Abstract
Corneal epithelial thickness (CET) and the regional variations in response to changes in corneal architecture and biomechanics have recently drawn the interest of corneal surgeons. Corneal epithelium possesses the tremendous capability of remodeling and changing its thickness. This remodeling of corneal epithelium takes place in response to underlying stromal irregularities which can result from a variety of corneal disorders including corneal ectasia. Measurement of CET can reveal the underlying stromal abnormalities and supplement in early diagnosis of corneal disorders especially corneal ectasia which has been one of the leading challenges in planning corneal refractive surgery. A significant number of patients ends up in ectasia after refractive surgery and the most common cause of this complication is the presence of preoperative subclinical keratoconus. Furthermore, postoperative complications of corneal refractive surgery are partly masked by epithelial remodeling and make the diagnosis and management difficult and extremely challenging. This leads not only to unpredictable visual and refractive outcome but also the need of multiple interventions to treat these complications. Although corneal tomography is considered as gold standard in the detection and diagnosis of corneal ectasia, a small number of subclinical cases may still go undetected. In this review, we have highlighted the underlying mechanism of epithelial remodeling, the devices and imaging modalities used to measure CET, and application of epithelial mapping in the diagnosis and management of various corneal disorders.
Keywords: Corneal epithelial thickness, Keratoconus, laser in situ keratomileusis, photo refractive keratectomy, phototherapeutic keratectomy, small incision lenticule extraction
Introduction
The corneal epithelium is a thin outermost layer of cornea that is considered essential in maintaining the corneal integrity. It plays a highly important role in protecting the rest of cornea and intraocular structures from external environment by providing a physical barrier and a regular smooth optical surface.[1] The epithelium has regenerating capability and therefore, constantly undergoing the process of renewal by proliferating limbal stem cells. It takes about 4–7 days for the whole corneal epithelium to completely regenerate itself.[2,3]
In normal population, the thickness of corneal epithelium is remarkably uniform, and the thickness profile remains the same under the influence renewal and regeneration by limbal stem cells. However, the thickness of corneal epithelium is not uniform in corneal disorders. The corneal epithelium is considered highly responsive to the underlying stromal irregularities and asymmetries.[4-6] It is believed that the corneal epithelium remodels itself by changing its thickness to mask the corneal curvature irregularities. These changes have been studied and documented in various corneal conditions such as corneal ectasia, post-laser refractive surgery, corneal dystrophies, and post-corneal collagen cross-linking (CXL).[7,8] The significant changes in the corneal epithelial thickness (CET) have drawn keen interest toward this subject over the recent years. Various studies have been published on the significance of measuring CET in diagnosis and management of ectatic disorders, postrefractive surgery complications, and ocular surface disorders.[9,10] Thickness of corneal epithelium determines the shape of air-tear film interface which can affect the total corneal dioptric power and the difference of refractive index between corneal epithelium and corneal stroma (1.401 vs. 1.377) can further potentiate this effect.[11]
In the light of published literature, we have evaluated the importance of CET and its application in the diagnosis and management of various corneal disorders.
Mechanism of Corneal Epithelial Remodeling
Corneal epithelial cells originate from stem cells in palisade of Vogt at limbus and move centripetally toward center to replaces all the layers of corneal epithelium. The mean thickness of corneal epithelium is 54 ± 5 μm and it consists of 5–7 layers of cells. Considering the regional thickness, nasal and inferior thickness have been documented as thicker as compared to superior and temporal quadrants.[12,13] In contrast to normal individuals, the thickness of corneal epithelium varies significantly in corneal disorders and after refractive procedures. The underlying mechanism for this corneal epithelial remodeling has been a debatable topic and there have been various hypotheses about the initiating mechanism for this change.
The most reliable proposed underlying mechanism is the rate of change in corneal curvature which drives the corneal epithelium to modify thickness and resist this change. The greater the rate of change in curvature leads to greater remodeling and greater corneal thickness variation to resist the change and keep the cornea smooth. Authors have supported this mechanism by concluding that a smaller optical zone in photorefractive keratectomy was associated with greater epithelial thickness changes due to a greater rate of change in corneal curvature while a larger optical zone was associated with less epithelial thickness changes and less regression.[14,15]
Other hypotheses that was considered by Kanellopoulos and Asimellis, proposed that the corneal epithelial remodeling occurs due to change in corneal thickness and corneal biomechanics. They concluded in their study that greater corneal epithelial change was seen when myopic LASIK was performed for a higher spherical equivalent.[16] However, this does not explain the increased epithelial thickness of the central cornea associated with central flattening after radial keratotomy where the central corneal thickness remains constant. Furthermore, this hypothesis goes against the study where a larger optical zone was associated with a smaller change in CET after photorefractive keratectomy (PRK) despite the fact that more tissue was ablated with larger optical zone.[14]
Another study which supports the hypothesis of curvature change as initiating mechanism for corneal epithelial remodeling has concluded that there is thinning of corneal epithelium at corneal apex after hyperopic laser refractive surgery where the peripheral cornea is ablated and central corneal thickness remains unscathed.[17]
Measuring Devices for Corneal Epithelial Thickness
There are number of available devices for the measurement of CET, the most important among them is very high-frequency digital ultrasound (VHFDU), optical coherence tomography (OCT), and confocal microscopy.[18]
Artemis 2 (ArcScan, Inc., Morrison, CO) is a commercially available VHFDU that uses a disposable eyepiece centered on the corneal vertex. It allows normal saline fluid coupling the transducer and patient’s eye to match the radius of curvature of the patient’s cornea. The measurements are acquired along different meridians from the surface of epithelium down to Bowmans’s membrane to generate a scanned map of 10 mm.
