Abstract
Isolated dystonia is a neurological disorder of diverse etiology, multifactorial pathophysiology, and wide spectrum of clinical presentations. We review the recent neuroimaging advances that led to the conceptualization of dystonia as a neural network disorder and discuss how current knowledge is shaping the identification of biomarkers of dystonia and the development of novel pharmacological therapies.
Keywords: Network disorder, Functional connectivity, Structural networks, Connectome, Neurotransmission
Introduction
Isolated dystonia is a hyperkinetic movement disorder manifesting as involuntary sustained or intermittent muscle contractions causing abnormal, often repetitive movements, postures, or both [2, 42]. It is the third most common movement disorder after Parkinson’s disease and essential tremor. While the incidence of isolated dystonia is underestimated due to the clinical challenges in timely diagnosing the disorder [1], it is known to affect up to 35.1 per 100,000 cases [56], with a higher prevalence among white females [27, 50, 102]. The clinical presentations of dystonia are diverse. The topographic distribution of symptoms classifies dystonia into five main categories: (i) focal dystonia, affecting a single region (e.g., hand dystonia, cervical dystonia, blepharospasm, laryngeal dystonia, oromandibular dystonia); (ii) segmental dystonia, affecting two or more continuous regions; (iii) multifocal dystonia, affecting two or more nonadjacent regions; (iv) hemidystonia, dominantly affecting regions on one side of the body; and (v) generalized dystonia, affecting the trunk and at least two other sites [2, 105].
The pathophysiology of isolated dystonia is multifactorial. Diverging from the historical tenet that considered dystonia a basal ganglia disorder, the majority of current reports refer to dystonia as a neural network disorder. Various environmental stressors and underlying genetic factors interact with and influence abnormal reorganization of neural networks, further shaping the diversity of its clinical characteristics. However, despite the substantial progress in understanding the disorder pathophysiology, therapeutic approaches in dystonia are primarily geared toward symptom management. Botulinum toxin injections into the affected muscles are the “gold”-standard treatment for patients with focal dystonia. Pharmacological therapy (primarily, anticholinergics, dopaminergic, and GABAergic drugs) and deep brain stimulation (DBS) are available in severe cases of generalized or segmental dystonias. Treatment responses are known to be highly variable across patients, and their effectiveness may be limited due to side effects or other factor of therapeutic inefficiency [5, 86, 106]. Recent estimates suggest that nearly 40% of patients with focal dystonia do not receive any treatment [86]. To improve clinical management of patients with dystonia, a recent workshop organized by the National Institute of Neurological Disorders and Stroke (NINDS/NIH) on research priorities in dystonia stressed the urgent need to design effective therapeutic interventions based on the novel evidence of network-level dysfunction in dystonia [71].
In this chapter, we review the experimental evidence that led to the conceptualization of dystonia as a neural network disorder and discuss the impact of this current view of dystonia pathophysiology on the clinical management of patients affected by this disorder.
From the Historical Tenet of a Basal Ganglia Disorder to the Modern Consensus of Dystonia as a Neural Network Disorder
The understanding of the neural underpinnings of dystonia has considerably evolved in the past decade (Fig. 1). One of the first mentions of dystonia as a condition arising from a basal ganglia pathology due to mineral accumulations dates back to the 1949 case report of two patients [10]. Over the following decades, several other reports of patients with dystonia secondary to brain lesions provided further evidence for the involvement of the basal ganglia in symptom development (e.g., [82, 110]). These observations culminated in the landmark paper by Marsden and colleagues [77], which reviewed 28 patients with focal or hemidystonia secondary to brain lesions due to tumors, arteriovenous malformations, infarcts, or hemorrhages. The authors concluded that the “abnormal input from the thalamus to the premotor cortex, due to lesions either of the thalamus itself or the striatum projecting by way of the globus pallidus to the thalamus” may be causative in dystonia pathophysiology [77]. Despite the outlined prominence of the thalamus, the primary focus shifted to the basal ganglia and their presumed pathophysiological role in both secondary and primary (isolated) dystonias. This study thus cemented the notion that dystonia is a basal ganglia disorder and paved the way for decades of research to understand the role of this structure in the disorder pathophysiology. According to the basal ganglia model of dystonia, the imbalance of the direct and indirect pathways underlies bottom-up abnormal decreases of thalamic and intracortical inhibition and subsequently abnormal increases of motor cortical excitability, leading to the dystonic output of motor behaviors (e.g., [51, 53, 98]).
Fig. 1.
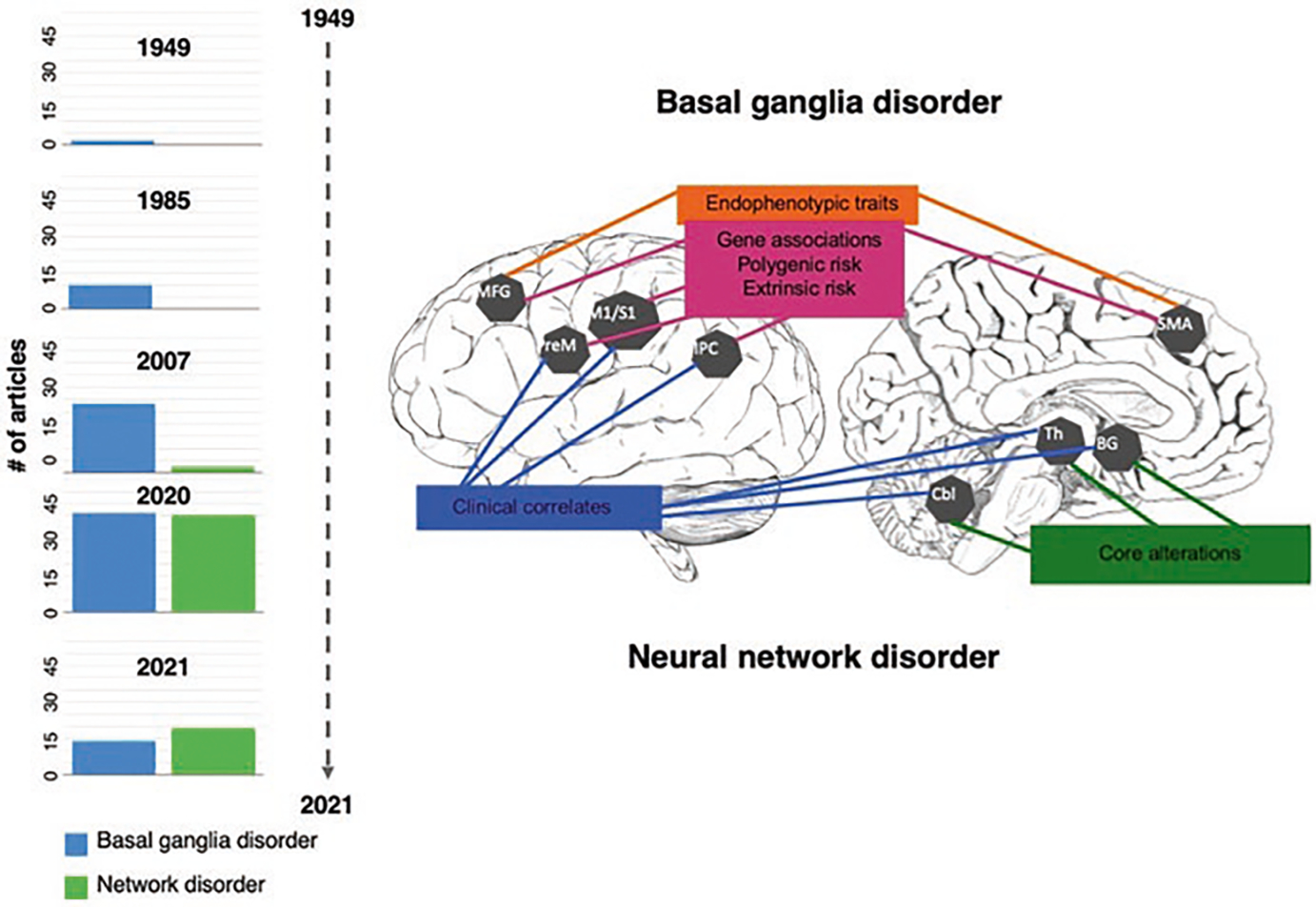
Imaging signatures of dystonia and timeline of the basal ganglia vs. neural network disorder evolution. Schematic representation of the main regions of abnormal brain function, structure, and metabolism in dystonia and their associations with genes, endophenotypic traits, clinical features, and extrinsic/environmental risk (Adapted from Simonyan et al. [101]). The bar graphs of the timeline show how the view of the pathophysiology of dystonia changed over the years from a basal ganglia to a neural network disorder. Based on the literature search in PubMed, bars graphs show the number of articles published across the years considering dystonia a basal ganglia disorder, or a network disorder. The terms used for the search included: “idiopathic dystonia OR primary dystonia OR isolated dystonia AND brain AND basal ganglia disorder” and “idiopathic dystonia OR primary dystonia OR isolated dystonia AND brain AND network disorder.” Abbreviations: MGF middle frontal gyrus, PreM premotor cortex, M1/S1 primary sensorimotor cortex, IPC inferior parietal cortex, SMA supplementary motor area, BG basal ganglia, Th thalamus, Cbl cerebellum
In the early 1990s, leveraging the breakthroughs in in-vivo investigations of human brain function, neuroimaging studies started unveiling more complex brain disorganization in patients with dystonia. Early studies, predominantly, in hereditary forms of dystonia caused by DYT1, DYT6, and DYT11 gene mutations relied heavily on the use of positron emission tomography (PET) with [15O] H2O and [18F]-fluorodeoxyglucose (FDG) radiotracers to investigate cerebral blood flow and glucose metabolism, respectively, as a proxy of neuronal activity [19, 38–40, 58, 59, 66, 74]. These studies identified abnormalities not only in the basal ganglia and thalamus but also in the cerebellum and sensorimotor cortex, suggesting a wider range of regional alterations and their interactions. Collectively, these findings led to the formulation of the metabolic network model of dystonia.
