Highlights
-
•
The extract of Z. joazeiro Mart. leaves (EEFZJ) showed the presence of flobabenic tannins, leucoanthocyanidins, flavonois, flavonones.
-
•
There was no clinically relevant antibacterial activity, but the extract showed synergistic.
-
•
This extract was non toxic and evidenced sedative effect in adult zebrafish.
-
•
That Z. joazeiro has potential as an alternative plant-derived of phytomedicines against resistant bacterial infections and anxiolytic therapy.
Keywords: Ziziphus joazeiro, Anxiolytic-like activity, Antibiotic activity, Bacterial resistance, Secondary metabolites
Abstract
Ziziphus joazeiro Mart. is an endemic plant of the Caatinga that presents a great socioeconomic importance for the Northeast and Semiarid Region of Brazil. In view of this, this study aimed to evaluate the antibacterial activity and anxiolytic-like effects of Ziziphus joazeiro Mart leaves in adult zebrafish (Danio rerio). The characterization of the main classes of metabolites was performed through chemical reactions. The antibacterial and antibiotic potentiating activity was evaluated by broth microdilution assays. The 96 h acute toxicity, open field test and anxiety models test was evaluated in vivo on adult zebrafish. The results obtained in the phytochemical prospection evidenced the presence of flobabenic tannins, leucoanthocyanidins, flavonois, flavonones, catechins, alkaloids, steroids, and triterpenoids. EEFZJ did not show antibacterial activity for all microorganism tested (MIC ≥ 1024 µg/mL), but reduced the concentration required for bacterial growth inhibition in combination with gentamicin and norfloxacin against multidrug-resistant strains of S. aureus (SA10) and E. coli (EC06), exhibiting synergistic effect with these antibiotics (p<0.0001). In the tests in vivo, EEFZJ was found to be nontoxic, performing reduced locomotor activity and demonstrated an anxiolytic-like effect in adult zebrafish via GABAergic and Serotoninergic systems (5-HT1, 5-HT2A/2C and 5-HT3A/3B).
Introduction
The use of medicinal plants is part of popular culture and has always been present in medical practices inherited from popular knowledge, being employed for curing and treating diseases [1]. Its use by the population dates back more than sixty thousand years and the first discoveries were made by archeological studies in ruins in Iran [2]. According to Albuquerque [3], this practice continues to exist due to the high costs of traditional medicines, among other factors, however, these customs have been lost over time.
Chacon [4], describes that sustainability emerges as a new order of survival and balance and no longer appears as a utopian model, but as a small-scale reality, so that communities are experiencing sustainable models that need to be studied and disseminated. In this follow-up, the use of bioactive plants can be aligned to achieve goals of dimensions for Regional Sustainable Development.
Ziziphus joazeiro Mart. popularly known as juazeiro or juá, is endemic to the caatinga [5] and has socioeconomic importance for the northeastern and semi-arid regions of Brazil, with its parts (stem, bark, leaf, fruit and root) used in popular human and veterinary medicine, animal feed, rural construction and obtaining firewood [6,7]. This plant has wide popular use in the treatment of skin problems, such as dermatitis and mycoses [8], in the treatment of bronchitis, as an expectorant, in gastric ulcers, in the manufacture of cosmetics, anti-dandruff shampoos and toothpaste [9]. In addition, it is known to have calming properties according to popular knowledge ([10]).
The juazeiro has been employed in the management of phytopathogens, due to compounds present in its secondary metabolism. In the bark of this plant the presence of steroids, tannins and triterpenes is cited, while in the leaves the presence of betulinic acid, lupeol, caffeine, amphibin-D and saponins, also called jujubosides, considered the main constituent, stands out [11].
According to [12], this plant has several medicinal uses, as an oral antiseptic, against dermatological problems (dandruff, scabies, seborrhea dermatitis and itching), of the respiratory system (asthma, cough, pneumonia, tuberculosis, bronchitis inflammation of the throat and flu) and digestive system (constipation, stomatitis, gastric ulcers and maldigestion), being also reported healing property, antifungal, antibacterial, antioxidant, antipyretic, anti-inflammatory and astringent [13,14].
The study of medicinal plants based on their use by communities can provide useful information for the elaboration of pharmacological and phytochemical studies, aiming at the development of phytotherapies or isolation of active substances that can be synthesized by the pharmaceutical industry [15]
Bacterial infections have been a significant public health concern for decades, with their impact ranging from mild to life-threatening conditions. These infections occur when harmful bacteria invade the body, leading to a host immune response that can result in localized or systemic symptoms. In addition to the pathogenicity of bacteria being a problem in bacterial infections, there is also resistance to antibiotics, which is becoming increasingly present in bacteria. Bacterial resistance refers to the ability of bacteria to withstand the effects of antibiotics or other antimicrobial agents that were once effective in eliminating them. This resistance can occur through various mechanisms, including genetic mutations, acquisition of resistance genes from other bacteria, or the production of enzymes that deactivate antibiotics. These adaptive changes in bacteria allow them to survive and proliferate, rendering conventional treatment options less effective or completely ineffective. The emergence and spread of antibiotic-resistant bacteria have been attributed to several factors, including the overuse and misuse of antibiotics in healthcare settings and agricultural practices. In addition, the global interconnectedness facilitated by travel and trade contributes to the rapid dissemination of resistant strains across borders [16].
Anxiety is a common mental health disorder that can have various effects on the body's overall well-being. While the primary focus of anxiety research has been on its impact on psychological health, emerging studies suggest a potential link between anxiety and susceptibility to bacterial infections. It is known that anxiety can decrease the immune response, making men more sensitive to bacterial infections. Therefore, the search for antibacterial agents with anxiolytic potential becomes a relevant strategy for the development of a dual and simultaneous therapy [17,18].
Recent studies have shown that adult zebrafish are appropriate for modeling certain aspects of complex behavior. The examination of reward behavior, learning and memory, aggression, anxiety, and sleep indicates that there are shared regulatory processes governing behavior in both zebrafish and mammals. The current initiation of isolating and conducting molecular analysis on zebrafish behavioral mutants enables the discovery of new genes responsible for controlling behavior [19].
In view of the above, this study aimed to assess the antibacterial activity and anxiolytic-like (in the zebrafish model organism) effect of the ethanolic extract of the leaves of Ziziphus joazeiro Mart.
Material and method
Plant material and extract preparation
The leaf collections were performed on specimens of Ziziphus joareizo Mart., located in Sítio Ipueiras, in the rural zone of Brejo Santo municipality, southern Ceará, Brazil, at the foot of Chapada do Araripe (geographic coordinates, south latitude and west longitude of Greenwich: 1 - 442 m, 07° 28′54.4 "S/39°01′47.2 "W). The collection was authorized by the Biodiversity Authorization and Information System (SISBIO), under protocol number 55,064. An exsicata of the species Ziziphus joazeiro was deposited in the Dárdano de Andrade Lima Herbarium of the Universidade Regional do Cariri - URCA, under number 13.346.
To prepare the ethanolic extract of Z. joazeiro leaves (EEFZJ), the maceration method was used with cold extraction of absolute ethanol P.A. [20]. At the end EEFZZJ was produced with a yield of 4.15%.
Photochemical prospecting
The chemical assays qualitatively analyzed the presence of secondary metabolites. The detection tests were performed according to the method described by [21], to assess the presence of phenols, tannins, flavonoids, alkaloids, steroids, and triterpenoids. These assays were based on visual observation of color modifications and precipitate formation after the addition of specific reagents. The Liebermann - Burchard test was used for the detection of steroids and/or triterpenoids according to the method adapted from [22].
