Abstract
An alternate synthesis route was developed to prepare norbornene-functionalized poly(ethylene glycol) (PEG) from reacting multi-arm PEG with carbic anhydride. The macromer, PEGNBCA, permits photocrosslinking of thiol-norbornene hydrogels with kinetics comparable to conventional PEGNB macromer. In addition, PEGNBCA provides an additional carboxylate group for further conjugation with amine-bearing molecules. Interestingly, PEGNBCA thiol-norbornene hydrogels are highly susceptible to hydrolytic degradation through enhanced ester hydrolysis. The ester linkage is further weakened after the secondary conjugation, resulting in extremely rapid degradation of PEGNB hydrogels. More importantly, the degradation can be readily adjusted via tuning macromer compositions, with complete degradation time ranging from hours to weeks. The PEGNBCA hydrogels are also highly cytocompatible toward various cell types, providing opportunities for future applications in tissue engineering and advanced biofabrication.
Graphical Abstract
Poly(ethylene glycol) (PEG)-based hydrogels are widely used in tissue engineering and biofabrication for their excellent biocompatibility,1 adaptable crosslinking mechanisms, and readily tunable physiochemical properties. PEG-based hydrogels are particularly desirable in tissue regeneration,2,3 controlled release,4 and biosensor applications.5 Conjugation of functional groups (e.g., acrylate, norbornene, vinylsulfone, maleimide) on the termini of PEG chains is necessary to permit their crosslinking into hydrogels. On the other hand, labile motifs (e.g., poly(lactic acid), PLA)6 are routinely copolymerized with functionalized PEG to render the otherwise stable network hydrolytically-labile. Alternative, hydrolytic degradation of PEG-based hydrogels may be engineered by synthesizing ester-containing crosslinkers with different hydrolytic susceptibility, such as groups with steric hindrance, local hydrophobicity, and electron-withdrawing moieties.3,7
Among the various crosslinking chemistries, PEG-based hydrogels fabricated by orthogonal thiol-norbornene photopolymerization are increasingly used in drug delivery and tissue engineering applications.8 In particular, multi-arm PEG-norbornene (PEGNB) can be crosslinked into an idealized network using multi-functional thiol crosslinker through radical-medicated thiol-norbornene reaction initiated by ultraviolet light,9–12 visible light,13,14 or enzymatic reaction15. We and others have prepared biomimetic PEG-based thiol-norbornene hydrogels for a variety of biomedical applications.16–20 In all prior studies utilizing PEGNB macromers, NB group functionalization was typically achieved between 5-norbornene-2-carboxylic acid (NB-acid) and either PEG hydroxyls (i.e., esterification) or PEG amines (i.e., carbodiimide chemistry).21 The former reaction produces ester linkages (i.e., PEGeNB), whereas the later yields stable amide bonds (i.e., PEGaNB). Compared with hydrogels crosslinked by PEGaNB, the more hydrolytically-labile PEGeNB (referring to PEGNB thereafter) hydrogels improved survival, proliferation, and spreading of human mesenchymal stem cells (hMSCs).
To functionalize hydrolytically-labile PEGNB (Figure 1A, X=H) through Steglich esterification,22 current synthesis requires that NB-acid being activated with N,N’-dicyclohexylcarbodiimide (DCC) to form O-acylisourea intermediate (Figure 1A-I). The intermediate is filtered into PEG-hydroxyls in the presence of pyridine and 4-dimethylaminopyridine (DMAP),9 followed by overnight reaction and precipitation in cold ether. To maximize the degree of substitution (DS), multiple efforts were developed, such as extended refluxing PEG in toluene to remove excess bond water, blanketing the reaction with inert gas, conducting back-to-back reactions, and limiting reaction to smaller batches (< 5 g). Unfortunately, none of these lengthy and laborious steps (~5 days) eliminates the pungent and extremely unpleasant odor of NB-acid. Stringent containment efforts are taken during and post synthesis to minimize the unpleasant order of NB-acid. While PEGNB macromers are commercially available, they are pricy and hence not a sustainable option. These disadvantages significantly limit the widespread usage of this powerful and diverse macromer.
Figure 1. Synthesis and characterization of PEGNBCA hydrogels.

(A) Reaction and purification routes for synthesis of norbornene-functionalized PEG. I: X=H (PEGNB); II: X=COOH (PEGNBCA). (B) Light and radical initiated thiol-norbornene gelation by lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP). (C) In situ photorheometry of 2.5 wt% PEGNBCA with DTT (R = 1). (D) Shear moduli of hydrogels crosslinked by PEGNB or PEGNBCA with DTT. PI: 1 mM LAP, 2 min 365 nm light exposure (5 mW/cm2).