OCT on the other hand allows measurements in an easier and noncontact way, however, the main disadvantage of OCT-based devices is that thickness of tear film affects the measurements. These devices measure the air-tear film interface down to the front of Bowman’s membrane. Commercially, there are number of anterior segment OCT imaging devices available. RTVue XR Avanti (Optovue, Inc., Fremont, CA) uses a spectral-domain OCT (SD-OCT) with infrared light source of 840 nm wavelength. It has scan speed of 70,000 with an axial resolution of <10 μm and transverse resolution of 30 μm. It acquires 8 meridional scans at 22.5° intervals and generates a map of 9 mm with an acquisition time of 0.31 s. Artemis (ArcScan, Inc., Morrison, CO) and RTVue SD-OCT (Optovue, Inc., Fremont, CA) were considered to be highly correlated, but the CET measurements were statistically different, and the values measured by Artemis-2 values were systematically thicker than RTVue by 1.7 μm.[19]
Anterion (Heidelberg Engineering) uses swept-source (SS) OCT with an infrared light source of 1300 nm wavelength. It has scan speed of 50,000 with an axial resolution of <10 um and transverse resolution of 30 um. It can perform 65 radial scans with 256 A-scan lines centered on the corneal vertex over a 7-mm diameter with an acquisition time of <1 s. Mean corneal thickness is displayed at 41 points. The comparison of Anterion and Avanti showed that the repeatability of the Anterion device was higher than that of the Avanti.[20] Moreover, it was further concluded by Feng et al. that the readings of these devices cannot be interchanged as Anterion was consistently giving a thinner CET values than that of the Avanti.[21]
Cirrus HDOCT (Carl Zeiss, Jana, Germany) is another available device which can obtain 24 radial OCT scans and produces a 9 mm CET map. The reliability and reproducibility of this device in measuring CET was concluded by Prakash et al. in their study.[22]
MS39 (Costruzione Strumenti Oftalmici, Florence, Italy) which is yet another device that combines high resolution SD OCT based tomography with Placido disk corneal topography. It uses infrared light source of 845 nm wavelength. It has scan speed of 102,400 scans with axial resolution of 3.6 um and transverse resolution of 35 um. It produces an 8 mm epithelial map with an acquisition time of approximately 1 s. The advent of these devices has contributed to the accurate and precise measurement of CET.
Epithelial Mapping and Keratoconus
Keratoconus is a condition characterized by progressive corneal steepening, thinning, and apical protrusion.[20] The prevalence of this disorder varies in different regions of the globe with a range of 1 per 100,000–229 per 100,000.[23] Early diagnosis of this disorder holds critical importance in the better management and long-term visual outcome. Late diagnosis means, the availability of fewer treatment options and suboptimum visual outcome due to limited availability of donor corneas for keratoplasty.[24] In the context of refractive surgery, the leading cause of iatrogenic ectasia after laser LASIK is unrecognized subclinical keratoconus. Detection of this condition has got paramount importance in preoperative screening and workup of patients seeking refractive surgery.[22,25]
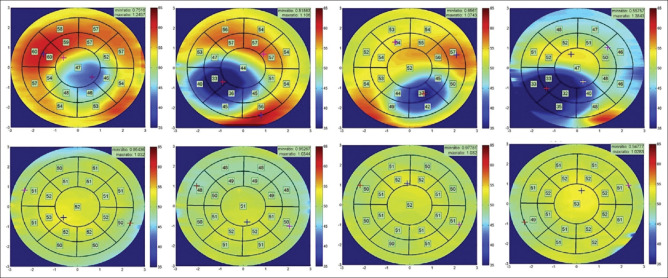
Out of various investigative modalities, corneal tomography is currently considered as gold standard diagnostic tool for early diagnosis of keratoconus. The diagnosis of KC is made by multiple tomographic indices based on corneal steepening, thinning, and asymmetry.[26,27] However, Reinstein et al. documented the role of corneal epithelial remodeling which can mask the corneal steepening in early keratoconus, and this may lead to poor detection of keratoconus. The corneal epithelium is found to be thinner over the corneal apex (steepest area) and thicker concentrically around it, creating a typical “Donut pattern” on the epithelial map.[28] CET is not only important in the early diagnosis of keratoconus but also useful in differentiating mimicking conditions such as asymmetric astigmatism and contact lens warpage.[29] Many authors documented the fact that CET map remains homogeneous in healthy individuals, while it shows a typical donut profile in early keratoconus as shown in Figure 1.
Figure 1.
Right and left eyes of keratoconus patients (top) and healthy individuals (bottom)
In addition to the typical donut profile in keratoconus, various topographic patterns of the epithelial map have been studied and it has been found that as the keratoconus progresses to more advanced form, the corneal epithelium is no more capable of masking the underlying stromal steepness and the typical donut shape or crater shape changes to more irregular patterns with multiple areas of thinning and thickening as shown in Figure 2. Patient with regular astigmatism shows regular epithelial thickness profile with no significant regional variation, while significant variability is seen in patients with corneal ectasia.[25,30] The epithelium is thicker centrally and thinner in the periphery in normal individuals, while in keratoconus patients, the corneal epithelium is thinner centrally and over the cone with surrounding thickening as shown in Figure 3.[30]
Figure 2.