Antibacterial tests
Bacterial strains
Three species of standard and multidrug-resistant bacterial strains were used, Staphylococcus aureus ATCC 25,923 and SA10, Pseudomonas aeruginosa ATCC 9027 and PA24 and Escherichia coli ATCC 25,922 and EC6, provided by the Laboratory of Microbiology and Molecular Biology (LMBM) of the Regional University of Cariri (URCA). To perform the tests, each sample was subcultured in Brain Heart Infusion Agar (BHIA) and incubated for 24 h at 37 °C. The bacterials strains were prepared in sterile buffered saline solution and the bacterial suspension was determined by the transmittance corresponding to that of a standard 0.5 McFarland scale solution (approximately 1.5 × 108 bacterial cells per mL) [23].
Solution of extracts, drugs and reagents
EEFZJ and the antibiotics gentamicin, norfloxacin and ampicillin (Sigma Co, St. Louis, USA), were used at a concentration of 1.024 μg/mL. The resazurin sodium reagent (Sigma-Aldrich, St. Louis, MO) was used for reading the tests as a colorimetric indicator of bacterial growth by oxide-reduction [24]. The antibiotics used were Gentamicin, Norfloxacin and Ampicillin, all antibiotics were obtained from sigma and prepared in distilled water before assays.
Determination of the minimum inhibitory concentration (MIC)
The minimum inhibitory concentration (MIC) is defined as the lowest concentration capable of inhibiting microbial growth. This was determined by the microdilution method in BHI broth, using 96-well plates, assay was performed in triplicate and repeated twice (CLSI 2018). Eppendorfs® were prepared containing liquid BHI culture medium and bacterial inoculum. Subsequently, microdilution plates were filled, then serial microdilution of the extract was performed. The extract concentrations ranged from 512 to 0.5 μg/mL. The plates were incubated in a microbiological incubator for 24 h at a temperature of 37 °C. The reading was performed with sodium resazurin, which indicates microbial growth.
Potentiating activity
To verify the potentiating effect, EEFZJ was used in subinhibitory concentration (MIC/8) and combined with antibiotics against multidrug-resistant bacteria. For that, eppendorfs® were filled with BHI culture medium, bacterial inoculum, and the extract. Subsequently, microdilution plates were filled, and then serial microdilution was performed with the antibiotics. The plates were incubated for 24 h at 37 °C and then the reading was performed with resazurin [25].
Adult zebrafish (Danio rerio) (aZF)
The animals (aZF), wild-type, of both sexes and with ages of > 90 days, measuring 3.5 ± 0.5 cm in length and weighing 0.4 ± 0.1 g were obtained from Agroquímica: Comércio de Produtos Veterinários LTDA (Fortaleza, Brazil). A Group of 50 fish were acclimatized for 24 h in glass aquariums (30 × 15 × 20 cm) containing dechlorinated water (ProtecPlus®) and air pumps with submerged filters, at 25 °C and pH 7.0, with a circadian rhythm of 14:10 h light/dark. Fish received were fed ad libitum 24 h prior to the experiments. After the experiments, the animals were sacrificed by immersion in ice water (2–4 °C) for 10 min until they lost opercular movements [26]. Experimental procedures were approved by the Ethics Committee for Animal Use of Universidade Estadual do Ceará (CEUA-UECE, #7,210,149/ 2016).
Non-clinical safety evaluation
Open field test
The open field test was performed [27] to evaluate alteration or not of motor coordination of the animals, either by sedation and/or muscle relaxation. Initially, the animals (n = 6/group) were treated, via intraperitoneal (i.p.), with EEFZJ (10 or 100 or 500 mg/kg; 20 µL), Diazepam (DZP; 100 mg/kg; 20 µL) or vehicle (DMSO 3%; 20 µL).
A group of animals without treatments was included (Naive). After 1 hour of the treatments, the animals were added to glass Petri dishes (10 × 15 cm), containing the same water as the aquarium, marked with four quadrants and the locomotor activity was analyzed by counting the number of line crossings (CL). Using the CL value of the Naive group as a baseline (100%), the locomotor activity (AL) was analysed individually for 0–5 min.
Acute toxicity 96 h
In the study carried out by Batista et al., it was described the method acute toxicity study was performed in adult zebrafish. Animals (n = 6/each) were treated, via intraperitoneal (i.p.), with EEFZJ (10 or 100 or 500 mg/kg; 20 µL) or negative control (CN) (DMSO 3%; 20 µL). After the treatments, the animals were left at rest to analyze the mortality rate. Every 24 h and up to 96 h the number of dead fish in each group was noted and the lethal dose capable of killing 50% of animals (LD50) was determined using the Trimmed Spearman-Karber mathematical method with 95% confidence interval [28].
Anxiolytic-like effect (Light & Dark Test)
The Light & Dark Test was performed according to the method described by Gebauer et al. [29]. The experiment was performed in a glass aquarium (30 × 15 × 20 cm) filled with distilled water to a maximum height of 3 cm, divided into two equal vertical sections and each section was covered externally, according to the dimensions of the aquarium, one section being transparent and the other with a black coating designated as Light/Dark.
Subsequently, animals were treated with 20 μL, via intraperitoneal, with solution of EEFZJ (10 or 100 or 500 mg/kg), vehicle (Control, DMSO 3%) or Diazepam (DZP; 100 mg/kg). A group without treatments (Naive) was included. After 30 min of the treatments, animals were, individually, added to the clear zone of the aquarium and the anxiolytic-like effect was quantified as percentage of stay in the clear zone (% PZC), for 5 min of analysis.
Analysis parameters are latency to first entry (in seconds) on the dark side, time spent in the light compartment, and number of alternations of the animal (exploratory activity) between sides of the aquarium [29].
GABAergic system involvement
GABAergic system involvement was investigated, according to the methodology proposed by Benneh et al. [30]. Animals (n = 6/group) were pretreated with 20 μL via intraperitoneal with EEFZJ (10 or 100 or 500 mg/kg) or vehicle (Control, DMSO 3%) or Diazepam (DZP; 100 mg/kg) or flumazenil (4.0 mg/kg; i.p.) and subjected to the Light & Dark Test [29].
A glass aquarium (30 × 15 × 20 cm) with a light and dark zone was filled with tap water pretreated with antichlorine until it reached a height of 3 cm. After 30 min of treatment, the animals were individually placed in the light zone of the aquarium and the anxiolytic effect was quantified based on the time the fish remained in the light zone for 5 min of analysis. A naive group was included.
To evaluate the GABAergic system involvement, other groups of animals (n = 6/each) received (4.0 mg/kg; i.p.) the GABAA antagonist flumazenil 15 min before EEFZJ (10 mg/kg; i.p.) or diazepam (DZP; 100 mg/kg; i.p.); and the test was performed in the same manner as described above.
Serotoninergic system involvement (5-HT2A, 5-HT1 and 5-HT2A/2C, 5-HT3A/3B)
The serotoninergic system involvement was investigated according to the methodology proposed by Benneh et al. [30]. Cyprohepatadine (Cipro; 5-HT2A antagonist), pizotifen (Piz; 5-HT1 and 5-HT2A/2C antagonist) and granisetron (Gran; 5-HT3A/3B antagonist) were used as antagonists. The lowest effective dose of the anxiolytic sample was employed in the light-dark. Animals (n = 6/group) were treated intraperitoneally (i.p.) with 20 µL vehicle (Control, DMSO 3%) or antagonist cyprohepatadine or pizotifen, both (32 mg/kg), or granisetron (20 mg/kg) or EEFZJ (10 mg/kg) or fluoxetine (Flx; 0.05 mg/kg).
To evaluate the serotoninergic system involvement, other groups of animals (n = 6/each) received 20 µL via oral (p.o.) of the antagonists (cyprohepatadine or pizotifen), both 32 mg/kg, or granisetron (20 mg/kg) 15 min before EEFZJ (10 mg/kg; 20 µL; i.p.) or fluoxetine (Flx; 0.05 mg/kg; 20 µL; i.p.). After 45 min of the treatments, animals were, individually, added to the clear zone of the aquarium and the anxiolytic-similar effect was quantified as percentage of stay in the clear zone (% PZC), for 5 min of analysis [29].