We sought to circumvent the cumbersome steps in the current PEGNB synthesis by adopting an alternative reaction protocol using carbic anhydride (CA), an odorless norbornene derivative containing diacid anhydride group (Figure 1A-II). CA is chosen as we have previously used it to synthesize gelatin-modified norbornene (e.g., GelNB).23,24 In this work, we established a user-friendly CA-based synthesis protocol for introducing norbornene to multi-arm PEG-hydroxyls. The reaction was catalyzed by DMAP25 in tetrahydrofuran (THF) (Figure 1A-II), affording a macromer PEGNBCA (Figure 1A, X=COOH). Importantly, no odor containment effort was needed for the PEGNBCA synthesis and the method shortened the overall synthesis time from 5 days to 2 days. We verified high NB substitution on PEGNBCA via quantifying thiol consumption using thiol-norbornene reaction with cysteine (Cys) (Figure S1). Moreover, the PEGNBCA macromer contains an additional carboxylic acid moiety, which may be exploited for subsequent conjugation of functional groups via standard carbodiimide chemistry.
We tested thiol-norbornene photo-gelation (Figure 1B) using the PEGNBCA and dithiothreitol (DTT) at a stoichiometric ratio (i.e., Rthiol/ene = 1). The gelation kinetics as evaluated by in situ photorheometry revealed a rapid gel point (~10 s) typical to the UV-initiated thiol-norbornene gelation (Figure 1C). However, compared with hydrogels crosslinked by conventional PEGNB, the shear moduli (G’) of the hydrogels crosslinked by PEGNBCA were consistently lower at equivalent polymer contents (Figure 1D), suggesting a lower reaction efficiency of PEGNBCA towards thiols. The reduced thiol-norbornene reaction efficiency of PEGNBCA may be a result from the additional carboxylic acid group that reduces the local pH value. This is consistent with a recent report by Colak et al., where it was demonstrated that thiol-norbornene reaction efficiency was lower with a carbic anhydride derivative functionalized with hexane-1,6-diamine.26 Nonetheless, this protocol offers a greener and more user-friendly route to the synthesis of norbornene functionalized PEG for orthogonal thiol-norbornene hydrogel crosslinking.
Ester-linked PEGNB hydrogels are known to undergo slow and predictable hydrolytic degradation (Figure 2A).9 Indeed, 4 wt% of PEGNB-DTT hydrogels degraded partially (G’/G’0 ~ 25%) after 8 days of incubation (Figure 2B), a result in agreement with our earlier work.9 To our surprise, however, hydrogels crosslinked by the PEGNBCA degraded much faster than the PEGNB counterpart (Figure 2B and S3). After further analysis of the hydrolytic degradation kinetics with a pseudo-first order degradation kinetics (G’/G’0 = exp(−khydt)),9 we obtained a hydrolysis kinetic rate constant (khyd) of 0.035 d−1 and 0.164 d−1 for PEGNB and PEGNBCA gels, respectively (Figure 2C). As the conventional thiol-norbornene hydrogels did not degrade sufficiently in the first few days, the fitting for PEGNB gel was not ideal (R2=0.42). However, the khyd value (0.035 d−1) was within the range of previous results with longer term degradation (i.e., khyd = 0.02 to 0.07 d−1).9 The R2 value for curve fitting of PEGNBCA degradation, on the other hand, reached over 0.95, indicating that the degradation of PEGNBCA gels was a result of ester bond hydrolysis.27 Compared with PEGNB-DTT hydrogels, PEGNBCA-DTT hydrogels degraded much faster and reached complete degradation within 2 weeks (data not shown). In addition to the moduli change, the degradation was also tracked by the swelling ratio (Q) (Figure S3). In particular, the swelling ratio of PEGNB-DTT and PEGNBCA-DTT hydrogels was comparable (Q~30) on day 1. However, the swelling ratio of PEGNBCA-DTT hydrogels continuously increased while that of PEGNB-DTT hydrogels remained relatively unchanged during the 8 days incubation course. The accelerated hydrolysis of PEGNBCA gels was likely due to the presence of an additional carboxylic acid group that destabilizes the ester bonds. The faster degradation kinetics of PEGNBCA hydrogels present opportunities for future applications when controllable hydrogel degradation is desired.
Figure 2. Hydrolytic degradation of PEGNB/PEGNBCA hydrogels.
