BACKGROUND:
Intracranial pressure (ICP) monitoring is widely practiced, but the indications are incompletely developed, and guidelines are poorly followed.
OBJECTIVE:
To study the monitoring practices of an established expert panel (the clinical working group from the Seattle International Brain Injury Consensus Conference effort) to examine the match between monitoring guidelines and their clinical decision-making and offer guidance for clinicians considering monitor insertion.
METHODS:
We polled the 42 Seattle International Brain Injury Consensus Conference panel members' ICP monitoring decisions for virtual patients, using matrices of presenting signs (Glasgow Coma Scale [GCS] total or GCS motor, pupillary examination, and computed tomography diagnosis). Monitor insertion decisions were yes, no, or unsure (traffic light approach). We analyzed their responses for weighting of the presenting signs in decision-making using univariate regression.
RESULTS:
Heatmaps constructed from the choices of 41 panel members revealed wider ICP monitor use than predicted by guidelines. Clinical examination (GCS) was by far the most important characteristic and differed from guidelines in being nonlinear. The modified Marshall computed tomography classification was second and pupils third. We constructed a heatmap and listed the main clinical determinants representing 80% ICP monitor insertion consensus for our recommendations.
CONCLUSION:
Candidacy for ICP monitoring exceeds published indicators for monitor insertion, suggesting the clinical perception that the value of ICP data is greater than simply detecting and monitoring severe intracranial hypertension. Monitor insertion heatmaps are offered as potential guidance for ICP monitor insertion and to stimulate research into what actually drives monitor insertion in unconstrained, real-world conditions.
KEY WORDS: Algorithms, Consensus development, Intracranial hypertension, Intracranial pressure monitoring, Neurocritical care, Practice guidelines, Traumatic brain injury
ABBREVIATIONS:
- ARDS
adult respiratory distress syndrome
- BTF
Brain Trauma Foundation
- CPP
cerebral perfusion pressure
- CWG
consensus working group
- DI
diffuse injury
- EML
evacuated mass lesions
- NEML
nonevacuated mass lesions
- PbtO2
brain tissue oxygen
- SIBICC
Seattle International Brain Injury Consensus Conference
- sTBI
severe traumatic brain injury
- TBI
traumatic brain injury.
Intracranial pressure (ICP) monitoring is the “monitor of choice” in severe traumatic brain injury (sTBI), but indications for insertion remain nebulous. Edition 3 of the Brain Trauma Foundation (BTF) Guidelines1 provided ICP monitoring recommendations, and the latest revision2 defaulted to those recommendations in the absence of new evidence. Practice surveys, however, have demonstrated tremendous variability in monitoring on individual and institutional levels,3-9 with poor penetrance of BTF recommendations. This implies that the decision process is inconsistent and poorly understood—a critical problem when examining the efficacy of ICP monitor–based care in noncontrolled studies. As well, some providers may consider ICP monitoring data of little additional clinical utility in ambiguous situations.
A tacit but unproven assumption of monitoring recommendations is that the sole/primary purpose is treating intracranial hypertension. Monitoring for the purpose of triage and timing of extracranial procedures is not subsumed under such an assumption. The value of monitoring to establish that ICP is acceptable is unaddressed.
Following the recent Seattle Severe Traumatic Brain Injury Consensus Conference (SIBICC) recommendations,10,11 the consensus working group (CWG) saw value in demonstrating how a large group of experienced, multidisciplinary TBI specialists make decisions about ICP monitor insertion based on common admission characteristics. Such an amalgamated assessment should reflect the perceived balance between having quantitative ICP values and the risks of monitoring, based on extensive, hands-on clinical management in a variety of environments (eg, “medicine-based evidence”). Therefore, the CWG members have constructed heatmaps demonstrating the likelihood of initial ICP monitor insertion in patients with TBI based on a matrix of clinical examination variables and computed tomography (CT) findings.
Our goals are to (1) examine the amalgamated monitoring results (“perceived utility”) of very experienced neurotraumatologists over the entire spectrum of acute patients with TBI and (2) to offer consensus-based guidance potentially useful in making or understanding monitoring decisions.
METHODS
The 42 SIBICC CWG10,11 physicians performed this work, including 10 neurointensivists, 23 neurosurgeons, 5 neurologist/neurointensivists, 2 trauma surgeons, and 2 emergency medicine specialists. The panel selection was based on the following: (1) >10 years clinical sTBI experience; (2) current, active involvement in acute sTBI management; (3) representation of involved disciplines; and (4) geographic diversity.
We performed this exercise by internet using Excel-based matrices (Microsoft Corporation) concatenating admission values for the Glasgow Coma Scale (GCS examination,12 pupillary status, and the modified Marshall CT Classification scheme13). We used the total GCS score (GCSt) or just the motor component (GCSm) for situations where the GCS examination is limited. Patients were posited to be adults, where advanced age (unspecified) was not a determinant factor. Pupillary status reflected size/reactivity and symmetry, with an “unexaminable” option. We reused the Marshall CT Classification modification used for SIBICC I11 and CREVICE,14 wherein postsurgical CT scans after mass lesion evacuation (EML) were additionally classified by adding the Diffuse Injury Scale (DI I-IV) to the EML label (eg, EML/DI IV). This is intended to increase the sensitivity of postoperative images to those indicators of intracranial hypertension used for nonoperative CTs.
CWG members received Excel-based GCSt and GCSm matrices and indicated their likelihood of monitoring for each combination of the 3 variables. We used a traffic light model, wherein green represented definite placement, red represented no monitoring, and yellow represented uncertainty. Entering G, Y, or R into a cell resulted in the corresponding color being displayed, providing “live” visual feedback.
The response period was from 08 October to 10 December, 2020. Subsequently, we combined responses and displayed the composite monitoring likelihood using a continuous color spectrum from pure green through yellow to pure red.
To demonstrate decision-making coherence, we reproduced heatmaps containing actual voting frequencies within each cell. In addition, we developed heatmaps using only primary green and red labels representing cells meeting ≥80% consensus (the SIBICC threshold10,11). We also developed heatmaps using 50% thresholds, representing simple majority decisions.