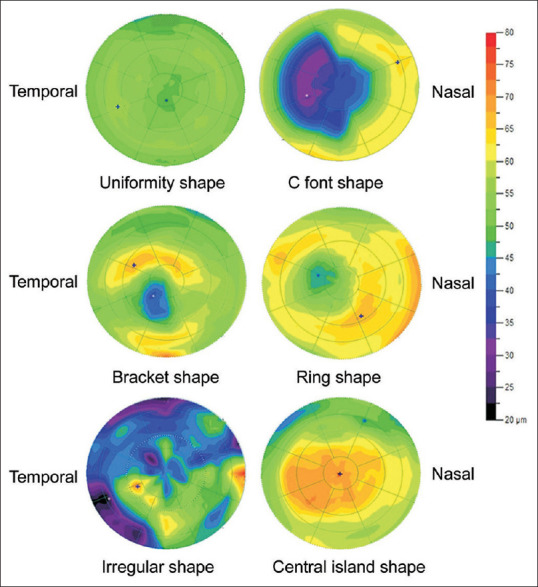
The maps shows normal and donut profile (top two images) of normal and Forme fruste keratoconus. The middle two images were seen in early Keratoconus and the multiple irregular pattern were seen in advanced cases of Keratoconus
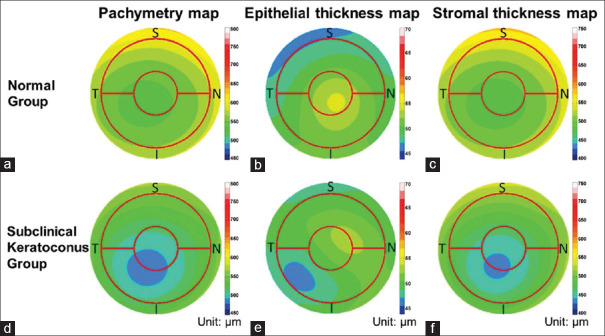
Figure 3.
Average pachymetric, corneal epithelial, and stromal thickness maps of normal (a-c) and subclinical keratoconus eyes (d-f). Maps of the left eyes that were included were mirrored before averaging. The red circles overlaid on the map had diameters of 2.0 mm and 5.0 mm. The color scale represents the thickness in microns. I: Inferior, N: Nasal, S: Superior, T: Temporal
The coincident corneal epithelial thinning over or adjacent to the thinnest pachymetry is an important finding which is seen before the appearance of the donut profile in early keratoconus detection.[31] The thinnest epithelial location has a strong agreement with the location of the steepest cornea. This fact can differentiate keratoconus from contact lens warpage, where the corneal epithelium is thicker over the steepest location.[31,32]
Corneal collagen CXL, which is considered an important treatment modality in halting progression of keratoconus, increases the corneal stiffness by inducing cross-linkage between collagen fibers. In addition to stopping the progression, it also regularizes the cornea curvature which is evidenced by decreased variability of regional CET after CXL. The pattern deviation of epithelial thickness decreases over 1–3 months after CXL which can be one of the factors responsible for the improvement of vision in patients of keratoconus.[33] The role of epithelial remodeling and epithelial thickness in combined procedures of CXL with topography-guided customized ablation such as phototherapeutic keratectomy (PTK) or PRK cannot be emphasized enough. As the thickness of epithelium is variable and thinnest over the cone, the ablation depth of these procedures should be customized to individual patients on the basis of epithelial thickness. This will lead to better visual outcome with the least tissue ablation.[34] The role corneal epithelial remodeling is also observed after the insertion of intracorneal ring segments (ICRS). CET increases adjacent to ICRS to compensate for the induced ridge and also over the cone to regularize the corneal surface.[35]
Corneal epithelial remodeling plays an important role in stromal lenticule addition keratoplasty (SLAK), a procedure whereby femtosecond laser-assisted stromal pocket is created in selected patients of keratoconus, followed by implantation of negative meniscus shaped lenticule.[36] The thin hyper prolate corneas of these patients undergo corneal epithelial and stromal remodeling. The thin epithelium over the center of the cone remodels itself and reaches almost a normal CET by 3 months postoperatively and this increase in epithelial thickness not only regularizes the corneal curvature for future contact lens fitting but also improves the corrected distance visual acuity in these patients.[37]
Epithelial Mapping and Refractive Surgery
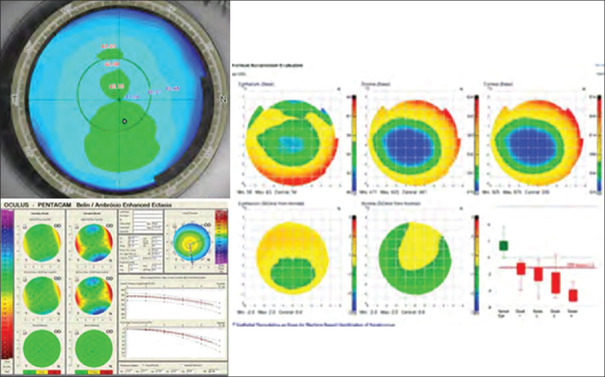
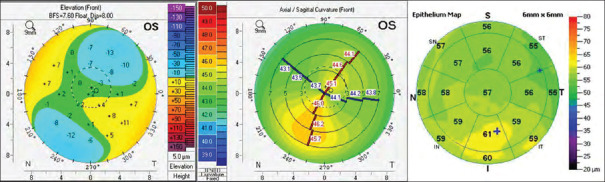
Three commonly performed laser procedures to treat myopia include PRK, laser in situ keratomileusis (LASIK), and small incision lenticule extraction (SMILE). The assessment of the epithelial thickness map can assist in the inclusion or exclusion of patients seeking refractive surgery. As depicted in Figure 4, the patient was included in the surgery as there was mild asymmetry and normal BAD, however, the epithelial map showed a typical “Donut pattern” and hence, the patient was finally excluded from surgery due to the presence of subclinical KC. In another patient, there was inferior steepening on the axial map, however, the epithelial map showed thickening over the steep part, and this patient was included for refractive surgery as shown in Figure 5.[28]
Figure 4.