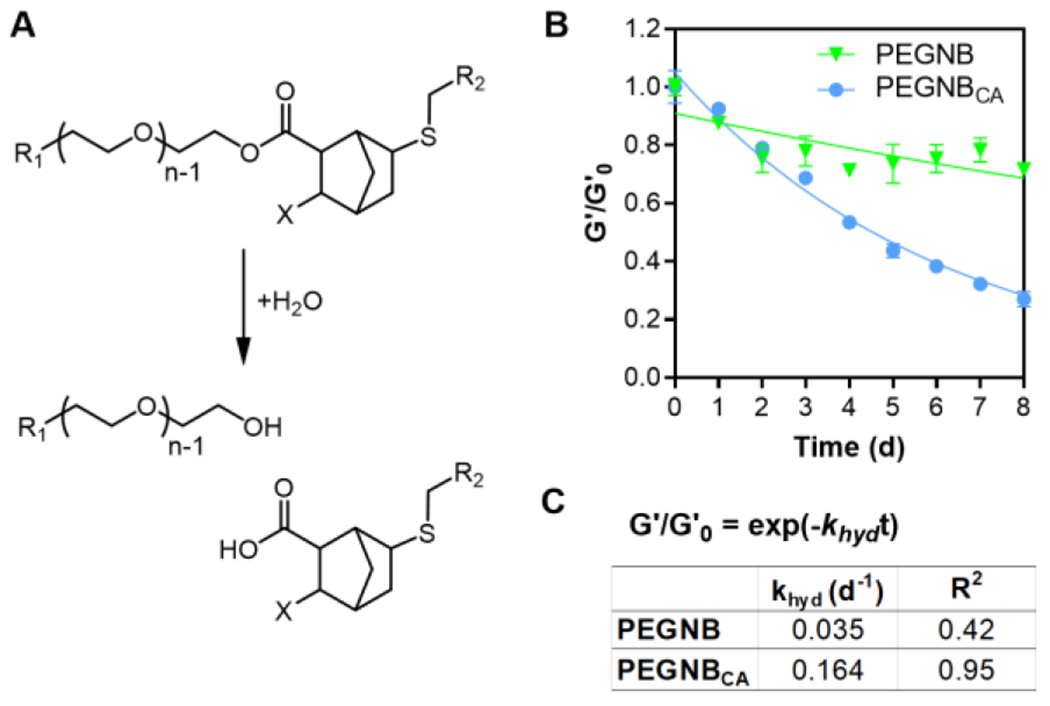
(A) Schematic of ester bond hydrolysis. (B) Characterization of hydrogel shear moduli changes (i.e., G’/G’0) as a function of time. (C) Parameters of pseudo-first order hydrolytic degradation kinetics.
PEG-based thiol-norbornene hydrogels are known for their high cytocompatibility for in situ cell encapsulation.8,12,16,28 The newly synthesized PEGNBCA hydrogels also showed excellent cytocompatibility, as exemplified by the encapsulation of human induced pluripotent stem cells (hiPSC), dental pulp stem cells (DPSC), and PANC-1 human pancreatic cancer cells (Figure 3). All hydrogels were crosslinked by matrix metalloproteinase (MMP)-sensitive peptide linker (i.e., KCGPQG*IWGQCK, *cleavage site) to permit cell-mediated matrix remodeling and immobilized with 1mM RGDS peptide to support cell survival. Live/Dead staining results showed that very limited number of dead cells were visible for all cell types following encapsulation. After 7 days of in vitro culture, hiPSC and PANC-1 cells proliferated to form multi-cellular spheroids, while DPSC exhibited spreading morphology, indicating extensive cell-mediated matrix cleavage. These results have established PEGNBCA hydrogels as an attractive alternative material for in situ encapsulation of a variety of cells.
Figure 3.
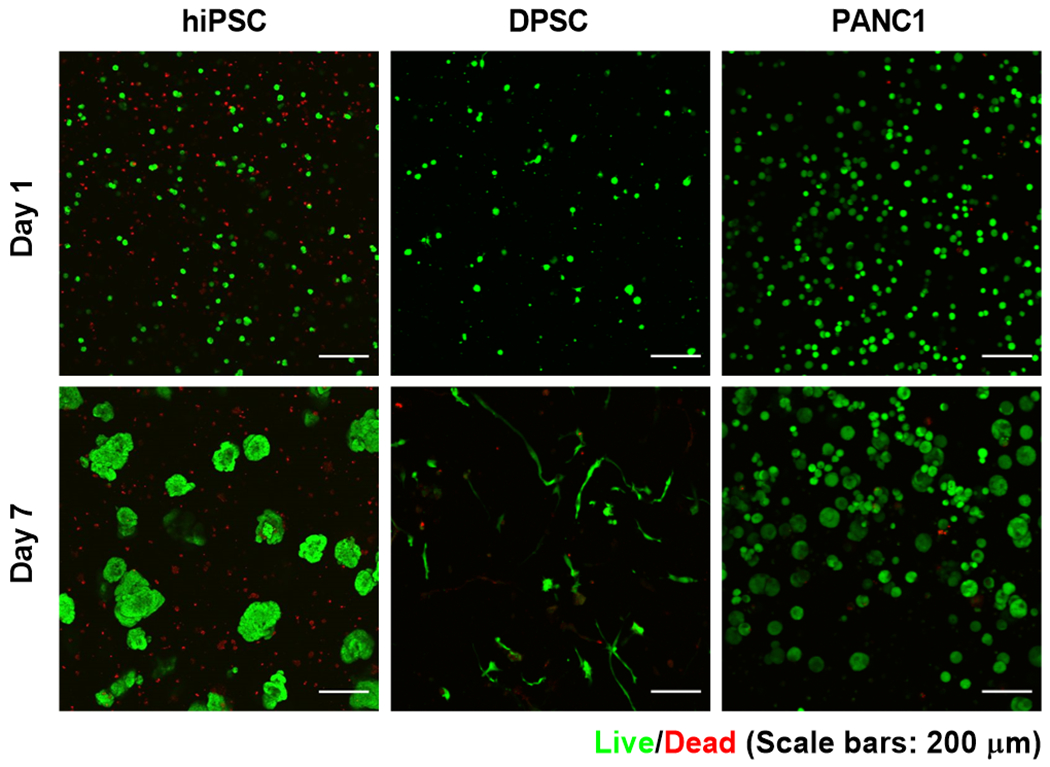
Live/dead staining and confocal imaging of cell-laden PEGNBCA-peptide hydrogels.