We aggregately modeled response tendencies using a fractional logit model, allowing us to model the “unsure” category as exactly halfway between “yes” and “no.” We modeled GCS, Marshall grade, and pupils as categorical fixed effects, as was survey respondent. We used SAS software version 9.4.
Neither IRB/ethics committee approval nor consent was required.
RESULTS
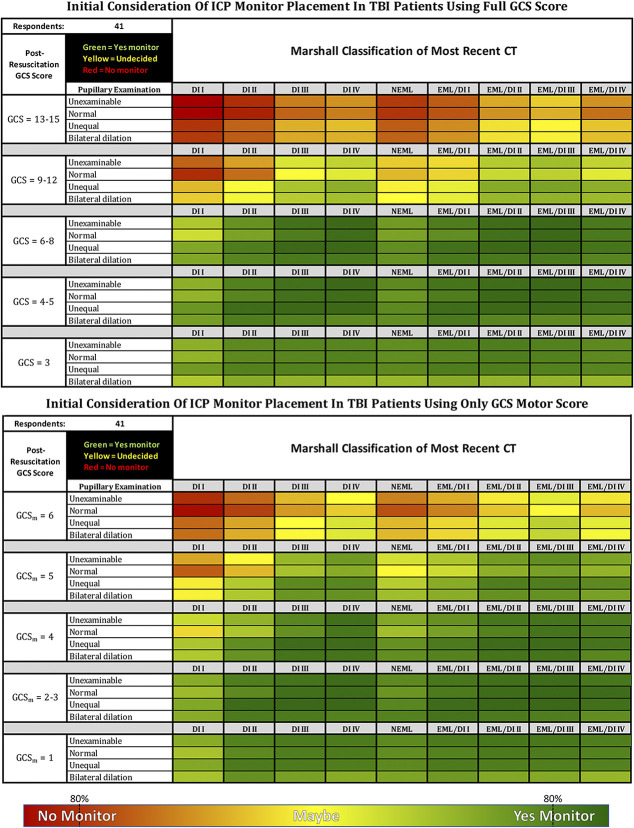
Forty-one CWG participants (98%) completed surveys, producing heatmaps for GCSt and GCSm (Figure 1). Both revealed a preponderance of green, although the frequency of intermediate shades (“greenish”) represents lack of comprehensive consensus for most choices. Red and “reddish” cells (not monitored) were much less common, limited to the highest GCSt/GCSm scores with normal or minimally pathological CTs.
FIGURE 1.
Summed responses of the 41 consensus working group respondents voting either to monitor (green), not to monitor (red), or maybe to monitor (yellow—depending on other issues such as evolution of examination, repeat CT, other injuries, etc). The table on the left represents a clinical examination allowing the full GCS; the table on the right is restricted to GCS motor score alone. Cell colors represent the combination using a Pantone gradient such that the purer the color, the higher the consensus as shown in the gradient below the heatmaps. The 80% indicators represent the consensus thresholds. Patients are assumed to be resuscitated (eg, not hypotensive, hypoxic, or hypothermic), not coagulopathic, and available for serial examination for the near future. The examination is assumed to have been conducted with minimal or no sedation. Unexaminable = Unable to examine pupils due to local trauma or other non-neurological causes. EML = evacuated mass lesion(s); NEML = nonevacuated mass lesion(s) >25 cc; EML/DI “X” = evacuated mass lesion(s) appended with Marshall diffuse injury classification (DI I-IV) of immediate postoperative CT (for patients where initial ICP monitor placement decision occurs after mass lesion evacuation—see text); CT classification modified from Marshall et al13. CT, computed tomography; GCS, Glasgow Coma Scale; ICP, intracranial pressure; TBI, traumatic brain injury.
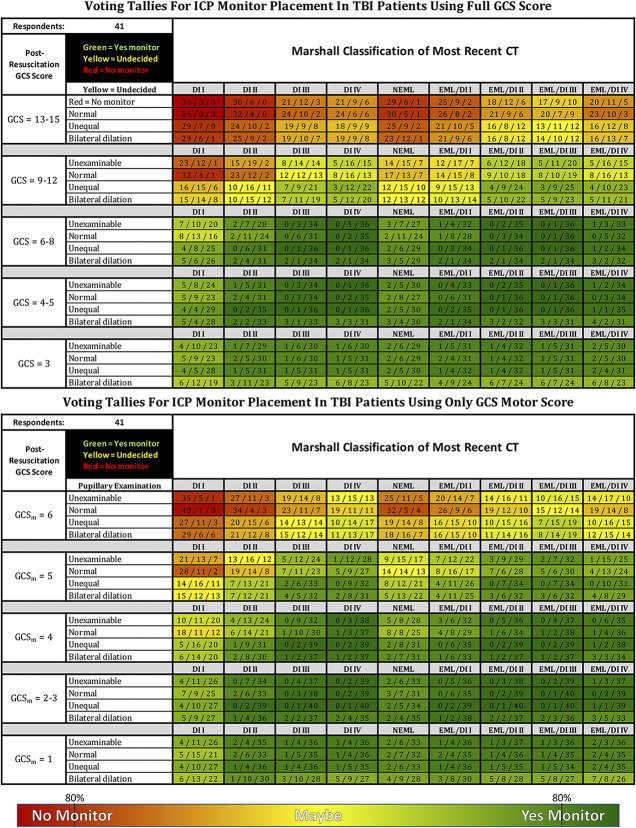
To explore these gradients, we examined the response distribution within individual cells. Figure 2 presents Figure 1 with voting tallies for red, yellow, and green within each cell. Most cells with a strongly yellow cast represent wide variations in voting that included all 3 options—indicating substantial lack of consensus rather than equipoise within individuals.
FIGURE 2.