Normal looking axial curvature, with normal Bellin Ambrosio enhanced display, epithelial map shows donut profile
Figure 5.
Epithelial hyperplasia on steep location. I: Inferior, N: Nasal, S: Superior, T: Temporal
In the treatment of Myopia, laser refractive procedures lead to the flattening of the central cornea, hence reducing the corneal power, and correcting the myopia.[38] The structural changes secondary to epithelial remodeling after laser refractive surgery occurs as a result of epithelial hyperplasia. This effect is mediated by the activation of corneal stromal keratocytes after laser-induced injury to the corneal stroma. The formation of fibroblasts and myofibroblasts induces the corneal epithelial remodeling and an excessive response can lead to subepithelial corneal haze after surface ablation and regression after refractive procedures.[39]
Surface ablation procedures such as PRK entails the removal of corneal epithelium, followed by ablation of the bowman membrane and corneal stroma. The corneal epithelium healing is complete within 4–7 days; however, the remodeling continues for months after the treatment. Corneal epithelial remodeling is also seen after LASIK and SMILE, although the procedure does not involve the removal of the epithelium. The hyperplasia of corneal epithelium is more marked at 3 months postoperatively coinciding with the regeneration of corneal nerves. Some authors believe that corneal nerves also play a role in corneal remodeling. After 3 months postoperatively, the corneal epithelium remains stable thereafter and statistically insignificant change is noted in CET thereafter.[40,41] Considering the regional CET after myopic treatment, the corneal epithelium is significantly thicker in the central and paracentral regions as compared to the periphery in post-myopic PRK.[42] The shape of the cornea changes from prolate to oblate after myopic treatment and this oblateness is said to be more if the ablation depth or preoperative spherical equivalent is higher. Corneal remodeling is believed to be partly responsible for the oblateness postoperatively. CET changes are positively related to the preoperative spherical equivalent and ablation depth, while negatively related to the optical zone. Therefore, in higher myopic correction and with a smaller optical zone, the postoperative corneal epithelial response is more aggressive, and the thickness is higher. Postoperative regression of myopia is still an important concern after laser refractive surgery and corneal epithelial remodeling is believed to be an important factor in inducing regression.[40] The CET is found to be higher in patients who developed regression. Higher CET in the center makes it steeper and this may lead to re-appearance of myopic refractive error postoperatively. Almost 10 μm increase in thickness of corneal epithelium induces an error of 1 diopter.[41]
LASIK for hypermetropia is also followed by epithelial remodeling and the changes in CET. The cornea is thinner in the center and thicker in the periphery. The typical pattern of “donut profile” seen in keratoconus patients is also seen after hyperopic LASIK.[39] The changes in thickness are more pronounced as compared to myopic LASIK, and this effect is attributed to the abrupt changes in curvature. The central thinning and paracentral thickening try to flatten the cornea centrally which can lead to a reversal of hyperopic correction. The excessive thinning (<25 μm) of apical corneal epithelium can result in apical syndrome. Therefore, in addition to steep k values, the epithelial thickness over corneal apex can be a useful deciding factor in limiting the amount of hyperopic correction.[43]
Advances in corneal topography and ocular wavefront measurement have improved the diagnosis and management of patients seeking refractive surgery. However, the cause of visual symptoms in irregular astigmatism is not always easy to evaluate because irregular astigmatism leads to the irregular epithelial thickness and the masking effect. Reinstein et al. utilized trans-epithelial phototherapeutic keratectomy (TE-PTK) to treat the irregularities that are masked by the corneal epithelium. TE-PTK is based on digital subtraction pachymetry which considers both CET and corneal pachymetry in account to generate an ablation profile that can deal with the masking effects of corneal epithelium. The limitation observed with this procedure was that it could only treat the proportion of irregularities that are compensated for by the corneal epithelium and it induced refractive error in the patients.[20,31,41,42]
Epithelial Thickness and Other New Modalities
Preoperative assessment of patients seeking refractive surgery is primarily based on the amount of refractive error and the corneal parameters such as central corneal thickness and corneal curvature. However, the risk of iatrogenic ectasia is the most important concern which needs a detailed assessment and a higher threshold for the commencement of treatment.[20,24,25] Various modalities have been developed to rule of risk of postoperative ectasia risk in addition to measurement of CET such as assessment of corneal biomechanics, Brillouin spectroscopy, optical coherence elastography (OCE) and biomarkers in tear film and serum. Devices such as ocular response analyzer designed for the measurement of intraocular pressure primarily and CORVIS STL which employs a noncontact air puff along with a Scheimpflug camera to measure the corneal bimechanical properties with the background that cause (biomechanics weakening of cornea) occurs much earlier than the effect (topographic changes) in keratoconus. Despite their usage in clinical practice for the measurement of intraocular pressure and corneal thickness, the utilization for early detection of keratoconus is still debatable and the results of corneal deformation and biomechanics parameters are variable.