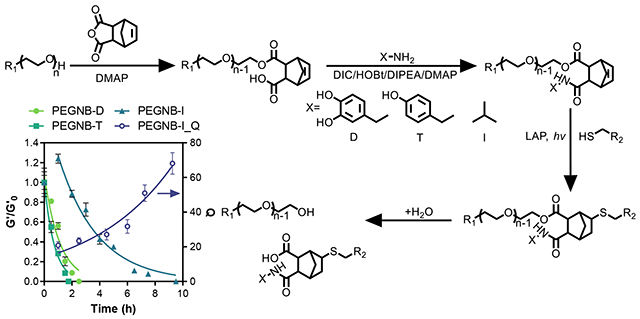
In addition to demonstrating an improvement in synthesis procedures and high cytocompatibility, the PEGNBCA affords a carboxylic acid moiety amenable for secondary conjugation with amine-bearing molecules (Figure 4A, PEGNB-X). The conversion of these X conjugates (e.g., L-Dopamine (D), Tyramine (T), or isopropylamine (I), Figure 4A) reached 83-100% depending on the reactants, as calculated from 1H NMR spectra (Figure S2) using a protocol modified from Zhang et al.29 These demonstrated X conjugates did not affect crosslinking efficiency of PEGNB-X/DTT hydrogels, as demonstrated by comparable G’0 to that of the PEGNBCA/DTT hydrogels (Figure 4B).
Figure 4. Crosslinking and degradation of PEGNB, PEGNBCA, and PEGNB-X hydrogels.
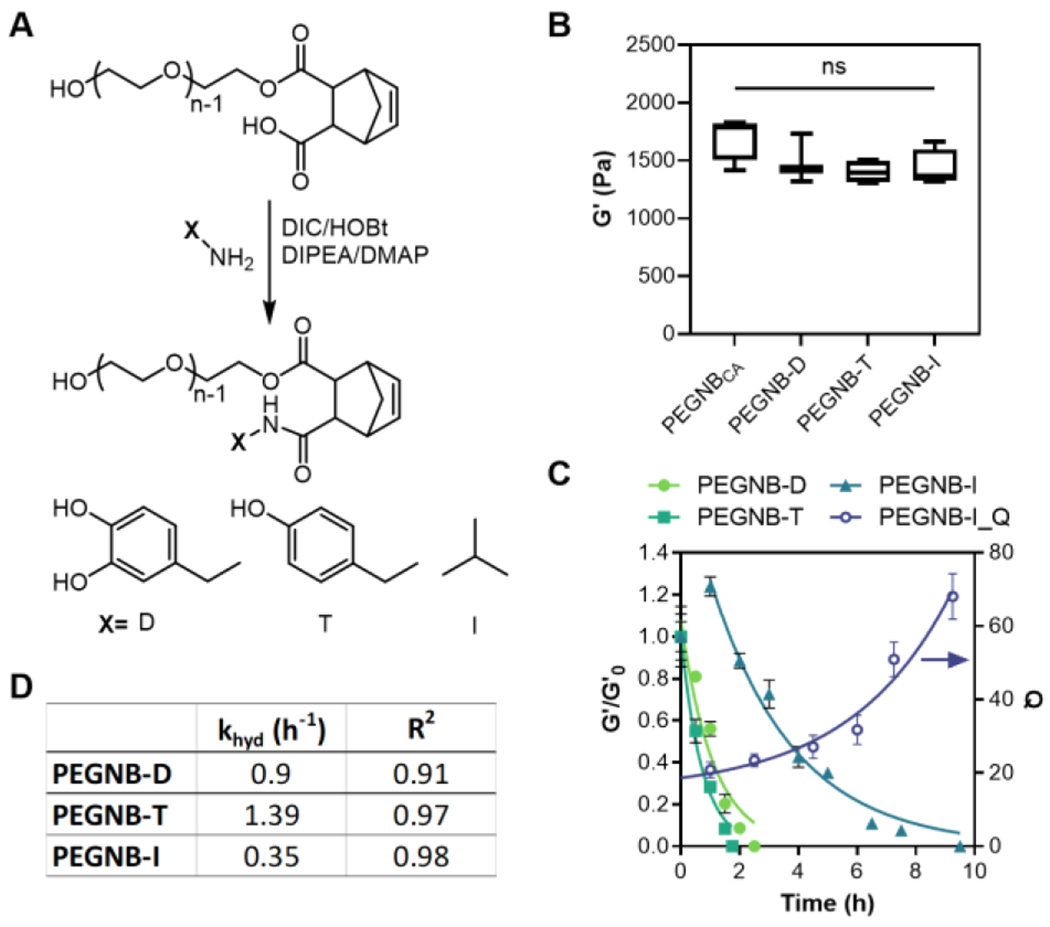
(A) Schematic of PEGNB-X synthesis. (B) Initial shear moduli of hydrogels crosslinked by 4 wt% PEGNBCA, PEGNB-D, PEGNB-T, and PEGNB-I with DTT. (C) Degradation of PEGNB-X hydrogels at 37°C by shear moduli changes (G’/G’0) and swelling ratio (Q). Curve fittings represent pseudo first order ester hydrolysis kinetics. (D) Parameters of pseudo-first order hydrolytic degradation kinetics. Al hydrogels were crosslinked by DTT (R = 1) with 1 mM LAP and 2 min 365 nm light exposure (5 mW/cm2).