Summed voting tallies for each choice within individual cells of the 41 consensus working group respondents voting either to monitor (green), not to monitor (red), or maybe to monitor (yellow—depending on other issues such as evolution of examination, repeat CT, other injuries, etc). The table on the left represents a clinical examination allowing the full GCS; the table on the right is restricted to GCS motor score alone. The 80% indicators represent the consensus thresholds. Patients are assumed to be resuscitated (eg, not hypotensive, hypoxic, or hypothermic), not coagulopathic, and available for serial examination for the near future. The examination is assumed to have been conducted with minimal or no sedation. Unexaminable = Unable to examine pupils due to local trauma or other non-neurological causes. EML = evacuated mass lesion(s); NEML = nonevacuated mass lesion(s) >25 cc; EML/DI “X” = evacuated mass lesion(s) appended with Marshall diffuse injury classification (DI I-IV) of immediate postoperative CT (for patients where initial ICP monitor placement decision occurs after mass lesion evacuation—see text); CT classification modified from Marshall et al13. CT, computed tomography; GCS, Glasgow Coma Scale; ICP, intracranial pressure; TBI, traumatic brain injury.
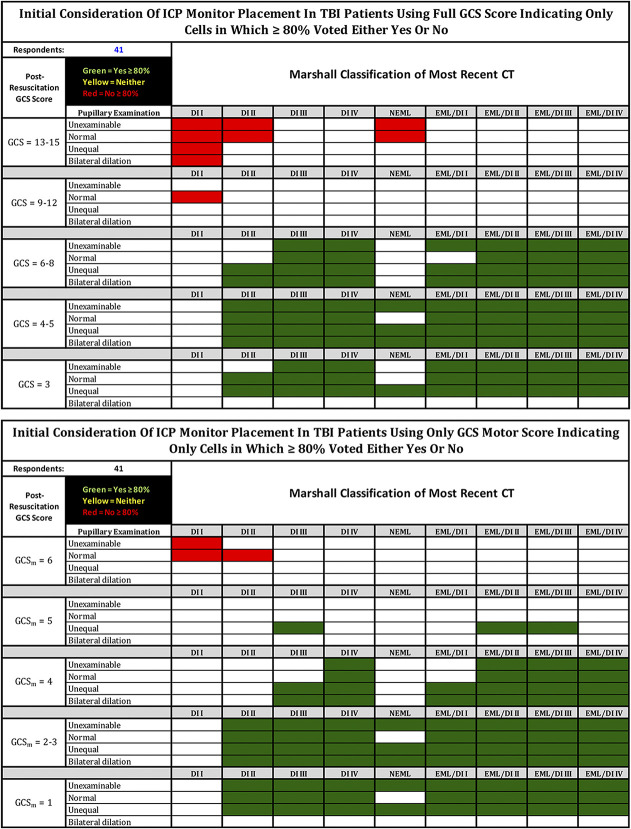
Figure 3 shows which cells represent 80% consensus for or against monitoring (the SIBICC threshold10,11). Supplemental Figure S1 (http://links.lww.com/NEU/D796) shows a threshold of ≥50% (“more probably than not”).
FIGURE 3.
Responses of the 41 CWG respondents voting either to monitor (green), not to monitor (red), or maybe to monitor (yellow—depending on other issues such as evolution of examination, repeat CT, other injuries, etc), with colored cells representing only those in which ≥80% of the CWG voted to monitor or not to monitor. The table on the left represents a clinical examination allowing the full GCS; the table on the right is restricted to GCS motor score alone. Patients are assumed to be resuscitated (eg, not hypotensive, hypoxic, or hypothermic), not coagulopathic, and available for serial examination for the near future. The examination is assumed to have been conducted with minimal or no sedation. Unexaminable = Unable to examine pupils due to local trauma or other non-neurological causes. EML = evacuated mass lesion(s); NEML = nonevacuated mass lesion(s) >25 cc; EML/DI “X” = evacuated mass lesion(s) appended with Marshall diffuse injury classification (DI I-IV) of immediate postoperative CT (for patients where initial ICP monitor placement decision occurs after mass lesion evacuation—see text); CT classification modified from Marshall et al13. CT, computed tomography; CWG, consensus working group; GCS, Glasgow Coma Scale; ICP, intracranial pressure; TBI, traumatic brain injury.
We used logistic regression modeling to characterize factors predicting ICP monitor placement. Regression modeling of the main effects in models without interactions is presented in Table 1. GCSt or GCSm (depth of coma) was dominant in decision-making. Compared with patients with GCSt 13 to 15, those with GCSt 9 to 12 had approximately 6 times the odds of being monitored, and patients with a GCSt of 6 to 8 were 99 times more likely (95% CI = 74, 132; P < .001). Despite current recommendation to monitor patients with GCSt score <9, the actual GCS value below this threshold influenced decision-making in a nonlinear fashion. Contrasted to patients with GCSt 6 to 8, those with a score of 4 to 5 had 34% higher odds of monitor placement, whereas patients with a score of 3 had lower odds (approximately half).
TABLE 1.