[42] Brillouin spectroscopy is a noncontact method that measures the frequency shift of photons when monochromatic laser light interact with the cornea. The frequency shift is mathematically related to the bulk elastic modulus of cornea. Despite the great future potential, the accuracy of this modality in vivo could not distinguish keratoconus from normal eyes. OCE is based on the depth-dependent analysis of cornea using the ultrasound elastography principle. One study has revealed that there is selective anterior stromal weakening in the eyes with keratoconus when assessed with OCE. The altered levels of biomarkers such as inflammatory cytokines and matrix metalloproteinase in the tear film and immunoglobulins in serum of patients with keratoconus have been documented and the noninflammatory nature of this disorder is questioned, however, long-term data and studies need further evidence to support this.[42,43]
Among all these new modalities for assessing ectasia risk, CET mapping is the only one that has been widely used and studied so far and it can be considered a reliable supplementary tool with tomography in preoperative workup of refractive surgery patients.
Epithelial Thickness and Contact Lenses
The role of contact lenses cannot be more emphasized in the management of refractive error and corneal ectasia. Based on the design, material, and usage, contact lenses are categorized as soft, rigid (Rigid Gas permeable), and hybrid lenses. Corneal epithelial remodeling does occur in response to contact lens wear and the measurement of CET confirms these changes. Hong et al. concluded that there is a significant reduction in the CET in central, para-central, and mid-peripheral zones after long-term wear (more than 2 years) of soft contact lenses in comparison to the normal population.[44] Another study regarding the daily disposable soft contact lenses showed that there is no statistically significant effect on CET in patients wearing daily disposable soft contact lenses.[45]
Epithelial Thickness and Orthokeratology
Orthokeratology is a well-documented modality used to correct refractive errors such as myopia and astigmatism. The rigid contact lens is worn overnight, and the compression effect of the lens induces a temporary change in the corneal curvature, thus treating the refractive error during the following day.[46] Epithelial thickness map plays a vital role in providing the explanation and mechanism of the effect of orthokeratology on the corneal epithelium. A reduction of CET in central zone with associated thickening in the mid-periphery was noticed in myopic patients.[46] On the other hand, the opposite effect was seen in hyperopic patients. Paul Gifford et al. concluded that there was higher CET and stromal thickness in the central zone along with thinning of CET in the mid-periphery in hyperopic Orthokeratology.[47]
Epithelial Thickness and Corneal Dystrophies
Epithelial basement membrane dystrophy (EBMD) is the most common type of dystrophy which is characterized by corneal epithelial irregularities and has a characteristic map dot fingerprint appearance.[48] Epithelial irregularities associated with EBMD can result in blurred vision and recurrent epithelial erosions. In a study by Buffault et al., CET was measured in patients suffering from EBMD. It was found that patients of EBMD have significantly higher CET.[49]
Epithelial Thickness and Dry Eyes
Dry eyes are a very common clinical problem and have a multifactorial etiology leading to significant visual morbidity. It has a significant role and impact on the preoperative workup of patients seeking treatment for refractive error and refractive surgery.[43] CET maps provide detailed information about epithelial behavior and remodeling in response to dryness. Kanellopoulos and Asimellis concluded that patients with dry eyes tend to have higher CET when compared to the normal population. Furthermore, this change in CET is reversible and revert back after treatment and resolution of the disease.[50]
Key Points
Changes in CET occur in response to changes in corneal curvature and thickness. The magnitude of these changes is directly related to the magnitude of change in curvature as well as the rate of change in curvature
Corneal epithelial remodeling masks the irregularities of the corneal stromal surface which can lead to miscalculation and misdiagnosis preoperatively and unpredictable results after laser refractive surgery. The masking effect occurs to a limited extent beyond which the irregularities become apparent over the corneal epithelial surface
CET profile can play an important role in the early diagnosis of keratoconus. The coincident presence of corneal epithelial thinning over or near to thinnest pachymetry and “Donut pattern” can augment early diagnosis of KC
CET map in conjunction with corneal topography can be utilized to include or exclude patients who are seeking corneal refractive surgery
TE-PTK procedure can effectively treat irregularly irregular astigmatism utilizing digital subtraction pachymetry, however this procedure can induce refractive error and a need for a second procedure later
CET remodeling has opened up a new dimension of customized ablation based on stromal surface topography instead of corneal topography alone. This will undermine the miscalculations and unpredictable refractive outcome in the management of refractive surgery complications as well as irregularly irregular astigmatism
Although new modalities for early detection of keratoconus are developing such as assessment of corneal biomechanics and biomarkers, none has gained as much utilization in clinical practice as did the measurement of CET.
