Abstract
A 30-year-old woman presented following a motor vehicle collision with a grade III blunt thoracic aortic injury and an aberrant right subclavian artery. Using intraoperative ultrasound and diagnostic subtraction angiography, we deployed an aortic endograft (cTAG; W.L. Gore & Associates), excluding the injury and aberrant right subclavian artery. The patient immediately lost arterial waveforms in her left arm, confirming incidental coverage of the left subclavian artery, likely due to the polytetrafluoroethylene sheath of the endograft. Her pulses returned after placement of a left subclavian chimney via retrograde brachial artery access.
Keywords: Aberrant right subclavian artery, Blunt traumatic aortic injury, BTAI, Left subclavian artery, TEVAR
Blunt thoracic aortic injury (BTAI) is the second most common cause of mortality after nonpenetrating traumatic injuries, behind only intracranial hemorrhage.1 Although thoracic endovascular aortic repair (TEVAR) has reduced procedure-related complications compared with traditional open repair, it can cause vertebrobasilar insufficiency and left upper extremity exertional fatigue secondary to left subclavian artery (LSA) coverage. Routine revascularization is rarely necessary if adequate collateralization is present to the vertebral artery (from the contralateral subclavian artery) and the extremity. Fewer than 10% of patients require an extremity intervention.2,3 However, an aberrant right subclavian artery (RSA) is a rare anatomic variant (0.16%-2%) that complicates aortic repair.4,5 In this variant, the RSA exits the descending aorta, usually in zone IV, and routinely requires endograft coverage, decreasing the perfusing pressures to the aforementioned collateral beds.
Case report
A 30-year-old woman presented as a level 1 trauma patient via air transport following a motor vehicle crash with ejection at 70 mph. The patient provided written informed consent for the report of her case details and imaging studies, which is available on request.
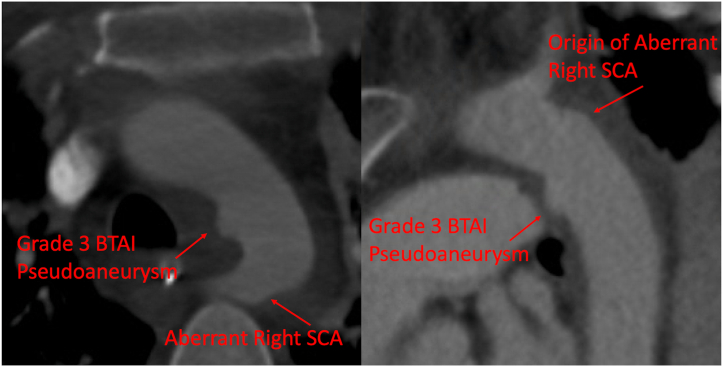
The patient was intubated because of a Glasgow coma scale score of 3 at the accident scene and transfused because of hemodynamic instability. The physical and ultrasound examination findings were significant for bilateral chest ecchymosis and free fluid in the right upper quadrant. Her hemodynamics improved with additional resuscitation, allowing for computed tomography angiography (CTA) using our trauma protocol of the head, chest, abdomen, and pelvis. CTA revealed a thoracic aortic injury, multiple cervical spinal fractures, a grade III liver laceration, and multiple rib fractures with pulmonary contusions. She was taken to the operating room for exploratory laparotomy and stabilization of her intra-abdominal injuries. Preoperative imaging revealed a zone 3, grade III BTAI measuring 6 × 3 × 8 mm with an aberrant RSA, originating posteriorly from the proximal descending thoracic aortic pseudoaneurysm, with a proximal landing zone diameter of 21.5 mm and ∼10 to 11 mm of healthy appearing aorta between the edge of the LSA and the beginning of the pseudoaneurysm (Figs 1 and 2).
Fig 1.
Axial and sagittal computed tomography angiography (CTA) of the aortic arch demonstrating zone 4 blunt traumatic aortic pseudoaneurysm with an aberrant right subclavian artery (RSA; SCA). BTAI, Blunt thoracic aortic injury.