Statistical analysis
The results of the tests in vivo were expressed as mean ± standard error of the mean for each group of 6 animals. After the confirmation of the data distribution normality and data homogeneity, the differences between the groups were submitted to analysis of variance (one-way ANOVA), followed by Tukey test. All analyses were performed using GraphPad Prism, v. 5.01.
Results and discussion
Phytochemical prospection
Among the classes of secondary metabolites, polyphenols (flobabenic tannins), flavonoids (leucoanthocyanidins, flavonols, flavonones, catechins), steroids and triterpenoids were evidenced in the composition of the ethanolic extract of Ziziphus joazeiro leaves (Table 1).
Table 1.
Phytochemical prospection of the ethanolic extract of Ziziphus joazeiro leaves.
| Classes of secondary metabolites | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Extracts | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| EtFoZj | – | – | + | – | + | – | – | + | – | – | – | + | + | + |
1 - Phenols; 2 - Pyrogallic tannins; 3 - Flobabenic tannins; 4 - Anthocyanins; 5 - Leucoanthocyanidins; 6 - Flavones; 7 – Flavonois; 8 - Flavonones; 9 - Xanthones; 10 - Aurones; 11 - Chalcones; 12 - Catechins; 13 - Alkaloids; 14 - Steroids and triterpenoids. (+) presence; (-) absence.
Xavier [31] performed phytochemical analysis of Z. joazeiro leaves extract and found the presence of flobabenic tannins, flavones, flavonois, xanthones, flavonols, chalcomas, aurones, leucoanthocyanidins and catechins. In another research, Brito [32] reported the presence of saponins, tannins, polyphenols, and flavonoids in the same extract. While in Melo et al. [11], the phytochemical prospection of the Z. joazeiro stem bark extract evidenced the presence of saponins, steroids, and triterpenes.
In a study by Andrade [33], the phytochemical characterization of the aqueous extract of the leaf and stem of Z. joazeiro found the presence of secondary metabolites common to both extracts, such as flavonoids (leucoanthocyanidins, flavones, flavonols, flavonols, flavonones, xanthones, catechins), steroids and saponins. In particular, phenols, condensed tannins and alkaloids were observed in the extract of Z. joazeiro leaves. The chromatographic analysis evidenced the presence of 12 compounds in the leaf extract, being seven saponins belonging to the terpenoid class, while in the stem extract, 12 compounds were also identified, including four terpenoids, seven flavonoids and one phenolic acid.
According to Gobbo-Neto and Lopes [34], the number of secondary metabolites varies from one plant to another due to several factors to which plant species are subjected, such as seasonality, temperature, rainfall index, UV radiation, atmospheric composition, circadian rhythm, plant age, water, nutrients, herbivory, and pathogen attack.
Determination of the minimum inhibitory concentration (MIC)
EEFZJ did not show antibacterial activity for all microorganisms tested (MIC ≥ 1024 µg/mL). The results of this study are in accord with other authors who did not observe or verified mild antibacterial activity for Z. joazeiro leaf extract [11,32,35,36].
EEFZJ has in its composition the presence of saponins and flavonol derivatives that have prominent antimicrobial activity [33,37,38]. However, despite the presence of these compounds, the extract did not show antibacterial activity, which can be explained by the low concentration of these constituents in Z. joazeiro leaf.
Silva et al. [35] determined the antimicrobial activity of different extracts of Z. joazeiro and observed MIC for the bark extract between 500 and 1000 µg/mL against Mycobacterium smegmatis. For the same bacteria, the leaf extract showed MIC between 125 and 250 µg/mL, being considered a strong inhibitor. The leaves extract also showed better activity against Micrococcus luteus, exhibiting MIC between 250 and 500 µg/mL. For Enterococcus faecalis and Enterobacter aerogenes, the extracts of joazeiro bark and leaves were considered weak inhibitors, presenting MICs above 5000 µg/mL.
In a study conducted by Alviano et al. [39], the aqueous extract of Z. joazeiro bark demonstrated activity against bacteria of the oral microbiota associated with peridental diseases, such as Prevotella intermadia, Porphyromonas gingivalis, Fusobacterium nucleatum, Streptococcus mutans and Lactobacillus casei, with MIC between 1000 and 16,000 μg/mL.
Potentiating activity
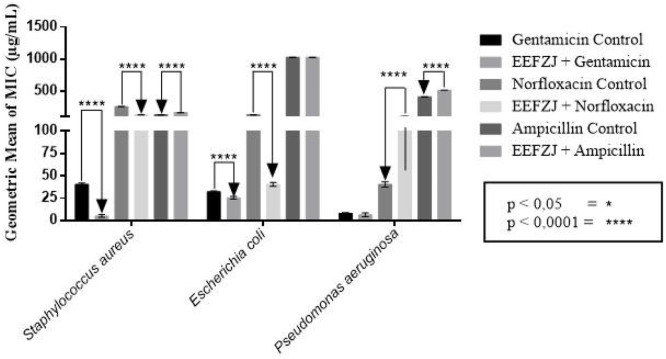
Potentiating activity assay was performed to evaluate the influence of EEFZJ on the action of conventional antibiotics against multidrug-resistant bacterial strains. The results showed that EEFZJ reduced the concentration required for bacterial growth inhibition in combination with gentamicin and norfloxacin against multidrug-resistant strains of S. aureus (SA10) and E. coli (EC06), exhibiting synergistic effect with these antibiotics (p<0.0001). No significant modulatory activity of EEFZJ was observed on P. aeruginosa (PA24) and neither in combination with ampicillin for the three strains evaluated, as described in Fig. 1.
Fig. 1.
Modulatory action of ethanolic extract of Ziziphus joazeiro Mart. leaves with gentamicin, norfloxacin and ampicillin antibiotics against multidrug-resistant bacterial species of S. aureus 10 (SA10), E. coli 06 (EC06) and P. aeruginosa 24 (PA24).
The synergistic effect obtained by the EEFZJ may be related to the presence of secondary metabolites that alter the permeability or rupture the cell membrane of microorganisms, enhancing the antimicrobial action, such as flavonoids [32]. Another metabolite found in EEFZJ that exerts antibacterial action are saponins. According to Fang et al. [37] and Simões et al. [38], saponins have detergent and emulsifying action through the formation of complexes with steroids, proteins, and phospholipids present in the plasma membrane, changing the permeability and breaking the cell membrane of microorganisms.
In agreement with the results of this study, several authors have demonstrated modulatory activity of Z. joazeiro leaf extract against different Gram-negative and Gram-positive bacteria. Brito et al. [32] evaluated the influence of hydroalcoholic extract of Z. joazeiro leaves (HELZJ) on the action of conventional antibiotics against multidrug-resistant bacterial strains and observed that the combination of gentamicin and amikacin antibiotics with HELZJ reduced the concentration required for growth inhibition of Enterobacter aerogenes from 78 to 19 μg/mL and 78 μg/mL to 9.7 μg/mL, respectively, exhibiting synergistic effects.
Andrade et al. [14] investigated the antibacterial action of extracts of the leaf and stem bark of Z. joazeiro in combination with antibiotics and observed significant modulatory activity on strains of Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus faecalis, Streptococcus mutans, Pseudomonas aeruginosa and E. coli, especially for the leaf extract in combination with the antibiotic gentamicin and norfloxacin. Unlike the findings of these authors, in our study no modulatory activity on Pseudomonas aeruginosa was observed.
When evaluating the activity of EEFZJ in combination with ampicillin on the tested multidrug resistant isolates it was possible to observe antagonism. This finding can be attributed to the presence of compounds with antioxidant potential present in Z. joazeiro. According to Kalghatgi et al. [40], some antibiotics induce a common oxidative pathway that damages bacterial cells by producing reactive oxygen species. In this context, the antagonism observed for ampicillin can be explained by the capture of these radicals by antioxidant compounds, intercepting the active oxygen and forming stable radicals, contributing to a decrease in antibiotic action [33,38].