Conclusion
The role of epithelial thickness in response to changes in corneal stromal surface and advancements in the imaging of the anterior segment has led to new dimensions in the management of corneal disorders and refractive errors. The utilization of CET profile will further lead to achieve perfection in the management of corneal refractive surgery and its complications. Futuristic research should focus on the factors that influence CET, utilizing the improved imaging modalities to generate laser ablation profile based on the corneal stromal surface along with CET profile.
Literature Search and Methodology
We used the search engine PubMed (www.pubmed.org) for the literature search. Terms used for this purpose included corneal epithelial remodeling, corneal epithelial thickness, anterior segment OCT, Confocal Microscopy, Very High-Frequency Digital Ultrasound, Keratoconus, Subclinical Keratoconus, Corneal collagen Cross-Linking (CXL), LASIK, PRK, SMILE, ICRS, Topo-guided customized ablation treatment (TCAT) and Trans-epithelial PTK (TE-PTK). After the initial segregation of the published literature, articles published in English were further sorted out. The titles and abstracts were assessed, and the relevant publications were selected to be reviewed and studied in detail. The references of selected articles were further explored to expand the review on the subject. All potential articles were reviewed in a comprehensive way and the most relevant ones were finally included. The literature referred to in this review may not be all inclusive, however, we intended to include the most recent and relevant articles on this subject.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Gauthier CA, Holden BA, Epstein D, Tengroth B, Fagerholm P, Hamberg-Nyström H. Factors affecting epithelial hyperplasia after photorefractive keratectomy. J Cataract Refract Surg. 1997;23:1042–50. doi: 10.1016/s0886-3350(97)80078-8. [DOI] [PubMed] [Google Scholar]
- 2.Ruan Y, Jiang S, Musayeva A, Pfeiffer N, Gericke A. Corneal epithelial stem cells-physiology, pathophysiology and therapeutic options. Cells. 2021;10:2302. doi: 10.3390/cells10092302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells:Past, present, and future. Stem Cell Res Ther. 2019;10:68. doi: 10.1186/s13287-019-1165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocha KM, Perez-Straziota CE, Stulting RD, Randleman JB. SD-OCT analysis of regional epithelial thickness profiles in keratoconus, postoperative corneal ectasia, and normal eyes. J Refract Surg. 2013;29:173–9. doi: 10.3928/1081597X-20130129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanellopoulos AJ, Asimellis G. OCT corneal epithelial topographic asymmetry as a sensitive diagnostic tool for early and advancing keratoconus. Clin Ophthalmol. 2014;8:2277–87. doi: 10.2147/OPTH.S67902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverman RH, Urs R, Roychoudhury A, Archer TJ, Gobbe M, Reinstein DZ. Epithelial remodeling as basis for machine-based identification of keratoconus. Invest Ophthalmol Vis Sci. 2014;55:1580–7. doi: 10.1167/iovs.13-12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reinstein DZ, Archer TJ, Gobbe M. Corneal epithelial thickness profile in the diagnosis of keratoconus. J Refract Surg. 2009;25:604–10. doi: 10.3928/1081597X-20090610-06. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Tan O, Brass R, Weiss JL, Huang D. Corneal epithelial thickness mapping by Fourier-domain optical coherence tomography in normal and keratoconic eyes. Ophthalmology. 2012;119:2425–33. doi: 10.1016/j.ophtha.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Utsunomiya T, Hanada K, Muramatsu O, Ishibazawa A, Nishikawa N, Yoshida A. Wound healing process after corneal stromal thinning observed with anterior segment optical coherence tomography. Cornea. 2014;33:1056–60. doi: 10.1097/ICO.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 10.Tang M, Li Y, Huang D. Corneal epithelial remodeling after LASIK measured by Fourier-Domain optical coherence tomography. J Ophthalmol. 2015;2015:860313. doi: 10.1155/2015/860313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel S, Marshall J, Fitzke FW., 3rd Refractive index of the human corneal epithelium and stroma. J Refract Surg. 1995;11:100–5. doi: 10.3928/1081-597X-19950301-09. [DOI] [PubMed] [Google Scholar]
- 12.Le Q, Chen Y, Yang Y, Xu J. Measurement of corneal and limbal epithelial thickness by anterior segment optical coherence tomography and in vivo confocal microscopy. BMC Ophthalmol. 2016;16:163. doi: 10.1186/s12886-016-0342-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reinstein DZ, Yap TE, Archer TJ, Gobbe M, Silverman RH. Comparison of corneal epithelial thickness measurement between Fourier-Domain OCT and very high-frequency digital ultrasound. J Refract Surg. 2015;31:438–45. doi: 10.3928/1081597X-20150623-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Brart DP, Corbett MC, Verma S, Heacock G, Oliver KM, Lohmann CP, et al. Effects of ablation diameter, depth, and edge contour on the outcome of photorefractive keratectomy. J Refract Surg. 1996;12:50–60. doi: 10.3928/1081-597X-19960101-12. [DOI] [PubMed] [Google Scholar]
