Abstract
Attention is biased in favor of stimuli that signal either threat or reward; this experience-dependent attentional bias develops via associative learning and persists into extinction. Physically salient yet task-irrelevant stimuli are also prioritized by the attention system, but the attentional priority of a physically salient distractor can be suppressed when it appears in a location in which it has been frequently encountered in the past. Similar effects of statistical learning on distractor suppression have been observed for distractors appearing in a predictable color. A pair of recent studies demonstrate that statistically learned distractor suppression and valence-based attentional biases combine additively, suggesting independent influences of learning on attentional priority. One limitation of these prior studies, however, is that the effects of statistical learning were defined with respect to spatial attention and the effects of associative learning with respect to feature-based attention. A strong version of the independence account would predict additive influences on attention even when both sources of priority are represented within a single domain of attentional control, which we tested in the present study. The attentional priority of a distractor was elevated when its color was previously associated with electric shock and reduced when its shape was frequently encountered as a distractor in a prior training phase, with these two influences on priority combining additively. Our findings provide strong evidence for the idea that statistical learning and valance-based associative learning exert independent influences on the control of attention, which has implications for contemporary theories of selection history.
Keywords: Attentional capture, Selection history, Statistical learning, Signal suppression
Introduction
Selective attention involves upweighting pertinent sensory information while simultaneously downweighting irrelevant and potentially distracting information (Desimone & Duncan, 1995). The attention system is fundamentally capacity limited, supported by control mechanisms that select which stimuli are allocated attentional priority (i.e., upweighted), and which are subject to suppression (i.e., downweighted). Contemporary theories of attentional control now recognize three distinct classes of attentional control mechanisms, one driven by the relationship between stimulus features and current task-specific goals (e.g., Folk et al., 1992; Wolfe et al., 1989), another driven by the physical salience or conspicuity of stimuli (Itti & Koch, 2001; Theeuwes, 1992, 2010), and a third driven by what has come to be referred to as selection history (Anderson et al., 2021; Awh et al., 2012).
Broadly, selection history reflects an influence of how attentional priority has been allocated in the past and the outcomes that have been experienced in relation to such attention allocation. With respect to outcomes, it has been shown that stimuli previously predictive of reward (e.g., Anderson, 2016; Anderson et al., 2011; Kim & Anderson, 2019a) and aversive outcomes (e.g., Anderson & Britton, 2020; Kim & Anderson, 2021b; Schmidt et al., 2015) are prioritized by the attention system, even when they are currently task-irrelevant and physically nonsalient. Both behavioral and neurophysiological evidence supports the idea that previously reward-associated and aversively conditioned stimuli bias attention through a common underlying mechanism driven by the motivational salience of a cue (e.g., Kim & Anderson, 2019a, 2021b; Kim et al., 2021; Liao et al., 2020; see Anderson et al., 2021, for a review). A second component of selection history that drives attentional priority reflects history as a former target or stimulus–response habit learning, which independently contributes to attentional priority (Anderson et al., 2017; Anderson, Laurent, & Yantis, 2014a; Anderson, Leal, et al., 2014b; Anderson & Britton, 2019; Kim & Anderson, 2019a, b). Although there is some evidence that the influence of history as a former target, reward learning, and aversive conditioning on attention can encompass the downweighting or suppression of stimuli (Anderson & Kim, 2020; Gregoire et al., 2022), motivational salience and history as a former target are generally characterized as components of selection history that serve to elevate the priority of particular stimuli.
Statistical learning, in contrast, reflects a component of selection history that is well established to contribute to signal suppression. When physically salient distractors appear more frequently at a particular spatial location, the attentional priority of stimuli appearing at this location is downweighted (e.g., Kim & Anderson, 2022; Wang et al., 2019; Wang & Theeuwes, 2018a, b), an effect that persists into an extinction period in which the spatial regularities become unbiased (Britton & Anderson, 2020). Similar effects have been observed in the context of distractor features, such that when a distractor is more likely to be rendered in a particular color, stimuli appearing in that color are to some degree suppressed (e.g., Failing et al., 2019; Stilwell et al., 2019; Vatterott & Vecera, 2012).