Non-clinical safety evaluation
The use of Zebrafish presents itself as a promising advantage for evaluating the toxicity and therapeutic potential of certain drugs on a large scale [41].
Open field test
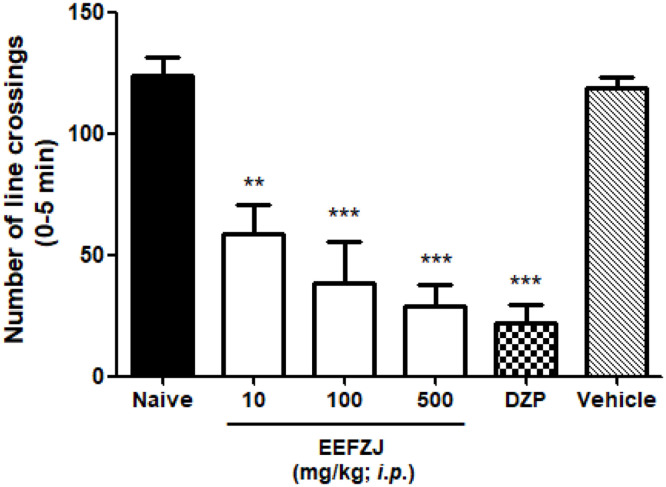
EEFZJ (10 or 100 or 500 mg/kg; i.p.) significantly (p<0.01; p<0.001 vs. Naive and Vehicle) caused locomotor impairment in adult Zebrafish (Danio rerio), just like the anxiolytic compound diazepam (DZP; 100 mg/kg; i.p.; p<0.001 vs. Naive and Vehicle), as observed in Fig. 2.
Fig. 2.
Effect of EEFZJ on locomotor activity of adult Zebrafish (Danio rerio), individually analyzed during 0–5 min in the Open Field Test. DZP - diazapam (Positive control; 100 mg/kg; i.p.). Control - Vehicle (DMSO 3%; 20 μL; i.p.). Naive - untreated group. Each column represents the mean ± standard deviation (n = 6/group). Numbers above the bars indicate the percentage of locomotor activity. One-way ANOVA with Tukey's post-hoc test (*p<0.01; **p<0.001 vs. Control).
Concordant with the results of this study, Gonçalves et al. [42] evaluated the effect of an enriched protein fraction from breadfruit pulp (PFBp) on the locomotor system in zebrafish and observed locomotor dysfunction in all concentrations tested. At the concentration of 5.0 mg/mL, PFBp induced the maximum reduction in locomotor activity to 39.3% (p<0.001 vs. Naïve).
In another study, Lira et al. [43] found that Pitaya (Hylocereus polyrhizus) pulp at doses of 0.1, 0.5 or 1.0 mg/mL did not alter the locomotor apparatus of fish, however the peeling at concentrations of 0.5 and 1.0 mg/mL demonstrated activity, significantly (p<0.01, p < 0.05, respectively) reducing locomotor activity compared to the vehicle and naive group.
Locomotor activity is a behavioral analysis parameter used to evaluate chemical compounds that have action on the central nervous system (SNC) of zebrafish [44,45].
In this sense, the decrease in locomotion caused by EEFZJ suggests a possible sedative action. Similar results were observed for the control drug diazepam, which is a benzodiazepine with anxiolytic effects, as highlighted by Gupta et al. [44] and Benneh et al. [30]. Based on the results presented, we selected EEFZJ to investigate possible anxiolytic-liker effect.
Acute toxicity 96 h
EEFZJ (10 or 100 or 500 mg/kg; i.p.) was not toxic to adult Zebrafish (Danio rerio) (LD50 ˃ 500 mg/kg), according to Table 2.
Table 2.
Acute 96 h toxicity test for EEFZJ against adult zebrafish (Danio rerio).
| Samples | Mortality of adult ZF | LD50 (mg/kg) / CI (95%) | |||
|---|---|---|---|---|---|
| CN | D1 | D2 | D3 | ||
| EtFoZj | 1 | 1 | 1 | 2 | > 500 / nd |
Treatments: 20 µL (i.p.). CN - Negative control group: DMSO 39%; D1 - dose 1 (10 mg/kg); D2 - dose 2 (100 mg/kg); D3 - dose 3 (500 mg/kg); LD50- lethal dose to kill 50% of adult ZF; CI - confidence interval; nd - not determined.
Adult zebrafish have been employed for a long time in toxicological monitoring tests of environmental contaminants [46], and are also useful in toxicity evaluation of pharmaceutical compounds [47], toxicological biomonitoring in drug development [48] and toxicity of plant extracts [30,49]. In this study, EEFZJ caused low mortality of Zebrafish, indicating that Z. joazeiro leaves at the tested concentrations were not toxic.
Anxiolytic-like effect
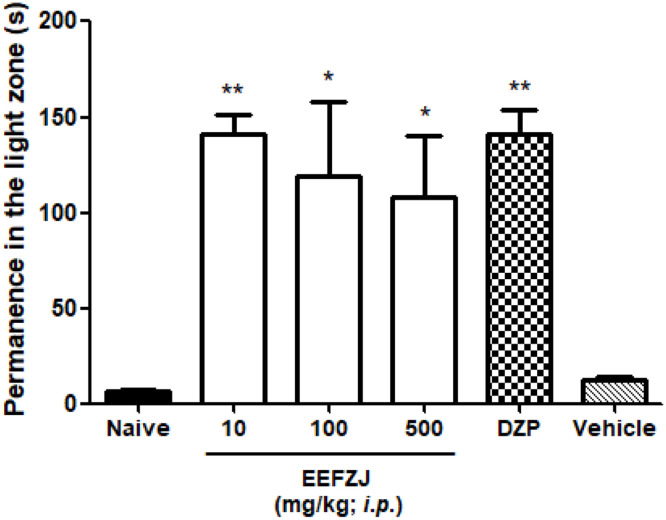
EEFZJ (10 or 100 or 500 mg/kg; i.p.) exhibited anxiolytic-similar effect to that of Diazepam (500 mg/kg; i.p.; p< 0.01 vs. Naive or vehicle), increasing the amount of time fish spent in the light compartment. The intensity of the effect was inversely proportional to the concentration of EEFZJ, so that the lowest dose of the extract (10 mg/kg) exerted maximum effect on Zebrafish (p<0.01), surpassing the dosages of 100 and 500 mg/kg (Fig. 3).
Fig. 3.
Anxiolytic-similar effect of EEFZJ in adult Zebrafish in the Light & Dark Test (0–5 min). Data above each column indicate percentage of stay in the light zone. Naive-group-no treatment. Control - Vehicle (DMSO 3%; 20 μL; i.p.); Dzp - Diazepam (100 mg/kg; i.p.). Values represent the mean ± standard error of the mean (S.P.E.) for 6 animals/group; ANOVA followed by Tukey (*p< 0.05; **p<0.01 vs. Control).
According to Gonçalves et al. (2020), the light-dark test is commonly applied to evaluate the anxiolytic effect in rodents and, recently, it has been validated in Zebrafish, being considered the gold standard in anxiety studies. Both animals prefer dark rather than light environments, an approach-avoidance conflict between the preference for safe areas (dark zones) and the innate exploration of new environments, such as light compartments.
Research with the same methodology using other substances and extracts from different plants showed similarities with this study, reporting the anxiolytic-similar effect. Ferreira et al. [50] evaluated the anxiolytic effect of the synthetic chalcone 4′-[(2E)−3-(3-nitrophenyl)−1-(phenyl) prop‑2-en-1-one] acetamide (PAAMNBA) and demonstrated the anxiolytic-simile effect (40 mg/kg: i.p.) on zebrafish, increasing the permanence of the fish in the clear zone, exerting a similar effect to Diazepam (p<0.0001 vs naive or vehicle).