Regression Modeling of the Association of the 3 Main Variables with ICP Monitor Insertion
| Effect | OR | P | Lower | Upper |
|---|---|---|---|---|
| Regression modeling for GCStotal matrix—likelihood of placing an ICP monitor | ||||
| Survey ID | <.001 | |||
| GCS total | <.001 | |||
| 9 to 12 (vs 13-15) | 5.85 | <.001 | 4.78 | 7.16 |
| 6 to 8 (vs 13-15) | 98.79 | <.001 | 74.06 | 131.78 |
| 4 to 5 (vs 13-15) | 133.09 | <.001 | 98.06 | 180.60 |
| 3 (vs 13-15) | 49.59 | <.001 | 38.41 | 64.04 |
| Marshall | <.001 | |||
| DI II (vs DI I) | 2.67 | <.001 | 1.99 | 3.59 |
| DI III (vs DI I) | 6.42 | <.001 | 4.70 | 8.76 |
| DI IV (vs DI I) | 8.02 | <.001 | 5.85 | 11.01 |
| NEML (vs DI I) | 2.43 | <.001 | 1.82 | 3.26 |
| EML/DI I (vs DI I) | 4.40 | <.001 | 3.25 | 5.95 |
| EML/DI II (vs DI I) | 9.99 | <.001 | 7.23 | 13.79 |
| EML/DI III (vs DI I) | 11.74 | <.001 | 8.47 | 16.29 |
| EML/DI IV (vs DI I) | 6.91 | <.001 | 5.05 | 9.45 |
| Pupils | <.001 | |||
| Unexaminable (vs normal) | 1.30 | .013 | 1.06 | 1.60 |
| Unequal (vs normal) | 2.27 | <.001 | 1.84 | 2.81 |
| Bilateral (vs normal) | 1.31 | .011 | 1.06 | 1.61 |
| Regression modeling for GCSMotor matrix—likelihood of placing an ICP monitor | ||||
| Survey ID | <.001 | |||
| GCS motor | <.001 | |||
| 5 (vs 6) | 7.11 | <.001 | 5.85 | 8.64 |
| 4 (vs 6) | 27.89 | <.001 | 22.02 | 35.33 |
| 2 to 3 (vs 6) | 63.98 | <.001 | 48.49 | 84.41 |
| 1 (vs 6) | 29.17 | <.001 | 22.99 | 37.00 |
| Marshall | <.001 | |||
| DI II (vs DI I) | 3.25 | <.001 | 2.49 | 4.24 |
| DI III (vs DI I) | 8.93 | <.001 | 6.67 | 11.95 |
| DI IV (vs DI I) | 13.23 | <.001 | 9.75 | 17.95 |
| NEML (vs DI I) | 3.43 | <.001 | 2.62 | 4.48 |
| EML/DI I (vs DI I) | 6.26 | <.001 | 4.72 | 8.29 |
| EML/DI II (vs DI I) | 12.51 | <.001 | 9.24 | 16.95 |
| EML/DI III (vs DI I) | 16.92 | <.001 | 12.34 | 23.20 |
| EML/DI IV (vs DI I) | 9.56 | <.001 | 7.13 | 12.83 |
| Pupils | <.001 | |||
| Unexaminable (vs normal) | 1.53 | <.001 | 1.26 | 1.85 |
| Unequal (vs normal) | 2.69 | <.001 | 2.20 | 3.30 |
| Bilateral (vs normal) | 1.74 | <.001 | 1.43 | 2.11 |
DI, diffuse injury; EML, evacuated mass lesions; GCS, Glasgow Coma Scale; ICP, intracranial pressure; NEML, nonevacuated mass lesions
DI I, diffuse injury I (“no visible intracranial pathology seen on CT scan”)13; DI II, diffuse injury II (“cisterns are present with midline shift of 0 to 5 mm and/or: lesion densities present; no high-density or mixed-density lesion >25 cc; may include bone fragments and foreign bodies”)13; DI III, diffuse injury III (“cisterns compressed or absent with midline shift 0 to 5 mm, no high-density or mixed-density lesion >25 cc”)13; DI IV, diffuse injury IV (“midline shift >5 mm, no high-density or mixed-density lesion >25 cc”)13; EML, evacuated mass lesion (“any lesion surgically evacuated”)13; NEML, nonevacuated mass lesion (“high-density or mixed-density lesion >25 cc, not surgically evacuated”)13
The absence of cisterns (DI III and EML/DI III) and midline shift >5 mm (DI IV and EML/DI IV) predict monitoring at 6 to 17 times greater probability for both the GCS groups. Nonevacuated mass lesions (NEMLs) is associated with monitoring probabilities around three times that of DI I. CT classes of DI II and DI III interact with a history of surgical management (EML); odds are at least 1.9 times higher if that DI class was seen after surgery. Those with DI IV after surgery had slightly lower odds of monitoring than those with DI IV without surgery. Pupillary abnormality increases the odds of monitoring 1.3 to 2.7 times.
For monitoring, 11 (28%) preferred an external ventricular drain, 9 (23%) preferred an intraparenchymal device (IP), 14 (35%) would choose the device at the time, and 6 (15%) would insert both or a combination monitor.
DISCUSSION
Precise ICP monitoring indications are unavailable. Suggested guidelines have low penetrance.3,5-7,9 Lacking new evidence, edition 4 of the guidelines2 defaulted to edition three's recommendations1 based on the incidence of intracranial hypertension in sTBI. They cited Class III papers,15-19 reporting low incidence of intracranial hypertension with a normal CT to recommend monitoring patients with abnormal imaging. For patients with normal scans, they cited a single retrospective study of 207 patients18 reporting that the presence of 2 of 3 adverse features (older than 40 years, motor posturing, or systolic BP <90 mm Hg) was associated with an intracranial hypertension incidence similar to patients with abnormal scans.
Studies such as Alali et al20 examine the sensitivity and specificity of models in predicting intracranial hypertension; however, they are limited to the models tested and are not useful in creating new indicators. In addition, no studies have addressed the value of knowing that ICP is normal regarding directing triage and treatment. It often seems that ICP monitoring frequency is as dependent on an institution's academic focus and resident availability as it is on suspicion of intracranial hypertension.4
After the BEST TRIP trial21 and its interpretation consensus conference,22 an international group of 14 multidisciplinary TBI experts developed the Milan consensus document on applications of ICP monitoring in TBI.23 They considered scenarios including diffuse injury, contusions, mass lesion evacuation, and secondary decompressive craniectomy. Consensus was that comatose patients with a normal CT should not undergo monitoring unless the clinical examination was unreliable or unavailable. They recommended monitoring after secondary decompressive craniectomy or mass lesion evacuation, as both are associated with the risk of ICP elevation. Notably, that meeting's focus reflected the literature in concentrating on the likelihood of intracranial hypertension as the sole indication for ICP monitoring. This differs from the question asked here, which does not specify why a monitor is placed but only whether the information from monitoring would be considered useful.