In parallel, cerebellar dysfunction and atrophy were reported in heterogeneous cohorts of patients and animal models of dystonia [23, 37, 60, 70]. This line of research prompted the theory that cerebellar alterations, similar to those in the basal ganglia, may also be causative in the disorder pathophysiology, conceptualizing the cerebellar model of dystonia.
In the past decade, the further advancements of neuroimaging techniques and analytical tools permitted in-depth investigations of different properties of brain structure and function. Rather than focusing on a single structure or network as a primary contributor to dystonia pathophysiology, a new line of research took an unbiased, data-driven approach to examining brain alterations in patients with dystonia. Mapping large-scale brain organization in dystonia demonstrated the existence of shared and divergent patterns of alterations in multiple neural networks across various forms of dystonia (for review, [94]). While the basal ganglia, thalamus, and cerebellum were found to be at the core of neural network disorganization across all forms of dystonia, the distinct patterns of functional and structural alterations were determined in cortical and subcortical sensorimotor regions responsible for multisensory processing, sensorimotor integration, and motor execution dependent on a particular form of dystonia. Collectively, these findings provided an updated view on the pathophysiology of isolated dystonia, establishing the neural network model of the disorder.
A PubMed review of the literature1 shows that most articles published until 2006 considered dystonia as a basal ganglia disorder (Fig. 1). Starting from 2007, the body of literature on the involvement of neural networks in dystonia pathophysiology steadily grew, balancing the articles referring to dystonia as a basal ganglia disorder by 2020 and surpassing these in 2021. The currently prevailing view is that dystonia is a functional and structural neural network disorder, not limited to the basal ganglia and cerebellar circuitries. This updated concept is crucial for identifying both shared and unique pathophysiological mechanisms in the various clinical manifestations of the disorder and informing the development of advanced diagnostics and the design of targeted therapeutics.
Clinical Implications of Dystonia as a Functional Neural Network Disorder
Among the most used functional neuroimaging methods in clinical research are task-based and resting-state functional magnetic resonance imaging (fMRI) paradigms. The former relies on changes in the blood oxygen level-dependent (BOLD) signal during the performance of a specific task or a behavior. The latter uses the measurement of low-frequency physiological fluctuations in the BOLD signal to examine regional correlations within intrinsic brain networks [16]. In the resting-state fMRI session, participants do not perform any cognitive tasks but are instead instructed to lay in the scanner, relax, and let their minds wander. This technique identifies multiple functional networks relevant to the salient states and behaviors in a single experimental session. In patients with dystonia, the use of resting-state fMRI circumvents the challenges associated with implementing a symptomatic (dystonic) task-based experimental design that needs to be customized according to muscles affected by the disorder, making direct comparisons between different forms of disorder not feasible. The independent component analysis (ICA) is one of the common techniques used to investigate the resting-state signal that defines functional networks by determining a set of statistically independent spatial maps and associated time courses.
Functional neuroimaging studies in dystonia demonstrate abnormal (typically increased) sensorimotor activity during the performance of symptomatic tasks and altered regional connectivity within sensorimotor and frontoparietal networks [7, 17, 18, 32, 33, 36, 55, 59, 64, 72, 76, 81, 95]. Task-specific dystonias, such as laryngeal, focal hand and musician’s dystonias, are further characterized by significant alterations in cortical areas compared to prevalently subcortical changes in non-task-specific dystonias, such as cervical dystonia and blepharospasm [14, 89, 108]. External risk factors appear to specifically influence the altered function of the basal ganglia, premotor and parietal cortices [26], while subclinical features of dystonia, such as abnormal temporal discrimination, are associated with abnormalities in primary somatosensory and middle frontal cortices [106]. Vulnerable functional connectivity of premotor and parietal regions is linked to the polygenic risk of dystonia [88], whereas functional and structural abnormalities in prefrontal-parietal cortices, thalamus, and basal ganglia represent the intermediate endophenotype of dystonia penetrance, with additional alterations in the cerebellum contributing to the secondary endophenotype of dystonia manifestation [67]. Altered functional connectivity of the thalamus, basal ganglia, premotor, and parietal cortices correlates with clinical measures associated with disease severity and age of onset (e.g., [54, 96]).
Another powerful technique for delineating the architecture of brain networks is graph-theoretical analysis, which examines global and local features of large-scale functional and structural networks (connectomes) [91, 103]. Important properties of these connectomes are the integration and segregation of nodes in neural communities and the configuration of hubs necessary for the most efficient organization of the overall network. These essential nodes of information transfer may be subdivided into provincial hubs that control within-community activity and connector hubs that control between-communities activity. Studies employing graph theoretical analysis provided the ultimate experimental evidence of dystonia as a neural network disorder. Specifically, an investigation of the large-scale architecture of the functional connectome in four different forms of focal dystonia (laryngeal dystonia, writer’s cramp, cervical dystonia, and blepharospasm) compared to healthy individuals revealed the disorganization of neural communities, including a breakdown of the basal ganglia-thalamo-cerebellar community and abnormal loss or gain of network hubs that impacted the network hierarchy necessary for information processing [8]. The follow-up research showed that the functional network kernel and community structure associated with motor execution, sensorimotor processing, and motor planning are differentially affected in different forms of dystonia [45, 93]. Further experimental evidence exists that large-scale neural network alterations are shaped by the clinical subtypes of dystonia (e.g., adductor vs. abductor laryngeal dystonia, simple vs. complex writer’s cramp), affected body part (e.g., hand vs. larynx), affected motor behavior (e.g., musician’s dystonia vs. nonmusician’s dystonia), and putative genotypes (e.g., familial vs. sporadic dystonia) [14, 15, 44, 93].
The current understanding of functional networks in dystonia presents a window of opportunity for developing novel interventions that selectively target and modulate the pathophysiologically abnormal functional neural network. One of such pathophysiologically based oral treatments showing promising potential is sodium oxybate, which is FDA-approved for cataplexy, excessive daytime sleepiness in narcolepsy, and idiopathic hypersomnia. Sodium oxybate is a centrally acting derivate of gamma-hydroxybutyric acid, which mimics the effects of alcohol. Notably, up to 55% of patients with dystonia report symptom improvement after alcohol intake [63, 68] (Fig. 2a). Alcohol modulates gamma-hydroxybutyric acid (GABA)-ergic function, which is decreased in dystonia contributing to the loss of inhibition within the dystonic network [52]. The recent open-label study in laryngeal dystonia (NCT01961297) showed that sodium oxybate modulates pathophysiological hyperactivity of brain regions associated with dystonic speech production, including the primary and secondary sensorimotor cortices, inferior frontal and superior temporal gyri, supplementary motor area, thalamus, and cerebellum [99] (Fig. 2a). This central effect translates into dystonic symptom improvement in 82.2% of alcohol-responsive patients [92]. The ongoing phase 2/3 double-blind, randomized, placebo-controlled, cross-over study of sodium oxybate in laryngeal dystonia (NCT03292458) is expected to provide concrete recommendations for its use in alcohol-responsive patients.
Fig. 2.