- 15.O'Brart DP, Gartry DS, Lohmann CP, Muir MG, Marshall J. Excimer laser photorefractive keratectomy for myopia:Comparison of 4.00- and 5.00-millimeter ablation zones. J Refract Corneal Surg. 1994;10:87–94. [PubMed] [Google Scholar]
- 16.Kanellopoulos AJ, Asimellis G. Longitudinal postoperative lasik epithelial thickness profile changes in correlation with degree of myopia correction. J Refract Surg. 2014;30:166–71. doi: 10.3928/1081597X-20140219-01. [DOI] [PubMed] [Google Scholar]
- 17.Vinciguerra P, Camesasca FI, Morenghi E, Azzolini C, Pagano L, Trazza S, et al. Corneal apical scar after hyperopic excimer laser refractive surgery:Long-term follow-up of treatment with sequential customized therapeutic keratectomy. J Refract Surg. 2018;34:113–20. doi: 10.3928/1081597X-20171214-01. [DOI] [PubMed] [Google Scholar]
- 18.Reinstein DZ, Silverman RH, Raevsky T, Simoni GJ, Lloyd HO, Najafi DJ, et al. Arc-scanning very high-frequency digital ultrasound for 3D pachymetric mapping of the corneal epithelium and stroma in laser in situ keratomileusis. J Refract Surg. 2000;16:414–30. doi: 10.3928/1081-597X-20000701-04. [DOI] [PubMed] [Google Scholar]
- 19.Urs R, Lloyd HO, Reinstein DZ, Silverman RH. Comparison of very-high-frequency ultrasound and spectral-domain optical coherence tomography corneal and epithelial thickness maps. J Cataract Refract Surg. 2016;42:95–101. doi: 10.1016/j.jcrs.2015.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Randleman JB, Woodward M, Lynn MJ, Stulting RD. Risk assessment for ectasia after corneal refractive surgery. Ophthalmology. 2008;115:37–50. doi: 10.1016/j.ophtha.2007.03.073. [DOI] [PubMed] [Google Scholar]
- 21.Feng Y, Reinstein DZ, Nitter T, Archer TJ, McAlinden C, Chen X, et al. Heidelberg anterion swept-source OCT corneal epithelial thickness mapping:Repeatability and agreement with optovue Avanti. J Refract Surg. 2022;38:356–63. doi: 10.3928/1081597X-20220414-01. [DOI] [PubMed] [Google Scholar]
- 22.Prakash G, Agarwal A, Mazhari AI, Chari M, Kumar DA, Kumar G, et al. Reliability and reproducibility of assessment of corneal epithelial thickness by Fourier domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:2580–5. doi: 10.1167/iovs.11-8981. [DOI] [PubMed] [Google Scholar]
- 23.Gordon-Shaag A, Millodot M, Shneor E, Liu Y. The genetic and environmental factors for keratoconus. Biomed Res Int. 2015;2015:795738. doi: 10.1155/2015/795738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colin J, Cochener B, Savary G, Malet F. Correcting keratoconus with intracorneal rings. J Cataract Refract Surg. 2000;26:1117–22. doi: 10.1016/s0886-3350(00)00451-x. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Chamberlain W, Tan O, Brass R, Weiss JL, Huang D. Subclinical keratoconus detection by pattern analysis of corneal and epithelial thickness maps with optical coherence tomography. J Cataract Refract Surg. 2016;42:284–95. doi: 10.1016/j.jcrs.2015.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim HB, Tan GS, Lim L, Htoon HM. Comparison of keratometric and pachymetric parameters with Scheimpflug imaging in normal and keratoconic Asian eyes. Clin Ophthalmol. 2014;8:2215–20. doi: 10.2147/OPTH.S66598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shetty R, Rao H, Khamar P, Sainani K, Vunnava K, Jayadev C, et al. Keratoconus screening indices and their diagnostic ability to distinguish normal from ectatic corneas. Am J Ophthalmol. 2017;181:140–8. doi: 10.1016/j.ajo.2017.06.031. [DOI] [PubMed] [Google Scholar]
- 28.Reinstein DZ, Archer TJ, Gobbe M, Silverman RH, Coleman DJ. Stromal thickness in the normal cornea:Three-dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg. 2009;25:776–86. doi: 10.3928/1081597X-20090813-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hwang ES, Schallhorn JM, Randleman JB. Utility of regional epithelial thickness measurements in corneal evaluations. Surv Ophthalmol. 2020;65:187–204. doi: 10.1016/j.survophthal.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Yang XL, Wang Y, Luo BG, Xu Y, Zhang XF. Corneal epithelial thickness analysis of forme fruste keratoconus with optical coherence tomography. Int J Ophthalmol. 2021;14:89–96. doi: 10.18240/ijo.2021.01.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang M, Li Y, Chamberlain W, Louie DJ, Schallhorn JM, Huang D. Differentiating keratoconus and corneal warpage by analyzing focal change patterns in corneal topography, pachymetry, and epithelial thickness maps. Invest Ophthalmol Vis Sci. 2016;57:T544–9. doi: 10.1167/iovs.15-18938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schallhorn JM, Tang M, Li Y, Louie DJ, Chamberlain W, Huang D. Distinguishing between contact lens warpage and ectasia:Usefulness of optical coherence tomography epithelial thickness mapping. J Cataract Refract Surg. 2017;43:60–6. doi: 10.1016/j.jcrs.2016.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rocha KM, Perez-Straziota CE, Stulting RD, Randleman JB. Epithelial and stromal remodeling after corneal collagen cross-linking evaluated by spectral-domain OCT. J Refract Surg. 2014;30:122–7. doi: 10.3928/1081597X-20140120-08. [DOI] [PubMed] [Google Scholar]