We next asked if the secondary conjugation would affect the hydrolytic degradation of the hydrogels. Surprisingly, the ester linkage is further weakened by the second conjugation. After overnight swelling at 37°C, all PEGNB-X/DTT hydrogels completely degraded, suggesting that the secondary conjugation substantially decreased stability of the ester bonds. In a previous study, Fairbanks et al. reported a similar norbornene functionalized macromer (mPEG5KNorbA).30 The additional carboxylic group was further reacted with 5-norbornene-2-methylamine, yielding two norbornene groups for forming miktoarm star copolymers. However, the study did not explore the application of this macromer for hydrogel fabrication, nor did it examine hydrolysis kinetics of the ester linkages. Nevertheless, PEGNB-Xs and PEGNB-X hydrogels behaved quite differently in aqueous environment. We determined the degradation rate of PEGNB-D, PEGNB-T, and PEGNB-I hydrogels (Figure 4C). At 37°C, PEGNB-D and PEGNB-T hydrogels fully degraded within 2 h, while PEGNB-I hydrogels degraded in about 10 h (Figure 4C). It is worth noting that the three sets of gels were crosslinked to have similar initial shear moduli (G’0 ~ 1.5 kPa, Figure 4B). Hydrogels crosslinked by PEGNB-D and PEGNB-T degraded extremely fast, with khyd = 0.9 and 1.39 h−1, respectively (Figure 4D). These represented ~50 to ~200-fold faster degradation rate than that with PEGNBCA hydrogels (Figure 2C. khyd = 0.164 d−1 or 0.0068 h−1). Interestingly, compared with T or D conjugate, I conjugation slowed gel degradation considerably (Figure 4C, 4D, khyd = 0.35 h−1). Note that the first data point of PEGNB-I was neglected in the exponential fitting, since G’ slightly increased in the first hour of incubation, potentially due to intermolecular hydrophobic interactions between isopropyl moieties. Therefore, we measured the swelling ratio (Q) of these hydrogels to gain insight into their degradation behavior. There was limited increase in swelling ratio in the first 2 hours, after which the swelling increased substantially (Figure 4C). The limited initial swelling and increase in the initial G’ could be attributed to the hydrophobic interactions between isopropyl groups. Nonetheless, the degradation of PEGNB-I hydrogels could still be described by a pseudo-first order degradation kinetics (R2=0.98. Figure 4D). Furthermore, the hydrolytic degradation of PEGNB-X hydrogels appeared to be temperature-dependent and the degradation rates were reduced at room temperature (Figure S4).
Besides L-dopamine, tyramine, and isopropylamine, we also conjugated longer aliphatic amines, such as hexylamine and stearamine, as well as aromatic benzylamine to the PEGNBCA. However, the conjugation of these long or bulky hydrophobic amines were not as successful and the norbornene concentration was drastically decreased after switching the dialysis solvent from methanol to water (data not shown). As a result, hydrogel crosslinking using PEGNB-hexylamine, PEGNB-stearamine, and PEGNB-benzylamine was not feasible since the norbornene moiety on these PEGNB-X was not sufficiently high (data not shown).
The facile crosslinking and rapid degradation of the PEGNB-X hydrogels offer an opportunity to tune hydrogel degradation precisely by crosslinking with different crosslinkers and/or mixing PEGNB-X with PEGNBCA while fixing the total PEGNB content (Figure 5). It is well-know that thiol-ene hydrogel crosslinking efficiency and hydrolytic degradation kinetics are both significantly affected by the functionality and structure of the crosslinkers.31 To demonstrate this, we compared the crosslinking and degradation of PEGNB-D hydrogels crosslinked by either tetra-functional (i.e., PEG4SH) or bi-functional thiol (i.e., DTT). As expected, at an identical PEGNB-D macromer content (i.e., 4 wt%), hydrogels crosslinked by PEG4SH were considerably stiffer (G’0~14 kPa) than that formed with DTT (G’0~1.6 kPa, Figure 5A). While these two sets of gels were crosslinked with identical amount of norbornene ester bonds, the degradation kinetics were significantly different, with PEG4SH-crosslinked gels degraded roughly 3-times slower than that with DTT (Figure 5B, Table S3). These results implied that the degradation of the hydrogels was primarily governed by the bulk hydrogel properties (e.g., swelling, crosslinking density), rather than by the kinetics of ester bond hydrolysis.
Figure 5. Controlling degradation of PEGNB hydrogels via crosslinking with different crosslinkers and mixing in PEGNB-D.