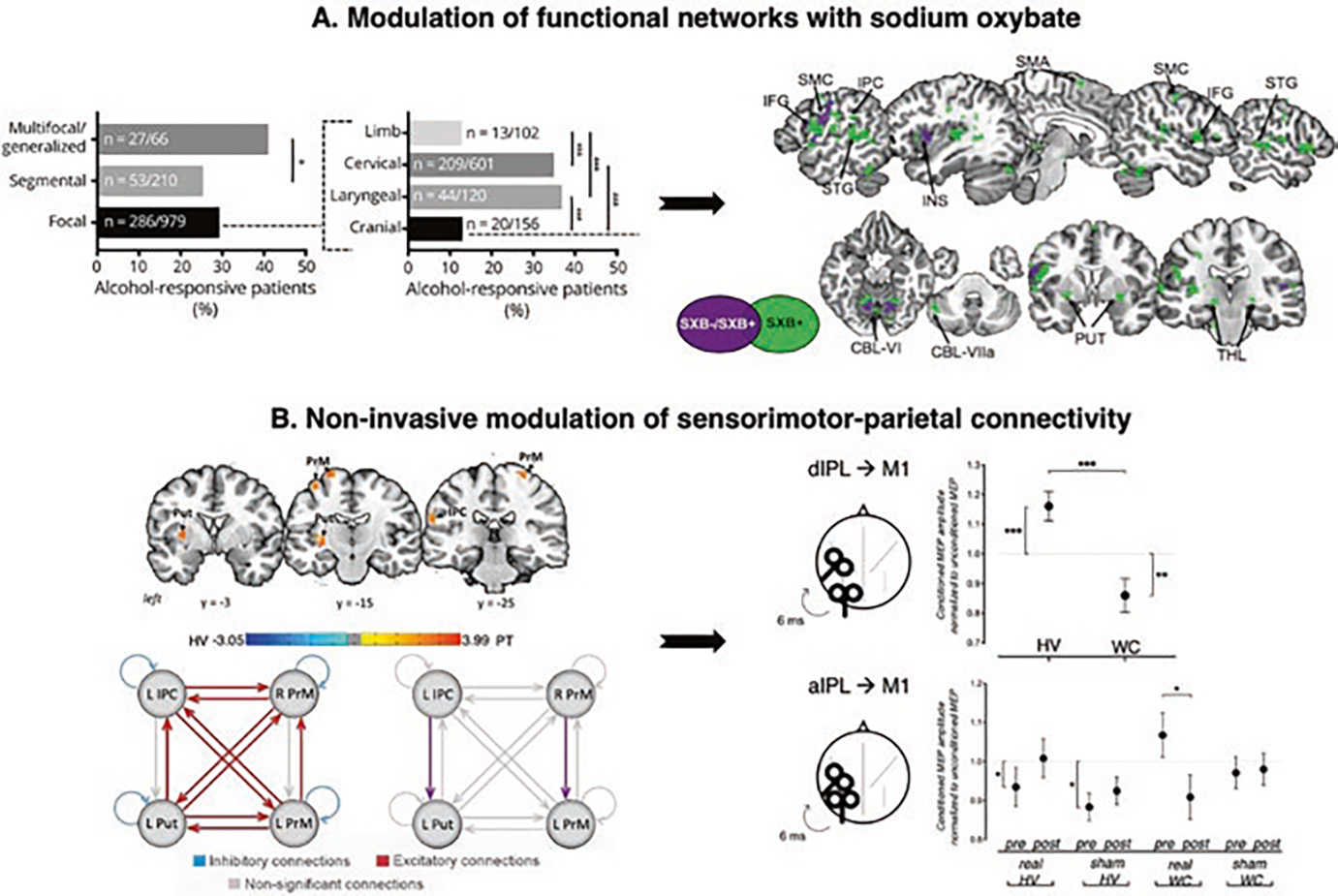
Clinical implications of dystonia as a functional neural network disorder. (a) Alcohol responsiveness in dystonia and the effects of sodium oxybate on brain activity. The left panel shows the alcohol responsiveness across different forms of dystonia (in % of patients in the examined cohort). The black arrow indicates how the understanding of alcohol responsiveness in dystonia influenced the design of the open-label study in laryngeal dystonia using sodium oxybate, a gamma-hydroxybutyric acid that mimics the effects of alcohol. The right panel shows a series of sagittal and axial slices of regions of common (in purple) and distinct (in green) brain activity in drug responders vs. nonresponders during symptomatic speech production. (b) Modulation of sensorimotor-parietal connectivity in focal dystonia. The left panel shows series of coronal brain images with regional alterations in resting-state functional connectivity in patients with laryngeal dystonia compared to healthy subjects. The schematic representation of the results of dynamic causal modeling reveals the direction of abnormal information flow between these altered regions in patients. Excitatory connections (red), inhibitory connections (blue), nonsignificant connections (gray), differences between laryngeal dystonia patients and healthy subjects (purple). The black arrow shows how imaging studies of functional and effective connectivity in dystonia informed the design of noninvasive neuromodulation in these patients. The right panel summarizes the transcranial magnetic stimulation experiment in healthy subjects and patients with writer’s cramp. The panel shows changes in sensorimotor-inferior parietal electrophysiological interaction introduced by real or sham continuous theta-burst stimulation. Abbreviations: M1 motor cortex, dIPL dorsal inferior parietal lobule, aIPL anterior inferior parietal lobule. (Panel (a) adapted from Simonyan et al. [99]. Panel (b) adapted from Battistella G. and K. Simonyan [6] and Merchant et al. [80])
The existing knowledge of dystonia as a functional network disorder may also accelerate the development and implementation of new approaches for noninvasive therapeutic neuromodulation of brain networks in dystonia. As discussed above, ample evidence supports the role of premotor-parietal regions in dystonia, particularly in task-specific dystonias. Departing from the traditional view that dystonic symptoms are generated from pure motor cortical and/or basal ganglia dysfunction, a recent study using dynamic causal modeling in laryngeal dystonia demonstrated that abnormal functional connectivity is driven by the increased top-down influence of the left inferior parietal cortex onto the putamen and increased interhemispheric right-to-left influence of the premotor cortex [6] (Fig. 2b). These findings indicated that the network disruption may be staged well before the primary motor cortex produces the dystonic behavior. In line with this, recent research in focal hand dystonia employed noninvasive neuromodulation to probe premotor and inferior parietal regions as candidate therapeutic targets. Improved dystonic symptoms were observed following active vs. sham transcranial direct current stimulation (tDCS) of the parietal cortex coupled with behavioral retraining in patients with musician’s dystonia [90]. Transcranial magnetic stimulation (TMS) with continuous theta-burst pulses over premotor and parietal regions was reported in another study to transiently decrease the parietal-premotor excitability and restore the motor cortical excitability in patients with writer’s cramp [80] (Fig. 2b). In contrast, many prior studies on noninvasive stimulation of the primary motor cortex reported a range of clinical benefits but offered limited mechanistic explanations of these effects [24]. Building on this knowledge, the currently ongoing phase 1 clinical trial (NCT04421365) is using brain–computer interface (BCI) that specifically targets parietal-premotor alterations for rehabilitation of dystonic symptoms in patients with laryngeal dystonia.
Clinical Implications of Dystonia as a Structural Neural Network Disorder
It is well known that conventional brain MRI scans of patients with isolated dystonia do not show any gross structural abnormalities. However, advances in in-vivo high-resolution MRI-based neuroimaging techniques using measures of gray matter volume, cortical thickness, and white matter microstructural properties allowed the identification of fine-grained patterns of structural alterations across the clinical spectrum of dystonias. These studies reported predominantly increased gray matter volume and cortical thickness and decreased white matter integrity across different forms of dystonia [reviewed in [94]] (Fig. 3a). In parallel with research on functional abnormalities, studies focusing on the gray matter organization determined core alterations in the basal ganglia, thalamus, and cerebellum across different forms of dystonia [30, 34, 41, 83, 84, 96, 111]. The capability of neuroimaging techniques to investigate the whole brain in a data-driven fashion further allowed the identification of abnormalities in primary sensorimotor, supplementary motor, and frontoparietal areas associated with altered motor execution, sensorimotor processing, and integration [30, 48, 49, 57, 78, 84, 89, 96, 109, 111]. The specific location of sensorimotor changes was shown to vary according to the clinical phenotype. Increased gray matter volume in the hand area in focal hand dystonia [14, 30, 48] but the larynx area in laryngeal dystonia [15, 69, 96,] are examples of this differential involvement. Dystonia gene mutations also impact the extent of these abnormalities within the sensorimotor dystonic network. For instance, non-manifesting DYT1 mutation carriers and patients without the DTY1 genetic mutation have increased gray matter volume in the putamen compared to manifesting DYT1 carriers [35], and specific patterns of gray matter structural changes in the supplementary motor area and superior temporal gyrus are present in patients with a familial history of dystonia [15, 67].
Fig. 3.