Extensive experience balancing such concerns in the setting of having or having not monitored ICP over a long, active practice produces heuristic knowledge on which individual experts base their current monitoring decisions. However, generalizability may be clouded by idiosyncrasies arising from individual biases, technical peculiarities, and situational eccentricities (eg, population demographics and health care policies). Summing the experiences of many such clinicians allows analysis of where they agree/disagree while minimizing the influence of such idiosyncrasies. Our heatmaps should, therefore, be viewed more as indicators of when ICP monitoring is considered valuable than when it is purely indicated for treating intracranial hypertension. Such a process provides a snapshot of clinical practice that may be useful to those who not routinely practicing neurotrauma care.
Analyzing “conventional monitoring indicators,” basic regression modeling without interactions (Table 2), confirms that the consciousness examination (GCSt/GCSm) is strongly most determinant in deciding whether to monitor. This supports obtaining a reliable examination with minimal sedation in a resuscitated patient as early as possible by the physician responsible for monitoring decisions.
TABLE 2.
Factors Relevant to the Decision-Making Process in Deciding to Monitor ICP in TBI
|
● The risks of monitor insertion and maintenance |
|
● The suspicion that ICP is elevated |
|
● The concern that the degree of suspected intracranial hypertension will restrict recovery if not actively managed |
|
● The concern for neuroworsening if quantitative ICP is not known |
|
● The prognostic value of knowing that intracranial hypertension is extreme/refractory to treatment as a sign of very severe primary disease |
|
● The value of quantitative ICP in facilitating evaluation of treatment escalation (eg, tier 3 intervention) |
|
● The risks of overtreating ICP (with and without quantitative ICP data) |
|
● The value of quantitative ICP in |
|
○ Guiding resuscitation (eg, CPP maintenance) |
|
○ Calculating CPP and facilitating CPP-directed therapy |
|
○ Enabling determination of a patient's autoregulatory status |
|
○ Assisting in the understanding of other cranial monitors (pupillometry, PbtO2, etc.) |
|
● The value of a quantitative monitor in providing an early warning system for potential neuroworsening in a busy or nonspecialist environment |
|
● The value of knowing that ICP is NOT elevated |
|
○ Allowing other nonurgent systemic surgeries to be performed early |
|
○ Allowing other nonurgent cranial surgeries to be performed early |
|
○ Facilitating treatment of systemic abnormalities (eg, proning for ARDS) |
|
○ Facilitating extubation and transfer from ICU |
ARDS, adult respiratory distress syndrome; CPP, cerebral perfusion pressure; ICP, intracranial pressure; ICU, intensive care unit; PbtO2, brain tissue oxygen; TBI, traumatic brain injury.
Absent or compressed basal cisterns or a midline shift of >5 mm were also strongly associated with monitoring, as was an abnormal pupillary examination (with less magnitude). For patients with GCSt/GCSm scores below the 80% thresholds, imaging and pupil data moderated the monitoring decision. This is demonstrated in the decision to monitor patients with lower GCSt/GCSm scores and a normal (DI I) CT scan or the classification of NEML as well as the decision to not monitor in patients with higher GCSt/GCSm scores. The magnitude of the effects of these variables underlies the “better safe than sorry” theme permeating this study. Apparently, the “time is brain” concept makes monitoring seem preferable to simple observation in marginal cases where waiting for signs of intracranial hypertension might produce treatment delay. This is exemplified in the NEML group, which, as a pot-pouri, supports the importance of individualized decision-making.
These data reveal a strong predilection toward monitoring a much wider spectrum of patients with TBI, as represented in the full heatmaps (Figure 1), and emphasized by the regression coefficients (Table 2) and the 50% threshold heatmaps (Figure S1). The monitoring frequencies in “mild or moderate” patients are particularly notable and, certainly, more aggressive than either the guidelines1,2 or the Milan consensus statements.23 This monitoring frequency undoubtedly surpasses the actual incidence of ICP elevation in these patients,1-3,23 suggesting that the CWG places value on sensitivity over specificity (not missing intracranial hypertension) in addition to roles for other, nonthreshold decision-making factors, such as those suggested in Table 2. Notably, some of these broader goals might be achieved using less invasive or semiquantitative methods.
Figure 2 presents “yellowish” cells to consist of a mixture of yes, no, and maybe votes—more indicative of controversy than indecision. Voters made definite decisions but in opposite directions. This might partially represent the clinical tendency to make a decision rather than remain undecided. Predominantly yellow cells probably further exemplify situations where factors other than GCS score, pupillary examination, and CT classification would influence decision-making. This also suggests possible utility of noninvasive monitoring.
ICP efficacy investigations compare outcomes between monitored and nonmonitored patients. Outside of the BEST TRIP RCT,21 studies have not controlled the initial monitoring decision. Efforts to overcome this include either matching groups using propensity analysis or adjusting for differences using regression statistics. Both methods require defining the variables relevant to such decision-making, which routinely consist of measures associated with a high probability of intracranial hypertension. Our findings suggest that other variables, not entered into such adjustments, are important to the monitoring decision, implying that some of the variability in these efficacy studies might result from nonadjusted differences between study groups and not from the influence of monitoring per se. Our results do not delineate these “occult” variables (eg, Table 2), but they strongly support their existence.
Our second goal was to provide a tool that might be useful in monitor insertion decision-making. The CWG believe that examining the consensus regarding the decision to place an ICP monitor within the group responsible for the SIBICC documents would be useful to nonexpert practitioners who less frequently manage TBI and are faced with such a decision. Consistent with the SIBICC approach, the 80% consensus heatmaps (Figure 3) and their textual summary (Table 3) represent the CWG's recommendations regarding monitor insertion based on their combined monitoring tendencies. The 50% threshold heatmaps (Figure S1) are included simply to demonstrate most decisions. None of these should be considered insertion rules. They are offered to assist in decision-making where desired and to perhaps spawn clinicians to question the basis on which they make decisions that vary from the heatmaps (eg Table 2).
TABLE 3.