- 34.Shetty R, Vunnava K, Khamar P, Choudhary U, Sinha Roy A. Topography-based removal of corneal epithelium for keratoconus:A novel and customized technique. Cornea. 2018;37:923–5. doi: 10.1097/ICO.0000000000001580. [DOI] [PubMed] [Google Scholar]
- 35.David C, Reinstein DZ, Archer TJ, Kallel S, Vida RS, Goemaere I, et al. Postoperative corneal epithelial remodeling after intracorneal ring segment procedures for keratoconus:An optical coherence tomography study. J Refract Surg. 2021;37:404–13. doi: 10.3928/1081597X-20210225-02. [DOI] [PubMed] [Google Scholar]
- 36.Mastropasqua L, Nubile M, Salgari N, Mastropasqua R. Femtosecond laser-assisted stromal lenticule addition keratoplasty for the treatment of advanced keratoconus:A preliminary study. J Refract Surg. 2018;34:36–44. doi: 10.3928/1081597X-20171004-04. [DOI] [PubMed] [Google Scholar]
- 37.Nubile M, Salgari N, Mehta JS, Calienno R, Erroi E, Bondì J, et al. Epithelial and stromal remodelling following femtosecond laser-assisted stromal lenticule addition keratoplasty (SLAK) for keratoconus. Sci Rep. 2021;11:2293. doi: 10.1038/s41598-021-81626-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schuh A, Kolb CM, Mayer WJ, Vounotrypidis E, Kreutzer T, Kohnen T, et al. Comparison of changes in corneal volume and corneal thickness after myopia correction between LASIK and SMILE. PLoS One. 2021;16:e0250700. doi: 10.1371/journal.pone.0250700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montorio D, Cennamo G, Menna F, Donna P, Napolitano P, Breve MA, et al. Evaluation of corneal structures in myopic eyes more than twenty-two years after photorefractive keratectomy. J Biophotonics. 2020;13:e202000138. doi: 10.1002/jbio.202000138. [DOI] [PubMed] [Google Scholar]
- 40.Hou J, Wang Y, Lei Y, Zheng X, Zhang Y. Corneal epithelial remodeling and its effect on corneal asphericity after transepithelial photorefractive keratectomy for myopia. J Ophthalmol. 2016;2016:8582362. doi: 10.1155/2016/8582362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen X, Stojanovic A, Liu Y, Chen Y, Zhou Y, Utheim TP. Postoperative changes in corneal epithelial and stromal thickness profiles after photorefractive keratectomy in treatment of myopia. J Refract Surg. 2015;31:446–53. doi: 10.3928/1081597X-20150623-02. [DOI] [PubMed] [Google Scholar]
- 42.Reinstein DZ, Archer TJ, Gobbe M. Change in epithelial thickness profile 24 hours and longitudinally for 1 year after myopic LASIK:Three-dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg. 2012;28:195–201. doi: 10.3928/1081597X-20120127-02. [DOI] [PubMed] [Google Scholar]
- 43.Reinstein DZ, Archer TJ, Gobbe M, Silverman RH, Coleman DJ. Epithelial thickness after hyperopic LASIK:Three-dimensional display with Artemis very high-frequency digital ultrasound. J Refract Surg. 2010;26:555–64. doi: 10.3928/1081597X-20091105-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desai RU, Jain A, Manche EE. Long-term follow-up of hyperopic laser in situ keratomileusis correction using the Star S2 excimer laser. J Cataract Refract Surg. 2008;34:232–7. doi: 10.1016/j.jcrs.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 45.Hong J, Qian T, Yang Y, Jiang C, Liu Z, Sun X, et al. Corneal epithelial thickness map in long-term soft contact lenses wearers. Optom Vis Sci. 2014;91:1455–61. doi: 10.1097/OPX.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 46.Turhan SA, Yigit DD, Toker E. Corneal epithelial thickness and corneal curvature changes during the day:The effects of daily disposable contact lens wear. Cont Lens Anterior Eye. 2020;43:389–94. doi: 10.1016/j.clae.2019.11.017. [DOI] [PubMed] [Google Scholar]
- 47.Kim WK, Kim BJ, Ryu IH, Kim JK, Kim SW. Corneal epithelial and stromal thickness changes in myopic orthokeratology and their relationship with refractive change. PLoS One. 2018;13:e0203652. doi: 10.1371/journal.pone.0203652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buffault J, Zéboulon P, Liang H, Chiche A, Luzu J, Robin M, et al. Assessment of corneal epithelial thickness mapping in epithelial basement membrane dystrophy. PLoS One. 2020;15:e0239124. doi: 10.1371/journal.pone.0239124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sambhi RS, Sambhi GDS, Mather R, Malvankar-Mehta MS. Dry eye after refractive surgery:A meta-analysis. Can J Ophthalmol. 2020;55:99–106. doi: 10.1016/j.jcjo.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Kanellopoulos AJ, Asimellis G. In vivo 3-dimensional corneal epithelial thickness mapping as an indicator of dry eye:Preliminary clinical assessment. Am J Ophthalmol. 2014;157:63–8.e2. doi: 10.1016/j.ajo.2013.08.025. [DOI] [PubMed] [Google Scholar]