A question arises concerning how the influence of statistical learning and the influence of motivational salience relate to each other in the control of attention. Like motivational salience and stimulus–response habit learning, they could reflect dissociable sources of experience-dependent prioritization. On the other hand, as learning associations between stimuli and outcomes and learning the frequency of different stimuli in different situations both involve tracking and forming a memory for the likelihood of different events and updating attentional priory accordingly, it is possible that motivational salience and statistical learning rely on a common memory-dependent mechanism of attentional control. One prediction that arises from the dissociable or independent mechanisms account is that elevated attentional priority driven by motivational salience and signal suppression driven by statistical learning should combine additively in the computation of attentional priority. In contrast, a common mechanism underlying these two sources of priority would predict an interaction driven by competition or mutual interference tied to capacity limitations in the context of attentional control. Two studies have sought to adjudicate between these competing possibilities, with each providing evidence in support of additive effects and therefore the dissociable mechanisms hypothesis (Kim & Anderson, 2021a; Le Pelley et al., 2022; see also Stankevich & Geng, 2014).
Although the idea that motivational salience and stimulus– response habit learning reflect dissociable components of experience-dependent attentional control is now supported programmatically by a range of different studies, the idea that motivational salience and statistically learning reflect dissociable components of experience-dependent attention rests largely on these two recent studies (see Anderson et al., 2021). Complicating matters, the strength of the evidence provided by these two studies can be called into question. In both Kim and Anderson (2021a) and Le Pelley et al. (2022), motivational salience was defined with respect to a stimulus feature (color) and statistical learning to a location in space. The idea that feature-based attention and spatial attention are supported by dissociable neural architectures is well-established (e.g., Giesbrecht et al., 2003; Moore & Zirnsak, 2017), and although all sources of priority are to some degree integrated in the control of attention (see Anderson et al., 2021), it is possible that a common underlying mechanism of selection history separately influences space-based and feature-based priority computations. Stronger evidence for the idea that motivational salience and statistical learning influence attention via dissociable underlying mechanisms would come from a study in which both were examined within the same domain of attentional control. In such a context, the dissociable underlying mechanisms hypothesis would make the strong prediction that prioritization of one feature based on its motivational salience and suppression of another feature based on statistical learning would combine to have an additive influence on the overall attentional priority of an object possessing both features.
In the present study, we put this strong prediction to the test. Participants first performed a visual search for a shape-defined target while ignoring a physically salient distractor rendered in one of two shapes (square or diamond). One distractor shape appeared much more frequently than the other. Participants then performed a second task in which the target could now appear in one of two colors, one of which predicted the deliverance of an aversive electric shock. Finally, participants performed a third task in which the target was never rendered in either of the two colors from the prior task and no shocks were delivered; the critical distractor was equally likely to appear in one of the two colors used for targets in the second task and in one of the two shapes used for the critical distractors in the first task. The dissociable mechanisms hypothesis would predict a significant slowing of responses when the distractor was rendered in the previously shock-associated versus neutral former target color and a speeding of responses when the shape of the distractor was the previously more frequent versus less frequent distractor shape, the influence of which would combine additively to determine response time, whereas a common underlying mechanism would predict an interaction between these two sources of attentional priority evident in behavior.
Methods
Participants
Thirty-one participants (15 male, 16 female) were recruited through Texas A&M University. Participants were all between the ages of 18 and 35 inclusive (M = 19.29y, SD = 0.94y), were English-speaking, reported normal or corrected-to-normal visual acuity, and reported normal color vision. All procedures were approved by the Texas A&M University Institutional Review Board and conformed with the principles expressed in the Declaration of Helsinki. Written informed consent was obtained for each participant prior to the study.
Apparatus
A Dell OptiPlex 7040 (Dell, Round Rock, TX, USA) equipped with MATLAB software (The MathWorks, Natick, MA, USA) and Psychophysics Toolbox extensions (Brainard, 1997) were used to present the stimuli on a Dell P2717H monitor. The eye-to-screen distance was approximately 70 cm and participants completed the experiment in a dimly lit room. Responses were entered using a MiliKey response box. An isolated linear stimulator (BIOPAC) was utilized to calibrate and to administer the shocks while in current mode.
Shock calibration
Shocks were administered via two electrodes attached to the participant’s left forearm. The intensity of the shock was calibrated by gradually increasing until a level was reached that was perceived as uncomfortable but not painful (as in, e.g., Anderson & Britton, 2020; Kim & Anderson, 2021a, b; Liao et al., 2020). The electrodes were then physically removed from the participants’ forearm throughout the entire experiment, except in the shock training run where participants learned the color–shock association.