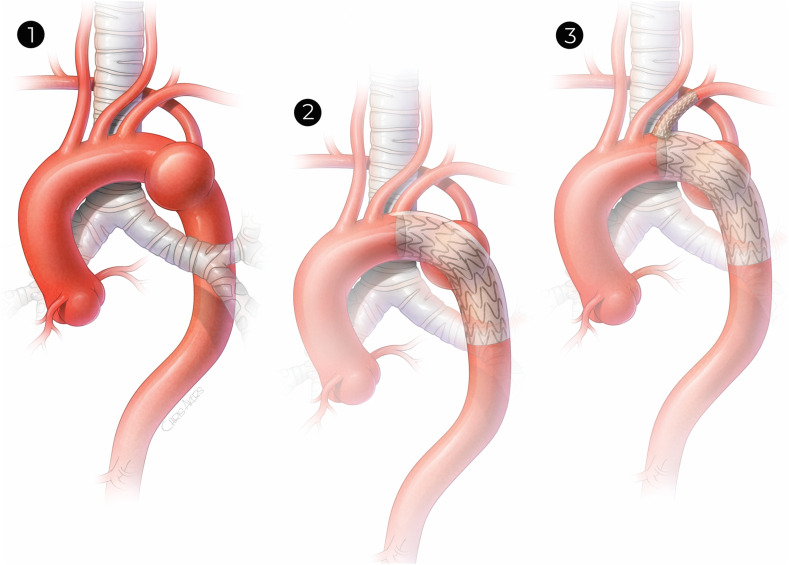
Fig 2.
1, Grade III blunt traumatic aortic injury (BTAI) zone 4 with aberrant right subclavian artery (RSA). 2, Excluded BTAI with incidental coverage of left subclavian artery (LSA) by polytetrafluoroethylene (PTFE) sheath. 3, LSA revascularization after parallel endovascular graft placement.
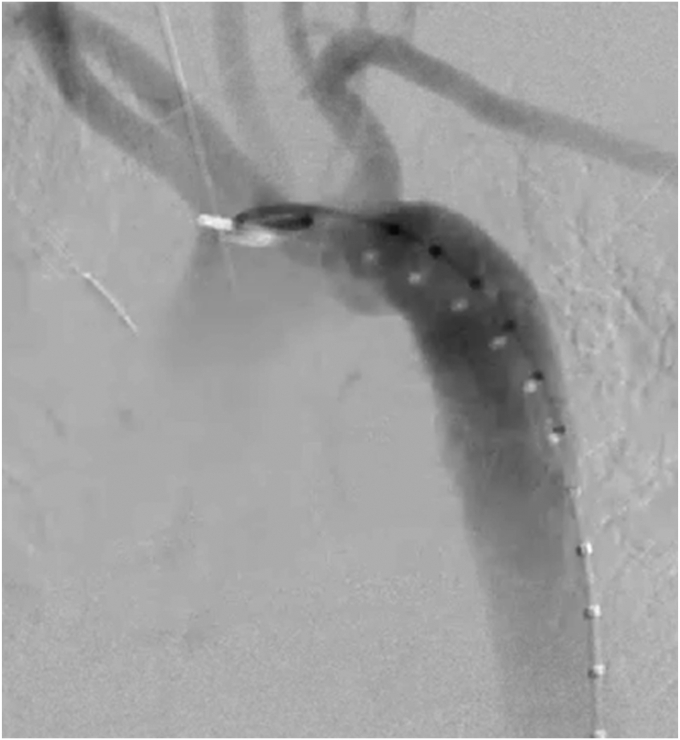
Because of the patient's additional injuries, including, but not limited to, the recent exploratory laparotomy, solid organ injures, multiple fractures, and unstable cervical vertebrae—and her inability to tolerate prolonged systemic heparinization—we elected not to perform prophylactic carotid subclavian bypass at that time. Instead, we treated the BTAI with TEVAR, including planned deployment in zone 3 distal to the LSA with coverage of the grade III BTAI with the aberrant RSA. The right common femoral artery was accessed under direct ultrasound guidance, and preclosure devices were deployed. After heparinization, a pigtail catheter was placed in the ascending aorta, and a diagnostic aortogram was performed to confirm the landing zones and ensure exclusion of injury with preservation of the LSA (Fig 3). As stated, preoperative CTA demonstrated a healthy proximal landing area in zone 3, with an aortic diameter of 21.5 mm. Using intraoperative ultrasound and diagnostic angiography to confirm the size and landing zone after resuscitation, we deployed a 26-mm × 10-cm Gore cTAG stent graft, successfully excluding the grade III BTAI and aberrant RSA. However, the patient immediately lost arterial waveforms in her left upper extremity, and angiography confirmed accidental coverage of the LSA.
Fig 3.