Consensus-Based Recommendations for ICP Monitor Insertion Based on 80% Agreementa
|
When total GCS score is available: |
|
● Patients not having undergone cranial surgery should be monitored if: |
|
○ GCS score ≤8 with an abnormal CT other than NEML |
|
▪ If CT classification is DI II and no pupillary abnormality consensus was <80% |
|
○ The CT classification is NEML and the GCS score is ≤4 with abnormal pupils |
|
● Postoperative patients should be monitored if: |
|
○ GCS ≤8 |
|
○ If CT classification is DI II and the pupillary examination is normal, consensus was < 80% |
|
● Patients do not require ICP monitor insertion if: |
|
○ GCS 13 to 15 |
|
▪ With a normal CT scan |
|
▪ With a CT classification of DI II or NEML and no pupillary abnormality |
|
When only the GCSm score is reliably available: |
|
● Patients not having undergone cranial surgery should be monitored if: |
|
○ GCSm < 3 with an abnormal CT other than NEML |
|
○ GCSm = 4 with: |
|
▪ A CT classification of DI III and abnormal pupils |
|
▪ A CT classification of DI IV |
|
○ The CT classification is NEML and the GCSm score is ≤3 with abnormal pupils |
|
● Postoperative patients should be monitored if: |
|
▪ GCSm ≤4 unless the CT is normal and there is no pupillary abnormality |
|
● Patients do not require ICP monitor insertion if: |
|
○ GCSm = 6, the CT is normal and there is no pupillary abnormality |
|
○ GCSm = 6, the CT classification is DI II and the pupils are normal |
GCS, Glasgow Coma Scale12; GCSm, Glasgow Coma Scale motor component12; ICP, intracranial pressure; CT, computed tomographic imaging of the brain; DI I, diffuse injury I (“no visible intracranial pathology seen on CT scan”)13; DI II, diffuse injury II (“cisterns are present with midline shift of 0 to 5 mm and/or: lesion densities present; no high-density or mixed-density lesion >25 cc; may include bone fragments and foreign bodies”)13; DI III, diffuse injury III (“cisterns compressed or absent with midline shift 0 to 5 mm, no high-density or mixed-density lesion >25 cc”)13; DI IV, diffuse injury IV (“midline shift >5 mm, no high-density or mixed-density lesion >25 cc”)13; EML, evacuated mass lesion (“any lesion surgically evacuated”)13; NEML, nonevacuated mass lesion (“high-density or mixed-density lesion >25 cc, not surgically evacuated”)13.
Limitations
Our approach is artificial. Many CWG comments addressed the improbability of many combinations (eg, bilateral pupillary abnormalities with GCS 15 and a DI I CT) as well as the artificial lack of myriad other factors present when making such decisions in actual practice. We acknowledge that factors unaddressed here, such as age, history of hypotension or hypoxia, severe extracranial trauma, and unavailability for repeated clinical examination, will influence monitoring decisions. Although it is impossible to weigh all such factors in a single exercise, this would better represent the actual clinical situation and would modify the CWG's recommendations based solely on GCS, CT diagnosis, and pupils. One example of such apparent in the heatmaps is the lower tendency to monitor patients with GCS 3 and dilated pupils (Figure 1), likely reflecting futility. These limitations are inherent in the model and should be considered by the reader in interpreting the results. Finally, the hypothetical nature of this exercise may not necessarily reflect the panelist's actual real-world behavior, although this should not detract from the value of the consensus as simple recommendations.
These heatmaps represent an effort toward assisting practitioners with limited TBI experience (although all may find them useful). “They are not a standard of care or even the best treatment approach in a given instance. They are not legally binding or designed as quality assurance tools. They do not represent the approach of any individual CWG member and should never be substituted for thoughtful judgment. There is no weight of evidence here that implies any necessity of changing practice.”11 As a first foray into investigating the collective perceived utility of ICP monitoring in guiding TBI care, they are for information only regarding clinical care and further scientific investigation.
Although exact indications and management details remain ill-defined, ICP monitoring is a cornerstone of TBI treatment. When uncontrollable monitoring constraints are removed, there is obviously a strong desire for ICP data in TBI, encompassing more than the basic goal of correcting intracranial hypertension. Recognizing that desire and minimizing such constraints present important goals for optimizing global TBI management.
CONCLUSION
The perceived utility of ICP monitoring in TBI, as expressed by experienced, international neurotraumatologists, is underrepresented in current monitoring guidelines. Knowing the ICP seems desirable in a broader spectrum of patients with TBI than simply those with intracranial hypertension. We provide consensus-based insertion heatmaps and recommendations for use in clinical decision-making and in analyzing survey-based ICP monitoring efficacy studies.
Supplementary Material
Acknowledgments
All authors contributed to the study conception and contributed equally to the consensus process. Material preparation, data collection, and analysis were performed by Randall M Chesnut, with the kind assistance of Nancy Temkin and Jason Barber. The first draft of the manuscript was written by Randall M Chesnut, and all authors commented on the three iterations of the manuscript. All authors read and approved the final manuscript. The authors wish to acknowledge Nancy Temkin, PhD, for assistance with statistical analyses; Peter Hendrickson, PhD, for managing our web-based surveys; Jason Barber MS for assistance with statistical analyses and matrix design; and Kelley Chaddock, BA, for organizational and managerial help. We also wish to thank our financial supporters for the original SIBICC consensus conference effort who include Adler/Geirsch Attorney at Law, the American Association of Neurological Surgeons/Congress of Neurological Surgeons Section on Neurotrauma and Critical Care, Bard, the Brain Trauma Foundation, DePuy, Hemedex, Integra, the Neurointensive Care Section of the European Society of Intensive Care Medicine, Neurosurgery Society of Australasia, Medtronic, Moberg Research, Natus, Neuroptics, Raumdic, Sophysa, Stryker, and Zoll. PJH is supported by the NIHR (Research Professorship, Cambridge BRC and Global Health Research Group on Neurotrauma), and DKM is an Emeritus Senior Investigator of the National Institute of Health Research (UK).