Stimuli, design, and procedure
Participants initially completed a 32-trial practice session where they learned the general procedure of the task. They then completed four runs that comprised a total of 920 trials. Each trial contained a fixation display (400–600 ms), a search display (1,500 ms or until response), a blank display (1,000 ms) and sometimes a feedback display (1,000 or 1,500 ms) and a blank display (500 ms) depending on which task they were performing (see below). During the search display on each trial (see Fig. 1), an array of eight shapes (each 3.1° × 3.1°) was presented on an imaginary circle with a radius of 9.1°. Each of these search displays contained one target pentagon and seven non-target distractor shapes. These non-target shapes were circles and each contained a line tilted 45-degrees to the left or right (randomly determined for each shape). Each of these shapes were rendered in one of 10 colors randomly assigned without replacement on each trial (purple, white, yellow, orange, cyan, blue, red, green. and grey). On distractor-present trials, either a square or diamond replaced one of the nontarget circles, with one of them serving as a high-probability shape distractor. Whether the square or diamond served as the high-probability shape alternated across participants. The pentagon target could either be right-side-up or upside down and contained either a horizontal or vertical line inside. The position of the target and high/low probability distractor were counterbalanced across runs. Trials were presented in a random order. Participants were instructed to search for the pentagon on each trial and determine if the line inside was horizontal or vertical by pressing the corresponding button on the response box. The colors used for the target and distractor varied by run as described below.
Fig. 1.
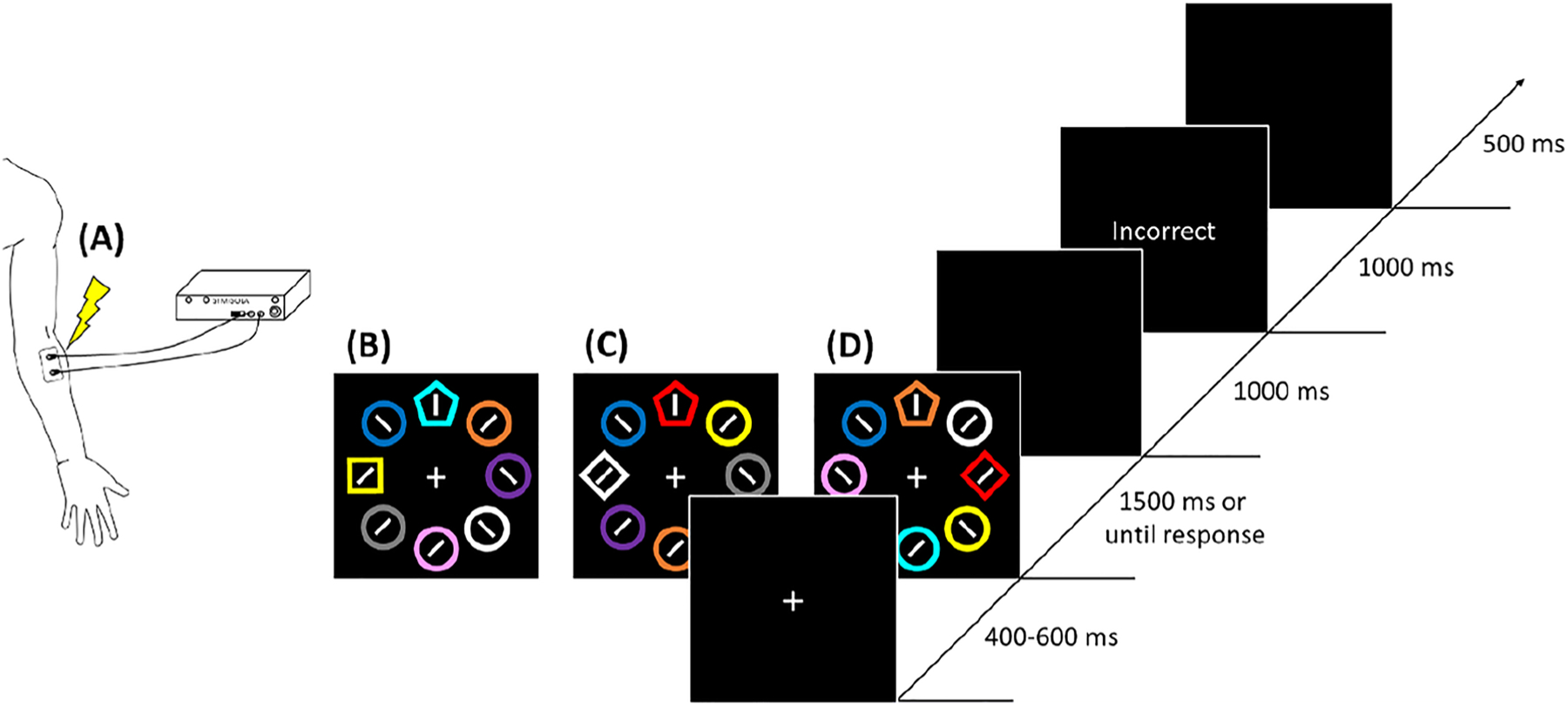
General sequence of trial events. a Shocks were delivered via two electrodes attached to the participant’s left forearm. The intensity was calibrated by gradually increasing it to a level perceived as uncomfortable but not painful. b In the shape suppression training run (Runs 1 and 2), performance feedback was provided for 1,000 ms only if participants responded incorrectly or too slowly. c In the shock training run (Run 3), performance feedback was provided after every trial for 1,500 ms, with a simultaneous shock delivery on shock target trials. d In the test phase (Run 4), performance feedback was again provided for 1,000 ms only if participants responded incorrectly or too slowly. (Color figure online)
Training phase
Shape suppression training
Runs 1 and 2 were identical and consisted of 264 trials each; 84.8% of those trials were distractor-present and 15.2% were distractor-absent. The high-probability distractor was present on 87.5% of distractor-present trials and the low-probability distractor on 12.5% of distractor-present trials. All types of stimuli in these runs, including the target pentagon and high/low probability distractors, could appear in any of the 10 aforementioned colors. A feedback display (1,000 ms) appeared only if participants responded incorrectly or too slowly (i.e., the trial timed out). The target and distractor were drawn from the same set of eight colors used for the non-target circles.
Shock training