Aortic arch angiogram demonstrating grade III blunt traumatic aortic injury (BTAI) and aberrant right subclavian artery (RSA).
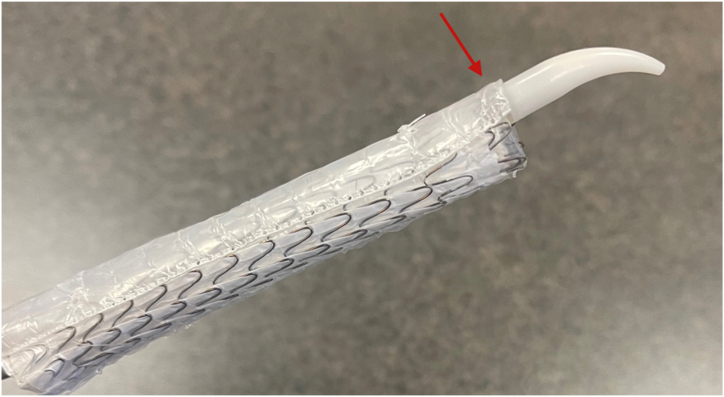
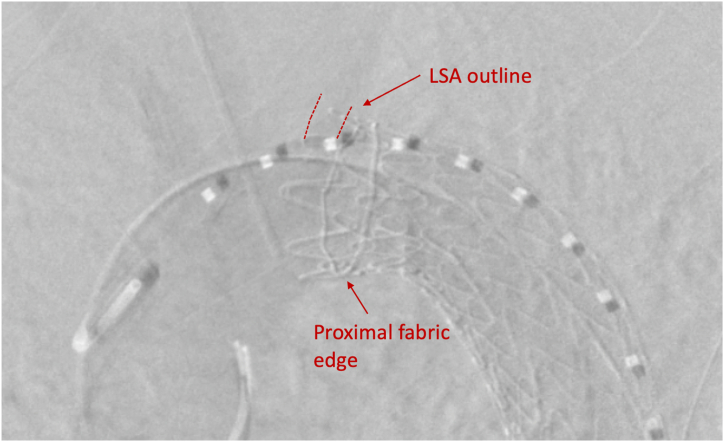
An aortic arch angiogram confirmed correct deployment of the thoracic endograft with fabric beginning distal to the ostia of the LSA. The etiology of the incidental coverage was likely retropulsion of the polytetrafluoroethylene (PTFE) sleeve (Fig 4). This sleeve is radiolucent and often sandwiched along the greater curvature of the aorta after stent graft deployment without complications. However, in the present case, the sleeve had migrated forward, covering the ostia of the LSA (Figs 2 and 5).
Fig 4.
Ex vivo deployed cTAG (W.L. Gore & Associates). (Arrow demonstrates polytetrafluoroethylene [PTFE] sleeve.)
Fig 5.
Aortic arch angiogram demonstrating successful exclusion of blunt traumatic aortic injury (BTAI) with nonopacification of left subclavian artery (LSA).
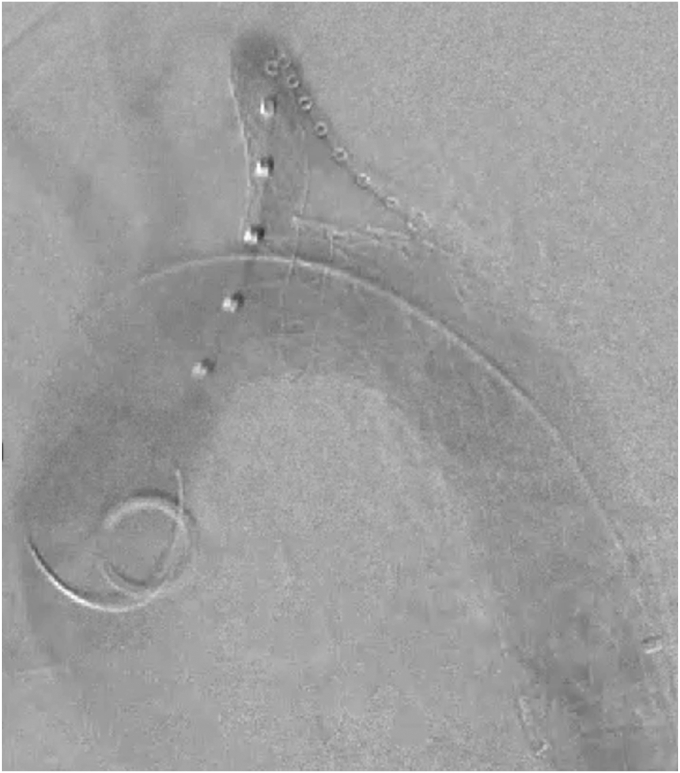
Because of the patient's dominant left vertebral artery and new coverage of the aberrant RSA, we decided to reposition the stent graft—or perform parallel stenting to revascularize the LSA. After unsuccessful retraction using a Coda balloon (Cook Medical Inc) inflated within the cTAG graft, the left brachial artery was exposed and accessed, and a Glidewire was directed to cannulate the ascending aorta. An 8F destination sheath was advanced before deploying an 8-mm VBX stent (W.L. Gore & Associates) to support the ostia of the LSA, which returned pulsatile flow to the left upper extremity. Completion angiography demonstrated a patent LSA stent and exclusion of the BTAI without an endoleak (Figs 2 and 6). Postoperatively, the patient did well without any upper extremity sequela and was discharged with an antiplatelet regimen.