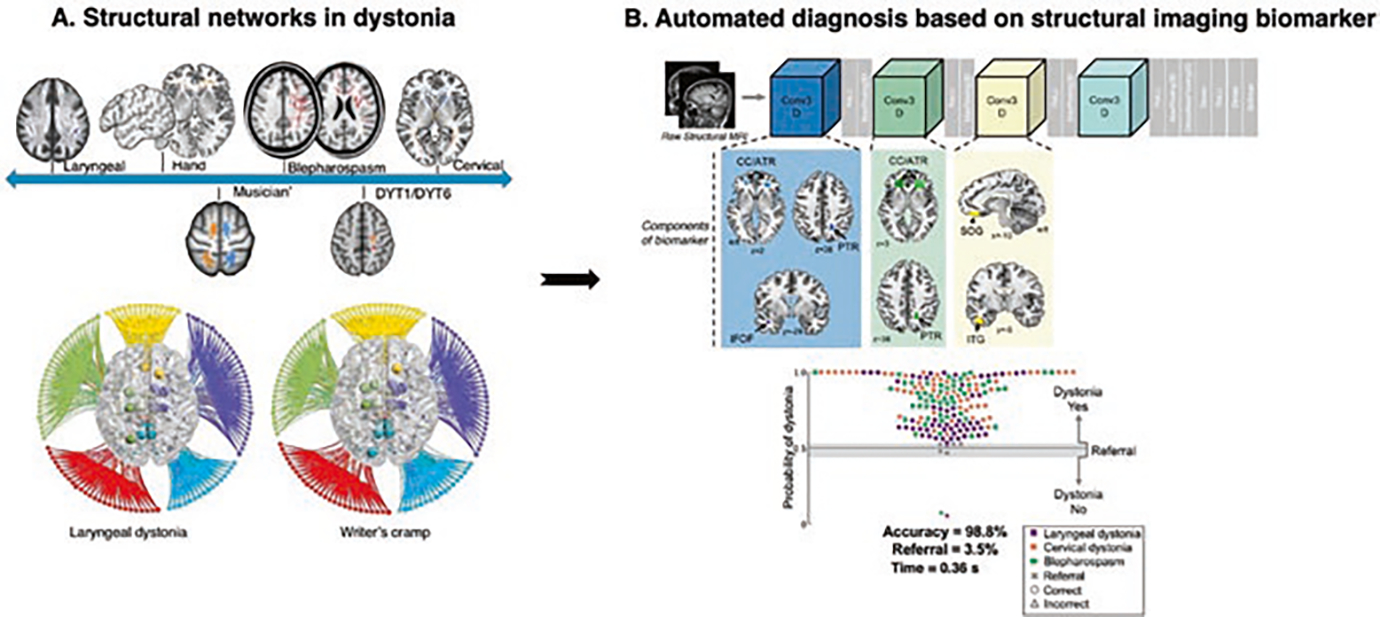
Clinical implications of dystonia as a structural neural network disorder. (a) Structural abnormalities in dystonia: the top panel shows the major microstructural abnormalities across different forms of dystonia. The bottom panel shows the large-scale connectome in focal dystonias. (b) Automated diagnosis of dystonia using DystoniaNet. The automated algorithm identified gray and white matter regions classifying patients with different forms of focal dystonia. The scatterplot shows the accuracy of the algorithm. (Panel (a) adapted from Hanekamp and Simonyan [54]. Panel (b) adapted from Valeriani and Simonyan [109])
Investigations of white matter integrity across different forms of dystonia revealed a consistent pattern of shared and phenotype-specific abnormalities along fiber tracts connecting regions of altered functional connectivity and gray matter volume/cortical thickness responsible for motor control and sensorimotor processing [3, 15, 20, 21, 31, 75, 89, 100, 108]. More recently, the investigation of the large-scale structural connectome in focal dystonias using white matter tractography demonstrated large-scale alterations, involving the abnormal organization of neural communities and hubs. This study also determined abnormal prefrontal-parietal connectivity and altered hubs in the basal ganglia, prefrontal, parietal, and insular cortices influencing the whole-brain structural reorganization [54] (Fig. 3a). An earlier in-vivo diffusion MRI study combined with postmortem neuropathology in laryngeal dystonia revealed the potential cause of microstructural changes by showing focal axonal degeneration and demyelination within the corticospinal/corticobulbar tract and clusters of mineral precipitates in the parenchyma of putamen and cerebellum [100]. Mineral accumulations are known to lead to the generation of free radicals and lipid peroxidation, causing oxidative stress, cell membrane damage, ferroptosis, and a subsequent damage to neuronal function [9, 22, 43, 104]. Future studies are warranted to characterize abnormal processes leading to mineral accumulations, the results of which may be crucial for determining the biological signatures underlying structural and functional alterations in dystonia.
The findings of abnormal structural networks in dystonia have recently paved the way for identifying reliable neural network biomarkers of diagnostic potential. Despite being the third most common movement disorder, the diagnosis of isolated dystonia remains clinically challenging, with up to half of the cases misdiagnosed at the first encounter and the final diagnosis extended up to 10 years [25, 26, 61, 62, 73, 87, 107]. The current diagnostic criteria are based purely on clinical syndrome characteristics, while the vast phenotypical variability of the disorder, the presence of conditions mimicking dystonia, and the experience and expertise of the clinician contribute to the misdiagnosis or delays in final diagnosis. Overall, this diagnostic approach is not reliable, as its specificity and sensitivity are not established, and the validity of the clinical diagnosis without a biomarker cannot be assessed [5, 28, 29, 56, 71, 86].
Based on the current knowledge of structural network abnormalities and motivated by the clinical need for an accurate and timely diagnosis of isolated dystonia, a deep learning algorithm, DystoniaNet, has been recently developed to objectively diagnose focal dystonia [109] (Fig. 3b). Using an automated, data-driven approach in a large cohort of 392 patients and 1770 healthy individuals, the DystoniaNet algorithm correctly identified gray and white matter regions frequently reported as microstructurally abnormal across the entire clinical spectrum of dystonia. Using this microstructural network biomarker, DystoniaNet achieved 98.8% accuracy in classifying patients with laryngeal dystonia, cervical dystonia, and blepharospasm while referring 3.5% of cases with an uncertain diagnosis for additional evaluations. Importantly, this algorithmic diagnostic decision was achieved in less than one second, significantly shortening the time from symptom evaluation to its diagnosis. Compared to the current diagnostic procedures, which often require various evaluations during multiple visits to multiple specialists, DystoniaNet-assisted diagnosis that is based on automatically determined pathophysiological neuroimaging signatures of the disorder may be critical in increasing the clinical accuracy and shortening the time to diagnosis.
Clinical Implications of Dystonia as a Neurotransmission Network Disorder
The last aspect of neuroimaging alterations in dystonia that may have widespread clinical implications relates to abnormal neurotransmission. The in vivo investigation of neurotransmission relies on PET neuroreceptor mapping with the use of specific radioligands to quantify dopamine, GABA, and other receptor bindings. Ample evidence demonstrates decreased striatal dopamine D2/D3 receptor binding during rest in focal and generalized forms of dystonia [4, 11, 12, 19, 85, 97]. Literature on focal dystonias also reports abnormally decreased phasic nigrostriatal dopamine release during symptomatic tasks and increased dopamine release during asymptomatic motor tasks [12, 98] (Fig. 4a). An earlier PET study including a patient cohort with different forms of focal dystonia reported no changes in striatal dopamine D1 receptor binding [65]. However, a subsequent study that carefully stratified patients based on their clinical presentations identified increased striatal dopamine D1 receptor [98]. Findings of D1 receptor binding increases and D2 receptor decreases are somatotopically distributed according to the affected body region in the sensorimotor and associative striatal subdivisions, pointing to highly specialized alterations of dopaminergic neurotransmission in the pathophysiology of dystonia. Reduced availability of striatal dopamine D2/D3 receptors decreases the inhibitory activity within the indirect basal ganglia pathway, while increased availability of striatal dopamine D1 receptors increases the excitatory activity within the direct basal ganglia pathway. This imbalance between the direct and indirect basal ganglia pathways likely contributes to hyperexcitability of the thalamus and, subsequently, the bottom-up thalamo-cortical projections to sensorimotor and parietal areas [98].
Fig. 4.