Run 3 was comprised of 128 total trials; 87.5% of those trials were distractor-present and 12.5% were distractor-absent. The high-probability distractor was present on 89.3% of distractor-present trials and the low-probability distractor on 10.7% of distractor-present trials. The color of the distractor was drawn from the same set of colors except for red and green, while the color of the target pentagon was equally often red and green. Either red or green served as the shock-associated color, which alternated across participants. Following a trial on which the color of the target was associated with shock, a shock was delivered at the onset of the feedback display with 100% contingency. A feedback display (1,500 ms) occurred after every trial, including feedback when participants were correct, to make it explicit that shocks were not related to performance. Shock was not delivered immediately after a response was made to allow time for participants to generate predictions concerning whether shock would be delivered (see Kim & Anderson, 2021b; Sali et al., 2014), avoid emphasizing stimulus–response bindings in the delivery of shock, and avoid creating a situation in which participants might develop an aversion to making a button response, in keeping with prior studies (e.g., Kim et al., 2021; Kim & Anderson, 2021a, b).
Test phase
Run 4 contained 264 total trials; 84.8% of those trials were distractor present and 15.2% were distractor absent. The color and shape of the distractor were fully crossed and counterbalanced such that it was equally often red and green and equally often a square and diamond, with each combination of color and shape occurring equally often. As in the first two runs, the target pentagon was drawn from the same set of ten colors used for the shapes on distractor-absent trials and set of eight colors except for red and green on distractor-present trials. No shocks were administered during this run, and participants were explicitly informed that this would be the case.
Data analysis
Response times (RTs) were measured from the onset of the search display. Only correct responses were used in the analyses of RT, and RTs that were either faster than 200 ms or exceeded 2.5 standard deviations of the conditional mean for a given participant were eliminated from analysis. Analyses were carried out using SPSS with the exception of the Bayesian analysis of variance (ANOVA), which was carried out using JASP. For the Bayesian ANOVA, a Bayes factor in favor of the alternative hypothesis (BF10) < 0.333 was taken as evidence against the alternative hypothesis and in support of the null.
Results
Training phase
Shape suppression training
RTs and accuracy were analyzed using a repeated-measures ANOVA with trial type as a factor (high-probability distractor, low-probability distractor, and distractor-absent). There was a main effect for RT, F(2, 60) = 128.87, p < .001. ηp2= 0.811 (see Table 1). Planned comparisons revealed that RT was significantly slowed by each of the distractors compared with distractor-absent trials, ts > 12.71, ps < .001, dzs > 2.28, and most critically, RT was faster for the high-probability compared with the low-probability distractor, t(30) = 4.34, p < .001, dz = 0.78. There was also a main effect for accuracy, F(2, 30) = 12.00, p < .001. ηp2= 0.286 (see Table 1). Accuracy was reduced in the presence of each of the two distractors compared with distractor-absent trials, ts > 3.49, ps < 0.002, dzs > 0.63, while the difference between the two distractor conditions was not significant, t(30) = 0.84, p = .409.