Fig 6.
Aortic arch angiogram demonstrating successful endovascular revascularization of left subclavian artery (LSA) via parallel stent grafting.
Discussion
Recent attempts to demonstrate improved outcomes for TEVAR vs open repair through systematic reviews have failed owing to the challenging natural history of BTAIs precluding any randomized control trials.6 Although long-term data are lacking, recent studies have suggested outcomes for TEVAR better than or equal to those with open repair.7,8 Despite the low quality of evidence, the Society for Vascular Surgery (SVS) has recommended TEVAR over open repair as early as 2011.9 Considering the patient's emergent need for surgical repair and anatomic abnormalities, our team decided the patient was a suitable candidate for TEVAR.
Even with typical anatomy, TEVAR for BTAI carries mortality of ∼6% and morbidity of 6%.8,10 A significant contributor to TEVAR morbidity is coverage of the LSA, which is often necessary for successful exclusion of the diseased aorta with landing in healthy proximal tissue. The value of revascularizing the LSA was demonstrated by a 2018 systematic review and meta-analysis by Huang et al.11 The review included 16 cohort studies and 2591 patients, with results suggesting LSA revascularization significantly lowered the perioperative stroke rate (relative risk [RR], 0.61; 95% confidence interval [CI], 0.45-0.82; I2 = 20%) and perioperative spinal cord ischemia rate (RR, 0.59; 95% CI, 0.39-0.90; I2 = 0). No significant difference was found in the mortality rate with vs without LSA revascularization (RR, 0.86; 95% CI, 0.60-1.21; I2 = 35%).11
Because the patient's aberrant RSA originated from the injured and aneurysmal portion of the descending thoracic aorta, the thoracic stent graft inadvertently covered the ostia of the LSAs and RSAs. We concluded that her risk of perioperative complications would be significantly increased without subclavian artery revascularization. Although the cTAG is a fairly ubiquitous device, the interventionalist should be familiar with the potential shortcomings of the design, including the radiolucent PTFE sleeve, which can become problematic during precise delivery.
An aberrant RSA is a rare anatomic variant with a reported prevalence of 0.16% to 2%.4,5 It correlates with higher rates of aortic dissection and coarctation and is common enough that vascular surgeons will likely treat a patient with this variant at least once in their career.12 These patients can still be acceptable candidates for TEVAR. Subclavian revascularization can help decrease the incidence of perioperative complications made more likely by coverage of both subclavian arteries. However, our patient was not an ideal candidate for bypass revascularization. Revascularization of the LSA was achieved through a parallel stenting technique, a minimally invasive, but equally effective, alternative to open revascularization.13,14
Long-term follow-up and surveillance guidelines for BTAI treated with TEVAR are not well established. Similar to the SVS guidelines pertaining to aortic pathology, our institution recommends daily aspirin of 81 mg for all patients undergoing endovascular stenting. The trauma cohort has been associated with lower compliance and higher loss to follow-up, with some reporting one of four patients receiving TEVAR for BTAI lost to follow-up after 1 year.15 Our department follows the SVS follow-up guidelines with plans to repeat chest CTA 1 month after discharge. However, our patient was from another state—only passing through Houston at the time of her motor vehicle collision. As with many trauma patients, she failed to follow-up with us. She has followed up with a cardiologist in another state and informed us that her last computed tomography scan was “normal.”