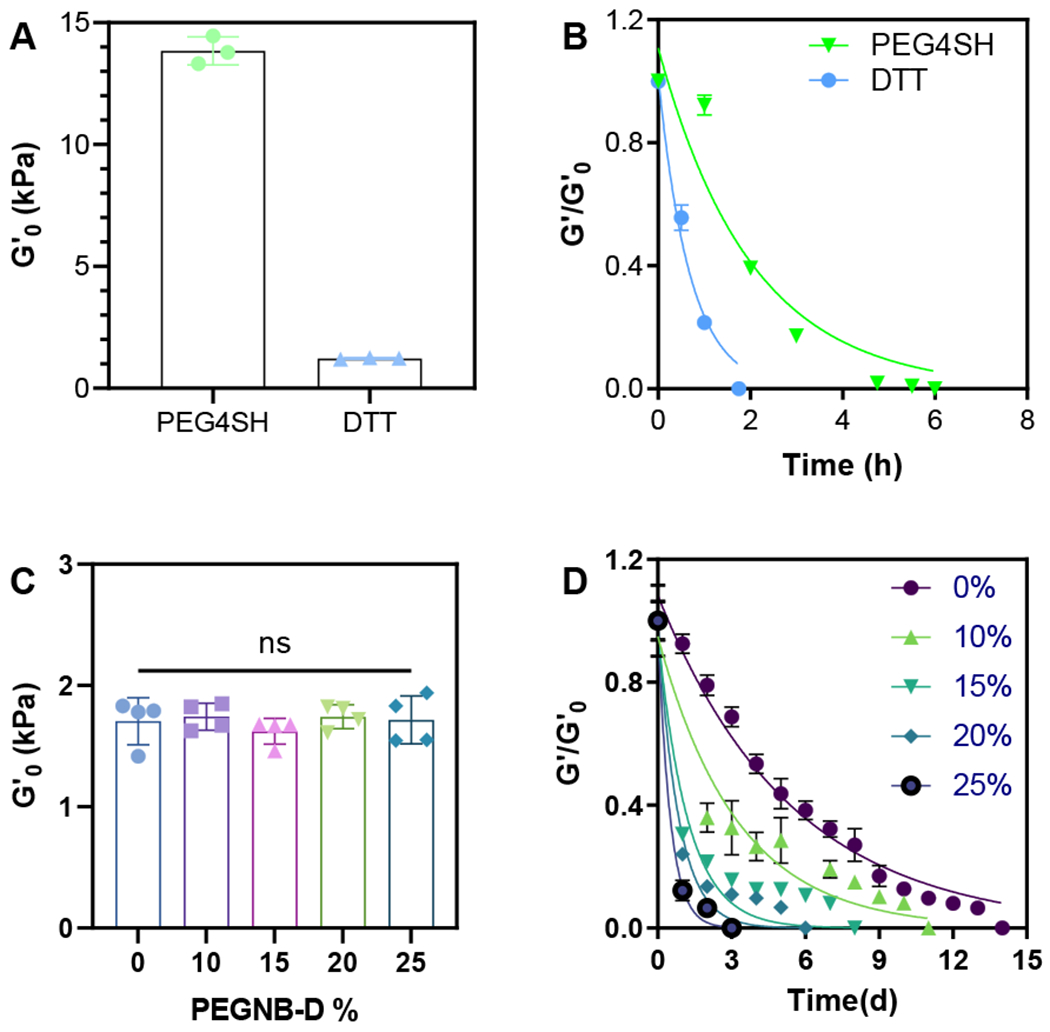
(A) Initial shear moduli and degradation (B)of 4 wt% PEGNB-D crosslinked with DTT and 4 arms-thiolated PEG (PEG4SH). (C) Initial shear moduli and (D) degradation of soft (4 wt%) PEGNBCA/PEGNB-D-DTT hydrogels with different PEGNB-D content. Curve fittings represent pseudo first order ester hydrolysis kinetics and listed in Table S3 and S4. Hydrogels were crosslinked with either DTT (R = 1) or PEG4SH (R = 1) with 1 mM LAP and 2 min 365 nm light exposure (5 mW/cm2).
In addition to tuning hydrogel degradation by varying functionality of the crosslinker, the degradation can be further regulated by mixing PEGNB-D with PEGNBCA at different ratios during hydrogel fabrication. At a fixed total PEGNB content (e.g., 4 wt% or 10 wt%), we showed that the inclusion of PEGNB-D did not alter hydrogel initial crosslinking (G’0~1.6 kPa and 17 kPa for 4 wt% (soft) and 10 wt% (stiff) hydrogels, respectively, Figure 5C and S5A). However, the degradation kinetics were decoupled from the degree of network connectivity (Figure 5D, and S5B). For soft hydrogels, the degradation period ranged from 3 to 14 days (Figure 5B). Notably, there was a significant drop in G’ early on (1-2 days) when PEGNB-D was included. For soft hydrogels incorporated with more than 25% PEGNB-D, complete gel degradation occurred overnight (data not shown). These results implied that the rapid ester hydrolysis of PEGNB-D quickly loosened the hydrogel network, leading to accelerated degradation in the nearby PEGNBCA macromer. Similar degradation was observed in stiff hydrogels. With these highly crosslinked networks, the degradation was further prolonged to 25 days (Figure S5B). Interestingly, stiff hydrogels still completed degradation overnight when PEGNB-D was higher than 50%. During this extended incubation, hydrogels notably swelled and discolored, likely due to oxidation of the residual dopamine (Figure S6). The cytocompatibility of soft PEGNBCA/PEGNB-D hydrogels at different PEGNB-D and PEGNBCA ratios were further demonstrated by encapsulation of PANC-1 cells (Figure S7 and S8). It is worth noting the hydrogels with higher than 25% PEGNB-D degraded overnight following encapsulation. Hence, cell viability staining was performed right after the encapsulation. For 25% PEGNB-D hydrogels, the cell viability was tracked over 3 days as the gels degraded in a slower rate. In all conditions, PEGNB-D hydrogels demonstrated exceptional cytocompatibility. Overall, this study established that it is possible to decouple the initial hydrogel moduli with their degradation rate, a unique feature not easily achievable by conventional covalent hydrogels.