Table 1.
Mean response times and accuracies from the shape suppression training
| Distractor condition | RT (ms) | Accuracy (%) |
|---|---|---|
| High probability | 798(101) | 91.7 (4.3) |
| Low probability | 839 (93) | 91.1 (6.3) |
| Absent | 701 (88) | 94.6(3.8) |
Note. Numbers in parentheses represent the standard deviations.
Shock training
RT was marginally faster for the shock-associated target (M = 698 ms, SD = 113 ms) compared with the neutral target (M = 715 ms, SD = 105 ms), t(30)= 2.01, p = .054, dz = 0.36. Accuracy did not differ between the shock-associated target (M = 95.3%, SD = 5.5%) and the neutral target (M = 95.5%, SD = 4.9%); t(30)= 0.20, p = .844.
Test phase
Pairwise comparisons demonstrated that RT was slower in all four of the distractor conditions compared with distractor-absent trials (M = 663 ms, SD = 89 ms), ts > 7.74, ps < .001, dzs > 1.39, confirming robust attentional capture by the distractors. Although accuracy was numerically highest on distractor-absent trials, none of the pairwise comparisons against any of the four distractor conditions were significant, ts < 2.02, ps > .052 (Table 2). Subsequent analyses focus on comparisons among the distractor conditions using a 2 × 2 ANOVA with distractor valence (shock, neutral) and probability (high, low) as factors. For RT, there was both a main effect of valence in which RT was slower when the distractor color was previously associated with shock, F(1, 30) = 5.71, p = .023, ηP2 = 0.160, and a main effect of distractor shape in which RT was faster when the distractor shape appeared with higher probability during training, F(1, 30) = 13.28, p < .001, ηp2 = 0.307. Importantly, there was no evidence for an interaction between these two factors, F(1, 30) = 0.04, p = .849 (Fig. 2). A Bayesian ANOVA provided moderate evidence again a model that included the two main effects and an interaction term relative to a null model that included only the two main effects, BF10 = 0.243. For accuracy, neither of the two main effects, Fs < 2.33, ps > .13, nor the interaction were significant, F(1, 30) = 0.78, p = .386.
Table 2.
Mean response times and accuracies from the test phase
| No-distractor condition | Distractor valence | RT (ms) | Accuracy (%) |
|---|---|---|---|
| High probability | Shock | 744 (98) | 94.5 (6.4) |
| Neutral | 732 (95) | 94 (4.9) | |
| Low probability | Shock | 772 (102) | 94.7 (4.7) |
| Neutral | 762 (104) | 95.4 (5.2) | |
| Absent | - | 663 (89) | 95.8 (6.9) |
Note. Numbers in parentheses represent the standard deviations.
Fig. 2.
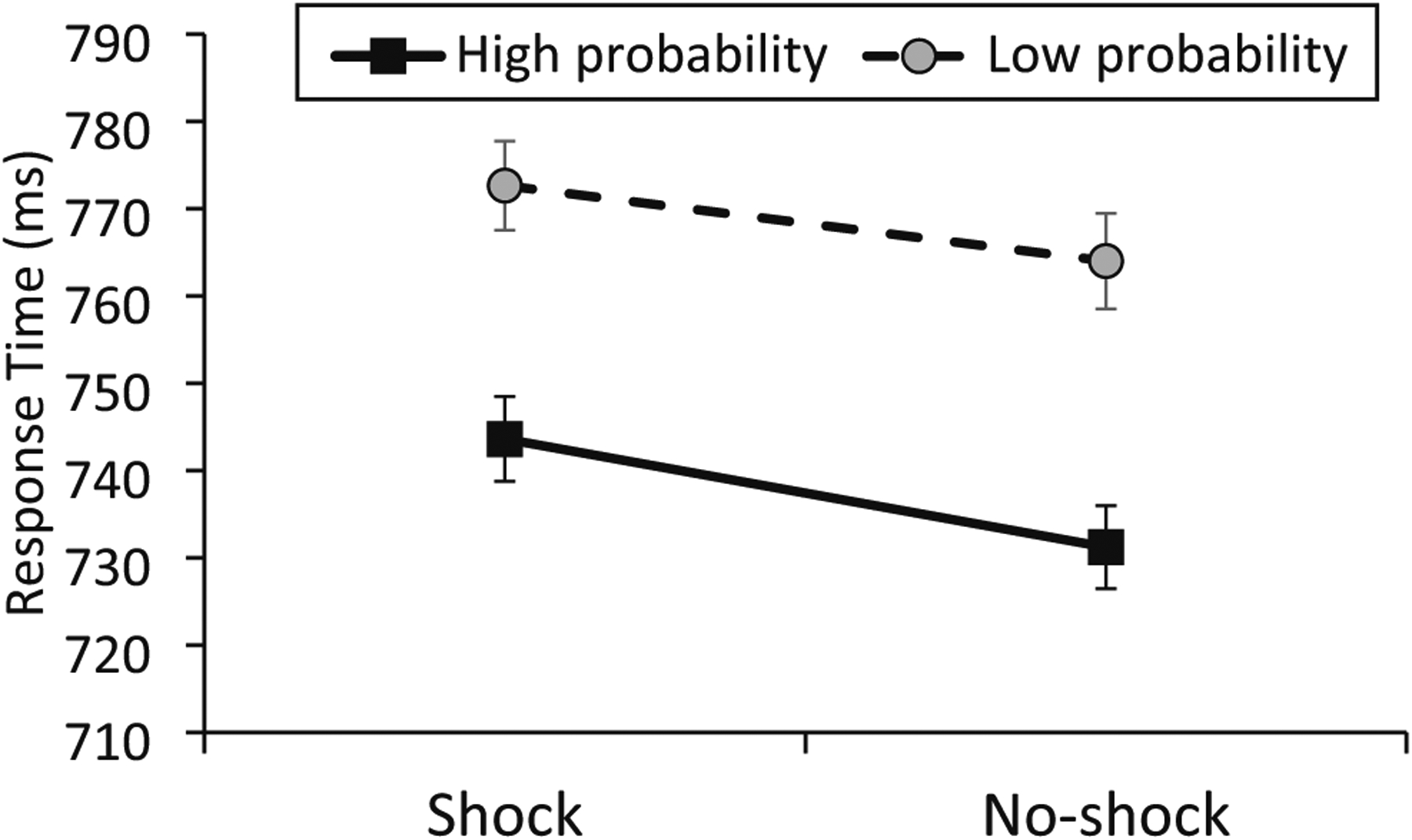
Response time on distractor-present trials in the test phase as a function of whether the distractor color was previously associated with shock (x-axis) and whether the distractor shape previously appeared with high or low probability during the initial phase of the experiment