Conclusions
We report the successful treatment of a patient with an aberrant RSA and a grade III BTAI. The anatomic variation and inadvertent proximal PTFE sleeve retropulsion during deployment resulted in bilateral subclavian artery coverage. Bailout LSA revascularization via retrograde brachial artery access and parallel subclavian stenting was successfully performed. Despite the anatomic variation, the patient was successfully treated without perioperative complications.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Mouawad N.J., Paulisin J., Hofmeister S., Thomas M.B. Blunt thoracic aortic injury—concepts and management. J Cardiothorac Surg. 2020;15:62. doi: 10.1186/s13019-020-01101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bashir M., Bailey D., Jones W.D., White R.D., Williams I.M. The fate of the left subclavian artery in TEVAR for aortic arch pathology. J Card Surg. 2021;36:3547–3553. doi: 10.1111/jocs.15831. [DOI] [PubMed] [Google Scholar]
- 3.Matsumura J.S., Rizvi A.Z. Left subclavian artery revascularization: Society for Vascular Surgery® practice guidelines. J Vasc Surg. 2010;52:65S–70S. doi: 10.1016/j.jvs.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Aortic arch with four vessels: aberrant right subclavian artery - Chaoui - 2008 - ultrasound in Obstetrics & Gynecology - Wiley online Library. https://obgyn.onlinelibrary.wiley.com/doi/10.1002/uog.5240 [DOI] [PubMed]
- 5.Choi Y., Chung S.B., Kim M.S. Prevalence and anatomy of aberrant right subclavian artery evaluated by computed tomographic angiography at a single institution in Korea. J Korean Neurosurg Soc. 2019;62:175–182. doi: 10.3340/jkns.2018.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pang D., Hildebrand D., Bachoo P. Thoracic endovascular repair (TEVAR) versus open surgery for blunt traumatic thoracic aortic injury. Cochrane Database Syst Rev. 2019;2:CD006642. doi: 10.1002/14651858.CD006642.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erben Y., Trejo G., Brownstein A.J., et al. Endovascular thoracic aortic transection repair has equivalent survival to open repair after blunt thoracic aortic injury. Int Angiol. 2018;37:155–159. doi: 10.23736/S0392-9590.17.03902-5. [DOI] [PubMed] [Google Scholar]
- 8.Shackford S.R., Dunne C.E., Karmy-Jones R., et al. The evolution of care improves outcome in blunt thoracic aortic injury: a Western Trauma Association multicenter study. J Trauma Acute Care Surg. 2017;83:1006–1013. doi: 10.1097/TA.0000000000001555. [DOI] [PubMed] [Google Scholar]
- 9.Lee W.A., Matsumura J.S., Mitchell R.S., et al. Endovascular repair of traumatic thoracic aortic injury: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2011;53:187–192. doi: 10.1016/j.jvs.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 10.Piffaretti G., Benedetto F., Menegolo M., et al. Outcomes of endovascular repair for blunt thoracic aortic injury. J Vasc Surg. 2013;58:1483–1489. doi: 10.1016/j.jvs.2013.05.096. [DOI] [PubMed] [Google Scholar]
- 11.Huang Q., Chen X.M., Yang H., Lin Q.N., Qin X. Effect of left subclavian artery Revascularisation in thoracic endovascular aortic repair: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2018;56:644–651. doi: 10.1016/j.ejvs.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 12.Chowdhury Y., Shaikh S.A., Salman A., Marmur J.D., McFarlane Isabel M. Aberrant right subclavian artery and stanford Type B aortic dissection. Am J Med Case Rep. 2020;8:247–249. [Google Scholar]
- 13.Li J., Xue Y., Li S., et al. Outcomes of thoracic endovascular aortic repair with chimney technique for aortic arch diseases. Front Cardiovasc Med. 2022;9:868457. doi: 10.3389/fcvm.2022.868457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter R., Wee I.J.Y., Petrie K., Syn N., Choong A.M. Chimney parallel grafts and thoracic endovascular aortic repair for blunt traumatic thoracic aortic injuries: a systematic review. Vascular. 2019;27:204–212. doi: 10.1177/1708538118812548. [DOI] [PubMed] [Google Scholar]
- 15.Kidane B., Plourde M., Chadi S.A., et al. The effect of loss to follow-up on treatment of blunt traumatic thoracic aortic injury. J Vasc Surg. 2015;61:1624–1634. doi: 10.1016/j.jvs.2015.02.017. [DOI] [PubMed] [Google Scholar]