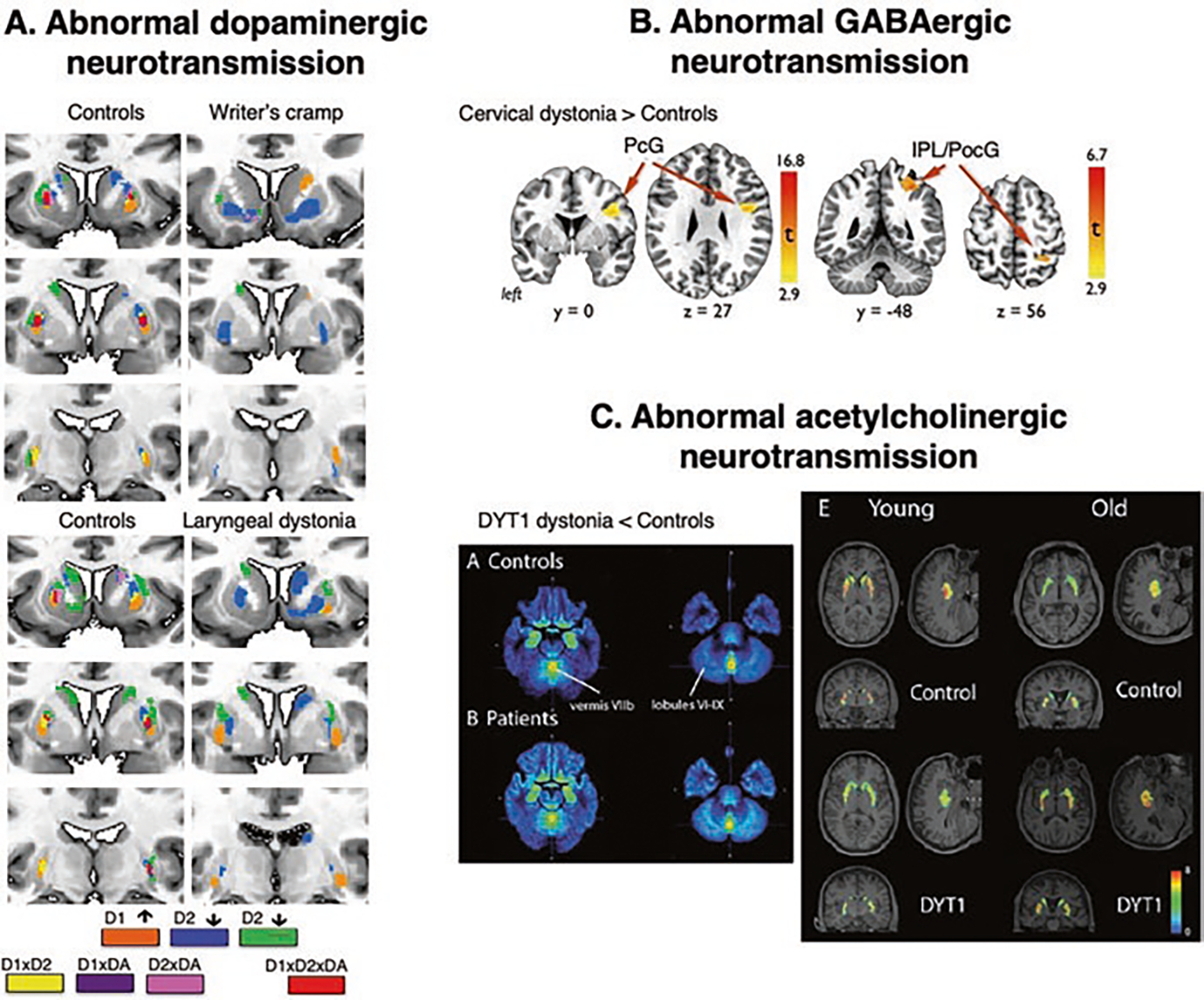
Clinical implications of dystonia as a neurotransmission network disorder. (a) Topological distribution of phasic striatal dopamine in healthy subjects and patients with writer’s cramp and laryngeal dystonia during finger tapping (for hand dystonia) and sentence production (for laryngeal dystonia). Different colors represent receptor-binding regions (D1, D2), dopamine release (DA), and their significant interactions. (b) Distribution of increased GABAA receptor binding in cervical dystonia compared to healthy subjects using [11C] flumazenil radiotracer. (c) Parametric map of decreased vesicular acetylcholine transporter in patients with DYT1 dystonia compared to healthy subjects using 18F-FEOBV-binding ratio and average binding in controls and patients stratified by age. (Panel (a) adapted from Simonyan et al. [98]. Panel (b) adapted from Berman et al. [13]. Panel (c) adapted from Mazere et al. [79])
In line with this, studies of GABAergic neurotransmission in different forms of dystonia showed decreased receptor binding within the dystonic network, including reduced GABAA receptor availability in premotor, primary sensorimotor, somatosensory, inferior parietal, insular cortices, caudate nucleus, and cerebellum [13, 46, 47, 98] (Fig. 4b). These abnormalities, albeit with minor variations across different clinical presentations of the disorder, are consistent across focal and generalized dystonias, including patients with the DYT1 gene mutation. Furthermore, reduced GABAA receptor binding correlates with increased gray matter volume and brain activity in the inferior parietal cortex [46, 98], reiterating the crucial role of this region in the pathophysiology of dystonia.
Adding to the landscape of abnormal neurotransmission in dystonia, a recent study in patients with DYT1 reported decreased vesicular acetylcholine transporter (VAChT) in the striatum and cerebellum, impacting the organization of functional connectivity within the motor network [79] (Fig. 4c). Interestingly, striatal VAChT expression was abnormal in young but not older patients, pointing to potential age-related compensatory changes. Collectively, these studies updated the basal ganglia model by including subtle alterations of the balance of major neurotransmitters within the basal ganglia-thalamo-cortical network.
The knowledge of abnormal neurotransmission in dystonia represents a powerful feature for designing novel pharmacological therapies. One such therapy includes the repurposing of sodium oxybate in laryngeal dystonia, which improves dystonic symptoms by normalizing the neural network activity via the modulation of GABAergic neurotransmission, as described above. Other well-designed randomized, blinded clinical trials of novel oral drugs in patients with different forms of dystonia should represent one of the primary research efforts in the field. These may include novel formulations and the repurposing of existing drugs that leverage the current knowledge of pathophysiologically altered neurotransmission.
Summary
The advancement of in vivo data acquisition protocols, neuroimaging techniques, and analytical tools permitted the investigation of different properties of brain microstructure and function that, collectively, transformed the understanding of dystonia pathophysiology over the past decade. These investigations were instrumental in identifying large-scale neural signatures of dystonia and defining the disorder as a network disorder, including alterations in brain function, structure, and neurotransmission. Both commonly shared and phenotype/genotype-specific changes are identified in different forms of dystonia. The basal ganglia with the thalamus and cerebellum are at the core of large-scale network disorganization in all forms of dystonia. In contrast, cortical abnormalities are characteristic of task-specific forms of dystonia in contrast to predominantly subcortical involvement in non-task-specific forms of dystonia. Cortical sensorimotor and parietal changes are linked to genetic predisposition and environmental triggers of dystonic symptoms. Understanding the pathophysiology of dystonia through the lens of impaired neural networks paved the way for the development of novel strategies to diagnostics and therapy of these patients, including the targeting of dystonia-specific neuroimaging changes in brain function and neurotransmission with new oral drugs and non-invasive neuromodulation and using microstructural changes as an objective biomarker for dystonia diagnosis.
Footnotes
The review conducted on 07/19/2021. The terms used for the search were: idiopathic dystonia OR primary dystonia OR isolated dystonia AND brain AND basal ganglia disorder & idiopathic dystonia OR primary dystonia OR isolated dystonia AND brain AND network disorder.
Contributor Information
Giovanni Battistella, Department of Otolaryngology – Head and Neck Surgery, Massachusetts Eye and Ear and Harvard Medical School, Boston, MA, USA.
Kristina Simonyan, Department of Otolaryngology – Head and Neck Surgery, Massachusetts Eye and Ear and Harvard Medical School, Boston, MA, USA; Department of Neurology, Massachusetts General Hospital, and Harvard Medical School, Boston, MA, USA.