In another study, Silva et al. [51] observed that the saponifiable fraction of the ethanolic extract of Azadirachta indica (Neem) bark showed anxiolytic-similar effect, since fish treated with this preparation (1.0 or 2.5 or 5.0 mg/mL; 20 µL; i.p.) remained most of the time of analysis in the clear zone, showing a significantly similar effect (p<0.05) to Diazepam (2.5 mg/mL; 20 µL; i.p.), an anxiolytic control.
Some secondary metabolites present in EEFZJ as saponins, terpenoids and alkaloids due to their calming, sedative, stimulant, analgesic, and anesthetic properties [52] may be responsible for the anxiolytic-similar effect observed, being necessary studies to prove this assumption. The effective dose of EEFZJ (10 mg/kg; i.p.) was chosen to investigate possible mechanisms of anxiolytic action.
GABAergic system involvement
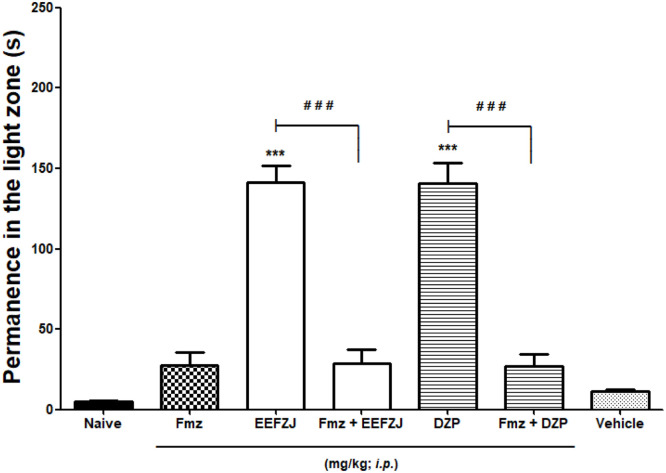
Pretreatment with flumazenil (4 mg/kg; i.p.) significantly (p<0.001) prevented the anxiolytic-like effect of EEFZJ (10 mg/kg; i.p.) and diazepam (100 mg/kg; i.p.), as shown in Fig. 4.
Fig. 4.
Effect of flumazenil on the anxiolytic-similar action of EEFZJ (10 mg/kg; i.p.) in adult Zebrafish (Danio rerio) in the Light & Dark Test (0–5 min). Naive - untreated animals; Dzp - Diazepam (100 mg/kg; i.p.). Fmz - Flumazenil (4.0 mg/kg; i.p.). Control - Vehicle (DMSO 3%; 20 μL; i.p.). Values represent the mean ± standard error of the mean (S.E.P.M.) for 6 animals/group. ANOVA followed by Tukey (***p<0.001 vs. Control; # # #p<0.001 vs. EEFZJ or DZP).
Many studies have been using flumazenil as a tool to identify possible endogenous and exogenous ligands at benzodiazepine receptors in rodents, Viswanatha et al. [53] as well as in adult zebrafish [30].
Administration of flumazenil, a competitive GABAA/Benzodiazepine receptor antagonist can prevent the anxiolytic effects of diazepam and drugs with similar mechanisms of action [54]. Since flumazenil binds in both (neutral) states, it does not affect GABA-A receptor function, but blocks agonist access. In this perspective, the experiments showed that the flumazenil antagonist reversed the anxiolytic effect of EEFZJ as well as diazepam, suggesting that the anxiolytic-similar effect of this extract is dependent on the GABAergic system and can be partially explained by the interaction with this receptor.
In a similar study by Lira et al. [43], intraperitoneal pretreatment with flumazenil (0.1 mg/mL, 20 μL) significantly (p<0.001) reversed the entire anxiolytic effect of Hylocereus polyrhizus (pitaya) pulp and bark extracts and diazepam, indicating that both preparations have anxiolytic-type effect through the GABAergic system. Similar results were obtained by Benneh et al. [30], when they observed that flumazenil significantly reversed the anxiolytic effects of Maerua angolensis stem bark extract (p<0.001) and diazepam (p<0.0001) in zebrafish, proving interaction with GABA-A receptor.
Serotoninergic system involvement
5-HTR2A system involvement
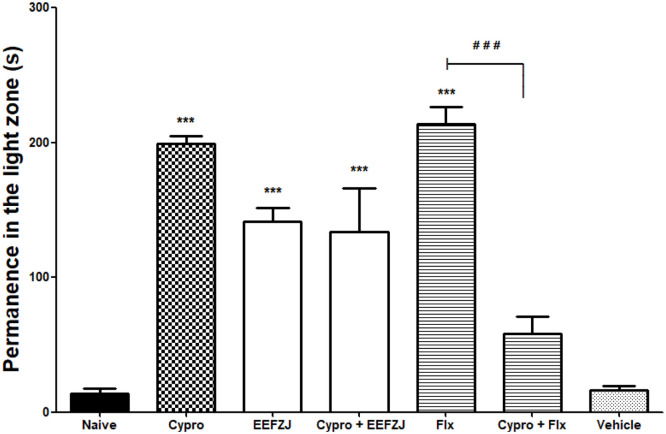
Serotonin (5-HT) plays an essential role in modulating anxiety, aggression, and depression [55]. Pretreatment with cyproheptadine (32 mg/kg; p.o.) did not prevent the anxiolytic-simile effect of EtFoZj (10 mg/kg; i.p.), however, this drug exerted a significant effect (p<0.001) on the anxiolytic-like effect of fluoxetine (Flx) (0.05 mg/kg; i.p.) (Fig. 5).
Fig. 5.
Effect of cyproheptadine on the anxiolytic-similar action of EEFZJ (10 mg/kg; i.p.) in adult Zebrafish (Danio rerio) in the Light & Dark Test (0–5 min). Naive - untreated animals; Cipro - cyproepatadine (32 mg/kg; p.o.). Flx - Fluoxetine (0.05 mg/kg; 20 µL; i.p.). Control - Vehicle (DMSO 3%; 20 μL; i.p.). Values represent the mean ± standard error of the mean (S.E.P.M.) for 6 animals/group. ANOVA followed by Tukey (***p<0.001 vs. Control; # # #p<0.001 vs. Flx).
In a similar study by Gonçalves et al. [42], the anxiolytic potential of the enriched protein fraction of breadfruit pulp (PFBp) was evaluated after pretreatment with the antagonist cyproheptadine (a 5-HTR2A antagonist). The authors observed that cyproheptadine (0.8 mg/mL; vo.) did not reverse the anxiolytic-like effect of PFBp (1.0 mg/mL; p.o.), suggesting that the mechanism of action of PFBp does not involve 5-HTR2A. In addition, the antagonist cyproheptadine abolished the effects of fluoxetine (p<0.001).
Fluoxetine (ISRS) is widely used for the treatment of anxiety and depression disorders. This drug promotes inhibition of serotonin reuptake in the presynaptic cell, increasing its extracellular level. Acute treatment with fluoxetine is a useful neuropharmacological tool to evaluate the effect of the serotoninergic system on stress and aggressive behavior in adult Zebrafish [56].
5-HT1 and 5-HT2A/2C system involvement
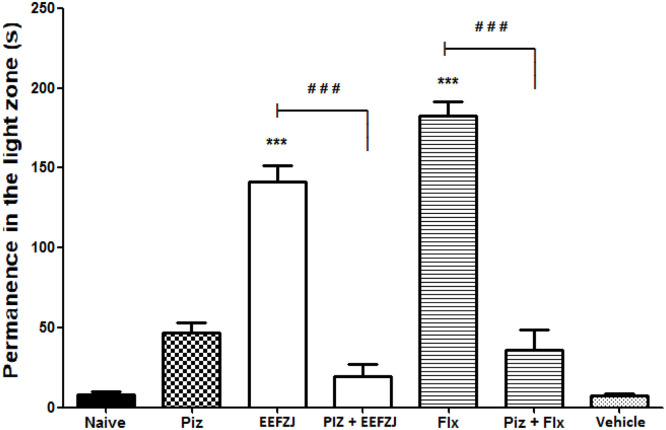
Pretreatment with pizotifen (32 mg/kg; p.o.) significantly (p<0.001) prevented the anxiolytic-similar effect of EtFoZj (10 mg/kg; i.p.) and fluoxetine (Flx; 0.05 mg/kg; i.p.). The reversal of anxiolysis with pretreatment using pizotifen suggests a possible involvement of the 5-HT1 and 5-HT2A/2C serotoninergic system in the anxiolytic effects of EEFZJ (Fig. 6).