Discussion
Prior studies have suggested that attentional biases driven by motivational salience and statistically learned distractor suppression independently contribute to attentional priority computations, consistent with dissociable underlying mechanisms of attentional control. When the color of a distractor is previously associated with either reward or aversive electric shock and the distractor appears in location at which distractors have been frequently encountered in the past, the influence of these two contingencies on distractor interference is additive (Kim & Anderson, 2021a; Le Pelley et al., 2022; see also Stankevich & Geng, 2014). A limitation of these studies, however, is that motivational salience was tied to stimulus feature and statistical learning to location. It may be that selection history independently influences spatial and feature-based attention, but within one of these domains, motivational salience and statistical learning do in fact interact, consistent with some degree of competition or mutual interference resulting from mechanistic overlap in the computation of attentional priority.
The present study overcomes this limitation by associating one stimulus feature (color) with a valent outcome (shock) while manipulating distractor frequency in a different feature dimension (shape). When an object possesses both a previously outcome-predictive and previously more frequent distractor feature, the joint influence of these features on the attentional priority of the stimulus reflects additive contributions from each. Our findings affirm the conclusions of Kim and Anderson (2021a) and Le Pelley et al. (2022), ruling out the possibility that the additivity observed in those prior studies was the result of distinctions between spatial and feature-based attention.
The idea that different feature dimensions can be differently weighted is well precedented in the context of goal-directed attentional control (e.g., Müller et al., 1995). Here, we show that involuntary attentional biases across color and shape can be independently modulated by selection history. One open question raised by our findings is the degree to which the influence of selection history is feature specific. One possibility is that associative learning only influenced the processing of stimulus color and statistical learning only influenced the processing of stimulus shape, with these two influences combining additively at the level of a priority map (Anderson, 2019). Another possibility is that the effects of each type of learning “spilled over” to the other feature, the influence of which combined additively to the same effect. Adjudicating between these possibilities would likely require the implementation of neuroimaging technology to decode feature-specific representations in the brain.
