273.14273. [DOI] [PMC free article] [PubMed]
- [28].Sun ES, Molini BJ, Barrett LK, Centurion-Lara A, Lukehart SA, Van Voorhis WC. Subfamily I Treponema pallidum repeat protein family: sequence variation and immunity. Microbes Infect 2004;6(8):725–37. 10.1016/j.micinf.2004.04.001. [DOI] [PubMed] [Google Scholar]
- [29].Morgan CA, Lukehart SA, Van Voorhis WC. Immunization with the N-terminal portion of Treponema pallidum repeat protein K attenuates syphilitic lesion development in the rabbit model. Infect Immun 2002;70(12):6811–6. 10.1128/IAI.70.12.6811-6816.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Centurion-Lara A, Castro C, Barrett L, Cameron C, Mostowfi M, Van Voorhis WC, et al. Treponema pallidum major sheath protein homologue Tpr K is a target of opsonic antibody and the protective immune response. J Exp Med 1999;189 (4):647–56. 10.1084/jem.189.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Centurion-Lara A, Giacani L, Godornes C, Molini BJ, Reid TB, Lukehart SA. Fine analysis of genetic diversity of the tpr gene family among treponemal species, subspecies and strains. PLoS Negl Trop Dis 2013;7(5):. 10.1371/journal.pntd.0002222e2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lithgow KV, Church B, Gomez A, Tsao E, Houston S, Anne Swayne Leigh, et al. Identification of the neuroinvasive pathogen host target, LamR, as an endothelial receptor for the Treponema pallidum adhesin Tp0751. mSphere 2020;5(2). 10.1128/mSphere.00195-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kao W-C, Pětrošová H, Ebady R, Lithgow KV, Rojas P, Zhang Y, et al. Identification of Tp0751 (pallilysin) as a Treponema pallidum vascular adhesin by heterologous expression in the Lyme disease spirochete. Sci Rep 2017;7(1):1538. 10.1038/s41598-017-01589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lu S, Wang J, He Z, He S, Zheng K, Xu M, et al. Treponema pallidum Tp0751 alters the expression of tight junction proteins by promoting bEnd3 cell apoptosis and IL-6 secretion. Int J Med Microbiol 2022;312(4):151553. 10.1016/j.ijmm.2022.151553. [DOI] [PubMed] [Google Scholar]
- [35].Haynes AM, Godornes C, Ke W, Giacani L. Evaluation of the protective ability of the Treponema pallidum subsp. pallidum Tp0126 OmpW homolog in the rabbit model of syphilis. Infect Immun 2019;87(8). 10.1128/IAI.00323-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Houston S, Hof R, Francescutti T, Hawkes A, Boulanger MJ, Cameron CE. Bifunctional role of the Treponema pallidum extracellular matrix binding adhesin Tp0751. Infect Immun 2011;79(3):1386–98. 10.1128/IAI.01083-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Stills HF Jr. Adjuvants and antibody production: dispelling the myths associated with Freund’s complete and other adjuvants. ILAR J 2005;46 (3):280–93. 10.1093/ilar.46.3.280. [DOI] [PubMed] [Google Scholar]
- [38].Giacani L, Molini B, Godornes C, Barrett L, Van Voorhis W, Centurion-Lara A, et al. Quantitative analysis of tpr gene expression in Treponema pallidum isolates: Differences among isolates and correlation with T-cell responsiveness in experimental syphilis. Infect Immun 2007;75(1):104–12. 10.1128/IAI.01124-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Godornes C, Leader BT, Molini BJ, Centurion-Lara A, Lukehart SA. Quantitation of rabbit cytokine mRNA by real-time RT-PCR. Cytokine 2007;38(1):1–7. 10.1016/j.cyto.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Schnupf P, Sansonetti PJ. Quantitative RT-PCR profiling of the rabbit immune response: assessment of acute Shigella flexneri infection. PLoS ONE 2012;7(6): e36446. 10.1371/journal.pone.0036446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Turner TB, Hardy PH, Newman B. Infectivity tests in syphilis. Br J Vener Dis 1969;45(3):183–96. 10.1136/sti.45.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Turner TB, Hollander DH. Biology of the Treponematoses Geneva: World Health Organization; 1957. [PubMed] [Google Scholar]
- [43].Lukehart SA. Prospects for development of a treponemal vaccine. Rev Infect Dis 1985;7(Suppl 2):305–13. 10.1093/clinids/7-supplement_2.s30. [DOI] [PubMed] [Google Scholar]