References
- 1.Albanese A, Barnes MP, Bhatia KP, Fernandez-Alvarez E, Filippini G, Gasser T, Krauss JK, Newton A, Rektor I, Savoiardo M, Valls-Solè J. A systematic review on the diagnosis and treatment of primary (idiopathic) dystonia and dystonia plus syndromes: report of an EFNS/MDS-ES Task Force. Eur J Neurol. 2006;13:433–44. [DOI] [PubMed] [Google Scholar]
- 2.Albanese A, Bhatia K, Bressman SB, Delong MR, Fahn S, Fung VS, Hallett M, Jankovic J, Jinnah HA, Klein C, Lang AE, Mink JW, Teller JK. Phenomenology and classification of dystonia: a consensus update. Mov Disord. 2013;28:863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Argyelan M, Carbon M, Niethammer M, Ulug AM, Voss HU, Bressman SB, Dhawan V, Eidelberg D. Cerebellothalamocortical connectivity regulates penetrance in dystonia. J Neurosci. 2009;29:9740–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asanuma K, Ma Y, Okulski J, Dhawan V, Chaly T, Carbon M, Bressman SB, Eidelberg D. Decreased striatal D2 receptor binding in non-manifesting carriers of the DYT1 dystonia mutation. Neurology. 2005;64:347–9. [DOI] [PubMed] [Google Scholar]
- 5.Balint B, Mencacci NE, Valente EM, Pisani A, Rothwell J, Jankovic J, Vidailhet M, Bhatia KP. Dystonia. Nat Rev Dis Primers. 2018;4:25. [DOI] [PubMed] [Google Scholar]
- 6.Battistella G, Simonyan K. Top-down alteration of functional connectivity within the sensorimotor network in focal dystonia. Neurology. 2019;92:e1843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Battistella G, Fuertinger S, Fleysher L, Ozelius LJ, Simonyan K. Cortical sensorimotor alterations classify clinical phenotype and putative genotype of spasmodic dysphonia. Eur J Neurol. 2016;23:1517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Battistella G, Termsarasab P, Ramdhani RA, Fuertinger S, Simonyan K. Isolated focal dystonia as a disorder of large-scale functional networks. Cereb Cortex. 2017;27:1203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beauvais G, Bode NM, Watson JL, Wen H, Glenn KA, Kawano H, Harata NC, Ehrlich ME, Gonzalez-Alegre P. Disruption of protein processing in the endoplasmic reticulum of DYT1 knock-in mice implicates novel pathways in dystonia pathogenesis. J Neurosci. 2016;36:10245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.BENDA CE. Chronic rheumatic encephalitis; torsion dystonia and Hallervorden-Spatz disease. Arch Neurol Psychiatr. 1949;61:137–63. [DOI] [PubMed] [Google Scholar]
- 11.Berger HJ, van der Werf SP, Horstink CA, Cools AR, Oyen WJ, Horstink MW. Writer’s cramp: restoration of striatal D2-binding after successful biofeedback-based sensorimotor training. Parkinsonism Relat Disord. 2007;13:170–3. [DOI] [PubMed] [Google Scholar]
- 12.Berman BD, Hallett M, Herscovitch P, Simonyan K. Striatal dopaminergic dysfunction at rest and during task performance in writer’s cramp. Brain. 2013;136:3645–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berman BD, Pollard RT, Shelton E, Karki R, Smith-Jones PM, Miao Y. GABA. Front Neurol. 2018;9:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bianchi S, Fuertinger S, Huddleston H, Frucht SJ, Simonyan K. Functional and structural neural bases of task specificity in isolated focal dystonia. Mov Disord. 2019;34:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bianchi S, Battistella G, Huddlestone H, Scharf R, Fleysher L, Rumbach AF, Frucht SJ, Blitzer A, Ozelius LJ, Simonyan K. Phenotype- and genotype-specific structural alterations in spasmodic dysphonia. Mov Disord. 2017;32:560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–41. 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 17.Burciu RG, Hess CW, Coombes SA, Ofori E, Shukla P, Chung JW, McFarland NR, Wagle Shukla A, Okun MS, Vaillancourt DE. Functional activity of the sensorimotor cortex and cerebellum relates to cervical dystonia symptoms. Hum Brain Mapp. 2017;38:4563–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butterworth S, Francis S, Kelly E, McGlone F, Bowtell R, Sawle GV. Abnormal cortical sensory activation in dystonia: an fMRI study. Mov Disord. 2003;18:673–82. [DOI] [PubMed] [Google Scholar]
- 19.Carbon M, Eidelberg D. Abnormal structure-function relationships in hereditary dystonia. Neuroscience. 2009;164:220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carbon M, Kingsley PB, Tang C, Bressman S, Eidelberg D. Microstructural white matter changes in primary torsion dystonia. Mov Disord. 2008;23:234–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carbon M, Kingsley PB, Su S, Smith GS, Spetsieris P, Bressman S, Eidelberg D. Microstructural white matter changes in carriers of the DYT1 gene mutation. Ann Neurol. 2004;56:283–6. [DOI] [PubMed] [Google Scholar]
- 22.Casanova MF, Araque JM. Mineralization of the basal ganglia: implications for neuropsychiatry, pathology and neuroimaging. Psychiatry Res. 2003;121:59–87. [DOI] [PubMed] [Google Scholar]
- 23.Ceballos-Baumann AO, Brooks DJ. Basal ganglia function and dysfunction revealed by PET activation studies. Adv Neurol. 1997;74:127–39. [PubMed] [Google Scholar]
- 24.Cho HJ, Hallett M. Non-invasive brain stimulation for treatment of focal hand dystonia: update and future direction. J Mov Disord. 2016;9:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Creighton FX, Hapner E, Klein A, Rosen A, Jinnah HA, Johns MM. Diagnostic delays in spasmodic dysphonia: a call for clinician education. J Voice. 2015;29:592–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Lima Xavier L, Simonyan K. The extrinsic risk and its association with neural alterations in spasmodic dysphonia. Parkinsonism Relat Disord. 2019;65:117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Defazio G, Berardelli A, Hallett M. Do primary adult-onset focal dystonias share aetiological factors? Brain. 2007;130:1183–93. [DOI] [PubMed] [Google Scholar]
- 28.Defazio G, Albanese A, Pellicciari R, Scaglione CL, Esposito M, Morgante F, Abbruzzese G, Bentivoglio AR, Bono F, Coletti Moja M, Fabbrini G, Girlanda P, Lopiano L, Pacchetti C, Romano M, Fadda L, Berardelli A. Expert recommendations for diagnosing cervical, oromandibular, and limb dystonia. Neurol Sci. 2019;40:89–95. [DOI] [PubMed] [Google Scholar]
- 29.Defazio G, et al. The Italian Dystonia Registry: rationale, design and preliminary findings. Neurol Sci. 2017;38:819–25. [DOI] [PubMed] [Google Scholar]
- 30.Delmaire C, Krainik A, Tézenas du Montcel S, Gerardin E, Meunier S, Mangin JF, Sangla S, Garnero L, Vidailhet M, Lehéricy S. Disorganized somatotopy in the putamen of patients with focal hand dystonia. Neurology. 2005;64:1391–6. [DOI] [PubMed] [Google Scholar]
- 31.Delmaire C, Vidailhet M, Wassermann D, Descoteaux M, Valabregue R, Bourdain F, Lenglet C, Sangla S, Terrier A, Deriche R, Lehéricy S. Diffusion abnormalities in the primary sensorimotor pathways in writer’s cramp. Arch Neurol. 2009;66:502–8. [DOI] [PubMed] [Google Scholar]
- 32.Delnooz CC, Helmich RC, Toni I, van de Warrenburg BP. Reduced parietal connectivity with a premotor writing area in writer’s cramp. Mov Disord. 2012;27:1425–31. [DOI] [PubMed] [Google Scholar]
- 33.Delnooz CC, Pasman JW, Beckmann CF, van de Warrenburg BP. Task-free functional MRI in cervical dystonia reveals multi-network changes that partially normalize with botulinum toxin. PLoS One. 2013;8:e62877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Draganski B, Thun-Hohenstein C, Bogdahn U, Winkler J, May A. “Motor circuit” gray matter changes in idiopathic cervical dystonia. Neurology. 2003;61:1228–31. [DOI] [PubMed] [Google Scholar]
- 35.Draganski B, Schneider SA, Fiorio M, Klöppel S, Gambarin M, Tinazzi M, Ashburner J, Bhatia KP, Frackowiak RS. Genotype-phenotype interactions in primary dystonias revealed by differential changes in brain structure. NeuroImage. 2009;47:1141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dresel C, Haslinger B, Castrop F, Wohlschlaeger AM, Ceballos-Baumann AO. Silent event-related fMRI reveals deficient motor and enhanced somatosensory activation in orofacial dystonia. Brain. 2006;129:36–46. [DOI] [PubMed] [Google Scholar]
- 37.Eidelberg D Abnormal brain networks in DYT1 dystonia. Adv Neurol. 1998;78:127–33. [PubMed] [Google Scholar]