Footnotes
This material was presented in part at the Neurocritical Care Society 2022 meeting in Santiago, Chile, on September 7, 2022.
Supplemental digital content is available for this article at neurosurgery-online.com.
Contributor Information
Sergio Aguilera, Email: aguilera71@gmail.com.
Andras Buki, Email: 2saturn@gmail.com.
Eileen M. Bulger, Email: ebulger@uw.edu.
Giuseppe Citerio, Email: giuseppe.citerio@unimib.it.
D. Jamie Cooper, Email: jamie.cooper@monash.edu.
Ramon Diaz Arrastia, Email: ramon.diaz-arrastia@uphs.upenn.edu.
Michael Diringer, Email: diringerm@neuro.wustl.edu.
Anthony Figaji, Email: anthony.figaji@uct.ac.za.
Guoyi Gao, Email: gao3@sina.com.
Romergryko G. Geocadin, Email: rgeocad1@jhmi.edu.
Jamshid Ghajar, Email: jghajar@stanford.edu.
Odette Harris, Email: oharris@stanford.edu.
Gregory W. J. Hawryluk, Email: gregoryhawryluk79@gmail.com.
Alan Hoffer, Email: SAlan.Hoffer@UHhospitals.o`rg.
Peter Hutchinson, Email: pjah2@cam.ac.uk.
Mathew Joseph, Email: mjoseph@cmcvellore.ac.in.
Ryan Kitagawa, Email: Ryan.S.Kitagawa@uth.tmc.edu.
Geoffrey Manley, Email: manleyg@ucsf.edu.
Stephan Mayer, Email: stephanamayer@gmail.com.
David K Menon, Email: dkm13@wbic.cam.ac.uk.
Geert Meyfroidt, Email: geert.meyfroidt@uzleuven.be.
Daniel B. Michael, Email: danielm@mhsi.us.
Mauro Oddo, Email: mauro.oddo@chuv.ch.
David O. Okonkwo, Email: okonkwodo@upmc.edu.
Mayur B. Patel, Email: mayur.b.patel@vumc.org.
Claudia Robertson, Email: claudiar@bcm.tmc.edu.
Jeffrey V. Rosenfeld, Email: J.Rosenfeld@alfred.org.au.
Andres M. Rubiano, Email: andresrubiano@aol.com.
Juain Sahuquillo, Email: sahuquillo@neurotrauma.net.
Franco Servadei, Email: franco.servadei@gmail.com.
Lori Shutter, Email: shutterla@upmc.edu.
Deborah M. Stein, Email: dstein@som.umaryland.edu.
Nino Stocchetti, Email: nino.stocchetti@policlinico.mi.it.
Fabio Silvio Taccone, Email: Fabio.Taccone@ulb.be.
Shelly D. Timmons, Email: stimmons@mac.com.
Eve C. Tsai, Email: etsai@ottawahospital.on.ca.
Jamie S. Ullman, Email: jamieu@aol.com.
Walter Videtta, Email: wvidetta2@icloud.com.
David W. Wright, Email: david.wright@emory.edu.
Christopher Zammit, Email: Christopher_Zammit@urmc.rochester.edu.
Funding
This study did not receive any funding or financial support.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. Giuseppe Citerio has financial relationships with Integra and Neuroptics. Jamshid Ghajar is President of the Brain Trauma Foundation. Ramon Diaz Arrastia is supported by NIH/NINDS and the US Department of Defense. Peter Hutchinson is support by the UK NIHR (Senior Investigator Award, Biomedical Research Centre, Brain Injury Medtech Co-operative and Research Group on Global Neurotrauma) and the Royal College of Surgeons of England. David K. Menon has personal fees from Lantmannen AB, GlaxoSmithKline plc, Calico Life Sciences LLC, PressSura Neuro, Integra Neurosciences, and NeuroTrauma Sciences, LLC, has grant support from GlaxoSmithKline plc, and a shared National Institutes of Health grant from Gryphon Collaborators on a grant application outside the presented work. Daniel B. Michael declares support from the European Commission under the seventh Framework Programme (FP7-270259-TBIcare; and grant 602150—CENTER-TBI), Medical Research Council (UK) Program Grant [Acute brain injury: heterogeneity of mechanisms, therapeutic targets and outcome effects (G9439390 ID 65883)], and the UK National Institute of Health Research (NIHR) through the Biomedical Research Centre at Cambridge. Mayur B. Patel received travel support for the SIBICC meeting, and has multiple federal grants related to brain injury.