Fig. 6.
Effect of pizotifen (Piz) on the anxiolytic-similar action of EEFZJ (10 mg/kg; i.p.) in adult Zebrafish (Danio rerio) in the Light & Dark Test (0–5 min). Naive - untreated animals; Piz - pizotifen (32 mg/kg; p.o.). Flx - Fluoxetine (0.05 mg/kg; i.p.). Control - Vehicle (DMSO 3%; 20 μL; i.p.). Values represent the mean ± standard error of the mean (S.E.P.M.) for 6 animals/group. ANOVA followed by Tukey (***p<0.001 vs. Control; # # #p<0.001 vs. EEFZJ or Flx).
5-HT3A/3B system involvement
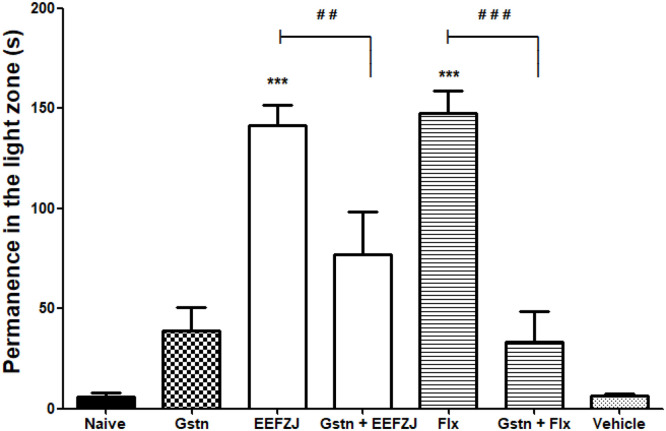
Pretreatment with granisetron (20 mg/kg; p.o.) significantly prevented the anxiolytic-similar effect of EEFZJ (10 mg/kg; i.p.; p<0.01) and fluoxetine (0.05 mg/kg; i.p.; p<0.001). The uniform reversal of the anxiolytic effect of the extract is suggestive of a possible activation of the serotoninergic system (Fig. 7).
Fig. 7.
Effect of granisetron (Gstn) under the anxiolytic-similar action of EEFZJ (10 mg/kg; i.p.) in adult Zebrafish (Danio rerio) in the Light & Dark Test (0–5 min). Naive - untreated animals; Gstn - Granisetron (20 mg/kg; p.o.). Flx - Fluoxetine (0.05 mg/Kg; i.p.). Control - Vehicle (DMSO 3%; 20 μL; i.p.). Values represent the mean ± standard error of the mean (S.E.P.M.) for 6 animals/group. ANOVA followed by Tukey (***p<0.001 vs. Control; # #p<0.01 vs. EEFZF; # #p<0.001 vs. Flx).
In a study conducted by Benneh et al. [30], the anxiolytic effect of Maerua angolensis extract was significantly reversed by pretreatment with granisetron (p<0.05), moreover, the anxiolytic action of fluoxetine was similarly reversed after treatment with this same drug (p<0.05).
Conclusion
The extract of Z. joazeiro Mart. leaves (EZJ) showed the presence of flobabenic tannins and other secondary metabolites, that were the chemical components, essential for the obtained results. There was no clinically relevant antibacterial activity, but the extract showed synergistic effect in combination with gentamicin and norfloxacin against multidrug-resistant strains of S. aureus and E. coli. This extract was nontoxic and evidenced sedative effect in adult zebrafish. EEFZJ demonstrated anxiolytic-like effect via GABAergic and Serotoninergic systems (5-HT1, 5-HT2A/2C and 5-HT3A/3B). These findings suggest that Z. joazeiro has potential as an alternative plant-derived of phytomedicines against resistant bacterial infections and anxiolytic therapy.
Funding (Financial statement)
Cearense Foundation for Scientific and Technological Development - FUNCAP, Coordination for the Improvement of Higher Education Personnel - CAPES and National Council for Scientific and Technological Development - CNPq.
CRediT authorship contribution statement
Antônio Barros de Souza: Writing – original draft. Jacqueline Cosmo Andrade Pinheiro: Resources. Juliete Bezerra Soares: Investigation. José Ismael Feitosa de Araújo: Validation. Sandra Maria Barbosa de Araújo: Methodology. Francisco Lucas Alves Batista: Conceptualization. Kalina Kelma Oliveira de Sousa: Writing – review & editing. Saulo Relison Tintino: Writing – review & editing. Isaac Moura Araujo: Writing – original draft. Francisco Ernani Alves Magalhães: Project administration. Laura Hévila Inocencio Leite: Funding acquisition. Francisco Roberto de Azevedo: Supervision.
Declaration of Competing Interest
The authors declare no conflict of interest, in manuscript: Antibacterial activity and Anxiolytic-like Effect of Ziziphus joazeiro Mart. Leaves in Adult Zebrafish (Danio rerio).
Acknowledgments
We thank the Programa de Pós-Graduação em Desenvolvimento Regional Sustentável, Universidade Federal do Cariri (UFCA), Crato, Ceará, Brasil, Universidade Estadual do Ceará (Campus CECITEC, Tauá-CE).
Data availability
The authors are unable or have chosen not to specify which data has been used.