In summary, we present a robust and user-friendly protocol to synthesize norbornene-functionalized PEG via using carbic anhydride. The method eliminates the use of extremely pungent odor of norbornene acid. The additional carboxylic group of PEGNBCA was further conjugated with amino-moieties, giving rise to PEGNB-X macromers. PEGNBCA and PEGNB-X were readily crosslinked by dithiols into thiol-norbornene hydrogels liable to accelerated yet tunable hydrolytic degradation. The facile synthesis and crosslinking, excellent cytocompatibility, and fast yet highly tunable degradation of PEGNBCA and PEGNB-X hydrogels provide opportunities in future tissue engineering applications. Furthermore, these hydrogels preserve all benefits of thiol-norbornene photo-gelation, including the spatiotemporally tunable crosslinking. This property has the potential in advanced biofabrication since the highly cytocompatible PEGNB-X hydrogels can be exploited as sacrificial hydrogels to control cell distribution by means of microfluidics and 3D bioprinting.
Supplementary Material
ACKNOWLEDGMENT
This project was supported by the National Cancer Institute of the National Institutes of Health (R01CA227737). The authors thank Mr. Matthew Arkenberg for assistance in encapsulation of iPSCs and Dr. Chandler Walker for providing DSPCs.
Footnotes
SUPPORTING INFORMATION
This material is available free of charge via the Internet at http://pubs.acs.org.
Experimental section, norbornene substitution ratio quantification, and PEGNB-R 1H NMR spectra
REFERENCES
- (1).Bjugstad KB; Lampe K; Kern DS; Mahoney M Biocompatibility of Poly(Ethylene Glycol)-Based Hydrogels in the Brain: An Analysis of the Glial Response across Space and Time. J. Biomed. Mater. Res. - Part A 2010, 95 (1), 79–91. [DOI] [PubMed] [Google Scholar]
- (2).Zhu J Bioactive Modification of Poly(Ethylene Glycol) Hydrogels for Tissue Engineering. Biomaterials 2010, 31 (17), 4639–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Zustiak SP; Leach JB Hydrolytically Degradable Poly(Ethylene Glycol) Hydrogel Scaffolds with Tunable Degradation and Mechanical Properties. Biomacromolecules 2010, 11 (5), 1348–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Peppas NA; Keys KB; Torres-Lugo M; Lowman AM Poly(Ethylene Glycol)-Containing Hydrogels in Drug Delivery. J. Control. Release 1999, 62 (1–2), 81–87. [DOI] [PubMed] [Google Scholar]
- (5).Tavakoli J; Tang Y Hydrogel Based Sensors for Biomedical Applications: An Updated Review. Polymers (Basel). 2017, 9 (8), 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Bryant SJ; Anseth KS Controlling the Spatial Distribution of ECM Components in Degradable PEG Hydrogels for Tissue Engineering Cartilage. J. Biomed. Mater. Res. Part A 2002, 64, 70–79. [DOI] [PubMed] [Google Scholar]
- (7).Kroger SM; Hill L; Jain E; Stock A; Bracher PJ; He F; Zustiak SP Design of Hydrolytically Degradable Polyethylene Glycol Crosslinkers for Facile Control of Hydrogel Degradation. Macromol. Biosci 2020, 20 (10), 1–13. [DOI] [PubMed] [Google Scholar]
- (8).Lin CC; Ki CS; Shih H Thiol-Norbornene Photoclick Hydrogels for Tissue Engineering Applications. J. Appl. Polym. Sci 2015, 132 (8), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Shih H; Lin CC Cross-Linking and Degradation of Step-Growth Hydrogels Formed by Thiol-Ene Photoclick Chemistry. Biomacromolecules 2012, 13 (7), 2003–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Fairbanks BD; Schwartz MP; Halevi AE; Nuttelman CR; Bowman CN; Anseth KS A Versatile Synthetic Extracellular Matrix Mimic via Thiol-Norbornene Photopolymerization. Adv. Mater 2009, 21 (48), 5005–5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Lin CC; Raza A; Shih H PEG Hydrogels Formed by Thiol-Ene Photo-Click Chemistry and Their Effect on the Formation and Recovery of Insulin-Secreting Cell Spheroids. Biomaterials 2011, 32 (36), 9685–9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Anderson SB; Lin CC; Kuntzler DV; Anseth KS The Performance of Human Mesenchymal Stem Cells Encapsulated in Cell-Degradable Polymer-Peptide Hydrogels. Biomaterials 2011, 32 (14), 3564–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Shih H; Lin C Visible-Light-Mediated Thiol-Ene Hydrogelation Using Eosin-Y as the Only Photoinitiator. Macromol. Rapid Commun 2013, 34, 269–273. [DOI] [PubMed] [Google Scholar]