SUPPLEMENTAL DIGITAL CONTENT
Figure S1. Responses of the 41 CWG respondents voting either to monitor (Green), not to monitor (Red), or maybe to monitor (Yellow—depending on other issues such as evolution of exam, repeat CT, other injuries, etc), with colored cells representing only those in which ≥50% of the CWG voted to monitor or not to monitor. The table on the left represents a clinical exam allowing the full GCS; the table on the right is restricted to GCS motor score alone. Patients are assumed to be resuscitated (eg, not hypotensive, hypoxic, or hypothermic), not coagulopathic and available for serial examination for the near future. The exam is assumed to have been done with minimal or no sedation. Unexaminable = Unable to examine pupils due to local trauma or other non-neurological causes. EML = evacuated mass lesion(s); NEML = nonevacuated mass lesion(s) >25 cc; EML/DI “X” = evacuated mass lesion(s) appended with Marshall diffuse injury classification (DI I-IV) of immediate postoperative CT (for patients where initial ICP monitor placement decision occurs following mass lesion evacuation—see text). CT classification modified from Marshall et al13
REFERENCES
- 1.Bratton S, Bullock R, Carney N, et al. Guidelines for the management of severe brain injury: 2007 revision. J Neurotrauma. 2007;24(Suppl 1):s1–s106. [DOI] [PubMed] [Google Scholar]
- 2.Carney N, Totten AM, O'Reilly C, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017;80(1):6-15. [DOI] [PubMed] [Google Scholar]
- 3.Alali AS, Fowler RA, Mainprize TG, et al. Intracranial pressure monitoring in severe traumatic brain injury: results from the American College of Surgeons Trauma Quality Improvement Program. J Neurotrauma. 2013;30(20):1737-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bulger EM, Nathens AB, Rivara FP, et al. Management of severe head injury: institutional variations in care and effect on outcome. Crit Care Med. 2002;30(8):1870-1876. [DOI] [PubMed] [Google Scholar]
- 5.Hesdorffer DC, Ghajar J. Marked improvement in adherence to traumatic brain injury guidelines in United States trauma centers. J Trauma Inj Infect Crit Care. 2007;63(4):841-848;discussion 847-848. [DOI] [PubMed] [Google Scholar]
- 6.Myburgh JA, Cooper DJ, Finfer SR, et al. Epidemiology and 12-month outcomes from traumatic brain injury in Australia and New Zealand. J Trauma Inj Infect Crit Care. 2008;64(4):854-862. [DOI] [PubMed] [Google Scholar]
- 7.Sahjpaul R, Girotti M. Intracranial pressure monitoring in severe traumatic brain injury--results of a Canadian survey. Can J Neurol Sci. 2000;27(2):143-147. [PubMed] [Google Scholar]
- 8.Sivakumar S, Taccone FS, Rehman M, Hinson H, Naval N, Lazaridis C. Hemodynamic and neuro-monitoring for neurocritically ill patients: an international survey of intensivists. J Crit Care. 2017;39:40-47. [DOI] [PubMed] [Google Scholar]
- 9.Stocchetti N, Penny KI, Dearden M, et al. Intensive care management of head-injured patients in Europe: a survey from the European brain injury consortium. Intensive Care Med. 2001;27(2):400-406. [DOI] [PubMed] [Google Scholar]
- 10.Chesnut R, Aguilera S, Buki A, et al. A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: the Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2020;46(5):919-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawryluk GWJ, Aguilera S, Buki A, et al. A management algorithm for patients with intracranial pressure monitoring: the Seattle international severe traumatic brain injury consensus conference (SIBICC). Intensive Care Med. 2019;45(12):1783-1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teasdale G, Jennett B. Assessment of coma and impaired consciousness: a practical scale. Lancet. 1974;304(7872):81-84. [DOI] [PubMed] [Google Scholar]
- 13.Marshall LF, Marshall SB, Klauber MR, et al. A new classification of head injury based on computerized tomography. J Neurosurg. 1991;75(Supplement):s14–s20. [Google Scholar]
- 14.Chesnut RM, Temkin N, Videtta W, et al. Consensus-based management protocol (CREVICE Protocol) for the treatment of severe traumatic brain injury based on imaging and clinical examination for use when intracranial pressure monitoring is not employed. J Neurotrauma. 2020;37(11):1291-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenberg HM, Gary HE, Jr., Aldrich EF, et al. Initial CT findings in 753 patients with severe head injury. A report from the NIH Traumatic Coma Data Bank. J Neurosurg. 1990;73(5):688-698. [DOI] [PubMed] [Google Scholar]
- 16.Lobato RD, Sarabia R, Rivas JJ, et al. Normal computerized tomography scans in severe head injury. Prognostic and clinical management implications. J Neurosurg. 1986;65(6):784-789. [DOI] [PubMed] [Google Scholar]
- 17.Miller MT, Pasquale M, Kurek S, et al. Initial head computed tomographic scan characteristics have a linear relationship with initial intracranial pressure after trauma. J Trauma Inj Infect Crit Care. 2004;56(5):967-973;discussion 972-963. [DOI] [PubMed] [Google Scholar]
- 18.Narayan R, Kishore P, Becker D, et al. Intracranial pressure: to monitor or not to monitor? A review of our experience with head injury. J Neurosurg. 1982;56(5):650-659. [DOI] [PubMed] [Google Scholar]
- 19.Poca MA, Sahuquillo J, Baguena M, Pedraza S, Gracia RM, Rubio E. Incidence of intracranial hypertension after severe head injury: a prospective study using the Traumatic Coma Data Bank classification. Acta Neurochir Suppl. 1998;71:27-30. [DOI] [PubMed] [Google Scholar]
- 20.Alali AS, Temkin N, Barber J, et al. A clinical decision rule to predict intracranial hypertension in severe traumatic brain injury. J Neurosurg 2018;131(2):612-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chesnut RM, Temkin N, Carney N, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012;367(26):2471-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chesnut RM, Bleck TP, Citerio G, et al. A consensus-based interpretation of the benchmark evidence from South American trials: treatment of intracranial pressure trial. J Neurotrauma. 2015;32(22):1722-1724. [DOI] [PubMed] [Google Scholar]
- 23.Stocchetti N, Picetti E, Berardino M, et al. Clinical applications of intracranial pressure monitoring in traumatic brain injury: report of the Milan consensus conference. Acta Neurochir (Wien). 2014;156(8):1615-1622. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Responses of the 41 CWG respondents voting either to monitor (Green), not to monitor (Red), or maybe to monitor (Yellow—depending on other issues such as evolution of exam, repeat CT, other injuries, etc), with colored cells representing only those in which ≥50% of the CWG voted to monitor or not to monitor. The table on the left represents a clinical exam allowing the full GCS; the table on the right is restricted to GCS motor score alone. Patients are assumed to be resuscitated (eg, not hypotensive, hypoxic, or hypothermic), not coagulopathic and available for serial examination for the near future. The exam is assumed to have been done with minimal or no sedation. Unexaminable = Unable to examine pupils due to local trauma or other non-neurological causes. EML = evacuated mass lesion(s); NEML = nonevacuated mass lesion(s) >25 cc; EML/DI “X” = evacuated mass lesion(s) appended with Marshall diffuse injury classification (DI I-IV) of immediate postoperative CT (for patients where initial ICP monitor placement decision occurs following mass lesion evacuation—see text). CT classification modified from Marshall et al13