References
- 1.Bruning M.C.R., Mosegui G.B.G., de C.M., Vianna M. A utilização da fitoterapia e de plantas medicinais em unidades básicas de saúde nos municípios de Cascavel e Foz do Iguaçu-Paraná: a visão dos profissionais de saúde. Cien. Saude Colet. 2012;17:2675–2685. doi: 10.1590/S1413-81232012001000017. [DOI] [PubMed] [Google Scholar]
- 2.da Silva A.C., Lobato F.H.S., Ravena-Canete V. Plantas medicinais e seus usos em um quilombo amazônico: o caso da comunidade Quilombola do Abacatal, Ananindeua (PA) Rev. Do NUFEN. 2019;11:113–136. doi: 10.26823/RevistadoNUFEN.vol11.no03artigo61. [DOI] [Google Scholar]
- 3.de Albuquerque W.R., Filho W.F. Centro de Estudos Afro-Orientais; 2006. Uma História Do Negro No Brasil. [Google Scholar]
- 4.Chacon S.S. Banco do Nordeste do Brasil; 2007. O Sertanejo e o Caminho Das águas: Políticas públicas, Modernidade e Sustentabilidade No Semi-Árido. [Google Scholar]
- 5.Giulietti A.M., Bocage Neta A.L., Castro A., Gamarra-Rojas C.F.L., Sampaio E., Virgínio J.F., Queiroz L.P., Figueiredo M.A., Rodal M.J.N., Barbosa M.R.V. Diagnóstico da vegetação nativa do bioma Caatinga. Biodiversidade Da Caatinga Áreas e Ações Prioritárias Para a Conserv. 2004 [Google Scholar]
- 6.de ALMEIDA M.A.O., Botura M.B., Santos M.M., Almeida G.N., Domingues L.F., Costa S.L., Batatinha M.J.M. Efeitos dos extratos aquosos de folhas de Cymbopogon citratus (DC.) Stapf (capimsanto) e de Digitaria insularis (L.) Fedde (Capim-açu) sobre cultivos de larvas de nematóides gastrintestinais de caprinos. Rev. Bras. Parasitol. Vet. 2003;12:125–129. [Google Scholar]
- 7.de Lucena R.F.P., do Nascimento V.T., de Lima Araújo E., de Albuquerque U.P. Local uses of native plants in an area of Caatinga vegetation (Pernambuco, NE Brazil) Ethnobot. Res. Appl. 2008;6:3–14. [Google Scholar]
- 8.Cruz M.C.S., Santos P.O., Barbosa Jr A.M., De Mélo D., Alviano C.S., Antoniolli A.R., Alviano D.S., Trindade R.C. Antifungal activity of Brazilian medicinal plants involved in popular treatment of mycoses. J. Ethnopharmacol. 2007;111:409–412. doi: 10.1016/j.jep.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 9.de Oliveira A.K., Diógenes F.É.P., de F.B. Coelho M., Maia S.S.S. Alelopatia em extratos de frutos de juazeiro (Ziziphus joazeiro Mart.-Rhamnaceae) Acta Bot. Brasilica. 2009;23:1186–1189. doi: 10.1590/S0102-33062009000400029. [DOI] [Google Scholar]
- 10.do Nascimento A.M., da Fonseca T.S., Campos M.F., Moreira L.O., Marques C.A., Tavares E.S., Mendonça S.C., Leitão G.G., Simas R.C., Leitão S.G. Ziziphus joazeiro, a saponin-rich Brazilian medicinal plant: pharmacognostic characterization of bark and leaves. Rev. Bras. Farmacogn. 2020;30:756–764. [Google Scholar]
- 11.Melo M.do S.F., Rocha C.Q., Santos M.H., Chavasco J.M., Chavasco J.K. Pesquisa de bioativos com atividade antimicrobiana nos extratos hidroetanólicos do fruto, folha e casca de caule do Zizyphus joazeiro mart. Rev. Da Univ. Val. Do Rio Verde. 2012;10:43–51. doi: 10.5892/ruvrv.2012.102.4351. [DOI] [Google Scholar]
- 12.De Albuquerque U.P., De Medeiros P.M., De Almeida A.L.S., Monteiro J.M., de F.L. Neto E.M., de Melo J.G., Dos Santos J.P. Medicinal plants of the caatinga (semi-arid) vegetation of NE Brazil: a quantitative approach. J. Ethnopharmacol. 2007;114:325–354. doi: 10.1016/j.jep.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Romão M., Costa A., Terra F.D.S., Boriollo M.F.G., Soares E.A. Assessment of gastric protective of rasp juá extract. Rev. Da Soc. Bras. Clínica Médica. 2010;8:222–227. [Google Scholar]
- 14.Andrade J.C., Silva A.R.P., Santos A.T.L., Freitas M.A., Carneiro J.N.P., Gonçalo M.I.P., de Souza A., Freitas T.S., Ribeiro P.R.V, Brito E.S. UPLC-MS-ESI-QTOF characterization and evaluation of the antibacterial and modulatory antibiotic activity of Ziziphus joazeiro Mart. aqueous extracts. South African J. Bot. 2019;123:105–112. doi: 10.1016/j.sajb.2019.02.001. [DOI] [Google Scholar]
- 15.Brasileiro B.G., Pizziolo V.R., Matos D.S., Germano A.M., Jamal C.M. Plantas medicinais utilizadas pela população atendida no" Programa de Saúde da Família", Governador Valadares, MG, Brasil. Rev. Bras. Ciências Farm. 2008;44:629–636. doi: 10.1590/S1516-93322008000400009. [DOI] [Google Scholar]
- 16.Fair R.J., Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect. Medicin. Chem. 2014;6:PMC–S14459. doi: 10.4137/PMC.S14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rohrmann S., Hennig J., Netter P. Trait anxiety–possible consequences for health. Ger. J. Psychiatry. 2000;3:19–25. [Google Scholar]
- 18.Picardi A., Amerio P., Baliva G., Barbieri C., Teofoli P., Bolli S., Salvatori V., Mazzotti E., Pasquini P., Abeni D. Recognition of depressive and anxiety disorders in dermatological outpatients. Acta Derm. Venereol. 2004;84 doi: 10.1080/00015550410025264. [DOI] [PubMed] [Google Scholar]
- 19.Norton W., Bally-Cuif L. Adult zebrafish as a model organism for behavioural genetics. BMC Neurosci. 2010;11:1–11. doi: 10.1186/1471-2202-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.F.J.A. MATOS, F. Vivas, Universidade Federal do Ceará, (2002).
- 21.F.J.A. Matos, Introdução à Fitoquímica Experimental, 3a, Edição, UFC, Fortaleza. (2009).
- 22.Campbell M.K., Shawn O.F. Thomson Asia pte Ltd; Singapore: 2005. Biochemistry. [Google Scholar]
- 23.CLSI, Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Eleventh Edition: M07, (2018).
- 24.Sales G.W.P., Batista A.H.M., Rocha L.Q., Nogueira N.A.P. Efeito antimicrobiano e modulador do óleo essencial extraído da casca de frutos da Hymenaea courbaril L. Rev. Ciências Farm. Básica e Apl. 2014;35 [Google Scholar]
- 25.Coutinho H.D.M., Costa J.G.M., Siqueira-Júnior J.P., Lima E.O. In vitro anti-staphylococcal activity of Hyptis martiusii Benth against methicillin-resistant Staphylococcus aureus: MRSA strains. Rev. Bras. Farmacogn. 2008;18:670–675. [Google Scholar]
- 26.de C.N.de C., Concea E.A. Resolução normativa No 37, DE 15 DE Fevereiro de 2018. Diário Of. Da União. 2018 [Google Scholar]
- 27.Magalhães F.E.A., Sousa C.Á.P.B., Santos S.A.A.R., Menezes R.B., Batista F.L.A., Abreu A.O., Oliveira M.V., Moura L.F.W.G., Raposo R.S., Campos A.R. Adult zebrafish (Danio rerio): an alternative behavioral model of formalin-induced nociception. Zebrafish. 2017;14:422–429. doi: 10.1089/zeb.2017.1436. [DOI] [PubMed] [Google Scholar]
- 28.Arellano-Aguilar O., Solis-Angeles S., Serrano-García L., Morales-Sierra E., Montero-Montoya R. Use of the zebrafish embryo toxicity test for risk assessment purpose: case study. J. Fish. Com. 2015;9:0. [Google Scholar]
- 29.Gebauer D.L., Pagnussat N., Piato Â.L., Schaefer I.C., Bonan C.D., Lara D.R. Effects of anxiolytics in zebrafish: similarities and differences between benzodiazepines, buspirone and ethanol. Pharmacol. Biochem. Behav. 2011;99:480–486. doi: 10.1016/j.pbb.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 30.Benneh C.K., Biney R.P., Mante P.K., Tandoh A., Adongo D.W., Woode E. Maerua angolensis stem bark extract reverses anxiety and related behaviours in zebrafish—Involvement of GABAergic and 5-HT systems. J. Ethnopharmacol. 2017;207:129–145. doi: 10.1016/j.jep.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 31.R.A.B. XAVIER, Atividade Antibacteriana dos extratos etanólico e hexânico das folhas do Ziziphus joazeiro, 2015.