- (14).Shih H; Greene T; Korc M; Lin CC Modular and Adaptable Tumor Niche Prepared from Visible Light Initiated Thiol-Norbornene Photopolymerization. Biomacromolecules 2016, 17 (12), 3872–3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Nguyen HD; Liu HY; Hudson BN; Lin CC Enzymatic Cross-Linking of Dynamic Thiol-Norbornene Click Hydrogels. ACS Biomater. Sci. Eng 2019, 5 (3), 1247–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Lin CC; Raza A; Shih H PEG Hydrogels Formed by Thiol-Ene Photo-Click Chemistry and Their Effect on the Formation and Recovery of Insulin-Secreting Cell Spheroids. Biomaterials 2011, 32 (36), 9685–9695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Brown A; He H; Trumper E; Valdez J; Hammond P; Griffith LG Engineering PEG-Based Hydrogels to Foster Efficient Endothelial Network Formation in Free-Swelling and Confined Microenvironments. Biomaterials 2020, 243, 119921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Liu HY; Nguyen HD; Lin CC Dynamic PEG-Peptide Hydrogels via Visible Light and FMN-Induced Tyrosine Dimerization. Adv. Healthc. Mater 2018, 7 (22), e1800954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Ki CS; Lin TY; Korc M; Lin CC Thiol-Ene Hydrogels as Desmoplasia-Mimetic Matrices for Modeling Pancreatic Cancer Cell Growth, Invasion, and Drug Resistance. Biomaterials 2014, 35 (36), 9668–9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Lin TY; Ki CS; Lin CC Manipulating Hepatocellular Carcinoma Cell Fate in Orthogonally Cross-Linked Hydrogels. Biomaterials 2014, 35 (25), 6898–6906. [DOI] [PubMed] [Google Scholar]
- (21).Raza A; Lin CC The Influence of Matrix Degradation and Functionality on Cell Survival and Morphogenesis in PEG-Based Hydrogels. Macromol. Biosci 2013, 13 (8), 1048–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Neises B; Steglich W Simple Method for the Esterification of Carboxylic Acids. Angew. Chemie Int. Ed. English 1978, 17 (7), 522–524. [Google Scholar]
- (23).Liu HY; Korc M; Lin CC Biomimetic and Enzyme-Responsive Dynamic Hydrogels for Studying Cell-Matrix Interactions in Pancreatic Ductal Adenocarcinoma. Biomaterials 2018, 160, 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Mũnoz Z; Shih H; Lin CC Gelatin Hydrogels Formed by Orthogonal Thiol-Norbornene Photochemistry for Cell Encapsulation. Biomater. Sci 2014, 2 (8), 1063–1072. [DOI] [PubMed] [Google Scholar]
- (25).Sakakura A; Kawajiri K; Ohkubo T; Kosugi Y; Ishihara K Widely Useful DMAP-Catalyzed Esterification under Auxiliary Base- and Solvent-Free Conditions. J. Am. Chem. Soc 2007, 129 (47), 14775–14779. [DOI] [PubMed] [Google Scholar]
- (26).Colak B; Wu L; Cozens EJ; Gautrot JE Modulation of Thiol–Ene Coupling by the Molecular Environment of Polymer Backbones for Hydrogel Formation and Cell Encapsulation. ACS Appl. Bio Mater 2020, 3, 6497–6509. [DOI] [PubMed] [Google Scholar]
- (27).Metters A; Hubbell J Network Formation and Degradation Behavior of Hydrogels Formed by Michael-Type Addition Reactions. Biomacromolecules 2004, 6 (1), 290–301. [DOI] [PubMed] [Google Scholar]
- (28).Arkenberg MR; Dimmitt NH; Johnson HC; Koehler KR; Lin CC Dynamic Click Hydrogels for Xeno-Free Culture of Induced Pluripotent Stem Cells. Adv. Biosyst 2020, 2000129, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Zhang K; Wei Z; Xu X; Feng Q; Xu J; Bian L Efficient Catechol Functionalization of Biopolymeric Hydrogels for Effective Multiscale Bioadhesion. Mater. Sci. Eng. C 2019, 103, 109835. [DOI] [PubMed] [Google Scholar]
- (30).Fairbanks BD; Love DM; Bowman CN Efficient Polymer-Polymer Conjugation via Thiol-Ene Click Reaction. Macromol. Chem. Phys 2017, 218 (18), 1700073. [Google Scholar]
- (31).Metters A; Hubbell J Network Formation and Degradation Behavior of Hydrogels Formed by Michael-Type Addition Reactions. Biomacromolecules 2005, 6 (1), 290–301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