- 38.Eidelberg D, Moeller JR, Ishikawa T, Dhawan V, Spetsieris P, Przedborski S, Fahn S. The metabolic topography of idiopathic torsion dystonia. Brain. 1995;118(Pt 6):1473–84. [DOI] [PubMed] [Google Scholar]
- 39.Eidelberg D, Moeller JR, Antonini A, Kazumata K, Nakamura T, Dhawan V, Spetsieris P, deLeon D, Bressman SB, Fahn S. Functional brain networks in DYT1 dystonia. Ann Neurol. 1998;44:303–12. [DOI] [PubMed] [Google Scholar]
- 40.Esmaeli-Gutstein B, Nahmias C, Thompson M, Kazdan M, Harvey J. Positron emission tomography in patients with benign essential blepharospasm. Ophthalmic Plast Reconstr Surg. 1999;15:23–7. [DOI] [PubMed] [Google Scholar]
- 41.Etgen T, Mühlau M, Gaser C, Sander D. Bilateral grey-matter increase in the putamen in primary blepharospasm. J Neurol Neurosurg Psychiatry. 2006;77:1017–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fahn S, Bressman SB, Marsden CD. Classification of dystonia. Adv Neurol. 1998;78:1–10. [PubMed] [Google Scholar]
- 43.Fraga CG, Oteiza PI. Iron toxicity and antioxidant nutrients. Toxicology. 2002;180:23–32. [DOI] [PubMed] [Google Scholar]
- 44.Fuertinger S, Simonyan K. Connectome-wide phenotypical and genotypical associations in focal dystonia. J Neurosci. 2017;37:7438–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuertinger S, Simonyan K. Task-specificity in focal dystonia is shaped by aberrant diversity of a functional network kernel. Mov Disord. 2018;33:1918–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gallea C, Herath P, Voon V, Lerner A, Ostuni J, Saad Z, Thada S, Solomon J, Horovitz SG, Hallett M. Loss of inhibition in sensorimotor networks in focal hand dystonia. Neuroimage Clin. 2018;17:90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garibotto V, Romito LM, Elia AE, Soliveri P, Panzacchi A, Carpinelli A, Tinazzi M, Albanese A, Perani D. In vivo evidence for GABA(A) receptor changes in the sensorimotor system in primary dystonia. Mov Disord. 2011;26:852–7. [DOI] [PubMed] [Google Scholar]
- 48.Garraux G, Bauer A, Hanakawa T, Wu T, Kansaku K, Hallett M. Changes in brain anatomy in focal hand dystonia. Ann Neurol. 2004;55:736–9. [DOI] [PubMed] [Google Scholar]
- 49.Granert O, Peller M, Gaser C, Groppa S, Hallett M, Knutzen A, Deuschl G, Zeuner KE, Siebner HR. Manual activity shapes structure and function in contralateral human motor hand area. NeuroImage. 2011;54:32–41. [DOI] [PubMed] [Google Scholar]
- 50.Guiry S, Worthley A, Simonyan K. A separation of innate and learned vocal behaviors defines the symptomatology of spasmodic dysphonia. Laryngoscope. 2019;129:1627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hallett M Physiology of basal ganglia disorders: an overview. Can J Neurol Sci. 1993;20:177–83. [DOI] [PubMed] [Google Scholar]
- 52.Hallett M Neurophysiology of dystonia: the role of inhibition. Neurobiol Dis. 2011;42:177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hallett M, Khoshbin S. A physiological mechanism of bradykinesia. Brain. 1980;103:301–14. [DOI] [PubMed] [Google Scholar]
- 54.Hanekamp S, Simonyan K. The large-scale structural connectome of task-specific focal dystonia. Hum Brain Mapp. 2020;41:3253–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haslinger B, Altenmüller E, Castrop F, Zimmer C, Dresel C. Sensorimotor overactivity as a pathophysiologic trait of embouchure dystonia. Neurology. 2010;74:1790–7. [DOI] [PubMed] [Google Scholar]
- 56.Hellberg C, Alinder E, Jaraj D, Puschmann A. Nationwide prevalence of primary dystonia, progressive ataxia and hereditary spastic paraplegia. Parkinsonism Relat Disord. 2019;69:79–84. [DOI] [PubMed] [Google Scholar]
- 57.Horovitz SG, Gallea C, Najee-Ullah M, Hallett M. Functional anatomy of writing with the dominant hand. PLoS One. 2013;8:e67931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hutchinson M, Nakamura T, Moeller JR, Antonini A, Belakhlef A, Dhawan V, Eidelberg D. The metabolic topography of essential blepharospasm: a focal dystonia with general implications. Neurology. 2000;55:673–7. [DOI] [PubMed] [Google Scholar]
- 59.Ibáñez V, Sadato N, Karp B, Deiber MP, Hallett M. Deficient activation of the motor cortical network in patients with writer’s cramp. Neurology. 1999;53:96–105. [DOI] [PubMed] [Google Scholar]
- 60.Jinnah HA, Hess EJ. A new twist on the anatomy of dystonia: the basal ganglia and the cerebellum? Neurology. 2006;67:1740–1. [DOI] [PubMed] [Google Scholar]
- 61.Jinnah HA, Berardelli A, Comella C, Defazio G, Delong MR, Factor S, Galpern WR, Hallett M, Ludlow CL, Perlmutter JS, Rosen AR, Dystonia Coalition I. The focal dystonias: current views and challenges for future research. Mov Disord. 2013;28:926–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jog M, Chouinard S, Hobson D, Grimes D, Chen R, Bhogal M, Simonyi S. Causes for treatment delays in dystonia and hemifacial spasm: a Canadian survey. Can J Neurol Sci. 2011;38:704–11. [DOI] [PubMed] [Google Scholar]
- 63.Junker J, Brandt V, Berman BD, Vidailhet M, Roze E, Weissbach A, Comella C, Malaty IA, Jankovic J, LeDoux MS, Berardelli A, Barbano R, Reich SG, Perlmutter JS, Jinnah HA, Brüggemann N. Predictors of alcohol responsiveness in dystonia. Neurology. 2018;91:e2020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kadota H, Nakajima Y, Miyazaki M, Sekiguchi H, Kohno Y, Amako M, Arino H, Nemoto K, Sakai N. An fMRI study of musicians with focal dystonia during tapping tasks. J Neurol. 2010;257:1092–8. [DOI] [PubMed] [Google Scholar]
- 65.Karimi M, Moerlein SM, Videen TO, Su Y, Flores HP, Perlmutter JS. Striatal dopamine D1-like receptor binding is unchanged in primary focal dystonia. Mov Disord. 2013;28:2002–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kerrison JB, Lancaster JL, Zamarripa FE, Richardson LA, Morrison JC, Holck DE, Andreason KW, Blaydon SM, Fox PT. Positron emission tomography scanning in essential blepharospasm. Am J Ophthalmol. 2003;136:846–52. [DOI] [PubMed] [Google Scholar]
- 67.Khosravani S, Chen G, Ozelius LJ, Simonyan K. Neural endophenotypes and predictors of laryngeal dystonia penetrance and manifestation. Neurobiol Dis. 2021;148:105223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kirke DN, Frucht SJ, Simonyan K. Alcohol responsiveness in laryngeal dystonia: a survey study. J Neurol. 2015;262:1548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kostic VS, Agosta F, Sarro L, Tomić A, Kresojević N, Galantucci S, Svetel M, Valsasina P, Filippi M. Brain structural changes in spasmodic dysphonia: A multimodal magnetic resonance imaging study. Parkinsonism Relat Disord. 2016;25:78–84. 10.1016/j.parkreldis.2016.02.003. Epub 2016 Feb 4. [DOI] [PubMed] [Google Scholar]
- 70.Le Ber I, Clot F, Vercueil L, Camuzat A, Viémont M, Benamar N, De Liège P, Ouvrard-Hernandez AM, Pollak P, Stevanin G, Brice A, Dürr A. Predominant dystonia with marked cerebellar atrophy: a rare phenotype in familial dystonia. Neurology. 2006;67:1769–73. [DOI] [PubMed] [Google Scholar]
- 71.Lungu C, Ozelius L, Standaert D, Hallett M, Sieber BA, Swanson-Fisher C, Berman BD, Calakos N, Moore JC, Perlmutter JS, Pirio Richardson SE, Saunders-Pullman R, Scheinfeldt L, Sharma N, Sillitoe R, Simonyan K, Starr PA, Taylor A, Vitek J, Dystonia paootNWoRPi. Defining research priorities in dystonia. Neurology. 2020;94:526–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Løkkegaard A, Herz DM, Haagensen BN, Lorentzen AK, Eickhoff SB, Siebner HR. Altered sensorimotor activation patterns in idiopathic dystonia-an activation likelihood estimation meta-analysis of functional brain imaging studies. Hum Brain Mapp. 2016;37:547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Macerollo A, Superbo M, Gigante AF, Livrea P, Defazio G. Diagnostic delay in adult-onset dystonia: data from an Italian movement disorder center. J Clin Neurosci. 2015;22:608–10. [DOI] [PubMed] [Google Scholar]
- 74.Magyar-Lehmann S, Antonini A, Roelcke U, Maguire RP, Missimer J, Meyer M, Leenders KL. Cerebral glucose metabolism in patients with spasmodic torticollis. Mov Disord. 1997;12:704–8. [DOI] [PubMed] [Google Scholar]