In interpreting the overall magnitude of attentional capture in the present study, it is worth noting that the target and distractor were defined within the same feature dimension— namely, shape. Although participants knew the specific shape they were tasked with searching for, it is more difficult to ignore distractors defined within the same dimension as the target compared with distractors defined in a different dimension (e.g., Liesefeld & Müller, 2019). Although the modulation of attentional capture by the specific distractor color and shape can only be explained by selection history tied to the prior training phases, the overall magnitude of distractor costs observed in the present study was likely influenced by the limitations of within-dimension selectivity in the control of attention.
Our findings lend further support to the idea that selection history reflects a constellation of dissociable underlying mechanisms of learning-dependent attentional control (Anderson et al., 2021). There appear to be multiple pathways through which selection history shapes attentional priority, likely tied to distinct learning systems that modulate attention through distinct neural processes, potentially via the midbrain and striatal dopamine system in the case of motivational salience and the medial temporal lobe and hippocampal memory system in the case of statistical learning. Future research should examine the nature of these distinctions more thoroughly, with the ultimate goal of constructing an integrative account of how different sources of attentional priority combine to influence information processing.
Acknowledgements
This study was supported by a grant from the NIH [R01-DA046410] to B.A.A.
Footnotes
Declaration
Conflicts of interest The authors declare no conflict of interest.
Open practices statement The experiments reported in this article were not formally preregistered. Neither the data nor the materials have been made available on a permanent third-party archive; requests for the data or materials can be sent via email to the lead author at Alexogden999@gmail.com.
References
- Anderson BA (2016). The attention habit: How reward learning shapes attentional selection. Annals of the New York Academy of Sciences, 1369, 24–39. [DOI] [PubMed] [Google Scholar]
- Anderson BA (2019). Neurobiology of value-driven attention. Current Opinion in Psychology, 29, 27–33. [DOI] [PubMed] [Google Scholar]
- Anderson BA, & Britton MK (2019). Selection history in context: Evidence for the role of reinforcement learning in biasing attention. Attention, Perception, & Psychophysics, 81, 2666–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, & Britton MK (2020). On the automaticity of attentional orienting to threatening stimuli. Emotion, 20, 1109–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, & Kim AJ (2020). Selection history-driven signal suppression. Visual Cognition, 28, 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, & Yantis S (2011). Value-driven attentional capture. Proceedings of the National Academy of Sciences of the United States of America, 108, 10367–10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Laurent PA, & Yantis S (2014a). Value-driven attentional priority signals in human basal ganglia and visual cortex. Brain Research, 1587, 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Leal SL, Hall MG, Yassa MA, & Yantis S (2014b). The attribution of value-based attentional priority in individuals with depressive symptoms. Cognitive, Affective, & Behavioral Neuroscience, 14, 1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Chiu M, DiBartolo MM, & Leal SL (2017). On the distinction between value-driven attention and selection history: Evidence from individuals with depressive symptoms. Psychonomic Bulletin & Review, 24, 1636–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BA, Kim H, Kim AJ, Liao M-R, Mrkonja L, Clement A, & Gregoire L (2021). The past, present, and future of selection history. Neuroscience and Biobehavioral Reviews, 130, 326–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Belopolsky AV, & Theeuwes J (2012). Top-down versus bottom-up attentional control: A failed theoretical dichotomy. Trends in Cognitive Sciences, 16, 437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH (1997). The psychophysics toolbox. Spatial Vision, 10, 433–436. [PubMed] [Google Scholar]
- Britton MK, & Anderson BA (2020). Specificity and persistence of statistical learning in distractor suppression. Journal of Experimental Psychology: Human Perception and Performance, 46, 324–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, & Duncan J (1995). Neural mechanisms of selective visual attention. Annual Review of Neuroscience, 18, 193–222. [DOI] [PubMed] [Google Scholar]
- Failing M, Feldmann-Wustefeld T, Wang B, Olivers C, & Theeuwes J (2019). Statistical regularities induce spatial as well as feature-specific suppression. Journal of Experimental Psychology: Human Perception and Performance, 45, 1291–1303. [DOI] [PubMed] [Google Scholar]