- [44].Cullen PA, Cameron CE . Progress towards an effective syphilis vaccine: the past, present and future. Expert Rev Vaccines 2006;5(1):67–80. 10.1586/14760584.5.1.67. [DOI] [PubMed] [Google Scholar]
- [45].Miller JN. Immunity in experimental syphilis. VI. Successful vaccination of rabbits with Treponema pallidum, Nichols strain, attenuated by γ- irradiation. J Immunol 1973;110(5):1206–15. [PubMed] [Google Scholar]
- [46].Alexander LJ, Schoch AG, Mantooth WB. Abortive treatment of syphilis; results obtained in the incubation, primary, and secondary stages of syphilis. Am J Syph Gonorrhea Vener Dis 1949;33(5):429–36. [PubMed] [Google Scholar]
- [47].Schroeter AL, Turner RH, Lucas JB, Brown WJ. Therapy for incubating syphilis. Effectiveness of gonorrhea treatment JAMA 1971;218(5):711–3. [PubMed] [Google Scholar]
- [48].Lukehart SA, Baker-Zander SA, Lloyd RM, Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. II. Nature of cellular infiltration and Treponema pallidum distribution in testicular lesions. J Immunol 1980;124(1):461–7. [PubMed] [Google Scholar]
- [49].Sell S, Baker-Zander S, Powell HC. Experimental syphilitic orchitis in rabbits: ultrastructural appearance of Treponema pallidum during phagocytosis and dissolution by macrophages in vivo. Lab Invest 1982;46(4):355–64. [PubMed] [Google Scholar]
- [50].Leader BT, Godornes C, VanVoorhis WC, Lukehart SA. CD4+ lymphocytes and gamma interferon predominate in local immune responses in early experimental syphilis. Infect Immun 2007;75(6):3021–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Van Voorhis WC, Barrett LK, Koelle DM, Nasio JM, Plummer FA, Lukehart SA. Primary and secondary syphilis lesions contain mRNA for Th1 cytokines. J Infect Dis 1996;173(2):491–5. 10.1093/infdis/173.2.491. [DOI] [PubMed] [Google Scholar]
- [52].Van Voorhis WC, Barrett LK, Nasio JM, Plummer FA, Lukehart SA. Lesions of primary and secondary syphilis contain activated cytolytic T cells. Infect Immun 1996;64(3):1048–50. 10.1128/iai.64.3.1048-1050.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Stary G, Klein I, Bruggen MC, Kholhofer S, Brunner PM, Spazierer D, et al. Host defense mechanisms in secondary syphilitic lesions: a role for IFN-gamma-/IL-17-producing CD8+ T cells? Am J Pathol 2010;177(5):2421–32. 10.2353/ajpath.2010.100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Houston S, Hof R, Honeyman L, Hassler J, Cameron CE. Activation and proteolytic activity of the Treponema pallidum metalloprotease, pallilysin. PLoS Pathog 2012;8(7):e1002822. 10.1371/journal.ppat. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lithgow KV, Tsao E, Schovanek E, Gomez A, Swayne LA, Cameron CE. Treponema pallidum disrupts VE-Cadherin intercellular junctions and traverses endothelial barriers using a cholesterol-dependent mechanism. Front Microbiol 2021;12:. 10.3389/fmicb.2021.691731691731. [DOI] [PMC free article] [PubMed]
- [56].Luthra A, Montezuma-Rusca JM, La Vake CJ, LeDoyt M, Delgado KN, Davenport TC, et al. Evidence that immunization with TP0751, a bipartite Treponema pallidum lipoprotein with an intrinsically disordered region and lipocalin fold, fails to protect in the rabbit model of experimental syphilis. PLoS Pathog 2020;16(9):e1008871. 10.1371/journal.ppat.1008871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Lukehart SA. Immunology and pathogenesis of syphilis. In: Quinn TC, Gallin JI, Fauci AS, editors. Sexually Transmitted Diseases New York: Raven Press; 1992. p. 141–63. [Google Scholar]
- [58].Centurion-Lara A, LaFond RE, Hevner K, Godornes C, Molini BJ, Van Voorhis WC, et al. Gene conversion: a mechanism for generation of heterogeneity in the tprK gene of Treponema pallidum during infection. Mol Microbiol 2004;52 (6):1579–96. 10.1111/j.1365-2958.2004.04086.x. [DOI] [PubMed] [Google Scholar]
- [59].Parker ML, Houston S, Pětrošová H, Lithgow KV, Hof R, Wetherell C, et al. The structure of Treponema pallidum Tp0751 (pallilysin) reveals a non-canonical lipocalin fold that mediates adhesion to extracellular matrix components and interactions with host cells. PLoS Pathog 2016;12(9):e1005919. 10.1371/journal.ppat.1005919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.