- 75.Mantel T, Altenmüller E, Li Y, Lee A, Meindl T, Jochim A, Zimmer C, Haslinger B. Structure-function abnormalities in cortical sensory projections in embouchure dystonia. Neuroimage Clin. 2020;28:102410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mantel T, Meindl T, Li Y, Jochim A, Gora-Stahlberg G, Kräenbring J, Berndt M, Dresel C, Haslinger B. Network-specific resting-state connectivity changes in the premotor-parietal axis in writer’s cramp. Neuroimage Clin. 2018;17:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marsden CD, Obeso JA, Zarranz JJ, Lang AE. The anatomical basis of symptomatic hemidystonia. Brain. 1985;108(Pt 2):463–83. [DOI] [PubMed] [Google Scholar]
- 78.Martino D, Di Giorgio A, D’Ambrosio E, Popolizio T, Macerollo A, Livrea P, Bertolino A, Defazio G. Cortical gray matter changes in primary blepharospasm: a voxel-based morphometry study. Mov Disord. 2011;26:1907–12. [DOI] [PubMed] [Google Scholar]
- 79.Mazere J, Dilharreguy B, Catheline G, Vidailhet M, Deffains M, Vimont D, Ribot B, Barse E, Cif L, Mazoyer B, Langbour N, Pisani A, Allard M, Lamare F, Guehl D, Fernandez P, Burbaud P. Striatal and cerebellar vesicular acetylcholine transporter expression is disrupted in human DYT1 dystonia. Brain. 2021;144:909–23. [DOI] [PubMed] [Google Scholar]
- 80.Merchant SHI, Frangos E, Parker J, Bradson M, Wu T, Vial-Undurraga F, Leodori G, Bushnell MC, Horovitz SG, Hallett M, Popa T. The role of the inferior parietal lobule in writer’s cramp. Brain. 2020;143:1766–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mohammadi B, Kollewe K, Samii A, Beckmann CF, Dengler R, Münte TF. Changes in resting-state brain networks in writer’s cramp. Hum Brain Mapp. 2012;33:840–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Narbona J, Obeso JA, Tuñon T, Martinez-Lage JM, Marsden CD. Hemi-dystonia secondary to localised basal ganglia tumour. J Neurol Neurosurg Psychiatry. 1984;47:704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Obermann M, Yaldizli O, De Greiff A, Lachenmayer ML, Buhl AR, Tumczak F, Gizewski ER, Diener HC, Maschke M. Morphometric changes of sensorimotor structures in focal dystonia. Mov Disord. 2007;22:1117–23. [DOI] [PubMed] [Google Scholar]
- 84.Pantano P, Totaro P, Fabbrini G, Raz E, Contessa GM, Tona F, Colosimo C, Berardelli A. A transverse and longitudinal MR imaging voxel-based morphometry study in patients with primary cervical dystonia. AJNR Am J Neuroradiol. 2011;32:81–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perlmutter JS, Stambuk MK, Markham J, Black KJ, McGee-Minnich L, Jankovic J, Moerlein SM. Decreased [18F]spiperone binding in putamen in idiopathic focal dystonia. J Neurosci. 1997;17:843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pirio Richardson S, Wegele AR, Skipper B, Deligtisch A, Jinnah HA, Investigators DC. Dystonia treatment: patterns of medication use in an international cohort. Neurology. 2017;88:543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Powell AT, Bidewell JW, Walker AC. Diagnosing idiopathic dystonia: must it take so long? Aust Health Rev. 1995;18:120–31. [PubMed] [Google Scholar]
- 88.Putzel GG, Battistella G, Rumbach AF, Ozelius LJ, Sabuncu MR, Simonyan K. Polygenic risk of spasmodic dysphonia is associated with vulnerable sensorimotor connectivity. Cereb Cortex. 2018;28:158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ramdhani RA, Kumar V, Velickovic M, Frucht SJ, Tagliati M, Simonyan K. What’s special about task in dystonia? A voxel-based morphometry and diffusion weighted imaging study. Mov Disord. 2014;29:1141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rosset-Llobet J, Fàbregas-Molas S, Pascual-Leone Á. Effect of Transcranial Direct Current Stimulation on Neurorehabilitation of Task-Specific Dystonia: A Double-Blind, Randomized Clinical Trial. Med Probl Perform Art. 2015;30(3):178–84. 10.21091/mppa.2015.3033. [DOI] [PubMed] [Google Scholar]
- 91.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. NeuroImage. 2010;52:1059–69. [DOI] [PubMed] [Google Scholar]
- 92.Rumbach AF, Blitzer A, Frucht SJ, Simonyan K. An open-label study of sodium oxybate in Spasmodic dysphonia. Laryngoscope. 2017;127:1402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schill J, Zeuner KE, Knutzen A, Tödt I, Simonyan K, Witt K. Functional neural networks in writer’s cramp as determined by graph-theoretical analysis. Front Neurol. 2021;12:744503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Simonyan K Neuroimaging applications in dystonia. Int Rev Neurobiol. 2018;143:1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Simonyan K, Ludlow CL. Abnormal activation of the primary somatosensory cortex in spasmodic dysphonia: an fMRI study. Cereb Cortex. 2010;20:2749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Simonyan K, Ludlow CL. Abnormal structure-function relationship in spasmodic dysphonia. Cereb Cortex. 2012;22:417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Simonyan K, Berman BD, Herscovitch P, Hallett M. Abnormal striatal dopaminergic neurotransmission during rest and task production in spasmodic dysphonia. J Neurosci. 2013;33:14705–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Simonyan K, Cho H, Hamzehei Sichani A, Rubien-Thomas E, Hallett M. The direct basal ganglia pathway is hyperfunctional in focal dystonia. Brain. 2017;140:3179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Simonyan K, Frucht SJ, Blitzer A, Sichani AH, Rumbach AF. A novel therapeutic agent, sodium oxybate, improves dystonic symptoms via reduced network-wide activity. Sci Rep. 2018;8:16111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Simonyan K, Tovar-Moll F, Ostuni J, Hallett M, Kalasinsky VF, Lewin-Smith MR, Rushing EJ, Vortmeyer AO, Ludlow CL. Focal white matter changes in spasmodic dysphonia: a combined diffusion tensor imaging and neuropathological study. Brain. 2008;131:447–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Simonyan K, Barkmeier-Kraemer J, Blitzer A, Hallett M, Houde JF, Jacobson Kimberley T, Ozelius LJ, Pitman MJ, Richardson RM, Sharma N, Tanner K; The NIH/NIDCD Workshop on Research Priorities in Spasmodic Dysphonia/Laryngeal Dystonia. Laryngeal Dystonia: Multidisciplinary Update on Terminology, Pathophysiology, and Research Priorities. Neurology. 2021;96(21):989–1001. 10.1212/WNL.0000000000011922. Epub 2021 Apr 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Soland VL, Bhatia KP, Marsden CD. Sex prevalence of focal dystonias. J Neurol Neurosurg Psychiatry. 1996;60:204–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sporns O, Tononi G, Kötter R. The human connectome: a structural description of the human brain. PLoS Comput Biol. 2005;1:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stockwell BR, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tarsy D, Simon DK. Dystonia. N Engl J Med. 2006;355:818–29. [DOI] [PubMed] [Google Scholar]
- 106.Termsarasab P, Ramdhani RA, Battistella G, Rubien-Thomas E, Choy M, Farwell IM, Velickovic M, Blitzer A, Frucht SJ, Reilly RB, Hutchinson M, Ozelius LJ, Simonyan K. Neural correlates of abnormal sensory discrimination in laryngeal dystonia. Neuroimage Clin. 2016;10:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tiderington E, Goodman EM, Rosen AR, Hapner ER, Johns MM 3rd, Evatt ML, Freeman A, Factor S, Jinnah HA. How long does it take to diagnose cervical dystonia? J Neurol Sci. 2013;335:72–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tomić A, Agosta F, Sarasso E, Svetel M, Kresojević N, Fontana A, Canu E, Petrović I, Kostić VS, Filippi M. Brain structural changes in focal dystonia-what about task specificity? A multimodal MRI study. Mov Disord. 2021;36:196–205. [DOI] [PubMed] [Google Scholar]
- 109.Valeriani D, Simonyan K. A microstructural neural network biomarker for dystonia diagnosis identified by a DystoniaNet deep learning platform. Proc Natl Acad Sci U S A. 2020;117:26398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zeman W, Whitlock C. In: Bruyn PJVGW, editor. Symptomatic dystonias. Amsterdam: North Holland; 1968. p. 544–66. [Google Scholar]
- 111.Zheng Z, Pan P, Wang W, Shang H. Neural network of primary focal dystonia by an anatomic likelihood estimation meta-analysis of gray matter abnormalities. J Neurol Sci. 2012;316:51–5. [DOI] [PubMed] [Google Scholar]