- Folk CL, Remington RW, & Johnston JC (1992). Involuntary covert orienting is contingent on attentional control settings. Journal of Experimental Psychology: Human Perception and Performance, 18, 1030–1044. [PubMed] [Google Scholar]
- Giesbrecht B, Woldorff MG, Song AW, & Mangun GR (2003). Neural mechanisms of top-down control during spatial and feature attention. NeuroImage, 19, 496–512. [DOI] [PubMed] [Google Scholar]
- Gregoire L, Britton MK, & Anderson BA (2022). Motivated suppression of value- and threat-modulated attentional capture. Emotion, 22, 780–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itti L, & Koch C (2001). Computational modelling of visual attention. Nature Reviews Neuroscience, 2, 194–203. [DOI] [PubMed] [Google Scholar]
- Kim H, & Anderson BA (2019a). Dissociable components of experience-driven attention. Current Biology, 29, 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, & Anderson BA (2019b). Dissociable neural mechanisms underlie value-driven and selection-driven attentional capture. Brain Research, 1708, 109–115. [DOI] [PubMed] [Google Scholar]
- Kim H, & Anderson BA (2021a). Combined influence of valence and statistical learning on the control of attention: Evidence for independent sources of bias. Cognition, 208, 104554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, & Anderson BA (2021b). How does the attention system learn from aversive outcomes? Emotion, 21, 898–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim AJ, & Anderson BA (2022). Systemic influence of selection history on learned ignoring. Psychonomic Bulletin & Review, 29(4), 1347–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Nanavaty N, Ahmed H, Mathur VA, & Anderson BA (2021). Motivational salience guides attention to valuable and threatening stimuli: Evidence from behavior and fMRI. Journal of Cognitive Neuroscience, 33, 2440–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pelley ME, Ung R, Mine C, Most SB, Watson P, Pearson D, & Theeuwes J (2022). Reward learning and statistical learning independently influence attentional priority of salient distractors in visual search. Attention, Perception, & Psychophysics, 84, 1446–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao M-R, Gregoire L, & Anderson BA (2020). The influence of threat and aversive motivation on conflict processing in the Stroop task. Attention, Perception, & Psychophysics, 82, 2802–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesefeld HR, & Müller HJ (2019). Distractor handling via dimension weighting. Current Opinion in Psychology, 29, 160–167. [DOI] [PubMed] [Google Scholar]
- Moore T, & Zirnsak M (2017). Neural mechanisms of selective visual attention. Annual Review of Psychology, 68, 47–72. [DOI] [PubMed] [Google Scholar]
- Müller HJ, Heller D, & Ziegler J (1995). Visual search for singleton feature targets within and across feature dimensions. Perception & Psychophysics, 57, 1–17. [DOI] [PubMed] [Google Scholar]
- Sali AW, Anderson BA, & Yantis S (2014). The role of reward prediction in the control of attention. Journal of Experimental Psychology: Human Perception and Performance, 40, 1654–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt LJ, Belopolsky AV, & Theeuwes J (2015). Attentional capture by signals of threat. Cognition and Emotion, 29, 687–694. [DOI] [PubMed] [Google Scholar]
- Stankevich BA, & Geng JJ (2014). Reward associations and spatial probabilities produce additive effects on attentional selection. Attention, Perception, & Psychophysics, 76, 2315–2325. [DOI] [PubMed] [Google Scholar]
- Stilwell BT, Bahle B, & Vecera SP (2019). Feature-based statistical regularities of distractors modulate attentional capture. Journal of Experimental Psychology: Human Perception and Performance, 45, 419–433. [DOI] [PubMed] [Google Scholar]
- Theeuwes J (1992). Perceptual selectivity for color and form. Perception & Psychophysics, 51, 599–606. [DOI] [PubMed] [Google Scholar]
- Theeuwes J (2010). Top-down and bottom-up control of visual selection. Acta Psychologica, 135, 77–99. [DOI] [PubMed] [Google Scholar]
- Vatterott DB, & Vecera SP (2012). Experience-dependent attentional tuning of distractor rejection. Psychonomic Bulletin & Review, 19, 871–878. [DOI] [PubMed] [Google Scholar]
- Wang B, & Theeuwes J (2018a). How to inhibit a distractor location? Statistical learning versus active, top-down suppression. Attention, Perception, & Psychophysics, 80, 860–870. [DOI] [PubMed] [Google Scholar]
- Wang B, & Theeuwes J (2018b). Statistical regularities modulate attentional capture. Journal of Experimental Psychology: Human Perception and Performance, 44, 13–17. [DOI] [PubMed] [Google Scholar]
- Wang B, Samara I, & Theeuwes J (2019). Statistical regularities bias overt attention. Attention, Perception, & Psychophysics, 81, 1813–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe JM, Cave KR, & Franzel SL (1989). Guided Search: An alternative to the feature integration model for visual search. Journal of Experimental Psychology: Human Perception and Performance, 15, 419–433. [DOI] [PubMed] [Google Scholar]