- 32.Brito S.M.O., Coutinho H.D.M., Talvani A., Coronel C., Barbosa A.G.R., Vega C., Figueredo F.G., Tintino S.R., Lima L.F., Boligon A.A. Analysis of bioactivities and chemical composition of Ziziphus joazeiro Mart. using HPLC–DAD. Food Chem. 2015;186:185–191. doi: 10.1016/j.foodchem.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 33.Andrade J.C., da Silva A.R.P., dos Santos A.T.L., Freitas M.A., de Matos Y.M.L.S., Braga M.F.B.M., Bezerra C.F., Gonçalo M.I.P., Gomez M.C.V., Rolóm M. Chemical composition, antiparasitic and cytotoxic activities of aqueous extracts of Ziziphus joazeiro Mart. Asian Pac. J. Trop. Biomed. 2019;9:222. doi: 10.4103/2221-1691.259003. [DOI] [Google Scholar]
- 34.Gobbo-Neto L., Lopes N.P. Medicinal plants: factors of influence on the content of secondary metabolites. Quim. Nova. 2007;30:374–381. [Google Scholar]
- 35.Silva T.C.L., Almeida C., Veras Filho J., Sobrinho T.J.S.P., Amorim E.L.C., Costa E.P., Araújo J.M. Atividades antioxidante e antimicrobiana de Ziziphus joazeiro mart.(Rhamnaceae): avaliação comparativa entre cascas e folhas. Rev. Ciências Farm. Básica e Apl. 2011;32 [Google Scholar]
- 36.Sousa I.J.O., Silva M.C.P., Leopoldino G.L., Agostinho L.S. Estudo fitoquímico, avaliação da capacidade hemolítica e antimicrobiana de um extrato bruto da casca do caule de Ziziphus joazeiro Mart.(Rhamnaceae) J. Biol. Pharm. Agric. Manag. 2018;14:208–225. [Google Scholar]
- 37.Fang Y.-S., Cai L., Li Y., Wang J.-P., Xiao H., Ding Z.-T. Spirostanol steroids from the roots of Allium tuberosum. Steroids. 2015;100:1–4. doi: 10.1016/j.steroids.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 38.Simões C.M.O., Schenkel E.P., de Mello J.C.P., Mentz L.A., Petrovick P.R. Artmed Editora; 2016. Farmacognosia: Do Produto Natural Ao Medicamento. [Google Scholar]
- 39.Alviano W.S., Alviano D.S., Diniz C.G., Antoniolli A.R., Alviano C.S., Farias L.M., Carvalho M.A.R., Souza M.M.G., Bolognese A.M. In vitro antioxidant potential of medicinal plant extracts and their activities against oral bacteria based on Brazilian folk medicine. Arch. Oral Biol. 2008;53:545–552. doi: 10.1016/j.archoralbio.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Kalghatgi S., Spina C.S., Costello J.C., Liesa M., Morones-Ramirez J.R., Slomovic S., Molina A., Shirihai O.S., Collins J.J. Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in mammalian cells. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3006055. 192ra85-192ra85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Resende R.R. Editora Blucher; 2015. Biotecnologia Aplicada à saúde-vol. 1: Fundamentos e Aplicações. [Google Scholar]
- 42.Gonçalves N.G.G., de Araújo J.I.F., Magalhães F.E.A., Mendes F.R.S., Lobo M.D.P., de O.M. Moreira A.C., de Azevedo Moreira R. Protein fraction from Artocarpus altilis pulp exhibits antioxidant properties and reverses anxiety behavior in adult zebrafish via the serotoninergic system. J. Funct. Food. 2020;66 doi: 10.1016/j.jff.2019.103772. [DOI] [Google Scholar]
- 43.Lira S.M., Dionísio A.P., Holanda M.O., Marques C.G., da Silva G.S., Correa L.C., Santos G.B.M., de Abreu F.A.P., Magalhães F.E.A., de Lima Rebouças E. Metabolic profile of pitaya (Hylocereus polyrhizus (FAC Weber) Britton & Rose) by UPLC-QTOF-MSE and assessment of its toxicity and anxiolytic-like effect in adult zebrafish. Food Res. Int. 2020;127 doi: 10.1016/j.foodres.2019.108701. [DOI] [PubMed] [Google Scholar]
- 44.Gupta P., Khobragade S.B., Shingatgeri V.M., Rajaram S.M. Assessment of locomotion behavior in adult Zebrafish after acute exposure to different pharmacological reference compounds. Drug Dev. Ther. 2014;5:127–133. [Google Scholar]
- 45.Taylor J.C., Dewberry L.S., Totsch S.K., Yessick L.R., DeBerry J.J., Watts S.A., Sorge R.E. A novel zebrafish-based model of nociception. Physiol. Behav. 2017;174:83–88. doi: 10.1016/j.physbeh.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 46.Huang Y., Zhang J., Han X., Huang T. The use of zebrafish (Danio rerio) behavioral responses in identifying sublethal exposures to deltamethrin. Int. J. Environ. Res. Public Health. 2014;11:3650–3660. doi: 10.3390/ijerph110403650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hill A.J., Teraoka H., Heideman W., Peterson R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- 48.Caballero M.V., Candiracci M. Zebrafish as screening model for detecting toxicity and drugs efficacy. J. Unexplored Med. Data. 2018;3:4. doi: 10.20517/2572-8180.2017.15. [DOI] [Google Scholar]
- 49.Ekambaram S.P., Perumal S.S., Pavadai S. Anti-inflammatory effect of Naravelia zeylanica DC via suppression of inflammatory mediators in carrageenan-induced abdominal oedema in zebrafish model. Inflammopharmacology. 2017;25:147–158. doi: 10.1007/s10787-016-0303-2. [DOI] [PubMed] [Google Scholar]
- 50.Ferreira M.K.A., da Silva A.W., Silva F.C.O., Holanda C.L.A., Barroso S.M., dos Reis Lima J., Neto A.E.V., Campos A.R., Bandeira P.N., Dos Santos H.S. Anxiolytic-like effect of chalcone N-{(4′-[(E)-3-(4-fluorophenyl)-1-(phenyl) prop-2-en-1-one]} acetamide on adult zebrafish (Danio rerio): involvement of the GABAergic system. Behav. Brain Res. 2019;374 doi: 10.1016/j.bbr.2019.03.040. [DOI] [PubMed] [Google Scholar]
- 51.Silva A.W.D., Ferreira M.K.A., Reboucas E.L., Silva F.C.O., Holanda C.L.A., Barroso S.M., Lima JdR B.F.L.A., Mendes F.R.S., Campos A.R., Menezes JESAD M.F.E.A. Anxiolytic-like effect of Azadirachta indica A. Juss.(Neem, Meliaceae) bark on adult zebrafish (Danio rerio): participation of the serotoninergic and GABAergic systems. Pharm. Pharmacol. Int. J. 2020;8:256–263. [Google Scholar]
- 52.Passos C.S., Arbo M.D., Rates S.M.K., von Poser G.L. Terpenoids with activity in the Central Nervous System (CNS) Rev. Bras. Farmacogn. 2009;19:140–149. doi: 10.1590/S0102-695X2009000100024. [DOI] [Google Scholar]
- 53.Viswanatha G.L., Venkataranganna M.V., Prasad N.B.L. Ameliorative potential of Colebrookea oppositifolia methanolic root extract against experimental models of epilepsy: possible role of GABA mediated mechanism. Biomed. Pharmacother. 2017;90:455–465. doi: 10.1016/j.biopha.2017.03.078. [DOI] [PubMed] [Google Scholar]
- 54.de Almeida A.A.C., Costa J.P., de Carvalho R.B.F., de Sousa D.P., de Freitas R.M. Evaluation of acute toxicity of a natural compound (+)-limonene epoxide and its anxiolytic-like action. Brain Res. 2012;1448:56–62. doi: 10.1016/j.brainres.2012.01.070. [DOI] [PubMed] [Google Scholar]
- 55.de Boer S.F., Koolhaas J.M. 5-HT1A and 5-HT1B receptor agonists and aggression: a pharmacological challenge of the serotonin deficiency hypothesis. Eur. J. Pharmacol. 2005;526:125–139. doi: 10.1016/j.ejphar.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 56.Theodoridi A., Tsalafouta A., Pavlidis M. Acute exposure to fluoxetine alters aggressive behavior of zebrafish and expression of genes involved in serotonergic system regulation. Front. Neurosci. 2017;11:223. doi: 10.3389/fnins.2017.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors are unable or have chosen not to specify which data has been used.